-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Many Roles of Molecular Chaperones and Co-chaperones in Tumour Biology
Role molekulárních chaperonů a ko-chaperonů v biologii nádorů
Molekulární chaperony (heat-shock proteiny, Hsps) jsou proteiny, které udržují intracelulární homeostázu skládáním a stabilizací konformace jiných proteinů. Díky schopnosti chránit proteom před špatně složenými a agregovanými proteiny jsou chaperony nezbytné pro přežití buněk vystavených stresu. Kromě základní funkce v udržování buněčné homeostázy a ochraně před vnějšími stresovými faktory hrají některé molekulární chaperony důležitou roli i při transformaci nádorové buňky. Zvýšená hladina chaperonů byla detekována u mnoha solidních nádorů a hematopoetických malignit. Nárůst aktivity chaperonů v nádorových buňkách odráží jejich schopnost kompenzovat stresové podmínky způsobené hypoxií, zvýšenou proteosyntézou a přítomností mutantních a potenciálně nestabilních proteinů. Chaperony navíc umožňují nádorovým buňkám tolerovat genetické změny stabilizováním terciární struktury mutantních proteinů – typicky onkoproteinů –, které by jinak byly pro buňku letální. Z tohoto pohledu chaperony zprostředkovávají fenotypové vyjádření onkogeních mutací a přispívají k získání všech základních znaků nádorové buňky. Kvůli jejich nezbytné funkci v nádorech ovlivňující současně několik esenciálních onkogenních drah se chaperony staly atraktivním cílem nádorové terapie.
Klíčova slova:
molekulární chaperony – ko-chaperony – Hsp90 – nádorové onemocnění
Authors: M. Ďurech; B. Vojtesek; P. Müller
Authors place of work: Regional Centre for Applied Molecular Oncology, Masaryk Memorial Cancer Institute, Brno, Czech Republic
Published in the journal: Klin Onkol 2012; 25(Supplementum 2): 45-49
Práce byla podpořena granty IGA MZ ČR NT/13794-4/2012, GAČR P206/12/G151 a Evropským fondem pro regionální rozvoj a státním rozpočtem České republiky (OP VaVpI –RECAMO, CZ.1.05/2.1.00/03.0101).
Autoři deklarují, že v souvislosti s předmětem studie nemají žádné komerční zájmy.
Redakční rada potvrzuje, že rukopis práce splnil ICMJE kritéria pro publikace zasílané do bi omedicínských časopisů.
Obdrženo: 11. 10. 2012
Přijato: 24. 10. 2012Summary
Molecular chaperones (heat-shock proteins, Hsps) are proteins that maintain intracellular homeostasis through folding and stabilisation of the conformation of other proteins. Molecular chaperones are critical for survival of cells that undergo cellular stress due to their ability to guard the proteome against misfolded proteins and aggregation. In addition to their canonical role in basic cellular homeostasis and protection against external stress, several molecular chaperones play a fundamental role in malignant cell transformation. The level of molecular chaperones is increased in many solid tumours and haematological malignancies. The increased activity of Hsps in cancer cells reflects the ability of chaperones to compensate for stress caused by hypoxia, increased protein turnover and the presence of numerous mutated and potentially unstable proteins. In addition, chaperones allow tumour cells to tolerate genetic alterations by stabilising tertiary structure of mutated unstable proteins – typically oncoproteins that would otherwise be lethal. From this perspective, chaperones mediate the phenotypic expression of oncogenic mutations and contribute to all the hallmarks of cancer cells. Due to their indispensable roles for cancer cells, chaperones became an attractive group of targets for novel cancer therapies affecting several essential oncogenic pathways simultaneously.
Key words:
molecular chaperones – co-chaperones – Hsp90 – cancerChaperones Act in Multichaperone Complexes
Although chaperones are relatively abundant, they rarely, if ever, function alone [1]. They typically create large multiprotein complexes that contain other chaperones, co-chaperones and various accessory proteins. Chaperone assisted folding is a complex multistep process based on non-covalent interactions between chaperones and their substrates, called “clients”. The folding cycle of Hsp90 (heat-shock protein of 90-kDa) is driven by ATP hydrolysis which enables conformational changes and the recruitment of different co-chaperones. The mechanism of the Hsp90 folding cycle was described for the maturation of steroid-hormone receptors (SHR) by Smith et al [2] (Fig. 1): The chaperone cycle starts when the newly synthesised or denaturated client protein associates with Hsp70 (heat-shock protein of 70-kDa), Hsp40 (heat-shock protein of 40-kDa) and the adapter HIP (Hsp70-interacting protein) to form an early complex [3,4]. Then adapter protein HOP (Hsp70/Hsp90-organising protein), that binds both Hsp70 and Hsp90 chap-erones simultaneously, shifts the client protein to Hsp90 dimer and displaces Hsp40 to form an intermediate complex. In an ATP-dependent manner, the Hsp90 dimer binds the client protein and Hsp70, HOP and HIP are replaced by co-chaperones p23 and CYP40 (cyclophilin 40) to complete the mature complex. Hormone binding to SHR in the mature complex leads to a conformational change of SHR driven by ATP hydrolysis. Finally, SHR is dissociated and transferred to the nucleus to regulate gene transcription. The spectrum of folded clients is also influenced by association of Hsp90 with different co-chaperones. For example, Cdc37 (cell division cycle 37) is a co-chaperone which binds to the N-terminal domain of Hsp90 and facilitates the recruitment of various kinases to the Hsp90 machinery [5,6]. The mechanism and function of co-chaperones will be discussed in more details below.
Fig. 1. Maturation of steroid-hormone receptor (SHR) in chaperone cycle driven by ATP hydrolysis, where Hsp90 conformational state is influenced by interaction with specific co-chaperones. 
Altered Chaperone Function in Cancer
Many client proteins of chaperones are unstable oncoproteins, which are highly dependent on chaperone-mediated stabilisation of their structure. Due to its’ broad spectrum of clients, Hsp90 activity is essential for manifestation of all cancer hallmarks [7]. Hsp90 thus participates in self-sufficiency in growth signals, insensitivity to growth-inhibitory signals, evasion of apoptosis, limitless replicative potential, sustained angiogenesis and tissue invasion and metastasis. Therefore, the inhibition of Hsp90 can affect all major attributes of cancer simultaneously by targeting the clients for degradation. Inhibition of Hsp90 or Hsp70 may provide a broader, more effective anti-cancer therapy than inhibition of single oncogenic pathways. Moreover, inhibition of Hsp90 prevents development of new oncogenic mutations and thus decreases resistance against this therapy. Another reason making Hsp90 a unique therapeutic target is the fact that the majority of its’ clients are regulatory proteins responsible for cell growth, cell cycle and survival [8,9]. Tab. 1 provides an insight into contributions of many Hsp90 client proteins to the malignant phenotype [10].
Tab. 1. Hsp90 clients and the malignant phenotype. 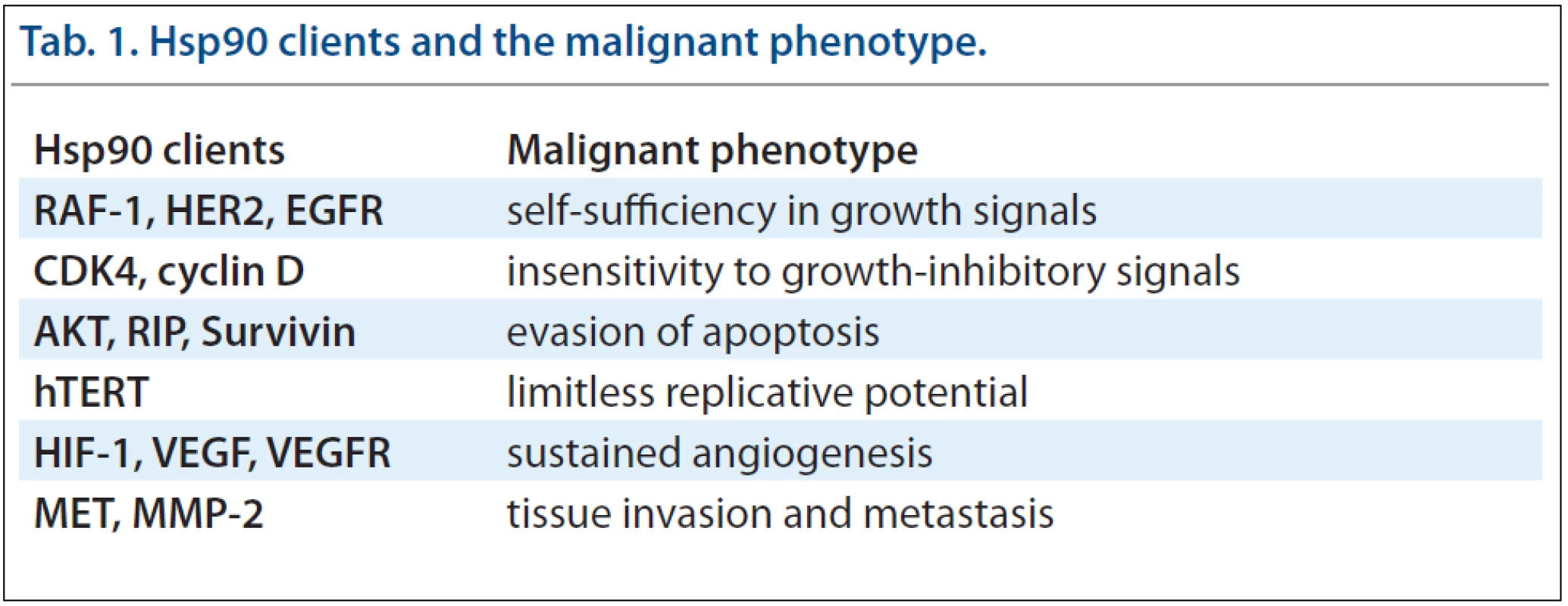
The earliest and perhaps most dramatic example of this phenomenon is provided by the SRC tyrosine kinase which is involved in several signal transduction pathways that regulate cell growth and proliferation. Most oncogenic mutations of SRC involve truncation of the C-terminal part of the protein which leads to a constitutively active but unstable protein [11]. Normal c-SRC requires only limited assistance of Hsp90 chaperone machinery [12] in contrast to mutated v-SRC that exhibits an unusually stable association with Hsp90 [13,14]. Other prominent client proteins of Hsp90 connected to cancer evolution are receptor tyrosine kinases (EGFR, HER2, IGF1R and FLT3), serine/threonine kinases (RAF-1, AKT and CDK4), mutant fusion kinases (BCR-ABL), transcription factors (p53, androgen and estrogen receptor, HSF-1 and HIF-1) and telomerase (hTERT) [9]. More proteins known to interact with Hsp90 can be viewed on http://www.picard.ch//downloads/Hsp90interactors.pdf maintained by Picard laboratory [15].
Hsp90 Inhibitors
The discovery of the antitumour activity of the Hsp90 inhibitors geldanamycin and its’ analogues opened a new field in anticancer therapy employing the inhibition of chaperones. Hsp90 inhibitors are now being actively pursued by the pharmaceutical industry, with 17 agents having entered clinical trials [16,17]. One of the first inhibitors of Hsp90, 17AAG, is undergoing Phase III clinical trials with an improved formulation that overcomes several toxicities that were common in earlier trials. 17AAG binds to the N-terminal ATP-binding pocket of Hsp90 and alters many of its’ normal functions [18]. Inhibition of Hsp90 results in recruitment of E3 ubiquitin ligases such as CHIP (C-terminus of Hsp70-interacting protein) that affects the multichaperone complex and leads to increased proteasome-mediated degradation of the client proteins and depletion of their cellular levels [19]. Recent evidence indicates that Hsp90 has an approximately 100-fold higher affinity for 17AAG in cancer cells than in normal cells, leading to accumulation of drug selectively in tumour cells [20]. The difference results from the presence of Hsp90 in multichaperone complexes in cancer cells, probably due to increased levels of unstable oncogenic proteins and higher rates of genetic instability [21]. In contrast to cancer cells, normal cells contain a substantial pool of free Hsp90 dimer with lower affinity to the drug.
Hsp90 Inhibition Induces Compensatory Overexpression of Hsp70 Chaperone
The effectiveness of Hsp90 inhibitors is limited by compensatory stress response mediated by heat-shock factor (HSF-1). The Hsp90 inhibitors disrupt a complex between Hsp90 and HSF-1 which results in activation of HSF-1 [22]. HSF-1 then triggers gene expression of other chaperones (e.g. Hsp70 or Hsp27) [23] that compensate for the effect of Hsp90 inhibition and enable cell survival.
Recent studies have shown that HSF-1 depletion decreased viability of multiple human cancer cell lines, but had no effect on normal cells [24]. HSF-1 seems to provide another critical element in maintaining cellular homeostasis in the stressful tumour microenvironment. In addition, recent reports suggest that HSF-1 supports malignancy not only by facilitating the induction of Hsps, but also by orchestrating a broad network of heterogeneous cellular functions that include proliferation, survival, protein synthesis and energy metabolism [24,25]. Hence, non-oncoproteins like HSF-1, whose functions are critical for cancer cells but dispensable for normal cells, may also be an attractive target for cancer therapy [26].
Another way to increase the effectiveness of Hsp90 inhibition is the combination of Hsp90 and Hsp70 specific inhibitors. The potentiation between Hsp70 and Hsp90 inhibition in cancer cells was demonstrated by co-administration of siRNA against Hsp70 and the Hsp90 inhibitor 17AAG [27]. Powers et al [28] have also shown that simultaneous suppression of two cytosolic Hsp70s (Hsc70 and Hsp72) sensitised tumour cells to 17AAG. This insight has led to the suggestion that simultaneous inhibition of Hsp90 and Hsp70 might increase the efficacy of Hsp90 inhibitors, but so far only a few compounds that are able to inhibit Hsp70 activity have been reported [29].
Co-chaperones Modulate Chaperone Activity
The most important regulators of the Hsp90 machinery are the co-chaperones and post-translational modifications of the Hsp90 protein itself, e.g. acetylation, nitrosylation and phosphorylation [30]. For example, acetylation of Hsp90 can inhibit the binding of client proteins to Hsp90 and enhance their proteasomal degradation.
Co-chaperones have diverse effects on the Hsp90 chaperone machinery. Mostly, they modulate the state of the Hsp90 cycle by affecting Hsp90 conformation and modulating its’ affinity to client proteins [30,31]. It was shown that interaction of Hsp90 with co-chaperones such as p23 or AHA1 (activator of Hsp90 ATPase homologue 1) influences the ATPase activity of Hsp90 and its’ sensitivity to Hsp90 inhibitors [32,33]. Therefore, the altered expression of these co-chaperones could be responsible for diverse sensitivity of cancer cells to anti-Hsp90 therapy [34].
Some co-chaperones serve as adaptors that deliver specific client proteins to Hsp90. For instance, Cdc37 delivers protein kinase clients [35], while HOP participates in delivering steroid hormone receptor clients from Hsp70 to Hsp90 [36]. Steroid hormone receptor function is then modified by other co-chaperones, including FKBP51 and FKBP52 (FK506-binding protein 51 and 52) [37]. The most extensive group of co-chaperones are those with TPR (tetratricopeptide repeat) domains that interact with the C-terminal EEVD motif of Hsp70 and/or Hsp90, including co-chaperones HOP, TOMM34 (34 kDa-translocase of outer mitochondrial membrane), CHIP, FKBPs, CYP40 and PP5 (protein phosphatase 5) [1]. The co-chaperone AHA1 interacts with the central domain of Hsp90, co-chaperones Cdc37 and p23 bind at the N-terminal domain.
As mentioned above, co-chaperone expression affects cancer cell sensiti-vity to Hsp90 inhibitors. The deletion of p23 in yeast causes hypersensitivity to geldanamycin (an antibiotic that inhibits N-terminal ATPase binding domain of Hsp90 in a similar way as 17AAG) [32] and overexpression of this co-chaperone is seen in cancers [38]. Similarly, silencing of Cdc37 and AHA1, which are also overexpressed in many cancers [35], sensitised cancer cells to both geldanamycin and 17AAG [33,39]. HOP has been shown to be overexpressed in colonic carcinoma cells [40], hepatocellular carcinoma [41], invasive pancreatic cancer cell lines and malignant tissues of pancreatic cancer patients [42], suggesting an important role in the malignant progression. Additionally, HOP knockdown by siRNA decreases expression of the downstream target matrix metalloproteinases-2 (MMP-2) and reduces the invasion of pancreatic cancer cells [43]. Knockdown of HOP expression also reduced expression levels of Hsp90 client proteins, HER2, BCR-ABL, c-MET and v-SRC. These data show that the attenuation of HOP expression inactivates key signal transducers possibly through the modulation of Hsp90 activity. Another study revealed accumulation of the co-chaperone TOMM34 in colorectal carcinoma tissues compared to corresponding non-cancerous mucosae [44]. Moreover, transfection of colon cancer HCT116 cells with siRNA specific to TOMM34 drastically inhibited cell growth. These findings suggest that TOMM34 is also involved in the growth of cancer cells and may contribute to the development of novel anti-cancer drugs and/or diagnosis for colorectal cancer.
Chaperones maintain protein homeostasis not only by maturation of newly synthesised proteins and stabilisation of unstable proteins, but also by recognition and transport of defective proteins to the degradation pathway. This needs the recruitment of another co-chaperone, CHIP (an E3 ubiquitin ligase), into the Hsp90 chaperone machinery [45]. It was shown that CHIP suppresses tumour progression in human breast cancer by enhancing the degradation of several oncogenic proteins, e.g. SRC-3 [46]. Moreover, knockdown of CHIP in breast cancer cells results in rapid tumour growth and metastatic phenotypes in mice. The machanisms regulating the protein folding/degradation balances involve chaperone binding to CHIP and HOP that depends on a phosphorylation state of Hsp90 and Hsp70 C-termini [47]. The phosphorylation of these chaperones prevents binding to CHIP and enhances binding to HOP. Proliferating cells express lower levels of CHIP and higher HOP, Hsp70 and Hsp90 levels compared to non-proliferating cells [48]. Decreased CHIP expression in proliferative cells supports its’ proposed tumour suppressor properties, while overexpression of HOP may contribute to excessive Hsp90 activity and stabilisation of client proteins in cancer cells. These reports reflect elevated protein folding environment in cancer cells regulated by the action of co-chaperone expression and chaperone modifications.
Taken together, these findings suggest that targeting co-chaperones may be therapeutically beneficial, especially in combination with Hsp90 inhibitors [33].
Conclusion
Molecular chaperones are proteins that guide normal protein folding and degradation of many key regulators of cell growth, differentiation and survival. In contrast to normal non-stressed cells, cancer cells are dependent on high activity of chaperones which must compensate for the stress caused by tumour microenviroment and genetic instability. The difference in expression level of specific co-chaperones in different cancers possibly influences Hsp90 affinity to Hsp90 inhibitors (e.g. 17AAG) suggesting co-chaperones as a new target for cancer therapy. Since Hsp90 inhibition also causes compensatory overexpression of Hsp70, the simultaneous inhibition of Hsp90 and Hsp70 chaperones might increase the efficacy of Hsp90 inhibitors. Thus, targeting the most abundant molecular chaperones Hsp70 and Hsp90 seems to be a powerful approach in cancer therapy in the future.
This work was supported by grant of Internal G rant Agency of the Czech Ministry of Health No. NT/13794-4/2012, by grant of Czech Science foundation No. P206/12/G151 and by the European Regional Development Fund and the State Budget of the Czech Republic (RECAMO, CZ.1.05/2.1.00/03.0101).
The authors declare they have no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Petr Muller, M.D., Ph.D.
Masaryk Memorial Cancer Institute
Regional Centre for Applied Molecular Oncology
Zluty kopec 7
656 53 Brno
Czech Republic
e-mail: muller@mou.cz
Submitted: 11. 10. 2012
Accepted: 24. 10. 2012
Zdroje
1. Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol 2010; 11(7): 515–528.
2. Smith DF, Whitesell L, Nair SC et al. Progesterone receptor structure and function altered by geldanamycin, an hsp90-binding agent. Mol Cell Biol 1995; 15(12): 6804–6812.
3. Prodromou C, Panaretou B, Chohan S et al. The ATPase cycle of Hsp90 drives a molecular ‚clamp‘ via transient dimerization of the N-terminal domains. EMBO J 2000; 19(16): 4383–4392.
4. Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer 2005; 5(10): 761–772.
5. Lee P, Shabbir A, Cardozo C et al. Sti1 and Cdc37 can stabilize Hsp90 in chaperone complexes with a protein kinase. Mol Biol Cell 2004; 15(4): 1785–1792.
6. Roe SM, Ali MM, Meyer P et al. The Mechanism of Hsp90 regulation by the protein kinase-specific cochaperone p50(cdc37). Cell 2004; 116(1): 87–98.
7. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100(1): 57–70.
8. Nathan DF, Vos MH, Lindquist S. In vivo functions of the Saccharomyces cerevisiae Hsp90 chaperone. Proc Natl Acad Sci U S A 1997; 94(24): 12949–12956.
9. Pratt WB. The hsp90-based chaperone system: involvement in signal transduction from a variety of hormone and growth factor receptors. Proc Soc Exp Biol Med 1998; 217(4): 420–434.
10. Ruckova E, Muller P, Vojtesek B. [Hsp90 – a target for anticancer therapy]. Klin Onkol 2011; 24(5): 329–337.
11. Falsone SF, Leptihn S, Osterauer A et al. Oncogenic mutations reduce the stability of SRC kinase. J Mol Biol 2004; 344(1): 281–291.
12. Xu Y, Singer MA, Lindquist S. Maturation of the tyrosine kinase c-src as a kinase and as a substrate depends on the molecular chaperone Hsp90. Proc Natl Acad Sci U S A 1999; 96(1): 109–114.
13. Brugge J, Yonemoto W, Darrow D. Interaction between the Rous sarcoma virus transforming protein and two cellular phosphoproteins: analysis of the turnover and distribution of this complex. Mol Cell Biol 1983; 3(1): 9–19.
14. Oppermann H, Levinson W, Bishop JM. A cellular protein that associates with the transforming protein of Rous sarcoma virus is also a heat-shock protein. Proc Natl Acad Sci U S A 1981; 78(2): 1067–1071.
15. Picard.ch [online]. Department of Cell Biology. University of Geneva, Switzerland; c2011 [updated 2012 August; cited 2012 October]. Available from: http://www.picard.ch/downloads/Hsp90interactors.pdf.
16. Kim YS, Alarcon SV, Lee S et al. Update on Hsp90 inhibitors in clinical trial. Curr Top Med Chem 2009; 9(15): 1479–1492.
17. Neckers L, Workman P. Hsp90 molecular chaperone inhibitors: are we there yet? Clin Cancer Res 2012; 18(1): 64–76.
18. Roe SM, Prodromou C, O‘Brien R et al. Structural basis for inhibition of the Hsp90 molecular chaperone by the antitumor antibiotics radicicol and geldanamycin. J Med Chem 1999; 42(2): 260–266.
19. Xu W, Marcu M, Yuan X et al. Chaperone-dependent E3 ubiquitin ligase CHIP mediates a degradative pathway for c-ErbB2/Neu. Proc Natl Acad Sci U S A 2002; 99(20): 12847–12852.
20. Kamal A, Boehm MF, Burrows FJ. Therapeutic and diagnostic implications of Hsp90 activation. Trends Mol Med 2004; 10(6): 283–290.
21. Kamal A, Thao L, Sensintaffar J et al. A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 2003; 425(6956): 407–410.
22. Whitesell L, Bagatell R, Falsey R. The stress response: implications for the clinical development of hsp90 inhibitors. Curr Cancer Drug Targets 2003; 3(5): 349–358.
23. Kim HR, Kang HS, Kim HD. Geldanamycin induces heat shock protein expression through activation of HSF1 in K562 erythroleukemic cells. IUBMB Life 1999; 48(4): 429–433.
24. Dai C, Whitesell L, Rogers AB et al. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell 2007; 130(6): 1005–1018.
25. Birch-Machin I, Gao S, Huen D et al. Genomic analysis of heat-shock factor targets in Drosophila. Genome Biol 2005; 6(7): R63.
26. Solimini NL, Luo J, Elledge SJ. Non-oncogene addiction and the stress phenotype of cancer cells. Cell 2007; 130(6): 986–988.
27. Guo F, Rocha K, Bali P et al. Abrogation of heat shock protein 70 induction as a strategy to increase antileukemia activity of heat shock protein 90 inhibitor 17-allylamino-demethoxy geldanamycin. Cancer Res 2005; 65(22): 10536–10544.
28. Powers MV, Clarke PA, Workman P. Dual targeting of HSC70 and HSP72 inhibits HSP90 function and induces tumor-specific apoptosis. Cancer Cell 2008; 14(3): 250–262.
29. Williamson DS, Borgognoni J, Clay A et al. Novel adenosine-derived inhibitors of 70 kDa heat shock protein, discovered through structure-based design. J Med Chem 2009; 52(6): 1510–1513.
30. Wandinger SK, Richter K, Buchner J. The Hsp90 chaperone machinery. J Biol Chem 2008; 283(27): 18473–18477.
31. Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem 2006; 75 : 271–294.
32. Forafonov F, Toogun OA, Grad I et al. p23/Sba1p protects against Hsp90 inhibitors independently of its intrinsic chaperone activity. Mol Cell Biol 2008; 28(10): 3446–3456.
33. Holmes JL, Sharp SY, Hobbs S et al. Silencing of HSP90 cochaperone AHA1 expression decreases client protein activation and increases cellular sensitivity to the HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin. Cancer Res 2008; 68(4): 1188–1197.
34. Usmani SZ, Bona R, Li Z. 17 AAG for HSP90 inhibition in cancer – from bench to bedside. Curr Mol Med 2009; 9(5): 654–664.
35. Smith JR, Workman P. Targeting CDC37: an alternative, kinase-directed strategy for disruption of oncogenic chaperoning. Cell Cycle 2009; 8(3): 362–372.
36. Hernandez MP, Sullivan WP, Toft DO. The assembly and intermolecular properties of the hsp70-Hop-hsp90 molecular chaperone complex. J Biol Chem 2002; 277(41): 38294–38304.
37. Wochnik GM, Ruegg J, Abel GA et al. FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem 2005; 280(6): 4609–4616.
38. McDowell CL, Bryan Sutton R, Obermann WM. Expression of Hsp90 chaperone [corrected] proteins in human tumor tissue. Int J Biol Macromol 2009; 45(3): 310–314.
39. Gray PJ Jr, Stevenson MA, Calderwood SK. Targeting Cdc37 inhibits multiple signaling pathways and induces growth arrest in prostate cancer cells. Cancer Res 2007; 67(24): 11942–11950.
40. Kubota H, Yamamoto S, Itoh E et al. Increased expression of co-chaperone HOP with HSP90 and HSC70 and complex formation in human colonic carcinoma. Cell Stress Chaperones 2010; 15(6): 1003–1011.
41. Sun W, Xing B, Sun Y et al. Proteome analysis of hepatocellular carcinoma by two-dimensional difference gel electrophoresis: novel protein markers in hepatocellular carcinoma tissues. Mol Cell Proteomics 2007; 6(10): 1798–1808.
42. Walsh N, O‘Donovan N, Kennedy S et al. Identification of pancreatic cancer invasion-related proteins by proteomic analysis. Proteome Sci 2009; 7 : 3.
43. Walsh N, Larkin A, Swan N et al. RNAi knockdown of Hop (Hsp70/Hsp90 organising protein) decreases invasion via MMP-2 down regulation. Cancer Lett 2011; 306(2): 180–189.
44. Shimokawa T, Matsushima S, Tsunoda T et al. Identification of TOMM34, which shows elevated expression in the majority of human colon cancers, as a novel drug target. Int J Oncol 2006; 29(2): 381–386.
45. Murata S, Minami Y, Minami M et al. CHIP is a chaperone-dependent E3 ligase that ubiquitylates unfolded protein. EMBO Rep 2001; 2(12): 1133–1138.
46. Kajiro M, Hirota R, Nakajima Y et al. The ubiquitin ligase CHIP acts as an upstream regulator of oncogenic pathways. Nat Cell Biol 2009; 11(3): 312–319.
47. Muller P, Ruckova E, Halada P et al. C-terminal phosphorylation of Hsp70 and Hsp90 regulates alternate binding to co-chaperones CHIP and HOP to determine cellular protein folding/degradation balances. Oncogene. In press 2012.
48. Ruckova E, Muller P, Nenutil R et al. Alterations of the Hsp70/Hsp90 chaperone and the HOP/CHIP co-chaperone system in cancer. Cell Mol Biol Lett 2012; 17(3): 446–458.
Štítky
Detská onkológia Chirurgia všeobecná Onkológia
Článok vyšiel v časopiseKlinická onkologie
Najčítanejšie tento týždeň
2012 Číslo Supplementum 2- Metamizol jako analgetikum první volby: kdy, pro koho, jak a proč?
- Nejasný stín na plicích – kazuistika
- Kombinace metamizol/paracetamol v léčbě pooperační bolesti u zákroků v rámci jednodenní chirurgie
- Antidepresivní efekt kombinovaného analgetika tramadolu s paracetamolem
- Fixní kombinace paracetamol/kodein nabízí synergické analgetické účinky
-
Všetky články tohto čísla
- p63 – an Important Player in Epidermal and Tumour Development
- Detection of Cancer Stem Cell Markers in Sarcomas
- NKT-like Cells are Expanded in Solid Tumour Patients
- Cancer as a Metabolic Disease and Diabetes as a Cancer Risk?
- PThe Regulation of p53 Synthesis
- Protein Quality Control and Cancerogenesis
- The Many Roles of Molecular Chaperones and Co-chaperones in Tumour Biology
- RECAMO – ...through Cancer Research towards Applied Molecular Oncology; Where, Why and How
- The Role of Platelets in Tumour Growth
- Circulating Levels of B-cell Activating Factor in Paediatric Patients with Malignancy With or without Cancer-Related Cachexia
- A Combined Immunoprecipitation and Mass Spectrometric Approach to Determine ΔNp63-Interacting Partners
- Identification and Characterisation of Pro-metastatic Targets, Pathways and Molecular Complexes Using a Toolbox of Proteomic Technologies
- The Biobanking Research Infrastructure BBMRI_CZ: a Critical Tool to Enhance Translational Cancer Research
- RECAMO – ...through Cancer Research towards Applied Molecular Oncology; Where, Why and How
- Development and Use of Non-FDG PET Radiopharmaceuticals at the Masaryk Memorial Cancer Institute
- New Mechanisms for an Old Drug; DHFR- and non-DHFR-mediated Effects of Methotrexate in Cancer Cells
- Stereotactic Body Radiation Therapy for Colorectal Cancer Liver Metastases; Early Results
- Phase I Trial in Oncology – Theory and Practice
- Klinická onkologie
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- p63 – an Important Player in Epidermal and Tumour Development
- NKT-like Cells are Expanded in Solid Tumour Patients
- The Many Roles of Molecular Chaperones and Co-chaperones in Tumour Biology
- Phase I Trial in Oncology – Theory and Practice
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



