-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Duration of Adulthood Overweight, Obesity, and Cancer Risk in the Women’s Health Initiative: A Longitudinal Study from the United States
In a longitudinal study, Melina Arnold and colleagues assess the relationship between adulthood overweight and obesity duration and cancer risks in postmenopausal women.
Published in the journal: Duration of Adulthood Overweight, Obesity, and Cancer Risk in the Women’s Health Initiative: A Longitudinal Study from the United States. PLoS Med 13(8): e32767. doi:10.1371/journal.pmed.1002081
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002081Summary
In a longitudinal study, Melina Arnold and colleagues assess the relationship between adulthood overweight and obesity duration and cancer risks in postmenopausal women.
Introduction
High body mass index (BMI) has become the leading risk factor for disease burden in high-income countries and has been offsetting or surpassing the decreasing disease burden attributable to tobacco smoking [1]. In the US, currently about 70% of all adults are considered overweight (BMI ≥ 25 kg/m2) and 36% obese (BMI ≥ 30 kg/m2, according to WHO classification), making the US one of the countries with the highest prevalence of obesity [2]. This development has also been found to contribute substantially to the country’s poor international ranking in longevity [3,4]. Continuing increases in obesity prevalence seen over the past decades remain of great public health concern.
Overweight and obesity have been associated with an increased risk of and mortality from cancer and other chronic diseases such as type 2 diabetes and cardiovascular disease [5]. In the US, more than 100,000 cancer cases were attributed to high BMI in the year 2012 alone [6]. Cancers previously linked to high BMI include postmenopausal breast cancer, adenocarcinoma of the esophagus, and pancreatic, colorectal, renal, endometrial, ovarian, and gallbladder cancer [7,8]. To date, most studies of these associations have used cross-sectional exposure information on BMI, i.e., BMI measured at one point in time and typically obtained at recruitment. Yet, insights into the dose-response relationship of the cumulative impact of overweight and obesity during the life course on cancer risk remain scarce. While age-dependent and cumulative effects of weight change have previously been reported to affect the risk of postmenopausal breast cancer [9–11], only a few studies have investigated the association between overweight duration and cancer outcomes [9,12]. It is thus still unclear how different exposure durations of overweight and obesity are associated with cancer development.
In this study, we assessed the impact of adulthood overweight and obesity duration and cumulative intensity on cancer risk in US women, using data from the observational study cohort of the Women’s Health Initiative (WHI). In secondary analyses, we investigated the influence of important effect modifiers and confounders, such as smoking status, postmenopausal hormone use, and ethnicity.
Methods
Ethics
WHI procedures and protocols were approved by the institutional review boards at each participating institution, and all participants provided written informed consent.
Study Population
The WHI is a large, multi-center prospective cohort study of postmenopausal women. The WHI was designed to have a clinical trial arm and an observational study cohort [13], and recruited postmenopausal women aged 50–79 y at 40 clinical centers nationwide between October 1, 1993, and December 21, 1998, to be followed for the development of diseases that are the most common causes of death, including cardiovascular disease and cancer. Details of the study design and methods have been published elsewhere [13–16]. In total, 93,676 women were enrolled in the observational study, and 68,132 were enrolled in the clinical trial arm (n = 161,808) [16]. For this study, we included all participants from the observational study cohort, except those who reported cancer prior to or at baseline or without data on cancer history (n = 12,827) and women with incomplete follow-up information (n = 411) (Fig 1).
Fig. 1. Flowchart of participant inclusion. 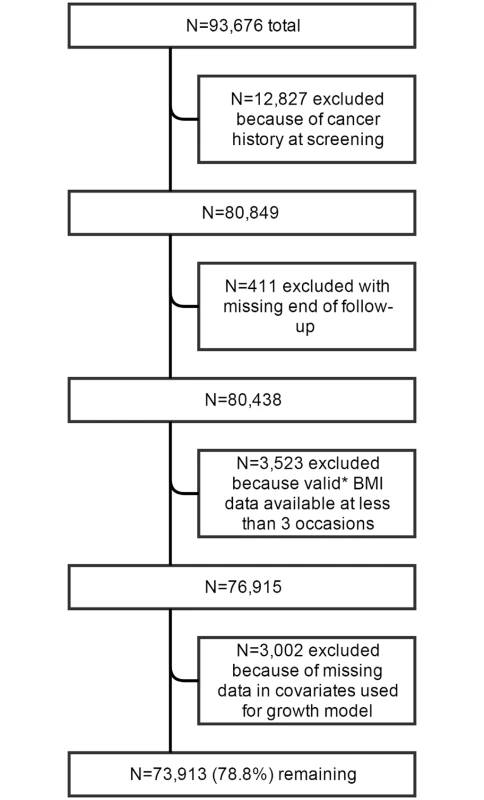
*Including data at baseline and excluding data from the year preceding cancer diagnosis; including BMI from self-reported as well as measured height and weight; BMI values lower <15 or >70 kg/m2 were excluded. Anthropometric Assessment
Information on BMI for was obtained from retrospective self-reports at baseline for ages 18, 35, and 50 y, from weight and height measurements at baseline and at 3-y follow-up, and from self-reports at follow-up years 4–8. BMI was calculated by dividing weight in kilograms by height in meters squared. For inclusion in the study, women were required to have valid body weight information from at least three occasions and a valid baseline measurement of body weight and height.
Covariate Assessment
Covariates included baseline information on age (continuous), ethnicity (six categories), education (11 categories), energy intake (in kilocalories, continuous), diet quality (Alternate Mediterranean Diet Score [17], nine categories), physical activity (frequency of moderate exercise per week, four categories), smoking status (never/past/current smoker), and outcome-specific covariates—for pancreatic cancer: diabetes (never/ever diabetic); for colon cancer: diabetes and red meat intake (servings/day); for postmenopausal breast, ovarian, and endometrial cancer: postmenopausal hormone use (never/past/current user), number of full-term pregnancies (continuous), age at first term pregnancy (<20, 20–24, 25–29, 30–34, 35–39, 40–44, 45+ y), and age at menopause (continuous).
Case Ascertainment
Adjudication and outcome ascertainment for the WHI have been described elsewhere [18]. In brief, all outcomes were self-reported annually in the observational study arm. Only first invasive cancers confirmed by adjudication were included in these analyses. All analyses were conducted for the following cancers with convincing evidence of a positive relation with excess BMI [7,8]: colon, rectum, liver, gallbladder, pancreas, postmenopausal breast, endometrium, ovary, kidney, and thyroid, as well as the combination of all of these, termed “all obesity-related cancers.”
Statistical Analysis
The analysis was carried out in two steps. In the first step, BMI was modeled across all ages using a quadratic growth model with random intercept and random slope, incorporating all available BMI information from all included participants [19]. No random coefficient was included for the quadratic term.
BMI information in the year preceding cancer diagnosis for those who developed an invasive malignancy was excluded. This model was developed and adjusted in a stepwise manner by adding ethnicity, education, and baseline physical activity, smoking status, energy intake, and diet quality score to the model. Using this approach, we allowed individuals to have their own BMI trajectory. Using the full model, BMI was predicted from age 18 y until the age at study exit for every cohort member. The obtained predicted age-specific BMI data were then used to compute the following parameters: overweight (BMI ≥ 25 kg/m2) and obesity (BMI ≥ 30 kg/m2) duration in years and weighted cumulative overweight years (OWY) and obese years (OBY). OWY and OBY were calculated by multiplying the duration of overweight and obesity in years by the difference (in BMI units) above normal BMI (≥25 kg/m2) for overweight and above overweight (≥30 kg/m2) for obesity for each age. This allowed us to take the degree of each participant’s overweight and obesity over time into account. An individual with a BMI of 35 kg/m2 for 10 y would thus contribute 100 (= 10 × [35 − 25]) OWY and 50 (= 10 × [35 − 30]) OBY units. The merit of this approach as a better predictor than cross-sectional BMI information alone for many obesity-related outcomes has been described earlier [20]. Overweight and obesity duration were assessed per 10-y increments and OWY and OBY per 100 units.
In the second step of the analysis, Cox proportional hazard models with time since enrollment as the underlying time metric were fitted to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for the relationship between BMI overweight/obesity duration, OWY/OBY, and the risk of developing specific cancers. Overweight/obesity duration and OWY/OBY were treated as continuous, time-varying covariates. Participants were censored at study exit due to death, loss to follow-up, any cancer diagnosis, or end of follow-up (August 29, 2014), whichever occurred first. Three models were fitted for all outcomes with adjustments for several established risk factors for obesity-related cancers. In model 1, adjustment was made for age. Model 2 was additionally adjusted for ethnicity and education. In model 3, smoking status, physical activity, energy intake, and diet quality score were additionally introduced into the model. Cancer-specific adjustments were made in model 4, introducing age at first birth, age at menopause, parity, and postmenopausal hormone use for breast, endometrial, and ovarian cancer; red meat intake and diabetes status for colon cancer; and diabetes status for pancreatic cancer. Possible interactions were tested by fitting models with and without the interaction term, and corresponding p-values were computed from likelihood ratio tests comparing the two models. In order to assess the potentially nonlinear dose-response relationships between overweight duration, OWY, and cancer risk, we used restricted cubic splines with three knots to model those relationships [21]. Nonlinearity was evaluated by testing the null hypothesis that the coefficients of the splines were the same.
In secondary analyses, we assessed a priori interactions by stratifying by postmenopausal hormone use, hysterectomy and/or oophorectomy, ethnicity, diabetes, and smoking status. We also tested the robustness of our findings by predicting BMI trajectories using self-reported height and weight assessments only.
All analyses were carried out using Stata 13.
Results
The final sample for analyses included 73,913 women with a mean follow-up of 12.6 y (standard deviation [SD] = 5.1), during which a total of 6,301 invasive obesity-related cancer cases occurred. Out of all included study participants, 40% (n = 29,770) were never overweight (BMI < 25 kg/m2) during their adult life (age 18 y until study exit), and 60% (n = 44,143) were ever overweight (BMI ≥ 25 kg/m2), almost half of whom (n = 19,654) were also ever obese (BMI ≥ 30 kg/m2) (Table 1). These values were estimated based on BMI data from on average >9 occasions (both measured and self-reported) per study participant. Women who were ever overweight were on average overweight for 31.3 y; those who were ever obese were, on average, obese for 20.6 y. Compared with women who were never overweight, those who were ever overweight or obese were slightly younger at baseline, had a lower education, and were more likely to be African-American (Table 1). While no differences existed in smoking status, women who were ever overweight or obese were less physically active, consumed more calories, had a lower diet quality score, and more often reported ever being diabetic than women who were never overweight. Women who were never overweight were more likely to be using postmenopausal hormones and less often had a hysterectomy.
Tab. 1. Cohort characteristics at recruitment by adulthood overweight and obesity. 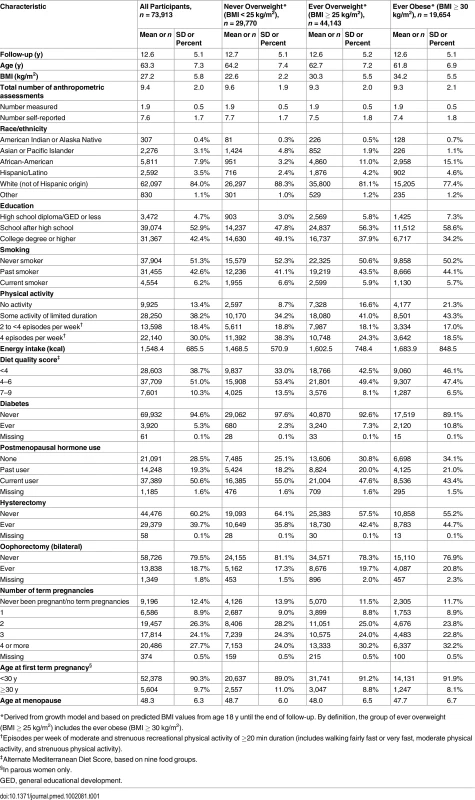
*Derived from growth model and based on predicted BMI values from age 18 y until the end of follow-up. By definition, the group of ever overweight (BMI ≥ 25 kg/m2) includes the ever obese (BMI ≥ 30 kg/m2). A longer overweight duration significantly increased the risk of all obesity-related cancers combined (multivariable-adjusted HR per 10-y increment: 1.07, 95% CI 1.06–1.09) (Table 2). The strongest associations were observed for endometrial (HR 1.17, 95% CI 1.12–1.22) and kidney cancer (HR 1.16, 95% CI 1.07–1.26), while no significant associations were found for rectal, liver, gallbladder, pancreatic, ovarian, and thyroid cancer. When taking into account the degree of overweight over time, the association became stronger for all obesity-related cancers combined, with an increase in risk of 12% for every 100 units of OWY. For endometrial cancer, the HR per 100 OWY was 1.37 (95% CI 1.29–1.46). The results for obesity duration were even more pronounced and showed a significant association for all obesity-related cancers combined (multivariable-adjusted HR per 10-y increment: 1.10, 95% CI 1.08–1.12) and individually for colon, breast, endometrial, and kidney cancer, with HRs for every 10 y of obesity ranging from 1.07 (95% CI 1.04–1.10) for breast cancer to 1.23 (95% CI 1.18–1.28) for endometrial cancer. Risks associated with OBY were similar. Further cancer-specific adjustments (for pancreatic cancer: diabetes; for colon cancer: diabetes and red meat intake; for postmenopausal breast, ovarian, and endometrial cancer: postmenopausal hormone use, number of full-term pregnancies, age at first term pregnancy, and age at menopause) did not materially change these results (Table A in S1 Appendix).
Tab. 2. Hazard ratios of specific cancers related to overweight (BMI ≥ 25 kg/m2) and obesity (BMI ≥ 30 kg/m2) duration and intensity. 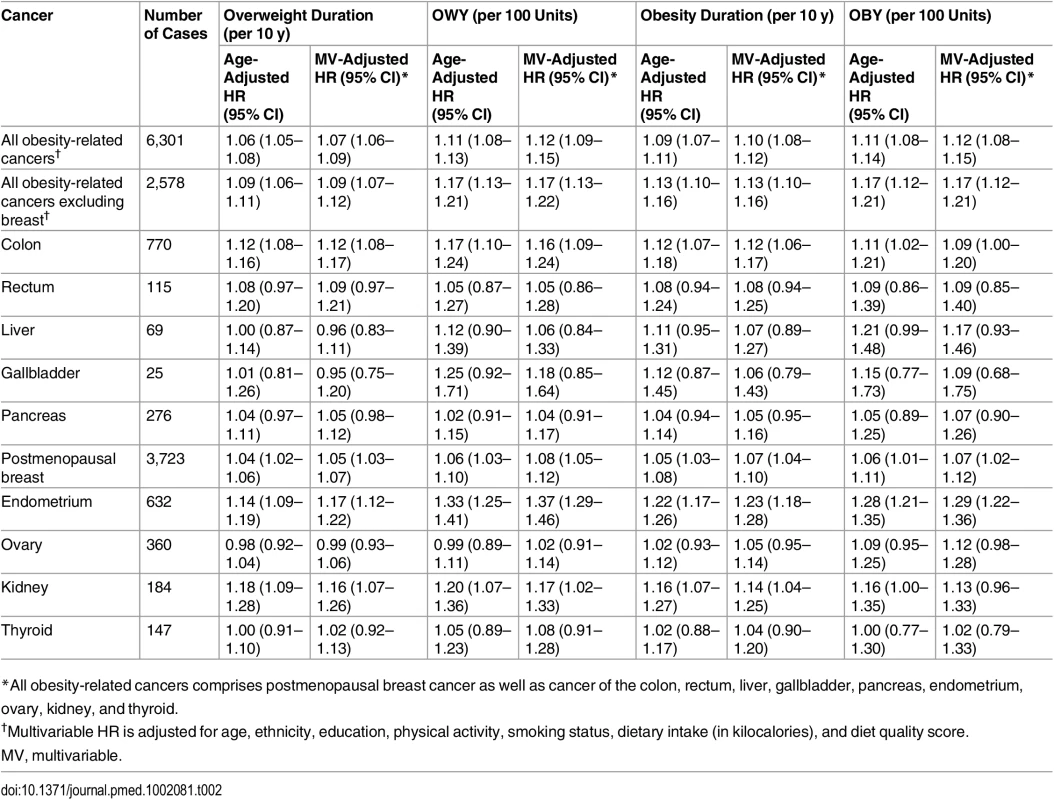
*All obesity-related cancers comprises postmenopausal breast cancer as well as cancer of the colon, rectum, liver, gallbladder, pancreas, endometrium, ovary, kidney, and thyroid. A dose-response relationship with increasing overweight duration was found for almost all cancers (Fig 2). The risk of endometrial cancer associated with increasing overweight duration rose in an exponential fashion, but became statistically significant only after 26 y of being overweight. This threshold effect disappeared when the degree of overweight over time was taken into account (Fig A in S1 Appendix). In contrast, the association of overweight duration with colon and postmenopausal breast cancer was linear.
Fig. 2. Association between overweight (BMI ≥ 25 kg/m2) duration since age 18 y and risk of specific cancers, allowing for non-linear effects, with 95% CIs. 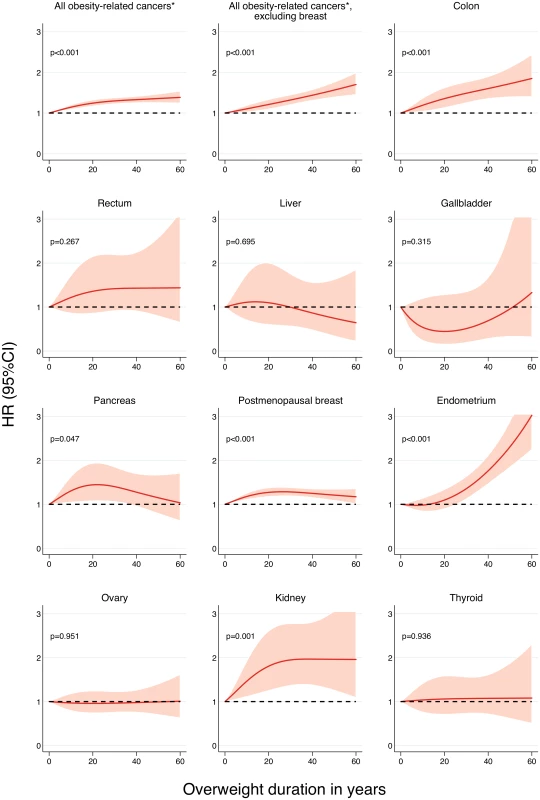
HRs are adjusted for age, ethnicity, education, physical activity, smoking status, dietary intake (in kilocalories), and diet quality score. Restricted cubic splines were fitted with knots at 0, 8, and 40 y. p-Values are for nonlinearity. *All obesity-related cancers comprises postmenopausal breast cancer as well as cancer of the colon, rectum, liver, gallbladder, pancreas, endometrium, ovary, kidney, and thyroid. When stratifying the analyses by postmenopausal hormone use, for which statistically significant interactions were found, stronger associations of breast and endometrial cancer with all measures of overweight and obesity duration emerged in women who were never or past users (Table B in S1 Appendix). While the risk of endometrial cancer was statistically nonsignificant in current users, the risk of breast cancer was still elevated in this group, although at a much lower level than in never users (Fig 3). In line with these findings, the incidence rate of postmenopausal breast cancer was high in women who were ever overweight, regardless of their hormone use (Fig B in S1 Appendix). In contrast, among women who were never overweight in adulthood, current hormone users had a higher incidence rate of postmenopausal breast cancer than never or past postmenopausal hormone users. This pattern was somewhat different for endometrial cancer, where the incidence rate in ever overweight current users was similar to that in women who were never overweight and never or past hormone users.
Fig. 3. Association between overweight (BMI ≥ 25 kg/m2) duration since age 18 y and the risk of postmenopausal breast and endometrial cancer by postmenopausal hormone use, allowing for nonlinear effects, with 95% CIs. 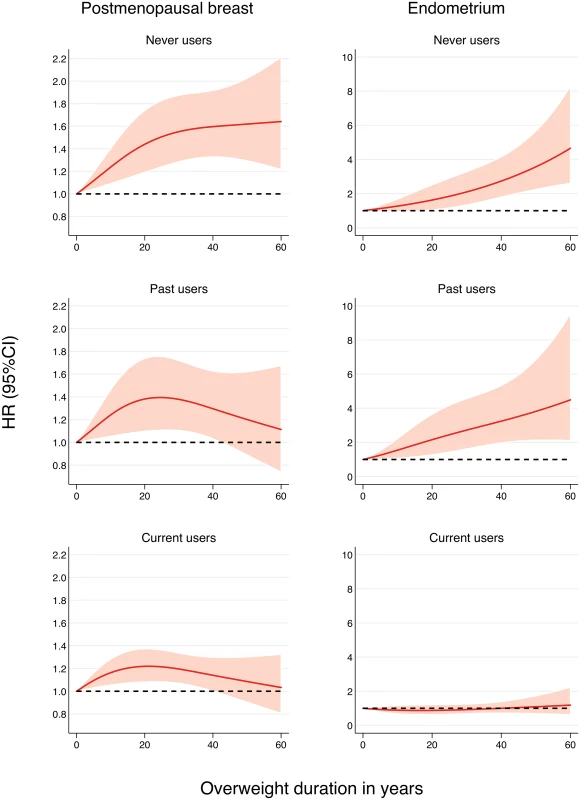
HRs are adjusted for age, ethnicity, education, physical activity, smoking status, dietary intake (in kilocalories), diet quality score, age at menopause, age at first birth, and parity. Restricted cubic splines were fitted with knots at 0, 15, and 44 y in never users; 0, 12, and 42 y in past users; and 0, 3, and 37 y in current users. Similar risks of postmenopausal breast, endometrial, and ovarian cancer associated with increasing overweight and obesity duration and intensity were found in women who had ever had a hysterectomy or oophorectomy compared to women who never underwent either of these procedures (Table C in S1 Appendix). Cancer risk related to increasing overweight and obesity duration was slightly higher in non-Hispanic white women than in African-American women for breast cancer (Table D in S1 Appendix), more pronounced in women who ever reported diabetes for colon cancer (Table E in S1 Appendix), and highest in current smokers compared to past and never smokers for colon and pancreatic cancer (Table F in S1 Appendix). Additional analyses using only self-reported BMI assessments confirmed the main findings (Table G in S1 Appendix).
Discussion
Many previous studies have reported on the association between obesity and cancer, but, to our knowledge, this study is the first to assess the impact of adulthood overweight and obesity duration on the risk of various types of cancer in a large cohort of postmenopausal women. We found that longer durations of overweight and obesity were significantly associated with an increased incidence of obesity-related cancers, postmenopausal breast cancer, and colon, endometrial, and kidney cancer. Taking into account the intensity of overweight over time further increased the risks, and clear dose-response relationships were found. These findings are in line with previous studies on other chronic diseases, which showed that obesity duration is an important and independent predictor of type 2 diabetes [11], cardiovascular disease [10], and all-cause mortality [9]. Underlying biological mechanisms explaining these associations include a higher risk of developing hypertension and insulin resistance [10,11]. Earlier and long-term exposure to overweight and obesity may also increase the risk and severity of chronic inflammation, oxidative DNA damage, and alterations in endogenous hormone metabolism, three key mechanisms that have been found to be associated with increased risk of cancer [22].
We also observed that the risks of postmenopausal breast and endometrial cancer related to overweight and obesity duration were modified by postmenopausal hormone use and were largely attenuated or even eliminated among postmenopausal hormone users. Very high levels of estrogen in women using postmenopausal hormones have been postulated to obscure the effect of obesity and might explain this finding. While similar effect modification was reported in earlier analyses of the WHI observational study [23,24], in the Million Women Study [25–27], and in several other prospective cohorts [28–30], a recent analysis of the WHI clinical trial suggested a remaining positive association between obesity and postmenopausal cancer risk, independent of postmenopausal hormone use [31]. This finding was attributed to the often self-reported height, weight, and hormone use information in observational studies as well as outcome ascertainment bias. Strong associations found for endometrial cancer and its exponential dose-response relationship with overweight duration and intensity confirm risk patterns observed in previous studies [32,33].
Long-term follow-up and many repeated measurements of weight and height are necessary in order to fully capture lifetime overweight and obesity exposure and to quantify corresponding health effects. In our study, we used data from a large cohort of women with numerous repeated measurements and self-reports of weight and height for each individual, and applied a novel approach to estimate adulthood overweight and obesity duration. By modeling individual BMI trajectories across age using growth curves, all observed BMI information for an individual could be used, and unbalanced repeated measurements accommodated. Compared to traditional approaches to handling incomplete time-dependent data, such as linear interpolation and last observation carried forward methods, which may not adequately model BMI trajectories, growth models can incorporate any covariate data that might be important when predicting an individual’s BMI during life course. Using this approach, we were able to quantify the risk associated with both the duration and the intensity of overweight and obesity, as opposed to most previous studies, which focused on cross-sectional BMI information only.
Our study has limitations because of variation in how BMI information was collected and used. Some of this information was obtained from retrospective self-reports, which might be subject to measurement error. Yet, including only self-reported height and weight information when modeling BMI trajectories only marginally altered the results. Data from longitudinal studies with long-term follow-up and repeated anthropometric measures are typically subject to missing data at various time points due to periodical measurements, which was also the case in this study. Using growth curve models, we were able to impute data at missing time points for each participant. In a previous small-scale simulation study focusing on the effects of measurement errors, it was reported that using predicted biomarker values from growth curve models as a time-dependent covariate in Cox regression models—instead of applying a more naïve approach based on periodically observed biomarker values—yielded much less biased estimates for the association between the biomarker and the clinical outcome, especially when the variance in measurement errors was large [34].
BMI is not an ideal measure of body fatness as it does not differentiate tissue type (fatty, lean, bone). It has been suggested that other anthropometric measures such as waist circumference or waist-to-hip ratio better predict obesity-related health outcomes than BMI does [35,36]. These measures were, however, not available in a repeated fashion in the WHI. Additionally, BMI has limitations with regard to its application across different race/ethnic groups and ages [37]. In the US, African-American and Hispanic women have been reported to be more likely to be obese than non-Hispanic white and Asian women, while Asian women have been shown to have a higher percentage of body fat than non-Hispanic white women at similar BMI levels [38]. It might thus be appropriate to use different BMI thresholds for overweight and obesity in different race/ethnic groups. In our study, the risks for postmenopausal breast cancer associated with a longer overweight duration were higher in non-Hispanic white women than in African-American women. The fairly small number of women with an ethnicity other than non-Hispanic white did not allow further investigation of this issue. Furthermore, it is important to note that BMI serves as a proxy for many internal physiological processes that are the actual correlates of cancer development and that these also depend on many other lifestyle and environmental factors. Any residual confounding can therefore not be ruled out.
In summary, this study showed that the risk of cancer associated with overweight and obesity compounds over time, and a longer duration of overweight and obesity during adulthood is associated with increased risks of several cancers. Furthermore, not only the duration but also the degree of overweight seems to play an important role in the risk of developing cancer, especially for endometrial cancer. Although the observational nature of our study precludes inferring causality or making clinical recommendations, our findings suggest that reducing overweight duration in adulthood could reduce cancer risk. If this is true, health care teams should recognize the potential of obesity management in cancer prevention and that excess body weight in women is important to manage regardless of the age of the patient.
Supporting Information
Zdroje
1. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380 : 2224–2260. doi: 10.1016/S0140-6736(12)61766-8 23245609
2. Stevens GA, Singh GM, Lu Y, Danaei G, Lin JK, Finucane MM, et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 2012;10 : 22. doi: 10.1186/1478-7954-10-22 23167948
3. Preston SH, Stokes A. Contribution of obesity to international differences in life expectancy. Am J Public Health. 2011;101 : 2137–2143. doi: 10.2105/AJPH.2011.300219 21940912
4. Chang SH, Pollack LM, Colditz GA. Life years lost associated with obesity-related diseases for U.S. non-smoking adults. PLoS ONE. 2013;8:e66550. doi: 10.1371/journal.pone.0066550 23823705
5. Prospective Studies Collaboration, Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373 : 1083–1096. doi: 10.1016/S0140-6736(09)60318-4 19299006
6. Arnold M, Pandeya N, Byrnes G, Renehan AG, Stevens GA, Ezzati M, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16 : 36–46. doi: 10.1016/S1470-2045(14)71123-4 25467404
7. Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371 : 569–578. doi: 10.1016/S0140-6736(08)60269-X 18280327
8. World Cancer Research Fund, American Institute for Cancer Research. Food, nutrition, physical activity and the prevention of cancer: a global perspective. Washington (District of Columbia):American Institute for Cancer Research; 2007. 517 p.
9. Abdullah A, Wolfe R, Stoelwinder JU, de Courten M, Stevenson C, Walls HL, et al. The number of years lived with obesity and the risk of all-cause and cause-specific mortality. Int J Epidemiol. 2011;40 : 985–996. doi: 10.1093/ije/dyr018 21357186
10. Abdullah A, Amin FA, Stoelwinder J, Tanamas SK, Wolfe R, Barendregt J, et al. Estimating the risk of cardiovascular disease using an obese-years metric. BMJ Open. 2014;4:e005629. doi: 10.1136/bmjopen-2014-005629 25231490
11. Abdullah A, Stoelwinder J, Shortreed S, Wolfe R, Stevenson C, Walls H, et al. The duration of obesity and the risk of type 2 diabetes. Public Health Nutr. 2011;14 : 119–126. doi: 10.1017/S1368980010001813 20587115
12. Stolzenberg-Solomon RZ, Schairer C, Moore S, Hollenbeck A, Silverman DT. Lifetime adiposity and risk of pancreatic cancer in the NIH-AARP Diet and Health Study cohort. Am J Clin Nutr. 2013;98 : 1057–1065. doi: 10.3945/ajcn.113.058123 23985810
13. Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19 : 61–109. 9492970
14. Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13(9 Suppl):S18–S77. 14575939
15. Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9 Suppl):S107–S121.
16. Phipps AI, Chlebowski RT, Prentice R, McTiernan A, Stefanick ML, Wactawski-Wende J, et al. Body size, physical activity, and risk of triple-negative and estrogen receptor-positive breast cancer. Cancer Epidemiol Biomarkers Prev. 2011;20 : 454–463. doi: 10.1158/1055-9965.EPI-10-0974 21364029
17. Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med. 2003;348 : 2599–2608. doi: 10.1056/NEJMoa025039 12826634
18. Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, et al. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Ann Epidemiol. 2003;13(9 Suppl):S122–S128. 14575944
19. Heo M, Faith MS, Mott JW, Gorman BS, Redden DT, Allison DB. Hierarchical linear models for the development of growth curves: an example with body mass index in overweight/obese adults. Stat Med. 2003;22 : 1911–1942. doi: 10.1002/sim.1218 12754724
20. Abdullah A, Wolfe R, Mannan H, Stoelwinder JU, Stevenson C, Peeters A. Epidemiologic merit of obese-years, the combination of degree and duration of obesity. Am J Epidemiol. 2012;176 : 99–107. doi: 10.1093/aje/kwr522 22759723
21. Orsini N, Greenland S. A procedure to tabulate and plot results after flexible modeling of a quantitative covariate. Stata J. 2011;11 : 1–29.
22. Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3 : 565–574. 12217794
23. Morimoto LM, White E, Chen Z, Chlebowski RT, Hays J, Kuller L, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women’s Health Initiative (United States). Cancer Causes Control. 2002;13 : 741–751. 12420953
24. Chlebowski RT, Manson JE, Anderson GL, Cauley JA, Aragaki AK, Stefanick ML, et al. Estrogen plus progestin and breast cancer incidence and mortality in the Women’s Health Initiative Observational Study. J Natl Cancer Inst. 2013;105 : 526–535. doi: 10.1093/jnci/djt043 23543779
25. Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335 : 1134. doi: 10.1136/bmj.39367.495995.AE 17986716
26. Beral V, Bull D, Reeves G, Million Women Study Collaborators. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2005;365 : 1543–1551.
27. Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362 : 419–427. 12927427
28. Brinton LA, Richesson D, Leitzmann MF, Gierach GL, Schatzkin A, Mouw T, et al. Menopausal hormone therapy and breast cancer risk in the NIH-AARP Diet and Health Study Cohort. Cancer Epidemiol Biomarkers Prev. 2008;17 : 3150–3160. doi: 10.1158/1055-9965.EPI-08-0435 18990757
29. Hou N, Hong S, Wang W, Olopade OI, Dignam JJ, Huo D. Hormone replacement therapy and breast cancer: heterogeneous risks by race, weight, and breast density. J Natl Cancer Inst. 2013;105 : 1365–1372. doi: 10.1093/jnci/djt207 24003037
30. Chen WY, Hankinson SE, Schnitt SJ, Rosner BA, Holmes MD, Colditz GA. Association of hormone replacement therapy to estrogen and progesterone receptor status in invasive breast carcinoma. Cancer. 2004;101 : 1490–1500. doi: 10.1002/cncr.20499 15378477
31. Neuhouser ML, Aragaki AK, Prentice RL, Manson JE, Chlebowski R, Carty CL, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the Women’s Health Initiative randomized clinical trials. JAMA Oncol. 2015;1 : 611–621. doi: 10.1001/jamaoncol.2015.1546 26182172
32. Reeves KW, Carter GC, Rodabough RJ, Lane D, McNeeley SG, Stefanick ML, et al. Obesity in relation to endometrial cancer risk and disease characteristics in the Women’s Health Initiative. Gynecol Oncol. 2011;121 : 376–382. doi: 10.1016/j.ygyno.2011.01.027 21324514
33. Bhaskaran K, Douglas I, Forbes H, Dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5·24 million UK adults. Lancet. 2014;384 : 755–765. doi: 10.1016/s0140-6736(14)60892-8 25129328
34. Dafni UG, Tsiatis AA. Evaluating surrogate markers of clinical outcome when measured with error. Biometrics. 1998;54 : 1445–1462. 9883544
35. Moore LL, Bradlee ML, Singer MR, Splansky GL, Proctor MH, Ellison RC, et al. BMI and waist circumference as predictors of lifetime colon cancer risk in Framingham Study adults. Int J Obes Relat Metab Disord. 2004;28 : 559–567. doi: 10.1038/sj.ijo.0802606 14770200
36. Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr. 2004;79 : 379–384. 14985210
37. Decaria JE, Sharp C, Petrella RJ. Scoping review report: obesity in older adults. Int J Obes (Lond). 2012;36 : 1141–1150. doi: 10.1038/ijo.2012.29
38. Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29 : 6–28. doi: 10.1093/epirev/mxm007 17510091
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2016 Číslo 8- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Dementia across the Lifespan and around the Globe—Pathophysiology, Prevention, Treatment, and Societal Impact: A Call for Papers
- Progress and Challenges in Scaling Up Laboratory Monitoring of HIV Treatment
- Facility-Based Delivery during the Ebola Virus Disease Epidemic in Rural Liberia: Analysis from a Cross-Sectional, Population-Based Household Survey
- Availability and Use of HIV Monitoring and Early Infant Diagnosis Technologies in WHO Member States in 2011–2013: Analysis of Annual Surveys at the Facility Level
- Duration of Adulthood Overweight, Obesity, and Cancer Risk in the Women’s Health Initiative: A Longitudinal Study from the United States
- Improving Clinical Risk Stratification at Diagnosis in Primary Prostate Cancer: A Prognostic Modelling Study
- Genetically Predicted Body Mass Index and Breast Cancer Risk: Mendelian Randomization Analyses of Data from 145,000 Women of European Descent
- Social Dancing and Incidence of Falls in Older Adults: A Cluster Randomised Controlled Trial
- Genetic and Environmental Risk for Chronic Pain and the Contribution of Risk Variants for Major Depressive Disorder: A Family-Based Mixed-Model Analysis
- Long-Term Outcomes Associated with Traumatic Brain Injury in Childhood and Adolescence: A Nationwide Swedish Cohort Study of a Wide Range of Medical and Social Outcomes
- On Risk Estimation versus Risk Stratification in Early Prostate Cancer
- Make Data Sharing Routine to Prepare for Public Health Emergencies
- Assessment of Adverse Events in Protocols, Clinical Study Reports, and Published Papers of Trials of Orlistat: A Document Analysis
- Concussions and Repercussions
- South Asia as a Reservoir for the Global Spread of Ciprofloxacin-Resistant : A Cross-Sectional Study
- Building from the HIV Response toward Universal Health Coverage
- Transitioning to Country Ownership of HIV Programs in Rwanda
- Accelerating the Uptake and Timing of Antiretroviral Therapy Initiation in Sub-Saharan Africa: An Operations Research Agenda
- Glycemic Control and the Risk of Tuberculosis: A Cohort Study
- Adjuvant Trastuzumab in HER2-Positive Early Breast Cancer by Age and Hormone Receptor Status: A Cost-Utility Analysis
- Uptake of Home-Based HIV Testing, Linkage to Care, and Community Attitudes about ART in Rural KwaZulu-Natal, South Africa: Descriptive Results from the First Phase of the ANRS 12249 TasP Cluster-Randomised Trial
- An Audit and Feedback Intervention for Reducing Antibiotic Prescribing in General Dental Practice: The RAPiD Cluster Randomised Controlled Trial
- Multidrug-Resistant Tuberculosis Treatment in North Korea: Is Scale-Up Possible?
- Associations between Mental Health and Ebola-Related Health Behaviors: A Regionally Representative Cross-sectional Survey in Post-conflict Sierra Leone
- Pancreatic Cancer Surgical Resection Margins: Molecular Assessment by Mass Spectrometry Imaging
- Integrated Delivery of Antiretroviral Treatment and Pre-exposure Prophylaxis to HIV-1–Serodiscordant Couples: A Prospective Implementation Study in Kenya and Uganda
- Measuring Burden of Unhealthy Behaviours Using a Multivariable Predictive Approach: Life Expectancy Lost in Canada Attributable to Smoking, Alcohol, Physical Inactivity, and Diet
- Comparison of Outcomes before and after Ohio's Law Mandating Use of the FDA-Approved Protocol for Medication Abortion: A Retrospective Cohort Study
- Core Outcomes for Colorectal Cancer Surgery: A Consensus Study
- Adverse Renal, Endocrine, Hepatic, and Metabolic Events during Maintenance Mood Stabilizer Treatment for Bipolar Disorder: A Population-Based Cohort Study
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Glycemic Control and the Risk of Tuberculosis: A Cohort Study
- Transitioning to Country Ownership of HIV Programs in Rwanda
- Dementia across the Lifespan and around the Globe—Pathophysiology, Prevention, Treatment, and Societal Impact: A Call for Papers
- Social Dancing and Incidence of Falls in Older Adults: A Cluster Randomised Controlled Trial
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



