A study of the compaction process and the properties of tablets from two types of directly compressible sorbitol
Studium lisovacího procesu a vlastností tablet ze dvou typů přímo lisovatelného sorbitolu
Práce se zabývá studiem energetických poměrů při lisování a vlastností tablet ze dvou typů přímo lisovatelného sorbitolu Sorbitabu™ SD 250 a Sorbitabu™ SD 500. Testovanými vlastnostmi byla pevnost tablet v tahu a doba rozpadu tablet, sledované v závislosti na lisovací síle, přídavku dvou typů mazadel ve dvou koncentracích a dvou typů léčivých látek v jedné koncentraci. Z porovnání obou typů přímo lisovatelného sorbitolu vyplynulo, že negativním momentem použití Sorbitabu SD 500 je vliv přítomnosti mazadel na pevnost výlisků, dále také významnější prodloužení doby rozpadu tablet vlivem mazadel na rozdíl od Sorbitabu SD 250. Z energetické bilance lisování vyplynula vyšší hodnota celkové energie lisování pro Sorbitab SD 500 díky vyšším hodnotám energie potřebné na tření a energie akumulované tabletou.
Klíčová slova:
přímo lisovatelný sorbitol – mazadla – pevnost tablet v tahu – doba rozpadu tablet – záznam „síla-dráha“
Authors:
J. Mužíková; E. Zemanová
Authors‘ workplace:
Charles University in Prague, Faculty of Pharmacy in Hradec Králové, Department of Pharmaceutical Technology, Czech Republic
Published in:
Čes. slov. Farm., 2010; 59, 59-66
Category:
Original Articles
Overview
The paper deals with the study of energy conditions in the course of compaction and the properties of tablets from two types of the directly compressible sorbitol Sorbitab™ SD 250 and Sorbitab™ SD 500. The tested properties included tensile strength of tablets and disintegration time of tablets, examined in dependence on compression force, addition of two types of lubricants in two concentrations, and two types of active ingredients in one concentration. A comparison of both types of directly compressible sorbitol revealed that the negative moment in the use of Sorbitab SD 500 is the effect of the presence of lubricants on the strength of the compacts, and furthermore a more significant prolongation of disintegration time of tablets by the action of lubricants in contrast to Sorbitab SD 250. The energy balance of compression showed a higher value of total energy of compression for Sorbitab SD 500 due to higher values of energy required for friction and the energy accumulated by the tablet.
Key words:
directly compressible sorbitol – lubricants – tensile strength of tablets – disintegration time – force-displacement profile
Introduction
Sorbitol was discovered already in the 19th century and has become the principal representative of sugar alcohols; it is most widely used in food and pharmaceutical industries. In nature it is found in ripe fruits of many trees and plants, in 1872 it was isolated from the fruits of the plant Sorbus americana (American Mountai-ash). It is a hexahydric alcohol, a relative of mannose, chemically an isomer of mannitol1). These substances differ from each other in hygroscopicity and solubility in water; sorbitol, in contrast to mannitol, is hygroscopic and better soluble in water. It is also non-cariogenic and better tolerated by diabetic patients than sucrose2). It exists in four crystalline polymorphic forms (α, β, γ, δ) and one amorphous form3). The properties of these forms are different and there exist considerable differences in the shape and structure of different products of sorbitol, which is reflected in their compressibility4). It has been found that the most stable form is γ sorbitol, which has the best compression properties, but the tablets show a longer time of disintegration and dissolution5). In his study, Schmidt6) compared two types of γ-sorbitol: type A sorbitol (instant sorbitol) and type B sorbitol (powder sorbitol). Type A sorbitol is produced by spray drying of sorbitol solutions. The spray-dried product contains more than 90% of γ-sorbitol and it is marketed as Sorbitol Instant, or Sorbitab™. Type B sorbitol is prepared by crystallization of the melt and it is manufactured, e.g., under the firm name Neosorb® DC. Microscopically, type A sorbitol is an agglomerate of irregular particles with scabrous, furrowed surfaces, whereas type B sorbitol exists as spherical particles with smooth surfaces, possessing greater particle distribution and also greater flowability. For tableting, particle distribution corresponding to type A sorbitol is more advantageous. Both types are plastically deformable.
This paper deals with the study of energy conditions in the course of compressing and the properties of tablets from the spray-dried product Sorbitab™ in two size degrees, SD 250 and SD500, i.e. differing in the average particle sizes of 250 and 500 μm. In these products, the firm literature reports excellent compressibility, spherical shape of particles and thus excellent flowability and high solubility7).
EXPERIMENTAL PART
Materials
- Sorbitab™ SD 250 – spray-dried sorbitol (SPI Pharma, USA),
- Sorbitab™ SD 500 – spray-dried sorbitol (SPI Pharma, USA),
- Pruv® – sodium stearyl fumarate (J. Rettenmaier & Söhne GmbH + Co, Rosenberg, Germany),
- magnesium stearate (Acros Organics, New Jersey, USA),
- ascorbic acid (Northeast General Pharmaceutical Factory, China),
- acetylsalicylic acid (Merck KgaA, Darmstadt, Germany).
Preparation of tableting materials
Tests included tableting materials from dry binders without lubricants, mixtures with lubricants, and mixtures with active ingredients. Altogether 18 tableting materials of the following composition were used (table 1 and 2).
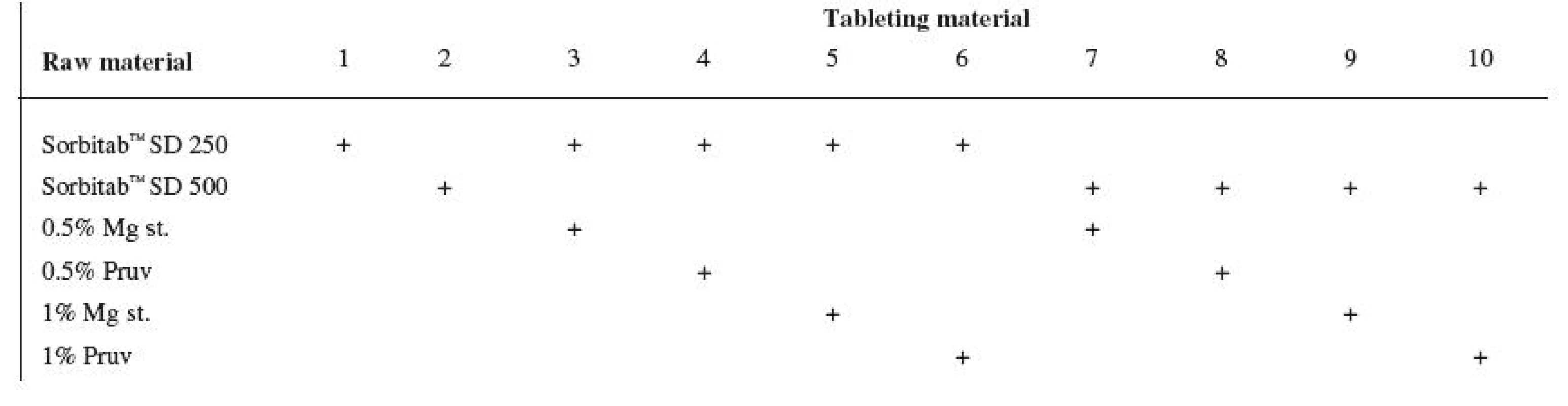
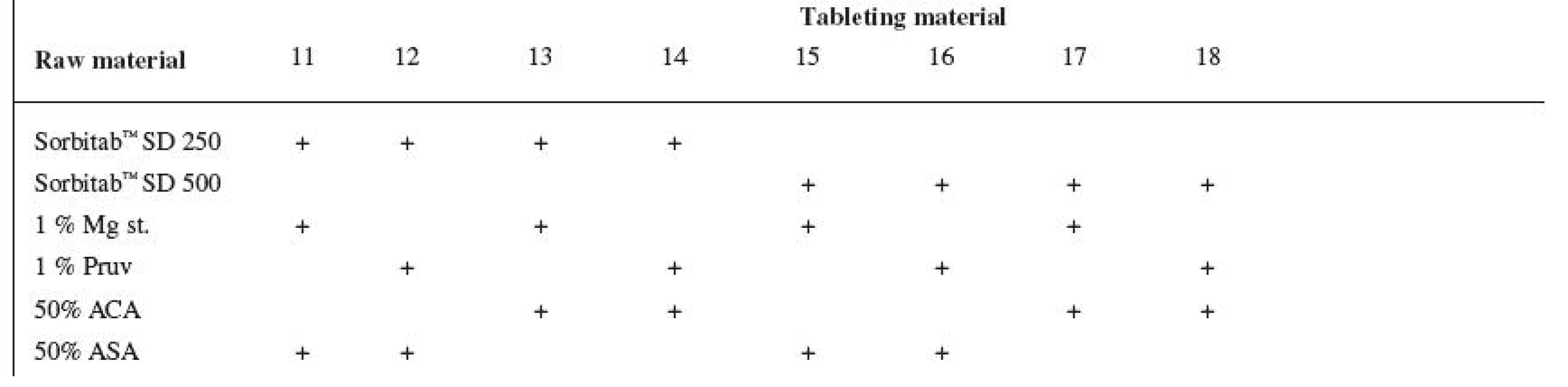
Dry binders with lubricants were mixed for 5 minutes in a stainless steel cube KB 15S (Erweka GmbH, Hausenstamm, Germany). Mixtures with active ingredients were prepared by mixing the dry binder and the active ingredient for 5 minutes, then the lubricant was added and the material was mixed for 5 minutes again. The rotation rate of the mixing cube was always 17 revolutions/minute. The amount of prepared tableting materials without active ingredients was always 30 g, with active ingredients, 20 g.
Preparation of tablets and energy evaluation of process of compaction
All tableting materials were used to produce 16 tablets compressed with the use of a special die with an upper and a lower punch on a material testing equipment T1-FRO 50 TH.A1K Zwick/Roell (Zwick GmbH & Co, Ulm, Germany). Proper compaction took place by applying the pressure on the upper punch. The tablets were of a cylindrical shape without facets with a diameter of 13 mm and weight of 0.5 ± 0.0010 g. Compression velocity was 1 mm/s and compression forces 3, 4.5 and 6 kN, in the case of pure dry binders, 4.5 kN. Mixtures including active ingredients were compressed using only a compression force of 6 kN. In 10 tablets from each group, the “force-displacement” plot was drawn by means of a computer programme testXpert V 9.01 and the compression process was evaluated as far as energy was concerned, i.e. the energies E1, E2 and E3 were expressed numerically. Energy E1 is the energy consumed by friction, energy E2 is the energy accumulated by the tablet in the course of compression, and energy E3 is the energy released during decompression (Fig. 1)8).

Measurement of tensile strength of tablets and evaluation of the lubricant sensitivity of tableting materials
Tensile strength was always evaluated in 10 tablets, first no sooner than 24 hours after compaction. Measurements were performed on a Schleuniger apparatus (Dr. Schleuniger Pharmatron AG, Solothurn, Switzerland), which measured tablet sizes accurate to 0.01 mm and destruction force in N. Tensile strength of tablets was calculated according to Eq. [1]:
where P is the tensile strength of tablets (MPa), F is the destruction force (N), d is the tablet diameter (mm), and h is the thickness of the tablet (mm)9).
LSR (lubricant sensitivity ratio) values, which make it possible to quantify and mutually compare the lubricant sensitivity of tableting materials, were calculated according to Eq. [2]
where Csu is the crushing strength of tablets without an added lubricant and Csl is the crushing strength with a lubricant. The more this value approaches 1, the more the dry binder is sensitive to an added lubricant from the viewpoint of decreased strength of tablets10). In the present paper, the values of tensile strength, not those of crushing strength, are used in the equation.
Measurement of disintegration time of tablets
Disintegration times of tablets were evaluated earliest 24 hours after compaction always in 6 tablets. Measurements were performed on an apparatus for the determination of disintegration time of tablets Erweka ZT 301 (Erweka GmbH, Hausenstamm, Germany) following the method described in the chapter Pharmaceutical Technical Procedures in the Ph. Eur. 2005. The test was carried out without discs in the medium of purified water tempered to 37 °C ± 1 °C. The tablet was considered disintegrated at the moment when there was no remainder on the net.
The results of strengths and disintegration times were statistically processed by means of the computer programmes Excel and Qcexpert. Elementary data analysis yielded the mean values with standard deviations, which were plotted into dependences on compression force. In the cases of unclear significance of differences in the values, unpaired t-test at a level of significance of 0.05 was employed.
RESULTS AND DISCUSSION
The paper aimed to compare the energy conditions in the course of compaction and the properties of tablets made of two types of directly compressible sorbitol Sorbitab™ SD 250 and Sorbitab™ SD 500. The tested properties included tensile strength of tablets and disintegration time of tablets, examined in dependence on compression force, addition of two types of lubricants in two concentrations, and two types of active ingredients in one concentration. Compression forces were selected in such a way to make it possible for the strength of the compacts without active ingredients to oscillate as much as possible within the range of the optimal strength of tablets, i.e. 0.56–1.11 MPa11). In particular, the compression forces were 3, 4.5 and 6 kN. The lubricants were magnesium stearate and sodium stearylfumarate in the concentration of 0.5 and 1%. The model active ingredients were acetylsalicylic acid and ascorbic acid, differing in the mechanism of compaction and solubility in water. Ascorbic acid on compression is fragmented and well soluble. Acetylsalicylic acid is prevalently plastically deformed and is badly soluble in water. Besides the properties of tablets, energy profiles of compaction were also examined, which may be useful as material compression characteristics in pre-formulation studies of compression of various substances. Their contribution primarily consists in a possibility of subsequent correlation of energy inputs or work of compression with deforming and tablets-forming properties of various materials8).
Figure 2 shows the dependence of tensile strength of tablets on compression force for Sorbitab SD 250 with lubricants. In the case of the compression force of 4.5 kN, the value without the lubricant is also shown. The results show an increase in strength due to the action of the increasing compression force in the cases of all tableting materials. A decrease in strength in comparison with the strength of compacts from pure Sorbitab SD 250 occurs only by the action of an addition of 1% of magnesium stearate. There is no statistically significant difference between the values of tensile strength of tablets from pure Sorbitab SD 250 and a mixture of the dry binder with 1% of Pruv. However, it holds true that the mixtures of the dry binder with a lower concentration of lubricants produce stronger tablets. On the basis of the fact that sorbitol is plastically deformable, also a decrease in tensile strength due to 0.5% concentration of lubricants could be assumed12). Nevertheless, as the values of strength in these mixtures are, on the other hand, higher than in the case of pure Sorbitab SD 250, the LSR values thus reach even negative values (see Table 3).

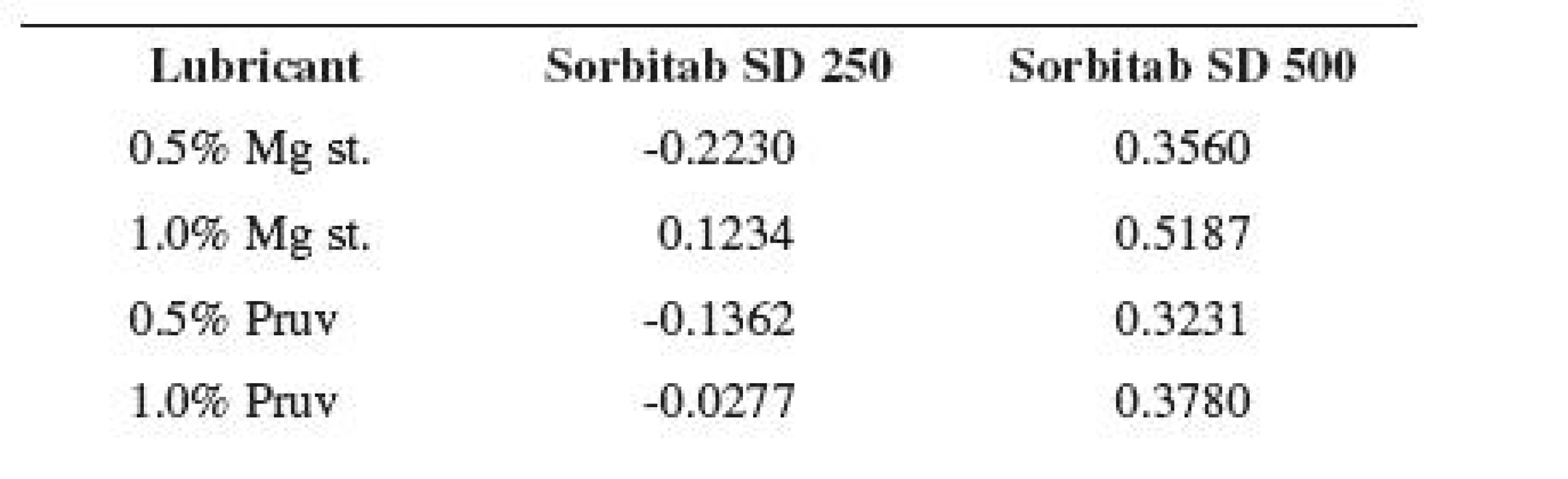
Figure 3 shows the same dependence for Sorbitab SD 500. This substance possesses a larger average particle size, i.e. a smaller specific surface, and thus a more perfect film of the lubricant in the same concentration developed and the theoretical assumption of decreased tensile strength of compacts by the action of lubricants, already with a 0.5% addition, was confirmed. In this concentrations of lubricants there is no statistically significant difference between the values of strength. Higher concentrations of lubricants produce a deeper decrease in strength, excepting a 1% concentration of Pruv at the compression force of 3 kN. The least strong tablets are again those made from a mixture of the dry binder with 1% of magnesium stearate; in the case of the compression force of 6 kN there is no statistically significant difference with the value for the mixture with 1% Pruv. A decrease in tensile strength of tablets by the action of lubricants is also indicated by the positive LSR values in Table 3. The strength of compacts increases with compression force in all cases.

Figure 4 presents the dependence of disintegration time on compression force for Sorbitab SD 250. The results for the pure substance compressed by the force of 4.5 kN show a clear intervention of the addition of lubricants whose hydrophobic character causes prolongation of disintegration time. At this compression force, there is no statistically significant difference in the values for mixtures with both lubricants in both concentrations. At the compression force of 3 kN, the disintegration time was most prolonged with a 1% addition of Pruv, in the compression force of 6 kN it was a 1% addition of magnesium stearate. Disintegration time is more significantly increased by the action of compression force only between the compression forces of 4.5 and 6 kN.

Figure 5 shows the same dependence for Sorbitab SD 500. Here a more significant effect of added lubricants can be seen at the compression force of 4.5 kN, the lowest value is for the pure substance. The disintegration time is prolonged more in the cases of all compression forces by the more hydrophobic magnesium stearate. Disintegration time increases with compression force, more markedly again between the compression forces of 4.5 and 6 kN.

A comparison of both figures clearly shows longer disintegration times for Sorbitab SD 500 in the mixtures with lubricants. There is no statistically significant difference between the values for pure substances.
Tensile strength of tablets at the compression force of 6 kN for tableting materials with active ingredients is shown in Figure 6. The results show a higher strength of tablets containing acetylsalicylic acid due to plastic deformability of the tableting material. The tablets with ascorbic acid are stronger in the mixture with Sorbitab SD 250, and in both dry binders no statistically significant difference within the type of the lubricant used was recorded. In the tablets with acetylsalicylic acid, the strength of the compacts with Sorbitab SD 500 is also lower, but not in such a marked manner as in the tablets containing ascorbic acid. In addition, there is a difference within the framework of the type of the lubricant used; the tablets with Pruv are stronger.

Figure 7 presents the disintegration times of tablets containing active ingredients at the compression force of 6 kN. The tablets containing ascorbic acid disintegrate more quickly because of good solubility of the active ingredient in water and a lower strength. A longer disintegration time is found in the tablets with Sorbitab SD 500, there is no statistically significant difference within the framework of the type of the lubricant used. In the case of Sorbitab SD 250, the tablets with stearate disintegrate longer. The tablets with acetylsalicylic acid would be practically unusable as they possess the disintegration times high above the pharmacopoeial limits, which is due to bad solubility of the active ingredient. In the case of the mixture with Sorbitab SD 250, the compacts with stearate disintegrated more quickly, and so did those with Pruv in the case of the mixture with Sorbitab SD 500. If these tablets were used in practice, an addition of a disintegrating agent would be necessary.

The energy inputs of compression are summarized in Table 4. The total energy of compression (Emax) is the sum of the energy for friction (E1), the energy accumulated by the tablet (E2) and the energy released during decompression (E3). The values of total energy of compression show a higher energy input in the case of Sorbitab SD 500 and furthermore a decrease in the total energy of compression after the lubricants are added. The values of energy for friction in the case of Sorbitab SD 250 and the values after adding the lubricants, which decrease friction, are again significantly lower. Another type of energy is the energy which is consumed directly for compression and which is accumulated in the tablet after compression. Its values are again higher for Sorbitab SD 500 and only in the case of this Sorbitab they are decreased by an addition of the lubricant. The third type of energy, which was examined in the course of compression, is the energy which is released during decompression, when the compact is slightly relaxed. The values show that in this component of energy there is no marked difference between Sorbitabs and that in the tableting materials with lubricants this value is minutely higher, just due to the presence of lubricants, which decrease friction during compression and decompression too. The analysis of energies shows that the total energy needed for compression is higher in the case of Sorbitab SD 500 due to a higher energy consumed for friction and for the compression of the tablet itself. It holds true also for the mixtures with active ingredients. Within the framework of the comparison of active ingredients, higher total energies of compression are found in the tableting materials with acetylsalicylic acid, first of all due to a higher energy consumed for friction and the energy released during decompression.
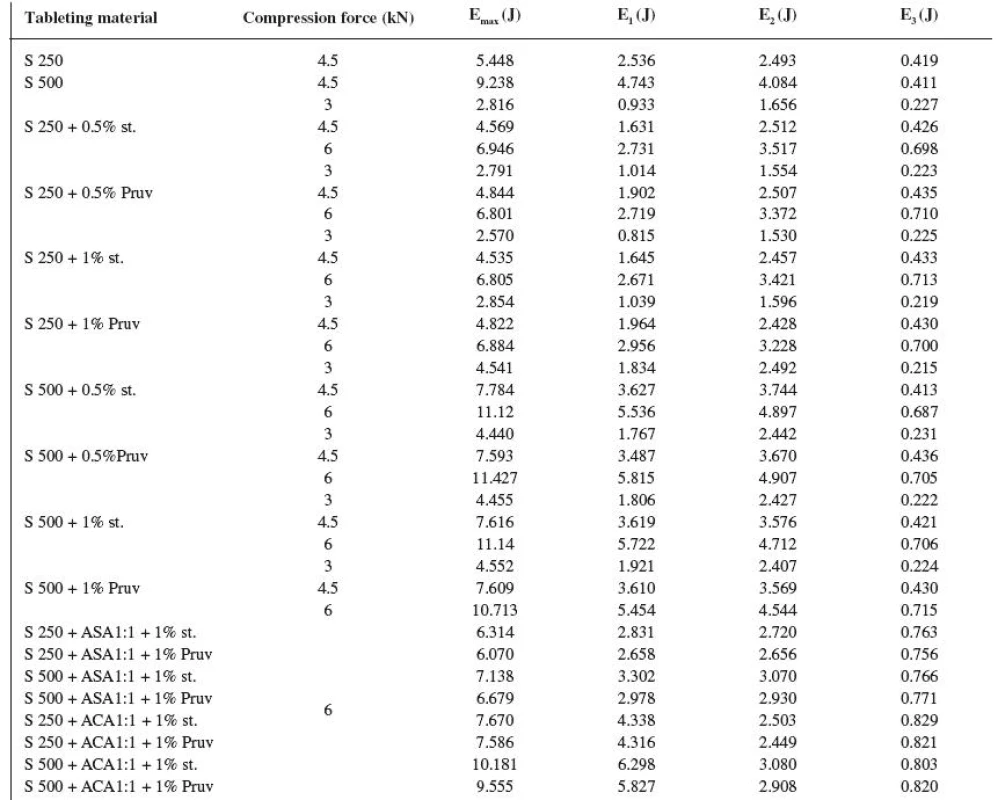
The comparison of both types of directly compressible sorbitol resulted in the conclusion that a negative moment for the use of Sorbitab SD 500 is the effect of the presence of lubricants on the strength of the compacts, then further also a more significant prolongation of disintegration time of tablets by the action of the lubricant in contrast to Sorbitab SD 250. The energy balance of compression produced a higher value of total energy of compression for Sorbitab SD 500 due to higher values of energy required for friction and the energy accumulated by the tablet.
The study was supported by the grant MSM 0021620822 and by the firm SPI Pharma, which supplied the samples of the dry binders tested.
Address
for correspondence:
PharmDr.
Jitka Mužíková, Ph.D.
Charles University in Prague, Faculty of Pharmacy in Hradec Králové
Department
of Pharmaceutical Technology, Czech Republic
Heyrovského
1203, 500 05 Hradec Králové
e-mail:
muzikova@faf.cuni.cz
Sources
1. Kibbe, A. H.: Handbook of pharmaceutical excipients. 3rd Ed. London: APhA Washington and PhP 2000; 515–518.
2. Bolhuis, G. K., Chowhan, Z. T.: Materials for direct compaction. In Alderborn, G, Nystrőm, Ch. (eds.) Pharmaceutical Powder Compaction Technology, Inc. New York: Marcel Dekker 1996, 419–500.
3. Du Ross, J. W.: Modification of the crystalline structure of sorbitol and its effect on tableting characteristics. Pharm. Technol., 1984; 8, 42–53.
4. Schangraw, R. F., Wallace, J. W., Bowers F. M.: Morphology and functionality in tablet excipients for direct compression: part I. Pharm. Technol., 1981; 5, 69–78.
5. Guyot-Hermann, A.M., Leblanc, D., Draguet-Brughmans, M.: Gamma sorbitol as a diluent in tablets. Drug Dev. Ind. Pharm., 1985; 11, 551–564.
6. Schmidt, P. C.: Tableting characteristics of sorbitol. Pharm. Technol., 1983; 7, 65–74.
7. SPI Pharma: Sorbitab™ SD 250 and Sorbitab™ SD 500 Spray Dried Sorbitol. Fir.Lit., Web: http://www. spipharma.com/ProductsFolder/SD250_SD500/SD250_SD500.html (2. 11. 2007).
8. Ragnarsson, G.: Force-displacement and network measurements. In Alderborn, G@ Nystrőm, Ch., eds. Pharmaceutical Powder Compaction Technology, Inc. NeyYork: Marcel Dekker 1996, 77–132.
9. Fell, J. T., Newton, J. M.: Determination of tablet strength by diametral-compression test.J. Pharm. Sci., 1970; 59, 688–691.
10. Bos, C. E., Bolhuis, H., Van Doorne, Lerk, C. F.: Native starch in tablet formulations: Properties on compaction. Pharm. Weekbl. 1987; Sci. Ed. 9, 274–282.
11. Belousov, V. A.: K voprosu o vybore optimalnikh davlenii pressovania pri tabletirovanii lekarstvennykh poroshkov. Khim. Farm. Zh., 1976; 10, 105–111.
12. Jarosz, P. J., Parrot, E. L.: Effect of lubricants on tensile strengths of tablets. Drug Dev. Ind. Pharm., 1984; 10, 259–273.
Labels
Pharmacy Clinical pharmacologyArticle was published in
Czech and Slovak Pharmacy

2010 Issue 2
-
All articles in this issue
- Manufacture of granulates containing high potency drugs
- Microparticles on the oxycellulose base – influence of processual variables on encapsulation efficiency
- Implementation of radio-frequency identification (RFID) in the process of preparation and administration of drugs – part 2
- Identification and susceptibility evaluation of Candida yeasts due to the optimization of ciclopiroxolamine release from mucoadhesive oral tablets
- Medicinal preparations in Czech pharmacies at the end of the 17th century
- A study of the compaction process and the properties of tablets from two types of directly compressible sorbitol
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Identification and susceptibility evaluation of Candida yeasts due to the optimization of ciclopiroxolamine release from mucoadhesive oral tablets
- Manufacture of granulates containing high potency drugs
- Medicinal preparations in Czech pharmacies at the end of the 17th century
- Microparticles on the oxycellulose base – influence of processual variables on encapsulation efficiency


