-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Specification of Corpora Cardiaca Neuroendocrine Cells from Mesoderm Is Regulated by Notch Signaling
Drosophila neuroendocrine cells comprising the corpora cardiaca (CC) are essential for systemic glucose regulation and represent functional orthologues of vertebrate pancreatic α-cells. Although Drosophila CC cells have been regarded as developmental orthologues of pituitary gland, the genetic regulation of CC development is poorly understood. From a genetic screen, we identified multiple novel regulators of CC development, including Notch signaling factors. Our studies demonstrate that the disruption of Notch signaling can lead to the expansion of CC cells. Live imaging demonstrates localized emergence of extra precursor cells as the basis of CC expansion in Notch mutants. Contrary to a recent report, we unexpectedly found that CC cells originate from head mesoderm. We show that Tinman expression in head mesoderm is regulated by Notch signaling and that the combination of Daughterless and Tinman is sufficient for ectopic CC specification in mesoderm. Understanding the cellular, genetic, signaling, and transcriptional basis of CC cell specification and expansion should accelerate discovery of molecular mechanisms regulating ontogeny of organs that control metabolism.
Published in the journal: Specification of Corpora Cardiaca Neuroendocrine Cells from Mesoderm Is Regulated by Notch Signaling. PLoS Genet 7(8): e32767. doi:10.1371/journal.pgen.1002241
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002241Summary
Drosophila neuroendocrine cells comprising the corpora cardiaca (CC) are essential for systemic glucose regulation and represent functional orthologues of vertebrate pancreatic α-cells. Although Drosophila CC cells have been regarded as developmental orthologues of pituitary gland, the genetic regulation of CC development is poorly understood. From a genetic screen, we identified multiple novel regulators of CC development, including Notch signaling factors. Our studies demonstrate that the disruption of Notch signaling can lead to the expansion of CC cells. Live imaging demonstrates localized emergence of extra precursor cells as the basis of CC expansion in Notch mutants. Contrary to a recent report, we unexpectedly found that CC cells originate from head mesoderm. We show that Tinman expression in head mesoderm is regulated by Notch signaling and that the combination of Daughterless and Tinman is sufficient for ectopic CC specification in mesoderm. Understanding the cellular, genetic, signaling, and transcriptional basis of CC cell specification and expansion should accelerate discovery of molecular mechanisms regulating ontogeny of organs that control metabolism.
Introduction
Recent work has revealed multiple features of evolutionary conservation in endocrine regulation of glucose metabolism. For example, in the fruit fly Drosophila melanogaster, insulin-producing cells (IPCs) in the brain and adipokinetic hormone-producing corpora cardiaca (CC) cells in the neuroendocrine ring gland are the respective functional orthologues of mammalian pancreatic β-cells and α-cells [1]–[4]. Insect CC cells resemble neurons in multiple ways; CC cells are peptidergic secretory cells [5] that harbor dense core vesicles [6], and have axon-like projections to vascular, gut and brain targets [3], [4], [7]. Similar to pancreatic islet cells and neuronal cell subsets, CC cells also use KATP channels to regulate AKH secretion [3]. Targeted CC ablation results in marked hypoglycemia [3], [4], demonstrating their role in glucose homeostasis. Thus, the molecular and physiological mechanisms governing CC endocrine function are strikingly similar to those of vertebrate pancreatic islets and neuroendocrine cells.
Despite their crucial role in regulating systemic glucose balance, the embryonic origin of CC cells remains unclear. Based, in part, on their emergence near embryonic foregut, CC cells were initially proposed to originate from a placode in the foregut that produces the stomatogastric nervous system [8]. The CC cell anlage was later inferred to be the most anterior part of mesoderm, based on studies of gene expression in the embryonic head region [9], [10]. Most recently, it was proposed that the CC cells originate from neuroectoderm-derived neuroblasts [11]. This latest study concluded that CC precursors originate from the same placode in which insulin producing neurons are born, and suggested that the developmental relationship between IPC and CC cells may be similar to that of hypothalamus and neuronal pituitary gland. Likewise, while a survey of candidate mutations revealed several genes required for CC development based on ontogenic similarities to pituitary development [9], a systematic, unbiased mutant screen to identify genetic regulators of CC development has not been previously reported.
Here we used genetic screens and gain-of-function studies to investigate specification of CC cell lineage. From a genetic deficiency screen, we discovered that Notch signaling factors are essential regulators of CC development. Our studies demonstrate that Notch signaling controls the number of emerging CC precursor cells. We unexpectedly found that CC cells develop from head mesoderm. Expression of tinman in head mesoderm is regulated by Notch signaling and the combination of tinman and daughterless is sufficient to specify programs leading to ectopic development of CC cell precursors and their AKH+ progeny. Thus our studies reveal genetic and cellular mechanisms underlying precursor specification and expansion of neuroendocrine cells crucial for metabolic homeostasis in Drosophila.
Results
A deficiency screen identifies novel regulators of corpora cardiaca development
To identify regulators of corpora cardiaca development, we screened 292 lines from the DrosDel deficiency collection [12], corresponding to approximately 50% of the genome. We generated strains harboring the akh-RedHStinger (akh-RHS) reporter gene which marks the nuclei of CC cells at embryonic stage 17 (see Materials and Methods). We observed that akh-RHS+ cells were undetectable in 39 deficiency lines, and successfully identified mutations in 18 lines that mapped to 14 genes using publicly available mutant alleles (Table S1). In agreement with the previous study [9], we found that mutations in giant (gt), short gastrulation (sog), sine oculis (so), and glass (gl) prevented embryonic development of akh-RHS+ cells. These findings validated our strategy to screen the DrosDel deficiency collection. In addition, we discovered that mutations in crooked neck (crn), spitz (spi), dimmed (dimm), phyllopod (phyl), double parked (dup), three rows (thr), Polycomblike (Pcl), ETS-domain lacking (edl), and heartless (htl) also result in the complete loss of AKH-expressing cells (Table S1). Thus, our deficiency line screen has revealed new regulators required for CC development.
Corpora cardiaca cell expansion from Notch signaling disruption
In contrast to loss of akh-RHS+ cells in 39 deficiency lines, analysis revealed expansion of akh-RHS+ cells in the Df(3R)ED5942 line. The deficiency in this line included the Delta gene, which encodes an essential conserved activator of Notch signaling. We subsequently confirmed that Delta mutations resulted in the CC cell expansion phenotype observed in Df(3R)ED5942. We detected an average of 14.0±0.8 akh-RHS+ cells in stage 17 control embryos (n = 16; Figure 1A and 1C), while in Delta mutants we detected an average of 110.2±23.7 akh-RHS+ cells (n = 16; Figure 1B and 1C). In situ hybridization and immunostaining revealed expansion of cells expressing akh mRNA (Figure 1E) and AKH protein (Figure 1F) in Delta mutants, demonstrating expanded CC cells in these mutants. Thus, Delta is required for regulating CC cell number.
Fig. 1. Disruption of Notch signaling results in the expansion of CC cells. 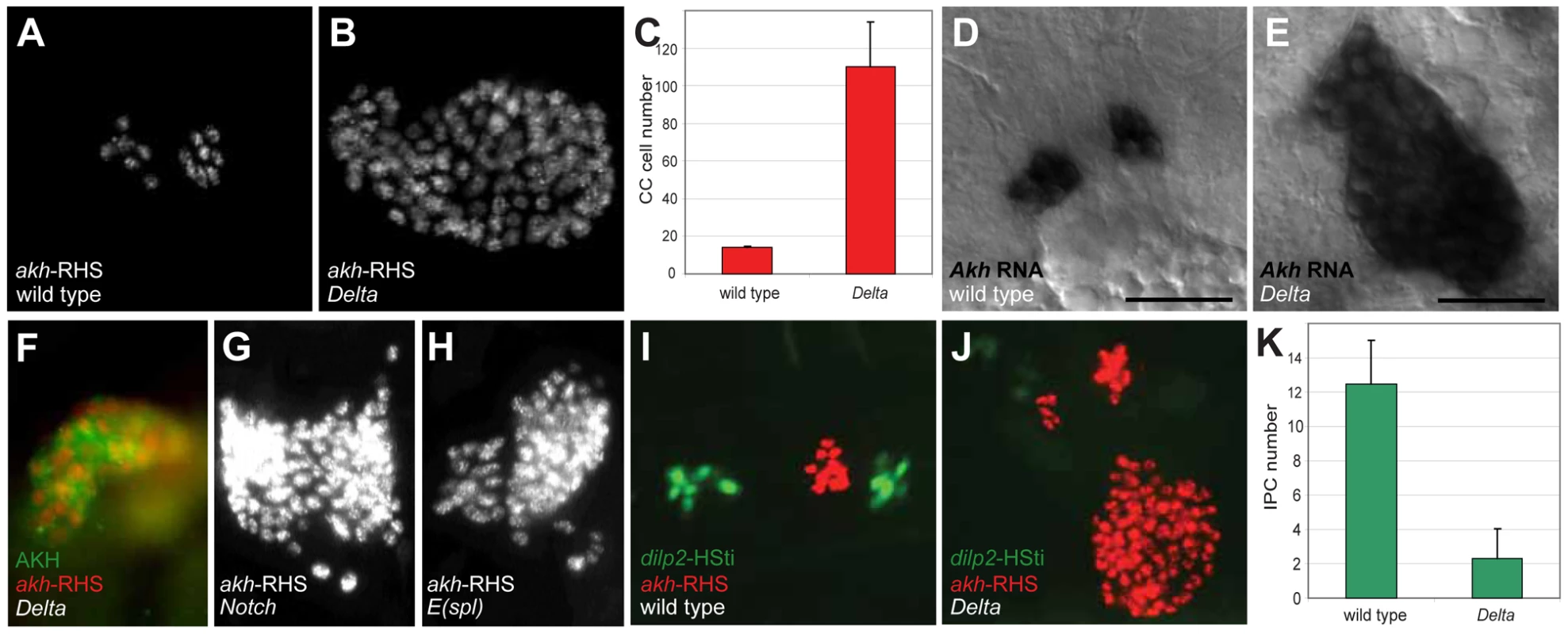
(A) Late stage 17 wild type embryo showing 14 CC cells marked by akh-RHS. (B) Late stage 17 Delta embryo with 93 CC cells marked by akh-RHS. (C) Quantification of CC cells in wild type and Delta mutants. Average CC cells in wild type embryos is 14.0±0.8 (n = 16) while Delta mutants show 110.2±23.7 (n = 16). (D) Akh mRNA in situ hybridization in stage 17 wild type embryo. Scale bar is equal to 10 µm. (E) Akh mRNA in situ in stage 17 Delta mutant embryo shows expanded CC cells. (F) Stage 17 Delta mutant showing expanded CC cells marked by AKH antibody staining (green) and akh-RHS reporter (red). (G–H) Both Notch (G) and E(spl) (H) mutants at stage 17 show CC cell expansion. (I) Stage 17 wild type embryo show 12 IPCs marked by dilp2-HSti reporter (green). (J) Stage 17 Delta mutant embryo exhibits reduced IPCs to 4 cells (pale green). (K) Quantification of IPCs in wild type and Delta mutants. Average IPCs in wild type embryos is 12.5±2.5 (n = 16) while Delta mutants show 2.3±1.7 (n = 16). Where indicated, data represent the mean ± standard deviation. See also Figure S1. To identify additional conserved Notch signaling factors required for CC development, we examined akh-RHS reporter expression in Notch, Enhancer of split (E(spl)), Serrate (Ser), and Suppressor of Hairless (Su(H)) mutant embryos. Notch (Figure 1G) and E(spl) (Figure 1H) mutant embryos had CC cell expansion indistinguishable from that in Delta mutants, while Ser and Su(H) mutants had no detectable change in CC cell number (data not shown). Together, these findings suggest that Notch signaling restrains development of Drosophila CC cells.
A prior study suggested that precursors of CC cells and Drosophila insulin producing cells (IPCs) are adjacent in anterior neuroectoderm [11]. To assess the effect of mutations disrupting Notch signaling on IPC development, we generated a dilp2-HStinger reporter (dilp2-HSti; see Materials and Methods) to mark IPC nuclei and facilitate IPC counting in stage 17 embryos. We detected an average of 12.5±2.5 IPCs (n = 16; Figure 1I and 1K) in control embryos, which was significantly different from the average of 2.3±1.7 IPCs in Delta mutants (n = 16; Figure 1J and 1K). Thus, Delta mutants have CC cell expansion accompanied by IPC hypoplasia, and these distinct outcomes suggest that Notch signaling has distinct roles in regulating developmental programs of CC cells and IPCs.
Delta is required before embryonic stage 11 to restrain corpora cardiaca development
To determine when Delta function is required to restrict CC cell number, we inactivated Delta function at specific embryonic stages using the temperature sensitive DeltaRF allele. During continuous development at 18°C, CC cell number was normal in DeltaRF mutants (13.3±3.1, n = 5; Figure 2B and 2H). However, during development at 29°C, CC cell number quadrupled in DeltaRF embryos (67.2±27.2, n = 13; Figure 2B and 2C), indicating that Delta function was efficiently inactivated at 29°C. Based on these findings, we next used temperature shift from 18°C to 29°C at specific developmental stages in DeltaRF embryos (summarized in Figure 2A). Delta inactivation at 7 or 8 hours after egg lay (hAEL) resulted in CC cell expansion (79.3±20.9, n = 11 and 74.3±19, n = 30, respectively; Figure 2D). By contrast, lesser CC cell expansion resulted from temperature shift to 29°C at 9 hAEL (59.2±17.6, n = 18; Figure 2E) or 10 hAEL (21.3±11.1, n = 7; Figure 2F). Shift from 18°C to 29°C at 11 hAEL (corresponding to embryonic stage 11) or thereafter produced CC cell numbers indistinguishable from those observed during continuous development at 18°C (Figure 2B and 2G). These results suggest that Delta function is essential for restricting CC cell number before stage 11. To better define better the period when Delta restricts CC cell number, we also performed temperature ‘down-shift’ studies at specific stages during DeltaRF embryonic development. When the temperature was shifted down from 29°C to 18°C at stage 10, CC cells were not expanded, although their position appeared to be more anterior (Figure S1E). However, temperature shift to 18°C at early stage 11 or thereafter led to CC cell expansion (Figure S1F–S1H). Together, our up - and down-shift experiments suggest that Delta is required in a brief period from the end of embryonic stage 10 to the beginning of stage 11 to regulate CC cell number, but may be dispensable before or after.
Fig. 2. Delta regulates CC cell number before embryonic stage 11. 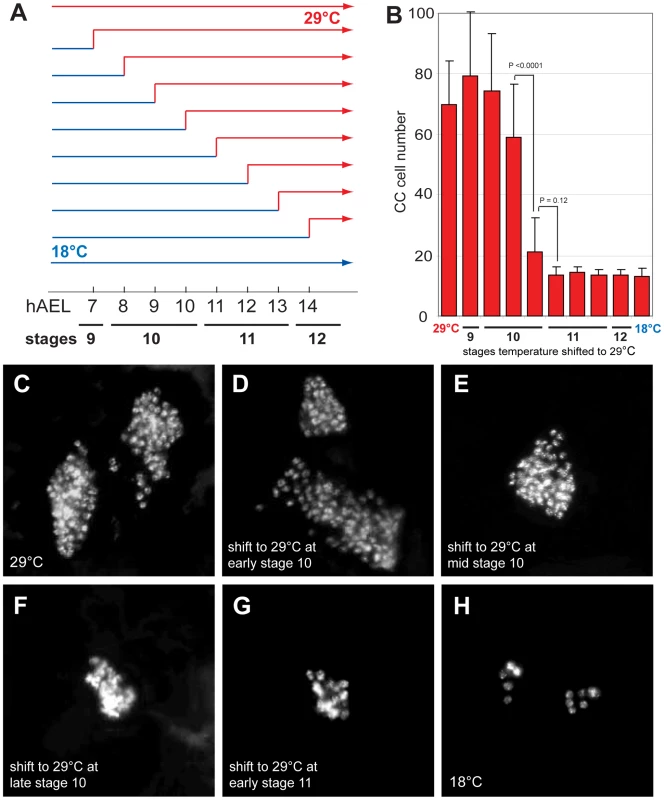
(A) Temperature shift conditions applied to DeltaRF mutants at different time points following 1-hour egg lay. hAEL is hours after egg lay. (B) CC cell number quantification in DeltaRF mutants resulting from the different temperature shift conditions shown in (A). (C) DeltaRF mutant grown at 29°C exhibits akh-RHS+ CC cell expansion. (D) DeltaRF mutant grown at 18°C for 8 hours followed by a shift to 29°C until stage 17 shows a similar CC cell expansion. (E) DeltaRF mutant shifted from 18 to 29°C at 9 hAEL showing moderate CC cell expansion. (F) DeltaRF mutant with a temperature shift at 10 hAEL shows a slight increase in CC cell number. (G) DeltaRF mutant with a temperature shift at 11 hAEL exhibits normal CC cell number. (H) DeltaRF mutant grown at 18°C shows normal CC cell number. Error bars are ± the standard deviation of the mean. The emergence of multiple Glass+ CC precursors in Notch mutants
The earliest known CC cell lineage marker glass is detected at embryonic stage 11 [9]; thus, we postulated that the requirement for Delta prior to this stage indicated that Notch signaling specifies the number of Glass+ CC precursors. Glass protein is first detected in AKHneg CC cell precursors, a pair of single cells emerging near the dorsal head midline at early stage 11 (red arrowheads in Figure 3B) [11]. Between stages 11 and 13, the number of Glass+ CC precursors increases to 14–16 cells (comprised of two clusters of 7–8 cells; Figure 5A) that migrate posteriorly to become AKH+ CC cells [11]. To investigate the basis of CC cell expansion in Delta, Notch, and E(spl) mutants, we first examined the emergence of Glass+ CC precursors near the head midline. At stage 10, in both wild type and Notch mutant embryos, no Glass+ CC precursors were detectable (Figure 3A and 3E). Thus, the CC cell lineage did not develop precociously in Notch mutants. In early stage 11 (see Materials and Methods), the first pair of midline Glass+ CC precursors emerged in wild type embryos (red arrowheads in Figure 3B). In contrast, up to 6 Glass+ CC precursors were detectable in Notch mutants at early stage 11 (white arrowhead and insert in Figure 3F). We observed variant increases in left and right groups of Glass+ CC precursors at this stage (2 cells indicated by red arrowhead in Figure 3F). In mid stage 11, a pair of Glass+ CC precursors remained as single cells in wild type embryo (red arrowheads in Figure 3C) while clusters of 4–6 Glass+ CC precursors were detectable in Notch mutants (white and red arrowheads in Figure 3G). By late stage 11 in wild type embryo, CC precursors commenced division to increase the number of Glass+ cells from 1 to 2 (white arrowhead and insert in Figure 3D). Likewise, the number of Glass+ CC precursors increased from 6–7 to an average of 14 cells in late stage 11 Notch mutant embryos (18 cells indicated by white arrowhead and insert in Figure 3H). These findings suggest that the increase of AKH-expressing CC cells found in Delta, Notch and E(spl) mutants reflects emergence of extra Glass+ CC precursors at early stage 11.
Fig. 3. The emergence of multiple Glass+ CC precursors in Notch mutants. 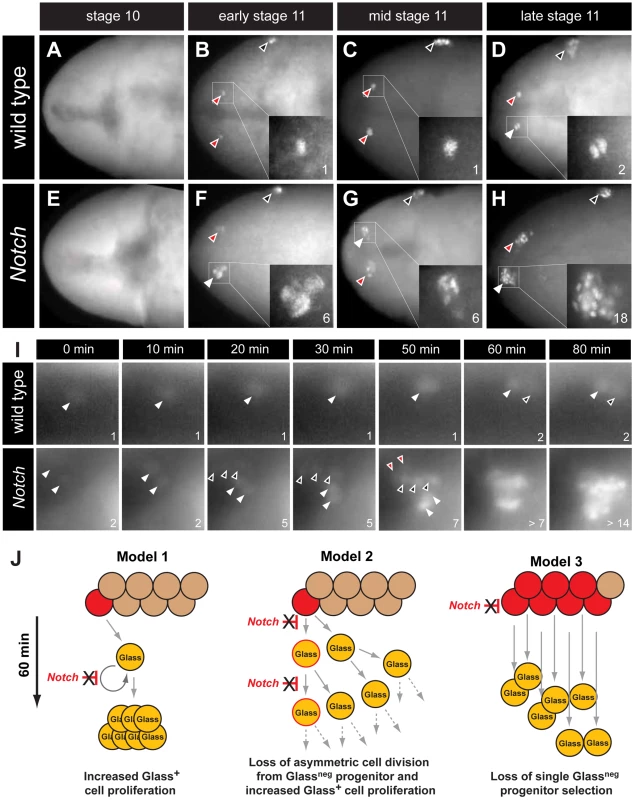
(A–D) CC precursor development in wild type embryo during embryonic stages 10 and 11. Early, mid, and late embryonic stages 11 are determined by Bolwig's organ precursor number (black arrowheads, Material and Methods). (A) Glass expression is not detected in stage 10 wild type embryo. Glass+ CC precursors increase from single cell in early-mid stage 11 embryo (red arrowheads and inserts in B and C) to 2 cells in late stage 11 embryo (white arrowhead and insert in D). (E–H) Glass+ CC precursors are expanded in Notch mutant embryos during embryonic stage 11. (E) Glass expression is not detected in stage 10 Notch mutant embryo. (F) Notch mutant at early stage 11 showing 2 (red arrowhead) or 6 (white arrowhead and insert) Glass+ CC precursors. (G) Notch mutant at mid stage 11 showing 4 (red arrowhead) or 6 (white arrowhead and insert) Glass+ CC precursors. (H) Notch mutant at late stage 11 showing 18 (white arrowhead and insert) Glass+ CC precursors. All embryos are dorsal views with anterior to the left. (I) Glass+ CC precursors in live wild type and Notch mutant embryos are identified by glass5.2-RHS expression. The time at which the first precursor is detected is set to 0 minutes. The number of cells counted is labeled in the lower right corner of each image. In wild type, the first CC precursor (white arrowhead) divides at 60 minutes as shown by the emergence of the second precursor (black arrowhead). In Notch mutants, two CC precursors first emerge (white arrowheads) at 0 minutes. After 20 minutes, three precursors (back arrowheads) appear without apparent cell division. Two additional precursors (red arrowheads) arise at 50 minutes. The older precursors begin to divide at 60 minutes, indicating that in both the wild type and Notch mutants the rate of CC precursor cell division is 60 minutes/division (See also Videos S1 and S2). (J) Models for Glass+ CC precursor expansion in Notch mutants. Model 1 depicts the possibility that Notch signaling regulates proliferation of Glass+ CC precursors. In Notch mutants, the speed of this proliferation is increased, resulting in appearance of multiple Glass+ CC precursors. Model 2 depicts that Notch signaling normally regulates asymmetric cell division of a Glassneg CC progenitor (red), resulting in one Glassneg progenitor and one Glass+ CC precursor followed by subsequent proliferation of the Glass+ daughter. In Notch mutants, both daughter cells become Glass+ CC precursors followed by additional rapid symmetric divisions. Model 3 depicts the possibility that Notch signaling restricts the development of Glassneg CC progenitors. In Notch mutants, increased numbers of Glassneg CC progenitors (red) produce multiple Glass+ cells that are CC precursors. Our analysis of static images did not preclude that a single Glassneg progenitor or Glass+ CC precursor might continuously proliferate in early stage 11 to produce an expanded number of Glass+ CC precursors (Figure 3J, models 1 and 2). To evaluate this possibility, we continuously imaged live embryos expressing a glass5.2-RedHStinger reporter (glass5.2-RHS) with fluorescence microscopy (Video S1). Nuclear localized fluorescent protein produced from this reporter permitted detection and counting of emerging glass-expressing CC precursors in early stage 11 wild type embryos (Figure 3I ‘wild type’, white arrowhead at t = 0 minutes). The signal intensity of the glass5.2-RHS reporter continuously increased until late stage 11 when the CC precursor divided to produce two adjacent progeny cells with equivalent reporter emission intensity (Figure 3I, t = 60 and 80 minutes). In Notch mutants, we observed a different sequence of cell appearance and reporter labeling (Video S2). Two glass5.2-RHS+ cells initially emerged (Figure 3I ‘Notch’, white arrowheads at t = 0 minutes). 20 minutes later, three additional glass5.2-RHS+ cells appeared (Figure 3I, black arrowheads at t = 20 minutes in panels labeled ‘Notch’). The three glass5.2-RHS+ cells appearing at this later time are not adjacent to the first two glass5.2-RHS+ cells. The emission intensity of these ‘new’ cells is fainter than that of the initial two cells. Thus, it is unlikely these new cells which appeared within 10 minutes represent progeny of the first two glass5.2-RHS+ cells. At 50 minutes, two additional glass5.2-RHS+ cells appeared (Figure 3I ‘Notch’, red arrowheads at t = 50 minutes), resulting in seven CC precursors. As in wild type embryos, CC precursor division begins at 60 minutes, and by 80 minutes the number of glass5.2-RHS+ cells in the Notch mutant was doubled. The number and density of glass5.2-RHS+ cells in the Notch mutant precluded further imaging and analysis. Thus, we did not detect accelerated proliferation by the first CC precursors appearing in Notch mutants. Rather, these data suggest that emergence of excess Glass+ CC precursors from Glassneg progenitors is the basis for CC cell expansion following disruption of Notch signaling (Figure 3J, model 3).
Corpora cardiaca precursors originate from head mesoderm
A recent study suggested CC cells develop from neuroectoderm [11] (site marked ‘2’ in Figure 5G), based on immunohistochemical detection of Glass in a subset of ectodermal cells labeled by a giant1-lacZ reporter (gt1-lacZ) [13]. With the goals of confirming this developmental origin, and controlling Notch signaling in progenitors of the CC cell lineage, we generated a gt1-GAL4 transgenic line (with an enhancer identical to the reported gt1-lacZ construct; see Materials and Methods). Anterior head expression of β-galactosidase (β-gal) in our gt1-GAL4; UAS-lacZ.NLS embryos (Figure S2D) was identical to the expression of gt1-lacZ expression reported previously (Figure S2A) [11]. However, the cytoplasmic β-gal signal from gt1-lacZ appeared diffuse, and was difficult to discern at single cell resolution (Figure S2A). Nuclear β-gal expression in gt1-GAL4; UAS-lacZ.NLS marked several cells near the Glass+ CC precursors (Figure 4A), but to our surprise we did not detect nuclear β-gal in Glass+ CC cell precursors in stage 11 embryos (Figure 4D). gt1-GAL4 cell lineage marking using FLP-recombinase (see Materials and Methods) traced gt1-GAL4 expression to third instar larval IPCs marked by the dilp2-HSti reporter (arrowheads in Figure 4H). This result is consistent with the reported origin of IPCs from gt1-lacZ expressing cells [11], and validates use of gt1-GAL4 for lineage tracing. However, gt1-GAL4 cell lineage marking did not trace to larval CC cells expressing the akh-RHS reporter (red in Figure 4H), showing that CC cells do not originate from gt1-expressing head neuroectoderm.
Fig. 4. Copora cardiaca precursors originate from head mesoderm. 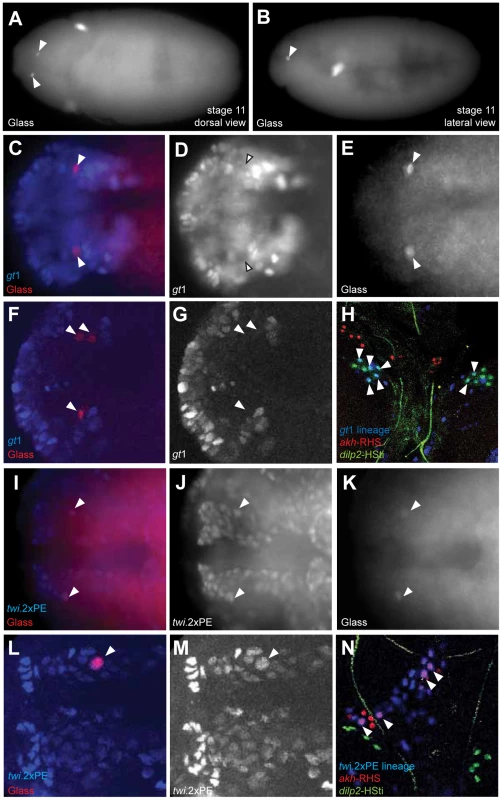
(A–B) Locations of Glass+ CC precursor in stage 11 embryos in the dorsal view (A) and the lateral view (B). (C–E) gt1-GAL4; UAS-lacZ.NZ embryo expresses β-gal in anterior head neuroectoderm. gt1-GAL4 is expressed in the anterior head and several cells posterior to Glass+ CC precursors. gt1-GAL4 does not express in CC precursors as shown by the arrowheads. (F, G) Single confocal plane image of gt1-GAL4; UAS-lacZ.NZ embryonic head region stained for β-gal. Glass+ CC precursors (F, arrowheads) are not co-localized with gt1-GAL4 expression. (H) Lineage tracing of gt1-GAL4+ cells in third instar larval IPC and CC cells. IPC and CC cells are marked by dilp2-HSti (green) and akh-RHS (red) respectively. β-gal expression (blue) from gt1-GAL4; UAS-FLP; Act5C(FRT.polyA)lacZ.nls1 is co-localized with a subset of IPCs (green), resulting in IPCs with cyan (arrowheads). CC cells (red) are not labeled by gt1-GAL4 lineage tracing, and therefore do not show any cells in magenta. (I–K) Glass+ CC precursors in stage 11 embryo (arrowheads) are a part of the dorsal mesoderm marked by twi.2×PE-GAL4; UAS-lacZ.NZ. (I) Merged image shows that Glass+ cells are located in outer part of the head mesoderm. (J) β-gal expression marks the dorsal head mesoderm. (L, M) Single confocal plane image of twi.2×PE-GAL4 (M, β-gal) expression in a stage 11 embryo showing Glass+ CC precursor (L, arrowhead) originates from head mesoderm. (N) Lineage tracing of twi.2×PE-GAL4 expressing cells in third instar larval CC cells. Lineage was traced by β-gal expression (blue) in twi.2×PE-GAL4; UAS-FLP; Act5C(FRT.polyA)lacZ.nls1 larvae. twi.2×PE-GAL4 lineage-traced in several CC cells (magenta with arrowhead), but not in IPCs (green). All embryo images except (B) are stage 11 dorsal views with anterior to the left. Based on expression and mutant phenotype analysis of genes that expressed in embryonic head, De Velasco et al [9], [10] suggested that CC cells originate from cells adjacent to the anterior ventral furrow (site marked ‘1’ in Figure 5G). To test if CC cells derive from twist-expressing mesoderm cells at this anterior junction between embryonic endoderm and mesoderm, we used the twi.2×PE-GAL4 line to label progeny of 12–14 ventral most mesodermal cells, as previously described [14]. Nuclei of the mesodermal cells and their progeny were labeled with β-gal through stage 11 in twi.2×PE-GAL4; UAS-lacZ.NLS embryos (Figure 4J). A subset of these β-gal+ mesodermal progeny co-expressed Glass (Figure 4I). Thus, ventral twist-expressing mesodermal cells invaginate and migrate toward the dorsal midline where Glass+ CC precursors are specified (blue domain in Figure 5G). In third instar larvae, lineage tracing of twi.2×PE-GAL4+ cells using FLP-recombinase revealed nuclear localization of β-gal in the majority of akh-RHS+ CC cells (arrowheads in Figure 4N). By contrast, IPCs were always β-galneg (green nuclei in Figure 4N). We also used Mef2-GAL4 line to trace embryonic and larval lineages derived from all muscle lineages beginning at stage 7 embryos [15] (purple domain in Figure 5G). Similar to our findings with twi.2×PE-GAL4, we observed labeling of Glass+ CC precursors with Mef2-GAL4; UAS-lacZ.NLS at stage 11 (Figure S3A–S3C), and labeling of mature larval AKH+ CC cells with Mef2 lineage tracing (arrowheads in Figure S3D). These results demonstrate that Glass+ CC precursors originate from head mesoderm, and that IPC and CC cells derive from distinct germ layers in Drosophila.
Fig. 5. Ectopic activation of Notch signaling prior to Glass expression in head mesoderm disrupts CC precursor development. 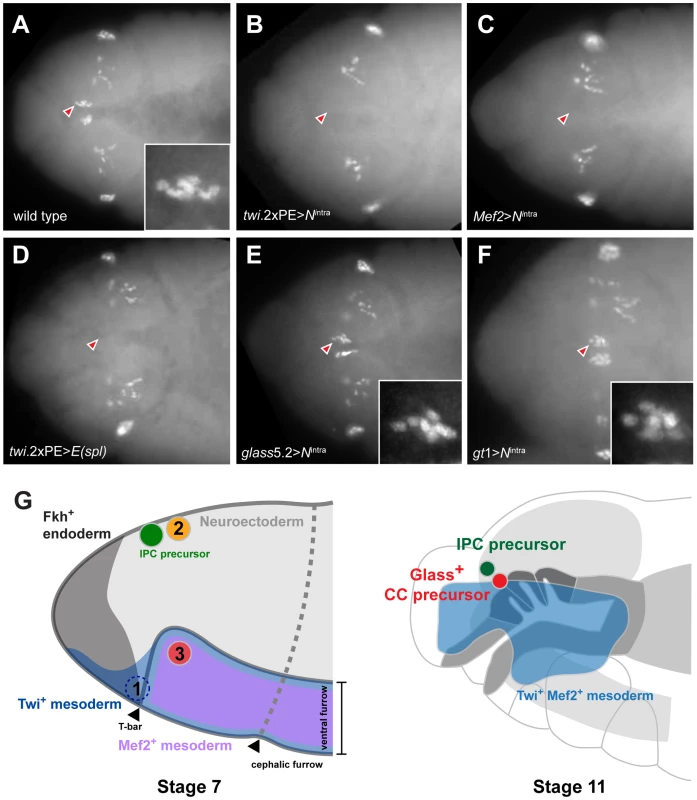
(A) Wild type embryo shows two clusters of Glass+ CC precursors, each one with 6 precursors (arrowhead and insert) in stage 12 embryo. Other Glass+ cells are larval eye precursors and brain primordia. (B) Activation of Notch signaling in mesoderm by twi.2×PE-GAL4; UAS-Nintra removes the CC precursors (arrowhead) in stage 12 embryo. (C) Activation of Notch signaling in muscle lineage by Mef2-GAL4; UAS-Nintra removes the CC precursors (arrowhead) in stage 12 embryo. (D) Ectopic expression of bHLH repressor E(spl) in mesoderm by twi.2×PE-GAL4; UAS-E(spl) also removes Glass+ CC precursors (arrowhead) in stage 12 embryo. (E) Activation of Notch signaling after Glass+ expression initiates in CC cell lineage does not perturb their development. glass5.2-GAL4; UAS-Nintra embryo in stage 12 maintains normal number of Glass+ CC precursors (arrowhead and insert show 6 cells). (F) Activation of Notch signaling in neuroectoderm by gt1-GAL4; UAS-Nintra does not disrupt the development of CC precursors (arrowhead and insert show 6 cells) in stage 12 embryo. All embryo images are dorsal views with anterior to the left. (G) Relative locations of CC and IPC precursors at stage 7 and 11 embryos. Embryos are drawn in the lateral view with anterior to the left. (1) twi+ gt+ cells, located in front of ventral furrow were proposed as an origin of CC cells by De Velasco et al [9], [10]. (2) Neighboring cells from the anterior neuroectoderm were proposed as origins for IPC and CC cells by Wang et al [11]. (3) The origin of CC cells identified by lineage tracing from Twist and Mef2 expressing head mesoderm in this study. By stage 11, Twi+ Mef2+ mesoderm has generated Glass+ CC precursors that are located near IPC precursors (green) and the endodermal foregut invagination (outlined by dark grey), where cells comprising the stomatogastric nervous system are born. To test this conclusion further, we asked if impaired CC development resulted from Notch signaling disruption in head mesoderm that expressed twist or Mef2. Based on our disruption of Notch signaling using loss-of-function or conditional mutations, we postulated that head mesodermal expression of the Nintra allele, which encodes a constitutively activate form of Notch [16], or E(spl) prior to stage 11 should reduce or eliminate development of embryonic stage 12 Glass+ CC precursors. By contrast, Notch signaling activation after formation of Glass+ CC precursors should not impair subsequent CC development. In twi.2×PE-GAL4; UAS-Nintra embryos and in Mef2-GAL4; UAS - Nintra embryos, we failed to detect Glass+ CC precursors (Figure 5B and 5C), confirming that CC cells originate from mesoderm that expresses twist and Mef2. Likewise, we observed elimination of Glass+ CC precursors in stage 12 twi.2×PE-GAL4, UAS-E(spl) embryos (Figure 5D). In contrast to these results, the number of Glass+ CC precursors at stage 12 was not detectably altered in glass5.2-GAL4; UAS-Nintra embryos compared to control embryos (Figure 5E). Thus, consistent with our studies of the conditional DeltaRF mutants, these results indicate that Notch signaling may be dispensable after glass-expressing CC precursors are established at the early stage 11. To test if activation of Notch signaling in neuroectoderm affects CC development in the adjacent mesoderm, Nintra was expressed in head neuroectoderm by gt1-GAL4. The number of Glass+ CC precursors at stage 12 was not altered in gt1-GAL4; UAS-Nintra embryos (Figure 5F), suggesting that Glass+ CC precursors develop independently of Notch signaling in neuroectoderm. Taken together, these results argue that CC cells originate from head mesoderm.
daughterless and tinman are required for CC cell development
During trunk mesoderm development, bHLH transcription factors, encoded by daughterless (da) and twist (twi), are necessary for the allocation of mesodermal cells to specific fates [17]–[19]. Prior study showed Twist is required for CC development [9], but it was not known if daughterless or specific Twist targets were required for CC development. Thus, we assessed requirements for Daughterless and Twist targets in CC cell development from head mesoderm. In late stage 12 wild type embryos, two groups of 6–7 Glass+ CC precursors are detectable near the dorsal midline (red arrowhead in Figure 6A). In stage 12 mutants lacking daughterless or twist, these Glass+ precursors are absent (red arrowheads in Figure 6B, 6C). Twist regulates expression of several transcription factors required for trunk mesoderm differentiation, including Zn finger homeodomain 1 (zfh1), Myocyte enhancer factor 2 (Mef2), held out wings (how), and tinman (tin) [20]–[25]. To test if these transcription factors are also required for CC development, we assessed CC precursor development in mutant embryos. Normal numbers of CC precursors were detected in embryos harboring mutations in zfh1, Mef2, and how (data not shown). By contrast, Glass+ CC precursors were not detected in stage 12 embryos with mutations in tinman (red arrowheads, Figure 6D). Since proneural genes are required for stomatogastric nerve cell precursor formation [8] and specification of muscle progenitors [26], we also tested mutant embryos deficient for the genes achaete, scute, lethal of scute, and asense, which encode proneural bHLH factors. However, the number of CC precursors was not altered in mutant embryos (Figure 6E), suggesting these proneural genes are not required for CC development. Thus, our mutant analysis revealed a specific requirement for twist, daughterless, and tinman transcription factors during CC precursor specification from head mesoderm.
Fig. 6. Tinman expression in head mesoderm is regulated by Notch signaling. 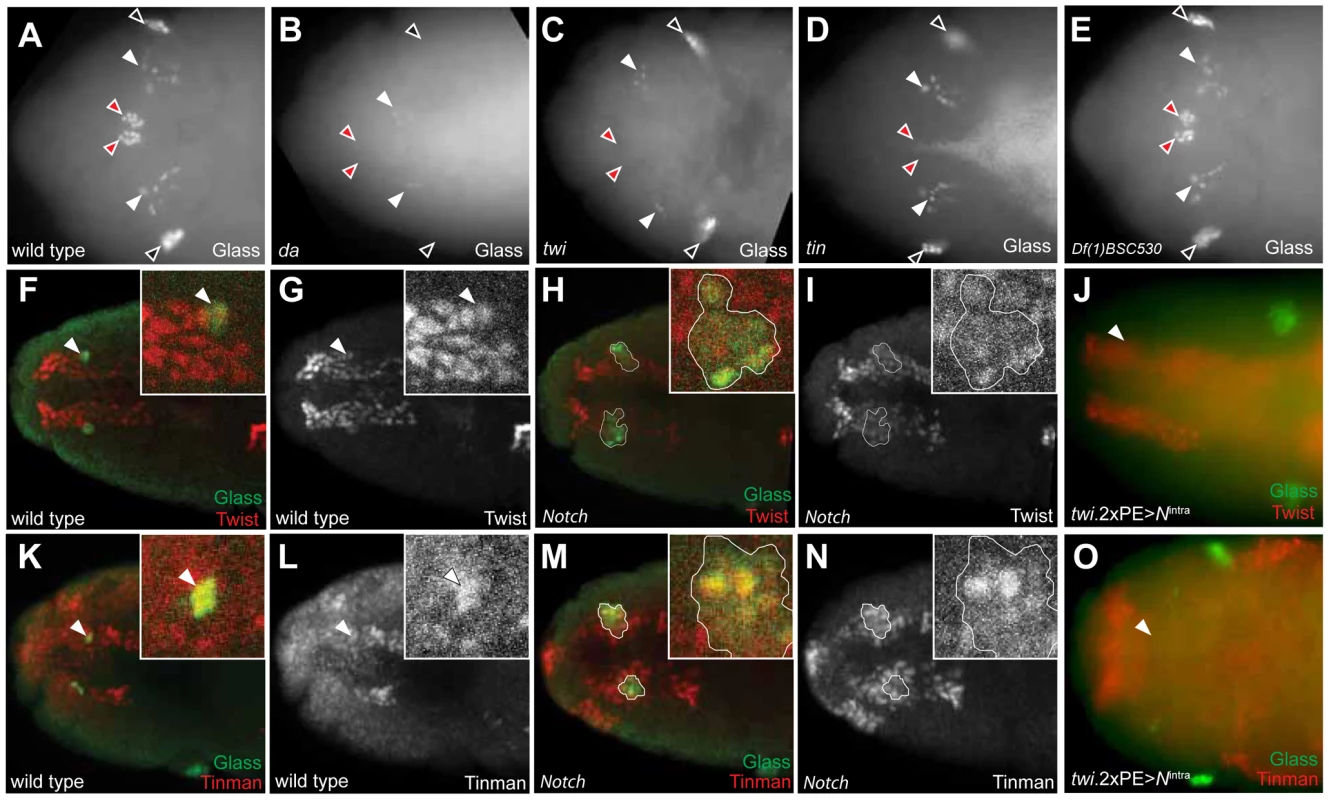
(A) Glass expression in stage 12 wild type embryonic head region. CC precursors are located in the dorsal midline (red arrowheads). Black arrowheads indicate Glass+ larval eye precursors while white arrowheads indicate Glass+ brain primordia. (B) In da mutant at stage 12, both CC (red arrowheads) and eye precursors (black arrowheads) are missing, but Glass expression in the brain primordia (white arrowheads) is maintained. (C) In twi mutant at stage 12, CC precursors are missing in the dorsal midline (red arrowheads) while brain (white arrowheads) and eye precursors (black arrowheads) are intact. (D) In tin mutant at stage 12, CC precursors are missing in the dorsal midline (red arrowheads). (E) In stage12 Df(1)BSC530 mutant in which proneural genes are removed, CC precursors (red arrowheads) are intact. (F, G) Dorsal view of stage 11 wild type embryonic head shows Glass+ CC precursors (arrowhead and green in insert of F) co-localized with Twist+ cells (red). (H, I) Dorsal view of stage 11 Notch mutant shows multiple Glass+ CC precursors (outlined and green in insert) co-localized with Twist+ cells (red). (J) Twi expression in head mesoderm is maintained in stage 11 embryo when Notch signaling is activated in mesoderm by twi.2×PE-GAL4; UAS-Nintra. (K, L) Dorsal view of stage 11 wild type embryonic head shows Glass+ CC precursors (arrowhead and green in K) co-localized with Tinman+ cells (red). (M, N) Dorsal view of stage 11 Notch mutant shows multiple Glass+ CC precursors (outlined and green in insert) co-localized with Tinman+ cells (red). (O) Both Glass and Tinman expression in head mesoderm is abolished at stage 11 embryo when Notch signaling is activated in mesoderm by twi.2×PE-GAL4; UAS-Nintra. All embryo images are dorsal views with anterior to the left. Tinman expression in head mesoderm is regulated by Notch signaling
We first postulated that regulation of twist expression by Notch signaling in head mesoderm, like in trunk mesoderm [27], might underlie CC cell expansion in Notch mutant flies. In wild type embryos, Twist expression by immunostaining was restricted to head mesoderm, and Glass+ CC precursors co-expressed Twist (inserts and arrowheads in Figure 6F, 6G). In stage 11 Notch mutants, we observed that two clusters of multiple Glass+ CC precursors co-localized with these Twist+ cells (one cluster enlarged in Figure 6H, 6I), indicating that the multiple Glass+ CC precursors in Notch mutants originate also from Twist+ head mesoderm. To test whether head mesodermal Twist expression may be regulated by Notch signaling, we asked if the ectopic activation of Notch signaling in head mesoderm in stage 11 twi.2×PE-GAL4; UAS-Nintra embryos results in loss or reduction of Twist expression. A normal pattern of Twist expression in head mesoderm was observed in these embryos but Glass+ CC precursors were absent (arrowhead in Figure 6J), providing additional evidence that the level of Twist expression in head mesoderm may not be regulated by Notch signaling.
Tinman expression is restricted to the anterior dorsal region of head mesoderm in stage 11 wild type embryos (Figure 6L for dorsal view and Figure S4C for lateral view), and Tinman+ head mesodermal cells at stage 11 include Glass+ CC precursors (arrowheads and enlarged in Figure 6K, 6L, and Figure S4A). In stage 12 embryos, Tinman expression in Glass+ CC precursors was extinguished, while adjacent Glassneg cells - which include the procephalic vascular rudiment [10] - maintained Tinman expression (Figure S4E–S4H). In Notch mutants, the number of Glass+ Tinman+ CC precursors in head mesoderm increased (outlined in Figure 6M and 6N). In addition, Glassneg Tinman+ cells adjacent to Glass+ CC precursors also appear expanded (brackets in Figure S4J and S4L), suggesting that Tinman expression in head mesoderm may be regulated by Notch signaling, To test this possibility, we asked if ectopic Notch signaling activation in head mesoderm resulted in loss of Tinman expression. Expression of both Glass and Tinman was abolished in the head mesoderm of twi.2×PE-GAL4; UAS-Nintra embryos (arrowhead in Figure 6O). Together, these results show that Tinman expression is regulated by Notch signaling in head mesoderm, and suggest the possibility that tinman mis-expression in this context underlies CC lineage expansion in Notch signaling disruption.
Co-expression of Tinman and Daughterless in mesoderm is sufficient for CC cell lineage specification
Since expanded Tinman expression in Notch mutant head mesoderm accompanied CC lineage expansion, we investigated if ectopic expression of tinman might be sufficient to expand CC cells. However, in twi.2×PE-GAL4 UAS-tinman embryos, the population of Glass+ CC precursors was not expanded (Figure 7A), suggesting that additional factors may be required to specify the CC cell lineage in head mesoderm. During trunk mesoderm differentiation, Twist activity is inhibited by its dimerization partner Daughterless to allocate mesodermal cells to various tissue fates [19]. Therefore, we next investigated effects of mis-expressing daughterless or twist in mesoderm. In twi.2×PE-GAL4 UAS-daughterless embryos, Glass+ CC precursors do not increase in head mesoderm (Figure 7B), although we reproducibly observed appearance of ectopic Glass+ cells in the trunk of these embryos (black arrowhead in Figure 7B). In contrast, Glass+ CC precursors were absent in twi.2×PE-GAL4 UAS-twist embryos (Figure 7C), suggesting that CC precursor specification is inhibited by excessive Twist activity. Ectopic CC cell development from mis-expression of daughterless or tinman in head mesoderm was also eliminated by co-expression of twist (Figure 7D, 7E), supporting the view that excess Twist activity can suppress CC development.
Fig. 7. Co-expression of Daughterless and Tinman in mesoderm is sufficient for ectopic CC cell lineage specification in mesoderm. 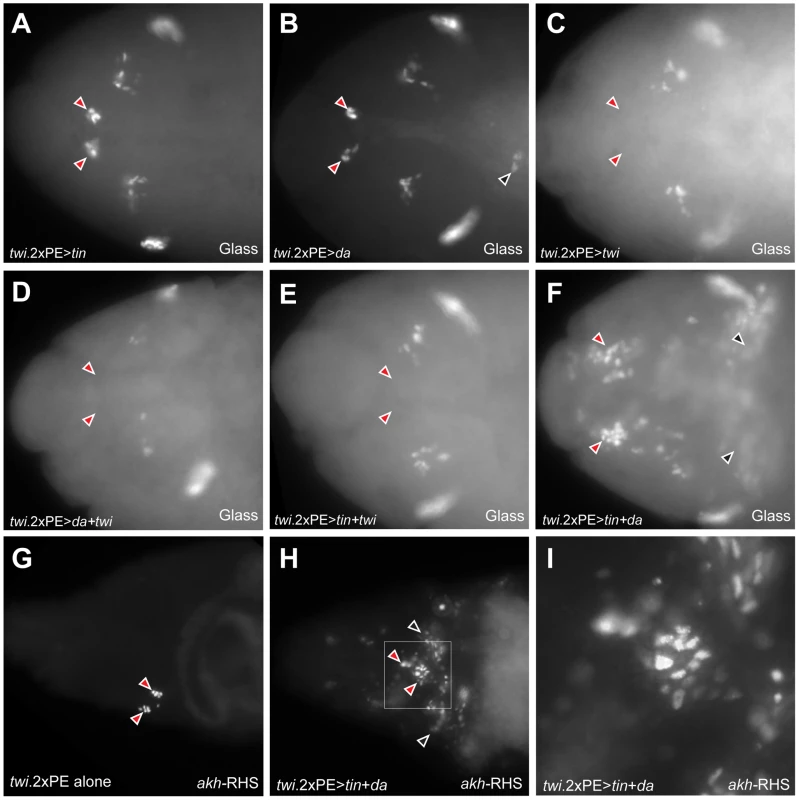
(A) Glass+ CC precursors are not expanded (red arrowheads) in stage 12 twi.2×PE-GAL4 UAS-tinman embryo. (B) CC precursors (red arrowheads) are developed normally in stage 12 twi.2×PE-GAL4 UAS-daughterless embryos. Ectopic Glass expressing cells in trunk region is marked by black arrowhead. (C–E) CC precursors are absent (read arrowheads) when twist is over-expressed alone (C) or twist is co-expressed with daughterless (D) or tinman (E) in head mesoderm. (F) Glass+ CC precursors in head mesoderm are expanded in stage 12 twi.2×PE-GAL4 UAS-tinman UAS-daughterless embryo (red arrowheads). Ectopic Glass expressing cells are also detected in trunk region (black arrowheads). (G) CC cells are marked by akh-RHS expression in stage 17 control embryo. (H) Expansion of CC cells (red arrowheads) and ectopic CC cells in trunk region (black arrowheads) are detected in stage 17 twi.2×PE-GAL4 UAS-tinman UAS-daughterless embryo. (I) Magnified view of a box marked in (H) to show the expansion of akh-RHS expressing CC cells. All embryo images are dorsal views with anterior to the left. Daughterless protein contains a repression domain, and can heterodimerize with Twist to regulate Twist activity [28]. Thus, we postulated that CC cell lineage specification may be regulated by tinman in mesodermal cells with increased Daughterless activity. To test this possibility, we co-expressed tinman with daughterless in mesoderm. In twi.2×PE-GAL4 UAS-tinman UAS-daughterless embryos, the number of Glass+ CC precursors in head mesoderm was markedly expanded (red arrowheads in Figure 7F). In addition to extra Glass+ cells in head mesoderm, we also detected ectopic Glass+ cells in the trunk (black arrowheads in Figure 7F). To test if these Glass+ cells developed further toward a fate resembling CC cells, we assessed akh-RHS marker expression at stage 17. Compared to normal akh-RHS+ cell numbers in twi.2×PE-GAL4 control embryos at stage 17 (Figure 7G), we detected increased numbers of akh-RHS+ cells in twi.2×PE-GAL4 UAS-daughterless UAS-tinman embryos at this stage (red arrowheads in Figure 7H). Unexpectedly, we also detected ectopic akh-RHS+ cells in embryonic trunk of these embryos at stage 17 (black arrowheads in Figure 7H). Taken together, these results show that co-expression of Daughterless and Tinman is sufficient to activate CC cell developmental programs and to promote CC cell lineage expansion both in head mesoderm and ectopic sites. Collectively, the results strongly suggest that the CC cell lineage is specified by a combinatorial transcription code in embryonic mesoderm.
Discussion
Identification of novel genes required for CC cell development
Although DrosDel deficiency lines used in this study cover only ∼50% of Drosophila genome, we successfully identified several genes previously not implicated in CC cell development. Mutations in crooked neck (crn), spitz (spi), dimmed (dimm), phyllopod (phyl), double parked (dup), three rows (thr), Polycomblike (Pcl), ETS-domain lacking (edl), and heartless (htl) result in the complete loss of Akh expression. Expression of dimm in CC cells has been previously reported [29], and dimm is required for the differentiation of central and peripheral neuroendocrine cells. Thus, dimm may be required for CC cell maturation. spi, edl, and phyl are components of the Epidermal Growth Factor signaling pathway and htl encodes a Drosophila Fibroblast Growth Factor Receptor. Thus, these results suggest that MAPK signaling pathways regulate CC cell development. thr, dup, and crn are required for the cell cycle control, suggesting that the regulation of cell cycle control is also important for proper CC cell development.
Disruption of Notch signaling leads to the expansion of neuroendocrine precursor cells
Prior studies suggest that development of stomatogastric endocrine cells from endoderm, and IPCs from neuroectoderm is regulated by Notch and MAPK signaling [30]–[32]. Here, we found that Notch signaling disruption from mutations in Notch, Delta or E(spl) led to expansion of CC cells, reminiscent of the expansion of endocrine islet α-cells during mammalian pancreas development of Dll1 or Hes1 mutant mice [33], [34]. Notch signaling is required to maintain undifferentiated mammalian pituitary progenitors (reviewed in [35]), and mutations disrupting Notch signaling also result in the expansion of specific pituitary cell types [36]. Thus, signaling pathways controlling CC cell development may reflect ancient conserved genetic programs for endocrine cell specification. Using time-lapse in vivo imaging, we detected the emergence of multiple Glass+ CC precursors in stage 11 Notch mutants. The most rapid mitotic divisions in Drosophila occur prior to embryonic cellularization, and require approximately 10 minutes [37]. Thus, we calculate that the emergence of 7 Glass+ CC precursors within 20 minutes in Notch mutant embryos is unlikely to result from accelerated division of a single Glass+ CC precursor or loss of asymmetric cell division from Glassneg CC progenitor. Rather, our data suggest that Notch signaling restricts head mesodermal fate specification possibly by a lateral inhibition mechanism (model 3 in Figure 3J). After the initial Glass+ CC precursors are formed, maturation process from a single Glass+ CC precursor to a cluster of 7–8 AKH+ CC cells appears to be Notch signaling independent. Conditional mutant studies using a temperature sensitive allele of Delta, or using Notch signaling activation in Glass+ CC precursor cells further support this possibility.
Mesodermal origin of neuroendocrine cells
Prior studies suggested that corpora cardiaca neuroendocrine cells in Drosophila may derive from the most anterior region of head mesoderm expressing twist and gt [9], [10]. Recently, an alternate neuroectodermal origin for CC cells was proposed [11]. CC cells manifest neuron-like features, lending plausibility to the suggestion that CC cells derived from neuroectoderm expressing gt1-lacZ. However our study identified that the corpora cardiaca originates from head mesoderm expressing twist, Mef2 and tinman. The absence of CC precursors in twist and tinman mutants also strongly support this view. Lineage tracing studies by cell marking with gt1-GAL4 here confirmed a neuroectodermal origin for insulin-producing neurons in the protocerebrum; however, we did not detect tracing of CC precursors or mature CC cells from gt1-expressing cells. Thus, CC cells and IPCs have distinct embryonic origins and our data provide conclusive evidence from lineage tracing that neuroendocrine CC cells derive from mesoderm. The origins of IPCs and CC cells from different germ layers is consistent with the observation that mutations preventing CC cell development do not detectably impair IPC formation [38]. Thus, cell interactions between IPCs and CC cells may not be essential for development of these two cell types. A prior study speculated that corpus allatum cells in the larval ring gland, which produce juvenile hormone, derive from gnathal mesoderm [9], but this origin has not been demonstrated with methods like lineage tracing. Thus, to our knowledge, CC cells may represent the sole example, thus far, of neuroendocrine cell development from mesoderm in Drosophila.
Vascular access and dispersion of hormones is a defining feature of endocrine organs. In mammals, signaling between vascular and endocrine progenitors is an important mechanism for regulating development of organs like the pancreas [39]. Tinman+ cells in Drosophila head mesoderm also form the procephalic vascular rudiment [10], whose progeny establish the contractile dorsal vessel (Drosophila heart), and prior studies have demonstrated that axon-like projections from larval CC cells terminate on the dorsal vessel [3]. In addition, similar to the posterior migration of head-mesodermal rudimentary vascular cells, Glass+ AKHneg CC progenitors migrate posteriorly during their maturation into AKH+ cells. De Velasco and colleagues have previously speculated that developing CC precursors might interact with other head mesoderm cells [9] during CC development. Our demonstration that CC cells originate from Tinman+ Glassneg head mesoderm further supports this possibility. The proximity of embryonic CC cell progenitors to dorsal vessel progenitors may enhance cell-cell interactions that govern hallmark CC cell properties, including AKH expression and physical connections to their vascular targets. Together, these observations suggest that key morphogenetic and developmental signaling relationships between endocrine and vascular precursors may be conserved from flies to mammals.
Encoding neuroendocrine lineage specification by transcription factor combinations
Many human diseases result from excessive or inadequate endocrine cell mass or function. Thus, there is intense interest in identifying evolutionarily-conserved transcriptional codes for neuroendocrine cell development and expansion. Our study identified a unique cell signaling context in mesoderm where neuroendocrine precursor cells can be specified by the two transcription factors Tinman and Daughterless. Allocation of trunk mesodermal fates is regulated by Twist and Daughterless activity [19], [28], and here we showed that CC cell specification in head mesoderm is also regulated by a combination of transcription factors. tinman expression in a small subset of head mesoderm is regulated by Notch signaling, reminiscent of tinman regulation in trunk cardiogenic mesoderm by Notch signaling [40]. However, only two cells within Tinman+ domain in head mesoderm develop into Glass+ CC progenitors. These observations suggest that other factors, in addition to Tinman, are required to specify the CC cell lineage. Consistent with this possibility, we show that Tinman mis-expression is not sufficient to expand CC development. By contrast, co-expression of Tinman and Daughterless led to increased development of head mesoderm into CC cells; thus, Tinman and Daughterless collaborate to specify the CC lineage. The combination of Tinman and Daughterless also induced ectopic AKH+ cells in the embryonic trunk, suggesting that trunk mesodermal cells may also be competent to develop into CC cells. We speculate that over-expression of Daughterless in mesoderm suppresses Twist activity, and the mesodermal cells in this context are competent to become CC lineage upon Tinman expression, but further studies are required to test this possibility. Our study identified a transcription factor combination whose reconstitution is sufficient for differentiation by a subset of mesodermal cells toward a neuroendocrine fate. However, most embryonic mesodermal cells failed to express Glass or Akh upon mis-expression of Tinman and Daughterless, suggesting additional factors are likely required to re-specify mesoderm into CC cells. Moreover, additional studies are needed to determine how Daughterless, which is ubiquitously expressed, might regulate Twist activity in differentiating mesoderm to give rise to distinct cell fates.
In summary, work here reveals embryonic and molecular mechanisms regulating development of Drosophila CC cells. We demonstrated that Notch signaling restricts CC precursor cell fate in head mesoderm and regulates Tinman expression. We used cell lineage tracing and genetic analysis to demonstrate that CC cells originate from embryonic mesoderm. We also showed that a combination of the transcription factors Tinman and Daughterless is necessary and sufficient to specify CC cell lineage in mesoderm. Findings from this study should accelerate advances in our understanding of the conserved molecular mechanisms controlling differentiation and expansion of endocrine organs essential for metabolic regulation.
Materials and Methods
Drosophila strains
y1 w1118 strain was used as the wild type stock. DrosDel deficiency lines were obtained from Bloomington Stock Center. The following mutant alleles and transgenic lines were used in this study: Dl9P, DlRF, N264-39, E(spl)rv1, SerRX82, Su(H)IB115, da10, twi1, zfh100865, Mef2X1, howstru-3R-3, Df(1)BSC530, twi.2×PE-GAL4, GAL4-Mef2.R and UAS-lacZ.NZ (Bloomington Stock Center). gt1-lacZ was provided by Dr. Stephen Small (New York University) [13]. tin346, tinEC40 and UAS-tin were provided by Dr. Rolf Bodmer (Burnham Institute) [41]. UAS-da and UAs-twi were provided by Dr. Mary K. Baylies (Sloan-Kettering Institute) [19]. UAS-Nintra was a gift from Dr. Margaret Fuller (Stanford University). Kr-GAL4 UAS-GFP or twi-GAL4 UAS-GFP harboring balancer chromosomes were used to identify hemi - or homozygous mutant embryos. For lineage tracing experiments, flies carrying UAS-FLP; dilp2-HSti, akh-RHS; Act5C(FRT.polyA)lacZ.nls1 were crossed to GAL4 lines.
In situ hybridization and immunohistochemistry
Antisense riboprobe for Akh was derived from pBS2KSP-Akh cDNA clone. RNA in situ hybridization was carried out as described [42]. Immunostaining of embryos was performed as described [42] with the following modifications; all embryos were manually devitellinized to avoid methanol exposure, late stage 17 embryos with cuticle were sonicated for 6 seconds under the lowest output setting in Branson Sonifier 450, primary antibodies were detected with Alexa488, 547, or 647 (Invitrogen) secondary antibodies, and embryos were mounted in 100% glycerol. Embryonic developmental stages were morphologically determined according to Campos-Ortega and Hartenstein [43]. During our studies, we found that the development of Glass+ larval eye precursors in Bolwig's organ lineage was unaffected in Notch mutants, and we quantified Glass+ larval eye precursors to determine embryonic stage accurately within stage 11 embryos. In both wild type and mutant embryos, we detected 1–3 precursors at early stage 11 (black arrowheads in Figure 3B and 3F), 4–7 precursors at mid stage 11 (black arrowheads in Figure 3C and 3G) and 8–11 cells at late stage 11(black arrowheads in Figure 3D and 3H), respectively. The following primary antibodies were used: rabbit anti-AKH (1∶300) [3], rabbit anti-Twist (1∶500; Dr. Maria Leptin, Universität Köln) [17], rabbit anti-Tinman (1∶300; Dr. Manfred Frasch, Mount Sinai School of Medicine) [24], mouse 9B2.1c anti-Glass (1∶10; Developmental Studies Hybridoma Bank under the auspices of NICHD and maintained by The University of Iowa, Department of Biology) [44] and chicken anti-β-gal (1∶1000; Abcam). Immunostaining of CC cells in larval brains was performed as described [3]. Imaging of RNA in situ hybridizations was performed on a Zeiss Axio Imager DIC microscope. Immunofluorescence microscopy was performed on a Zeiss Axio Imager or a Zeiss LSM510 confocal microscope. Z-projections of confocal stacks were generated using ImageJ with sum slice option.
Generation of reporter and GAL4 driver lines
The enhancer sequences used in this study were amplified from y1 w1118 genomic DNA. pAkhp1016 Red H-Stinger (akh-RHS) and pAkhp1016 Green H-Pelican (akh-GHP) were constructed by subcloning the 1016 bp sequence upstream of the Akh start codon [3] into the pRed H-Stinger and pGreen H-Pelican vectors [45], respectively. pDilp215-1-H-Stinger (dilp2-HSti) was generated by subcloning the 541 bp sequence upstream of the dilp2 transcription start site [2] into the pH-Stinger vector. pGlass5.2 Red H-Stinger (glass5.2-RHS) was constructed by subcloning the 5197 bp sequence upstream of the glass start codon [46] into the pRed H-Stinger vector. pGt1-GAL4 (gt1-GAL4) was constructed by subcloning the 787 bp gt1 CRM fragment [13] into pPTGAL. pGlass5.2-GAL4 (glass5.2-GAL4) was constructed by subcloning this 5197 bp sequence into the pPTGAL vector. P-element mediated germline transformations were carried out as described [47]. For all transgenic strains, at least two independently-derived transgenic lines with transgenes mapping to the second or third chromosome were evaluated.
Live embryo imaging
To capture fluorescent reporter signals in developing embryos, stage 7 or 8 embryos were mounted between two cover glasses spaced with 0.1% agarose blocks. Z-stack images (35×1 µm) were captured every 2 minutes for 3 hours in the Zeiss Axio Imager fluorescent microscope. Conversions of Z-stacks to projection images and time-lapse movies were performed in ImageJ software.
Supporting Information
Zdroje
1. RulifsonEJKimSKNusseR 2002 Ablation of insulin-producing neurons in flies: growth and diabetic phenotypes. Science 296 1118 1120
2. IkeyaTGalicMBelawatPNairzKHafenE 2002 Nutrient-dependent expression of insulin-like peptides from neuroendocrine cells in the CNS contributes to growth regulation in Drosophila. Curr Biol 12 1293 1300
3. KimSKRulifsonEJ 2004 Conserved mechanisms of glucose sensing and regulation by Drosophila corpora cardiaca cells. Nature 431 316 320
4. LeeGParkJH 2004 Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics 167 311 323
5. ParkDTaghertPH 2009 Peptidergic neurosecretory cells in insects: organization and control by the bHLH protein DIMMED. Gen Comp Endocrinol 162 2 7
6. AggarwalSKKingRC 1971 An electron microscopic study of the corpus cardiacum of adult Drosophila melanogaster and its afferent nerves. J Morphol 134 437 445
7. CognigniPBaileyAPMiguel-AliagaI 2010 Enteric neurons and systemic signals couple nutritional and reproductive status with intestinal homeostasis. Cell Metab 13 92 104
8. HartensteinVTepassUGruszynski-DefeoE 1994 Embryonic development of the stomatogastric nervous system in Drosophila. J Comp Neurol 350 367 381
9. De VelascoBShenJGoSHartensteinV 2004 Embryonic development of the Drosophila corpus cardiacum, a neuroendocrine gland with similarity to the vertebrate pituitary, is controlled by sine oculis and glass. Dev Biol 274 280 294
10. De VelascoBMandalLMkrtchyanMHartensteinV 2006 Subdivision and developmental fate of the head mesoderm in Drosophila melanogaster. Dev Genes Evol 216 39 51
11. WangSTulinaNCarlinDLRulifsonEJ 2007 The origin of islet-like cells in Drosophila identifies parallels to the vertebrate endocrine axis. Proc Natl Acad Sci U S A 104 19873 19878
12. RyderEAshburnerMBautista-LlacerRDrummondJWebsterJ 2007 The DrosDel deletion collection: a Drosophila genomewide chromosomal deficiency resource. Genetics 177 615 629
13. Ochoa-EspinosaAYucelGKaplanLPareAPuraN 2005 The role of binding site cluster strength in Bicoid-dependent patterning in Drosophila. Proc Natl Acad Sci U S A 102 4960 4965
14. JiangJLevineM 1993 Binding affinities and cooperative interactions with bHLH activators delimit threshold responses to the dorsal gradient morphogen. Cell 72 741 752
15. RanganayakuluGSchulzRAOlsonEN 1996 Wingless signaling induces nautilus expression in the ventral mesoderm of the Drosophila embryo. Dev Bio 176 143 148
16. LieberTKiddSAlcamoECorbinVYoungMW 1993 Antineurogenic phenotypes induced by truncated Notch proteins indicate a role in signal transduction and may point to a novel function for Notch in nuclei. Genes Dev 7 1949 1965
17. LeptinM 1991 twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev 5 1568 1576
18. BayliesMKBateM 1996 twist: a myogenic switch in Drosophila. Science 272 1481 1484
19. CastanonIVon StetinaSKassJBayliesMK 2001 Dimerization partners determine the activity of the Twist bHLH protein during Drosophila mesoderm development. Development 128 3145 3159
20. LaiZCFortiniMERubinGM 1991 The embryonic expression patterns of zfh-1 and zfh-2, two Drosophila genes encoding novel zinc-finger homeodomain proteins. Mech Dev 34 123 134
21. LillyBGalewskySFirulliABSchulzRAOlsonEN 1994 D-MEF2: a MADS box transcription factor expressed in differentiating mesoderm and muscle cell lineages during Drosophila embryogenesis. Proc Natl Acad Sci U S A 91 5662 5666
22. TaylorMVBeattyKEHunterHKBayliesMK 1995 Drosophila MEF2 is regulated by twist and is expressed in both the primordia and differentiated cells of the embryonic somatic, visceral and heart musculature. Mech Dev 50 29 41
23. ZaffranSAstierMGratecosDSemerivaM 1997 The held out wings (how) Drosophila gene encodes a putative RNA-binding protein involved in the control of muscular and cardiac activity. Development 124 2087 2098
24. YinZXuXLFraschM 1997 Regulation of the twist target gene tinman by modular cis-regulatory elements during early mesoderm development. Development 124 4971 4982
25. LeeYMParkTSchulzRAKimY 1997 Twist-mediated activation of the NK-4 homeobox gene in the visceral mesoderm of Drosophila requires two distinct clusters of E-box regulatory elements. J Biol Chem 272 17531 17541
26. CarmenaABateMJimenezF 1995 lethal of scute, a proneural gene participates in the specification of muscle progenitors during Drosophila embryogenesis. Genes Dev 9 2373 2383
27. Tapanes-CastilloABayliesMK 2004 Notch signaling patterns Drosophila mesodermal segments by regulating the bHLH transcription factor twist. Development 131 2359 2372
28. WongMCastanonWBayliesMK 2008 Daughterless dictates Twist activity in a context-dependent manner during somatic myogenesis. Dev Biol 317 417 429
29. HewesRSParkDGauthierSASchaeferAMTaghertPH 2003 The bHLH protein Dimmed controls neuroendocrine cell differentiation in Drosophila. Development 130 1771 1781
30. HartensteinVTepassUGruszynski-deFeoE 1996 Proneural and neurogenic genes control specification and morphogenesis of stomatogastric nerve cell precursors in Drosophila. Dev Bio 173 213 227
31. Gonzalez-GaitanMJackleH 2000 Tip cell-derived RTK signaling initiates cell movement in the Drosophila stomatogastric nervous system anlage. EMBO Rep 1 366 371
32. HwangHJRulifsonE 2011 Serial specification of diverse neuroblast identities from a neurogenic placode by Notch and Egfr signaling. Development 138 2883 2893
33. ApelqvistALiHSommerLBeatusPAndersonDJ 1999 Notch signalling controls pancreatic cell differentiation. Nature 400 877 881
34. JensenJPedersenEEGalantePHaldJHellerRS 2000 Control of endodermal endocrine development by Hes-1. Nat Genet 24 36 44
35. KelbermanDRizzotiKLovell-BadgeRRobinsonICAFDattaniMT 2009 Genetic regulation of pituitary gland development in human and mouse. Endocr Rev 30 790 829
36. DuttaSDietrichJEWesterfieldMVargaZM 2008 Notch signaling regulates endocrine cell specification in the zebrafish anterior pituitary. Dev Biol 319 248 257
37. FoeVEAlbertsBM 1983 Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J Cell Sci 61 31 70
38. De VelascoBErclikTShyDSclafaniJLipshitzH 2007 Specification and development of the pars intercerebralis and pars lateralis, neuroendocrine command centers in the Drosophila brain. Dev Biol 302 309 323
39. LammertECleaverOMeltonD 2001 Induction of pancreatic differentiation by signals from blood vessels. Science 294 564 567
40. MandalLBanerjeeUHartensteinV 2004 Evidence for a fruit fly hemangioblast and similarities between lymph-gland hematopoiesis in fruit fly and mammal aorta-gonadal-mesonephros mesoderm. Nat Genet 36 1019 1023
41. QianLBodmerR 2009 Partial loss of GATA factor Pannier imparis adult heart function in Drosophila. Hum Mol Genet 19 3153 3163
42. Torres-VazquezJParkSWarriorRAroraK 2001 The transcription factor Schnurri plays a dual role in mediating Dpp signaling during embryogenesis. Development 128 1657 1670
43. Campos-OrtegaJAHartensteinV 1985 The embryonic development of Drosophila melanogaster Berlin Springer-Verlag
44. EllisMCO'NeillEMRubinGM 1993 Expression of Drosophila glass protein and evidence for negative regulation of its activity in non-neuronal cells by another DNA-binding protein. Development 119 855 865
45. BaroloSCastroBPosakonyJW 2004 New Drosophila transgenic reporters: insulated P-element vectors expressing fast-maturing RFP. Biotechniques 36 436 440, 442
46. LiuHMaCMosesK 1996 Identification and functional characterization of conserved promoter elements from glass: a retinal development gene of Drosophila. Mech Dev 56 73 82
47. DolezalTDolezelovaEZurovecMBryantPJ 2005 A role for adenosine deaminase in Drosophila larval development. PLoS Biol 3 e201 doi:10.1371/journal.pbio.0030201
Štítky
Genetika Reprodukčná medicína
Článek The T-Box Factor MLS-1 Requires Groucho Co-Repressor Interaction for Uterine Muscle SpecificationČlánek B Chromosomes Have a Functional Effect on Female Sex Determination in Lake Victoria Cichlid FishesČlánek Distinct Cdk1 Requirements during Single-Strand Annealing, Noncrossover, and Crossover RecombinationČlánek Ongoing Phenotypic and Genomic Changes in Experimental Coevolution of RNA Bacteriophage Qβ and
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2011 Číslo 8- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Polo, Greatwall, and Protein Phosphatase PP2A Jostle for Pole Position
- Genome-Wide Association Analysis of Incident Coronary Heart Disease (CHD) in African Americans: A Short Report
- The T-Box Factor MLS-1 Requires Groucho Co-Repressor Interaction for Uterine Muscle Specification
- B Chromosomes Have a Functional Effect on Female Sex Determination in Lake Victoria Cichlid Fishes
- Analysis of DNA Methylation in a Three-Generation Family Reveals Widespread Genetic Influence on Epigenetic Regulation
- PP2A-Twins Is Antagonized by Greatwall and Collaborates with Polo for Cell Cycle Progression and Centrosome Attachment to Nuclei in Drosophila Embryos
- Discovery of Sexual Dimorphisms in Metabolic and Genetic Biomarkers
- Pervasive Sharing of Genetic Effects in Autoimmune Disease
- DNA Methylation and Histone Modifications Regulate Shoot Regeneration in by Modulating Expression and Auxin Signaling
- Mutations in and Reveal That Cartilage Matrix Controls Timing of Endochondral Ossification by Inhibiting Chondrocyte Maturation
- Variance of Gene Expression Identifies Altered Network Constraints in Neurological Disease
- Frequent Beneficial Mutations during Single-Colony Serial Transfer of
- Increased Gene Dosage Affects Genomic Stability Potentially Contributing to 17p13.3 Duplication Syndrome
- Distinct Cdk1 Requirements during Single-Strand Annealing, Noncrossover, and Crossover Recombination
- Hunger Artists: Yeast Adapted to Carbon Limitation Show Trade-Offs under Carbon Sufficiency
- Suppression of Scant Identifies Endos as a Substrate of Greatwall Kinase and a Negative Regulator of Protein Phosphatase 2A in Mitosis
- Temporal Dynamics of Host Molecular Responses Differentiate Symptomatic and Asymptomatic Influenza A Infection
- MK2-Dependent p38b Signalling Protects Hindgut Enterocytes against JNK-Induced Apoptosis under Chronic Stress
- Specification of Corpora Cardiaca Neuroendocrine Cells from Mesoderm Is Regulated by Notch Signaling
- Genome-Wide Gene-Environment Study Identifies Glutamate Receptor Gene as a Parkinson's Disease Modifier Gene via Interaction with Coffee
- Identification of Functional Toxin/Immunity Genes Linked to Contact-Dependent Growth Inhibition (CDI) and Rearrangement Hotspot (Rhs) Systems
- Genomic Analysis of the Necrotrophic Fungal Pathogens and
- Celsr3 Is Required for Normal Development of GABA Circuits in the Inner Retina
- Genetic Architecture of Aluminum Tolerance in Rice () Determined through Genome-Wide Association Analysis and QTL Mapping
- Predisposition to Cancer Caused by Genetic and Functional Defects of Mammalian
- Regulation of p53/CEP-1–Dependent Germ Cell Apoptosis by Ras/MAPK Signaling
- and but Not Interact in Genetic Models of Amyotrophic Lateral Sclerosis
- Gamma-Tubulin Is Required for Bipolar Spindle Assembly and for Proper Kinetochore Microtubule Attachments during Prometaphase I in Oocytes
- Ongoing Phenotypic and Genomic Changes in Experimental Coevolution of RNA Bacteriophage Qβ and
- Genetic Architecture of a Reinforced, Postmating, Reproductive Isolation Barrier between Species Indicates Evolution via Natural Selection
- -eQTLs Reveal That Independent Genetic Variants Associated with a Complex Phenotype Converge on Intermediate Genes, with a Major Role for the HLA
- The GATA Factor ELT-1 Works through the Cell Proliferation Regulator BRO-1 and the Fusogen EFF-1 to Maintain the Seam Stem-Like Fate
- and Control Optic Cup Regeneration in a Prototypic Eye
- A Comprehensive Map of Mobile Element Insertion Polymorphisms in Humans
- An EMT–Driven Alternative Splicing Program Occurs in Human Breast Cancer and Modulates Cellular Phenotype
- Evidence for Hitchhiking of Deleterious Mutations within the Human Genome
- A Broad Brush, Global Overview of Bacterial Sexuality
- Global Chromosomal Structural Instability in a Subpopulation of Starving Cells
- A Pre-mRNA–Associating Factor Links Endogenous siRNAs to Chromatin Regulation
- Glutamine Synthetase Is a Genetic Determinant of Cell Type–Specific Glutamine Independence in Breast Epithelia
- The Repertoire of ICE in Prokaryotes Underscores the Unity, Diversity, and Ubiquity of Conjugation
- Genome-Wide Association Analysis of Autoantibody Positivity in Type 1 Diabetes Cases
- Natural Polymorphism in BUL2 Links Cellular Amino Acid Availability with Chronological Aging and Telomere Maintenance in Yeast
- Chromosome Painting Reveals Asynaptic Full Alignment of Homologs and HIM-8–Dependent Remodeling of Chromosome Territories during Meiosis
- Ku Must Load Directly onto the Chromosome End in Order to Mediate Its Telomeric Functions
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- An EMT–Driven Alternative Splicing Program Occurs in Human Breast Cancer and Modulates Cellular Phenotype
- Chromosome Painting Reveals Asynaptic Full Alignment of Homologs and HIM-8–Dependent Remodeling of Chromosome Territories during Meiosis
- Discovery of Sexual Dimorphisms in Metabolic and Genetic Biomarkers
- Regulation of p53/CEP-1–Dependent Germ Cell Apoptosis by Ras/MAPK Signaling
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



