-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Unexpected Role for DNA Polymerase I As a Source of Genetic Variability
Helicobacter pylori, a human pathogen infecting about half of the world population, is characterised by its large intraspecies variability. Its genome plasticity has been invoked as the basis for its high adaptation capacity. Consistent with its small genome, H. pylori possesses only two bona fide DNA polymerases, Pol I and the replicative Pol III, lacking homologues of translesion synthesis DNA polymerases. Bacterial DNA polymerases I are implicated both in normal DNA replication and in DNA repair. We report that H. pylori DNA Pol I 5′ - 3′ exonuclease domain is essential for viability, probably through its involvement in DNA replication. We show here that, despite the fact that it also plays crucial roles in DNA repair, Pol I contributes to genomic instability. Indeed, strains defective in the DNA polymerase activity of the protein, although sensitive to genotoxic agents, display reduced mutation frequencies. Conversely, overexpression of Pol I leads to a hypermutator phenotype. Although the purified protein displays an intrinsic fidelity during replication of undamaged DNA, it lacks a proofreading activity, allowing it to efficiently elongate mismatched primers and perform mutagenic translesion synthesis. In agreement with this finding, we show that the spontaneous mutator phenotype of a strain deficient in the removal of oxidised pyrimidines from the genome is in part dependent on the presence of an active DNA Pol I. This study provides evidence for an unexpected role of DNA polymerase I in generating genomic plasticity.
Published in the journal: Unexpected Role for DNA Polymerase I As a Source of Genetic Variability. PLoS Genet 7(6): e32767. doi:10.1371/journal.pgen.1002152
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002152Summary
Helicobacter pylori, a human pathogen infecting about half of the world population, is characterised by its large intraspecies variability. Its genome plasticity has been invoked as the basis for its high adaptation capacity. Consistent with its small genome, H. pylori possesses only two bona fide DNA polymerases, Pol I and the replicative Pol III, lacking homologues of translesion synthesis DNA polymerases. Bacterial DNA polymerases I are implicated both in normal DNA replication and in DNA repair. We report that H. pylori DNA Pol I 5′ - 3′ exonuclease domain is essential for viability, probably through its involvement in DNA replication. We show here that, despite the fact that it also plays crucial roles in DNA repair, Pol I contributes to genomic instability. Indeed, strains defective in the DNA polymerase activity of the protein, although sensitive to genotoxic agents, display reduced mutation frequencies. Conversely, overexpression of Pol I leads to a hypermutator phenotype. Although the purified protein displays an intrinsic fidelity during replication of undamaged DNA, it lacks a proofreading activity, allowing it to efficiently elongate mismatched primers and perform mutagenic translesion synthesis. In agreement with this finding, we show that the spontaneous mutator phenotype of a strain deficient in the removal of oxidised pyrimidines from the genome is in part dependent on the presence of an active DNA Pol I. This study provides evidence for an unexpected role of DNA polymerase I in generating genomic plasticity.
Introduction
Phenotypic selection from a pool of genetic variants present in their population allows prokaryotes to successfully adapt to specific niches and changing environments. The gram-negative bacterium Helicobacter pylori is one of the most successful human pathogens. Indeed, it colonises the stomach mucosa of about half the human population, triggering pathologies that span asymptomatic chronic gastritis, peptic ulcers and cancer [1]. The study of natural isolates suggests that the genetic diversity of H. pylori exceeds that recorded in all other bacterial species studied. Moreover, it is now clear that even in the course of infection of a single individual, H. pylori strains display high rates of allelic variation [2], [3]. This enhanced ability to change and the consequent advantage of counting upon a large pool of variants from which to select the most-fit combinations have been proposed to facilitate adaptation within a host as well as colonisation of new hosts [4], [5]. Therefore, besides its clinical importance, the amazing genetic variability of H. pylori makes it an excellent model for the analysis of the molecular mechanisms underlying microbial phenotype evolution.
At the origin of the allelic variability are nucleotide changes that can arise from replication errors either spontaneous or induced by DNA lesions. H. pylori displays a high rate of mutations, accounted not only by the lack of mismatch repair system [6], [7] but also by the exposure to genotoxic stresses during infection leading to the formation of DNA lesions [8], [9]. While replicative DNA polymerases are highly accurate and efficient in replicating undamaged DNA, the presence of abasic sites or modified bases will often impede the progression of the replication fork. It is now well established that in most organisms DNA polymerases exist that are capable of substituting for the replicative polymerase and facilitate translesion synthesis (TLS) allowing the replication machinery to overcome the blockage. In many cases, TLS is associated with the acquisition of heritable mutations induced by the incorporation by the TLS polymerase of an incorrect nucleotide opposite the lesion [10].
In agreement with the low functional redundancy in its DNA repair pathways, analysis of the H. pylori genome sequences predicts the presence of only two putative DNA polymerases. Indeed, six genes code for the replicative polymerase subunits, while one gene, HP1470 in the reference strain 26695, codes for a putative DNA Polymerase I. E. coli Pol I was the first DNA polymerase discovered and is the most abundant one [11]. The Pol I bacterial DNA polymerases are multifunctional proteins. In most bacterial species Pol I presents two distinct functional domains, a 5′ - 3′ exonuclease N-terminal domain and a larger C-terminal domain (Klenow fragment) harbouring the polymerase and the associated proofreading 3′ – 5′ exonuclease catalytic sites [12]. The 5′-3′ exonuclease activity allows the removal of the RNA primers of the Okazaki fragments during DNA replication [13]. The gap-filling capacity of Pol I not only participates in the replication of the lagging strand but also in DNA excision repair and in recombination.
The absence of other predicted DNA polymerases in H. pylori, in particular of those capable of TLS, raises several questions regarding the distribution of roles between the two DNA polymerases during replication and repair. To investigate these issues we characterised the protein coded by H. pylori polA gene and showed that it is able to bypass blocking lesions. Based on our genetic results we conclude that H. pylori DNA polymerase I, albeit its important role in cell viability and DNA repair, contributes to mutagenesis during normal chromosome replication and therefore to the plasticity of the genome.
Results
HP1470 codes for a bona fide DNA polymerase I devoid of proofreading activity
The inferred absence of specialised TLS polymerases coded by the H. pylori genome [14], [15] prompted us to study the characteristics of the putative DNA polymerase I, one of the two predicted DNA polymerases of this pathogen. The protein sequence deduced for HP1470 suggested that the protein is a bona fide DNA Polymerase I orthologue. However, closer inspection of the amino acid sequence shows that even though the overall structure of the 3′ – 5′ exonuclease domain is likely to be preserved, at least three conserved residues - Asp355, Asp424 and Asp501 in E. coli DNA polymerase I - involved in metal binding and essential for the exonuclease catalytic activity [16]–[18] are missing (Figure S1). In order to verify the activities of the H. pylori DNA Polymerase I (herein Pol I), the protein was expressed in E. coli and purified to apparent homogeneity (Text S1 and Figure S2). As expected, Pol I displayed DNA–dependent polymerase (Figure 1A) and 5′ – 3′ exonuclease (Figure 1B) activities. In the polymerisation assays (Figure 1A), extensions beyond the expected full-length product could be detected. This has previously been shown to be characteristic of some DNA polymerases lacking a proofreading activity [19]. To verify the prediction of a lack of 3′ - 5′ exonuclease activity, we then tested Pol I mismatch editing capacity. In conditions in which the E. coli Klenow fragment efficiently removed a mispaired base from the primer 3′-end, Pol I showed no exonuclease activity on substrates with different 3′-mismatches (Figure 1C). Moreover, with the possible exception of a G:A after which only one or two bases were incorporated, Pol I was able to extend primers with various mismatches at their 3′-end (Figure 1D).
Fig. 1. Pol I enzymatic activities. 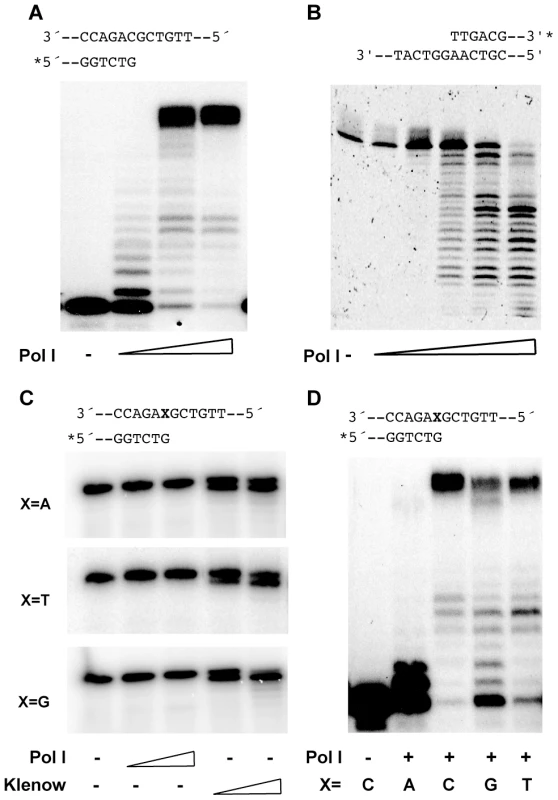
A. Primer extension. Increasing concentrations (0.1; 1 and 5 nM) of Pol I were used. B. 5′ – 3′ exonuclease. Concentrations of Pol I used were 10, 50, 100, 150 and 200 nM. C. 3′ – 5′ exonuclease on mismatched primers. Concentrations of E. coli Klenow fragment and Pol I were 25 and 100 nM. D. Mismatch extension. 5 nM Pol I was used. Substrates in A, C and D consisted in a 34-mer template oligonucleotide paired to 18-mer primers as described on top of each gel (Table S1). For B, the duplex was formed by a 62-mer (XV82) paired to a 31-mer (XV101) oligonucleotide (Table S1). In all cases representative gels of at least three independent experiments are shown. An essential role for the 5′ – 3′ exonuclease domain
In order to explore the role of Pol I in vivo, we generated H. pylori strains deficient in this protein. The constructs used to disrupt the gene were designed to replace the 2 kb central region of the gene with an antibiotic resistance cassette, leaving only 300 bp of the gene at each extremity. Interestingly, very few clones were obtained and in all cases analyzed, the cassette was inserted downstream of the expected site. Sequencing of five independent insertions showed that the first kilobase of the coding sequence was always preserved (Figure S3), potentially allowing the 5′ – 3′ exonuclease to be expressed. To rule out a sequence context bias for the insertion, we performed transformations with the same disruption cassette in a strain carrying an extra copy of the polA gene at the ureA locus. In this case the number of clones recovered was several orders of magnitude higher compared to those obtained from transformation of the wild type strain. Analysis of 22 independent insertions showed that 4 were in the ectopic gene and 18 in the hp1470 locus. Interestingly, in all cases the insertion resulted in the expected product, a deletion starting 300 bp from the initiation codon, leading to the truncation of two thirds of the 5′ – 3′ exonuclease domain. Taken together, these observations strongly suggest that the 5′ – 3′ exonuclease activity coded by the N-terminal domain of Pol I is essential for viability.
Pol I DNA polymerase activity is required for DNA repair but promotes mutagenesis
We then assessed the capacity of strains defective in DNA polymerase activity of Pol I (polA) to survive to various genotoxic treatments. The polA strains used correspond to those strains where the 3′ – 5′ exonuclease and polymerase domains are replaced by an antibiotic resistance cassette, leaving an intact 5′ – 3′ exonuclease domain, shown above to be essential. polA mutants are extremely sensitive to agents such as ionising radiation, UV light, hydrogen peroxide and the alkylating agent methyl-methanesulfonate (MMS) (Figure 2A–2D), all inducing different types of DNA damage. Interestingly, when polA was disrupted in a strain deficient in AP-endonuclease activity (xth) [20] there was an additive effect on the sensitivity to MMS, suggesting that Pol I participates in another pathway besides base excision repair. These results underscore the crucial role of Pol I in various DNA repair systems.
Fig. 2. polA mutants are sensitive to genotoxic agents. 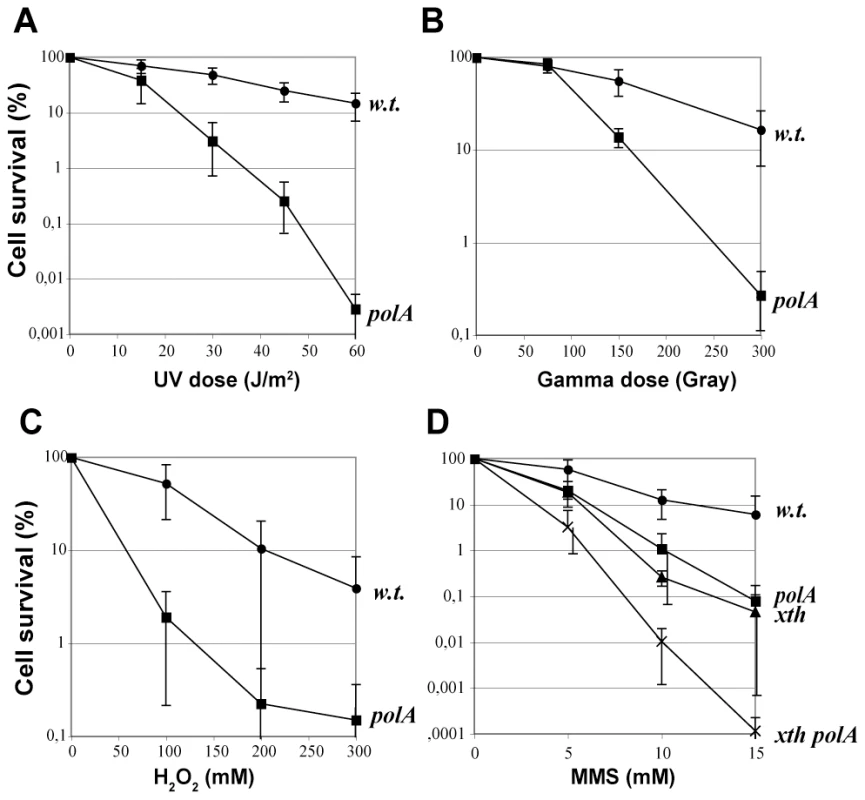
Values correspond to the average of at least 4 independent determinations and their SD. To better characterise the in vivo functions of the protein, we tested the effect of the deficiency in Pol I on H. pylori spontaneous mutagenesis. The rate of base-pair substitutions was determined by monitoring the appearance of rifampicin-resistant (Rifr) colonies [9] (Figure 3A). Surprisingly, even though DNA polymerases I are involved in excision repair, inactivation of the polymerase activity of Pol I not only failed to increase base-pair mutation rates but resulted in a modest but significant hypo-mutator phenotype. Moreover, overexpression of Pol I driven by the strong ureA promoter resulted in a hyper-mutator phenotype thus indicating that Pol I in vivo generates base-substitutions.
Fig. 3. Pol I contributes to mutagenesis. 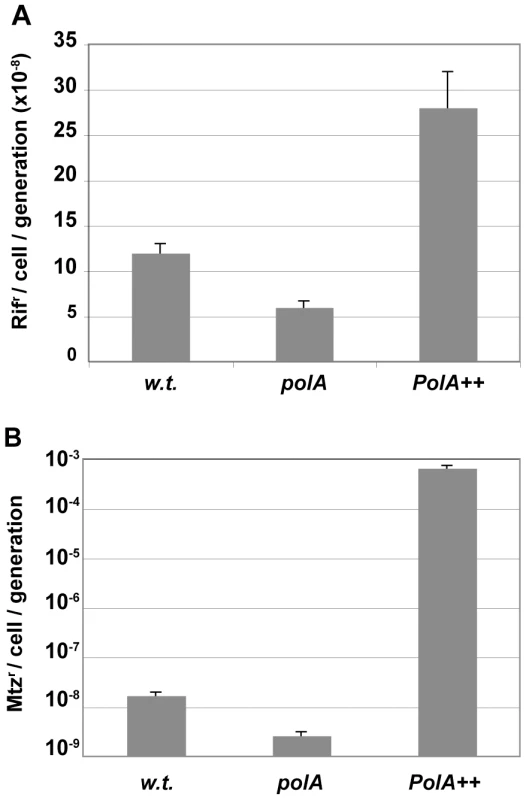
A. Rates of spontaneous mutations to rifampicin resistance. B. Rates of spontaneous mutations to metronidazole (Mtz) resistance. Rates and SD were calculated by the method of the median from N independent cultures for wild type, polA and PolA++. N (rif) = 76, 50 and 11 and N (Mtz) = 20, 36 and 22 cultures, respectively. Since the Rifr mutagenesis test is limited to the detection of specific base substitutions, we used a forward mutation assay to explore a larger spectrum of genetic alterations. For such purpose, we determined the rate of mutations in the rdx gene, leading to resistance to metronidazole (Mtzr) [21]. The results showed a much more pronounced effect of Pol I on the Mtzr mutation rates than in the case of Rifr. Indeed, inactivation of polA resulted in a 4-fold decrease in spontaneous mutations while its overexpression increased the rate of mutation by 500-fold (Figure 3B). Sequencing of Mtzr isolates showed that the spectrum of mutations also changed. Indeed, sequencing of Mtzr isolates showed that the 10-fold excess of mutants obtained in a wild type with respect to polA strain was due to 72 - and 9-fold increases in the frequency of base substitutions and one base-pair frameshifts respectively, while the frequency of larger deletions or insertions was essentially unmodified (Table 1). The same trend was observed for the Pol I over-expressing strain where the increase in mutations was accounted for by the increase in base substitutions and one base pair frameshifts, with only 1 out of 36 clones analyzed displaying a change involving more than one base-pair (−2 deletion). Interestingly, in the overproducing strain the enhanced rate of base pair substitutions could essentially be accounted for by the increase in transversions. In conclusion, the excess mutations observed in strains expressing Pol I were essentially base substitutions or one nucleotide frameshifts. Taken together these data confirm that Pol I, although important for DNA repair, contributes to genetic variability mainly through the generation of single nucleotide polymorphisms.
Tab. 1. Sequence alterations in metronidazole-resistant mutants. 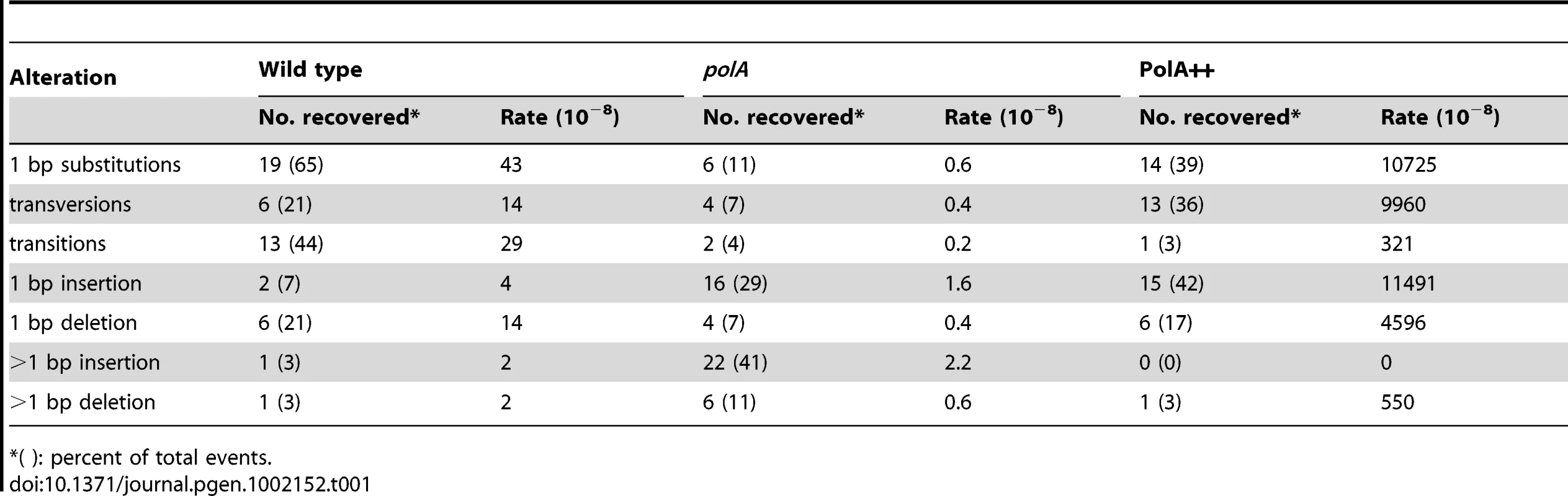
*( ): percent of total events. Pol I has an accurate DNA polymerase activity
The results presented above prompted us to analyse whether, besides the lack of proofreading, Pol I harboured an intrinsic error-prone DNA polymerase activity. The fidelity of Pol I was determined during synthesis to fill a 407-nt single-stranded gap within a circular duplex M13mp2 DNA substrate. The gap contains the lacZ α-complementation sequence that serves as the target for detecting polymerisation errors that are detected as light blue and colourless plaques among blue plaques resulting from correct synthesis [22]. The DNA products of gap filling by Pol I yielded a lacZ mutant frequency of 0.15%. This frequency is lower than values obtained after gap filling by several other exonuclease-deficient family A polymerases, including 0.57% Klenow fragment polymerase [23], 0.75% for Thermus aquaticus polymerase [24], 1.6% for exonuclease-deficient T7 polymerase [23], [24], and 0.62% for exonuclease-deficient pol γ [25]. Thus H. pylori Pol I is among the most accurate exonuclease–deficient members of the family A polymerases when copying an undamaged DNA template in vitro.
Translesion synthesis by Pol I
The apparent contradiction between the fidelity of the Pol I DNA polymerase activity on undamaged DNA and its role in the generation of mutations prompted us to further investigate the enzymatic characteristics of Pol I. The lack of proofreading activity, the consequent capacity to elongate from mismatches and the spectrum of mutations it generates are reminiscent of TLS polymerases. We directly addressed this possibility by determining the ability of purified Pol I to bypass DNA lesions present in the template strand. Among the lesions tested, some, like the abasic (AP) site analogue tetrahydrofurane (THF) and thymine glycol (Tg) are known to impose a blockage to normal DNA replication [26], [27] while others like 8-oxoguanine (8-oxoG) do not, but have a miscoding potential [28]. In the case of E. coli Klenow fragment, DNA synthesis is indeed strongly blocked opposite Tg and THF residues present in the template strand (estimated bypass efficiencies: 5 and 11% respectively). Conversely, albeit also partially blocked, H. pylori Pol I is able to synthesise through these lesions (Figure 4A) (estimated bypass efficiencies: 61 and 48%, for Tg and THF respectively).
Fig. 4. Translesion synthesis by Pol I. 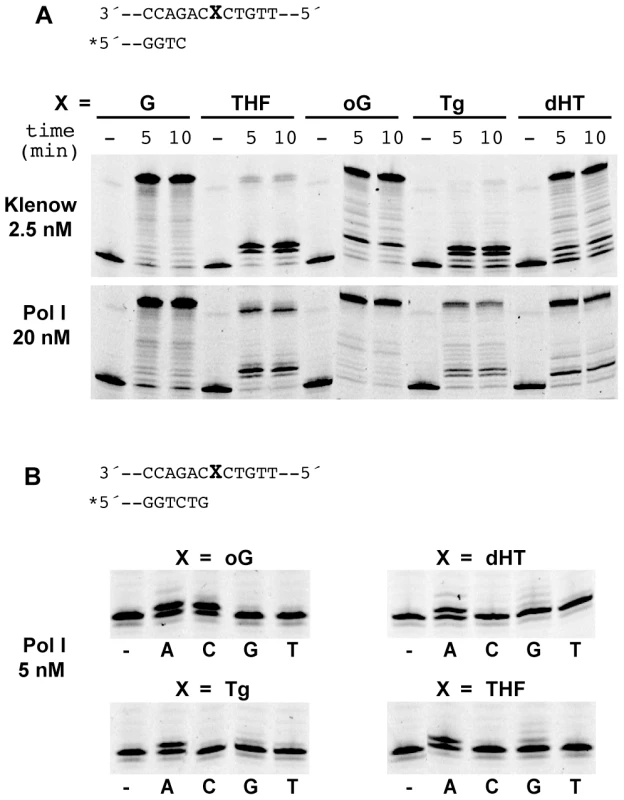
A. Translesion synthesis by E. coli Klenow fragment (2,5 nM) and Pol I (20 nM) on 34-mer templates harbouring a single lesion (X) at position 16. The polymerases have to add two nucleotides before encountering the lesion. oG = 8-oxoguanine; dHT = di-hydrothymidine; Tg = thymine-glycol and THF = tetra-hydrofurane, an abasic site analogue. The concentrations used for the two DNA polymerases were chosen for yielding the same activity on undamaged DNA. B. Nucleotide selection. Extension reactions directly on a lesion were carried out for 5 min in the presence of a single dNTP (0.1 mM) as indicated for each lane. We next examined the single nucleotide insertion profile promoted by Pol I opposite the various lesions. Consistently with the described coding capacity of 8-oxoG, Pol I introduces both A and C opposite the oxidised guanine (Figure 4B). For the other lesions tested DNA Pol I follows the A-rule, introducing preferentially adenine opposite the lesion. In the case of the abasic site analogue addition of G opposite THF is also observed (Figure 4B).
To confirm that the Pol I-dependent in vivo mutagenesis could be at least partly related to the presence of DNA lesions, we analysed the effect of Pol I on the spontaneous mutagenesis in strains lacking Nth, the DNA glycosylase responsible for the removal of oxidised pyrimidines from DNA. As previously reported [9], an nth mutant has a 4-fold higher mutation rate than its parental strain (Table 2). Inactivation of polA in an nth background resulted in partial reduction of the mutator phenotype induced by the lack of Nth, strongly suggesting that a fraction of unrepaired Nth substrate lesions are normally bypassed by the DNA Pol I. This result is consistent with a contribution of Pol I to mutagenesis through TLS.
Tab. 2. Rates (×10<sup>−8</sup>) of spontaneous mutation to Rif<sup>r</sup>. 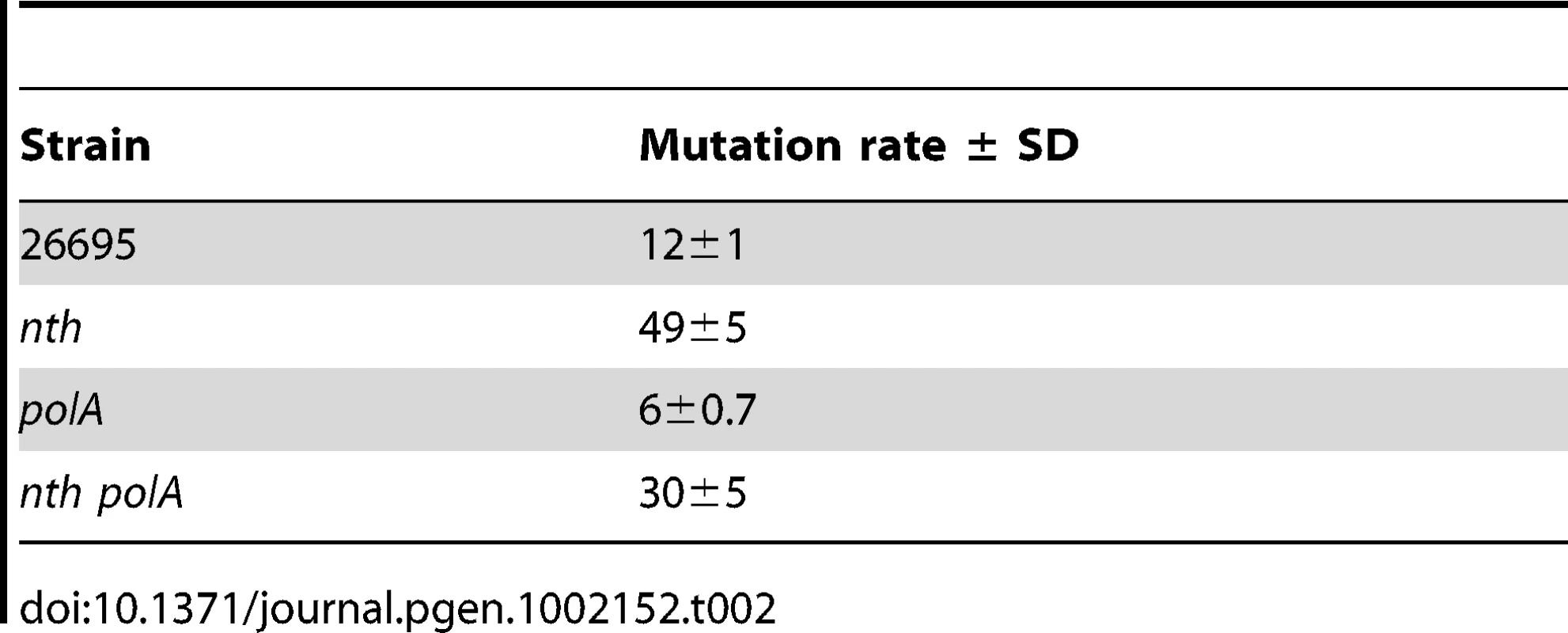
Discussion
The success of H. pylori in colonising a large fraction of the human population has been attributed to its adaptation capacity based, in turn, on the genetic diversity of the species. At the basis of the remarkable genetic variation found in H. pylori are nucleotide polymorphisms that can be rapidly propagated by recombination between strains [29]. In vivo generation of allelic diversity is driven by mutation rates significantly higher than those of most other bacteria [6]. Several mechanisms have been suggested as contributing to the high mutation frequency of this pathogen, starting with the lack of homologues of many DNA repair genes known to be involved in maintaining the genetic stability in other bacterial species [4]. Among the most remarkable absences is probably that of a mismatch repair system [6], [7] capable of removing incorrect bases introduced during replication.
The work presented here unveiled another mechanism contributing to H. pylori high mutation rates. We showed that H. pylori strains deficient in DNA Pol I polymerase activity have reduced mutation rates indicating that DNA polymerase I actively participates in generating allelic diversity. This constitutes a surprising role for a protein associated with DNA repair and replication in all the studied bacterial models. The sensitivity of polA strains to various genotoxic agents confirms that H. pylori Pol I is involved in various DNA repair pathways such as recombination and base excision repair. However, their hypomutator phenotype is in contrast with the 7 - to 10-fold-higher spontaneous mutation frequency in E. coli Pol I-deficient strains [30]. Our results, together with the absence of DNA polymerases other than DNA Pol I and Pol III, suggest that the polA gene in this species has been selected for coding a DNA Pol I capable of fulfilling extra functions allowing increased mutation rates. Indeed, we showed that DNA Pol I can not only extend mismatched primers, but also bypass DNA lesions that would normally block the replicative polymerase as well as DNA Pol I homologues from other bacteria. How can those characteristics contribute to increase mutagenesis?
Spontaneous or induced DNA damage is constantly generated in the genome [31]. In most organisms TLS polymerases allow replication across damaged DNA avoiding the blockage of the replication machinery. In E. coli, the SOS response includes the expression of TLS polymerases that allow survival in such situations at the expense of induced mutations. In spite of the lack of evidence for an SOS response system, it is now clear that H. pylori takes advantage of stress-induced DNA damage to mutate [8], [9]. Such a response necessitates a DNA polymerase capable of performing mutagenic TLS [10]. Unlike what was shown in B. subtilis, where Pol I, also lacking the proofreading function, acts in concert with TLS polymerases PolY1 and PolY2 to bypass lesions [32], the biochemical activities of H. pylori Pol I unveiled in this work would be sufficient to accomplish the task. The hypo-mutator phenotype of the polA strains and the lack of specialised TLS polymerases are consistent with this view. So is the increased proportion of transversions among base substitutions found in the Pol I overproducing strain with respect to the wild-type (13/14 versus 6/19), as expected for the bypass of non-coding lesions. The partial dependence on a functional DNA Pol I of the increased mutagenesis of an nth strain provides further support for this hypothesis. The oxidative stress generated by infection-induced inflammation, the acidic medium of the stomach together with the limited set of H. pylori DNA glycosylases involved in base excision repair [20], [33] favour the formation and persistence of stress-induced damage in DNA, including modified bases and abasic sites [9], [34]. Therefore, it is likely that beyond its functions in DNA repair, DNA Pol I plays an important role in the survival of the bacteria during infection by allowing the replication of damaged DNA and, concomitantly, by contributing to generate allelic diversity in response to the stress.
From the enzymatic point of view, H. pylori polymerase I combines two antagonistic properties not usually found within the same enzyme. Although it exhibits high accuracy on undamaged DNA, it is able to efficiently bypass several types of lesions and can extend mismatched primers. How can such a plasticity be understood in the context of a single enzyme? Regarding other Pol I homologues, accuracy was shown to be exquisitely controlled through a closing mechanism of the fingers domain involving a tight packing between the active site residues and the nucleotide to be inserted. Residues in the so-called O-helix were shown to actively disfavour misincorporation [35], [36]. Mutation Y766S within the O-helix of E. coli Klenow polymerase led to a more open active site and favoured lesion bypass at the expense of fidelity [37], [38]. Similarly, the more open active site of TLS polymerases, such as that of yeast Polη or the archaeal Dpo4, is a major determinant to account for their ability to accommodate bulky lesions [39], [40]. As a first hypothesis, we thought that H. pylori Pol I active site might have a special open structure particularly tolerant to mispairs insertions. However, mapping the conservation of the sequences of both Taq and H. pylori Pol I at the structure of Taq polymerase showed that both sequences are strictly conserved all along the active site groove (Figure S4). In particular, residues of the O-helix involved in the steric-gate mechanism are identical. Consequently, both the high accuracy of PolA on undamaged DNA and the conserved nature of the active site support that, unlike other TLS polymerases, H. pylori Pol I permissiveness is not due to a more open cavity. Consistently we have not been able to detect a significant mutagenesis induced by UV (data not shown). One might suppose that subtle dynamical properties of the enzyme allow accommodation of small lesions but not necessarily bulky lesions. A large body of evidence suggests that this is the case for other members of the Pol A family, including E. coli Klenow fragment. Indeed, these polymerases were shown to be able to incorporate a nucleotide opposite AP sites and products of cytosine or thymine oxidation (Tg, urea, uracyl-glycol and others) although not always to elongate from it [19], [26], [41]–[44]. Moreover, inactivation of the proofreading activity of Klenow allows the bypass of most of these lesions [19], [43], [45]–[47]. Interfestingly, two higher-eukaryote members of the family lacking a 3′-5′ exonuclease domain, DNA polymerases θ and ν, have been shown to be proficient for bypassing Tg and abasic sites [48]–[50]. Taking into account the strong structural conservation predicted for the active sites of H. pylori Pol I and the other members of the family (Figure S4), the work cited above supports the notion that the loss of proofreading activity can account for the capacity of Pol I to bypass AP sites and non-bulky damaged bases. In the case of AP sites this activity will contribute to mutagenesis either by incorporating in three out of four events the wrong nucleotide or by inducing frameshifts [47].
Further support for a role of TLS by Pol I in H. pylori mutagenesis, comes from the results showing that inactivation of Pol I partially complements the mutator phenotype of an nth strain (Table 2). H. pylori Nth is the only DNA glycosylase in this organism capable of removing oxidised pyrimidines from DNA [9]. Many of the products of thymine and cytosine oxidation are pre-mutagenic lesions. In particular, oxidised derivatives from cytosine as 5′-hydroxycytosine, uracyl-glycol and 5′-hydroxyuracyl [46], [51]–[54] but also from thymine [46], [51]–[54] have been shown to be bypassed by proofreading-deficient DNA polymerases and to be mutagenic [55], [56].
Besides TLS, another, non-exclusive, mechanism can be invoked for the role of H. pylori Pol I in mutagenesis. The essential character of the 5′ - 3′ exonuclease domain of H. pylori Pol I strongly suggest that this activity is required for Okazaki fragment processing, even in the absence of the other protein activities. Recently, elegant genetic experiments established that E. coli Pol I proofreading activity plays a crucial role in chromosomal replication fidelity [57]. The model put forward by the authors proposes that Pol I performs 1–2% of lagging strand synthesis. They show that inactivation of the 3′ - 5′ exonuclease activity leads to a mutator phenotype with a strong bias towards lagging strand mutations. The mutator phenotype observed in polA strains even in the absence of all three TLS polymerases is also consistent with a proposed role for E. coli Pol I proofreading activity during replication [58]. In the case of H. pylori, despite accurate polymerase activity of Pol I, its lack of proofreading capacity could contribute to mutagenesis during Okazaki fragment processing. In the absence of Pol I DNA polymerase activity, the replicative polymerase is the only candidate to perform lagging strand synthesis. Because of its high fidelity, lower mutation rates are expected. Conversely, over-expression of Pol I can lead to a more extensive processing of Okazaki fragments, therefore increasing the fraction of lagging strand synthesis performed by this enzyme and leading to a higher level of replication error rates.
In conclusion, independently of the relative contributions of Pol I to TLS and lagging strand synthesis, the results presented here strongly support the hypothesis by which in H. pylori the loss of proofreading activity of this DNA polymerase has been selected for increasing genome plasticity.
Materials and Methods
H. pylori strains and growth conditions
All H. pylori strains used were in the 26695 genetic background [15]. To generate gene-specific mutants, the corresponding open-reading frame (ORF) cloned into pILL570 was disrupted, leaving 5′ and 3′ ends (300 bp) of the gene, by a nonpolar kanamycin - (Km), apramycin - (Apr) or chloramphenicol (Cm) resistance cassette [59], [60]. To generate the Pol I over-expressing strain, the HP1470 ORF was inserted into pADC vector, downstream of the ureA promoter, as described [61]. Plasmids were introduced into H. pylori strains by natural transformation and recombinants were selected after 3 to 5 days of growth on either 20 µg/ml Km, 12.5 µg/ml Apr or 8 µg/ml Cm. Allelic replacement was verified by PCR. As described in the Results section, the polA mutants used correspond, unless specified, to the replacement of the equivalent of the Klenow fragment by the resistance cassette, leaving the 5′ to 3′ exonuclease domain intact. Double mutants were obtained by plasmid or genomic DNA transformation of single mutant or by mixing two mutant strains together before plating the mix on double selection. H. pylori cultures were grown at 37°C under a microaerobic atmosphere on BAB, blood agar base medium supplemented with an antibiotic mix and 10% defibrillated horse blood.
Sensitivity and mutagenesis assays
For all experiments, H. pylori strains were initially grown for 24 hr on plates with BAB medium. UV, MMS and gamma irradiation sensitivity assays were performed as described [33], [62]. For chemical oxidative stress treatment, H pylori (OD600 = 1) cell suspensions were incubated with different concentrations of hydrogen peroxide (100, 200 and 300 mM). Cells were washed 10 min later, diluted with peptone broth and plated on BAB plates. Survival was determined as the number of cells forming colonies on plates after a given treatment divided by the number of colonies from non-treated cells. Assays to determine spontaneous mutation rates were performed as described [7].
Activity assays
All assays were performed at 37°C for 30 min in 20 µl reactions containing 10 mM Tris-HCl (pH 7.9), 50 mM NaCl, 10 mM MgCl2, 1 mM Dithiothreitol, 2.5 nM DNA substrate (see Text S1 and Table S1) and variable concentrations of Pol I (specified in the figure legends). 0.1 mM of either all four dNTPs or each dNTP individually was included in the reactions except for the exonuclease activity assays. When required as a control, Klenow fragment DNA polymerase (Roche) was used. Reactions were stopped by adding loading buffer (10 mM EDTA, 95% (v/v) formamide, 0.03% (w/v) bromophenol blue, 0.03% (w/v) xylene cyanol) and subjected to electrophoresis in 8 M urea-containing 20% polyacrylamide gels. Gels were visualised and quantified using a Molecular Dynamics PhosphorImager. According to Koskoska et al. [63], bypass probabilities were calculated as the proportion of DNA synthesis products extended beyond the lesion. Bypass efficiencies were then calculated for each enzyme and each substrate as the ratio of the bypass probability of a specific damaged base with respect to that of an undamaged nucleotide in the same position.
M13mp2 fidelity assay
The assay was performed as described previously [22]. Gap-filling DNA synthesis was performed in a reaction mixture (25 µl) containing 50 mM Tris (pH 6.8), 50 mM NaCl, 10 mM MgCl2, 1 mM DTT, 0.2 mM each of dNTP and 0.2 nM of gapped M13mp2 DNA substrate. Reactions were initiated by adding Pol I, incubated at 37°C for 30 min, and terminated by adding EDTA to 20 mM. When DNA products were analyzed by agarose gel electrophoresis [22], the majority of the gapped molecules were filled to completion. However, a minority of DNA products migrated as if synthesis had paused at the palindrome just upstream of the open reading frame of the LacZ gene. In this minority population, only about 75% of the template used to score errors had been copied. As a consequence, the lacZ mutant frequency observed for the ensemble reaction products may slightly underestimate the error rate of H. pylori Pol I.
Supporting Information
Zdroje
1. SuerbaumSMichettiP 2002 Helicobacter pylori infection. N Engl J Med 347 1175 1186
2. FalushDKraftCTaylorNSCorreaPFoxJG 2001 Recombination and mutation during long-term gastric colonization by Helicobacter pylori: estimates of clock rates, recombination size, and minimal age. Proc Natl Acad Sci U S A 98 15056 15061
3. IsraelDASalamaNKrishnaURiegerUMAthertonJC 2001 Helicobacter pylori genetic diversity within the gastric niche of a single human host. Proc Natl Acad Sci U S A 98 14625 14630
4. KangJBlaserMJ 2006 Bacterial populations as perfect gases: genomic integrity and diversification tensions in Helicobacter pylori. Nat Rev Microbiol 4 826 836
5. TenaillonOToupanceBLe NagardHTaddeiFGodelleB 1999 Mutators, population size, adaptive landscape and the adaptation of asexual populations of bacteria. Genetics 152 485 493
6. BjorkholmBSjolundMFalkPGBergOGEngstrandL 2001 Mutation frequency and biological cost of antibiotic resistance in Helicobacter pylori. Proc Natl Acad Sci U S A 98 14607 14612
7. PintoAVMathieuAMarsinSVeauteXIelpiL 2005 Suppression of homologous and homeologous recombination by the bacterial MutS2 protein. Mol Cell 17 113 120
8. KangJMIovineNMBlaserMJ 2006 A paradigm for direct stress-induced mutation in prokaryotes. FASEB J 20 2476 2485
9. O'RourkeEJChevalierCPintoAVThibergeJMIelpiL 2003 Pathogen DNA as target for host-generated oxidative stress: role for repair of bacterial DNA damage in Helicobacter pylori colonization. Proc Natl Acad Sci U S A 100 2789 2794
10. TippinBPhamPGoodmanMF 2004 Error-prone replication for better or worse. Trends Microbiol 12 288 295
11. KornbergABakerTA 1992 DNA Replication New York Freeman
12. JoyceCMGrindleyND 1984 Method for determining whether a gene of Escherichia coli is essential: application to the polA gene. J Bacteriol 158 636 643
13. OkazakiRArisawaMSuginoA 1971 Slow joining of newly replicated DNA chains in DNA polymerase I-deficient Escherichia coli mutants. Proc Natl Acad Sci U S A 68 2954 2957
14. AlmRALingLSMoirDTKingBLBrownED 1999 Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397 176 180
15. TombJFWhiteOKerlavageARClaytonRASuttonGG 1997 The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388 539 547
16. DerbyshireVFreemontPSSandersonMRBeeseLFriedmanJM 1988 Genetic and crystallographic studies of the 3′,5′-exonucleolytic site of DNA polymerase I. Science 240 199 201
17. DerbyshireVPinsonneaultJKJoyceCM 1995 Structure-function analysis of 3′→5′-exonuclease of DNA polymerases. Methods Enzymol 262 363 385
18. LiuXHouJLiuJ 2006 Chlamydial DNA polymerase I can bypass lesions in vitro. Biochem Biophys Res Commun 345 1083 1091
19. ClarkJMBeardsleyGP 1989 Template length, sequence context, and 3′-5′ exonuclease activity modulate replicative bypass of thymine glycol lesions in vitro. Biochemistry 28 775 779
20. MathieuAO'RourkeEJRadicellaJP 2006 Helicobacter pylori genes involved in avoidance of mutations induced by 8-oxoguanine. J Bacteriol 188 7464 7469
21. JeongJYMukhopadhyayAKAkadaJKDailidieneDHoffmanPS 2001 Roles of FrxA and RdxA nitroreductases of Helicobacter pylori in susceptibility and resistance to metronidazole. J Bacteriol 183 5155 5162
22. BebenekKKunkelTA 1995 Analyzing fidelity of DNA polymerases. Methods Enzymol 262 217 232
23. BebenekKJoyceCMFitzgeraldMPKunkelTA 1990 The fidelity of DNA synthesis catalyzed by derivatives of Escherichia coli DNA polymerase I. J Biol Chem 265 13878 13887
24. EckertKAKunkelTA 1990 High fidelity DNA synthesis by the Thermus aquaticus DNA polymerase. Nucleic Acids Res 18 3739 3744
25. LongleyMJNguyenDKunkelTACopelandWC 2001 The fidelity of human DNA polymerase gamma with and without exonucleolytic proofreading and the p55 accessory subunit. J Biol Chem 276 38555 38562
26. IdeHKowYWWallaceSS 1985 Thymine glycols and urea residues in M13 DNA constitute replicative blocks in vitro. Nucleic Acids Res 13 8035 8052
27. StraussBRabkinSSagherDMooreP 1982 The role of DNA polymerase in base substitution mutagenesis on non-instructional templates. Biochimie 64 829 838
28. GrollmanAPMoriyaM 1993 Mutagenesis by 8-oxoguanine: an enemy within. Trends Genet 9 246 249
29. SuerbaumSJosenhansC 2007 Helicobacter pylori evolution and phenotypic diversification in a changing host. Nat Rev Microbiol 5 441 452
30. BatesHRandallSKRayssiguierCBridgesBAGoodmanMF 1989 Spontaneous and UV-induced mutations in Escherichia coli K-12 strains with altered or absent DNA polymerase I. J Bacteriol 171 2480 2484
31. LindahlT 1993 Instability and decay of the primary structure of DNA [see comments]. Nature 362 709 715
32. DuigouSEhrlichSDNoirotPNoirot-GrosMF 2005 DNA polymerase I acts in translesion synthesis mediated by the Y-polymerases in Bacillus subtilis. Mol Microbiol 57 678 690
33. O'RourkeEJChevalierCBoiteuxSLabigneAIelpiL 2000 A novel 3-methyladenine DNA glycosylase from helicobacter pylori defines a new class within the endonuclease III family of base excision repair glycosylases. J Biol Chem 275 20077 20083
34. EutseyRWangGMaierRJ 2007 Role of a MutY DNA glycosylase in combating oxidative DNA damage in Helicobacter pylori. DNA Repair (Amst) 6 19 26
35. KieferJRMaoCBramanJCBeeseLS 1998 Visualizing DNA replication in a catalytically active Bacillus DNA polymerase crystal. Nature 391 304 307
36. LiYKorolevSWaksmanG 1998 Crystal structures of open and closed forms of binary and ternary complexes of the large fragment of Thermus aquaticus DNA polymerase I: structural basis for nucleotide incorporation. EMBO J 17 7514 7525
37. BellJBEckertKAJoyceCMKunkelTA 1997 Base miscoding and strand misalignment errors by mutator Klenow polymerases with amino acid substitutions at tyrosine 766 in the O helix of the fingers subdomain. J Biol Chem 272 7345 7351
38. LoneSRomanoLJ 2003 Mechanistic insights into replication across from bulky DNA adducts: a mutant polymerase I allows an N-acetyl-2-aminofluorene adduct to be accommodated during DNA synthesis. Biochemistry 42 3826 3834
39. RechkoblitOKolbanovskiyAMalininaLGeacintovNEBroydeS 2010 Mechanism of error-free and semitargeted mutagenic bypass of an aromatic amine lesion by Y-family polymerase Dpo4. Nat Struct Mol Biol 17 379 388
40. WashingtonMTPrakashLPrakashS 2001 Yeast DNA polymerase eta utilizes an induced-fit mechanism of nucleotide incorporation. Cell 107 917 927
41. IdeHPetrulloLAHatahetZWallaceSS 1991 Processing of DNA base damage by DNA polymerases. Dihydrothymine and beta-ureidoisobutyric acid as models for instructive and noninstructive lesions. J Biol Chem 266 1469 1477
42. MatrayTJHaxtonKJGreenbergMM 1995 The effects of the ring fragmentation product of thymidine C5-hydrate on phosphodiesterases and klenow (exo-) fragment. Nucleic Acids Res 23 4642 4648
43. Paz-ElizurTTakeshitaMLivnehZ 1997 Mechanism of bypass synthesis through an abasic site analog by DNA polymerase I. Biochemistry 36 1766 1773
44. SagherDStraussB 1983 Insertion of nucleotides opposite apurinic/apyrimidinic sites in deoxyribonucleic acid during in vitro synthesis: uniqueness of adenine nucleotides. Biochemistry 22 4518 4526
45. HatahetZZhouMReha-KrantzLJIdeHMorricalSW 1999 In vitro selection of sequence contexts which enhance bypass of abasic sites and tetrahydrofuran by T4 DNA polymerase holoenzyme. J Mol Biol 286 1045 1057
46. PurmalAALampmanGWBondJPHatahetZWallaceSS 1998 Enzymatic processing of uracil glycol, a major oxidative product of DNA cytosine. J Biol Chem 273 10026 10035
47. ShibutaniSGrollmanAP 1993 On the mechanism of frameshift (deletion) mutagenesis in vitro. J Biol Chem 268 11703 11710
48. AranaMESekiMWoodRDRogozinIBKunkelTA 2008 Low-fidelity DNA synthesis by human DNA polymerase theta. Nucleic Acids Res 36 3847 3856
49. AranaMETakataKGarcia-DiazMWoodRDKunkelTA 2007 A unique error signature for human DNA polymerase nu. DNA Repair (Amst) 6 213 223
50. SekiMMasutaniCYangLWSchuffertAIwaiS 2004 High-efficiency bypass of DNA damage by human DNA polymerase Q. EMBO J 23 4484 4494
51. FeigDISowersLCLoebLA 1994 Reverse chemical mutagenesis: identification of the mutagenic lesions resulting from reactive oxygen species-mediated damage to DNA. Proc Natl Acad Sci U S A 91 6609 6613
52. KreutzerDAEssigmannJM 1998 Oxidized, deaminated cytosines are a source of C→T transitions in vivo. Proc Natl Acad Sci U S A 95 3578 3582
53. NajranaTSaitoYUrakiFKuboKYamamotoK 2000 Spontaneous and osmium tetroxide-induced mutagenesis in an Escherichia coli strain deficient in both endonuclease III and endonuclease VIII. Mutagenesis 15 121 125
54. PurmalAAKowYWWallaceSS 1994 Major oxidative products of cytosine, 5-hydroxycytosine and 5-hydroxyuracil, exhibit sequence context-dependent mispairing in vitro. Nucleic Acids Res 22 72 78
55. KamiyaH 2003 Mutagenic potentials of damaged nucleic acids produced by reactive oxygen/nitrogen species: approaches using synthetic oligonucleotides and nucleotides: survey and summary. Nucleic Acids Res 31 517 531
56. WallaceSS 2002 Biological consequences of free radical-damaged DNA bases. Free Radic Biol Med 33 1 14
57. Makiela-DzbenskaKJaszczurMBanach-OrlowskaMJonczykPSchaaperRM 2009 Role of Escherichia coli DNA polymerase I in chromosomal DNA replication fidelity. Mol Microbiol 74 1114 1127
58. TagoYImaiMIharaMAtofujiHNagataY 2005 Escherichia coli mutator (Delta)polA is defective in base mismatch correction: the nature of in vivo DNA replication errors. J Mol Biol 351 299 308
59. HeuermannDHaasR 1998 A stable shuttle vector system for efficient genetic complementation of Helicobacter pylori strains by transformation and conjugation. Mol Gen Genet 257 519 528
60. SkouloubrisSThibergeJMLabigneADe ReuseH 1998 The Helicobacter pylori UreI protein is not involved in urease activity but is essential for bacterial survival in vivo. Infect Immun 66 4517 4521
61. KangJHuangSBlaserMJ 2005 Structural and functional divergence of MutS2 from bacterial MutS1 and eukaryotic MSH4-MSH5 homologs. J Bacteriol 187 3528 3537
62. MarsinSMathieuAKortulewskiTGueroisRRadicellaJP 2008 Unveiling novel RecO distant orthologues involved in homologous recombination. PLoS Genet 4 e1000146 doi:10.1371/journal.pgen.1000146
63. KokoskaRJMcCullochSDKunkelTA 2003 The efficiency and specificity of apurinic/apyrimidinic site bypass by human DNA polymerase eta and Sulfolobus solfataricus Dpo4. J Biol Chem 278 50537 50545
Štítky
Genetika Reprodukčná medicína
Článek Local Absence of Secondary Structure Permits Translation of mRNAs that Lack Ribosome-Binding SitesČlánek Independent Chromatin Binding of ARGONAUTE4 and SPT5L/KTF1 Mediates Transcriptional Gene SilencingČlánek Trade-Off between Bile Resistance and Nutritional Competence Drives Diversification in the Mouse GutČlánek FGF Signaling Regulates the Number of Posterior Taste Papillae by Controlling Progenitor Field SizeČlánek Mammalian BTBD12 (SLX4) Protects against Genomic Instability during Mammalian SpermatogenesisČlánek Identification of Nine Novel Loci Associated with White Blood Cell Subtypes in a Japanese PopulationČlánek Differential Effects of and Risk Variants on Association with Diabetic ESRD in African AmericansČlánek Dynamic Chromatin Localization of Sirt6 Shapes Stress- and Aging-Related Transcriptional Networks
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2011 Číslo 6- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Local Absence of Secondary Structure Permits Translation of mRNAs that Lack Ribosome-Binding Sites
- Statistical Inference on the Mechanisms of Genome Evolution
- Revisiting Heterochromatin in Embryonic Stem Cells
- A Two-Stage Meta-Analysis Identifies Several New Loci for Parkinson's Disease
- Identification of a Sudden Cardiac Death Susceptibility Locus at 2q24.2 through Genome-Wide Association in European Ancestry Individuals
- Genomic Prevalence of Heterochromatic H3K9me2 and Transcription Do Not Discriminate Pluripotent from Terminally Differentiated Cells
- Epistasis between Beneficial Mutations and the Phenotype-to-Fitness Map for a ssDNA Virus
- Recurrent Chromosome 16p13.1 Duplications Are a Risk Factor for Aortic Dissections
- Telomere DNA Deficiency Is Associated with Development of Human Embryonic Aneuploidy
- Genome-Wide Association Study of White Blood Cell Count in 16,388 African Americans: the Continental Origins and Genetic Epidemiology Network (COGENT)
- Unexpected Role for DNA Polymerase I As a Source of Genetic Variability
- Transportin-SR Is Required for Proper Splicing of Genes and Plant Immunity
- How Chromatin Is Remodelled during DNA Repair of UV-Induced DNA Damage in
- Independent Chromatin Binding of ARGONAUTE4 and SPT5L/KTF1 Mediates Transcriptional Gene Silencing
- Two Evolutionary Histories in the Genome of Rice: the Roles of Domestication Genes
- Natural Allelic Variation Defines a Role for : Trichome Cell Fate Determination
- Multiple Common Susceptibility Variants near BMP Pathway Loci , , and Explain Part of the Missing Heritability of Colorectal Cancer
- Pathogenic Mechanism of the FIG4 Mutation Responsible for Charcot-Marie-Tooth Disease CMT4J
- A Functional Variant in Promoter Modulates Its Expression and Confers Disease Risk for Systemic Lupus Erythematosus
- Drift and Genome Complexity Revisited
- Chromosomal Macrodomains and Associated Proteins: Implications for DNA Organization and Replication in Gram Negative Bacteria
- Trade-Off between Bile Resistance and Nutritional Competence Drives Diversification in the Mouse Gut
- Pathways of Distinction Analysis: A New Technique for Multi–SNP Analysis of GWAS Data
- Web-Based Genome-Wide Association Study Identifies Two Novel Loci and a Substantial Genetic Component for Parkinson's Disease
- Chk2 and p53 Are Haploinsufficient with Dependent and Independent Functions to Eliminate Cells after Telomere Loss
- Exome Sequencing Identifies Mutations in High Myopia
- Distinct Functional Constraints Partition Sequence Conservation in a -Regulatory Element
- CorE from Is a Copper-Dependent RNA Polymerase Sigma Factor
- A Single Sex Pheromone Receptor Determines Chemical Response Specificity of Sexual Behavior in the Silkmoth
- FGF Signaling Regulates the Number of Posterior Taste Papillae by Controlling Progenitor Field Size
- Maps of Open Chromatin Guide the Functional Follow-Up of Genome-Wide Association Signals: Application to Hematological Traits
- Increased Susceptibility to Cortical Spreading Depression in the Mouse Model of Familial Hemiplegic Migraine Type 2
- Differential Gene Expression and Epiregulation of Alpha Zein Gene Copies in Maize Haplotypes
- Parallel Adaptive Divergence among Geographically Diverse Human Populations
- Genetic Analysis of Genome-Scale Recombination Rate Evolution in House Mice
- Mechanisms for the Evolution of a Derived Function in the Ancestral Glucocorticoid Receptor
- Mammalian BTBD12 (SLX4) Protects against Genomic Instability during Mammalian Spermatogenesis
- Interferon Regulatory Factor 8 Regulates Pathways for Antigen Presentation in Myeloid Cells and during Tuberculosis
- High-Resolution Analysis of Parent-of-Origin Allelic Expression in the Arabidopsis Endosperm
- Specific SKN-1/Nrf Stress Responses to Perturbations in Translation Elongation and Proteasome Activity
- Graded Nodal/Activin Signaling Titrates Conversion of Quantitative Phospho-Smad2 Levels into Qualitative Embryonic Stem Cell Fate Decisions
- Genome-Wide Analysis Reveals PADI4 Cooperates with Elk-1 to Activate Expression in Breast Cancer Cells
- Trait Variation in Yeast Is Defined by Population History
- Meiosis-Specific Loading of the Centromere-Specific Histone CENH3 in
- A Genome-Wide Survey of Imprinted Genes in Rice Seeds Reveals Imprinting Primarily Occurs in the Endosperm
- Multiple Regulatory Mechanisms to Inhibit Untimely Initiation of DNA Replication Are Important for Stable Genome Maintenance
- SIRT1 Promotes N-Myc Oncogenesis through a Positive Feedback Loop Involving the Effects of MKP3 and ERK on N-Myc Protein Stability
- Bacteriophage Crosstalk: Coordination of Prophage Induction by Trans-Acting Antirepressors
- Role of the Single-Stranded DNA–Binding Protein SsbB in Pneumococcal Transformation: Maintenance of a Reservoir for Genetic Plasticity
- Genomic Convergence among ERRα, PROX1, and BMAL1 in the Control of Metabolic Clock Outputs
- Genome-Wide Association of Bipolar Disorder Suggests an Enrichment of Replicable Associations in Regions near Genes
- Identification of Nine Novel Loci Associated with White Blood Cell Subtypes in a Japanese Population
- DNA Ligase III Promotes Alternative Nonhomologous End-Joining during Chromosomal Translocation Formation
- Differential Effects of and Risk Variants on Association with Diabetic ESRD in African Americans
- Finished Genome of the Fungal Wheat Pathogen Reveals Dispensome Structure, Chromosome Plasticity, and Stealth Pathogenesis
- Dynamic Chromatin Localization of Sirt6 Shapes Stress- and Aging-Related Transcriptional Networks
- Extracellular Matrix Dynamics in Hepatocarcinogenesis: a Comparative Proteomics Study of Transgenic and Null Mouse Models
- Integrating 5-Hydroxymethylcytosine into the Epigenomic Landscape of Human Embryonic Stem Cells
- Vive La Différence: An Interview with Catherine Dulac
- Multiple Loci Are Associated with White Blood Cell Phenotypes
- Nuclear Accumulation of Stress Response mRNAs Contributes to the Neurodegeneration Caused by Fragile X Premutation rCGG Repeats
- A New Mutation Affecting FRQ-Less Rhythms in the Circadian System of
- Cryptic Transcription Mediates Repression of Subtelomeric Metal Homeostasis Genes
- A New Isoform of the Histone Demethylase JMJD2A/KDM4A Is Required for Skeletal Muscle Differentiation
- Genetic Determinants of Lipid Traits in Diverse Populations from the Population Architecture using Genomics and Epidemiology (PAGE) Study
- A Genome-Wide RNAi Screen for Factors Involved in Neuronal Specification in
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Recurrent Chromosome 16p13.1 Duplications Are a Risk Factor for Aortic Dissections
- Statistical Inference on the Mechanisms of Genome Evolution
- Genome-Wide Association Study of White Blood Cell Count in 16,388 African Americans: the Continental Origins and Genetic Epidemiology Network (COGENT)
- Chromosomal Macrodomains and Associated Proteins: Implications for DNA Organization and Replication in Gram Negative Bacteria
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



