Oligoclonal IgG and free light chains – comparison between agarose and polyacrylamide isoelectric focusing
Authors:
D. Zeman 1,2; P. Kušnierová 1; P. Hradílek 2; M. Čábal 2; O. Zapletalová 2
Authors‘ workplace:
Ústav laboratorní diagnostiky, FN Ostrava
1; Neurologická klinika FN Ostrava
2
Published in:
Cesk Slov Neurol N 2019; 82(1): 68-75
Category:
Original Paper
doi:
https://doi.org/10.14735/amcsnn201968
Overview
Aim:
To compare agarose and polyacrylamide isoelectric focusing for oligoclonal imunoglobulin G (o-IgG) and oligoclonal free light chains (o-fLC) detection.
Patients and methods:
Oligoclonal bands were detected in 106 (o-IgG) and 48 (o-fLC), resp. consecutive paired cerebrospinal fluid and serum samples. Kappa statistics was used for method comparison and inter-observer agreement.
Results:
When results were expressed as negative or positive, only three samples (2.8%) for o-IgG, three (6.2%) for o-fLC kappa and one (2.1%) for o-fLC lambda were evaluated differently. Maximum difference between methods was three bands in these discrepant cases. Inter-observer agreement for o-fLC was very good (κ = 0.906–1.000).
Conclusions:
Although polyacrylamide gel might be advantageous due to smaller pore size and hence better resolution, results for both o-IgG and o-fLC were very similar to agarose isoelectric focusing in our series. Both methods performed equally well and discrepances were restricted to borderline positive cases only.
Key words:
cerebrospinal fluid – free light chains – immunoglobulin G – isoelectric focusing – agarose – polyacrylamide
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
寡克隆IgG和自由光链。琼脂糖和聚丙烯酰胺等电聚焦的比较
目的:
比较琼脂糖和聚丙烯酰胺等电聚焦检测寡克隆免疫球蛋白G (o-IgG)和寡克隆自由轻链(o-fLC)的效果。
患者和方法:
在106例(o-IgG)和48例(o-fLC)脑脊液和血清样品中检测到寡克隆条带。采用Kappa统计量进行方法比较和观察者之间的一致性。
结果:
当结果表达为阴性或阳性时,只有3个样本(2.8%)的o-IgG, 3个(6.2%)的o-fLC kappa和1个(2.1%)的o-fLC lambda得到不同的评价。在这些差异的情况下,方法之间的最大差异是三个波段。Inter-observer协议o-fLC非常好(κ= 0.906 - -1.000)。
结论:
虽然聚丙烯酰胺凝胶由于孔径较小,因此分辨率较高,但在我们的系列中,o-IgG和o-fLC的结果与琼脂糖等电聚焦非常相似。两种方法均表现良好,差异仅局限于阳性的边缘病例。
关键词:
无脑脊液轻链-免疫球蛋白G -等电聚焦-琼脂糖-聚丙烯酰胺
Introduction
Oligoclonal IgG (o-IgG) is a principal test used to demonstrate intrathecal antibody synthesis in chronic inflammatory CNS diseases, MS in particular [1]. Although replacing the o-IgG test by a more convenient free kappa light chains (fKLC) quantitation [2,3] has been suggested, o-IgG gained on formal importance for MS diagnosis in the recent revision of McDonald diagnostic criteria [4], therefore it is likely to be performed whenever CSF analysis is required for the support of MS diagnosis.
Besides fKLC quantitation, cerebrospinal fluid (CSF)-restricted fKLC oligoclonality showed to be a sensitive marker of intrathecal inflammation, perhaps even slightly more sensitive than o-IgG [5–7]. However, no between-method comparison is available for this test that is rarely performed due to its labouriousness, especially compared to new automated quantitative methods. Free lambda light chains (fLLC) intrathecal synthesis also occurs in MS and although being less frequent than that of IgG or fKLC [5,7,8], recent studies have shown that simultaneous analysis of fKLC and fLLC may be of prognostic relevance [9–11].
Oligoclonal free light chain (o-fLC) tests gained popularity among the clinicians sending samples to our laboratory, that did not diminish after the introduction of quantitative fLC tests. After the discontinuation of the Multiphor II apparatus production (GE Healthcare Life Sciences, Buckinghamshire, United Kingdom), we looked for an adequate substitute and chose the EDC Flatbed Professional (Electrophoresis Development and Consulting, Tübingen, Germany). Then we decided to assess the performance of the manufacturer´s polyacrylamide gels (PAG) for o-IgG and o-fLC separation. Finally, we aimed at evaluating inter-observer agreement on o-fLC results.
Materials and methods
Clinical specimens
Consecutive paired CSF and serum samples were examined for o-IgG (N = 106) and o-fLC (N = 48) according to a clinician´s request. No clinical data were available for patients followed in other hospitals whose samples had been sent for routine CSF analyses.
Patients followed in our hospital with available clinical data (N = 42) were diagnosed MS (N = 12, six of them after a clinically isolated syndrome according to the latest revision of McDonald diagnostic criteria), clinically isolated syndrome (CIS) (N = 3, not fulfilling MS diagnostic criteria), CNS inflammatory diseases (one case each of herpetic encephalitis, varicella zoster meningoencephalitis, and neuromyelitis optica), peripheral nervous system inflammatory diseases (one case each of acute inflammatory demyelinating polyneuropathy, multifocal motor neuropathy, and paraproteinaemic neuropathy), non-inflammatory neurological diseases (dementia, N = 4; vertigo, N = 2; migraine, N = 2; cryptogenic polyneuropathy, N = 2; and one case each of psychosis, stroke, Parkinson disease, spinal canal stenosis, cervical myelopathy, steroid myopathy) or as symptomatic controls (N = 5).
Oligoclonal IgG and fLC analyses
Agarose isoelectric focusing (IEF) o-IgG detection with immunofixation (IF) was performed with commercially available Hydragel 9 CSF Isofocusing Kit (Sebia, Evry Cedex, France) using Hydrasys apparatus according to the manufacturer´s instructions.
Polyacrylamide IEF was performed on commercially available gels pH 6-11 40S (EDC, Tübingen, Germany) using EDC Flatbed Professional apparatus under the conditions recommended by the manufacturer (Step 1 : 500 V, 25 mA, 10 W, 20 min; Step 2 : 1350 V, 25 mA, 22 W, 90 min; Step 3 : 1750 V, 20 mA, 26 W, 20 min). CSF and serum samples were diluted to 10 mg/L IgG with 0.1% NaCl, and 10 µL were applied into slots in the gel. Sensitive immunodetection based on the principle of a single alkaline-phosphatase labelled anti-IgG antibody method described by Sádaba et al. [12] was performed. Briefly, after capillary blotting for 50 min, the membrane was blocked in 3% bovine serum albumin (Serva Electrophoresis, Heidelberg, Germany) for 45 min and incubated with an alkaline-phosphatase labelled goat anti-human IgG Fc antibody (Bio-Rad, Prague, Czech Republic) for 75 min. Colour reaction was developed using BCIP/NBT substrate (Vector Laboratories, Burlingame, USA).
Agarose IEF for the demonstration of o-fLC was performed using Multiphor II apparatus as described previously [7,8]. PAG fLC IEF was performed on gels pH 3-10 on the Flatbed Professional device under the conditions recommended by the manufacturer (Step 1 : 500 V, 12 mA, 10 W, 30 min; Step 2 : 1700 V, 12 mA, 18 W, 90 min; Step 3 : 2000 V, 8 mA, 20 W, 30 min). For both agarose and PAG fLC IEF, serum samples were diluted 1/80 with 0.7% NaCl and 7 μl of paired samples were applied using the application mask. Subsequent blotting and immunodetection procedure were identical as those for agarose gels.
Oligoclonal IgG evaluation was performed by one of us, whereas inter-observer agreement between two of the authors was studied for o-fLC. IgG bands without serum counterparts or clearly stronger in the CSF than in serum were considered intrathecally synthesized (‘CSF-restricted’); in the latter case, their faint serum counterparts were disregarded for the five-pattern consensus classification. On the contrary, fLC bands were considered intrathecally synthesized only if no serum counterpart was visible because the fLC amounts applied in paired CSF and serum samples were not equalized. The gels and membranes were evaluated in a blinded fashion. Numbers and percentages of discrepantly classified cases were evaluated; chi-squared test and kappa statistics were used for between-method and inter-observer agreement. Spearman´s rank correlation, weighted κ and Wilcoxon test were used to compare the number of CSF-restricted bands. Statistical analyses were performed using the MedCalc Statistical Software version 18.5 (MedCalc Software bvba, Ostend, Belgium).
Albumin, IgG and free light chain quantitation
Cerebrospinal fluid and serum albumin and IgG were measured on BN ProSpec nephelometer (Siemens Healthcare, Prague, Czech Republic). fLC were quantitated by turbidimetry on SPAPLUS analyser using kits certified for CSF and serum measurements (LK016.L.S and LK018.L.S) (The Binding Site, Birmingham, United Kingdom).
Ethical approval
Informed consent was obtained from all patients followed in our hospital that were included in the study. The study has been approved by the local Ethics Committee as a part of the project ‘CSF biomarkers of multiple sclerosis’ (Ref. No. 400/2017).
Results
Oligoclonal IgG
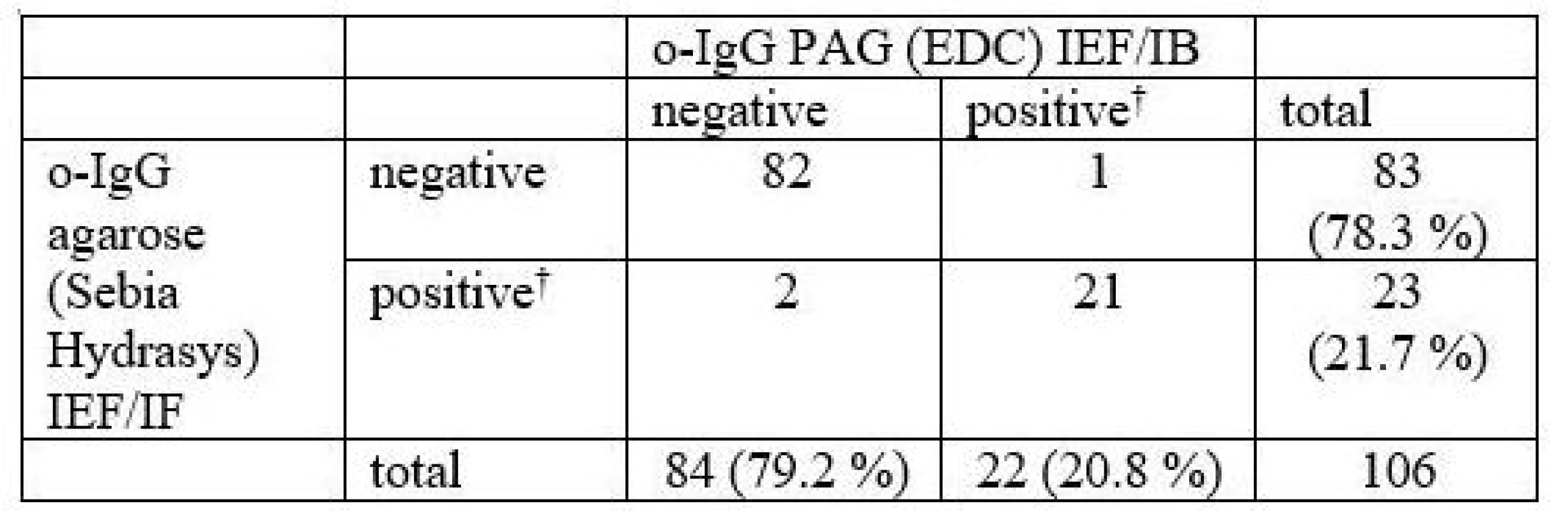
106 consecutive samples were compared. The results are presented in Tab. 1 and a representative example is provided in Fig. 1.
CSF – cerebrospinal fluid; IB – immunoblotting; IEF – isoelectric focusing; IF – imunofixation; PAG – polyacrylamide gel; o-IgG – oligoclonal immunoglobulin G
In total, 3 samples (2.8 %) were classified differently. Numbers of CSF-restricted IgG bands in these cases are presented in Tab. 2.
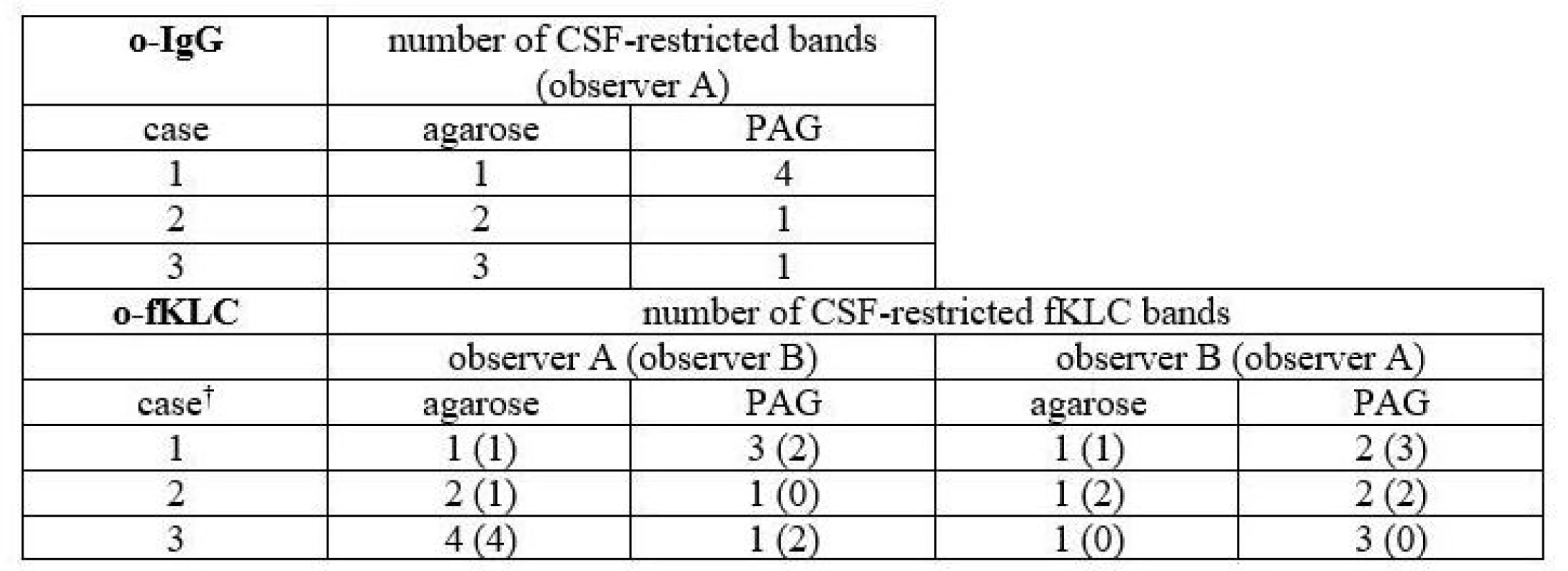
1 CSF-restricted IgG band was found in 7/106 (6.6%) samples on Sebia IEF/IF. On PAG IEF/IB, no band was seen in 4 of these cases, 1 band in 2, and 4 bands in 1 case. An example of such a borderline result is provided in Fig. 2.
On PAG IEF/IB, 1 CSF-restricted IgG band was found in 10 cases (9.4%). On Sebia IEF/IF, no band was seen in 6 of these cases, 1 band in 2, and 2 and 3 bands in one case each.
A more elaborate o-IgG pattern classification containing five types according to two consensus statements [13,14] was compared as well. An agreement was reached on the o-IgG pattern in 75 samples (70.8 %). κ statistics was 0.503, i.e. only moderate agreement. It can be seen that pattern 1 using one of the methods was classified as pattern 4 using another method in 19 cases, and analogous exchange of patterns 2 and 3 contributed to another 8 differently classified patterns. Detailed results are presented in Tab. 3.
![Oligoclonal IgG patterns 1–5 according to consensus classification [13,14]. Chi-squared 106.504, P < 0.0001; κ = 0.5032, 95% CI 0.3669–0.6395.](https://www.prelekara.sk/media/cache/resolve/media_object_image_small/media/image_pdf/880b768bd261020bc8d71487f53fabe7.jpeg)
The number of CSF-restricted IgG bands was compared by Spearman´s rank correlation coefficient (ρ 0.861; 95% CI 0.802–0.903, P < 0.0001) and weighted κ statistics (0.795; 95% CI 0.7432–0.8473) indicating good between-method agreement. No systematic difference was revealed by the Wilcoxon test in the number of CSF-restricted IgG bands between the two methods (16 positive and 17 negative differences, P = 0.8442).
Oligoclonal fLC
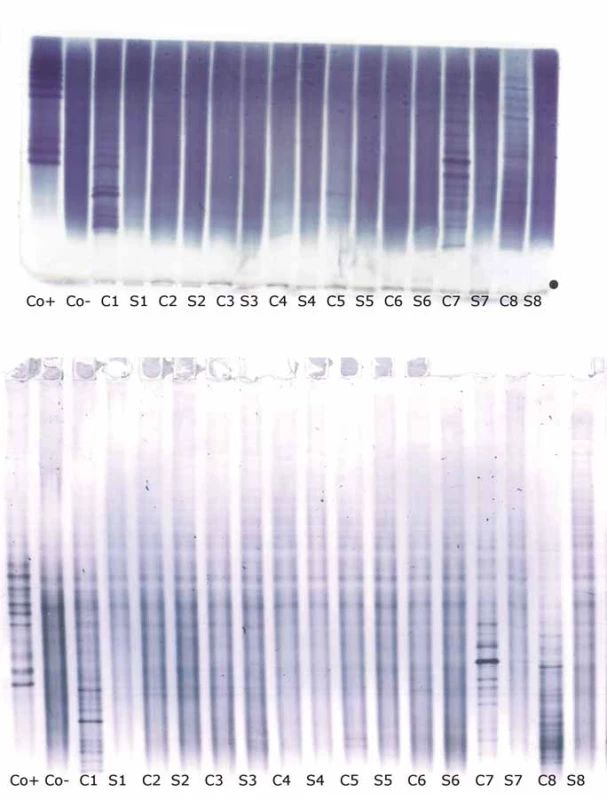
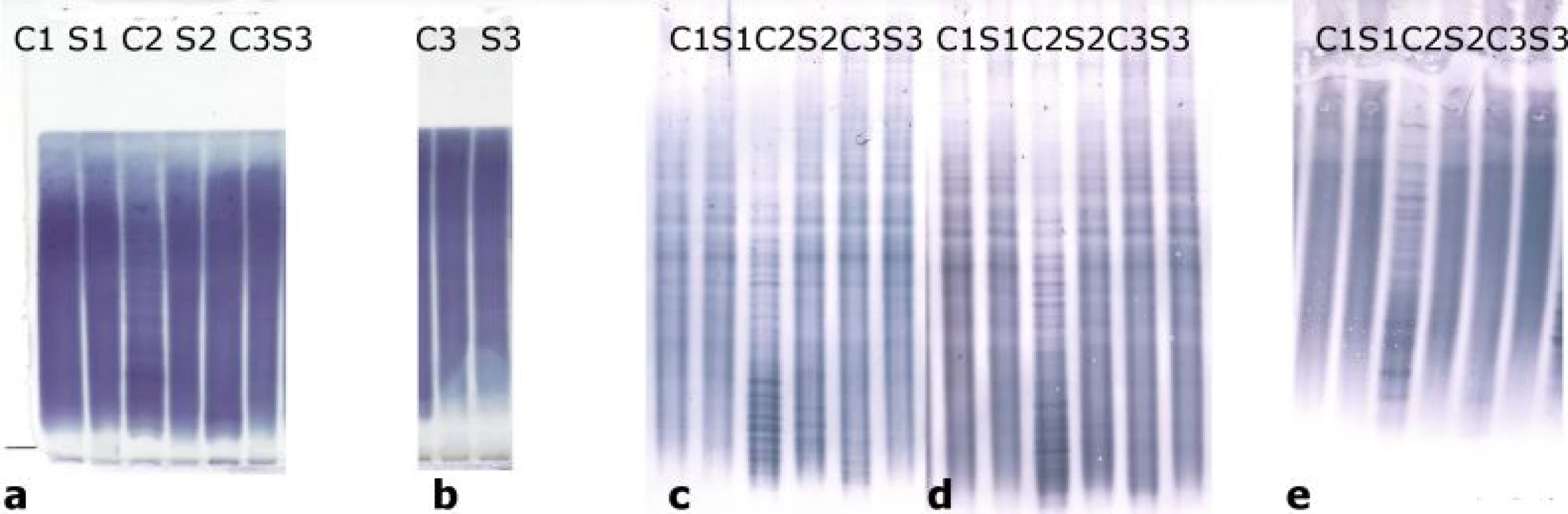
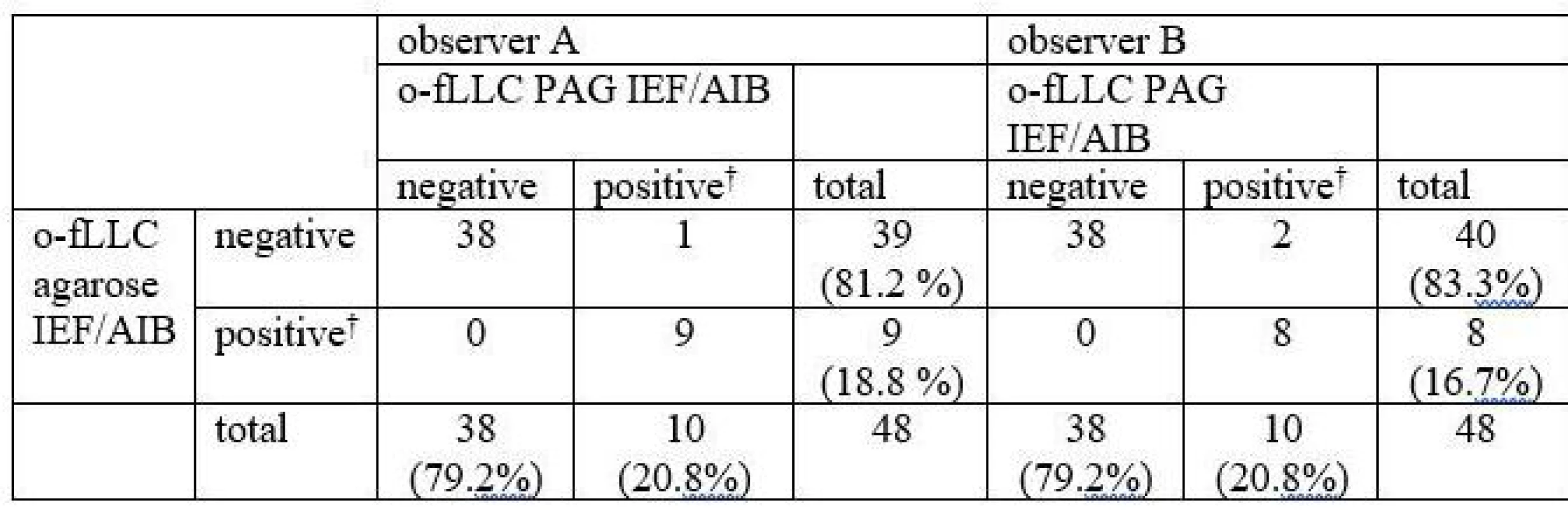
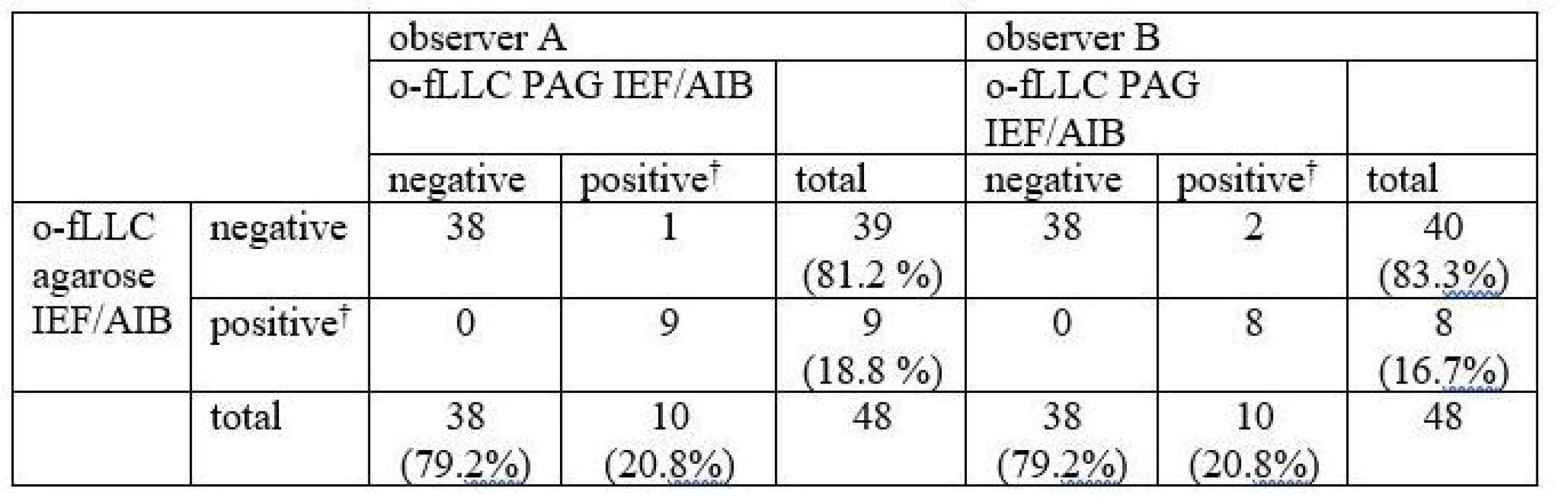
Oligoclonal-fLC were detected in 48 samples. Results are presented in Tab. 4 and 5 and a representative example is provided in Fig. 3. Both between-methods and inter-observer agreement were very good. Inter-observer κ values were 0.9064 and 0.9091 for agarose and PAG o-fKLC and 0.9286 and 1.000 for agarose and PAG o-fLLC.
![Oligoclonal free light chains.
A) Concordant results between agarose and PAG IEF/AIB. C1, C2 and C3 are clearly positive for oligoclonal fKLC while only C2 is clearly positive for oligoclonal fLLC. In C1 sample, two faint cerebrospinal fl uid-restricted fLLC bands were found on the membrane after PAG IEF/AIB, while the evaluation was discrepant for agarose IEF/IF (two bands by observer A, one band by observer B). Sample C4 is negative.
AIB – affinity immunoblotting; C1–4 – paired undiluted cerebrospinal fl uid samples; C1d – cerebrospinal fl uid 1 diluted 1/10 (for fKLC only); Co+ – positive control (monoclonal free light chains [Bio-Rad – AbD Serotec, Prague, Czech Republic] diluted to 0.25 and 0.10 mg/l for fKLC and 1.0, 0.5, 0.25 and 0.10 mg/l for fLLC); Co– – negative control (intravenous immunoglobulin G remedy at a concentration of 250 mg/lIgG); fKLC – free kappa light chains; fLLC – free lambda light chains; IEF – isoelectric focusing; IF – immunofi xation; PAG – polyacrylamide gel; S1–4 – paired serum samples diluted 1/80 (except for S1 next to neat C1 that was diluted 1/20 for fKLC analysis)
(B) Example of a discordant result of fLLC between agarose and PAG IEF/AIB. There are numerous fKLC bands in sample C1, whereas faint fLLC bands were only found on PAG IEF/AIB (two bands by observer A, three bands by observer B). Co+ is diluted to 0.25 and 0.10 mg/L for fKLC and 1.0 and 0.25 mg/L for fLLC. AIB – affinity immunoblotting; fKLC – free kappa light chains; fLLC – free lambda light chains; IEF – isoelectric focusing; PAG – polyacrylamide gel](https://www.prelekara.sk/media/cache/resolve/media_object_image_small/media/image_pdf/41370eea486ab375ebda6d42819a028e.jpeg)
AIB – affinity immunoblotting; CSF – cerebrospinal fluid; IEF – isoelectric focusing; fKLC – free kappa light chains; PAG – polyacrylamide gel
AIB – affinity immunoblotting; CSF – cerebrospinal fluid; IEF – isoelectric focusing; fLLC – free lambda light chains; PAG – polyacrylamide ge
A different classification was observed in 3 samples (6.2%) for o-fKLC and 1 (2.1%; observer A) or 2 (4.2%) samples (observer B) for fLLC. The number of CSF-restricted fKLC bands in discrepant cases is presented in Tab. 2. For fLLC, the only discrepant case had no fLLC bands in agarose but 2 CSF-restricted bands on PAG.
Only one CSF-restricted fLC band was noted in one to four samples (2.1–8.3%) depending on the observer and separation method used. The number of CSF-restricted fLC bands was compared by Spearman´s rank correlation coefficient (ρ 0.847–0.978; P < 0.0001 for all comparisons) and weighted κ (0.804–0.912) indicating very good agreement. Notably we observed a higher number of CSF-restricted fKLC bands using PAG compared to agarose (Wilcoxon test, P = 0.0063 and P = 0.0023 for observers A and B, respectively), and a tendency for a higher number of CSF-restricted fLLC bands (Wilcoxon test significant only for observer B, P = 0.0137).
Comparison with quantitative measures of intrathecal IgG and fLC synthesis
Comparing quantitative IgG measurements and calculations with agarose IEF/IF, there were no false positives using the formulas of Reiber [15], Auer et al. [16] or Öhman et al. [17], while there was one false positive result for IgG index (using a conventional criterion > 0.7). However, the sensitivity of all the calculations was low (43.5% for IgG index and the formulas of Reiber and Auer et al., and 52.2% for the formula of Öhman et al.).
All 10 samples with intrathecal IgG synthesis according to the formulas of Reiber and Auer et al. displayed CSF-restricted oligoclonal IgG bands using both methods, while one sample with intrathecal IgG synthesis, according to the formula of Öhman, was positive for o-IgG by agarose IEF/IF but negative by PAG IEF/IB. Notably, the optimum cut-off value for IgG index obtained from the analysis of ROC curve (not shown) was > 0.589 (sensitivity: 69.6% and 72.7%; specificity: 96.4% and 96.4% compared to agarose IEF/IF and PAG IEF/IB, resp.), while the conventional criterion > 0.7 resulted in sensitivity of 43.5% and 45.5% and specificity of 98.8% and 98.8% compared to agarose IEF/IF and PAG IEF/IB, resp..
Although fLC quantitation was requested in 10 samples only, we consider the results to be interesting enough to warrant their presentation (Tab. 6). Using the cut-offs for the presence of intrathecal fLC synthesis previously determined in our laboratory (CSF fKLC 0.54 mg/L; CSF fLLC 0.30 mg/L; fKLC index 6.07; fLLC index 6.27), there was 100% agreement between quantitative and qualitative values for fKLC, 90% for CSF fLLC and 80% for fLLC index. One patient with normal CSF fLLC yet increased fLLC index had no CSF-restricted fLLC bands, while one patient with normal CSF fLLC and fLLC index had 2 CSF-restricted fLLC bands in PAG and 1 (observer B) or 2 (observer A) faint bands in agarose. Notably, while 100% agreement was obtained for fKLC using both non-linear formulas for intrathecal synthesis [18,19], the recently proposed cut-off of 4.2 for fLLC index or the formula for intrathecal fLLC synthesis [19] resulted in 3 and 2 presumably false positives, resp.
Correlation with clinical data
Although this study has not been aimed at correlations of o-IgG and o-fLC findings with clinical data, we analysed the results of patients with available clinical diagnoses (N = 42 for o-IgG; N = 22 for o-fLC) as an additional test for the plausibility of our laboratory findings. All 12 patients diagnosed with MS were positive for o-IgG on agarose IEF/IF; one of them was negative on PAG IEF/IB (1 CSF-restricted IgG band only). This patient displayed 3 CSF-restricted o-IgG bands on agarose IEF/IF but was negative for o-fKLC and o-fLLC. All 3 patients diagnosed as CIS were positive for o-IgG using both methods. 27 non-MS patients were uniformly negative for o-IgG. Out of 6 patients with MS evaluated for o-fLC, o-fKLC were positive in 5 and negative in 1 MS patient. 3 of these patients were also positive for o-fLLC. Both CIS patients evaluated for o-fLC were positive for o-fKLC as well as o-fLLC (in one case, o-fLLC were visible only on PAG; otherwise, there was complete agreement between methods and observers). Out of 14 non-MS patients, 3–5 were positive for o-fKLC (maximum of 5 bands on PAG and 7 bands on agarose in a patient with multifocal motor neuropathy; one and two patients were discrepantly classified by the two observers using PAG and agarose IEF/AIB, resp.) but only one patient (with a diagnosis of cryptogenic polyneuropathy) was positive for o-fLLC. Interestingly, using a higher cut-off of ≥ 6 CSF-restricted o-fKLC bands, as proposed in an earlier study [7], would only result in 1 non-MS patient, but at the same time only 3 MS patients classified as o-fKLC positive for agarose IEF/AIB. By contrast, the same cut-off value applied to PAG IEF/AIB would preserve the o-fKLC positivity in all 5 MS patients, whereas leaving all 14 non-MS patients o-fKLC negative. There was 100% inter-observer agreement on these cases.
Discussion
Comparison of various o-IgG separation and specific detection methods in contemporary use has been reported in several studies [12,20–23], but the use of agarose versus polyacrylamide IEF was not systematically studied in this context. For CSF o-fLC analysis, we were not able to find a single comparative study.
We aimed to compare two commercially available methods for o-IgG and separation of o-fLC in agarose vs. PAG, followed by identical detection procedure established for o-fLC [7,8]. The strength of our study lies in the recruitment of consecutive samples sent for o-IgG and o-fLC analysis, preventing any possible bias, and potentially providing some estimate of an expected proportion of positive samples. We admit that the absence of clinical information for more than 50% of the studied patients might be considered a weakness of our study. Nevertheless, the correlations between various tests aimed at detection of intrathecal immunoglobulin synthesis were very good, and results for a subgroup of patients with known diagnoses were consistent with previous reports. Hence, we consider our results to be reliable. However, clinical-laboratory correlations observed in our study should be interpreted with great caution since the o-IgG and o-fLC results were available to clinicians and could thus influence their diagnostic decisions. Although it should be kept in mind that intrathecal immunoglobulin synthesis is not disease-specific and may occur in many inflammatory CNS diseases, it appears that the o-IgG test is not only highly sensitive, but also quite specific for MS in an unselected series of patients indicated for o-IgG testing.
A much larger sample size would be required to reveal subtle but significant differences. Using κ statistics, however, it can be concluded that there was very good agreement (κ > 0.80) in all comparisons except for o-IgG pattern classification. Moreover, the differences concerning positivity/negativity of the o-IgG test as well as o-fLC tests were observed in borderline cases only.
The agreement on o-IgG patterns according to international consensus classification was less pronounced, in agreement with earlier studies [20,22,23]. The significance of this more elaborate classification is controversially discussed [1,24,25]. We speculate that only moderate agreement on these five patterns (opposed to very good agreement on a simple negative/positive classification) might be the main reason why this classification is of rather limited clinical utility.
Borderline results are important since when reported, they may contribute to uncertainty and resulting stress of a patient. Single CSF-restricted band may represent a ‘tip of the iceberg’ of the monoclonal as well as oligoclonal response [25]. In routine practice, the presence of only 1 CSF-restricted band is considered either negative or borderline. For o-IgG, we have noted such a result in 7 and 9% of samples, an incidence higher than reported in previous studies [26–29]. We classify such a result as negative; however, unlike others [1] but in accordance with the results of a recent multicentre study [29], we do report such a finding. If MS is suspected in these cases, close follow-up is recommended since it has been shown that a certain proportion of these patients develop oligoclonal profile over time [26,27,29]. At least 2 bands are required for the statement of o-IgG positivity and similar cut-off is used for o-fLC. In several reports, even higher cut-offs of three [30,31] or four [3,7,28,32] CSF-restricted IgG bands or six [7] fKLC bands have been proposed. Further studies might warrant comparing contemporary o-IgG and o-fLC separation/detection methods, perhaps including comparison with quantitative fLC results and focusing on ‘critical samples’ with one or a few CSF-restricted bands.
It has been proven once again that calculation methods are less sensitive for intrathecal IgG synthesis detection. No definite conclusions can be made for quantitative analysis of fLC in this study due to a low number of analysed samples. However, our results suggest apossibility of both false negative and false positive results of the quantitative fLC test. fLC quantitation using different reagents/instruments might not provide comparable values to allow the universal use of cut-off values for intrathecal fLC synthesis calculations. Discrepant results between quantitative and qualitative fKLC tests were also reported in a recent study [33]. In general, o-fLC tests, although previously shown to be slightly more sensitive for the demonstration of intrathecal inflammatory response than o-IgG, might be less specific for MS than o-IgG [5-8]. We therefore agree with Bayart et al. [33] that fLC tests might be used as a complement to rather than a substitute for o-IgG analysis that is still listed as a key element of CSF analysis in published local recommendations for CSF analysis [34,35].
Polyacrylamide IEF might be particularly advantageous for smaller fLC molecules because of a smaller pore size. Indeed, we observed a higher number of CSF-restricted fLC bands using PAG compared to agarose. In addition, we felt that fLC bands were more even and the overall o-fLC pattern was sharper in PAG compared to agarose where distorted bands were common. We admit, however, that these differences might have been influenced not only by the separation medium, but also by different programmes used for the IEF run. In any case, these minor differences seem not to be critical for the final qualitative evaluation of the o-fLC results.
In conclusion, the agreement between agarose and polyacrylamide IEF for o-IgG and o-fLC detection was very good and we consider both methods to be suitable for routine practice. Inter-observer agreement on o-fLC results was as good as demonstrated earlier for o-IgG.
Accepted for review: 4. 10. 2018
Accepted for print: 10. 12. 2018
Acknowledgements
We wish to thank laboratory technicians L. Fürstová, I. Faruzelová, R. Malečková, R. Výtisková, B. Strakošová and O. Michnová for their skillful technical assistance; to Prof. D. Stejskal, Assoc. Prof. K. Šafarčík and Dr. Z. Švagera (Institute of Laboratory Diagnostics, University Hospital Ostrava), Dr. J. Jurčíková and Dr. K. Vítková (Dept. for Science and Research, University Hospital Ostrava) for their continuous support; and to M. Hradecká for language corrections.
Supporting agencies
The study was supported in part by an Institutional grant From Ministry of Health, Czech Republic (07/RVO-FNOs/2017).
Ing. MUDr. David Zeman, Ph.D.
Ústav laboratorní diagnostiky FN Ostrava
17. Listopadu 1790
708 52 Ostrava
e-mail: david.zeman@fno.cz
Sources
1. Link H, Huang YM. Oligoclonal bands in multiple sclerosis cerebrospinal fluid: an update on methodology and clinical usefulness. J Neuroimmunol 2006; 180(1–2): 17–28. doi: 10.1016/ j.jneuroim.2006.07.006.
2. Presslauer S, Milosavljevic D, Huebl W et al. Validation of kappa free light chains as a diagnostic biomarker in multiple sclerosis and clinically isolated syndrome: a multicenter study. Mult Scler 2016; 22(4): 502–510. doi: 10.1177/ 1352458515594044.
3. Gurtner KM, Shosha E, Bryant SC et al. CSF free light chain identification of demyelinating disease: comparison with oligoclonal banding and other CSF indexes. Clin Chem Lab Med 2018; 56(7): 1071–1080. doi: 10.1515/ cclm-2017-0901.
4. Thompson AJ, Banwell BL, Barkhof F et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17(2): 162–173. doi: 10.1016/ S1474-4422(17)30470-2.
5. Sindic CJM, Laterre E. Oligoclonal free kappa and lambda bands in the cerebrospinal fluid of patients with multiple sclerosis and other neurological diseases. An immunoaffinity-mediated capillary blot study. J Neuroimmunol 1991; 33(1): 63–72.
6. Goffette S, Schluep M, Henry H et al. Detection of oligoclonal free kappa chains in the absence of oligoclonal IgG in the CSF of patients with suspected multiple sclerosis. J Neurol Neurosurg Psychiatry 2004; 75(2): 308–310.
7. Zeman D, Hradílek P, Kušnierová P et al. Oligoclonal free light chains in cerebrospinal fluid as markers of intrathecal inflammation. Comparison with oligoclonal IgG. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2015; 159(1): 104–113.
8. Zeman D, Kušnierová P, Švagera Z et al. Assessment of intrathecal free light chain synthesis: Comparison of different quantitative methods with the detection of oligoclonal free light chains by isoelectric focusing and affinity-mediated immunoblotting. PloS One 2016; 11(11): e0166556. doi: 10.1371/ journal.pone.0166556.
9. Voortman MM, Stojakovic T, Pirpamer L et al. Prognostic value of free light chains lambda and kappa in early multiple sclerosis. Mult Scler 2017; 23(11): 1496–1505. doi: 10.1177/ 1352458516681503
10. Ganelin-Cohen E, Golderman S, Yeskaraev R et al. Search for new biomarkers of pediatric multiple sclerosis: application of immunoglobulin free light chain analysis. Clin Chem Lab Med 2018; 56(7): 1081–1089. doi: 10.1515/ cclm-2017-0911.
11. Rathbone E, Durant L, Kinsella J et al. Cerebrospinal fluid immunoglobulin light chain ratios predict disease progression in multiple sclerosis. J Neurol Neurosurg Psychiatry 2018; 89(10): 1044–1049. doi: 10.1136/ jnnp-2018-317947.
12. Sádaba MC, González Porqué P, Masjuan J et al. An ultrasensitive method for the detection of oligoclonal IgG bands. J Immunol Methods 2004; 284(1–2): 141–145.
13. Andersson M, Alvarez-Cermeño J, Bernardi G et al. Cerebrospinal fluid in the diagnosis of multiple sclerosis: a consensus report. J Neurol Neurosurg Psychiatry 1994; 57(8): 897–902.
14. Freedman MS, Thompson EJ, Deisenhammer F et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis. A consensus statement. Arch Neurol 2005; 62(6): 865–870. doi: 10.1001/ archneur.62.6.865.
15. Reiber H. Flow rate of cerebrospinal fluid (CSF) – a concept common to normal blood-CSF barrier function and to dysfunction in neurological diseases. J Neurol Sci 1994; 122(2): 189–203.
16. Auer M, Hegen H, Zeileis A et al. Quantitation of intrathecal immunoglobulin synthesis – a new empirical formula. Eur J Neurol 2016; 23(4): 713–721. doi: 10.1111/ ene.12924.
17. Öhman S, Forsberg P, Nelson N et al. An improved formula for the judgement of intrathecally produced IgG in the presence of blood brain barrier damage. Clin Chim Acta 1989; 181(3): 265–272.
18. Presslauer S, Milosavljevic D, Huebl W et al. Kappa free light chains: diagnostic and prognostic relevance in MS and CIS. PLoS One 2014; 9: e89945. doi: 10.1371/ journal.pone.0089945.
19. Hegen H, Milosavljevic D, Schnabl C et al. Cerebrospinal fluid free light chains as diagnostic biomarker in neuroborreliosis. Clin Chem Lab Med 2018; 56(8): 1383–1391. doi: 10.1515/ cclm-2018-0028.
20. Sellebjerg F, Christiansen M. Qualitative assessment of intrathecal IgG synthesis by isoelectric focusing and immunodetection: interlaboratory reproducibility and interobserver agreement. Scand J Clin Lab Invest 1996; 56(2): 135–143.
21. Abraira V, Alvarez-Cermeño JC, Arroyo R et al. Utility of oligoclonal IgG band detection for MS diagnosis in daily clinical practice. J Immunol Methods 2011; 371(1–2): 170–173. doi: 10.1016/ j.jim.2011.06.009.
22. Nováčková L, Zeman D. Detection of oligoclonal IgG bands in cerebrospinal fluid and serum: comparison between commercial immunofixation method and home-made affinity immunoblotting method and evaluation of interobserver agreement. Klin Biochem Metab 2011; 19(4): 229–233.
23. Dlouhy O, Kusnierova P, Kurasova I et al. Chemiluminescent detection of oligoclonal immunoglobulins after isoelectric focusing and affinity-mediated immunoblotting. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2018; 162(2): 107–115.
24. Franciotta D, Lolli F. Interlaboratory reproducibility of isoelectric focusing in oligoclonal band detection. Clin Chem 2007; 53(8): 1557–1558. doi: 10.1373/ clinchem.2007.089052.
25. Gastaldi M, Zardini E, Franciotta D. An update on the use of cerebrospinal fluid analysis as a diagnostic tool in multiple sclerosis. Expert Rev Mol Diagn 2017; 17(1): 31–46.doi: 10.1080/ 14737159.2017.1262260.
26. Davies G, Keir G, Thompson EJ et al. The clinical significance of an intrathecal monoclonal immunoglobulin band. A follow-up study. Neurology 2003; 60(7): 1163–1166.
27. Franciotta D, Zardini E, Lolli F. The clinical significance of an intrathecal monoclonal immunoglobulin band: a follow-up study. Neurology 2004; 62(4): 675–676.
28. Wurster U. The clinical significance of an intrathecal monoclonal immunoglobulin band: a follow-up study. Neurology 2004; 62(7): 1237.
29. Ferraro D, Franciotta D, Bedin R et al. A multicenter study on the diagnostic significance of a single cerebrospinal fluid IgG band. J Neurol 2017; 264(5): 973–978. doi: 10.1007/ s00415-017-8480-5.
30. Bourahoui A, De Seze J, Guttierez R et al. CSF isoelectrofocusing in a large cohort of MS and other neurological diseases. Eur J Neurol 2004; 11(8): 525–529. doi: 10.1111/ j.1468-1331.2004.00822.x.
31. Mayringer I, Timeltaler B, Deisenhammer F. Correlation between the IgG index, oligoclonal bands in the CSF, and the diagnosis of demyelinating diseases. Eur J Neurol 2005; 12(7): 527–530. doi: 10.1111/ j.1468-1331.2005.00997.x.
32. Fortini AS, Sanders EL, Weinshenker BG et al. Cerebrospinal fluid oligoclonal bands in the diagnosis of multiple sclerosis. Am J Clin Pathol 2003; 120(5): 672–675. doi: 10.1309/ EM7K-CQR4-GLMH-RCX4.
33. Bayart JL, Muls N, van Pesch V. Free kappa light chains in neuroinflammatory disorders: complement rather than substitute? Acta Neurol Scand 2018; 138(4): 352–358. doi: 10.1111/ ane.12969.
34. Sobek O, Adam P, Koudelková J et al. Algoritmus vyšetření likvoru v návaznosti na doporučení Sekce neuroimunologie a likvorologie České neurologické společnosti JEP. Cesk Slov Neurol N 2012; 75/ 108(2): 159–163.
35. Mrázová K, Zeman D, Bořecká K. Doporučení k vyšetřování mozkomíšního moku. Klin Biochem Metab 2017; 25(1): 43–47.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2019 Issue 1
- Advances in the Treatment of Myasthenia Gravis on the Horizon
- Memantine in Dementia Therapy – Current Findings and Possible Future Applications
- Memantine Eases Daily Life for Patients and Caregivers
-
All articles in this issue
- Ketogenic diet – effective treatment of childhood and adolescent epilepsies
- Can we accurately diagnose the dyskinetic form of cerebral palsy? YES
- Sub signum coma – current view of chronic disorders of consciousness
- Chronic subdural haematoma
- Iatrogenesis of patients with psychogenic non-epileptic seizures – possible solutions
- Genetics of neurodegenerative dementias in ten points – what can a neurologist expect from molecular genetics?
- Mild traumatic brain injury management – consensus statement of the Czech Neurological Society CMS JEP
- Contact heat evoked potentials – impact of physiological variables
- Laboratory efficacy testing of acetylsalicylic acid treatment in secondary prevention of ischemic stroke
- Magnetic resonance imaging showing parietal atrophy of the brain in late-onset Alzheimer’s disease
- Transcranial magnetic stimulation in borderline personality disorder – case series
- TNFα and microRNA-15b expression changes in experimental model of subarachnoid haemorrhage
- A Rasch analysis of the Q-LES-Q-SF questionnaire in a cohort of patients with neuropathic pain
- Oligoclonal IgG and free light chains – comparison between agarose and polyacrylamide isoelectric focusing
- New possibilities of ultrasound in predicting low back pain in adolescent males – pilot study
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Mild traumatic brain injury management – consensus statement of the Czech Neurological Society CMS JEP
- Chronic subdural haematoma
- Oligoclonal IgG and free light chains – comparison between agarose and polyacrylamide isoelectric focusing
- Ketogenic diet – effective treatment of childhood and adolescent epilepsies
