-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Glypican-3 immunostaining significantly improves histological diagnosis of hepatocellular carcinoma
Imunohistochemická detekce glypicanu-3 zpřesňuje diagnózu hepatocelulárního karcinomu
Východisko:
Hepatocelulární karcinom je jedním z nejčastěji se vyskytujících maligních nádorů, který vzniká hlavně u pacientů s chronickými onemocněními jater. Pro léčbu je velmi důležitá brzká diagnóza karcinomu. Časné hepatocelulární karcinomy jsou malé, klinicky bez příznaků a nemají ještě vyvinutá diagnostická radiologická kritéria. Také histopatologická diagnóza je často velmi komplikovaná s neshodami i mezi experty v hepatopatologii.Metody:
Ve 138 bioptických vzorcích jater jsme studovali expresi glypicanu-3. Šlo o 86 hepatocelulárních karcinomů, 10 adenomů jater, 12 fokálních nodulárních hyperplasií, 25 vzorků cirhóz bez nádoru a 5 metastáz neuroendokrinního karcinomu.Výsledky.
Pozitivních bylo 80 hepatocelulárních karcinomů (93 %; tj. všech 11 jehlových biopsií, 12 z 15 resekátů, 57 z 60 nádorů v explantovaných játrech při transplantaci). Exprese glypicanu-3 byla nezávislá na diferenciaci a velikosti nádoru. Šest případů (6,9 %; 3 v resekátech a 3 v explantovaných játrech) mělo negativní průkaz glypicanu-3. Všechny benigní nodulární léze a vzorky s cirhózou bez nádoru byly negativní.Závěry:
Imunohistochemická detekce glypicanu-3 významně zlepšuje komplikovanou histopatologickou diagnostiku hepatocelulárních karcinomů, a to i v malých časných nádorech v jehlových biopsiích.Klíčová slova:
glypican-3, hepatocelulární karcinom, jehlové biopsie.
Authors: Eva Honsová 1; Alena Lodererová 1; Soňa Fraňková 2; Martin Oliverius 3; Pavel Trunečka 2
Authors place of work: Institute for Clinical and Experimental Medicine Prague, Department of Clinical and Transplant Pathology, Czech Republic 1; Institute for Clinical and Experimental Medicine Prague, Department of Hepatology, Czech Republic 2; Institute for Clinical and Experimental Medicine Prague, Department of Transplant Surgery, Czech Republic 3
Published in the journal: Čas. Lék. čes. 2011; 150: 37-40
Category: Původní práce
Summary
Background:
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors which occur mainly in patients with chronic liver disease. Early detection of HCC is critically important for treatment of the patients. However, most early HCC cases are asymptomatic clinically with the lack of typical radiological findings. Also histological diagnosis is often very difficult with the lack of agreement even among expert pathologists.Methods:
We studied the expression of Glypican-3 in 138 liver biopsy samples; 86 HCC, 10 hepatocellular adenomas, 12 focal nodular hyperplasias, 25 samples with liver cirrhosis without tumor, and 5 liver metastases of neuroendocrine carcinomas.Results:
HCC showed positive staining in 80 nodules (93%; all of the 11 needle biopsy samples, 12 out of 15 liver resection specimens, 57 out of 60 nodules in explanted livers). Glypican-3 expression was independent of the differentiation and size of the HCC. Six cases (6.9%), 3 HCC in liver resection specimens and 3 in the explanted liver were negative for Glypican-3. However, all cases with benign nodular lesions and cirrhosis without tumors were negative for Glypican-3.Conclusions:
Immunohistochemical detection of Glypican-3 significantly improves the complicated routine histological diagnosis of HCC even in early lesions in needle biopsy samples.Key words:
glypican-3, hepatocellular carcinoma, needle biopsy sample.Introduction
Hepatocellular carcinoma (HCC) is one of the most common types of malignant tumors worldwide. The incidence of HCC has been increasing in both Asian and Western countries (1, 2). Cirrhosis of different etiologies represents the major risk factor for the development of HCC. Guidelines for surveillance of patients with cirrhosis were recommended with the goals of reducing morbidity and mortality (3). These guidelines indicate that nodules more than 2 cm in diameter with typical features in imaging methods which develop in a cirrhotic patient do not require biopsy confirmation. If lesions reveal atypical features on imaging, then a biopsy is required to establish the diagnosis. Several studies showed that only around 44% of the nodules between 1 and 2 cm met the criteria of HCC using imaging methods (4, 5). The study of Forner et al. showed that the sensitivity of non-invasive criteria was 33%, and a biopsy was required to confirm the diagnosis. However, most early HCC are clinically asymptomatic, negative for serum markers, and lack typical radiological findings. Also, evaluation of a biopsy sample has several limitations. Unfortunately, no strict objective histologic criteria have been established to distinguish small well-differentiated hepatocellular carcinoma, high-grade dysplastic nodules or adenoma. It is well known that there is lack of agreement even among expert pathologists in the diagnostic interpretation of these lesions. Immunohistochemical or other markers that could help in the diagnostic process have been intensively studied. Recently, Glypican-3 (GPC3) has been reported by several groups as one of the most promising serum and histological markers (6–8). GPC3 is an oncofetal protein which is expressed in the embryo and involved in morphogenesis and growth control during development. GPC3 has been detected in the fetal but not in the normal adult liver. It reappears in HCC and its immunohistochemical detection can improve the complicated histological diagnosis of small HCC especially in needle biopsy samples.
Material and methods
Patients and tumor samples: A total of 138 liver biopsy samples were included in this study; 86 with histological diagnosis of HCC, 10 hepatocellular adenomas, 12 focal nodular hyperplasias, 25 samples with liver cirrhosis without tumor, and 5 liver metastases of neuroendocrine carcinomas.
The diagnosis of HCC was confirmed by two pathologists, and 11 needle biopsy samples, 15 HCC in liver resection specimens, and 60 HCC nodules of 17 patients in explanted liver during transplantation were included. The staining protocol included H & E, Vgel, reticulum, and immunohistochemical detection of Carcinoembryonic Antigen (polyclonal antibody, Dako, Glostrup, Denmark), CD34 (clone QBEnd 10, Dako, Glostrup, Denmark), and Alpha-Smooth Muscle Actin (clone 1A4, Dako, Glostrup, Denmark).
Immunohistochemical staining for GPC3 was performed in accordance with the protocol of our laboratory. Briefly, immunohistochemistry was performed on four-micrometer-thick paraffin sections. Slides were deparaffinized in xylene and rehydrated in graded ethanols. After deparaffinization and rehydration slides were cooked in a microwave oven using an EDTA buffer pH 8.0 for target retrieval. Endogenous peroxidase was blocked by 0.3 % H2O2 in 70% methanol. The sections were then incubated with primary antibody (anti-Glypican-3, clone 1G12, Santa Cruz Biotechnology CA, USA), diluted 100×. Antibody was detected by Histofine Simple Stain MAX PO (Nichirei, Japan). The immunoreaction was visualized by 3-amino-9-ethylcarbazole. Finally, the tissues were counterstained with Mayer’s hematoxylin and embedded in Entellan.
Results
The demographic data and causes of liver diseases are summarized in table 1. Based on histological examination, 70 HCCs (81.3%) occurred in cirrhotic livers; 15 (17.4%) were well differentiated, 27 (31.3%) were moderately, and 44 (51.1%) were poorly differentiated. The majority of poorly differentiated HCC were diagnosed in liver resection specimens (p < 0.05). There were no statistically significant differences in age or sex among groups of patients with needle biopsy, liver resection or explanted liver. Three different immunostaining patterns of GPC3 were observed: predominantly cytoplasmatic, predominantly canalicular, and membranous (Fig. 1, 2). Strong staining for GPC3 was observed in 42 (48.8%) of HCCs, and 38 (44.1%) tumors showed weak GPC3 expression. All GPC3 positive surgical specimens showed a focal pattern of staining. Six cases (6.9%), 3 HCC in liver resection specimens and 3 in the explanted liver were negative for GPC3. However, all cases with focal nodular hyperplasias, adenomas, and cirrhosis without tumor were negative for GPCs.
Tab. 1. Underlying causes and demographic data for HCC cases 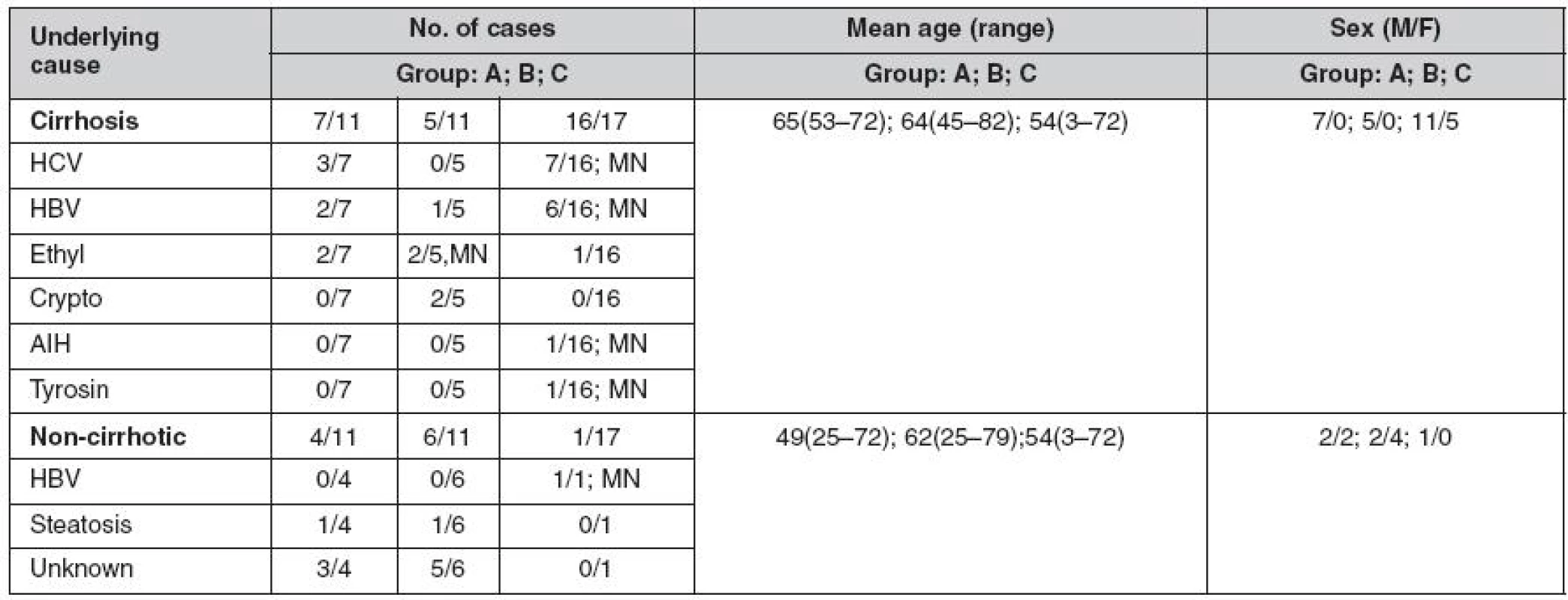
Group A – needle biopsy samples, Group B – liver resection specimens, Group C – explanted livers, HCV– hepatitis C virus infection, HBV– hepatitis B virus infection, AIH – autoimmune hepatitis, Ethyl – alcoholic cirrhosis, Crypto – cryptogenic cirrhosis, unknown – no evidence of chronic liver disease, MN– multiple nodules Fig. 1. HCC – GPC3 immunohistochemical staining shows different grades of predominantly cytoplasmatic positive expression 
Fig. 2. HCC – GPC3 positive immunohistochemical staining with predominantly membranous pattern 
Discussion
According to international guidelines, when radiological features of liver nodules are not typical for HCC, a liver biopsy should be performed to establish the diagnosis.
Histological diagnosis of early HCC is often very difficult, because no strict objective histologic criteria have been established to distinguish small well-differentiated hepatocellular carcinoma, high-grade dysplastic nodules (DN) or sometimes adenoma. Some high-grade DN can be misinterpreted as malignant, while other well-differentiated HCC may be consider as adenoma or steatotic livers; both of these situations can have severe clinical implications. To improve the complicated diagnostic process, several histological and serological markers were recently studied by different groups. Moreover, standard histological criteria for early HCC have been more precisely defined by the international consensus group for hepatocellular neoplasia (9). The experts in this group had no serious difficulty in distinguishing low-grade dysplastic nodules from early HCC. However, the differentiation between high-grade DN and early HCC represented a problem. Also, to distinguish between low-grade DN and large regenerative nodules was difficult or impossible. Stromal invasion (tumor cell invasion into the remaining intranodular portal tracts) remains the most helpful in distinguishing between malignant and benign lesions. Unfortunately, stromal invasion is frequently focal, and does not necessarily appear in a needle biopsy sample. Supportive immunohistochemical markers have been studied. Alpha-fetoprotein is a well-established serum marker for advanced HCC, but in early cases elevated levels are rarely found. In histology, alpha fetoprotein is not helpful because of its low sensitivity.
Detection of CD34 can be more useful. Cirrhotic liver and benign lesions show CD34 positive staining in areas which receive increased arterial blood, so they tend to be positive only around the periphery in sinusoids near the fibrous septa. On the other hand, HCC frequently shows diffuse, regular CD34 positive staining probably due to the intensive newly formed arterial blood supply, which is represented in histology by so-called unpaired arteries (arteries not accompanied by the bile duct as in the portal tract, which reflect neoangiogenesis in tumors). There still remain problems in interpretation, CD34 staining in hepatocellular adenoma is variable, and in needle biopsy samples only periseptal hepatocytes can appear. Thus positive results of CD34 do not necessarily indicate malignancy.
HepPar1 (Hepatocyte Paraffin 1) is very useful in distinguishing HCC from other malignancies and can be helpful in cases of poorly differentiated tumors. HepPar1 is a monoclonal antibody which reacts with an epitope of liver mitochondria, with a typical granular cytoplasmatic pattern. It is not completely specific for hepatocytes because it sometimes reacts with renal tubules and also with the intestinal epithelium. Moreover, HepPar1 does not discriminate benign from malignant hepatocytes, and therefore it is not useful in differential diagnosis of hepatocellular tumors. Therefore, we perform HepPar1 staining in cases with uncertain histogenesis, where positive staining can help to differentiate between HCC and other malignant tumors.
In our study, we evaluated the diagnostic value of GPC3 immunostaining in a large group of needle and surgical biopsy specimens with HCC, and also in benign liver lesions and cirrhotic liver without tumor. There are only 2 studies which examined GPC3 expression in HCC in needle biopsy specimens (10, 11). In the study by Libbrecht et al., GPC3 positive expression was observed in 10 out of 12 cases of HCC, and in Anatelli’s study only one-half of the HCC showed positive staining. We included 11 needle biopsy samples, and all showed positive results, in 7 samples with strong positive staining in more than half of the tumor cells.
All HCC in surgical specimens showed focal pattern of GPC3 staining. We observed 6 cases negative for GPC3. All of which were surgical specimens, where we assumed inadequate fixation. Takai et al. showed in their study different results dependent on the time of fixation and type of antigen retrieval. According to their results, the fixation time should not be longer than 24 hours, and protease should be used for the antigen retrieval treatment (12).
In addition to HCC, expression of GPC3 has been reported in several other types of human tumors, including melanoma, and yolk sac germ tumor (13, 14). In the current study of Coston et al. only 7 out of 225 cases of non-HCC tumors showed focal positive GPC3 staining (15). These tumors include ovarian, endometrial and clear cell adenocarcinomas, neuroendocrine tumors, and testicular yolk sac tumor. In our study, all neuroendocrine metastatic lesions in the liver were GPC3 negative.
Our data demonstrate that GPC3 is a very useful diagnostic marker in the process of histological diagnosis of HCC. Positive results of this staining can help to distinguish between benign nodules and HCC in cirrhotic liver not only in surgical resection specimens, but also in needle biopsies with a small number of neoplastic cells.
Abbreviation
- CD34 – endothelial cell marker
- GPC3 – glypican-3
- HCC – hepatocellular carcinoma
This study was supported by the Institute for Clinical and Experimental Medicine institutional grant MZO 00023001.
Address for correspondence:
Eva Honsová, MD, PhD.
Department of Pathology, IKEM
Vídeňská 1958/9, 140 21 Prague 4, Czech Republic
fax: +420 236 053 076, e-mail: eva.honsova@ikem.cz
Zdroje
1. Bosh FX, Ribes J, Cleries R, Diaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis 2005; 9 : 191–211.
2. Okuda K. Hepatocellular carcinoma. J Hepatol 2000; 32 : 225–237.
3. Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005; 42 : 1208–1236.
4. Bolondi L, Gaiani S, Celli N, et al. Characterization of small nodules in cirrhosis by assessment of vascularity: the problem of hypovascular hepatocellular carcinoma. Hepatology 2005; 42 : 27–34.
5. Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20mm or smaller in cirrhosis: prospective validation of noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008; 47 : 97–104.
6. Di Tommaso L, Franchi G, Park YN, et al. Diagnostic value of HSP70, glypican 3, and glutamine synthetase in hepatocellular nodules in cirrhosis. Hepatology 2007; 45 : 725–34.
7. Shirakawa H, Suzuki H, Shimomura M, et al. Glypican-3 expression is correlated with poor prognosis in hepatocellular carcinoma. Cancer Sci 2009; 100 : 1403–1407.
8. Nassar A, Cohen C, Siddiqui MT. Utility of glypican-3 and surviving in differentiating hepatocellular carcinoma from benign and preneoplastic hepatic lesions and metastatic carcinomas in fine-needle aspiration biopsies. Diagnostic Cytopathol 2009; 37 : 629–635.
9. Korio M, Wanless I, Alves V, et al. Pathological diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology 2009; 49 : 658–664.
10. Libbrecht L, Severi T, Cassiman D, et al. Glypican-3 expression distinguishes small hepatocellular carcinomas from cirrhosis, dysplastic nodules, and focal nodular hyperplasia-like nodules. Am J Surg Pathol 2006; 30 : 1405–1411.
11. Anatelli F, Chuang ST, Yang XJ, Wang HL. Value of glypican 3 immunostaining in the diagnosis of hepatocellular carcinoma on needle biopsy. Am J Clin Pathol 2008; 130 : 219–223.
12. Takai H, Kato A, Ishiguro T, et al. Optimization of tissue processing for immunohistochemistry for the detection of human glypican-3. Acta Histochem 2010; 112 : 240–250.
13. Nakatsura T, Kageshita T, Ito S, et al. Identification of glypican-3 as a novel marker for melanoma. Clin Cancer Res 2004; 10 : 6612–6621.
14. Zynger DL, Everton MJ, Dimov ND, et al. Expression of glypican 3 in ovarian and extragonadal germ cell tumors. Am J Clin Pathol 2008; 130 : 224–230.
15. Coston WM, Loera S, Lau SK,et al. Distinction of hepatocellular carcinoma from benign hepatic mimickers using Glypican-3 and CD34 immunohistochemistry. Am J Surg Pathol 2008; 32 : 433–444.
Štítky
Adiktológia Alergológia a imunológia Angiológia Audiológia a foniatria Biochémia Dermatológia Detská gastroenterológia Detská chirurgia Detská kardiológia Detská neurológia Detská otorinolaryngológia Detská psychiatria Detská reumatológia Diabetológia Farmácia Chirurgia cievna Algeziológia Dentální hygienistka
Článok vyšiel v časopiseČasopis lékařů českých
Najčítanejšie tento týždeň
- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Subkutánne vs. intravenózne imunoglobulíny u pacientov s CLL
- Facilitovaná subkutánna imunoglobulínová terapia u seniorov s imunodeficienciami v reálnej praxi
-
Všetky články tohto čísla
- Catheter ablation in atrial fibrillation
- Hyperglycemia and its control in the critically ill patient
- Acute liver failure: Present recommendations
- IKEM – Institut klinické a experimentální medicíny – na prahu páté dekády úspěšné existence
- Plánované akce odborných složek ČLS JEP
- Specific program for perioperative care in paediatric liver transplantation
- Cadaver organ donor in transplantology
- Glypican-3 immunostaining significantly improves histological diagnosis of hepatocellular carcinoma
- Eversion carotid endarterectomy: evaluation of results after changing the operation technique
- Benefit of paracorporeal pulsatile assist device in multiorgan failing patients in terminal stage of heart failure
- Islet transplantation for treatment of type-1 diabetes mellitus
- IKEM – odborné, politické a další souvislosti vzniku a dosavadního vývoje
- Kidney transplantation at the Institute for Clinical and Experimental Medicine
- Long-term follow-up of the first 500 liver transplant recipients transplanted at the Institute for Clinical and Experimental Medicine in Prague
- Determination of liver triglycerides by 1H MR spectroscopy
- Molecular diagnosis of hereditary canalicular cholestasis and familial hyperbilirubinemias
- Liver transplantation in patients with portal vein thrombosis
- The influence of surgical complications on renal graft function
-
Molecular Diagnostic Europe
Hannover, 4. až 7. října 2010 -
Konference Tabák a zdraví po jedenácté v Lékařském domě
Praha, 5. listopadu 2010 - Prof. MUDr. Václav Čepelák, DrSc. osmdesátníkem
- Spolek lékařů českých v Praze
-
Strouhal E, Vachala B, Vymazalová H.
Lékařství starých Egypťanů
I. staroegyptská chirurgie, Péče o ženu a dítě - CHARLES BRENTON HUGGINS
- Časopis lékařů českých
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Acute liver failure: Present recommendations
- Hyperglycemia and its control in the critically ill patient
- Prof. MUDr. Václav Čepelák, DrSc. osmdesátníkem
- Eversion carotid endarterectomy: evaluation of results after changing the operation technique
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



