-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
CD8+ T Cell Control of HIV—A Known Unknown
article has not abstract
Published in the journal: CD8+ T Cell Control of HIV—A Known Unknown. PLoS Pathog 6(1): e32767. doi:10.1371/journal.ppat.1000728
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1000728Summary
article has not abstract
The former US Secretary of Defense, Donald Rumsfeld, once famously divided our areas of understanding into three categories: “known knowns … things we know we know”, “known unknowns … [things we] know we … do not know”, and “unknown unknowns—the ones we don't know we don't know” [1]. In HIV immunity, the role of CD8+ cytotoxic T lymphocytes (CTLs) in controlling virus has been thought to be “known” for over two decades. The recognition of viral peptides by CD8+ T cells was initially demonstrated by showing that CTLs could lyse chromium-labeled target cells presenting viral epitopes [2]. Multiple lines of evidence have subsequently supported the important role for CTLs in controlling HIV replication [3],[4]. However, most of these studies have not explicitly examined the mechanisms by which viral control is achieved. Because of the name (“cytotoxic”) and the early assays for detection (measuring cytolysis using chromium release in vitro), it is usually assumed that CD8+ T cells control HIV infection by killing HIV-infected cells. Indeed, the use of the terms CD8+ T lymphocyte and cytotoxic T lymphocyte have become almost interchangeable in HIV. Thus, the mechanism of CTL control of infection through the cytolysis of infected cells has fallen into the category of a “known known”.
Two papers in this issue of PLoS Pathogens present a major challenge to the assumption that CD8+ T cells kill HIV-infected cells [5],[6]. Using very similar methodology, Klatt et al. and Wong et al. used an approach of depleting CD8+ T cells during simian immunodeficiency virus (SIV) infection of macaques to study the mechanisms of CD8+ T cell control of virus. What makes their studies different from previous studies of CD8+ T cell depletion is that they also treated animals with anti-retroviral therapy (ART) after CD8+ T cell depletion. Because ART blocks subsequent rounds of viral infection, it has been used extensively over the last decade to study the turnover of HIV-infected cells [7],[8]. Combining CD8 depletion and ART treatment allowed the two groups to study the death rate of SIV-infected cells in the presence and absence of CD8+ T cells. Remarkably, the groups found no difference in the decay rate of virus in CD8-depleted compared to control animals. This indicates that CD8+ T cells had no significant impact on the death rate of infected cells, suggesting that a high death rate is an intrinsic property of infected cells. Since CD8+ T cells have a clear role in viral control, they must act via some mechanism other than lysis of infected cells.
On the surface, we could simply accept that CD8+ T cell control of HIV infection acts via non-cytolytic mechanisms such as the production of cytokines and/or chemokines, and make that our new credo. Such a shift in beliefs would be supported by the demonstration that the ability of CD8+ T cells to produce multiple cytokines is associated with good viral control in HIV, suggesting an important role for cytokines [9],[10]. However, it is difficult to reconcile the results of the Klatt and Wong papers with other recent in vivo and in vitro results (see Table 1). First amongst these are studies of CD8+ T cell depletion in acute SIV infection [3],[11]. These studies demonstrate that in the absence of CD8+ T cells, the virus level rises to its “normal” peak in acute infection, but then does not decline from the peak for a prolonged period. The simplest interpretation of this result is that, in the absence of CD8+ T cells, the peak number of infected cells does not decline, but remains constant because these cells are not dying. However, two other observations make this unlikely: First, the increased loss of CD4+ T cells in CD8-depleted animals indicates that many cells are dying. Second, the Klatt and Wong results show that infected cells die at the same rate independent of the presence of CD8+ T cells in chronic infection, so why would things be so different in primary infection? How can viral load stay so high in CD8-depleted animals in acute infection if infected cells are dying at the same rate? One explanation would be that CD8+ T cell depletion leads to activation of CD4+ T cells; since SIV preferentially infects activated cells, the infected CD4+ T cells are dying, but these dying cells are being replaced because of the high level of CD4+ T cell activation. However, a recent study of CD8+ T cell depletion in acute SIV infection has excluded this possibility, by showing that CD4+ T cell activation is neither necessary nor sufficient to produce the prolonged high viral loads observed in vivo [11].
Tab. 1. Evidence for and against Cytolytic Control of HIV. 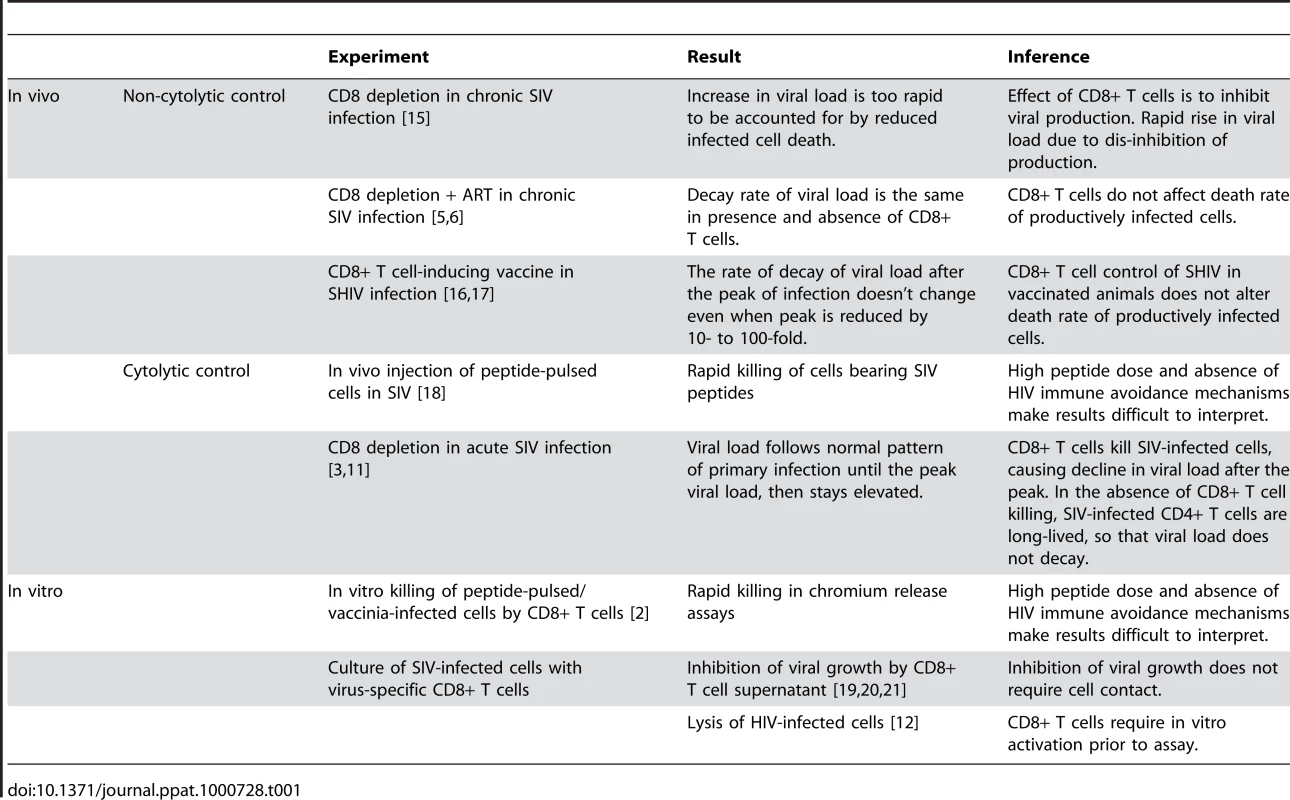
A non-cytolytic mechanism of CD8+ T cell control in vivo is also difficult to reconcile with in vitro results directly demonstrating CD8+ T cell lysis [2],[12]. Early studies of in vitro lysis relied on pulsing target cells with HIV peptides, or infecting cells with vaccinia virus bearing HIV proteins [2]. These experiments may not reflect the normal levels of peptide-MHC present on HIV-infected cells, both because they may involve unphysiologically high doses of peptide, and because HIV Nef is known to down-regulate MHC class I expression in infected cells. However, recent studies have demonstrated the recognition and lysis of HIV-infected primary target cells in vitro, presumably bearing physiological levels of peptide, and suffering any effects of Nef and other proteins on antigen presentation [12]. Notably, these studies required prior activation of the CD8+ T cells to demonstrate high levels of killing, but still suggest a role for direct killing of infected cells. A compromise between the cytolytic and non-cytolytic camps is the idea that infected cells may be killed in a “window period” before they start to produce virus [13],[14]. However, this still fails to explain all of the experimental observations.
The Klatt and Wong papers provide a direct challenge to the assumption that CD8+ T cell cytolysis of infected cells is the mechanism of viral control in HIV. However, it is difficult to reconcile the combination of in vivo and in vitro results investigating CD8+ T cell activity in HIV. Given the enormous effort invested in the development of HIV vaccines specifically directed at inducing CD8+ T cell responses, it is somewhat disconcerting to admit that CD8+ T cell function in HIV remains a “known unknown”.
Zdroje
1. US Department of Defense 2002 DoD news briefing - Secretary Rumsfeld and Gen. Myers. Available: http://www.defense.gov/transcripts/transcript.aspx?transcriptid=2636. Accessed 29 December 2009
2. NixonDF
TownsendAR
ElvinJG
RizzaCR
GallweyJ
1988 HIV-1 gag-specific cytotoxic T lymphocytes defined with recombinant vaccinia virus and synthetic peptides. Nature 336 484 487
3. SchmitzJE
KurodaMJ
SantraS
SassevilleVG
SimonMA
1999 Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283 857 860
4. PhillipsRE
Rowland-JonesS
NixonDF
GotchFM
EdwardsJP
1991 Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature 354 453 459
5. WongJK
StrainMC
PorrataR
ReayE
Sankaran-WaltersS
2010 In vivo CD8+ T-cell suppression of SIV viremia is not mediated by CTL clearance of productively infected cells. PLoS Pathog 6 e1000748 doi:10.1371/journal.ppat.1000748
6. KlattNR
ShudoE
OrtizAM
EngramJC
PaiardiniM
2010 CD8+ lymphocytes control viral replication in SIVmac239-infected rhesus macaques without decreasing the lifespan of productively infected cells. PLoS Pathog 6 e1000747 doi:10.1371/journal.ppat.1000747
7. HoDD
NeumannAU
PerelsonAS
ChenW
LeonardJM
1995 Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373 123 126
8. WeiX
GhoshSK
TaylorME
JohnsonVA
EminiEA
1995 Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373 117 122
9. BettsMR
NasonMC
WestSM
De RosaSC
MiguelesSA
2006 HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107 4781 4789
10. AlmeidaJR
PriceDA
PapagnoL
ArkoubZA
SauceD
2007 Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med 204 2473 2485
11. OkoyeA
ParkH
RohankhedkarM
Coyne-JohnsonL
LumR
2009 Profound CD4+/CCR5+ T cell expansion is induced by CD8+ lymphocyte depletion but does not account for accelerated SIV pathogenesis. J Exp Med 206 1575 1588
12. MiguelesSA
OsborneCM
RoyceC
ComptonAA
JoshiRP
2008 Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity 29 1009 1021
13. SachaJB
ChungC
RakaszEG
SpencerSP
JonasAK
2007 Gag-specific CD8+ T lymphocytes recognize infected cells before AIDS-virus integration and viral protein expression. J Immunol 178 2746 2754
14. DavenportMP
RibeiroRM
ZhangL
WilsonDP
PerelsonAS
2007 Understanding the mechanisms and limitations of immune control of HIV. Immunol Rev 216 164 175
15. JinX
BauerDE
TuttletonSE
LewinS
GettieA
1999 Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med 189 991 998
16. DavenportMP
RibeiroRM
PerelsonAS
2004 Kinetics of virus-specific CD8+ T cells and the control of human immunodeficiency virus infection. J Virol 78 10096 10103
17. DavenportMP
ZhangL
BagchiA
FridmanA
FuTM
2005 High-potency human immunodeficiency virus vaccination leads to delayed and reduced CD8+ T-cell expansion but improved virus control. J Virol 79 10059 10062
18. CheaS
DaleCJ
De RoseR
RamshawIA
KentSJ
2005 Enhanced cellular immunity in macaques following a novel peptide immunotherapy. J Virol 79 3748 3757
19. Geiben-LynnR
KursarM
BrownNV
KerrEL
LusterAD
2001 Noncytolytic inhibition of X4 virus by bulk CD8(+) cells from human immunodeficiency virus type 1 (HIV-1)-infected persons and HIV-1-specific cytotoxic T lymphocytes is not mediated by beta-chemokines. J Virol 75 8306 8316
20. WalkerCM
EricksonAL
HsuehFC
LevyJA
1991 Inhibition of human immunodeficiency virus replication in acutely infected CD4+ cells by CD8+ cells involves a noncytotoxic mechanism. J Virol 65 5921 5927
21. TomarasGD
LaceySF
McDanalCB
FerrariG
WeinholdKJ
2000 CD8(+) T cell-mediated suppressive activity inhibits HIV-1 after virus entry with kinetics indicating effects on virus gene expression. Proc Natl Acad Sci U S A 97 3503 3508
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of MetastasisČlánek Nutrient Availability as a Mechanism for Selection of Antibiotic Tolerant within the CF AirwayČlánek Type I Interferon Induction Is Detrimental during Infection with the Whipple's Disease Bacterium,
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 1- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- CD8+ T Cell Control of HIV—A Known Unknown
- The Deadly Chytrid Fungus: A Story of an Emerging Pathogen
- Characterization of the Oral Fungal Microbiome (Mycobiome) in Healthy Individuals
- Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of Metastasis
- Within-Host Evolution of in Four Cases of Acute Melioidosis
- The Type III Secretion Effector NleE Inhibits NF-κB Activation
- Protease-Sensitive Synthetic Prions
- Histone Deacetylases Play a Major Role in the Transcriptional Regulation of the Life Cycle
- Parasite-Derived Plasma Microparticles Contribute Significantly to Malaria Infection-Induced Inflammation through Potent Macrophage Stimulation
- β-Neurexin Is a Ligand for the MSCRAMM SdrC
- Structure of the HCMV UL16-MICB Complex Elucidates Select Binding of a Viral Immunoevasin to Diverse NKG2D Ligands
- Nutrient Availability as a Mechanism for Selection of Antibiotic Tolerant within the CF Airway
- Like Will to Like: Abundances of Closely Related Species Can Predict Susceptibility to Intestinal Colonization by Pathogenic and Commensal Bacteria
- Importance of the Collagen Adhesin Ace in Pathogenesis and Protection against Experimental Endocarditis
- N-glycan Core β-galactoside Confers Sensitivity towards Nematotoxic Fungal Galectin CGL2
- Two Plant Viral Suppressors of Silencing Require the Ethylene-Inducible Host Transcription Factor RAV2 to Block RNA Silencing
- A Small-Molecule Inhibitor of Motility Induces the Posttranslational Modification of Myosin Light Chain-1 and Inhibits Myosin Motor Activity
- Temporal Proteome and Lipidome Profiles Reveal Hepatitis C Virus-Associated Reprogramming of Hepatocellular Metabolism and Bioenergetics
- Marburg Virus Evades Interferon Responses by a Mechanism Distinct from Ebola Virus
- B Cell Activation by Outer Membrane Vesicles—A Novel Virulence Mechanism
- Killing a Killer: What Next for Smallpox?
- PPARγ Controls Dectin-1 Expression Required for Host Antifungal Defense against
- TRIM5α Modulates Immunodeficiency Virus Control in Rhesus Monkeys
- Immature Dengue Virus: A Veiled Pathogen?
- Panton-Valentine Leukocidin Is a Very Potent Cytotoxic Factor for Human Neutrophils
- In Vivo CD8+ T-Cell Suppression of SIV Viremia Is Not Mediated by CTL Clearance of Productively Infected Cells
- Placental Syncytiotrophoblast Constitutes a Major Barrier to Vertical Transmission of
- Type I Interferon Induction Is Detrimental during Infection with the Whipple's Disease Bacterium,
- The M/GP Glycoprotein Complex of Porcine Reproductive and Respiratory Syndrome Virus Binds the Sialoadhesin Receptor in a Sialic Acid-Dependent Manner
- Social Motility in African Trypanosomes
- Melanoma Differentiation-Associated Gene 5 (MDA5) Is Involved in the Innate Immune Response to Infection In Vivo
- Protection of Mice against Lethal Challenge with 2009 H1N1 Influenza A Virus by 1918-Like and Classical Swine H1N1 Based Vaccines
- Upregulation of xCT by KSHV-Encoded microRNAs Facilitates KSHV Dissemination and Persistence in an Environment of Oxidative Stress
- Persistent ER Stress Induces the Spliced Leader RNA Silencing Pathway (SLS), Leading to Programmed Cell Death in
- Evolutionary Trajectories of Beta-Lactamase CTX-M-1 Cluster Enzymes: Predicting Antibiotic Resistance
- Nucleoporin 153 Arrests the Nuclear Import of Hepatitis B Virus Capsids in the Nuclear Basket
- CD8+ Lymphocytes Control Viral Replication in SIVmac239-Infected Rhesus Macaques without Decreasing the Lifespan of Productively Infected Cells
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Panton-Valentine Leukocidin Is a Very Potent Cytotoxic Factor for Human Neutrophils
- CD8+ T Cell Control of HIV—A Known Unknown
- Polyoma Virus-Induced Osteosarcomas in Inbred Strains of Mice: Host Determinants of Metastasis
- The Deadly Chytrid Fungus: A Story of an Emerging Pathogen
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



