-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Global Gene Expression in Urine from Women with Urinary Tract Infection
Murine models of urinary tract infection (UTI) have provided substantial data identifying uropathogenic E. coli (UPEC) virulence factors and assessing their expression in vivo. However, it is unclear how gene expression in these animal models compares to UPEC gene expression during UTI in humans. To address this, we used a UPEC strain CFT073-specific microarray to measure global gene expression in eight E. coli isolates monitored directly from the urine of eight women presenting at a clinic with bacteriuria. The resulting gene expression profiles were compared to those of the same E. coli isolates cultured statically to exponential phase in pooled, sterilized human urine ex vivo. Known fitness factors, including iron acquisition and peptide transport systems, were highly expressed during human UTI and support a model in which UPEC replicates rapidly in vivo. While these findings were often consistent with previous data obtained from the murine UTI model, host-specific differences were observed. Most strikingly, expression of type 1 fimbrial genes, which are among the most highly expressed genes during murine experimental UTI and encode an essential virulence factor for this experimental model, was undetectable in six of the eight E. coli strains from women with UTI. Despite the lack of type 1 fimbrial expression in the urine samples, these E. coli isolates were generally capable of expressing type 1 fimbriae in vitro and highly upregulated fimA upon experimental murine infection. The findings presented here provide insight into the metabolic and pathogenic profile of UPEC in urine from women with UTI and represent the first transcriptome analysis for any pathogenic E. coli during a naturally occurring infection in humans.
Published in the journal: Global Gene Expression in Urine from Women with Urinary Tract Infection. PLoS Pathog 6(11): e32767. doi:10.1371/journal.ppat.1001187
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001187Summary
Murine models of urinary tract infection (UTI) have provided substantial data identifying uropathogenic E. coli (UPEC) virulence factors and assessing their expression in vivo. However, it is unclear how gene expression in these animal models compares to UPEC gene expression during UTI in humans. To address this, we used a UPEC strain CFT073-specific microarray to measure global gene expression in eight E. coli isolates monitored directly from the urine of eight women presenting at a clinic with bacteriuria. The resulting gene expression profiles were compared to those of the same E. coli isolates cultured statically to exponential phase in pooled, sterilized human urine ex vivo. Known fitness factors, including iron acquisition and peptide transport systems, were highly expressed during human UTI and support a model in which UPEC replicates rapidly in vivo. While these findings were often consistent with previous data obtained from the murine UTI model, host-specific differences were observed. Most strikingly, expression of type 1 fimbrial genes, which are among the most highly expressed genes during murine experimental UTI and encode an essential virulence factor for this experimental model, was undetectable in six of the eight E. coli strains from women with UTI. Despite the lack of type 1 fimbrial expression in the urine samples, these E. coli isolates were generally capable of expressing type 1 fimbriae in vitro and highly upregulated fimA upon experimental murine infection. The findings presented here provide insight into the metabolic and pathogenic profile of UPEC in urine from women with UTI and represent the first transcriptome analysis for any pathogenic E. coli during a naturally occurring infection in humans.
Introduction
Animal models of infection have provided valuable insight into diverse mechanisms of bacterial pathogenesis. Application of microarray technology to these models has further enabled analysis of bacterial global gene expression during infection of a specific host. These studies have included transcriptional profiles of pathogenic Escherichia coli in macrophages [1], host epithelial cells [2], and mice [3], [4]. More recently, a limited number of groups have measured genome-wide expression of bacterial pathogens during infections of a human host, including Vibrio cholerae in rice water stool of cholera patients [5], [6], Pseudomonas aeruginosa in sputum from cystic fibrosis patients [7], and M. tuberculosis in resected lung specimens [8]. When these data were compared to results of animal model transcriptional studies, host-specific differences were observed [8].
The urinary tract is among the most common sites of bacterial infection in humans, and E. coli is by far the most common species infecting this site, accounting for more than 80% of community-acquired infections [9]. Uncomplicated UTIs include cystitis infections in adult women who are not pregnant and do not suffer from structural or neurological dysfunction [10]. Cystitis, a clinical diagnosis presumed to represent infection of the bladder, is defined by the presence of ≥103 bacteria/ml in a midstream, clean-catch urine sample from a patient with symptoms including dysuria, urinary urgency, and increased frequency [11], [12]. Forty percent of adult women will experience symptoms of cystitis during their lifetime and there is a 25% risk that a second symptomatic episode will occur within 6–12 months [13].
Uropathogenic E. coli (UPEC) represent a specific subset of E. coli capable of colonizing the urinary tract and eliciting the symptoms of cystitis and pyelonephritis. Genetically distinct from commensal E. coli found in the intestinal tract, these strains contain numerous genomic insertions into the “backbone” E. coli chromosome, both as pathogenicity-associated islands (PAIs) [14], [15], [16] and shorter islet sequences. In pyelonephritis isolate CFT073, for example, genomic islands and islets comprise over 20% of the genome [14]. Acquired by horizontal gene transfer, PAIs often encode proteins that contribute to pathogenesis; loss of these regions may attenuate virulence [17], [18].
An array of virulence and fitness factors has been described that allow UPEC to access and persist in the urinary tract niche. Flagellin-dependent motility is required for ascension to the kidneys [19] and secreted toxins including hemolysin, cytotoxic necrotizing factor 1, and secreted autotransporter toxin elicit damage to the host epithelium [20], [21], [22]. Polysaccharide capsule [23] and immunosuppressive proteins [24] also contribute to urinary tract colonization and may allow immune evasion. Finally, as the urinary tract represents a unique nutritional niche, TonB-dependent metal acquisition systems are required for UPEC survival in this iron-limited environment [25] and recent evidence suggests that these pathogens metabolize peptides and amino acids as a primary carbon source [26]. Transcriptome analysis of strain CFT073 during murine experimental UTI demonstrated that many of these fitness factors are upregulated during infection [4].
Perhaps the most well-defined UPEC virulence factors are type 1 fimbriae, adhesive structures required for complete colonization of the murine urinary tract [27], [28], [29]. Encoded by virtually all E. coli strains, type 1 fimbriae mediate urinary tract adherence via the FimH fimbrial tip adhesin, which binds to mannosylated uroplakins located on the uroepithelium surface [30]. This interaction elicits a host response, including induction of pro-apoptotic and epithelial differentiation factors [31], as well as secretion of the pro-inflammatory cytokines interleukin-6 and IL-8 [32]. Expression of type 1 fimbriae is phase variable, controlled by an invertible DNA element that contains the promoter for the major structural subunit gene, fimA [33]. During murine experimental UTI, fimA was the fourth most highly-expressed gene and the other fim operon genes were also upregulated in vivo as compared to in vitro culture [4].
To date, much of the work in this field has used E. coli strains isolated from patients with UTI. The investigation of UPEC pathogenesis, however, has largely been conducted using in vitro models and the well established murine model of ascending UTI [34]. Volunteer colonization studies utilizing a non-pathogenic asymptomatic bacteriuria strain have shed light into mechanisms that promote E. coli survival within the human urinary tract [35], [36], but do not represent UTIs caused by E. coli with full pathogenic potential. If we wish to fill gaps in our understanding of this widespread human pathogen, it is crucial to focus on UPEC gene expression during naturally-occurring human UTI.
In this study, we measure gene expression in E. coli isolated immediately following collection from the urine of eight women experiencing symptoms of UTI. The data presented provide insight into the metabolic and virulence profile of multiple UPEC strains during human infection. We propose that E. coli utilizes an array of iron acquisition systems and has access to plentiful carbon sources during human UTI, allowing for robust replication and may downregulate or transiently express a major virulence factor, type 1 fimbria.
Results
Transcriptome analysis of E. coli UTI in urine from women with UTI
Thirty-six urine samples were collected from 34 female patients (ages 21–89, mean = 47) attending a urology clinic with presumptive bacteriuria. Nineteen urine samples were culture-negative and six specimens were culture-positive for bacterial species other than E. coli, including coagulase-negative Staphylococcus sp. (n = 2), Acinetobacter baumannii (n = 1), Enterobacter cloacae (n = 1), Enterococcus sp. (n = 1), and Klebsiella pneumoniae (n = 1). Eleven women were culture-positive for E. coli and 10 of these specimens were suitable for our study, as poor RNA yield prevented analysis of one E. coli specimen. Of these ten E. coli-positive samples, two urine specimens contained mixed infections of two different E. coli strains. O and H serotyping conducted on these 12 E. coli isolates indicated that O6 and O25, which are frequently associated with UTI isolates [37], were the most common serogroups, representing 7 of 12 strains (Table S1). Two isolates (121 and 361), obtained from the same patient on separate clinic visits (5 months apart), had identical serotypes, but could not be conclusively identified as the same strain. Antibiotic susceptibility testing indicated that these clinical isolates also showed high frequencies (up to 7 of 12 isolates) of resistance to common UTI therapies, including trimethoprim-sulfamethoxazole (Table S2).
Of the eight isolates ultimately used in our study (see below), all were collected from pyuria-positive patients who were not catheterized at the time of collection and 6 of 8 were collected from patients reporting a previous UTI. Although these isolates were obtained from urology patients, some with histories of UTI or kidney stones, extensive genotype analysis compared against a collection of over 300 E. coli isolates indicated that the virulence gene profiles of these strains most closely matched other cystitis isolates and not fecal-commensal E. coli (P. Vigil and H. Mobley, in preparation).
CFT073-specific microarrays were used to measure transcript levels from the eight E. coli isolates obtained from single-strain infections, both immediately from the urine of infected women and following static culture to mid-exponential phase in pooled human urine ex vivo. Under each condition, 20–30% of all genes measured (5379 ORFs) were classified as expressed for each isolate (Table S3). It is important to note that genes classified as not expressed may be absent or divergent in these strains.
UPEC replicates rapidly during human UTI
In all strains, the most highly expressed genes in urine from women with UTI were those encoding ribosomal subunits. Indeed, ribosomal genes comprised 24–54% of the top 50 most highly expressed genes in each clinical isolate. It is well established that E. coli rRNA and ribosomal protein mRNA synthesis increases proportionally to growth rate [38], [39]. Consistent with this, the most highly expressed non-ribosomal genes also suggest rapid bacterial growth in vivo (Table 1). Genes encoding transcription and translation machinery (infC, yfiA, rpoA, rhoI, tufA, fusA, tufB, efp), F0F1 ATPase components (atpE, atpF), fatty acid biosynthesis factors (acpP, fabI), protein folding and secretion apparatus (slyD, secG, prlA), and outer membrane components (ompA) were among the most highly expressed during human infection.
Tab. 1. Genes among the top 50 non-ribosomal genes expressed by at least half of clinical isolates in vivoa. 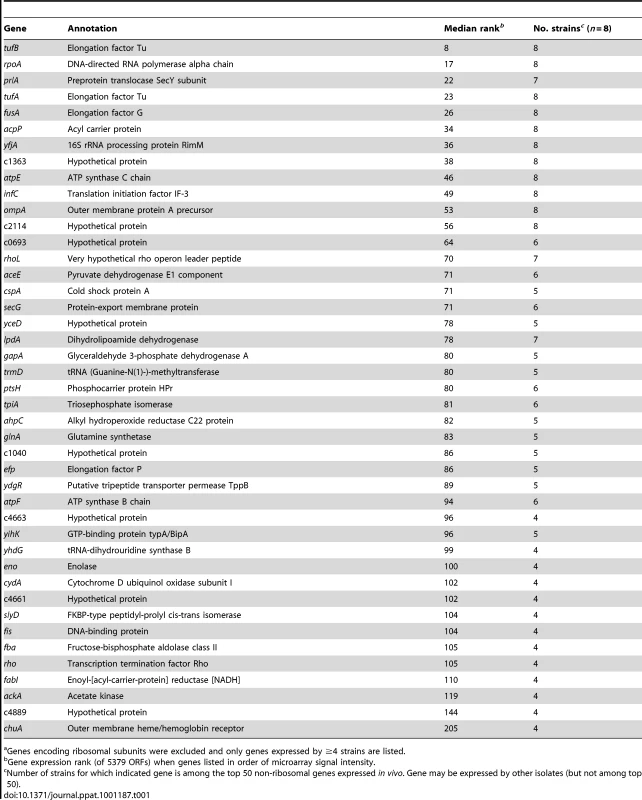
Genes encoding ribosomal subunits were excluded and only genes expressed by ≥4 strains are listed. Because variable host nucleic acid present in the in vivo samples prevented direct comparison between in vivo and in vitro conditions, a more conservative, relative comparison was derived. To identify expression differences between E. coli growth in urine during cystitis and growth in urine ex vivo, genes were ranked in order of microarray signal intensity, yielding an estimate of relative gene expression. For each isolate, these rank values were compared between in vivo and in vitro conditions. Genes for which the expression rank significantly changed between these conditions were considered differentially expressed.
Although we noted that two cold-shock-associated genes, cspA and deaD, were upregulated in human urine samples (Table 2), expression of cspA is known to occur during non-cold shock stress conditions [40], [41]. Moreover, both deaD and cspA were also upregulated by UPEC [4] and an ABU strain [35] following expulsion from the murine and human bladders, respectively. Nevertheless, to assess the effect of temperature downshift that may have occurred during sample processing, transcript levels of a representative virulence gene, fimA, were measured after a urine culture of strain CFT073 was moved from 37°C to room temperature (Fig. S1). fimA transcript levels did not significantly change after 10 min (or up to 60 min) at room temperature, suggesting that sample processing was not responsible for these results.
Tab. 2. Genes upregulated in vivo compared to growth in human urine in vitro. 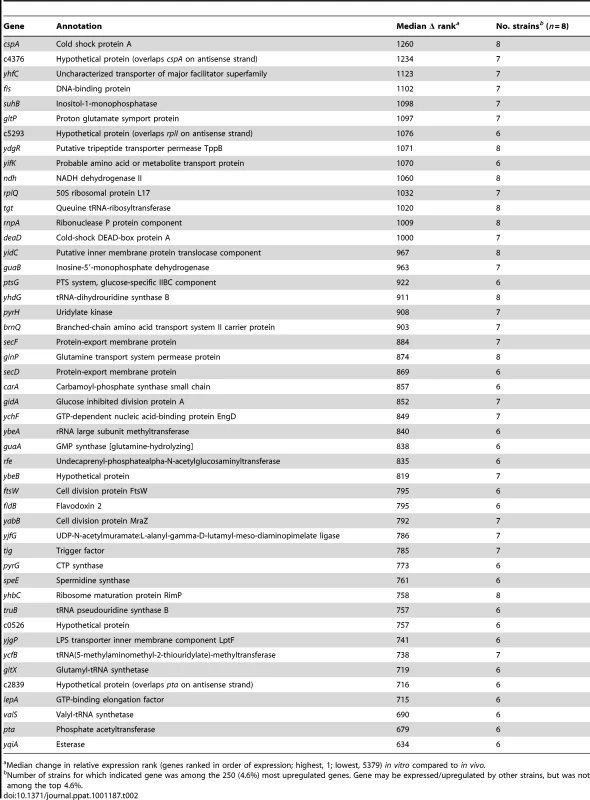
Median change in relative expression rank (genes ranked in order of expression; highest, 1; lowest, 5379) in vitro compared to in vivo. Cell division factors (ftsW, mraZ), tRNA processing and translation machinery (rnpA, tgt, rplQ, rimP, truB, valS, gltX, yhdG, ybeA, ycfB, lepA, ychf), and protein secretion components (secD, secF, yidC) were among genes upregulated in vivo by the majority of strains, suggesting that E. coli may be replicating faster in the human urinary tract than in vitro (Table 2). Similarly, genes involved in nucleotide synthesis (guaA, guaB, pyrG, pyrH), lipopolysaccharide assembly (lptF), peptidoglycan recycling (yjfG), and enterobacterial common antigen synthesis (rfe) were also upregulated. Taken together, these data indicate that E. coli was likely replicating very rapidly during symptomatic UTI in women.
Microarray correlates with qPCR
To confirm these microarray findings, qPCR was performed using in vivo - and in vitro-derived cDNA templates of two representative E. coli strains isolated in this study, AL151 and AL371. Strains were selected based on availability of in vivo cDNA. The threshold cycle (Ct) values of the 13 genes measured (gapA, hma, fyuA, fimA, papA_2, fliC, sat, sisA, glnA, gdhA, ackA, acs, and rplD) correlated (P<0.001 or r<−0.8431) with the normalized microarray signal intensities of each gene (Fig. 1A). Similarly, genes identified as differentially expressed between in vivo and in vitro conditions were consistently found to be up - or down-regulated in these two strains by qPCR measurement (Fig. 1B). Finally, PCR using in vivo-derived cDNA generally confirmed expression of select virulence genes (Fig. 1C).
Fig. 1. E. coli gene expression in voided urine from cystitis patients and culture in urine ex vivo. 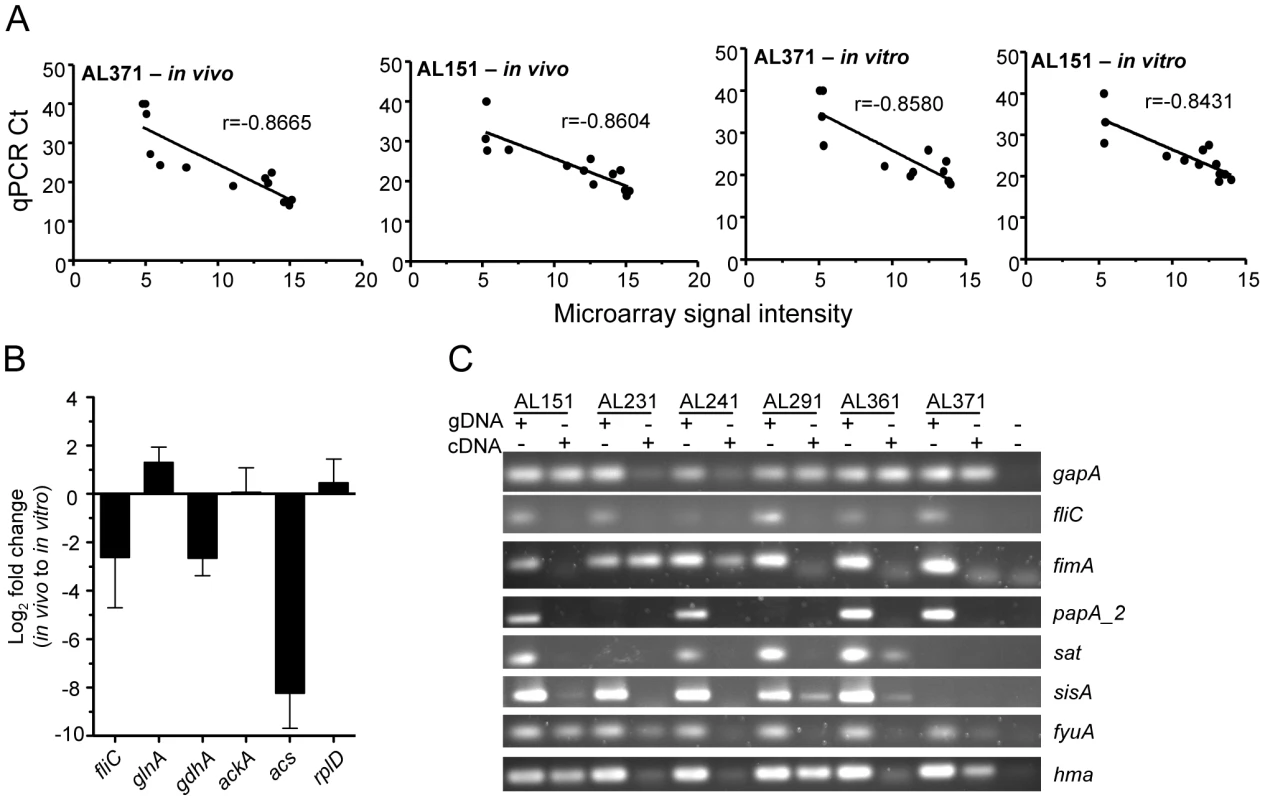
(A) Correlation of gene expression levels obtained by microarray and qPCR. Ct values determined by qPCR are plotted for 13 genes (see text) versus normalized microarray signal intensity for in vivo (left two panels) and in vitro (right two panels) cDNA samples from patient isolates 371 (first and third panel) and 151 (second and last panel). Correlation coefficient (r) values are shown and P<0.001 for all correlations. (B) Genes differentially expressed during UTI in women and culture in urine ex vivo. Log2 fold changes in vivo, compared to in vitro expression, were measured by qPCR and are normalized to gapA transcript. (C) Confirmation of in vivo gene expression by clinical isolates as shown by PCR using genomic DNA or cDNA template. E. coli undergoes aerobic or microaerobic respiration during human UTI
Genes involved in aerobic respiration tended to be highly expressed by most strains assessed immediately after expulsion from the human bladder. Encoding a component of cytochrome d complex, which is maximally expressed during microaerobiosis, cydA was among the top 50 most expressed non-ribosomal genes by four isolates (Table 1) and was in the top 3% of genes expressed by all strains in vivo and was similarly expressed during urine culture in vitro. Expression of the cytochrome o oxidase genes cyoABCDE, which are expressed in oxygen-rich conditions, varied among patients. These genes were strongly expressed by 5 of 8 strains in vivo (top 7%), more weakly expressed by 2 of 8 strains (top 20–30%), and just below background in one strain. This is in contrast to a recent E. coli transcriptome study for an asymptomatic bacteriuria strain, which noted consistent downregulation of cyoABCD during intentional colonization of the human bladder [35]. For all strains, expression of cyoABCD and cydAB did not appear to differ between in vivo and in vitro samples or from expression by E. coli CFT073 during experimental infection of the murine urinary tract [4], suggesting similar oxygenation in these conditions.
Genes that encode a terminal electron acceptor pathway used during anaerobiosis were not uniformly expressed by all strains. For example, the genes encoding nitrate reductase I, narGHJIK, were only expressed in vivo by two strains, AL121 and AL151. Formate dehydrogenase N (fdnGHI), induced by nitrate [42] and anaerobic conditions [43], was expressed by E. coli in half of UTI patients (isolates AL121, AL151, AL291, and AL361). Nitrite-inducible genes nirBDC were also expressed in half of patients (isolates AL121, AL151, AL291, and AL371). Interestingly, the three patient isolates that only weakly expressed cyoABCDE also expressed nitrate reductase, formate dehydrogenase N, and nitrite reductase, suggesting that these isolates likely experienced a more anaerobic and nitrate/nitrite-rich environment and may be using these products for anaerobic respiration. Together, these data indicate that oxygen and/or nitrate levels in voided urine vary by patient and suggest that pathogenic E. coli adapts to utilize either form of respiration.
E. coli experiences nitrogen limitation in vivo
Previous transcriptomic analysis of E. coli indicated that the murine urinary tract is nitrogen-limiting for this pathogen, despite a high urea concentration in urine [4]. Glutamine synthetase (glnA), which assimilates ammonia with high affinity in an energy-dependent manner and is transcriptionally induced by nitrogen-limited growth [44], was among the most highly expressed genes in urine from 5 of 8 cystitis patients (Table 1). By qPCR, glnA was upregulated in vivo (2.9-fold) in the two strains tested (AL151 and AL371), while the low affinity energy-independent glutamate dehydrogenase gdhA, which is transcriptionally repressed in low nitrogen conditions [45], was downregulated 8.3-fold (Fig. 1B). This indicates that E. coli similarly experiences nitrogen limitation during infection of the human urinary tract. High concentrations of nitrogen in urea are not available to the urease-negative E. coli.
Carbon metabolism suggests abundant carbon sources in vivo
The gene encoding acetyl-CoA synthetase, acs, was one of the most strongly downregulated genes in all E. coli strains during growth in urine from women with UTI as compared to culture in urine ex vivo (Table 3). Involved in acetate assimilation by conversion to pyruvate, expression of this enzyme is activated by decreasing oxygen and increasing cAMP levels [46]. In contrast, genes involved in acetate excretion, shown to contribute to urovirulence in a murine model [47], were strongly expressed in vivo. The phosphate acetyltransferase (pta) gene was among the top 100 genes upregulated in vivo in 6 of 8 isolates (Table 2) and acetate kinase (ackA) was one of the most highly expressed non-ribosomal genes in 50% of the strains (Table 1). By qPCR, acs was downregulated nearly 600-fold in vivo, while ackA was relatively unchanged (Fig. 1B). Acetate-induced genes [48] glcBGD and the glyoxylate shunt genes aceAB were also downregulated (Table 3). Furthermore, components of the pyruvate dehydrogenase complex, which functions upstream of AckA-Pta to convert pyruvate to acetyl-CoA, aceE and lpdA were among the most highly in vivo expressed genes in the majority of patient isolates (Table 1). These expression patterns imply that E. coli produces, but does not assimilate acetate during growth in the human bladder.
Tab. 3. Genes downregulated in vivo compared to growth in human urine in vitro. 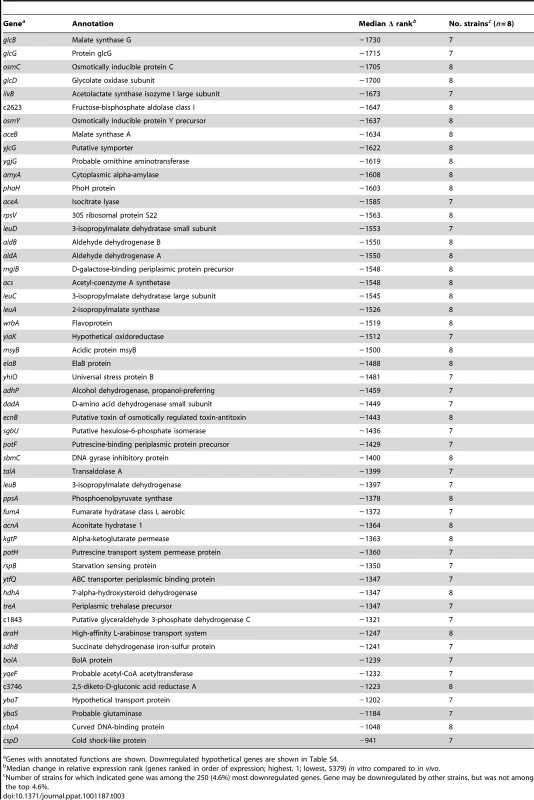
Genes with annotated functions are shown. Downregulated hypothetical genes are shown in Table S4. In bacteria, the acetate switch occurs when cells transition from acetate dissimilation to assimilation. The expression of dissimilatory genes by E. coli during infection of the human urinary tract indicates not only that the bacteria have not yet reached stationary phase in vivo, but also that they may be utilizing acetogenic carbon sources. Acetogenesis occurs during mixed acid fermentation under anaerobic conditions or aerobically when rapid growth on excess carbon sources limits flux through the tricarboxylic acid (TCA) cycle by excessive NADH production (reviewed in [49]). Because data presented here support rapid aerobic or microaerobic growth of E. coli during human UTI, this suggests that the observed acetogenesis is due to “overflow metabolism” and not mixed acid fermentation. This further implies that easily assimilable carbon sources are available to UPEC during symptomatic UTI.
Peptides and amino acids have been previously implicated as primary carbon sources for UPEC during colonization of the murine urinary tract [26]. Di - and oligopeptide transport components dppA and oppA, shown previously to contribute to UPEC virulence [26], were strongly expressed by all strains during UTI in women (Fig. 2A). Additionally, a putative tripeptide transporter, ygdR (tppB), was upregulated in vivo by all E. coli isolates (Table 2) and was among the top 50 most highly expressed non-ribosomal genes in 5 of 8 strains (Table 1). Other amino acid or metabolite transporters, yifK, yhfC, and brnQ were also upregulated in vivo. These findings suggest that E. coli may use peptides as an energy source during human UTI, as well.
Fig. 2. E. coli virulence factor expression in urines from UTI patients. 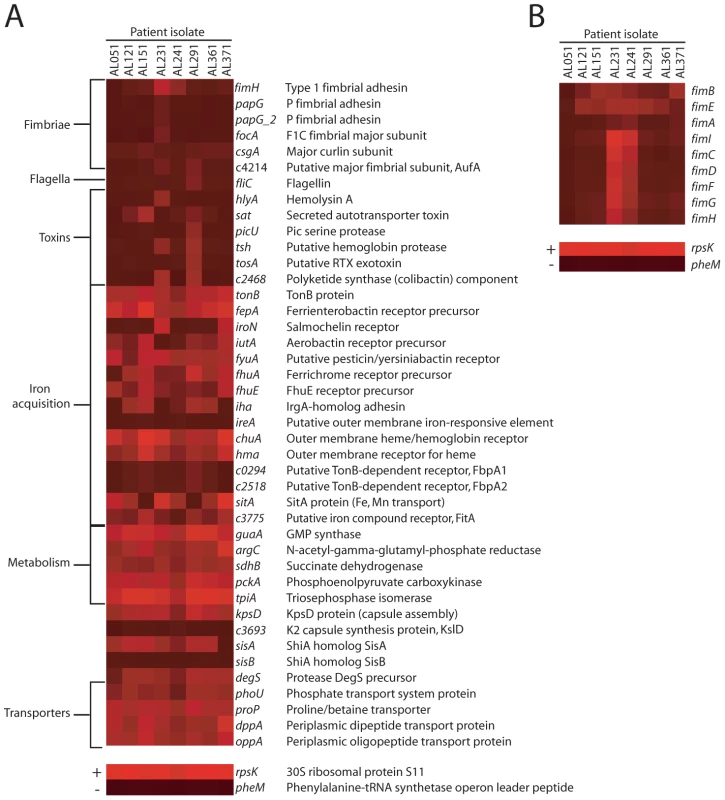
Heat maps indicate normalized microarray signal intensities for genes encoding (A) UPEC fitness factors and (B) genes in the fim locus in eight E. coli isolates in urine collected from women with cystitis. For reference, the overall most (rpsK, +) and least (pheM, −) expressed genes are shown in the bottom panels, representing average signal intensities of 15.821 and 3.881, respectively. When compared to culture in urine ex vivo, E. coli in voided urine from UTI patients downregulated a number of genes involved in metabolic functions (Table 3). The talA gene, which encodes one of two transaldolases in the E. coli pentose phosphate pathway and does not to contribute to UPEC fitness in a murine model [26], was downregulated. Consistent with rapid growth and TCA cycle saturation, genes in this pathway (sdhB, acnA, and fumA) as well as α-ketoglutarate permease (kgtP), which imports a TCA cycle intermediate, were also downregulated. Indeed, growth on excess glucose (an easily assimilable carbon source) has long been appreciated to inhibit expression of sdhAB [50] and the succinyl-CoA synthetase complex (reviewed in [49]). Overall, dehydrogenase-type enzymes were frequently downregulated in vivo compared to growth in vitro, consistent with the hypothesis that rapid growth in vivo requires E. coli to adapt by modifying the TCA cycle to optimally regenerate NAD+. Together, the finding that genes involved in acetogenic growth, peptide import, and the TCA cycle are expressed or modulated during human cystitis is consistent with the requirement of these pathways for UPEC virulence and support the current model of UPEC metabolism during UTI [26], [47].
Virulence gene expression in vivo
While a number of studies have assessed expression of UPEC virulence genes during experimental infection of animal models [3], [4], [51], much less is known about expression patterns during human infection. These microarray data show most strikingly that the expression of genes involved in adherence to host tissues that are highly expressed during murine infection [4], was not detected in the majority of patient isolates (Fig. 2A). Genes encoding P, F1C, or Auf fimbriae were not expressed (i.e., below background) in any E. coli strains after expulsion from the human urinary tract or gene absence/divergence otherwise prevented detection of these transcripts. Furthermore, the type 1 fimbrial adhesin fimH, which is required for virulence during murine infection [27], [28] and invasion [29], was only expressed at detectable levels by E. coli in 2 of 8 patients. These strains, AL231 and AL241, also expressed the remaining genes in the fim locus, while the other six strains did not (Fig. 2B). To validate these microarray findings, PCR was performed on in vivo-isolated cDNA from all strains except AL051 and AL121 (for which no in vivo-derived cDNA sample remained). This confirmed the expression of the major structural subunit fimA only by strains AL231 and AL241, as well as lack of P fimbrial (papA_2) gene expression by strains AL151, AL241, AL361, and AL371 (Fig. 1C). Strains AL231 and AL241, which were shown to express fimA in vivo by RT-PCR (Fig. 1C), encoded genes that were 90% identical to fimACFT073 (Fig. S4). Because fimA transcripts were not detected by microarray for these strains (Fig. 2), we can conclude that our microarray hybridization conditions required greater than 90% sequence identity to yield signal significantly above background. PCR results for additional virulence genes were also generally consistent with their expression by microarray (Fig. 1C, Fig. 2A).
Few patient isolates appeared to express toxin-encoding genes with significant sequence identity to CFT073 toxins above background levels in vivo (Fig. 2A). Two isolates expressed sat or genes encoding the non-ribosomal peptide/polyketide colibactin significantly above background and expression of both colibactin and tsh was only detected in a single isolate (strain AL291). Consistent with transcriptome data from murine UTI [4], flagellin (fliC) gene expression was downregulated in vivo, as compared to in vitro culture (Fig. 1B).
As alluded to above, metabolic pathways have also been implicated in UPEC virulence. The central metabolic pathways of gluconeogenesis and the TCA cycle are required for UPEC fitness in the murine urinary tract [26] and sdhB, pckA and tpiA, encoding enzymes in these pathways, although downregulated compared to growth in vitro, were nonetheless expressed in all patient isolates (Fig. 2A). Other metabolic genes implicated in urovirulence, including guaA and argC [52] were also expressed in vivo by all E. coli strains and guaA was upregulated relative to culture in urine ex vivo (Table 2). Expression of D-serine dehydratase (dsdA), which processes the putative metabolite signal D-serine [53], was detected in half of the bladder-expelled E. coli strains measured.
Genes involved in siderophore production and iron acquisition were globally the most highly expressed fitness determinants across all eight isolates following infection of the human urinary tract. All isolates robustly expressed tonB, fepA, and fyuA, although genes for the synthesis of enterobactin and yersiniabactin siderophores were sporadically detected, possibly due to inherently lower transcript levels for these enzymes (Fig. 2A). Nearly all (7 of 8) strains expressed the heme receptor-encoding chuA and in half of patients, this gene was among top 50 most highly expressed non-ribosomal genes (Table 1). In contrast, the heme receptor hma was expressed above background in just 4 of 8 patient isolates. In vivo expression of genes for the salmochelin receptor iroN and aerobactin receptor iutA was only observed in two isolates, while receptor genes ireA, fpbA1 and fbpA2 were not expressed by any strains, either in vivo or in vitro. Despite these differences, all strains were capable of growth in vitro under iron-limiting culture conditions in the presence of the chelator 2′2-dipyridyl (200 µM), although strains AL231 and AL241 could not replicate under more stringent iron-limitation (400 µM) (Fig. S2). These data provide further support for the well-established model of the human urinary tract as an iron-limiting environment [4], [25], [35].
Interestingly, in vivo and in vitro expression of iron uptake systems did not always correlate and isolates occasionally expressed a specific system in only one condition. For example, patient isolates AL121 and AL241 expressed genes coding for yersiniabactin production only during in vitro culture, while strain AL231 expressed these genes strictly during in vivo conditions (Fig. S3). For strain AL231, poor in vitro expression of these and other genes involved in iron acquisition (including fep/ent and iro loci) correlated with the strain's inability to replicate under stringent iron limitation (Fig. S2). This apparent differential expression of iron uptake genes implies that the extent of iron limitation (and by implication, Fur regulation) differs among individual patients, as well as between the human bladder and urine ex vivo. Furthermore, it suggests additional regulation of iron acquisition systems during E. coli infection; indeed oxygen tension has been shown to affect expression of iron uptake genes by other pathogens [54].
Pathogen-specific genes expressed during human cystitis
A subset of fitness genes expressed by E. coli following infection of the human urinary tract was specific to pathogenic E. coli. That is, these genes are absent from the fecal-commensal E. coli strain K12, suggesting that they represent horizontally-acquired, putative fitness genes. Iron acquisition components (fyuA, chuA, chuS, chuW, chuX), capsule synthesis genes (kpsF, kpsD, kpsU, kpsC), an inflammatory suppressor (sisA), a PAI-associated prophage integrase gene (c2418), and several hypothetical genes were expressed by the majority of UPEC patient isolates in vivo (Table 4). These data indicate that the patient isolates examined here express an array of pathogen-specific genes in vivo and further imply that they indeed represent pathogenic E. coli strains.
Tab. 4. Pathogen-specifica genes expressed in the majority of urines from women with UTI. 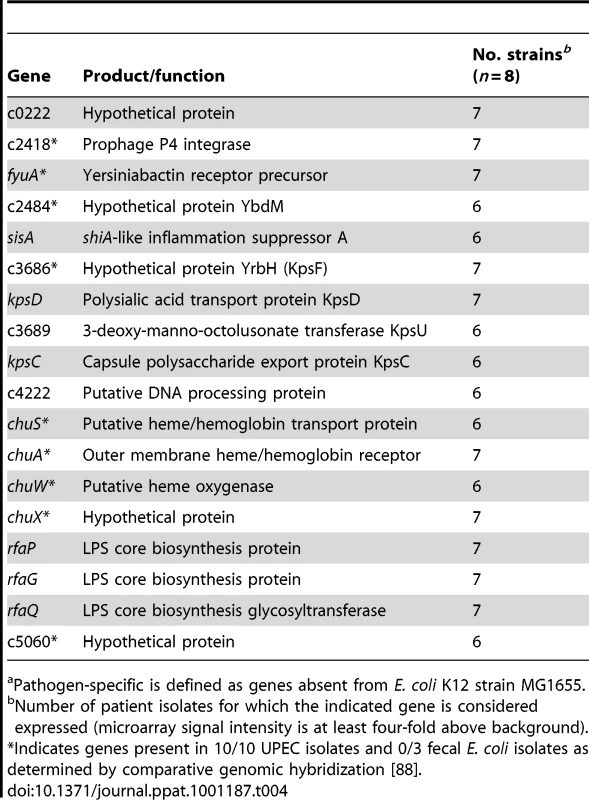
Pathogen-specific is defined as genes absent from E. coli K12 strain MG1655. Expression of iron uptake and metabolic genes, but not adhesin genes, is similar in voided urine from murine and human UTI
To compare UPEC virulence gene expression in different mammalian hosts, relative expression levels of 46 fitness genes by CFT073 following experimental murine UTI (derived from [4]) were compared to the average relative expression levels (average expression rank) by E. coli patient isolates following human UTI. Overall, relative UPEC virulence gene expression in mice positively correlated with expression in a human host (Spearman r = 0.5890; P<0.0001). Genes involved in iron acquisition and metabolism correlated most strongly between the two data sets, with most having less than 10% difference in relative (ranked) expression (Fig. 3). fyuA was the exception, due to its poor expression by CFT073 [4], likely attributed to mutations preventing yersiniabactin production [15], [55]. Expression of toxin-encoding genes was moderately similar in expelled urine of the two hosts, differing by only 30–50%. In contrast, adhesin and fimbriae expression was quite different between human and murine urine, with most genes having more than 50% relative expression difference between the two hosts. For example, fimA was the fourth most highly expressed gene (relative expression value: 5375) by E. coli CFT073 during murine infection, while it had an average rank of 3873 out of 5379 ORFs in women with UTI (relative expression value: 1506).
Fig. 3. Host-specific virulence gene expression by E. coli. 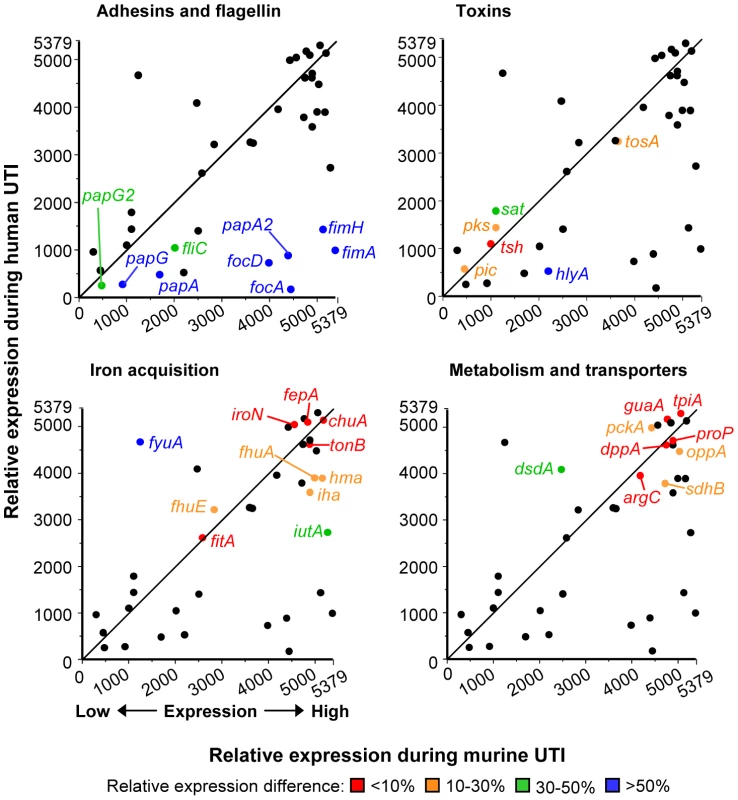
Relative virulence and fitness gene expression by E. coli isolates in urine from human UTI plotted versus relative expression by E. coli CFT073 in urine from experimental murine UTI. Line shows the theoretical perfect correlation. Panels highlight fitness genes encoding adherence and motility factors (top left), toxins (top right), iron acquisition systems (bottom left), and metabolic and transport proteins (bottom right). Relative expression is based on normalized microarray signal intensity rank (1, gene with lowest signal; 5379, gene with highest signal). Relative human expression is the median rank of all eight isolates, except for fimA, papA, papA_2, hlyA, sat, pic, tsh, tosA, chuA, iutA, iroN, fyuA, hma, iha, and sitA, which are medians from isolates positive for given gene by PCR (P. Vigil and H. Mobley, in preparation). Highlighted genes are colored to indicate the expression rank difference of each gene between human and murine UTI: red, less than 10% difference in expression rank; orange, 10–30% difference; green, 30–50% difference; blue, greater than 50% difference. Spearman rank correlation coefficient for all genes is r = 0.5890 (P<0.0001). Murine data were derived from our previously-published transcriptome study [4]. Clinical UPEC isolates express type 1 fimbriae in vitro
Surprisingly, we found that most (6 of 8) E. coli isolates did not express type 1 fimbriae in the urine of patients with UTI. One possible explanation is that these strains do not encode intact type 1 fimbrial genes or that expression of these genes is defective. To distinguish among these possibilities, we examined expression of type 1 fimbriae in vitro by the 8 clinical isolates. Using a PCR-based assay (Fig. 4A), we determined the orientation of the fim invertible element, the 314 bp region that contains the promoter for the major structural subunit fimA and is responsible for phase variation of type 1 fimbriae [33]. As expected, after two 48 h static passages to enrich for type 1 fimbriae expression, all strain populations consisted of bacteria in both the phase-on and phase-off orientations (Fig. 4B). In contrast, when isolates were cultured with aeration for 4 h, only the phase-off orientation could be detected. Invertible element orientations correlated with detection of FimA by western blot (Fig. 4C). Except for strain AL051, from which neither fimA nor the invertible element could be PCR-amplified, all strains expressed an approximately14 kDa protein that reacted with antiserum raised against FimACFT073. Band intensity may reflect differences in expression level among strains or, more likely, differences in antibody reactivity to diverse FimA antigens. Indeed, the nucleotide sequences of the fimA genes of the clinical isolates are 90–99% identical to fimACFT073, with strain AL371 having the highest identity (Fig. S4).
Fig. 4. In vitro expression of type 1 fimbriae by clinical E. coli isolates. 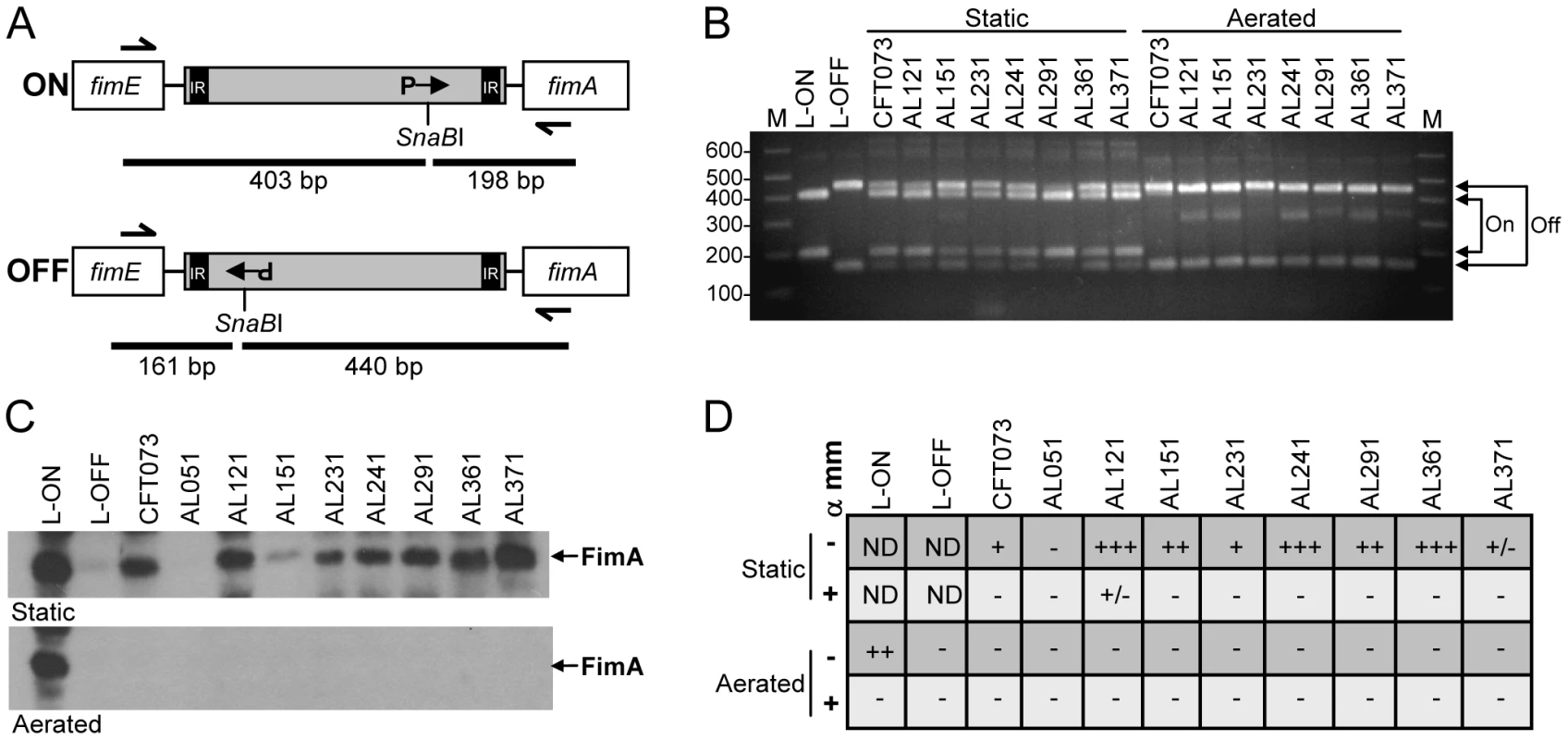
(A) Invertible element (IE) assay for type 1 fimbriae [73]. IE is shown in gray; inverted repeats (IR) located within the IE; orientation of the fim promoter (P) indicated by arrow in phase-on (top panel) and phase-off (bottom panel) positions. Expected fragment sizes following PCR (half arrows show primer sites) and asymmetrical SnaBI digest are indicated by black bars. (B) IE orientation determined as described in (A) for strains cultured statically under type 1 fimbriae-enriching conditions or with aeration. Arrows indicate expected sizes for phase-on and phase-off. CFT073 strains with mutations preventing IE switching and locking the “phase-on” (L-ON) or “phase-off” (L-OFF) orientation are also included. M, molecular mass standards; sizes (in kb) are indicated. (C) Western blot using anti-FimA polyclonal antibody. Strains were cultured as in (B) and acid-treated whole cell lysate separated on 15% SDS-PAGE gels. Top panel, type 1 fimbriae-enriching static culture; bottom panel, aerated culture. Arrows indicate the ∼12 kDa FimA band. (D) Mannose-sensitive hemagglutination. Strains were cultured as in (B) (statically, top two rows; aeration, bottom two rows) and their ability to agglutinate guinea pig erythrocytes in the presence (+) or absence (−) of alpha-methyl-mannoside (α mm) was assessed. −, no agglutination; +/−, weak agglutination; +, agglutination with undiluted or 1∶2 dilution of bacterial suspension; ++, agglutination with 1∶4 or 1∶8 dilution; +++, agglutination with 1∶16 or 1∶32 dilution; ND, not determined. Data represent median agglutination reactions of three independent experiments. Finally, to assess the assembly of functional fimbriae, the ability of these isolates to agglutinate guinea pig erythrocytes in a mannose-sensitive manner was measured. With the exception again of AL051, all strains exhibited some degree of mannose-sensitive hemagglutination, indicative of type 1 fimbrial production (Fig. 4D). Strain AL371 consistently displayed weak, but detectable hemagglutination. Together, these data demonstrate that, although expression of type 1 fimbrial genes was generally not detected in the urines of cystitis patients, 7 of 8 clinical E. coli isolates obtained from women with UTI are capable of appropriately expressing functional type 1 fimbriae in vitro.
Clinical UPEC isolates upregulate fimA during murine UTI
The transcriptome analyses presented in this study identified genes differentially expressed in the urine of different mammalian hosts (Fig. 3). However, these data were obtained by comparing the expression of E. coli clinical isolates following collection from human UTI with E. coli strain CFT073 expression during murine experimental UTI. As a result, the expression incongruencies could be due to inherent strain-specific differences that exist between the model E. coli strain CFT073 and the clinical isolates collected from infected women. To address this, the isolates (except for Fim− strain AL051) were tested in the murine model of ascending UTI. Unlike fecal strain EFC4, which was shown previously to have low infectivity in mice [56], all isolates except AL241 colonized the bladders of CBA/J mice (Fig. 5A and data not shown).
Fig. 5. Murine colonization and type 1 fimbriae expression by clinical isolates. 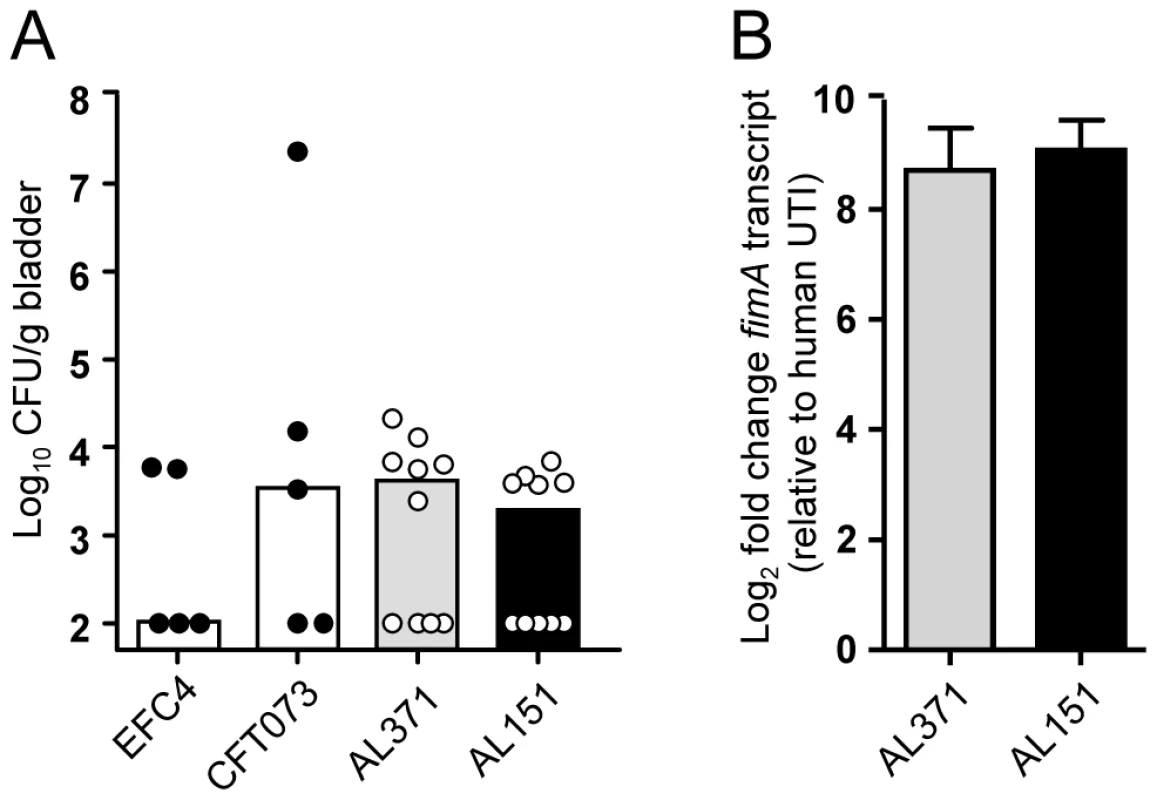
CBA/J mice were transurethrally inoculated with 108 CFU E. coli EFC4 (fecal strain), CFT073 (model pyelonephritis strain), or clinical isolates AL371 or AL151. (A) Bladder colonization at 48 hpi. Symbols represent data from individual animals and bars indicate the median. (B) fimA expression by isolates AL371 (gray bars) and AL151 (black bars) during murine UTI. Urine was collected from infected mice 6–48 hpi and transcript levels from expelled bacteria measured by qPCR. For each strain, urines were pooled from five mice (n = 10) and represent two biological replicates. Expression fold change following expulsion from the murine bladder relative to expression following expulsion from the human bladder is shown. To directly compare the gene expression of a single strain after its colonization of the human and murine urinary tracts, urine was collected and pooled throughout the 48 h infection for strains AL151 and AL371 and transcripts isolated from these pooled samples were quantified by qPCR. In contrast to human infection, both clinical isolates strongly upregulated fimA in the urine of infected mice (Fig. 5B). Relative to expression during human UTI, fimA was upregulated 660 - and 640-fold in the murine bladder by strains AL151 and AL371, respectively. These data, which reflect the change in relative expression rank between murine and human infection (Fig. 3), indicate not only that strains AL151 and AL371 are capable of expressing type 1 fimbriae in vitro, but that they robustly express fimA during murine UTI. Thus, it appears that these E. coli strains had downregulated type 1 fimbriae in the urine of women with cystitis.
Discussion
Here we report, for the first time, the transcriptional profile for any pathogenic E. coli following a naturally-occurring human infection. This study represents the largest number of subjects for any human transcriptome study and includes the largest number of bacterial strains studied in such experiments. Our data suggest that E. coli had access to abundant carbon sources prior to expulsion from the human bladder and replicates rapidly, expressing genes involved in nitrogen assimilation, iron acquisition, and virulence, while variably expressing or downregulating at least one major adhesin as assessed in voided urine at the time of sample collection.
Although the women sampled have a history of recurrent UTI, our data suggest that the E. coli strains measured in this study represent UPEC strains with full pathogenic potential. All strains were able to colonize a mouse model of ascending UTI, unlike fecal-commensal E. coli, and genotype analysis classified them with normal cystitis strains (P. Vigil and H. Mobley, in preparation). All patients in the present study had pyuria, indicating the presence of a robust inflammatory response, which is generally absent in patients with asymptomatic bacteriuria [57]. Nonetheless, it is important to note that the data presented here indeed represent a limited sampling of patients and future studies should expand this analysis to a larger population.
Iron acquisition systems were the most highly expressed virulence determinants across all eight patient isolates in vivo. In addition to bacteria isolated from urine, we and other groups have observed expression of outer membrane iron receptors by bladder cell-associated UPEC, both in vitro [58] and in vivo [51]. Iron acquisition is required for urinary tract colonization [25] and UPEC isolates produce siderophores that are not synthesized by most non-pathogenic fecal E. coli strains [59]. Consequently, outer membrane iron receptors have been examined as targets of a vaccine to protect against E. coli UTI [60], [61]. Data presented here indicate that at least one protective antigen, Hma, is expressed by at least half of E. coli populations during expulsion from the human urinary tract. Of note, the antigen that generated the highest IgA titer, IreA, was not expressed by any patient isolate examined, while the non-protective antigen ChuA was among the most highly expressed genes in all strains. It is interesting to speculate that pressure from the host adaptive immune response may represent negative selection against these genes and/or their regulatory elements.
Despite the iron - and nitrogen-limiting conditions within the human urinary tract, our data support a model of robust UPEC replication during infection. When rapid growth and excess reducing equivalents (NADH and FADH2) impose a limitation on the oxidative-dependent TCA cycle, acetate can be excreted to maintain redox homeostasis and recycle coenzyme A [49]. Recently, Welch and colleagues demonstrated that UPEC is better adapted to acetogenic growth than E. coli K12 and showed that mutants defective in acetate dissimilation (pta and ackA) had reduced fitness during murine UTI, while a mutant defective in acetate assimilation (acs) did not [47]. Our data support these findings and suggest that UPEC is undergoing similar growth and metabolism during human cystitis. Furthermore, this implies that acetyl-phosphate, which accumulates during acetogenic growth and can act as an intracellular signal, could play a role in UPEC pathogenesis during UTI in women. While we indeed observed differential expression of a number of metabolic genes, flux through these pathways is often regulated posttranscriptionally by enzyme activity and allosteric mechanisms [62], [63], so it is not surprising that a complete metabolic profile cannot be detailed solely from transcriptional data.
Our data suggest that growth of E. coli in the human urinary tract is similar to its replication in a chemostat culture. Urine has been described as a mixture of small peptides and amino acids [64] and urine production by the kidneys assures that this medium is continuously replenished. Thus, in contrast to culture in urine ex vivo, E. coli in the human urinary tract likely has constant access to easily assimilable carbon sources. These results also imply that, although nitrogen and iron are limiting in this environment, E. coli acquires adequate quantities of these elements for robust replication.
Whether E. coli present in voided urine accurately represent the physiological state of the bacteria attached to and within the bladder mucosa is unclear [65]. Because the majority of UTI pathogenesis occurs on the bladder epithelium, the critical contribution of adherent bacteria is apparent. In contrast, the pathogenic contribution of luminal E. coli, which are diagnostic for UTI, is largely undefined. While several groups have measured global gene expression by various E. coli strains in urine from infected mice [3], [4] and humans [35], the transcriptome of bladder-associated UPEC has not yet been described and is obviously not feasible in human patients. However, genes identified as highly expressed or upregulated in the urine of infected mice, such as type 1 fimbriae and iron acquisition systems [4], frequently have roles in colonization [25], [28], [66], [67], suggesting that these genes are expressed at some point during association with the murine bladder. Furthermore, qPCR analysis of laser-capture microdissected UPEC from within urothelial cells of infected mice showed increased expression of ferric iron acquisition genes, including the heme receptor chuA [51]. These data are consistent with our transcriptome studies of experimental [4] and natural UTI; both identified chuA as one of the most highly expressed genes in the urine of infected mice and cystitis patients. Taken together, these studies strongly suggest that, at least in mice, voided urine represents a reasonable estimate of virulence gene expression during cystitis.
Surprisingly, none of the major adherence factors described for UPEC were appreciably expressed in bacteria in urine voided from the human bladder. Because surface structures like fimbriae are known to vary antigenically, sequence dissimilarities between the clinical isolates tested and the UPEC genome represented on the microarray likely contributed at least partially to the low fimbrial detection. Indeed, for type 1 fimbriae, sequence divergence appeared to account for the low detection of the major structural subunit gene fimA, as transcript could not be detected for any isolate by microarray, while strains AL231 and AL241 were shown to express this gene by qPCR. However, expression of the remaining fim locus was indeed detected for these two patient isolates (AL231 and AL241), indicating that these genes may be more conserved. Similarly, at least four patient isolates encode the major P fimbrial subunit papA_2 (AL151, AL241, AL361, AL371), but neither microarray nor qPCR could detect papA_2 transcript in the corresponding in vivo samples. Although not critical for virulence in a murine model of infection [68], P fimbriae have long been associated with pyelonephritis in humans [37]. Indeed, E. coli that react with anti-P fimbrial antibodies can be isolated from the urine of patients with UTI [69], [70], [71]. The fact that P fimbrial gene expression was not detected in our patient isolates may be due to temporal or localized expression of these genes, or a result of our focus on patients with cystitis, rather than pyelonephritis.
Data from previous studies have implied expression of type 1 fimbriae by a small or variable subset of E. coli in human urine. Indirect immunofluorescence of bacteria present in urine of patients with acute UTI has yielded varied results with respect to type 1 fimbrial detection. Several studies identified type 1 fimbriate cells in less than 38% [70] or 45% [72] of urine samples, but nearly in 100% of the same isolates following in vitro culture, while another group observed type 1 fimbriate cells in 76% of urine specimens [69]. Experiments from our laboratory quantified the type 1 fimbrial invertible element switch orientation in a bacterial population collected directly from the urine of 11 women with E. coli UTI. In that study, the switch was primarily in the “off” position within this population; for all 11 cases, bacteria in patient urine averaged only 4% “on” [73]. While there appears to be some variation among patient populations, these findings are overall consistent with our observation that only 25% of E. coli isolates expressed type 1 fimbrial genes in urine collected from cystitis patients.
Several models could account for our finding that the majority of E. coli are not transcriptionally active for type 1 fimbriae in urine collected from cystitis patients. First, it is possible that E. coli present in voided urine represent the nonadherent “losing” fraction of the population that is expelled from the bladder. However, the samples used in our microarray were not processed to remove exfoliated epithelial cells, so it is likely that both planktonic and adherent bacteria were present to some extent and that neither population significantly expressed type 1 fimbriae. Moreover, measurement of the UPEC transcriptome during murine UTI also relied on the collection of expelled urine from infected animals and those data identified fimA as the fourth most highly in vivo-expressed gene. Nonetheless, adhesin gene expression differences between adherent and planktonic E. coli during human infection likely contributed to our results and should be further examined in future studies.
The duration of infection likely also contributed at least somewhat to our variable detection of fimbriae expression. Urine was collected from mice infected with strains AL151 and AL371 from 6–48 hpi (Fig. 5), while it is unknown how long the women in this study were colonized at the time of sample collection. Although it is well-established that type 1 fimbriae are critical for the establishment of infection in mice [28], [74], their role during bacterial persistence has not been characterized. Consequently, the human urine samples collected in this study may have represented later stages of UTI, during which type 1 fimbrial genes may not be expressed or after fimbriated cells had been cleared by the immune system. In a mouse model of UTI, the orientation of the fim invertible element varied throughout the course of infection and by strain, with cystitis strains generally maintaining their IEs in the phase-on position throughout the infection (up to 96 hpi) [75]. Similarly, expression of type 1 fimbriae was shown to be required for UPEC fitness subsequent to the initial attachment/invasion event in a murine model of intracellular replication [76]. Furthermore, transcriptome analysis of UPEC during murine UTI analyzed urine collected up to 10 days post-infection (with a reinfection at day 6) and still observed a high level of fim expression [4], so it is unclear whether infection duration alone can explain our results. Nevertheless, future studies should attempt to correlate UTI symptom duration with type 1 fimbrial gene expression of E. coli collected from human urine.
Given the abundance of data from our and other laboratories demonstrating the importance of type 1 fimbriae for UTI [27], [28], [29], [77] and positive selection for fimH among UTI isolates [16], a likely explanation for our findings is that expression of these genes may be a transient or regulated event during human infection. Analogous to flagellin, which is tightly regulated and only maximally expressed during ascension to the kidneys [19], fim expression might be temporally or spatially controlled. We may speculate that, while phase-on bacteria would be primed to adhere to and invade the bladder epithelium, perhaps switching to the phase-off orientation allows dispersal or immune avoidance. Thus, future delineation of the molecular basis for the apparent variable type 1 fimbriae expression detected in our samples, as well as distinction between global gene expression in planktonic versus adherent bacteria in urine voided during human UTI will be necessary.
Differences in urinary tract environments among patients and between mammalian hosts are also expected to account for some of the variable expression patterns observed in this study. Diet, hydration, and genetic factors all influence urine composition, urinary tract physiology and, most likely, gene expression by colonizing bacteria. For example, amino acids, temperature [78], sialic acid [79], oxygenation [80], pH and osmolarity [81] are known to affect the orientation of the invertible element region and thus, fimbrial expression. As the present study represents an initial investigation of UPEC gene expression during human UTI, further analysis of additional patient samples will be needed to more completely assess potential correlations between urine chemistry and UPEC gene expression in urine from patients with UTI.
The data presented in this study provide the first insights into pathogenic E. coli gene expression within the human host. Our findings are generally consistent with data generated using murine models and support the current model of UPEC pathogenesis. In urine from women with cystitis, E. coli express metabolic genes consistent with rapid replication and acetate excretion, actively scavenge iron, express known virulence genes, and may modulate expression of genes involved in motility and adherence. Continued investigation of UPEC gene expression in the urine of UTI patients will contribute both to our understanding of UPEC pathogenesis and to the development of effective UTI therapies.
Materials and Methods
Ethics statement
Non-pregnant women over the age of 18 years with symptoms indicative of a UTI were invited to participate in our study by A.L.L. or G.J.F. Written consent was obtained from all subjects prior to enrollment and patient samples were assigned arbitrary identification based on the order of enrollment in our study. All human subject protocols were approved by the Institutional Review Boards of the University of Michigan Medical School (HUM00011155). All animal procedures were conducted in accordance with the guidelines of the University Committee on Use and Care of Animals at the University of Michigan Medical School and following protocols approved by UCUCA.
Bacterial strains
Clinical E. coli isolates were cultured from the urine of women with suspected UTIs using standard methods [82]. Antimicrobial susceptibility testing was performed on all clinical E. coli isolates cultured from the urine of the women participating in our study by the Clinical Microbiology Laboratory at the University of Michigan Health System using the VITEK 2 system (bioMerieux, Durham, NC). Clinical isolates were serotyped by the E. coli Reference Center at the Pennsylvania State University using antisera against O1-O181 (except O31, O47, O72, O93, O94, and O121, which were not designated) and PCR-restriction fragment length polymorphism analysis of the fliC gene.
E. coli CFT073 was isolated from the blood and urine of a patient with acute pyelonephritis and E. coli EFC4 was isolated from the feces of a healthy woman with no history of a UTI or antibiotic use in the previous six months [56]. E. coli K12 is the prototypical commensal strain, MG1655 [83]. CFT073 type 1 fimbriae phase locked-on (L-ON) and locked-off (L-OFF) mutants were constructed as previously described by our laboratory [84]. Strains were routinely cultured in Luria broth (10 g/L tryptone, 5 g/L yeast extract, 0.5 g/L NaCl) at 37°C with aeration, unless otherwise noted.
Sample collection and in vivo RNA isolation
Fresh mid-stream urine was collected from consenting women with presumptive bacteriuria attending the University of Michigan Urology clinic. A diagnosis of presumptive bacteriuria was made based on symptoms of urgency and frequency and/or a history of previous UTI. Volumes collected ranged from 28 to 187 ml, with a median volume of 70 ml (average = 78.8 ml). Urine was collected from 34 women in order to obtain 10 E. coli-positive samples that were suitable for our study. Of these, two samples contained multiple E. coli strains and were not analyzed further. For the eight patients from whom single strains of E. coli were isolated and studied in this report, no patient was catheterized. Seven of eight patients reported a previous UTI. Two patients were taking one antibiotic (ciprofloxacin or nitrofurantoin); however, each respective E. coli strain was resistant to that antibiotic.
Collected urine was immediately tested by urinalysis and analyzed by wet-mount microscopy for the presence of bacteria. Specimens positive for leukocyte esterase and/or nitrites, and/or those containing visible bacteria by microscopy were immediately stabilized (within 10 min of sample collection) by the addition of 2 volumes of RNAprotect (Qiagen) and incubated at 25°C for at least 10 min. Stabilized urine specimens were collected by centrifugation (3000×g, 30 min, 25°C) and stabilized bacterial pellets were stored at −80°C for up to four weeks. Upon receipt of the clinical culture and sensitivity results, the E. coli-positive samples were processed for RNA isolation using the RNeasy Mini system (Qiagen) according to the manufacturer's instructions. DNA was removed from the preparation using TURBO DNase (Ambion) and, where necessary, RNA samples were concentrated using MinElute columns (Qiagen). It is important to note that samples likely also contained human RNA, which was not quantified or removed.
In vitro RNA isolation
Clinical E. coli isolates were cultured overnight in LB, washed twice in pooled, filter-sterilized human urine (pooled from 5 healthy donors) and adjusted to OD600 = 4.0. Standardized bacterial suspension was inoculated 1∶100 into 25 ml human urine (starting OD600 = 0.004) and cultured statically at 37°C until OD600 = 0.2±0.02. Culture aliquots (5 ml) were stabilized with 10 ml RNAprotect and total RNA was isolated using the RNeasy Mini procedure described above.
All RNA and cDNA preparations were analyzed using the Agilent 2100 Bioanalyzer (Agilent Technologies) to verify sample quality and integrity. Each sample met the criteria A260/A280≥1.7 and A260/A230≥1.5. Concentrations of total RNA and cDNA samples were determined using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific).
cDNA synthesis, labeling and microarray hybridization
cDNA was synthesized from total RNA isolated using the Superscript Double-Stranded cDNA Synthesis system (Invitrogen) according to the manufacturer's instructions. The only modifications to the protocol were an increase in random primer concentration (3 μg) and extension of the reverse transcriptase reaction (42°C, 90–120 min). cDNA was labeled and hybridized by Roche NimbleGen (Madison, WI) according to their standard protocols. Briefly, cDNA was labeled with Cy3 using the One-Color DNA Labeling protocol (Roche NimbleGen) and 15 μg labeled cDNA was prepared for each sample (10 in vivo samples and 12 in vitro samples, each in triplicate microarrays). Following hybridization, microarrays were washed and scanned using a GenePix 4000B Scanner (Axon Instruments). Data were extracted and analyzed using the Roche NimbleScan software, which normalizes expression data using quantile normalization [85] and generates gene calls using the Robust Multichip Average algorithm [86].
E. coli CFT073 gene-specific microarray and data normalization
The E. coli CFT073 microarray (Roche NimbleGen) contains 14 60-mer perfect match probes (no mismatch) for each of the 5379 open reading frames in the annotated CFT073 genome [14], as well as random probes with similar G+C content. Each array value for the 5379 genes was derived from the automated normalization of 5 replicates of probes printed on each slide. The expression value for each potential gene was obtained by hybridizing triplicate samples to the E. coli CFT073 microarray. Biological replicates were not performed on the in vivo specimens as patients commenced antibiotic therapy following consultation with G.J.F. and the opportunity to collect multiple urine specimens was not possible, therefore in vivo microarrays represent technical replicates.
The median value of the three replicates was obtained for each ORF and was compared to the median value of the random probes on each chip. The median value of the random probes on each array was used as a correction factor for the remaining signal on the chip, normalizing the hybridization and RNA quality of each preparation. The resulting absolute intensity values were transformed into log2 values and subsequent analysis utilized these log transformed normalized values to determine the potential expression for any given gene represented on the array. The gene was considered to be expressed if the intensity was four-fold above the value of the randomized control values. It is important to note that, as these isolates are uncharacterized, a lack of hybridization does not always indicate that a gene is not expressed, only that under the conditions examined there was not sufficient signal to indicate expression. Gene absence or genetic divergence between gene sequences of the isolates and gene sequences of the reference strain, E. coli CFT073 may also explain poor hybridization. Gene expression heat maps were generated using TreeView 1.60. Microarray data have been deposited in NCBI Gene Expression Omnibus [87] and are accessible through GEO Series accession number GSE24478 (http://www.ncbi.nlm.nih.gov/geo/).
Invertible element assay
The orientation of the fim invertible element was determined as described [73] using the primers listed in Table 5. Clinical isolates and strain CFT073 were inoculated into 5 ml LB, incubated statically at 37°C for 48 h, passaged 1∶100 into fresh medium, and incubated at 37°C for an additional 48 h. Aerated cultures were similarly inoculated and incubated at 37°C for ∼4 h. All cultures were adjusted to OD600 = 1.0 and 500 µl was centrifuged (10,000×g, 1 min) for western blotting (see below). Standardized culture (50 µl) was added to 50 µl water and boiled 10 min. PCR was performed to amplify the invertible element and 2 µl of crude lysate as template. PCR product was digested with SnaBI and separated on a 2% agarose gel.
Tab. 5. Primers used in this study. 
All primers are listed 5′→3′. Antisera production and western blotting
The fimA gene was cloned into the commercially-available expression vector pBAD-myc-HisA (Invitrogen) in-frame with a C-terminal His6 tag. Expression was induced by addition of L-arabinose to 100 µM and recombinant protein was isolated on nickel-nitriloacetic acid-agarose columns (Qiagen). Antibodies were raised in rabbits against recombinant FimA-His6 excised from SDS-PAGE gels by Rockland Immunochemicals, Inc. (Gilbertsville, PA).
Bacterial pellets collected as described above were resuspended 100 µl acidified water (pH 1.8) and boiled for 10 min. Lysate was mixed with 20 µl 6× SDS-PAGE loading buffer, neutralized with 1 N NaOH, and separated on 15% SDS-PAGE gels. Proteins were transferred to PVDF membrane and blocked with 5% milk in TBS +0.01% Tween-20. FimA was detected with rabbit anti-FimA (1∶2000), followed by anti-rabbit-HRP (1∶25,000) and ECL Plus detection reagents (GE Healthcare).
Hemagglutination
Strains were passaged statically or cultured with aeration as described above and adjusted to OD600 = 0.8. One ml of culture was pelleted (2500×g, 2 min, 25°C) and resuspended in 200 µl PBS and serial dilutions of this bacterial suspension (25 µl) were added to the wells of a microtiter plate. A 3% suspension of PBS-washed guinea pig erythrocytes (v/v) was prepared in PBS on ice and 25 µl added to each well. For testing mannose sensitivity, erythrocyte suspension mixed with α-methyl mannoside (Sigma) at 1 mg/ml was added to wells containing undiluted bacterial suspension. Plates were gently rocked and incubated at 25°C for 30–45 min. Hemagglutination titer was defined as the highest dilution of bacterial suspension that yielded a positive reaction.
Mouse model of ascending UTI
Six - to eight-week female CBA/J mice were transurethrally inoculated as previously described [34]. Clinical isolates were cultured overnight in LB, collected by centrifugation (3000×g, 30 min, 25°C) and resuspended in PBS to 2×109 CFU/ml. Bacterial suspension (50 µl/mouse) was delivered directly into the bladders of anesthetized mice via a sterile 0.28 mm inner diameter polyethylene catheter connected to an infusion pump (Harvard Apparatus), with a total inoculum of 1×108 CFU/mouse. At two-hour intervals beginning at 5 h post-inoculation, urine was collected and pooled from each cage of animals (5 mice). Immediately after collection, cold 5% phenol-ethanol stop solution was added, samples centrifuged (10,000×g, 1 min, 4°C), and pellets stored at −80°C for RNA isolation. For CFU determination, mice were sacrificed at 48 hpi and urinary tract organs homogenized with a GLH homogenizer (Omni International) in 3 ml PBS. Homogenate was plated on LB agar using an Autoplate 4000 spiral plater (Spiral Biotech) and colonies enumerated with a QCount plate reader (Spiral Biotech). Significance was determined using the two-tailed Mann-Whitney test.
qPCR
For microarray validation, real-time qPCR was performed with cDNA isolated above for microarray hybridization. Reactions were conducted in a Mx300P instrument (Stratagene), using 30 ng cDNA template, 0.1 µM primers (Table 1), and Brilliant SYBR Green reagents (Stratagene). Data were normalized to gapA and analyzed with MxPro 4.0 software (Stratagene). For fimA measurement at room temperature, CFT073 wildtype and L-ON were cultured in 70 ml pooled, filter-sterilized human urine for 6 h at 37°C. Cultures were decanted into 120 ml urine collection cups with lids and incubated at room temperature for 60 min. At intervals, 1 ml culture aliquots were mixed with 125 µl cold 5% phenol-ethanol stop solution, centrifuged (10,000×g, 1 min, 4°C), and stabilized pellets stored at −80°C. Thawed pellets were resuspended in 100 µl 1 mg/ml lysozyme in TE and RNA isolated using the RNeasy protocol as described above. cDNA was synthesized using SuperScriptII First-Strand Synthesis reagents according to the manufacturer's instructions and qPCR was performed as described.
Supporting Information
Zdroje
1. PoirierK
FaucherSP
BelandM
BrousseauR
GannonV
2008 Escherichia coli O157:H7 survives within human macrophages: global gene expression profile and involvement of the Shiga toxins. Infect Immun 76 4814 4822
2. JanduN
HoNK
DonatoKA
KarmaliMA
MascarenhasM
2009 Enterohemorrhagic Escherichia coli O157:H7 gene expression profiling in response to growth in the presence of host epithelia. PLoS One 4 e4889
3. HaugenBJ
PellettS
RedfordP
HamiltonHL
RoeschPL
2007 In vivo gene expression analysis identifies genes required for enhanced colonization of the mouse urinary tract by uropathogenic Escherichia coli strain CFT073 dsdA. Infect Immun 75 278 289
4. SnyderJA
HaugenBJ
BucklesEL
LockatellCV
JohnsonDE
2004 Transcriptome of Uropathogenic Escherichia coli during Urinary Tract Infection. Infect Immun 72 6373 6381
5. LarocqueRC
HarrisJB
DziejmanM
LiX
KhanAI
2005 Transcriptional profiling of Vibrio cholerae recovered directly from patient specimens during early and late stages of human infection. Infect Immun 73 4488 4493
6. MerrellDS
ButlerSM
QadriF
DolganovNA
AlamA
2002 Host-induced epidemic spread of the cholera bacterium. Nature 417 642 645
7. SonMS
MatthewsWJJr
KangY
NguyenDT
HoangTT
2007 In vivo evidence of Pseudomonas aeruginosa nutrient acquisition and pathogenesis in the lungs of cystic fibrosis patients. Infect Immun 75 5313 5324
8. TimmJ
PostFA
BekkerLG
WaltherGB
WainwrightHC
2003 Differential expression of iron-, carbon-, and oxygen-responsive mycobacterial genes in the lungs of chronically infected mice and tuberculosis patients. Proc Natl Acad Sci U S A 100 14321 14326
9. KuninCM
1987 Detection, Prevention and Management of Urinary Tract Infections, 4th ed. Philadelphia Lea & Febiger 125 193
10. BachellerCD
BernsteinJM
1997 Urinary tract infections. Med Clin North Am 81 719 730
11. FalzanoL
FiorentiniC
DonelliG
MichelE
KocksC
1993 Induction of phagocytic behaviour in human epithelial cells by Escherichia coli cytotoxic necrotizing factor type 1. Mol Microbiol 9 1247 1254
12. WarrenJW
AbrutynE
HebelJR
JohnsonJR
SchaefferAJ
1999 Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA). Clin Infect Dis 29 745 758
13. FoxmanB
1990 Recurring urinary tract infection: incidence and risk factors. Am J Public Health 80 331 333
14. WelchRA
BurlandV
PlunkettG3rd
RedfordP
RoeschP
2002 Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc Natl Acad Sci U S A 99 17020 17024
15. BrzuszkiewiczE
BruggemannH
LiesegangH
EmmerthM
OlschlagerT
2006 How to become a uropathogen: comparative genomic analysis of extraintestinal pathogenic Escherichia coli strains. Proc Natl Acad Sci U S A 103 12879 12884
16. ChenSL
HungCS
XuJ
ReigstadCS
MagriniV
2006 Identification of genes subject to positive selection in uropathogenic strains of Escherichia coli: a comparative genomics approach. Proc Natl Acad Sci U S A 103 5977 5982
17. KnappS
HackerJ
JarchauT
GoebelW
1986 Large, unstable inserts in the chromosome affect virulence properties of uropathogenic Escherichia coli O6 strain 536. J Bacteriol 168 22 30
18. LloydAL
HendersonTA
VigilPD
MobleyHL
2009 Genomic islands of uropathogenic Escherichia coli contribute to virulence. J Bacteriol 191 3469 3481
19. LaneMC
AlteriCJ
SmithSN
MobleyHL
2007 Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc Natl Acad Sci U S A 104 16669 16674
20. SmithYC
RasmussenSB
GrandeKK
ConranRM
O'BrienAD
2008 Hemolysin of uropathogenic Escherichia coli evokes extensive shedding of the uroepithelium and hemorrhage in bladder tissue within the first 24 hours after intraurethral inoculation of mice. Infect Immun 76 2978 2990
21. MillsM
MeysickKC
O'BrienAD
2000 Cytotoxic necrotizing factor type 1 of uropathogenic Escherichia coli kills cultured human uroepithelial 5637 cells by an apoptotic mechanism. Infect Immun 68 5869 5880
22. GuyerDM
RadulovicS
JonesFE
MobleyHL
2002 Sat, the secreted autotransporter toxin of uropathogenic Escherichia coli, is a vacuolating cytotoxin for bladder and kidney epithelial cells. Infect Immun 70 4539 4546
23. BucklesEL
WangX
LaneMC
LockatellCV
JohnsonDE
2009 Role of the K2 capsule in Escherichia coli urinary tract infection and serum resistance. J Infect Dis 199 1689 1697
24. LloydAL
SmithSN
EatonKA
MobleyHL
2009 Uropathogenic Escherichia coli Suppresses the host inflammatory response via pathogenicity island genes sisA and sisB. Infect Immun 77 5322 5333
25. TorresAG
RedfordP
WelchRA
PayneSM
2001 TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect Immun 69 6179 6185
26. AlteriCJ
SmithSN
MobleyHL
2009 Fitness of Escherichia coli during urinary tract infection requires gluconeogenesis and the TCA cycle. PLoS Pathog 5 e1000448
27. Bahrani-MougeotFK
BucklesEL
LockatellCV
HebelJR
JohnsonDE
2002 Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol Microbiol 45 1079 1093
28. ConnellI
AgaceW
KlemmP
SchembriM
MarildS
1996 Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci U S A 93 9827 9832
29. MulveyMA
Lopez-BoadoYS
WilsonCL
RothR
ParksWC
1998 Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282 1494 1497
30. WuXR
SunTT
MedinaJJ
1996 In vitro binding of type 1-fimbriated Escherichia coli to uroplakins Ia and Ib: relation to urinary tract infections. Proc Natl Acad Sci U S A 93 9630 9635
31. MysorekarIU
MulveyMA
HultgrenSJ
GordonJI
2002 Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J Biol Chem 277 7412 7419
32. SamuelssonP
HangL
WulltB
IrjalaH
SvanborgC
2004 Toll-like receptor 4 expression and cytokine responses in the human urinary tract mucosa. Infect Immun 72 3179 3186
33. AbrahamJM
FreitagCS
ClementsJR
EisensteinBI
1985 An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc Natl Acad Sci U S A 82 5724 5727
34. HagbergL
EngbergI
FreterR
LamJ
OllingS
1983 Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun 40 273 283
35. RoosV
KlemmP
2006 Global gene expression profiling of the asymptomatic bacteriuria Escherichia coli strain 83972 in the human urinary tract. Infect Immun 74 3565 3575
36. HancockV
SeshasayeeAS
UsseryDW
LuscombeNM
KlemmP
2008 Transcriptomics and adaptive genomics of the asymptomatic bacteriuria Escherichia coli strain 83972. Mol Genet Genomics 279 523 534
37. O'HanleyP
LowD
RomeroI
LarkD
VostiK
1985 Gal-Gal binding and hemolysin phenotypes and genotypes associated with uropathogenic Escherichia coli. N Engl J Med 313 414 420
38. GausingK
1977 Regulation of ribosome production in Escherichia coli: synthesis and stability of ribosomal RNA and of ribosomal protein messenger RNA at different growth rates. J Mol Biol 115 335 354
39. NomuraM
GourseR
BaughmanG
1984 Regulation of the synthesis of ribosomes and ribosomal components. Annu Rev Biochem 53 75 117
40. BrandiA
SpurioR
GualerziCO
PonCL
1999 Massive presence of the Escherichia coli ‘major cold-shock protein’ CspA under non-stress conditions. Embo J 18 1653 1659
41. YamanakaK
InouyeM
2001 Induction of CspA, an E. coli major cold-shock protein, upon nutritional upshift at 37 degrees C. Genes Cells 6 279 290
42. WangH
GunsalusRP
2003 Coordinate regulation of the Escherichia coli formate dehydrogenase fdnGHI and fdhF genes in response to nitrate, nitrite, and formate: roles for NarL and NarP. J Bacteriol 185 5076 5085
43. BergBL
StewartV
1990 Structural genes for nitrate-inducible formate dehydrogenase in Escherichia coli K-12. Genetics 125 691 702
44. ReitzerLJ
MagasanikB
1985 Expression of glnA in Escherichia coli is regulated at tandem promoters. Proc Natl Acad Sci U S A 82 1979 1983
45. CamarenaL
PoggioS
GarciaN
OsorioA
1998 Transcriptional repression of gdhA in Escherichia coli is mediated by the Nac protein. FEMS Microbiol Lett 167 51 56
46. KumariS
BeattyCM
BrowningDF
BusbySJ
SimelEJ
2000 Regulation of acetyl coenzyme A synthetase in Escherichia coli. J Bacteriol 182 4173 4179
47. AnforaAT
HalladinDK
HaugenBJ
WelchRA
2008 Uropathogenic Escherichia coli CFT073 is adapted to acetatogenic growth but does not require acetate during murine urinary tract infection. Infect Immun 76 5760 5767
48. PellicerMT
FernandezC
BadiaJ
AguilarJ
LinEC
1999 Cross-induction of glc and ace operons of Escherichia coli attributable to pathway intersection. Characterization of the glc promoter. J Biol Chem 274 1745 1752
49. WolfeAJ
2005 The acetate switch. Microbiol Mol Biol Rev 69 12 50
50. ParkSJ
TsengCP
GunsalusRP
1995 Regulation of succinate dehydrogenase (sdhCDAB) operon expression in Escherichia coli in response to carbon supply and anaerobiosis: role of ArcA and Fnr. Mol Microbiol 15 473 482
51. ReigstadCS
HultgrenSJ
GordonJI
2007 Functional genomic studies of uropathogenic Escherichia coli and host urothelial cells when intracellular bacterial communities are assembled. J Biol Chem 282 21259 21267
52. RussoTA
JodushST
BrownJJ
JohnsonJR
1996 Identification of two previously unrecognized genes (guaA and argC) important for uropathogenesis. Mol Microbiol 22 217 229
53. AnforaAT
HaugenBJ
RoeschP
RedfordP
WelchRA
2007 Roles of serine accumulation and catabolism in the colonization of the murine urinary tract by Escherichia coli CFT073. Infect Immun 75 5298 5304
54. BouletteML
PayneSM
2007 Anaerobic regulation of Shigella flexneri virulence: ArcA regulates Fur and iron acquisition genes. J Bacteriol 189 6957 6967
55. BultreysA
GheysenI
de HoffmannE
2006 Yersiniabactin production by Pseudomonas syringae and Escherichia coli, and description of a second yersiniabactin locus evolutionary group. Appl Environ Microbiol 72 3814 3825
56. MobleyHL
GreenDM
TrifillisAL
JohnsonDE
ChippendaleGR
1990 Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun 58 1281 1289
57. HootonTM
ScholesD
StapletonAE
RobertsPL
WinterC
2000 A prospective study of asymptomatic bacteriuria in sexually active young women. N Engl J Med 343 992 997
58. HaganEC
MobleyHL
2007 Uropathogenic Escherichia coli outer membrane antigens expressed during urinary tract infection. Infect Immun 75 3941 3949
59. HendersonJP
CrowleyJR
PinknerJS
WalkerJN
TsukayamaP
2009 Quantitative metabolomics reveals an epigenetic blueprint for iron acquisition in uropathogenic Escherichia coli. PLoS Pathog 5 e1000305
60. AlteriCJ
HaganEC
SivickKE
SmithSN
MobleyHL
2009 Mucosal immunization with iron receptor antigens protects against urinary tract infection. PLoS Pathog 5 e1000586
61. RussoTA
McFaddenCD
Carlino-MacDonaldUB
BeananJM
OlsonR
2003 The Siderophore receptor IroN of extraintestinal pathogenic Escherichia coli is a potential vaccine candidate. Infect Immun 71 7164 7169
62. Daran-LapujadeP
JansenML
DaranJM
van GulikW
de WindeJH
2004 Role of transcriptional regulation in controlling fluxes in central carbon metabolism of Saccharomyces cerevisiae. A chemostat culture study. J Biol Chem 279 9125 9138
63. PerrenoudA
SauerU
2005 Impact of global transcriptional regulation by ArcA, ArcB, Cra, Crp, Cya, Fnr, and Mlc on glucose catabolism in Escherichia coli. J Bacteriol 187 3171 3179
64. BrooksT
KeevilCW
1997 A simple artificial urine for the growth of urinary pathogens. Lett Appl Microbiol 24 203 206
65. HultgrenSJ
PorterTN
SchaefferAJ
DuncanJL
1985 Role of type 1 pili and effects of phase variation on lower urinary tract infections produced by Escherichia coli. Infect Immun 50 370 377
66. JohnsonJR
JelacicS
SchoeningLM
ClabotsC
ShaikhN
2005 The IrgA homologue adhesin Iha is an Escherichia coli virulence factor in murine urinary tract infection. Infect Immun 73 965 971
67. RussoTA
McFaddenCD
Carlino-MacDonaldUB
BeananJM
BarnardTJ
2002 IroN functions as a siderophore receptor and is a urovirulence factor in an extraintestinal pathogenic isolate of Escherichia coli. Infect Immun 70 7156 7160
68. MobleyHL
JarvisKG
ElwoodJP
WhittleDI
LockatellCV
1993 Isogenic P-fimbrial deletion mutants of pyelonephritogenic Escherichia coli: the role of alpha Gal(1-4) beta Gal binding in virulence of a wild-type strain. Mol Microbiol 10 143 155
69. KisieliusPV
SchwanWR
AmundsenSK
DuncanJL
SchaefferAJ
1989 In vivo expression and variation of Escherichia coli type 1 and P pili in the urine of adults with acute urinary tract infections. Infect Immun 57 1656 1662
70. LichodziejewskaM
TopleyN
SteadmanR
MackenzieRK
JonesKV
1989 Variable expression of P fimbriae in Escherichia coli urinary tract infection. Lancet 1 1414 1418
71. PereA
LeinonenM
Vaisanen-RhenV
RhenM
KorhonenTK
1985 Occurrence of type-1C fimbriae on Escherichia coli strains isolated from human extraintestinal infections. J Gen Microbiol 131 1705 1711
72. PereA
NowickiB
SaxenH
SiitonenA
KorhonenTK
1987 Expression of P, type-1, and type-1C fimbriae of Escherichia coli in the urine of patients with acute urinary tract infection. J Infect Dis 156 567 574
73. LimJK
GuntherNWt
ZhaoH
JohnsonDE
KeaySK
1998 In vivo phase variation of Escherichia coli type 1 fimbrial genes in women with urinary tract infection. Infect Immun 66 3303 3310
74. MartinezJJ
MulveyMA
SchillingJD
PinknerJS
HultgrenSJ
2000 Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. Embo J 19 2803 2812
75. GuntherNWt
LockatellV
JohnsonDE
MobleyHL
2001 In vivo dynamics of type 1 fimbria regulation in uropathogenic Escherichia coli during experimental urinary tract infection. Infect Immun 69 2838 2846
76. WrightKJ
SeedPC
HultgrenSJ
2007 Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell Microbiol 9 2230 2241
77. GuntherNWt
SnyderJA
LockatellV
BlomfieldI
JohnsonDE
2002 Assessment of virulence of uropathogenic Escherichia coli type 1 fimbrial mutants in which the invertible element is phase-locked on or off. Infect Immun 70 3344 3354
78. GallyDL
BoganJA
EisensteinBI
BlomfieldIC
1993 Environmental regulation of the fim switch controlling type 1 fimbrial phase variation in Escherichia coli K-12: effects of temperature and media. J Bacteriol 175 6186 6193
79. El-LabanyS
SohanpalBK
LahootiM
AkermanR
BlomfieldIC
2003 Distant cis-active sequences and sialic acid control the expression of fimB in Escherichia coli K-12. Mol Microbiol 49 1109 1118
80. LaneMC
LiX
PearsonMM
SimmsAN
MobleyHL
2009 Oxygen-limiting conditions enrich for fimbriate cells of uropathogenic Proteus mirabilis and Escherichia coli. J Bacteriol 191 1382 1392
81. SchwanWR
LeeJL
LenardFA
MatthewsBT
BeckMT
2002 Osmolarity and pH growth conditions regulate fim gene transcription and type 1 pilus expression in uropathogenic Escherichia coli. Infect Immun 70 1391 1402
82. PezzloM
YorkMK
2004 Urine cultures.
IsenbergHD
Clinical Microbiology Procedures Handbook 2nd ed Washington, D.C. American Society for Microbiology 3.12.11 13.12.31
83. BlattnerFR
PlunkettG3rd
BlochCA
PernaNT
BurlandV
1997 The complete genome sequence of Escherichia coli K-12. Science 277 1453 1474
84. GuntherIN
SnyderJA
LockatellV
BlomfieldI
JohnsonDE
2002 Assessment of virulence of uropathogenic Escherichia coli type 1 fimbrial mutants in which the invertible element is phase-locked on or off. Infect Immun 70 3344 3354
85. BolstadBM
IrizarryRA
AstrandM
SpeedTP
2003 A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19 185 193
86. IrizarryRA
BolstadBM
CollinF
CopeLM
HobbsB
2003 Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res 31 e15
87. EdgarR
DomrachevM
LashAE
2002 Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res 30 207 210
88. LloydAL
RaskoDA
MobleyHL
2007 Defining genomic islands and uropathogen-specific genes in uropathogenic Escherichia coli. J Bacteriol 189 3532 3546
89. HaganEC
MobleyHL
2009 Haem acquisition is facilitated by a novel receptor Hma and required by uropathogenic Escherichia coli for kidney infection. Mol Microbiol 7 79 91
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease AgentČlánek TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease SusceptibilityČlánek The Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive ParasiteČlánek Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 11- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Patients with Discordant Responses to Antiretroviral Therapy Have Impaired Killing of HIV-Infected T Cells
- A Molecular Mechanism for Eflornithine Resistance in African Trypanosomes
- Tyrosine Sulfation of the Amino Terminus of PSGL-1 Is Critical for Enterovirus 71 Infection
- Autoimmunity as a Predisposition for Infectious Diseases
- The Subtilisin-Like Protease AprV2 Is Required for Virulence and Uses a Novel Disulphide-Tethered Exosite to Bind Substrates
- Structural Analysis of HIV-1 Maturation Using Cryo-Electron Tomography
- Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease Agent
- Interferon-Inducible CXC Chemokines Directly Contribute to Host Defense against Inhalational Anthrax in a Murine Model of Infection
- TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease Susceptibility
- The Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive Parasite
- CO Acts as a Signalling Molecule in Populations of the Fungal Pathogen
- SV2 Mediates Entry of Tetanus Neurotoxin into Central Neurons
- MAP Kinase Phosphatase-2 Plays a Critical Role in Response to Infection by
- Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
- Potentiation of Epithelial Innate Host Responses by Intercellular Communication
- Fcγ Receptor I Alpha Chain (CD64) Expression in Macrophages Is Critical for the Onset of Meningitis by K1
- ANK, a Host Cytoplasmic Receptor for the Cell-to-Cell Movement Protein, Facilitates Intercellular Transport through Plasmodesmata
- Analysis of the Initiating Events in HIV-1 Particle Assembly and Genome Packaging
- Evolution of Linked Avirulence Effectors in Is Affected by Genomic Environment and Exposure to Resistance Genes in Host Plants
- Structural Basis of HIV-1 Neutralization by Affinity Matured Fabs Directed against the Internal Trimeric Coiled-Coil of gp41
- Hepatitis C Virus (HCV) Evades NKG2D-Dependent NK Cell Responses through NS5A-Mediated Imbalance of Inflammatory Cytokines
- Host Cell Invasion and Virulence Mediated by Ssa1
- Global Gene Expression in Urine from Women with Urinary Tract Infection
- Should the Human Microbiome Be Considered When Developing Vaccines?
- HapX Positively and Negatively Regulates the Transcriptional Response to Iron Deprivation in
- Enhancing Oral Vaccine Potency by Targeting Intestinal M Cells
- Herpes Simplex Virus Reorganizes the Cellular DNA Repair and Protein Quality Control Machinery
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- Cryo Electron Tomography of Native HIV-1 Budding Sites
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- Modelling the Evolution and Spread of HIV Immune Escape Mutants
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
- Platelet-Activating Factor Receptor Plays a Role in Lung Injury and Death Caused by Influenza A in Mice
- Genetic and Structural Basis for Selection of a Ubiquitous T Cell Receptor Deployed in Epstein-Barr Virus Infection
- Ubiquitin-Regulated Nuclear-Cytoplasmic Trafficking of the Nipah Virus Matrix Protein Is Important for Viral Budding
- Pneumolysin Activates the NLRP3 Inflammasome and Promotes Proinflammatory Cytokines Independently of TLR4
- Immune Evasion by : Differential Targeting of Dendritic Cell Subpopulations
- Survival in Selective Sand Fly Vector Requires a Specific -Encoded Lipophosphoglycan Galactosylation Pattern
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



