-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Inter-pregnancy Weight Change and Risks of Severe Birth-Asphyxia-Related Outcomes in Singleton Infants Born at Term: A Nationwide Swedish Cohort Study
Using data from the Swedish Medical Birth Register, Martina Persson and colleagues examine the associations between inter-pregnancy weight change and risks of severe birth asphyxia-related outcomes in singleton infants born at term.
Published in the journal: Inter-pregnancy Weight Change and Risks of Severe Birth-Asphyxia-Related Outcomes in Singleton Infants Born at Term: A Nationwide Swedish Cohort Study. PLoS Med 13(6): e32767. doi:10.1371/journal.pmed.1002033
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002033Summary
Using data from the Swedish Medical Birth Register, Martina Persson and colleagues examine the associations between inter-pregnancy weight change and risks of severe birth asphyxia-related outcomes in singleton infants born at term.
Introduction
Maternal overweight and obesity during pregnancy increase the risks of severe maternal and infant complications [1–4]. In Sweden, the proportion of women with overweight and obesity (body mass index [BMI] ≥ 25 kg/m2) in early pregnancy increased from 26% in 1992 to 38% in 2010 [5]. In the US, 58% of women between 20 and 39 y of age were overweight or obese in 2011–2012 [6]. As recently stated by WHO, the prevalence of maternal obesity must be reduced in order to improve maternal, fetal, and neonatal health [7].
Maternal overweight and obesity increase the risks of severe neonatal complications, including major malformations, preterm birth, neonatal morbidities, and low Apgar score (0–6) [3,8–11]. In term non-malformed infants, low Apgar score is commonly caused by birth asphyxia [12].
We have previously demonstrated a linear relationship between maternal BMI in early pregnancy and the risks of low Apgar scores at 5 and 10 min and birth-asphyxia-related neonatal morbidity in infants born at term [8]. Furthermore, we have found that the risk of asphyxia-related infant mortality in term infants increases with the degree of maternal obesity [5]. Risks being influenced by a change of exposure (i.e., maternal BMI) over time would be consistent with a causal relationship between maternal BMI and adverse outcomes of the offspring.
In this nationwide Swedish cohort study, we examined whether changes in maternal BMI between first and second pregnancies influenced the risks of birth-asphyxia-related outcomes in the second-born offspring, including low Apgar score (0–6) at 5 min, neonatal seizures, and meconium aspiration.
Methods
Study Design and Population
Ethics approval for this study was obtained from the Research Ethics Committee at Karolinska Institutet in Stockholm, Sweden (number 2012/4 : 9). Informed consent was not required as all data were anonymous.
Between January 1992 and December 2012, the nationwide Swedish Medical Birth Register included 533,535 mothers with first and second live singleton term births (≥37 completed weeks). We excluded 165 mothers with no information on inter-pregnancy interval and 512 mothers with no information on mothers’ country of birth. In analyses of low Apgar score in the second infant, we excluded another 6,423 mothers (1.2%) where either the first - or the second-born infant had missing information on Apgar score at 1 or 5 min.
The Swedish Medical Birth Register started in 1973 and contains prospectively collected data on more than 98% of all births in Sweden. The quality of data is considered high [13]. Information on sociodemographic factors, maternal and infant anthropometry, and Apgar scores are collected on standardized forms used in antenatal, obstetric, and neonatal care in Sweden. Maternal and neonatal diagnoses, including pregnancy complications and neonatal morbidities, are classified by physicians according the Swedish version of the International Classification of Diseases (ICD). The ninth version (ICD-9) was used between 1992 and 1996, and the tenth version (ICD-10) thereafter. The standardized forms are forwarded to the Swedish Medical Birth Register when the mother and infant are discharged from hospital. Information on the mother’s country of birth and level of education were obtained from the Swedish Register of Total Population and the Swedish Register of Education, respectively. Individual cross-linkage of registries was possible using the personal identification number [14], a person-unique identifier assigned to all Swedish citizens at birth or at naturalization.
Exposures
Maternal BMI (kg/m2) was calculated for each of the two consecutive pregnancies. At the first antenatal visit, which occurs in the first trimester in 90% of pregnancies [13], the woman´s weight is measured wearing light indoor clothes and barefoot, and data on self-reported height are recorded. Inter-pregnancy change in BMI was calculated as the difference in BMI between the second and first pregnancies. We categorized the inter-pregnancy BMI change as <−2 kg/m2 (i.e., BMI loss greater than 2 kg/m2), −2 to <−1 kg/m2, −1 to <1 kg/m2 (stable weight), 1 to <2 kg/m2, 2 to <4, and ≥4 kg/m2. A 1-kg/m2 change in BMI corresponds to change of 2.8 kg in a woman of average height (167 cm). Based on BMI in the first pregnancy, mothers were categorized as underweight (BMI < 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), obese grade I (30–34.9 kg/m2), and obese grade II-III (≥35 kg/m2) [15].
The inter-pregnancy interval was calculated as the time difference between the date of birth of the first infant and estimated date of conception of the second infant (i.e., date of birth of the second infant minus gestational age + 14 d). Gestational age was estimated primarily based on early second trimester ultrasound, which is offered to all pregnant women and which 95% of women accept [16]. When dating from an ultrasonic scan was not available, gestational age was estimated using information on date of last menstrual period. Obesity-related disorders were identified in the Swedish Medical Birth Register based on ICD codes: preeclampsia—ICD-9 codes 642E–642G, ICD-10 codes O14–O15; chronic hypertension—ICD-9 codes 401–405, 642C, 642M, ICD-10 codes O10–O11; gestational diabetes—ICD-9 code 648W, ICD-10 code O244; pregestational diabetes—ICD-9 code 250, ICD-10 codes E10–E14, O241–O243. Covariates were categorized according to Table 1.
Tab. 1. Maternal characteristics and rates of low Apgar scores (0–6), meconium aspiration, and neonatal seizures: live-born singleton second term infants of women in Sweden 1992–2012. 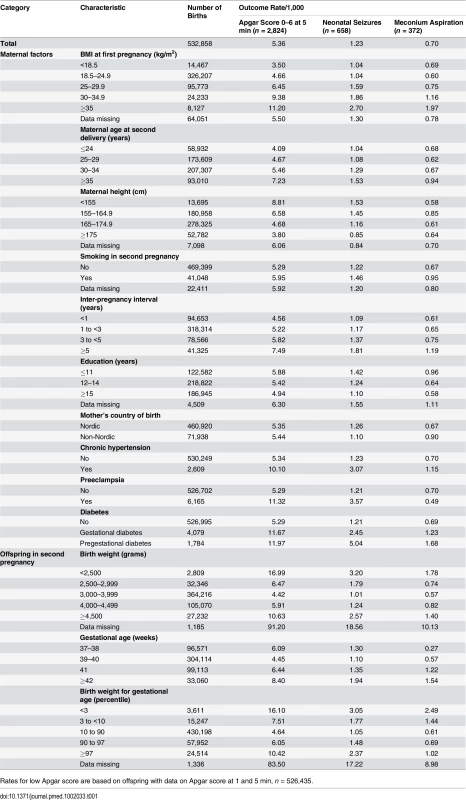
Rates for low Apgar score are based on offspring with data on Apgar score at 1 and 5 min, n = 526,435. Outcomes
We estimated the risks of severe asphyxia-related outcomes, including low Apgar score, meconium aspiration, and neonatal seizures, at the second birth by change in BMI from the first to the second pregnancy. A low Apgar score was defined as a score between 0 and 6 points at 5 min after birth. A diagnosis of neonatal seizures was based on ICD-9 code 779.0 or ICD-10 code P90, and a diagnosis of meconium aspiration was based on ICD-9 code 770.1 or ICD-10 code P24.0.
Statistical Analyses
Rates of low Apgar score, meconium aspiration, and neonatal seizures were calculated as the number of infants with these outcomes per 1,000 births. Logistic regression analyses were used to calculate odds ratios (ORs) with 95% confidence intervals for all outcomes. Mothers whose first infant had a low Apgar score, meconium aspiration, or neonatal seizures were excluded from the analysis of the respective outcome in the second pregnancy to avoid bias in case of a tendency to repeat adverse pregnancy outcomes. Inter-pregnancy weight change was categorized as presented above in the regression model, but also treated as a continuous variable. Multivariate models were restricted to second births with complete data on inter-pregnancy weight change and covariates. We adjusted for maternal BMI in the first pregnancy, maternal height, maternal age at second delivery, smoking habits in the second pregnancy, inter-pregnancy interval, mother´s education, mother’s country of birth, and year of second birth, categorized according to Table 1. Given the long study period, spanning over 21 y, we categorized year of second birth (not shown in Table 1) into intervals as 1992–1996, 1997–2001, 2002–2006, and 2007–2012.
In sensitivity analyses (S1 Table), mothers with obesity-related disorders (chronic hypertension, preeclampsia, or any type of diabetes) were excluded from the regression analyses. A sensitivity analysis was also performed to explore whether pregnancies of mothers with missing data on inter-pregnancy weight change differed from those with information on inter-pregnancy weight change (S2 Table). To explore any effect modification by first pregnancy BMI on the association between exposure and outcomes, analyses were also stratified by maternal BMI in the first pregnancy (BMI < 25 or BMI ≥ 25 kg/m2). Interaction terms were introduced in the multivariate models, and a p-value of less than 0.05 for the interaction term was considered statistically significant.
Results
The total number of infants with a low Apgar score (0–6 points) at 5 min was 2,824 (rate 4.08/1,000). Corresponding numbers (rates) for neonatal seizures and meconium aspiration were 658 (1.23/1,000) and 372 (0.70/1,000), respectively (Table 1). Rates of all birth-asphyxia-related outcomes increased with maternal BMI in the first pregnancy, inter-pregnancy interval, smoking, chronic hypertension, preeclampsia, any type of diabetes, and generally also with maternal age at second delivery (Table 1). Maternal education was inversely correlated with all outcomes. There was a U-shaped relationship between birth weight and gestational age and rates of low Apgar score and neonatal seizures, while the rate of meconium aspiration increased with gestational age. Rates of low Apgar score, neonatal seizures, and meconium aspiration in second-born offspring were essentially similar in offspring of mothers with missing data on BMI in the first pregnancy and the total population.
Rates of low Apgar score, neonatal seizures, and meconium aspiration increased with inter-pregnancy weight gain (Table 2). Compared with mothers with stable weight, the risk of a low Apgar score was 26% increased in offspring of mothers who gained 2 to <4 kg/m2 and 33% increased in offspring of mothers who gained ≥4 kg/m2. Risk of neonatal seizures was increased by more than 40% in offspring of mothers who gained 2 to <4 and ≥4 kg/m2. The risk of meconium aspiration was 78% higher in offspring of mothers who gained ≥4 kg/m2 compared with offspring of mothers with stable weight.
Tab. 2. Maternal inter-pregnancy weight change and risks of low Apgar score (0–6) at 5 min, neonatal seizures, and meconium aspiration: live singleton second term infants of women in Sweden 1992–2012. 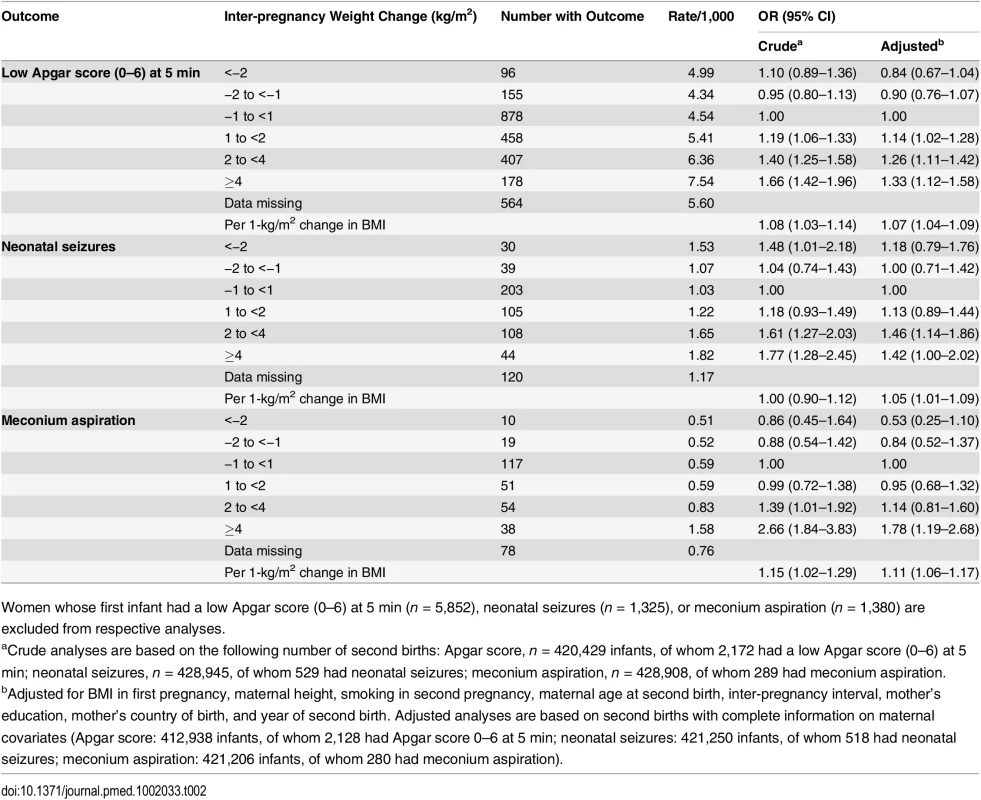
Women whose first infant had a low Apgar score (0–6) at 5 min (n = 5,852), neonatal seizures (n = 1,325), or meconium aspiration (n = 1,380) are excluded from respective analyses. We investigated whether the effect of weight change on asphyxia-related outcomes differed between offspring of mothers who were underweight/normal weight and overweight/obese in the first pregnancy (BMI <25 and ≥25 kg/m2, respectively) (Table 3). In underweight/normal weight mothers in the first pregnancy, the risk of low Apgar score increased with weight gain, while the corresponding association was less evident in infants of mothers who were overweight/obese in the first pregnancy. However, the test for an interaction between maternal BMI and low Apgar was not significant (p = 0.12).
Tab. 3. Maternal inter-pregnancy weight change and risk of low Apgar score (0–6) at 5 min, neonatal seizures, and meconium aspiration, stratified by maternal BMI in first pregnancy: live singleton second term infants of women in Sweden 1992–2012. 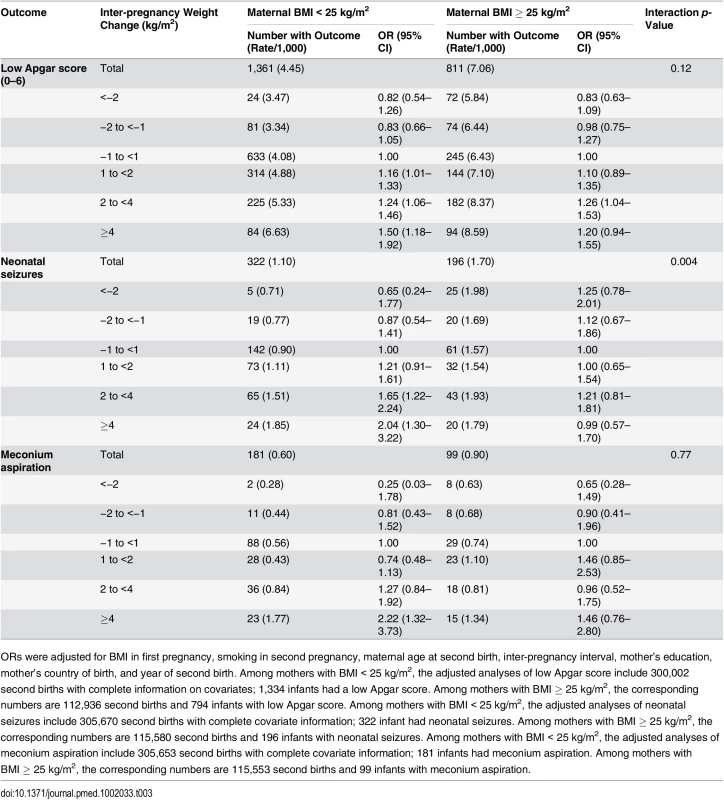
ORs were adjusted for BMI in first pregnancy, smoking in second pregnancy, maternal age at second birth, inter-pregnancy interval, mother’s education, mother’s country of birth, and year of second birth. Among mothers with BMI < 25 kg/m2, the adjusted analyses of low Apgar score include 300,002 second births with complete information on covariates; 1,334 infants had a low Apgar score. Among mothers with BMI ≥ 25 kg/m2, the corresponding numbers are 112,936 second births and 794 infants with low Apgar score. Among mothers with BMI < 25 kg/m2, the adjusted analyses of neonatal seizures include 305,670 second births with complete covariate information; 322 infant had neonatal seizures. Among mothers with BMI ≥ 25 kg/m2, the corresponding numbers are 115,580 second births and 196 infants with neonatal seizures. Among mothers with BMI < 25 kg/m2, the adjusted analyses of meconium aspiration include 305,653 second births with complete covariate information; 181 infants had meconium aspiration. Among mothers with BMI ≥ 25 kg/m2, the corresponding numbers are 115,553 second births and 99 infants with meconium aspiration. The risk of neonatal seizures increased with weight gain in offspring of mothers who were underweight/normal weight in the first pregnancy, but not in offspring of mothers who were overweight/obese in the first pregnancy (test for interaction; p = 0.004). In underweight/normal weight mothers, offspring of mothers with a weight gain of ≥4 kg/m2 had a doubled risk of neonatal seizures compared with offspring of mothers with stable BMI.
Stratifying the analyses by maternal BMI in the first pregnancy (<25 and ≥25 kg/m2) demonstrated a more than doubled risk of meconium aspiration in offspring of underweight/normal weight mothers who gained ≥4 kg/m2. In contrast, in overweight/obese mothers, weight gain was not associated with increased risk of meconium aspiration in offspring. However, the test for interaction was not significant (p = 0.77).
In order to investigate whether associations between maternal weight gain and asphyxia-related outcomes were influenced by obesity-related disorders, we repeated the analyses after excluding offspring of mothers with chronic hypertension, preeclampsia, or any type of diabetes. Restricting the analyses to offspring of mothers without obesity-related disorders did not change the risks of low Apgar score, neonatal seizures, or meconium aspiration (S1 Table).
We also investigated weight gain and the risks of birth-asphyxia-related outcomes in the offspring of mothers who were underweight/normal weight (BMI below 25 kg/m2) in the second pregnancy (S2 Table). Compared with the offspring of mothers with stable weight, the risks of low Apgar score and neonatal seizures were more than doubled in offspring of mothers who were normal weight in the second pregnancy but who had gained ≥4 kg/m2 between pregnancies.
Finally, we investigated whether mothers with missing information on inter-pregnancy weight gain, and hence not included in the analyses, differed from mothers with information on weight gain with respect to other maternal characteristics or birth outcomes. The proportions of women who were overweight, obesity grade I, and obesity grade II-III in the first pregnancy were 20.5%, 5.2%, and 1.7%, respectively, for mothers with known weight change and 19.3%, 4.9%, and 1.9%, respectively, for mothers with unknown weight change (due to missing data on second pregnancy BMI) (S3 Table). Also, the distributions of maternal covariates (as listed in Table 1) were comparable between mothers with missing data on inter-pregnancy weight change and mothers with an inter-pregnancy weight change of −1 and <1 kg/m2 (the reference group) or an inter-pregnancy weight gain of 1 to <2 kg/m2. The proportions of mothers with obesity-related diseases and rates of birth-asphyxia-related outcomes were similar in mothers with and without data on inter-pregnancy weight change (S3 Table).The proportions of women who were overweight, obesity grade I, and obesity grade II-III in the second pregnancy were 25.3%, 7.6%, and 1.4%, respectively, for mothers with information on inter-pregnancy weight change and 24.2%, 7.1%, and 1.4%, respectively, for mothers with unknown weight change (due to missing data on first pregnancy BMI).
Discussion
Principal Findings
In this population-based cohort study we found that the risks of severe birth-asphyxia-related outcomes in the second offspring born at term increased with inter-pregnancy maternal weight gain. The risk increases were primarily found in offspring of mothers with BMI < 25 kg/m2 in the first pregnancy. The observed increments in risks of low Apgar score, neonatal seizures, and meconium aspiration remained essentially the same after exclusion of offspring of mothers with obesity-related diseases.
Findings in Comparison with Other Studies
Data from epidemiological studies support that maternal BMI and changes in maternal BMI influence the risks of maternal complications, preterm delivery, and infant mortality [8–11,17–23], We have previously reported that the risks of severe birth-asphyxia-related complications increase with maternal overweight and obesity [8]. To our knowledge, this is the first study to assess whether these risks are influenced by changes of exposure (i.e., change in weight) over time.
The pathophysiology underlying the associations between maternal overweight/obesity and birth-asphyxia-related outcomes in offspring is likely to be complex and multifactorial. Obesity in pregnant women is accompanied by inflammation in maternal and placental tissues, impaired microvascular function, oxidative stress, and marked insulin resistance [24,25], changes that may contribute to the increased risk of birth asphyxia and other complications. It has also been proposed that an altered gut microbiota in obese women adversely influences maternal metabolism, which in turn may affect fetal health [26].
Fetal macrosomia is the most prevalent complication in pregnancies with maternal obesity [4]. There is a linear association between maternal BMI and fetal macrosomia and between maternal BMI and measures of hyperinsulinemia in cord blood [27], independent of maternal glucose values. Results from both experimental and clinical studies strongly suggest that fetal hyperinsulinemia is a risk factor for fetal hypoxia [28–31]. Thus, it is possible that fetal hyperinsulinemia is of pathophysiological importance for the increased risk of birth asphyxia in pregnancies with maternal obesity. Fetal macrosomia also increases the risks of traumatic delivery and shoulder dystocia, which increase the risk of birth asphyxia [32]. Obesity-related disorders, including chronic hypertension, preeclampsia, and diabetic diseases, are associated with increased risks of fetal hypoxia and low Apgar score [33,34]. However, excluding offspring of mothers with obesity-related diseases did not substantially change the weight-gain-related risks of asphyxia-related outcomes in our study.
Compared with offspring of normal weight mothers, offspring of overweight and obese mothers are at increased risks of birth-asphyxia-related neonatal outcomes [8]. However, increments in risks associated with inter-pregnancy weight gain were primarily restricted to offspring of mothers with BMI < 25 kg/m2 in the first pregnancy. The same pattern has also been demonstrated for complications during pregnancy [17]. Interestingly, in offspring of mothers with BMI < 25 kg/m2 in the second pregnancy, risks of low Apgar score and neonatal seizures were more than doubled in offspring of mothers who gained ≥4 kg/m2 between pregnancies (S2 Table). One could speculate that the less pronounced effect of weight gain in women with established overweight/obesity may reflect a metabolic adaption over time to an increased fat mass. Furthermore, women with overweight or obesity in early pregnancy accumulate less fat in pregnancy than underweight/normal weight women [25]. Thus, the same absolute increase in weight would reflect a larger relative increase in fat mass in women of underweight/normal weight compared with women who already were overweight. It is also possible that the distribution of added fat mass between pregnancies differs between lean women and overweight women, with a relatively larger increment in visceral fat in lean than in overweight women. However, rates of birth-asphyxia-related outcomes also increased with weight gain in offspring among women who were overweight or obese in the first pregnancy. Thus, the statistical power to detect risk increments could have been insufficient.
Strengths and Limitations of the Present Study
The primary strengths of our study are the population-based design, including a large number of births, and the prospectively recorded data on exposures and outcomes. The large cohort enabled us to investigate the impact of a range of inter-pregnancy weight change categories on the risks of neonatal outcomes related to birth asphyxia. The prospectively collected data limited the risks of selection and information bias. We were also able to analyze these risks stratified by maternal BMI in the first pregnancy and to adjust for several potential confounders. We used maternal BMI as a proxy for maternal fat mass. This assumption is justified as there is a strong correlation (r2 = 0.84) between BMI and fat mass in early pregnancy [35].
Some limitations of the present study should be noted. In the present study, inter-pregnancy weight change was calculated as the difference in early pregnancy BMI between the two first consecutive pregnancies. We did not have information on gestational weight gain and do not know when the weight gain occurred. The distribution of fat may differ if weight is gained during or after pregnancy. Longitudinal studies demonstrate that women with high gestational weight gain are likely to retain this weight into subsequent pregnancies [36] and that high weight gain in the first pregnancy is an important risk factor for future overweight and obesity [37].
In spite of having data on many key confounders, we cannot rule out the possibility of residual confounding by unmeasured maternal factors driving the relationship between inter-pregnancy weight change and risk of birth asphyxia. For example, women who gain weight between pregnancies may also have a less healthy life style in other aspects than women with stable weight. We also lacked specific information on obstetric interventions and neonatal resuscitation efforts. Therefore, the impact of these factors on the risk of birth asphyxia could not be investigated.
We studied neonatal conditions related to birth asphyxia. Severe birth asphyxia is commonly defined as an Apgar score of 0–3 at 5 min in combination with cord blood acidosis and neurological symptoms like neonatal seizures [38]. However, in epidemiological studies, a frequently used definition of birth asphyxia is an Apgar score of 0–6 at 5 min [39,40]. The validity of this definition is supported by the fact that an infant with an Apgar score of 4–6 at 5 min has a 45 times higher risk of neonatal death and a 31 times higher risk of cerebral palsy compared to infants with an Apgar score of 7–10 at 5 min [41,42]. We studied only infants born at term, as preterm birth itself is a common reason for low Apgar score [43]. Maternal BMI in early pregnancy also influences risk of preterm birth [10]. In addition, the possibility of selection bias should be considered given that the analyses were restricted to offspring of mothers with two children.
Information on inter-pregnancy weight change was missing in 19% of the study population. If pregnancies with information on inter-pregnancy weight change differ from pregnancies without this information, results could be biased. However, the distributions of maternal covariates, overweight, and obesity were similar in the first and second pregnancies of women with and without information on inter-pregnancy weight change, and rates of asphyxia-related outcomes were also similar.
Summary
Given the high prevalence of maternal overweight and the possible long-term consequences of birth asphyxia, our results have substantial public health relevance, as even modest weight increases in normal weight women may impact offspring outcomes on a population level. However, our finding that inter-pregnancy weight gain influences the risks of birth-asphyxia-related outcomes should be confirmed in other populations. Encouraging women to normalize BMI before pregnancy and to avoid weight gain between pregnancies is likely to be an important measure to improve infant health.
Supporting Information
Zdroje
1. Heslehurst N, Simpson H, Ells LJ, Rankin J, Wilkinson J, et al. (2008) The impact of maternal BMI status on pregnancy outcomes with immediate short-term obstetric resource implications: a meta-analysis. Obes Rev 9 : 635–683. doi: 10.1111/j.1467-789X.2008.00511.x 18673307
2. Yogev Y, Catalano PM (2009) Pregnancy and obesity. Obstet Gynecol Clin North Am 36 : 285–300. doi: 10.1016/j.ogc.2009.03.003 19501314
3. Marchi J, Berg M, Dencker A, Olander EK, Begley C (2015) Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 16 : 621–638. doi: 10.1111/obr.12288 26016557
4. Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, et al. (2001) Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord 25 : 1175–1182. 11477502
5. Johansson S, Villamor E, Altman M, Bonamy AK, Granath F, et al. (2014) Maternal overweight and obesity in early pregnancy and risk of infant mortality: a population based cohort study in Sweden. BMJ 349: g6572. doi: 10.1136/bmj.g6572 25467170
6. Ogden CL, Carroll MD, Kit BK, Flegal KM (2013) Prevalence of obesity among adults: United States, 2011–2012. NCHS Data Brief 131 : 1–8. 24152742
7. Food and Agriculture Organization of the United Nations, World Health Organization (2014) Second international conference on nutrition. Rome, 19–21 November 2014. Conference outcome document: Rome declaration on nutrition. Available: http://www.fao.org/3/a-ml542e.pdf. Accessed 5 May 2015.
8. Persson M, Johansson S, Villamor E, Cnattingius S (2014) Maternal overweight and obesity and risks of severe birth-asphyxia-related complications in term infants: a population-based cohort study in Sweden. PLoS Med 11: e1001648. doi: 10.1371/journal.pmed.1001648 24845218
9. Persson M, Pasupathy D, Hanson U, Westgren M, Norman M (2012) Pre-pregnancy body mass index and the risk of adverse outcome in type 1 diabetic pregnancies: a population-based cohort study. BMJ Open 2: e000601. doi: 10.1136/bmjopen-2011-000601 22334581
10. Cnattingius S, Villamor E, Johansson S, Edstedt Bonamy AK, Persson M, et al. (2013) Maternal obesity and risk of preterm delivery. JAMA 309 : 2362–2370. doi: 10.1001/jama.2013.6295 23757084
11. Nohr EA, Vaeth M, Bech BH, Henriksen TB, Cnattingius S, et al. (2007) Maternal obesity and neonatal mortality according to subtypes of preterm birth. Obstet Gynecol 110 : 1083–1090. 17978123
12. Hogan L, Ingemarsson I, Thorngren-Jerneck K, Herbst A (2007) How often is a low 5-min Apgar score in term newborns due to asphyxia? Eur J Obstet Gynecol Reprod Biol 130 : 169–175. 16621222
13. Centre for Epidemiology (2003)The Swedish Medical Birth Register—a summary of content and quality. Stockholm: Swedish National Board of Health and Welfare.
14. Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, Ekbom A (2009) The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol 24 : 659–667. doi: 10.1007/s10654-009-9350-y 19504049
15. World Health Organization (2015) Global database on body mass index. Available: http://apps.who.int/bmi/. Accessed 5 May 2015.
16. Hogberg U, Larsson N (1997) Early dating by ultrasound and perinatal outcome. A cohort study. Acta Obstet Gynecol Scand 76 : 907–912. 9435727
17. Villamor E, Cnattingius S (2006) Interpregnancy weight change and risk of adverse pregnancy outcomes: a population-based study. Lancet 368 : 1164–1170. 17011943
18. Getahun D, Ananth CV, Peltier MR, Salihu HM, Scorza WE (2007) Changes in prepregnancy body mass index between the first and second pregnancies and risk of large-for-gestational-age birth. Am J Obstet Gynecol 196 : 530.e1–8.
19. Getahun D, Ananth CV, Oyelese Y, Chavez MR, Kirby RS, et al. (2007) Primary preeclampsia in the second pregnancy: effects of changes in prepregnancy body mass index between pregnancies. Obstet Gynecol 110 : 1319–1325. 18055727
20. Getahun D, Kaminsky LM, Elsasser DA, Kirby RS, Ananth CV, et al. (2007) Changes in prepregnancy body mass index between pregnancies and risk of primary cesarean delivery. Am J Obstet Gynecol 197 : 376.e1–7.
21. Bogaerts A, Van den Bergh BR, Ameye L, Witters I, Martens E, et al. (2013) Interpregnancy weight change and risk for adverse perinatal outcome. Obstet Gynecol 122 : 999–1009. doi: 10.1097/AOG.0b013e3182a7f63e 24104777
22. Whiteman VE, Rao K, Duan J, Alio A, Marty PJ, et al. (2011) Changes in prepregnancy body mass index between pregnancies and risk of preterm phenotypes. Am J Perinatol 28 : 67–74. doi: 10.1055/s-0030-1262905 20640971
23. Cnattingius S, Villamor E (2015) Weight change between successive pregnancies and risks of stillbirth and infant mortality: a nationwide cohort study. Lancet 387 : 558–565. doi: 10.1016/S0140-6736(15)00990-3 26651225
24. Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, et al. (2002) Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab 87 : 4231–4237. 12213876
25. Jarvie E, Hauguel-de-Mouzon S, Nelson SM, Sattar N, Catalano PM, et al. (2010) Lipotoxicity in obese pregnancy and its potential role in adverse pregnancy outcome and obesity in the offspring. Clin Sci (Lond) 119 : 123–129.
26. Santacruz A, Collado MC, Garcia-Valdes L, Segura MT, Martin-Lagos JA, et al. (2010) Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr 104 : 83–92. doi: 10.1017/S0007114510000176 20205964
27. HAPO Study Cooperative Research Group (2010) Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index. BJOG 117 : 575–584. doi: 10.1111/j.1471-0528.2009.02486.x 20089115
28. Philipps AF, Widness JA, Garcia JF, Raye JR, Schwartz R (1982) Erythropoietin elevation in the chronically hyperglycemic fetal lamb. Proc Soc Exp Biol Med 170 : 42–47. 7043470
29. Stonestreet BS, Widness JA, Berard DJ (1995) Circulatory and metabolic effects of hypoxia in the hyperinsulinemic ovine fetus. Pediatr Res 38 : 67–75. 7478799
30. Teramo KA, Widness JA (2009) Increased fetal plasma and amniotic fluid erythropoietin concentrations: markers of intrauterine hypoxia. Neonatology 95 : 105–116. doi: 10.1159/000153094 18776724
31. Widness JA, Teramo KA, Clemons GK, Voutilainen P, Stenman UH, et al. (1990) Direct relationship of antepartum glucose control and fetal erythropoietin in human type 1 (insulin-dependent) diabetic pregnancy. Diabetologia 33 : 378–383. 2199280
32. Zhang X, Decker A, Platt RW, Kramer MS (2008) How big is too big? The perinatal consequences of fetal macrosomia. Am J Obstet Gynecol 198 : 517.e1–6.
33. Persson M, Norman M, Hanson U (2009) Obstetric and perinatal outcomes in type 1 diabetic pregnancies: a large, population-based study. Diabetes Care 32 : 2005–2009. doi: 10.2337/dc09-0656 19675195
34. Ferrazzani S, Luciano R, Garofalo S, D’Andrea V, De Carolis S, et al. (2011) Neonatal outcome in hypertensive disorders of pregnancy. Early Hum Dev 87 : 445–449. doi: 10.1016/j.earlhumdev.2011.03.005 21497462
35. Sewell MF, Huston-Presley L, Amini SB, Catalano PM (2007) Body mass index: a true indicator of body fat in obese gravidas. J Reprod Med 52 : 907–911. 17977164
36. Linne Y, Neovius M (2006) Identification of women at risk of adverse weight development following pregnancy. Int J Obes (Lond) 30 : 1234–1239.
37. Amorim AR, Rossner S, Neovius M, Lourenco PM, Linne Y (2007) Does excess pregnancy weight gain constitute a major risk for increasing long-term BMI? Obesity (Silver Spring) 15 : 1278–1286.
38. Carter BS, Haverkamp AD, Merenstein GB (1993) The definition of acute perinatal asphyxia. Clin Perinatol 20 : 287–304. 8358952
39. Scott-Pillai R, Spence D, Cardwell CR, Hunter A, Holmes VA (2013) The impact of body mass index on maternal and neonatal outcomes: a retrospective study in a UK obstetric population, 2004–2011. BJOG 120 : 932–939. doi: 10.1111/1471-0528.12193 23530609
40. Chen M, McNiff C, Madan J, Goodman E, Davis JM, et al. (2010) Maternal obesity and neonatal Apgar scores. J Matern Fetal Neonatal Med 23 : 89–95. doi: 10.3109/14767050903168440 19670044
41. Moster D, Lie RT, Irgens LM, Bjerkedal T, Markestad T (2001) The association of Apgar score with subsequent death and cerebral palsy: a population-based study in term infants. J Pediatr 138 : 798–803. 11391319
42. Iliodromiti S, Mackay DF, Smith GC, Pell JP, Nelson SM (2014) Apgar score and the risk of cause-specific infant mortality: a population-based cohort study. Lancet 384 : 1749–1755. doi: 10.1016/S0140-6736(14)61135-1 25236409
43. Catlin EA, Carpenter MW, Brann BSt, Mayfield SR, Shaul PW, et al. (1986) The Apgar score revisited: influence of gestational age. J Pediatr 109 : 865–868. 3772665
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2016 Číslo 6- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Direct-to-consumer Marketing to People with Hemophilia
- Geographical Inequalities and Social and Environmental Risk Factors for Under-Five Mortality in Ghana in 2000 and 2010: Bayesian Spatial Analysis of Census Data
- Phosphodiesterase Type 5 Inhibitors and Risk of Malignant Melanoma: Matched Cohort Study Using Primary Care Data from the UK Clinical Practice Research Datalink
- Age, Spatial, and Temporal Variations in Hospital Admissions with Malaria in Kilifi County, Kenya: A 25-Year Longitudinal Observational Study
- Obesity and Multiple Sclerosis: A Mendelian Randomization Study
- Inter-pregnancy Weight Change and Risks of Severe Birth-Asphyxia-Related Outcomes in Singleton Infants Born at Term: A Nationwide Swedish Cohort Study
- Impact Evaluation of a System-Wide Chronic Disease Management Program on Health Service Utilisation: A Propensity-Matched Cohort Study
- Early Childhood Developmental Status in Low- and Middle-Income Countries: National, Regional, and Global Prevalence Estimates Using Predictive Modeling
- Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies
- Exclusive Breastfeeding and Cognition, Executive Function, and Behavioural Disorders in Primary School-Aged Children in Rural South Africa: A Cohort Analysis
- Investigating the Causal Relationship of C-Reactive Protein with 32 Complex Somatic and Psychiatric Outcomes: A Large-Scale Cross-Consortium Mendelian Randomization Study
- Agreements between Industry and Academia on Publication Rights: A Retrospective Study of Protocols and Publications of Randomized Clinical Trials
- Weighing Evidence from Mendelian Randomization—Early-Life Obesity as a Causal Factor in Multiple Sclerosis?
- A Global Champion for Health—WHO’s Next?
- Malaria Epidemiology in Kilifi, Kenya during the 21st Century: What Next?
- Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement
- Strengthening the Reporting of Observational Studies in Epidemiology—Nutritional Epidemiology (STROBE-nut): An Extension of the STROBE Statement
- Why Most Clinical Research Is Not Useful
- Delinking Investment in Antibiotic Research and Development from Sales Revenues: The Challenges of Transforming a Promising Idea into Reality
- Novel Three-Day, Community-Based, Nonpharmacological Group Intervention for Chronic Musculoskeletal Pain (COPERS): A Randomised Clinical Trial
- Prediction of Bladder Outcomes after Traumatic Spinal Cord Injury: A Longitudinal Cohort Study
- The Effect of Sitagliptin on Carotid Artery Atherosclerosis in Type 2 Diabetes: The PROLOGUE Randomized Controlled Trial
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Why Most Clinical Research Is Not Useful
- Agreements between Industry and Academia on Publication Rights: A Retrospective Study of Protocols and Publications of Randomized Clinical Trials
- Inter-pregnancy Weight Change and Risks of Severe Birth-Asphyxia-Related Outcomes in Singleton Infants Born at Term: A Nationwide Swedish Cohort Study
- Strengthening the Reporting of Observational Studies in Epidemiology—Nutritional Epidemiology (STROBE-nut): An Extension of the STROBE Statement
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



