Prospects of сomplex pharmaceutical composition application for pharmacological correction of metabolic syndrome
Možnosti využití komplexní farmaceutické kompozice pro farmakologickou korekci metabolického syndromu
Metabolický syndrom (MetS) je symptomatický komplex charakterizovaný inzulinovou rezistencí, poruchou prooxidačně-antioxidační rovnováhy organismu s rozvojem subchronického zánětu a dyslipidemií. Cílem studie je prozkoumat vlivkomplexní farmaceutické kompozice (CPC) (antioxidanty a metabolitotropní látky), která se v lékařské praxi na Ukrajině široce používá jako multivitaminový komplex, na experimentální metabolický syndrom u potkanů. Byl hodnocen vliv CPC na korekci experimentálního MetS u potkanů, vyvolaného vysokým obsahem sacharidů a tuků ve stravě. MetS u potkanů byl charakterizován snížením citlivosti buněk na inzulin, zvýšeným obsahem glukózy a porušením její utilizace, a prooxidačně-antioxidační dysbalancí. Výsledky provedených studií naznačují pozitivní vliv CPC, který obsahuje etylestery omega-3 kyselin, vitamin E, koenzym Q10, zinek, vitamin A, biotin a selen, na citlivost buněk k inzulinu, utilizaci glukózy, trvání hyperglykémie a ukazatele oxidačních procesů volných radikálů a antioxidačníchobranných systémů u potkanů s experimentálním MetS. Tyto výsledky dokazují možnost využití CPC k korekci metabolického syndromu.
Klíčová slova:
metabolický syndrom – hyperglykemie – citlivost buněk na inzulin – prooxidačně-antioxidační rovnováha
Authors:
Kateryna Kalko 1; Nadia Dukhnich 2; Oksana Mishchenko 2; Viktor Khomenko 3; Olena Toziuk 4
Authors‘ workplace:
Department of Pharmacology and Drug Technology of I. I. Mechnikov Odesa National University
1; Department of Clinical Pharmacology of the National University of Pharmac, Kharkiv, Ukraine
2; Department of Pharmacology and Pharmacy of the Donetsk National Medical University, Lyman, Ukraine
3; Department of Pharmacy, National Pirogov Memorial Medical University, Vinnytsya, Ukraine
4
Published in:
Čes. slov. Farm., 2023; 72, 287-296
Category:
Original Articles
doi:
https://doi.org/10.5817/CSF2023-6-287
Overview
Metabolic syndrome (MetS) is a symptomatic complex characterized by insulin resistance, impaired prooxidant-antioxidantbalance of the body with the development of subchronic inflammation, and dyslipidemia. The aim of the study is to investigate the effect of a complex pharmaceutical composition (CPC) (antioxidants and metabolitotropic agents), which is widely used in medical practice in Ukraine as a multivitamin complex, on experimental metabolic syndrome in rats. The effectof CPC on the correction of experimental MetS in rats, induced by a high content of carbohydrates and fats in the diet, was assessed. MetS in rats was characterized by a decrease in the sensitivity of cells to insulin, increased glucose content, and aviolation of its utilization, prooxidant-antioxidant disbalance. The results of the conducted studies indicate the positive effect of CPC, which contains ethyl esters of omega-3 acids, vitamin E, coenzyme Q10, zinc, vitamin A, biotin, and selenium, on the sensitivity of cells to insulin, glucose utilization, duration of hyperglycemia and indicators of free radical oxidation processes and antioxidant defense systems in rats with experimental MetS. These results prove the feasibility of using CPC to correct metabolic syndrome.
Keywords:
metabolic syndrome – hyperglycemia – sensitivity of cells to insulin – prooxidant-antioxidant balance
Introduction
Metabolic syndrome (MetS) is a symptomatic complex characterized by insulin resistance and impaired prooxidant-antioxidant balance of the body with the development of subchronic inflammation and dyslipidemia. It predictscardiovascular diseases and type 2 diabetes mellitus (T2DM)1–3). It was found that MetS causes a 5-fold increase in the riskof developing T2DM, a 2-fold risk of developing cardiovascular diseases over the next 5 to 10 years, 2-4 times increased risk ofstroke, 3–4 times myocardial infarction and twice the risk of death1, 4). Given the above, timely prevention and treatment of MetS is an important task.
Insulin sensitizers biguanide derivatives – metformin, a drug with proven efficacy for treating MetS, are used as drugs for the pharmacological correction of MetS. Considering the importance of oxidative stress in the development of MetS, using agents with antioxidant activity is advisable to prevent MetS5–7). These are plant phenolic substances (quercetin), as well as vitamins(A, E, C) and trace elements (zinc, selenium), correctors of mitochondrial function (coenzyme Q)8–13). The described datahighlight the positive effect of the individual compounds, but the impact of their combined use is unknown.
The aim of the study is to investigate the effect of a complex pharmaceutical composition (CPC) (containing antioxidantsand metabolitotropic agents), which is widely used in medical practice in Ukraine as a multivitamin complex, on the course of experimental metabolic syndrome in rats.
Experimental part
Materials and methods
The research was conducted based on the Educational and Scientific Training Center for Biomedical Research of the Educational and Scientific Institute of Applied Pharmacy of the National University of Pharmacy (NUPh). During theexperiment, the animals were kept in the vivarium of the NUPh training center at an air temperature of 20-22 C, a natural day-night light regime, in standard cages, on a standard diet. All manipulations with animals were carried out following the requirements of GLP, recommendations of the State Expert Center of the Ministry of Health of Ukraine, “General Ethical Principles of Experiments on Animals” (Ukraine, 2001), Law of Ukraine dated February 21, 2006 No. 3447-IV withamendments “On the protection of animals from cruel treatment,” the decree and the National Congress on Bioethics (Kyiv, 2007), and the European Convention for the Protection of Vertebrate Animals Used for Experimental or other Scientific Purposes14).
The effect of CPC on the correction of experimental MetS in rats, induced by a high content of carbohydrates and fats in the diet, was assessed by the sensitivity of cells to insulin, glucose utilization, duration of glycemia, and indicators of free radical oxidation and antioxidant defense systems.
Research object and comparison drugs
Complex pharmaceutical composition (CPC) (AEVIT PREMIUM produced by the Joint Stock Company Kyiv Vitamin Plant)(ethyl esters of omega-3 acids – 280 mg, vitamin E – 65 mg coenzyme Q10 – 30 mg zinc (as part of zinc oxide) – 15 mg, vitamin A – 1765 μg biotin – 150 μg selenium (as part of sodium selenite) – 100 mg and excipients)15). Metformin in the example of the drug Siofor® tab. 500 mg produced by Berlin-Chemie/A. Menarini Ukraine GmbH16). Vitamin E by theexample of the preparation “Vitamin E” 100 mg/ml, 20 ml produced by the Joint Stock Company Lekhim17).
Animals and Experimental Protocol
An experimental model of metabolic syndrome (EMetS) in rats was caused by a high content of carbohydrates and fats in the diet by enriching the diet with fructose (adding fructose to the feed and replacing the drink with a 10.0% fructose solution – in total in the diet up to 20.0% of the daily caloric value) and animal fats (total lard and fat in the diet up to 20.0%of daily calories) for 18 weeks18).
Male albino Wistar rats weighing 220–240 g were used. The conduct of the experiment was coordinated with the Bioethics Commission of the National University of Pharmacy (Protocol dated June 25, 2021, No. 5). The animals were divided into 4 groups of 6 rats:
- – intact control (IC), animals that were kept on a standard vivarium diet and consumed a diet balanced in terms of the setof proteins, fats, carbohydrates, essential microelements, and vitamins (composition: cereal grains; high-protein components, skimmed milk powder, meat, salcium P (monogastric), apple pectin, coccidiostat, antioxidant, PF VITA, Ukraine);
- – not treated animals with EMetS (control pathology group, CP), in which the diet was enriched with fructose and fats (as indicated above);
- – animals with EMetS, which were treated with CPC at a dose of 25.8 mg/kg intragastrically (w/w)19);
- – animals with EMetS that were treated with vitamin E at a dose of 100 mg/kg intragastrically (w/w)20);
- – EMetS animals that were treated with metformin at a dose of 60 mg/kg intragastrically (w/w)18).
CPC, metformin, and vitamin E were used in a therapeutic regimen, starting from the 15th week of modeling the control pathology for four weeks (28 days).
Assessment of glucose metabolism
Glucose metabolism in rats with EMetS was assessed by the test of insulin resistance and glucose tolerance bydetermining the level of basal glycemia and insulinemia in the blood serum with subsequent calculation of the homeostatic model assessment of insulin resistance (HOMA-IR) index21). The duration of glycemia was assessed by the content of glycosylated hemoglobin (HbA1C)18). The insulin content in the blood serum was determined by the enzyme immunoassay using the DRC1 Insulin Elisa reagent kit (Germany). Serum glucose and HbA1C levels were determined spectrophotometrically using Filisit-Diagnostics kits(Ukraine). The sensitivity of cells to insulin was determined by a short insulin test, assessing the % decrease in the basal glycemic content 30 min after administering insulin (1 U/ kg of the rat body)22). We also investigated the glycemic response under the action of CPC and reference drugs by assessing the area under the glycemic curve during the intraperitoneal glucose tolerance test (IGTT) (glucose 2 g/kg rat body)22) (blood sampling from the tail vein to glucose load 0 value (output level) and at 15, 30 and 45 minutes after glucose administration) and during the oral glucose tolerance test (OGTT) (glucose 3 g/kg rat body)22) (blood sampling was carried out from the tail vein to load with glucose 0 value (baseline) and at 15, 30, 60 and 120 minutes after glucose administration. Blood samples were collected from the tail vein through a temporary surgicalcannula. The animals were under general anesthesia caused by introducing thiopental sodium (Thiopentalum natriсumlyophilisate for solution for injection 0.5 g bottle; PJSC Kyivmedpreparat) at a dose of 40 mg/kg. The selected whole blood in avolume of 1–2 drops was immediately applied to the test strip glucometer Contour Plus (Ascensia Diabetes Care).
Determination of the activity of the processes of free radical oxidation (FRO), lipid peroxidation (LPO), and oxidative modification of proteins (OMP)
The state of the antioxidant defense system (AOD) was determined by the content of SH-groups and the index of the total antioxidant activity of blood serum in %. Total antioxidant activity was determined by the spectrophotometric method,measuring the degree of inhibition of the formation of peroxidation products in a suspension of egg yolk lipoproteins with the addition of whey. A suspension of yolk lipoproteins (YLP) was obtained by homogenizing chicken egg yolk in an equal volume of phosphate buffer (40 mM KH2PO4, 105 mM KCl, pH 7.45). The resulting suspension was diluted 25 times with the same buffer before use. The optical density of the control (without the addition of serum) and samples (with the addition of serum) was measured against the blank at a wavelength of 532 nm on a Scolar PV spectrophotometer (Spectronic CamSpec M550, UK; UV-1800 UV Spectrophotometer Shimadzu, Japan).
Total antioxidant activity was calculated using the formula:
TAA = ((ΔODes – ΔODcs) / ΔODes) × 100%
where TAA – total antioxidant activity; ΔODes = ODesa – ODes; ΔODcs = ODcsa – ODcs; ODes Determination of the activi optical density of the experimental sample before incubation; ODcs Determination of the activioptical density of control samples before incubation; ODesa – optical density of the experimental sample after incubation; ODcsa – optical density of control samples after incubation.
The measurement of ΔODes and ΔODcs is necessary to consider the initial degree of oxidation of the liquid lipid suspension and test samples.
Determination of the concentration of SH groups is based on the reaction of sulfhydryl groups with 5,5-dithiobis-(2-nitrobenzoic acid) to form yellow-colored thionitrophenyl anion (in equimolar concentration), with an absorption maximum at 412 nm. Optical density measurements were carried out using a Scolar PV spectrophotometer.
The content of thiobarbituric acid of active products (TBA-AP) and diene conjugates (DC) were indicators of LPO. The content of TBA-AP was determined by measuring the concentration of malondialdehyde (MDA), which, when heated, interacts with 2-thiobarbituric acid, forming a colored complex with an absorption maximum at λ = 533 nm; measurements were carried out on a Scolar-PV spectrophotometer. Determination of diene conjugates (DC) was done by measuring the concentration of fatty acids in a heptane extract from blood serum containing conjugated double bonds and having an absorption maximum at λ = 233 nm. Measurements were carried out on a Scolar PV spectrophotometer.
Carbonylation of proteins was an indicator of oxide modification of proteins (OMP). To determine carbonylated proteins, we measured the amount of oxidized amino acid residues in protein molecules, which, in reaction with 2,4-dinitrophenylhydrazine, form 2,4-dinitrophenylhydrazones with an absorption maximum at λ = 363 nm. Measurementswere carried out on a Scolar PV spectrophotometer.
Statistical analysis
Statistical processing was performed using the Statistica 6.0 software (StatSoft, Inc., USA), and the normal distribution was checked using the W-Shapiro-Wilsey test. The data were found to be subject to an abnormal distribution, so a nonparametric Mann-Whitney U-test was used, and the results were presented as median (Me) and interquartile range (25–75 percentiles). The accepted level of significance was p < 0.05. To obtain statistical conclusions, we used the standard Statistica software package23).
Results and discussion
Long-term feeding of rats with a diet enriched with fructose and animal fats led to the development of insulin resistance and impaired glucose utilization, as evidenced by an increase in the level of basal glycemia by 36.0% (p < 0.05),insulinemia by 1.38 times (p < 0.05) and changes in the HOMA-IR index increase by a factor of 1.83 (p < 0.05) at animalswith untreated EMetS compared to those in IR rats (Table 1). Disruption of glucose utilization processes was evidenced by the results of the performed glucose tolerance tests.
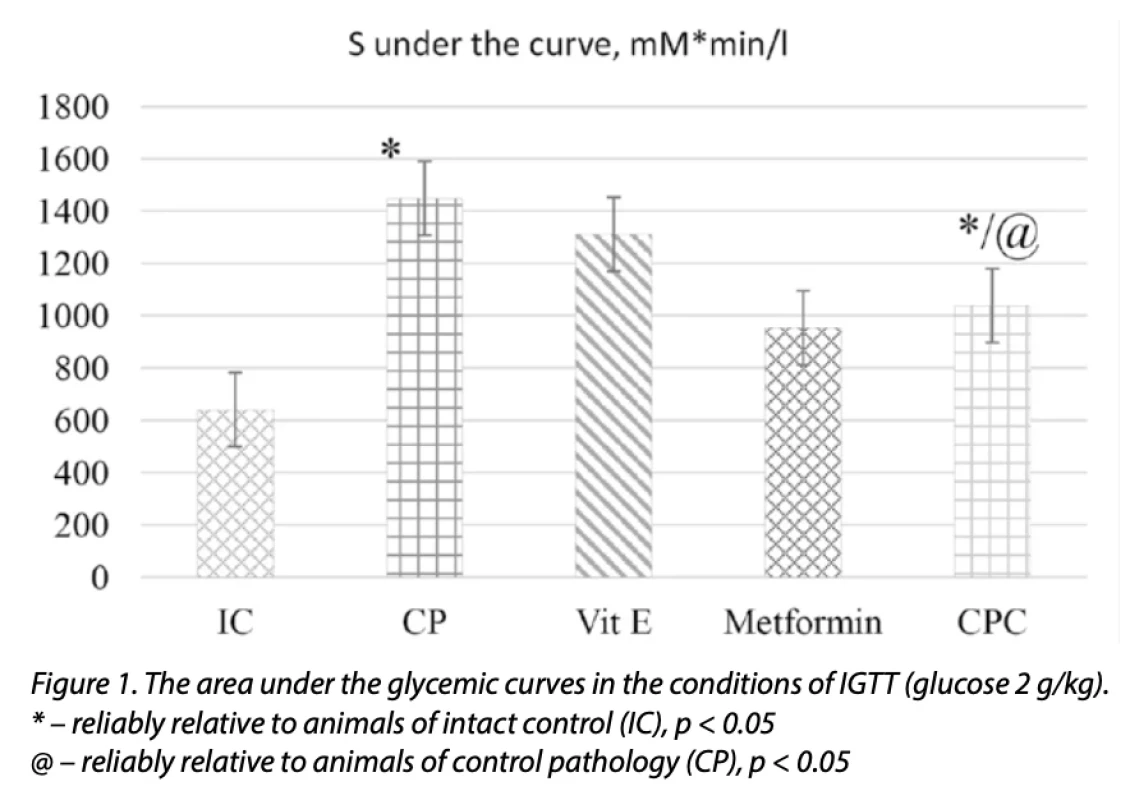
When IGTT was reproduced, glycemia increased by 1.62, 3.02, 2.44, and 2.12 times (p < 0.05), respectively, in all periods: 0, 15, 30, and 45 min (Table 2), also reflects an increase in the area under the glycemic curve (2.27 times; p <0.05) in animals of the CP group (Fig. 1).
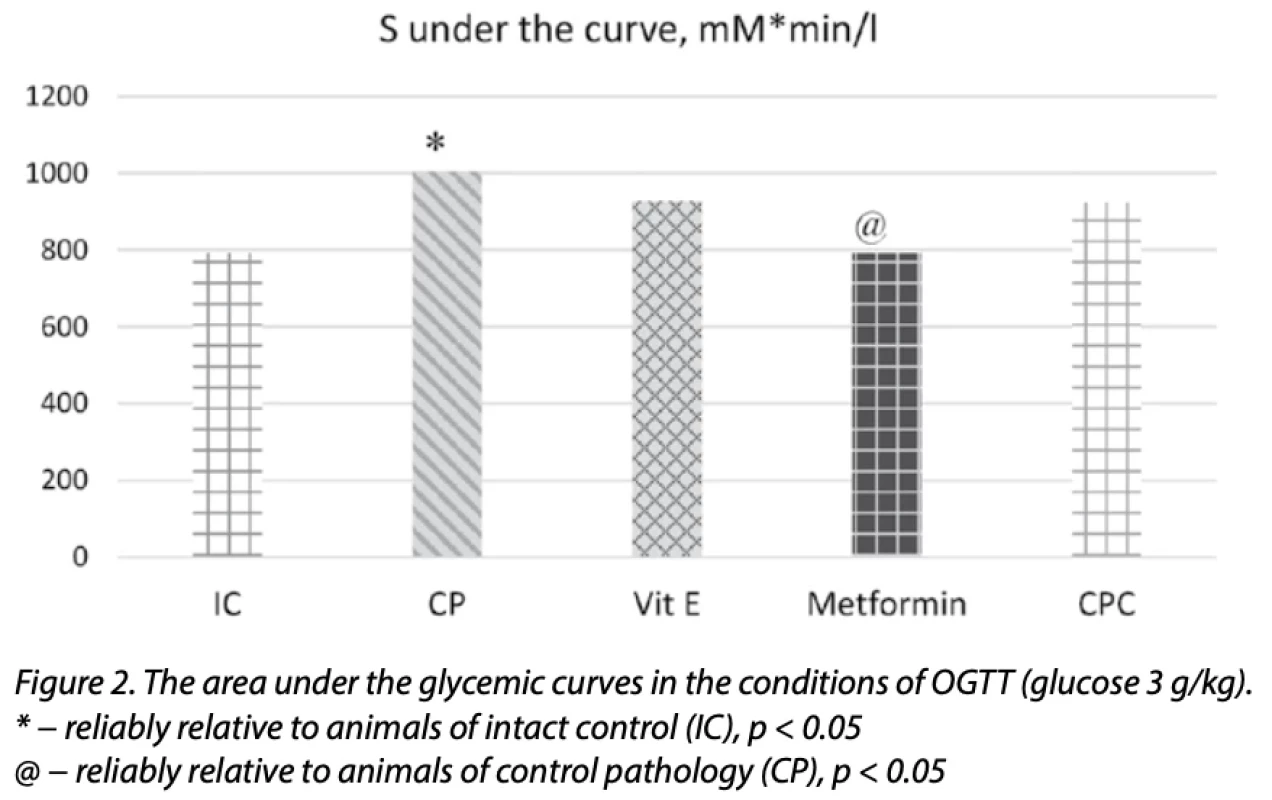
Under the conditions of OGTT reproduction, glucose increased at 1.59, 1.08,1.39, 1.25, and 1.22 times (p < 0.05) in all periods: 0, 15, 30, 50, and 120 min, respectively (Table 3), which also reflects an increase in the area underthe glycemic curve (1.27 times; p < 0.05) in animals of the CP group (Fig. 2).
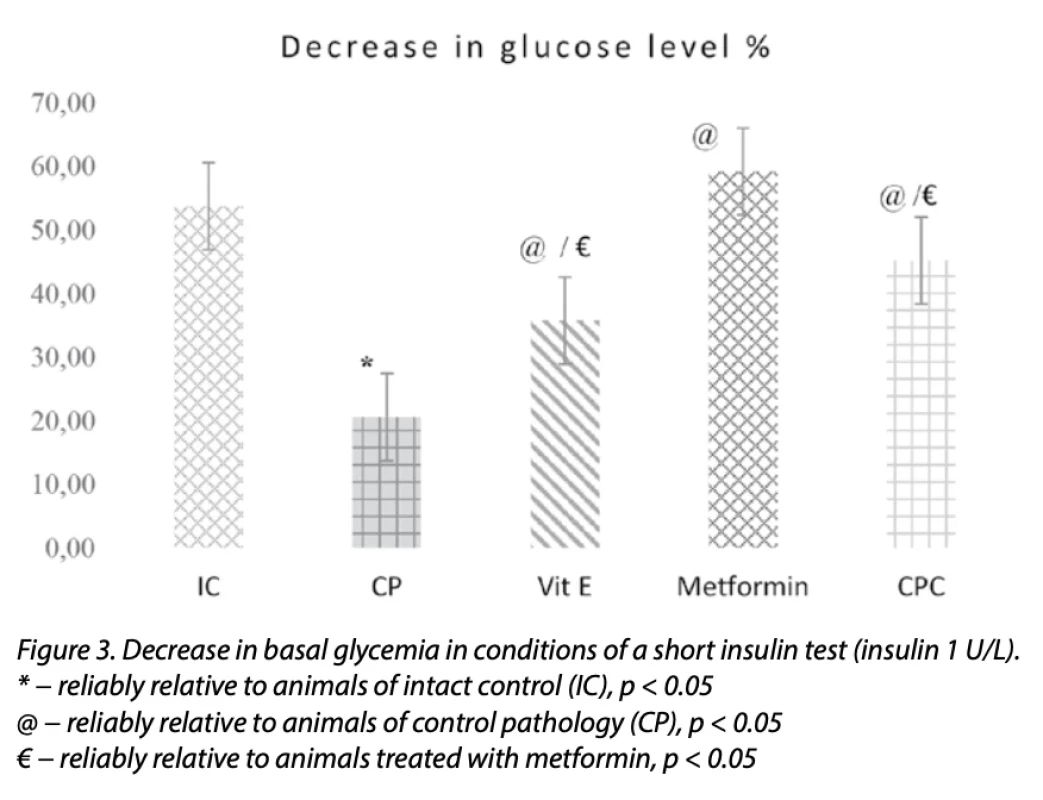
One possible way of disrupting glucose utilization processes is the insensitivity of body cells to the action of insulin). It wasfound that under the conditions of a short insulin test, there was a decrease in the ability of cells to utilize glucose: thereduction in glucose content in rats from the control pathology group was 20.55%, which was 2.6 times less (p < 0.05) than it (53, 70%) in intact control animals (Fig. 3).
An increase in the duration of hyperglycemia was confirmed by the rise in the content of glycosylated hemoglobin HbA1C by 2.28 times (p < 0.05) relative to intact control animals (Table 1).
The use of CPC in a therapeutic mode for 28 days, starting from the 15th week of EMetS modeling, contributed to the prevention of the development of insulin resistance, as evidenced by the low glucose level and the value of the HOMA-IR index by 11 and 22.3% (p < 0.05) relative to this in animals of CP. A decrease in glycemia under IGTT conditions of 1.22, 1.66 and 1.43 times (p < 0.05) was observed under CPC at 0, 15 and 30 minutes, respectively, also reflected by a decrease in the area under the glycemic curve by 1.4 times (p < 0.05) concerning the animals of the CP group (Fig. 1). A similar trend was observed under the conditions of OGTT under the influence of CPC; the level of glucose at 0, 30, and 60 minutes was lower than in animals of CP, respectively, by 1.2; 1.3 and 1.28 times (p < 0.05) (Table 3), which is also reflected by a decrease in the area under the glycemic curve (1.1 times; p < 0.05) relative to the CP (Fig. 2).
When using CPC under short insulin test conditions, the glucose content decreased by 45.16% compared to 20.55% against the background of CP (Fig. 3). A decrease in the duration of glycemia under the conditions of the use of CPC was confirmed by a reduction in the content of HbA1C by 1.18 times (p < 0.05) relative to animals of CP (Table 1).
For all the studied parameters, CPC was inferior to the comparison drug, the highly effective insulin sensitizer metformin, but exceeded the effect of vitamin E in its ability to improve the sensitivity of cells to insulin (HOMA-IR under the influence of CPK was 0.17 versus 0.22 under the influence of vitamin E, p < 0.05), in terms of the effect on basal and induced blood glucose (subject to HTTTG and OGTTG in certain periods), the duration of glycemia (the HbA1C content under the action of CPK was 2.43 versus 2.72 under the action of vitamin E, p < 0.05).
Table 1. Influence of CPC and reference drugs on the indicators of glucose metabolism in rats with EMetS Me (LQ; UQ)
|
Experimental conditions/investigatedindicator |
Insulin, IU/l |
Glucose, mM/l |
HOMA-IR |
HbA1C, % |
|
IC |
0.61 (0.58; 0.65) |
4.33 (4.23; 4.51) |
0.12 (0.12; 0.12) |
1.26 (1.23; 1.29) |
|
CP (untreated EMetS) |
0.83*(0.79;0.86) |
5.97* (5.86;6.10) |
0.22* (0.20;0.23) |
2.88* (2.70;3.00) |
|
EMetS + CPC 25,8 mg/kg |
0.74*/ # (0.68; 0.75) |
5.96*/€(5.68; 6.12) |
0.17*/@/# (0.16; 0.19) |
2.43*/@/€/# (2.39; 2.47) |
|
EMetS + Vitamin E, 100 mg/kg |
0.82*(0.80;0.83) |
6.12*/€(5.53; 6.14) |
0.22* (0.20;0.22) |
2.72*/€(2.65; 2.85) |
|
EMetS + Metformin, 60 mg/kg |
0.85*(0.79;0.93) |
4.59@ (4.54;4.61) |
0.19* (0.19;0.20) |
1.45*/@ (1.40;1.47) |
EMetS – experimental metabolic syndrome
* − reliably relative to animals of intact control (IC), р < 0.05
@ − reliably relative to animals of control pathology (CP), р < 0.05
€ − reliably relative to animals treated with metformin, р < 0.05
# − reliably relative to animals treated with Vitamin Е, р < 0.05
Table 2. Influence of CPC and reference drugs on the level of glycemia in conditions of IGTT to glucose (2 g/kg) Me (LQ; UQ)
|
Groups |
Glucose level, mM/l |
|||
|
Initial |
15 min |
30 min |
45 min |
|
|
IC |
3.6 (3.5; 3.6) |
5.6 (5.4; 6.3) |
5.4 (5.3; 6.8) |
4.2 (3.2; 4.6) |
|
CP (untreated EMetS) |
5.85*(5.8; 6.12) |
16.9* (6.5;17.52) |
13.2*(12.6; 14.77) |
8.9*(8.55;9.1) |
|
EMetS + CPC, 25,8 mg/kg |
4.8*/@/# (4.6;4.9) |
10.2*/@/# (9.8; 10.8) |
9.2*/@/# (9.1; 10.0) |
8.5*(8.3;8.7) |
|
EMetS + Vitamin Е, 100 mg/kg |
5.55*/€(5.45; 5.82) |
13.9 */@/€ (13.32; 14.35) |
12.2*/@/€(12.00; 12.62) |
8.7*(8.17;9.07) |
|
EMetS + Metformin,60 mg/kg |
4.45 */@ (4.25; 4.57) |
9.2 */@ (9.12; 9.95) |
8.45 */@ (8.17; 8.57) |
7.45 (6.72; 7.87) |
EMetS – experimental metabolic syndrome
* − reliably relative to animals of intact control (IC), р < 0.05
@ − reliably relative to animals of control pathology (CP), р < 0.05
€ − reliably relative to animals treated with metformin, р < 0.05
# − reliably relative to animals treated with Vitamin Е, р < 0.05
Table 3. Influence of CPC and reference drugs on the level of glycemia under conditions of OGTT (glucose – 3 g/kg) Me (LQ; UQ)
|
Groups |
Glucose level, mM/l |
||||
|
initial |
15 min |
30 min |
60 min |
120 min |
|
|
IC |
3.8 (3.75; 3.87) |
6.4 (6.35; 6.42) |
6.95 (6.7; 7.45) |
7.1 (6.67; 7.87) |
5.15 (4.82; 5.25) |
|
CP (untreated EMetS) |
6.05 * (5.75; 6.22) |
6.95 * (6.85; 7.05) |
9.65 * (9.57; 9.82) |
8.9 * (8.65; 9.1) |
6.3 (6.12; 6.42) |
|
EMetS + CPC, 25,8 mg/kg |
5.05 */@ (4.95; 5.25) |
9.15 */@ (8.15; 10.22) |
7.45 */@/# (7.00; 7.72) |
6.95 @/# (6.35; 7.47) |
6.3 (6.05; 6.47) |
|
EMetS + Vitamin Е,100 mg/kg |
5.6 */@ (5.35; 5.8) |
8.3 */@ (7.47; 9.00) |
8.45 */@ (8.07; 8.67) |
7.05 @ (6.82; 7.25) |
6.35 (6.22; 6.45) |
|
EMetS +Metformin, 60mg/kg |
4.8 */@ (4.65; 4.8) |
5.4 @ (5.3; 5.45) |
8 @ (7.8; 8.1) |
6.5 @ (6.4; 6.5) |
6.3 (6.25; 7.1) |
EMetS – experimental metabolic syndrome
* − reliably relative to animals of intact control (IC), р < 0.05
@ − reliably relative to animals of control pathology (CP), р < 0.05
# − reliably relative to animals treated with Vitamin Е, р < 0.05
Table 4. Influence of CPC and reference drugs on indicators of lipid peroxidation and oxidative modification of proteinsMe (LQ;UQ)
|
Experimental conditions/investigatedindicator |
TBA-AP, μmol/l |
Diene conjugates μmol/l |
Protein carbonylation, % |
|
IC |
1.69 (1.65; 1.74) |
49.43 (49.18; 49.72) |
0.12 (0.11; 0.13) |
|
CP (untreated EMetS) |
3.56* (3.47;3.62) |
65.14* (65.02;65.34) |
0.27* (0.25;0.28) |
|
EMetS + CPC, 25,8 mg/kg |
2.06*/@/€/# (2.02; 2.10) |
56.07*/€(54.64; 56.30) |
0.14*/€(0.13; 0.15) |
|
EMetS + Vitamin E,100 mg/kg |
2.46*/@ (2.44;2.50) |
58.23*/@ (58.10;58.98) |
0.16*/@ (0.15;0.17) |
|
EMetS + Metformin,60 mg/kg |
2.88*/@ (2.80;2.92) |
65.15* (61.88;62.90) |
0.24* (0.23;0.25) |
EMetS – experimental metabolic syndrome
* − reliably relative to animals of intact control (IC), р < 0.05
@ − reliably relative to animals of control pathology (CP), р < 0.05
€ − reliably relative to animals treated with metformin, р < 0.05
# − reliably relative to animals treated with Vitamin Е, р < 0.05
Table 5. Influence of CPC on the indicators of the AOD system Me (LQ;UQ)
|
Experimentalconditions/investigated indicator |
SH-groups, mmol/l |
Total antioxidant activity of blood serum, % |
|
IC |
13.73 (13.20; 14.02) |
50.86 (50.23; 51.32) |
|
CP (untreated EMetS) |
8.77* (8.55; 8.95) |
37.57*(37.20; 37.97) |
|
EMetS + CPC, 25.8 mg/kg |
11.42*/@/€(11.23; 12.06) |
49.41@/€(49.12; 50.20) |
|
EMetS + Vitamin E,100 mg/kg |
10.91*/@ (10.36;11.25) |
46.97@ (46.69;47.13) |
|
EMetS + Metformin,60 mg/kg |
9.24* (8.95;9.40) |
43.84*/@ (42.24;45.96) |
EMetS – experimental metabolic syndrome
* − reliably relative to animals of intact control (IC), р < 0.05
@ − reliably relative to animals of control pathology (CP), р < 0.05
€ − reliably relative to animals treated with metformin, р < 0.05
The activation of FRO processes against the background of EMetS is confirmed by an increase in the content of secondary and tertiary LPO DC products and TBA-AP by 1.32 (p < 0.05) and 2.11 times (p < 0.05), respectively, as well as the OMP marker carbonylated proteins by 2.25 times (p < 0.05) (Table 4).
At the same time, against the background of CP, there was a decrease in the total antioxidant activity of blood serum by 1.35 (p < 0.05) and the content of SH-groups by 1.56 times (p < 0.05) relative to animals of the IC (Table 5).
These results confirm the leading role of oxidative stress activation in the pathogenesis of metabolic syndrome5, 7, 8).
The use of CPC contributed to the suppression of the FRO processes, as evidenced by the decrease in the content of DC,TBA-AP, and protein carbonylations in 1.73 (p < 0.05), 1.16 (p < 0.05) and 1.93 (p < 0, 05) times relative to CP animals (Table 4). The effect of CPC promoted an increase by 30% (p < 0.05) in the content of SH-groups in relation to CP animals and the restoration of the total antioxidant activity of blood serum almost to the level of animals IC (Table 5). Regarding influence on the FRO processes and the AOD system, CPC exceeded the activity of the reference drugs vitamin E and metformin.
The manifestation of the positive pharmacological effect of CPC on the course of the metabolic syndrome is probably due to the implementation of the direct and indirect antioxidant action of its components, highlighted in many works8, 10, 25) and the ability to influence the processes of impaired lipid metabolism24) and inflammation of adipose tissue, which are decisive in theformation of insulin resistance2). In particular, the ability to be involved in the modulation of lipid metabolism, regulation of adipokines, alleviating inflammation of adiposetissue, and promoting adipogenesis with changes in epigenetic mechanisms have been established for ethyl esters of omega-3 acids, along with an antioxidant effect26–28). Vitamin E is characterized by a direct antioxidant effect and the ability to suppress the activity of 3-hydroxy-3-methylglutaryl-coenzyme A-reductase, which limits the rate of cholesterol biosynthesis29). Coenzyme Q10 can attenuate mitochondrial dysfunction caused by oxidative stress in metabolic syndrome30, 31). The ability of zinc and biotin to improve cells’ sensitivity to insulin action is well known32–34). The data on the preventive effect of selenium and vitamin A on the development of metabolic syndrome are contradictory35, 36).
Conclusion
The results of the conducted studies indicate a positive effect of CPC, which contains ethyl esters of omega-3 acids, vitamin E, coenzyme Q10, zinc, vitamin A, biotin, and selenium, on the sensitivity of cells to insulin, glucose utilization,duration of hyperglycemia and indicators of free radical oxidation processes and antioxidant defense systems in rats withEMetS. These results prove the feasibility of using CPC for the pharmaco-correction of metabolic syndrome.
Conflicts of interest: none.
Assistant Professor Kateryna Kalko, PhD
Department of Pharmacology and Drug Technology of I. I. Mechnikov Odesa National University
Yelisavetynska st. (Schepkina) 14, 65026 Odesa, Ukraine
e-mail: ketrin27kalko@gmail.com
Sources
- Bovolini A, Garcia J, Andrade MA, Duarte JA. Metabolic Syndrome Pathophysiology and Predisposing Factors. Int. J. Sports Med. 2021; 42(3), 199–214. doi: 10.1055/a-1263-0898
- Kalko K. O. «Korvitin®» – new therapeutic opportunities Pharmacologyonline 2021; 2, 1310–1316. Avaiable from:https://pharmacologyonline.silae.it/files/archives/2021/ vol2/PhOL_2021_2_A144_Kalko.pdf
- Gurka MJ, Guo Y, Filipp SL, DeBoer MD. Metabolic syndrome severity is significantly associated with future coronary heart disease in Type 2 diabetes. Cardiovasc. Diabetol. 2018; 17, 17. doi: 10.1186/s12933-017-0647-y
- Lopez-Candales A, Hernández Burgos PM, Hernandez-Suarez DF, Harris D. Linking chronic inflammation with cardiovascular disease: From normal aging to the metabolic syndrome. J. Nat. Sci. 2017; 3, e341
- Castillo-Díaz LA, Ruiz-Pacheco JA, Elsawy MA, ReyesMartínez JE, Enríquez-Rodríguez AI. Self-Assembling Peptides as anEmerging Platform for the Treatment of Metabolic Syndrome. Int. J. Nanomedicine 2020; 15, 10349–10370. doi: 10.2147/IJN.S278189
- Carrier A. Metabolic Syndrome and Oxidative Stress: A Complex Relationship. Antioxid. Redox Signal 2017; 26(9), 429–431. doi: 10.1089/ars.2016.6929
- McCracken E, Monaghan M, Sreenivasan S. Pathophysiology of the metabolic syndrome. Clin. Dermatol 2018; 36(1), 14–20. doi: 10.1016/j.clindermatol.2017.09.004
- Gregório BM, De Souza DB, de Morais Nascimento FA, Pereira LM, Fernandes-Santos C. The potential role of antioxidants in metabolic syndrome. Curr. Pharm. Des. 2016; 22(7), 859–869. doi: 10.2174/13816128226661512 09152352
- Alam MA, Subhan N, Rahman MM, Uddin SJ, Reza HM, Sarker SD. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv. Nutr. 2014; 5(4), 404–417. doi: 10.3945/ an.113.005603.
- Dludla PV, Nyambuya TM, Orlando P, Silvestri S, Mxinwa V, Mokgalaboni K, Nkambule BB, Louw J, Muller CJF, Tiano L. Theimpact of coenzyme Q10 on metabolic and cardiovascular disease profiles in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Endocrinol. Diabetes Metab. 2020; 3(2), e00118. doi: 10.1002/edm2.118
- Dakshinamurti K. Vitamins and their derivatives in the prevention and treatment of metabolic syndrome diseases (diabetes). Can J. Physiol. Pharmacol. 2015; 93(5), 355–362. doi: 10.1139/cjpp-2014-0479
- Beydoun MA, Chen X, Jha K, Beydoun HA, Zonderman AB, Canas JA. Carotenoids, vitamin A, and their association with the metabolic syndrome: a systematic review and meta-analysis. Nutr. Rev. 2019; 77(1), 32–45. doi: 10.1093/nutrit/nuy044
- Farajbakhsh A, Mazloomi SM, Mazidi M, Rezaie P, Akbarzadeh M, Ahmad SP, Ferns GA, Ofori-Asenso R, Babajafari S. Sesame oil and vitamin E co-administration may improve cardiometabolic risk factors in patients with metabolic syndrome: a randomized clinical trial. Eur. J. Clin. Nutr. 2019; 73(10), 1403–1411. doi: 10.1038/ s41430-019-0438-5
- Directive 2010/63/EU of the European Parliament and of the Council of September 22 2010 on the protection of animals used for scientific purposes. Official Journal of the European Union 2010; 33–79.
- AEVIT PREMIUM: instructions for medical use [Electronic resource] – Access mode: https://compendium.com.ua/ info/350158/aevit-premium/
- Siofor: instructions for medical treatment [Electronic resource] – Access mode: https://mozdocs.kiev.ua/likiview. php?id=38861
- Vitamin E instructions for medical treatment [Electronic resource] – Access mode: https://www.lekhim.ua/ru/alfa-tokoferola-acetat-vitamin-e_dose-100-mgml-po-20ml-no-1
- Kalko K. O., Drogovoz S. M., Mishchenko O. Ya., Komisarenko M. A., Komissarenko A. M., Bondariev Y. V., Moeen Dababneh. Administration of «CORVITIN®» as an off-label agent for pharmacological correction of metabolic syndrome. Problems of endocrine pathology 2021; 1, 97–102. https://www.jpep.endocrinology.org.ua/index.php/1/article/view/124
- Rybolovlev Yu. R., Sidlyarov D. P., Afonin N. I. Predictive assessment of the safety of substances for humans by the constants oftheir biological activity. Toxicological aspects of the safety of finished dosage forms: astract report scientific conf. Moscow, December 16–18, 1981. M., 1981. P. 9–10.
- Geddawy A, Hussian M, Kamel MY, Kamal R, Ibrahim MA. Effects of Liraglutide and Vitamin E in Fructose-Induced MetabolicSyndrome in Rats. Pharmacology 2017; 9 (1–2), 48-56. doi: 10.1159/000449429.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulinresistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28, 412–419.
- Stefanov O. V. Preclinical studies of drugs (methodological recommendations). For order. Corresponding Member NAMS of Ukraine Kyiv: VD Avicenna, 2001.
- Truhacheva N. V. Mathematical statistics in medical-biological researches using the package statistics. Moscow, Russian Federation: GEOTAR-Media 2012.
- Russo B, Picconi F, Malandrucco I, Frontoni S. Flavonoids and Insulin-Resistance: From Molecular Evidences to Clinical Trials. Int. J. Mol. Sci. 2019; 20(9), 2061. doi: 10.3390/ijms20092061
- Dakshinamurti K. Vitamins and their derivatives in the prevention and treatment of metabolic syndrome diseases (diabetes). Can. J. Physiol. Pharmacol. 2015; 93(5), 355–362. doi: 10.1139/cjpp-2014-0479
- Albracht-Schulte K, Kalupahana NS, Ramalingam L, Wang S, Rahman SM, Robert-McComb J, Moustaid-Moussa N. Omega-3 fatty acids in obesity and metabolic syndrome: a mechanistic update. J. Nutr. Biochem. 2018; 58, 1–16. doi:10.1016/j.jnutbio.2018.02.012
- Tortosa-Caparrós E, Navas-Carrillo D, Marín F, Orenes-Piñero E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food. Sci. Nutr. 2017; 57(16), 3421–3429. doi: 10.1080/10408398.2015.1126549
- Kawada T. Omega-3 polyunsaturated fatty acids and metabolic syndrome. Clin. Nutr. 2020; 39(7), 2319. doi:10.1016/j.clnu.2020.05.033
- Geddawy A, Hussian M, Kamel MY, Kamal R, Ibrahim MA. Effects of Liraglutide and Vitamin E in Fruc tose-Induced MetabolicSyndrome in Rats. Pharmacology. 2017; 99(1–2), 48–56. doi: 10.1159/000449429
- Zozina VI, Covantev S, Goroshko OA, Krasnykh LM, Kukes VG. Coenzyme Q10 in Cardiovascular and Metabolic Diseases:Current State of the Problem. Curr. Cardiol. Rev. 2018; 14(3), 164–174. doi: 10.2174/1573403X146 66180416115428
- Dludla PV, Orlando P, Silvestri S, Marcheggiani F, Cirilli I, Nyambuya TM, Mxinwa V, Mokgalaboni K, Nkambule BB, Johnson R, Mazibuko-Mbeje SE, Muller CJF, Louw J, Tiano L. Coenzyme Q10 Supplementation Improves Adipokine Levels and Alleviates Inflammation and Lipid Peroxidation in Conditions of Metabolic Syndrome: A Meta-Analysis of RandomizedControlled Trials. Int. J. Mol. Sci. 2020; 21(9), 3247. doi: 10.3390/ijms21093247.
- Aguilera-Mendez A, Hernández-Equihua MG, Rueda-Rocha AC, Guajardo-López C, Nieto-Aguilar R, Serrato-Ochoa D, Ruíz Herrera LF, Guzmán-Nateras JA. Protective effect of supplementation with biotin against high-fructose-induced metabolic syndromein rats. Nutr. Res. 2018; 57, 86–96. doi: 10.1016/j.nutres.2018.06.007
- Miao X, Sun W, Fu Y, Miao L, Cai L. Zinc homeostasis in the metabolic syndrome and diabetes. Front. Med. 2013; 7(1), 31–52. doi: 10.1007/s11684-013-0251-9
- Aguilera-Mendez A, Hernández-Equihua MG, Rueda-Rocha AC, Guajardo-López C, Nieto-Aguilar R, Serrato-Ochoa D, Ruíz Herrera LF, Guzmán-Nateras JA. Protective effect of supplementation with biotin against high-fructose-induced metabolic syndrome in rats. Nutr. Res. 2018; 57, 86–96. doi: 10.1016/j. nutres.2018.06.007
- Retondario A, Fernandes R, Rockenbach G, Alves MA, Bricarello LP, Trindade EBSM, Vasconcelos FAG. Selenium intake and metabolic syndrome: A systematic review. Clin. Nutr. 2019; 38(2), 603–614. doi: 10.1016/j. clnu.2018.02.021
- Beydoun MA, Chen X, Jha K, Beydoun HA, Zonderman AB, Canas JA. Carotenoids, vitamin A, and their association with the metabolic syndrome: a systematic review and meta-analysis. Nutr. Rev. 2019; 7(1), 32–45. doi: 10.1093/nutrit/nuy044.
Labels
Pharmacy Clinical pharmacologyArticle was published in
Czech and Slovak Pharmacy

2023 Issue 6
-
All articles in this issue
- Ethical aspects of conducting clinical trials of human medicinal products
- Brief insight into the in silico properties, structure–activity relationships and biotransformation of fruquintinib, an anticancer drug of a new generation containing a privileged benzofuran scaffold
- Potential impact on mental health in patients with treatment-resistant schizophrenia – clozapine augmentation with long-acting parenteral antipsychotics: a case series
- Prospects of сomplex pharmaceutical composition application for pharmacological correction of metabolic syndrome
- Increasing the efficiency of hypolipidemic therapy with the combined use of quercetin in patients with non-alcoholic fatty liver disease on the background of the metabolic syndrome
- Analysis of medication administration in relation to food and beverages in inpatients
- On the history and development of some changes in pharmacy practice – work safety in pharmacies
- Beauty in books: pharmaceutical bookplates in the territory of the present Czech Republic
- Úvodník
- Czech and Slovak Pharmacy
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Analysis of medication administration in relation to food and beverages in inpatients
- Potential impact on mental health in patients with treatment-resistant schizophrenia – clozapine augmentation with long-acting parenteral antipsychotics: a case series
- Ethical aspects of conducting clinical trials of human medicinal products
- Brief insight into the in silico properties, structure–activity relationships and biotransformation of fruquintinib, an anticancer drug of a new generation containing a privileged benzofuran scaffold