-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Role for Sumoylation in Systemic Inflammation and Immune Homeostasis in Larvae
To counter systemic risk of infection by parasitic wasps, Drosophila larvae activate humoral immunity in the fat body and mount a robust cellular response resulting in encapsulation of the wasp egg. Innate immune reactions are tightly regulated and are resolved within hours. To understand the mechanisms underlying activation and resolution of the egg encapsulation response and examine if failure of the latter develops into systemic inflammatory disease, we correlated parasitic wasp-induced changes in the Drosophila larva with systemic chronic conditions in sumoylation-deficient mutants. We have previously reported that loss of either Cactus, the Drosophila (IκB) protein or Ubc9, the SUMO-conjugating enzyme, leads to constitutive activation of the humoral and cellular pathways, hematopoietic overproliferation and tumorogenesis. Here we report that parasite infection simultaneously activates NF-κB-dependent transcription of Spätzle processing enzyme (SPE) and cactus. Endogenous Spätzle protein (the Toll ligand) is expressed in immune cells and excessive SPE or Spätzle is pro-inflammatory. Consistent with this function, loss of Spz suppresses Ubc9− defects. In contrast to the pro-inflammatory roles of SPE and Spätzle, Cactus and Ubc9 exert an anti-inflammatory effect. We show that Ubc9 maintains steady state levels of Cactus protein. In a series of immuno-genetic experiments, we demonstrate the existence of a robust bidirectional interaction between blood cells and the fat body and propose that wasp infection activates Toll signaling in both compartments via extracellular activation of Spätzle. Within each organ, the IκB/Ubc9-dependent inhibitory feedback resolves immune signaling and restores homeostasis. The loss of this feedback leads to chronic inflammation. Our studies not only provide an integrated framework for understanding the molecular basis of the evolutionary arms race between insect hosts and their parasites, but also offer insights into developing novel strategies for medical and agricultural pest control.
Published in the journal: Role for Sumoylation in Systemic Inflammation and Immune Homeostasis in Larvae. PLoS Pathog 6(12): e32767. doi:10.1371/journal.ppat.1001234
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001234Summary
To counter systemic risk of infection by parasitic wasps, Drosophila larvae activate humoral immunity in the fat body and mount a robust cellular response resulting in encapsulation of the wasp egg. Innate immune reactions are tightly regulated and are resolved within hours. To understand the mechanisms underlying activation and resolution of the egg encapsulation response and examine if failure of the latter develops into systemic inflammatory disease, we correlated parasitic wasp-induced changes in the Drosophila larva with systemic chronic conditions in sumoylation-deficient mutants. We have previously reported that loss of either Cactus, the Drosophila (IκB) protein or Ubc9, the SUMO-conjugating enzyme, leads to constitutive activation of the humoral and cellular pathways, hematopoietic overproliferation and tumorogenesis. Here we report that parasite infection simultaneously activates NF-κB-dependent transcription of Spätzle processing enzyme (SPE) and cactus. Endogenous Spätzle protein (the Toll ligand) is expressed in immune cells and excessive SPE or Spätzle is pro-inflammatory. Consistent with this function, loss of Spz suppresses Ubc9− defects. In contrast to the pro-inflammatory roles of SPE and Spätzle, Cactus and Ubc9 exert an anti-inflammatory effect. We show that Ubc9 maintains steady state levels of Cactus protein. In a series of immuno-genetic experiments, we demonstrate the existence of a robust bidirectional interaction between blood cells and the fat body and propose that wasp infection activates Toll signaling in both compartments via extracellular activation of Spätzle. Within each organ, the IκB/Ubc9-dependent inhibitory feedback resolves immune signaling and restores homeostasis. The loss of this feedback leads to chronic inflammation. Our studies not only provide an integrated framework for understanding the molecular basis of the evolutionary arms race between insect hosts and their parasites, but also offer insights into developing novel strategies for medical and agricultural pest control.
Introduction
Drosophila are hosts to a range of pathogens, and in the wild, they likely encounter a large number of pathogen species [1], [2]. The survival of natural population structures of Drosophila spp. is expected to depend on the distribution of their pathogens, occurrence of co-infection, and specific host-pathogen interactions. One class of natural fly enemies is the parasitoid (parasitic) wasps, which, although free-living as adults, have an obligate relationship with their hosts for pre-imaginal development. Females inject 100 µm size eggs through the larval cuticle, directly into the host hemocoel, bypassing barrier tissues (cuticle, trachea, and gut). Because insects have an open circulatory system (there is no distinction between interstitial fluid and blood), the hemolymph bathes all internal organs in the hemocoel. Wasp egg recognition thus activates both systemic responses, blood cell proliferation and activation [3], [4], and the production of humoral factors from the fat body into the hemolymph [4], [5].
Blood cells recognize, surround, and melanize the parasite egg to sequester it from the host tissues. All three blood cells types, namely, the abundant phagocytic plasmatocytes, larger adhesive lamellocytes, and few melanin-producing crystal cells, are called into action [2], [3], [4], [5]. The encapsulation reaction is resolved within a day and is reminiscent of effector cell activation and resolution in mammals (e.g., tuberculosis granulomas [6]).
While cell-based immunity in Drosophila appears sufficient to restrain parasite development, it remains unclear why metazoan parasite infections trigger the Toll-dependent humoral arm in the fat body that is activated (and has been characterized) in response to microbial infections [2], [5]. Antimicrobial peptides, lysozymes and activation of the pro-phenoloxidase cascade (melanization) make up the humoral reactions. Both, the cellular and humoral immune reactions in healthy animals are regulated by conserved NF-κB, JAK-STAT and pro-phenoloxidase cascades [5], [7], [8]. Not surprisingly, many parasitic wasp species that infect Drosophila have evolved mechanisms to evade or suppress their host's immune responses [4], [5].
The goal of this study was to (1) identify the key mechanisms in Drosophila that contribute to both, the activation and resolution of parasitic wasp-induced acute inflammation, and (2) examine if the aberrant regulation of the latter sustains systemic chronic inflammation in hosts. We were guided by the paradigm of acute inflammation in mammals, which is characterized by proliferation, differentiation, and recruitment of blood cells to the site of injury. Acute-phase gene expression is self-limiting, being quickly resolved within minutes to hours after infection [9], [10]. Innate effector cells aggregate to sequester the invading parasite or microbe. Examples of such reactions include granulomas around parasitic helminthes, which involve neutrophils, macrophages or T cells [11] and inflammatory granulomas of tuberculosis-inducing Mycobacterium tuberculosis by foamy macrophages [6]. Inflammatory cells produce pro-inflammatory cytokines (TNF-α and interleukins), chemokines, and prostaglandins, which help initiate and mediate the inflammatory processes by activation of NF-κB signaling [9], [10].
The aberrant regulation of this inflammatory response in humans has been identified as a common denominator underlying many conditions such as cancer, diabetes, and heart disease. Chronic inflammation is caused by, among others, the continuous activation of acute inflammatory pathways [12]. At times, acute inflammation can spiral out of control and cause major organ failure and death.
To understand how activation and resolution of acute inflammation are coordinated in the fly, we examined patterns of expression in genome-wide microarray data of Drosophila infected by distantly-related parasitic wasps (Leptopilina boulardi [5] and Asobara tabida [8]). We found that the transcription of two core components of the Toll pathway, Spätzle Processing Enzyme (SPE) and cactus (cact), is strongly activated in the first six hours after infection. The SPE protease is constitutively expressed in the larval fat body and blood cells. It cleaves and activates the Toll ligand pro-Spätzle (encoded by spz) [13], [14]. Spz, is a cysteine-knot protein; the spz locus itself encodes multiple alternatively-spliced isoforms [15]. SPE transcription is induced by injury via a Toll-dependent positive feedback loop. Loss or depletion of SPE impairs the induction of Drosomycin (Drs), a target gene of the Toll pathway [13], [14].
Loss of function of the fly IκB, cact, [16] or the SUMO conjugase, Ubc9 (also called lesswright, lwr [17], [18]), results in hyperactive Toll/NF-κB signaling. Mutant larvae show constitutive activation of humoral (antimicrobial and immune peptide gene expression in the fat body, [19]) and cellular (blood cell proliferation, aggregation and microtumor formation) reactions [17], [18]. Transcriptional activation of cactus after bacterial challenge, like that of SPE, is under the control of the Toll-Dorsal pathway. High levels of Cactus protein terminate signaling [20]. Several components in the mammalian NF-κB pathway, including IκBα, are regulated by sumoylation. The SUMO (small ubiquitin-like modifier) modification system utilizes SUMO-activating and -conjugating enzymes, Uba2/Aos1 and Ubc9, respectively [21]. Sumoylation plays a significant role in host defense in plants, insects and mammals [22], [23].
With this backdrop, we hypothesized that the encapsulation of the wasp egg is akin to the mammalian acute inflammatory reaction, whereas hyperactive Toll signaling in Ubc9− mutants is a deregulated chronic condition. We sought to systematically define this parallel in the fly larva and discovered the central role of Ubc9 in the regulation of the core, primordial NF-κB immuno-genetic circuit, where it governs the balance between pro-inflammatory (SPE/Spz) and anti-inflammatory (Cactus) molecules. Our findings provide a molecular model for understanding the immune physiology underlying the evolutionary arms race between insect hosts and their parasitic wasps.
Results
Sumoylation restrains systemic inflammation
There are striking parallels between wasp-induced activation of cellular and humoral immunity in control larvae and hyperactive immune phenotypes of Ubc9− larvae. First, parasite infection induces some blood cells to divide, differentiate, and aggregate. Blood cells are recruited to surround the melanized wasp egg to form the capsule structure itself (Figure 1A, B–B2). Loss of sumoylation via RNA interference in blood cells and fat body (Cg>Uba2RNAi Figure 1C; [24]), or in Ubc9− mutants (Figure 1D1, D2) leads to similar changes. Second, while Drs-GFP, a transgenic Toll pathway readout is normally induced only after parasite infection of control larvae (Figure 1E–F'), Ubc9− fat body cells constitutively express this reporter (Figure 1 G–H').
Fig. 1. Inflammatory responses in the fly larvae. 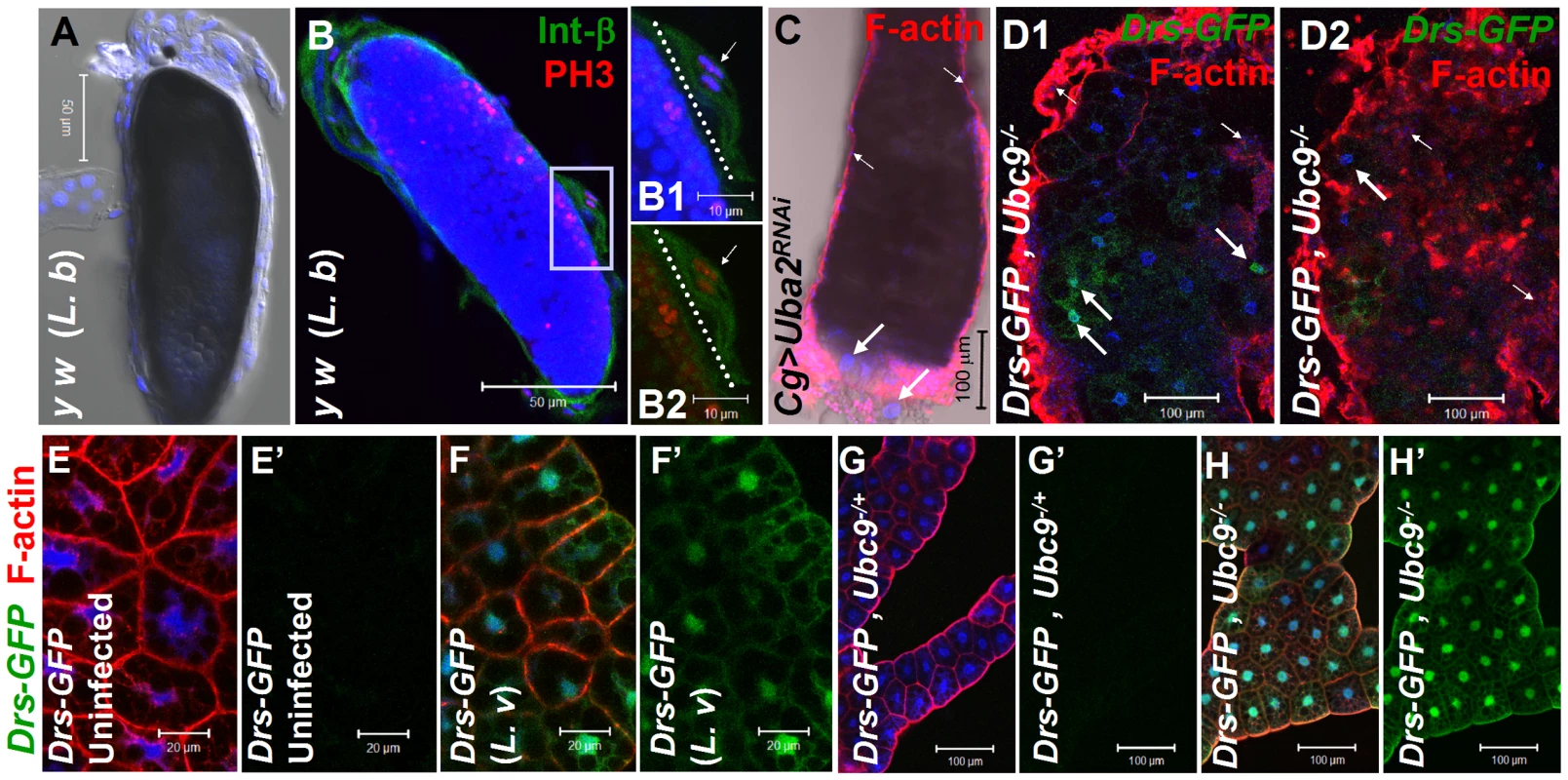
(A) Melanized wasp (L. boulardi) egg encapsulated by blood cells. (B) Integrin β PS (green) is expressed in most blood cells of the capsule. Some blood cells are mitotically active, where the phospho-histone H3 (PH 3, red) signal overlaps with DNA (B1, B2). Inset shows higher magnification of wasp embryo to which integrin β PS-positive (blood cells, [52]) and some PH 3-positive blood cells adhere (short arrows in B1–B2). PH 3-positive cells are also detected within the developing wasp embryo. Broken line shows the wasp embryo/host capsule interface (B1, B2). (C) Melanized microtumor showing fat body encapsulated by blood cells in Cg>Uba2RNAi larva. Microtumors are structures larger than aggregates of more than 50 blood cells, but smaller than 1 mm3 in volume. Long arrows point to fat body and short arrows to blood cells adhering to the fat body. Blood cells are diploid and significantly smaller than the larger, endopolyploid fat body cells. (D1, D2) Confocal sections of a microtumor (D1, tumor interior; D2 tumor periphery) showing blood cells (short arrows) infiltrating fat body cells. Some fat body cells show constitutive Drs-GFP expression (long arrows). (E–H) Drs-GFP expression in fat body before (E, E'), or after (F, F') wasp (L. victoriae) infection. (G–H) Drs-GFP expression in heterozygous (G, G'), and Ubc9− (H, H') fat body. Panels E', F', G' and H' show Drs-GFP transgene expression alone. (A–H) Cells counterstained for DNA with Hoechst (blue). We hypothesized that the fat body infiltration by blood cells in sumoylation-deficient Cg>Uba2RNAi or Ubc9− animals represents a chronic version of the egg recognition/encapsulation reaction. To understand the nature of the blood cell-fat body interaction, we stained cells of 6-day-old control and Ubc9− mutants with anti-Collagen IV antibody [25]. Collagen IV, a component of the basement membrane, is expressed uniformly around the cells of control fat body (Figure 2A1, A2). However, the staining signal for Collagen IV around the fat body of 6-day (Figure 2B1, B2) and 8-day-old (Figure 2C) mutant larvae is discontinuous. In 6-day-old samples, the Collagen IV-deficient regions coincide with low or undetectable F-actin signal, suggesting loss of tissue integrity. Aggregates of blood cells around such collagen-deficient regions are frequently observed in 8-day-old fat body (Figure 2C, D). These results suggest that mutant blood cells are actively recruited to regions of the fat body with irregular basement membrane, in a process that is likely to be similar to infiltration and aggregation of blood cells around the wasp egg. The Collagen IV staining signal in control and mutant blood cells is high and primarily cytoplasmic (Figure 2E, F), consistent with the high promoter activity in the Cg-Gal4 strain.
Fig. 2. Loss of sumoylation enzymes induces loss of fat body integrity, hematopoietic defects, infiltration, and Drs activation. 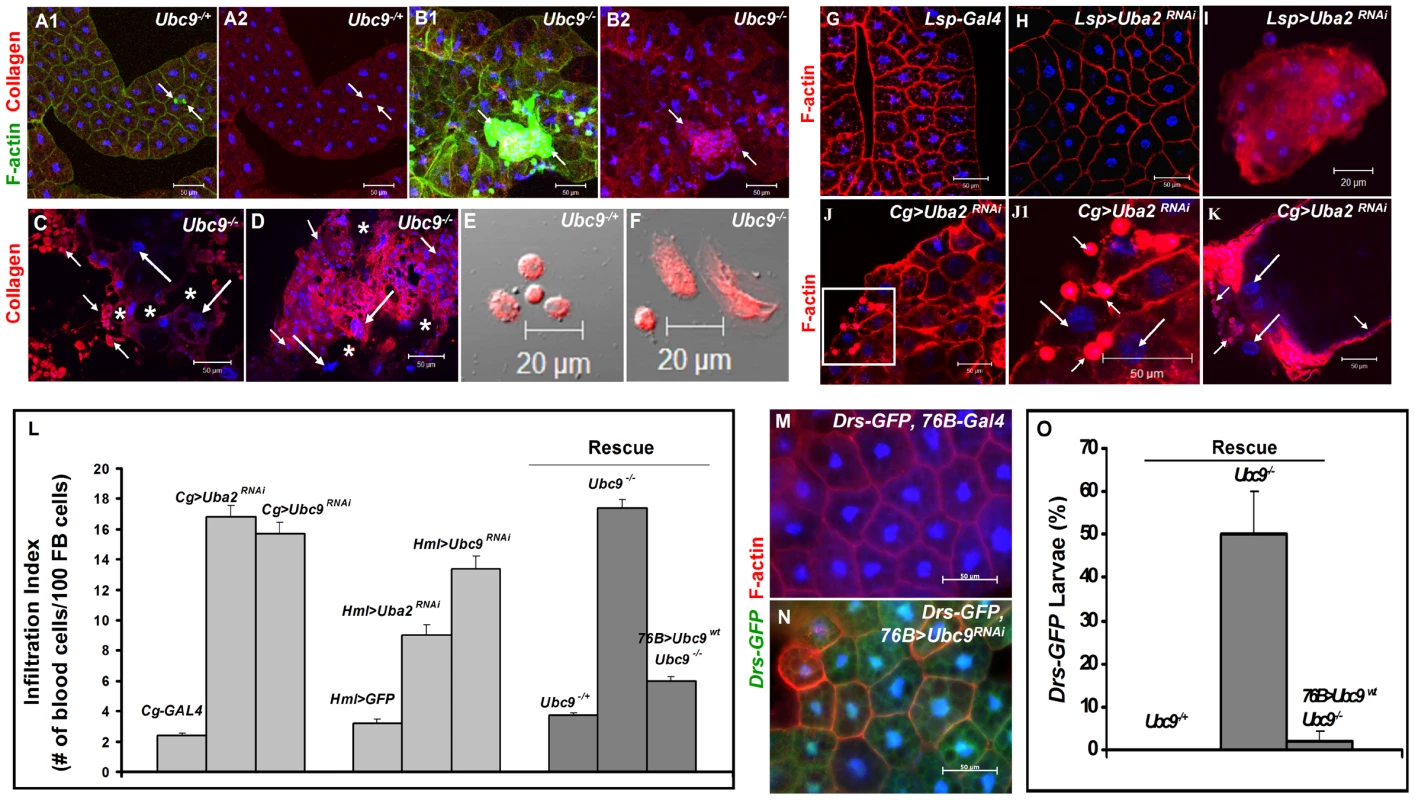
(A–B) Merged Z-stack sections of 6-day-old heterozygous (A1, A2), or Ubc9− (B1, B2) fat body stained for Collagen IV. Short arrows point to single blood cells or clusters of blood cells in contact with the fat body tissue. Panels A2 and B2 show the anti-Collagen IV antibody signal only. (C–D) Anti-Collagen IV antibody staining of 8-day-old mutant fat body (C) or microtumors (D). Regions of the fat body with no Collagen IV signal are marked with an asterisk. Blood cells (short arrows) infiltrating the fat body (long arrows). (E–F) Blood cells from control and mutant larvae stained for Collagen IV. (G–K) Fat body from control Lsp-Gal4 (G) and Lsp>Uba2RNAi (H). A hematopoietic aggregate from Lsp>Uba2RNAi hemolymph (I). Fat body from Cg>Uba2RNAi larva (J, J1). Inset in panel J (white square) is shown at high magnification in panel J1. Microtumor from a Cg>Uba2RNAi larva (K). Blood cells (short arrows) encapsulating fat body (long arrows) in Cg>Uba2RNAi larva (J1, K). (L) Infiltration indices in third instar larvae (see Methods). n>20 larvae for all genotypes except Hml>Uba2RNAi, Hml>Ubc9RNAi, and Cg>Ubc9RNAi, where n = 8 larvae. Bars show standard error. (M–N) No Drs-GFP expression in control (76B-Gal4) larval fat body (M). Drs-GFP expression is induced in the experimental (76B>Ubc9RNAi) fat body (N). (O) Rescue of constitutive Drs-GFP expression in Ubc9− mutants with 76B>Ubc9WT transgenes n>90 animals. Cells in A–B, E–K and M–N are counterstained for DNA with Hoechst (blue). To examine the relative contributions of fat body and blood cells to blood cell activation and infiltration, we compared the effects of sumoylation knockdown (Uba2RNAi) in fat body (Lsp-Gal4), blood cells (Hml-Gal4), or both these tissues (Cg-Gal4). Whereas knockdown of Uba2 in the larval fat body alone did not yield strong fat body defects (i.e., loss of tissue integrity, Figure 2G, H), it resulted in weak but reproducible differentiation and aggregation of blood cells in the hemocoel (Figure 2I). In contrast, Cg>Uba2RNAi, Cg>Ubc9RNAi, Hml>Uba2RNAi or Hml>Ubc9RNAi larvae mimic defects of Ubc9 mutants, significantly promoting infiltration (number of single hemocytes per 100 fat body cells; Figure 2J, J1, L) and tumorogenesis (Figure 1C and Figure 2K). Whereas 60% of the Cg>Uba2RNAi or Cg>Ubc9RNAi animals developed microtumors, none of the Cg-Gal, UAS-Uba2RNAi, or UAS-Ubc9RNAi parents were tumorous in third-instar larval stages (n>30 larvae for all genotypes; data not shown).
To check if sumoylation-deficient blood cells can signal the wild type fat body to activate Drs-GFP, we examined the 76B>Ubc9RNAi animals and found that deficiency in even a few blood cells of the lymph gland is sufficient to trigger Drs-GFP activation (Figure 2M, N). The ability of blood cells to activate Drs-GFP in wild type fat body was confirmed in 76B>Aos1RNAi animals (data not shown). (The 76B driver is expressed in few cells of the lymph gland [26], the multi-lobed larval hematopoietic organ [2]. Aos1 is a SUMO-activating enzyme subunit.).
These results suggest that (1) the sumoylation of target proteins in the fat body inhibits the release of pro-inflammatory factors, and (2) blood cells lacking this protein modification pathway become inflammatory, i.e., they divide, differentiate, release pro-inflammatory signals and infiltrate the fat body in the absence of infection. Loss of sumoylation in both immune tissues amplifies these defects.
If deficiency of sumoylation in blood cells of Ubc9− mutants or Aos1/Ubc9 knockdown animals elicits factors that can activate pathways in the fat body, then supplying wild type Ubc9 protein in blood cells should, to a large extent, restore immune homeostasis in both tissues. We tested this idea in a rescue experiment in which wild type Ubc9 protein was expressed only in a subset of the lymph gland cells (76B>Ubc9WT) in Ubc9− mutants. Not only was the infiltration index significantly reduced in 76B>Ubc9WT mutants (Figure 2L), but surprisingly, the Drs-GFP reporter in the fat body was no longer expressed [i.e., while 50.3±9.6% of mutants (n>35 animals) show Drs-GFP expression, only 2.1±2.1% of mutants expressing Ubc9WT transgene (n>35 animals) are Drs-GFP-positive, Figure 2O].
All together, these results support the idea that Ubc9 plays an anti-inflammatory role in both, the fat body and blood cells. Loss of Ubc9-dependent inhibition leads to failure of immune homeostasis and the development of chronic inflammation. This interpretation implies that each immune tissue can activate the other by secretion of cell non-autonomous factors and thus, mutually alter the patterns of gene expression and cell decision processes.
Identification of pro - and anti-inflammatory factors
To clarify the underlying molecular parallels between infection and the genetic loss of sumoylation, we utilized microarray datasets to examine patterns of gene expression in parasite-infected fly larvae and identified genes with acute-phase expression profile, typical of mammalian inflammation [12]. In hosts infected by A. tabida and L. boulardi [5], [8], we identified 81 genes with acute-phase expression pattern (Figure S1A, B; see Table S1 for the identity of genes). Forty genes were activated after A. tabida infection (Wertheim study [8]), and 51 genes were induced by L. boulardi infection (Schlenke study [5]). Ten genes were induced by infection with either wasp, and thus, identified in data sets from both studies.
A majority of the 81 genes are linked to the immune response of the fly (Irc and Idgf family members) and roughly a quarter of genes encode components or targets of the Toll (PGRP-SD, SPE, nec, Mtk, Tl, cactus, IM2, IM3, IM23), Imd (PGRP-LB, Relish, AttA, B, mtk), JAK-STAT (dome, hop) or melanization (yellow-f, Dox-A3) pathways (Table S1). A handful of genes, not implicated in immune, development, metabolism, or other processes (e.g., Ugt86Dd, CG32687, CG7896, CG9095) were also identified (Table S1). Significantly however, the 81 putative acute inflammation genes include components that act upstream (PGRP-SD, SPE and Tl) and downstream (cact) of Ubc9 itself. Since the expression of SPE and cactus was previously shown to be regulated by Toll-dependent feedback [13], [14], [20], we hypothesized that their coordinate regulation is essential for both activation and termination of acute inflammation.
The levels of SPE and cactus depend on NF-κB proteins Dorsal and Dorsal-related immunity factor
We quantified SPE, spz, cactus and Drs transcription in parasite-infected (Figure 3A, B) and mutant animals (Figure 3C, D). Parasite infection activates SPE 10-fold higher than uninfected controls in the first six hours of infection, subsequent to which, its expression is reduced as RNA levels return below 5-fold at 12 and 24 hours. Unlike SPE, spz RNA levels remain low. However, RNA levels of cact increase significantly (up to 5-fold) over 24 hours post-infection. Transcription of two other immune genes (not core components of the Toll pathway), hop and Irc, is activated (2-3-fold), but RNA levels remain relatively constant at all time points tested (Figure 3A).
Fig. 3. Inflammatory responses are dependent on Dorsal/Dif. 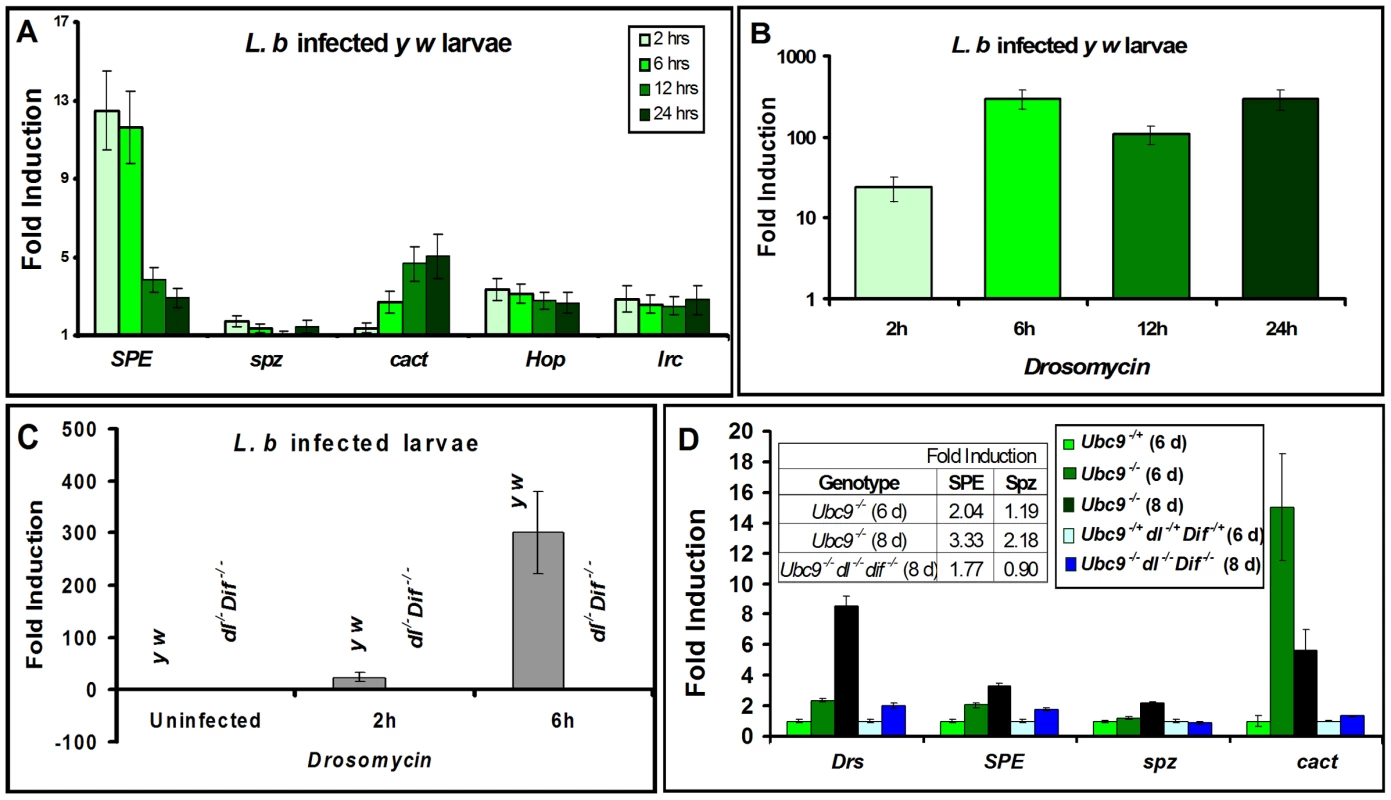
(A–D) Real-time PCR on RNA from whole larvae. (A) Time course of post infection (L. boulardi) of SPE, spz, cact, hopscotch and immune-regulated catalase activation relative to uninfected wild type (y w) animals. (B) Drs activation at different time points after L. boulardi infection in wild type y w animals. (C) Drs activation at 2 h or 6 h after L. boulardi infection in wild type (y w) or Dif− dl− animals. (D) Gene expression in single or triple mutants compared to respective heterozygotes. Table (D, inset) shows numeric fold increase for SPE and spz in single and triple mutants. Bars represent standard errors for results from triplicate measurements. Toll pathway activation was confirmed by quantifying expression of Drs. Parasite infection of control y w larvae activates Drs more than 100-fold (Figure 3B, C) and its constitutive expression in Ubc9− mutants is roughly 8-fold higher relative to heterozygous controls (Figure 3D). Like Drs, both SPE (2 fold) and cact (15 fold), but not spz, are constitutively expressed in 6-day old Ubc9− mutants (Figure 3D). Eight-day-old mutants exhibit stronger cellular immune defects [17], and RNA levels of Drs (8 fold), SPE (3 fold), and spz (2 fold) are higher relative to their 6-day-old counterparts (Figure 3D). This observation suggests a progressive loss of gene regulation in older animals.
To formally test the dependence of both acute (parasite-induced Toll activation in wild type) and chronic (constitutively-active Toll signaling in Ubc9− mutants) inflammatory responses on the transcription factors Dorsal (dl) and Dorsal-related immunity factor (Dif), we quantified Drs expression in animals lacking Dif and dl (Figure 3C, D). Under both conditions of infection (Figure 3C) and mutation (Figure 3D), Drs expression is almost completely dependent on these NF-κB proteins.
Like Drs, the constitutive expression of cact (at 6 day), SPE (at 6 and 8 day) and spz (at 8 day) in Ubc9− animals also depends on Dorsal/Dif as their normal transcription is completely or partially restored in Ubc9− Dif− dl− triple mutants (Figure 3D). Consistent with these findings, high confidence Dorsal-binding sites are present in genomic regions of SPE and cact ([20]; Figure S2).
Parasite infection promotes the nuclear localization of both NF-κB proteins Dif and Dorsal in wild type fat body (Cg>CFP-Dif, Cg>GFP-Dorsal; Figure 4A–D) and blood cells (Figure 4E–F). Constitutive nuclear localization of both fusion proteins is observed in Ubc9− animals (Figure 4G–J). Nuclear localization of endogenous Dorsal was confirmed by antibody staining. Mutant fat body cells have higher Dorsal protein levels (compare Figure 4K' with L'), an observation that is in agreement with higher steady state dl RNA levels in Ubc9− mutants relative to heterozygotes (data not shown). Finally, a higher proportion (> 40%) of mutant blood cells exhibit nuclear Dorsal protein relative to heterozygous (<20%) controls (our unpublished results and [18]).
Fig. 4. Nuclear localization of Dorsal and Dif in inflammation. 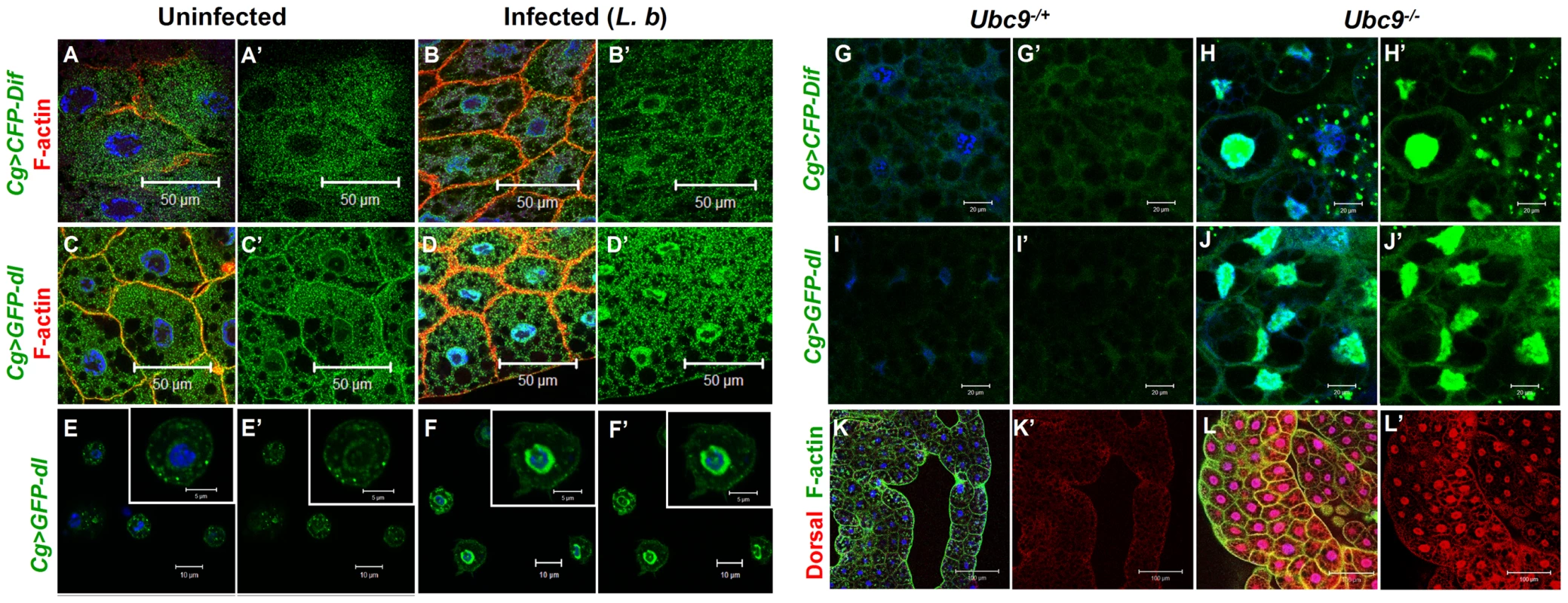
(A–D) Fat body cells from uninfected y w; Cg>CFP-Dif and yw; Cg>GFP-dl larvae (A, A', C, C') show abundant vesicular localization of fusion proteins in the cytoplasm. After infection, these proteins are also nuclear (B, B', D, D'). (E–F') Cytoplasm-to-nuclear relocalization of GFP-Dorsal in blood cells after infection. (G–J) CFP-Dif and GFP-Dorsal in heterozygous (G, G', I, I') and Ubc9− (H, H', J, J') fat body cells. (K, L) Endogenous Dorsal protein expression (red) in heterozygous (K, K') and Ubc9− (L, L') fat body. Cells in panels A–L are counterstained with Hoechst to visualize nuclei (blue). Panels A'–J' show the green channel only. Panels K' and L' show the expression of Dorsal alone. The parallels (with respect to NF-κB-dependent gene expression changes and hematopoietic activation, infiltration and associated changes) and differences (in regulation) defined in the preceding experiments in infected and mutant larvae support the notion that sumoylation of specific proteins coordinates the activation and deactivation of canonical Toll signaling in fat body and blood cells in part by regulating the steady state levels of SPE and Cactus. Furthermore, the Ubc9− animals suffer from chronic inflammatory phenotypes because of their inability to properly terminate Toll signaling.
SPE and Spätzle are proinflammatory
To understand its role in systemic inflammation, we next examined the expression of Spätzle in larval blood cells and fat body. Polyclonal anti-Spätzle antibodies [14] reveal Spz expression in the cytoplasm of control (y w or spz−/+), but not in spz−/− blood cells (Figure 5A, B; Figure S3A–C). Spz protein level is high after parasite infection of y w animals, particularly in circulating plasmatocytes (Figure 5B, B', C, C'). Spz protein is also clearly detected in plasmatocytes and lamellocytes layered around the wasp egg (Figure 5D–D1').
Fig. 5. Spätzle expression in larval blood cells. 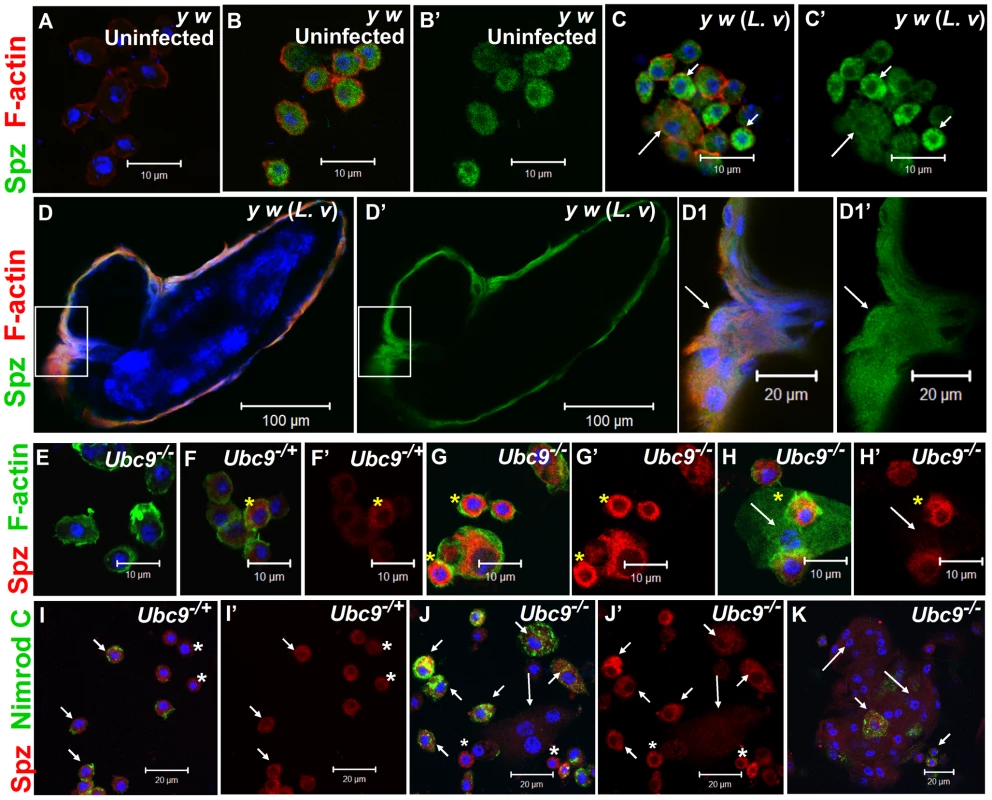
(A–C') Infection: Blood cells from control larvae not treated with primary antibody (A). Spätzle expression (green) in blood cells from uninfected y w (B, B') and L. victoriae-infected y w (C, C') larvae. Lamellocytes (long arrow) in panel C show low but detectable Spz expression that is higher compared to plasmatocytes (short arrows). (D–D1') Spz expression (green) in blood cells surrounding the wasp (L. victoriae) egg (D). At high magnification (D1, D1'), Spz expression (green) in lamellocytes (arrow) colocalizes with F-actin (red). (E–H') Mutants: Ubc9− blood cells not treated with primary antibody (E). Spz signal (red) in heterozygous cells (F, F') is lower than in mutant (G, G', H, H') cells. Spz is less abundant in lamellocytes (H, H' arrow) than plasmatocytes. Asterisk denotes cells in which there is partial colocalization of the Spz (red) and F-actin (green) signals. (I–K) Spz (red) colocalizes with Nimrod C (green) in Ubc9−/+ plasmatocytes (short arrows, I) and Ubc9− cells in circulation (J) or in a small aggregate (K). Small round blood cells (I, I', J, J'; asterisk) are Nimrod-negative, but express Spz. Long arrows point to lamellocytes. (A–K) Cells counterstained for DNA with Hoechst (blue). All the panels with a prime letter show the expression of Spz alone. Spz levels are high in uninfected Ubc9− blood cells (Figure 5G, G', H, H', J, J', K) compared to heterozygotes (5F, F', I, I'). In some cells, the Spz signal overlaps with F-actin (Figure 5F–H, asterisk) or Nimrod C (plasmatocyte marker) in circulating (Figure 5I–K), tumorous (Figure 6B, B, B1', short arrows), or fat body-infiltrating plasmatocytes (Figure 6D, D1, short arrows). Spz protein levels are higher in fat body of infected animals (data not shown) and mutant fat body cells relative to uninfected heterozygous cells (Figure 6C, C, D, D'' and Figure S3D–F'). High levels of Spz protein in cells of infected animals (or in mutant blood cells engaged in infiltration of mutant fat body) suggest that Spz serves a novel pro-inflammatory role in the activation of parasite-induced immune responses.
Fig. 6. Spätzle protein expression in immune tissues of Ubc9 mutants. 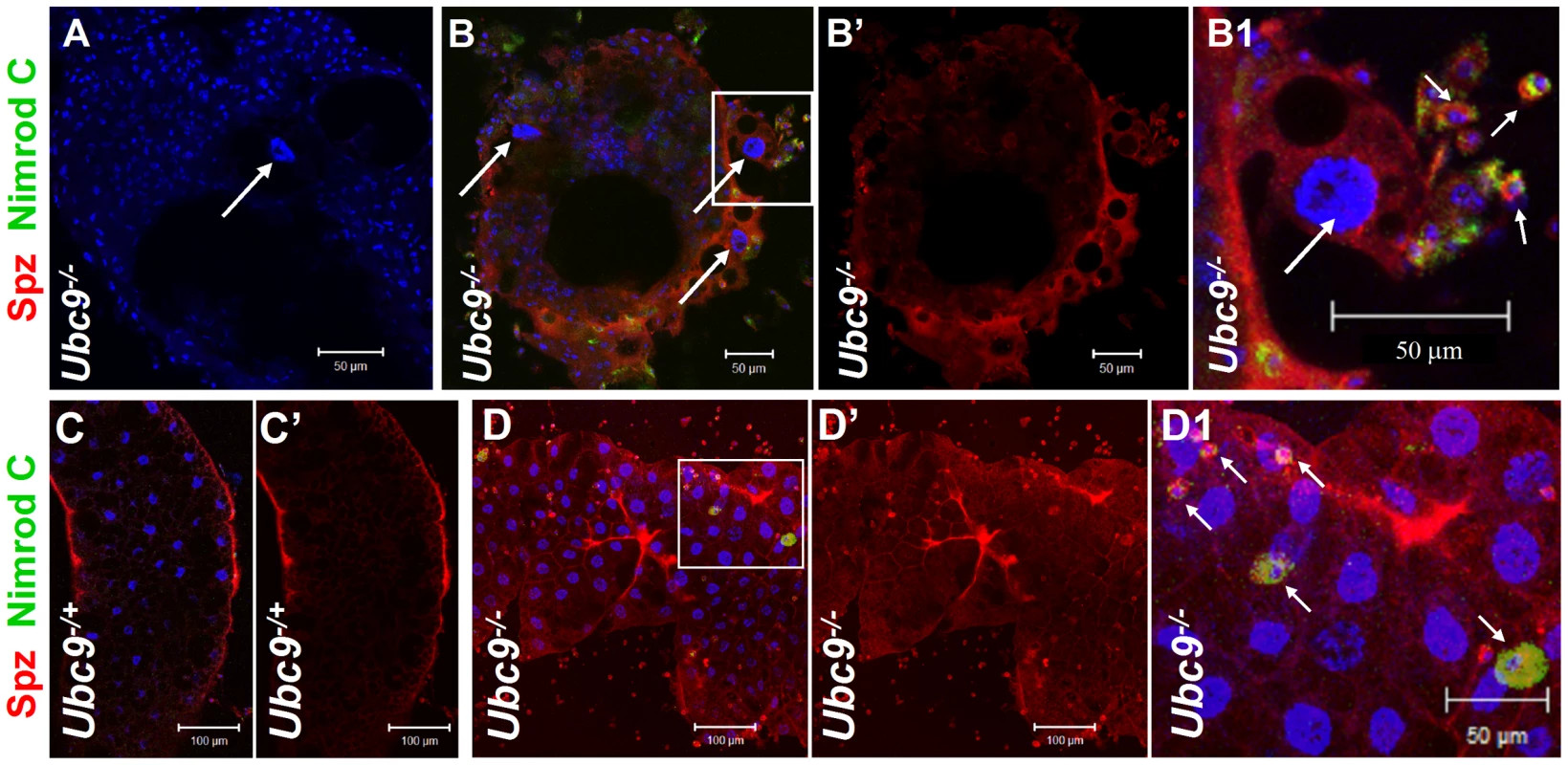
(A–B1) Ubc9− samples. Primary antibody omitted (A). Plasmatocytes (Nimrod C, green, short arrows) around fat body cell (long arrow) express high levels of Spz (red). Inset in panel B is shown at high magnification in panel B1. (C–D1) Heterozygous (C, C') or Ubc9− (D, D') fat body stained with anti-Spz antibody. Infiltrating mutant plasmatocytes (D1, short arrows) express high levels of Spz. Panels B', C' and D' show the red channel alone. (A–D) All cells counterstained for DNA with Hoechst (blue). We next tested the effects of ectopic expression of full-length Spz and activated SPE (SPE-Act) with various drivers. Experimental animals with excessive Spz or SPE-Act either in the fat body (Lsp-Gal4, [27]) or hematopoietic (Hml-Gal4, [28]) or both (Cg-Gal4, [24]) compartments exhibit variable hematopoietic defects similar to those in Ubc9− and cact− third instar larvae (Figure 7A–H, [16], [17]). A similar outcome is observed upon over-expression of Dorsal or Dif ([18], [29], our unpublished results).
Fig. 7. Excessive SPE, Spz and Dorsal proteins activate inflammation in the larva. 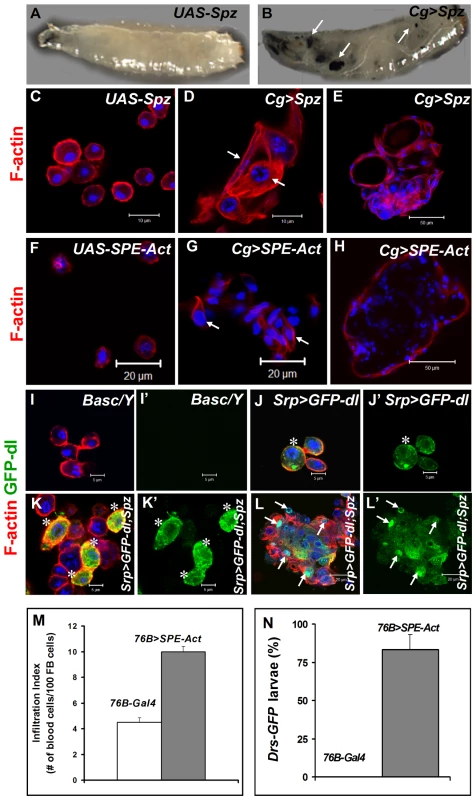
(A, B) Misexpression of Spz induces blood cell aggregation and melanization. Control third instar larva with only the UAS-Spz transgene (A). Experimental Cg>Spz larva with excessive Spz in blood cells and fat body (B). Arrows point to melanized microtumors visible through the transparent cuticle. (C–H) Either Spz (D, E) or activated SPE (G, H) expressed via Gal4 drivers as shown. Wild type plasmatocytes in parental (UAS-Spz; C and UAS-SPE-Act; F) control animals. Arrows point to blood cells of lamellocyte morphology within a hematopoietic aggregate (D, G). Small microtumors from experimental animals (E, H). (I–L) High levels of Spz synergize with GFP-Dorsal to promote localization of the latter to the nucleus. Blood cells from control (Basc/Y, I, I') larva, without any transgene, lack GFP expression. GFP-Dorsal in blood cells from Srp>GFP-dl (J, J') larva is predominantly cytoplasmic (asterisk). Addition of excessive Spätzle (Srp>GFP-dl; Spz, K, K', L, L') promotes blood cell aggregation. GFP-Dorsal levels are high and the protein is both cytoplasmic and nuclear (K, K' asterisk) or predominantly nuclear (L, L' arrows). (C–L) Cells were counterstained with Hoechst (blue). Panels I', J', K' and L' show the expression of GFP-Dorsal alone. (M) Infiltration index in third instar larvae. Genotypes are shown. Bars show standard error. (N) Drs-GFP expression in 76-Gal4 (control) and 76B>SPE-Act animals (n>45). To determine if ectopic Spz can promote the nuclear localization of Dorsal, we monitored the subcellular distribution of GFP-Dorsal expressed in all blood cells (Srp>GFP-dl) with added Spz (UAS-Spz). In the absence of ectopic Spz, GFP-Dorsal is predominantly cytoplasmic in circulating blood cells (Figure 7J). Additional Spz expression promotes lamellocyte differentiation and some, but not all, blood cells show high levels of nuclear GFP-Dorsal, especially in microtumors (Figure 7K–L', arrows). Together, these observations suggest that Spz is a proinflammatory cytokine and its regulated activation after infection is an important step for the onset and resolution of the immune responses in Drosophila.
To examine if SPE derived from blood cells is sufficient to activate them and induce Drs-GFP in the fat body, we examined 76B>SPE-Act carrying the Drs-GFP. Infiltration index in experimental (76B>SPE-Act) animals was significantly higher than in control (76B-Gal4) animals (Figure 7M). Furthermore, while the transgene remains inactive in the fat body of 76B-Gal4 control animals at both second and third instar stages, the experimental 76B>SPE-Act larvae strongly express the Drs-GFP transgene at both stages (Figure 7N).
Loss of SPE/spz suppress systemic inflammation
The preceding results, i.e., (1) acute-phase activation of SPE after parasite infection (Figure 3A); (2) its elevated expression in Ubc9− mutants (Figure 3D); (3) high levels of Spätzle (Figure 5, 6) and SPE [13], [14] in immune cells; and (4) systemic inflammatory effects of the misexpression of either protein (Figure 7) suggest that a positive feedback loop through SPE/Spz supports the activation and resolution of infiltration, encapsulation and tumorogenesis. Consistent with this idea, we found that loss of spz function relieves the chronic effects of the Ubc9 mutation (Figure 8A). Wandering third-instar Ubc9−; spz− double mutant larvae are almost free of microtumors and show a significant reduction in fat body infiltration index (number of single hemocytes/100 fat body cells, Figure 8A).
Fig. 8. Inflammatory phenotypes depend on levels of Spz/SPE and Cactus. 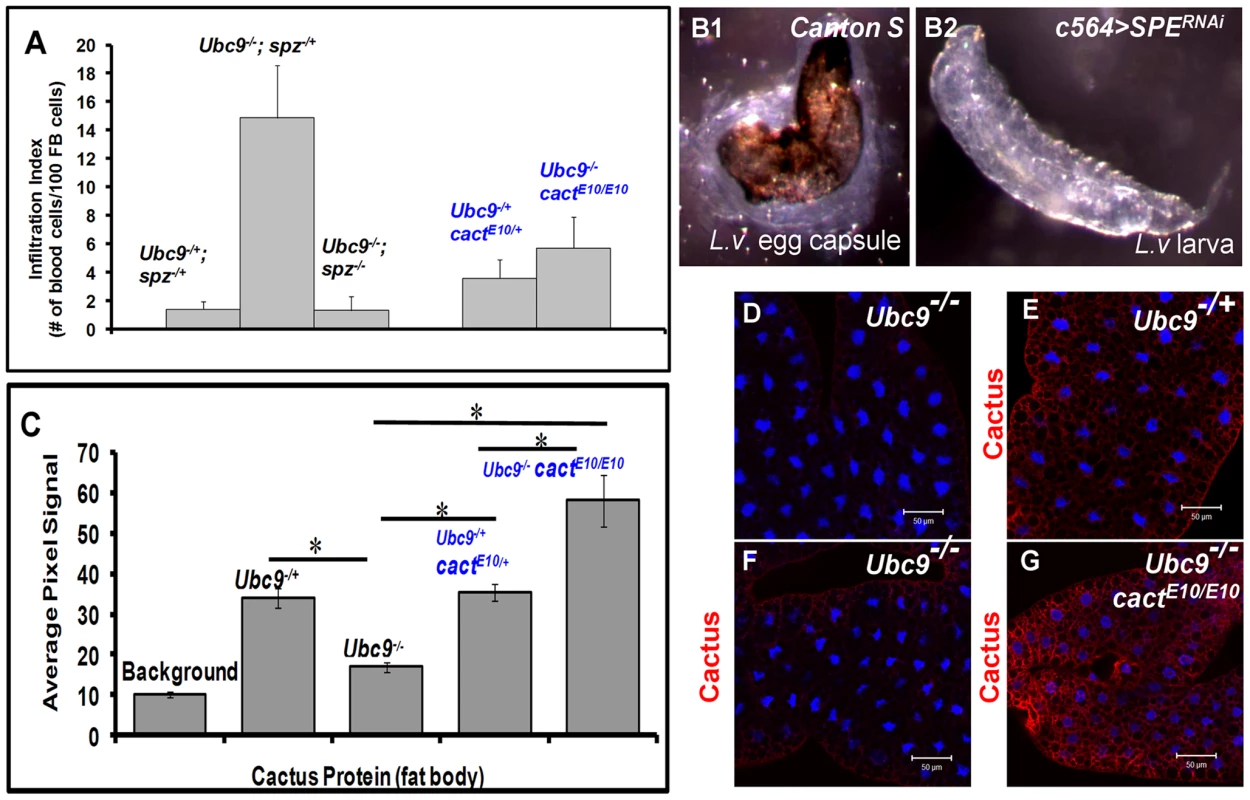
(A) Infiltration index in different genetic backgrounds. For all genotypes, n = 20, except for double mutants (Ubc9−/−; spz−/−), where n = 5 animals. Bars show standard error. (B1, B2) Outcome of L victoriae infection in Canton S (control) and c564>SPERNAi hosts. (C) Pixel signal quantification (see Methods) of Cactus protein levels in larval fat body. Genotypes are shown. Bars show standard error. Asterisk indicates significant difference (p<0.05). (D–G) Sample in panel D was not treated with anti-Cactus antibody (background). Cactus levels in fat body cells of Ubc9−/+ (E), Ubc9−/− (F), or Ubc9−/− cactE10/E10 (G) animals. (D–G) Cells were counterstained with Hoechst (blue). To test the specific contribution of SPE for normal encapsulation of the wasp egg, we infected c564>SPERNAi animals (c564-Gal4 is expressed in larval fat body and blood cells [30]). While experimental animals were completely immune compromised (0/153 infected animals showed encapsulation), control Canton S larvae efficiently (83.3%; n = 120 animals) encapsulated L. victoriae eggs (Figure 8B1, B2). These results strongly support the notion that high levels of SPE present in the hemolymph after infection are essential for encapsulation.
Ubc9 controls stability of Cactus protein
To further examine how transcriptional and translational regulation of cactus modulates systemic inflammation and delineate the function of Ubc9 in this process, we first confirmed the transcriptional activation of the cact255-lacZ allele [31] in immune cells after parasite infection. Consistent with the temporal profile observed in whole larvae (Figure 3A), the fat body (Figure S4A–C) and blood cells (plasmatocytes, Figure S4D, F, H; lamellocytes; Figure S4G, G') of cactus255/+ larvae showed significant activation of cact transcription. Consistent with this regulation, fat body and blood cells from parasitized y w animals showed increased level of Cactus protein (Figure S4I–L and data not shown). Thus, Cactus-dependent feedback is important for the resolution of wasp-induced acute inflammation.
In contrast to increased Cactus mRNA and protein after wasp infection, we were surprised to find that although cact RNA is higher in Ubc9− mutants relative to their heterozygous siblings (Figure 3A and S4), Cactus protein levels in mutant fat body are roughly half of that found in heterozygous fat body (Figure 8C–F), although this reduction is not as pronounced in the mutant blood cells (data not shown). Additionally, we examined Cactus levels in Ubc9− cactE10 double mutant fat body. This cact allele encodes a degradation-insensitive form of Cactus [32]. We found that, whereas single or double heterozygotes show roughly equal levels of Cactus (twice of that in Ubc9− fat body), the levels in Ubc9−/− cactE10/E10 fat body are roughly twice in comparison to controls (Figure 8C, G).
These results suggest that systemic chronic inflammation in Ubc9− larvae is, in part, sustained by reduced stability of endogenous Cactus in fat body cells. Significantly, the stable form of CactusE10 protein suppresses blood cell infiltration, tumorogenesis, and expression of Drs-GFP in Ubc9− mutants (Figure 8A, C).
Discussion
Coordination and calibration of immune responses
Parasitic wasps are a large group of insects that typically attack other insects. Because of the absolute dependence on their insect hosts, parasitic wasps are of enormous commercial interest and can replace insecticides to control insect pests. The motivation of this study was to gain a clearer understanding of how insect larvae respond to attacks of these natural enemies. Using an immuno-genetic approach in Drosophila, we found that the same Toll-dependent NF-κB mechanism that rids Drosophila of microbial infections also defends the host against metazoan parasites. However, because of critical differences in their size and mode of entry, the combination of immune responses summoned in the two cases is different. While phagocytosis and systemic humoral responses (the latter originating from the fat body and in the gut) are the principal mechanisms of host defense against bacteria and fungi [2], [33], the development of parasitic wasp eggs is blocked primarily by encapsulation response [1], [2], [3], [4].
We present data that for the first time demonstrate the critical requirement of the humoral arm in both the activation and resolution of egg encapsulation (Figures 4 and 8). We show that the bi-directional interaction between the blood cells and the fat body occurs via cell non-autonomous effects of SPE/Spz, where these secreted proteins synthesized in one compartment can activate immune signaling in the other (Figure 9A). Recent reports corroborate a signaling role for Spz derived from blood cells in the expression of antimicrobial peptides from the larval fat body in response to microbes [34], [35]. Because activation/deactivation of both immune arms is accomplished via the IκB/Ubc9-dependent feedback loop that has both, cell autonomous and cell non-autonomous effects (Figure 9A, also see below), we propose that this shared mechanism allows efficient coordination between the immune organs and helps restore normal immune homeostasis within the infected host.
Fig. 9. Regulatory immune circuit for host defense against wasp encapsulation. 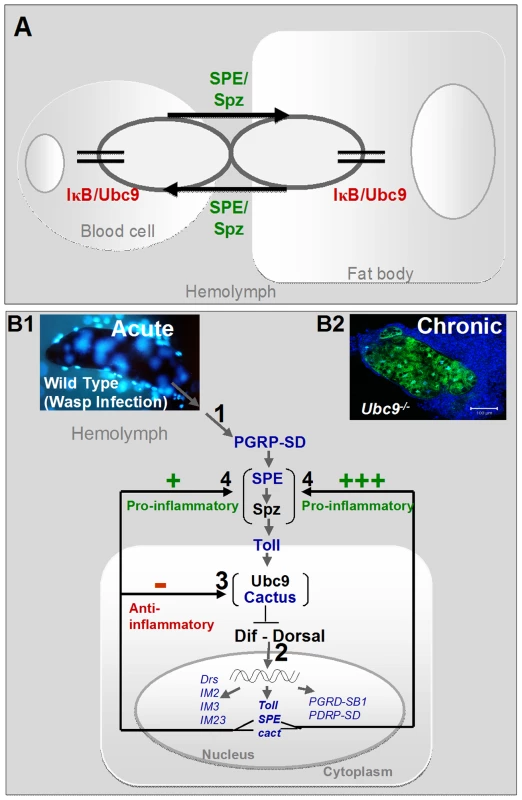
(A) A bi-directional cross-organ interaction involves a common regulatory circuit shown in detail below. Active Spätzle (SPE/Spz) acts cell non-autonomously to mediate this interaction. In each organ, sumoylation plays a regulatory role in restoring immune homeostasis. (B1) Acute infection by parasite activates the Toll-NF-κB cascade (step 1). Infection results in activation of, among others, pro- (SPE) and anti-inflammatory (cactus) targets (step 2). In a conserved feedback mechanism, Cactus protein is stabilized by Ubc9 (step 3). (Transcription of components in blue was identified to be activated in the microarray datasets; Table S1.) High SPE levels activate Spz in the extracellular compartment (step 4). SPE/Spz stimulates pro-inflammatory reactions via Toll (step 4). Sustained Cactus inhibition dampens the SPE/Spz cues and inflammation is resolved. (B2) In the absence of negative regulation via loss of Ubc9 (step 3), Cactus transcript levels are high, but protein levels remain low. Thus, signaling persists even in the absence of infection (step 1) because high levels of SPE/Spz build up (step 4). The amplification of this positive feedback step leads to chronic inflammation observed in Ubc9− mutants. Sumoylation balances activation and resolution of inflammation in vivo
The mechanism that coordinates the activation and resolution of both immune arms after parasite infection involves a balance between the positive (SPE) and negative (Cactus) components. Infection induces nuclear localization of Dorsal and Dif, and the transcription of both SPE (which resolves over time) and cactus (transcription levels off) (Figures 3 and S4). This Cactus-dependent regulation is essential for the downregulation of SPE transcription and the termination of the encapsulation response (Figure 9B). The negative feedback loop of Cactus in flies is similar to the one identified for IκBα in mammalian cells [36].
In Ubc9 mutants, the stability of Cactus protein is compromised, and Toll signaling persists during the extended larval life. Accordingly, knockdown of Cactus in blood cells (Hml>cactusRNAi) promotes inflammation, aggregation and melanization (our unpublished results). We propose that loss of immune homeostasis leads to constitutive SPE expression and activation of Spätzle, which promotes the development of chronic inflammation (Figures 8 and 9B). Thus, sumoylation serves an anti-inflammatory function in the fly larva.
We have identified at least two distinct biological roles of sumoylation: first, an essential role in blood cells, where the post-translational modification curbs proliferation in the lymph gland in the absence of infection [17]. This conclusion is also strongly supported by restoration of normal hematopoietic complement in mutants expressing wild type Ubc9 only within a limited lymph gland population (Figure 2L, O, and data not shown). Second, sumoylation is essential to sustain significant, steady state levels of Cactus (Figure 8). In mammalian cells, sumoylation of IκBα protects it from antagonistic, ubiquitination-mediated degradation [21]. Our results are consistent with the mammalian model where Cactus sumoylation would be expected to modulate its half-life. We are testing this idea in ongoing experiments.
Distinct roles for Spz in hematopoiesis and immunity
Cytokine activation and function are hallmarks of the normal inflammatory response in mammals. A key finding of our study is that active Spz serves a pro-inflammatory function in fly larvae. This first report of any pro-inflammatory molecule in the fly confirms that cytokines activate inflammation across phyla. As with mammalian cytokines that act as immuno-stimulants, Spz is expressed, and is therefore likely to activate the blood cells surrounding the parasite capsule (Figure 5). Active Spz promotes blood cell division, migration and infiltration (Figure 7) much like high levels of Dorsal and Dif, suggesting that the cell biological changes triggered by SPE/Spz are mediated by target genes of Dorsal and Dif. It is intriguing that the integrity of the basement membrane (as visualized by Collagen IV expression pattern) appears to be important for orchestrating blood cells to the site of “diseased self” (here, mutant fat body) in a manner that may be similar to recognition of the non-self parasitic egg, underscoring the parallel roles of basement membrane proteins in the origin and development of inflammation in both flies and mammals [10].
Although excessive (active) Spz is proinflammatory, its loss leads to reduction in the hematopoietic complement. For example mutants lacking spz (spzrm7/spzrm7) exhibit a 40% reduction in circulating blood cell concentration and these animals do not encapsulate wasp eggs as efficiently as their heterozygous siblings [7]. These observations suggest that active Spz's normal proliferative/pro-survival functions, required for maintaining the normal hematopoietic complement, are fundamentally linked to its immune function for the activation and recruitment of blood cells to target sites. Thus, the autocrine and paracrine hematopoietic and inflammatory effects of Spz are amplified in the presence of hyperactive Toll receptor, excessive Dorsal/Dif, or the loss of Cactus/Ubc9 inhibition, resulting in production of hematopoietic tumors [18], [29]. It is possible that mutations in other, unrelated, genes that yield similar inflammatory tumors arise due to the loss of Toll-NF-κB dependent immune homeostasis.
Our results highlight the central role of the Dorsal/Dif proteins not only in immune activation, but also in the resolution of these responses. Recent proteomic studies [37] have confirmed that Dorsal is a bona fide SUMO target and its transcriptional activity is affected by sumoylation [38]. Dorsal and Dif exhibit genetic redundancy in both the humoral and cellular responses [17], [18], [39]. It is possible that this redundancy ensures that immune reactions against microbes and parasites are efficiently resolved to allow proper host development.
Implications
In nature, parasitic wasps are continually evolving to evade or suppress the immune responses of their hosts. To this end, they secrete factors or produce protein complexes with specific molecular activities to block encapsulation. Our studies provide the biological context in which the effects of virulence factors produced by pathogens and parasites on primordial immune pathways can be more clearly interpreted. The molecular identity of wasp factors which actively suppress humoral and cellular responses (e.g., those in L. heterotoma [7]) remains largely unknown [4]. Such virulence factors are likely to be “anti-inflammatory” as they clearly interfere with host physiology [4], [7] that ultimately disrupts the central regulatory immune circuit defined in our studies.
Encapsulation reactions of non-self (wasp egg) or diseased self tissues (fat body) of the kind in the Drosophila larva are not only reported in other insects [40], but the reaction is likely to be similar to mammalian granulomas, which are characterized by different forms of localized nodular inflammation [11]. Furthermore, the phenotypes arising from persistent signaling in mutants recapitulate the key features of mammalian inflammation: i.e., reliance on conserved signaling mechanism, the requirement for cytokines, and sensitivity to aspirin (our unpublished results). Our studies also reveal a clear link between innate immunity and the development and progression of hematopoietic cancer in flies, as has been hypothesized from work in mammalian systems [9], [41], [42], [43]. In the past, genetic approaches in Drosophila have served well to dissect signaling mechanisms governing developmental processes in animals. The fly model with hallmarks of acute and chronic mammalian inflammatory responses will provide deep insights into signaling networks and feedback regulatory mechanisms in human infections and disease. It can also be used to test the potency and mechanism of action of pesticides, anti-inflammatory and anti-cancer agents in vivo.
Materials and Methods
Microarray data analysis
Published [5], [8] microarray datasets were formatted and compared to compile an initial list of putative inflammation genes in Drosophila. First, from the Wertheim dataset, 162 probe sets that were differentially regulated after A. tabida infection, 53 (classified as defense and/or immune related) were selected based on gene expression profiles. In the Schlenke dataset, 589 probe sets were differentially regulated after L. boulardi infection, of which 95 (classified as defense related) were selected, also based on gene expression profiles. We manually selected only those probe sets with acute-phase kinetics whose expression profile showed strong activation minutes to hours after infection, but followed a downward trend 12 hours after infection. This exercise resulted in 81 putative inflammation genes (40 genes from the Wertheim study and 51 genes from the Schlenke study, with 10 genes common to both studies).
Drosophila stocks
UAS lines: UAS-Ubc9WT [17], UAS-Ubc9RNAi (TRiP Valium 1), UAS-Aos1RNAi (Vienna Stock Center) and UAS-Uba2RNAi (Valium 10 stock), UAS-EGFP-Dorsal and UAS-CFP-Dif (from T. Ip; [44]); UAS-SPE-Act (amino acid 135–400 from B. Lemaitre; [14]); UAS-Spz (From B. Lemaitre); UAS-Spz-Myc, UAS-Spz-V5 (from T. Ip; [45]), C564-Gal, UAS-SPERNAi (from B. Lemaitre, C564 is expressed in the fat body and the hemocytes; [30]). Full-length Spätzle is 326 amino acids long and the above transgenic lines contain the entire coding region of the protein. When overexpressed in immune tissues, all three transgenic lines produced the same biological effects.
Gal4 lines: Gal4 lines, their sources and expression patterns are as follows: SerpentHemo-Gal4 (srp is expressed in all blood cells; [46]; stock obtained from Dr. M. Meister), Cg-Gal4 (Cg is expressed in fat body, circulating hemocytes and some cells of the lymph gland; [24]; Bloomington line 7011), 76B-Gal4 (76B is expressed in some cells of the medullary zone of the lymph gland, ring gland, genital disc, salivary gland; [26]; obtained from Dr. D. Harrison; Lsp2-Gal4 (Lsp is expressed in the fat body; [27] Dr. Hao Li), Hml-Gal4 (Hml is expressed in circulating hemocytes and some cells in the lymph gland; [28]; obtained from Dr. S. Bhattacharya). The y w pUAST-EGFP-Dorsal line was recombined into Srp-Gal4 background and was balanced with Basc to generate y w Srp-Gal4 UAS GFP-dl/Basc stock.
Ubc9 stocks and recombinants: Drosophila Ubc9 is synonymous to lesswright (lwr). Mutant Ubc94-3/Ubc95 animals are developmentally-delayed and most mutants die by day 10 as larvae.
Ubc9 stocks y w; Ubc94-3 FRT40A/CyO y+, y w; Ubc95 FRT40A/CyO y+, y w; Drs-GFP, Ubc94-3/CyO y+, y w; Drs-GFP Ubc95/CyO y+ are described previously [17]. The Ubc9 alleles were recombined or crossed into the Gal4 or UAS backgrounds to produce: y w; Ubc95, Cg-Gal4/CyO y+, y w, UAS-CFP-Dif; Ubc94-3/CyO y+, y w; Ubc94-3, UAS-EGFP-dl/CyO y+.
For rescue, a 76B-Gal4 insert [26] was recombined with the Ubc95 mutation on a chromosome carrying Drs-GFP transgene [47]. Males from this stock were crossed with UAS - Ubc9WT; Drs-GFP, Ubc94-3/CyO y+ females [17]. Mutants carrying 76B>Ubc9WT were compared to mutants without either UAS - Ubc9WT or 76B-Gal4 transgene.
Dif, dorsal, cactus stocks: Stocks lacking Dif and Dorsal, y w; Df(2L)J4/CyO y+ and y w; b Df(2L)119/CyO y+ and cactus stocks yw; Drs-GFP, Ubc94-3 cactE10/CyO y+, y w; Drs-GFP Ubc95 cactE10/CyO y+ are described previously [17]. P{FZ}-cact255/CyO is a P-lacZ insertion allele [31]. hsp83-lacZ line 25 has high levels of ubiquitous β-galactosidase expression [48] and was used to compare β-galactosidase expression in the cact255 stock.
spätzle mutant: The Ubc9 alleles were crossed into the original line rut h s tri roe pp e spzrm7/TM3 Sb Tb [7] to produce double mutant stocks of the genotype w; Ubc95/CyO actin GFP; spzrm7/TM6 Tb and w; Ubc94-3/CyO actin GFP; spzrm7/TM6 Tb. Double mutants, Ubc94-3/Ubc95; spzrm7/spzrm7 or Ubc94-3 cactE10/Ubc95 cactE10 are lethal during late larval stages.
Wasp infections
Standard protocol [3] was used for rearing wasps on the y w fly strain. Wasp stocks used were L. victoriae [49] and L. boulardi strain 17 [5]. Infections were performed on 3-day-old larvae (after 72 hrs of egg-laying). For real time PCR experiment, 50 early third-instar y w larvae were exposed to 10 L. boulardi-17 females for 2 hours. To assess Drs-GFP expression, third-instar y w; P{w+ Drs-GFP} larvae were exposed for 24 hours. As a positive control, larvae were challenged by poking with a sterile glass needle. In all cases, success of infection was confirmed by dissection of parasite eggs from the host. To examine the role of SPE in wasp encapsulation, second instar c564>SPERNAi larvae were superinfected with L. victoriae. Third instar larvae were dissected to document the number of wasp larvae, melanized aggregates and capsules per host larva. The experiment was done twice and more than 120 animals; in each attempt more than 60 infected hosts were examined.
RNA collection and real time-PCR
Fifty developmentally-synchronized animals of the appropriate genotypes (6 or 8 days after egg lay) or y w larvae (2, 6, 12 or 24 hours after infection) were collected for RNA extraction (Trizol method, GibcoBRL, Invitrogen, Carlsbad, CA). RNA was quantified by a Smartspec 3000 (Bio-Rad Laboratories, Hercules, CA). 2 µg of total RNA served as template for cDNA synthesis (Protoscript first strand synthesis kit; New England BioLabs, Ipswich, MA). Real time PCR was performed running the standard two-step PCR program: 0.5 µl of the cDNA sample was mixed with iQ SYBR Green Supermix (Bio-Rad Laboratories) and primers to set up a 25-µl reaction mix. Transcript levels detected were normalized to rp49 mRNA values. Primers used:
Drosomycin: Forward primer (5′) ATC CTG AAG TGC TGG TGC GAA GGA (3′); Reverse primer (5′) ACG TTC ATG CTA ATT GCT CAT GG (3′)
spätzle: Forward primer (5′) GGA GCG GAT CAA CCC TGT G (3′); Reverse primer (5′) TTG GAT TAT AGC TCT GCG GAA AG (3′)
SPE: Forward primer (5′) CTT TTC GCT GAT CGC ATT TT (3′); Reverse primer (5′) CAC CGG ATT TGT CCA GTT CT (3′)
cactus: Forward primer (5′) CTG CTC AAC ATC CAG AAC GA (3′); Reverse primer (5′) GCC GAA CTT CTC TGT CAA GG (3′)
hopscotch: Forward primer (5′) AAT AAT CCA CGG CTC GTC AG (3′); Reverse primer (5′) ACG CTT GCT TTT CGC ATA GT (3′)
Irc: Forward primer (5′) TGG CTG AAA AAT CCG AGT TC (3′); Reverse primer (5′) GTC CAA CGC CGT TTC TAC AT (3′)
rp49: Forward primer (5′) GAC GCT TCA AGG GAC AGT ATC TG (3′); Reverse primer (5′) AAA CGC GGT TCT GCA TGA G (3′)
Infiltration index, tumor penetrance, and Drs-GFP expression
Fat body from control, mutant and experimental animals was dissected, fixed, stained with Hoechst and TRITC labeled phalloidin, and then mounted with 50% glycerol in PBS. The number of single blood cells adhering to the top and bottom of the fat body were scored using a Zeiss Axioscope fluorescence microscope. Aggregates attached to the fat body were not scored. Whole larvae were scored for melanized microtumors through the cuticle. Transcriptional activity of the Drs-GFP promoter was analyzed in whole larvae under a Leica stereomicroscope equipped with GFP-compatible fluorescence. Student t-test was performed using SAS (SAS, Inc., Cary, NC) to determine statistical significance.
Immunostaining, microscopy, data collection and analysis
Developmentally synchronized larvae were dissected to collect fat bodies or blood cells. Samples were fixed with 4% paraformaldehyde, prepared in 2% sucrose in PBS at pH 7.6. Fixed samples were blocked in 3% bovine serum albumin, followed by an overnight incubation with primary antibody (mouse monoclonal anti-dorsal (7A4, 1∶10; [50]), mouse monoclonal anti-Cactus (3H12, 1∶20; [50]), mouse monoclonal anti-integrin-β PS (1∶50, [51]) all obtained from Developmental Studies Hybridoma Bank, University of Iowa). Integrin-β PS is expressed in blood cells [52]. Rabbit polyclonal anti-Collagen IV (1∶500, [25], gift of Dr. L. Fessler), rabbit polyclonal anti-Spätzle (1∶100, [14], gift of Dr. C. Hashimoto), plasmatocyte-specific monoclonal mouse anti-Nimrod C (1∶10, [53], gift of Dr. I. Ando), rabbit polyclonal anti-phospho histone H3 (1∶200, Upstate Cell Signaling Solutions), and rabbit anti-β-galactosidase (1∶1, MP Biomedicals, LLC). Washed samples were incubated for 3 hrs at room temperature with commercially available secondary antibody (TRITC/FITC-conjugated donkey anti-rabbit, 1∶50 or TRITC/FITC-conjugated donkey anti-mouse, 1∶50, Jackson Immuno Research Laboratories). After three washes, samples were counterstained with nuclear dye Hoechst 33258 (Invitrogen Molecular Probes, Eugene, OR) and/or TRITC (Tetramethyl Rhodamine Iso-Thiocyanate)-labeled phalloidin (0.5 µg/ml, Invitrogen Molecular Probes, Eugene, OR), and then mounted in 50% glycerol in PBS or Vectashield (Vector Laboratories, Inc., Burlingame, CA). To assess Dorsal and Dif subcellular localization, CFP-Dif and GFP-Dorsal, driven by Cg-Gal4, were monitored in fat body of 6 or 7 day-old third instar larvae, after a six hour egg-lay from animals reared at 28°C. Images were obtained using a Zeiss LSM510 confocal microscope or Zeiss Axioplan 2 equipped with fluorescence optics. Images for control and experimental samples were taken at identical settings. Images were processed identically for quantitative analysis of signals.
Quantification of the fluorescence signal after anti-Cactus antibody staining was done in Adobe Photoshop CS3 (Adobe Systems Inc., San Jose, CA). At least 300 cells from 9 larvae were analyzed for each genotype. A one-way ANOVA analysis was performed in SAS (SAS Inc., Cary, NC). We partitioned and contrasted the different genotypes to identify significant pair-wise differences by comparing p-values on a PDIFF matrix and applying sequential bon Ferroni corrections to adjust for accumulated error.
Dorsal and Dif/Relish binding sites
We queried both Dif/Relish (GGGAWTCMC; [54]) and Dorsal binding sites GGGWDWWWCCM or GGGWWWWCCM; [55]) consensus sequences in the fly genome using Fly Enhancer (opengenomics.org). To yield all possible binding sites for these consensus sequences, we queried one cluster per 400 bp frame. The Dif/Relish consensus sequence query obtained close to 3,000 predicted target binding sites, but none mapped to either the cactus or SPE regions. Search for the Dorsal consensus sequences yielded over 10,000 predicted target binding sites, with multiple hits mapping to either the cactus or SPE regions (Figure S2).
Approximately 6 kb of SPE (∼5 kb flanking) and 17 kb of cact (∼2 kb flanking) genomic sequences containing coding and flanking sequences were retrieved in FASTA file format from Fly Base Gbrowser [56]. Known exons and untranslated regions were annotated on the cactus and SPE genomic sequences using NCBI Sequence Viewer (http://www.ncbi.nlm.nih.gov). To confirm dorsal consensus sequence predictions, we processed consensus sequences through GeneQuest (DNAStar, Inc., Madison, WI) keeping the threshold at 100% with bi-directionality match.
Supporting Information
Zdroje
1. GovindS
2008 Innate immunity in Drosophila: Pathogens and pathways. Insect Science 15 29 43
2. LemaitreB
HoffmannJ
2007 The host defense of Drosophila melanogaster. Annu Rev Immunol 25 697 743
3. SorrentinoRP
CartonY
GovindS
2002 Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev Biol 243 65 80
4. LeeMJ
KalamarzME
PaddibhatlaI
SmallC
RajwaniR
2009 Virulence factors and strategies of Leptopilina spp.: selective responses in Drosophila hosts. Adv Parasitol 70 123 145
5. SchlenkeTA
MoralesJ
GovindS
ClarkAG
2007 Contrasting infection strategies in generalist and specialist wasp parasitoids of Drosophila melanogaster. PLoS Pathog 3 1486 1501
6. PeyronP
VaubourgeixJ
PoquetY
LevillainF
BotanchC
2008 Foamy macrophages from tuberculous patients' granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog 4 e1000204
7. SorrentinoRP
MelkJP
GovindS
2004 Genetic analysis of contributions of dorsal group and JAK-Stat92E pathway genes to larval hemocyte concentration and the egg encapsulation response in Drosophila. Genetics 166 1343 1356
8. WertheimB
KraaijeveldAR
SchusterE
BlancE
HopkinsM
2005 Genome-wide gene expression in response to parasitoid attack in Drosophila. Genome Biol 6 R94
9. KarinM
LawrenceT
NizetV
2006 Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell 124 823 835
10. MedzhitovR
2008 Origin and physiological roles of inflammation. Nature 454 428 435
11. AnthonyRM
RutitzkyLI
UrbanJFJr
StadeckerMJ
GauseWC
2007 Protective immune mechanisms in helminth infection. Nat Rev Immunol 7 975 987
12. LozaMJ
McCallCE
LiL
IsaacsWB
XuJ
2007 Assembly of inflammation-related genes for pathway-focused genetic analysis. PLoS One 2 e1035
13. MulinariS
HackerU
Castillejo-LopezC
2006 Expression and regulation of Spatzle-processing enzyme in Drosophila. FEBS Lett 580 5406 5410
14. JangIH
ChosaN
KimSH
NamHJ
LemaitreB
2006 A Spatzle-processing enzyme required for toll signaling activation in Drosophila innate immunity. Dev Cell 10 45 55
15. BodianDL
LeungS
ChiuH
GovindS
2004 Cytokines in Drosophila hematopoiesis and cellular immunity. Prog Mol Subcell Biol 34 27 46
16. QiuP
PanPC
GovindS
1998 A role for the Drosophila Toll/Cactus pathway in larval hematopoiesis. Development 125 1909 1920
17. ChiuH
RingBC
SorrentinoRP
KalamarzM
GarzaD
2005 dUbc9 negatively regulates the Toll-NF-kappa B pathways in larval hematopoiesis and drosomycin activation in Drosophila. Dev Biol 288 60 72
18. HuangL
OhsakoS
TandaS
2005 The lesswright mutation activates Rel-related proteins, leading to overproduction of larval hemocytes in Drosophila melanogaster. Dev Biol 280 407 420
19. LemaitreB
MeisterM
GovindS
GeorgelP
StewardR
1995 Functional analysis and regulation of nuclear import of dorsal during the immune response in Drosophila. EMBO J 14 536 545
20. NicolasE
ReichhartJM
HoffmannJA
LemaitreB
1998 In vivo regulation of the IkappaB homologue cactus during the immune response of Drosophila. J Biol Chem 273 10463 10469
21. MabbAM
MiyamotoS
2007 SUMO and NF-kappaB ties. Cell Mol Life Sci 64 1979 1996
22. MiuraK
JinJB
HasegawaPM
2007 Sumoylation, a post-translational regulatory process in plants. Curr Opin Plant Biol 10 495 502
23. StulemeijerIJ
JoostenMH
2008 Post-translational modification of host proteins in pathogen-triggered defence signalling in plants. Mol Plant Pathol 9 545 560
24. AshaH
NagyI
KovacsG
StetsonD
AndoI
2003 Analysis of Ras-induced overproliferation in Drosophila hemocytes. Genetics 163 203 215
25. FesslerLI
CondicML
NelsonRE
FesslerJH
FristromJW
1993 Site-specific cleavage of basement membrane collagen IV during Drosophila metamorphosis. Development 117 1061 1069
26. HarrisonDA
BinariR
NahreiniTS
GilmanM
PerrimonN
1995 Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J 14 2857 2865
27. CherbasL
HuX
ZhimulevI
BelyaevaE
CherbasP
2003 EcR isoforms in Drosophila: testing tissue-specific requirements by targeted blockade and rescue. Development 130 271 284
28. SinenkoSA
Mathey-PrevotB
2004 Increased expression of Drosophila tetraspanin, Tsp68C, suppresses the abnormal proliferation of ytr-deficient and Ras/Raf-activated hemocytes. Oncogene 23 9120 9128
29. GovindS
1996 Rel signalling pathway and the melanotic tumour phenotype of Drosophila. Biochem Soc Trans 24 39 44
30. KambrisZ
BrunS
JangIH
NamHJ
RomeoY
2006 Drosophila immunity: a large-scale in vivo RNAi screen identifies five serine proteases required for Toll activation. Curr Biol 16 808 813
31. GeislerR
BergmannA
HiromiY
Nusslein-VolhardC
1992 cactus, a gene involved in dorsoventral pattern formation of Drosophila, is related to the I kappa B gene family of vertebrates. Cell 71 613 621
32. BergmannA
SteinD
GeislerR
HagenmaierS
SchmidB
1996 A gradient of cytoplasmic Cactus degradation establishes the nuclear localization gradient of the dorsal morphogen in Drosophila. Mech Dev 60 109 123
33. CharrouxB
RoyetJ
2010 Drosophila immune response: From systemic antimicrobial peptide production in fat body cells to local defense in the intestinal tract. Fly (Austin) 4 40 47
34. CharrouxB
RoyetJ
2009 Elimination of plasmatocytes by targeted apoptosis reveals their role in multiple aspects of the Drosophila immune response. Proc Natl Acad Sci U S A 106 9797 9802
35. ShiaAK
GlittenbergM
ThompsonG
WeberAN
ReichhartJM
2009 Toll-dependent antimicrobial responses in Drosophila larval fat body require Spatzle secreted by haemocytes. J Cell Sci 122 4505 4515
36. ScottML
FujitaT
LiouHC
NolanGP
BaltimoreD
1993 The p65 subunit of NF-kappa B regulates I kappa B by two distinct mechanisms. Genes Dev 7 1266 1276
37. NieM
XieY
LooJA
CoureyAJ
2009 Genetic and proteomic evidence for roles of Drosophila SUMO in cell cycle control, Ras signaling, and early pattern formation. PLoS One 4 e5905
38. StielowB
SapetschnigA
KrugerI
KunertN
BrehmA
2008 Identification of SUMO-dependent chromatin-associated transcriptional repression components by a genome-wide RNAi screen. Mol Cell 29 742 754
39. ManfruelliP
ReichhartJM
StewardR
HoffmannJA
LemaitreB
1999 A mosaic analysis in Drosophila fat body cells of the control of antimicrobial peptide genes by the Rel proteins Dorsal and DIF. EMBO J 18 3380 3391
40. StanleyD
MillerJ
TunazH
2009 Eicosanoid actions in insect immunity. J Innate Immun 1 282 290
41. CoussensLM
WerbZ
2002 Inflammation and cancer. Nature 420 860 867
42. KarinM
GretenFR
2005 NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 5 749 759
43. MantovaniA
AllavenaP
SicaA
BalkwillF
2008 Cancer-related inflammation. Nature 454 436 444
44. BettencourtR
AshaH
DearolfC
IpYT
2004 Hemolymph-dependent and -independent responses in Drosophila immune tissue. J Cell Biochem 92 849 863
45. HuX
YagiY
TanjiT
ZhouS
IpYT
2004 Multimerization and interaction of Toll and Spatzle in Drosophila. Proc Natl Acad Sci U S A 101 9369 9374
46. BrucknerK
KockelL
DuchekP
LuqueCM
RorthP
2004 The PDGF/VEGF receptor controls blood cell survival in Drosophila. Dev Cell 7 73 84
47. FerrandonD
JungAC
CriquiM
LemaitreB
Uttenweiler-JosephS
1998 A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. EMBO J 17 1217 1227
48. GovindS
WhalenAM
StewardR
1992 In vivo self-association of the Drosophila rel-protein dorsal. Proc Natl Acad Sci U S A 89 7861 7865
49. MoralesJ
ChiuH
OoT
PlazaR
HoskinsS
2005 Biogenesis, structure, and immune-suppressive effects of virus-like particles of a Drosophila parasitoid, Leptopilina victoriae. J Insect Physiol 51 181 195
50. WhalenAM
StewardR
1993 Dissociation of the dorsal-cactus complex and phosphorylation of the dorsal protein correlate with the nuclear localization of dorsal. J Cell Biol 123 523 534
51. BrowerDL
WilcoxM
PiovantM
SmithRJ
RegerLA
1984 Related cell-surface antigens expressed with positional specificity in Drosophila imaginal discs. Proc Natl Acad Sci U S A 81 7485 7489
52. KwonSY
XiaoH
GloverBP
TjianR
WuC
2008 The nucleosome remodeling factor (NURF) regulates genes involved in Drosophila innate immunity. Dev Biol 316 538 547
53. KuruczE
MarkusR
ZsambokiJ
Folkl-MedzihradszkyK
DarulaZ
2007 Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr Biol 17 649 654
54. SengerK
ArmstrongGW
RowellWJ
KwanJM
MarksteinM
2004 Immunity regulatory DNAs share common organizational features in Drosophila. Mol Cell 13 19 32
55. MarksteinM
MarksteinP
MarksteinV
LevineMS
2002 Genome-wide analysis of clustered Dorsal binding sites identifies putative target genes in the Drosophila embryo. Proc Natl Acad Sci U S A 99 763 768
56. TweedieS
AshburnerM
FallsK
LeylandP
McQuiltonP
2009 FlyBase: enhancing Drosophila Gene Ontology annotations. Nucleic Acids Res 37 D555 559
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 12- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Is Adherence to Erythrocytes a Factor in Extrapulmonary Dissemination?
- Identifying the Age Cohort Responsible for Transmission in a Natural Outbreak of
- Blockade of Immunosuppressive Cytokines Restores NK Cell Antiviral Function in Chronic Hepatitis B Virus Infection
- Metaeffector Exploits Host Proteasome to Temporally Regulate Cognate Effector
- The p53-Target Gene Drives Neutrophil-Mediated Protection against Lethal Bacterial Sepsis
- Development of an RNAi Protocol to Investigate Gene Function in the Filarial Nematode,
- Lysine Acetyltransferase GCN5-A Functions in the Cellular Response to Alkaline Stress and Expression of Cyst Genes
- Structural Basis for Apoptosis Inhibition by Epstein-Barr Virus BHRF1
- Molecular Architectures of Trimeric SIV and HIV-1 Envelope Glycoproteins on Intact Viruses: Strain-Dependent Variation in Quaternary Structure
- Interaction of c-Cbl with Myosin IIA Regulates Bleb Associated Macropinocytosis of Kaposi's Sarcoma-Associated Herpesvirus
- Glacial Refugia in Pathogens: European Genetic Structure of Anther Smut Pathogens on and
- Role for Sumoylation in Systemic Inflammation and Immune Homeostasis in Larvae
- Inflammasome Sensor Nlrp1b-Dependent Resistance to Anthrax Is Mediated by Caspase-1, IL-1 Signaling and Neutrophil Recruitment
- Noise Cancellation: Viral Fine Tuning of the Cellular Environment for Its Own Genome Replication
- Infectious Speciation Revisited: Impact of Symbiont-Depletion on Female Fitness and Mating Behavior of
- Eis Regulates Autophagy, Inflammation, and Cell Death through Redox-dependent Signaling
- NleC, a Type III Secretion Protease, Compromises NF-κB Activation by Targeting p65/RelA
- Early Myeloid Dendritic Cell Dysregulation is Predictive of Disease Progression in Simian Immunodeficiency Virus Infection
- HIV Capsid is a Tractable Target for Small Molecule Therapeutic Intervention
- Structural and Functional Studies of Nonstructural Protein 2 of the Hepatitis C Virus Reveal Its Key Role as Organizer of Virion Assembly
- Coming of Age—Sexual Reproduction in Species
- HIV-1 Envelope Subregion Length Variation during Disease Progression
- Compartmentation of Redox Metabolism in Malaria Parasites
- Evidence That Intracellular Stages of Utilize Amino Sugars as a Major Carbon Source
- Rapid End-Point Quantitation of Prion Seeding Activity with Sensitivity Comparable to Bioassays
- Dimeric 2G12 as a Potent Protection against HIV-1
- Hypoxia Induces an Immunodominant Target of Tuberculosis Specific T Cells Absent from Common BCG Vaccines
- CD4 Natural Regulatory T Cells Prevent Experimental Cerebral Malaria via CTLA-4 When Expanded In Vivo
- The Killing of African Trypanosomes by Ethidium Bromide
- H2A.Z Demarcates Intergenic Regions of the Epigenome That Are Dynamically Marked by H3K9ac and H3K4me3
- Large-Scale Field Application of RNAi Technology Reducing Israeli Acute Paralysis Virus Disease in Honey Bees (, Hymenoptera: Apidae)
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- HIV-1 Envelope Subregion Length Variation during Disease Progression
- Coming of Age—Sexual Reproduction in Species
- Evidence That Intracellular Stages of Utilize Amino Sugars as a Major Carbon Source
- Compartmentation of Redox Metabolism in Malaria Parasites
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



