Giant frontal sinus pneumocele with massive intracranial spread imitating lumboperitoneal shunt malfunction in a predisposed patient with Marfan syndrome
Objemná pneumokéla frontálního sinu s rozsáhlým intrakraniálním šířením imitujícím dysfunkci lumboperitoneální drenáže u predisponovaného pacienta s Marfanovým syndromem
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Authors:
M. Formánek 1; P. Matoušek 1
; T. Hrbáč 2; R. Lipina 2; Pavel Komínek 1
Authors place of work:
Department of Otorhinolaryngology and Head and Neck Surgery, University Hospital Ostrava, Czech Republic
1; Department of Neurosurgery, University Hospital Ostrava, Czech Republic
2
Published in the journal:
Cesk Slov Neurol N 2019; 82(4): 458-460
Category:
Dopis redakci
doi:
https://doi.org/10.14735/amcsnn2019458
Summary
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do biomedicínských časopisů.
Dear editorial office,
Frontal sinus pneumocele (FSP) was first described in 1898 [1]. It is characterized by excessive pneumatization of the frontal sinus, extending beyond the frontal bone margins, with bony wall thinning and defects, and extension of air into the surrounding soft tissue. Pneumocele is often confused with pneumosinus dilatans, from which it can be distinguished based on the bony wall effects in FSP [2]. FSP is a rare condition, with only a few cases reported in literature [2–8].
The pathophysiologic mechanism behind FSP development remains unclear. Proposed causes include spontaneous mucocele drainage, the presence of gas-producing microorganisms, post-traumatic involvement, benign and malignant neoplasms, hormonal abnormalities, congenital factors and ostiomeatal complex pathology [2–8]. FSP is usually asymptomatic, or presents symptoms typical for paranasal sinus pathology [2].
Here we report a unique case in which a predisposed patient with Marfan syndrome presented with a giant left FSP showing massive intracranial spread. This FSP caused frontal lobe compression and brain midline shift, imitating lumboperitoneal (LP) shunt malfunction. To our knowledge, such a case has not previously been reported in literature.
The patient was a 32-year-old man with Marfan syndrome, who had a history of Bentall aortic procedure and repeated LP shunt placements due to repeated spinal epidural haematoma following lumbar puncture and revision surgeries for adhesions. The lumbar puncture was performed for several years of recurring acute pain of sacrococcygeal symphysis radiating to the top of the head and behind the eyes lasting from 12 to 24 h. The patient had amaurosis on the right side after previous eye surgery. He was referred to the Department of Neurosurgery at a tertiary referral hospital due to headaches, vomiting and fatigue lasting 14 days. He had no nasal obstruction or secretion, no localized dull pain in the frontal region and no further change of the facial contours of Marfanoid habitus.
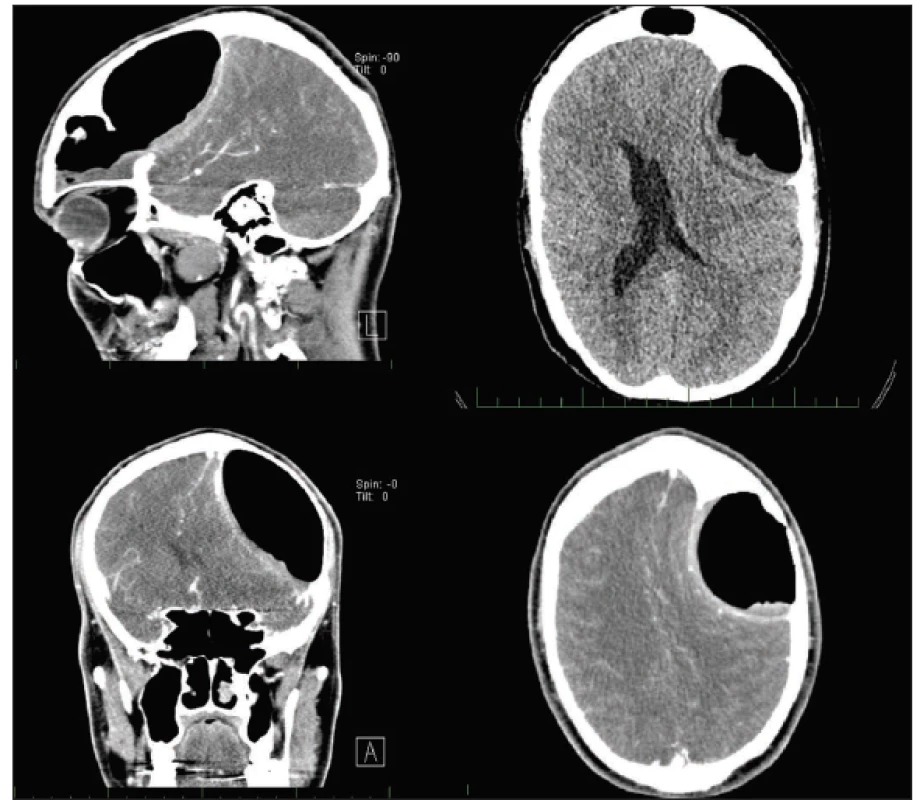
Clinical examination revealed no explanatory pathology – negative tests for meningitis, physiologic function of cranial nerves, muscle strength and present direct photoreaction on the left side. There were only Romberg III titubation and mild lower limbs hyperreflexia. CT was performed due to a suspicion of LP malfunction, considering the patient’s history of similar symptoms in every previous LP malfunction. CT scans revealed a giant FSP on the left side, eroding the dorsal bone wall, spreading into the intracranium, compressing the left frontal lobe, causing brain midline shift and lateral ventricle compression, and dislocating branches of the middle cerebral artery. Frontal sinus ostium obstruction was also evident (Fig. 1). The patient was referred to the Department of Otorhinolaryngology and Head and Neck Surgery for surgical intervention.
Obr. 1. Předoperační CT vyšetření s kontrastní látkou zobrazující objemnou pneumokélu frontálního sinu vlevo, která komprimuje levý
frontální lalok, způsobuje středočárový posun a kompresi postranní mozkové komory vlevo.
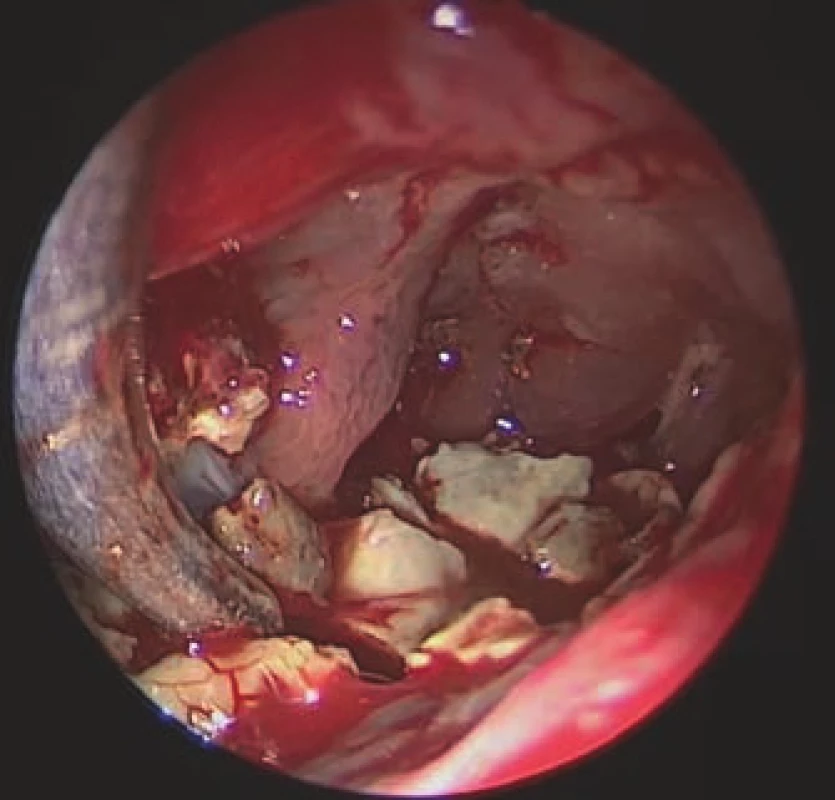
A combined procedure was performed on the left side using neuronavigation. First, an endoscopic endonasal Draf II frontal sinusotomy was performed. Due to the size of the cavity and the finding of a massive fungal infection therein (Fig. 2), this procedure had to be combined with endoscopic frontal sinus surgery via an external approach, from a small skin incision in the inner corner of the left eye and window in the caudal sinus wall (sec. Jansen-Ritter). The FSP dorsally exposed a large dura mater surface. Visualization of the whole cavity was challenging, requiring the use of 30° and 70° 4- mm nasal HD endoscopes (KARL STORZ SE & Co. KG, Tuttlingen, Germany). All the yellowish, hard stone-like masses were removed. At the end of surgery, the interfrontal sinus septum was partly removed to support proper drainage and prevent recurrence. The biopsy confirmed the presence of a fungal infection in the cavity, but cultivation was negative and no antifungal treatment was indicated. The LP shunt was left in place.
Obr. 2. Endoskopický pohled z externího
přístupu do levé frontální dutiny, ve které
jsou viditelné nažloutlé, kamenně tuhé,
mykotické hmoty.
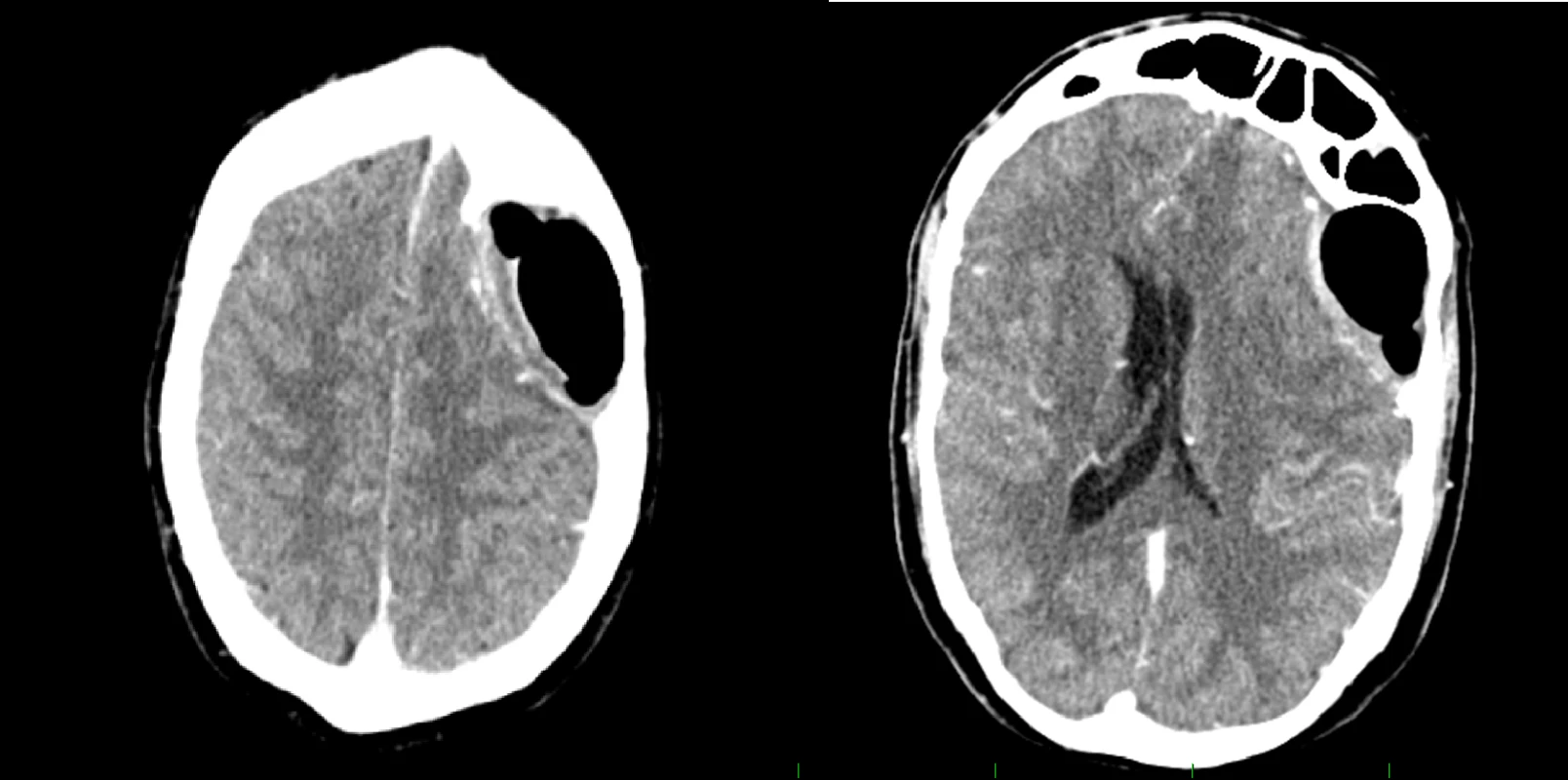
The procedure yielded complete symptomatic relief. At 12 months postoperatively, the patient remains free of symptoms. A postoperative CT scan revealed regression of the midline shift (Fig.3).
Obr. 3. Pooperační CT vyšetření s kontrastní látkou ukazující regresi nálezu.
Frontal sinus pneumocele is a rare condition. The most commonly mentioned cause is a unidirectional valve mechanism operating at the level of the frontal sinus ostium, which was also found in the presently reported case. In our patient, a massive fungal infection had caused near-total obstruction of the nasofrontal duct on the left side [2,7]. This obstruction acted as a one-way valve mechanism, allowing air to penetrate into the cavity but not exit from it, leading to increased pressure in the cavity and the development of potentially life-threatening FSP. Some prior reports have also mentioned the presence of gas-producing microorganisms in the sinus cavity as a possible cause of FSP; however, this would only be considered in cases involving bacterial infection [4]. In our present case, FSP development may have been facilitated by the patient’s primary diagnosis of Marfan syndrome, which is a systemic autosomal dominant disorder of the connective tissue caused by mutations on chromosome 15 in the FBN1 gene encoding fibrillin-1 and is generally associated with skeletal fragility and dural defects (predominantly progressive dural
ectasia) [9,10]. Fibrillin-1 together with other extra-cellular matrix proteins forms thread-like microfibrils, which provide elasticity and structural support to tissue. Mutated FBN1 leads to loss of extracellular integrity [10].
Frontal sinus pneumocele symptoms vary greatly and depend on pneumocele size and extent. FSPs commonly have an asymptomatic course, but may also be associated with dull pain in the affected area, change of facial contours, diplopia, or other local pressure symptoms. The presently reported patient uncharacteristically suffered from headaches, vomiting and fatigue due to the massive intracranial spread of the FSP. Although the FSP spread into the orbit has been described, this is the first reported case with such extensive and potentially life-threatening intracranial spread, with brain midline shift and lateral ventricle compression [7,8]. We assume that the presence of the LP shunt could contribute to this unique occurrence. We assume that partial unidirectional or pressure-dependent communication through adhesions was preserved considering the patient’s symptoms of intracranial hypertension in previous LP malfunctions. The shunt equalized the intracranial pressure by reducing the CSF volume and decreased resistance, promoting FSP growth. It also prevented earlier symptomatic manifestation, allowing additional time for massive growth before discovery. Notably, the patient did not report previous meningitis or cerebral infection, despite the presence of fungal infection in the sinus and the extensive communication between the frontal sinus and less solid dura mater due to the posterior sinus wall erosion.
The combined endoscopic procedure addressed the FSP cause and, despite the huge FSP volume, was successful and provided long-term relief with minimal cosmetic effect. However, the procedure was challenging and had to be performed by a highly experienced surgeon, especially when operating in close proximity to the patient’s only seeing eye.
Here we report a unique case in which an FSP exhibited a massive spread into the intracranium in a predisposed patient, causing potentially life-threatening changes. Despite the extensive spread, a successful endoscopic procedure was performed, addressing the cause of the FSP and achieving long-term relief.
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manu script met the ICMJE “uniform requirements” for biomedical papers.
Martin Formánek, MD, PhD
Department of Otorhinolaryngology and Head and Neck Surgery
University Hospital Ostrava
17. listopadu 1790
708 52 Ostrava
Czech Republic
e-mail: martin.formanek@fno.cz
Accepted for review: 17. 3. 2019
Accepted for print: 15. 5. 2019
Zdroje
1. Meyes W. Mitteilung eines Falles vermutlicher: pneumatocele des Sinus frontalis. Monatsschr Ohrenheilkd 2009; 32: 467–469.
2. Urken ML, Som PM, Lawson W et al. Abnormally large frontal sinus. II. Nomenclature, pathology, and symptoms. Laryngoscope 1987; 97(5): 606–611.
3. Benedikt RA, Brown DC, Roth MK et al. Spontaneous drainage of an ethmoidal mucocele: a possible cause of pneumosinus dilatans. AJNR Am J Neuroradiol 1991; 12(4): 729–731.
4. Mauri M, Oliveira CO, Krumenauer RC et al. Pneumosinus dilatans: uma doença de etiologia variada. Braz J Otorhinolaryngol 1999; 65: 529–533.
5. Gu Z, Cao Z. Frontal sinus pneumocele associated with respiratory epithelial adenomatoid hamartoma and nasal polyps. Otolaryngol Head Neck Surg 2012; 147(1): 177–178. doi: 10.1177/ 0194599811432232.
6. Karadag D, Calisir C, Adapinar B. Post-traumatic pneumocele of the frontal sinus. Korean J Radiol 2008; 9(4): 379–381. doi: 10.3348/ kjr.2008.9.4.379.
7. Abdel-Aal AK, Abayazeed AH, Raghuram K et al. Pneumocele of the frontal sinus producing orbital roof defect: case report and review of literature. Am J Otolaryngol 2010; 31(3): 202–204. doi: 10.1016/ j.amjoto.2009.01.001.
8. Sasaki T, Yamoto T, Fujita K et al. A Case of orbital emphysema associated with frontal sinus pneumocele. J Neurol Surg Rep 2013; 74(1): 54–56. doi: 10.1055/ s-0033-1347903.
9. McKusick V. The defect in Marfan syndrome. Nature 1991; 352(6333): 279–281. doi: 10.1038/ 352279a0.
10. Chavkin U, Brenner-Ullman A, Ungar OJ et al. Prevalence of temporal bone tegmen defects among patients with Marfan syndrome. Acta Otolaryngol 2019; 139(5): 421–424.
Štítky
Detská neurológia Neurochirurgia NeurológiaČlánok vyšiel v časopise
Česká a slovenská neurologie a neurochirurgie

2019 Číslo 4
- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- MUDr. Lenka Klimešová: Multiodborová vizita je kľúč k efektívnejšej perioperačnej liečbe chronickej bolesti
- Realita liečby bolesti v paliatívnej starostlivosti v Nemecku
Najčítanejšie v tomto čísle
- Multiple system atrophy
- Superior semicircular canal dehiscence
- Spina bifida in the Czech Republic – incidence and prenatal diagnostics
- Hearing loss after spinal anesthesia
