-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
An isolated metastasis to the heart from a malignant phyllodes tumor with osteosarcomatous differentiation
Izolovaná metastáza do srdce z maligního fyloidního nádoru s osteosarkomatózní diferenciací
74-letá žena byla přijata v těžkém stavu pro selhávající pravé srdce. CT vyšetření ukázalo tumor, který infiltrativně prorůstal mezikomorovým septem v pravé části srdce a šířil se od hrotu srdečního až pod trojcípou chlopeň. Nádor pronikal do konusu a. pulmonalis, kde se polokulovitě vyklenoval a výrazně zužoval lumen. V důsledku nádorové infiltrace pacientka zemřela na selhání srdeční.
Histologicky nádor tvořily atypické protáhlé buňky někde s velkými buňkami, které měly výrazně zvěšená hyperchromní a laločnatá jádra. V některých úsecích měl nádor bifazický vzhled. Nádorové buňky připomínaly epitel, ale imunohistologická vyšetření na průkaz cytokeratinů byla negativní. Protáhlé buňky exprimovaly desmin a hladkosvalový aktin. Převážná část nádoru byla tuhé až tvrdé konzistence a histologicky odpovídala osteosarkomu. Dodatečně bylo zjištěno, že u pacientky byla před třemi roky provedena pravostranná mastektomie pro maligní fyloidní nádor s osteosarkomatózní diferenciací. Metastáza do srdce byla jedinou metastázou zjištěnou při pitvě.Klíčová slova:
maligní fyloidní nádor s osteosarkomatózní diferenciací - metastáza do srdce - selhání srdce
Authors: Jirka Mačák 1; Pavel Hurník 1; Jana Dvořáčková 1,2; Jana Mačáková 3
Authors place of work: Department of Pathology, Faculty of Medicine, University of Ostrava and University Hospital Ostrava, Czech Republic 1; CGB Laboratory, Ostrava, Czech Republic 2; Department of Physiology and Pathophysiology, Faculty of Medicine, University of Ostrava and University Hospital Ostrava, Czech Republic 3
Published in the journal: Čes.-slov. Patol., 50, 2014, No. 4, p. 146-149
Category: Původní práce
Summary
A 74-year-old woman was admitted in a serious condition due to the failing right heart. A CT scan revealed a tumor infiltration through the interventricular septum in the right heart, spreading from the apex as far as under the tricuspid valve. The tumor penetrated into the conus of the pulmonary artery, bulging and markedly narrowing the lumen. As a result of the tumor infiltration, the patient died from cardiac failure.
Histological examination of the tumor revealed atypical elongated cells and areas of large cells with significantly enlarged hyperchromatic and lobulated nuclei. In some portions, the tumors had a biphasic appearance. The tumor cells resembled epithelial tissue but immunohistological analyses to detect cytokeratins yielded negative results. The elongated cells expressed desmin and smooth muscle actin. A vast majority of the tumor was solid or hard, histologically corresponding to osteosarcoma. Later, it was found that the patient undergone right-sided mastectomy for a malignant phyllodes tumor with osteosarcomatous differentiation three years previously. The metastasis to the heart was the only metastasis detected by the autopsy.Keywords:
malignant phyllodes tumor with osteosarcomatous differentiation - metastasis to heart - cardiac failure
Malignant phyllodes tumors (PTs) account for approximately 20 % of all PTs. They are also responsible for local recurrences (1-3). Approximately one-fifth of them metastasize but a lethal course is rare (2,3). Most frequently, hematogenous metastases to the lungs, soft tissues, bones and pleura are observed. Spread to the heart is rare and isolated metastases may mimic primary tumors of the heart (4,5). Occasionally, tumors exhibit heterologous elements, for instance, liposarcomatous, fibrosarcomatous, osteosarcomatous, chondrosarcomatous and rhabdomyosarcomatous (6). In such cases, the original biphasic features disappear and the tumors are sarcomatous.
We present a case of a patient who developed a tumor in the right heart three years after right-sided mastectomy. The tumor led to stenosis in the area of the tricuspid valve and conus arteriosus and, subsequently, to cardiac failure.
Material and Methods
Tissue samples from autopsy and mastectomy three years previously were fixed in 10 % formalin and processed by the paraffin technique. Then the tissues were stained with hematoxylin and eosin; immunohistochemical staining was performed with the avidin-biotin complex (ABC) method. The antibodies including clone numbers, working dilutions and results are shown in Table 1.
Case report
A 74-year-old woman was admitted due to progressive dyspnoea over several months and chest tightness. The examination revealed a tumor in the right ventricle causing stenosis in the tricuspid valve region and cardiac failure. When endomyocardial biopsy was attempted, iatrogenic cardiac tamponade and circulatory instability occurred. After drainage of the pericardial cavity, the patient’s condition improved but her dyspnoea at rest continued to be present. The condition gradually progressed, resulting in bradycardia and respiratory and circulatory failure.
It was ascertained from the patient’s history that at the age of 71 years, she underwent right-sided mastectomy for a tumor sized 10 x 8 x 7 cm in a health care facility outside the University Hospital Ostrava. In the center of the tumor, a cystic cavity was found. The tumor was solid to hard. Originally, it was diagnosed as a malignant PT with osseous metaplasia. Later, the diagnosis was revised to a malignant PT with osteosarcomatous differentiation. Histologically, the tumor comprised structures of a fibroadenoma transforming into a malignant PT (Fig. 1). Mesenchymal lesions markedly narrowed tubular epithelial structures. Some mesenchymal cells were atypical, with large irregular hyperchromatic nuclei. Some portions of the mesenchyme just under the epithelium of compressed tubules contained areas of osteoid and bone trabeculae transforming into larger segments with bone trabeculae and atypical osteoblasts in their close proximity. Adjacent to bone trabeculae there were elongated cellular elements with relatively large hyperchromatic cells. The mitotic index was 5 mitoses per 10 HPF. The finding was classified as a malignant PT with osteosarcomatous differentiation (Fig. 2).
Fig. 1. Malignant phyllodes tumor of the breast, HE. 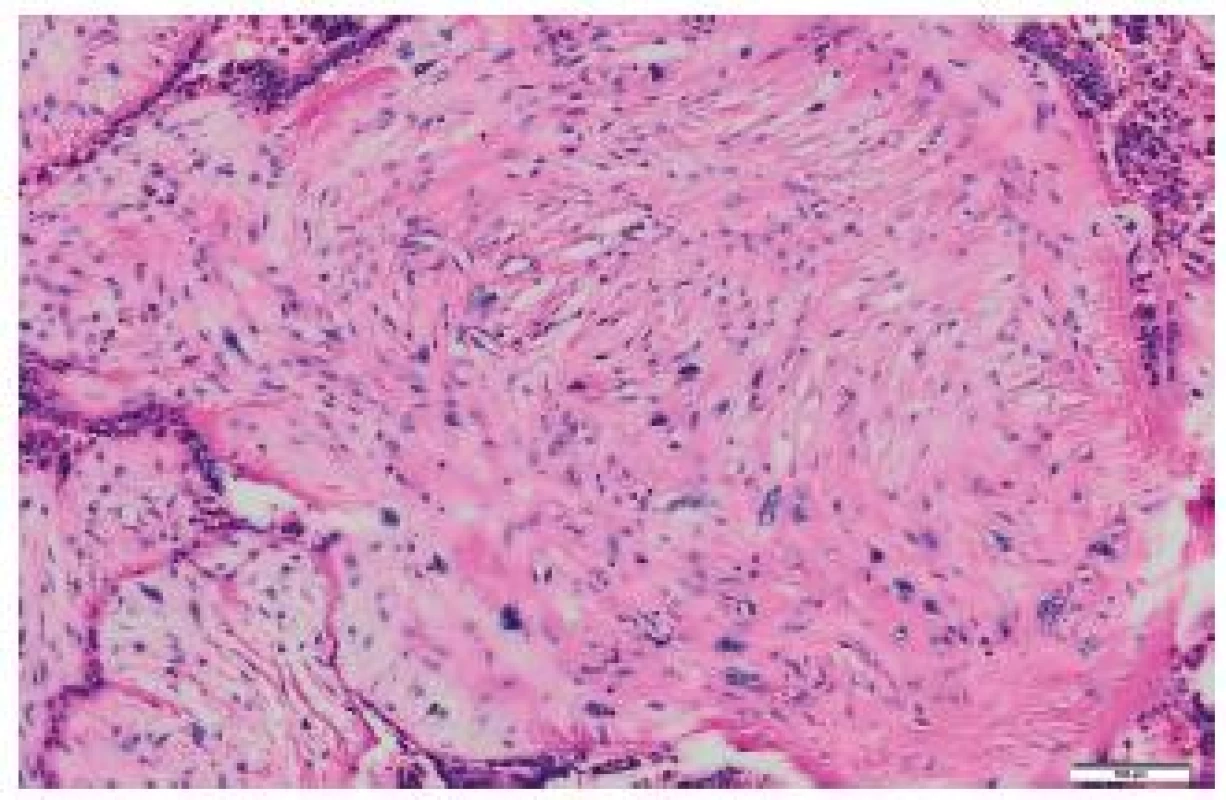
Fig. 2. Osteosarcomatous differentiation of a malignant phyllodes tumor of the right breast. HE, scale bar 20 μm. 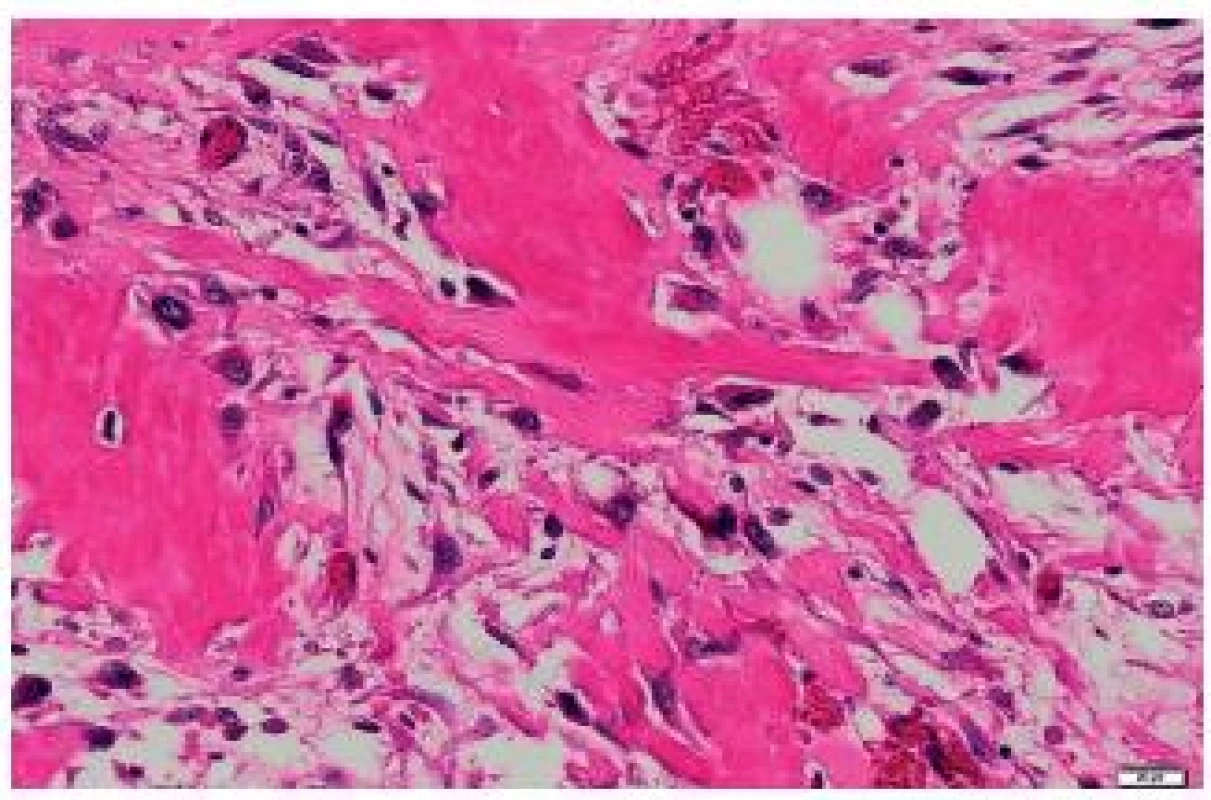
Fig. 3. Heart – a metastasis mostly infiltrating the right ventricular myocardium and ventricular septum. 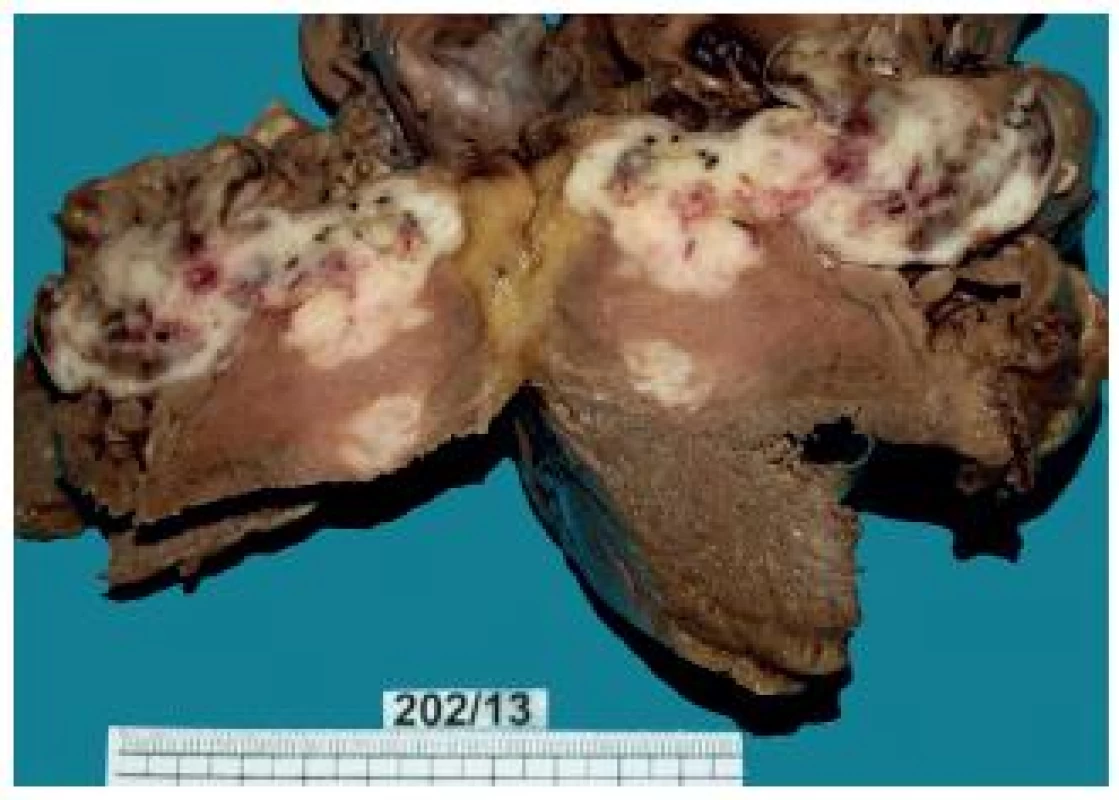
Fig. 4. Elongated tumor cells of the metastasis to the heart, with no signs of storiform arrangement. HE, scale bar 20 μm. 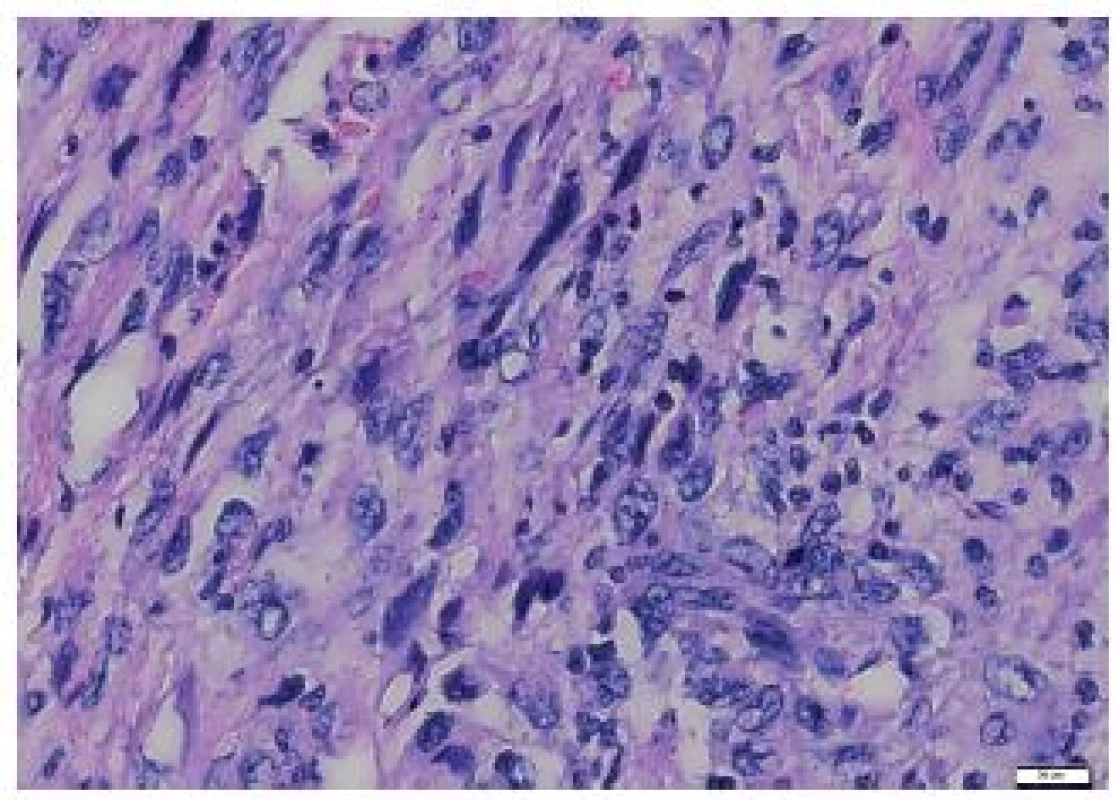
Fig. 5. Irregular bone trabeculae of the metastasis of a malignant phyllodes tumor with osteosarcomatous differentiation to the heart. Close to the trabeculae, elongated tumor cells are seen. HE, scale bar 20 μm. 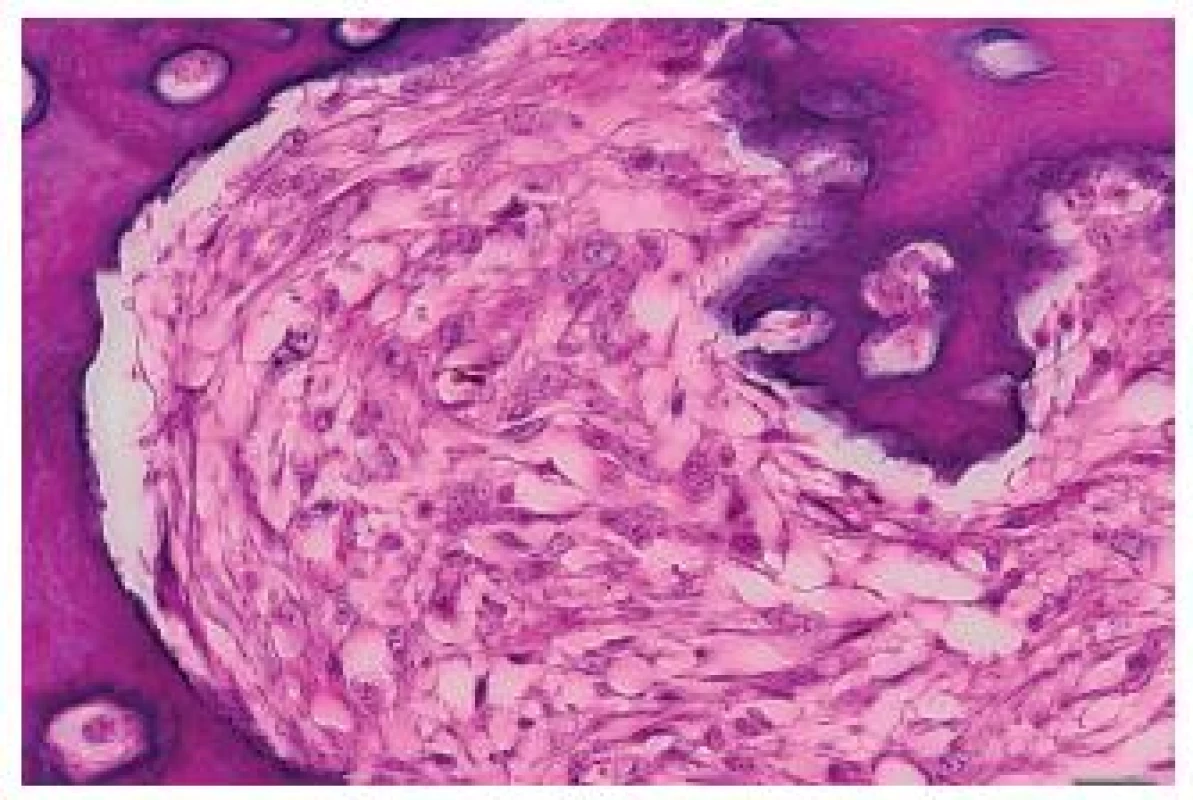
Fig. 6. Metastasis of the tumor to the heart – biphasic structures were present in some portions. HE, scale bar 100 μm. 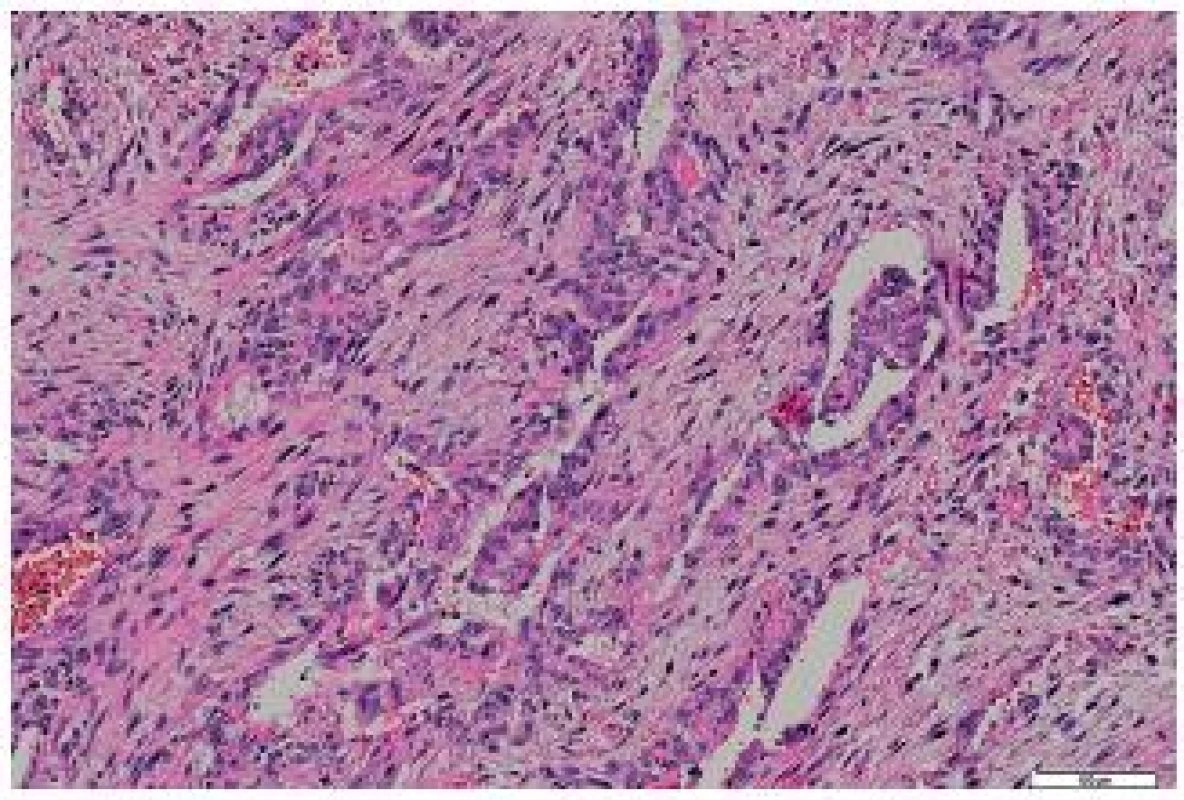
Tumor in the heart: macroscopic findings
The tumor was located in the region of the right ventricular apex. From there, it spread along the muscular ventricular septum to the tricuspid valve. Irregular tumor nodules bulging into the right ventricular lumen were covered with the endocardium. The tumor grew into the conus arteriosus, forming a bulging polypoid structure that narrowed the space under the pulmonary valve (Fig. 3). The size of the tumor from the right ventricular apex to the tricuspid valve region was 7.5 x 8 x 6 cm. In the region between the apex and the tricuspid valve, the tumor was solid to hard.
Histological pattern of the tumor in the heart
The tumor was made up of elongated cells with irregular hyperchromatic nuclei. The tumor cells formed intertwined fascicles (Fig. 4). The tumor contained necrotic deposits. These tumor structures were smoothly continuous with irregular bone trabeculae surrounded by elongated tumor cells (Fig. 5). In some portions, structures with a biphasic appearance were formed (Fig. 6). Immunohistochemical evaluation with antibodies against cytokeratins yielded negative results. The tumor was penetrated by numerous irregular thin-walled capillaries expressing the CD34 antigen. The tumor cells themselves were negative with an antibody against the antigen. Sporadically, small amounts of lipoblast-like cells were seen. The immunohistochemistry results are shown in Table 1. Positive results were achieved with antibodies against desmin in approximately 40 % of cells and against SMA in 50 - 60 % of cells. The other antibodies did not react with the tumor.
Discussion
PTs are classified into benign, borderline and malignant. Benign and borderline tumors have a very favorable prognosis and may be treated by surgical removal. Malignant tumors may recur and metastasize. Despite intensive research in this area, immunohistochemical methods have failed to predict behavior of malignant PTs (7-9) and attention has turned to molecular pathology methods (10).
Although malignant PTs mostly metastasize to the lungs and bones, distant metastases may occur, for example to the adrenals (11) and heart. Two cases of malignant PTs metastasizing to the heart have been published (4,5). In both cases, tumors occupied the right ventricle. In one case, a metastatic tumor was detected 3 years after mastectomy. This was similar to our case of a tumor metastasizing to the right ventricle and right atrium 3 years after mastectomy.
Tab. 1. Results of immunohistochemical examination of the metastasis to the heart. 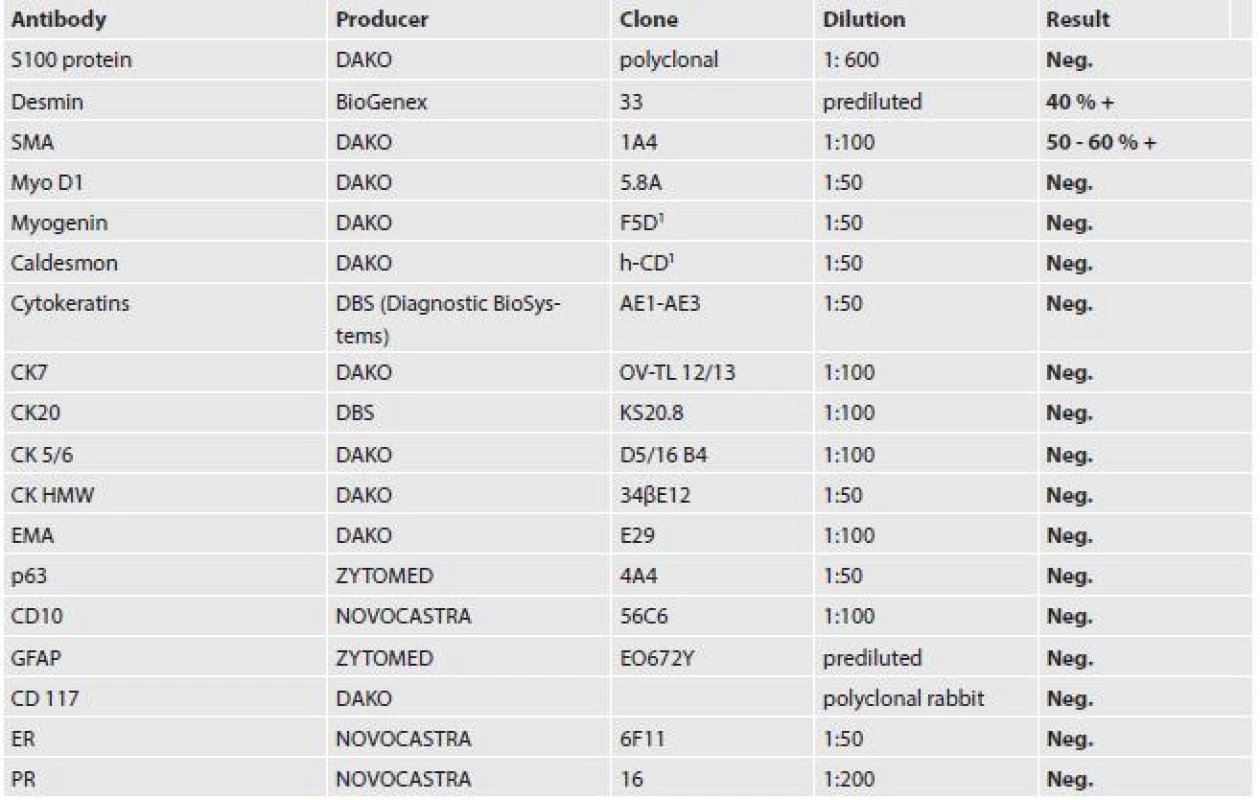
PTs with osteosarcomatous differentiation are estimated to account for approximately 1.3 % of all malignant PTs (6,12). As in other locations, osteosarcomas in PTs are classified based on prevailing morphological patterns into fibroblastic, osteoclastic and osteoblastic. According to Tavassoli (14), the osteosarcomatous component can occupy a variable percentage of a PT, ranging from 25 % to 100 %. Rare cases have been reported (13) of the osteosarcomatous component being detected not in the primary tumor but only in lung metastastases. In these cases, very rare primary pulmonary osteosarcoma must be ruled out. In another case, telangiectatic osteosarcoma was detected in a recurrent tumor but not in the primary mass (15).
Some PTs are quite large and tiny areas of osteosarcomatous differentiation cannot be completely ruled out. In the mammary gland, sarcoma may rarely occur as the primary tumor or as a part of a malignant PT. Silver et al. (6) studied Armed Forces Institute of Pathology material collected in the last 30 years to detect 22 malignant PTs with osteosarcomatous differentiation. In 11 cases, axillary lymph nodes were histologically examined and found to be free of tumor. The mean size of the neoplasms was 6.4 cm. According to the authors, neoplasms larger than 5 cm are potentially aggressive. Of the set of 22 PTs with osteosarcomatous differentiation, metastases were clinically apparent within 1 year of diagnosis in 8 patients and 7 died within 12 months of detection of initial metastases. Thus, biological behavior of these neoplasms is similar to that of soft tissue osteosarcomas.
In our case, transformation of connective tissue into osteosarcoma was observed just under the ductal epithelium. Other segments contained numerous irregular bone lamellae with atypical osteoblasts in their close proximity. Differential diagnosis must distinguish the tumor from other neoplasms potentially containing cartilaginous or osseous structures such as breast cancer or metaplastic carcinoma (16). In such cases, metaplastic cartilaginous or osseous segments are a part of, for example, invasive ductal carcinoma in the adjacent areas.
Rarely, the epithelial component may undergo malignant transformation in PTs. Such cases are very low in numbers, with fewer than 30 cases having been reported (17-19). Very sporadically, metaplastic spindle cell carcinoma was noticed in a PT (1).
In metaplastic carcinoma, elongated sarcomatous cells transforming into carcinoma structures may be observed, with immunohistochemical findings revealing cytokeratin-positive isolated elongated cells or clusters of such cells. Kinkor et al. (1) reported sarcomatoid (metaplastic) spindle cell carcinoma in a PT. The authors pointed to potential confusion with primary sarcoma of the mammary gland, especially in small or core-cut biopsies. Sarcomas, however, are always negative in immunohistochemical reactions for cytokeratin (12). It must be stressed that elongated cells in PTs may focally express cytokeratins (20). In such cases, immunohistochemistry results should be interpreted with caution. The prognosis of patients with metaplastic breast carcinoma containing osteocartilaginous heterologous elements is no worse than in infiltrating ductal carcinomas (21).
Treatment of primary osteosarcomas of the mammary gland is similar to that of malignant PTs with osteosarcomatous differentiation (22-24). These patients undergo radical mastectomy, simple mastectomy or complete resection of the tumor. In spite of hematogenous spread of the tumor, resection of axillary lymph nodes is recommended.
✉ Correspondence address:
Prof. MUDr. Jirka Mačák, CSc.
Ústav patologie, Fakultní nemocnice Ostrava
17. listopadu 1790, 708 52 Ostrava
e-mail: macak.jirka@seznam.cz
tel. 00420 737054581
Zdroje
1. Kinkor Z, Sticová E, Šach J, Rychtera J, Skálová A. Sarkomatoidní (metaplastický) vřetenobuněčný karcinom prsu vznikající ve fyloidním tumoru s rozsáhlou skvamózní metaplázií – kazuistika a přehled literatury. Cesk Patol 2012; 48 : 156-160.
2. Rosen PP. Fibroepithelial neoplasms. In: Rosen PP, Hoda SA, Breast Pathology. Diagnosis by needle core biopsy (2nd ed). Philadelphia, PA: Lippincott Williams & Wilkins 2006, 69-84.
3. Bellocq JP, Margo G. Tumours of the breast. Fibroepithelial tumors. In: Tavassoli FA, Devilee P. WHO classification of tumours. Pathology & Genetics. Tumours of the Breast and Female Genital Organs. Lyon: IARC Press 2003, 99-103.
4. Garg N, Moorthy N, Agrawal SK. Delayed cardiac metastasis from phyllodes breast tumor. Tex Heart Inst 2011; 38 : 441-444.
5. Myojin K, Murakami T, Ishii K, Kunihara T. An emergent operation for metastatic cardiac tumor of malignant cystosarcoma phyllodes. Jpn J Thorac Cardiovasc Surg 1998; 46 : 202-206.
6. Silver SA, Fattanen A, Tavassoli A. Osteosarcomatous differetiation in phyllodes tumors. Am J Surg Pathol 1999; 23 : 815-821.
7. Al-Masri M, Darwazeh G, Sawalmi S, Mughrabi A, Sughayer M, Al-Shatti M. Phyllodes tumor of the breast: role of CD10 in predicting metastasis. Ann Surg Oncol 2012; 19 : 1181-1184.
8. Spitaleri G, Toesca A, Botteri E et al. Breast phyllodes tumor: A review of literature and single retrospective series analysis. Oncol Haematol 2013; 88 : 427-436.
9. Noronha Y, Raza A, Hutchins B et al. CD34, CD117, and Ki-67 expression in phyllodes tumor of the breast: an immunohistochemical study of 33 cases. Inter J Surg Pathol 2011; 19 : 152-158.
10. Kwon JE, Jung WH, Koo JS. Molecules involved in epithelial-mesenchymal transition and epithelial-stromal interaction in phyllodes tumors: implications for histologic grade and prognosis. Tumour Biol 2012; 33 : 787-798.
11. Collin Y, Chagnon F, Mongeau C J, Gonzales-Amaya GL, Sideris L. Adrenal metastasis of phyllodes tumor of the breast: case report and review of the literature. Int J Surg Case Reports 2013; 4 : 687-689.
12. Küçük Ü, Bayol Ü, Etit D et al. Malignant phyllodes tumor of the breast with osteosarcomatous differentiation. J Breast Health 2013; 9 : 88-91.
13. Tsubochi H, Sato N, Kaimori M, Imai T. Osteosarcomatous differentiation in lung metastases from a malignant phyllodes tumour of the breast. J Clin Pathol 2004; 57 : 432-434.
14. Tavassoli FA. Pathology of the breast (2nd ed). Stamford Connecticut: Appleton & Lange 1999, 598-613.
15. Graadt van Roggen JF, Zordenland HM, Welvaart K, Peterse JL, Hogendoom PC. Local recurrence of a phyllodes tumor of the breast presenting with widespread differentiation to a teleangiectatic osteosarcoma. J Clin Pathol 1998; 51 : 706-708.
16. Yoichi T, Nagashma T, Yagata H et al. Breast cancer with cartilaginous and/or osseous metaplasia. Breast Cancer 2009; 16 : 234-237.
17. Abdul Aziz M, Sullivan F, Kerin MJ, Callogy G. Malignant phyllodes tumour with liposarcomatous differentiation, invasive tubular carcinoma, and ductal and tubular carcinoma in situ: case report and review of the literature. Patholog Res Int 2010; 2010 : 501274.
18. Kodama T, Kameyama K, Mukai M, Sugiura H, Ikeda T, Okada Y. Invasive lobular carcinoma arising in phyllodes tumor of the breast. Virchows Arch 2003; 442 : 614-616.
19. Nishimura R, Hasebe T, Imoto S, Mukai K. Malignant phyllodes tumour with a non-invasive ductal carcinoma component. Virchows Arch 1998; 432 : 89-93.
20. Chia Y, Thike AA, Cheak PY, Yong-Zheng Chong L, Man-Kit Tse G, Tan PH. Stromal keratin expression in phyllodes tumors of the breast: a comparison with other spindle cell breast lesions. J Clin Pathol 2012; 65 : 339-347.
21. Chhieng CH, Cranor M, Lesser MW, Rosen PP. Metaplastic carcinoma of the breast with osteocartilaginous heterologous elements. Am J Surg Pathol 1998; 22 : 188-194.
22. Minami H, Wakita N, Kawanishi Y, Kitano I, Sakata M, Shida T. Primary osteosarcoma of the heart with severe congestive heart failure. Jpn J Thorac Cardiovasc Surg 2000;48 : 607-609.
23. Koçak H, Karapolat S, Gündoğdu C, Bozkurt E, Ünlü Y. Primary cardiac osteosarcoma in a pregnant woman. Heart Vessels 2006; 21 : 56-58.
24. Takeuchi I, Kawaguchi T, Kimura Y, Kojima J, Shimamura H, Shimizu N, Izumi T. Primary cardiac osteosarcoma in a young man with severe congestive heart failure. Intern Med 2007; 46 : 649-651.
Štítky
Patológia Súdne lekárstvo Toxikológia
Článek Pokroky v uropatológiiČlánek Za všetkým hľadaj ženu...
Článok vyšiel v časopiseČesko-slovenská patologie

2014 Číslo 4-
Všetky články tohto čísla
- Pokroky v uropatológii
- Za všetkým hľadaj ženu...
- MONITOR aneb nemělo by vám uniknout, že...
- A concise update on prostate pathology
- Update on urinary bladder pathology
- International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia 2012
- Current status of urinary cytology in the evaluation of bladder neoplasms
- An isolated metastasis to the heart from a malignant phyllodes tumor with osteosarcomatous differentiation
- Morphological and electrophysiological changes of the heart atria in necropsy patients with atrial fibrillation – a pilot study
- Bart´s syndrome associated with epidermolysis bullosa junctionalis and with pyloric atresia. An autopsy case report
- Novinky v testování RAS u kolorektálního karcinomu - konsensus z pracovního setkání zástupců referenčních laboratoří
- Česko-slovenská patologie
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Current status of urinary cytology in the evaluation of bladder neoplasms
- Bart´s syndrome associated with epidermolysis bullosa junctionalis and with pyloric atresia. An autopsy case report
- International Society of Urological Pathology (ISUP) Vancouver Classification of Renal Neoplasia 2012
- A concise update on prostate pathology
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



