Composition of fatty acids in Centaurea cyanus (L.)
Složení mastných kyselin v Centaurea cyanus (L.)
Práce prezentuje výsledky studie mastných kyselin (FA) ve květech a nati volně rostoucí a pěstované chrpy (Centaurea cyanus (L.)). Analýza byla provedena metodou plynové chromatografie (GC) s vnitřní normalizací. V nati obou typů chrpy bylo identifikováno 13 mastných kyselin. V květech a nati pěstovaných chrp, stejně jako v květech volně rostoucích, převládaly nenasycené mastné kyseliny, zejména kyselina linolová, linolenová a olejová. Kyselina palmitová představovala nejhojněji se vyskytující nasycenou FA.
Klíčová slova:
Centaurea cyanus (L.) – Fatty acids – GC method
Authors:
Iryna B. Pietkova Liana M. Unhurian Liliia M. Horiacha Viktoriia S. Kyslychenko Iryna O. Zhuravel; Yu. Viiktoria Kuznietsova Leksandr I. Panasenko
Published in:
Čes. slov. Farm., 2020; 69, 194-197
Category:
Short communication
Overview
The presented study shows the composition of fatty acids (FA) in flowers and herb of wild and cultivated cornflower (Centaurea cyanus (L.)). The analysis was performed by gas chromatography (GC) with a method using internal normalization. Together 13 fatty acids were identified in both types of cornflower herb. Unsaturated fatty acids, particularly, linoleic, linolenic, and oleic acids, were prevailing in cultivated cornflower flowers and herb, as well as in wild cornflower flowers. Palmitic acid was the most abundant saturated FA.
Keywords:
fatty acids
Introduction
Centaurea cyanus (L.) is a plant of the Asteraceae family, originating from Europe and Middle East and now present all over the world in both wild and cultivated variants1). In traditional medicine, the cornflower herb is known as a diuretic, antimicrobial, anti-inflammatory, antioxidant, gastric mucosa protective, hypotensive agent1–8). Cornflower chemical components include flavonoids, anthocyanins, carbonic acids, sugars, amino acids, macro - and microelements, and tocopherols1, 5, 7, 9, 10). Fatty acids are not only vital macronutrients, but also promising pharmacological bioactive substances. Polyunsaturated fatty acids are known to be precursors of eicosanoids which act as lipid mediators, settling inflammations, immune and neuroprotective processes.
Fatty acids, such as saturated stearic and palmitic acids, unsaturated oleic, linoleic and linolenic acids, are potential antibacterial agents able to destabilize cell membranes of microorganisms, thus directly and indirectly suppressing their growth13, 14). Bioactive lipid mediators to which the most unsaturated fatty acids, such as linoleic and α-linolenic acids, belong settle pro-inflammatory and anti-inflammatory processes, stimulating enzymes and producing cytokines11).
Pharmacological research has proved that fatty acids, such as unsaturated linoleic, α-linolenic, eicosapentaenoic and docosahexaenoic acids, retard tumor growth, inducing apoptotic processes in tumor cells, inhibiting angiogenesis, as well as improving efficiency of chemical drugs and alleviating their side effects12, 15–19). Various bioactive substances in complexes with fatty acids, such as saturated palmitic acid and unsaturated lenolic, linolenic and docosahexaenoic acids, are known to possess improved lipophilic properties, becoming better compatible with lipophilic cell membranes. Such complexes are used to slow drug release; they are more bioaccessible12, 20–22).
Data on fatty acids in cornflower herb in the literature are quite few. Fernandes L. et al. studied the fatty acid composition of cornflower flowers which contained 45.0% saturated and 55.0% unsaturated fatty acids. The most abundant were linolenic (27.7%), palmitic (25.2%), and oleic (19.8%) acids9).
This paper is devoted to a study of fatty acids in Centaurea cyanus flowers and herb by GC method.
Experimental part
Materials and methods
Plant materials
The objects of study were wild cornflower and cultivated cornflower herb and flowers collected during blooming period in Kharkov Region, Ukraine, in 2018.
Identification of plants was carried out by the professor of the Department of Botany of National University of Pharmacy A. G. Serbin in comparison with voucher herbarium samples. The voucher herbarium samples are kept at the Department of Chemistry of Natural Compounds and Nutritionology of the National University of Pharmacy.
Determination of fatty acids
Methyl esters of fatty acids were studied using a Selmichrom-1 (Ukraine) gas chromatograph with a flame ionization detector. A gas chromatography column of stainless steel, 2.5 m long and 4 mm in the inner diameter, was filled with immobile phase – inert on treating with 10% diethylene glycol succinate (DEGS).
The following working parameters were established in the chromatograph: column thermostat temperature 180 °С, vaporizer temperature 230 °С, detector temperature 220 °С, carrier gas (nitrogen) flow velocity 30 cm3/min, sample volume 2 mm3 hexane solution of fatty acid methyl esters.
Reference samples were standards of saturated and unsaturated fatty acid methyl esters from Sigma.
Lipophilic fractions were obtained by exhaustive hexane extraction, they were hydrolyzed and then the developed fatty acid methyl esters determined.
Fatty acid methyl esters were obtained by the modified Peisker method which ensured complete methylation of fatty acids. Methylation was affected with a 100 : 100 : 1 mixture of chloroform with methanol and sulfuric acid. Lipophilic fraction in a volume of 30–50 μl was dosed into glass ampoules, 2.5 ml of methylating mixture were added, and then the ampoules were sealed. Then they were introduced to a thermostat at 105 °С for 3 hours. After methylation completed, the ampoules were unpacked, their contents moved to a test tube, powdered zinc sulfate added at a scalpel tip, 2 ml purified water and 2 ml hexane poured for extraction of methyl esters. After thorough stirring and settling, the hexane extract was filtered and subjected to chromatographic analysis23).
Fatty acid methyl esters were identified by peak retention time as compared to the standard mix. Composition of methyl esters was calculated by the interior normalization method.
Results and discussion
Thirteen fatty acids were identified in tested raw materials. Of them, seven fatty acids were saturated (С12–С24), four fatty acids were monounsaturated (С14–С20), and two fatty acids were polyunsaturated (С18).
Fatty acid composition of wild and cultivated cornflower flowers and herb lipophilic fractions was determined by the GC method (Fig.1). The GC-chromatogram on the example of fatty acids in cultivated cornflower herb is shown in Figure 1.
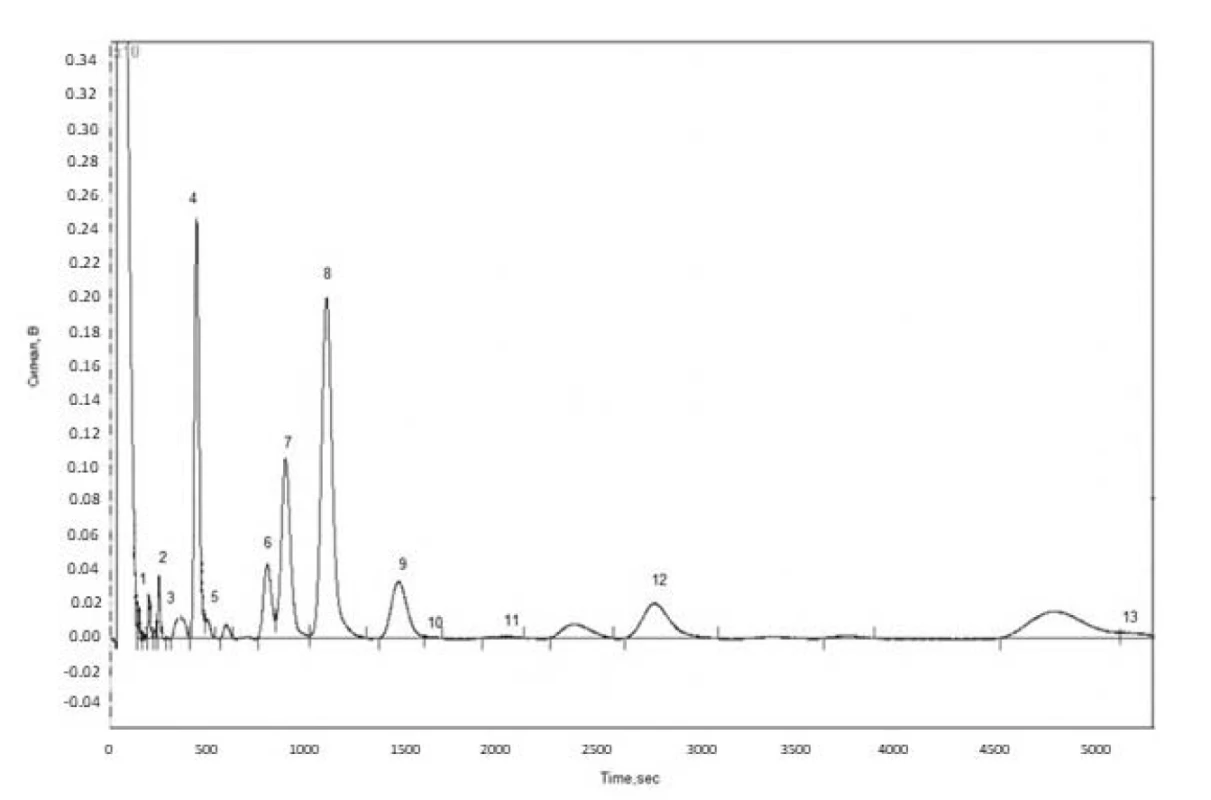
As the result of the study, 13 fatty acid methyl esters were identified in each of wild and cultivated cornflower flowers and herb lipophilic fractions (Table 1).
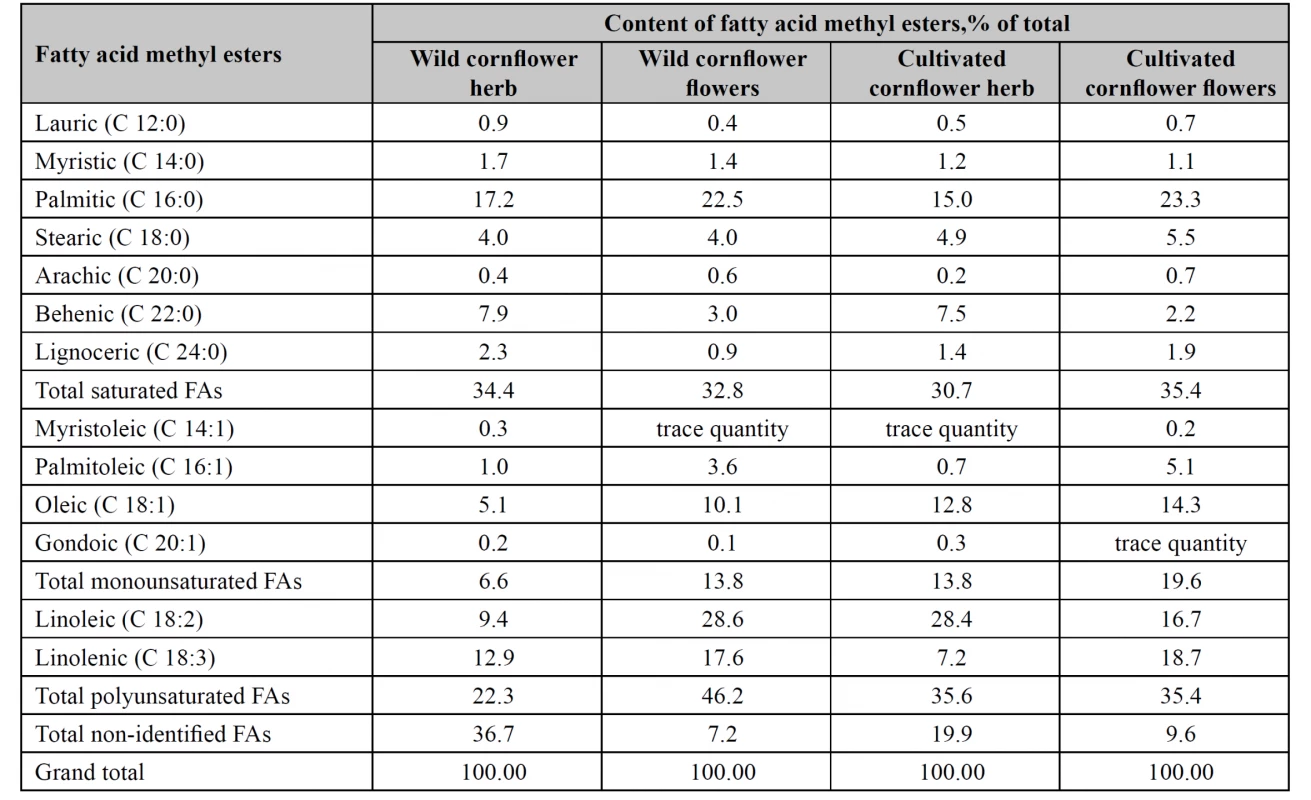
The sum of saturated FAs in tested raw materials was almost identical (30.7–35.4%). The highest content of monounsaturated FAs was found in the flowers of the cultivated cornflower (19.6%), the lowest content was found in the wild cornflower herb (6.6%). The content of the sum of monounsaturated FAs in the flowers of the wild cornflower and in the herb of the cultivated cornflower varied within the limits of 13.8%. Wild cornflower flowers accumulated most polyunsaturated FAs, particularly, linoleic and linolenic acids, whereas their content in the wild cornflower herb was the lowest.
The sum of non-identified fatty acids in the wild cornflower herb was 36.7%, in the cultivated cornflower herb, 19.9%, in the wild cornflower flowers, 7.2%, and in the cultivated cornflower flowers, 9.6%.
In the cultivated cornflower herb, wild and cultivated cornflower flowers, unsaturated fatty acids prevailed. Wild cornflower flowers contained 60.0% unsaturated fatty acids, cultivated cornflower flowers contained 55.0% unsaturated fatty acids, cultivated cornflower herb contained 49.4% unsaturated fatty acids, and wild cornflower herb contained only 28.9% unsaturated fatty acids.
In wild and cultivated cornflower flowers we identified 28.6% and 16.7% linoleic acid, 22.5% and 23.3% palmitic acid, 17.6% and 18.7% linolenic acid, 10.1% and 14.3% oleic acid.
Wild cornflower herb accumulated palmitic (17.2%) acid, linolenic (12.9%) acid, linoleic (9.4%) acid and behenic (7.9%) acid, whereas the cultivated cornflower herb contained linoleic (28.4%), palmitic (15.0%), oleic (12.8%), behenic (7.5%), linolenic (7.2%) acids.
Palmitoleic acid content in tested samples of cornflower flowers varied within the limits 3.6–5.1%, being much lower in the herb: 0.70–1.0%.
Tested cornflower herbs accumulated nearly identical amounts of saturated acids, namely, lauric, myristic, stearic, arachic, and gondoic acids.
Conclusions
In this study we compared the fatty acid composition of wild and cultivated cornflower herbs and flowers collected in Ukraine. Unsaturated oleic (5.1–14.3%), linoleic (9.4–28.6%), linolenic (7.2–18.7%) acids as well as saturated palmitic acid (15.0–23.3%) dominated in tested raw materials.
Our obtained results show only minor difference between the fatty acid compositions of wild and cultivated cornflower raw materials, thus, cultivated cornflower herbs may serve as a substitute for wild raw materials. The herb and flowers of the cornflower are a promising raw material for further phytochemical and pharmacological research.
Acknowledgments
The research was funded by the Ministry of Health of Ukraine from the government budget.
Conflicts of interest: none.
I. B. Pietkova • L. M. Unhurian
Department of Organization and Economics of Pharmacy
Odessa National Medicinal University, Odessa, Ukraine
Liliia M. Horiacha () • V. S. Kyslychenko • I. O. Zhuravel • V. Y.
Kuznietsova
Department of Chemistry of Natural Compounds and Nutritionology
National University of Pharmacy
Valentynivska 4, 61168 Kharkiv, Ukraine
e-mail: lilia4252@ukr.net
О. I. Panasenko
Department of Natural Sciences for Foreign Students and Toxicological
Chemistry
Zaporizhzhia State Medical University, Zaporizhzhia, Ukraine
Sources
1. Al-Snafi A. E. The pharmacological importance of Centaurea cyanus – a review. IJPRR 2015; 5(4), 379–384.
2. Garbacki N., Gloguen V., Damas J. Antiinflammatory and immunological effects of Centaurea cyanus flower-heads. J Ethnopharmacol. 1999; 68, 235–241.
3. Escher G. B., Santos J. S., Rosso N. D., Marques M. B., Azevedo L., do Carmo M. A. V., Daguer H., Molognoni L., Prado-Silva L. D., Sant’Ana A. S., da Silva M. C., Granato D. Chemical study, antioxidant, anti-hypertensive, and cytotoxic/cytoprotective activities of Centaurea cyanus L. petals aqueous extract. Food Chem Toxicol. 2018; 118, 439–453.
4. Klimas R., Rabiskovi M., Civinskiene G., Bernatoniene J. The diuretic effect of cornflower water extract. Medicina (Kaunas) 2007; 43(3), 221–225.
5. Nowicka P., Wojdyło A. Anti-hyperglycemic and anticholinergic efects of natural antioxidant contents in edible flowers. Antioxidants 2019; 8(308), 1–12.
6. Park J. B. Synthesis, biological activities and bioavailability of moschamine, a safflomide-type phenylpropenoic acid amide found in Centaurea cyanus. Natural Product Research: Formerly Natural Product Letters 2012; 26(16), 1465–1472.
7. Pirvu L., Armatu A., Rau I., Şchiopu S., Coprean D. Centaurea cyanus L. herba, chemical composition and therapeutic potential. Proceeding of the International Symposium 2008; 187–194.
8. Pirvu L., Dragomir C., Schiopu S., Mihul S. C. Vegetal extracts with gastroprotective activity. Part. I. Extracts obtained from Centaurea cyanus L. raw material. Romanian Biotechnological Letters 2012; 17(2), 7169–7176.
9. Fernandes L., Pereira J. A., Saraiva J. A., Ramalhosa E., Casal S. Phytochemical characterization of Borago officinalis L. and Centaurea cyanus L. during flower development. Food Res. Int. 2019; 123, 771–778.
10. Muraveva D. A., Bubenchikova V. N. Phenolcarboxylic acids of the flowers of Centaurea cyanus. Chem. Nat. Compd. 2007; 22(1), 102.
11. Johnson M., Bradford C. Omega-3, Omega-6 and Omega-9 fatty acids: implications for cardiovascular and other diseases. J Glycomics Lipidomics 2014; 4(4), 1–8.
12. Jóźwiak M., Filipowska A., Fiorino F., Struga M. Anticancer activities of fatty acids and their heterocyclic derivatives. Eur. J. Pharmacol. 2020; 871(172937), 1–13.
13. McGaw L. J., Jäger A. K., Staden van J. Antibacterial effects of fatty acids and related compounds from plants. S. Afr. J. Bot. 2002; 68, 417–423.
14. Yoon B. K., Jackman J. A., Valle-González E. R., Cho N.-J. Antibacterial free fatty acids and monoglycerides: biological activities, experimental testing, and therapeutic applications. Int. J. Mol. Sci. 2018; 19(1114), 1–40.
15. Chamras H., Ardashian A., Heber D., Glaspy J. A. Fatty acid modulation of MCF-7 human breast cancer cell proliferation, apoptosis and differentiation. J. Nutr. Biochem. 2002; 13(12), 711–716.
16. Serini S., Piccioni E., Merendino N., Calviello G. Dietary polyunsaturated fatty acids as inducers of apoptosis: implications for cancer. Apoptosis 2009; 14(2), 135–152.
17. Siddiqui R. A., Harvey K. A., Xu Z., Bammerlin E. M., Walker C., Altenburg J. D. Docosahexaenoic acid: a natural powerful adjuvant that improves efficacy for anticancer treatment with no adverse effects. Biofactors 2011; 37(6), 399–412.
18. Spencer L., Mann C., Metcalfe M., Webb M., Pollard C., Spencer D., Berry D., Steward W., Dennison A. The effect of omega-3 FAs on tumour angiogenesis and their therapeutic potential. Eur. J. Cancer 2009; 45(12), 2077–2086.
19. Wen B., Deutsch E., Opolon P., Auperin A., Frascogna V., Connault E., Bourhis1 J. n-3 Polyunsaturated fatty acids decrease mucosal/epidermal reactions and enhance antitumour effect of ionising radiation with inhibition of tumour angiogenesis. Br. J. Canc. 2003; 89(6), 1102–1107.
20. Bala V., Rao S., Li P., Wang S., Prestidge C. A. Lipophilic prodrugs of SN38: synthesis and in vitro characterization toward oral chemotherapy. Mol. Pharm. 2016; 13(1), 287–294.
21. Borkar N., Li B., Holm R., Hakansson A. E., Müllertz A., Yang M., Mu H. Lipophilic prodrugs of apomorphine I: preparation, characterisation, and in vitro enzymatic hydrolysis in biorelevant media. Eur. J. Pharm. Biopharm. 2015; 89, 216–223.
22. Tao Y., Yang F., Meng K., Chen D., Yang Y., Zhou K., Luo W., Qu W., Pan Y., Yuan Z., Xie S. Exploitation of enrofloxacin-loaded docosanoic acid solid lipid nanoparticle suspension as oral and intramuscular sustained release formulations for pig. Drug Deliv. 2019; 26(1), 273–280.
23. Pohodina L., Burda N., Kyslychenko V. Fatty acids composition study of birthwort dutchman’s pipe (Aristolochia clematitis L.) herb and roots. Norwegian Journal of development of the International Science 2019; 31, 53–57.
Labels
Pharmacy Clinical pharmacologyArticle was published in
Czech and Slovak Pharmacy

-
All articles in this issue
- Vaccines from the perspective of a pharmacist
- Collagen in combination with the acid form of carboxymethylcellulose in the form of a nonwoven textile as a modern wound dressing – formulation, preparation and evaluation
- Application of inhaled drugs from the pharmacist’s point of view
- Simultaneous determination of amoxicillin and potassium clavulanate in combined medicinal forms: procedure transfer from HPLC to UPLC
- Composition of fatty acids in Centaurea cyanus (L.)
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Vaccines from the perspective of a pharmacist
- Application of inhaled drugs from the pharmacist’s point of view
- Collagen in combination with the acid form of carboxymethylcellulose in the form of a nonwoven textile as a modern wound dressing – formulation, preparation and evaluation
- Composition of fatty acids in Centaurea cyanus (L.)
