-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
A -Acting Protein Effect Causes Severe Eye Malformation in the Mouse
Mp is an irradiation-induced mouse mutation associated with microphthalmia, micropinna and hind limb syndactyly. We show that Mp is caused by a 660 kb balanced inversion on chromosome 18 producing reciprocal 3-prime gene fusion events involving Fbn2 and Isoc1. The Isoc1-Fbn2 fusion gene (Isoc1Mp) mRNA has a frameshift and early stop codon resulting in nonsense mediated decay. Homozygous deletions of Isoc1 do not support a significant developmental role for this gene. The Fbn2-Isoc1 fusion gene (Fbn2Mp) predicted protein consists of the N-terminal Fibrillin-2 (amino acids 1–2646, exons 1–62) lacking the C-terminal furin-cleavage site with a short out-of-frame extension encoded by the final exon of Isoc1. The Mp limb phenotype is consistent with that reported in Fbn2 null embryos. However, severe eye malformations, a defining feature of Mp, are not seen in Fbn2 null animals. Fibrillin-2Mp forms large fibrillar structures within the rough endoplasmic reticulum (rER) associated with an unfolded protein response and quantitative mass spectrometry shows a generalised defect in protein secretion in conditioned media from mutant cells. In the embryonic eye Fbn2 is expressed within the peripheral ciliary margin (CM). Mp embryos show reduced canonical Wnt-signalling in the CM – known to be essential for ciliary body development - and show subsequent aplasia of CM-derived structures. We propose that the Mp “worse-than-null” eye phenotype plausibly results from a failure in normal trafficking of proteins that are co-expressed with Fbn2 within the CM. The prediction of similar trans-acting protein effects will be an important challenge in the medical interpretation of human mutations from whole exome sequencing.
Published in the journal: A -Acting Protein Effect Causes Severe Eye Malformation in the Mouse. PLoS Genet 9(12): e32767. doi:10.1371/journal.pgen.1003998
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003998Summary
Mp is an irradiation-induced mouse mutation associated with microphthalmia, micropinna and hind limb syndactyly. We show that Mp is caused by a 660 kb balanced inversion on chromosome 18 producing reciprocal 3-prime gene fusion events involving Fbn2 and Isoc1. The Isoc1-Fbn2 fusion gene (Isoc1Mp) mRNA has a frameshift and early stop codon resulting in nonsense mediated decay. Homozygous deletions of Isoc1 do not support a significant developmental role for this gene. The Fbn2-Isoc1 fusion gene (Fbn2Mp) predicted protein consists of the N-terminal Fibrillin-2 (amino acids 1–2646, exons 1–62) lacking the C-terminal furin-cleavage site with a short out-of-frame extension encoded by the final exon of Isoc1. The Mp limb phenotype is consistent with that reported in Fbn2 null embryos. However, severe eye malformations, a defining feature of Mp, are not seen in Fbn2 null animals. Fibrillin-2Mp forms large fibrillar structures within the rough endoplasmic reticulum (rER) associated with an unfolded protein response and quantitative mass spectrometry shows a generalised defect in protein secretion in conditioned media from mutant cells. In the embryonic eye Fbn2 is expressed within the peripheral ciliary margin (CM). Mp embryos show reduced canonical Wnt-signalling in the CM – known to be essential for ciliary body development - and show subsequent aplasia of CM-derived structures. We propose that the Mp “worse-than-null” eye phenotype plausibly results from a failure in normal trafficking of proteins that are co-expressed with Fbn2 within the CM. The prediction of similar trans-acting protein effects will be an important challenge in the medical interpretation of human mutations from whole exome sequencing.
Introduction
The accurate prediction of the phenotypic consequences of individual mutations is of increasing medical importance with the rapid development of whole genome sequencing technologies. Different mutations can have distinct effects on expression of the gene, its function, or localisation of its product. It is therefore not surprising that allelic heterogeneity can result in multiple distinct disorders from mutations in a single gene (e.g. LMNA [1], FLNA [2], FGFR1 [3]). Such heterogeneity, in combination with stochastic effects and genetic and/or environmental modifiers, also accounts for phenotypic variability within an individual disorder.
Particular mutations cause disease due to a failure of normal post-translational processing and protein folding. For example, the mutation-specific induction of endoplasmic reticulum (ER) stress responses seen in disorders including late onset neurodegenerative diseases [4], congenital hearing loss [5] and skeletal dysplasias [6]. The mechanisms proposed for pathogeneisis associated with ER stress include: loss of function of the mutant protein [7], alteration of the cell specific differentiation state [6] or uncharacterised generalised cytopathy [4].
Anophthalmia (absence of the eye) and microphthalmia (small eye) are important causes of congenital visual impairments in developed countries with a live birth prevalence rate of 0.2 and 1.7 per 10,000 live births respectively [8], [9]. In most cases the cause of anophthalmia/microphthalmia remains unknown [10] although several single gene causes have been discovered including SOX2 [11], [12], OTX2 [13], STRA6 [14], FOXE3 [15], SMOC1 [16], [17], [18] and PAX6 [19]. Of these, heterozygous loss-of-function mutations in SOX2 are the most common, accounting for 20–40% of bilaterally affected cases [20]. SOX2 functions at multiple stages during eye development, including during lens induction [21], [22] and formation and maintenance of the ciliary margin (CM) at the distal peripheral rim of the optic cup [23]. The CM forms the ciliary epithelium and the inner layer of the iris and controls ciliary muscle and stroma development [24]. At the boundary of the CM and the neural retina (NR), multipotent cells make a binary decision to commit to either CM or NR fate [23]. SOX2 expression, which marks the boundary between CM and NR, is dependent on canonical Wnt-signaling [25], [26]. Loss of either Wnt-signalling or SOX2 expression in the CM leads to failure of the development of the ciliary body structures and thinning and rosetting of the neural retina [23], [27], [28].
The Mp mouse was generated through the irradiation mutagenesis programme at the Oak Ridge National Laboratory in the 1960's. Mp homozygotes appeared to be anophthalmic and to have syndactylyyly of the hindlimbs [29]. Here, we identify the cause of Mp as a balanced 660 kb inversion on chromosome 18. The consequent gene fusion events result in the production of a C-terminally truncated fibrillin-2 protein that is retained in the rough-ER(rER) of expressing cells. Null mutations of Fbn2 accurately phenocopy the Mp limb anomalies but are not associated with any ocular malformation [30], [31], [32]. Fbn2Mp inclusions trigger the unfolded protein response (UPR) in a subset of cells within the CM resulting in aplasia of the ciliary apparatus with thinning and rosetting of the neural retina. The UPR seen in Mp is associated with signalling and patterning anomalies and a reduction in general protein secretion in Fbn2Mp expressing cells. This mechanism has broader significance in the interpretation of human disease-causing mutations. It suggests that the phenotypic effect of specific UPR-inducing mutations may not result from loss of that gene product but rather from loss of co-expressed gene products that are also processed through the ER.
Results
Main adult phenotypes of Mp
We bred Mp onto both the C57BL/6J inbred, and the CD1 outbred background strains. The phenotype in these mice was consistent with that documented in the previous report ([29]; Figure 1A–H), with small eyes and ears in adult heterozygotes (Figure 1B; Mp/+) and small ears, apparent anophthalmia and hindlimb syndactyly in homozygotes (Mp/Mp; Figure 1C). The eyelids in Mp/Mp animals never opened, however contrary to the original report, microphthalmia rather than anophthalmia was identified on dissection (Figure 1D). Further analyses revealed that the Mp/Mp limb phenotype ranged in severity from osseous fusions of the entire phalanges of digits 2–3–4 (Figure 1H), to simple soft tissue syndactyly affecting these digits (not shown). Digits 1 and 5 were never affected and no metatarsal abnormalities were identified. Consistent with the original report in which homozygotes were described as runted, we observed significant weight differences between mutant and Wt animals (Figure S1). Mp mutant phenotypes were fully penetrant throughout crosses of all genetic backgrounds tested (Figure S1).
Fig. 1. The Mp phenotype was characterised by severe ocular and limb abnormalities. 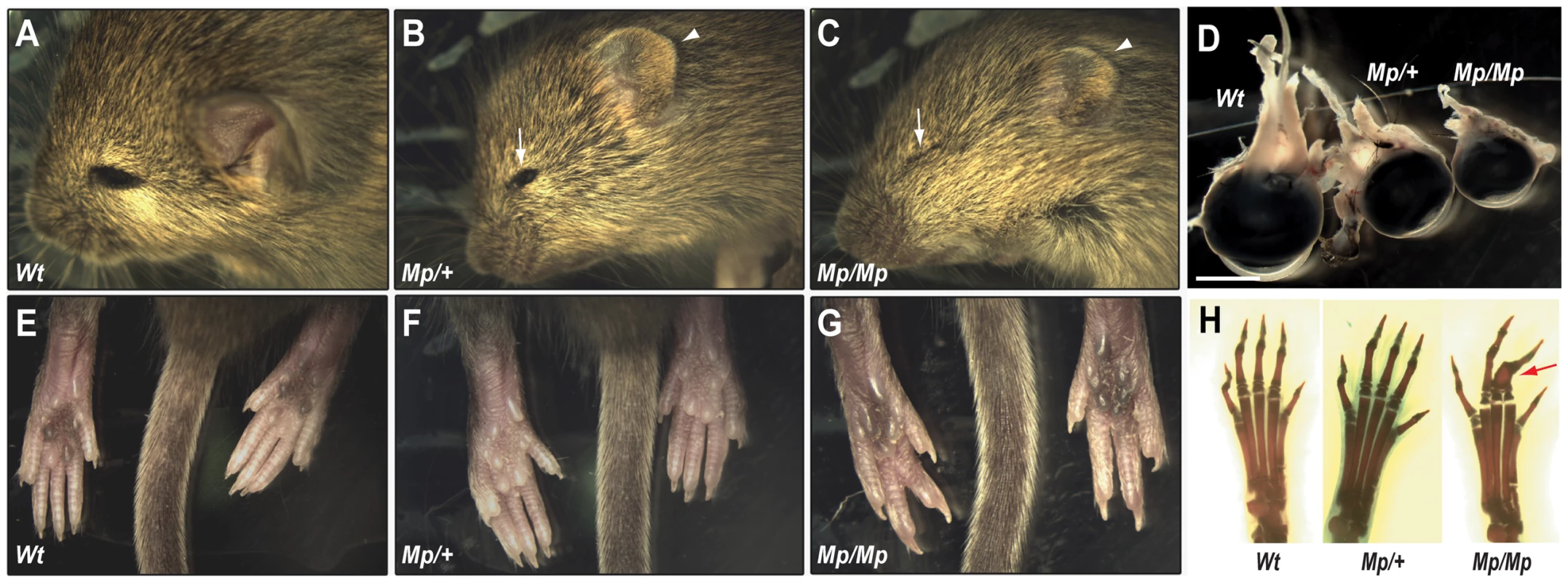
(A–C) Heterozygous (Mp/+) animals had apparent microphthalmia (arrow) and reduced ear size (arrowhead) compared to Wt. Homozygotes (Mp/Mp) were more severely affected with closed eyes, indicating anophthalmia (arrow), and further reduced ear size (arrowhead). (D) Dissection of adult eyes revealed microphthalmia in both Mp genotypes and revealed a graded reduction in size compared to Wt (scale bar = 3.0 mm). (E–G) Hindlimb syndactylyyly was identified in Mp/Mp animals with the presence of only 4 digits on each paw compared to Wt and Mp/+. (H) Skeletal analysis revealed the presence of all 5 complete digital rays in Wt and Mp/+ hindlimbs but revealed osseous fusions of phalanges (arrow) within Mp/Mp mid-axial digits (digits 2–4). Ciliary body malformation and aberrant developmental Wnt signalling in the Mp eye
Histological analysis at adult stages (P21) revealed pan-ocular structural defects in Mp (Figure 2A–C). In particular, the neural retina cell layers displayed severe rosetting and the vitreous was absent from the anterior chamber and posterior eye. Immunofluorescence studies revealed the nature of the retinal disruption with rosettes composed primarily of disorganised rod photoreceptors, and reduced numbers of cells at the inner nuclear layer (Figure S2). Furthermore, ectopic ganglion cells were identified in multiple regions of the Mp/Mp retina and expression of GFAP was observed, indicative of gross damage to the retina. In both Mp/M and Mp/+, the ciliary body was consistently absent however the iris appeared to form normally (Figure 2D). Although retinal lamination is not fully complete until approximately three weeks after birth, the ciliary body develops during mid gestation from non-pigmented ciliary epithelial cells. The anterior region of the early developing retina, and future ciliary epithelium, is marked specifically by canonical Wnt signalling and we therefore crossed Mp to BAT-gal [33], a Wnt-reporter strain that expresses beta-galactosidase in the presence of activated beta-catenin. In Mp/Mp-BAT-gal+/− E14.5 mice, we detected significantly reduced canonical Wnt signalling in the anterior retina as shown by the loss of positively stained cells (Figure 2E–F, and Figure S2). Furthermore, at E15.5 a marked reduction in the size of the developing non-pigmented ciliary was observed upon histological staining as well as staining for the anterior retina marker Pax6 (Figure 2G–J). Furthermore, we revealed reduced total retinal size in Mp/Mp eyes at the same developmental stage (Figure S2). We therefore concluded that the Mp eye phenotype resulted from perturbations to normal ocular development, and first identifiable at the region of the developing ciliary epithelium.
Fig. 2. Mp eyes displayed structural defects and abnormal ciliary development. 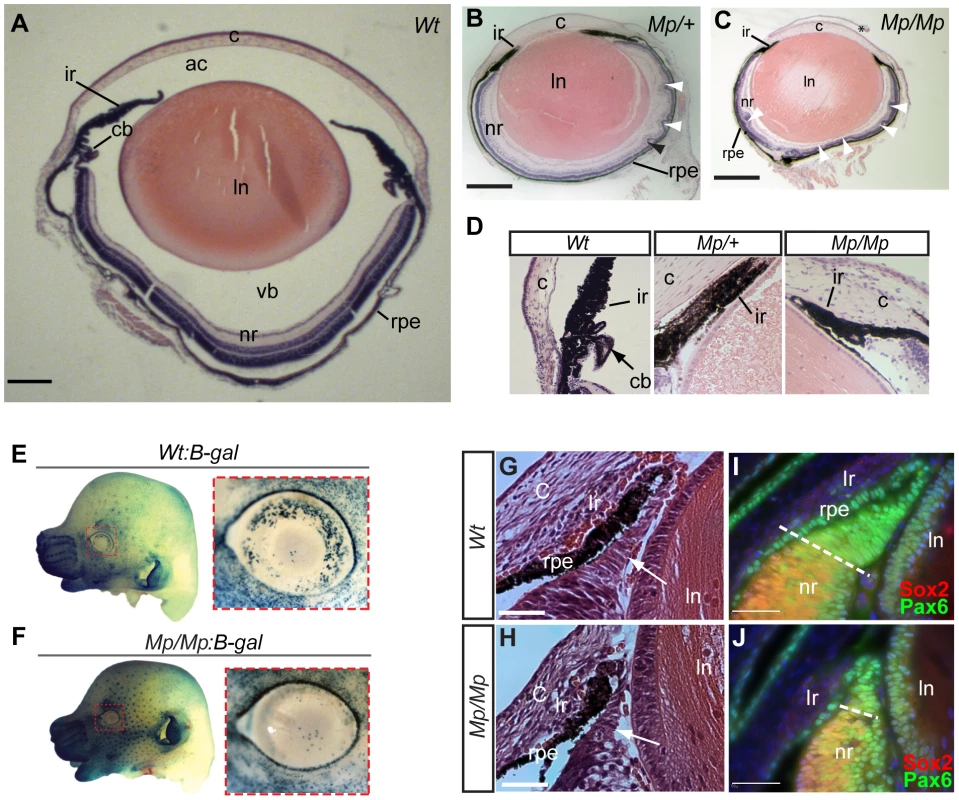
Histological comparison of eye tissues in Wt (A), Mp/+(B), and Mp/Mp (C) at P21 revealed severe pan-ocular defects. Mutant eyes displayed microphthalmia and retinal rosetting (arrowheads), together with loss of vitreous in the anterior chamber and between lens and retina. Lens size was also reduced in both mutant genotypes. Scale bars = 500 µm; sections are oriented in the sagittal plane. (D) Enlarged view of iris and the anterior region of retinas revealed the absence of ciliary body structures in both mutant types. (E–F) Genetic crosses of Mp with the Wnt-signalling reporter mouse line BAT-gal, revealed a significant reduction in galactosidase-positive cells in E14.5 Mp retinas compared to Wt, specifically in the dorsal and temporal regions of the anterior retina (n = 8 per genotype). In contrast, non-ocular tissue displayed slightly increased staining in Mp:B-gal compared to Wt:B-gal, due to the slight increase in staining time in these samples. Ventral regions had low expression in both genotypes and were used as reference for quantitative comparison (Figure S2). (G–H) Histological analysis of anterior retinas at E15.5 revealed a reduction in non-pigmented ciliary body tissue (arrows) in Mp/Mp compared to Wt. (I–J) Immunohistochemical staining of anterior retinal structures with antibodies specific for Pax6 and Sox2 at E15.5 revealed reduction in the Pax6-positive and Sox2-negative non-pigmented ciliary epithelial region in Mp. Scale bars, 50 µm. The asterisk in C indicates artefactual disruption to the corneal epithelium during sample processing. Abbreviations: ac, anterior chamber; c, cornea; cb, ciliary body; ir, iris; ln, lens; nr, neural retina; rpe, retinal pigmented epithelium; vb, vitreous body. Genetic mapping of Mp to the Fbn2 locus
We performed a whole-genome scan using microsatellite markers on heterozygous Mp mice backcrossed to C57BL/6J, and identified enrichment for background strain C3H-specific alleles co-segregating with Mp at three consecutive markers on chromosome 18 (data not shown). During fine mapping, no recombination events between markers D18Mit74 and D18Mit184 were found in 43 Mp/+ animals tested (Figure 3A). The Mp mutation was thus likely to be located between approximately 53 Mb and 67 Mb on chromosome 18. Fbn2 (RefSeq Gene NM_010181), an extracellular matrix protein, maps within this interval. The hind-limb phenotype of Mp was consistent with previously published loss of function Fbn2 mutations [30], [31], [32], [34]. Human mutations in FBN2 are associated with crumpled ear helices in affected patients [35], [36], [37] and comparable ear phenotypes were observed in Mp. However, heterozygous FBN2 mutations in humans do not cause ocular malformations and no ocular phenotype has been reported for Fibrillin-2 null mice [30], [31], [32], [34]. Furthermore, our histological examination of adult and neonatal Fbn2-null eyes revealed no ocular phenotype on genetic backgrounds analogous to Mp (Figure S3).
Fig. 3. Mp mapped to a balanced 660 kb inversion on chromosome 18 disrupting Fbn2 and Isoc1. 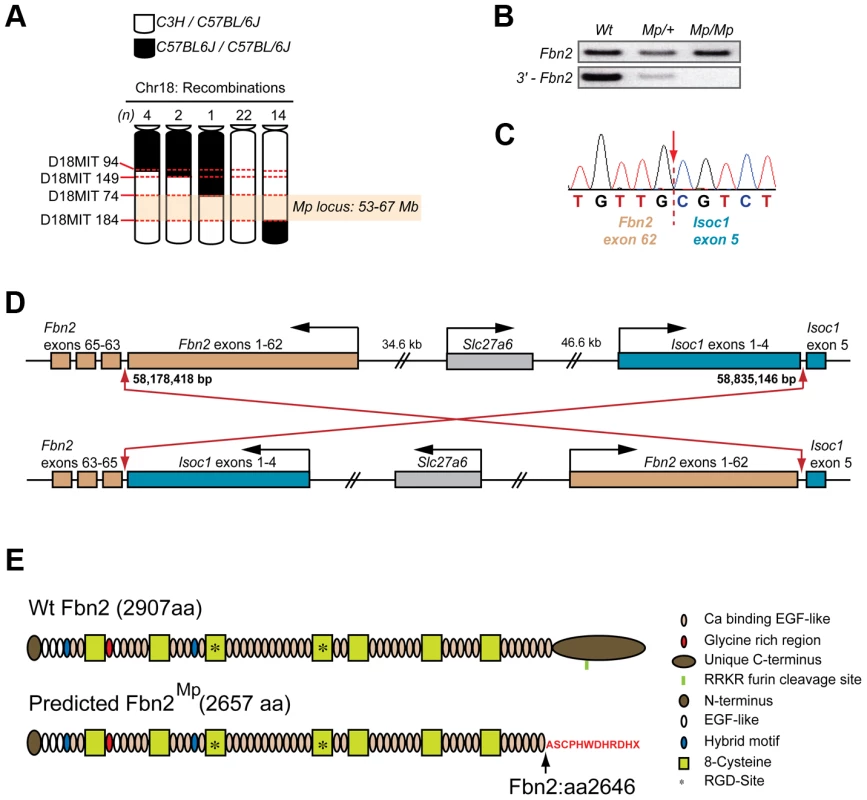
(A) Haplotype analysis using DNA samples from 3rd backcross (from parental strain C3H onto C57BL/6J) Mp/+ animals (n = 43) for four microsatellite markers on chromosome 18 revealed there had been no meiotic recombination events between markers D18MIT74 and D18MIT184, defining an Mp candidate loci between 53–67 Mb (NCBI37/mm9 mouse assembly). (B) Semi-quantitative RT-PCR analysis of the Fbn2 mRNA for each genotype revealed a failure to amplify the 3′-terminal region (bottom panel) of the transcript in Mp/Mp and an apparent reduction in Mp/+, compared to the normal amplification of a more central region of the Fbn2 mRNA (top panel). (C) Sequence chromatogram from 3′-RACE revealed cDNA sequence from Fbn2 exon 62 fused with sequence from Isoc1, a 5-exon gene positioned <1 Mb in cis from Fbn2 on chromosome 18. Fbn2 has 65 exons, Isoc1 has 5 exons. The Mp transcript was composed of Fbn2 exons 1–62 spliced directly to exon 5 of Isoc1 with 100% homology and no extra nucleotides added or removed. (D) The Mp genomic rearrangement comprised a balanced inversion of chromosome 18 with breakpoints positioned in the 3′-introns of Fbn2 (intron 62) and Isoc1 (intron 4). Breakpoints were located at Chr18:58,178,418 and Chr18:58,835,246. Note that Fbn2 is transcribed from the minus strand. (E) Fibrillin-2 Wt (top) and Fibrillin-2Mp (bottom) proteins with domains indicated. Fibrillin-2Mp is predicted to be missing the final calcium-binding EGF domain and its unique C-terminus, which have both been replaced by a sequence of 11 exogenous amino acids (red text), encoded out of frame from the Isoc1 terminal exon and followed by stop codon. Note also the removal of the endogenous RKKR furin cleavage domain. Nevertheless, we considered Fbn2 to be a good candidate for Mp. Standard RT-PCR was unable to amplify a 3′ - region of the Fbn2 cDNA (Figure 3B). Using 3′-RACE we identified a fragment present in Mp/Mp but absent from Wt mRNA, and a corresponding absence of the Wt Fbn2 amplicon in Mp/Mp (Figure S3). Cloning and sequence analysis of the Mp-specific Fbn2 amplicon identified that Fbn2Mp was transcribed up to and including all of exon 62, following which the mRNA contained the full sequence of the terminal exon from Isoc-1 (RefSeq ID NM_025478) (Figure 3C), a 5-exon gene of undetermined function located <1 Mb from Fbn2 on chromosome 18. Using combinations of Fbn2 and Isoc1 specific primers we discovered that this fusion was reciprocal and balanced so that the terminal Fbn2 exons (exons 63–65) replaced the final exon (exon 5) of the Isoc1 transcript (Figure S3), creating a 7-exon mRNA. Sequencing genomic DNA confirmed this to be a balanced inversion of chromosome 18 [NCBI37/mm9: Chr18 : 58,178,418–58,835,146] (Figure 3D). The predicted consequence for the translated Fibrillin-2Mp protein was replacement of the C-terminal 261 amino acids (aa) from position 2646, with an 11 aa out-of-frame extension followed by a termination codon, encoded by the out-of-frame final exon of Isoc1 (Figure 3E). Fibrillin-2Mp lacks the evolutionarily conserved predicted furin cleavage site present in the full-length protein (Fbn2 2769–73 aa; RRKR) [38].
In contrast, the inversion predicted nonsense-mediated decay of the Isoc1Mp transcript due to the frameshift-induced stop codon in the 5th of the 7 exons of this fusion gene [39], [40]. Consistent with this we observed approximately 50% reduction in Isoc1Mp mRNA levels by quantitative RT-PCR from developing eye tissue (Figure S3). The Slc27a6 gene, encoding a member of the fatty-acid transport family protein, is located between Fbn2 and Isoc1 and its orientation was changed by virtue of its position at the centre of the Mp genomic rearrangement, possibly affecting its cis-regulation. However the gene itself was not structurally altered and we observed no change in the low level of Slc27a6 expression that could be detected in Wt and Mp samples by RT-PCR (Figure S3). Additionally, signal was not detectable for Slc27a6 or Isoc1 at any stage when analysed by whole mount in situ hybridisation (WISH) in Wt and mutant samples (Figure S4). Although WISH is not sensitive enough provide conclusive proof, this data suggests that the developmental expression of these genes was not significantly changed or ectopically upregulated in Mp embryos. However, we could exclude loss of low-level expression of either of these genes as a cause of the ocular phenotype based on previous genetic studies. A chromosomal deletion causes the shaker-with-syndactylism (sy) phenotype extends at least between the microsatellite markers D18Mit124 at 57.61 Mb (NCBI37/mm9 C57BL/6J) and D18Mit205 at 58.95 Mb, encompassing Isoc1 (58.82–58.84 Mb) and Slc27a6 (58.72–58.77 Mb) as well as Slc12a2 and Fbn2 [34]. Mice heterozygous for sy have no morphological phenotype, and homozygotes have fused digits due to loss of Fbn2 and are deaf because they lack Slc12a2 [41]. The truncation of Fibrillin-2Mp thus remained the sole candidate and we therefore focussed our investigations on the Fbn2Mp gene product.
Fibrillin-2 distribution was disturbed in Mp
We performed whole-mount and tissue section in situ hybridisation to determine the embryonic expression patterns of Fbn2 Wt and Mp mouse embryos. Fbn2 showed site and stage specific expression in the developing eye, limb and tail in early embryos and gene expression patterns did not differ between Wt and Mp in any tissue or at stage analysed (Figure 4A & B; and Figure S4). Expression of Fbn2 has recently been well documented in the developing mouse eye, with strong expression observed in the developing non-pigmented ciliary epithelium at the anterior retina, with expression also observed in cells of the cornea and the periocular mesenchyme [42]. Similarly, we observed Fbn2 expression in our analysis of Wt and Mp/Mp eyes at E13.5, with no differences observed between genotypes (Figure 4C–D). Consistent with these sites of expression, using the Fibrillin-2 specific polyclonal pAb868 antibody, we observed Wt Fibrillin-2 protein in extracellular regions of the periocular mesenchyme and in the anterior region of the eye between the neural retina and lens (Figure 4E). In contrast, Fibrillin-2Mp was absent from these extracellular areas and instead was observed within cells of the anterior eye (Figure 4F), with the strongest signal detected in the non-pigmented ciliary region. Fibrillin-2Mp was also observed in the retinal pigmented epithelium (RPE) and in the corneal mesenchyme (consistent with Fbn2 mRNA distribution). We then looked at Fibrillin-2 distribution in other tissues known to express Fbn2 and revealed similarly marked differences in protein localisation between Wt and Mp (Figure S4). In all samples analysed (mouse embryonic fibroblasts (MEFs); femoral articular cartilage and skin), Wt Fibrillin-2 protein was detectable in typical extracellular microfibril structures, but mutant protein was confined to intracellular foci adjacent to cell nuclei and was not observed in extracellular regions.
Fig. 4. Fibrillin-2Mp aggregated into large intracellular inclusions within the rough endoplasmic reticulum. 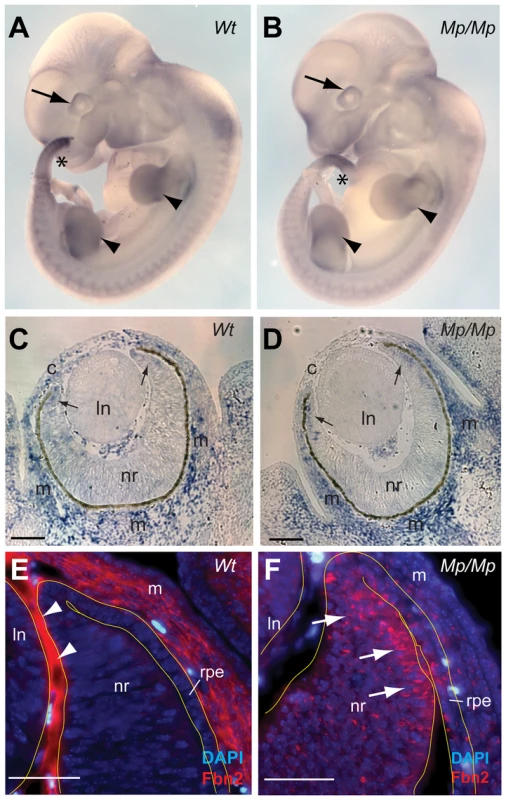
(A–B) Whole Mount In Situ Hybridisation to Fbn2 using antisense 3′-UTR riboprobes of Fbn2 for Wt and Isoc1 for Mp/Mp in early Wt and Mp/Mp embryos (E11.5) revealed the spaciotemporal expression of the variant Fbn2 alleles appeared unaffected by the genomic inversion and that cis-regulation of the genes was unchanged. Fbn2 was expressed in the developing eyes (arrows), limbs (arrowheads), and tail (asterisks), and expression was also observed in the somites. (C–D) Section In Situ Hybridisation for Fbn2 at E13.5 again showed that mutant Fbn2 expression was comparable to Wt in the eyes, with Fbn2 identified in the periocular (m) and corneal (c) mesenchyme, and faintly in the anterior retina (arrows). No expression was identified in the lens (ln). Scale bars in, 100 µm. (E–F) Immunohistochemical analysis of the anterior region of E13.5 Wt eyes illustrated that Fibrillin-2 was localised to extracellular regions in the corneal mesenchyme and in the region of apposition between the lens and neural retina (arrowheads). In contrast, Fibrillin-2Mp in Mp/Mp eyes was not observed in these extracellular locations but instead appeared to be retained within cells throughout the developing eye, with the anterior neural retina (arrows) and adjacent RPE displaying the most numbers of positive-cells. Scale bars, 50 µm. Fibrillin-2Mp formed stable fibrils within the endoplasmic reticulum
To further identify the consequence of the Mp truncation to Fibrillin-2 distribution, and to explore possible consequences to expressing cells, we performed transmission electron microscopy (TEM) of eye scleral cells on mutant samples. These revealed inclusions located within the enlarged rough endoplasmic reticulum (rER) lumen that were organised into thick fibril-like aggregates, with apparent structural periodicity (Figure 5A–B). The inclusions were confirmed as Fibrillin-2 positive by immuno-EM (Figure 5C) and by immunofluorescence (Figure 5D). Deposits of Fibrillin-1-containing fibrils with similar ultrastructural appearance to the Fibrillin-2Mp inclusions have been previously identified in extracellular normal human cartilage matrix [43], however the inclusions in our study did not contain Fibrillin-1 when tested by immunofluorescence (Figure S4), or by immuno-EM (not shown), and were exclusively intracellular. Nevertheless, both these results suggest that under some physiological conditions, Fibrillins can produce atypically banded fibrils. Immunofluorescence confirmed that the Fibrillin-2Mp inclusions were ER-specific, and did not localise within the golgi (figure 5E–G).
Fig. 5. Fibrillin-2Mp inclusions resulted in ER-stress and cell death in the developing Mp eye. 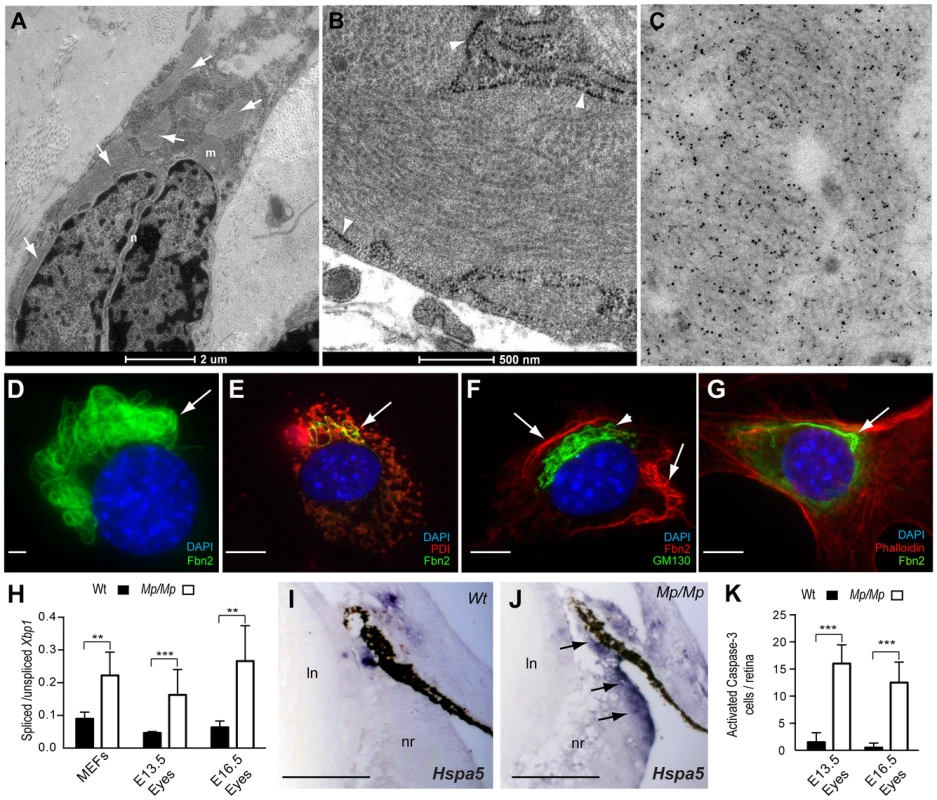
(A) TEM micrograph of Mp/Mp ocular scleral cells revealed multiple intracellular inclusions located within the enlarged rough endoplasmic reticulum (rER) membrane (arrows). Scale bar, 2 µm. (B) Enlarged micrograph illustrating the structural periodicity of the inclusions with regular banding, possibly representing heterotypic fibrils. Arrowheads indicate rER membrane. Scale bar, 500 nm. (C) Immuno-gold labelling of the inclusions in Mp/Mp scleral cells with polyclonal anti-Fibrillin-2 antibody pAb868 and beads conjugated to secondary antibody revealed the inclusions to be composed of Fibrillin-2. (D) Fibrillin-2 immunofluorescence in Mp MEF cultures illustrated the perinuclear localisation and bundle-like organisation of the mutant protein aggregates. Scale bar, 10 µm. (E) Co-immunofluorescence with anti-ER marker PDI (red) confirmed the Fibrillin-2Mp (green) inclusions colocalised within the ER (yellow stain; arrow). (F) Staining with the golgi-specific marker GM130 (green staining; arrowhead) showed that Fibrillin-2Mp (red staining, arrows) was not localised within the Golgi. (G) Non-overlapping staining of Fibrillin-2 (green) and phalloidin (red) indicated that the inclusions were not located in the cytoplasm. Scale bars in E–G, 100 µm. (H) The ratio of Xbp-1s to Xbp-1u was significantly increased in Mp/Mp compared to Wt in RNA samples collected from MEFs and from Mp/Mp eyes at E13.5 and E16.5. (I) Section In Situ for ER-stress marker Hspa5 mRNA revealed no staining in Wt retinas at E16.5, (J) however there was clear signal in the non-pigmented ciliary epithelium (arrows) in Mp/Mp. Scale bars, 50 µm. (K) (J) Activated-Caspase-3 stained cells from E13.5 and E16.5 Wt and Mp/Mp retinas were quantified and revealed significant increase in mutant eyes. Error bars are s.d. for H & K (**P<0.005; *** P<0.001). Fibrillin-2Mp inclusions triggered ER-stress
We hypothesised that the presence of large inclusions could disrupt normal ER mechanisms and secretory function. First, we analysed expression of Xbp1, a widely used marker ER-stress or the unfolded protein response (UPR)[44]. Specifically, a quantitative assay was devised to measure the ratios of unspliced versus UPR-specific Xbp1 transcripts. Validation was performed with cells cultured with increasing concentrations of tunicamycin (not shown). We then assayed Mp/Mp and Wt RNA samples from MEFs and from dissected E13.5 and E16.5 embryonic eye tissues, and found that relative levels of Xbp1s were significantly increased in all Mp/Mp samples compared to Wt (Figure 5H). We next analysed embryonic ocular expression of the UPR chaperone protein Hspa5, and observed expression in Mp, but not in Wt, specifically in the ciliary epithelium (Figure 5I–J). Immunostaining for Protein Disulphide Isomerase (PDI), revealed that Mp embryos had enhanced staining in the non-pigmented ciliary epithelium and adjoining RPE, co-localising with the Fibrillin-2Mp inclusion-positive cells, whereas equivalent Wt cells were negative for PDI expression (Figure S5). In combination, these data confirmed ER-stress in developing Mp eyes, in regions consistent with the distribution of Fibrillin-2Mp inclusions at the anterior developing ciliary margin.
We then investigated whether the presence of Fibrillin-2Mp inclusions resulted in an increase in apoptosis in the developing Mp eye. We reasoned this could provide a straightforward explanation for the loss of retinal and ciliary epithelial cells observed in Mp. Immunostaining for activated Caspase-3 revealed increased numbers of apoptotic cells in the embryonic mutant retinas compared to Wt (Figure 5K). The positive cells were evenly dispersed throughout the entire neural retina, but surprisingly these were not enriched in the areas with the highest number of Fibrillin-2Mp-positive cells, i.e. the non-pigmented ciliary epithelium and adjacent RPE (Figure S5). This suggested that additional or alternative pathological mechanisms account for the disruptions to the developing anterior eye in Mp.
Protein secretion defects in UPR-affected cells
We then considered whether the presence of Fibrillin-2Mp inclusions and the consequential induction of UPR prevented the secretion of other extra-cellular proteins expressed in the same cells. We took multiple independent primary Wt and Mp MEF cultures and analysed their conditioned media using quantitative label-free mass-spectrometry. We found reduction in the secretion of many proteins, of which the most severely affected were collagens (Col6a1; Col3a1; Col1a2; Col1a1; and Col12a1), Periostin and Follistatin-like 1 (Figure 6A). Confirmation of the MS data was performed by immunoblotting for one of the identified enriched proteins, Col6a1, which revealed elevated intracellular protein levels in Mp (Figure 6B). Albumin supplemented into the culture media was detected at equivalent levels by these analyses, and Coomassie-stained SDS-PAGE gels of total secreted proteins were used as a further experimental control. To ensure that transcriptional down-regulation was not responsible for the differences seen in protein levels, we assessed the relative mRNA levels of the affected proteins and found the expression of all the genes to be equivalent to Wt (Figure 6C). As a complementary assay, we transfected Wt human RPE1 cells with a plasmid encoding a tagged secreted protein (Wnt3A-FLAG) and added tunicamycin (Tn) to the cell culture medium to induce the UPR. Increased levels of Hspa5 in cell lysates confirmed that the Tn-treated cells were undergoing the UPR, and we saw a coincident reduction to secreted Wnt-FLAG in these cells compared to similarly transfected cells without Tn (Figure S6). We also observed a reduction in the mass of the migrating Wnt-FLAG protein, consistent with reduced glycosylation in the Tn-treated cells. Thus, Fibrillin-2Mp inclusions have a negative effect on protein secretion, and this is a likely consequence of ER-stress.
Fig. 6. Fbn2Mp inclusions affect the secretion of extracellular proteins. 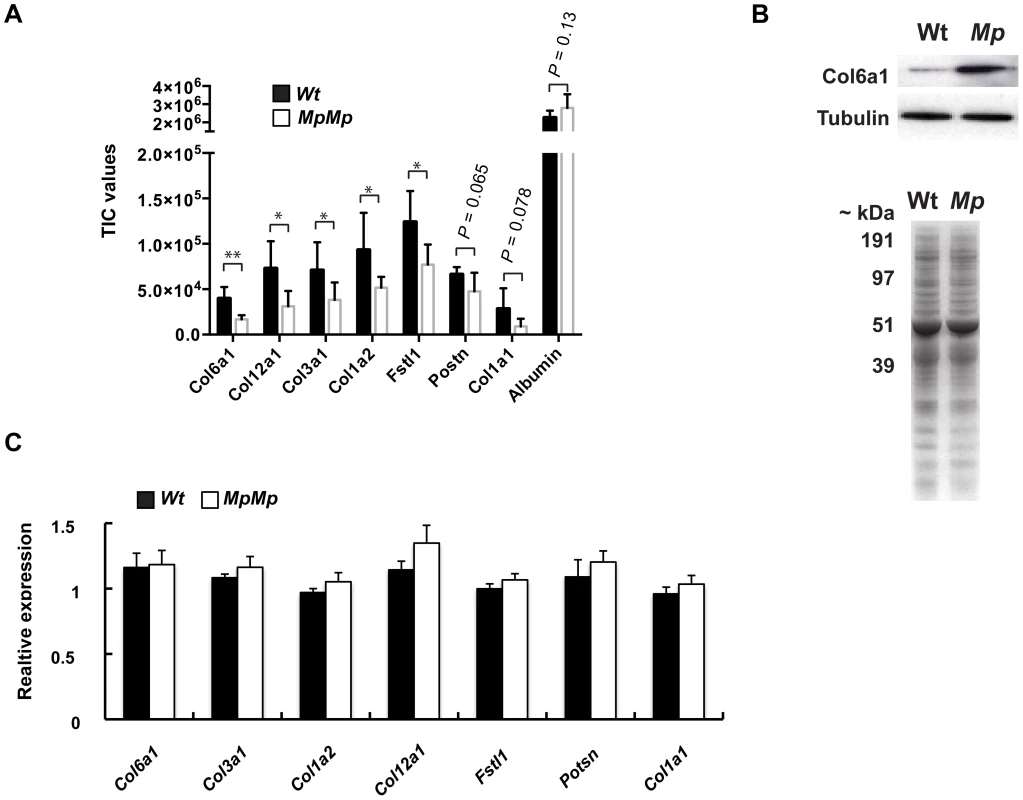
(A) Conditioned media samples from primary MEF cultures collected from independent embryos (n = 5 cell lines for each genotype) revealed reductions in secretion of five separate Collagen-family proteins, Periostin and Follistatin-like 1 by quantitative proteomics using comparative peptide TIC analysis. As a control, the detected levels of BSA supplemented into the culture media were equivalent (Plots: mean TIC values of proteins secreted from Mp/Mp and Wt cells; error bars, s.d. *P<0.05; **P<0.01). (B) Top: Immunoblot of total MEF cell lysates with anti-Col6a1 antibody revealed increased intracellular protein in Mp/Mp versus Wt. Below: Coomassie stained SDS-PAGE gel of conditioned media showed no observable total protein differences between samples. (C) Quantitative RT-PCR analysis of RNA from MEF samples revealed no significant reduction in mRNA levels of these genes between the two genotypes, indicating that transcription was not the primary cause of reduced secretion. RNA was extracted from the same cultures as the conditioned media was collected. Graph represents the mean cT values for Wt and Mp/Mp, divided by the respective mean cT values for Actin mRNA (All PCR reactions were carried out in triplicate, n = 5 per genotype, error bars, s.d. No differences between means reached significance, Students t-test). Discussion
In humans, mutations in the FIBRILLIN gene family result in fibrillinopathies, diseases that display a broad phenotypic spectrum consistent with their developmental expression patterns [45], [46]. Autosomal dominant (AD), and less commonly autosomal recessive, mutations in FBN1 result in the skeletal, cardiovascular and ocular features of Marfan syndrome (MIM #154700), whereas AD FBN2 mutations typically affect skeletal regions in arthrogryposis, in which patients usually present with long digits, distal joint contractures and crumpled ears, but rarely with ocular or cardiovascular complications. Haploinsufficiency for FBN1 appears to be the mechanism responsible for the majority of cases of Marfan syndrome [47]. However, dominant-negative effects appear to be responsible for the very severe phenotypes associated with neonatal and infantile Marfan syndrome. In these cases the causative mutations cluster within exons 24–32 [48]. The cognate region of FBN2 is the most commonly mutated in Beal syndrome and would plausibly be associated with a similar dominant negative effect [49]. An interesting phenotype of neonatal progeroid features, Marfan syndrome and generalised lipodytrophy (Wiedemann-Rautenstrauch syndrome) has recently been described in association with FBN1 mutations located in the C-terminal domain of the protein [50], [51], [52]. The pathogenic mechanism is not yet clear but it will be important to determine if the mutant protein is normally secreted.
Mp displayed phenotypic overlap with the human fibrillinopathies, including contractures, small ears and skeletal malformations in the limbs. Studies in mice have shown that the Fibrillins are differentially expressed through embryonic development, with Fbn2 transcripts appearing first and coinciding with early morphogenesis and elastic fibre assembly. The overlapping, but delayed expression of Fbn1 is consistent with a more structural role after defined organ structures have been established. Although both Fibrillin-1 and Fibrillin-2 are present during embryogenesis, neither is absolutely necessary, as microfibrils and elastic fibres are formed in the absence of either protein. To date, five separate loss of function mouse mutations in Fbn2 have been described, all with highly penetrant homozygous hind-limb syndactyly but no ocular phenotype [30], [31], [32]. Thus Mp is likely a Fibrillin-2 null mutation in limb development but its ocular phenotype clearly resulted from a separate mechanism, specific to its genomic inversion.
It cannot be ruled out that the extraneous amino acids added by the out-of-frame fusion to Isoc1 conferred a gain-of-function to this chimeric protein, preventing its secretion. Although no pro-protein cleavage by furin has previously been shown directly for Fibrillin-2 at the C-terminal domain, the inclusion data presented here, together with the high degree of conservation of furin-cleavage motifs among all Fibrillins [53], strongly suggests that this processing is common to all Fibrillins but is lost in Fibrillin-2Mp. It seems likely then that the failure of proprotein processing is the cause of the intracellular accumulations, and it is the failure of secretion of Fibrillin-2Mp that is the cause of the UPR.
In addition to the primary secretion failure, ER-stress is likely to reduce the protein synthesis and/or secretory capacity of affected cells [54]. We observed a significant reduction in several secreted proteins from Mp cultured primary cells. The cells in the developing Mp eye that contain the highest number of Fibrillin-2Mp inclusions and display a UPR are those at the anterior rim of the optic cup in the non-pigmented ciliary epithelium. These cells produce various secreted factors including collagen proteins required for the accumulation and maintenance of ocular vitreous [55], [56]. In this context it is interesting that the quantitative MS analysis of the Mp versus Wt MEFs identified several collagen molecules among the most altered of the secreted proteins. The reduction of posterior vitreous chamber is an early and prominent feature of the developmental pathology in the Mp eye. While this feature could be due to failure of collagen secretion, is it also plausibly the consequence of a failure in morphogenesis of the ciliary apparatus itself. Cells in the Fbn2-expressing region of the embryonic eye also secrete Wnt2b, which is of interest in the context of the reduction to BAT-gal reporter activity that we observed in Mp in this region. Disruption of Wnt2b production results in pan-ocular phenotypes overlapping with those observed in Mp, including retinal rosettes, lens abnormalities, iris hypoplasia and the absence of vitreous [27], [57]. Wnt2b from the anterior retina has also been shown to confer layer-organising properties to the central retina [58]. In Mp, a reduction of Wnt2b from the ciliary body may be predicted as a result of UPR-mediated apoptosis (i.e. fewer cells to produce), but may also result from reduction to the normal secretion of Wnt2b through the ER of Fbn2 expressing cells, thus reducing the amount of bioactive paracrine signal available to the developing eye.
One question that arises from this study is why are developing eyes the only tissues that are sensitive to Fibrillin-2Mp ER-stress, and not other expressing cells? If we trust the phenotype as an assay both in the Fbn2 null and Mp animals, this would suggest that Fibrillin-2 has few non - redundant roles (digit development) and perhaps in Mp only the developing retinal cells that normally secrete Fbn2 are co-expressing gene products that are also critical for development. Skin tissue appeared surprisingly lax compared to Wt (data not shown), and animals were runted. These data suggest there may also be pathogenic consequences to other tissues, but that these do not manifest as profoundly as in the developing eyes, indicating either elevated sensitivity to ER-stress, or intolerance to reductions of co-secreted proteins in the developing eye.
It is possible that there are other mechanisms mediating the protein secretion abnormalities observed in Mp in addition to the ER stress. One such mechanism could relate to the capacity for the ER-localised Fibrillin-2Mp to bind other proteins. Mutations in COMP cause human pseudoachondroplasia (PSACH; MIM#177170) and multiple epiphyseal dysplasia (EDM1; MIM#132400), diseases of short stature and osteoarthiritis, both of which are associated with retention of mutant COMP within the rER. The mutations cause a variety of plausibly pathological effects including: a decrease in cellular viability, failure of COMP to be secreted into the extracellular matrix, and a trans-acting effect on co-expressed proteins [59], [60], [61]. Mutant COMP accumulates within the enlarged rER of in vivo cartilage and associates with co-expressed type IX collagen, with the secretion and extracellular localisation of these factors correspondingly affected. However, recent analyses of PSACH mouse models have shown that these effects are not mediated via UPR but rather through a novel form of chondrocyte stress [62]. The best-known endogenous Fibrillin-2 binding partners are the family of latent TGFbeta binding proteins (LTBPs). In this regard, the observed reduction in secretion of the modulator of BMP signalling factor, Follistatin-like 1, may be interesting, although there is currently no direct evidence that this protein binds to either LTBPs or Fibrillin-2. Interestingly Fibrillin-1 is also a binding partner of Fibrillin-2 and is similarly processed through the ER, but we were not able to find any evidence of Fibrillin-1 colocalisation to the Mp inclusions (see Figure S4). However, we did observe increased intracellular retention of Col6a1, suggesting that intracellular association of Collagens to Fibrillin-2Mp may occur. Future work will be required to determine whether collagens, or Wnt2b, directly associate with Fibrillin-2Mp inclusions in the developing eye.
We have shown here that the ocular phenotypes in Mp are caused by a truncating mutation in Fbn2. Perturbation of normal ER function, via mutations similar to Mp in proteins that functionally pass through the ER, may lead to abnormal phenotypes that cannot be predicted from knowledge of the primary mutant allele. The terms antimorphic, and the more commonly used dominant-negative, are used to describe a worse than heterozygous effect on the function of the protein encoded by the mutated gene or on the cognate functional pathway. Here we propose that the Fbn2Mp mutation is disrupting one or more developmental functions in which the gene product itself plays no direct part. The compromised gene products are simply expressed in the same cell and share a secretory pathway. We suggest the term synodiporic (fellow traveller) effect for this phenomenon. Thus, the Mp mouse provides clues towards a genetic mechanism of activating disease mutation, which may be phenotypically more severe than those predicted simply by loss of protein function, or may elicit an entirely novel phenotype due to ER perturbation or any other mechanism affecting the production or availability of secondary co-expressed proteins. This has wide-ranging consequences for disease-gene mapping, and for disease management and therapy in human conditions.
Materials and Methods
Ethics statement
The Mp mouse was re-derived from frozen sperm obtained from the Jackson Lab (http://www.jax.org/mmdb/results/Sp_Mp.htmlx). All work was carried out under UK Home Office Project License 60/3785 (IJ Jackson, MRC Human Genetics Unit).
Rederivation and phenotyping of Mp
Heterozygote (Mp/+) sperm was used to perform intra-cytoplasmic sperm injection using oocytes from a donor female F1 (CBA x C57BL/6J). Phenotypically affected offspring were crossed to C57BL/6J to facilitate genetic mapping and to CD1 for phenotype and penetrance analysis. The Mp mutation phenotype has been described [29]. Heterozygote animals were identified by small eyes with/or without reduced pinnae size. Apparent anophthalmia (demonstrated by failure to open eyelids) in combination with distal limb syndactyly was used to identify homozygote animals. Records of each phenotype were accumulated for penetrance analysis in crosses to both C57BL/6J and CD1 strains.
Skeletal analysis
Adult animals were culled with lethal intravenous doses of Euthatal (Merial Animal Health Ltd. Essex, UK), treated in 95% ethanol for 24 hours; then 72 hours in 100% acetone; a minimum of 3 days in stain solution (1 part 0.3% alcian blue in 70% ethanol; 1 part 0.1% alazarin red in 95% ethanol; and 1 part acetic acid, in 17 parts 70% ethanol); 3 days in 1% potassium hydroxide (KOH); 3 days in 1% KOH/30% glycerol; and 24 hours in each 1% KOH/50% glycerol and 1% KOH/70% glycerol and finally stored in 100% glycerol.
Mouse eye processing for histology and immunofluorescence
Embryos and eyes were dissected and rinsed in cold phosphate buffered saline (PBS) and then fixed in 4% PFA overnight at 4°C; rinsed again in PBS and dehydrated in serial dilutions of ethanol and paraffin embedded. All adult eye sections were cut using a standard microtome at 12 µm and oriented in the sagittal plane; embryonic sections were cut at 6 µm and oriented in the coronal plane. Haemotoxylin and Eosin staining was performed according to standard methods.
qRT-PCR and PCR
Details of all primers and sequences used in this study are in Table S1. DNA was extracted according to established protocols. RNA was isolated from tissues or from cultured mouse embryonic fibroblast (MEF) cells using TRIzol Reagent (Invitrogen) according to the manufacturers instructions. For cDNA, 1st Strand cDNA Synthesis Kit for RT-PCR (Roche), or Superscript III (Invitrogen) were used according to the manufacturers instructions. All PCR reactions were carried out with primers at 0.2 µM. Cycle conditions: 95°C×3 min; followed by 30 cycles of 95°C×30 s; 58°C×45 s; 72°C×45 s; followed by 10 min at 72°C. Quantitative RT-PCR was performed as above using Brilliant II SYBR Green QPCR Master Mix (Agilent) and an ABI HT7900.
Genome-wide mapping of Mp
Microsatellite markers were used for PCR-based mapping using genomic DNA samples from heterozygous (n = 38) offspring from the 3rd generation of outcrosses to C57BL/6J (for Wt, n = 30). In total 73× markers were selected that were informative between C3H and C57BL/6J alleles. A list of the markers used and peak sizes for each allele are available in Table S1. Equimolar samples were pooled together as either wild type or heterozygote DNA. Amplicons were analysed on the ABI PRISM 310 Genetic Analyzer. Peak heights for each allele were recorded and the ratios of C57BL/6J:C3H were calculated. For the chromosome 18 locus, non-fluorescently labeled microsatellite PCR products were resolved on 4% agarose gels. Oligonucleotides for fine mapping chromosome 18 are listed in Table S1.
3′-End cDNA amplification
Rapid Amplification of cDNA Ends (RACE) was used for the identification of the 3′-end of the Fbn2 transcript using an adaptor oligonucleotide primer (5′-GACTCGAGTCGACATCGATTTTTTTTTTTTTTTTT-3′) as the only primer in separate reverse transcription reactions with RNA extracted from phenotypically mutant and Wt animals as described above, using 1st Strand cDNA Synthesis Kit for RT-PCR (Roche) with standard conditions. The single-stranded cDNA species (cDNA) were then used in gene-specific PCR reactions with an adaptor-only primer (5′-GACTCGAGTCGACATCG-3′) and the Fbn2-specific primer (5′-GTCTCAGCCTTCCCTCTGTG-3′). Products from these separate reactions were then gel electrophoresed and candidate bands were excised and TA-cloned into the pGEM-Easy vector system (Promega), according to the manufacturers instructions. Sequence analysis was performed using T7 and SP6 vector-specific primers and Sequencher Version 4.8 (Gene Codes Corp. MI, USA) and then used as query sequences in BLAT search alignments against the mouse genome (http://genome.ucsc.edu/cgi-bin/hgBlat). A PCR-based genotyping assay was devised based on the genomic rearrangement identified at the Mp locus.
Transmission-Electron Microscopy (TEM)
Tissues were collected from freshly culled neonates (P2 and P8) and placed into sterile serum-free DMEM (Gibco). For ultrastructural observation, the tissue was fixed for 1–3 hours in 1.5% Glutaraldehyde/1.5% Paraformaldehyde with 0.05% tannic acid in DMEM, rinsed in DMEM then immersed in 1% OsO4 in DMEM for one hour. Tissues were dehydrated in a graded series of ethanol to 100%, washed in propylene oxide, then infiltrated and embedded in Spurr's epoxy. 60–90 nm ultrathin sections were contrasted with saturated uranyl acetate in 50% ethanol for 15 minutes followed by lead citrate for 3 minutes. For immunocytochemistry, fresh tissue was fixed in 0.1% glutaraldehyde/4% paraformaldehyde for 30 minutes on ice, rinsed in DMEM then 0.15 M TRIS-HCl (pH 7.4) overnight. Tissues were then exposed to a graded series of ethanol dilutions to 90% at progressively lower temperature to −20°C, infiltrated in LR White embedding media at −20°C, then polymerized at 60°C in a nitrogen atmosphere. Ultrathin sections mounted on formvar coated 1×2 mm nickel slot grids were immunolabeled using antibody pAb0868 specific to Fbn2, pAb9543 specific to Fbn1 and also control antibodies of irrelevant specificity followed by a combination of −5 and −10 nm secondary gold conjugates as previously described [42]. Sections were examined on either a Philips EM410LS TEM or an FEI G20 TEM operated at 120 kV.
Primary MEF cultures
Genotyped embryos were collected at E13.5 and the limbs and skin from each embryo were then finely chopped and cultured in DMEM (Gibco) with 20% foetal calf serum and 1% penicillin/streptomycin at 37°C; 5% CO2; 3% O2. The Wnt-FLAG containing plasmid was a kind gift from Dr Angela Lee and modified from a PCMxGFP2FLAG vector. Transfection of RPE1 cells with Wnt-FLAG were performed using Lipofectamine-LTX (Invitrogen), with pcDNA3.1 containing enhanced-GFP to control for transfection efficiency. Tunicamycin (Sigma) was used at a concentration of 0.5 µg/ml and carried in DMSO. Non-Tn treated cells were exposed to carrier alone.
Xbp1 splicing assay
Alternatively spliced Xbp1transcripts were differentially identified using RT-PCR and the ABI PRISM 310 Genetic Analyzer, using a FAM-labeled forward, and an unlabelled reverse primers specific for the spliced region of Xbp1 (Table S1). RNA samples were collected from embryonic eyes and MEF cultures and cDNA was synthesized. The unspliced (Xbp1u) transcript was 289 bp and the spliced transcript (Xbp1s) was 263 bp. The assay was verified using tunicamycin to induce ER-stress in cultured MEFs (data not shown). Peak heights for each amplicon were measured using the ABI PRISM 310 Genetic Analyzer, and means were calculated and displayed graphically.
Immunohistochemistry
All antibodies used in this study are listed in Table S2. For immunofluorescence, primary MEFs were cultured on glass coverslips as described above for 72 hours and media was replaced with serum-free media for 16 hours. Coverslips were fixed (i) for PDI and GM130 staining - with 3.7% formaldehyde on ice for 10 minutes, or (ii) for Fbn2 with acetone at −20°C for 15 minutes, rinsed once in PBS and then permeabilised in PBS+0.5% Triton-X-100 (Sigma) for 10 minutes, blocked in PBS+10% serum for 1 hour, and incubated with primary antibodies overnight at 4°C. Coverslips were washed 3×5 minutes in PBS, and incubated in Alexa Fluor F(ab′) fragment (Invitrogen) secondary antibodies diluted 1∶1000 in blocking buffer for 1 hour and mounted with ProLong Gold Antifade reagent (Invitrogen). For immunohistochemistry, paraffin sections were de-waxed and antigen retrieval was performed by boiling slides in 0.1 M Citrate buffer (pH 6.0) for 5 minutes. Paraffin or cryosection samples were then rinsed in PBS and blocked for 1 hr in buffer containing 10% serum (heat inactivated at 60°C for 1 hr); 1% BSA; 0.1% Tween-20; 0.05% Triton-X-100, all in PBS. Primary and secondary antibodies were applied as before. Colorimetric immunohistochemistry was performed using Vectastain ABC kits (Vector Laboratories) with alkaline phosphatase detection and BM Purple (Roche). Imaging was performed using a Zeiss Axioplan II fluorescence microscope with Plan-neofluar objectives. Images were captured using a Coolsnap HQ CCD camera (Photometrics Ltd, Tucson, AZ). Image analysis was performed using IPLab Spectrum Software (Scanalytics Corp, Fairfax, VA).
RNA In Situ analysis and riboprobe synthesis
Riboprobe synthesis primers are listed in Table S1. Purified PCR products were incubated for 37°C for 2 h in a 20 µl reaction containing: 2 µl transcription buffer; 1 µl RNase inhibitor; 2 µl DIG RNA labelling mix and T7 RNA polymerase (all Roche); in ultra-pure H2O. Riboprobes were then DNAseI treated and Sodium-acetate/Ethanol precipitated. Whole-mount in situ hybridization to mouse embryos was carried out as previously described [63]. For section in situ, tissue sections were prepared as described for immunohistochemistry, and processed as described in J. Rainger's doctoral thesis (available on request). X-gal staining was performed on dissected embryos for beta-galactosidase activity as described in [18].
Secretion assays
For secretion assays, 1×105 cells were seeded into 6-well plates and cultured under normal conditions for 3 days. Five independent cell lines were used per genotype. Media was removed and replaced with 1 ml DMEM (Gibco) with reduced serum and cultured for a further 72 hours. The conditioned media was collected and 500 µl was concentrated 10 fold using an Amicon Ultra 30 K (Millipore) centrifugal filter according to the manufacturers instructions. Concentrated conditioned media samples were then both (i) run on 10% NuPAGE Novex Bis-Tris Mini Gel SDS polyacrylamide gel (Invitrogen) and stained with 0.05% Coomassie Brilliant Blue (40% methanol, 10% acetic acid), and (ii) measured for total protein by Bradford assay, to ensure equal protein content per sample. Their protein composition in these media were analysed using a label-free shotgun proteomics method according to a previous report [64] with some modifications. First, the database search was performed using Proteome Discover (version 1.2) or Peaks (Peaks Scientific, version 6.0) with peptide false discovery rate constrained at 1%. Second, parent ion mass tolerance and fragment ion mass tolerances were set at 5 ppm and 0.6 Da. Third, Sieve software (version 2.0; Thermo Scientific) and Peaks (Peaks Scientific) were used to compare protein abundance with a total ion current normalization. Only more than two peptides per protein detectable was considered and each protein was expressed as a ratio Mp/Mp:Wt according to Asara et al [65]. For transcript analyses, cells were dissociated and counted using a haemocytometer and total RNA extractions were performed with Trizol reagent (Invitrogen) and cDNA prepared. qRT-PCR was performed on ABI HT7900 using 0.5 M sequence-specific primers for Col6a1; Col3a1; Col1a2; Col12a1; Fstl1; Postn; and Actn1 with Brilliant II SYBR Green QPCR mix (Agilent) and 0.4 µl of cDNA template per 20 µl reaction. Each sample was run in triplicate per qRT-PCR reaction and the data presented represents the mean of 5 independent samples per genotype and three separate qRT-PCR technical repeat. For relative expression, all CT values were normalized to CT-ß-Actin.
Immunoblotting
Protein samples were run on NuPage SDS polyacrylamide gels and transferred to a PVDF membrane, blocked for 1 hour in 5% non-fat dry milk diluted in PBS and incubated overnight in primary antibodies diluted in block. Membranes were washed extensively in PBS and incubated in the relevant secondary IgG-HRP antibody diluted in block for 1 hour at room temperature. Membranes were then washed again and immuno-complexes were visualized with Amersham ECL Western Blotting Detection Reagent (GE Healthcare Life Sciences).
Supporting Information
Zdroje
1. WormanHJ, FongLG, MuchirA, YoungSG (2009) Laminopathies and the long strange trip from basic cell biology to therapy. J Clin Invest 119 : 1825–1836.
2. RobertsonSP (2005) Filamin A: phenotypic diversity. Curr Opin Genet Dev 15 : 301–307.
3. WilkieAO (2005) Bad bones, absent smell, selfish testes: the pleiotropic consequences of human FGF receptor mutations. Cytokine Growth Factor Rev 16 : 187–203.
4. Roussel BDKA, MirandaE, CrowtherDC, LomasDA, MarciniakSJ (2013) Endoplasmic reticulum dysfunction in neurological disease. Lancet Neurol 12 : 105–118.
5. XiaK, MaH, XiongH, PanQ, HuangL, et al. (2010) Trafficking abnormality and ER stress underlie functional deficiency of hearing impairment-associated connexin-31 mutants. Protein Cell 1 : 935–943.
6. TsangKY, ChanD, CheslettD, ChanWC, SoCL, et al. (2007) Surviving endoplasmic reticulum stress is coupled to altered chondrocyte differentiation and function. PLoS Biol 5: e44.
7. VijN, FangS, ZeitlinPL (2006) Selective inhibition of endoplasmic reticulum-associated degradation rescues DeltaF508-cystic fibrosis transmembrane regulator and suppresses interleukin-8 levels: therapeutic implications. J Biol Chem 281 : 17369–17378.
8. MorrisonD, FitzpatrickD, HansonI, WilliamsonK, van HeyningenV, et al. (2002) National study of microphthalmia, anophthalmia, and coloboma (MAC) in Scotland: investigation of genetic aetiology. Journal of Medical Genetics 39 : 16–22.
9. StollC, AlembikY, DottB, RothMP (1997) Congenital eye malformations in 212,479 consecutive births. Ann Genet 40 : 122–128.
10. FitzpatrickDR, van HeyningenV (2005) Developmental eye disorders. Curr Opin Genet Dev 15 : 348–353.
11. FantesJ, RaggeNK, LynchSA, McGillNI, CollinJR, et al. (2003) Mutations in SOX2 cause anophthalmia. Nat Genet 33 : 461–463.
12. RaggeNK, LorenzB, SchneiderA, BushbyK, de SanctisL, et al. (2005) SOX2 anophthalmia syndrome. Am J Med Genet A 135 : 1–7 discussion 8.
13. RaggeNK, BrownAG, PoloschekCM, LorenzB, HendersonRA, et al. (2005) Heterozygous mutations of OTX2 cause severe ocular malformations. Am J Hum Genet 76 : 1008–1022.
14. PasuttoF, StichtH, HammersenG, Gillessen-KaesbachG, FitzpatrickDR, et al. (2007) Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet 80 : 550–560.
15. ReisLM, TylerRC, SchneiderA, BardakjianT, StolerJM, et al. (2010) FOXE3 plays a significant role in autosomal recessive microphthalmia. Am J Med Genet A 152A: 582–590.
16. AbouzeidH, BoissetG, FavezT, YoussefM, MarzoukI, et al. (2011) Mutations in the SPARC-related modular calcium-binding protein 1 gene, SMOC1, cause waardenburg anophthalmia syndrome. Am J Hum Genet 88 : 92–98.
17. OkadaI, HamanoueH, TeradaK, TohmaT, MegarbaneA, et al. (2011) SMOC1 is essential for ocular and limb development in humans and mice. Am J Hum Genet 88 : 30–41.
18. RaingerJ, van BeusekomE, RamsayJK, McKieL, Al-GazaliL, et al. (2011) Loss of the BMP antagonist, SMOC-1, causes Ophthalmo-acromelic (Waardenburg Anophthalmia) syndrome in humans and mice. PLoS Genet 7: e1002114.
19. GlaserT, JepealL, EdwardsJG, YoungSR, FavorJ, et al. (1994) PAX6 gene dosage effect in a family with congenital cataracts, aniridia, anophthalmia and central nervous system defects. Nat Genet 7 : 463–471.
20. Gerth-Kahlert CWK, AnsariM, RaingerJ, HignstV, ZimmermannT, et al. (2013) Clinical and Mutation Analysis of 51 Probands with Anophthalmia and/or Severe Microphthalmia from a Single Centre. Molecular Genetics & Genomic Medicine 1 : 15–31.
21. FurutaY, HoganBL (1998) BMP4 is essential for lens induction in the mouse embryo. Genes Dev 12 : 3764–3775.
22. KamachiY, UchikawaM, CollignonJ, Lovell-BadgeR, KondohH (1998) Involvement of Sox1, 2 and 3 in the early and subsequent molecular events of lens induction. Development 125 : 2521–2532.
23. MatsushimaD, HeavnerW, PevnyLH (2011) Combinatorial regulation of optic cup progenitor cell fate by SOX2 and PAX6. Development 138 : 443–454.
24. BeebeDC (1986) Development of the ciliary body: a brief review. Trans Ophthalmol Soc U K 105 (Pt 2) 123–130.
25. AgathocleousM, IordanovaI, WillardsenMI, XueXY, VetterML, et al. (2009) A directional Wnt/beta-catenin-Sox2-proneural pathway regulates the transition from proliferation to differentiation in the Xenopus retina. Development 136 : 3289–3299.
26. Van RaayTJ, MooreKB, IordanovaI, SteeleM, JamrichM, et al. (2005) Frizzled 5 signaling governs the neural potential of progenitors in the developing Xenopus retina. Neuron 46 : 23–36.
27. ChoSH, CepkoCL (2006) Wnt2b/beta-catenin-mediated canonical Wnt signaling determines the peripheral fates of the chick eye. Development 133 : 3167–3177.
28. TaranovaOV, MagnessST, FaganBM, WuY, SurzenkoN, et al. (2006) SOX2 is a dose-dependent regulator of retinal neural progenitor competence. Genes Dev 20 : 1187–1202.
29. PhippsE (1964) Mp. Mouse News Letter 31 : 41.
30. Arteaga-SolisE, GayraudB, LeeSY, ShumL, SakaiL, et al. (2001) Regulation of limb patterning by extracellular microfibrils. J Cell Biol 154 : 275–281.
31. ChaudhrySS, GazzardJ, BaldockC, DixonJ, RockMJ, et al. (2001) Mutation of the gene encoding fibrillin-2 results in syndactyly in mice. Hum Mol Genet 10 : 835–843.
32. MillerG, NeilanM, ChiaR, GheryaniN, HoltN, et al. (2010) ENU mutagenesis reveals a novel phenotype of reduced limb strength in mice lacking fibrillin 2. PLoS One 5: e9137.
33. MarettoS, CordenonsiM, DupontS, BraghettaP, BroccoliV, et al. (2003) Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc Natl Acad Sci U S A 100 : 3299–3304.
34. JohnsonKR, CookSA, ZhengQY (1998) The original shaker-with-syndactylism mutation (sy) is a contiguous gene deletion syndrome. Mamm Genome 9 : 889–892.
35. LeeB, GodfreyM, VitaleE, HoriH, MatteiMG, et al. (1991) Linkage of Marfan syndrome and a phenotypically related disorder to two different fibrillin genes. Nature 352 : 330–334.
36. PutnamEA, ZhangH, RamirezF, MilewiczDM (1995) Fibrillin-2 (FBN2) mutations result in the Marfan-like disorder, congenital contractural arachnodactyly. Nat Genet 11 : 456–458.
37. WangM, ClericuzioCL, GodfreyM (1996) Familial occurrence of typical and severe lethal congenital contractural arachnodactyly caused by missplicing of exon 34 of fibrillin-2. Am J Hum Genet 59 : 1027–1034.
38. Piha-GossackA, SossinW, ReinhardtDP (2012) The evolution of extracellular fibrillins and their functional domains. PLoS One 7: e33560.
39. ChangYF, ImamJS, WilkinsonMF (2007) The nonsense-mediated decay RNA surveillance pathway. Annu Rev Biochem 76 : 51–74.
40. NagyE, MaquatLE (1998) A rule for termination-codon position within intron-containing genes: when nonsense affects RNA abundance. Trends Biochem Sci 23 : 198–199.
41. DixonMJ, GazzardJ, ChaudhrySS, SampsonN, SchulteBA, et al. (1999) Mutation of the Na-K-Cl co-transporter gene Slc12a2 results in deafness in mice. Hum Mol Genet 8 : 1579–1584.
42. ShiY, TuY, De MariaA, MechamRP, BassnettS (2013) Development, composition, and structural arrangements of the ciliary zonule of the mouse. Invest Ophthalmol Vis Sci 54 : 2504–2515.
43. KeeneDR, JordanCD, ReinhardtDP, RidgwayCC, OnoRN, et al. (1997) Fibrillin-1 in human cartilage: developmental expression and formation of special banded fibers. J Histochem Cytochem 45 : 1069–1082.
44. YoshidaH, MatsuiT, YamamotoA, OkadaT, MoriK (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107 : 881–891.
45. ZhangH, HuW, RamirezF (1995) Developmental expression of fibrillin genes suggests heterogeneity of extracellular microfibrils. J Cell Biol 129 : 1165–1176.
46. QuondamatteoF, ReinhardtDP, CharbonneauNL, PophalG, SakaiLY, et al. (2002) Fibrillin-1 and fibrillin-2 in human embryonic and early fetal development. Matrix Biol 21 : 637–646.
47. Hilhorst-HofsteeY, HamelBC, VerheijJB, RijlaarsdamME, ManciniGM, et al. (2011) The clinical spectrum of complete FBN1 allele deletions. Eur J Hum Genet 19 : 247–252.
48. FaivreL, Collod-BeroudG, CallewaertB, ChildA, BinquetC, et al. (2009) Clinical and mutation-type analysis from an international series of 198 probands with a pathogenic FBN1 exons 24–32 mutation. Eur J Hum Genet 17 : 491–501.
49. ParkES, PutnamEA, ChitayatD, ChildA, MilewiczDM (1998) Clustering of FBN2 mutations in patients with congenital contractural arachnodactyly indicates an important role of the domains encoded by exons 24 through 34 during human development. Am J Med Genet 78 : 350–355.
50. GoldblattJ, HyattJ, EdwardsC, WalpoleI (2011) Further evidence for a marfanoid syndrome with neonatal progeroid features and severe generalized lipodystrophy due to frameshift mutations near the 3′ end of the FBN1 gene. Am J Med Genet A 155A: 717–720.
51. Graul-NeumannLM, KienitzT, RobinsonPN, BaasanjavS, KarowB, et al. (2010) Marfan syndrome with neonatal progeroid syndrome-like lipodystrophy associated with a novel frameshift mutation at the 3′ terminus of the FBN1-gene. Am J Med Genet A 152A: 2749–2755.
52. HornD, RobinsonPN (2011) Progeroid facial features and lipodystrophy associated with a novel splice site mutation in the final intron of the FBN1 gene. Am J Med Genet A 155A: 721–724.
53. RittyTM, BroekelmannT, TisdaleC, MilewiczDM, MechamRP (1999) Processing of the fibrillin-1 carboxyl-terminal domain. J Biol Chem 274 : 8933–8940.
54. TsangKY, ChanD, BatemanJF, CheahKS (2010) In vivo cellular adaptation to ER stress: survival strategies with double-edged consequences. J Cell Sci 123 : 2145–2154.
55. BishopPN, TakanosuM, Le GoffM, MayneR (2002) The role of the posterior ciliary body in the biosynthesis of vitreous humour. Eye (Lond) 16 : 454–460.
56. HalfterW, DongS, SchurerB, RingC, ColeGJ, et al. (2005) Embryonic synthesis of the inner limiting membrane and vitreous body. Invest Ophthalmol Vis Sci 46 : 2202–2209.
57. LiuH, MohamedO, DufortD, WallaceVA (2003) Characterization of Wnt signaling components and activation of the Wnt canonical pathway in the murine retina. Dev Dyn 227 : 323–334.
58. NakagawaS, TakadaS, TakadaR, TakeichiM (2003) Identification of the laminar-inducing factor: Wnt-signal from the anterior rim induces correct laminar formation of the neural retina in vitro. Dev Biol 260 : 414–425.
59. MaddoxBK, KeeneDR, SakaiLY, CharbonneauNL, MorrisNP, et al. (1997) The fate of cartilage oligomeric matrix protein is determined by the cell type in the case of a novel mutation in pseudoachondroplasia. J Biol Chem 272 : 30993–30997.
60. MerrittTM, BickR, PoindexterBJ, AlcornJL, HechtJT (2007) Unique matrix structure in the rough endoplasmic reticulum cisternae of pseudoachondroplasia chondrocytes. Am J Pathol 170 : 293–300.
61. DinserR, ZauckeF, KreppelF, HultenbyK, KochanekS, et al. (2002) Pseudoachondroplasia is caused through both intra - and extracellular pathogenic pathways. J Clin Invest 110 : 505–513.
62. SulemanF, GualeniB, GregsonHJ, LeightonMP, PirogKA, et al. (2012) A novel form of chondrocyte stress is triggered by a COMP mutation causing pseudoachondroplasia. Hum Mutat 33 : 218–231.
63. HarewoodL, LiuM, KeelingJ, HowatsonA, WhitefordM, et al. (2010) Bilateral renal agenesis/hypoplasia/dysplasia (BRAHD): postmortem analysis of 45 cases with breakpoint mapping of two de novo translocations. PLoS One 5: e12375.
64. WilsonR, DisebergAF, GordonL, ZivkovicS, TatarczuchL, et al. (2010) Comprehensive profiling of cartilage extracellular matrix formation and maturation using sequential extraction and label-free quantitative proteomics. Mol Cell Proteomics 9 : 1296–1313.
65. AsaraJM, ChristofkHR, FreimarkLM, CantleyLC (2008) A label-free quantification method by MS/MS TIC compared to SILAC and spectral counting in a proteomics screen. Proteomics 8 : 994–999.
Štítky
Genetika Reprodukčná medicína
Článek Interaction between and during Mammalian Jaw Patterning and in the Pathogenesis of SyngnathiaČlánek Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of Genes in Developing LimbsČlánek Transcription Factor Occupancy Can Mediate Active Turnover of DNA Methylation at Regulatory RegionsČlánek Tay Bridge Is a Negative Regulator of EGFR Signalling and Interacts with Erk and Mkp3 in the Wing
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2013 Číslo 12- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Stressing the Importance of CHOP in Liver Cancer
- The AmAZI1ng Roles of Centriolar Satellites during Development
- Flies Get a Head Start on Meiosis
- Recommendations from Jane Gitschier's Bookshelf
- And Baby Makes Three: Genomic Imprinting in Plant Embryos
- Bugs in Transition: The Dynamic World of in Insects
- Defining the Role of ATP Hydrolysis in Mitotic Segregation of Bacterial Plasmids
- Synaptonemal Complex Components Promote Centromere Pairing in Pre-meiotic Germ Cells
- Cohesinopathies of a Feather Flock Together
- Genetic Recombination Is Targeted towards Gene Promoter Regions in Dogs
- Parathyroid-Specific Deletion of Unravels a Novel Calcineurin-Dependent FGF23 Signaling Pathway That Regulates PTH Secretion
- MAN1B1 Deficiency: An Unexpected CDG-II
- Phosphate Flow between Hybrid Histidine Kinases CheA and CheS Controls Cyst Formation
- Basolateral Mg Extrusion via CNNM4 Mediates Transcellular Mg Transport across Epithelia: A Mouse Model
- Truncation of Unsilences Paternal and Ameliorates Behavioral Defects in the Angelman Syndrome Mouse Model
- Autozygome Sequencing Expands the Horizon of Human Knockout Research and Provides Novel Insights into Human Phenotypic Variation
- Huntington's Disease Induced Cardiac Amyloidosis Is Reversed by Modulating Protein Folding and Oxidative Stress Pathways in the Heart
- Low Frequency Variants, Collapsed Based on Biological Knowledge, Uncover Complexity of Population Stratification in 1000 Genomes Project Data
- Targeted Ablation of and in Retinal Progenitor Cells Mimics Leber Congenital Amaurosis
- Genomic Imprinting in the Embryo Is Partly Regulated by PRC2
- Binary Cell Fate Decisions and Fate Transformation in the Larval Eye
- The Stress-Regulated Transcription Factor CHOP Promotes Hepatic Inflammatory Gene Expression, Fibrosis, and Oncogenesis
- A Global RNAi Screen Identifies a Key Role of Ceramide Phosphoethanolamine for Glial Ensheathment of Axons
- Functional Analysis of the Interdependence between DNA Uptake Sequence and Its Cognate ComP Receptor during Natural Transformation in Species
- Cross-Modulation of Homeostatic Responses to Temperature, Oxygen and Carbon Dioxide in
- Alcohol-Induced Histone Acetylation Reveals a Gene Network Involved in Alcohol Tolerance
- Molecular Characterization of Host-Specific Biofilm Formation in a Vertebrate Gut Symbiont
- CRIS—A Novel cAMP-Binding Protein Controlling Spermiogenesis and the Development of Flagellar Bending
- Dual Regulation of the Mitotic Exit Network (MEN) by PP2A-Cdc55 Phosphatase
- Expanding the Marine Virosphere Using Metagenomics
- Detection of Slipped-DNAs at the Trinucleotide Repeats of the Myotonic Dystrophy Type I Disease Locus in Patient Tissues
- Interaction between and during Mammalian Jaw Patterning and in the Pathogenesis of Syngnathia
- Mutations in the UQCC1-Interacting Protein, UQCC2, Cause Human Complex III Deficiency Associated with Perturbed Cytochrome Protein Expression
- Reactivation of Chromosomally Integrated Human Herpesvirus-6 by Telomeric Circle Formation
- Anoxia-Reoxygenation Regulates Mitochondrial Dynamics through the Hypoxia Response Pathway, SKN-1/Nrf, and Stomatin-Like Protein STL-1/SLP-2
- The Midline Protein Regulates Axon Guidance by Blocking the Reiteration of Neuroblast Rows within the Drosophila Ventral Nerve Cord
- Tomato Yield Heterosis Is Triggered by a Dosage Sensitivity of the Florigen Pathway That Fine-Tunes Shoot Architecture
- Selection on Plant Male Function Genes Identifies Candidates for Reproductive Isolation of Yellow Monkeyflowers
- Role of Tomato Lipoxygenase D in Wound-Induced Jasmonate Biosynthesis and Plant Immunity to Insect Herbivores
- Meiotic Cohesin SMC1β Provides Prophase I Centromeric Cohesion and Is Required for Multiple Synapsis-Associated Functions
- Identification of Sphingolipid Metabolites That Induce Obesity via Misregulation of Appetite, Caloric Intake and Fat Storage in
- Genome-Wide Screen Reveals Replication Pathway for Quasi-Palindrome Fragility Dependent on Homologous Recombination
- Histone Methylation Restrains the Expression of Subtype-Specific Genes during Terminal Neuronal Differentiation in
- A Novel Intergenic ETnII-β Insertion Mutation Causes Multiple Malformations in Mice
- The NuRD Chromatin-Remodeling Enzyme CHD4 Promotes Embryonic Vascular Integrity by Transcriptionally Regulating Extracellular Matrix Proteolysis
- A Domesticated Transposase Interacts with Heterochromatin and Catalyzes Reproducible DNA Elimination in
- Acute Versus Chronic Loss of Mammalian Results in Distinct Ciliary Phenotypes
- MBD3 Localizes at Promoters, Gene Bodies and Enhancers of Active Genes
- Positive and Negative Regulation of Gli Activity by Kif7 in the Zebrafish Embryo
- A Hereditary Spastic Paraplegia Mouse Model Supports a Role of ZFYVE26/SPASTIZIN for the Endolysosomal System
- The CCR4-NOT Complex Mediates Deadenylation and Degradation of Stem Cell mRNAs and Promotes Planarian Stem Cell Differentiation
- Reconstructing Native American Migrations from Whole-Genome and Whole-Exome Data
- Contributions of Protein-Coding and Regulatory Change to Adaptive Molecular Evolution in Murid Rodents
- Comprehensive Analysis of Transcriptome Variation Uncovers Known and Novel Driver Events in T-Cell Acute Lymphoblastic Leukemia
- A -Acting Protein Effect Causes Severe Eye Malformation in the Mouse
- Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of Genes in Developing Limbs
- Germline Progenitors Escape the Widespread Phenomenon of Homolog Pairing during Development
- Transcription Factor Occupancy Can Mediate Active Turnover of DNA Methylation at Regulatory Regions
- Somatic mtDNA Mutation Spectra in the Aging Human Putamen
- ESCRT-I Mediates FLS2 Endosomal Sorting and Plant Immunity
- Ethylene Promotes Hypocotyl Growth and HY5 Degradation by Enhancing the Movement of COP1 to the Nucleus in the Light
- The PAF Complex and Prf1/Rtf1 Delineate Distinct Cdk9-Dependent Pathways Regulating Transcription Elongation in Fission Yeast
- Dual Regulation of Gene Expression Mediated by Extended MAPK Activation and Salicylic Acid Contributes to Robust Innate Immunity in
- Quantifying Missing Heritability at Known GWAS Loci
- Smc5/6-Mms21 Prevents and Eliminates Inappropriate Recombination Intermediates in Meiosis
- Smc5/6 Coordinates Formation and Resolution of Joint Molecules with Chromosome Morphology to Ensure Meiotic Divisions
- Tay Bridge Is a Negative Regulator of EGFR Signalling and Interacts with Erk and Mkp3 in the Wing
- Meiotic Crossover Control by Concerted Action of Rad51-Dmc1 in Homolog Template Bias and Robust Homeostatic Regulation
- Active Transport and Diffusion Barriers Restrict Joubert Syndrome-Associated ARL13B/ARL-13 to an Inv-like Ciliary Membrane Subdomain
- An Regulatory Circuit Modulates /Wnt Signaling and Determines the Size of the Midbrain Dopaminergic Progenitor Pool
- Variants Induce Differential Protection to Viruses in : A Phenotypic and Phylogenomic Analysis
- Base Pairing Interaction between 5′- and 3′-UTRs Controls mRNA Translation in
- Evidence That Masking of Synapsis Imperfections Counterbalances Quality Control to Promote Efficient Meiosis
- Insulin/IGF-Regulated Size Scaling of Neuroendocrine Cells Expressing the bHLH Transcription Factor in
- Sumoylated NHR-25/NR5A Regulates Cell Fate during Vulval Development
- TATN-1 Mutations Reveal a Novel Role for Tyrosine as a Metabolic Signal That Influences Developmental Decisions and Longevity in
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The NuRD Chromatin-Remodeling Enzyme CHD4 Promotes Embryonic Vascular Integrity by Transcriptionally Regulating Extracellular Matrix Proteolysis
- Mutations in the UQCC1-Interacting Protein, UQCC2, Cause Human Complex III Deficiency Associated with Perturbed Cytochrome Protein Expression
- The Midline Protein Regulates Axon Guidance by Blocking the Reiteration of Neuroblast Rows within the Drosophila Ventral Nerve Cord
- Tomato Yield Heterosis Is Triggered by a Dosage Sensitivity of the Florigen Pathway That Fine-Tunes Shoot Architecture
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



