Placement Accuracy of Deep Brain Stimulation Electrodes using the NexFrame© Frameless System
Přesnost uložení elektrod pro hlubokou mozkovou stimulaci pomocí bezrámového systému NexFrame©
Úvod:
Hluboká mozková stimulace využívá k přesnému cílení jader v oblasti bazálních ganglií přímé zobrazení magnetickou rezonancí, intraoperační microrecording nebo dané anatomické koordináty. K zavedení elektrod lze využít rámový nebo bezrámový stereotaktický systém. Pro správnou funkci hluboké mozkové stimulace je zásadní co nejpřesnější uložení elektrody v daném jádru, a proto jsme provedli vyhodnocení souboru pacientů operovaných bezrámovým stereotaktickým systémem NexFrame©.
Metodika:
Koordináty plánovaného cílového bodu získáváme pomocí předoperační magnetické rezonance a finální pozici uložení modifikujeme na základě microrecordingu a klinického testování. Souřadnice pooperační pozice elektrod kontrolujeme na základě CT vyšetření počítačově fúzovaného s předoperační plánovací magnetickou rezonancí. K určení přesnosti uložení elektrody byla počítána celková chyba vč. chyb v laterální, vertikální a AP ose.
Výsledky:
Celkový počet 70 elektrod byl implantován pomocí systému NexFrame© v období červen 2013 až leden 2016 u 35 pacientů s diagnózou Parkinsonovy nemoci, dystonie nebo esenciálního třesu. Celková chyba byla 1,64 ± 0,81 mm, chyba v laterální ose byla 1,03 ± 0,79 mm, chyba v AP ose byla 1,14 ± 0,95 mm a chyba ve vertikální ose byla 1,05 ± 0,91 mm. Výsledky naší studie byly porovnány s publikovanými studiemi vč. výčtu možných chyb při implantaci při použití jak rámového, tak bezrámového systému. Výsledkem studie je závěr, že bezrámový systém NexFrame© je plně srovnatelný s rámovými systémy.
Klíčová slova:
Parkinsonova nemoc – dystonie – esenciální třes – hluboká mozková stimulace – bezrámový system
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Authors:
D. Krahulík 1
; M. Nevrlý 2; P. Otruba 2; J. Bardoň 2; L. Hrabálek 1; M. Vaverka 1; P. Kaňovský 2
Authors‘ workplace:
Department of Neurosurgery, Faculty of Medicine Palacky University and University Hospital Olomouc
1; Department of Neurology, Faculty of Medicine Palacky University and University Hospital Olomouc
2
Published in:
Cesk Slov Neurol N 2017; 80/113(2): 208-212
Category:
Short Communication
doi:
https://doi.org/10.14735/amcsnn2017208
Overview
Background:
Various methods are used to target nuclei of basal ganglia, including direct visualization of preoperative magnetic resonance images, intraoperative microelectrode recording and anatomical target coordinates. A frame-based stereotaxy or a frameless stereotactic system (NexFrame©) are used during electrode placement. Accurate electrode placement is necessary for correctly functioning deep brain stimulation (DBS). The objective of the study was to evaluate placement accuracy of DBS electrodes using the NexFrame© frameless navigation system in our department.
Methods:
Coordinates of the planned target point according to anterior and posterior commissural points are found using preoperative MRI of the brain and are usually modified intraoperatively according to microrecording and clinical examination. The coordinates of the actual position of the electrode are detected using a fusion of preoperative MRI with postoperative CT. To determine placement accuracy of the electrodes, the total error and lateral, anteroposterior, and vertical errors were calculated.
Results:
A total of 70 DBS electrodes were implanted using the NexFrame© system in 35 patients diagnosed with Parkinson΄s disease, essential tremor, or dystonia (mean age 62.1 ± 8.3) between June 2013 and January 2016. The mean total error was 1.64 ± 0.81 mm, the mean lateral error was 1.03 ± 0.79 mm, the mean anteroposterior error was 1.14 ± 0.95 mm, and the mean vertical error was 1.05 ± 0,91 mm. Results were compared to the results of other studies and we conclude that the frameless Nexframe© system is fully comparable to frame-based systems.
Key words:
Parkinson’s disease – dystonia – tremor – deep brain stimulation – frameless systém
Chinese summary - 摘要
使用NexFrame©无框系统的深部脑刺激电极的放置精度背景:
使用各种方法来靶向基底神经节,包括术前磁共振图像的直接可视化,术中微电极记录和解剖目标坐标。在电极放置期间使用基于框架的立体定向或无框架立体定向系统(NexFrame©)。精确的电极放置对于正确运作的深部脑刺激(DBS)是必需的。本研究的目的是使用我们部门的NexFrame©无框导航系统来评估DBS电极的放置精度。
方法:
根据大脑术前MRI计算前后连合点的目标点的坐标,并且通常根据微观和临床检查在术中进行修改。使用术前MRI与术后CT的融合来检测电极实际位置的坐标。为了确定电极的放置精度,计算出总误差和横向,前后和垂直误差。
结果:
在2013年6月至2016年1月期间,35名患有帕金森病,原发性震颤或肌张力障碍(平均年龄62.1±8.3岁)的患者中使用NexFrame©系统植入了70个DBS电极。平均总误差为1.64±0.81 mm,平均横向误差为1.03±0.79 mm,平均前后误差为1.14±0.95 mm,平均垂直误差为1.05±0.91 mm。将结果与其他研究的结果进行比较,我们得出结论,无框架的Nexframe©系统完全可与基于帧的系统相媲美。
关键词:
帕金森氏病 - 肌张力障碍 - 震颤 - 深层脑刺激 - 无框架系统
Introduction
Deep brain stimulation (DBS) is a widely used technique for modulation of subcortical brain structures in patients with Parkinson’s disease [1,2], essential tremor [3], dystonia [4,5] and some other movement disorders. Class I evidence supports its use in Parkinson’s disease, in comparison with best medical treatment [6]. The DBS is now being more frequently indicated also during earlier stages of Parkinson’s disease [7,8]. DBS electrodes have conventionally been placed using frame-based stereotaxy with microelectrode recording (MER) and physiological mapping of target structures. Frameless neuronavigation-guided implantation technique using a skull-mounted aiming devices (Nex-Frame©, STarFix, Clearpoint) is used in some centers in conjunction with bone-implanted fiducial markers. Targeting accuracy of frameless stereotactic system has been previously evaluated in laboratory and clinical settings with no significant differences compared to frame-based systems [9–11]. There are several advantages of the frameless system compared to the frame-based system for functional neurosurgical procedure, such as improved patient’s comfort, independent of the head, dissociation of imaging and surgical procedure, reduced surgical time, avoidance of technical difficulties associated with imaging of patients with a stereotactic frame [12–14].
In both techniques, brain images used for targeting (CT and/ or MRI) are obtained preoperatively. Surgical planning software is used to register brain targets in an image space (“stereotactic space”) defined by the frame geometry or by bone-implanted fiducial markers.
The optimal method for targeting the subthalamic nucleus (STN) and positioning the DBS electrode is still debated [15,16]. MER is widely used intraoperatively for precise electrode position [17,18], although there are some reports about verification of electrode position using intraoperative CT [19] or postoperative MRI [20].
The aim of the this project was to evaluate accuracy of DBS electrode placement using NexFrame© system in comparison with data obtained in previously published studies.
Methods
Patients
Thirty-five consecutive patients (total 70 electrodes) who underwent DBS using the NexFrame© were retrospectively included.
All patients met the UK-Brain Bank criteria for diagnosis of idiopathic Parkinson’s disease. Patients treated with DBS for dystonia who did not experience sufficient effects from pharmacological treatment, including applications of botulinum toxin type A. All subjects implanted for the diagnosis of tremor had pharmacoresistant essential tremor.
All patients were fully informed about the procedure and the procedure was performed by a single surgeon (K. D.) and neurologists (N. M., O. P.).
Imaging
Three MRI sets were obtained a few days before the surgery,:
- volumetric 3D Gd-enhanced gradient echo MRI sequence covering the whole brain in 1 mm axial slices, mainly for trajectory planning;
- T2 images turbo spin echo 2 mm slices;
- IR-FSE image set covering the basal ganglia region only, 2 mm axial slices, mainly for direct visualization of the borders of the GPI and surrounding structures.
Images were obtained using the Magnetom Avanto 1.5 Tesla unit (Siemens).
Surgical technique
Four to six stereotactic NexFrame© pins were placed one day before the surgery and CT scan with contrast was performed in 1 mm slices covering the whole brain. Both MRI and CT image sets were imported into stereotactic surgical planning software package (FramelinkMedtronic®), computationally fused, and reformatted to produce images orthogonal to the AC–PC line and mid-sagittal plane.
The target points for the tips of the electrodes were selected using a combination of direct (visualized) and indirect targeting in Parkinson΄s disease and dystonia, and with only indirect targeting in tremor. The trajectories were visualized on the volumetric MRI images using “navigation” views. Small adjustments were then made to avoid traversing the cortical veins and dural venous lakes (easily seen on Gd-enhanced images) and lateral ventricles.
Surgical procedures were carried out in two stages during the same day. The first stage, implantation of the DBS electrodes was on an awaken patient, and the second stage was implantation of the internal pulse generator, performed under general anesthesia.
Using a passive planar blunt probe, a non-sterile registration of the skull fiducial markers was then performed to link image and surgical spaces. The burr hole entry point of the predetermined electrode trajectory was then marked on the skin, and a small hole was drilled to mark that point on the skull. After we performed appropriate sterile preparation and draping, linear skin incisions were made and bur holes centered on the pilot hole were completed. The lead anchoring device (StimLoc, Medtronic, Inc.) and the NexFrame© base were attached to the skull (Fig. 1), and the second sterile registration was performed using the implanted fiducials (target registration error < 0.4 mm). The NexFrame© tower was then attached and aligned to the corresponding target using FrameLink software (Medtronic Inc.). Target depth was then calculated and set on the microTargeting™ Drive System (FHC) positioning device. The dura was opened and closed by fibrin glue to prevent CSF leak or pneumocefalus.

Intraoperative microelectrode registration (MER)
To perform MER in STN-DBS, four MER/macrostimulation needles were placed in an array with central, lateral, anterior and posterior to delineate the borders of the nucleus. Depending on preoperative MRI, it was decided in some cases to record with three or five microelectrodes rather than four. In GPi-DBS, based on the pre-operative MRI and the better visibility of the GP structures and internal capsule, usually three to four channel recordings were performed in the central, medial, posterior, and lateral channels to define the distance of the calculated target to the border between the GPi and the internal capsule. Starting for the STN and GPi, respectively, 10 mm above the MRI-based target, the microelectrodes were advanced in steps of 500 μm towards the target by an electric microdrive. When the needles were inside the STN, GPe (globus pallidus externus) and GPi at each depth, the spiking activity of the neurons lying close to the needle could be recorded. Depending on neuronal density, not more than three to five units were recorded simultaneously. More distant units could not be distinguished from the background level.
Macro-test stimulation
After MER, the tip of the microelectrode was retracted. Channels that showed significant multi-unit activity over a length longer than 3 mm were selected for intraoperative test stimulation (60 μs pulse-duration; 130 Hz pulse frequency). The complete electrode with the macro-tip was then advanced to be used for macro-test stimulation, and this was performed by an experienced neurologist at two or three depths with a 2mm interval, all within the boundaries of the target nucleus as determined by MER. After evaluating the selected channels by macro-test stimulation, the one with the largest therapeutic window, i.e., the lowest current threshold for improvement of symptoms and the highest threshold for side effects, was chosen for permanent electrode implantation. For dystonic patients, the threshold for capsular side effects was used to select the best electrode. In addition, improvement of mobile dystonia was sought when present. With respect to the depth of implantation of the electrodes in STN DBS, we are used to implanting contact number 1 at the point with the best stimulation parameters. For GPi DBS, we position the deepest contact point at the inferior border of the nucleus as determined by MER.
Lead Anchoring and Implantable Pulse Generator Placement
Leads were anchored to the skull with a lead anchoring device (Stimlock, Medtronic®). After scalp closure, the surgery continued under the general anesthesia and the lead extenders and pulse generators were placed. The duration of surgery (from initial skin incision until pulse generators placement) was 185 ± 9.2 min. Control CT imaging was performed the same day after surgery. The CT scan with contrast was performed in 1 mm slices covering whole brain and after that fused with preoperative planning to control the accuracy of the placement (Fig. 2).

Accuracy assessment
Precise location of the electrode within the STN is possible by calculating an error on the preoperative/ perioperative MRI/ CT fusion images. The entry point AC-PC coordinates (point A) and the target point AC-PC coordinates (point B) of the trajectory are found on the navigation device using preoperative MRI. The target is usually modified intraoperatively according to microrecording and clinical examination by a shift on the trajectory labeled as distance d. Knowing this distance and the AC-PC coordinates of both the starting point and the planned target, it is possible to calculate the AC-PC coordinates of the modified target (point C). The AC-PC coordinates of the actual position of the electrode (point D) are localized by placing a cursor manually at the end of the electrode visible on CT-MRI fusion on the navigation device. Using the equation for calculating the distance of two points in 3D space, it is possible to determine the total error (distance between the modified target and the actual position of the electrode) and by using the equation for the distance of two points in straight line, the placement errors in the lateral, anteroposterior, and vertical axes are identified (Fig. 3).

Results
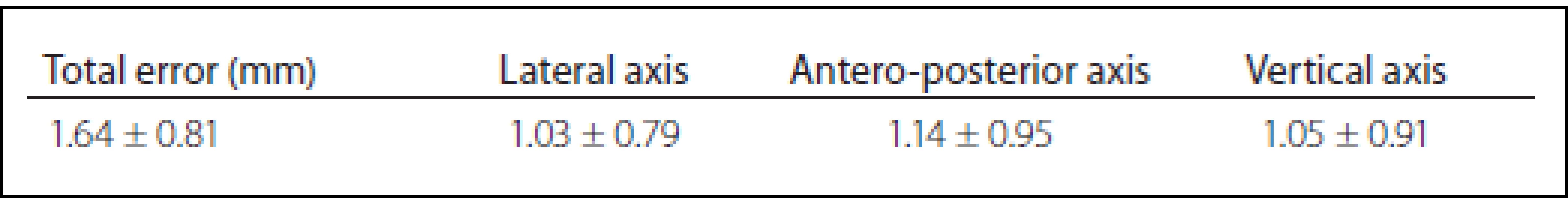
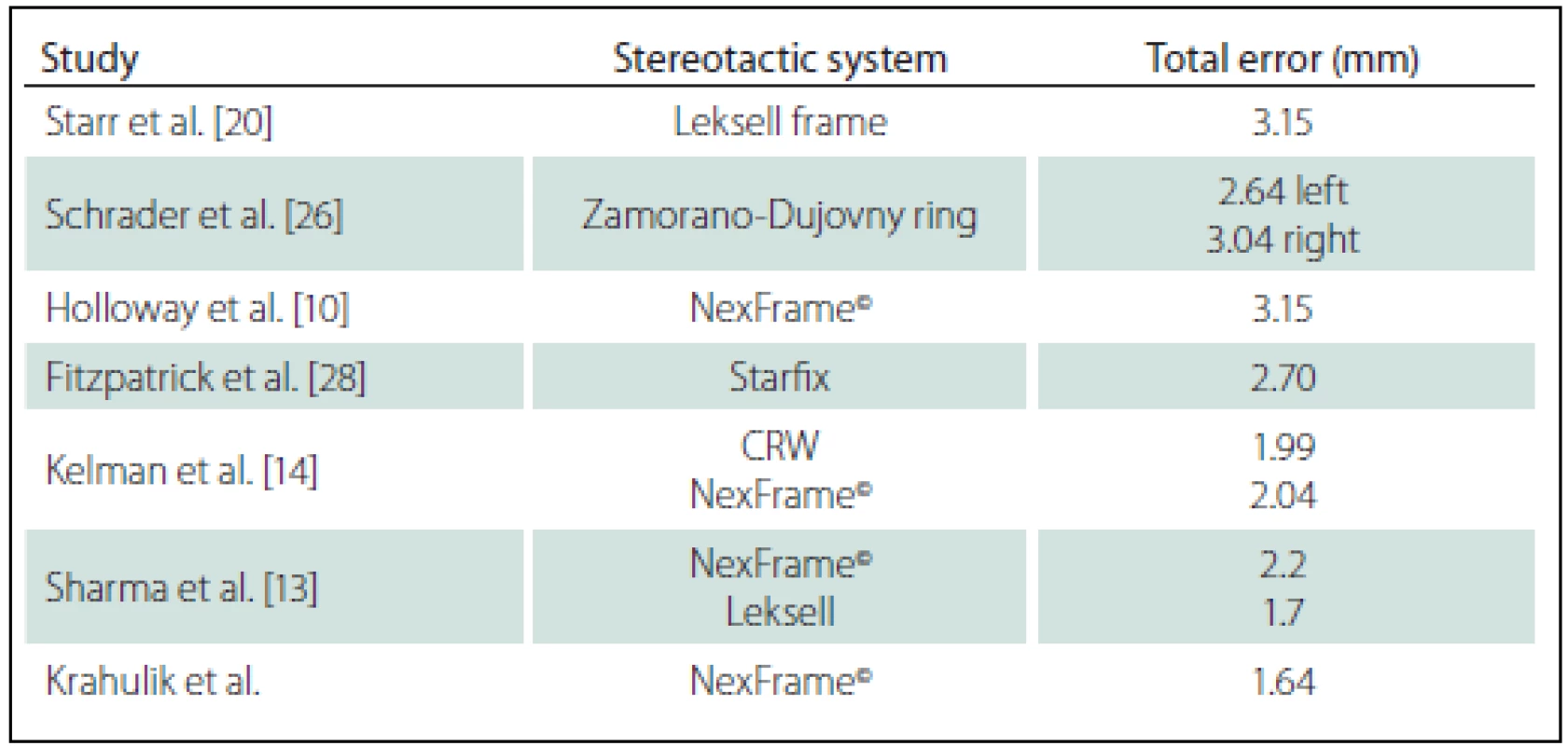
A total of 35 patients with Parkinson΄s disease, dystonia and tremor were implanted in two stages between June 2013 and January 2016. The mean age was 62.1 ± 8.3 years. The mean total operating room time was 185 ± 9.2 min. There were no hemorrhages (either symptomatic or asymptomatic) visible on CT images or any of the complication such as infection or alergic reaction. The mean total error was 1,64 ± 0.81 mm, the mean lateral error was 1.03 ± 0.79 mm, the mean anteroposterior error was 1.14 ± 0.95 mm, and the mean vertical error was 1.05 ± 0,91 mm (Tab. 1). Results of our study compared to other published studies are shown in Tab. 2.
Discussion
The authors are aware that the results of this study can be influenced by errors related to the measurement of accuracy. The MRI/CT fusion is used for placement accuracy measurement and the error might occur while merging preoperative MRI with postoperative CT and thus influence the results. Furthermore, the AC-PC coordinates of the electrode real position are found using manual placement of the cursor on the visible end of the electrode, as described in the material and methods section. Manual placement of the cursor can produce error as it is impossible to place the cursor exactly at the end of the electrode in all three axes. However, for the purposes of our study, we consider our methods to be accurate enough to evaluate placement accuracy in our department.
Phantom studies have demonstrated mean accuracy of the Cosman-Roberts-Wells and Leksell frames to be 1.7 ± 0.1 and 1.8 ± 0.11, resp. when using 1 - mm CT slice thickness and no weight bearing [21–23]. The increased error usually seen in a clinical situation is expected for several reasons, including weight bearing by the frame, mobility of the brain within the cranial cavity, loss of cerebrospinal fluid with subsequent brain shift, inaccuracies of localization introduced by selection of the lead tip and the AC–PC coordinates on postoperative imaging, and deviations of the microelectrode or DBS as it passes through the brain substance.
Starr and colleagues assessed postoperative coordinates of 76 STN DBS electrodes that had been placed using the Leksell frame and found a mean deviation of 3.15 mm from the expected target location [20]. The University of California Los Angeles group evaluated the discrepancy between expected and actual targets in 217 DBS cases. There was a mean vector error of 2.9 mm (range 0.1–6.44 mm) for VIM, 2.3 mm (range 0–7.61 mm) for STN, and 2.2 mm (range 0.03––4.5 mm) for GPI targets.
Urgosik et al. analyzed accuracy of DBS placement using the Leksel frame according to intraoperative monitoring with very good results and minimum complications [24].
Rohlfing et al. found reduced accuracy of stereotactic frames because of torque introduced by the effect of weight bearing on the frame [25]. They assessed the effects of mechanical loading of the frame and a change in patient position on localization error within the clinical situation. They chose to compare scans obtained while the patient was prone and supine, maximizing the adverse effect of linear mechanical loading. Computerized tomography scans were obtained in 14 patients placed in the Brown-Roberts-Wells frame while supine and then prone, and the registration transformations were compared. The mean error was 0.97 mm (standard deviation 0.38), but the registration error was greater than 1.5 mm in eight of 14 patients. The authors noted that the errors from positioning and mechanical loading were additive with other sources of error.
Other factors influencing localization accuracy include differences in scan slice thickness, MRI susceptibility artifact, and differences in scanners and fiducial marker placement [12,26]. Although errors could potentially be introduced on image fusion, these errors were usually less than 1 to 2 imaging voxels [27].
An additional category of error can be related directly to errors in lead placement. Some examples include deflection of the lead during implantation, slippage of the lead during anchoring, and various inaccuracies in stereotactic localization that have been described elsewhere [12,24].
Sharma and colleagues evaluated the accuracy of the NexFrame© system compared to the Leksell stereotactic frame [13]. The frame-based system had greater accuracy compared to the frameless system. The targeting accuracy of the NexFrame© using bone fiducials was not significantly different compared to a stereotactic frame-based system. The error in targeting using frameless system significantly improved over a period of time.
Kelman [14] analyzed accuracy of CRW frame compared to the NexFrame©. Ninety patient underwent microelectrode recording guided placement of 139 DBS leads using a CRW frame (n = 70) and the Nex-Frame© (n = 69) with equivalent accuracy of both systems.
Several advantages related to the use of a frameless device are described in literature. Patients were much less apprehensive when faced with skull fiducial marker placement compared to application of a stereotactic frame. The ability to apply fiducial markers one or more days prior to surgery allowed imaging and planning to be separated from the procedure, thus decreasing operating room time and enhancing patient comfort given the shorter periods spent without medication. Without rigid fixation to the operating table, patients were allowed greater mobility and appeared better able to tolerate lengthy procedures. Intraoperative examination of the patient was easier without the frame [13,14].
Conclusion
The NexFrame© system, using bone fiducial markers for DBS, is an accurate and safe procedure for frameless stereotaxy and it is easily tolerated by patients. The Nex-Frame© system for performing DBS should be considered as an alternative to frame-based systems.
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers‘ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/ or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
assoc. prof. David Krahulík, MD, Ph.D.
Department of Neurosurgery
Faculty of Medicine Palacky University
University Hospital Olomouc
I. P. Pavlova 6
779 00 Olomouc
e-mail: david.krahulik@fnol.cz
Accepted for review: 7. 6. 2016
Accepted for print: 23. 12. 2016
Sources
1. Deuschl G, Paschen S, Witt K. Clinical outcome of deep brain stimulation for Parkinson‘s disease. Handb Clin Neurol 2013;116 : 107–28. doi: 10.1016/ B978-0-444-53497-2.00010-3.
2. Weaver FM, Follett K, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA 2009;301(1):63–73. doi: 10.1001/ jama.2008.929.
3. Benabid AL, Pollak P, Gervason C, et al. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet 1991;337(8738):403–6.
4. Kupsch A, Benecke R, Müller J, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N Engl J Med 2006;355(19):1978–90.
5. Vidailhet M, Vercueil L, Houeto JL, et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 2005;352(5):459–67.
6. Benabid AL, Chabardes S, Mitrofanis J, et al. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson‘s disease. Lancet Neurol 2009;8(1):67–81. doi: 10.1016/ S1474-4422(08)70291-6.
7. Schuepbach WM, Rau J, Knudsen K, et al. EARLYSTIM Study Group. Neurostimulation for Parkinson‘s disease with early motor complications. N Engl J Med 2013;14;368(7):610–22. doi: 10.1056/ NEJMoa1205158.
8. Moro E, Schüpbach M, Wächter T, et al. Referring Parkinson‘s disease patients for deep brain stimulation: a RAND/ UCLA appropriateness study. J Neurol 2015;263(1):112–9. doi: 10.1007/ s00415-015-7942-x
9. Henderson JM. Frameless localization for functional neurosurgical procedures: a preliminary accuracy study. Stereotact Funct Neurosurg 2004;82(4):135–41.
10. Holloway KL, Gaede SE, Starr PA, et al. Frameless stereotaxy using bone fiducial markers for deep brain stimulation. J Neurosurg 2005;103(3):404–13.
11. Henderson JM, Holloway KL, Gaede SE, et al. The application accuracy of a skull-mounted trajectory guide system for image-guided functional neurosurgery. Comput Aided Surg 2004;9(4):155–60
12. Maciunas RJ, Galloway RL jr, Latimer JW. The application accuracy of stereotactic frames. Neurosurgery 1994;35(4):682–94.
13. Sharma M, Rhiew R, Deogaonkar M, et al. Accuracy and precision of targeting using frameless stereotactic system in deep brain stimulator implantation surgery. Neurology India 2014;62(5):503–9. doi: 10.4103/ 0028-3886.144442.
14. Kelman C, Ramakrishnan V, Davies A, et al. Analysis of stereotactic accuracy of the Cosman-Robert-Wells frame and nexframe frameless systems in deep brain stimulation surgery. Stereotact Funct Neurosurg 2010;88(5):288–95. doi: 10.1159/ 000316761.
15. Gross RE, Krack P, Rodriguez-Oroz MC, et al. Electrophysiological mapping for the implantation of deep brain stimulators for Parkinson’s disease and tremor. Mov Disord 2006;21(Suppl 14):S259–83.
16. Rezai AR, Kopell BH, Gross RE, et al. Deep brain stimulation for Parkinson’s disease: surgical issues. Mov Disord 2006; 21(Suppl 14):S197–218.
17. Benazzouz A, Breit S, Koudsie A, et al. Intraoperative microrecordings of the subthalamic nucleus in Parkinson’s disease. Mov Disord 2002;17(Suppl 3):145–9.
18. Pralong E, Villemure JG, Bloch J, et al. Quality index for the quantification of the information recorded along standard microelectrode tracks to the sub-thalamic nucleus in parkinsonian patients. Neurophysiol Clin 2004;34(5):209–15.
19. Burchiel KJ, McCartney S, Lee A, et al. Accuracy of deep brain stimulation electrode placement using intraoperative computed tomography without microelectrode recording. J Neurosurg 2013;119(2):301–6. doi: 10.3171/ 2013.4.JNS122324.
20. Starr PA, Martin AJ, Ostrem JL, et al. Subthalamic nucleus deep brain stimulator placement using high-field interventional magnetic resonance imaging and a skull-mounted aiming device: technique and application accuracy. J Neurosurg Mar 2010;112(3):479–90. doi: 10.3171/ 2009.6.JNS081161.
21. Bernardete EA, Leonard MA, Weiner HL. Comparison of frameless stereotactic systems: accuracy, precision, and applications. Neurosurgery 2001;49(6):1409–16.
22. Dorward NL, Alberti O, Palmer JD, et al. Accuracy of true frameless stereotaxy: in vivo measurement and laboratory phantom studies. Technical note. J Neurosurg 1999;90(1):160–8.
23. Helm PA, Eckel TS. Accuracy of registration methods in frameless stereotaxis. Comput Aided Surg 1998;3(2):51–6.
24. Urgošík D, Jech R, Růžička E. Hluboká mozková stimulace u nemocných s extrapyramidovými poruchami pohybu – stereotaktická procedura a intraoperační nálezy. Cesk Slov Neurol N 2011;74/ 107(2):175–86.
25. Rohlfing T, Maurer CR jr, Dean D et al. Effect of changing patient position from supine to prone on the accuracy of a Brown-Roberts-Wells stereotactic head frame system. Neurosurgery 2003;52(3):610–8.
26. Schrader B, Hamel W, Weinert D, et al. Documentation of electrode localization. Mov Disord 2002;17(Suppl 3):S167–74.
27. Hemler PF, Sumanaweera TS, van den Elsen PA, et al. A versatile system for multimodality image fusion. J Image Guid Surg 1995;1(1):35–45.
28. Fitzpatrick JM, Konrad PE, Nickele C. Accuracy of customized miniature stereotactic platforms. Stereotact Funct Neurosurg 2005;83(1):25–31.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2017 Issue 2
- Advances in the Treatment of Myasthenia Gravis on the Horizon
- Hope Awakens with Early Diagnosis of Parkinson's Disease Based on Skin Odor
- Memantine in Dementia Therapy – Current Findings and Possible Future Applications
-
All articles in this issue
- Ulnar Nerve
- Increased Muscle Tone in Pre-term Infants as a Sign of Neuromaturation and Options for its Assessment
- Options for Activation of Plastic and Adaptation Processes in the Central Nervous System using Physiotherapy in Multiple Sclerosis Patients
- Transcranial Magnetic Stimulation in the Research of Cortical Inhibition in Depressive Disorder and Schizophrenia, the Effect of Antipsychotics
- Toxic Effects of Pesticides
- The Role of the Cell-mediated Immunity in the Pathogenesis of Multiple Sclerosis with Focus on Th17 and Treg Lymfocytes
- Stroke Incidence in Europe – a Systematic Review
- Ocular Myasthenia Gravis in Slovak Republic
- Emotional Awareness in Adolescents – a Pilot Study of Psychometric Properties of the Czech Adaptation of the Levels of Emotional Awareness Scale for Children LEAS-C
- Fingolimod in Real Clinical Practice
- “Awake” Resection of Glioma in Semisitting – a Case Report
- Anti-NMDAR Encephalitis in Children – a Case Report
- Febrile Seizures – Guidelines for Examination of a Child with Simple Febrile Seizures, Adapted from the Guidelines of the American Academy of Pediatrics
- Guidelines for Diagnosis and Treatment of Lower Urinary Tract Symptoms in Patients with Multiple Sclerosis in the Czech Republic – Interdisciplinary Expert Consensus Using DELPHI Methodology
- Placement Accuracy of Deep Brain Stimulation Electrodes using the NexFrame© Frameless System
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Ulnar Nerve
- Stroke Incidence in Europe – a Systematic Review
- Anti-NMDAR Encephalitis in Children – a Case Report
- Febrile Seizures – Guidelines for Examination of a Child with Simple Febrile Seizures, Adapted from the Guidelines of the American Academy of Pediatrics
