Flush, rosacea or blushing – understanding the differences
Flush, rosacea, alebo červenanie – pochopenie rozdielov
Výraz „flush” je označenie začervenania pokožky pri rozličných fyziologických a patologických zdravotných stavoch. Flush však môže byť spôsobený aj závažnými príčinami. Veľké množstvo pacientov s nekarcinoidovým flushom bolo odoslaných na našu kliniku pre podozrenie na karcinoid. Väčšina z pacientov už v tom čase podstúpila mnohé biochemické a rádiologické vyšetrenia, bez praktického prínosu a finančne zaťažujúce. Sme si vedomí, že rozdiel medzi karcinoidovým a nekarcinoidovým flushom je stále viac zastretý.
Ciele:
V našom prehľade sme sa zamerali na diferenciáciu karcinoidového flushu a jeho odlíšenie od iných príčin, ktoré môžu imitovať karcinoidový syndróm, najmä rosacea a menopauzálny flush. Zameriavame sa stručne na základnú patofyziológiu flushu. Cieľom prehľadu je pomôcť lekárom stretávajúcim sa s flushom. Vymyslieť užitočný diagnostický algoritmus, ktorý pomôže objasniť jeho etiológiu a určiť nutnosť použitia multidisciplinárneho postupu. Zdôrazňujeme však, že lekár by mal vždy posudzovať každého pacienta individuálne.
Výsledky:
Na odlíšenie karcinoidového a nonkarcinoidového flushu sme sa pokúsili zaviesť diagnostický algoritmus za účelom zjednodušenia manažmentu týchto pacientov. Liečba je nad rozsah tejto práce.
Kľúčové slová:
flush – karcinoid – neuroendokrinný – menopauza – rosacea – patofyziológia – algoritmus
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Doručeno:
19. 5. 2016
Přijato:
6. 6. 2016
Authors:
S. M. Agouba; P. Hyrdel; R. Hyrdel
Authors place of work:
Gastroenterology Clinic, Department of Internal Medicine, Jessenius Faculty of Medicine and University Hospital Martin, Slovak Republic
Published in the journal:
Gastroent Hepatol 2016; 70(4): 346-352
Category:
Kapitoly z interní medicíny: přehledová práce
doi:
https://doi.org/10.14735/amgh2016346
Summary
The term “flush” is a misnomer for diverse physiological and pathological medical conditions; however, simple flush can signify the presence of a serious underlying medical condition. Many patients with non-carcinoid facial flush have been referred to our department as cases of carcinoid flush. Most of these patients had already undergone many biochemical and radiological workups, which were neither practical nor cost-effective. We acknowledge that the line between carcinoid and non-carcinoid flush is ever more blurred.
Aim:
In this review, we highlight the definition of carcinoid flush, and distinguish it from other conditions resembling those of carcinoid syndrome, mainly rosacea flush and menopausal flush. We briefly explore the basic pathophysiology of flush. Finally, in order to help guide referring physicians dealing with this condition, we have tried to devise a clinically useful algorithm for the diagnosis of carcinoid flush that takes into account the need for a multidisciplinary approach; nonetheless, physicians should always examine patients on a case-by-case basis.
Results:
To distinguish carcinoid flush from non-carcinoid flush, we designed an algorithm to help guide referring physicians dealing with these conditions. Treatment is beyond the scope of this review.
Key words:
flush – carcinoid – neuroendocrine – menopause – rosacea – pathophysiology – algorithm
Introduction
Flushing describes episodic attacks of redness of the skin, together with a sensation of warmth or burning. Flush is usually confined to the face and neck; flush can also affect the upper chest and back, however it could still appear elsewhere. Physicians dealing with flush should always take a careful and detailed medical history [1].
The term flush was originally used to describe climacteric flush by Dr. E. J. Tilt, who proposed a short and expressive word for this phenomenon in his publication on menopause in 1857 [2].
The incidence and prevalence of carcinoid flushing has not been determined, however over the past 30 years, the incidence of neuroendocrine tumours has increased more than five fold [3].
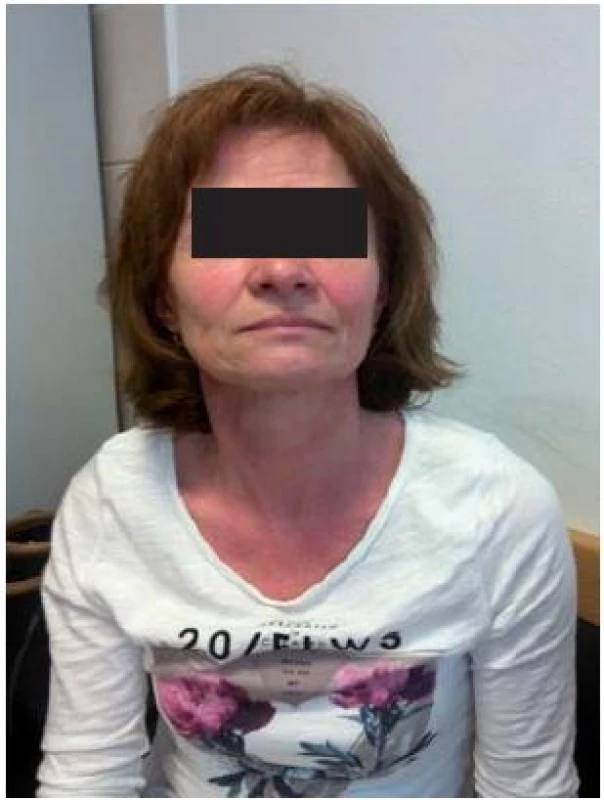
About 80% of postmenopausal women experience flushing which is associated with sweating. It can occur years before the menopause, lasting 1–5 years in most patients, and can continue for up to 10 years after the menopause [4]. There is considerable individual variation in the symptoms, frequency, intensity, and duration of hot flushes within and among patients [5].
Flush in menopausal women is considered part of the vasomotor symptoms of dysregulation and could include tachycardia and mood swings or depression [5].
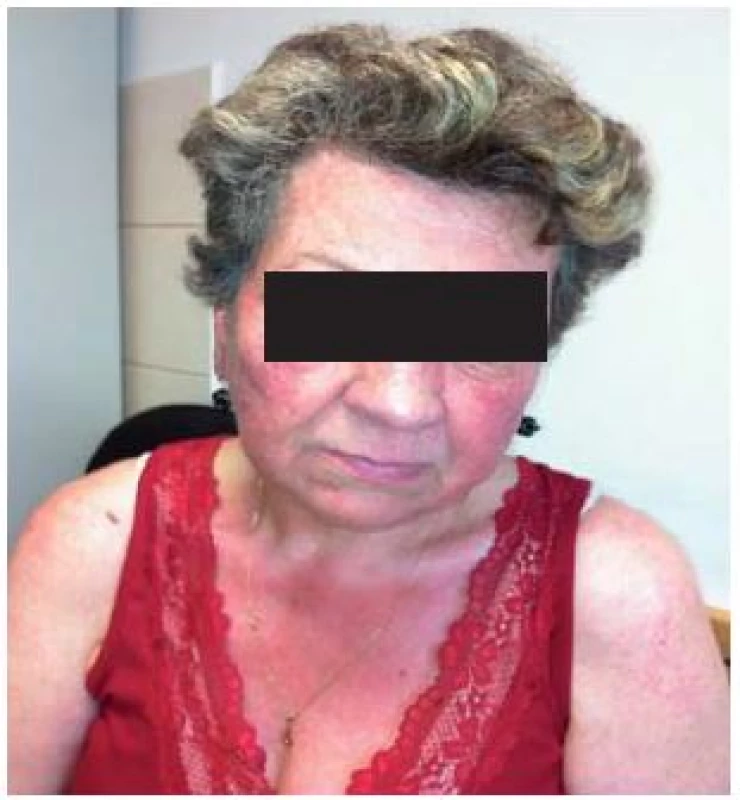
Rosacea or rosacea flush is usuallya centro-facial skin redness, affecting patients in their 40s to 60s [6]. It affects both sexes, but women are affected more frequently than men. It affects Celtic white patients more than others, and is rarely seen in Afro-American and Asian populations [7].
Rosacea is relatively common; the exact incidence is unknown but in one study conducted in Sweden it was found that the disease occurred in about 10–14% female, and 5% male [8].
Pathophysiology of flushing
Flush could be divided into: 1. physiologic flushing or blushing, or flushing associated with certain environmental triggers and 2. pathological facial flush; the latter could be further divided into wet flushing or dry flushing [1], although the general rule states that a typical carcinoid flush is always dry.
Climacteric flush could be considered physiological or pathological. One should note that nearly one in everyfive women with climacteric flush are so badly affected that they cannot tolerate these symptoms and seek treatment for them [7] in such instance flush. Flush associated with climacteric transitions should be considered pathological. This juxtaposition illustrates how the physio-pathological life courses are understood to be quite literally discernible in the female body.
Thermoregulation
Thermoregulation is a complex biological system of neuroendocrine and autonomic structures that may maintain the core body temperature within the threshold [9]. This mechanism of controlling core body temperature either by losing or producing heat is well studied [10,11]. The thermo-neutral zone is maintained within specified limits that can vary rhythmically during the day [9,12].
Three correcting variables play an important role in this bi-directional control circuit:
- the body core, with reporting of the body core temperature to the CNS via afferent thermo-sensitive signal pathways;
- the CNS with efferent lines to the spinal column, brain stem, preoptic area (POA), hypothalamus and limbic system;
- the peripheral vascular system that receives and transmits signals to the CNS [13].
Temperature sensors of body core temperature, among many organs, can be found in the gastrointestinal tract, in intra-abdominal veins and in the spinal cord [14,15]. Thermo-sensitive nerve fibres in the skin as well as in the internal tissues are involved as triggers of thermoregulatory reactions [16]. Thermal information is processed within the CNS, particularly in the anterior hypothalamus with the POA, having pivotal importance in regulating this function [14].
The POA is divided into the medial POA (MPA) and lateral POA [17,18]. The MPA function, among others, is related to the production of gonadotropic releasing hormone [17]. The innervation of MPA is mainly by catecholaminergic pathways, since many noradrenergic neurons and fibres are found in the MPA, which come from different parts of the brain [19], but also dopamine and serotonin activity is described in the MPA [16,20]. Furthermore, structural differences between the two sexes have been described in the MPA in many species of animals and in humans, which are known as sexual dimorphism of the MPA [21] variations that could explain females’ sensitivity to hormonal changes resulting in aggravated vasomotor symptoms. Furthermore, many luteinizing hormone-releasing hormone (LHRH), fibres and neurons were found in different parts of the preoptic hypothalamus, and a study in female rat models has found monomorphic and polymorphic (LHRH) neurons [22].
Warm-sensitive neurons in the POA control the release of heat; effectors in the lateral hypothalamus, periaqueductal grey and in the reticular formation are responsible for peripheral vasodilatation and sweating [23]. The functionally coupled elements of the thermoregulatory control circuit are under catecholaminergic and/or serotonergic control. Serotonergic neurons of the dorsal raphe nucleus of the brain stem project into the POA. Serotonin (5-HT – 5-hydroxytryptamin) 1A, 2A and 2C receptor mRNA is also localised in pre- and postsynaptic serotonin receptors in the POA of primates [24,25]. Noradrenaline signal pathways likewise lead to the POA so that this area receives noradrenergic signals from the solitary nucleus and the locus coeruleus. Alpha-adrenoceptor mRNA has been detected in the POA and in the hypothalamus in rats, as well as β-adrenergic receptors [26]. Vasomotor effectors, that control peripheral vasodilatation and vasoconstriction, are also modulated by noradrenergic and serotonergic signals [27].
Pathophysiology of wet flush
Hot-wet flushes represent an excessive reflex response to changes in the temperature control circuit. The classical vasomotor system (VMS) of a thermoregulatory dysfunction is typical in menopausal women. A variety of disorders can induce comparable symptoms [28]. With concurrent hypothalamic damage, post-traumatic hyperthermia may result [29– 31]. Flushes may occur in patients with breast cancer more frequently and severely than in healthy women [32,33]. It could occur with drugs that act on the oestrogen receptor, and could affect men with prostatic cancer, undergoing androgen ablation treatment [7,5].
Two factors have a significant effect on menopausal flush, the fluctuating level of oestrogen that has a direct effect on VMS and the high level of gonadotropic luteinizing hormone.
- Oestrogens are potent neuromodulators [28,34]. The hypothalamus and POA are rich with functional oestrogen and progesterone receptors [35,29,30]. Oestrogens control the functional activity of neurotransmitter systems in the POA through specific oestrogen receptors [31]. If the CNS cannot adapt itself quickly or efficiently enough to the fluctuating level of oestrogens, then thermoregulatory dysfunction could result [32]. Studies of POA in animal models have found that oestrogens affect the level of serotonin and noradrenaline, and its pre- and postsynaptic binding sites and the deactivation. It increases serotonin synthesis and slows its degradation [25,33,36]. Plasma noradrenaline levels, before and during the hot flush, are found to be increased [37,38,39].
- LH rises in serum LH secretion almost always coinciding with increases in core temperature and hot flushes [40]. Many other causes of wet flush have been identified [41].
Pathophysiology of dry flush (carcinoid flush)
Dry flushing is the most common symptom of carcinoid syndrome and occurs in more than 90% of patients, while carcinoid syndrome occurs in about 10% of patients [42,43].
Certain peptides have been suggested in the pathophysiology of carcinoid flush. During the flushing episode there is a significant increase of substance P-like immunoreactivity and a mild rise of neurokinin 1 (NK1). In contrast, there is no increase in the serotonin level, suggesting that serotonin may or may not be released into the circulation during flushing [44]. It is important to note that substance P and the NK1 receptor are widely distributed in the brain specifically (hypothalamus, amygdala, and the periaqueductal gray) [45] and they are found in close association with 5-HT and neurons containing norepinephrine [46].
Substance P is a potent vasodilator [47]. Substance P is involved in the axon reflex-mediated vasodilatation to local heating and wheal and flare reaction, and in skin through activation of human cutaneous mast cells by acting on G proteins and protein kinase C resulting in the release of histamine [48]. It has been shown that vasodilatation to substance P is dependent on the NK1 receptor located on the endothelium [49]. It also has bronchoconstrictive properties through the vagal system.
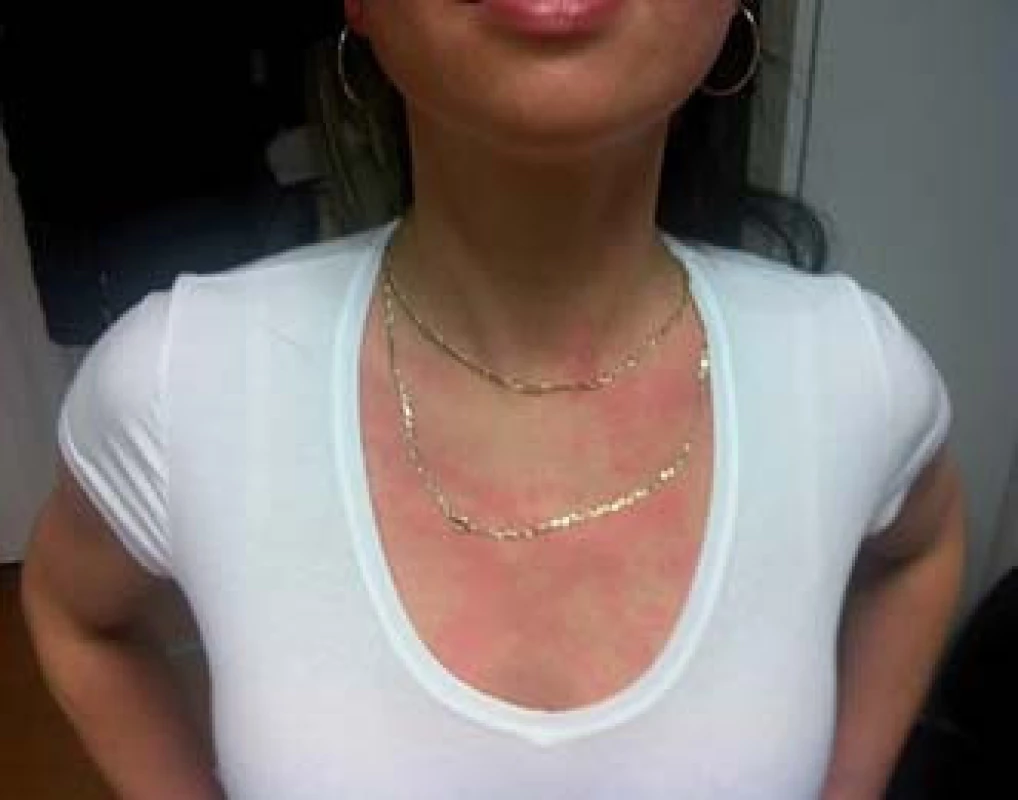
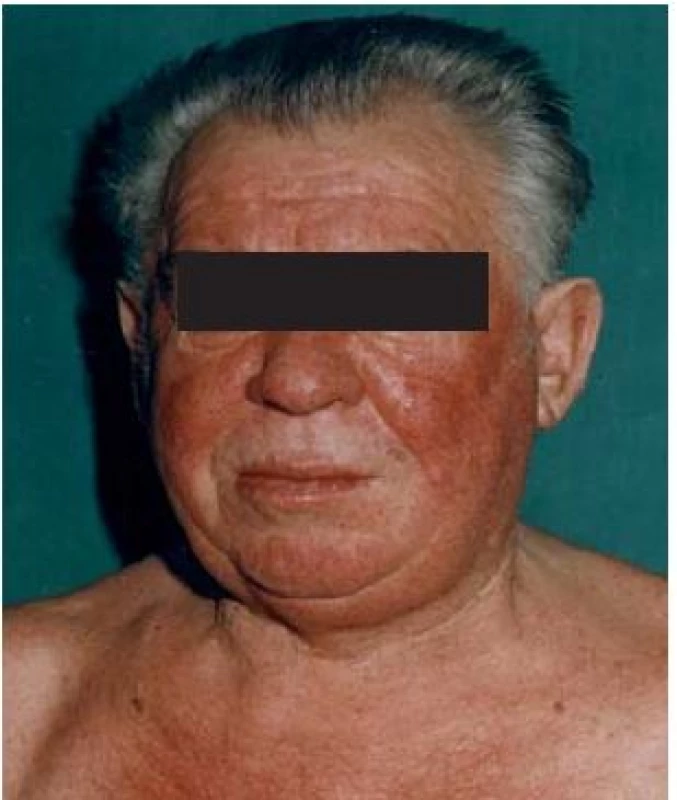
The variations in carcinoid symptoms are attributed to the neuropeptides released by the tumour [50–52]. Tumours of the foregut (stomach, lung bronchi, first part of the duodenum, pancreas) are associated with a brightred geographic flush of a more sustained duration, as well as lacrimation, wheezing, sweating, and a burning sensation. The flush is usually deeper in colour; the hormones involved are believed to be 5-HT, adrenocorticotropin, growth hormone, gastrin, and growth hormone releasing hormone [41,52].
In the midgut (the second part of the duodenum, jejunum, and ascending colon) the flush is believed to be pink red in colour. It is believed that the hormones involved are serotonin, kinins, prostaglandin and neuropeptides including (substance P, substance K and neuropeptide K). Ileal flush, however, cannot be explained solely by the production of serotonin. In ileal tumours, the flush is patchier, intermingled with areas of pallor, and does not last as long. Long-standing flushing may lead to telangiectasia and even facial rosacea [52].
In the hindgut (transverse colon, descending colon and rectum) there is no tendency to flushing [41,51]. Diarrhoea and other gastrointestinal manifestations may precede or coexist with flushing [53].
![Scheme 1. Patient with flush. Adapted from [52].
Schéma 1. Pacient s flushom. Prevzaté z [52].](https://pl-master.mdcdn.cz/media/image/55946c0d6084ad265f5528ea5fa57b59.jpg?version=1537794054)
Pathophysiology of rosacea flush
In the so-called pre-rosacea stage, there is frequent and intense vasodilatation of superficial facial vasculature in susceptible individuals. This brisk flushing can easily be induced by a number of non-specific triggers [54].
The incidence of rosacea is shown to be higher in females, and less frequently in those with skins of a darker colour [55,56]. Studies on the effect of oestrogen on the circulatory apparatus have shown changes in vascular reactivity and structural alterations of blood vessels that participate in vascular growth and remodelling [57]. Furthermore, the incidence of varices is shown to be higher in females, with female : male 6 : 1 ratio before the age of 50, after menopause 1.5 : 1 [5,58], such evidence suggesting a role for oestrogen in rosacea [59].
In the pathogenesis of rosacea, recent studies have suggested disturbances in an innate immune system in patients who are susceptible to rosacea [60]. Such patients usually have disrupted neurovascular communication, causing a lower threshold response to external triggers which leads to the activation of neuropeptides kallikrein 5, cathelicidin and active peptide, e. g. LL35 FA-29 [61], which in turn leads to increased angiogenesis and increased inflammatory response [62]. The hyper reactivity of innate immunity explains the direct influence of pathogens that have been attributed in the pathogenesis of rosacea [63–67].
Conditions that cause physiological flush are considered as triggers [54]. Overheating can cause physiologic flushing, due to the effect of the rise in blood temperature on the thermoregulatory centre in the anterior hypothalamus. A similar mechanism is responsible for facial flushing which is caused by hot drinks, causing rise in the temperature of blood in the oral cavity. Recent anatomical studies have shown that a large number of neurons in the upper cervical spinal cord and caudal medulla project directly to the hypothalamus; sensory signals that arise in the orofacial area are conveyed via the trigeminal nerve to the brain structure. Hence the centro-facial distribution of flush follows the trigeminal dermatome [52]. After years of transient, prolonged blushing, there is an eventual progression to the vascular stage of rosacea. Steinhoff et al. have illustrated the potential pathophysiology of rosacea integrating clinical, immunological, neurovascular, and molecular characteristics [61].
The differential diagnosis of flush represents a myriad of clinical conditions. Whether the diagnosis is as a result of common or less common causes [52], the cornerstone for diagnosis is based on considering a careful and detailed history.
Acknowledgment
All figures are published with courtesy of professor Hyrdel, Gastroenterology Clinic, University Hospital Martin.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE „uniform requirements“ for biomedical papers.
Submitted: 19. 5. 2016
Accepted: 6. 6. 2016
Sohaib Agouba, MD
Gastroenterology Clinic
Department of Internal Medicine
Jessenius Faculty of Medicine and University Hospital Martin
Kollarova 2
036 59 Martin
Slovak Republic
sohaiib@yahoo.com
MUDr. Sohaib Agouba
MU Dr. Sohaib Agouba se narodil v roce 1975 ve městě Chartum v Súdánu. Studium na 1. lékařské fakultě Univerzity Karlovy ukončil v roce 2001. Část studia absolvoval v rámci programu Internship na lékařské fakultě univerzity King Saud a nemocnice King Khalid v Saúdské Arábii a Univerzitní nemocnice Omdurman v Súdanu, kromě toho se účastnil několika dalších pre- a postgraduálních studijních pobytů a stáží, v letech 2003–2004 postgraduálních studií MSc. v oboru klinickė dermatologie v Londýně na londýnské univerzitě King’s College. Po studiu pracoval až do roku 2010 v dermatovenerologickém centru v Saúdské Arábii. V současné době vykonává praxi na Interním oddělení Univerzitní nemocnice Martin na Slovensku a externě spolupracuje s Gastroenterologickým oddělením v téže nemocnici. Pod vedením prof. Hyrdela se věnuje postgraduálnímu studiu v oboru gastroenterologie. Z klinické dermatologie atestoval v roce 2004 v Londýně, z venerologie v roce 2013 na Slovenské zdravotnické univerzitě a jeho první atestace z vnitřního lékařství je z roku 2014. Je autorem publikace o pyoderma gangrenosum a myloproliferativním onemocnění a spoluautorem práce Neuroendokrinné nádory horného tráviaceho traktu. Publikuje v domácím i sáudském odborném tisku.
Zdroje
1. Bouloux PG. Sweating and flushing: evaluation and management. Presented at: ENDO 2013. June 18th, 2013. Available from: http://sessions.endocrine.org/s/2013an/endo/M58
2. Wiel L. The time of the change: menopause’s medicalization and the gender politics of aging. International Journal of Feminist Approaches to Bioethics 2014; 7(1): 74– 98. doi: 10.2979/ intjfemappbio.7.1.74.
3. Hyrdel P, Agouba S, Režňák I et al. Neuroendokrinné nádory tráviaceho traktu. Gastroenterol. prax 2014; 13(4): 209– 216.
4. National Institutes of Health. National Institutes of Health State of the Science Conference statement: management of menopause-related symptoms. Ann Intern Med 2005; 142 (12 Pt 1): 1003– 1013.
5. Rossmanith WG, Ruebberdt W. What causes hot flushes? The neuroendocrine origin of vasomotor symptoms in the menopause. Gynecological Endocrinology 2009; 25(5): 303– 314.
6. Barankin B, Guenther L. Rosacea and atopic dermatitis. Two common oculocutaneous disorders. Can Fam Physician 2002; 48: 721– 724.
7. Taylor SC, David JN. Acne and Rosacea: a closer look at skin of color. [online]. Available from: http://www.medscape.org/viewarticle/770773.
8. Drake L. New studies show high incidence of rosacea and possible new causes. Rosacea Review 2007. [online]. Available from: www.rosacea.org/rr/2007/summer/article_1.php.
9. Deecher DC. Physiology of thermoregulatory dysfunction and current approaches to the treatment of vasomotor symptoms. Expert Opin Investig Drugs 2005; 14(4): 435– 448.
10. Freedman RR. Physiology of hot flashes. Am J Hum Biol 2001; 13(4): 453– 464.
11. Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc 2003; 78(5): 603– 612.
12. Kräuchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol 1994; 267 (3 Pt 2): R819– R829.
13. Boulant JA, Dean JB. Temperature receptors in the central nervous system. Annu Rev Physiol 1986; 48: 639– 654.
14. Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system.Am J Physiol Regul Integr Comp Physiol2007; 292(1): R37– R46.
15. Simon E. The enigma of deep-body thermosensory specificity. Int J Biometeorol 2000; 44(3): 105– 120.
16. Kyratsas C, Dalla C, Anderzhanova E. Experimental evidence for sildenafil’s action in the central nervous system: dopamine and serotonin changes in the medial preoptic area and nucleus accumbens during sexual arousal. J Sex Med 2013; 10(3): 719– 729. doi: 10.1111/ j.1743-6109.2012.03000.x.
17. Koutcherov Y, Mai JK, Paxinos G. Hypothalamus of the human fetus. J Chem Neuroanat 2003; 26(4): 253– 270.
18. Castañeyra-Perdomo A, Pérez-Delgado MM, Montagnese C et al. Brainstem projections to the medial preoptic region containing the luteinizing hormone-releasing hormone perikarya in the rat. An immunohistochemical and retrograde transport study. Neurosci Lett 1992; 139(1): 135– 139.
19. Dudas B, Merchenthaler I. Three-dimensional representation of the neurotransmitter systems of the human hypothalamus: inputs of the gonadotrophin hormone-releasing hormone neuronal system. J Neuroendocrinol 2006; 18(2): 79– 95.
20. Graham MD, Pfaus JG. Differential effects of dopamine antagonists infused to the medial preoptic area on the sexual behavior of female rats primed with estrogen and progesterone. Pharmacol Biochem Behav 2012; 102(4): 532– 539. doi: 10.1016/ j.pbb.2012.06.020.
21. Orikasa C, Sakuma Y. Estrogen configures sexual dimorphism in the preoptic area of C57BL/ 6J and ddN strains of mice. J Comp Neurol 2010; 518(17): 3618– 3629.
22. Castañeyra-Ruiz L, González-Marrero I, Castañeyra-Ruiz A. Luteinizing hormone-releasing hormone distribution in the anterior hypothalamus of the female rats. ISRN Anat 2013; 2013: 870721. doi: 10.5402/ 2013/ 870721.
23. Brück K, Zeisberger E. Adaptive changes in thermoregulation and their neuropharmacological basis. Pharmacol Ther 1987; 35(1– 2): 163– 215.
24. Gundlah C, Pecins-Thompson M, Schutzer WE et al. Ovarian steroid effects on serotonin 1A, 2A and 2C receptor mRNA in macaque hypothalamus. Brain Res Mol Brain Res 1999; 63(2): 325– 339.
25. Bethea CL, Lu NZ, Gundlah C et al. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol 2002; 23(1): 41– 100.
26. Karkanias GB, Ansonoff MA, Etgen AM. Estradiol regulation of alpha 1b-adrenoceptor mRNA in female rat hypothalamus-preoptic area. J Neuroendocrinol 1996; 8: 449– 455.
27. Martin GR. Vascular receptors for 5-hydroxytryptamine: distribution, function and classification. Pharmacol Ther 1994; 62(3): 283– 324.
28. Morissette M, Le Saux M, D’Astous M. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Mol Biol 2008; 108(3– 5): 327– 338.
29. Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol 1993; 336(2): 293– 306.
30. Schwarz JM, Nugent BM, McCarthy MM.Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology 2010; 151(10): 4871– 4881. doi: 10.1210/ en.2010-0142.
31. Malik KF, Feder HH, Morrell JI. Estrogen receptor immunostaining in the preoptic area and medial basal hypothalamus of estradiol benzoate- and prazosin-treated female guinea-pigs. J Neuroendocrinol 1993; 5(3): 297– 306.
32. Deecher DC, Dorries K. Understanding the pathophysiology of vasomotor symptoms (hot flushes and night sweats) that occur in perimenopause, menopause, and postmenopause life stages. Arch Womens Ment Health 2007; 10(6): 247– 257.
33. McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev 1999; 20(3): 279– 307.
34. Gould E, Woolley CS, Frankfurt M et al. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci 1990; 10(4): 1286– 1291.
35. Liu X, Shi H. Regulation of estrogen receptor α expression in the hypothalamus by sex steroids: implication in the regulation of energy homeostasis. Int J Endocrinol 2015; 2015: 949085. doi: 10.1155/ 2015/ 949085.
36. Lu NZ, Bethea CL. Ovarian steroid regulation of 5-HT1A receptor binding and G protein activation in female monkeys. Neuropsycho-pharmacology 2002; 27(1): 12– 24.
37. Serova LI, Maharjan S, Huang A et al. Response of tyrosine hydroxylase and GTP cyclohydrolase I gene expression to estrogen in brain catecholaminergic regions varies with mode of administration. Brain Res 2004; 1015(1– 2): 1– 8.
38. Karkanias GB, Etgen AM. Estradiol reduction of the agonist high affinity form of the alpha 2-adrenoceptor in the hypothalamus of female rats: identification as the alpha 2D subtype. Mol Pharmacol 1994; 45(3): 509– 516.
39. Gundlah C, Lu NZ, Bethea CL. Ovarian steroid regulation of monoamine oxidase-A and -B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology (Berl) 2002; 160(3): 271– 282.
40. Casper RF, Yen SS, Wilkes MM. Menopausal flushes: a neuroendocrine link with pulsatile luteninizing hormone secreation. Science 1979; 205(4408): 823– 825.
41. Family Allergy & Asthma Care of Montana. Do you experience flushing? Is it dry or wet flushing? [online]. Available from: www.familyallergyasthmacare.com/2014/01/do-you-experience-flushing-is-it-dry-or-wet-flushing.
42. Tebbi CK. Carcinoid tumor. [online]. Available from: http://emedicine.medscape.com/article/986050-overview.
43. Aldrich LB, Moattari AR, Vinik AI. Distinguishing features of idiopathic flushing and carcinoid syndrome. Arch Intern Med 1988; 148(12): 2614– 2618.
44. Geppetti P, Renzi D, Caleri C et al. Increase of plasma substance P- and neurokinin A-like immunoreactivity during carcinoid flushing. In: Henry JL, Couture R, Cuello AC (eds). Substance P and Neurokinins. New York: Springer-Verlag 1987: 220– 222.
45. Yip J, Loris A Chahl. Localization of NK1 and NK3 receptors in guinea-pig brain. Regulatory Peptides 2001; 98(1– 2): 55– 62. doi: 10.1016/ S0167-0115(00)00228-7.
46. Gobbi G, Cassano T, Radja F et al. Neurokinin 1 receptor antagonism requires norepinephrine to increase serotonin function. Eur Neu-ropsychopharmacol 2007; 17(5): 328– 338. doi: 10.1016/ j.euroneuro. 2006.07.004.
47. Bossaller C, Reither K, Hehlert-Friedrich C et al. In vivo measurement of endothelium-dependent vasodilation with substance P in man. Herz 1992; 17(5): 284– 290.
48. Columbo M, Horowitz EM, Kagey-Sobotka A et al. Substance P activates the release of histamine from human skin mast cells through a pertussis toxin-sensitive and protein kinase C-dependent mechanism. Clin Immunol Immunopathol 1996; 81(1): 68– 73.
49. Wong BJ, Tublitz NJ, Minson CT. Neurokinin-1 receptor desensitization to consecutive microdialysis infusions of substance P in human skin. J Physiol 2005; 568(3): 1047– 1056. doi: 10.1113/ jphysiol.2005.095 372.
50. Bouloux PM. What to do about flushing and sweating disorders. [online]. Available from: http://press.endocrine.org/doi/full/10.1210/MTP4.9781936704941.ch32.
51. Greaves MW. Flushing and flushing syndromes, rosacea and perioral dermatitis. In: Champion RH, Burton JL, Burns T et al (eds). Rook/Wilkinson/Ebling Textbook of Dermatology. 6th ed. Oxford: Blackwell Scientific 1998: 2099– 2104.
52. Izikson L, English JC 3rd, Zirwas MJ. The flushing patient: differential diagnosis, workup, and treatment. J Am Acad Dermatol 2006; 55(2): 193– 208.
53. Nasr C. Flushing. [online]. Available from: www.clevelandclinicmeded.com/medicalpubs/diseasemanagement/endocrinology/flushing/.
54. Scheinfeld NS. Rosacea. Skinmed 2006; 5(4): 191– 194.
55. Al Balbeesi AO, Halawani MR. Unusual features of rosacea in saudi females with dark skin. Ochsner J 2014; 14(3): 321– 327.
56. Pray WS, Pray JJ. Differentiating between rosacea and acne. US Pharmacist 2004; 29(4). [online]. Available from: www.medscape.com/viewarticle/475331.
57. Perrot-Applanat M. Effect of estrogens on vascular proliferation. Therapie 1999; 54(3): 333– 337.
58. Laing W. Chronic venous disease of the leg. London: Office of Health Economics 1992: 10– 12.
59. Özcan S, Tezcan O, Kurt T et al. Serum estradiol/ free testosterone ratio precipitate recurrent varicose veins in men. Int Angiol 2015; 34(6): 576– 581.
60. Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci 2009; 55(2): 77– 81.
61. Steinhoff M, Buddenkotte J, Aubert Jet al. Clinical, cellular, and molecular aspectsin the pathophysiology of rosacea. J Investig Dermatol Symp Proc 2011; 15(1): 2– 11.doi: 10.1038/ jidsymp.2011.7.
62. Coda AB, Hata T, Miller J et al. Cathelicidin, kallikrein 5, and serine protease activity is inhibited during treatment of rosacea with azelaic acid 15% gel. J Am Acad Dermatol 2013; 69(4): 570– 577. doi: 10.1016/ j.jaad.2013.05.019.
63. Nielsen PG. Metronidazole treatment in rosacea. Int J Dermatol 1988; 27(1): 1– 5.
64. Utaş S, Ozbakir O, Turasan A. Helicobacter pylori eradication treatment reduces the severity of rosacea. J Am Acad Dermatol 1999; 40(3): 433– 435
65. Gravina A, Federico A, Ruocco E. Helicobacter pylori infection but not small intestinal bacterial overgrowth may play a pathogenic role in rosacea. United European Gastroenterol J 2015; 3(1): 17– 24.
66. Bonnar E, Eustace P, Powell FC. The Demodex mite population in rosacea. J Am Acad of Dermatol 1993; 28(3): 443– 448.
67. Gollnick H. Current concepts of the pathogenesis of acne: implications for drug treatment. Drugs 2003; 63(15): 1579– 1596.
Štítky
Detská gastroenterológia Gastroenterológia a hepatológia Chirurgia všeobecnáČlánok vyšiel v časopise
Gastroenterologie a hepatologie
2016 Číslo 4
- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- MUDr. Lenka Klimešová: Multiodborová vizita je kľúč k efektívnejšej perioperačnej liečbe chronickej bolesti
- Realita liečby bolesti v paliatívnej starostlivosti v Nemecku
- Fixní kombinace paracetamol/kodein nabízí synergické analgetické účinky
- Tramadol a paracetamol v tlumení poextrakční bolesti
Najčítanejšie v tomto čísle
- A first evaluation of the Septin 9 test in the Czech Republic
- Gastric outlet obstruction and obstructive jaundice as the first symptoms of primary duodenal lymphoma
- Flush, rosacea or blushing – understanding the differences
- Oesophageal bronchogenic cyst

