-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Membrane Fusion Step of Vaccinia Virus Entry Is Cooperatively Mediated by Multiple Viral Proteins and Host Cell Components
For many viruses, one or two proteins allow cell attachment and entry, which occurs through the plasma membrane or following endocytosis at low pH. In contrast, vaccinia virus (VACV) enters cells by both neutral and low pH routes; four proteins mediate cell attachment and twelve that are associated in a membrane complex and conserved in all poxviruses are dedicated to entry. The aim of the present study was to determine the roles of cellular and viral proteins in initial stages of entry, specifically fusion of the membranes of the mature virion and cell. For analysis of the role of cellular components, we used well characterized inhibitors and measured binding of a recombinant VACV virion containing Gaussia luciferase fused to a core protein; viral and cellular membrane lipid mixing with a self-quenching fluorescent probe in the virion membrane; and core entry with a recombinant VACV expressing firefly luciferase and electron microscopy. We determined that inhibitors of tyrosine protein kinases, dynamin GTPase and actin dynamics had little effect on binding of virions to cells but impaired membrane fusion, whereas partial cholesterol depletion and inhibitors of endosomal acidification and membrane blebbing had a severe effect at the later stage of core entry. To determine the role of viral proteins, virions lacking individual membrane components were purified from cells infected with members of a panel of ten conditional-lethal inducible mutants. Each of the entry protein-deficient virions had severely reduced infectivity and except for A28, L1 and L5 greatly impaired membrane fusion. In addition, a potent neutralizing L1 monoclonal antibody blocked entry at a post-membrane lipid-mixing step. Taken together, these results suggested a 2-step entry model and implicated an unprecedented number of viral proteins and cellular components involved in signaling and actin rearrangement for initiation of virus-cell membrane fusion during poxvirus entry.
Published in the journal: The Membrane Fusion Step of Vaccinia Virus Entry Is Cooperatively Mediated by Multiple Viral Proteins and Host Cell Components. PLoS Pathog 7(12): e32767. doi:10.1371/journal.ppat.1002446
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002446Summary
For many viruses, one or two proteins allow cell attachment and entry, which occurs through the plasma membrane or following endocytosis at low pH. In contrast, vaccinia virus (VACV) enters cells by both neutral and low pH routes; four proteins mediate cell attachment and twelve that are associated in a membrane complex and conserved in all poxviruses are dedicated to entry. The aim of the present study was to determine the roles of cellular and viral proteins in initial stages of entry, specifically fusion of the membranes of the mature virion and cell. For analysis of the role of cellular components, we used well characterized inhibitors and measured binding of a recombinant VACV virion containing Gaussia luciferase fused to a core protein; viral and cellular membrane lipid mixing with a self-quenching fluorescent probe in the virion membrane; and core entry with a recombinant VACV expressing firefly luciferase and electron microscopy. We determined that inhibitors of tyrosine protein kinases, dynamin GTPase and actin dynamics had little effect on binding of virions to cells but impaired membrane fusion, whereas partial cholesterol depletion and inhibitors of endosomal acidification and membrane blebbing had a severe effect at the later stage of core entry. To determine the role of viral proteins, virions lacking individual membrane components were purified from cells infected with members of a panel of ten conditional-lethal inducible mutants. Each of the entry protein-deficient virions had severely reduced infectivity and except for A28, L1 and L5 greatly impaired membrane fusion. In addition, a potent neutralizing L1 monoclonal antibody blocked entry at a post-membrane lipid-mixing step. Taken together, these results suggested a 2-step entry model and implicated an unprecedented number of viral proteins and cellular components involved in signaling and actin rearrangement for initiation of virus-cell membrane fusion during poxvirus entry.
Introduction
Entry of enveloped viruses into cells can be divided into three steps: (i) close apposition of viral and cellular membranes, (ii) lipid mixing of the outer membrane leaflets leading to formation of a hemifusion intermediate, and (iii) formation and expansion of a fusion pore allowing entry of the viral nucleoprotein or core into the cytoplasm [1]. One or two glycoproteins that provide cell binding and membrane fusion are sufficient to mediate entry of many enveloped viruses [2]. The process is more complex for members of the herpesvirus family, which employ four to five glycoproteins for entry [3]. Poxviruses represent an extreme case, as at least sixteen unglycosylated vaccinia virus (VACV) proteins participate in this process (referenced below). The large number of poxvirus proteins and the absence of any that resemble conventional membrane fusion proteins by sequence suggest a novel entry mechanism. For mature virions (MVs), the basic and most abundant infectious VACV particle, entry can occur by fusion at the plasma membrane [4], [5] or in a low pH-dependent manner from within an intracellular vesicle, depending to some extent on the virus strain [6], [7] and cell type [7]–[9]. Endocytosis of MVs is believed to occur by macropinocytosis [10]–[15] or dynamin-mediated fluid phase uptake [16], consistent with a role for actin dynamics and cell signaling. Progeny virions that depart the cell by exocytosis contain an additional membrane that helps escape antibody neutralization and is ultimately ruptured to allow fusion of the enclosed MV with the plasma membrane or endocytic vesicle [17], [18].
Four VACV proteins are involved in attachment of MVs [19]–[22] and twelve, conserved in all members of the poxvirus family, participate in subsequent entry steps [23]–[34]. Initial binding to target cells occurs via interactions of the MV attachment proteins with cell surface glycosaminoglycans or laminin. A cellular protein, referred to as VACV penetration factor, appears to be important for entry but exactly how is not yet understood [16]. The twelve conserved VACV entry proteins are mostly small, ranging in size from 35 to 377 amino acids, and have a N - or C-terminal transmembrane domain. The proteins are all components of the MV membrane, which is formed within the cytoplasm by incompletely defined mechanisms rather than by budding as typically occurs with other viruses [35]. This feature, as well as the association of most or all the proteins in a complex [31], makes it difficult to investigate the roles of individual entry proteins. A useful approach has been to construct conditional lethal mutants, with one putative entry gene controlled by the Escherichia coli lac operator/repressor system and positively regulated by ß-D-isopropylthiogalactopyanoside (IPTG) inducer, or with an analogous tetracycline-inducible system. These mutants share similar phenotypes: in the presence of inducer, replication proceeds normally and the progeny virions contain the protein product of the inducible gene and are infectious; in the absence of inducer, progeny virions appear indistinguishable from wild type by electron microscopy and protein analysis (except for the missing entry protein) but have very low infectivity. Although the non-infectious virions bind to cells, immunofluorescence microscopy studies show reduced numbers of cores in the cytoplasm. With the exception of I2 [30], repressed expression of the individual proteins does not significantly reduce the trafficking of the others to the MV membrane. However, when expression of an individual component is repressed, the formation or stability of the complex is reduced, as determined by detergent extraction and immunoaffinity purification [31]. The proteins A16, A21, A28, G3, G9, H2, J5, L5 and O3, make up the central components of the so-called entry fusion complex (EFC). The L1 and F9 proteins are also required for entry; although they physically interact with the EFC, they are not required for assembly or stability of the complex, and consequently have been referred to as EFC-associated proteins [26], [32]. The overall structure of the EFC has not been elucidated, though several pair-wise protein interactions have been identified [36]–[38].
The mechanisms involved in poxvirus entry are poorly understood. Previous studies have depended on post-membrane fusion assays and a specific role of the EFC in fusion could only be inferred from the inability of cells infected with the mutant viruses made in the absence of IPTG to undergo low pH-induced syncytia formation. Thus, direct evidence for a role of EFC proteins in membrane fusion during entry of virions has been lacking. Here, we used a variety of approaches including cell binding, membrane lipid mixing, core entry and reporter gene expression (Figure 1) to evaluate the roles of host components and individual MV membrane proteins.
Fig. 1. Virion binding, lipid mixing and core entry assays. 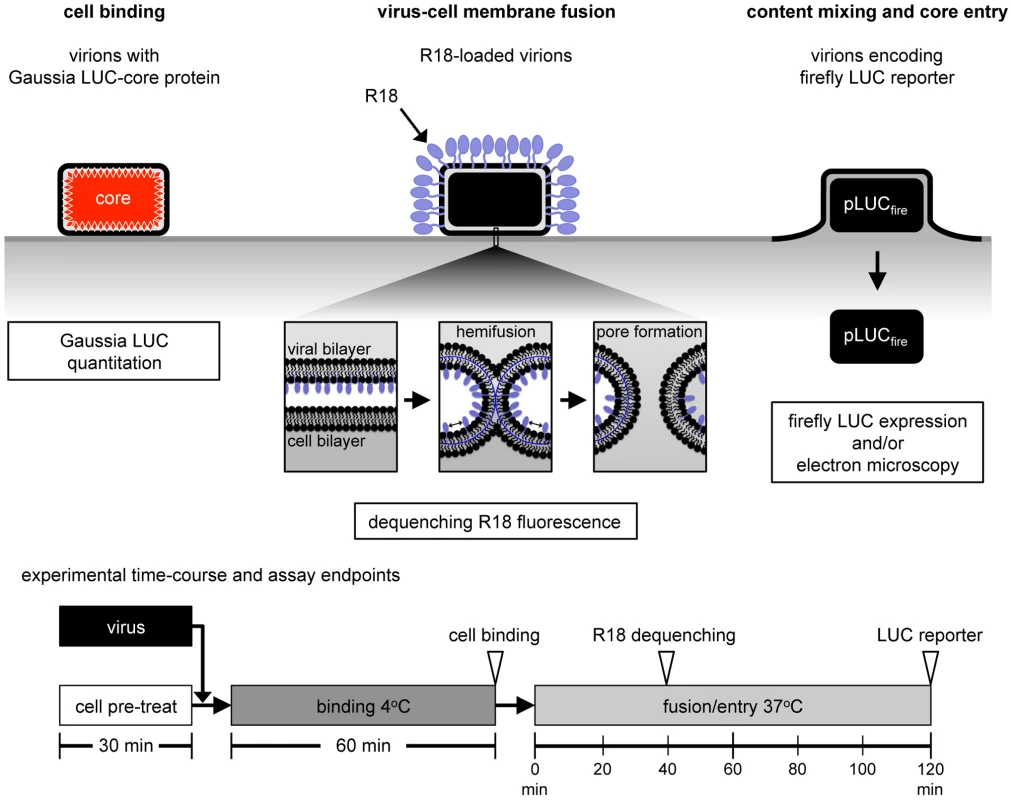
VACV Gauss-A4, a recombinant VACV with Gaussia LUC fused to a core protein, was used to measure the binding of virions at 4°C by assaying cell-associated LUC activity. For virus-cell membrane fusion, R18-loaded virions were bound to target cells at 4°C, shifted to 37°C, and the dequenching of R18 due to lipid mixing was measured by increased fluorescence. WRvFire, a recombinant VACV that expresses firefly LUC under an early promoter, was used to infect cells and newly synthesized LUC was measured. Direct visualization of virions fusing with the plasma membrane and quantification of viral cores in the cytosol were achieved by transmission electron microscopy. The times used for pretreatment, binding and entry are depicted at the bottom of the figure. Results
VACV-Cell Membrane Fusion and Core Entry
Fusion of viral and cellular membranes involves lipid mixing, which can be studied by loading a self-quenching fluorescent probe such as octadecylrhodamine (R18) into viral membranes (Figure 1). Fusion of viral and cell membranes results in dilution of the probe and increased fluorescence [39]. Dequenching does not require full fusion of the viral and cell membrane but can occur at the initial step in which only the outer leaflets of the viral and cellular membranes fuse, known as hemifusion [1]. Therefore, dequenching could signify the occurrence of hemifusion alone or full fusion with pore formation. In a 2-step membrane fusion model (see Discussion), inhibitors that prevent dequenching must operate at or prior to the hemifusion step, which precedes full fusion.
In the present experiments, sucrose gradient purified VACV MVs were incubated with R18 at room temperature for 20 min. Incorporation of R18 into MVs minimally affected infectivity as shown in Figure 2A. After removal of excess R18, the MVs were incubated with HeLa cells for 1 h at 4°C to allow adsorption and then the temperature was raised to permit fusion. R18 fluorescence was more rapid at the physiological temperature of 37°C than at 20°C (Figure 2B), consistent with an active transfer process. We used WRvFire, a recombinant VACV that expresses firefly luciferase (LUC) regulated by an early promoter, to compare the kinetics of fusion and reporter gene expression. Whereas fusion occurred within a few minutes after incubation of virus-bound cells, LUC expression was detected at 40 min (Figure 2C) and was routinely assayed after 1 or 2 h.
Fig. 2. Membrane fusion and core entry. 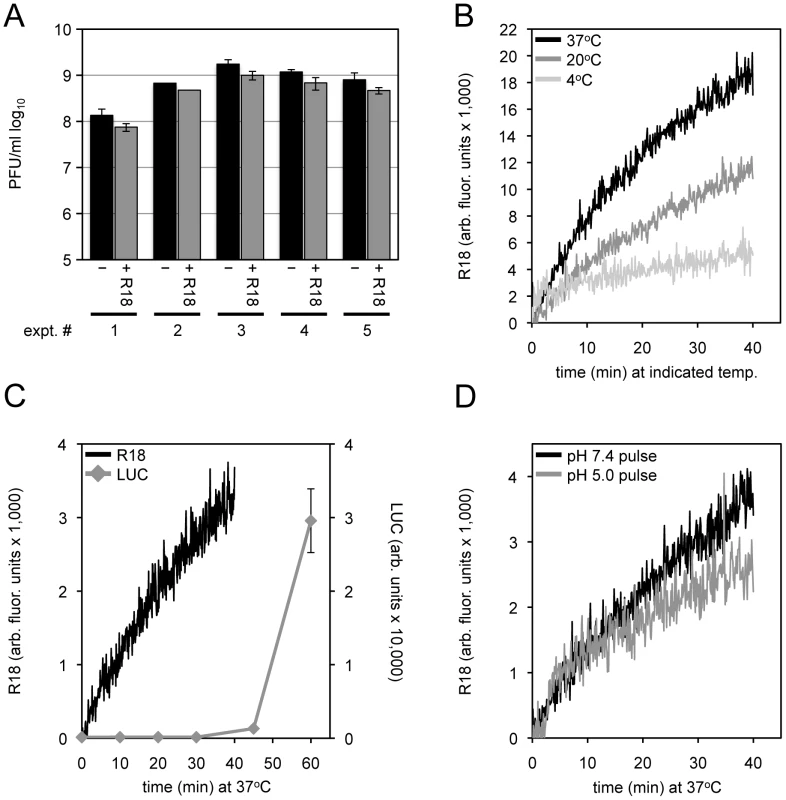
(A) Equivalent numbers of purified MVs were untreated or loaded with R18 for 20 min at room temperature. Unbound R18 was removed by pelleting and washing the virus. Control and R18-labeled virions were resuspended and serial dilutions made to assay virus infectivity (PFU/ml) by plaque assay. The results of five independent experiments with error bars are plotted. (B) Purified R18-loaded MV particles were bound to HeLa cells at 4°C for 60 min. The cells were then incubated at 4°C, 20°C, or 37°C for 40 min while R18 fluorescence was monitored and quantified as arbitrary fluorescent units. (C) R18-loaded MVs (recombinant WRvFire) were incubated with HeLa cells at 4°C to permit binding. Washed cells were then placed in a cuvette containing pre-warmed media at 37°C and fluorescence was monitored over time (black line; left y-axis). In parallel, unlabeled MVs were bound to cells in the cold and then shifted to 37°C. Cell lysates were prepared at indicated times and assayed for LUC activity (gray line; right y-axis). (D) An equivalent number of purified R18-loaded MVs were bound to HeLa cells in the cold for 60 min. Virus-bound cells were then placed at 37°C in a pre-warmed cuvette containing media adjusted to either pH 7.4 or 5.0 while R18 fluorescence was monitored. After 3 min, cell media was adjusted back to neutral \ and R18 fluorescence monitoring continued. The above results supported the use of the fluorescent R18 probe for analyzing VACV-cell membrane fusion. In subsequent experiments we compared the effects of inhibitors on binding of virions to cells, fusion, and core entry as measured by LUC expression and in some cases by transmission electron microscopy.
Fusion Was Not Enhanced by Low pH or Greatly Reduced by Cholesterol Depletion
An earlier study had shown that fusion of VACV strain WR was not enhanced at low pH [40], which in retrospect seemed surprising in view of the subsequent demonstration of low pH enhancement of core entry and reporter gene expression [6]. Nevertheless, we confirmed the similar rates of VACV WR fusion following a brief incubation with a pH 7.4 or pH 5.0 buffer and return to neutral pH (Figure 2D). Furthermore, we found that bafilomycin A1, which prevents endosomal acidification and reduces firefly LUC expression, had little effect on binding of MVs containing a Gaussia LUC core protein chimera or membrane fusion (Figure 3A), similar to previous findings of membrane fusion in the presence of ammonium chloride and chloroquine [40]. Thus, low pH promotes an entry step beyond membrane lipid mixing.
Fig. 3. Effects of inhibitors on VACV-cell attachment, membrane fusion and core entry. 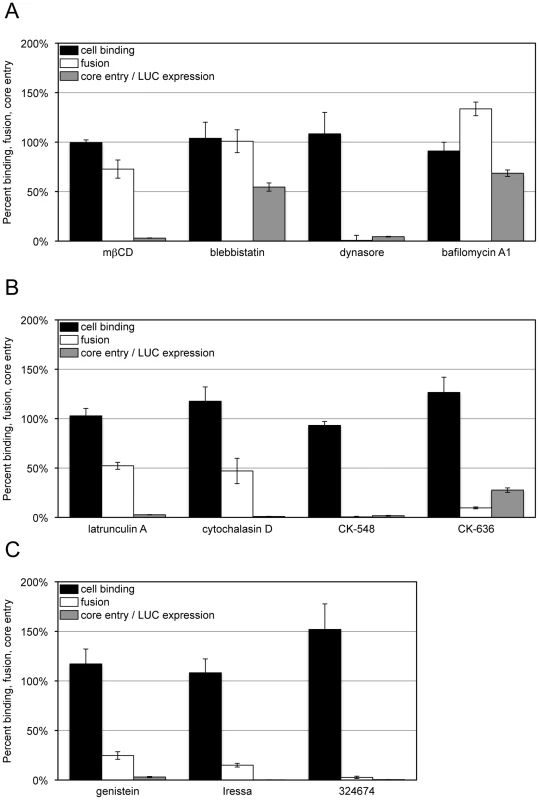
HeLa cells were left untreated or pre-treated for 30 min at 37°C with: (A) mßCD (10 mM), blebbistatin (75 µM), dynasore (100 µM) and bafilomycin A1 (50 nM); (B) latrunculin A (10 µM), cytochalasin D (10 µM), CK-548 (100 µM) and CK-636 (100 µM); (C) genestein (100 µM), Iressa (40 µM), and 32674 (40 µM). For cell binding (black bars), control and inhibitor-treated cells were incubated with equivalent numbers of VACV Gauss-A4 MVs at 4°C for 60 min. Unbound virions were removed by washing and cells lysed to measure cell-associated Gaussia LUC activity. For membrane fusion (white bars), control and inhibitor-treated cells were incubated with equivalent numbers of R18-loaded WRvFire particles at 4°C for 60 min. Washed cells were then incubated at 37°C for 40 min in the presence of the indicated inhibitor while R18 fluorescence was monitored. For core entry (gray bars), equivalent numbers of WRvFire MVs were adsorbed to control and inhibitor-treated cells at 4°C for 60 min. Cells were washed and incubated for 2 h at 37°C in the presence or absence of the indicated inhibitor. Cells were then lysed and firefly LUC activity in cell extracts measured. Data are represented as percent of the untreated cell control for each assay. Depletion of cellular cholesterol reversibly prevents the accumulation of VACV cores in the cytosol at a post-attachment step [41]. Treatment of HeLa cells with methyl - ß-cyclodextrin (mßCD) resulted in up to a 74% reduction in total cellular cholesterol levels (Figure S1A) without reducing cell viability over the time-course of the experiment (Figure S1B), although some cell rounding occurred. Nevertheless, MVs efficiently bound to cholesterol-depleted HeLa cells and R18 fluorescence was only mildly reduced, whereas LUC expression was greatly inhibited (Figure 3A). These data indicated that the lowered level of cellular cholesterol was sufficient for membrane lipid mixing but impaired a later step in entry or reporter gene expression.
Cellular Components Required for Virion Attachment, Membrane Fusion and Core Entry
Inhibitors targeting membrane blebbing, dynamin function, actin dynamics, and the activities of certain protein kinases have been shown to reduce VACV entry to varying extents as measured by reporter gene expression or detection of cytoplasmic cores [11]–[13], [16], [42]. In the present experiments, HeLa cells were preincubated for 30 min with inhibitors at previously used concentration ranges and the drugs were maintained in the medium during and after virus adsorption. Infection with VACV induces actin-enriched protrusions or cellular blebs [42] and entry can be partially reduced by blebbistatin, a small molecule specific inhibitor of myosin-II-dependent blebbing, virus movement along filopodia and macropinocytosis [11], [43], [44]. Blebbistatin was without effect on virion attachment but reduced LUC reporter expression by about 50% (Figure 3A), similar to the value previously reported for a GFP reporter assay [11]. However, we found little or no effect on dequenching of the R18 probe (Figure 3A), indicating that membrane fusion can occur independently of cell membrane blebbing.
Dynasore is a small molecule inhibitor of the GTPase activity of dynamin1, dynamin2 and the mitochondrial dynamin and is a rapid and potent inhibitor of dynamin-dependent endocytic pathways [45]. Dynamin also directly interacts with actin and regulates the actin cytoskeleton [46]–[48]. The effect of dynasore on VACV entry is ambiguous as it was reported not to influence entry in some studies [11] but to inhibit entry in another [16]. We found that dynasore had no effect on virion binding to HeLa cells but severely decreased LUC expression (Figure 3A). Moreover, dynasore potently inhibited membrane fusion (Figure 3B). These results implicated cellular dynamin as a critical factor in promoting VACV entry into HeLa cells at the membrane fusion step.
We also tested several specific inhibitors of actin dynamics: CK-636 and CK-548 bind to the Arp2/3 complex and prevent actin nucleation whereas latrunculins and cytochalasins bind actin and inhibit polymerization [49], [50]. These drugs had little effect on virion attachment but severely blocked LUC expression (Figure 3B). CK-548 and CK-636 were also very effective inhibitors of membrane fusion, whereas latrunculin A and cytochalasin D inhibited fusion by approximately 50% at the concentrations used (Figure 3B). These studies indicated a role for actin rearrangement in membrane fusion and raised the possibility that the effect of dynasore was related to its influence on the actin cytoskeleton rather than endocytosis.
Cell signaling has been reported to have a role in VACV entry at the stage of blebbing and macropinocytosis [11]. Genestein, gefitinib (Iressa) and 324674 (PD153035) are small molecule tyrosine kinase inhibitors [51], [52]. These drugs did not reduce virion binding but profoundly inhibited LUC expression (Figure 3C). Moreover, they also greatly inhibited membrane fusion (Figure 3C). The results could be related to the relative specificity of gefitinib and 324674 for epidermal growth factor receptor signaling, which causes rapid actin polymerization and rearrangement [53].
Based on a previous report [11], we attempted to bypass the effects of inhibitors of actin remodeling and signaling on entry by brief low pH treatment of cells with attached virions. However, in our hands, such treatments only alleviated the effects of drugs such as bafilomycin A1, concanamycin and monensin that prevented endosomal acidification [6] but did not bypass the effects of several other inhibitors on entry as measured by LUC expression or R18 dequenching (Figure S2).
Core entry steps were also analyzed by transmission electron microscopy. The results cannot be precisely compared to the above assays because a high virus multiplicity and spinoculation were used to allow counting of a sufficient number of virus particles in thin sections of infected cells. Hemifusion cannot be detected by this procedure and the earliest recognizable entry step consisted of full fusion of the viral and plasma membranes with an open pore allowing core entry (Figure 4A). Although MVs can be readily detected in vesicles, full fusion of viral and vesicle membranes are rarely seen (5). Cores that accumulate in the cytoplasm (Figure 4B) could have entered through the plasma membrane or an endocytic vesicle. In the absence of inhibitors, the number of plasma membrane full fusion images decreased and cores in the cytoplasm increased between 30 and 90 min (Figure 4C, D). At both times, the numbers of plasma membrane full fusion images (Figure 4C) and cytoplasmic cores (Figure 4D) were reduced when the cells were treated with blebbistatin, dynasore, latrunculin A or cytochalasin D. These observations confirmed the results obtained with the LUC assay for measuring core entry.
Fig. 4. Effects of inhibitors on entry determined by transmission electron microscopy. 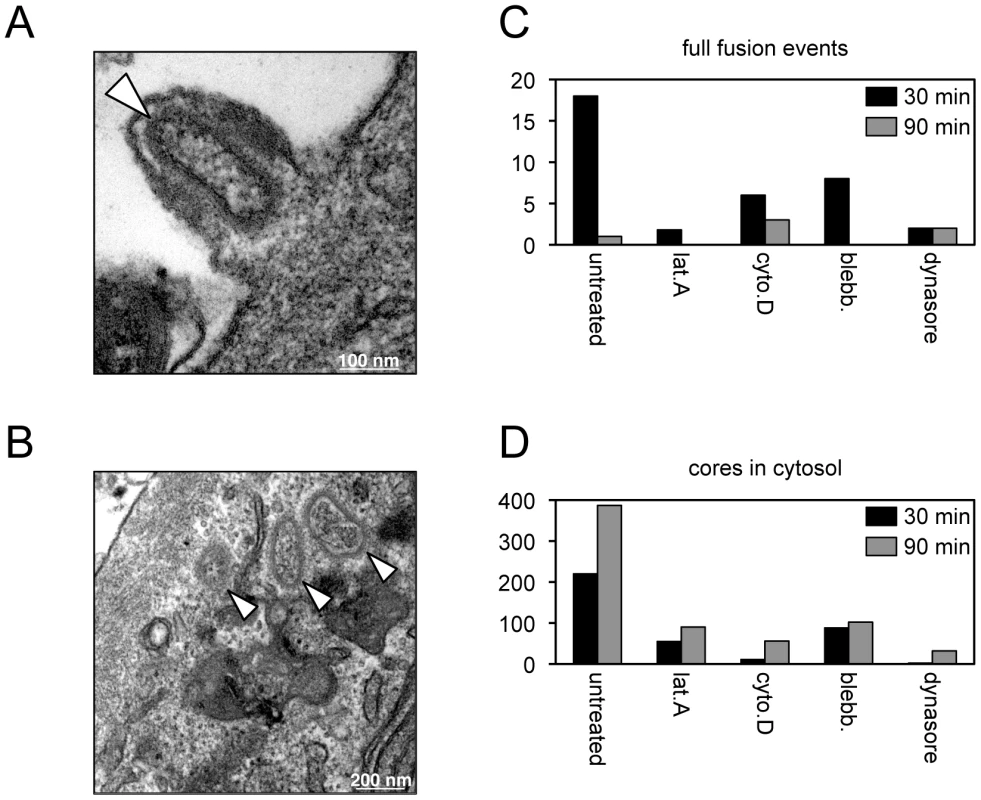
Purified MVs (350 PFU per cell) were spinoculated onto inhibitor-treated HeLa monolayers at 4°C for 60 min. Virus-bound cells were then incubated for either 30 or 90 min at 37°C in the presence or absence of the indicated inhibitor, fixed and processed for transmission electron microscopy. Representative images from untreated cells at 30 min showing full fusion of virion and plasma membranes resulting in pore formation (A) and cores in the cytosol (B). White arrowheads point to cores; scale bars indicate magnification. For each infection, a total of 90 randomly-selected cell sections were visualized and the number of plasma membrane full fusion events (C) and viral cores in the cytosol (D) were determined at 30 and 90 min. In summary, our data are generally consistent with other studies showing the importance of cell signaling and remodeling of the actin cytoskeleton on VACV entry [10]–[15], and importantly further demonstrate that these activities are necessary for the membrane fusion step. Low pH, cholesterol and membrane blebbing appear to be more important for entry steps beyond membrane lipid mixing.
Roles of EFC Proteins in Virus-Cell Membrane Fusion
Most or all of the MV membrane proteins required for entry, as distinguished from cell attachment, are components of the EFC (A16, A21, A28, G3, G9, H2, J5, L5, O3) or physically associated with the EFC (L1, F9). We employed conditional lethal mutants for all EFC and EFC-associated proteins except J5, for which a stringent mutant was unavailable. As a control, we tested a mutant with a deletion of the gene encoding the I5 MV membrane protein that is not required for entry [54]. The recombinant viruses were replicated in the presence or absence of the IPTG inducer and the MVs were purified by sucrose gradient sedimentation. For each mutant, the number of purified virions was determined from the optical density. In some cases, virions were inactivated at 56°C prior to adsorption to cells as an additional control [55]. Equivalent numbers of particles were loaded with R18 and washed by sedimentation to remove excess dye. Dye transfer to HeLa cells was determined by increased fluorescence as in the preceding sections. In addition parallel cultures were maintained for 48 h and the yield of infectious virus determined by plaque assay. As expected, R18-loaded MVs lacking the I5 protein (I5−) promoted R18 probe transfer as efficiently as wild type MV (I5+), whereas transfer was reduced with the heat-inactivated MVs (Figure 5A). Virions deficient in individual EFC and EFC-associated proteins had very low infectivity and except for A28, L1 and L5 mutants exhibited severely reduced R18 dequenching as well (Figure 5B-K), providing the first evidence of a direct role of EFC proteins in the membrane fusion step of virus entry. Previous studies had only shown that the EFC was required for fusion of infected cells.
Fig. 5. Effects of deficiencies of individual virion membrane proteins on membrane fusion and virus infectivity. 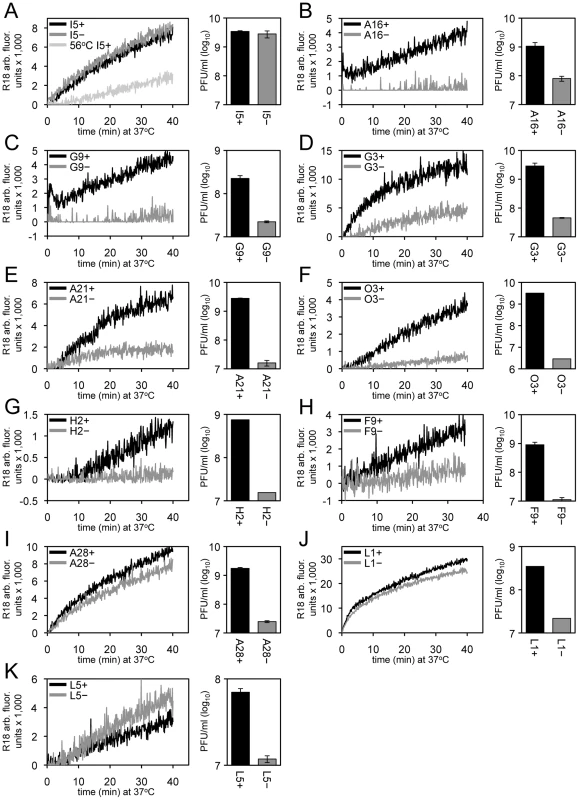
In each panel, equivalent numbers of purified R18-loaded MVs were bound to HeLa cells at 4°C for 60 min and unbound virions were removed by washing. Virus-bound cells were then incubated at 37°C for 40 min and R18 fluorescence was monitored and plotted as arbitrary units. Parallel cultures were incubated for 48 h and the yield of virus was determined by plaque assay. Recombinant viruses were as follows: (A) ΔI5L, (B) IPTG-inducible A16, (C) IPTG-inducible G9, (D) IPTG-inducible G3, (E) IPTG-inducible A21, (F) IPTG-inducible O3, (G) IPTG-inducible H2, (H) IPTG-inducible F9, (I) IPTG-inducible A28, (J) IPTG-inducible L1, and (K) IPTG-inducible L5. For panels B-K, the plus and minus signs in the upper left signifies the virus was grown in the presence or absence of IPTG, respectively. For panel A, the plus and minus refer to wild type virus and a deletion mutant, respectively. As a negative control, 56°C heat-inactivated I5+ virions (panel A) were assayed for hemifusion and infectivity (<105 PFU/ml; data not shown). We used transmission electron microscopy to monitor core entry steps, following attachment of H2+, H2−, A28+ and A28− virions. We chose H2 and A28 as examples of mutants that reduced and allowed R18 dequenching, respectively (Figure 5G, I). As indicated earlier, a high multiplicity and spinoculation was needed because of the thin cell sections. The lower numbers of full fusions with pore formation at the plasma membrane and cytoplasmic cores in cells infected with H2− virions compared to H2+ virions were expected in view of the inability of the former to mediate R18 dequenching (Figure 6A,B). However, there was a similar reduction in full fusion images at the plasma membrane and cytoplasmic cores after infection with A28− virions compared to A28+ virions (Figure 6C, D) despite the greater ability of the former to allow membrane fusion as determined by lipid mixing. Inhibition of core entry was previously shown using a confocal microscopy assay for virions deficient in L1 [32] and L5 [34] confirming an entry block despite their ability to allow lipid mixing as shown here.
Fig. 6. Entry of virions lacking H2 or A28 protein determined by transmission electron microscopy. 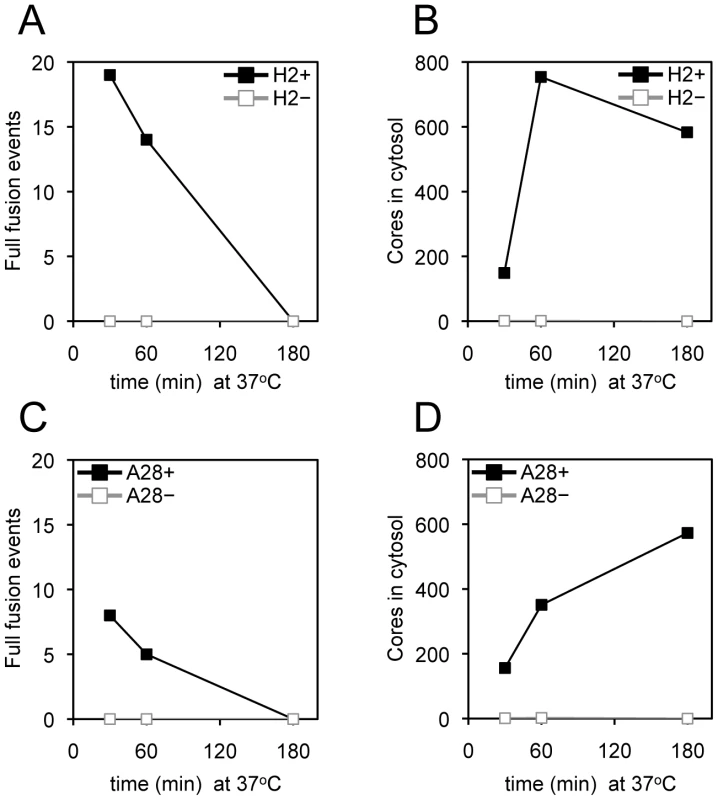
Purified MVs (350 PFU per cell or equivalent number of particles) possessing (+) or lacking (-) H2 or A28 protein as indicated were spinoculated onto pre-chilled HeLa cell monolayers at 4°C for 60 min. Virus-bound cells were then incubated for either 30, 60 or 180 min at 37°C, fixed and processed for electron microscopy. For each infection, a total of 90 randomly-selected cell sections were inspected and the number of full fusion events (A and C) and free viral cores in the cytosol (B and D) were determined as described in the legend to Figure 4. Neutralizing Antibody to L1 Inhibits Entry at a Step Beyond Membrane Lipid Mixing
The above results showing that L1-deficient virions allowed membrane fusion but not core entry led us to investigate the effect of a potent L1-neutralizing monoclonal antibody (MAb) [56]. We found that a concentration of L1 MAb that severely inhibited core entry as determined by LUC expression and formation of infectious virus had minimal effect on membrane fusion as determined by R18 dequenching (Figure 7). This result was confirmed by a flow cytometry-based 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine (DiD) lipid mixing assay using a wide-range of MAb concentrations (Figure S3).
Fig. 7. Effects of anti-L1 MAb on virus-cell membrane fusion, viral core entry and virus infectivity. 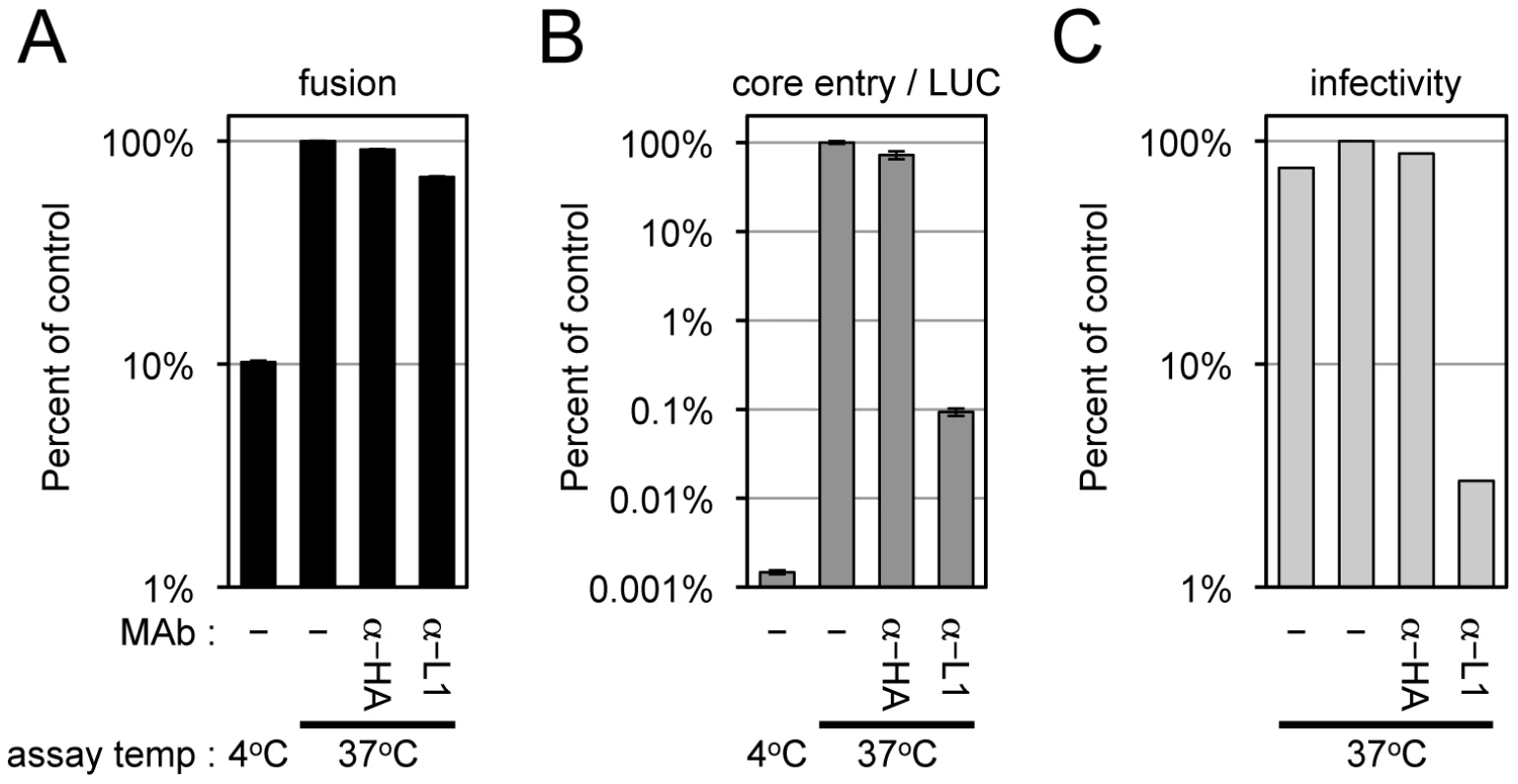
Equivalent numbers of R18-loaded virions (WRvFire) were incubated with or without 100 µg/ml of anti-L1 mouse MAb or control anti-HA mouse MAb for 30 min at room temperature. Virions were then assayed for their ability to mediate virus-cell membrane fusion by R18 dequenching (A) or core entry by LUC expression (B), at either 37°C or 4°C. Infectivity (C) was assayed by adsorbing each virus sample at 37°C to BS-C-1 monolayers for 60 min and enumerating plaque formation 48 h later. Data are represented as percent of the no MAb control at 37°C for each assay. Attempt to Trans-Complement an EFC Mutant
We still needed to consider the possibility that the role of the EFC is to activate the cell for virion entry rather than to directly participate in the entry step per se. In this context, Mercer and Helenius [11] had reported that very few VACV particles are needed to induce widespread blebbing and actin rearrangement. To further investigate the role of the EFC in entry, we coinfected cells with wild type VACV and either A28+ or A28− virions that expressed firefly LUC. We used a particle/cell multiplicity of approximately 200 for the A28+ and A28− virions and varied the multiplicity of the wild type virions from 9 to 1840 particles/cell (equivalent to 0.1 to 20 plaque forming units (PFU)/cell). Coinfection with wild type virions caused a two-fold increase in LUC expression by A28+ virions and raised expression about four-fold for A28− virions (Figure 8A). However, the latter was still only 3% of the value for A28+ virions indicating that efficient trans-complementation had not occurred. We also determined that soluble A28 protein [57] mixed with virions had no effect on entry of either the A28+ or A28− virions (Figure 8B).
Fig. 8. Attempt to trans-complement entry of virions lacking A28. 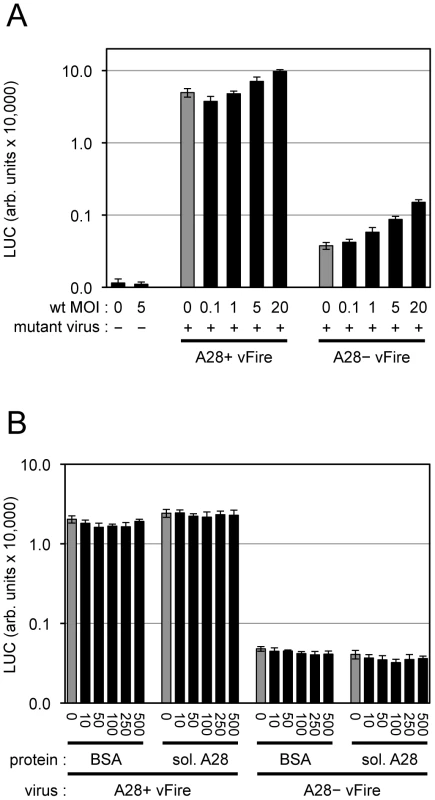
(A) Equivalent numbers of purified A28+ or A28− MVs expressing firefly LUC (WRvFire) were mixed with varying amounts of purified, wild type (wt) MVs as indicated. Virions were adsorbed to HeLa cell monolayers at 4°C for 60 min. Cells were washed and placed at 37°C for 2 h to allow virus entry. Cells were then lysed and firefly LUC activity measured. MOI (multiplicity of infection; PFUs per cell) of wt VACV is indicated. (B) Equivalent numbers of purified A28+ or A28− MVs expressing firefly LUC were mixed with varying amounts of bovine serum albumin (BSA) or soluble A28 protein as indicated (ng/ml). Virions were adsorbed to HeLa cell monolayers at 4°C for 60 min. Cells were washed and placed at 37°C for 2 h to allow for virus entry. Cells were then lysed and LUC activity measured. Discussion
Viral and cellular membranes each consists of two leaflets and in principal membrane fusion could occur by two different pathways as discussed by Chernomordik [1]. The direct fusion model posits that pores form in each of the apposing membranes and the pore rims join forming a fusion pore that allows lipid and content mixing in a single step. In contrast, the 2-step model posits fusion of the outer leaflets of the apposing membranes to form a hemifusion intermediate followed by merging of the inner leaflets to form the fusion pore. In the latter model, lipid mixing and content mixing occur sequentially. Evidence to support the second model involving a hemifusion intermediate has been obtained for several different viruses by demonstrating membrane lipid mixing without content mixing by mutation of viral fusion proteins, slowing or interrupting fusion with inhibitors and decreasing the surface density of viral fusion proteins [58]–[61]. In the present study of VACV, we showed that membrane lipid mixing could occur without core entry under three circumstances: depletion of certain EFC proteins (A28, L1 or L5), neutralization of VACV with a MAb to the L1 EFC-associated protein, and partial cholesterol depletion of the cell membrane. These findings are consistent with a 2-step entry model with a hemifusion intermediate for VACV.
In the first part of the Results, we described the effects of inhibitors of cell processes on virion attachment, membrane fusion and core entry. Most of the inhibitors had previously been shown to reduce entry as determined by reporter gene expression or detection of cytoplasmic cores [11]–[13], [16], [42]. We found that none of these inhibitors prevented binding of virions to cells, many reduced membrane fusion, while others only acted at the core entry step (Figure 9). The membrane fusion inhibitors were either directly involved with actin polymerization or remodeling (CK-636, CK-548, latrunculin A, cytochalasin D) or blocked tyrosine kinases that can modulate actin cytoskeletal changes (genestein, Iressa, 324674). The action of dynasore, a specific inhibitor of dynamin GTPase, could be due to its known effect on actin since there is evidence against a role for caveolae-mediated endocytosis in VACV entry [16]. Further evidence for dynamin2 in VACV core entry has been obtained with siRNA [16]. Extensive actin remodeling and mobilization has been observed during MV binding to cell surfaces [11], [16], [42] suggesting that actin-enriched membrane protrusions increase the intimacy of membrane contact and promote virus-cell membrane fusion. Actin remodeling has been suggested to facilitate fusion by forcing membranes together and enlarging pores in a variety of systems [62]–[64] including virus entry and viral protein-induced cell-cell fusion [65]–[70]. With human immunodeficiency virus, actin remodeling appears to have a more important role in pore expansion and content mixing than in hemifusion [71], [72]. We found that cytochalasin D and latrunculin A had a greater inhibitory effect on core entry (determined by LUC expression) than membrane fusion as determined by lipid mixing, suggesting that actin dynamics may be required for multiple steps in VACV entry.
Fig. 9. Summary of effects of inhibitors on VACV entry. 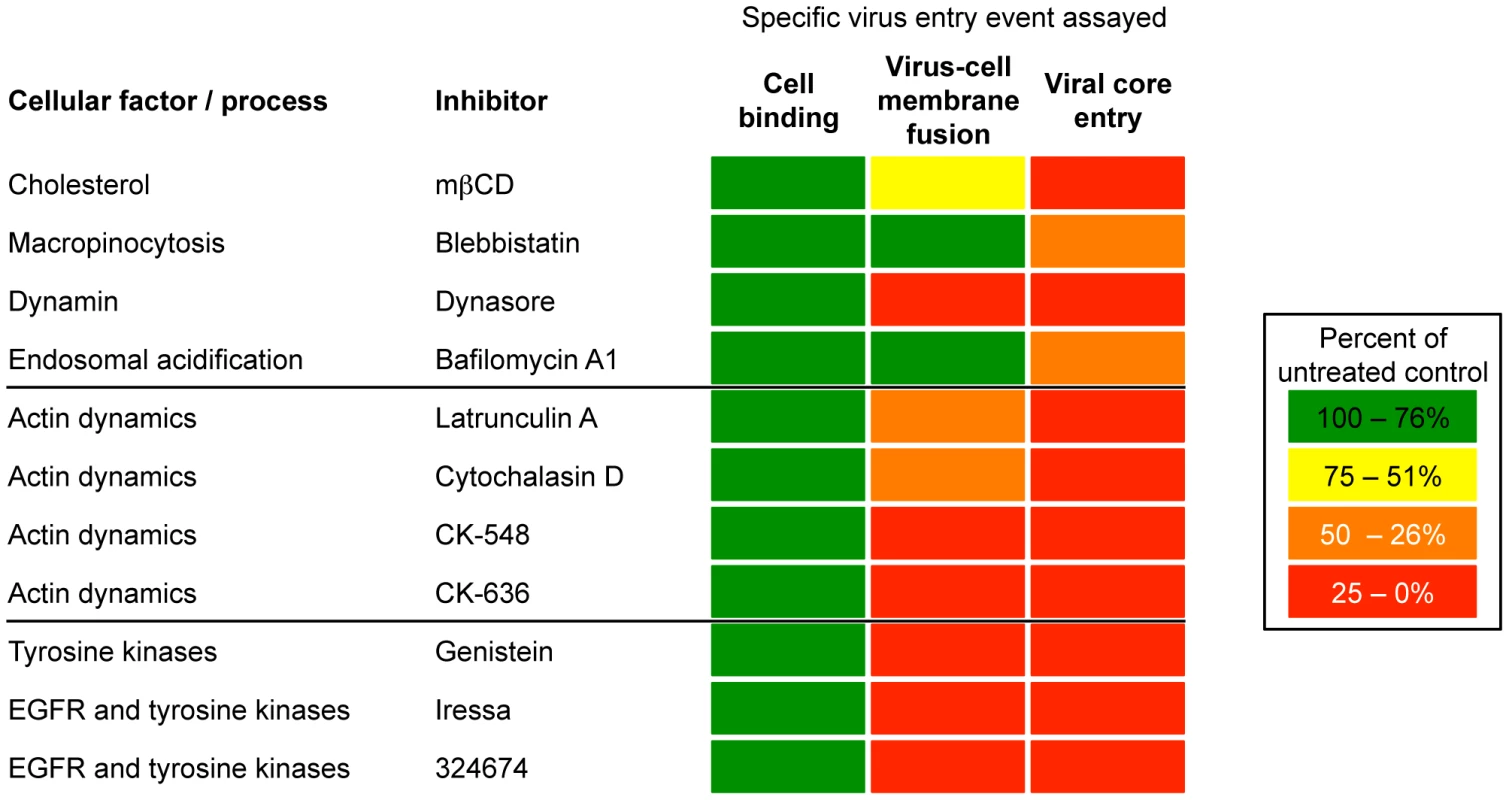
Cell binding, virus-cell membrane fusion, and viral core entry were assessed as described in the text and as depicted in Figures 1 and 3. The inhibitors are grouped according to their best-characterized effects but may also perturb cells in other ways. In contrast to the role of actin rearrangement, inhibitors that prevented membrane blebbing involved in virus surfing and macropinocytosis or that interfered with the reduction in pH of endosomes, had a much greater effect on core entry than membrane lipid mixing (Figure 9). It will be important to determine whether lipid mixing is occurring at the plasma membrane or in endosomes at neutral pH. Similarly, a 74% reduction of cellular cholesterol with mßCD had little effect on membrane fusion but had a major effect on core entry as measured by LUC expression. A previous study had shown that MVs associate with cholesterol-rich regions of the plasma membrane and that cholesterol depletion reduced VACV entry as measured by visualizing cores in the cytoplasm [41]. In studies with influenza virus and Semliki Forest virus in insect cells, which can be more stringently depleted of cholesterol than mammalian cells, both hemifusion and pore widening were affected [73], [74]. The cell surface receptors for certain viruses reside in cholesterol-rich lipid rafts, but receptors for VACV have not been identified.
The VACV EFC proteins were previously shown to be required for virus core entry and cell-cell fusion but evidence for a role in the fusion of viral and cell membranes had been indirect. Of the ten EFC or EFC-associated mutants tested in the present study, all were blocked in core entry as determined by infectivity or transmission electron microscopy and seven of these were unable to mediate membrane fusion. The three proteins apparently not required for membrane fusion were A28, L1, and L5. It is possible that these proteins have a specific role at a later step in entry such as pore formation. However, in other systems it has been shown that the density of activated fusion proteins has to be higher for the formation and expansion of a fusion pore than for hemifusion [1]. Although these three mutants each display stringent repression of EFC protein expression as shown by Western blotting, undetectable differences could affect the sensitive lipid-mixing assay. Therefore, our main conclusion is that the EFC is required for membrane fusion and that additional studies are required to conclude that A28, L1 and L5 have a specific role at a later step of entry such as pore formation.
The L1 protein is a target of potent neutralizing and protective antibodies [56], [75]. The structure of L1 alone and in association with a conformation-specific MAb has been solved to high resolution [76], [77]. The Fab fragment binds to a discontinuous epitope containing two loops that are held together by a disulfide bond. Here we showed that the MAb prevents VACV entry at a step beyond lipid mixing, consistent with the effect on entry of virions deficient in the L1 protein.
Since our inhibitor studies had shown that actin dynamics are required for membrane fusion and core entry, we considered the possibility that the EFC indirectly promotes entry by inducing cell signaling. Indeed, such a role could contribute to the need for multiple EFC proteins. Since Mercer and Helenius [11] had shown that cell signaling requires few virus particles, we tried to rescue EFC protein-deficient virions in trans by coinfecting with wild type VACV. Although wild type virus enhanced core entry by four-fold as measured by LUC expression, this value was still only 3% of that achieved by the control virus, suggesting that the EFC proteins have a direct role in membrane fusion and entry. Nevertheless, whether EFC protein interactions also cause signaling is an interesting question for future studies.
Why so many different proteins are needed for poxvirus entry remains an enigma. None of the proteins resemble type I or type II viral fusion proteins by sequence so that determination of the 3-dimensional structure of the VACV EFC may be needed to define putative fusion loops, if the mechanism of entry involves such structures. At this time, only the structure of the L1 EFC-associated protein has been solved [76].
Materials and Methods
Cells and Viruses
African green monkey kidney BS-C-1 and human HeLa cells were maintained in minimum essential medium with Earle's salts (EMEM) supplemented with 2.5% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin (Quality Biological). The recombinant VACV WRvFire expressing firefly LUC under a synthetic early/late VACV promoter was described previously [6]. Recombinant VACVs in which expression of individual EFC or EFC-associated proteins are IPTG-inducible have been previously constructed and characterized: A16 [23], A21 [24], A28 [25], G3 (A. Townsley and BM, unpublished), G9 [28], H2 [29], J5 [31], L5 [34], O3 [33], L1 [32], and F9 [26]. The recombinant VACV in which the I5L gene was deleted has been described [54]. The recombinant VACV Gauss-A4 (parental strain WRvFire), which expresses the Gaussia LUC enzyme fused to the A4 core protein was generated as follows. Overlap polymerase chain reaction (PCR) was utilized to generate a construct in which the Gaussia LUC gene (New England Biolabs) was appended to the N-terminal codon of the VACV A4L gene and the EGFP coding region (and accompanying synthetic early/late VACV promoter sequence) was placed downstream of the Gaussia-A4L region. To achieve homologous recombination, flanking genomic sequences of A4L (approximately 500 bp in length) were appended to the termini of the PCR product. HeLa cells were infected with 0.05 PFU of WRvFire per cell and at 2 h post infection were transfected with 400 ng of purified PCR product using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. At 24 h post infection, the infected cells were lysed by five freeze/thaw cycles and clonally purified five times by picking GFP positive plaques on BS-C-1 cells. The recombinant VACV in which A28L is IPTG-inducible and expresses firefly LUC under a synthetic early/late VACV promoter has been described [25].
Purification and Quantitation of Virus Particles
BS-C-1 cells were infected with VACV in the presence or absence of the inducer IPTG (Calbiochem) and at 48 to 72 h post infection MVs were isolated as described [78], [79]. Briefly, infected cells were subjected to Dounce homogenization and MVs were purified by sedimentation through two 36% (wt/vol) sucrose cushions followed by one sedimentation on a 25 to 40% (wt/vol) continuous sucrose gradient; the visible virus band was collected, and virus was pelleted and stored at −80°C. Upon thawing, virus was sonicated on ice for 1 min. The infectious viral titer (PFU per ml) for each purified MV stock of recombinant VACV was determined by plaque assay on BS-C-1 cells as described [80]. Additionally, the number of total virus particles obtained for each purified MV stock of recombinant VACV was estimated from the optical density at 260 nm [80].
R18 Loading of Virus Particles and Fusion Assay
Purified MVs (approximately 9.0×109 particles) were labeled with 3 ml of 1 mg/ml of R18 (Molecular Probes) in phosphate-buffered saline (PBS; Quality Biological) + 0.2% bovine serum albumin (BSA; Sigma-Aldrich) for 20 min at room temperature in the dark. Non-incorporated R18 was removed by pelleting virions (16,000 x g for 10 min at 4°C) and washing several times in PBS + 0.2% BSA. R18-labeled virions were re-suspended in PBS + 0.2% BSA, vortexed, and sonicated for 15 sec on ice. Virions sufficient to achieve a multiplicity of 1 to 5 PFU (or the equivalent number of non-infectious particles) per cell were then incubated with approximately 1.5×106 HeLa cells in suspension for 1 h at 4°C in cold fusion medium comprised of EMEM without phenol red and with 10 mM N-2-hydroxyethylpiperazine-N'-2-ethanesulfonicacid (HEPES) and 10 mM 2-(N-morpholino)ethanesulfonic acid (pH 7.4) in the dark. Virus-bound cells were washed twice with cold fusion medium following low-speed centrifugation (750 x g for 3 min at 4°C). Virus-bound cells were injected into a cuvette containing fusion medium pre-warmed to 37°C and kept in suspension utilizing a magnetic stir bar. R18 fluorescence (560 nm excitation and 590 nm emission) was monitored by use of a Fluoro-Max3 spectrofluorometer (Horiba Jobin Yvon) outfitted with a Peltier sample cooler (Horiba Jobin Yvon) and a temperature control unit (Wavelength Electronics model LFI-3751) to maintain the desired temperature within the chamber housing the sample cuvette. For graphical presentation, the raw fluorescence data were plotted versus time. For quantitative comparisons, we determined the percent fluorescence by dividing the value obtained at 40 min by the value obtained following addition of Triton X-100 (1% [wt/vol] final concentration).
LUC Core Entry Assay
HeLa cells seeded in 24-well plates (2.0×105 cells per well) were chilled to 4°C before virus adsorption. WRvFire MVs were adsorbed in cold EMEM + 2.5% FBS for 1 h at 4°C. Cells were washed with cold PBS to remove unbound virions and incubated with pre-warmed EMEM + 2.5% FBS for 2 h (unless indicated otherwise) at 37°C. Cells were washed with PBS and then incubated with Cell Culture Lysis Reagent (Promega) for 30 min at room temperature with gentle agitation. LUC activity in cellular extracts was measured according to the manufacturer's protocol (Promega) and quantified on a Berthold Sirius luminometer (Berthold Detection Systems).
Cholesterol Depletion of Target Cells
HeLa cells seeded in 24-well plates (2.0×105 cells per well) were left untreated or treated with 10 mM mßCD (Sigma-Aldrich) for 30 min in EMEM at 37°C. Cells were then washed with cold PBS and cold EMEM was added to cells prior to virus adsorption at 4°C for R18 hemifusion or LUC entry assays as described above. Cholesterol levels in HeLa cells were determined using the Amplex Red Cholesterol Assay Kit (Molecular Probes) and was performed according to the manufacturer's protocol. The viability of mßCD-treated cells was assayed using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega) and was performed according to the manufacturer's protocol.
Inhibitor Treatments
HeLa cells were left untreated or pre-treated with the indicated concentrations of inhibitors: Sigma-Aldrich: blebbistatin (75 µM), dynasore (100 µM), bafilomycin A1 (50 nM), latrunculin A (10 µM), cytochalasin D (10 µM), CK-548 (100 µM), CK-636 (100 µM), genistein (100 µM); LC Laboratories: Iressa (40 µM); EMD4Biosciences: 324674 (40 µM) for 30 min at 37°C. Cells were then chilled to 4°C prior to virus adsorption for virus-cell binding, R18 hemi-fusion, or LUC assays as described. The indicated drug concentrations were maintained throughout the assay.
Virus-Cell Binding Assay
Equivalent amounts of VACV Gauss-A4 virions (5 PFU per cell) were incubated with untreated or inhibitor-treated HeLa cells in 24-well plates at neutral pH for 1 h at 4°C. Cells were washed twice with cold PBS to remove unbound virus. Cells were then incubated with LUC assay lysis buffer (Promega) for 30 min at room temperature with gentle agitation. Gaussia LUC activity in cellular extracts was measured according to the manufacturer's protocol (Promega) and quantified on a Berthold Sirius luminometer (Berthold Detection Systems).
Stimulation of Virus Entry by Low pH Treatment
Low pH stimulation of virus entry was performed as described previously [6]. Following a wash to remove unbound virions, cells were incubated for 3 min in 37°C PBS with Ca2+ and Mg2+ at pH 7.4 or PBS with Ca2+ and Mg2+ supplemented with 1 mM 2-morpholinoethane-sulfonic acid adjusted to pH 5.0 with HCl. After removal of buffers, the pH was neutralized by one wash with EMEM + 2.5% FBS. Cells were incubated in pre-warmed EMEM + 2.5% FBS for 2 h at 37°C and then prepared for the LUC entry assay as described above.
Transmission Electron Microscopy
BS-C-1 cells in six-well tissue culture plates (1.0×105 cells per well) were pre-chilled at 4°C for 30 min prior to virus spinoculation. Purified MVs (350 PFU per cell or equivalent number of particles) in cold EMEM + 2.5% FBS were sedimented onto the BS-C-1 cells at 4°C for 1 h at 650 x g in a Legend RT centrifuge (Sorvall). Cells were washed with cold PBS to remove unbound virions and incubated with pre-warmed EMEM + 2.5% FBS for varying amounts of time at 37°C. At the indicated time, the samples were fixed on ice with 4% paraformaldehyde (Electron Microscopy Sciences) in 0.1 M phosphate buffer for 10 min and processed for transmission electron microscopy as described previously [6]. For quantitation of virus entry events, ninety randomly selected cell sections were visualized and particles therein counted.
MAb Neutralization
Equivalent numbers of R18-loaded MV particles (recombinant strain WRvFire) were incubated with 100 µg/ml of anti-L1 mouse MAb 7D11 [56] or control anti-HA mouse monoclonal (clone 16B12, Covance) for 30 min at room temperature. Virion and antibody mixtures were then divided and used for R18-based fusion, LUC core entry, or plaque formation assays as described above.
DiD Loading of Virus Particles and Fusion Assay
Purified MVs (approximately 9.0×109 particles) were labeled with 3 µl of DiD (Molecular Probes) in phosphate-buffered saline (PBS; Quality Biological) + 0.2% bovine serum albumin (BSA; Sigma-Aldrich) for 20 min at room temperature in the dark. Non-incorporated DiD was removed by pelleting virions (16,000 x g for 10 min at 4°C) and washing several times in PBS + 0.2% BSA. DiD-labeled virions were re-suspended in PBS + 0.2% BSA, vortexed, and sonicated for 15 sec on ice. Virions sufficient to achieve a multiplicity of 1 to 5 PFU per cell were then incubated with approximately 8.0×104 HeLa cells in a 48-well plate for 90 min at 37°C in minimum essential medium with Earle's salts (EMEM) supplemented with 2.5% FBS, 2 mM L-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin. Cells were washed with PBS, trypsinized, spun and fixed in 4% paraformaldehyde/PBS for 2 h at 4°C. DiD-positive cells were quantified using a FACSCalibur (BD Biosciences). DiD loading had minimal effect on virus infectivity as measured by plaque assay.
Supporting Information
Zdroje
1. ChernomordikLVKozlovMM 2005 Membrane hemifusion: crossing a chasm in two leaps. Cell 123 375 382
2. WhiteJMDelosSEBrecherMSchornbergK 2008 Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme. Crit Rev Biochem Mol Biol 43 189 219
3. HeldweinEEKrummenacherC 2008 Entry of herpesviruses into mammalian cells. Cell Mol Life Sci 65 1653 1668
4. ArmstrongJAMetzDHYoungMR 1973 The mode of entry of vaccinia virus into L cells. J Gen Virol 21 533 537
5. CarterGCLawMHollinsheadMSmithGL 2005 Entry of the vaccinia virus intracellular mature virion and its interactions with glycosaminoglycans. J Gen Virol 86 1279 1290
6. TownsleyACWeisbergASWagenaarTRMossB 2006 Vaccinia virus entry into cells via a low pH-dependent-endosomal pathway. J Virol 80 8899 8908
7. BengaliZTownsleyACMossB 2009 Vaccinia virus strain differences in cell attachment and entry. Virology 389 132 140
8. WhitbeckJCFooC-HPonce de LeonMEisenbergRJCohenGH 2009 Vaccinia virus exhibits cell-type-dependent entry characteristics. Virology 385 383 391
9. BengaliZSatheshkumarPSYangZWeisbergASParanN 2011 Drosophila S2 cells are non-permissive for vaccinia virus DNA replication following entry via low pH-dependent endocytosis and early transcription. PLoS One 6 e17248
10. MossB 2006 Poxvirus entry and membrane fusion. Virology 344 48 54
11. MercerJHeleniusA 2008 Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science 320 531 535
12. MercerJKnebelSSchmidtFICrouseJBurkardC 2010 Vaccinia virus strains use distinct forms of macropinocytosis for host-cell entry. Proc Natl Acad Sci U S A 107 9346 9351
13. MoserTSJonesRGThompsonCBCoyneCBCherryS 2010 A kinome RNAi screen identified AMPK as promoting poxvirus entry through the control of actin dynamics. PLoS Pathog 6 e1000954
14. SandgrenKJWilkinsonJMiranda-SaksenaMMcInerneyGMByth-WilsonK 2010 A differential role for macropinocytosis in mediating entry of the two forms of vaccinia virus into dendritic cells. PLoS Pathog 6 e1000866
15. VillaNYBarteeEMohamedMRRahmanMMBarrettJW 2010 Myxoma and vaccinia viruses exploit different mechanisms to enter and infect human cancer cells. Virology 401 266 279
16. HuangCYLuTYBairCHChangYSJwoJK 2008 A novel cellular protein, VPEF, facilitates vaccinia virus penetration into HeLa cells through fluid phase endocytosis. J Virol 82 7988 7999
17. LawMCarterGCRobertsKLHollinsheadMSmithGL 2006 Ligand-induced and non-fusogenic dissolution of a viral membrane. Proc Natl Acad Sci USA 103 5989 5994
18. IchihashiY 1996 Extracellular enveloped vaccinia virus escapes neutralization. Virology 217 478 485
19. ChungC-SHsiaoJ-CChangY-SChangW 1998 A27L protein mediates vaccinia virus interaction with cell surface heparin sulfate. J Virol 72 1577 1585
20. HsiaoJCChungCSChangW 1998 Cell surface proteoglycans are necessary for A27L protein - mediated cell fusion: Identification of the N-terminal region of A27L protein as the glycosaminoglycan-binding domain. J Virol 72 8374 8379
21. LinCLChungCSHeineHGChangW 2000 Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J Virol 74 3353 3365
22. ChiuWLLinCLYangMHTzouDLMChangW 2007 Vaccinia virus 4c (A26L) protein on intracellular mature virus binds to the extracellular cellular matrix laminin. J Virol 81 2149 2157
23. OjedaSSenkevichTGMossB 2006 Entry of vaccinia virus and cell-cell fusion require a highly conserved cysteine-rich membrane protein encoded by the A16L gene. J Virol 80 51 61
24. TownsleyASenkevichTGMossB 2005 Vaccinia virus A21 virion membrane protein is required for cell entry and fusion. J Virol 79 9458 9469
25. SenkevichTGWardBMMossB 2004 Vaccinia virus A28L gene encodes an essential protein component of the virion membrane with intramolecular disulfide bonds formed by the viral cytoplasmic redox pathway. J Virol 78 2348 2356
26. BrownESenkevichTGMossB 2006 Vaccinia virus F9 virion membrane protein is required for entry but not virus assembly, in contrast to the related l1 protein. J Virol 80 9455 9464
27. IzmailyanRAHuangCYMohammadSIsaacsSNChangW 2006 The envelope G3L protein is essential for entry of vaccinia virus into host cells. J Virol 80 8402 8410
28. OjedaSDomiAMossB 2006 Vaccinia virus G9 protein is an essential component of the poxvirus entry-fusion complex. J Virol 80 9822 9830
29. SenkevichTGMossB 2005 Vaccinia virus H2 protein is an essential component of a complex involved in virus entry and cell-cell fusion. J Virol 79 4744 4754
30. NicholsRJStanitsaEUngerBTraktmanP 2008 The vaccinia I2L gene encodes a membrane protein with an essential role in virion entry. J Virol 82 10247 10261
31. SenkevichTGOjedaSTownsleyANelsonGEMossB 2005 Poxvirus multiprotein entry-fusion complex. Proc Natl Acad Sci USA 102 18572 18577
32. BishtHWeisbergASMossB 2008 Vaccinia virus L1 protein is required for cell entry and membrane fusion. J Virol 82 8687 8694
33. SatheshkumarPSMossB 2009 Characterization of a newly Identified 35 amino acid component of the vaccinia virus entry/fusion complex conserved in all chordopoxviruses. J Virol 83 12822 12832
34. TownsleyASenkevichTGMossB 2005 The product of the vaccinia virus L5R gene is a fourth membrane protein encoded by all poxviruses that is requried for cell entry and cell-cell fusion. J Virol 79 10988 10998
35. ConditRCMoussatcheNTraktmanP 2006 In a nutshell: structure and assembly of the vaccinia virion. Adv Virus Res 66 31 124
36. NelsonGEWagenaarTRMossB 2008 A conserved sequence within the H2 subunit of the vaccinia virus entry/fusion complex is Important for interaction with the A28 subunit and infectivity. J Virol 82 6244 6250
37. WolfeCLMossB 2011 Interaction between the G3 and L5 proteins of the vaccinia virus entry-fusion complex. Virology 412 278 283
38. WagenaarTROjedaSMossB 2008 Vaccinia virus A56/K2 fusion regulatory protein interacts with the A16 and G9 subunits of the entry fusion complex. J Virol 82 5153 5160
39. LoyterACitovskyVBlumenthalR 1988 The use of fluorescence dequenching measurements to follow viral membrane fusion events. Methods Biochem Anal 33 129 164
40. DomsRWBlumenthalRMossB 1990 Fusion of intra - and extracellular forms of vaccinia virus with the cell membrane. J Virol 64 4884 4892
41. ChungCSHuangCYChangW 2005 Vaccinia virus penetration requires cholesterol and results in specific viral envelope proteins associated with lipid rafts. J Virol 79 1623 1634
42. LockerJKKuehnASchleichSRutterGHohenbergH 2000 Entry of the two infectious forms of vaccinia virus at the plasma membrane is signaling-dependent for the IMV but not the EEV. Mol Biol Cell 11 2497 2511
43. LimouzeJStraightAFMitchisonTSellersJR 2004 Specificity of blebbistatin, an inhibitor of myosin II. J Muscle Res Cell Motil 25 337 341
44. LehmannMJShererNMMarksCBPypaertMMothesW 2005 Actin - and myosin-driven movement of viruses along filopodia precedes their entry into cells. J Cell Biol 170 317 325
45. MaciaEEhrlichMMassolRBoucrotEBrunnerC 2006 Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell 10 839 850
46. GuCYaddanapudiSWeinsAOsbornTReiserJ 2010 Direct dynamin-actin interactions regulate the actin cytoskeleton. EMBO J 29 3593 3606
47. KruchtenAEMcNivenMA 2006 Dynamin as a mover and pincher during cell migration and invasion. J Cell Sci 119 1683 1690
48. PraefckeGJMcMahonHT 2004 The dynamin superfamily: universal membrane tubulation and fission molecules? Nat Rev Mol Cell Biol 5 133 147
49. BrownSSSpudichJA 1981 Mechanism of action of cytochalasin: evidence that it binds to actin filament ends. J Cell Biol 88 487 491
50. YarmolaEGSomasundaramTBoringTASpectorIBubbMR 2000 Actin-latrunculin A structure and function. Differential modulation of actin-binding protein function by latrunculin A. J Biol Chem 275 28120 28127
51. AkiyamaTIshidaJNakagawaSOgawaraHWatanabeS 1987 Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 262 5592 5595
52. FryDWKrakerAJMcMichaelAAmbrosoLANelsonJM 1994 A specific inhibitor of the epidermal growth factor receptor tyrosine kinase. Science 265 1093 1095
53. RijkenPJHageWJvan Bergen en HenegouwenPMVerkleijAJBoonstraJ 1991 Epidermal growth factor induces rapid reorganization of the actin microfilament system in human A431 cells. J Cell Sci 100 Pt 3 491 499
54. SoodCLWardJMMossB 2008 Vaccinia virus encodes a small hydrophobic virion membrane protein (I5) that enhances replication and virulence in mice. J Virol 82 10071 10078
55. DalesSKajiokaR 1964 The cycle of multiplication of vaccinia virus in Earle's strain L cells. I. Uptake and penetration. Virology 24 278 294
56. WolffeEJVijayaSMossB 1995 A myristylated membrane protein encoded by the vaccinia virus L1R open reading frame is the target of potent neutralizing monoclonal antibodies. Virology 211 53 63
57. NelsonGESislerJRChandranDMossB 2008 Vaccinia virus entry/fusion complex subunit A28 is a target of neutralizing and protective antibodies. Virology 380 394 401
58. KembleGWDanieliTWhiteJM 1994 Lipid-anchored influenza hemagglutinin promotes hemifusion, not complete fusion. Cell 76 383 391
59. ArmstrongRTKushnirASWhiteJM 2000 The transmembrane domain of influenza hemagglutinin exhibits a stringent length requirement to support the hemifusion to fusion transition. J Cell Biol 151 425 437
60. ZavorotinskayaTQianZFranksJAlbrittonLM 2004 A point mutation in the binding subunit of a retroviral envelope protein arrests virus entry at hemifusion. J Virol 78 473 481
61. ZaitsevaEMittalAGriffinDEChernomordikLV 2005 Class II fusion protein of alphaviruses drives membrane fusion through the same pathway as class I proteins. J Cell Biol 169 167 177
62. ZhengQAChangDC 1991 Reorganization of cytoplasmic structures during cell fusion. J Cell Sci 100 Pt 3 431 442
63. EitzenG 2003 Actin remodeling to facilitate membrane fusion. Biochim Biophys Acta 1641 175 181
64. MassarwaRCarmonSShiloBZSchejterED 2007 WIP/WASp-based actin-polymerization machinery is essential for myoblast fusion in Drosophila. Dev Cell 12 557 569
65. KallewaardNLBowenALCroweJEJr 2005 Cooperativity of actin and microtubule elements during replication of respiratory syncytial virus. Virology 331 73 81
66. GowerTLPasteyMKPeeplesMECollinsPLMcCurdyLH 2005 RhoA signaling is required for respiratory syncytial virus-induced syncytium formation and filamentous virion morphology. J Virol 79 5326 5336
67. PontowSEHeydenNVWeiSRatnerL 2004 Actin cytoskeletal reorganizations and coreceptor-mediated activation of rac during human immunodeficiency virus-induced cell fusion. J Virol 78 7138 7147
68. SchowalterRMWurthMAAguilarHCLeeBMoncmanCL 2006 Rho GTPase activity modulates paramyxovirus fusion protein-mediated cell-cell fusion. Virology 350 323 334
69. StantchevTSMarkovicITelfordWGClouseKABroderCC 2007 The tyrosine kinase inhibitor genistein blocks HIV-1 infection in primary human macrophages. Virus Res 123 178 189
70. HarmonBRatnerL 2008 Induction of the Galpha(q) signaling cascade by the human immunodeficiency virus envelope is required for virus entry. J Virol 82 9191 9205
71. HarmonBCampbellNRatnerL 2010 Role of Abl kinase and the Wave2 signaling complex in HIV-1 entry at a post-hemifusion step. PLoS Pathog 6 e1000956
72. MiyauchiKKimYLatinovicOMorozovVMelikyanGB 2009 HIV enters cells via endocytosis and dynamin-dependent fusion with endosomes. Cell 137 433 444
73. PhalenTKielianM 1991 Cholesterol is required for infection by Semliki Forest virus. J Cell Biol 112 615 623
74. BiswasSYinSRBlankPSZimmerbergJ 2008 Cholesterol promotes hemifusion and pore widening in membrane fusion induced by influenza hemagglutinin. J Gen Physiol 131 503 513
75. LustigSFoggCWhitbeckJCEisenbergRJCohenGH 2005 Combinations of polyclonal or monoclonal antibodies to proteins of the outer membranes of the two infectious forms of vaccinia virus protect mice against a lethal respiratory challenge. J Virol 79 13454 13462
76. SuHPGarmanSCAllisonTJFoggCMossB 2005 The 1.51-A structure of the poxvirus L1 protein, a target of potent neutralizing antibodies. Proc Natl Acad Sci USA 102 4240 4245
77. SuHPGoldenJWGittisAGHooperJWGarbocziDN 2007 Structural basis for the binding of the neutralizing antibody, 7D11, to the poxvirus L1 protein. Virology 368 331 341
78. EarlPLMossB 1998 Characterization of recombinant vaccinia viruses and their products. AusubelFMBrentRKingstonREMooreDDSeidmanJG Current Protocols in Molecular Biology New York Greene Publishing Associates & Wiley Interscience 16.18.11 16.18.11
79. EarlPLCooperNWyattLSMossBCarrollMW 1998 Preparation of cell cultures and vaccinia virus stocks. AusubelFMBrentRKingstonREMooreDDSeidmanJG Current Protocols in Molecular Biology New York John Wiley and Sons 16.16.11 16.16.13
80. EarlPLMossBWyattLSCarrollMW 1998 Generation of recombinant vaccinia viruses. AusubelFMBrentRKingstonREMooreDDSeidmanJG Current Protocols in Molecular Biology New York Greene Publishing Associates & Wiley Interscience 16.17.11 16.17.19
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Genesis of Mammalian Prions: From Non-infectious Amyloid Fibrils to a Transmissible Prion DiseaseČlánek Role of Permissive Neuraminidase Mutations in Influenza A/Brisbane/59/2007-like (H1N1) VirusesČlánek Allelic Variation on Murine Chromosome 11 Modifies Host Inflammatory Responses and Resistance toČlánek Multifaceted Regulation of Translational Readthrough by RNA Replication Elements in a TombusvirusČlánek Latent KSHV Infection of Endothelial Cells Induces Integrin Beta3 to Activate Angiogenic PhenotypesČlánek Controlling Viral Immuno-Inflammatory Lesions by Modulating Aryl Hydrocarbon Receptor Signaling
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2011 Číslo 12- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Inhibition of Apoptosis and NF-κB Activation by Vaccinia Protein N1 Occur via Distinct Binding Surfaces and Make Different Contributions to Virulence
- Genesis of Mammalian Prions: From Non-infectious Amyloid Fibrils to a Transmissible Prion Disease
- Kaposi's Sarcoma Herpesvirus microRNAs Target Caspase 3 and Regulate Apoptosis
- Nutritional Immunology: A Multi-Dimensional Approach
- Role of Permissive Neuraminidase Mutations in Influenza A/Brisbane/59/2007-like (H1N1) Viruses
- Vaccinomics and Personalized Vaccinology: Is Science Leading Us Toward a New Path of Directed Vaccine Development and Discovery?
- Symbiont Infections Induce Strong Cytoplasmic Incompatibility in the Tsetse Fly
- Allelic Variation on Murine Chromosome 11 Modifies Host Inflammatory Responses and Resistance to
- Computational and Biochemical Analysis of the Effector AvrBs2 and Its Role in the Modulation of Type Three Effector Delivery
- Granzyme B Inhibits Vaccinia Virus Production through Proteolytic Cleavage of Eukaryotic Initiation Factor 4 Gamma 3
- Association of Activating KIR Copy Number Variation of NK Cells with Containment of SIV Replication in Rhesus Monkeys
- Fungal Virulence and Development Is Regulated by Alternative Pre-mRNA 3′End Processing in
- versus the Host: Remodeling of the Bacterial Outer Membrane Is Required for Survival in the Gastric Mucosa
- Follicular Dendritic Cell-Specific Prion Protein (PrP) Expression Alone Is Sufficient to Sustain Prion Infection in the Spleen
- Autophagy Protein Atg3 is Essential for Maintaining Mitochondrial Integrity and for Normal Intracellular Development of Tachyzoites
- Longevity and Composition of Cellular Immune Responses Following Experimental Malaria Infection in Humans
- Sequential Adaptive Mutations Enhance Efficient Vector Switching by Chikungunya Virus and Its Epidemic Emergence
- Acquisition of Pneumococci Specific Effector and Regulatory Cd4 T Cells Localising within Human Upper Respiratory-Tract Mucosal Lymphoid Tissue
- The Meaning of Death: Evolution and Ecology of Apoptosis in Protozoan Parasites
- Deficiency of a Niemann-Pick, Type C1-related Protein in Is Associated with Multiple Lipidoses and Increased Pathogenicity
- Feeding Cells Induced by Phytoparasitic Nematodes Require γ-Tubulin Ring Complex for Microtubule Reorganization
- Eight RGS and RGS-like Proteins Orchestrate Growth, Differentiation, and Pathogenicity of
- Prion Uptake in the Gut: Identification of the First Uptake and Replication Sites
- Nef Decreases HIV-1 Sensitivity to Neutralizing Antibodies that Target the Membrane-proximal External Region of TMgp41
- Multifaceted Regulation of Translational Readthrough by RNA Replication Elements in a Tombusvirus
- A Temporal Role Of Type I Interferon Signaling in CD8 T Cell Maturation during Acute West Nile Virus Infection
- The Membrane Fusion Step of Vaccinia Virus Entry Is Cooperatively Mediated by Multiple Viral Proteins and Host Cell Components
- HIV-1 Capsid-Cyclophilin Interactions Determine Nuclear Import Pathway, Integration Targeting and Replication Efficiency
- Neonatal CD8 T-cell Hierarchy Is Distinct from Adults and Is Influenced by Intrinsic T cell Properties in Respiratory Syncytial Virus Infected Mice
- Two Novel Transcriptional Regulators Are Essential for Infection-related Morphogenesis and Pathogenicity of the Rice Blast Fungus
- Five Questions about Non-Mevalonate Isoprenoid Biosynthesis
- The Human Cytomegalovirus UL11 Protein Interacts with the Receptor Tyrosine Phosphatase CD45, Resulting in Functional Paralysis of T Cells
- Wall Teichoic Acids of Limit Recognition by the Drosophila Peptidoglycan Recognition Protein-SA to Promote Pathogenicity
- A Novel Role for the NLRC4 Inflammasome in Mucosal Defenses against the Fungal Pathogen
- Inflammasome-dependent Pyroptosis and IL-18 Protect against Lung Infection while IL-1β Is Deleterious
- CNS Recruitment of CD8+ T Lymphocytes Specific for a Peripheral Virus Infection Triggers Neuropathogenesis during Polymicrobial Challenge
- Latent KSHV Infection of Endothelial Cells Induces Integrin Beta3 to Activate Angiogenic Phenotypes
- A Receptor-based Switch that Regulates Anthrax Toxin Pore Formation
- Targeting of Heparin-Binding Hemagglutinin to Mitochondria in Macrophages
- Chikungunya Virus Neutralization Antigens and Direct Cell-to-Cell Transmission Are Revealed by Human Antibody-Escape Mutants
- Ce-Duox1/BLI-3 Generated Reactive Oxygen Species Trigger Protective SKN-1 Activity via p38 MAPK Signaling during Infection in
- Structural Elucidation and Functional Characterization of the Effector Protein ATR13
- Controlling Viral Immuno-Inflammatory Lesions by Modulating Aryl Hydrocarbon Receptor Signaling
- SAMHD1-Deficient CD14+ Cells from Individuals with Aicardi-Goutières Syndrome Are Highly Susceptible to HIV-1 Infection
- Acid Stability of the Hemagglutinin Protein Regulates H5N1 Influenza Virus Pathogenicity
- Cryo Electron Tomography of Herpes Simplex Virus during Axonal Transport and Secondary Envelopment in Primary Neurons
- A Novel Human Cytomegalovirus Locus Modulates Cell Type-Specific Outcomes of Infection
- Juxtamembrane Shedding of AMA1 Is Sequence Independent and Essential, and Helps Evade Invasion-Inhibitory Antibodies
- Pathogenesis and Host Response in Syrian Hamsters following Intranasal Infection with Andes Virus
- IRGM Is a Common Target of RNA Viruses that Subvert the Autophagy Network
- Epstein-Barr Virus Evades CD4 T Cell Responses in Lytic Cycle through BZLF1-mediated Downregulation of CD74 and the Cooperation of vBcl-2
- Quantitative Multicolor Super-Resolution Microscopy Reveals Tetherin HIV-1 Interaction
- Late Repression of NF-κB Activity by Invasive but Not Non-Invasive Meningococcal Isolates Is Required to Display Apoptosis of Epithelial Cells
- Polar Flagellar Biosynthesis and a Regulator of Flagellar Number Influence Spatial Parameters of Cell Division in
- Epstein-Barr Virus Nuclear Antigen 3C Stabilizes Gemin3 to Block p53-mediated Apoptosis
- The Enteropathogenic (EPEC) Tir Effector Inhibits NF-κB Activity by Targeting TNFα Receptor-Associated Factors
- Toward an Integrated Model of Capsule Regulation in
- A Systematic Screen to Discover and Analyze Apicoplast Proteins Identifies a Conserved and Essential Protein Import Factor
- A Host Small GTP-binding Protein ARL8 Plays Crucial Roles in Tobamovirus RNA Replication
- Comparative Pathobiology of Fungal Pathogens of Plants and Animals
- Synergistic Roles of Eukaryotic Translation Elongation Factors 1Bγ and 1A in Stimulation of Tombusvirus Minus-Strand Synthesis
- Engineered Immunity to Infection
- Inflammatory Monocytes and Neutrophils Are Licensed to Kill during Memory Responses
- Sialidases Affect the Host Cell Adherence and Epsilon Toxin-Induced Cytotoxicity of Type D Strain CN3718
- Eurasian-Origin Gene Segments Contribute to the Transmissibility, Aerosol Release, and Morphology of the 2009 Pandemic H1N1 Influenza Virus
- SARS Coronavirus nsp1 Protein Induces Template-Dependent Endonucleolytic Cleavage of mRNAs: Viral mRNAs Are Resistant to nsp1-Induced RNA Cleavage
- Identification and Characterization of a Novel Non-Structural Protein of Bluetongue Virus
- Functional Analysis of the Kinome of the Wheat Scab Fungus
- Norovirus Regulation of the Innate Immune Response and Apoptosis Occurs via the Product of the Alternative Open Reading Frame 4
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Controlling Viral Immuno-Inflammatory Lesions by Modulating Aryl Hydrocarbon Receptor Signaling
- Fungal Virulence and Development Is Regulated by Alternative Pre-mRNA 3′End Processing in
- Epstein-Barr Virus Nuclear Antigen 3C Stabilizes Gemin3 to Block p53-mediated Apoptosis
- Engineered Immunity to Infection
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



