-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Dynamic Chromatin Localization of Sirt6 Shapes Stress- and Aging-Related Transcriptional Networks
The sirtuin Sirt6 is a NAD-dependent histone deacetylase that is implicated in gene regulation and lifespan control. Sirt6 can interact with the stress-responsive transcription factor NF-κB and regulate some NF-κB target genes, but the full scope of Sirt6 target genes as well as dynamics of Sirt6 occupancy on chromatin are not known. Here we map Sirt6 occupancy on mouse promoters genome-wide and show that Sirt6 occupancy is highly dynamic in response to TNF-α. More than half of Sirt6 target genes are only revealed upon stress-signaling. The majority of genes bound by NF-κB subunit RelA recruit Sirt6, and dynamic Sirt6 relocalization is largely driven in a RelA-dependent manner. Integrative analysis with global gene expression patterns in wild-type, Sirt6−/−, and double Sirt6−/ − RelA−/− cells reveals the epistatic relationships between Sirt6 and RelA in shaping diverse temporal patterns of gene expression. Genes under the direct joint control of Sirt6 and RelA include several with prominent roles in cell senescence and organismal aging. These data suggest dynamic chromatin relocalization of Sirt6 as a key output of NF-κB signaling in stress response and aging.
Published in the journal: Dynamic Chromatin Localization of Sirt6 Shapes Stress- and Aging-Related Transcriptional Networks. PLoS Genet 7(6): e32767. doi:10.1371/journal.pgen.1002153
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002153Summary
The sirtuin Sirt6 is a NAD-dependent histone deacetylase that is implicated in gene regulation and lifespan control. Sirt6 can interact with the stress-responsive transcription factor NF-κB and regulate some NF-κB target genes, but the full scope of Sirt6 target genes as well as dynamics of Sirt6 occupancy on chromatin are not known. Here we map Sirt6 occupancy on mouse promoters genome-wide and show that Sirt6 occupancy is highly dynamic in response to TNF-α. More than half of Sirt6 target genes are only revealed upon stress-signaling. The majority of genes bound by NF-κB subunit RelA recruit Sirt6, and dynamic Sirt6 relocalization is largely driven in a RelA-dependent manner. Integrative analysis with global gene expression patterns in wild-type, Sirt6−/−, and double Sirt6−/ − RelA−/− cells reveals the epistatic relationships between Sirt6 and RelA in shaping diverse temporal patterns of gene expression. Genes under the direct joint control of Sirt6 and RelA include several with prominent roles in cell senescence and organismal aging. These data suggest dynamic chromatin relocalization of Sirt6 as a key output of NF-κB signaling in stress response and aging.
Introduction
Silent Information Regulator-2 (Sir2) encodes an NAD-dependent histone deacetylase that links chromatin regulation to genomic stability, gene silencing and lifespan in yeast. Sir2 deacetylates lysines in the amino terminal tails or histones H3 and H4 and in the globular core of histone H3 [1]–[4]. Mutations disrupting Sir2 mutation leads to global hyperacetylation of histones H3 and H4. Increased rDNA recombination and dysfunctional mating type loci silencing occurs, thus contributing to accelerated cellular aging and reduced replicative lifespan in yeast. Conversely, enhanced Sir2 function in several model organisms can increase lifespan [5].
Seven Sir2 homologues exist in the mammalian genome, termed sirtuins (SIRT1-7) [6]–[7]. The chromatin-associated sirtuin, SIRT6, is an important regulator of gene expression and genome integrity [8]–[13]. SIRT6 specifically deacetylates lysine 9 and 56 of histone H3 (H3K9Ac and H3K56Ac), and its deacetylase activity is involved in inhibition of gene expression [9]–[10], [12]. The NF-κB subunit RELA can recruit SIRT6 via direct protein-protein interaction to the promoters of several NF-κB target genes. SIRT6 then deacetylates H3K9Ac and destabilizes RELA occupancy, leading to termination of NF-κB-dependent gene expression [10]. It is unclear whether this model applies generally to most or to only select NF-κB target genes. Recently, it has been demonstrated that SIRT6 can act as a corepresser of the transcription factor, Hif1a, suggesting that SIRT6 may interact with additional regulators to modulate gene expression in a variety of contexts [12]. Additionally, the full set of genes targeted by SIRT6 remains to be determined.
NF-κB comprises a family of transcription factors that control the expression of genes involved in cells survival, senescence, inflammation, immunity and aging [14]. NF-κB proteins are responsive to stress signals including infection, inflammation, DNA damage, oxidative stress and metabolic stress. In response to such signals, the IκB kinase (IKK) complex phosphorylates the IκB proteins which bind and sequester NF-κB proteins in the cytoplasm. Phosphorylated IκB is subsequently ubiquitinated and degraded, and liberated NF-κB translocates into the nucleus to activate transcription of its target genes, including regulators of the NF-κB pathway [15]–[16]. As a result of negative feedback loops, NF-κB shuttles in and out of the nucleus, and target gene expression can be oscillatory. Intriguingly, subsequent rounds of NF-κB can activate different target genes due to chromatin changes induced by the pioneering round of NF-κB activation [17]–[18].
Disruption of Sirt6 in mice results in a degenerative phenotype resembling premature aging [11]. Importantly, concomitant heterozygous knockout of RelA allows a significant fraction of mice to overcome the degenerative phenotypes and avoid lethality [10]–[11]. This genetic epistasis supports a model where Sirt6 limits excessive NF-κB-dependent transcription in order to promote longevity. NF-κB activity also increases with age in mice and humans, and is required to enforce cellular senescence and tissue aging [19]. Genes jointly controlled by Sirt6 and NF-κB should include important contributors to aging, but to date, the identity of relevant target genes are not known. In this respect, we determine the targets of Sirt6 genome-wide, reveal dynamic movement of Sirt6 during stress signaling and identify joint target genes, many of which are linked to aging.
Results
Dynamic relocalization of Sirt6 genome-wide upon stress signaling
We hypothesized that Sirt6 is a stress-responsive chromatin modifier, and that Sirt6 itself may relocalize to distinct target genes upon stress signaling. We used genome-scale chromatin immunoprecipitation (ChIP)-chip assays with high-density oligonucleotide arrays to analyze the binding patterns of Sirt6 and RelA in mouse embryonic fibroblasts (MEF) before and after TNF-α addition. Because we and others have observed that histone acetylations and Sirt6 occupancy are clustered in promoter regions upstream of the transcriptional start site (TSS) [10], [12], [20], we used whole genome promoter arrays tiling 3.25 kb upstream to 0.75 kb downstream of the TSS. Wild-type MEFs were treated with TNF-α for 0, 15, 30 or 60 minutes, and chromatin was immunoprecipitated using an antibody recognizing Sirt6. Sirt6−/− MEFs were also similarly treated as a negative control (Figure S1A, Table S1). We identified sequences bound by SIRT6 with 90% confidence using Nimblegen's peak calling software and subtracted nonspecific targets identified in the Sirt6−/− MEFs. Altogether, Sirt6 can dynamically bind up to 5050 gene promoters (Figure 1A, Table S2). Sirt6 bound 1899 genes in unstimulated cells. Notably upon TNF-α signaling, Sirt6 moved away from a large percentage of these site (684 of 1899), and relocalized to a much expanded set of genes (4366). Sirt6 occupancy also showed a striking periodic pattern: Sirt6 inducibly bound to thousands of genes at 15 minutes after TNF-α treatment, disengaged most of these sites at 30 minutes, and then re-engaged but also bound new sites at 60 minutes after TNF-α treatment. Thus, Sirt6 occupancy on chromatin is surprisingly dynamic and is globally reconfigured upon a specific stimulus.
Fig. 1. Dynamic relocalization of Sirt6 genome-wide upon stress-signaling. 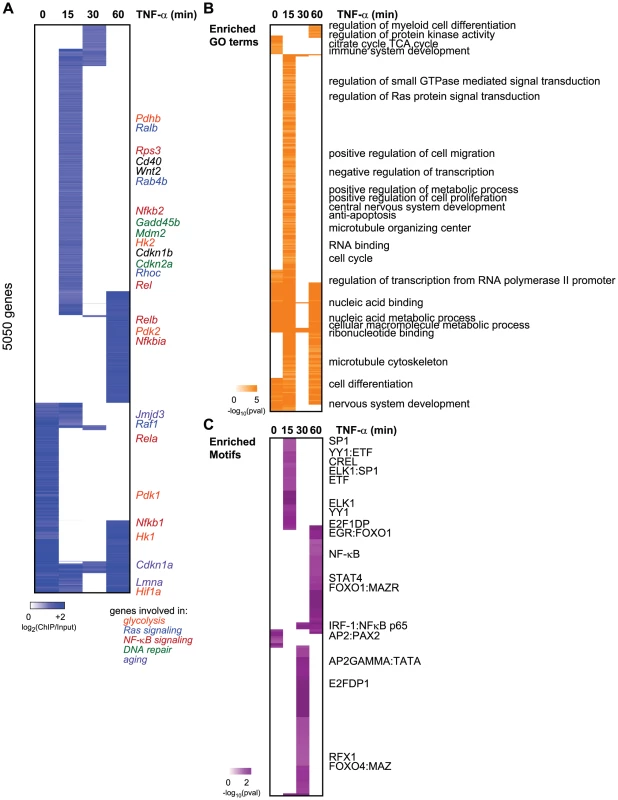
(A) Endogenous Sirt6 targets the promoter of 5050 genes in the presence or absence of TNF-α. The heatmap displays the binding data as the degree of enrichment of ChIP DNA over total genomic DNA. (B) Shown are the gene ontologies (GO) terms significantly induced (pval<.05, Benjamini) among Sirt6 targets in wild-type MEFs following treatment with TNF-α. Intensity of orange is representative of the degree of significance. (C) Shown are the motif modules significantly induced (pval<.05) among Sirt6 targets in wild-type MEFs following treatment with TNF-α. Intensity of purple is representative of the degree of significance [-ln(pval)]. The dynamic relocalization of Sirt6 at each time point is biologically coherent and enriched for specific functions and sequence motifs. Promoters bound by SIRT6 are enriched for genes with roles in the cell cycle, regulation of small GTP-ase activity, immune system development, nucleic acid binding and anti-apoptosis (FDR<0.05, Benjamini-Hochberg test) (Figure 1B). It has been previously shown that SIRT6 negatively regulates expression of glucose homeostasis by binding promoters of glycolytic genes and deacetylating H3K9Ac [12]. Consistent with this, we observed the citrate acid TCA cycle as a function highly enriched for among the genes bound by Sirt6 at baseline. We also observe Sirt6 binding the promoters of Pfk1 and Ldha, as previously reported [12]. Sirt6 bound the promoter of many other glycolysis-related genes including Pdk1, Pdk2 and Hif1α. In addition, Sirt6 has been shown to be required for Ras-mediated epigenetic silencing of the pro-apoptotic Fas gene [21]. Interestingly, we find that our TNF-α-induced Sirt6 targets are enriched for genes involved in regulation of Ras protein signal transduction. These genes include Ralb, Raf1, Rhoc, Rab4b and Rab30, Rasgrp2 and Nkiras1.
We next searched for transcription factor binding motifs enriched for among SIRT6 bound promoters using motif module map [19] (Figure 1C). The NF-κB RelA motif along with other three other NF-κB motifs were significantly enriched. Consistent with our previous genes expression analyses revealing Nfkbia, Nfkb1, and Nfkb2 as candidate target genes coregulated by RelA and Sirt6, these genes showed inducible SIRT6 binding to these targets upon stimulation with TNF-α [10]. Interestingly, we also detect the SP1, STAT1/3, ELK1, E2F1 and FOXO1/4 as highly enriched motifs (p<0.05, hypergeometric distribution).
RelA occupancy genome-wide shows similar dynamics as Sirt6
To compare the Sirt6 occupancy profile with that of NF-κB, we mapped RelA occupancy by ChIP-chip after TNF-α treatment for 0, 15, or 30 minutes. NF-κB is normally found in the cytoplasm, but upon TNF-α stimulation, NF-κBis transported to the nucleus where it binds to the promoter of target genes. Activation of NF-κBexhibits oscillatory behavior when stimulated by TNF-α [22]–[23]. Further studies have revealed that within a given population of cells, not all cells respond to TNF-α and at high doses of TNF-α (>0.5 ng/mL), NF-κBprotein peaks in the nucleus 20 minutes post stimulation [24]. We found that the 60 minute time point for RelA showed variable ChIP signal, possibly due to loss of synchrony RelA oscillation [10], [24], and for these reasons we did not analyze this time point by ChIP-chip. RelA−/− MEFs were also similarly treated as a negative control (Figure S1B). Altogether, RelA was found to occupy a total of 2738 genes with 80% confidence (Figure 2A). RelA bound 13% of genes in unstimulated cells and 91% of the 2738 genes in the presence of TNF-α. With our method, we were able to confirm the binding of RelA to 110 genes previously identified to be bound by RelA [25]–[26]. These genes include Bcl3, Bcl6, Rac2, Traf1, Il6st, Hoxa7, Elk1, Junb and Relb. It should be noted that previous studies interrogating genome-wide binding of RelA to DNA were carried out in a different species and in response to LPS, in which the periodic NF-κB response may be partially obscured by antiphasic feedback loops [22]–[23],. Importantly, RelA occupancy also peaks at 15 minutes and redistributes at 30 minutes after TNF-α treatment in a very similar pattern to Sirt6 occupancy.
Fig. 2. Binding of RelA resembles the occupany pattern of Sirt6. 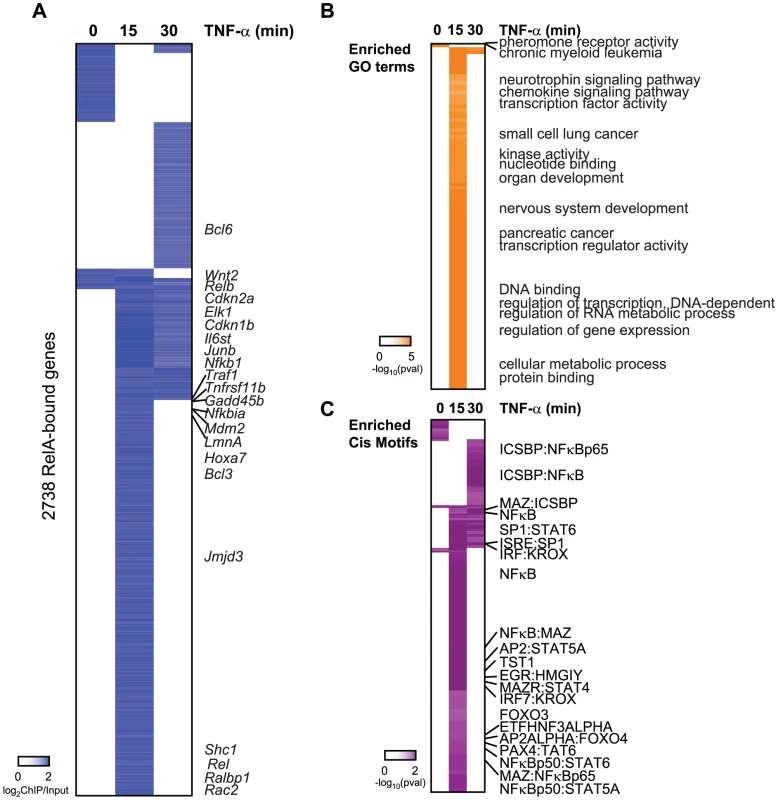
(A) Endogenous RelA targets the promoters of 2738 genes in the absence or presence of TNF-α. The heatmap displays the binding data as the degree of enrichment of ChIP DNA over total genomic DNA. (B) Shown are the gene ontologies (GO) terms significantly induced (pval<.05, Benjamini) among RelA targets in wild-type MEFs following treatment with TNF-α. Intensity of orange is representative of the degree of significance. (C) Shown are the motif modules significantly induced (pval<.05) among RelA targets in wild-type MEFs following treatment with TNF-α. Intensity of purple is representative of the degree of significance [-ln(pval)]. RelA target genes are enriched for genes with similar functions and motifs as SIRT6 targets. These include chemokine signaling, immune system development, nervous system development, and pathways in cancer (p<.05, Benjamini) (Figure 2B). Specific cancer pathways that were highly enriched for by our RelA targets include chronic myeloid leukemia, glioma, small cell lung cancer, pancreatic cancer. As anticipated, the NF-κB RelA motif along with 14 instances of gene sets containing related NF-κB motifs were significantly enriched (Figure 2C), highlighting the high quality of our ChIP-chip technique. We also detect motifs containing ICSBP, MAZ, SP1, KROX, PAX4, STAT4/5A/6, TST1 and EGR highly enriched for (p<.05, hypergeometric mean).
RelA drives dynamic relocalization of Sirt6 genome-wide
The direct physical interaction of RelA and Sirt6 raises the hypothesis that RelA can drive the stress-responsive relocalization of Sirt6 genome-wide, thereby allowing Sirt6 to deacetylate histones occupying the promoters of NF-κB target genes. The importance of RelA in Sirt6 function has been demonstrated by the ability of RelA haplo-insufficiency to rescue the lethality and degenerative phenotypes of Sirt6 knockout mice [10]. While genes occupied by Sirt6 independently of RelA may well be interesting from other perspectives [also suggested by our data (Figure 1C)], we reasoned that genes under the joint regulation of Sirt6 and RelA must be central to Sirt6 function in promoting organismal longevity and health. Joint occupancy by Sirt6 and RelA should be an efficient approach to prioritize functional target genes among the numerous Sirt6-bound genes.
Indeed, RelA and Sirt6 significantly colocalized to the same genomic sites (Figure 3A, 3B). RelA and Sirt6 bound to 2738 and 5050 promoters, respectively, of which 1481 promoters were in common (p<2.5×10−286, hypergeometric distribution) (Figure 3B, Table S3). This striking number represents 54% and 29% overlap of all RelA and Sirt6 targets, respectively, and suggests that Sirt6 and RelA collaborate to regulate a large fraction of their target genes. Moreover, when Sirt6 and RelA co-occupied the same promoters, Sirt6 and RelA bound at sites less than 500 base pairs apart—the shearing size of our chromatin fragments and limit of resolution — for the majority of targets (65%, Figure 3C). To provide direct genetic proof of the requirement of RelA for Sirt6 mobilization, we performed Sirt6 ChIP-chip in RelA−/− MEFs following induction with TNF-α for 0, 15, 30 and 60 minutes. Sirt6 and RelA do not influence each other's protein level [10]. We compared the binding of Sirt6 to the shared Sirt6 and RelA targets in RelA−/− cells to wild-type cells. Consistent with our hypothesis, Sirt6 occupancy was completely abrogated for 49% of shared targets (Figure 3D), most of which are inducibly targeted to chromatin by TNF-α treatment in wild-type cells. These genes include Nfkbia, Gadd45b, RelB, Ralbp1, Cdkn2a and Cdkn1b. For a number of canonical NF-κB target genes including Nfkb1, HoxA7 and Rel, however, Sirt6 binding was reduced but not abrogated, suggesting that other NF-κB family members can potentially compensate and target Sirt6 to the promoters of NF-κB targets. In genes bound by Sirt6 but not RelA, Sirt6 occupancy at many genes is also altered in RelA−/− cells, possibly due to indirect effects of RelA (Figure S2). Together, these data suggest that a substantial part of dynamic Sirt6 localization to chromatin is driven by RelA.
Fig. 3. RelA drives dynamic relocalization of Sirt6. 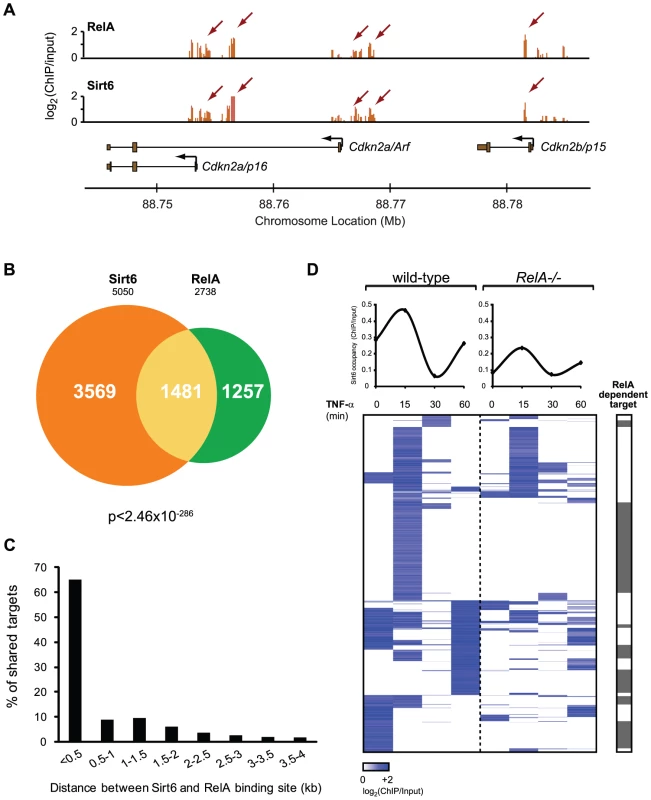
(A) Example of ChIP-chip data for Sirt6 and RelA show substantial similarity. (B) Venn diagram representing the overlap of Sirt6 and RelA promoter bound regions. (C) RelA and Sirt6 bind shared targets within 500 bp of each other. The distance between RelA and Sirt6 binding sites and the percentage of shared targets with the corresponding distance is plotted. (D) Sirt6 occupancy at promoters shared by RelA is largely dependent on RelA. Shown is a heat map displaying the ChIP binding data in wild-type and RelA−/− MEFs following TNF-α treatment for the indicated times, for 1481 gene promoters shared by RelA and Sirt6. The average binding of Sirt6 for each sample (column) is plotted above. The grey bar indicates that Sirt6 binding is completely abrogated in RelA−/− samples. 49% of shared Sirt6 targets are dependent on RelA. Gene expression consequences of interplay between Sirt6 and RelA
We next measured the global gene expression patterns of wild-type, Sirt6−/− or Sirt6-/-RelA−/− cells in the temporal response to TNF-α. We focused on genes with promoters bound by Sirt6 and RelA in order to understand the consequences of Sirt6 and RelA binding. A further goal is to identify candidate genes that may drive the aging process because genetic evidence suggests a critical role for genes jointly regulated by Sirt6 and RelA [10]. We were able to detect expression in 480 out of the 1481 targets shared by RelA and Sirt6 (Figure 4A). Out of these 480 genes, 301 genes exhibited an expression pattern consistent with antagonistic regulation by RelA versus Sirt6. Specifically, expression of these genes was elevated in Sirt6−/− cells compared to wild-type and correspondingly reduced in Sirt6−/ − RelA−/− cells. Hierarchical clustering of the gene expression patterns organized the genes into three distinct classes based on their differential expression in Sirt6−/ − cells (Figure 4B). The first class, termed “All-Up”, showed elevated gene expression in Sirt6−/− cells at baseline without TNF-α treatment and at every single time point after TNF-α-treatment. All-Up includes genes such as candidate aging regulators Shc1(encoding p66) [27], Cdkn2a (encoding the cell cycle inhibitor p16 that increases in expression with age) [28]–[29], Wnt2 [30]–[31], and stress responsive genes Gadd45b [32] and Mdm2 [33]. Adler et al. previously demonstrated that age-dependent accumulation of p16 protein in vivo requires ongoing NF-κB activity [19]. Our data further reinforce this observation, and suggest that p16 protein accumulation is due in part to direct RelA-mediated transcription of Cdkn2a (Figure 3A, Figure 4B).
Fig. 4. Gene expression consequences of interplay between RelA and Sirt6. 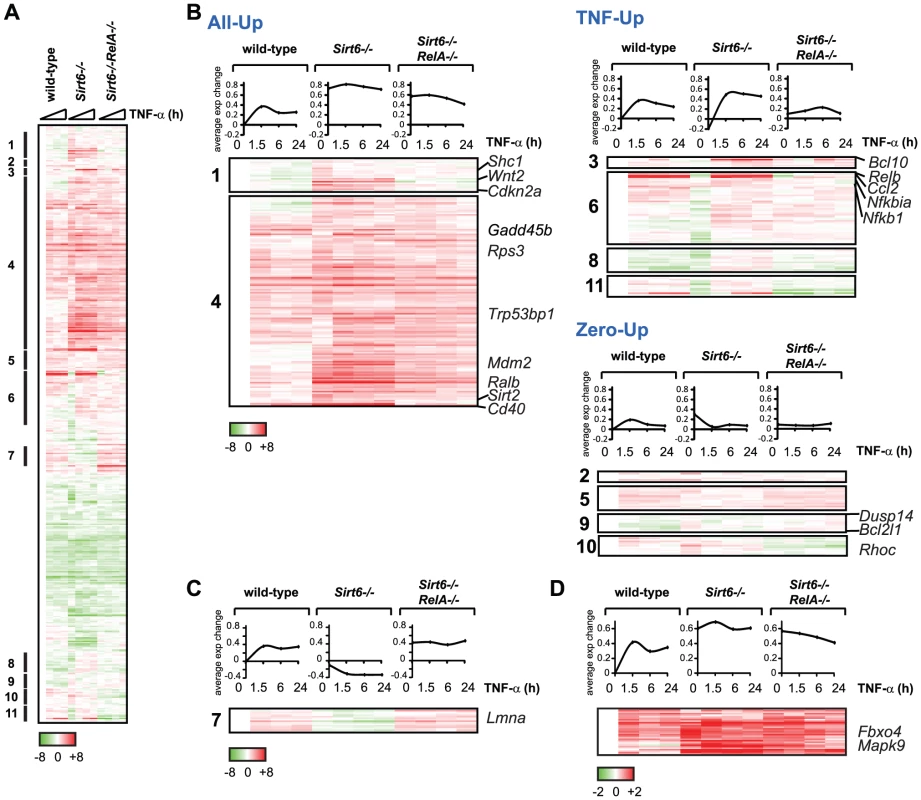
(A) Targeting of promoters by RelA and Sirt6 impacts gene expression. Shown is the gene expression analysis of 480 targeted by both RelA and Sirt6 following TNF-α treatment for the indicated times. (B) 301 genes exhibited an expression pattern consistent with antagonistic regulation by RelA and Sirt6. Based on the differential expression observed in Sirt6−/− cells, we separated these genes into 3 distinct classes called “All Up,” “TNF-Up or “Zero Up,”. (C) A surprising class of genes, in which expression is reduced upon Sirt6 knockout, behaves contrary to our model of Sirt6 repression of NF-κB target genes. Lmna, a gene thought to prevent aging, is in this class. (D) Class of genes in which Sirt6 targeting to promoters is inhibited by RelA. In the second class termed “TNF-Up”, expression in Sirt6−/ − cells is increased only upon stimulation with TNF-α, demonstrating the importance of stress signaling to elucidate transcriptional alterations in Sirt6−/− cells. The TNF-Up class includes genes such as Bcl10, Relb, Ccl2 and Nfkbia that encode both positive and negative feedback mechanisms to NF-κB signaling [17]. Third, the “Zero-Up” class is a smaller set of genes that showed increased gene expression in Sirt6−/− cells only in the absence of TNF-α, and this aberrant pattern is reversed in Sirt6−/−Rela−/− cells. This class includes genes encoding several signaling proteins such as protein phosphatase Dusp14, anti-apoptotic gene Bcl2l1 and actin regulator Rhoc. Within both All-Up and Zero-Up classes, there is a small subset of genes that are normally repressed upon treatment with TNF-α. In Sirt6−/− cells, however, stimulation with TNF-α induces expression, suggesting that Sirt6 is normally responsible for preventing expression of these genes in the presence of TNF-α. These genes include Sdc2, Tinagl, Pkia and Tnfrsf11b.
Furthermore, we found two interesting classes of genes which behave in a manner contrary to our model of Sirt6 repression of NF-κB target genes (Figure 4C, 4D). In one class, gene expression is repressed in Sirt6−/− cells compared to wild-type cells, and repression is relieved in Sirt6−/−RelA−/− cells to an even greater extent than in wild-type cells (“Inverse”, Figure 4C). Notably, one such gene is Lmna (encoding lamin A), which has been previously linked to aging [34]. In the second class of genes, dynamic localization of Sirt6 impacts gene expression, as Sirt6 is recruited to and away from its binding sites. Sirt6 occupies the promoters of a diverse set of genes in the basal state and its occupancy is linked with transcriptional repression. Upon the addition of TNF-α, Sirt6 is largely redistributed to new promoters, in part through its direct interaction with RelA [10]. As a result of relocation of Sirt6 to new targets, TNF-α treatment may induce gene de-repression. We identified a set of genes that fulfill these criteria and are bound by bound by Sirt6 only in the absence of TNF-α (Figure 4D). These genes include Mapk9 and Fbxo4. Since RelA inhibits Sirt6-dependent repression of these genes, they show an opposite epistatic relationship: They are derepressed in Sirt6−/− cells, but are not reverted in Sirt6−/−RelA−/− cells (Figure 4D, Figure 5). Meanwhile, TNF-α induces stabilization of Sirt6 at the promoters of genes including p16, Gadd45b and Nfkb2, titrating them away from the promoters of targets that are de-repressed.
Fig. 5. Validation of Chip-chip data and gene expression data. 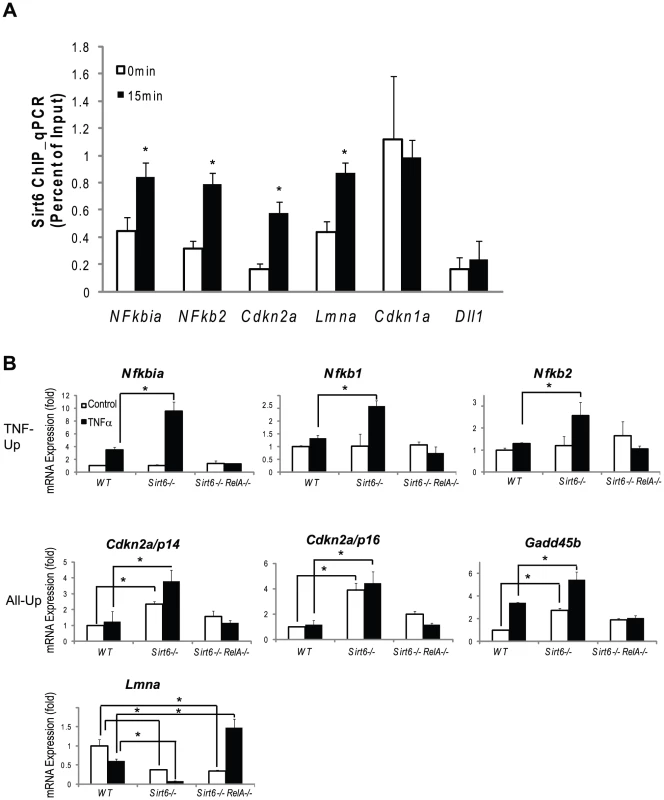
(A) SIRT6 is recruited to the promoters of canonical NF-κB target genes (Nfkbia, Nfkb1, Nfkb2) and to the promoters of genes involved in aging (Cdkn2a/p16 and Lmna). Independently derived wildtype MEFs were treated with TNF-α (20 ng/ml) for 0 or 15 minutes. ChIP with α-Sirt6 antibodies was preformed and percent Sirt6 occupancy is shown relative to input as measured by qPCR. Mean ±S.E. of three technical replicates is shown. (B) Gene expression validation by qRT-PCR. TNF-Up genes demonstrate increased expression in Sirt6 −/− cells in response to TNF-α which is abolished in Sirt6 RelA −/− cells. All-Up genes demonstrate increased expression in Sirt6 −/− cells in the presence and absence of TNF-α which is attenuated inSirt6 RelA −/− cells. Lmna expression is repressed in Sirt6 −/− cells and derepressed in Sirt6 RelA −/−in response to TNF-ã Wildtype, Sirt6 −/− and Sirt6 RelA−/− MEFs were treated with TNF-a (20 ng/mL) for 0 and 1.5 hrs. Quantitative Taqman real time RT-PCR of the indicated mRNAs is shown normalized to GAPDH levels. Mean ± S.D. is shown. *p<0.05, student's t-Test. Next, we validated our ChIP-chip results on a subset of known and novel Sirt6 target genes. Independently derived wildtype MEFs were stimulated with TNF-α for 0 and 15 minutes and Sirt6 ChIP was performed using a microfluidic device [35] followed by quantitative PCR to interrogate Sirt6 occupancy of the indicated promoters (Figure 5A). In response to TNF-α stimulation, Sirt6 was inducibly recruited to the promoters of canonical NF-κB target genes Nfkbia and Nfkb2. Sirt6 was also recruited to the promoters of two novel loci, Cdkn2a and Lmna, in response to TNF-α stimulation (p<0.05). Additionally, we found that Sirt6 is located at the promoter of Cdkn1a independent of TNF-α stimulation. As a negative control, we did not detect Sirt6 at the promoter of Dll1, consistent without ChIP-chip results. We also demonstrate the reproducibility of these experiments by perfoming ChIP on two additional independently derived non-littermate wildtype MEF lines on two targets, Nfkbia and Nfkb2 and show the inducibility of Sirt6 recruitment to the promoters of these genes in response to TNF-α stimulation (Figure S1).
We validated our expression array data by examining mRNA expression in wild type, Sirt6−/− and Sirt6−/− RelA−/− double knockout MEFs quantitative reverse transcription PCR. In wildtype cells, TNF-α stimulation led to the inducible expression of the “TNF-Up” genes, Nfkbia, Nfkb1 and Nfkb2 (p<0.05 vs uninduced for each, student's t-test). In Sirt6 knockout cells, no difference was seen at baseline, but TNF-α stimulation led to ∼2-fold greater induction of these genes when compared to wildtype at time 1.5 hours (p<0.05). In Sirt6 RelA double knockout cells the inducibility of the “TNF-Up” genes was abolished, demonstrating that these gene expression changes are dependent on RelA (Figure 5B).
Next, we tested genes in the “All-Up” class, including candidate aging regulator, Cdkn2a/p16, the coregulated transcript, Cdkn2a/p14, and stress response gene, Gadd45. Here we find that Cdkn2a/p16 and Cdkn2a/p14 are very weakly induced in response to TNF-α in wildtype cells. However, both of these transcripts are induced in the Sirt6 knockout cells in the absence of TNF - and Cdkn2a/p16 can be superinduced by TNF-α in Sirt6−/ − cells (p<0.05 for each comparison). Again, the inducibility of both transcripts was abolished in Sirt6−/−RelA−/− double knockout cells. On the other hand, Gadd45b expression is upregulated three-fold both in response to TNF-α in wildtype cells and in Sirt6 knockout cells at time 0 (p<0.05). Furthermore, induction with TNF-α in Sirt6 knockout cells led to a further two-fold superinduction of Gadd45b gene expression when compared to Sirt6 knockout at time 0 hours (p<0.05). In double knockout cells, Gadd45b is no longer responsive to TNF stimulation, indicating that the induction requires NF-κB (Figure 5B).
Finally, we tested the progeria causing gene, Lmna, which falls into the “inverse” category of genes that are repressed in Sirt6 knockout cells. In wildtype cells, TNF-α stimulation led to a 40% reduction in Lmna expression. In Sirt6 knockout cells at time 0, Lmna expression was reduced by 60% and was reduced about 10-fold after TNF-α stimulation when compared to wildtype at time zero (p<0.05). In Sirt6−/ − RelA−/− cells at time 0 gene expression was reduced to by 60% as in Sirt6 knockout cells, but was induced nearly 1.5 fold with TNF-α stimulation (p<0.05) (Figure 5B). Mutations in Lmna cause Hutchinson-Gilford progeria syndrome (HGPS) [36], a disorder with clinical features of premature aging. Normal aging is associated with accumulation of a spliced isoform of Lmna that impedes nuclear function. Lmna expression is decreased in Sirt6−/− cells but induced in Sirt6−/−RelA−/− cells in response to TNF-α, consistent with a role for Sirt6 in modulating NF-κB-dependent expression to prevent aging. The LmnaL530P/L530P mice display a progeroid phenotype that strikingly resembles the phenotype of Sirt6−/− mice: reduced size, loss of subcutaneous fat, reduced bone mineral density and lethality by 4–5 weeks of age. These results raise the possibility that some of the characteristics of Sirt6−/− mice could be driven by deregulated LmnA expression. Since Lmna expression is reduced rather than enhanced in Sirt6 knockout, however, Sirt6 and RelA may regulate a factor that represses Lmna expression. Whether Sirt6 alters Lmna splicing, lamin A processing, or nuclear architecture remains to be determined.
Together these data demonstrate that the interplay of chromatin localization of Sirt6 and RelA regulates the expression of many genes, many of which have roles in regulating aging, stress response and NF-κB feedback, via both direct and indirect mechanisms (Figure 6).
Fig. 6. Model for Sirt6 regulation of targeting and gene expression. 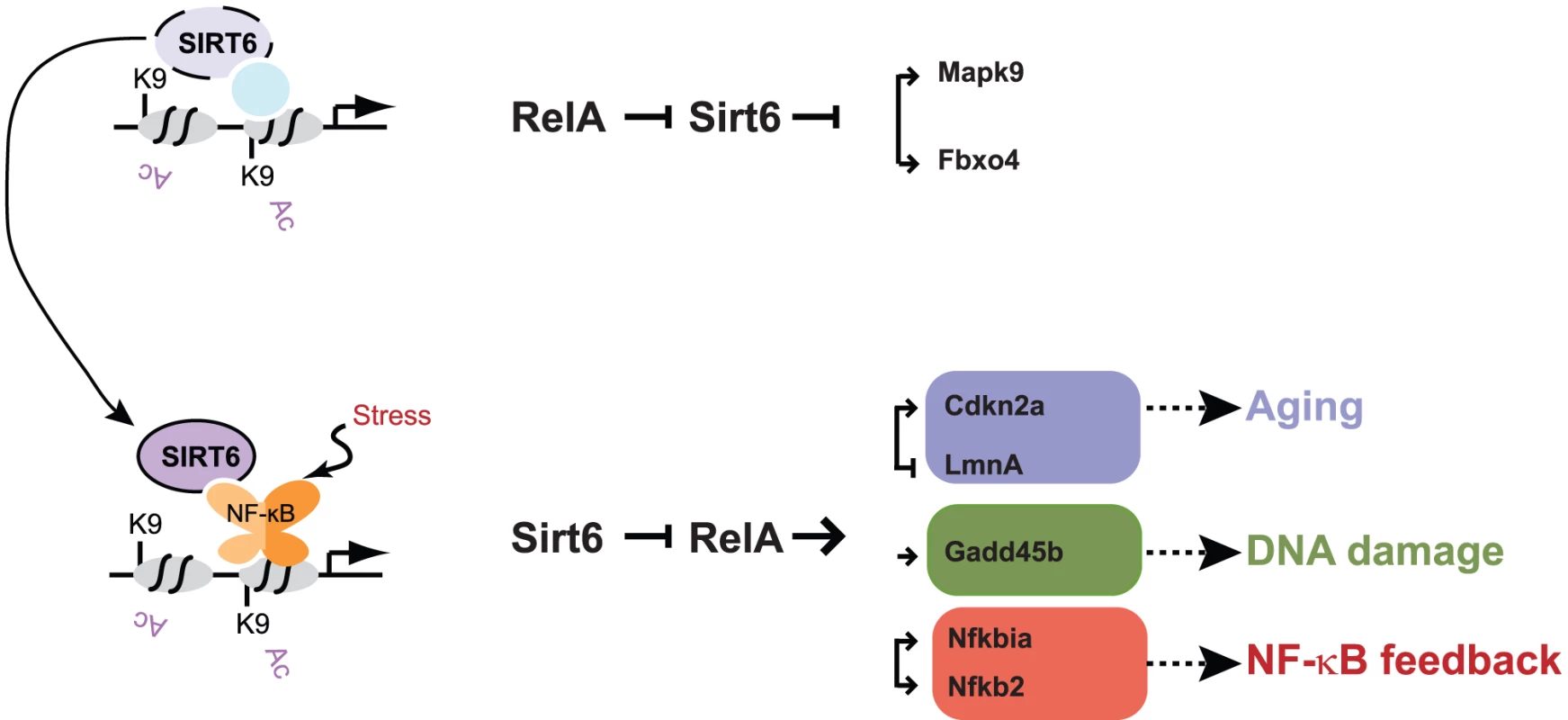
Sirt6 occupies the promoters of a wide array of genes, likely in conjunction with other transcription factors. We propose that upon the addition of a stress such as TNF-α, Sirt6 redistributes to promoters via its interaction with RelA. This redistribution enables derepression of a subset of genes (e.g. Mapk2, Fbxo4) and induction of repression of genes involved in pathways such as aging (Cdkn2a, Lmna), DNA damage response (Gadd45b), and NF-κB feedback (Nfkbia, Nfkb2). The experience of NF-κB activation may influence subsequent rounds of stress response by creating a new basal state of Sirt6 occupancy. Discussion
Deletion of Sirt6 results in a premature aging-like degenerative syndrome in mice, and heterozygosity of the NF-κB subunit RelA can rescue the degenerative and early lethality phenotype [10]–[11]. We and others also independently identified NF-κB as a positive regulator of aging-related gene expression programs and phenotypes [19], [37]–[38]. These findings suggest a model in which aging is caused in part by elevated NF-κB-dependent gene expression. In contrast, Sirt6, via its H3K9Ac deacetylase activity, restrains NF-κB dependent transcription and promotes longevity. Because this model is based on chromatin analysis of a small number of genes, its generality and the relevant target genes were not clear. Here, we have coupled genome-scale profiling of RelA - and Sirt6-bound sites with global gene expression analysis to identify direct targets of RelA and Sirt6. Sirt6 occupies the promoters of 54% of RelA targets. Of these genes, we demonstrate Sirt6-dependent repression of basal and/or TNF-α-induced expression of hundreds, many of which have been shown to regulating aging and longevity (Figure 6).
Dynamic localization of Sirt6
Dynamic relocalization of sirtuins is an important feature of both yeast Sir2 and mammalian Sirt1. Yeast Sir2 represses recombination or transcription at telomeres, silent mating type loci and ribosomal DNA. Upon the addition of DNA damage agents, Sir2 leaves telomeric sites and relocates to sites of DNA damage [39]. Similarly, Sirt1 was recently shown to occupy a diverse set of genes in the basal state, but in response to DNA damage, Sirt1 redistributes to these sites of DNA damage [40]. These findings are also consistent with recent work documenting inducible recruitment of Sirt6 to sites of DNA damage [8].
We now show that Sirt6 binds thousands of promoters in the mouse genome and that similar to RelA, this binding pattern is largely reconfigured in response to stress induced by TNF-α. 29% of Sirt6 targets are shared by RelA, and of these shared targets, 49% require RelA for Sirt6 occupancy. This suggests that in response to TNF-α or one of the many stressors that activate NF-κB, the stress-responsive nature of Sirt6 binding could be driven by its interaction with the stress-responsive transcription factor RelA. However, Sirt6 targets a large number of genes in the absence of RelA, which begs the question of whether another NF-κB family member or related transcription factor may also contribute to Sirt6 localization (Figure S2). In addition, the NF-κB pathway is involved in crosstalk with several pathways, including JNK, STAT3 and Foxo3a, and, these pathways will be altered in the absence of RelA [41]. It will be interesting to determine whether other stress-responsive transcription factors exist to guide the relocalization of Sirt6 and other Sir2-related deacetylases upon distinct stress signals such as DNA damage. Our identification of specific transcription factor motifs at Sirt6 bound sites provide a logical starting point for other regulators of Sirt6 localization.
Impacts on gene expression
Sirt6 and RelA share a large number of direct targets and co-regulate expression of these genes. Genes under the joint control of Sirt6 and RelA include several key regulators of aging, including CDKN2A (encoding p16), Shc1, Wnt, and LMNA. The interplay of Sirt6 and RelA occupancy can shape several patterns of stimulus-dependent gene expression programs, as revealed in single and double knockout MEFs. Although MEF cultures can be heterogeneous, we have previously shown that the NF-κB driven gene expression program is independent of anatomical origin and is instead responsive to stress and aging [19]. Further studies revealed that Sirt6 and RelA regulate a similar set of genes by acute RNAi depleton through a similar relationship [10].
While the majority of genes with joint occupancy of Sirt6 and RelA showed the expected epistatic relationships (Sirt6 --| RelA ◊ gene), there is a surprising diversity of stimulus-dependent NF-κB responses when Sirt6 is absent (Figure 4). In other words, chromatin regulation by Sirt6 is important to maintain precise coordination among NF-κB target genes. Notably, loss of coordinate expression of NF-κB target genes is a defining characteristic of aging tissues [37]. Understanding the context of additional transcription factors and chromatin states at these genes may help to explain these quantitative differences. Furthermore, we provide evidence that the stress-dependent movement of Sirt6 away from its basal binding sites can also de-repress gene expression, yielding a reversed regulatory hierarchy (RelA --| Sirt6 --| gene, Figure 6). This latter class of genes was only appreciated due to the comprehensive epigenomic studies and integrative analyses with global gene expression patterns. One important caveat of our study is that all of our measurements are based on populations of cells, and it is now clear that variation in behavior on a cell-by-cell basis (i.e. noise) also modulates the overall NF-κB response [23]-[24]. While the observed changes in promoter occupancy and gene expression strongly indicate that target genes are directly regulated by RelA and Sirt6, some of the observed changes may also be due to indirect effect of Sirt6 or RelA inactivation. Future studies addressing genome-wide binding of NF-κB family members as well as dynamic gene expression with high temporal precision and in single cells shed light on these outstanding questions.
Materials and Methods
Ethics statement
All animals were handled in strict accordance with good animal practice as defined by the relevant national and/or local animal welfare bodies. All animal work was approved by the Stanford University Institutional Animal Care and Use Committee.
Antibodies and cell lines
Antibodies specific for RELA (Abcam), SIRT6 (Abcam) and Actin (Santa Cruz) are from the indicated sources. MEFs were generated from 13.5-day-old embryos using standard methods and propagated in DMEM (Invitrogen) plus 15% FBS. MEFs were passaged a total of four times before TNF-α treatment.
Chromatin immunoprecipitation (ChIP)
Table S1 provides a summary of all data generated and analyzed in this paper. RelA −/− and Sirt6 −/− MEFS were generated from littermate embryos derived on the same day as wildtype embryos. Biological replicates from wildtype embryos were generated from independently derived non littermate embryos, grown at separate times and treated with TNF-α on separate days. See Table S1 for details on number of independent experiments. Cells were treated with TNF-α (20 ng/ml) for the indicated times. DNA was cross-linked for 10 minutes with 1% formaldehyde and stopped in 0.125 M glycine. Purified chromatin was sonicated to ∼500 bp using the Bioruptor (Diagenode, Inc) and incubated with the indicated antibodies as previously described [10]. Following reverse cross-linking and RNase treatment, DNA was purified with the Ziagen Mini-elute Reaction Cleanup Kit and amplified using the Whole Genome Amplification kit (Sigma) as described by the manufacturer.
ChIP-chip assays
Each amplified DNA sample was labeled according to the manufacturer's ChIP-chip protocol (Nimblegen). Briefly, each DNA sample (1 µg) was denatured in the presence of Cy5 - or Cy3-labeled randon nonamers and incubated with 100 units (exo-) Klenow fragment (NEB) and dNTP mix (6 mM each in TE buffer) for 2 hours at 37°C. Reactions were terminated by addition of 0.5 M EDTA (pH 8), precipitated with isopropanol, and resuspended in water. 12 µg of Cy5-labeled ChIP sample and 6 µg of Cy-3 labeled total sample were mixed, dried and resuspended in 40 µL of buffer including hybridization buffer (Nimblegen), alignment oligonucleotides (Nimblegen) and Component A (Nimblegen). The labeled DNA samples were next denatured and hybridized to “MM8” arrays overnight at 42°C. Samples were co-hybridized with input DNA as a reference, and the microarrays (Nimblegen) contained probes tiling a total of 4 kilobases of 21,249 mouse promoters. Fluorescence intensity raw data were obtained from scanned images of the oligonucleotide tiling arrays using NIMBLESCAN 3.0 extraction software (Nimblegen). For each spot on the array, log2-ratios of the Cy5-labeled test sample versus the Cy3-labeled reference sample were calculated. To normalize across samples, the biweight mean of this log2 ratio was subtracted from each point. ChIP-chip time courses were done twice or more for most time points; representative data from single time courses are shown.
ChIP-chip data analysis
Identification of targets: Raw Chip-chip data were normalized using RMA normalization algorithm in NimbleScan software. Peaks were called out using NimbleScan peak-calling package. We determined whether canonical NF-κBtargets identified by ChIP-seq [12], [42] and previously identified Sirt6 targets [10] were detected by for different threshold values (Figure S3). For RelA, an FDR ≤0.1 failed to retrieve 414 of 980 known canonical targets while an FDR ≤0.2 retrieved all of them. For Sirt6 an FDR ≤0.1 retrieved all the known Sirt6 targets previously identified [10]. An FDR ≤0.2 increased the number of Sirt6 targets by 5547 additional genes, of which 627 (11.3%) are also bound by RelA with an FDR ≤0.2. Compared to the 29% RelA co-occupancy with a Sirt6 FDR ≤0.1, this analysis shows that the higher confidence Sirt6 target genes tend to be co-occupied with RelA. Therefore, for Sirt6, data for all peaks with an FDR ≤0.1 were included and for RelA, data for all peaks with an FDR ≤0.2 were included. Promoter regions were defined as +/−4000 bp of RefSeq genes (mm8). Sirt6 and RelA Chip target genes were obtained by overlapping significant peaks with promoter coordinates in Galaxy, using functions of “getflanks”, “intersect”, “substract” and “join”. To determine the binding sites for Sirt6 and RelA, the list of targets was filtered to include only genes that were targets in wild-type cells and not in the negative control knockout cells (Sirt6−/− or RelA−/−, respectively).
Motif module map: To analyze cis-regulatory motifs enriched among Sirt6 and RelA direct targets, we identified promoters of genes bound by Sirt6 or RelA and tested for their enrichment (p<0.05, hypergeometric distribution) of sets of genes sharing the presence of cis-motifs, termed motif modules [19]. We then selected for motif modules that were induced among wild-type and Sirt6−/− MEFs. All motifs attributed to any of the five NF-κB family members are referred to as “NF-κB”.
GO term and KEGG pathway analyses: Functional annotations were performed using the program Database for Annotation, Visualization, and Integrated Discovery (DAVID). We used a Benjamini threshold of <0.05.
ChIP-qPCR
Sirt6 antibody was conjugated to magnetic Protein A beads (Invitrogen) using the manufacturer supplied protocol. Prior to IP, a volume of sonicated chromatin equivalent to four times the amount used for the IP was reserved as the input sample. Three technical replicates were done for each time point shown, using an equivalent of 10,000 cells of chromatin each time. ChIP was performed using sonicated chromatin on an automated microfluidic device (modified from that previously described [35], to be detailed in a future publication), for a total of two hours on a 4°C thermocycler block, with constant mixing. After IP, RIPA buffer (10 mM Tris-HCl pH 7.5, 1 mM EDTA, 0.5 mM EGTA, 1% Triton X-100, 0.1% SDS, 0.1% Na-deoxycholate, 140 mM NaCl) was used to wash the beads for 10 minutes, following which the beads were then eluted into thin-walled PCR tubes with a minimal amount of Dulbecco's phosphate buffered saline (DPBS). These PCR tubes were then immediately placed on a magnet and the DPBS removed with a pipette leaving only the magnetic beads. The DNA was then purified from the beads using the Chelex (Bio-Rad) resin extraction method described previously [43]. An ethanol precipitation was done on the input sample by adding 250 ul of 100% ethanol (Sigma-Aldrich), 2 ul of carrier glycoblue (Invitrogen), and 16 ul of 5 M NaCl to the sample and precipitating at −80°C for one hour. The precipitated sample was then centrifuged at 20,000 g for 15 minutes, and the supernatant discarded. The pellet was washed in 500 ul of freshly prepared and chilled 70% ethanol, and then centrifuged again at 20,000 g for 10 minutes. Finally, the supernatant was discarded and the pellet left to air dry. Once the pellet was dry, the same Chelex resin extraction was applied in parallel with the IP samples. The purified DNA was used directly in the real-time quantitative SYBR green PCR reactions. Sequences of PCR primers used are provided in the Table S4.
Microarray-based RNA expression assays
MEFs were derived from littermate embryos. Total RNA was extracted with TRIzol (Invitrogen) from mouse embryonic fibroblasts following TNF-α treatment (20 ng/mL) for the indicated times. RNA was labeled with Cy5 and hybridized to whole genome mouse bead arrays (Illumina). Genes that were induced with an absolute detection value of 100 in any sample during the time course were selected and normalized to the untreated wild-type sample. All genes and samples were next organized by hierarchical clustering [44].
Real-time quantitative RT-PCR
Total RNA was extracted with TRIzol. RT-qPCR was performed using total RNA (50 ng), Taqman One Step RT-PCR master mix, and one of the following Taqman assays: GAPDH (Mm99999915g1*), NFKB1 (Mm00476361_m1*), NFKB2 (Mm00479807_m1*), NFKBia (Mm00477798_m1*), Cdkn2a/p14 (Mm01257348_m1), Cdkn2a/p16 (Mm00494449_m1*), Gadd45b (Mm00435123_m1*) and Lmna (Mm00497787_g1). All Taqman reagents were from Applied Biosystems. Reactions were in triplicate for each sample and preformed a minimum of three times. Data were normalized to GAPDH levels.
URLs
Primary microarray data are available at Stanford Microarray Database (http://smd.stanford.edu) and GEO (http://www.ncbi.nlm.nih.gov/geo/) with accession GSE28641.
Supporting Information
Zdroje
1. ImaiSArmstrongCMKaeberleinMGuarenteL 2000 Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403 795 800
2. LandryJSuttonATafrovSTHellerRCStebbinsJ 2000 The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci U S A 97 5807 5811
3. SmithJSBrachmannCBCelicIKennaMAMuhammadS 2000 A phylogenetically conserved NAD+-dependent protein deacetylase activity in the Sir2 protein family. Proc Natl Acad Sci U S A 97 6658 6663
4. XuFZhangQZhangKXieWGrunsteinM 2007 Sir2 deacetylates histone H3 lysine 56 to regulate telomeric heterochromatin structure in yeast. Mol Cell 27 890 900
5. DenuJM 2003 Linking chromatin function with metabolic networks: Sir2 family of NAD(+)-dependent deacetylases. Trends Biochem Sci 28 41 48
6. FryeRA 1999 Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun 260 273 279
7. FryeRA 2000 Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun 273 793 798
8. McCordRAMichishitaEHongTBerberEBoxerLD 2009 SIRT6 stabilizes DNA-dependent protein kinase at chromatin for DNA double-strand break repair. Aging (Albany NY) 1 109 121
9. MichishitaEMcCordRABerberEKioiMPadilla-NashH 2008 SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature 452 492 496
10. KawaharaTLMichishitaEAdlerASDamianMBerberE 2009 SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell 136 62 74
11. MostoslavskyRChuaKFLombardDBPangWWFischerMR 2006 Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124 315 329
12. ZhongLDUrsoAToiberDSebastianCHenryRE The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell 140 280 293
13. TennenRIChuaKF Chromatin regulation and genome maintenance by mammalian SIRT6. Trends Biochem Sci 36 39 46
14. HaydenMSGhoshS 2008 Shared principles in NF-kappaB signaling. Cell 132 344 362
15. HaydenMSGhoshS 2004 Signaling to NF-kappaB. Genes Dev 18 2195 2224
16. GhoshSMayMJKoppEB 1998 NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 16 225 260
17. HoffmannABaltimoreD 2006 Circuitry of nuclear factor kappaB signaling. Immunol Rev 210 171 186
18. FosterSLHargreavesDCMedzhitovR 2007 Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 447 972 978
19. AdlerASSinhaSKawaharaTLZhangJYSegalE 2007 Motif module map reveals enforcement of aging by continual NF-kappaB activity. Genes Dev 21 3244 3257
20. WangZZangCCuiKSchonesDEBarskiA 2009 Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell 138 1019 1031
21. GazinCWajapeyeeNGobeilSVirbasiusCMGreenMR 2007 An elaborate pathway required for Ras-mediated epigenetic silencing. Nature 449 1073 1077
22. HoffmannALevchenkoAScottMLBaltimoreD 2002 The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science 298 1241 1245
23. CovertMWLeungTHGastonJEBaltimoreD 2005 Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science 309 1854 1857
24. TaySHugheyJJLeeTKLipniackiTQuakeSR Single-cell NF-kappaB dynamics reveal digital activation and analogue information processing. Nature 466 267 271
25. SchreiberJJennerRGMurrayHLGerberGKGiffordDK 2006 Coordinated binding of NF-kappaB family members in the response of human cells to lipopolysaccharide. Proc Natl Acad Sci U S A 103 5899 5904
26. LimCAYaoFWongJJGeorgeJXuH 2007 Genome-wide mapping of RELA(p65) binding identifies E2F1 as a transcriptional activator recruited by NF-kappaB upon TLR4 activation. Mol Cell 27 622 635
27. MigliaccioEGiorgioMMeleSPelicciGReboldiP 1999 The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402 309 313
28. KrishnamurthyJTorriceCRamseyMRKovalevGIAl-RegaieyK 2004 Ink4a/Arf expression is a biomarker of aging. J Clin Invest 114 1299 1307
29. JanzenVForkertRFlemingHESaitoYWaringMT 2006 Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature 443 421 426
30. BrackASConboyMJRoySLeeMKuoCJ 2007 Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 317 807 810
31. LiuHFergussonMMCastilhoRMLiuJCaoL 2007 Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317 803 806
32. De SmaeleEZazzeroniFPapaSNguyenDUJinR 2001 Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature 414 308 313
33. MendrysaSMMcElweeMKPerryME 2001 Characterization of the 5′ and 3′ untranslated regions in murine mdm2 mRNAs. Gene 264 139 146
34. MounkesLCKozlovSHernandezLSullivanTStewartCL 2003 A progeroid syndrome in mice is caused by defects in A-type lamins. Nature 423 298 301
35. WuARHiattJBLuRAttemaJLLoboNA 2009 Automated microfluidic chromatin immunoprecipitation from 2,000 cells. Lab Chip 9 1365 1370
36. ErikssonMBrownWTGordonLBGlynnMWSingerJ 2003 Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423 293 298
37. SouthworthLKOwenABKimSK 2009 Aging mice show a decreasing correlation of gene expression within genetic modules. PLoS Genet 5 e1000776 doi:10.1371/journal.pgen.1000776
38. AdlerASKawaharaTLSegalEChangHY 2008 Reversal of aging by NFkappaB blockade. Cell Cycle 7 556 559
39. MartinSGLarocheTSukaNGrunsteinMGasserSM 1999 Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell 97 621 633
40. OberdoerfferPMichanSMcVayMMostoslavskyRVannJ 2008 SIRT1 redistribution on chromatin promotes genomic stability but alters gene expression during aging. Cell 135 907 918
41. PerkinsND 2007 Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol 8 49 62
42. KasowskiMGrubertFHeffelfingerCHariharanMAsabereA Variation in transcription factor binding among humans. Science 328 232 235
43. DahlJACollasP 2008 A rapid micro chromatin immunoprecipitation assay (microChIP). Nat Protoc 3 1032 1045
44. EisenMBSpellmanPTBrownPOBotsteinD 1998 Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A 95 14863 14868
Štítky
Genetika Reprodukčná medicína
Článek Local Absence of Secondary Structure Permits Translation of mRNAs that Lack Ribosome-Binding SitesČlánek Independent Chromatin Binding of ARGONAUTE4 and SPT5L/KTF1 Mediates Transcriptional Gene SilencingČlánek Trade-Off between Bile Resistance and Nutritional Competence Drives Diversification in the Mouse GutČlánek FGF Signaling Regulates the Number of Posterior Taste Papillae by Controlling Progenitor Field SizeČlánek Mammalian BTBD12 (SLX4) Protects against Genomic Instability during Mammalian SpermatogenesisČlánek Identification of Nine Novel Loci Associated with White Blood Cell Subtypes in a Japanese PopulationČlánek Differential Effects of and Risk Variants on Association with Diabetic ESRD in African Americans
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2011 Číslo 6- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Local Absence of Secondary Structure Permits Translation of mRNAs that Lack Ribosome-Binding Sites
- Statistical Inference on the Mechanisms of Genome Evolution
- Revisiting Heterochromatin in Embryonic Stem Cells
- A Two-Stage Meta-Analysis Identifies Several New Loci for Parkinson's Disease
- Identification of a Sudden Cardiac Death Susceptibility Locus at 2q24.2 through Genome-Wide Association in European Ancestry Individuals
- Genomic Prevalence of Heterochromatic H3K9me2 and Transcription Do Not Discriminate Pluripotent from Terminally Differentiated Cells
- Epistasis between Beneficial Mutations and the Phenotype-to-Fitness Map for a ssDNA Virus
- Recurrent Chromosome 16p13.1 Duplications Are a Risk Factor for Aortic Dissections
- Telomere DNA Deficiency Is Associated with Development of Human Embryonic Aneuploidy
- Genome-Wide Association Study of White Blood Cell Count in 16,388 African Americans: the Continental Origins and Genetic Epidemiology Network (COGENT)
- Unexpected Role for DNA Polymerase I As a Source of Genetic Variability
- Transportin-SR Is Required for Proper Splicing of Genes and Plant Immunity
- How Chromatin Is Remodelled during DNA Repair of UV-Induced DNA Damage in
- Independent Chromatin Binding of ARGONAUTE4 and SPT5L/KTF1 Mediates Transcriptional Gene Silencing
- Two Evolutionary Histories in the Genome of Rice: the Roles of Domestication Genes
- Natural Allelic Variation Defines a Role for : Trichome Cell Fate Determination
- Multiple Common Susceptibility Variants near BMP Pathway Loci , , and Explain Part of the Missing Heritability of Colorectal Cancer
- Pathogenic Mechanism of the FIG4 Mutation Responsible for Charcot-Marie-Tooth Disease CMT4J
- A Functional Variant in Promoter Modulates Its Expression and Confers Disease Risk for Systemic Lupus Erythematosus
- Drift and Genome Complexity Revisited
- Chromosomal Macrodomains and Associated Proteins: Implications for DNA Organization and Replication in Gram Negative Bacteria
- Trade-Off between Bile Resistance and Nutritional Competence Drives Diversification in the Mouse Gut
- Pathways of Distinction Analysis: A New Technique for Multi–SNP Analysis of GWAS Data
- Web-Based Genome-Wide Association Study Identifies Two Novel Loci and a Substantial Genetic Component for Parkinson's Disease
- Chk2 and p53 Are Haploinsufficient with Dependent and Independent Functions to Eliminate Cells after Telomere Loss
- Exome Sequencing Identifies Mutations in High Myopia
- Distinct Functional Constraints Partition Sequence Conservation in a -Regulatory Element
- CorE from Is a Copper-Dependent RNA Polymerase Sigma Factor
- A Single Sex Pheromone Receptor Determines Chemical Response Specificity of Sexual Behavior in the Silkmoth
- FGF Signaling Regulates the Number of Posterior Taste Papillae by Controlling Progenitor Field Size
- Maps of Open Chromatin Guide the Functional Follow-Up of Genome-Wide Association Signals: Application to Hematological Traits
- Increased Susceptibility to Cortical Spreading Depression in the Mouse Model of Familial Hemiplegic Migraine Type 2
- Differential Gene Expression and Epiregulation of Alpha Zein Gene Copies in Maize Haplotypes
- Parallel Adaptive Divergence among Geographically Diverse Human Populations
- Genetic Analysis of Genome-Scale Recombination Rate Evolution in House Mice
- Mechanisms for the Evolution of a Derived Function in the Ancestral Glucocorticoid Receptor
- Mammalian BTBD12 (SLX4) Protects against Genomic Instability during Mammalian Spermatogenesis
- Interferon Regulatory Factor 8 Regulates Pathways for Antigen Presentation in Myeloid Cells and during Tuberculosis
- High-Resolution Analysis of Parent-of-Origin Allelic Expression in the Arabidopsis Endosperm
- Specific SKN-1/Nrf Stress Responses to Perturbations in Translation Elongation and Proteasome Activity
- Graded Nodal/Activin Signaling Titrates Conversion of Quantitative Phospho-Smad2 Levels into Qualitative Embryonic Stem Cell Fate Decisions
- Genome-Wide Analysis Reveals PADI4 Cooperates with Elk-1 to Activate Expression in Breast Cancer Cells
- Trait Variation in Yeast Is Defined by Population History
- Meiosis-Specific Loading of the Centromere-Specific Histone CENH3 in
- A Genome-Wide Survey of Imprinted Genes in Rice Seeds Reveals Imprinting Primarily Occurs in the Endosperm
- Multiple Regulatory Mechanisms to Inhibit Untimely Initiation of DNA Replication Are Important for Stable Genome Maintenance
- SIRT1 Promotes N-Myc Oncogenesis through a Positive Feedback Loop Involving the Effects of MKP3 and ERK on N-Myc Protein Stability
- Bacteriophage Crosstalk: Coordination of Prophage Induction by Trans-Acting Antirepressors
- Role of the Single-Stranded DNA–Binding Protein SsbB in Pneumococcal Transformation: Maintenance of a Reservoir for Genetic Plasticity
- Genomic Convergence among ERRα, PROX1, and BMAL1 in the Control of Metabolic Clock Outputs
- Genome-Wide Association of Bipolar Disorder Suggests an Enrichment of Replicable Associations in Regions near Genes
- Identification of Nine Novel Loci Associated with White Blood Cell Subtypes in a Japanese Population
- DNA Ligase III Promotes Alternative Nonhomologous End-Joining during Chromosomal Translocation Formation
- Differential Effects of and Risk Variants on Association with Diabetic ESRD in African Americans
- Finished Genome of the Fungal Wheat Pathogen Reveals Dispensome Structure, Chromosome Plasticity, and Stealth Pathogenesis
- Dynamic Chromatin Localization of Sirt6 Shapes Stress- and Aging-Related Transcriptional Networks
- Extracellular Matrix Dynamics in Hepatocarcinogenesis: a Comparative Proteomics Study of Transgenic and Null Mouse Models
- Integrating 5-Hydroxymethylcytosine into the Epigenomic Landscape of Human Embryonic Stem Cells
- Vive La Différence: An Interview with Catherine Dulac
- Multiple Loci Are Associated with White Blood Cell Phenotypes
- Nuclear Accumulation of Stress Response mRNAs Contributes to the Neurodegeneration Caused by Fragile X Premutation rCGG Repeats
- A New Mutation Affecting FRQ-Less Rhythms in the Circadian System of
- Cryptic Transcription Mediates Repression of Subtelomeric Metal Homeostasis Genes
- A New Isoform of the Histone Demethylase JMJD2A/KDM4A Is Required for Skeletal Muscle Differentiation
- Genetic Determinants of Lipid Traits in Diverse Populations from the Population Architecture using Genomics and Epidemiology (PAGE) Study
- A Genome-Wide RNAi Screen for Factors Involved in Neuronal Specification in
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Recurrent Chromosome 16p13.1 Duplications Are a Risk Factor for Aortic Dissections
- Statistical Inference on the Mechanisms of Genome Evolution
- Genome-Wide Association Study of White Blood Cell Count in 16,388 African Americans: the Continental Origins and Genetic Epidemiology Network (COGENT)
- Chromosomal Macrodomains and Associated Proteins: Implications for DNA Organization and Replication in Gram Negative Bacteria
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



