-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Host Cell Invasion and Virulence Mediated by Ssa1
Candida albicans Ssa1 and Ssa2 are members of the HSP70 family of heat shock proteins that are expressed on the cell surface and function as receptors for antimicrobial peptides such as histatins. We investigated the role of Ssa1 and Ssa2 in mediating pathogenic host cell interactions and virulence. A C. albicans ssa1Δ/Δ mutant had attenuated virulence in murine models of disseminated and oropharyngeal candidiasis, whereas an ssa2Δ/Δ mutant did not. In vitro studies revealed that the ssa1Δ/Δ mutant caused markedly less damage to endothelial cells and oral epithelial cell lines. Also, the ssa1Δ/Δ mutant had defective binding to endothelial cell N-cadherin and epithelial cell E-cadherin, receptors that mediate host cell endocytosis of C. albicans. As a result, this mutant had impaired capacity to induce its own endocytosis by endothelial cells and oral epithelial cells. Latex beads coated with recombinant Ssa1 were avidly endocytosed by both endothelial cells and oral epithelial cells, demonstrating that Ssa1 is sufficient to induce host cell endocytosis. These results indicate that Ssa1 is a novel invasin that binds to host cell cadherins, induces host cell endocytosis, and is critical for C. albicans to cause maximal damage to host cells and induce disseminated and oropharyngeal disease.
Published in the journal: Host Cell Invasion and Virulence Mediated by Ssa1. PLoS Pathog 6(11): e32767. doi:10.1371/journal.ppat.1001181
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001181Summary
Candida albicans Ssa1 and Ssa2 are members of the HSP70 family of heat shock proteins that are expressed on the cell surface and function as receptors for antimicrobial peptides such as histatins. We investigated the role of Ssa1 and Ssa2 in mediating pathogenic host cell interactions and virulence. A C. albicans ssa1Δ/Δ mutant had attenuated virulence in murine models of disseminated and oropharyngeal candidiasis, whereas an ssa2Δ/Δ mutant did not. In vitro studies revealed that the ssa1Δ/Δ mutant caused markedly less damage to endothelial cells and oral epithelial cell lines. Also, the ssa1Δ/Δ mutant had defective binding to endothelial cell N-cadherin and epithelial cell E-cadherin, receptors that mediate host cell endocytosis of C. albicans. As a result, this mutant had impaired capacity to induce its own endocytosis by endothelial cells and oral epithelial cells. Latex beads coated with recombinant Ssa1 were avidly endocytosed by both endothelial cells and oral epithelial cells, demonstrating that Ssa1 is sufficient to induce host cell endocytosis. These results indicate that Ssa1 is a novel invasin that binds to host cell cadherins, induces host cell endocytosis, and is critical for C. albicans to cause maximal damage to host cells and induce disseminated and oropharyngeal disease.
Introduction
The fungus, Candida albicans is a significant human pathogen. In hospitalized patients, this organism disseminates hematogenously and infects virtually all organs. Even with currently available therapy, bloodstream infections with C. albicans are associated with a 37% mortality [1]. C. albicans is also part of the normal oral flora and it usually grows as a harmless commensal. However, when local or systemic host defense mechanisms are impaired, this organism can proliferate and cause debilitating oropharyngeal candidiasis.
To persist within the human host and cause disease, C. albicans must be able to adhere to and invade host cells or tissues while resisting the stress caused by host-derived reactive oxygen intermediates and antimicrobial peptides [2]–[5]. In other organisms, heat shock proteins play an important role in each of these activities. For example, some heat shock proteins are expressed on the cell surface of microorganisms, where they function as adhesins [6]-[9]. Also, in some bacteria and parasites, members of the Hsp70 and Hsp100 family of heat shock proteins are required for resistance to host-induced stress [10]–[12].
Ssa1 and Ssa2 are the only two members of the Hsp70 family in C. albicans, and both proteins are expressed on the cell surface of yeast and hyphae [13], [14]. Previously, we found that histatin 5, one of the main antimicrobial proteins found in saliva, binds with high affinity to Ssa2 and with lower affinity to Ssa1. After histatin 5 is bound to Ssa proteins, it is transported into the cytoplasm, where it kills the fungal cell [15], [16]. C. albicans Ssa1 and Ssa2 are also required for maximal fungicidal activity of human β defensins 2 and 3 [17].
As reported here, we investigated the roles of Ssa1 and Ssa2 in C. albicans virulence in murine models of hematogenously disseminated and oropharyngeal candidiasis. We found that Ssa1, but not Ssa2 is essential for normal virulence in both models. Through in vitro experiments, we discovered that surface-expressed Ssa1 likely contributes to virulence by acting as an invasin and directly mediating C. albicans invasion of both endothelial and oral epithelial cells in vitro.
Results
Ssa1 is required for maximal virulence in a mouse model of hematogenously disseminated candidiasis
To evaluate the contribution of Ssa1 and Ssa2 to C. albicans pathogenicity, the virulence of ssa1Δ/Δ and ssa2Δ/Δ null mutants was tested in a murine model of disseminated candidiasis. Over the 21 day observation period, none of the mice infected intravenously with the ssa1Δ/Δ mutant died (Figure 1A). In contrast, the median survival of mice infected with the wild-type and ssa1Δ/Δ::SSA1Δ/Δ complemented strain was 7 to 8 days. Ssa2 did not appear to influence virulence in this model because the survival of mice infected with the ssa2Δ/Δ null mutant was similar to that of mice infected with the wild-type strain (p = 0.13).
Fig. 1. Ssa1 is required for normal virulence during hematogenously disseminated candidiasis. 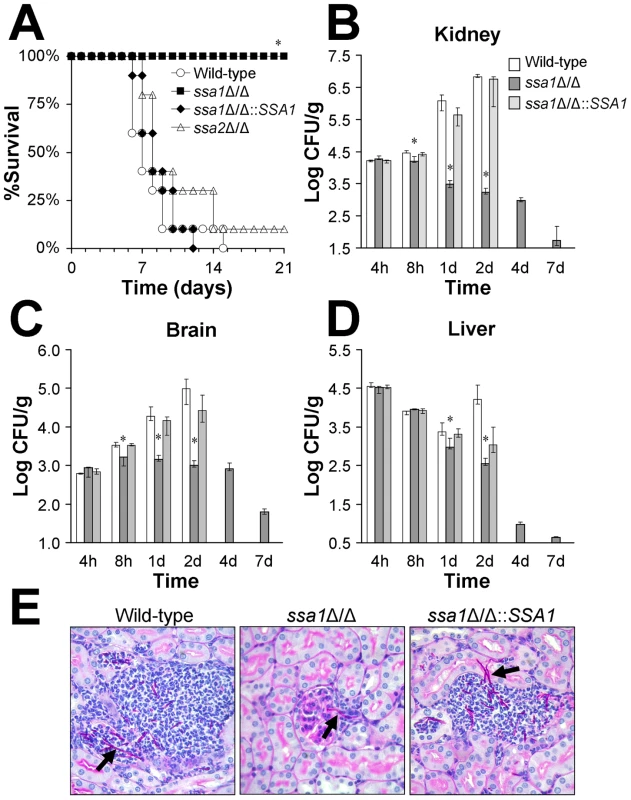
Mice were inoculated via the tail vein with 5×105 yeast-phase cells of the indicated strains of C. albicans. (A) Survival over 21 days (n = 10 mice per strain of C. albicans). *P<0.0001 compared to mice infected with either the wild-type or ssa1Δ/Δ::SSA1 complemented strain. (B–D) Fungal burden of the kidneys (B), brains (C), and livers (D) of mice infected with the wild-type, ssa1Δ/Δ and ssa1Δ/Δ::SSA1 complemented strains at the indicated time points. For each organ, the lower bound of the y-axis scale indicates the limits of detection. Results from the 1 day time point are the median ± interquartile range of two experiments with a total of 12 mice per strain of C. albicans. Results from the other time points are the median ± interquartile range of one experiment with 7 mice per strain *P<0.02 compared to mice infected with either the wild-type or ssa1Δ/Δ::SSA1 complemented strain. (E) Periodic acid Schiff stained thin sections of kidneys after 1 day of infection with the indicated strains of C. albicans. Arrows indicate C. albicans filaments in the tissues. Next, we compared the organ fungal burden at various time points in mice infected with the different strains. After 8 h of infection, the kidney and brain fungal burdens of mice inoculated with the ssa1Δ/Δ mutant were significantly lower than mice infected with either the wild-type or ssa1Δ/Δ::SSA1Δ/Δ complemented strains (Figures 1B–D). After 1 and 2 days of infection, the kidney and brain fungal burdens of mice challenged with the ssa1Δ/Δ mutant either declined or were stable. In contrast, the fungal burden of these organs steadily increased in mice infected with either the wild-type or ssa1Δ/Δ::SSA1Δ/Δ complemented strains. The fungal burdens of the livers of mice infected with the ssa1Δ/Δ mutant were also significantly lower than those of the control mice after 1 and 2 days of infection. These results indicate that SSA1 is required for normal levels of C. albicans infection in the kidney and brain as early as 8 h after inoculation. SSA1 also appears to be necessary for the organism to persist in the tissues at later time points.
To investigate why mice infected with the ssa1Δ/Δ mutant had no mortality over the course of the infection, we determined their organ fungal burden after 4, 7 and 14 days post-inoculation. We were not able to analyze the organ fungal burden of mice infected with either the wild-type strain or the ssa1Δ/Δ::SSA1Δ/Δ complemented strain at these later time points because a significant percentage of these mice had died. We found that mice infected with the ssa1Δ/Δ mutant progressively cleared the infection so that their organs became sterile by 14 days (Figure 1B–D and data not shown). This clearance provides an explanation for the prolonged survival of mice infected with the ssa1Δ/Δ mutant.
The low fungal burden in the kidneys of mice infected with the ssa1Δ/Δ mutant was verified by histopathology. After 1 day of infection, the kidneys of these mice contained very small foci of organisms. These foci usually consisted of a single fungal element surrounded by a few inflammatory cells (Figure 1E). Interestingly, many of the ssa1Δ/Δ cells were located in the glomeruli. The kidneys from mice infected with either the wild-type or ssa1Δ/Δ::SSA1Δ/Δ complemented strain contained the expected microabscesses, which contained numerous neutrophils surrounding multiple C. albicans filaments. It was not possible to determine whether the filaments of the various C. albicans strains were true hyphae or pseudohyphae in these histopathologic specimens. However, the filaments of the ssa1Δ/Δ mutant appeared to be similar in length to those of the wild-type and ssa1Δ/Δ::SSA1Δ/Δ complemented strain. Also, we have previously found that the ssa1Δ/Δ mutant forms hyphae similar to the wild-type strain in the presence of serum in vitro [16]. Therefore, the attenuated virulence of the ssa1Δ/Δ mutant was unlikely due to a defect in filamentation.
During the construction of the ssa1Δ/Δ mutant, URA3 was used as the selectable marker. The chromosomal locus at which URA3 is integrated can sometimes influence the virulence of C. albicans mutants in the mouse model of disseminated candidiasis [18]–[20]. In the ssa1Δ/Δ mutant, URA3 was integrated at the SSA2 locus [16]. However, in the ssa1Δ/Δ::SSA1 complemented strain, URA3 was integrated at the RPS10 locus, which is known to result in normal activity of the URA3 gene product, orotidine 5′-monophosphate decarboxylase [21]. To verify that the observed attenuated virulence of the ssa1Δ/Δ mutant was due to the absence of SSA1 and not the result of the chromosomal locus of URA3, we tested the virulence of a second ssa1Δ/Δ strain in which URA3 was integrated at the RPS10 locus. As expected, all mice infected intravenously with this mutant survived (Supplemental Figure S1A), thus confirming that SSA1 is required for the normal virulence of C. albicans.
The ssa1Δ/Δ mutant had attenuated virulence in the mouse model of oropharyngeal candidiasis
Next, we investigated the contributions of SSA1 and SSA2 to virulence in a mouse model of oropharyngeal candidiasis. There was a trend towards reduced oral fungal burden in mice infected with the ssa1Δ/Δ mutant after 1 day of infection, but this difference was only significant when compared to the ssa1Δ/Δ complemented strain (p = 0.04), but not the wild-type strain (p = 0.13) (Figure 2A). However, after 2 and 5 days of infection, mice infected with the ssa1Δ/Δ mutant had markedly lower oral fungal burdens compared to mice infected with either the wild-type or ssa1Δ/Δ::SSA1 complemented strain. In contrast, the oral fungal burden of mice infected with the ssa2Δ/Δ mutant was similar to that of mice infected with the wild-type strain (p = 0.57) (Figure 2B). Therefore, Ssa1 is required for maximal virulence during oropharyngeal candidiasis, but Ssa2 is dispensable for virulence during this infection.
Fig. 2. Attenuated virulence of the ssa1Δ/Δ mutant during oropharyngeal candidiasis. 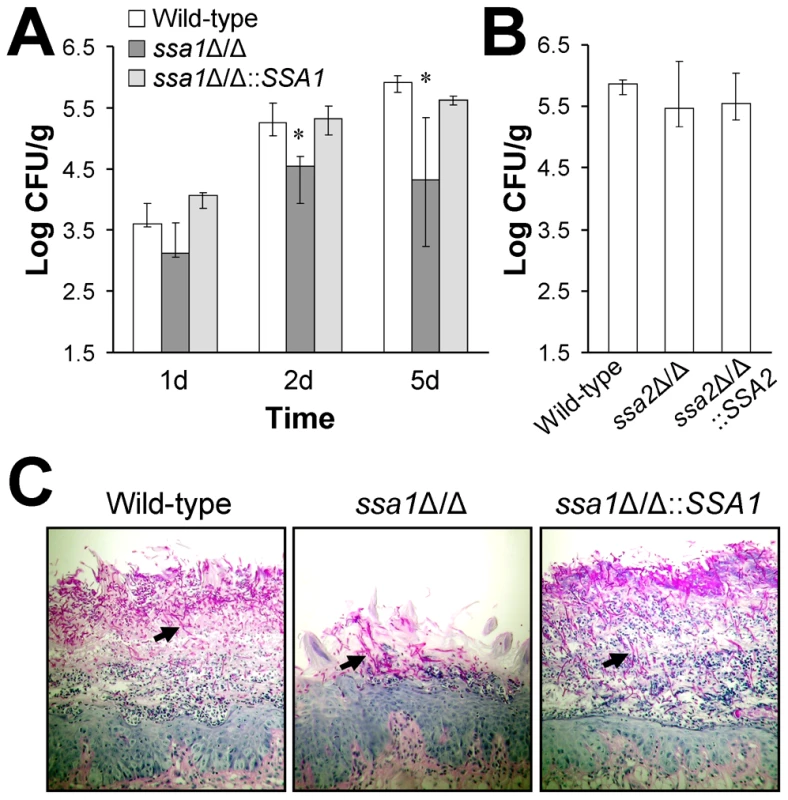
Mice were immunosuppressed with cortisone acetate and then orally inoculated with yeast-phase cells of the indicated strains of C. albicans. After 1, 2, and 5 days of infection, the mice were sacrificed and the tongues and attached tissues were excised. (A) Oral fungal burden of mice infected with the wild-type, ssa1Δ/Δ, and ssa1Δ/Δ::SSA1 complemented strains for the indicated times. Results from the 1 and 2 day time points are the median ± the interquartile range of one experiment with 7 mice per strain of C. albicans. Results from the 5 day time point are the median ± the interquartile range of two experiments with a total of 12 mice per strain. *P≤0.01 compared to both the wild-type and ssa1Δ/Δ::SSA1 strains. (B) Oral fungal burden of mice infected with the wild-type, ssa2, and ssa2Δ/Δ::SSA2 complemented strain for 5 days. Results are the median ± the interquartile range of one experiment with 7 mice per strain of C. albicans. (C) Periodic acid-Schiff stained thin sections of the tongues of mice infected with the indicated strains for 5 days. Arrows indicate the C. albicans filaments. The results of histopathologic examination of the tongues of the mice infected with the various strains verified the reduced virulence of the ssa1Δ/Δ mutant. The tongues of mice infected with the ssa1Δ/Δ mutant had relatively small lesions that contained fewer fungal cells and neutrophils than the lesions of mice infected with either the wild-type or the ssa1Δ/Δ::SSA1 complemented strain (Figure 2C). Importantly, the filaments of the ssa1Δ/Δ mutant were similar in length to those of the wild-type strain. Collectively, these results indicate that Ssa1 is necessary for maximal C. albicans virulence during both hematogenously disseminated and oropharyngeal candidiasis.
Ssa1 is required for C. albicans to cause maximal damage to both endothelial and oral epithelial cells in vitro
During hematogenously disseminated candidiasis, blood-borne C. albicans cells must adhere to and penetrate the endothelial cell lining of the blood vessels to invade the deep tissues [3]. C. albicans also adheres to and invades oral epithelial cells during oropharyngeal candidiasis [22]–[24]. After the organism invades endothelial or oral epithelial cells in vitro, it damages these cells. Moreover, C. albicans mutants with impaired capacity to cause host cell damage in vitro frequently have attenuated virulence in mice [25]–[27]. Therefore, we used a 51Cr release assay to determine the capacity of the ssa1Δ/Δ mutant to damage monolayers of human umbilical vein endothelial cells and the FaDu oral epithelial cell line. In this assay, the host cells are incubated with 51Cr, which is taken up by the cells and binds to cytoplasmic proteins. When the host cells are damaged, there is loss of membrane integrity and the labeled proteins leak out of the cells into the medium. The amount of 51Cr that is released is proportional to the extent of cellular damage [26]–[31]. We found that the ssa1Δ/Δ mutant caused 50% less damage to endothelial cells and 89% less damage to epithelial cells compared to the wild-type strain (Figure 3). Complementing the ssa1Δ/Δ mutant with a wild-type copy of SSA1 restored its capacity to damage these cells. As predicted by the virulence data, the ssa2Δ/Δ mutant caused the same extent of damage to the endothelial and epithelial cells as did the wild-type strain. All strains of C. albicans formed true hyphae that were of similar length on both endothelial and epithelial cells (data not shown). Therefore, the host cell damage defects of the ssa1Δ/Δ mutant were not due to impaired hyphal formation [27], [32], [33]. These results indicate that Ssa1 is necessary for C. albicans to cause maximal damage to endothelial and oral epithelial cells in vitro. This defective capacity to damage host cells likely contributed to the attenuated virulence of the ssa1Δ/Δ mutant.
Fig. 3. Ssa1 is necessary for C. albicans to cause maximal damage to endothelial cells and an oral epithelial cell line. 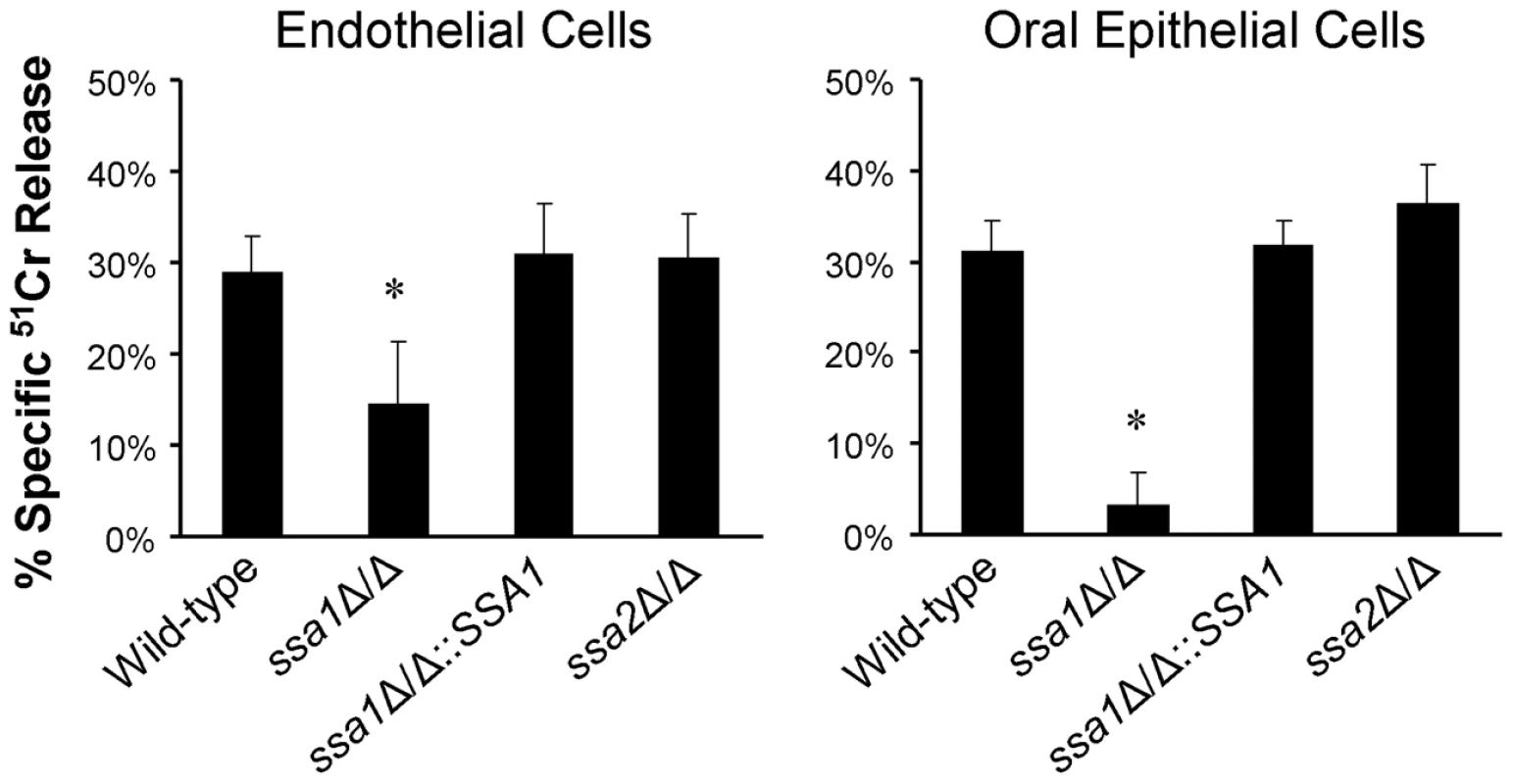
Endothelial cells and the FaDu oral epithelial cell line were incubated with the indicated strains of C. albicans for 3 h, after which the extent of host cell damage was determined using a 51Cr release assay. Results are the mean ± SD of three independent experiments, each performed in triplicate. *P<0.001 compared with the wild-type and ssa1Δ/Δ::SSA1 complemented strains. Although endothelial cells grow as a monolayer in vivo, epithelial cells grow in multiple, stratified layers in the oropharynx. Therefore, we tested the interactions of the ssa1Δ/Δ and ssa2Δ/Δ mutants with oral epithelial cells in a three-dimensional culture model, which more closely mimics the normal human oral mucosa and submucosa [34]. Consistent with the intraoral animal findings, the ssa1Δ/Δ mutant grew only in small foci and caused little visible epithelial cell damage, even after 2 days of infection (Figure 4A). There was only slight cellular edema in the epithelium, which otherwise maintained its barrier function and prevented the organisms from crossing the basal layer of cells into the collagen gel. In contrast, the ssa1Δ/Δ::SSA1 complemented strain formed a thick biofilm on the epithelial cells, which resulted in degradation of most of the epithelial layers and subsequent submucosal invasion. As predicted by our previous results, the ssa2Δ/Δ strain induced extensive epithelial destruction and invaded into the submucosal compartment similarly to the ssa1Δ/Δ::SSA1 complemented strain.
Fig. 4. The ssa1Δ/Δ mutant has impaired capacity to damage a three-dimensional organotypic model of the oral mucosa. 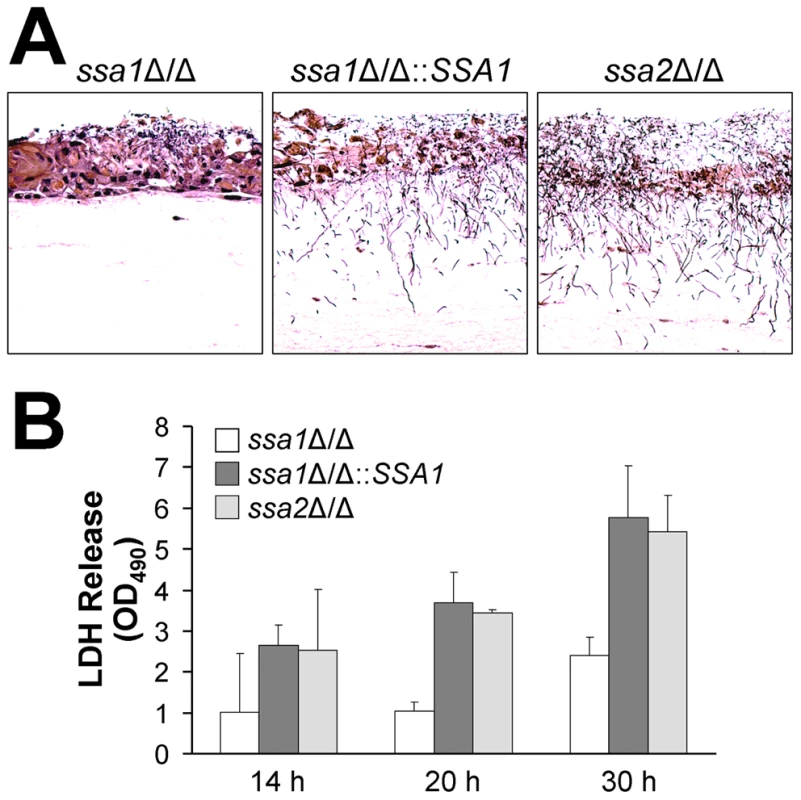
The three-dimensional model of the oral mucosa was infected by adding 105 yeast-phase cells of the indicated C. albicans strains to the apical surface. (A) Histopathology after 2 days of infection. Thin sections were stained with periodic acid-Schiff. The results are representative of one of three experiments. (B) Epithelial cell damage by the indicated strains was quantified by the release of lactate dehydrogenase (LDH) into the medium. Results are the mean ± SD of 2 experiments. To quantify the extent of epithelial cell damage caused by the various strains of C. albicans in the 3-dimensional model, we measured the leakage of lactate dehydrogenase (LDH) into the medium. The ssa1Δ/Δ mutant induced less LDH release than did the ssa1Δ/Δ::SSA1 complemented or ssa2Δ/Δ strains (Figure 4B). These results provide further support for the importance of Ssa1 in the capacity of C. albicans to damage oral epithelial cells.
Ssa1 governs the capacity of C. albicans to bind to cadherins and invade endothelial and oral epithelial cells
To cause maximal damage endothelial or oral epithelial cells, C. albicans must adhere to and then invade these host cells [27], [30], [31]. One mechanism by which C. albicans invades endothelial cells and oral epithelial cells is by inducing its own endocytosis [27], [31], [33], [35]. Therefore, we used our standard differential fluorescent assay to determine if the ssa1Δ/Δ mutant had a defect in its capacity to adhere to and/or be endocytosed by endothelial and FaDu epithelial cells in vitro. Compared to the wild-type strain, the ssa1Δ/Δ mutant was endocytosed poorly by both endothelial cells and oral epithelial cells (Figure 5A). The ssa1Δ/Δ::SSA1 complemented strain and the ssa2Δ/Δ mutant interacted with both the endothelial and epithelial cells similarly to the wild-type strain. In addition, endocytosis defects of the ssa1Δ/Δ mutant persisted when URA3 was integrated at the RPS10 locus (Supplemental Figure S1B). Therefore, Ssa1, but not Ssa2, is required for C. albicans to invade endothelial cells and oral epithelial cells in vitro.
Fig. 5. Ssa1 is necessary for C. albicans to adhere to and be endocytosed by endothelial cells and oral epithelial cells. 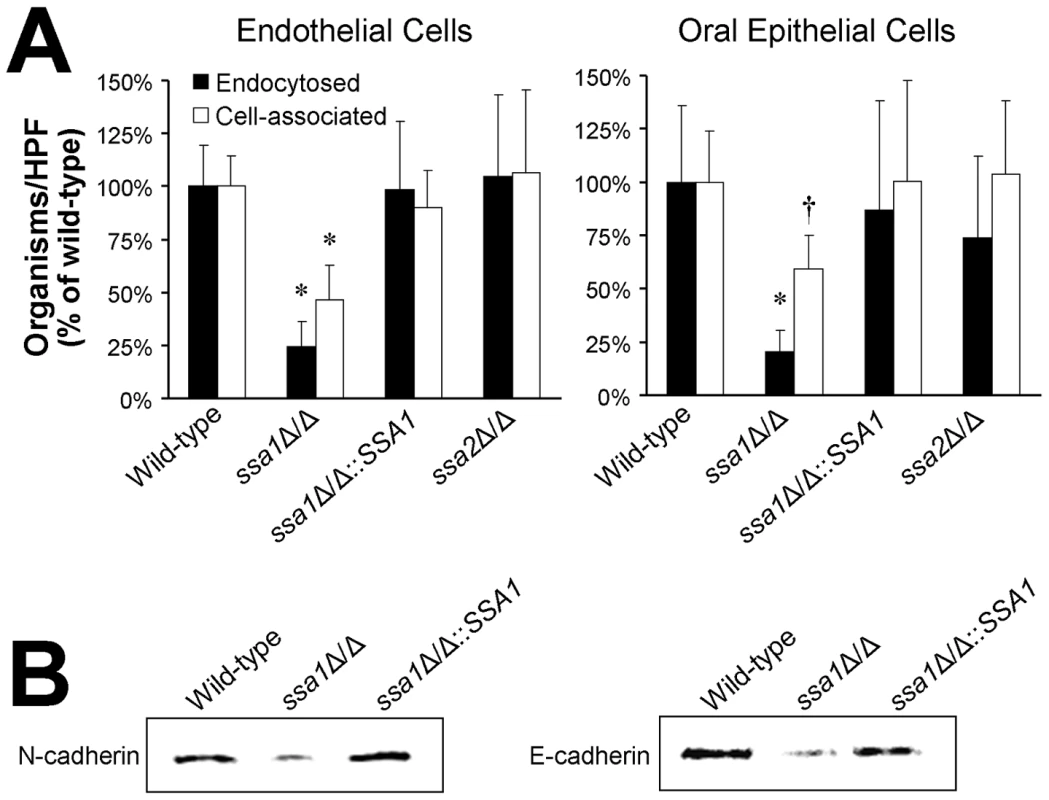
(A) Adherence and endocytosis assay. The indicated strains of C. albicans were incubated with endothelial cells or FaDu oral epithelial cells for 90 min, after which the number of endocytosed and cell-associated (endocytosed plus adherent) organisms was determined by a differential fluorescent assay. Results are the mean ± SD of three independent experiments, each performed in triplicate. *P<0.005 compared to the wild-type and ssa1ΔΔ::SSA1 complemented strains; †p<0.03 compared to the wild-type and ssa1ΔΔ::SSA1 complemented strains. (B) The capacity of the indicated strains to bind to N-cadherin in extracts of endothelial cell membrane proteins and E-cadherin in extracts of FaDu oral epithelial cell membrane proteins was determined using a whole-cell affinity purification approach. The immunoblot of endothelial cell proteins eluted from hyphae of the indicated strains was developed with an anti-N-cadherin antibody. The immunoblot of epithelial cell proteins eluted from hyphae of the indicated strains was developed with an anti-E-cadherin antibody. C. albicans induces its own endocytosis by endothelial cells in vitro by binding to N-cadherin on the endothelial cell surface [36]. The corresponding receptor for C. albicans on oral epithelial cells is E-cadherin [37]. To determine the mechanism of host cell invasion defects of the ssa1Δ/Δ mutant, we examined its capacity to bind to N-cadherin and E-cadherin in cell membrane extracts from endothelial cells and epithelial cells, respectively. Hyphae of the ssa1Δ/Δ mutant bound poorly to N-cadherin and E-cadherin relative to both the wild-type and ssa1Δ/Δ::SSA1 complemented strain, (Figure 5B). This reduced binding to cadherins likely contributed to the impaired host cell invasion and subsequent reduced host cell damage of the ssa1Δ/Δ mutant.
Ssa1 can directly mediate adherence to and endocytosis by host cells
Ssa1 is expressed either on or near the cell surface of both hyphae and yeast phase C. albicans [13]. Therefore, we investigated the possibility that Ssa1 by itself could mediate pathogenic host cell interactions. Latex beads were coated with recombinant Ssa1 (rSsa1), rSsa2, or bovine serum albumin (BSA), after which their endocytosis by host cells was measured. Both endothelial cells and epithelial cells endocytosed significantly more beads coated with either rSsa1 or rSsa2 compared to the control BSA-coated beads (Figure 6). These results suggest that both Ssa1 and Ssa2 can directly induce endocytosis by endothelial and epithelial cells.
Fig. 6. Ssa1 is sufficient to induce endocytosis by host cells. 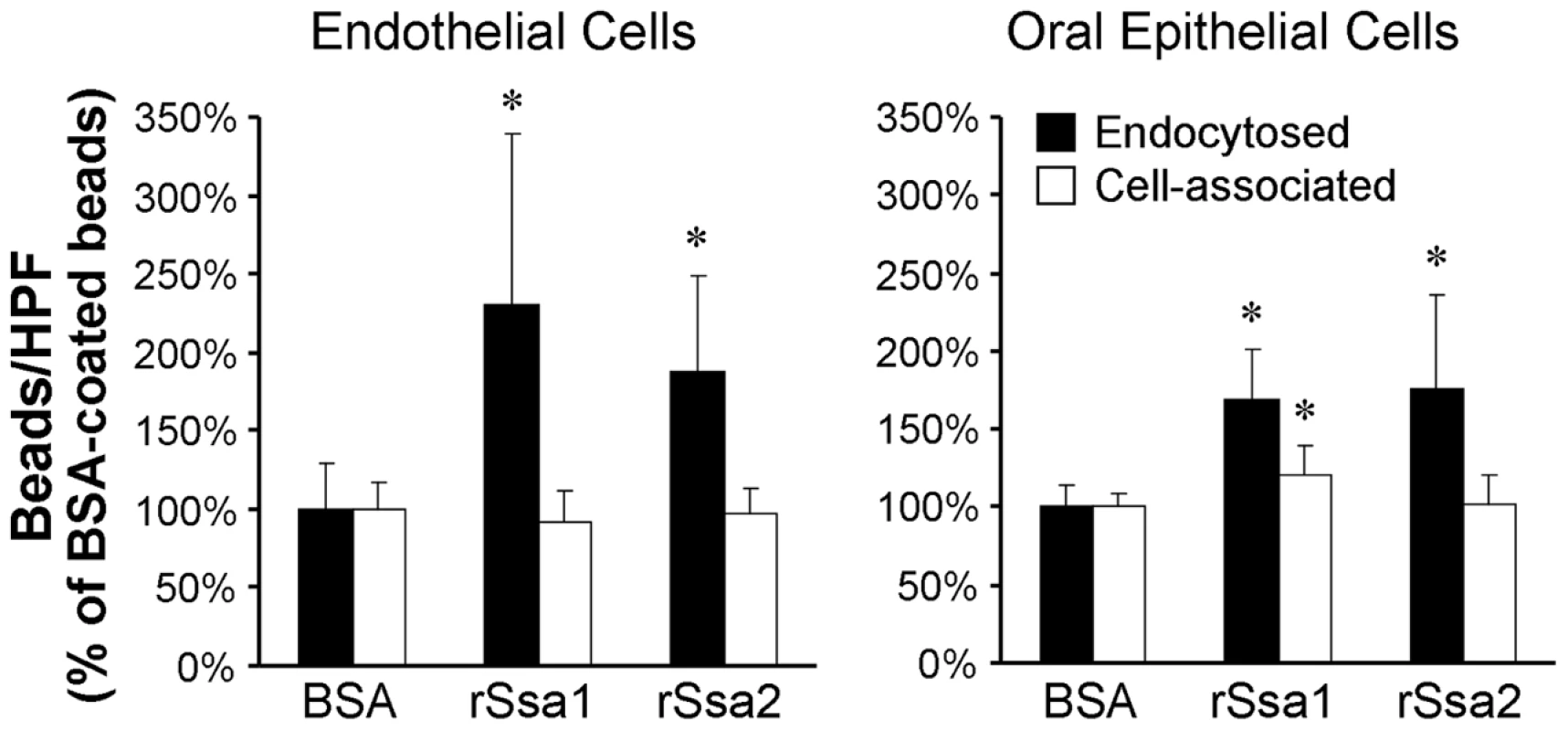
Latex beads were coated with bovine serum albumin (BSA), recombinant Ssa1 (rSsa1), or rSsa2. They were incubated for 45 min with endothelial cells and FaDu oral epithelial cells, after which the number of endocytosed and cell-associate beads was determined. Data are expressed as a percentage of the results with beads coated with BSA and are the mean ± SD of 3 experiments, each performed in triplicate. *P<0.01 compared to beads coated with BSA. To verify that Ssa1 was expressed on the surface of C. albicans hyphae that were interacting with host cells, we infected FaDu epithelial cells with a strain of C. albicans that expressed an Ssa1-GFP fusion protein. After a 90 min incubation, the cells were fixed, stained with an Alexa 594-conjugated anti-C. albicans antibody to label the cell surface, and then imaged by confocal microscopy. We found that Ssa1-GFP fluorescence was strongest at the periphery of the hyphae (Figure 7). Importantly, this fluorescence co-localized with that of the fluorescently-labeled anti-C. albicans antibody, indicating that Ssa1 was located in the outermost part of the cell wall. Interestingly, when a similar experiment was performed using organisms grown as yeast cells in suspension, Ssa1 was located slightly internal to the cell surface (Figure S2). These results suggest that the location of Ssa1 is dependent on the morphology and culture conditions of the organism. Importantly, when C. albicans hyphae are in contact with host cells, Ssa1 is likely expressed on the cell surface where it can interact with host cell receptors.
Fig. 7. Ssa1 is located on the cell surface of C. albicans hyphae during epithelial cell interaction. 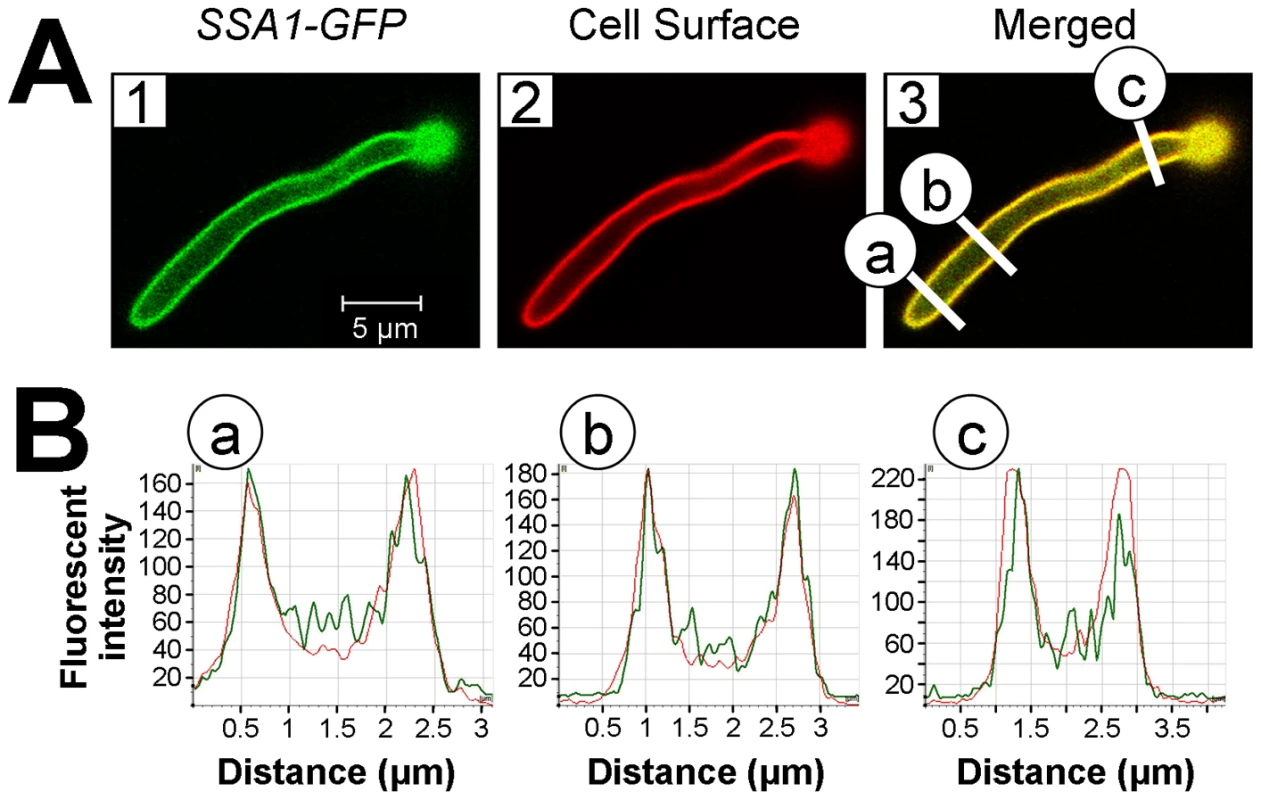
FaDu oral epithelial cells were infected with C. albicans expressing an Ssa1-GFP fusion protein for 90 min, after which the cells were fixed and stained with an Alexa 594-conjugated anti-C. albicans polyclonal antibody to label the cell surface. (A) Confocal microscopic images of Ssa1-GFP (1) and fluorescent-labeled anti-C. albicans antibody (2). The merged image is shown in (3) and the regions in yellow indicate areas of co-localization between Ssa1-GFP and the fluorescent-labeled anti-C. albicans antibody. (B) Graphs of fluorescent intensity at different cross sections of the hypha in panel (3). The green lines indicate the fluorescent intensity of the Ssa1-GFP and the red lines indicate the fluorescent intensity of the fluorescent-labeled anti-C. albicans antibody. The letters (a-c) correspond to those in panel (3) and indicate the locations along the hypha at which the fluorescent intensity was measured. Ssa1 and Als3 function in the same pathway(s) to induce endocytosis
One C. albicans ligand that binds to N-cadherin and E-cadherin is Als3, and als3Δ/Δ mutants have significantly reduced adherence to and invasion of both endothelial cells and oral epithelial cells in vitro [37]–[39]. Because heat shock proteins can potentially influence the expression of other proteins on the surface of C. albicans, we investigated the possibility that the host cell interaction defects of the ssa1Δ/Δ mutant were due to decreased surface expression of Als3. Flow cytometric analysis of hyphae stained with a polyclonal anti-Als3 antibody demonstrated that the ssa1Δ/Δ mutant expressed a similar amount of Als3 on its surface as did the wild-type and ssa1Δ/Δ::SSA1 complemented strains (Figure 8A). Furthermore, using indirect immunofluorescence with the anti-Als3 antibody, we determined that Als3 was distributed along the entire length of ssa1Δ/Δ mutant hyphae, similar to the wild-type and ssa1Δ/Δ::SSA1 complemented strains (Figure 8B). Thus, the host cell interaction defects of the ssa1Δ/Δ mutant are unlikely to be due to abnormal amount or location of Als3 on the cell surface.
Fig. 8. Interaction between Ssa1 and Als3. 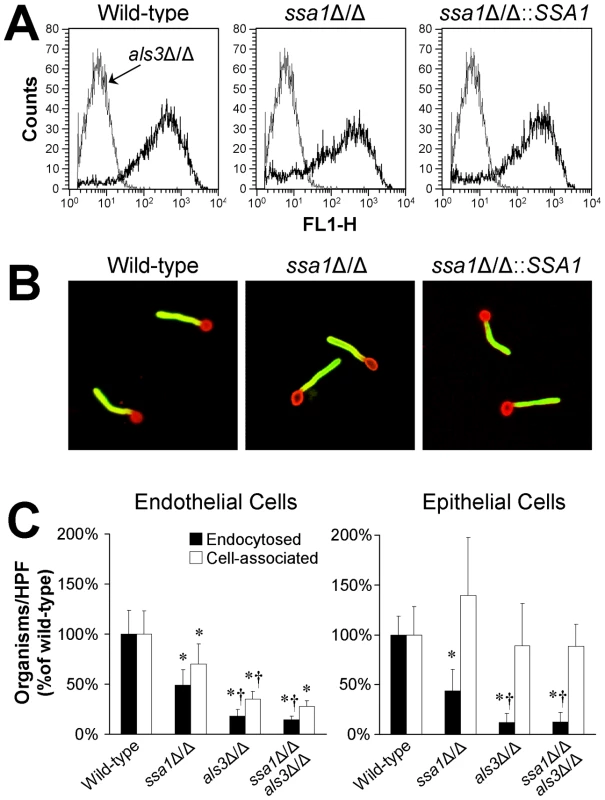
(A) The ssa1Δ/Δ mutant has normal amounts of Als3 on its surface. Hyphae of the indicated C. albicans strains were stained with a polyclonal anti-Als3 antibody, after which amount of surface exposed Als3 was determined by flow cytometric analysis of 10,000 cells per strain. (B) Normal surface distribution of Als3 on ssa1Δ/Δ hyphae. The distribution of Als3 on the surface of hyphae of the indicated C. albicans strains was determined by staining the organisms with a polyclonal anti-Als3 antibody (green fluorescence) and counterstaining with an anti-C. albicans polyclonal antibody (red fluorescence). (C) Effects of deletion of ALS3 in the ssa1Δ/Δ mutant background. Endothelial cells and FaDu oral epithelial cells were incubated with the indicated strains for 150 min, after which the number of endocytosed and cell-associated organisms were determined. Results are the mean ± SD of 3 experiments, each performed in triplicate. *P<0.01 compared to the wild-type strain, †p<0.005 compared to the ssa1Δ/Δ single mutant. To further investigate possible interactions between Ssa1 and Als3, we constructed an ssa1Δ/Δ als3Δ/Δ double mutant and compared its host cell interactions with those of ssa1Δ/Δ and als3Δ/Δ single mutants. When a 90 min incubation period was used, the host cell interaction defects of the both the ssa1Δ/Δ and als3Δ/Δ mutants were so large that it was not possible to detect any further reduction in endocytosis of the ssa1Δ/Δ als3Δ/Δ double mutant (Figure 5 and data not shown). Therefore, we increased the incubation period to 150 min. We found that at this time point, the als3Δ/Δ single mutant had a greater defect in inducing endocytosis by endothelial and epithelial cells than did the ssa1Δ/Δ single mutant (Figure 8C). However, the endocytosis defect of the ssa1Δ/Δ als3Δ/Δ double mutant was similar to that of the als3Δ/Δ single mutant. Collectively, these results indicate that Ssa1 and Als3 function in the same pathway(s) to induce endocytosis, perhaps by binding to the same host cell surface proteins and/or forming part of the same multiprotein complex.
SSA1 is expressed at higher levels than SSA2
It seemed paradoxical that Ssa2 was sufficient to induce endocytosis by host cells, yet the ssa2Δ/Δ mutant was endocytosed similarly to the wild-type strain. One potential explanation for these results is that SSA1 is expressed at a higher level than SSA2. Previously, we had found that, in yeast-phase C. albicans, the SSA1 mRNA transcript levels were significantly greater than those of SSA2, and the fungal cell wall contained 4 - to 5 - fold more Ssa1 than Ssa2 [16]. To determine if SSA1 was expressed greater than SSA2 in hyphae that were in contact with host cells, we infected FaDu oral epithelial cells with the various C. albicans strains and allowed them to germinate. SSA1 and SSA2 mRNA expression in these organisms was measured by real-time PCR. We found that in the wild-type strain, SSA1 was expressed 8-fold higher than SSA2 (Figure 9). As expected, SSA1 mRNA was not detectable in the ssa1Δ/Δ mutant. Importantly, SSA2 expression in this mutant was similar to that of the wild-type strain, indicating that SSA2 was not up-regulated in compensation for the absence of SSA1. Therefore, even though Ssa2 is capable of stimulating host cell endocytosis, the effects of SSA2 deletion are likely masked by the high level expression of SSA1.
Fig. 9. Relative transcript levels of SSA1 and SSA2. 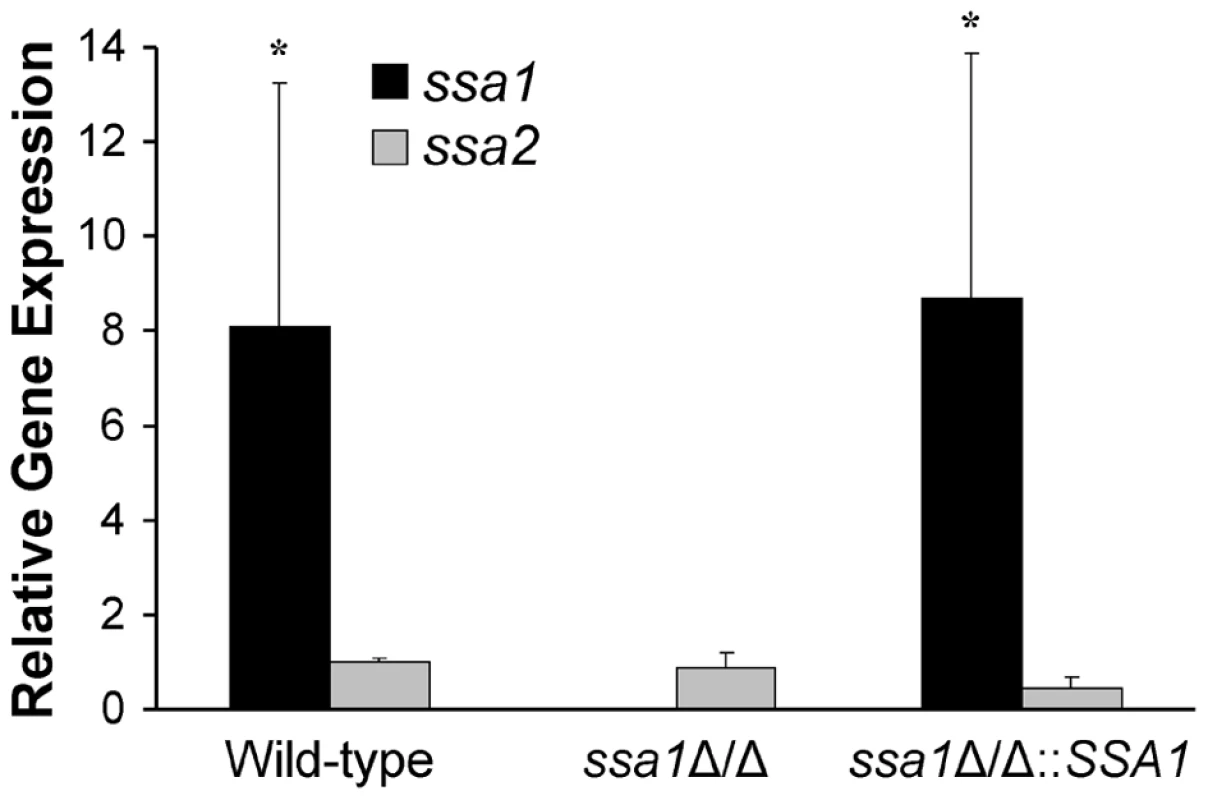
FaDu epithelial cells were infected with the indicated strains of C. albicans for 90 min, after which the C. albicans RNA was extracted. The relative transcript levels of SSA1 and SSA2 were measured by real-time PCR. Results are the mean ± SD of three biological replicates, each tested in duplicate. *P<0.01 compared to SSA2 transcript levels. Ssa1 is not required for secretion of phospholipases and proteases
C. albicans secretes phospholipases and aspartyl proteases, both of which contribute to the virulence of this organism, probably by damaging host cells [35], [40]–[46]. Heat shock proteins can influence protein secretion [47], [48]. Therefore, we investigated the possibility that the attenuated damage of endothelial and epithelial cells caused by the ssa1Δ/Δ mutant was due to reduced secretion of phospholipases or proteases. We grew the various strain on either egg yolk agar or BSA agar and measured the size of the zones of precipitation or clearance around the colonies to screen for total extracellular phospholipase and protease activity, respectively [43], [49]. The ssa1Δ/Δ mutant was similar to the wild-type and ssa1Δ/Δ::SSA1 complemented strains in these assays (Figure 10). These data suggest that the reduced virulence of the ssa1Δ/Δ mutant was unlikely the result of diminished secretion of phospholipases or proteases.
Fig. 10. C. albicans extracellular phospholipase and protease activities are independent of Ssa1 and Ssa2. 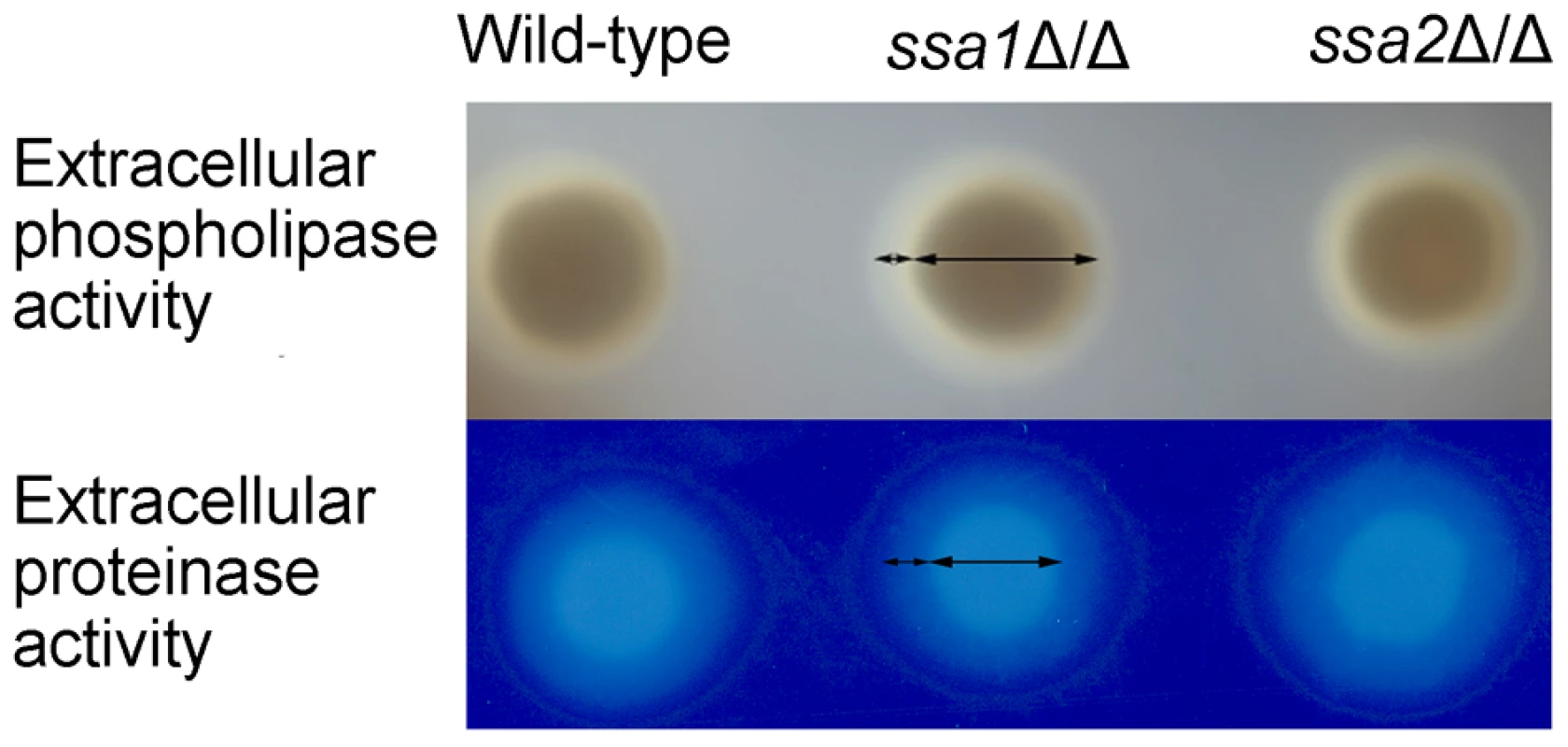
The total extracellular phospholipase activity of the indicated strains was determined by growing them on egg yolk agar at room temperature for 5 days and then measuring the width of the zone of precipitation around each colony (top panel). The total extracellular protease activity of the indicated strains was assessed by growing them on BSA agar at 37°C for 5 days and then staining the plates with 0.5% amido black. The extracellular protease activity was proportional to the width of the zone of clearance around the colonies (lower panel). C. albicans resistance to stress is independent of Ssa1 and Ssa2
Heat shock proteins are important for some microorganisms to resist the stressful conditions they encounter in the host [10]–[12]. Therefore, increased susceptibility to host-induced stress could have contributed to the reduced virulence of the ssa1Δ/Δ mutant. To evaluate this possibility, we tested the susceptibility of the ssa1Δ/Δ and ssa2Δ/Δ mutants to various stressors. Both the ssa1Δ/Δ and ssa2Δ/Δ mutants had wild-type susceptibility to oxidant (menadione and H2O2), cell wall (Calcofluor white), osmotic (NaCl), and plasma membrane (SDS) stress (Figure 11A). To verify that deletion of SSA1 or SSA2 did not affect stress resistance, we tested these stressors at higher concentrations. The growth of the ssa1Δ/Δ and ssa2Δ/Δ mutants was inhibited similarly to the wild-type strain under these conditions (data not shown). In addition, all strains had comparable susceptibility to nitric oxide (data not shown). We also tested the susceptibility of the ssa1Δ/Δ mutant to damage by the human neutrophil-like HL-60 cell line. This mutant was not more susceptible than the wild-type and ssa1Δ/Δ::SSA1 complemented strains (p≥0.12) (Figure 11B). Therefore, the reduced virulence of the ssa1Δ/Δ mutant was unlikely to be due to increased susceptibility to host induced stress.
Fig. 11. Ssa1 and Ssa2 are not required for resistance to stress. 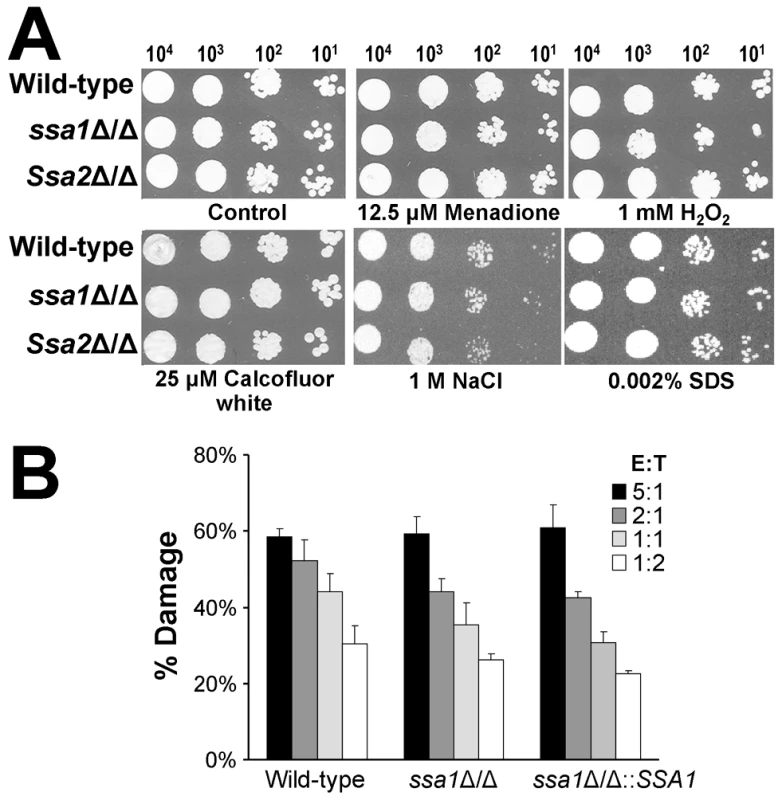
(A) Susceptibility to oxidant and cell wall stress. Serial 10-fold dilutions of the indicated strains were used to inoculate YPD plates containing menadione, H2O2, Calcofluor white, NaCl, or SDS. Images were obtained after growth at 30°C for 24 h. (B) Susceptibility to leukocyte induced damage. Yeast-phase cells of the indicated strains were incubated for 3 h with HL-60 cells that had been differentiated into neutrophil-like cells, after which the amount of damage to the organisms was determined by an XTT assay. Each strain was tested at an effector to target ratio (E:T) ranging from 5∶1 to 1∶2. Results are the mean ± SD of three independent experiments, each performed in triplicate. Discussion
Heat shock proteins play diverse roles in the pathogenicity of many microorganisms, including bacteria, protozoa, and fungi [6]–[12]. However, the contribution of members of the HSP70 family of heat shock proteins to C. albicans virulence has not been reported previously. Our results with the ssa1Δ/Δ mutant demonstrate that Ssa1 is require for maximal C. albicans virulence during both hematogenously disseminated and oropharyngeal candidiasis in mice.
The data from our in vitro studies indicated that the ssa1Δ/Δ mutant was defective in its capacity to adhere to, invade, and damage both endothelial cells and oral epithelial cells. The invasion defect of this mutant was likely due in part to its impaired capacity to bind to endothelial cell N-cadherin and epithelial cell E-cadherin, receptors that can mediate the endocytosis of C. albicans [36], [37], [39]. Other C. albicans mutants that are defective in invading and damaging endothelial cells or oral epithelial cells frequently have attenuated virulence in mouse models of candidiasis [25], [26], [32], [50]. Therefore, it is highly probable that the host cell interaction defects of the ssa1Δ/Δ mutant contributed to its attenuated virulence in mice.
The experiments with latex beads coated with Ssa1 or Ssa2 demonstrated that either protein can induce endocytosis by both endothelial cells and oral epithelial cells. However, in intact organisms, Ssa1 is likely more important than Ssa2 for inducing host cell endocytosis because the transcript levels of SSA1 are much greater. In addition, using GFP-labeled Ssa1, we verified that Ssa1 is expressed on the surface of hyphae that are in contact with epithelial cells. Thus, Ssa1 is located where it can bind to host cell receptors. Collectively, these results indicate that Ssa1 can function as an invasin and induce host cells to endocytose the organism. Members of the Hsp70 family have also been found to mediate the host cell adherence of bacteria such as Helicobacter pylori, Haemophilus influenza, and Mycobacterium avium [6]–[8]. In addition, Hsp60 is expressed on the surface of Histoplasma capsulatum and is the major ligand for CD11b/CD18 on macrophages [9]. Orthologs of Ssa1 and Ssa2 are also present in species of Candida other than C. albicans. Whether these orthologs also mediate pathogenic host cell interactions remains to be determined.
Ssa1 is functionally similar to the C. albicans invasin Als3, which also binds to cadherins on the surface of endothelial cells and epithelial cells, and thereby induces endocytosis [37], [39]. It is notable that ssa1Δ/Δ and als3Δ/Δ mutants both have defects in their capacity to invade and damage these cells in vitro. Furthermore, the host cell invasion defect of the ssa1Δ/Δ als3Δ/Δ double mutant was similar that of the als3Δ/Δ single mutant. The most likely explanation for this result is that Ssa1 and Als3 bind to same endothelial and epithelial cell surface proteins, either separately or perhaps as part of multiprotein complex. An alternative explanation for these results is that Ssa1 functions as a molecular chaperone that is required for appropriate folding or trafficking of Als3 [47], [48], [51], [52]. Although we cannot completely exclude the possibility that the folding or post-translational modification of Als3 was aberrant in the ssa1Δ/Δ mutant, we determined that deletion of SSA1 had no effect on the amount and surface distribution of Als3 on C. albicans hyphae. These results suggest that proper Als3 trafficking can occur in the absence of Ssa1. Furthermore, the localization of Ssa1 on the fungal cell surface and the finding that beads coated with rSsa1 were avidly endocytosed suggests the more likely scenario that Ssa1 and Als3 function cooperatively as invasins.
After C. albicans invades host cells, it damages them. Host cell damage requires at least some extent of fungal invasion because blocking invasion by treatment with cytochalasin D or infection with non-invasive mutants of C. albicans reduces the extent of host cell damage [27], [31], [32], [50]. Thus, the defective host cell invasion of the ssa1Δ/Δ mutant likely contributed to its reduced capacity to damage these cells. Although the exact mechanism by which C. albicans causes host cell damage is incompletely understood, it is probable that phospholipases and aspartyl proteases secreted by the organism participate in this process [35], [40]–[46]. Some heat shock proteins can assist with protein secretion [47], [48]. However, we found no evidence of impaired phospholipase or protease secretion in the ssa1Δ/Δ mutant. While these results indicate that bulk protein secretion is intact in the ssa1Δ/Δ mutant, we cannot completely rule out the possibility that there was reduced secretion or activity of a single phospholipase or protease isozyme in this strain.
During both hematogenously disseminated and oropharyngeal infection, C. albicans is exposed to significant stress induced by the host. These stresses include exposure to reactive oxygen intermediates and antimicrobial peptides. Furthermore, in the oral cavity, C. albicans is likely exposed to toxic secondary metabolites produced by the resident oral flora. In some microbial pathogens, such as Leishmania spp. and Francisella tularensis, members of the Hsp70 and Hsp100 family of heat shock proteins are required for them to tolerate host-induced stress and cause persistent infection in mice [10]–[12]. Here, we found that Ssa1 was dispensable for the resistance of C. albicans to oxidant, cell wall, and cell membrane stress. We also determined that ssa1Δ/Δ mutant cells did not have increased susceptibility to killing by neutrophil-like HL-60 cells. Thus, the reduced virulence of the ssa1Δ/Δ mutant was unlikely due to impaired ability to withstand host-induced stress.
Previously we have found that Ssa1 acts as receptor for some antimicrobial proteins such as histatin 5, and that the ssa1Δ/Δ mutant is more resistant to some defensins [17]. Thus, one might have predicted that the ssa1Δ/Δ mutant would be resistant to neutrophil killing and therefore hypervirulent. However, the ssa1Δ/Δ mutant is not resistant to some toxic products of neutrophils, such as human neutrophil defensin 1 [17], reactive oxygen intermediates, and nitric oxide. Therefore, it is probable that the wild-type susceptibility of the ssa1Δ/Δ mutant to these stressors was the reason why it was neither resistant to killing by the neutrophil-like HL-60 cells in vitro nor hypervirulent in mice.
Although induction of host cell endocytosis is an important virulence function of Ssa1, it is possible that this protein may also contribute to C. albicans pathogenicity in other ways. For example, in S. cerevisiae Ssa1 regulates the transcription of the multidrug resistant gene, PDR3 [53]. Furthermore, in Cryptococcus neoformans, Ssa1 functions as a transcriptional co-activator of laccase, and is required for normal melanin synthesis and virulence [54]. Thus, Ssa1 may either directly or indirectly govern the transcription of genes that also contribute to C. albicans pathogenicity. This possibility is currently being investigated.
Materials and Methods
Strains and growth conditions
The C. albicans strains used in these studies and their relevant genotypes are listed in Table 1. All strains were maintained on yeast extract/peptone/dextrose (YPD; Qbiogene) agar plates and re-cultured at least monthly from −80°C stock. For use in the experiments, yeast-phase cells of the various strains were grown YPD broth overnight in a rotary shaker at 30°C.
Tab. 1. Strains of <i>C. albicans</i> used in this study. 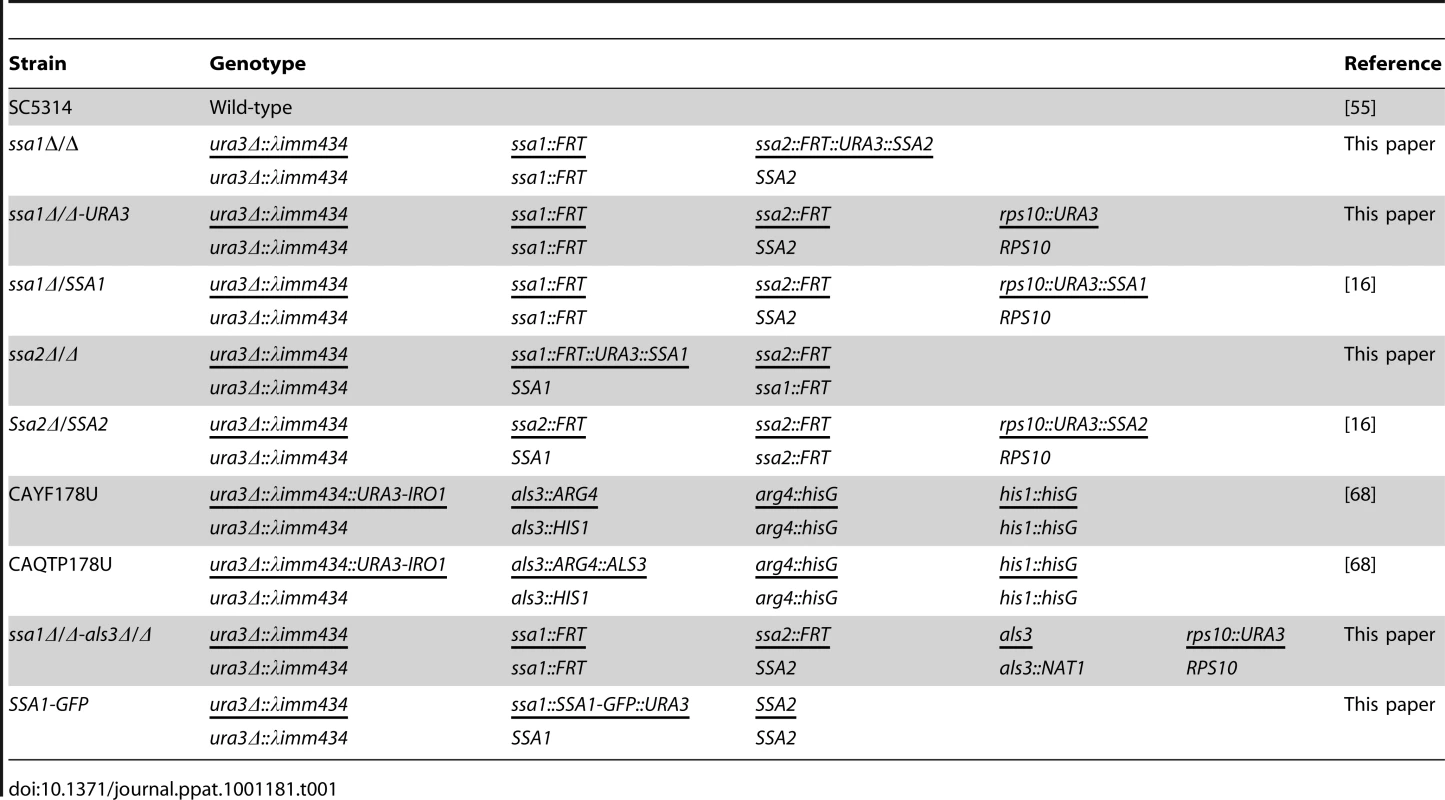
The initial ssa1Δ/Δ, ssa2Δ/Δ, ssa1Δ/Δ::SSA1, and ssa2Δ/Δ::SSA2 strains were constructed previously [16]. To verify that the phenotype of the ssa1Δ/Δ mutant was not the result of URA3 being integrated at the SSA2 locus, a Ura - strain was selected by growth on 5-fluoroorotic acid [55]. This strain was transformed by the lithium acetate procedure with the CIp10 vector that had been linearized with NcoI [56]. The resulting strain, ssa1Δ/Δ-URA3 contained URA3 at the neutral RPS10 locus [21].
To delete the entire protein coding region of ALS3 in the ssa1Δ/Δ mutant, the PCR-product-directed gene deletion approach was used [57]. Briefly, deletion cassettes containing ALS3 flanking regions and the URA3 or NAT1 selection markers were amplified by PCR with primers GATATTTTGAATATGGAAATAAATCGTGCATAAGAAAGTTTTGCTATGCACGTTCATACTTCCAAAAATTGTAATACGACTCACTATAGGGC and AAACTATAGAAACAAACTAATCAAATTAACAACACACCAAATTGGAGGTAATTAATCATACCGAAAATAGCTATGACCATGATTACGCCA, using pGEM-URA3 [57] and pJK795 [58] as templates, respectively. These PCR products were then used to successively transform the Ura - ssa1Δ/Δ strain [57]. The resulting ssa1Δ/Δ als3Δ/Δ mutant was plated on 5-fluoroorotic acid to select for a Ura - strain. This strain was transformed with NcoI-linearized CIp10 [56] to re-integrated URA3 at RPS10 locus.
Strains containing C-terminal GFP fusions were generated in strain CAF4-2 (wt) by substitution of one SSA1 allele with an SSA1-GFP tagged allele as described previously [16]. Plasmid pGFP-URA3 (PMG1602) was kindly provided by Dr. C. A. Gale, and was used as a template to PCR amplify the transformation cassettes using primers containing sequences flanking the SSA1 stop codon (67 bp) and homologous sequences from the vector (forward primer: 9 bp glycine linker and 21bp vector sequence; reverse primer: 23 bp vector sequence). PCR products were used to transform parental strains using frozen EZ yeast transformation II kit (Zymo Research) following the manufacturer's protocol and selected on URA3- YNB agar plates. To identify transformants carrying cassettes correctly integrated into the target gene sequence, genomic DNA was prepared and used as the template for PCR reactions using one primer for annealing within the transformation module and a second primer annealing with the target gene locus outside the altered region. Cells were examined using confocal microscopy and Western blotting with anti-GFP to confirm expression of Ssa1-GFP fusion protein.
Mouse models of disseminated and oropharyngeal candidiasis
The virulence of the various strains was tested in the mouse model of hematogenously disseminated candidiasis as described previously [26]. Briefly, 10 male BALB/c mice (20 g body weight; National Cancer Institute, Bethesda, MD) were infected via the lateral tail vein with 5×105 yeast-phase cells of each strain of C. albicans in 500 µl of PBS. All inocula were confirmed by quantitative culture. The mice were monitored at least three times daily, and moribund mice were euthanized. To determine the organ fungal burden, 5 to 7 mice were inoculated with each strain as in the survival experiments. After 4 h, 8 h, 1 day, 2 days, 4 days, 7 days, and 14 days of infection, the brain, liver, and one kidney from each mouse were harvested, weighed, homogenized, and quantitatively cultured. The remaining kidneys from the 1 day time point were fixed in zinc-buffered formalin followed by 70% ethanol and then embedded in paraffin. Thin sections were cut and stained with periodic acid-Schiff.
The different strains of C. albicans were also tested for virulence in our previously described mouse model of oropharyngeal candidiasis [25], [27], [59]. Briefly, male BALB/c mice were immunosuppressed with cortisone acetate (225 mg/kg; Sigma-Aldrich) administered subcutaneously on days -1, 1, and 3 relative to infection. For oral inoculation, each mouse was anaesthetized by intraperitoneal injection with ketamine and xylazine (both from Phoenix Pharmaceuticals), after which a calcium alginate swab (Type 4 Calgiswab; Puritan Medical Products Company LLC) saturated with 106 yeast/ml was placed sublingually for 75 min. The mice were subsequently provided food and water ad libitum and then sacrificed after 1, 2 and 5 days of infection. The tongue and adjacent hypoglossal tissue were excised and cut in half. One half was weighed and homogenized for quantitative culture and the other half was processed for histopathological analysis as described above.
Endothelial cells and oral epithelial cells
Endothelial cells were isolated from human umbilical cord veins by the method of Jaff et al. [60] and cultured as described [32] in M-199 medium supplemented with 10% fetal bovine serum and 10% defined bovine calf serum (Gemini Bio-Products), and containing 2 mM L-glutamine with penicillin and streptomycin (Irvine Scientific). Endothelial cells were used at the third passage. The FaDu oral epithelial cell line (American Type Culture Collection) was cultured in Eagle's minimum essential medium with Earle's balanced salt solution (Irvine Scientific) supplemented with 10% fetal bovine serum, 1 mM pyruvic acid, 2 mM l-glutamine, and 0.1 mM nonessential amino acids, with penicillin and streptomycin [25], [27]. Both cell types were maintained in a humidified incubator in 5% CO2 at 37°C.
Host cell damage assay
The extent of endothelial and epithelial cell damage caused by the different strains of C. albicans was measured using our previously described 51Cr release assay [25]–[27], [31], [32]. Briefly, endothelial cells or FaDu oral epithelial cells were grown to 95% confluency in 96 well tissue culture plates with detachable wells (Corning) and loaded with 5 µCi/ml Na251CrO4 (MP Biomedicals) overnight. After removing the unincorporated 51Cr by rinsing, the cells were then infected with yeast of the various C. albicans strains suspended in RPMI 1640 medium (Irvine Scientific). The inoculum was 4×104 organisms per well of endothelial cells and 1×105 organisms per well of oral epithelial cells. The infected host cells were incubated for 3 h, after which the amount of 51Cr released into the medium and retained by the cells was determined by γ-counting. Wells containing host cells, but no organism, were processed in parallel to determine the spontaneous release of 51Cr. After correcting for well-to-well differences in the incorporation of 51Cr, the percent specific release of 51Cr was calculated using the following formula: (experimental release - spontaneous release)/(total incorporation - spontaneous release). Experimental release was the amount of 51Cr released into the medium by cells infected with C. albicans. Spontaneous release was the amount of 51Cr released into the medium by uninfected host cells. Total incorporation was the sum of the amount of 51Cr released into the medium and remaining in the host cells. Each assay was performed in triplicate on three separate occasions.
Three-dimensional model of the human oral mucosa
The capacity of the various C. albicans strains to invade a three dimensional model of the oral mucosa in vitro was determined by our previously described method [34], [61]. OKF6/TERT-2 oral epithelial cells were grown on top of a feeder layer of collagen embedded NIH 3T3 cells in 30 mm diameter cell culture inserts (Millipore). They were infected via their apical surface by adding 105 C. albicans cells in 100 µl of airlift medium (DMEM with 4.5 g/l glucose [Fisher Scientific] and Ham's F-12 medium [Invitrogen] mixed 3∶1, and supplemented with 5 µg/ml insulin, 0.4 µg/ml hydrocortisone, 2×1011 M 3,3′,5-triiodo-L-thyronine, 1.8×10−4 M adenine, 5 µg/ml transferrin, 10−10 M cholera toxin, 2 mM L-glutamine; 5% FBS, and penicillin–streptomycin). For histopathology, the cultures were fixed after 2 days with 10% formaldehyde in PBS and embedded in paraffin. Thin sections were stained with periodic acid-Schiff and then evaluated by light microscopy. The extent of epithelial cell damage was quantified after 2 days of infection by measuring the accumulation of LDH in the medium using the CytoTox-96 assay (Promega).
Adherence and endocytosis assay
The capacity of the various C. albicans strains to adhere to and be endocytosed by endothelial and FaDu epithelial cells was quantified using our previously described differential fluorescence assay [25], [27], [32], [36]. The host cells were grown to 95% confluency on fibronectin-coated glass coverslips in a 24-well tissue culture plate. Each coverslip was infected with 105 organisms in RPMI 1640 medium. After a 90 or 150 min incubation, the medium was aspirated, non-adherent organisms were removed by rinsing the coverslips with Hank's balanced salt solution (HBSS; Irvine Scientific), and cells were fixed with 3% paraformaldehyde. The adherent and non-internalized portions of the organisms were stained with rabbit anti-C. albicans antiserum (Biodesign International) conjugated with Alexa 594 (Molecular Probes). Next, the host cells were permeabilized with 1% Triton X-100 for 15 min, and then all of the C. albicans cells were stained with rabbit anti-C. albicans antiserum conjugated with Alexa 488 (Molecular Probes). The coverslips were mounted inverted on a microscope slide and organisms were viewed under epifluorescence. The number of endocytosed organisms was determined by subtracting the number of non-endocytosed organisms (which fluoresced red) from the number of cell-associated organisms (endocytosed plus non-endocytosed organisms, which fluoresced green). Organisms that were partially internalized were considered to be endocytosed. At least 100 organisms were counted per coverslip, and each experiment was repeated in triplicate, at least three times. Results were expressed as the number of endocytosed and cell-associated organisms per high powered field.
C. albicans binding to endothelial cell N-cadherin and epithelial cell E-cadherin
The capacity of each strain to bind to N-cadherin and E-cadherin was determined using our affinity purification procedure as outlined previously [36], [37]. Germ tubes were prepared by incubating yeast-phase cells for 90 min at 37°C in 150 mm diameter Petri dishes containing RPMI 1640 medium buffered to pH 7.5 with 150 mM HEPES. The resulting germ tubes were removed by scraping and rinsed once with PBS containing calcium and magnesium. The germ tubes (2×108 cells) were incubated for 1 h on ice with 250 µg of cell membrane proteins of endothelial cells or FaDu epithelial cells in PBS with calcium and magnesium containing 1.5% octyl-glucopyranoside and protease inhibitors. The unbound proteins were removed by rinsing in the same buffer, after which the proteins that remained bound to the germ tube were eluted with 6 M urea. The eluted proteins were separated by SDS-PAGE. N-cadherin and E-cadherin were detected by immunoblotting with anti-N-cadherin murine monoclonal antibody (clone 32, Transduction Laboratories), and an anti-E-cadherin murine monoclonal antibody (clone HECD 1), respectively.
Host cell interactions of latex beads coated with rSsa1, rSsa2, or BSA
Recombinant Ssa1 (rSsa1) and rSsa2 were produced in S. cerevisiae as outlined previously [16]. Latex beads were coated with these proteins or biotinylated BSA as described [37], [62]. Briefly, 5×107 fluorescent, yellow-green, amine-modified, 2.0 µm diameter polystyrene latex beads (Sigma-Aldrich) were washed with PBS followed by coupling buffer (0.2 M Na2HCO3 [pH 8.5], 0.5 M NaCl). The beads were then incubated with rSsa1, rSsa2 (0.5 mg/ml) or coupling buffer at 37°C for 30 min. The beads that had been coated with Ssa proteins were incubated with 1% rabbit serum, while the control beads were incubated with biotinylated BSA (1 mg/ml). All beads were sonicated briefly, blocked with unlabeled BSA and rinsed PBS-BSA. They were suspended in PBS containing 2 mg BSA per ml. Binding of rSsa1 or rSsa2 to the beads was verified by indirect immunofluorescence with an anti-Xpress monoclonal antibody (Invitrogen) directed against the Xpress leader sequence of these recombinant proteins. Binding of biotinylated BSA to the beads was confirmed using Alexa 568 conjugated streptavidin (Invitrogen). The interactions of these beads with endothelial and FaDu epithelial cells were determined by the differential fluorescent assay described above.
Real-time PCR detection of SSA1 and SSA2 transcript levels
The relative transcript levels of SSA1 and SSA2 in hyphae of the various strains of C. albicans were determined by our previously described method [63]. Yeast cells were suspended in RPMI 1640 medium and added to FaDu epithelial cells at a final concentration of 5×105 cells/cm2. After a 90 min incubation, the non-adherent organisms were removed by rinsing with ice-cold distilled water. Next, ice-cold DEPC-treated water was added and the C. albicans and epithelial cells were removed with a cell scraper. The mixture was vortexed for 30 sec to lyse the epithelial cells and then the organisms were collected by a brief centrifugation at 4°C. These organisms were suspended in TES buffer (10 mM Tris, 10 mM EDTA, 0.5% SDS), and then snap frozen in liquid nitrogen. The total time from rinsing the organisms to freezing them in liquid nitrogen was less than 5 min. At a later time, the cells were thawed on ice and the fungal RNA was extracted by the hot phenol method [64]. For real-time PCR, the C. albicans RNA was treated with DNase I (Ambion), after which cDNA was synthesized using MMLV reverse transcriptase (Ambion). Quantitative real-time PCR was carried out using the SYBR green PCR kit (Applied Biosystems) and an ABI 7000 Real-Time PCR System (Applied Biosystems) following the manufacturer's protocol. The results were analyzed by the 2-ΔΔCT method [65] using ACT1 as the endogenous control. The SSA1 and SSA2 transcript levels were determined in three biological replicates, each tested in duplicate.
Assessment of surface expression of Als3 and Ssa1
Flow cytometry was used to quantify the amount of Als3 on the surface of the various strains using a slight modification of our previously described method [37]. Briefly, 3×10 106 yeast-phase cells in 15 ml RPMI 1640 medium buffered to pH 7.5 with 10 mM HEPES were added to 100 mm diameter Petri dishes and incubated in 5% CO2 at 37°C. After 90 min, the resulting germ tubes were scraped from the dishes with a cell scraper, fixed with 3% paraformaldehyde, blocked with 1% goat serum, and then stained with a rabbit polyclonal anti-Als3 antiserum [37] followed by an Alexa 488 conjugated goat anti-rabbit antibody (Molecular Probes). An als3Δ/Δ mutant was included in these experiments as a negative control. The amount of Als3 surface staining on 104 germ tubes per strain was analyzed by flow cytometry.
To analyze the surface distribution of Als3 on the various strains, 105 yeast-phase cells in 1 ml RPMI 1640 medium buffered to pH 7.5 with 10 mM HEPES were added to 12 mm diameter glass cover slips in a 24-well tissue culture plate. After a 90 min incubation in 5% CO2 at 37°C, the resulting germ tubes were fixed with 3% paraformaldehyde, and blocked with 1% goat serum, and then stained with the rabbit polyclonal anti-Als3 antiserum followed by an Alexa 488 conjugated goat anti-rabbit antibody. Next, the germ tubes were counter stained with the Alexa 594 conjugated anti-Candida antibody to label the cell surface. The coverslips were mounted inverted on microscope slides and imaged by confocal microscopy. Stacked images were acquired along the z-axis and then combined for the final images.
The surface expression of Ssa1 on C. albicans expressing Ssa1-GFP was determined by a similar process, except that FaDu oral epithelial cells were grown on the coverslips prior to adding the organisms and the anti-Als3 antiserum was omitted.
Screening for extracellular phospholipase and protease activities
The C. albicans strains were screened for phospholipase activity by the method of Samaranayake et al. [49]. Briefly, egg yolk agar consisting of Sabouraud's dextrose agar, NaCl, CaCl2 and egg yolk emulsion was prepared. A 10 µl of suspension of 107 yeast-phase cells per ml in 10 mM sodium phosphate buffer was plated on the agar and incubated at room temperature for 5 days. The phospholipase activity of each strain was determined by measuring the width of zone of precipitation around the colony.
The total extracellular protease activity of the various strains was assessed using BSA agar by the method of Ruchel et al. [66]. BSA agar (0.2% BSA, 1.17% yeast carbon base, and 0.01% yeast extract, pH 5) was spot inoculated with the various C. albicans strains and then incubated at 37°C. After 5 days the agar was stained with 0.5% amido black and the width of the zone of clearance around each colony was measured.
Susceptibility to stressors
The susceptibility of the various C. albicans strains to stressors were tested by spotting dilutions of 104 to 101 yeast-phase cells in a total volume of 7 µl onto YPD agar plates containing menadione (12.5 and 25 µM); H2O2 (1, 2 and 4 mM), Calcafluor white (25 and 50 µM), NaCl (1 and 2 M) and SDS (0.002%). The plates were incubated at 30°C for 24 hours and then imaged.
The susceptibility of the different strains to damage by a neutrophil-like cell line was determined by the 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide (XTT) assay, as previously described [67]. Briefly, HL-60 cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum and 25 mM HEPES. They were induced to differentiate into neutrophil-like cells by exposure to 1.25% dimethyl sulfoxide for 7 days. For the damage assay, C. albicans yeast suspended in Dulbecco's Modified Eagle Medium containing 10% fetal bovine serum were added to 96-well plates at concentrations ranging from 105 to 2×104 cells/well. There was a linear relationship between viable cell number and colorimetric signal (XTT activity) in this concentration range with all three strains (not shown). HL-60 cells were added to the C. albicans cells at effector to target cell ratios (E:T) ranging from 5∶1 to 1∶2. After incubation at 37°C in 5% CO2 for 3 hours, the medium was aspirated and the HL-60 cells were lysed with sterile H2O. To each well was added 100 µl of a mixture of XTT and coenzyme Q0 (0.25 mg/ml XTT and 40 µg/ml coenzyme Q0), after which the plate was incubated at 37°C in 5% CO2 for 2 h. Supernatants were transferred into new plates, and optical densities (OD) were measured by an Opsys Microplate Reader (Thermo Labsystems) at 450–490 nm, with a 630 nm reference filter. Antifungal activity was calculated according to the following formula: %fungal damage = (1−x/n)*100, where x is the OD450 of experimental wells (C. albicans with effectors) and n is the OD450 of control wells (C. albicans only). Each experiment was performed in triplicate and repeated 3 times.
Statistics
The results of the survival experiments were analyzed with the Log-Rank Test, and the organ fungal burden data were analyzed using the Log Rank Test. The results of the in vitro experiments were analyzed using the Student's T test. P values ≤0.05 were considered to be significant.
Ethics statement
All experiments were approved by the Los Angeles Biomedical Research Institute Animal Care and Use Committee and as outlined in the Guide for the Care and Use of Laboratory Animals of National Institutes of Health. The collection of umbilical cords for the harvesting of endothelial cells used in these studies was approved by the Institutional Review Board of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center.
Supporting Information
Zdroje
1. WisplinghoffH
BischoffT
TallentSM
SeifertH
WenzelRP
2004 Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39 309 317
2. FillerSG
2006 Candida-host cell receptor-ligand interactions. Curr Opin Microbiol 9 333 339
3. GrubbSE
MurdochC
SudberyPE
SavilleSP
Lopez-RibotJL
2008 Candida albicans-endothelial cell interactions: a key step in the pathogenesis of systemic candidiasis. Infect Immun 76 4370 4377
4. WalkerLA
MaccallumDM
BertramG
GowNA
OddsFC
2009 Genome-wide analysis of Candida albicans gene expression patterns during infection of the mammalian kidney. Fungal Genet Biol 46 210 219
5. RodakiA
BohovychIM
EnjalbertB
YoungT
OddsFC
2009 Glucose promotes stress resistance in the fungal pathogen Candida albicans. Mol Biol Cell 20 4845 4855
6. YamaguchiH
OsakiT
TaguchiH
HanawaT
YamamotoT
1996 Flow cytometric analysis of the heat shock protein 60 expressed on the cell surface of Helicobacter pylori. J Med Microbiol 45 270 277
7. HartmannE
LingwoodC
1997 Brief heat shock treatment induces a long-lasting alteration in the glycolipid receptor binding specificity and growth rate of Haemophilus influenzae. Infect Immun 65 1729 1733
8. RatnakarP
RaoSP
CatanzaroA
1996 Isolation and characterization of a 70 kDa protein from Mycobacterium avium. Microb Pathog 21 471 486
9. LongKH
GomezFJ
MorrisRE
NewmanSL
2003 Identification of heat shock protein 60 as the ligand on Histoplasma capsulatum that mediates binding to CD18 receptors on human macrophages. J Immunol 170 487 494
10. HubelA
KrobitschS
HoraufA
ClosJ
1997 Leishmania major Hsp100 is required chiefly in the mammalian stage of the parasite. Mol Cell Biol 17 5987 5995
11. FolgueiraC
CarrionJ
MorenoJ
SaugarJM
CanavateC
2008 Effects of the disruption of the HSP70-II gene on the growth, morphology, and virulence of Leishmania infantum promastigotes. Int Microbiol 11 81 89
12. MeibomKL
DubailI
DupuisM
BarelM
LencoJ
2008 The heat-shock protein ClpB of Francisella tularensis is involved in stress tolerance and is required for multiplication in target organs of infected mice. Mol Microbiol 67 1384 1401
13. UrbanC
SohnK
LottspeichF
BrunnerH
RuppS
2003 Identification of cell surface determinants in Candida albicans reveals Tsa1p, a protein differentially localized in the cell. FEBS Lett 544 228 235
14. Lopez-RibotJL
AlloushHM
MastenBJ
ChaffinWL
1996 Evidence for presence in the cell wall of Candida albicans of a protein related to the hsp70 family. Infect Immun 64 3333 3340
15. LiXS
ReddyMS
BaevD
EdgertonM
2003 Candida albicans Ssa1/2p is the cell envelope binding protein for human salivary histatin 5. J Biol Chem 278 28553 28561
16. LiXS
SunJN
Okamoto-ShibayamaK
EdgertonM
2006 Candida albicans cell wall Ssa proteins bind and facilitate import of salivary histatin 5 required for toxicity. J Biol Chem 281 22453 22463
17. VylkovaS
LiXS
BernerJC
EdgertonM
2006 Distinct antifungal mechanisms: beta-defensins require Candida albicans Ssa1 protein, while Trk1p mediates activity of cysteine-free cationic peptides. Antimicrob Agents Chemother 50 324 331
18. SundstromP
CutlerJE
StaabJF
2002 Reevaluation of the role of HWP1 in systemic candidiasis by use of Candida albicans strains with selectable marker URA3 targeted to the ENO1 locus. Infect Immun 70 3281 3283
19. ChengS
NguyenMH
ZhangZ
JiaH
HandfieldM
2003 Evaluation of the roles of four Candida albicans genes in virulence by using gene disruption strains that express URA3 from the native locus. Infect Immun 71 6101 6103
20. StaabJF
SundstromP
2003 URA3 as a selectable marker for disruption and virulence assessment of Candida albicans genes. Trends Microbiol 11 69 73
21. BrandA
MacCallumDM
BrownAJ
GowNA
OddsFC
2004 Ectopic Expression of URA3 can influence the virulence phenotypes and proteome of Candida albicans but can be overcome by targeted reintegration of URA3 at the RPS10 locus. Eukaryot Cell 3 900 909
22. EversoleLR
ReichartPA
FicarraG
Schmidt-WesthausenA
RomagnoliP
1997 Oral keratinocyte immune responses in HIV-associated candidiasis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 84 372 380
23. MontesLF
WilbornWH
1968 Ultrastructural features of host-parasite relationship in oral candidiasis. J Bacteriol 96 1349 1356
24. CawsonRA
RajasinghamKC
1972 Ultrastructural features of the invasive phase of Candida albicans. Br J Dermatol 87 435 443
25. ChiangLY
SheppardDC
BrunoVM
MitchellAP
EdwardsJEJr
2007 Candida albicans protein kinase CK2 governs virulence during oropharyngeal candidiasis. Cell Microbiol 9 233 245
26. SanchezAA
JohnstonDA
MyersC
EdwardsJEJr
MitchellAP
2004 Relationship between Candida albicans virulence during experimental hematogenously disseminated Infection and endothelial cell damage In vitro. Infect Immun 72 598 601
27. ParkH
MyersCL
SheppardDC
PhanQT
SanchezAA
2005 Role of the fungal Ras-protein kinase A pathway in governing epithelial cell interactions during oropharyngeal candidiasis. Cell Microbiol 7 499 510
28. ChopraJ
JoistJH
WebsterRO
1987 Loss of 51chromium, lactate dehydrogenase, and 111indium as indicators of endothelial cell injury. Lab Invest 57 578 584
29. RotrosenD
EdwardsJEJr
GibsonTR
MooreJC
CohenAH
1985 Adherence of Candida to cultured vascular endothelial cells: mechanisms of attachment and endothelial cell penetration. J Infect Dis 152 1264 1274
30. FillerSG
IbeBO
LuckettPM
RajJU
EdwardsJEJr
1991 Candida albicans stimulates endothelial cell eicosanoid production. J Infect Dis 164 928 935
31. FillerSG
SwerdloffJN
HobbsC
LuckettPM
1995 Penetration and damage of endothelial cells by Candida albicans. Infect Immun 63 976 983
32. PhanQT
BelangerPH
FillerSG
2000 Role of hyphal formation in interactions of Candida albicans with endothelial cells. Infect Immun 68 3485 3490
33. ZakikhanyK
NaglikJR
Schmidt-WesthausenA
HollandG
SchallerM
2007 In vivo transcript profiling of Candida albicans identifies a gene essential for interepithelial dissemination. Cell Microbiol 9 2938 2954
34. Dongari-BagtzoglouA
KashlevaH
2006 Development of a novel three-dimensional in vitro model of oral Candida infection. Microb Pathog 40 271 278
35. DalleF
WachtlerB
CoralieL
HollandG
BannertN
2010 Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol 12 248 271
36. PhanQT
FrattiRA
PrasadaraoNV
EdwardsJEJr
FillerSG
2005 N-cadherin mediates endocytosis of Candida albicans by endothelial cells. J Biol Chem 280 10455 10461
37. PhanQT
MyersCL
FuY
SheppardDC
YeamanMR
2007 Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol 5 e64
38. ZhaoX
OhSH
ChengG
GreenCB
NuessenJA
2004 ALS3 and ALS8 represent a single locus that encodes a Candida albicans adhesin; functional comparisons between Als3p and Als1p. Microbiology 150 2415 2428
39. Moreno-RuizE
Galan-DiezM
ZhuW
Fernandez-RuizE
d'EnfertC
2009 Candida albicans internalization by host cells is mediated by a clathrin-dependent mechanism. Cell Microbiol 11 1179 1189
40. LeidichSD
IbrahimAS
FuY
KoulA
JessupC
1998 Cloning and disruption of caPLB1, a phospholipase B gene involved in the pathogenicity of Candida albicans. J Biol Chem 273 26078 26086
41. IbrahimAS
MirbodF
FillerSG
BannoY
ColeGT
1995 Evidence implicating phospholipase as a virulence factor of Candida albicans. Infect Immun 63 1993 1998
42. TheissS
IshdorjG
BrenotA
KretschmarM
LanCY
2006 Inactivation of the phospholipase B gene PLB5 in wild-type Candida albicans reduces cell-associated phospholipase A2 activity and attenuates virulence. Int J Med Microbiol 296 405 420
43. De BernardisF
AranciaS
MorelliL
HubeB
SanglardD
1999 Evidence that members of the secretory aspartyl proteinase gene family, in particular SAP2, are virulence factors for Candida vaginitis. J Infect Dis 179 201 208
44. HubeB
SanglardD
OddsFC
HessD
MonodM
1997 Disruption of each of the secreted aspartyl proteinase genes SAP1, SAP2, and SAP3 of Candida albicans attenuates virulence. Infect Immun 65 3529 3538
45. SanglardD
HubeB
MonodM
OddsFC
GowNA
1997 A triple deletion of the secreted aspartyl proteinase genes SAP4, SAP5, and SAP6 of Candida albicans causes attenuated virulence. Infect Immun 65 3539 3546
46. SchallerM
KortingHC
SchaferW
BastertJ
ChenW
1999 Secreted aspartic proteinase (Sap) activity contributes to tissue damage in a model of human oral candidosis. Mol Microbiol 34 169 180
47. RakestrawA
WittrupKD
2006 Contrasting secretory processing of simultaneously expressed heterologous proteins in Saccharomyces cerevisiae. Biotechnol Bioeng 93 896 905
48. HolkeriH
PaunolaE
JamsaE
MakarowM
1998 Dissection of the translocation and chaperoning functions of yeast BiP/Kar2p in vivo. J Cell Sci 111 Pt 6 749 757
49. SamaranayakeLP
RaesideJM
MacFarlaneTW
1984 Factors affecting the phospholipase activity of Candida species in vitro. Sabouraudia 22 201 207
50. NobileCJ
SolisN
MyersCL
FayAJ
DeneaultJS
2008 Candida albicans transcription factor Rim101 mediates pathogenic interactions through cell wall functions. Cell Microbiol 10 2180 2196
51. YamAY
AlbaneseV
LinHT
FrydmanJ
2005 Hsp110 cooperates with different cytosolic HSP70 systems in a pathway for de novo folding. J Biol Chem 280 41252 41261
52. KimS
SchilkeB
CraigEA
HorwichAL
1998 Folding in vivo of a newly translated yeast cytosolic enzyme is mediated by the SSA class of cytosolic yeast Hsp70 proteins. Proc Natl Acad Sci U S A 95 12860 12865
53. ShahiP
GulshanK
Moye-RowleyWS
2007 Negative transcriptional regulation of multidrug resistance gene expression by an Hsp70 protein. J Biol Chem 282 26822 26831
54. ZhangS
HachamM
PanepintoJ
HuG
ShinS
2006 The Hsp70 member, Ssa1, acts as a DNA-binding transcriptional co-activator of laccase in Cryptococcus neoformans. Mol Microbiol 62 1090 1101
55. FonziWA
IrwinMY
1993 Isogenic strain construction and gene mapping in Candida albicans. Genetics 134 717 728
56. MuradAM
LeePR
BroadbentID
BarelleCJ
BrownAJ
2000 CIp10, an efficient and convenient integrating vector for Candida albicans. Yeast 16 325 327
57. WilsonRB
DavisD
MitchellAP
1999 Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol 181 1868 1874
58. ShenJ
GuoW
KohlerJR
2005 CaNAT1, a heterologous dominant selectable marker for transformation of Candida albicans and other pathogenic Candida species. Infect Immun 73 1239 1242
59. KamaiY
KubotaM
HosokawaT
FukuokaT
FillerSG
2001 New model of oropharyngeal candidiasis in mice. Antimicrob Agents Chemother 45 3195 3197
60. JaffeEA
NachmanRL
BeckerCG
MinickCR
1973 Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest 52 2745 2756
61. VillarCC
KashlevaH
NobileCJ
MitchellAP
Dongari-BagtzoglouA
2007 Mucosal tissue invasion by Candida albicans is associated with E-cadherin degradation, mediated by transcription factor Rim101p and protease Sap5p. Infect Immun 75 2126 2135
62. DerschP
IsbergRR
1999 A region of the Yersinia pseudotuberculosis invasin protein enhances integrin-mediated uptake into mammalian cells and promotes self-association. Embo J 18 1199 1213
63. ParkH
LiuY
SolisN
SpotkovJ
HamakerJ
2009 Transcriptional responses of Candida albicans to epithelial and endothelial cells. Eukaryot Cell 8 1498 1510
64. KohrerK
DomdeyH
1991 Preparation of high molecular weight RNA. Methods Enzymol 194 398 405
65. LivakKJ
SchmittgenTD
2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25 402 408
66. RuchelR
TegelerR
TrostM
1982 A comparison of secretory proteinases from different strains of Candida albicans. Sabouraudia 20 233 244
67. Dongari-BagtzoglouA
VillarCC
KashlevaH
2005 Candida albicans-infected oral epithelial cells augment the anti-fungal activity of human neutrophils in vitro. Med Mycol 43 545 549
68. NobileCJ
AndesDR
NettJE
SmithFJ
YueF
2006 Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog 2 e63
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease AgentČlánek TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease SusceptibilityČlánek The Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive ParasiteČlánek Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 11- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Patients with Discordant Responses to Antiretroviral Therapy Have Impaired Killing of HIV-Infected T Cells
- A Molecular Mechanism for Eflornithine Resistance in African Trypanosomes
- Tyrosine Sulfation of the Amino Terminus of PSGL-1 Is Critical for Enterovirus 71 Infection
- Autoimmunity as a Predisposition for Infectious Diseases
- The Subtilisin-Like Protease AprV2 Is Required for Virulence and Uses a Novel Disulphide-Tethered Exosite to Bind Substrates
- Structural Analysis of HIV-1 Maturation Using Cryo-Electron Tomography
- Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease Agent
- Interferon-Inducible CXC Chemokines Directly Contribute to Host Defense against Inhalational Anthrax in a Murine Model of Infection
- TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease Susceptibility
- The Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive Parasite
- CO Acts as a Signalling Molecule in Populations of the Fungal Pathogen
- SV2 Mediates Entry of Tetanus Neurotoxin into Central Neurons
- MAP Kinase Phosphatase-2 Plays a Critical Role in Response to Infection by
- Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
- Potentiation of Epithelial Innate Host Responses by Intercellular Communication
- Fcγ Receptor I Alpha Chain (CD64) Expression in Macrophages Is Critical for the Onset of Meningitis by K1
- ANK, a Host Cytoplasmic Receptor for the Cell-to-Cell Movement Protein, Facilitates Intercellular Transport through Plasmodesmata
- Analysis of the Initiating Events in HIV-1 Particle Assembly and Genome Packaging
- Evolution of Linked Avirulence Effectors in Is Affected by Genomic Environment and Exposure to Resistance Genes in Host Plants
- Structural Basis of HIV-1 Neutralization by Affinity Matured Fabs Directed against the Internal Trimeric Coiled-Coil of gp41
- Hepatitis C Virus (HCV) Evades NKG2D-Dependent NK Cell Responses through NS5A-Mediated Imbalance of Inflammatory Cytokines
- Host Cell Invasion and Virulence Mediated by Ssa1
- Global Gene Expression in Urine from Women with Urinary Tract Infection
- Should the Human Microbiome Be Considered When Developing Vaccines?
- HapX Positively and Negatively Regulates the Transcriptional Response to Iron Deprivation in
- Enhancing Oral Vaccine Potency by Targeting Intestinal M Cells
- Herpes Simplex Virus Reorganizes the Cellular DNA Repair and Protein Quality Control Machinery
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- Cryo Electron Tomography of Native HIV-1 Budding Sites
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- Modelling the Evolution and Spread of HIV Immune Escape Mutants
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
- Platelet-Activating Factor Receptor Plays a Role in Lung Injury and Death Caused by Influenza A in Mice
- Genetic and Structural Basis for Selection of a Ubiquitous T Cell Receptor Deployed in Epstein-Barr Virus Infection
- Ubiquitin-Regulated Nuclear-Cytoplasmic Trafficking of the Nipah Virus Matrix Protein Is Important for Viral Budding
- Pneumolysin Activates the NLRP3 Inflammasome and Promotes Proinflammatory Cytokines Independently of TLR4
- Immune Evasion by : Differential Targeting of Dendritic Cell Subpopulations
- Survival in Selective Sand Fly Vector Requires a Specific -Encoded Lipophosphoglycan Galactosylation Pattern
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



