-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Basolateral Mg Extrusion via CNNM4 Mediates Transcellular Mg Transport across Epithelia: A Mouse Model
Transcellular Mg2+ transport across epithelia, involving both apical entry and basolateral extrusion, is essential for magnesium homeostasis, but molecules involved in basolateral extrusion have not yet been identified. Here, we show that CNNM4 is the basolaterally located Mg2+ extrusion molecule. CNNM4 is strongly expressed in intestinal epithelia and localizes to their basolateral membrane. CNNM4-knockout mice showed hypomagnesemia due to the intestinal malabsorption of magnesium, suggesting its role in Mg2+ extrusion to the inner parts of body. Imaging analyses revealed that CNNM4 can extrude Mg2+ by exchanging intracellular Mg2+ with extracellular Na+. Furthermore, CNNM4 mutations cause Jalili syndrome, characterized by recessive amelogenesis imperfecta with cone-rod dystrophy. CNNM4-knockout mice showed defective amelogenesis, and CNNM4 again localizes to the basolateral membrane of ameloblasts, the enamel-forming epithelial cells. Missense point mutations associated with the disease abolish the Mg2+ extrusion activity. These results demonstrate the crucial importance of Mg2+ extrusion by CNNM4 in organismal and topical regulation of magnesium.
Published in the journal: Basolateral Mg Extrusion via CNNM4 Mediates Transcellular Mg Transport across Epithelia: A Mouse Model. PLoS Genet 9(12): e32767. doi:10.1371/journal.pgen.1003983
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003983Summary
Transcellular Mg2+ transport across epithelia, involving both apical entry and basolateral extrusion, is essential for magnesium homeostasis, but molecules involved in basolateral extrusion have not yet been identified. Here, we show that CNNM4 is the basolaterally located Mg2+ extrusion molecule. CNNM4 is strongly expressed in intestinal epithelia and localizes to their basolateral membrane. CNNM4-knockout mice showed hypomagnesemia due to the intestinal malabsorption of magnesium, suggesting its role in Mg2+ extrusion to the inner parts of body. Imaging analyses revealed that CNNM4 can extrude Mg2+ by exchanging intracellular Mg2+ with extracellular Na+. Furthermore, CNNM4 mutations cause Jalili syndrome, characterized by recessive amelogenesis imperfecta with cone-rod dystrophy. CNNM4-knockout mice showed defective amelogenesis, and CNNM4 again localizes to the basolateral membrane of ameloblasts, the enamel-forming epithelial cells. Missense point mutations associated with the disease abolish the Mg2+ extrusion activity. These results demonstrate the crucial importance of Mg2+ extrusion by CNNM4 in organismal and topical regulation of magnesium.
Introduction
Magnesium is an essential element involved in a wide variety of biological activities. Homeostasis of the magnesium level is strictly regulated by intestinal absorption and renal reabsorption, in which epithelia function as a barrier that permits selective and regulated transport of Mg2+ from apical to basolateral surfaces. Genomic analyses of familial cases of hypomagnesemia have identified key molecules directly involved in these processes. CLDN16, encoding claudin-16/paracellin-1, and CLDN19, encoding claudin-19, are mutated in recessive familial hypomagnesemia with hypercalciuria and nephrocalcinosis [1], [2]. These genes are highly expressed in the thick ascending limb of Henle's loop in the kidney and encode tight junction proteins, which form a cation-selective paracellular channel and drive the flux of Mg2+ between adjacent epithelial cells [3]. Another key molecule is TRPM6; mutations of TRPM6 cause recessive hypomagnesemia with secondary hypocalcemia [4], [5].
TRPM6 is a member of the transient receptor potential melastatin-related (TRPM) protein family and constitutes a Mg2+-permeable ion channel that localizes to the apical membrane of epithelial cells in the intestine and kidney [6]. In addition, it has also been shown that TRPM7, a close relative of TRPM6, plays an essential role in magnesium homeostasis in mice [7]. Therefore, TRPM6/TRPM7 plays a primary role in the apical entry of Mg2+ into cells, which is the first step in transcellular Mg2+ absorption across the epithelial barrier, another major Mg2+ transport pathway. To accomplish Mg2+ absorption, epithelial cells need to extrude Mg2+ via their basolateral membrane by opposing the inward-oriented driving force on Mg2+ imposed by the electrical membrane potential. Such a transcellular Mg2+ transport mechanism, involving both apical entry and basolateral extrusion, is evolutionarily conserved from Caenorhabditis elegans [8], [9], but molecules involved in basolateral Mg2+ extrusion have not been identified.
Ancient conserved domain protein/cyclin M (CNNM) constitutes a family of 4 integral membrane proteins that possess an evolutionarily conserved but uncharacterized domain from bacteria [10]. Recent genomic analyses have revealed a link between CNNM genes and magnesium homeostasis. Several single nucleotide polymorphisms in CNNM genes are associated with the serum magnesium level [11] and mutations in CNNM2 cause familial dominant hypomagnesemia [12]. The bacterial ortholog of these proteins in Salmonella, CorC, has been suggested to participate in Mg2+ efflux [13], while ectopically expressed CNNM2 in Xenopus oocytes showed voltage-dependent transport of several divalent cations, including Mg2+ [14]. Moreover, expression of a splice-variant of CNNM2 could restore the growth of a Mg2+-deficient Salmonella strain [15]. However, a study on CNNM2 expressed in HEK293 cells showed that it mediates a Na+ current [12]. Therefore, the importance of CNNMs in Mg2+ transport still remains unknown. Moreover, it has been reported that mutations in CNNM4 cause Jalili syndrome, which is characterized by recessive amelogenesis imperfecta (AI) and cone-rod dystrophy (CRD) [16], [17]. However, the molecular mechanism that links CNNM4 dysfunction to these pathological conditions and its relationship with magnesium homeostasis remain to be determined.
In this study, we generated CNNM4-knockout mice; these mice showed defects in amelogenesis and intestinal Mg2+ absorption. Endogenous CNNM4 is highly expressed in the mature ameloblasts and intestinal epithelia, and localizes at their basolateral membrane. Functional analyses at the molecular and organismal levels revealed a common role for CNNM4 in mediating transcellular Mg2+ transport by basolateral Mg2+ extrusion.
Results
Generation of CNNM4-knockout mice
To reveal the physiological function of CNNM4, we generated CNNM4-knockout mice. For this purpose, we used a commercially available embryonic stem (ES) cell clone, which possesses the neomycin-resistance gene cassette inserted in the genomic region between the first and second exons of CNNM4 by homologous recombination (Figure 1A). Chimeric heterozygous mice were obtained by blastocyst injection of the ES cells, and CNNM4-knockout mice were obtained by breeding. Successful recombination in the genomic DNA obtained from CNNM4+/− and CNNM4−/− mice was confirmed by Southern blotting (Figure 1B) and routine genotyping was done by PCR (Figure 1C). The gene cassette contains the splice acceptor sequence that forces mRNA splicing to occur artificially at the acceptor sequence, and the resulting mRNA is truncated after the second exon. Indeed, immunoblotting analyses with the anti-CNNM4 antibody (Figure S1) confirmed that CNNM4−/− mice lack expression of endogenous CNNM4 protein (Figure 1D). Both CNNM4+/− and CNNM4−/− mice were viable, with no gross abnormalities.
Fig. 1. Generation of CNNM4-knockout mice. 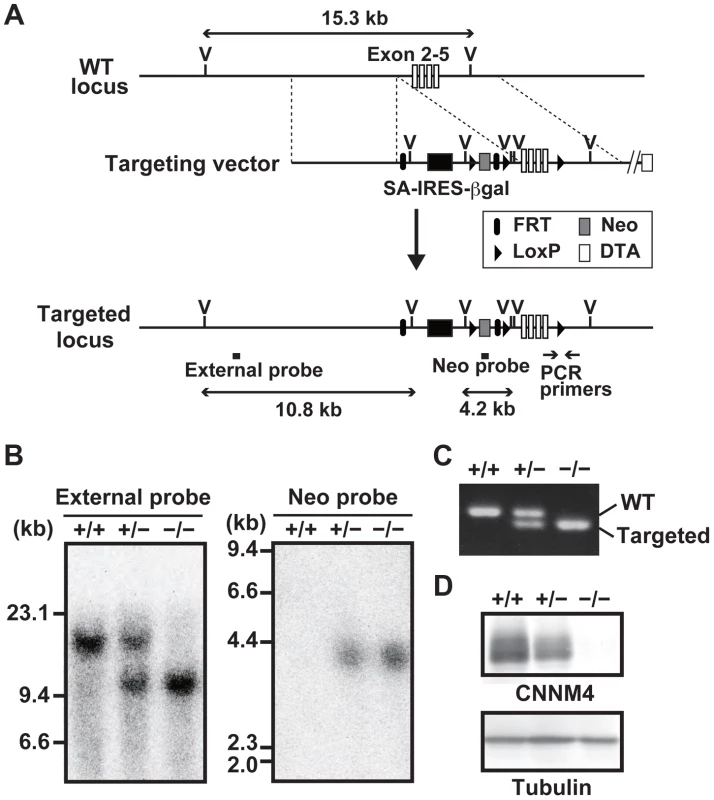
(A) Targeting strategy. βgal, β-galactosidase gene; DTA, diphtheria toxin A; FRT, Flp recombination target; IRES, internal ribosomal entry site; LoxP, locus of crossing over P1; Neo, neomycin resistance gene; SA, splice acceptor; V, EcoRV endonuclease-recognition site. (B) Genomic DNA, isolated from the tails of CNNM4+/+, CNNM4+/−, and CNNM4−/− mice was digested with EcoRV and hybridized with the external or neo probes, as schematically shown in (A). (C) PCR was performed using the genomic DNA as a template with the oligonucleotide primers schematically shown in (A). (D) Lysates of the colon were subjected to immunoblotting analyses with the anti-CNNM4 antibody. Basolateral localization of CNNM4 in the intestinal epithelia
Immunoblotting analyses of lysates obtained from various organs showed that CNNM4 is highly expressed in the small intestine and colon (Figure 2A), consistent with the previously reported analyses at mRNA level [18]. We next performed immunohistochemical staining to examine the expression pattern in the colon. As shown in Figure 2B, positive CNNM4 signals were specifically observed at the mucosal epithelial layer, with no significant signals at the muscular layer. Counterstaining of the tissue samples obtained from CNNM4−/− mice showed no positive signals, thus confirming that the signal at the mucosal epithelia properly reflects the localization of endogenous CNNM4.
Fig. 2. Basolateral localization of CNNM4 in the intestinal epithelia. 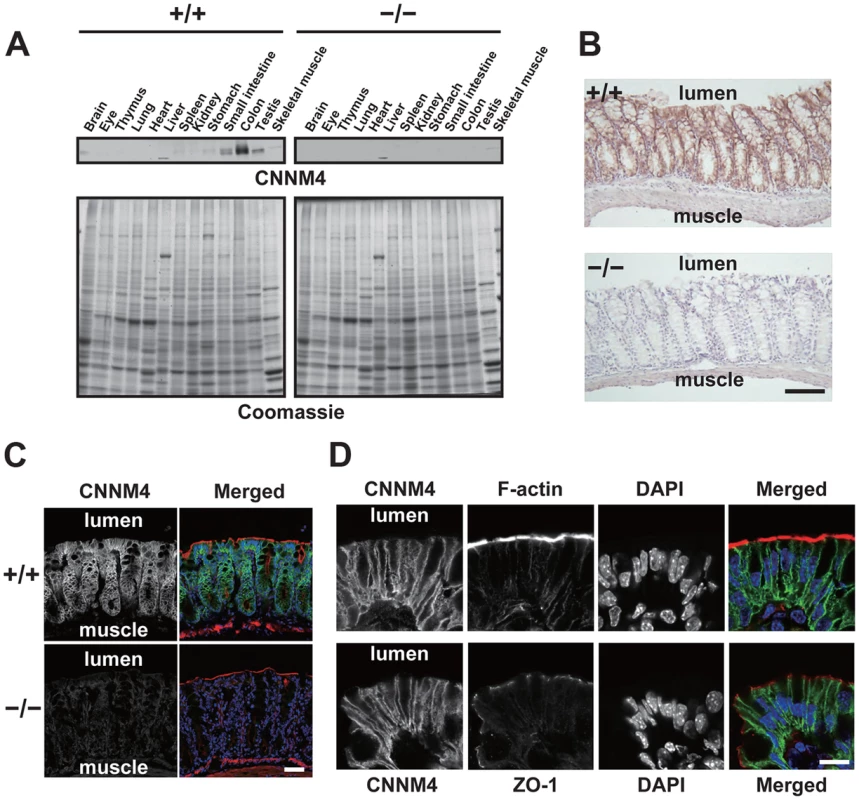
(A) Lysates of various organs obtained from 2-month-old CNNM4+/+ and CNNM4−/− mice were subjected to immunoblotting analyses with the anti-CNNM4 antibody. Coomassie-stained images are also indicated. (B) Cryosections of the colon were subjected to immunohistochemical staining with the anti-CNNM4 antibody. Bar, 100 µm. (C) Cryosections of the colon were subjected to immunofluorescence staining with the anti-CNNM4 antibody (green), phalloidin (red), and DAPI (blue). Monochrome images for CNNM4 are also indicated. Bar, 50 µm. (D) Colonic epithelia facing the lumen were subjected to immunofluorescence staining with anti-CNNM4 antibody (green), DAPI (blue), and phalloidin (red, upper panels), or anti-ZO-1 antibody (red, lower panels). Monochrome images for each signal are also indicated. Bar, 10 µm. To precisely determine the subcellular localization of CNNM4, we also performed immunofluorescence microscopy. Low-magnification images confirmed the specific expression of CNNM4 in the mucosal epithelia (Figure 2C). In the high-magnification images, positive signals for CNNM4 were mostly observed at the plasma membrane, but were clearly separated from those for F-actin, immediately beneath those for ZO-1 (Figure 2D). F-actin staining strongly labels the apical membrane of the intestinal epithelia [19], and ZO-1 is a marker for tight junctions in the colonic mucosa [20], which form a physical border between the apical and the basolateral membranes. Thus, these results imply a basolateral localization of CNNM4 in the colon epithelia.
To further confirm the basolateral localization of CNNM4, we ectopically expressed CNNM4-FLAG in MDCK cells, which maintain a highly polarized epithelial character in culture. As shown in Figure S2, the expressed CNNM4-FLAG proteins co-localized with Na+/K+ ATPase (basolateral marker), immediately beneath ZO-1.
Malabsorption of magnesium in CNNM4-knockout mice
The fact that CNNM4, a putative Mg2+ transporter, localizes to the basolateral membrane of the intestinal epithelia suggests the involvement of CNNM4 in the regulation of magnesium homeostasis. To explore this possibility, we analyzed the magnesium levels in CNNM4−/− mice maintained on a normal diet (CLEA Rodent Diet CE-2 containing 0.34% magnesium). Magnesium quantitation, using the colorimetric reagent Xylidyl Blue-I, showed that CNNM4−/− mice had a significantly lower serum magnesium concentration: an approximately 18% decrease was observed in comparison to CNNM4+/+ mice (Figure 3A). Moreover, the magnesium level in urine was drastically reduced, by approximately 71% (Figure 3A). These results demonstrate that CNNM4−/− mice have altered magnesium regulation. To examine whether this alteration was specific to magnesium, we used inductively coupled plasma-emission spectroscopy (ICP-ES) to examine the levels of several major metal elements in serum. As shown in Figure 3B, the levels of sodium, potassium, and calcium were not affected in CNNM4−/− mice, whereas the magnesium level was significantly reduced. We then observed mice fed a magnesium-deficient diet (containing 0.0027% magnesium) and found a significant increase in mortality in CNNM4−/− mice (Figure 3C), indicating that CNNM4−/− mice have abnormal magnesium homeostasis.
Fig. 3. Malabsorption of magnesium in CNNM4-KO mice. 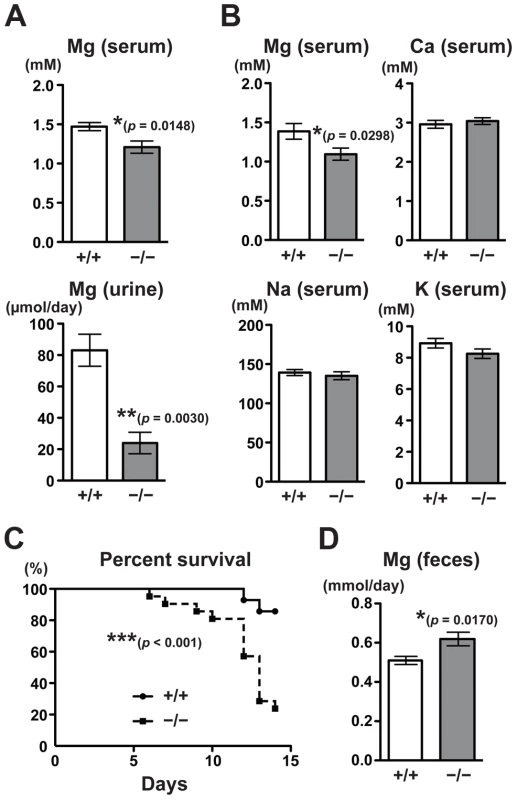
(A) Magnesium quantitation in serum (n = 9) and urine (n = 4) obtained from 2-month-old CNNM4+/+ and CNNM4−/− mice. The data are shown as mean ± s.e.m.. P-values were determined by Student's two-tailed t-test (unpaired). *p<0.05, **p<0.01. (B) Serum samples were subjected to elemental analyses using ICP-ES. The data are shown as mean ± s.e.m. (n = 14). P-values were determined by Student's two-tailed t-test (unpaired). *p<0.05. (C) Survival of CNNM4+/+ (n = 11) and CNNM4−/− (n = 18) mice on a magnesium-deficient diet. ***p<0.001; p-values were determined using the log-rank test. (D) Magnesium quantitation in feces. The data are shown as mean ± s.e.m. (n = 8). P-values were determined by Student's two-tailed t-test (unpaired). *p<0.05. Magnesium homeostasis is regulated by the balance between intestinal absorption and renal excretion. The decrease in renal excretion can be considered to reflect a compensatory response to maintain magnesium levels during hypomagnesemia caused by intestinal malabsorption. To directly measure the effect on intestinal absorption, we analyzed the magnesium content in feces. As shown in Figure 3D, there was significantly higher excretion of magnesium in feces in CNNM4−/− mice (22% increase compared to CNNM4+/+ mice), without a significant difference in the quantity of food ingested. These symptoms are very similar to those of the TRPM7-mutant mice, which have defects in intestinal magnesium absorption [7]. Collectively, these results indicate that CNNM4-deficiency results in malabsorption of magnesium at the intestine.
Mg2+ extrusion by CNNM4
To clarify the molecular function of CNNM4, we first examined the effect of CNNM4-overexpresion on the intracellular levels of major metal elements by using ICP-ES. As shown in Figure 4A, HEK293 cells transfected with CNNM4-FLAG contained more sodium and less magnesium in comparison to control vector-transfected cells, consistent with the occurrence of Mg2+ extrusion. Other analyzed elements (potassium, calcium, and zinc) showed no significant differences. We next performed imaging analyses with Magnesium Green, a fluorescent indicator for Mg2+. HEK293 cells transfected with CNNM4-FLAG were first loaded with Mg2+ by bathing them in a solution containing 40 mM Mg2+, which was then exchanged with a Mg2+-free solution to artificially promote Mg2+ extrusion. As shown in Figure 4B, the intensity of fluorescent signals in cells expressing CNNM4-FLAG (confirmed by immunofluorescence microscopy, performed after the imaging analyses) rapidly decreased immediately after Mg2+ depletion, whereas only a very subtle decrease was observed in empty vector-transfected cells. Thus, CNNM4 is able to stimulate Mg2+ extrusion.
Fig. 4. Mg2+ extrusion by CNNM4. 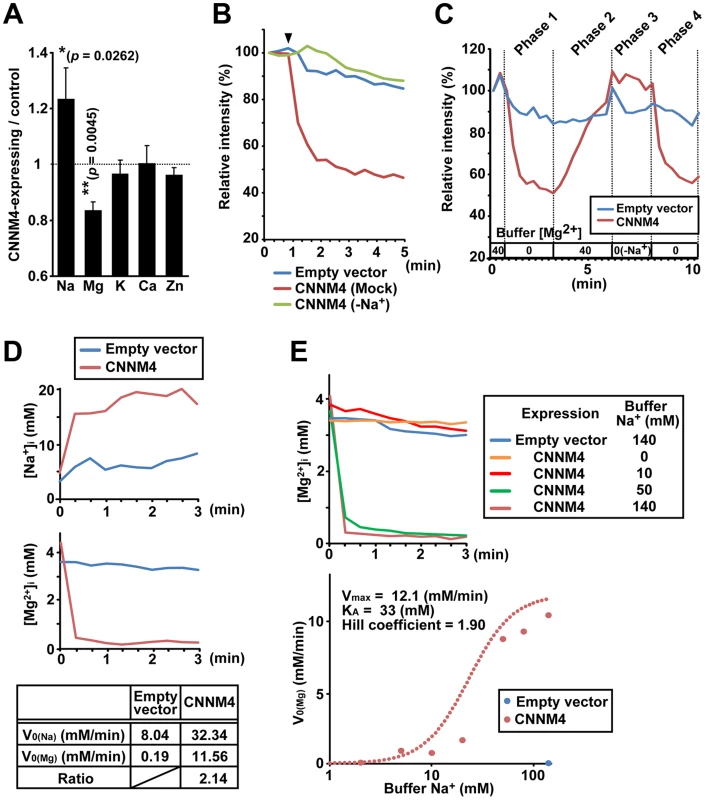
(A) Lysates of HEK293 cells transfected with CNNM4-FLAG were subjected to ICP-ES analyses. Relative amount of each element (CNNM4-expressing cells/control cells) is shown as mean ± s.e.m. (n = 7). P-values were determined by Student's two-tailed t-test (paired). *p<0.05, **p<0.01. (B) HEK293 cells expressing CNNM4-FLAG were loaded with Magnesium Green, and then subjected to Mg2+ depletion at the indicated time point (arrowhead). The experiment was repeated in a Na+-depleted extracellular solution (−Na+) by replacing NaCl with NMDG-Cl. The means of relative fluorescence intensities of 10 cells are indicated. (C) HEK293 cells expressing CNNM4-FLAG were loaded with Magnesium Green and then subjected to time-lapse imaging analyses under various extracellular solutions. The Mg2+ concentration in the extracellular solution is indicated (−Na+: NaCl in the buffer was replaced with NMDG-Cl). The means of relative fluorescence intensities of 10 cells are indicated. (D) HEK293 cells expressing CNNM4-FLAG were loaded with SBFI or Mag-fura2, and then subjected to Mg2+ depletion at 0 min. The data are shown as the means of [Na+]i (SBFI-loaded cells, top) and [Mg2+]i (Mag-fura2-loaded cells, middle) from 6 independent experiments (10 cells for each experiment). Initial velocities of Na+ influx (V0 (Na)) and Mg2+ efflux (V0 (Mg)), and the ratio of CNNM4-dependent Na+ influx versus Mg2+ efflux are also indicated (bottom). See Materials and Methods for details. (E) HEK293 cells expressing CNNM4-FLAG were loaded with Mag-fura2, and subjected to Mg2+ depletion at 0 min with extracellular Mg2+-free buffers containing various concentrations of Na+. Top: Time course of [Mg2+]i (means of 3 independent experiments, and 10 cells for each experiment). Bottom: Values for V0 (Mg) are plotted against Na+ concentrations in the buffer. Hill-type curve is also indicated (dotted line). The electrical potential across the plasma membrane forces Mg2+ to move inward into cells, and thus, energy supply is needed to actively extrude Mg2+ to the outside. Many proteins involved in active transport across the plasma membrane utilize the large electrochemical potential of Na+. To determine the importance of extracellular Na+ in Mg2+ extrusion, we first performed Mg2+ extrusion assays by replacing Na+ in the medium with another cation, N-methyl-D-glucamine (NMDG). In this case, Mg2+ extrusion was completely abolished (“−Na+” in Figure 4B). We also performed time-lapse imaging analyses for 10 min (Figure 4C and Video S1). Mg2+ depletion in the medium caused Mg2+ extrusion in CNNM4-expressing cells (Phase 1) and addition of 40 mM Mg2+ restored intracellular Mg2+ (Phase 2). In the absence of extracellular Na+, Mg2+ depletion did not induce Mg2+ extrusion (Phase 3), but restoration of Na+ instantaneously caused Mg2+ extrusion (Phase 4). Such tight coupling between the presence of extracellular Na+ and the occurrence of Mg2+ extrusion further supports the notion that CNNM4 stimulates Na+/Mg2+ exchange; this is also consistent with the sodium increase observed in CNNM4-expressing cells (Figure 4A).
To determine whether the rapid restoration of intracellular Mg2+ in 40 mM Mg2+ media is caused by the reverse action of CNNM4, we performed similar time-lapse imaging analyses using cells treated with Cobalt (III) hexammine (CoHex), which broadly inhibits channel-mediated Mg2+ influx [21], [22]. CoHex treatment significantly inhibited the Mg2+ recovery (Figure S3A), suggesting that some Mg2+ channels are involved in the Mg2+ recovery process. For more detailed characterization of the Mg2+ uptake in CNNM4-expressing cells, we performed a quantitative imaging analyses by using a less-sensitive, but ratiometric fluorescent probe Mag-fura2. Cells were bathed in extracellular solutions containing various concentrations of Mg2+ and Na+. Unlike 40 mM extracellular Mg2+, 10 mM Mg2+ was not sufficient to load CNNM4-expressing cells when extracellular Na+ was set to 78.1 mM (Figure S3B). However, when extracellular Na+ was depleted (0 mM), CNNM4-expressing cells incorporated significant amount of Mg2+ even at 10 mM. Furthermore, we observed that even though the Mg2+ level in CNNM4-expressing cells was lower than that in the control cells before loading, it became much higher after the loading procedure with 10 mM Mg2+, 0 mM Na+ solution, and then returned to the basal level when extracellular Mg2+ was removed. These data strongly suggest the occurrence of the reverse action of CNNM4 and corroborate our notion that CNNM4 stimulates Na+/Mg2+ exchange.
Electroneutral Na+/Mg2+ exchange by CNNM4
To characterize the molecular function of CNNM4 in more detail, we next performed electrophysiological analyses on CNNM4 expressed in HEK293 cells. As shown in Figure S4A–C, CNNM4 expression induced no significant electronic currents, while CNNM2 expression generated an inward current of Na+, as reported previously [12]. To directly measure Mg2+ extrusion, we next performed simultaneous Mg2+ imaging and electrophysiological recording experiments. The exchange of the extracellular solution with an Mg2+-free solution stimulated rapid Mg2+ decrease without inducing significant electronic currents in CNNM4-expressing cells (Figure S4D–E). These results suggest the possibility that CNNM4 might exchange 2 Na+ and 1 Mg2+, and thus, it is electroneutral. Therefore, we performed quantitative imaging analyses of intracellular Na+ and Mg2+ by using ratiometric fluorescent probes, sodium-binding benzofuran isophthalate (SBFI) and Mag-fura2, respectively. As shown in Figure 4D, Mg2+ depletion from the extracellular medium induced not only the decrease of intracellular Mg2+ but also the increase of intracellular Na+. In addition, the molar ratio of increased Na+ and decreased Mg2+ was calculated to be 2.14∶1, which is roughly consistent with the electroneutral exchange of Na+ and Mg2+ (2∶1). To quantitatively assess the dependency of Mg2+ extrusion on the presence of extracellular Na+, we performed Mg2+ extrusion assays by changing the concentration of extracellular Na+. Extracellular Na+ accelerated Mg2+ extrusion in a dose-dependent manner, and the Hill coefficient was calculated to be 1.90, a value close to 2 (Figure 4E). This result suggests that there are 2 or more Na+-binding sites in CNNM4, which also agrees with the characteristic of 2 Na+/1 Mg2+ exchanger.
Hypomineralization of the tooth enamel in CNNM4-knockout mice
One of the common features of Jalili syndrome, which is caused by mutations in CNNM4, is AI, the malformation of tooth enamel [16], [17]. We noticed that CNNM4−/− mice displayed abnormal teeth with chalky-white discoloration (Figure 5A), which is typically observed in mice with defective amelogenesis. This phenotype was apparent as early as 3 weeks of age and was observed in all CNNM4−/− mice examined. To characterize the abnormality in amelogenesis, we subjected maxillary incisors to analyses with scanning electron microscopy (SEM). The low-magnification images showed that the thickness of the enamel layer in CNNM4−/− mice was not so different from that in CNNM4+/+ mice (Figure 5B). However, the high-magnification images showed that the enamel rods were immature and the inter-rod area was increased in CNNM4−/− mice (Figure 5C). We then subjected the samples to composition analyses using energy dispersive X-ray spectrometry (EDX). As shown in Figure 5D, the levels of both calcium and phosphorus were significantly decreased in CNNM4−/− mice, confirming the occurrence of hypomineralization.
Fig. 5. Hypomineralization of the tooth enamel in CNNM4-knockout mice. 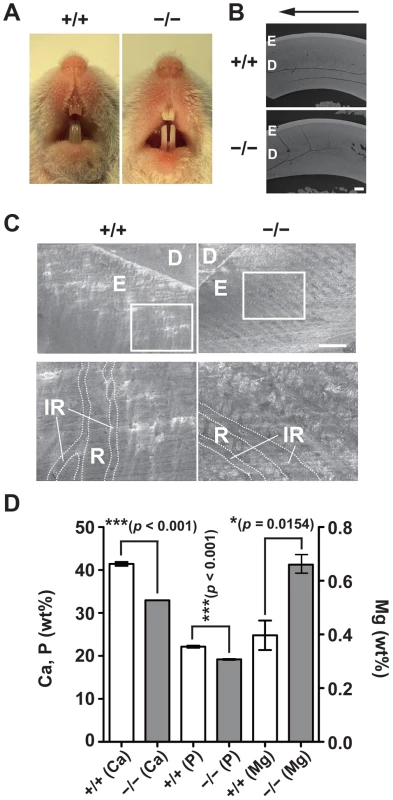
(A) Oral photographs showing incisors of 2-month-old CNNM4+/+ and CNNM4−/− mice. (B) Backscattered SEM images showing incisors of 2-month-old CNNM4+/+ and CNNM4−/− mice. An arrow shows incisal direction. E, enamel; D, dentine. Bar, 200 µm. (C) SEM images showing the mature enamel regions of incisors. Magnified images of the boxed areas are also indicated. E, enamel; D, dentine; R, enamel rod; IR, inter-rod area. Bar, 20 µm. (D) The mineral content of the incisor enamel. The mineral content is expressed as a weight percent (wt%). The data are shown as mean ± s.e.m. (n = 3). P-values were determined by Student's two-tailed t-test (unpaired). *p<0.05, ***p<0.001. Basolateral localization of CNNM4 in the ameloblasts
To explore the role of CNNM4 in amelogenesis, we performed immunohistochemical staining to examine the localization of CNNM4 in the enamel-forming tissue. Enamel formation occurs in the area covered by ectodermally-derived epithelial cells, so-called ameloblasts [23]. The ameloblasts first deposit a complex extracellular matrix composed of enamel proteins (secretory stage), and then come to maturity, with a shortened morphology, and promote mineralization of the enamel (maturation stage). During the secretory stage, positive signals of CNNM4 were observed specifically at the stratum intermedium (SI) layer, but not in the ameloblasts (Figure 6A–B). However, the expression pattern significantly changes at the maturation stage, with strong positive signals in the ameloblasts themselves.
Fig. 6. Basolateral localization of CNNM4 in the ameloblasts. 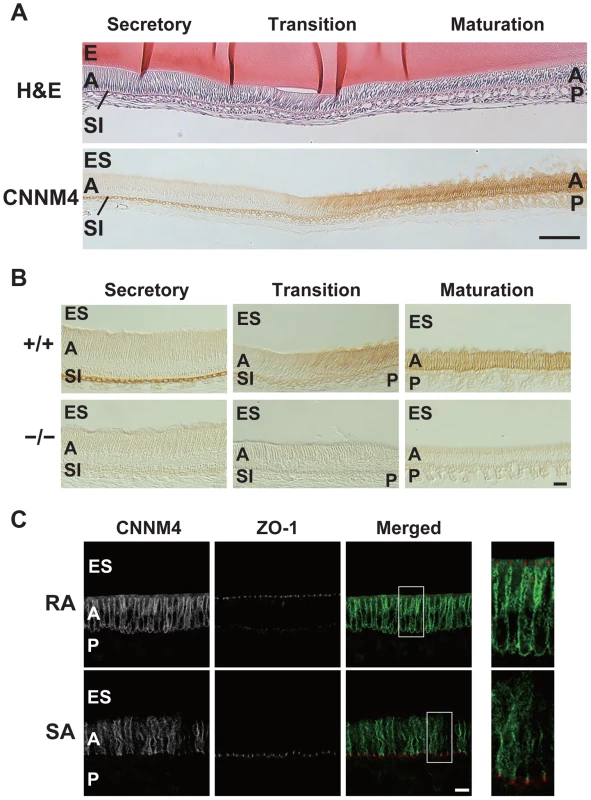
(A, B) The enamel-forming tissues of 6-week-old mice were subjected to H&E staining (A) and immunohistochemical staining with the anti-CNNM4 antibody. The specimens were observed with DIC microscope (B). E, enamel; ES, enamel space; A, ameloblast; SI, stratum intermedium; P, papillary layer. Bar, 100 µm (A), 20 µm (B). (C) Cryosections were double-stained with anti-CNNM4 (green) and anti-ZO-1 (red) antibodies, and subjected to immunofluorescence microscopy. RA, ruffle-ended ameloblast; SA, smooth-ended ameloblast; ES, enamel space; A, ameloblast; P, papillary layer. Magnified images of the boxed areas in the merged images are also indicated. Bar, 10 µm. Mature ameloblasts are known to undergo repetitive cycles of transdifferentiation between ruffle-ended (RA) and smooth-ended (SA) ameloblasts, which can be discerned by ZO-1-staining [24]. Immunofluorescence staining showed that CNNM4 exists throughout the basolateral membrane immediately beneath the ZO-1 signals in the RA-type ameloblasts, which possess dot-like accumulations of ZO-1 at the cell-cell contact sites facing the enamel-forming area (Figure 6C). It should be noted that this basolateral localization pattern of CNNM4 in RA-type ameloblasts is quite similar to that observed in intestinal epithelia (Figure 2D), suggesting that CNNM4 promotes Mg2+ removal from the maturing enamel. Indeed, the elemental analyses of the mature enamel with EDX indicated that the magnesium levels were significantly increased in CNNM4−/− mice (Figure 5D).
To ascertain the functional importance of Mg2+ extrusion by CNNM4, we examined whether missense point mutations in CNNM4, which have been reported to occur in the patients of Jalili syndrome [16], [17], have any effects on Mg2+ extrusion activity. We tested the effect of two different point mutations, viz., S200Y and L324P, both of which occur in the evolutionarily conserved DUF21 domain (Figure 7A). When these mutants were expressed in HEK293 cells, they localized to the plasma membrane, similarly to wild-type (WT) CNNM4 (Figure 7B). However, both mutants showed very weak, if any, Mg2+ extrusion activity in comparison to WT CNNM4 (Figure 7C). Therefore, a dysfunction in Mg2+ extrusion, caused by mutations in this gene, probably underlies this little understood human disease.
Fig. 7. Mutations associated with Jalili syndrome abolish Mg2+ extrusion. 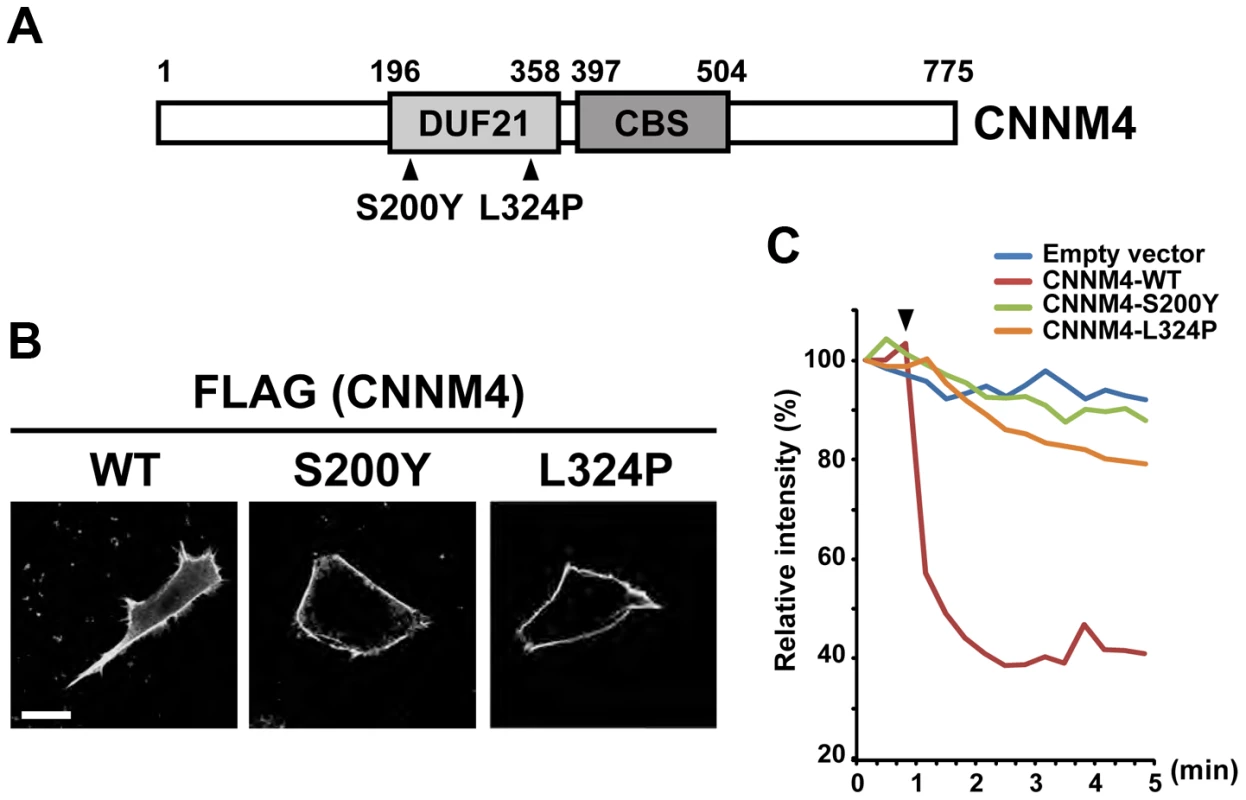
(A) Schematic illustration of CNNM4 and point mutations found in patients with Jalili syndrome. The evolutionarily conserved DUF21 and CBS domains are boxed and the amino acid residue numbers are indicated. (B) HEK293 cells transfected with the WT and mutant CNNM4-FLAG constructs were subjected to immunofluorescence staining with the anti-FLAG antibody. Bar, 10 µm. (C) HEK293 cells transfected with the WT and mutant CNNM4-FLAG constructs were subjected to Mg2+ extrusion assays. The arrowhead indicates the starting point of Mg2+ depletion. The means of relative fluorescence intensities of 10 cells are indicated. No symptoms of CRD in CNNM4-knockout mice
Another feature of Jalili syndrome is CRD, which is characterized with the degeneration of rod and cone photoreceptors in the retina [16], [17]. To investigate the integrity of retinal function of CNNM4−/− mice, we performed histological and electroretinogram (ERG) analyses. To observe the retinal histology, we stained retinal sections from 2-month-old (young adult) CNNM4−/− mice with toluidine blue. We found that the retinal layers were normal and no symptom of retinal degeneration was observed in the retina of CNNM4−/− mice (Figure S5A). We also performed immunofluorescent analysis in the CNNM4−/− retina, using markers of photoreceptor, bipolar, and horizontal cells. Outer segments of rod and cone photoreceptors stained with anti-rhodopsin and cone opsins (M-opsin and S-opsin) are normal in the CNNM4−/− retina (Figure S5B). Cone photoreceptor synaptic terminals stained with Peanut Agglutinin (PNA) are also localized normally in the outer plexiform layer (OPL). Photoreceptor synaptic ribbons stained with the anti-Ctbp2 antibody showed horseshoe-like structure in the vicinity of dendritic tips of bipolar cells stained with the anti-mGluR6 antibody both in CNNM4+/+ and CNNM4−/− mice, and dendrites of rod ON-bipolar cells stained with anti-PKC-α antibody and processes of horizontal cells stained with the anti-Calbindin antibody were properly extended into the OPL in the CNNM4−/−retina. To evaluate the retinal function, we recorded ERGs from CNNM4−/− mice. As shown in Figure S5C, no obvious difference was observed between 2-month-old CNNM4+/+ and CNNM4−/− mice in their ERGs under both scotopic and photopic conditions, which reflects the functions of rods and cones, respectively (a-wave in scotopic condition 1.0 log stimuli: +/+, 280±57 µV; −/−, 251±23; unpaired t-test: p = 0.6204; a-wave in photopic condition 1.0 log stimuli: +/+, 11.3±1.9 µV; −/−, 8.1±0.9; p = 0.1966; b-wave in scotopic condition 1.0 log stimuli: +/+, 619±115 µV; −/−, 563±44; p = 0.6366; b-wave in photopic condition 1.0 log stimuli: +/+, 163±24 µV; −/−, 126±23; p = 0.2937; +/+, n = 5; −/−, n = 6).
Retinal dysfunction occasionally becomes evident with age. Indeed, knockout mice for RP3, one of causative genes of human hereditary retinal diseases [25], do not show an apparent loss of the retinal cells at 1 month of age, but degeneration of photoreceptor cells has occurred at 6 months [26]. Therefore, histological analyses of the retina of 6-month-old CNNM4−/− mice were performed. However, we did not observe any signs of histological abnormalities (Figure S5A–B). We also recorded ERGs from 6-month-old CNNM4−/− mice and again observed normal ERGs under both scotopic and photopic conditions (Figure S5C).
Discussion
In this study, we have shown that CNNM4 localizes to the basolateral membrane of epithelial cells and extrudes Mg2+. Theoretically, Mg2+ extrusion requires an energy supply to overcome the inward-oriented force on Mg2+ diffusion imposed by the membrane potential. A Na+-coupling Mg2+ extrusion mechanism has long been suggested, and indeed, various types of mammalian cells possess Na+/Mg2+ exchange activity [27], [28]. It was recently reported that SLC41A1 can biochemically function as a Na+/Mg2+ exchanger when expressed in HEK293 cells [29]. It is expressed ubiquitously [30], and the ectopically expressed SLC41A1 in MDCK cells localizes at the basolateral membrane [31]. Therefore, SLC41A1 may also be involved in the regulation of directional Mg2+ transport across the intestinal epithelia. However, it should be noted that the speed of Mg2+ extrusion by CNNM4 (reaching plateau after 1∼2 min) is much faster than that by SLC41A1 (after ∼10 min) [29]. Such a rapid Mg2+ extrusion has not been reported in the previous studies characterizing the endogenous Mg2+ extrusion systems in non-intestinal cells [27], [28]. Thus, CNNM4 appears to be a qualitatively different, high capacity type of Mg2+ extrusion molecule, which may have a specialized role in the intestinal epithelia. Magnesium absorption from the intestine is essential for magnesium homeostasis, and 100–150 mg magnesium is daily absorbed from the intestine in humans [32]. To absorb such a large amount of magnesium through the intestinal epithelia, the magnesium transport system in the intestine should be highly active. It is known that both paracellular and transcellular pathways are functional and play important roles in the intestinal magnesium absorption [33]. In the transcellular pathway, Mg2+ entry into the intestinal epithelial cells is mediated by apically localized Mg2+-permeable channels TRPM6/7 that can rapidly incorporate Mg2+ [6], [7]. Therefore, it is very reasonable that Mg2+ extrusion from the basolateral membrane is mediated by high capacity transporters, such as CNNM4, to achieve efficient transcellular Mg2+ transport through intestinal epithelia.
CNNM4−/− mice showed a defect in magnesium absorption, but were viable, without any significant observable phenotype when fed a normal diet. CNNM proteins comprise a family of 4 related proteins, CNNM1–4 [10], and thus, the mild phenotype of CNNM4−/− mice can be ascribed to the functional complementation by other CNNM family proteins. CNNM4 is expressed in the intestine, but not in the kidney, and thus, it will not affect renal reabsorption, the other key process in the regulation of magnesium homeostasis. The amount of magnesium reabsorbed from the glomerular filtrate is estimated to be about 10 times that absorbed from digested food. Therefore, the absence of CNNM4 in the kidney raises the next important question of what molecule is responsible for Mg2+ extrusion from distal convoluted tubule (DCT) cells in the kidney, where TRPM6 is expressed at the apical membrane and where transcellular Mg2+ transport occurs [34]. Two previous papers have reported strong expression and localization of CNNM2 at the basolateral membrane of the DCT cells [12], [18]. Therefore, it can be assumed that CNNM2 plays an important role in renal reabsorption of magnesium at the DCT by mediating transcellular Mg2+ transport cooperatively with TRPM6. It should be noted here that SLC41A1 is also expressed in the DCT cells and its gene mutation causes nephronophthisis-related disorder [31]. Because the affected patients did not exhibit any abnormalities in serum or urine magnesium level, the authors speculated that the disease phenotype might result from perturbed intracellular magnesium homeostasis [31]. Future studies using gene knockout mice and detailed analyses of the biochemical properties of these molecules, CNNM2 and SLC41A1, will grant more insight into the individual roles in renal magnesium control.
CNNM4 is mutated in Jalili syndrome, which is characterized by recessive AI and CRD [16], [17]. Our CNNM4−/− mice showed no signs of abnormalities in the retinal tissue architecture and function (Figure S5). In contrast, we observed a clear amelogenesis-defective phenotype. In the enamel-forming tissue, CNNM4 is strongly expressed at the basolateral membrane in RA-type ameloblasts. Such a basolateral localization is similar to that observed in the intestinal epithelia and suggests that CNNM4 is involved in the vectorial transport of Mg2+ from the enamel-forming areas through the ameloblasts. Indeed, RA-type ameloblasts have tight junctions in the region adjacent to the enamel-forming areas, and form a niche in which active ion transport occurs [24]. The precise role of Mg2+ in the enamel-forming process remains unknown, but the striking expression of CNNM4 in RA-type mature ameloblasts suggests that Mg2+ needs to be removed from the enamel tissue to promote mineralization of enamel. Indeed, it has been reported that the magnesium content of the enamel is inversely correlated with the extent of mineralization [35]. Further characterization of CNNM4-knockout mice will contribute to a better understanding of this intriguing process in which the most solid tissue in the body is generated.
Materials and Methods
Ethics statement
We appropriately treated mice to ameliorate suffering, according to the guidelines for proper conduct of animal experiments (issued by the Science Council of Japan), and received approval for this study from the institutional review board of Osaka University.
Generation of CNNM4-knockout mice
We purchased an ES clone (ID: EPD0426_1_C08) from EUCOMM, in which the neomycin-resistant gene cassette had been inserted in the genomic region between the first and second exons of CNNM4 by homologous recombination. The ES cells were used to generate germline chimeras that were bred with C57BL/6J females to generate CNNM4-knockout mice. Southern blot analyses were performed to confirm appropriate recombination. Genomic DNA of mice was digested with EcoRV and hybridized with the external or neo probes. Genotyping PCR was performed using the following primer set: 5′-TAACTGTTGGAAGGCTGAGG-3′ and 5′-AGGCAGGGGCTCCCTTTCAT-3′. Mice were maintained under standard specific pathogen-free conditions.
cDNA and antibody
Human CNNM4 cDNA was purchased from Invitrogen (IMAGE: 30340626). Amino acid substituted mutants S200Y and L324P were generated with the QuickChange Site-Directed Mutagenesis Kit (Agilent). An anti-CNNM4 rabbit polyclonal antibody was raised in rabbits immunized with bacterially expressed His-CNNM4 proteins (amino acids 546–775) and purified with corresponding GST-tagged recombinant proteins. Anti-ZO-1 mouse monoclonal antibody was generated in the previous study [36] and provided by Dr. Masahiko Itoh (Dokkyo Medical University) and Dr. Mikio Furuse (Kobe University). Anti-mGluR6 guinea pig polyclonal antibody was described previously [37]. Anti-Na+/K+ ATPase mouse monoclonal antibody (#05-369) and anti-M-opsin rabbit polyclonal antibody (AB5405) were purchased from Merck Millipore. Anti-FLAG rabbit polyclonal antibody (F7425) and anti-PKCα rabbit polyclonal antibody (P4334) were purchased from Sigma-Aldrich. Anti-Ctbp2 mouse monoclonal antibody (612044) was purchased from BD Biosciences. Anti-Rhodopsin (LB-5597) and anti-Calbindin (PC253L) rabbit polyclonal antibodies were purchased from LSL and Calbiochem, respectively. Anti-S-opsin goat polyclonal antibody (sc-14363) was purchased from Santa Cruz Biotechnology. Alexa Fluor 488-conjugated anti-rabbit IgG was purchased from Invitrogen and Sigma-Aldrich. Alexa Fluor 488-conjugated anti-mouse IgG was purchased from Sigma-Aldrich. Alexa Fluor 568-conjugated anti-mouse IgG, and rhodamine-labelled phalloidin were purchased from Invitrogen. Cy3-conjugated anti-rabbit, -goat and -guinea pig IgGs were purchased from Jackson ImmunoResearch Laboratories. Rhodamine-labeled PNA (RL1072) was purchased from Vector Laboratories.
Expression and RNAi knockdown in culture cells
HEK293 cells and MDCK cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics. Transient expression and knockdown were achieved using LipofectAmine2000 (Invitrogen) to transfect cells with plasmids or siRNAs according to the manufacturer's instruction. Plasmid constructs in the pCMV-Tag 4 vector (Agilent Technologies) were used for expression of CNNM4. For knockdown experiments, duplex siRNAs against human CNNM4 (Invitrogen), which target the following sequence: CNNM4-siRNA, 5′-GCGAGAGCAUGAAGCUGUAUGCACU-3′, were used. As control, we used siRNA representing a scrambled sequence of CNNM4-siRNA, 5′-GCGACGAAAGUGUCGGUAUCGAACU-3′.
Immunohistochemistry
For intestine preparation, intestines were dissected from 2-month-old mice, embedded in OCT compound (Sakura Finetechnical), frozen in liquid nitrogen, and then sectioned into at 10-µm sections using a cryostat (Leica). The sections were mounted on glass slides, air-dried, and fixed with phosphate-buffered saline (PBS) containing 4% paraformaldehyde (PFA) for 10 min at 4°C. For mandible preparation, 6-week-old mice were anesthetized and fixed by perfusion with PBS containing 4% PFA. Mandibles were dissected out, fixed with PBS containing 4% PFA for 12 h at 4°C, decalcified with 10% EDTA for 2 weeks, dehydrated with xylene through a graded ethanol series, and embedded in paraffin. Sections (4-µm thick) were cut using a microtome (Leica), and then mounted on glass slides. Slides were heat-treated in Pascal, a pressure chamber (Dako) and cooled at room temperature after deparaffinization and rehydration. Both frozen and paraffin-embedded sections were then incubated with PBS containing 0.3% H2O2. After blocking with PBS containing 3% fetal bovine serum and 10% bovine serum albumin for 1 h at room temperature, specimens were incubated with the primary antibodies overnight at 4°C, followed by incubation with the peroxidase-conjugated secondary antibodies for 1 h at room temperature. Immunostaining was developed with diaminobenzidine and counterstained with Mayer's haematoxylin. The specimens were observed under a microscope (BX41 equipped with a DP20 camera; Olympus). Differential Interference Contrast (DIC) images were collected using an inverted microscope (IX71 equipped with a DP20 camera; Olympus).
Immunofluorescence microscopy
Cells cultured on coverglasses were washed with PBS and fixed with 1% formaldehyde for 15 min at room temperature. When stained for ZO-1, cells were permeabilized with 0.5% TritonX-100 in PBS for 10 min at room temperature. When stained for Na+/K+-ATPase, cells were permeabilized with 0.1% TritonX-100 for 5 min at room temperature. After blocking with PBS containing 3% fetal bovine serum and 10% bovine serum albumin (blocking buffer) for 1 h, cells were incubated for 12 h with the primary antibody diluted in blocking buffer. After 3 washes with PBS, cells were incubated for 30 min with the appropriate secondary antibodies diluted in blocking buffer. Cryosections of intestines and paraffin-embedded sections were prepared as described above. When stained for ZO-1, sections were permeabilized with ice-cold acetone for 3 min after fixation. Fixed sections were blocked and incubated with the primary and secondary antibodies as for cultured cells. After washing with PBS, coverglasses were mounted with Aqueous Mounting Medium PermaFluor (Thermo SCIENTIFIC) and observed with a confocal scanning laser microscope (FLUOVIEW FV1000; Olympus). The procedure of immunofluorescent analysis of retinas was described previously [38], [39]. Mouse eyes were fixed with PBS containing 4% PFA for 30 min or 5 min, embedded in OCT compound, frozen, and sectioned. Frozen 20 µm sections were blocked with PBS containing 5% normal goat serum and 0.5% Triton X-100 for 30 min, and then incubated with primary antibodies for 4 h at room temperature. Slides were washed with PBS three times for 5 min each time and incubated with secondary antibodies for 2 h at room temperature. The specimens were observed with a confocal scanning laser microscope (LSM510; Carl Zeiss).
ERG recordings
ERG responses were measured after overnight dark adaptation using PuREC system with LED electrodes (Mayo Corporation) [40]. 2 - and 6-month-old mice were anesthetized with an intraperitoneal injection of ketamine and xylazine. The mice were stimulated with stroboscopic stimuli of 1.0 log cd-s/m2 (photopic units) maximum intensity. 4 levels of stimulus intensities ranging from −4.0 to 1.0 log cd-s/m2 were used for the scotopic ERG recordings, and 4 levels of stimuli ranging from −0.5 to 1.0 log cd-s/m2 were used for the photopic ERGs. Animals were light adapted for 10 min before the photopic ERG recordings. 8 and 16 responses were averaged for photopic (−4.0 and −3.0 log) and all scotopic recordings, respectively.
Colorimetric quantitation of magnesium
Mice were fed either a normal diet containing 0.34% magnesium (CLEA Rodent Diet CE-2, CLEA Japan) or a magnesium-deficient diet containing 0.0027% magnesium (CLEA Japan). Blood samples were obtained from 8-week-old mice. These were incubated at 4°C overnight, and serum was then collected by centrifugation at 1,000× g for 20 min at 4°C. Urine and feces samples were collected from 2-month-old mice by using metabolic cages (CLEA Japan). Feces were air-dried, incubated with 1 N nitric acid (1∶10; wt∶volume) overnight, and then centrifuged. The magnesium concentration of the supernatant was determined using Xylidyl Blue-I (Wako) according to the manufacturer's instructions.
ICP-ES
Serum samples were mixed with HCl at a final concentration of 1% and incubated at 95°C for 2 h. Samples were then subjected to elementary analysis with ICPS-8100 (Shimadzu), according to the manufacturer's instructions. The mean of triplicate measurements was used to represent the result of a single sample. The results were normalized to total protein levels, which were determined by the Bradford method.
Mg2+-imaging analyses with Magnesium Green
Mg2+-imaging analyses with Magnesium Green were performed as follows. HEK293 cells were incubated with Mg2+-loading buffer (78.1 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 40 mM MgCl2, 5.5 mM glucose, 5.5 mM HEPES-KOH, pH 7.4), including 2 µM Magnesium Green-AM (Invitrogen), for 45 min at 37°C. The cells were rinsed once with loading buffer and viewed using a microscope (IX81 equipped with a DP30BW camera and a USH-1030L mercury lamp; Olympus). Fluorescence was measured every 20 sec (excitation at 470–490 nm and emission at 505–545 nm) under the control of the Metamorph software (Molecular Devices). Then, the buffer was changed to −Mg2+ buffer (MgCl2 in the loading buffer was replaced with 60 mM NaCl), or to −Mg2+−Na+ buffer (NaCl in −Mg2+ buffer was replaced with NMDG-Cl). The data are presented as line plots (mean of 10 cells). After imaging analyses, cells were fixed with PBS containing 3.7% formaldehyde and subjected to immunofluorescence microscopy to confirm protein expression. Cobalt (III) hexammine was purchased from SIGMA.
Electrophysiological recordings
pIRES-HcRed plasmids [41] for expressing CNNM2 or CNNM4 were transfected into HEK293 cells with FuGENE6 (Roche). After 24 h, cells were plated on glass coverslips coated with poly-L-lysine (SIGMA) and maintained in normal culture media plus 40 mM MgCl2 until use. Patch-clamp experiments under the whole-cell configuration were performed according to Stuiver et al., [12] with minor modifications. The experiments were performed with Axopatch 200B amplifier and Clampex 9.2 data acquisition system (Molecular Devices), and borosilicate patch pipettes had resistances of 5–10 MΩ after filled with the intracellular solution. Voltage steps (1 sec in duration) from the holding potential of 0 mV to potentials between −120 to +70 mV with 10 mV increment were delivered every 4 sec. The density current was obtained from the peak current at −110 mV and was normalized with the membrane capacitance of the cell. The extracellular solutions was 80 mM Na-gluconate, 0 or 20 mM MgSO4, 10 mM HEPES (pH 7.35 adjusted with Tris). The intracellular solution was 120 mM NMDG, 120 mM 2-(N-morpholino)-ethanesulfonic acid hydrate, 2 mM MgSO4, 10 mM HEPES (pH 7.2 adjusted with H2SO4). All solutions were adjusted to 295–305 mOsm with sucrose.
Simultaneous Mg2+-imaging and electrophysiological recording experiments were performed with IX71 microscope (Olympus) equipped with iXon EM-CCD camera (Andor Technology) and a xenon lamp in Lambda DG-4 illumination system (Sutter Instrument). Borosilicate patch pipettes had resistances of 3–5 MΩ after filled with the intracellular solution containing 2 µM Magnesium Green (non-AM form, Invitrogen). Cells were voltage clamped to −10 mV, and the imaging was started after the fluorescent intensities from the cell became stabilized (20–35 min after the establishment of the whole-cell configuration). The fluorescence was measured every 20 sec (excitation at 470–490 nm and emission at 505–545 nm). Mg2+-loading buffer and −Mg2+ buffer were used as extracellular solutions. The intracellular solution was 2 mM MgCl2, 2 mM NaCl, 5 mM EGTA, 140 mM KCl, 5 mM HEPES (pH 7.25 adjusted with KOH). All solutions were adjusted to 295–305 mOsm with sucrose.
Ratiometric imaging of Mg2+ and Na+
HEK293 cells were transfected with expression plasmids for CNNM4, and maintained in normal culture media plus 40 mM MgCl2 until use. Mg2+ extrusion assays were performed with the abovementioned protocol, with following modifications. Cells were loaded with 2 µM Mag-fura2-AM or 3 µM SBFI-AM (Invitrogen) and viewed using the IX81 microscope (Olympus) equipped with ORCA-Flash 4.0 CMOS camera (Hamamatsu Photonics) and USH-1030L mercury lamp (Olympus). The fluorescence was measured every 20 sec (excitation at 330–350 nm and 370–390 nm, and emission at 505–545 nm), and −Mg2+ buffer with various Na+ concentrations (prepared by replacing NaCl with NMDG-Cl) was used to stimulate Mg2+ efflux.
Intracellular concentrations of free Mg2+ and Na+ ([Mg2+]i and [Na+]i, respectively) were determined from the following equation:
R: the ratio of the signal intensity with 330–350 nm excitation (F1) to that with 370–390 nm excitation (F2) (R = F1/F2). Rmax: the maximum value of R. Rmin: the minimum value of R. Q: the ratio of the signal intensity with 370–390 nm excitation under minimum Mg2+ or Na+ concentration to the signal intensity with 370–390 nm excitation under maximum Mg2+ or Na+ concentration (F2min/F2max). Kd: 1.5 mM for Mag-fura2 [42] and 11.3 mM for SBFI [43], respectively. Rmin, Rmax, Fmin, Fmax were obtained after each experiment. For Mag-fura2, Rmin, Fmin were recorded by addition of 6 µM 4-Bromo-A23187 (Wako) and 10 mM EDTA, and Rmax, Fmax were recorded by incubating the cells under −Mg2+ buffer plus 6 µM 4-Bromo-A23187 and 50 mM MgCl2. For SBFI, Rmax, Fmax were recorded by incubating the cells under −Mg2+ buffer supplemented with 5 µM Gramicidin (Wako), and Rmin, Fmin were recorded by incubating the cells under the Na+-depleted buffer (a −Mg2+ buffer which NaCl is replaced with KCl) with 5 µM Gramicidin. The cells were fixed with PBS containing 3.7% formaldehyde after fluorescence measurement and subjected to immunofluorescence microscopy to confirm protein expression. Difference of [Na+]i and [Mg2+]i just after Mg2+ depletion (between time = 0 and 20 sec) was used to determine the initial velocity of Na+ influx (V0 (Na)) and Mg2+ efflux (V0 (Mg)), respectively. The ratio of CNNM4-dependent Na+ influx versus Mg2+ efflux was calculated as follows: Vmax, KA, and Hill coefficient were determined by SigrafW software [44].Mg2+ loading assays
HEK293 cells were transfected with expression plasmids for CNNM4, and maintained in normal culture media until use. Mg2+ loading assays were performed with the abovementioned protocol for ratiometric imaging, with following modifications. Cells were incubated in −Mg2+ buffer with 2 µM Mag-fura2-AM for 10 min, 37°C. The cells were once rinsed with −Mg2+ buffer and viewed using the same apparatuses. Then, the extracellular solution was changed to buffers with various Mg2+ and Na+ concentrations (buffer with low Na+ concentrations were prepared by replacing NaCl with NMDG-Cl) and incubated for 4 min to load Mg2+. Finally, the extracellular solution was changed to −Mg2+ buffer to stimulate Mg2+ efflux.
SEM analyses
Maxillae dissected from 2-month-old mice were fixed with 70% ethanol for 5 days, dehydrated in ascending alcohol series, and embedded in methyl methacrylate. After embedding, cutting specimen, and surface polishing, the samples were then coated with room-temperature ionic liquid (1-butyl-3-methylimidazolium tetrafluoroborate), which work as an electric conductor and enables the observation of biological specimen by an SEM [45]. Samples were mounted with carbon adhesion tape on a specimen holder for SEM. Backscattered and secondary electron images were obtained with SEM (VE-9800: Keyence). The composition changes were analyzed with EDX (VE9800: EDAX) attached to the SEM at an accelerating voltage of 8 keV.
Supporting Information
Zdroje
1. SimonDB, LuY, ChoateKA, VelazquezH, Al-SabbanE, et al. (1999) Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science 285 : 103–106.
2. KonradM, SchallerA, SeelowD, PandeyAV, WaldeggerS, et al. (2006) Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet 79 : 949–957.
3. HouJ, PaulDL, GoodenoughDA (2005) Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci 118 : 5109–5118.
4. SchlingmannKP, WeberS, PetersM, Niemann NejsumL, VitzthumH, et al. (2002) Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet 31 : 166–170.
5. WalderRY, LandauD, MeyerP, ShalevH, TsoliaM, et al. (2002) Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet 31 : 171–174.
6. VoetsT, NiliusB, HoefsS, van der KempAW, DroogmansG, et al. (2004) TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem 279 : 19–25.
7. RyazanovaLV, RondonLJ, ZierlerS, HuZ, GalliJ, et al. (2010) TRPM7 is essential for Mg(2+) homeostasis in mammals. Nat Commun 1 : 109.
8. TeramotoT, LambieEJ, IwasakiK (2005) Differential regulation of TRPM channels governs electrolyte homeostasis in the C. elegans intestine. Cell Metab 1 : 343–354.
9. TeramotoT, SternickLA, Kage-NakadaiE, SajjadiS, SiembidaJ, et al. (2010) Magnesium excretion in C. elegans requires the activity of the GTL-2 TRPM channel. PLoS One 5: e9589.
10. WangCY, ShiJD, YangP, KumarPG, LiQZ, et al. (2003) Molecular cloning and characterization of a novel gene family of four ancient conserved domain proteins (ACDP). Gene 306 : 37–44.
11. MeyerTE, VerwoertGC, HwangSJ, GlazerNL, SmithAV, et al. (2010) Genome-wide association studies of serum magnesium, potassium, and sodium concentrations identify six Loci influencing serum magnesium levels. PLoS Genet 6: e1001045.
12. StuiverM, LainezS, WillC, TerrynS, GünzelD, et al. (2011) CNNM2, encoding a basolateral protein required for renal Mg2+ handling, is mutated in dominant hypomagnesemia. Am J Hum Genet 88 : 333–343.
13. GibsonMM, BaggaDA, MillerCG, MaguireME (1991) Magnesium transport in Salmonella typhimurium: the influence of new mutations conferring Co2+ resistance on the CorA Mg2+ transport system. Mol Microbiol 5 : 2753–2762.
14. GoytainA, QuammeGA (2005) Functional characterization of ACDP2 (ancient conserved domain protein), a divalent metal transporter. Physiol Genomics 22 : 382–389.
15. SponderG, SvidovaS, SchweigelM, VormannJ, KolisekM (2010) Splice-variant 1 of the ancient domain protein 2 (ACDP2) complements the magnesium-deficient growth phenotype of Salmonella enterica sv. typhimurium strain MM281. Magnes Res 23 : 105–114.
16. ParryDA, MighellAJ, El-SayedW, ShoreRC, JaliliIK, et al. (2009) Mutations in CNNM4 cause Jalili syndrome, consisting of autosomal-recessive cone-rod dystrophy and amelogenesis imperfecta. Am J Hum Genet 84 : 266–273.
17. PolokB, EscherP, AmbresinA, ChoueryE, BolayS, et al. (2009) Mutations in CNNM4 cause recessive cone-rod dystrophy with amelogenesis imperfecta. Am J Hum Genet 84 : 259–265.
18. de BaaijJH, StuiverM, MeijIC, LainezS, KopplinK, et al. (2012) Membrane topology and intracellular processing of cyclin M2 (CNNM2). J Biol Chem 287 : 13644–13655.
19. CasalettoJB, SaotomeI, CurtoM, McClatcheyAI (2011) Ezrin-mediated apical integrity is required for intestinal homeostasis. Proc Natl Acad Sci USA 108 : 11924–11929.
20. SuL, ShenL, ClayburghDR, NalleSC, SullivanEA, et al. (2009) Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology 136 : 551–563.
21. KucharskiLM, LubbeWJ, MaguireME (2000) Cation hexaammines are selective and potent inhibitors of the CorA magnesium transport system. J Biol Chem 275 : 16767–16773.
22. KolisekM, LaunayP, BeckA, SponderG, SerafiniN, et al. (2008) SLC41A1 is a novel mammalian Mg2+ carrier. J Biol Chem 283 : 16235–16247.
23. SmithCE (1998) Cellular and chemical events during enamel maturation. Crit Rev Oral Biol Med 9 : 128–161.
24. InaiT, SengokuA, HiroseE, IidaH, ShibataY (2008) Differential expression of the tight junction proteins, claudin-1, claudin-4, occludin, ZO-1, and PAR3, in the ameloblasts of rat upper incisors. Anat Rec 291 : 577–585.
25. MeindlA, DryK, HerrmannK, MansonF, CiccodicolaA, et al. (1996) A gene (RPGR) with homology to the RCC1 guanine nucleotide exchange factor is mutated in X-linked retinitis pigmentosa (RP3). Nat Genet 13 : 35–42.
26. HongDH, PawlykBS, ShangJ, SandbergMA, BersonEL, et al. (2000) A retinitis pigmentosa GTPase regulator (RPGR)-deficient mouse model for X-linked retinitis pigmentosa (RP3). Proc Natl Acad Sci USA 97 : 3649–3654.
27. GüntherT (2006) Mechanisms, regulation and pathologic significance of Mg2+ efflux from erythrocytes. Magnes Res 19 : 190–198.
28. GüntherT (2007) Na+/Mg2+ antiport in non-erythrocyte vertebrate cells. Magnes Res 20 : 89–99.
29. KolisekM, NestlerA, VormannJ, Schweigel-RöntgenM (2012) Human gene SLC41A1 encodes for the Na+/Mg2+ exchanger. Am J Physiol Cell Physiol 302: C318–326.
30. WabakkenT, RianE, KveineM, AasheimHC (2003) The human solute carrier SLC41A1 belongs to a novel eukaryotic subfamily with homology to prokaryotic MgtE Mg2+ transporters. Biochem Biophys Res Commun 306 : 718–724.
31. HurdTW, OttoEA, MishimaE, GeeHY, InoueH, et al. (2013) Mutation of the Mg2+ transporter SLC41A1 results in a nephronophthisis-like phenotype. J Am Soc Nephrol 24 : 967–977.
32. Navarro-GonzálezJF, Mora-FernándezC, García-PérezJ (2009) Clinical implications of disordered magnesium homeostasis in chronic renal failure and dialysis. Semin Dial 22 : 37–44.
33. KonradM, SchlingmannKP, GudermannT (2004) Insights into the molecular nature of magnesium homeostasis. Am J Physiol Renal Physiol 286: F599–605.
34. DimkeH, HoenderopJG, BindelsRJ (2011) Molecular basis of epithelial Ca2+ and Mg2+ transport: insights from the TRP channel family. J Physiol 589 : 1535–1542.
35. JälevikB, OdeliusH, DietzW, NorénJ (2001) Secondary ion mass spectrometry and X-ray microanalysis of hypomineralized enamel in human permanent first molars. Arch Oral Biol 46 : 239–247.
36. ItohM, YonemuraS, NagafuchiA, TsukitaS, TsukitaS (1991) 220-kD undercoat-constitutive protein: its specific localization at cadherin-based cell-cell adhesion sites. J Cell Biol 115 : 1449–1462.
37. KoikeC, ObaraT, UriuY, NumataT, SanukiR, et al. (2010) TRPM1 is a component of the retinal ON bipolar cell transduction channel in the mGluR6 cascade. Proc Natl Acad Sci USA 107 : 332–337.
38. OmoriY, ChayaT, KatohK, KajimuraN, SatoS, et al. (2010) Negative regulation of ciliary length by ciliary male germ cell-associated kinase (Mak) is required for retinal photoreceptor survival. Proc Natl Acad Sci USA 107 : 22671–22676.
39. SatoS, OmoriY, KatohK, KondoM, KanagawaM, et al. (2008) Pikachurin, a dystroglycan ligand, is essential for photoreceptor ribbon synapse formation. Nat Neurosci 11 : 923–931.
40. FujitaT, HirookaK, NakamuraT, ItanoT, NishiyamaA, et al. (2012) Neuroprotective effects of angiotensin II type 1 receptor (AT1-R) blocker via modulating AT1-R signaling and decreased extracellular glutamate levels. Invest Ophthalmol Vis Sci 53 : 4099–4110.
41. HibinoH, KurachiY (2007) Distinct detergent-resistant membrane microdomains (lipid rafts) respectively harvest K(+) and water transport systems in brain astroglia. Eur J Neurosci 26 : 2539–2555.
42. GüntherT (2006) Concentration, compartmentation and metabolic function of intracellular free Mg2+. Magnes Res 19 : 225–236.
43. SchillingT, EderC (2004) A novel physiological mechanism of glycine-induced immunomodulation: Na+-coupled amino acid transporter currents in cultured brain macrophages. J Physiol 559 : 35–40.
44. LeoneFA, BaranauskasJA, FurrielRP, BorinIA (2005) SigrafW: An easy-to-use program for fitting enzyme kinetic data. Biochem Mol Biol Educ 33 : 399–403.
45. TsudaT, NemotoN, KawakamiK, MochizukiE, KishidaS, et al. (2011) SEM observation of wet biological specimens pretreated with room-temperature ionic liquid. Chembiochem 12 : 2547–2550.
Štítky
Genetika Reprodukčná medicína
Článek Interaction between and during Mammalian Jaw Patterning and in the Pathogenesis of SyngnathiaČlánek Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of Genes in Developing LimbsČlánek Transcription Factor Occupancy Can Mediate Active Turnover of DNA Methylation at Regulatory RegionsČlánek Tay Bridge Is a Negative Regulator of EGFR Signalling and Interacts with Erk and Mkp3 in the Wing
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2013 Číslo 12- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Stressing the Importance of CHOP in Liver Cancer
- The AmAZI1ng Roles of Centriolar Satellites during Development
- Flies Get a Head Start on Meiosis
- Recommendations from Jane Gitschier's Bookshelf
- And Baby Makes Three: Genomic Imprinting in Plant Embryos
- Bugs in Transition: The Dynamic World of in Insects
- Defining the Role of ATP Hydrolysis in Mitotic Segregation of Bacterial Plasmids
- Synaptonemal Complex Components Promote Centromere Pairing in Pre-meiotic Germ Cells
- Cohesinopathies of a Feather Flock Together
- Genetic Recombination Is Targeted towards Gene Promoter Regions in Dogs
- Parathyroid-Specific Deletion of Unravels a Novel Calcineurin-Dependent FGF23 Signaling Pathway That Regulates PTH Secretion
- MAN1B1 Deficiency: An Unexpected CDG-II
- Phosphate Flow between Hybrid Histidine Kinases CheA and CheS Controls Cyst Formation
- Basolateral Mg Extrusion via CNNM4 Mediates Transcellular Mg Transport across Epithelia: A Mouse Model
- Truncation of Unsilences Paternal and Ameliorates Behavioral Defects in the Angelman Syndrome Mouse Model
- Autozygome Sequencing Expands the Horizon of Human Knockout Research and Provides Novel Insights into Human Phenotypic Variation
- Huntington's Disease Induced Cardiac Amyloidosis Is Reversed by Modulating Protein Folding and Oxidative Stress Pathways in the Heart
- Low Frequency Variants, Collapsed Based on Biological Knowledge, Uncover Complexity of Population Stratification in 1000 Genomes Project Data
- Targeted Ablation of and in Retinal Progenitor Cells Mimics Leber Congenital Amaurosis
- Genomic Imprinting in the Embryo Is Partly Regulated by PRC2
- Binary Cell Fate Decisions and Fate Transformation in the Larval Eye
- The Stress-Regulated Transcription Factor CHOP Promotes Hepatic Inflammatory Gene Expression, Fibrosis, and Oncogenesis
- A Global RNAi Screen Identifies a Key Role of Ceramide Phosphoethanolamine for Glial Ensheathment of Axons
- Functional Analysis of the Interdependence between DNA Uptake Sequence and Its Cognate ComP Receptor during Natural Transformation in Species
- Cross-Modulation of Homeostatic Responses to Temperature, Oxygen and Carbon Dioxide in
- Alcohol-Induced Histone Acetylation Reveals a Gene Network Involved in Alcohol Tolerance
- Molecular Characterization of Host-Specific Biofilm Formation in a Vertebrate Gut Symbiont
- CRIS—A Novel cAMP-Binding Protein Controlling Spermiogenesis and the Development of Flagellar Bending
- Dual Regulation of the Mitotic Exit Network (MEN) by PP2A-Cdc55 Phosphatase
- Expanding the Marine Virosphere Using Metagenomics
- Detection of Slipped-DNAs at the Trinucleotide Repeats of the Myotonic Dystrophy Type I Disease Locus in Patient Tissues
- Interaction between and during Mammalian Jaw Patterning and in the Pathogenesis of Syngnathia
- Mutations in the UQCC1-Interacting Protein, UQCC2, Cause Human Complex III Deficiency Associated with Perturbed Cytochrome Protein Expression
- Reactivation of Chromosomally Integrated Human Herpesvirus-6 by Telomeric Circle Formation
- Anoxia-Reoxygenation Regulates Mitochondrial Dynamics through the Hypoxia Response Pathway, SKN-1/Nrf, and Stomatin-Like Protein STL-1/SLP-2
- The Midline Protein Regulates Axon Guidance by Blocking the Reiteration of Neuroblast Rows within the Drosophila Ventral Nerve Cord
- Tomato Yield Heterosis Is Triggered by a Dosage Sensitivity of the Florigen Pathway That Fine-Tunes Shoot Architecture
- Selection on Plant Male Function Genes Identifies Candidates for Reproductive Isolation of Yellow Monkeyflowers
- Role of Tomato Lipoxygenase D in Wound-Induced Jasmonate Biosynthesis and Plant Immunity to Insect Herbivores
- Meiotic Cohesin SMC1β Provides Prophase I Centromeric Cohesion and Is Required for Multiple Synapsis-Associated Functions
- Identification of Sphingolipid Metabolites That Induce Obesity via Misregulation of Appetite, Caloric Intake and Fat Storage in
- Genome-Wide Screen Reveals Replication Pathway for Quasi-Palindrome Fragility Dependent on Homologous Recombination
- Histone Methylation Restrains the Expression of Subtype-Specific Genes during Terminal Neuronal Differentiation in
- A Novel Intergenic ETnII-β Insertion Mutation Causes Multiple Malformations in Mice
- The NuRD Chromatin-Remodeling Enzyme CHD4 Promotes Embryonic Vascular Integrity by Transcriptionally Regulating Extracellular Matrix Proteolysis
- A Domesticated Transposase Interacts with Heterochromatin and Catalyzes Reproducible DNA Elimination in
- Acute Versus Chronic Loss of Mammalian Results in Distinct Ciliary Phenotypes
- MBD3 Localizes at Promoters, Gene Bodies and Enhancers of Active Genes
- Positive and Negative Regulation of Gli Activity by Kif7 in the Zebrafish Embryo
- A Hereditary Spastic Paraplegia Mouse Model Supports a Role of ZFYVE26/SPASTIZIN for the Endolysosomal System
- The CCR4-NOT Complex Mediates Deadenylation and Degradation of Stem Cell mRNAs and Promotes Planarian Stem Cell Differentiation
- Reconstructing Native American Migrations from Whole-Genome and Whole-Exome Data
- Contributions of Protein-Coding and Regulatory Change to Adaptive Molecular Evolution in Murid Rodents
- Comprehensive Analysis of Transcriptome Variation Uncovers Known and Novel Driver Events in T-Cell Acute Lymphoblastic Leukemia
- A -Acting Protein Effect Causes Severe Eye Malformation in the Mouse
- Clustering of Tissue-Specific Sub-TADs Accompanies the Regulation of Genes in Developing Limbs
- Germline Progenitors Escape the Widespread Phenomenon of Homolog Pairing during Development
- Transcription Factor Occupancy Can Mediate Active Turnover of DNA Methylation at Regulatory Regions
- Somatic mtDNA Mutation Spectra in the Aging Human Putamen
- ESCRT-I Mediates FLS2 Endosomal Sorting and Plant Immunity
- Ethylene Promotes Hypocotyl Growth and HY5 Degradation by Enhancing the Movement of COP1 to the Nucleus in the Light
- The PAF Complex and Prf1/Rtf1 Delineate Distinct Cdk9-Dependent Pathways Regulating Transcription Elongation in Fission Yeast
- Dual Regulation of Gene Expression Mediated by Extended MAPK Activation and Salicylic Acid Contributes to Robust Innate Immunity in
- Quantifying Missing Heritability at Known GWAS Loci
- Smc5/6-Mms21 Prevents and Eliminates Inappropriate Recombination Intermediates in Meiosis
- Smc5/6 Coordinates Formation and Resolution of Joint Molecules with Chromosome Morphology to Ensure Meiotic Divisions
- Tay Bridge Is a Negative Regulator of EGFR Signalling and Interacts with Erk and Mkp3 in the Wing
- Meiotic Crossover Control by Concerted Action of Rad51-Dmc1 in Homolog Template Bias and Robust Homeostatic Regulation
- Active Transport and Diffusion Barriers Restrict Joubert Syndrome-Associated ARL13B/ARL-13 to an Inv-like Ciliary Membrane Subdomain
- An Regulatory Circuit Modulates /Wnt Signaling and Determines the Size of the Midbrain Dopaminergic Progenitor Pool
- Variants Induce Differential Protection to Viruses in : A Phenotypic and Phylogenomic Analysis
- Base Pairing Interaction between 5′- and 3′-UTRs Controls mRNA Translation in
- Evidence That Masking of Synapsis Imperfections Counterbalances Quality Control to Promote Efficient Meiosis
- Insulin/IGF-Regulated Size Scaling of Neuroendocrine Cells Expressing the bHLH Transcription Factor in
- Sumoylated NHR-25/NR5A Regulates Cell Fate during Vulval Development
- TATN-1 Mutations Reveal a Novel Role for Tyrosine as a Metabolic Signal That Influences Developmental Decisions and Longevity in
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The NuRD Chromatin-Remodeling Enzyme CHD4 Promotes Embryonic Vascular Integrity by Transcriptionally Regulating Extracellular Matrix Proteolysis
- Mutations in the UQCC1-Interacting Protein, UQCC2, Cause Human Complex III Deficiency Associated with Perturbed Cytochrome Protein Expression
- The Midline Protein Regulates Axon Guidance by Blocking the Reiteration of Neuroblast Rows within the Drosophila Ventral Nerve Cord
- Tomato Yield Heterosis Is Triggered by a Dosage Sensitivity of the Florigen Pathway That Fine-Tunes Shoot Architecture
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy





