Sequestration of MBNL1 Protein by Mutant ZNF9 mRNA in Lymphocytes of Patients with Myotonic Dystrophy Type 2
Sekvestrace MBNL1 proteinu mutovaným ZNF9 mRNA v lymfocytech pacientů s myotonickou dystrofií 2. typu
Myotonická dystrofie 2. typu (DM2) je způsobena (CCTG)n expanzí v ZNF9 genu lokalizovaném na dlouhém raménku chromozomu 3. Patogeneze DM2 zahrnuje sekvestraci muscleblind-like (MBNL) proteinu patologicky expandovaným transkriptem ZNF9 mRNA, což vede k abnormálnímu sestřihu cílových pre-mRNA a následně k rozvoji patologického fenotypu. V naší práci demonstrujeme expresi transkriptu ZNF9, proteinu ZNF9 a proteinu MBNL1 v lymfocytech periferní krve jak u non-DM2 kontrolních pacientů, tak u DM2 pacientů. V lymfocytech pacientů s DM2 jsme prokázali částečnou kolokalizaci a sekvestraci proteinu MBNL1 na expandovanou ZNF9 mRNA. Předpokládáme, že i nesvalové tkáně exprimující transkript ZNF9 mohou být u pacientů s DM2 postiženy podobným molekulárním mechanismem jako kosterní sval. Přítomnost expandovaného transkriptu může být užitečná v rychlé diagnostice z nátěrů periferní krve u pacientů s podezřením na DM2, nabízí se i možnost sledování efektu molekulární terapie.
Klíčová slova:
myotonická dystrofie – lymfocyty – sekvestrace MBNL1 – expandovaný transkript ZNF9
Authors:
O. Soucek 1; S. Voháňka 2; J. Zaoralkova 1; I. Falková 1,3; R. Hrabálková 1; M. Falk 3; Z. Lukas 1
Authors‘ workplace:
Institute of Pathology, Faculty Hospital Brno and Masaryk University, Brno
1; Institute of Neurology, Faculty Hospital Brno and Masaryk University, Brno
2; Institute of Biophysics, Academy of Sciences of the Czech Republic, Brno
3
Published in:
Cesk Slov Neurol N 2012; 75/108(5): 606-609
Category:
Short Communication
Overview
Myotonic dystrophy type 2 (DM2) results from the (CCTG)n expansion in the ZNF9 gene located on the long arm of chromosome 3. The pathogenesis of DM2 includes sequestration of muscleblind-like (MBNL) proteins by pathological CCUGexp ZNF9 mRNA transcripts, leading to abnormal splicing of target pre-mRNAs and, consequently, to the development of the pathological phenotype. In this report, we demonstrate expression of the ZNF9 transcript, ZNF9 protein as well as MBNL1 protein in lymphocytes of non-DM controls and DM2 patients. In DM2 patients lymphocytes, MBNL1 protein is co-localized and partly sequestered in CCUGexp ZNF9 mRNA intranuclear foci. We suppose that non-muscle tissues expressing ZNF9 transcript in DM2 patients might be affected by a similar molecular mechanism as the skeletal muscle. The presence of the expanded ZNF9 transcript in peripheral blood lymphocytes may be useful for rapid diagnosis of DM2 from blood smears of suspected patients, or for a follow-up of patients treated with molecular therapy.
Key words:
myotonic dystrophy – lymphocytes – MBNL1 sequestration – ZNF9 expanded transcript
Introduction
Both myotonic dystrophy type 1 (DM1) and 2 (DM2) are autosomal dominant, multisystem disorders sharing a similar pathogenetic pathway that begins with an expansion of CTG and CCTG nucleotide repeats in the genes encoding dystrophia myotonica protein kinase (DMPK) and zinc-finger protein 9 (ZNF9), respectively [1,2]. The current pathogenetic model of myotonic dystrophy (DM) involves an interaction of CUG/CCUGexp mRNA of the DMPK/ZNF9 genes with CUG/CCUG--binding proteins. Transcripts with CUGexp and CCUGexp repeats are retained in the nuclei and sequester CUG-binding proteins, leading to an abnormal splicing of their target pre-mRNAs [3,4]. There are two groups of CUG-binding proteins. The MBNL1 protein from the MBNL family promotes transition of splicing from fetal to adult exons. The CUG-binding protein (CUG-BP1), on the other hand, helps to retain fetal exons. In DM2, MBNL1 is sequestered on CCUGexp ZNF9 mRNA that accumulates as ribonuclear foci. Sequestration of the MBNL proteins then prevents their activity at specific targets and this results in aberrant transcription and splicing of a selected group of pre-mRNAs transcripts. Recently, we demonstrated sequestration of the MBNL1 protein by an expanded ZNF9 transcript in soft tissue of DM2 patients and expression of ZNF9 transcript and MBNL1 protein in non-DM controls [12]. In this report, we extend the study by a molecular genetic examination of peripheral blood lymphocytes from DM2 patients and non-DM controls. So far, an interaction of CUG/CCUGexp mRNA of the DMPK/ZNF9 genes with CUG/CCUG-binding proteins in blood cells has not been studied. We aimed to demonstrate that sequestration of MBNL1 protein also occurs in blood cells (lymphocytes). We suppose that non-muscle tissues expressing ZNF9 transcript in DM2 patients might be affected by similar molecular mechanism as the skeletal muscle.
Material and methods
Whole human peripheral blood samples from five patients with DM2 and three non-DM controls were diluted 1 : 1 (with saline). Lymphocytes were isolated using the LymphoprepTM (Axis-Shield PoC AS, Oslo, Norway) solution according to the manufacturer’s instructions.
Muscle biopsies containing skeletal muscle fibers, vascular endothelia and smooth muscle cells from both DM2 patients and non-DM controls were tested as controls.
MB1a monoclonal antibody supplied by Professor Glenn Morris, MDA, Monoclonal antibody resource, Wolfson CIND, RJAH Orthopaedic Hospital, Oswestry, UK was used to detect MBNL1 protein.
The probe to the expanded CCUGexp transcript (5’-CAGG CAGG CAGG CAGG CAGG CAGG CAGG-3’2’-O-Me-NA5’Fl, scale 200 nmol) and to the CUGexp transcript (5’-CAG CAG CAG CAG CAG CAG CAG-3’2’-O-Me-RNA5’Fl) were supplied by Generi Biotech s.r.o., Czech Republic.
Probe to ZNF9 transcript: for the exon in situ hybridization studies, PCR products specific to either exon 1 or exon 5 were generated using the following primers: ZNF9 exon 1F, ZNF9 exon 1R; ZNF9 exon 5F and ZNF9 exon 5R were prepared by Generi Biotech s.r.o., Czech Republic. In this study, we used probes specific to exon 5, conjugated with Cy3 fluorochrome.
Polyclonal anti-ZNF9 protein antibody (Moravian-Biotech, Czech Republic) was obtained by immunization of a rabbit with a 20 amino-acid peptide from the C-terminus of human ZNF9 (CYRCGESGLHARECTIEATA) that includes the seventh zinc finger.
Fluorescence in situ hybridization (FISH) for ZNF9 RNA and immunofluorescence for ZNF9 and MBNL1 proteins in the blood smears and frozen muscle sections were performed as described by Holt et al [5].
Results
In various tissues from non-DM controls, immunoblotting of the ZNF9 protein detected by the polyclonal anti-ZNF9 antibody showed a typical band at the level of 19 kDa, corresponding to the ZNF9 protein molecular weight (Fig. 1). ZNF9 mRNA was found as granular cytoplasmic deposits in non-DM blood lymphocytes (Fig. 2a) and strong reactivity was identified in the sarcoplasm of skeletal muscle controls (Fig. 2b). The ZNF9 protein also displayed fine granular deposits in the sarcoplasm of muscle fibers (Fig. 2c). Positive immunoreactivity was also found in the cytoplasm of the vascular endothelia and smooth muscle cells, Schwann cells, and adipocytes (not demonstrated) [12]. A weak reactivity – a thin chain of perinuclear granules – was observed in peripheral blood lymphocytes (Fig. 2d). The MBNL1 protein from non-DM controls was found as irregular deposits in the nucleoplasm (not demonstrated).


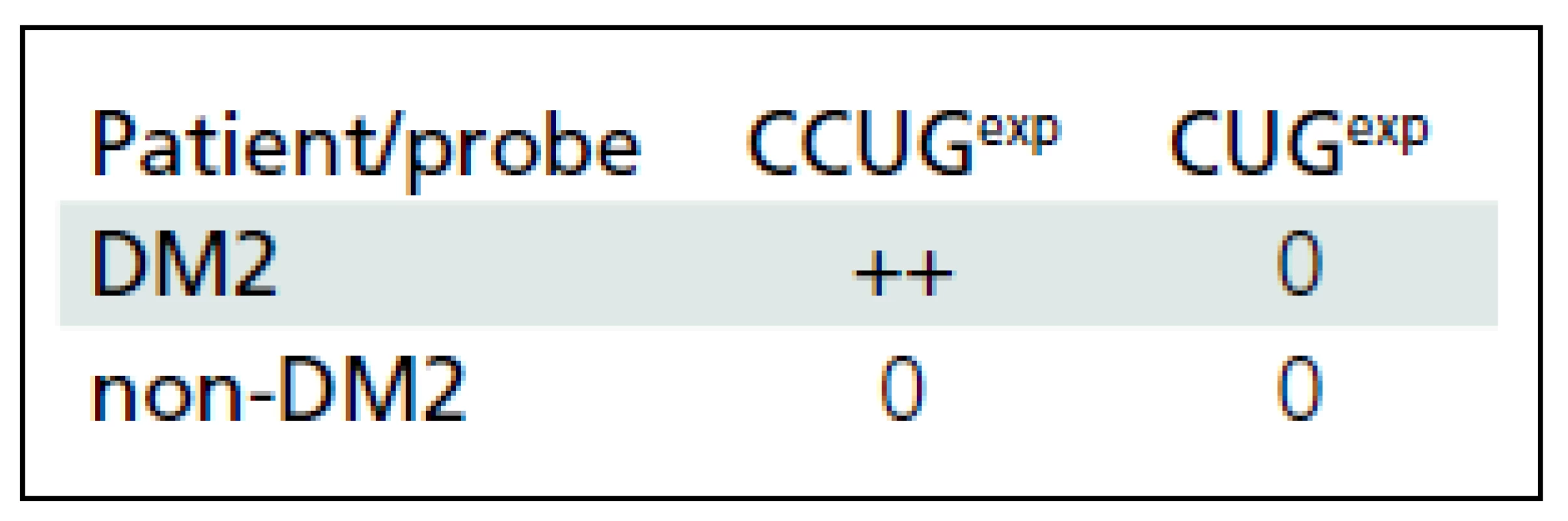
CCUGexp ZNF9 mRNA was found in lymphocytes of DM2 patients in a form of 1 to 4 distinct intranuclear foci (Fig. 3a). The MBNL protein from DM2 patients (Fig. 3b) was also present as intranuclear foci. Double label tests (immunofluorescence and FISH) demonstrated that only a portion of intranuclear MBNL1 signals were sequestered in the ribonuclear foci, while the rest of the protein was localized as extrafocal, finely granular deposits (Fig. 4). No foci were identified in lymphocytes of non-DM2 control patients (Tab. 1).


Discussion
The ZNF9 gene is a ubiquitously expressed gene but the level of expression of the ZNF9 transcript, splicing of ZNF9 pre-mRNA, ZNF9 protein expression or post-transcriptional modification in human tissues is largely unknown. ZNF9 protein is highly conserved at the amino acid and nucleotide levels in human, mouse, rat, chicken, and frog tissues. It is expressed in a variety of animal tissues [6]. In humans, expression of CCUGexp ZNF9 mRNA or co-localization of CCUGexp ZNF9 mRNA foci with MBNL1 protein was reported in muscle tissue and cell cultures [7,8] and the level of expression was extensively studied in skeletal muscle and myoblast cell lines only [8]. Recently, expression of the ZNF9 transcript and protein and sequestration of the MBNL1 protein by expanded CCUGexp ZNF9 mRNA transcript in soft tissues from DM2 patients and expression of the ZNF9 transcript and MBNL1 protein in non-DM controls were analyzed in human soft tissue and epidermal derivatives [12].
To address the issue of the role of ZNF9 in DM2, the effects of (CCTG)n expansion on ZNF9 expression in lymphoblastoid cell lines from DM2 patients were analyzed by Botta et al [9] but ZNF9 expression or MBNL1 protein sequestration in peripheral blood cells were not reported. On the other hand, DMPK gene expression was identified in lymphocytes of adult-onset patients with DM1 and normal controls [10]. These results suggest that expression of the two alleles at the DMPK locus in lymphocytes is coordinated and reduction at mutant-allele transcript levels is compensated by an increase in wild-type allele mRNA levels. It would be interesting to know whether similar compensatory mechanisms take place in lymphocytes from DM2 patients.
MBNL1 regulates terminal differentiation of cells through alternative splicing control and it also participates in differentiation of photo-receptors, neurons, adipocytes and blood cell types [11]. Our double label tests (immunofluorescence and FISH) demonstrated that only a portion of intranuclear MBNL1 signals were sequestered in the ribonuclear foci, while the rest of the protein was localized as extrafocal deposits. Sequestration of the MBNL1 protein is the core feature in the development of the myotonic dystrophy phenotype. In this report, we demonstrated that sequestration of MBNL1 protein also takes place in the blood cells (lymphocytes). It is a question whether, and to what extent, sequestration of MBNL1 in blood cells may contribute to DM2 pathology.
Conclusion
The ZNF9 gene is expressed in peripheral blood lymphocytes: the presence of ZNF9 transcript as well as MBNL1 protein were demonstrated by in situ hybridization and immunofluorescence/immunoblotting methods, respectively. The MBNL1 protein colocalizes with and is partially sequestered in intranuclear CCUGexp foci. Possible toxic effect of MBNL1 sequestration in lymphocytes on the DM2 phenotype is currently unknown. Detection of CCUGexp foci in peripheral blood smears of suspected patients may be useful in the diagnosis of DM2 – or during follow-up of patients treated with molecular therapy.
This report was supported by grant No. NS/9877-4, Ministry of Health of the Czech Republic.
Ondrej Soucek, MD
Institute of Pathology, Faculty Hospital Brno and Masaryk University
Jihlavska 20
625 00 Brno
e-mail: osoucek@fnbrno.cz
Accepted for review: 19. 1. 2012
Accepted for print: 5. 3. 2012
Sources
1. Taneja KL, McCurrach M, Schalling M, Housman D, Singer RH. Foci of trinucleotide repeat transcripts in nuclei of myotonic dystrophy cells and tissues. J Cell Biol 1995; 128(6): 995–1002.
2. Liquori CL, Ricker K, Moseley ML, Jacobsen JF, Kress W, Naylor SL et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science 2001; 293(5531): 864–867.
3. Day JW, Ranum LP. RNA pathogenesis of the myotonic dystrophies. Neuromuscul Disord 2005; 15(1): 5–16.
4. Osborne RJ, Thornton CA. RNA-dominant diseases. Hum Mol Genet 2006; 15(2): R162–R169.
5. Holt I, Jacquemin V, Fardaei M, Sewry CA, Butler-Browne GS, Furling D et al. Muscleblind-like proteins: similarities and differences in normal and myotonic dystrophy muscle. Am J Pathol 2009; 174(1): 216–227.
6. Botta A, Vallo L, Rinaldi F, Bonifazi E, Amati F, Biancolella M et al. Gene expression analysis in myotonic dystrophy: indications for a common molecular pathogenic pathway in DM1 and DM2. Gene Expr 2007; 13(6): 339–351.
7. Lucchiari S, Pagliarani S, Corti S, Mancinelli E, Servida M, Fruguglietti E et al. Colocalization of ribonuclear inclusions with muscle blind like-proteins in a family with myotonic dystrophy type 2 associated with a short CCTG expansion. J Neurol Sci 2008; 275(1–2): 159–163.
8. Cardani R, Baldassa S, Botta A, Rinaldi F, Novelli G, Mancinelli E et al. Ribonuclear inclusions and MBNL1 nuclear sequestration do not affect myoblast differentiation but alter gene splicing in myotonic dystrophy type 2. Neuromuscul Disord 2009; 19(5): 335–343.
9. Botta A, Caldarola S, Vallo L, Bonifazi E, Fruci D, Gullotta F et al. Effect of the [CCTG]n repeat expansion on ZNF9 expression in myotonic dystrophy type II (DM2). Biochim Biophys Acta 2006; 1762(3): 329–334.
10. Depardon F, Cisneros B, Alonso-Vilatela E, Montañez C. Myotonic dystrophy protein kinase (DMPK) gene expression in lymphocytes of patients with myotonic dystrophy. Arch Med Res 2001; 32(2): 123–128.
11. Pascual M, Vicente M, Monferrer L, Artero R. The Muscle blind family of proteins: an emerging class of regulators of developmentally programmed alternative splicing. Differentiation 2006; 74(2–3): 65–80.
12. Lukáš Z, Falk M, Feit J, Souček O, Falková I, Štefančíková L et al. Sequestration of MBNL1 in tissues of patients with myotonic dystrophy type 2. Neuromuscul Disord 2012; 22(7): 604
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2012 Issue 5
- Advances in the Treatment of Myasthenia Gravis on the Horizon
- Memantine in Dementia Therapy – Current Findings and Possible Future Applications
- Memantine Eases Daily Life for Patients and Caregivers
-
All articles in this issue
- Can Intracranial Venous Reflux Be Detected from Transcondylar Approach? The Results of a Fusion Imaging Study
- Closure of Calvarial Defect with Custom-made Biocompatible Implant in a Pediatric Patient – a Case Report
- Motor Stereotypies in Childhood – Case Reports
- Therapy of Spinal Paragangliomas – Case Reports
- Amyotrophic Lateral Sclerosis in a Palliative Hospice Care Setting – a Case Report
- Idiopathic Aqueductal Stenosis and Developmental Speech Disorder in Children with Neurofibromatosis von Recklinghausen type 1 – Two Case Reports
- Neurological Complications Associated with Assisted Reproductive Technology – a Case Report
- The Effect of Small Amounts of Wine or Other Alcohol Drinks on Human Health and Longevity
- Emotional Memory – Pathophysiology and Clinical Associations
- Endovascular Therapy of Intracranial Aneurysms – Methods, Indications, Complications
- Validity and Predictive Value of Screening Tests in Prediabetic and Early Diabetic Polyneuropathy
- Incidence and Risk Factors of Postoperative Delirium
- Safety and Efficacy of Intravenous Thrombolytic Therapy of Cerebral Infarction in Patients over 80 Years of Age
- Cerebrospinal Fluid Triplet in the Diagnosis of Alzheimer-Fischer disease
- Cognitive Deficit And Contralateral Frontal Hypoperfusion In Patients With Cerebellar Lesions
- Baha as a Solution for Single-Sided Deafness after Vestibular Schwannoma Surgery
- Sequestration of MBNL1 Protein by Mutant ZNF9 mRNA in Lymphocytes of Patients with Myotonic Dystrophy Type 2
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Motor Stereotypies in Childhood – Case Reports
- Emotional Memory – Pathophysiology and Clinical Associations
- Neurological Complications Associated with Assisted Reproductive Technology – a Case Report
- Cerebrospinal Fluid Triplet in the Diagnosis of Alzheimer-Fischer disease
