Simultaneous determination of amoxicillin and potassium clavulanate in combined medicinal forms: procedure transfer from HPLC to UPLC
Současné stanovení amoxicilinu a klavulanátu draselného v kombinovaných lékových formách: přenos postupu z HPLC na UPLC
Článek představuje výsledky přenosu postupu vysoce účinné kapalinové chromatografie (HPLC) pro stanovení amoxicilinu a klavulanátu draselného v tabletách do podmínek ultravysokoúčinné kapalinové chromatografie (UPLC). Protože Státní lékopis Ukrajiny (SPhU) neobsahuje monografii pro simultánní analýzu amoxicilinu a kyseliny klavulanové, byl použitý postup podle Britského lékopisu. Parametry postupu byly optimalizovány tak, aby vyhovovaly UPLC a zlepšily výkon. Přenos metody do podmínek UPLC umožnil zkrátit dobu chodu z 15 minut na 7,5 minuty, což činí proces méně časově náročným a nákladově efektivnějším. Vylepšený postup byl dále ověřen. Validace obou metod byla provedena z hlediska linearity, přesnosti, správnosti, specificity a stability. Metoda HPLC byla ověřena pro pozdější implementaci do monografie SPhU. Poté byly metody porovnány z hlediska jejich dopadu na životní prostředí pomocí ekologické stupnice, která zahrnovala rizika řešení, množství vyprodukovaných odpadů, dopad na pracovníky životního prostředí a laboratoře atd. Obě metody se ukázaly být ekologické s mírnou výhodou pro metodu UPLC. Statistické srovnání bylo navíc provedeno regresní metodou Passing Bablok. Ukázalo se, že obě metody jsou statisticky srovnatelné.
Klíčová slova:
UPLC – HPLC – amoxicillin/clavulanate formulations – method comparison – green chemistry
Authors:
Anna O. Dobrova; Olga S. Golovchenko; Ivan V. Bezruk; Liudas Ivanauskas; Victoriya Georgiyants
Published in:
Čes. slov. Farm., 2020; 69, 186-193
Category:
Original article
Overview
This article presents the results of transferring a high-performance liquid chromatography (HPLC) procedure for the assay of amoxicillin and potassium clavulanate in tablets to the ultra-performed liquid chromatography (UPLC) conditions. Since the State Pharmacopoeia of Ukraine (SPhU) does not contain the monograph for the simultaneous analysis of amoxicillin and clavulanic acid, the British Pharmacopoeia procedure was used. Parameters of the procedure were optimized to fit the UPLC and to make a better performance. Transfer of the method to the UPLC conditions allowed to shorten the run time from 15 min to 7.5 min, which makes the process less time-consuming and more cost-effective. The upgraded procedure was further validated. Validation of both methods was performed in terms of linearity, precision, accuracy, specificity and stability. HPLC method was verified to later implementation into the SPhU’s monograph. Afterwards, the methods were compared in terms of their impact on the environment using the eco-scale that included hazards of the solution, the amount of produced wastes, the impact on environmental and laboratory staff, etc. Both methods appeared to be eco-friendly with a moderate advantage of UPLC method. Moreover, the statistical comparison was performed using Passing Bablok regression method. It showed that both methods are statistically comparable.
Introduction
Amoxicillin is a broad-spectrum semi-synthetic β-lactam antibiotic. Recently, it has been widely used in combination with clavulanic acid or its derivative potassium clavulanate. Clavulanic acid is a β-lactamase inhibitor, which prevents the enzymatic destruction of the β-lactam ring of amoxicillin by β-lactamase, and has also shown minimal antibacterial activity1). According to the World Health Organisation’s (WHO) latest list of Critically Important Antimicrobials for Human Medicine, β-lactam antibiotics with β-lactamase inhibitors are the high priority drugs since they have shown a lower resistance potential and have a high frequency of use for various indications. Thereby, it is recommended as a first-choice medicine for treating upper and lower respiratory tract diseases, hospital and community-acquired pneumonia, exacerbations of chronic obstructive pulmonary disease (COPD), etc. There is also a possibility of its usage in the treatment of complications of novel virus infections of the respiratory tract and to prevent secondary infection2–4). Amoxicillin still plays a significant role in the treatment of Helicobacter pylori associated gastritis according to the last Maastricht V consensus since its moderate resistance5). It is also used in the treatment of complicated intraabdominal infections, lower urinary tract infections, skin and soft tissue infections, etc. The chemical structures of amoxicillin and clavulanic acid are presented in Figure 1.
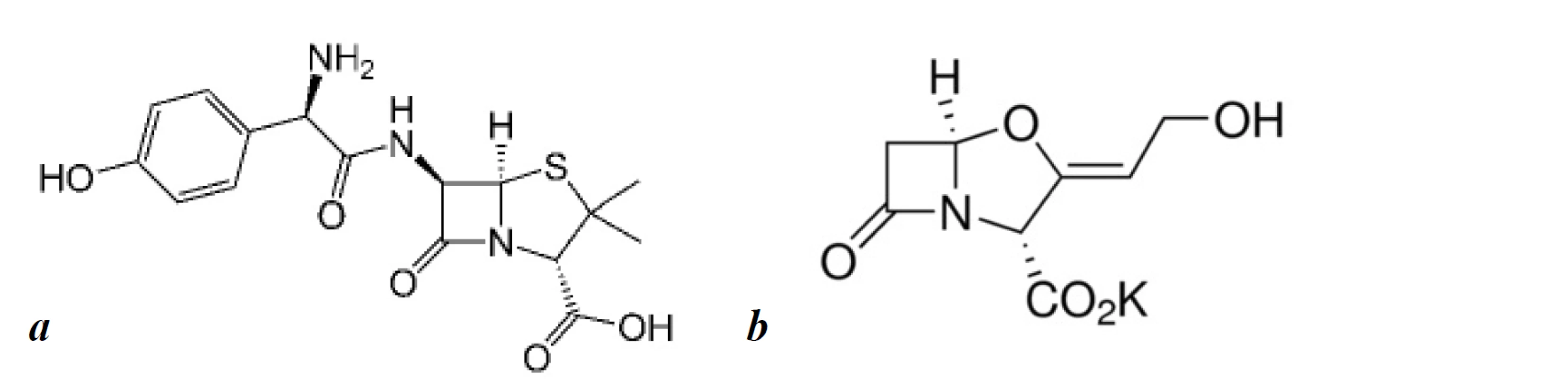
Effectiveness of the therapy depends on the quality of active pharmaceutical ingredients which are components of dosage forms. The State Pharmacopoeia of Ukraine does not have any requirements for quality control of medicines that contain amoxicillin and potassium clavulanate. Thus verification of the method is necessary to deliver qualitative treatment for patients in Ukraine.
European Pharmacopoeia as well as the United States Pharmacopoeia recommend estimating the quality of amoxicillin and potassium clavulanate combined medicinal forms by high-performance liquid chromatography (HPLC)6, 7). With technology improvement, ultra-performance liquid chromatography (UPLC) method provides some notable advantages such as speed, while maintaining high sensitivity and resolution comparable to HPLC. These advantages can be useful for manufacturers to reduce costs during analysis. The UPLC analysis is less time-consuming, which makes the analysts’ time more efficient and productive.
The analytical method selection should not be only in terms of accuracy or precision but also its environmental and health safety. Whereas the UPLC method takes fewer volumes of solvent, it can be hypothetically assumed to be eco-friendlier and less harmful to analysts’ health8).
In this study, we aimed to transfer HPLC procedures to UPLC to check the equality of measurements and compare their environmental and health impact.
Experimental part
Chemicals and reagents
Amoxicillin and potassium clavulanate standards were purchased from Sigma-Aldrich GmbH (Steinheim, Germany). All solvents were HPLC grade, methanol was purchased from Honeywell (Germany) and purified water was obtained from a Mili-Q purification system (Millipore, Burlington, MA, USA). Five manufacturing batches (184153, 187489, 184111, 185691, 184117) of combined amoxicillin and clavulanic acid tablets – AMOXIL-K625 (Arterium Corporation, Ukraine) were purchased in the local pharmacy and taken as study samples.
Equipment
Liquid chromatography separation was performed using a Shimadzu Nexera X2 LC-30AD HPLC system (Shimadzu, Japan) composed of a quaternary pump, an on-line degasser, a column temperature controller, a SIL-30AC autosampler (Shimadzu, Japan), a CTO-20AC thermostat (Shimadzu, Japan) as well as a SPD-M20A diode array detector (DAD). Other instruments such as an Ultrasonic Cleaner Set for ultra-sonication use (Wise Clean WUC-A06H, Witeg Labortechnik GmbH, Germany), a Libra UniBloc AUW120D (Shimadzu Analytical Scale, Japan); “A” class volumetric flasks that meet requirements of the State Pharmacopoeia of Ukraine were used in the investigation9).
Stock and working solutions
Buffer solution pH 4.4. 7.8 g of sodium phosphate monobasic was dissolved in 950 ml of water R and adjusted to pH 4.4 ± 0.05 with phosphoric acid diluter, then filled up to the volume of 1000.0 mL with the same solvent, and mixed.
The test solution. To 500 mg (accurate weight) of Amoxil-K625 tablets powder (equivalent to 250 mg of amoxicillin) 400 ml of water were added, shaken at a speed of 400 rpm (Shaker SM 30 B (Edmund Buhler, Germany)) for 20 minutes, and filled up to the volume of 500 mL.
Standard solution. 100 mg (accurate weight) of amoxicillin trihydrate analytical standard and 80 mg (accurate weight) of potassium clavulanate analytical standard were placed into a 200 mL volumetric flask, dissolved in water, filled up to the mark, and mixed.
Placebo solution. Accurate weight of placebo (500 mg) was placed in a volumetric flask of a volume of 500 mL, 400 mL of water were added, shaken at a speed of 400 rpm (Shaker SM 30 B; Edmund Buhler, Germany) for 20 minutes, filled up to the volume of 500 mL, and mixed.
All solutions were filtered through a membrane filter with a pore diameter of 0.45 µm.
Chromatographic conditions for HPLC analysis
The separation of components was performed on a RP column ACE C18 (250 mm × 4.6 mm, with particle size of 5 µm, Pennsylvania, USA). The composition of the mobile phase was buffer solution pH 4.4 and methanol in the ratio 95 : 5, respectively. The flow rate was set at 1.0 mL/min. The wavelength was set at 220 nm. 20 µL was injected into the chromatographic system.
Chromatographic conditions for UPLC method
The analysis was performed with an ACQUITY UPLC BEH C18 column (50 mm × 2.1 mm, with particles size 1.7 µm, Milford, USA). The composition of the mobile phase was buffer solution with pH 4.4 and methanol in the ratio 98 : 2, respectively. The detection was performed at 220 nm with a flow rate at 0.1 mL/min. The volume of injection was 0.2 µL.
Method validation
Both HPLC and UPLC procedures were validated according to the requirements of ICH guidelines and the State Pharmacopoeia of Ukraine in terms of linearity, precision, accuracy, specificity, and stability9, 10).
Assessment of methods influence on the environment
Parameters of the analytical methods such as the number of reagents, hazards, energy, and waste define its impact on the environment11, 12). Determination of method greenness was performed using Eco-scale, where the ideal value is 100. If some parameters of analysis differ from the principles of green chemistry, penalty points are assigned. The sum of penalty points obtained after revision is taken into account for Eco-scale calculation using the following formula:
• Analytical Eco-scale = 100 – penalty points
• > 75 represents an excellent green method
• > 50 represents an acceptable green method
• < 50 represents an inadequate green method
Globally Harmonized System (GHS) of Classification and Labeling of Chemicals provides full information about the determination of safety class of reagent based on physical, environmental, and health hazards13). M. Tobiszewski et al. proposes determination of penalty points for each reagent by multiplying the amount of GHS hazard pictograms by a hazard degree (for instance, a warning is 1, and danger, 2)14). Also, the energy consumptions for HPLC and UPLC are ≤ 1.5 kWh per sample and < 0.1kWh per sample, respectively14). Besides, hazards and generation of wastes were taken into account.
Method comparison between HPLC and UPLC
Five batches (27 samples) of combined amoxicillin/potassium clavulanate tablets previously analyzed by HPLC were re-analyzed by the described UPLC method. For all analytes, the correlation between the methods was tested by a Passing-Bablok fit. It was chosen since the preliminary check of the data samples for compliance with the normal distribution showed that data samples of amoxicillin (measured by UPLC method) and potassium clavulanate (measured by HPLC) did not correspond to a normal distribution (р < 0.01). This limits the use of ordinary least squares method for regression analysis. Therefore, the non-parametric Passing-Bablok method was taken15). Comparison procedure was performed by Microsoft Excel 2013.
Results and discussion
Verification of HPLC Pharmacopoeial method
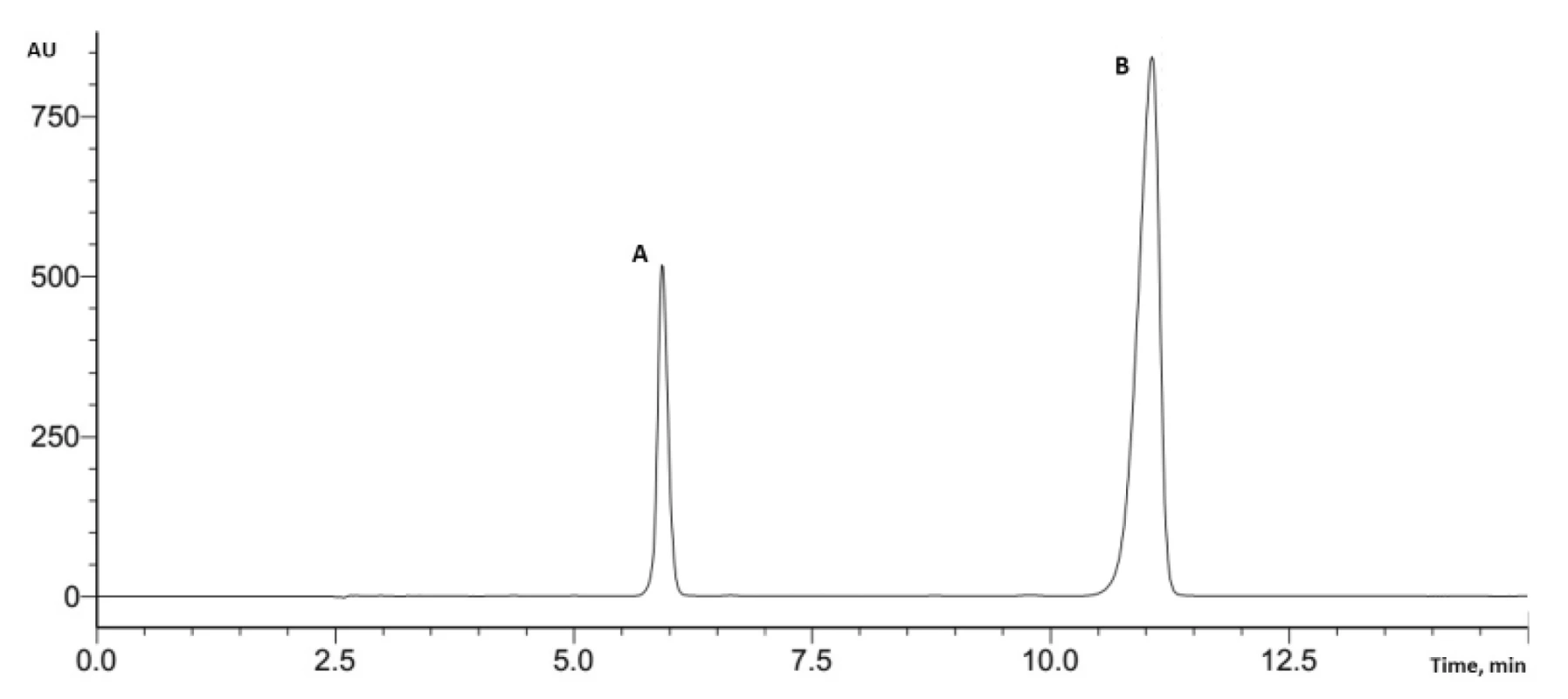
The British Pharmacopoeia proposes to use the HPLC method for the assay of medicines containing potassium clavulanate and amoxicillin6). For separation of components, a stainless steel column (250 mm × 4.6 mm, with a particle size of 5 µm) packed with octadecylsilyl silica gel for chromatography is required. The mobile phase is a mixture of 5 volumes of methanol and 95 volumes of a 0.78% solution of sodium phosphate monobasic adjusted to pH 4.4 with phosphoric acid. The flow rate of the mobile phase should be 2 mL/min. The volume of injection is 20 µL, and the detection wavelength is set at 220 nm.
When the parameters on the HPLC system were applied, a high pressure was observed at this flow rate. Considering that, the flow rate was reduced to 1 mL/min. These changed parameters were used in further analysis of tablets containing potassium clavulanate and amoxicillin (Fig. 2).
Transfer of HPLC method to UPLC
Initially, the flow rate was reduced to 0.2 mL/min, and a smaller volume of the injection was applied (1 µL). The analysis showed no separation between the components; therefore, the flow rate was changed to 0.1 mL/min.
After reducing the flow rate, the coefficient of separation between components was 2.02 but still, the peak of potassium clavulanate was close to the void volume.
To increase separation, different buffer pH was tried in the range of 4.0–6.0. Changed conditions had no improvements for compounds separation, thus the buffer with 4.4 pH was left.
In the next step, we changed the ratio between buffer and methanol to 97 : 3 and reduced the volume of injection to 0.5 µL. As a result, the separation between peaks became greater. Moreover, retention time for the peak of potassium clavulanate increased significantly and moved from the void volume.
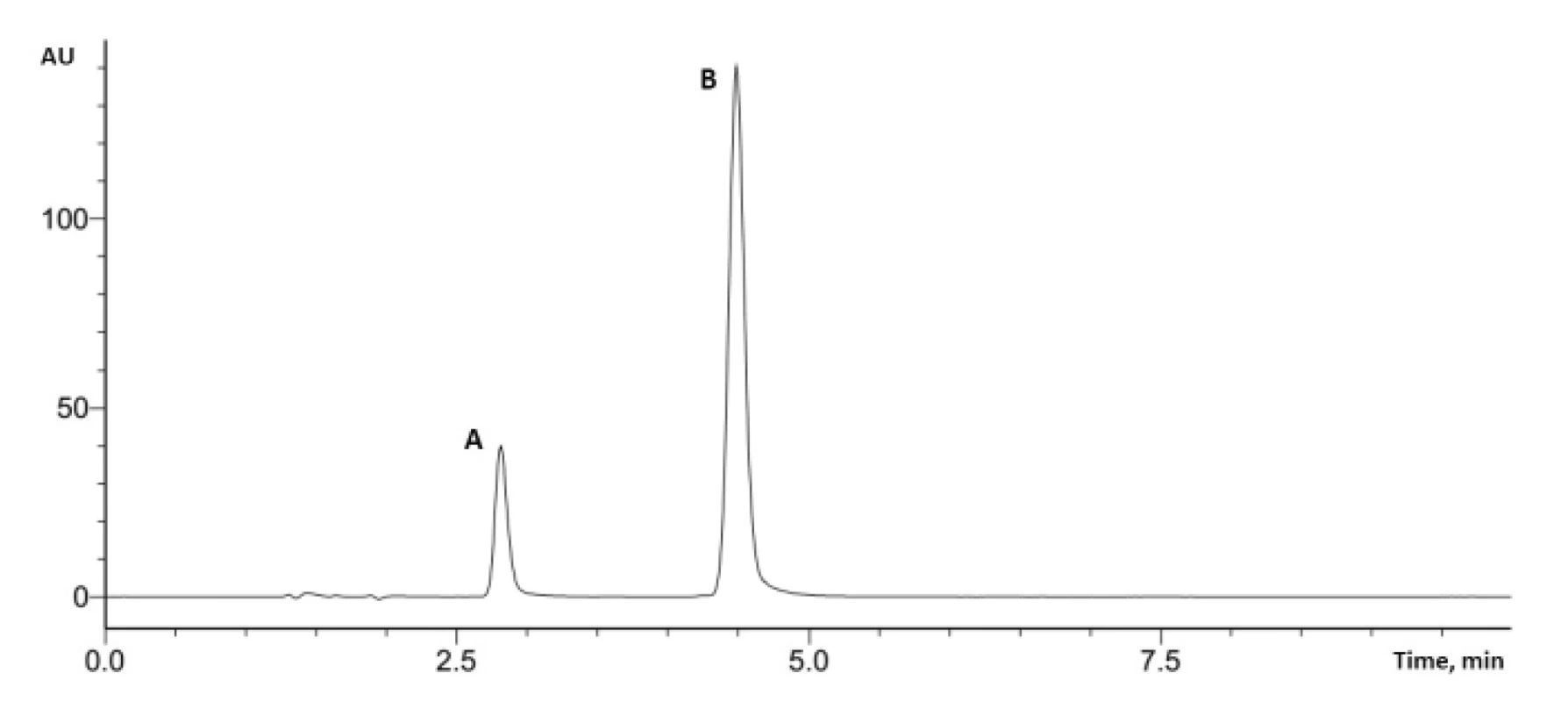
And at the final step, we changed the ratio between buffer and methanol to 98 : 2 and set a volume of injection at 0.2 µL.
These conditions were used in the assay of potassium clavulanate and amoxicillin in tablets (Fig.3).

Suitability of chromatographic methods
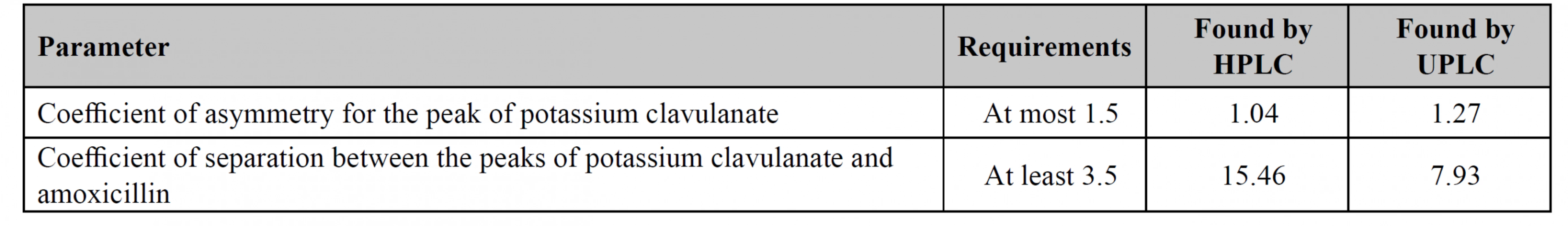
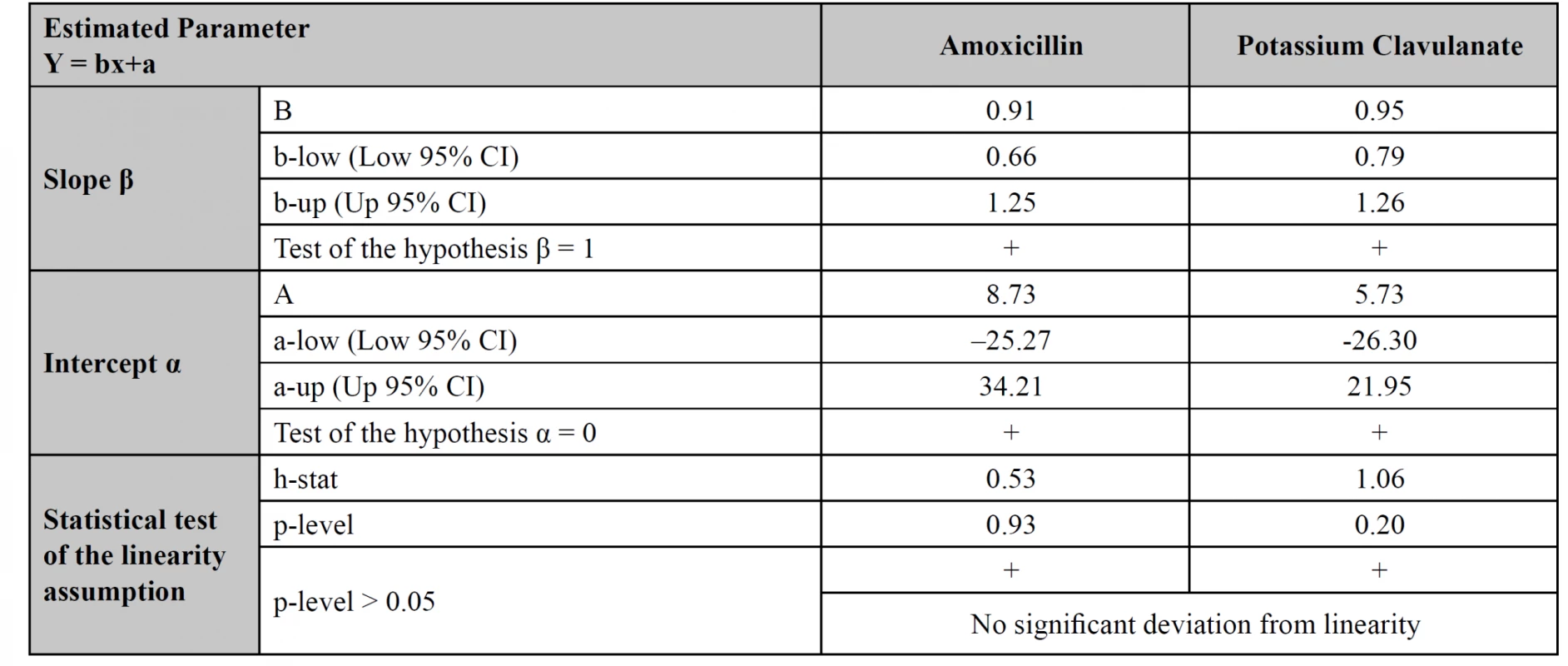
The main purpose of the system suitability test is to prove the reproducibility of the chromatographic system and to examine applied procedures, equipment, reagents, etc. The British Pharmacopoeia requires to control two parameters: the coefficient of asymmetry for potassium clavulanate and the coefficient of separation between potassium clavulanate and amoxicillin. Both methods have met all requirements, thus they can be recommended for applying in the analysis of medicines containing amoxicillin and potassium clavulanate (Table 1).
Validation
Specificity
Specificity studies were performed for both HPLC and UPLC methods by analyzing blank, placebo (containing all components except potassium clavulanate and amoxicillin), standard, and test solutions. According to the obtained results, no interference in the analysed components quantification from blank or placebo solutions was found. Thus, these procedures can be applied in the analysis of tablets contain potassium clavulanate and amoxicillin.
Linearity, LOD, LOQ
The assay procedure should be linear within the range of application, which should overlap the possible values of concentrations of the active substance. Linearity studies were performed in the range of 80–120% (step – 5%). For this purpose, 9 model solutions were prepared, the concentration of which varied uniformly within the range of application (Table 2).
The LOD and LOQ were calculated by the following formulas:
where σ is the standard deviation of the response, and S is the slope of the calibration curve9, 10).
The values of LOD and LOQ are presented in Table 2.
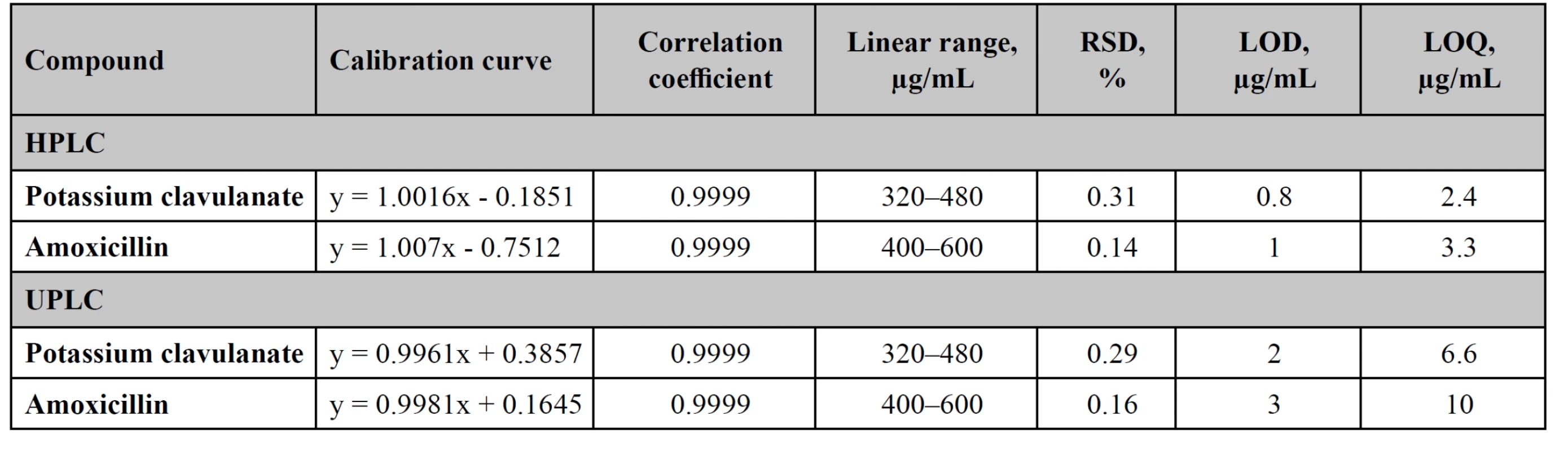
Precision
The precision study was performed within 2 days by different analysts. Test solution contained 100% of potassium clavulanate and amoxicillin. The results of the studies are shown in Table 3.
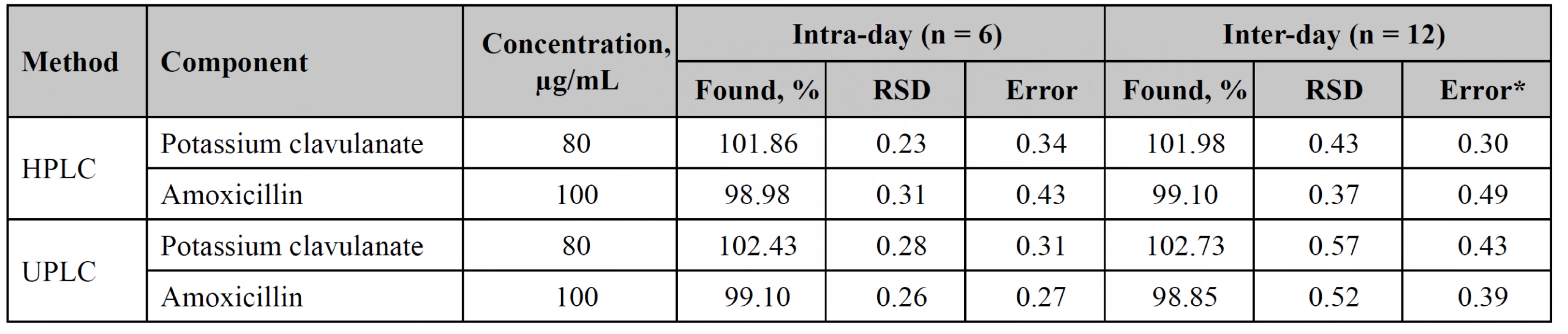
Stability
Stability studies have been performed for both procedures within 24 hours for a standard solution. It was found that the solutions were stable. Deviations of analyte peaks areas in the HPLC method for potassium clavulanate and amoxicillin were 0.338% and 0.362%, respectively. In the case of UPLC, potassium clavulanate and amoxicillin showed deviations of 0.391% and 0.164%, respectively.
Accuracy
To test accuracy within the range of analytical procedure, 9 test solutions with a known amount of potassium clavulanate (PC) and amoxicillin (AC) were prepared, following all stages of the analytical procedure. The measured solutions were in the range of 80–120% (Table 4). Both procedures have satisfactory results, thus they can be used in the analysis of medicines.
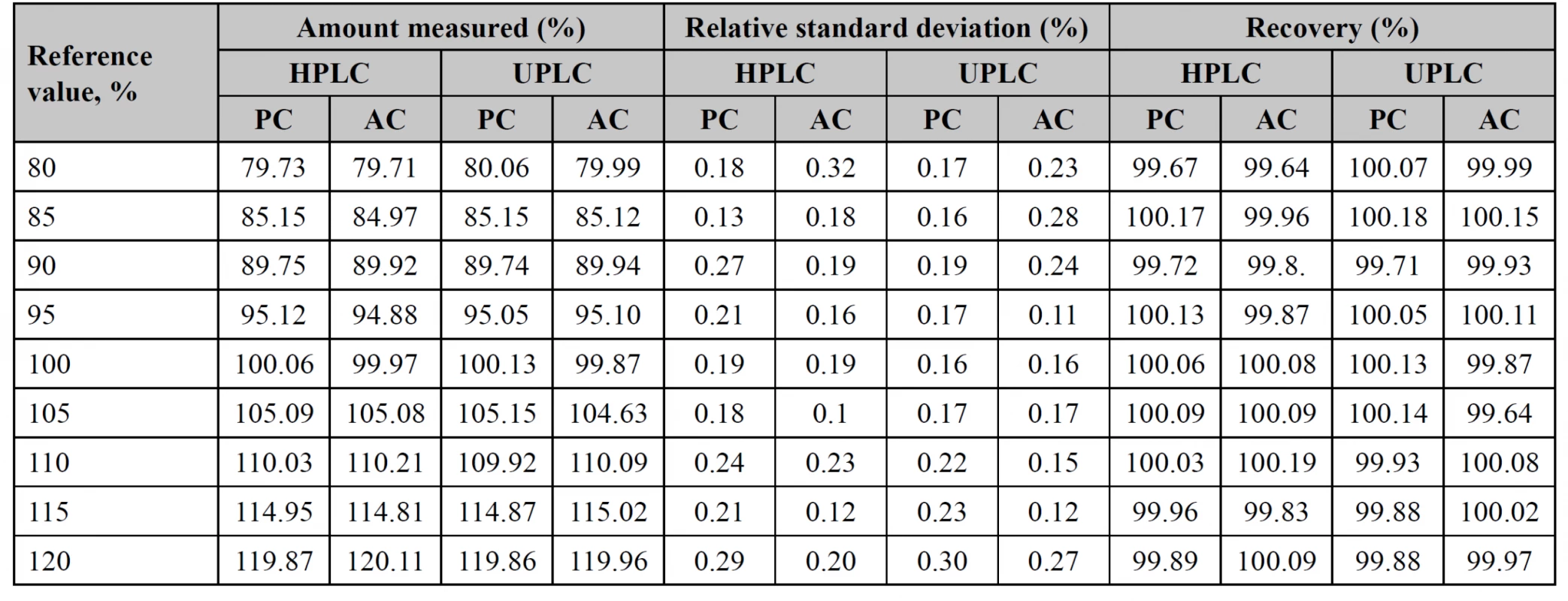
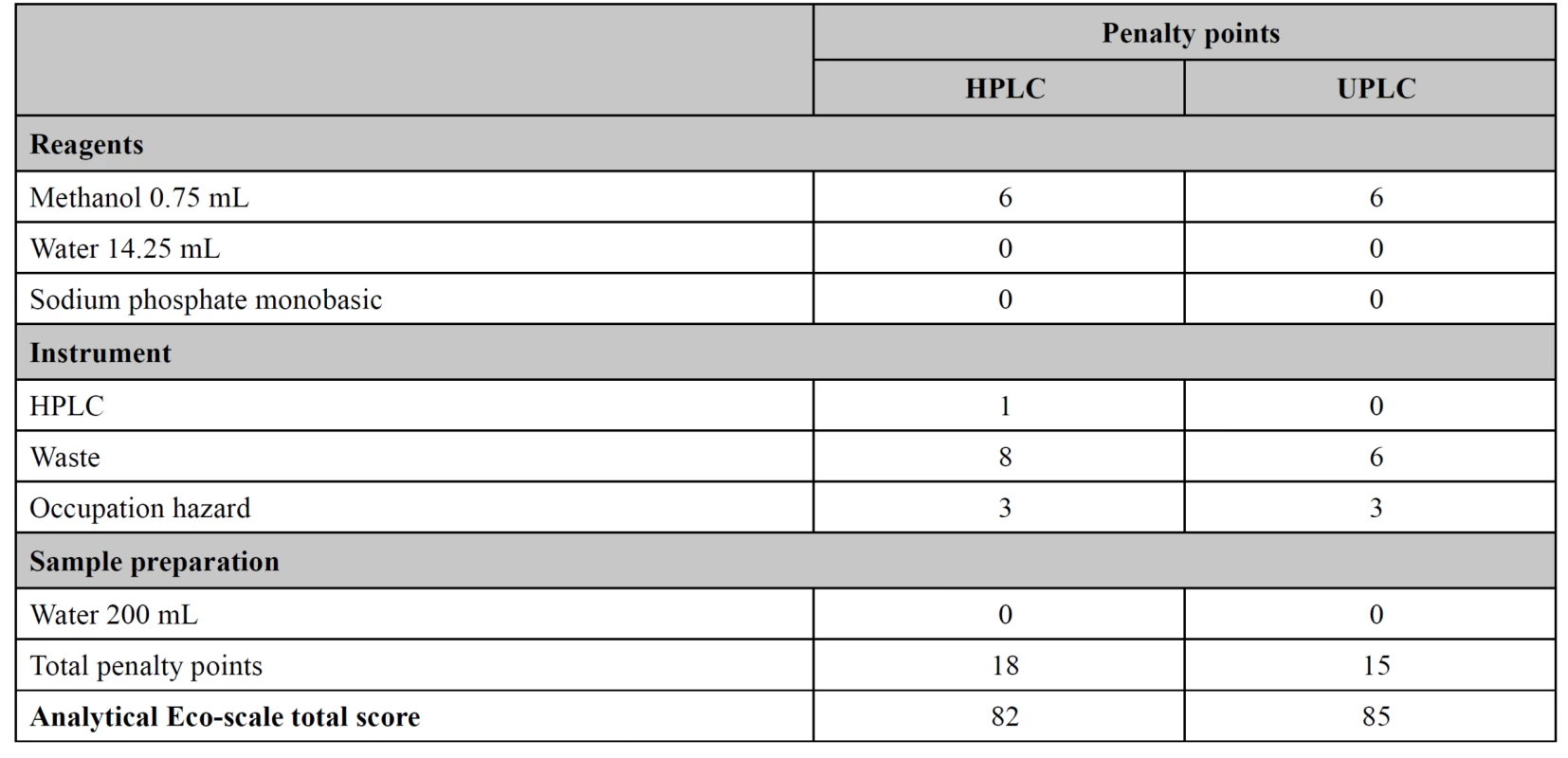
Eco-scale calculation
Both methods for simultaneous assay of amoxicillin and potassium clavulanate were assessed by their “greenness” using the Eco-scale to find a method with less environmental impact. The UPLC method showed a slightly higher Eco-scale value than the HPLC. The total scores for HPLC and UPLC were 82 and 85 respectively (Table 5).
Method comparison between HPLC and UPLC
The methods comparison was performed using the values obtained in the quantitative assessment of 27 tablets samples, measured by both HPLC and UPLC methods. The assessment was done using Passing-Bablok regression equations (estimation of α, β), 95% CI for a, b (test of hypothesis β = 1 and α = 0), a test of linearity assumption (Table 6) and corresponding regression plots with real measurement data (Fig. 4)15). In plots, the regression lines (continuous) are in the range of amoxicillin and potassium clavulanate estimated quantitative contents from 98 to 104%. The real measurement data are presented by dots. The statistical test of the linearity assumption proved linearity between the HPLC and UPLC methods for both amoxicillin and potassium clavulanate (Table 6). The 95% CI included the value 1 for the slope and the value 0 for the intercept for both studied substances, which demonstrated that there is no statistically significant difference between the old and the new method.
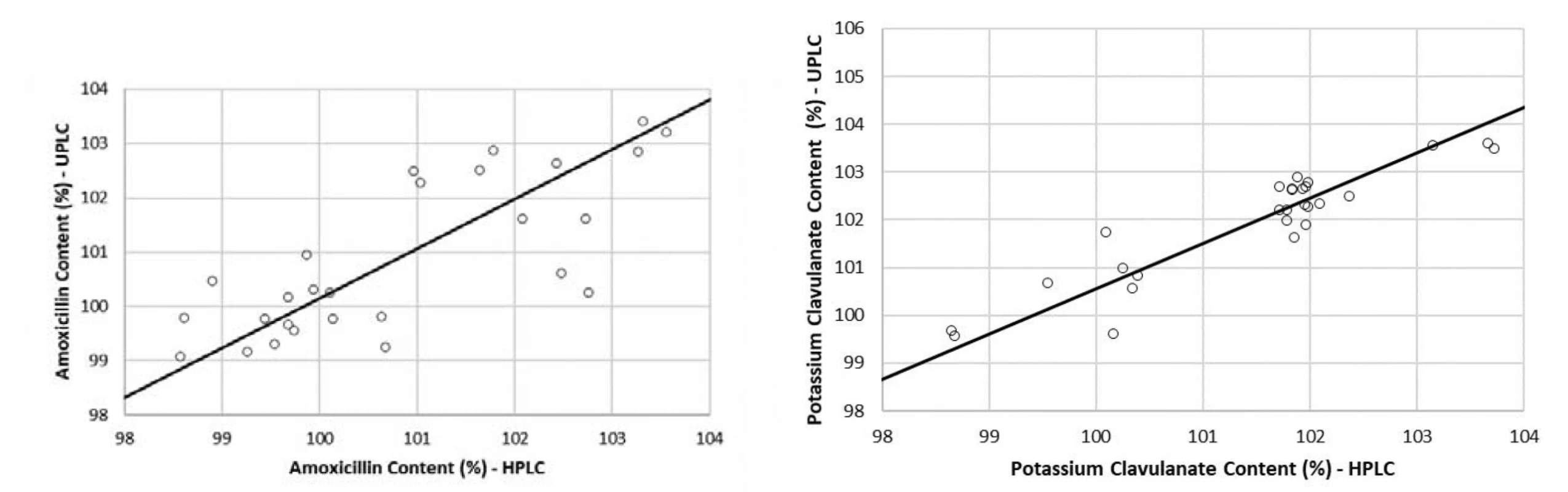
Conclusions
The HPLC procedure for the simultaneous assay of amoxicillin and potassium clavulanate was successfully transferred to the UPLC. To optimize the conditions of the analysis, we have changed the ratio of mobile phase components, flow rate, and injection volume. Furthermore, the run time was shortened from 15 min to 7.5 min. The HPLC procedure was verified to further implementation into the SPhU monograph, and the UPLC procedure was fully validated. Both methods have met all requirements, and could be recommended for applying in the analysis of medicines containing amoxicillin and potassium clavulanate. The methods were compared in terms of environmental friendliness. In general, both methods have shown themselves to be eco-friendly, although the UPLC method showed a slightly better result. A Passing-Bablok regression method comparison showed the similarity of methods in obtained results. Both methods are reliable and could be used in laboratories during the quality control process.
Conflict of interest: none.
Anna O. Dobrova – PhD student ) • O. S. Golovchenko • I. V. Bezruk
• V. Georgiyants
Department of Pharmaceutical Chemistry
National University of Pharmacy
61100 Valentynivska Str. 4, Kharkiv, Ukraine
e-mail: anna.dobrova08@gmail.com
L. Ivanauskas
Department of Analytical and Toxicological Chemistry
Lithuanian University of Health Sciences
A. Mickevičiaus Str. 9, LT 44307 Kaunas, Lithuania
Sources
1. Finlay J, Miller L, Poupard JA. A review of the antimicrobial activity of clavulanate. J. Antimicrob. Chemother. 2003; 52(1), 18–23.
2. World Health Organization Model List of Essential Medicines, 21st List, 2019. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO. https://apps.who.int/iris/handle/10665/325771 (28.07.2020).
3. WHO latest list of Critically Important Antimicrobials for Human Medicine 5th rev. Geneva: World Health Organization; 2019. https://www.who.int/foodsafety/publications/antimicrobials-sixth/en/ (28.07.2020).
4. Liang T. Handbook of COVID-19 Prevention and Treatment. The First Affiliated Hospital. Zhejiang University School of Medicine. https://gmcc.alibabadoctor.com/prevention-manual/detail?content_id=0 (28.07.2020).
5. Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon ATR, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, El-Omar EM, Kuipers EJ, European Helicobacter Study Group. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017; 66(1), 6–30.
6. British Pharmacopoeia Commission. British Pharmacopoeia 2017: Volume III. London: TSO 2017.
7. United States Pharmacopeia and National Formulary (USP 40-NF 35). Rockville, MD: United States Pharmacopeial Convention 2017.
8. Raynie D, Driver JL. Green assessment of chemical methods. In: Proceedings of the 13th Green Chemistry & Engineering Conference. USA 2009.
9. State Pharmacopoeia of Ukraine 2nd ed. 1st supplement. Kharkiv: State Enterprise “Scientific-and-expert Pharmacopeial Centre” 2015; 1128 pp.
10. ICH harmonized tripartite guideline Q2(R1). Validation of analytical procedures: text and methodology Q2(R1). In: Proceedings of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. Switzerland 2005.
11. Gałuszka A, Migaszewski ZM, Konieczka P, Namieśnik J. Analytical Eco-Scale for assessing the greenness of analytical procedures. TrAC Trends in Anal. Chem. 2012; 37, 61–72.
12. Bezruk I, Kotvitska A, Korzh I, Materiienko A, Gubar S, Budanova L, Ivanauskas L, Vyshnevsky I, Georgiyants V. Combined Approach to the Choice of Chromatographic Methods for Routine Determination of Hederacoside C in Ivy Leaf Extracts, Capsules, and Syrup. Sci. Pharm. 2020; 88(2), 24.
13. United Nations. Globally Harmonized System of Classification and Labeling of Chemicals (GHS, Rev. 4). USA 2011. https://digitallibrary.un.org/record/704047?ln=en (28.07.2020).
14. Tobiszewski M, Marć M, Gałuszka A, Namieśnik J. Green Chemistry Metrics with Special Reference to Green Analytical. Chemistry Molecules 2015; 20(6), 10928–10946.
15. Passing H, Bablok W. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I. J. Clin. Chem. Clin. Biochem. 1983; 21(11), 709–720.
Labels
Pharmacy Clinical pharmacologyArticle was published in
Czech and Slovak Pharmacy

-
All articles in this issue
- Vaccines from the perspective of a pharmacist
- Collagen in combination with the acid form of carboxymethylcellulose in the form of a nonwoven textile as a modern wound dressing – formulation, preparation and evaluation
- Application of inhaled drugs from the pharmacist’s point of view
- Simultaneous determination of amoxicillin and potassium clavulanate in combined medicinal forms: procedure transfer from HPLC to UPLC
- Composition of fatty acids in Centaurea cyanus (L.)
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Vaccines from the perspective of a pharmacist
- Application of inhaled drugs from the pharmacist’s point of view
- Collagen in combination with the acid form of carboxymethylcellulose in the form of a nonwoven textile as a modern wound dressing – formulation, preparation and evaluation
- Composition of fatty acids in Centaurea cyanus (L.)
