-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
ImmunoChip Study Implicates Antigen Presentation to T Cells in Narcolepsy
Recent advances in the identification of susceptibility genes and environmental exposures provide broad support for a post-infectious autoimmune basis for narcolepsy/hypocretin (orexin) deficiency. We genotyped loci associated with other autoimmune and inflammatory diseases in 1,886 individuals with hypocretin-deficient narcolepsy and 10,421 controls, all of European ancestry, using a custom genotyping array (ImmunoChip). Three loci located outside the Human Leukocyte Antigen (HLA) region on chromosome 6 were significantly associated with disease risk. In addition to a strong signal in the T cell receptor alpha (TRA@), variants in two additional narcolepsy loci, Cathepsin H (CTSH) and Tumor necrosis factor (ligand) superfamily member 4 (TNFSF4, also called OX40L), attained genome-wide significance. These findings underline the importance of antigen presentation by HLA Class II to T cells in the pathophysiology of this autoimmune disease.
Published in the journal: ImmunoChip Study Implicates Antigen Presentation to T Cells in Narcolepsy. PLoS Genet 9(2): e32767. doi:10.1371/journal.pgen.1003270
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003270Summary
Recent advances in the identification of susceptibility genes and environmental exposures provide broad support for a post-infectious autoimmune basis for narcolepsy/hypocretin (orexin) deficiency. We genotyped loci associated with other autoimmune and inflammatory diseases in 1,886 individuals with hypocretin-deficient narcolepsy and 10,421 controls, all of European ancestry, using a custom genotyping array (ImmunoChip). Three loci located outside the Human Leukocyte Antigen (HLA) region on chromosome 6 were significantly associated with disease risk. In addition to a strong signal in the T cell receptor alpha (TRA@), variants in two additional narcolepsy loci, Cathepsin H (CTSH) and Tumor necrosis factor (ligand) superfamily member 4 (TNFSF4, also called OX40L), attained genome-wide significance. These findings underline the importance of antigen presentation by HLA Class II to T cells in the pathophysiology of this autoimmune disease.
Introduction
Narcolepsy is a life-long sleep disorder caused by the autoimmune-mediated loss of 70,000–90,000 hypocretin (orexin)-producing neurons in the hypothalamus. Prevalence is approximately 0.02–0.03% in Caucasian populations, and somewhat higher in Japanese (0.16%). Family and twin studies support the importance of genetic (10–40 fold increased risk in first degree relatives) as well as environmental factors (25% concordance in identical twins) [1]. Onset is typically around puberty and displays a seasonal pattern of incidence, with highest rates in spring and summer. Likely triggering factors are influenza A, notably the pandemic H1N1 2009 variant, and Streptococcus Pyogenes infections [2]–[5]. Unique among autoimmune diseases, the condition is almost completely associated with Human HLA DQ0602, a heterodimeric protein encoded by the DQA1*01 : 02-DQB1*06 : 02 haplotype (90% versus 25% frequency in European ancestry cases and controls, respectively). The overwhelming effect of this haplotype on risk suggests the importance of antigen presentation by DQ0602. As seen in other autoimmune diseases, additional HLA alleles carried in trans of this haplotype also confer modulatory effects [6], [7]. Most notably, DQA1*01 : 02-DQB1*06 : 02 homozygosity increases predisposition by 2–4 fold. Further, DQA1 and DQB1 alleles known to heterodimerize with DQA1*01 : 02 or DQB1*06 : 02 reduce susceptibility, likely through allelic competition with DQ0602 [8]. However, non-HLA related genes play important roles. In addition to these well-established HLA class II effects, recent genome-wide association studies (GWAS) have identified variants in the T Cell receptor alpha locus (TRA@), on chromosome 14q11.2, and in the region containing P2RY11-DNMT1 on chromosome 19p13.2, as additional susceptibility loci. Finally, exome sequencing in families with a rare autosomal dominant syndrome including cerebellar ataxia, narcolepsy and deafness (ADCA-DN) also indicate an important role for DNMT1 in survival of hypocretin neurons [9].
Based on the recognition that considerable overlap exists in risk loci for various autoimmune diseases, the Immunochip consortium was formed to create a single nucleotide polymorphism (SNP) array for targeted finemapping of these loci [10], [11]. The ImmunoChip was designed for deep replication of signals from large-scale meta-analyses in nine autoimmune diseases, and for finemapping of loci reaching genome-wide significance (2009). Approximately 200,000 rare and common variants were selected to cover intervals with established genome-wide significant association to autoimmune and seronegative diseases, and at selected loci of known importance in major immune-related diseases, including the major histocompatibility (MHC) and KIR/LILR loci. Using this platform, we conducted a GWAS to identify genetic risk factors for narcolepsy in addition to HLA DQ0602. We analyzed 111,240 high quality SNP markers of minor allele frequency ≥1%, located outside the extended HLA region on chromosome 6, in 1,886 narcolepsy cases and 10,421 controls of European ancestry sampled across global populations including the European Union, Canada and North America (Table 1). To test for potential confounding effects of population stratification in our study cohort, we performed principal component analysis (PCA) of cases and controls (Figure S1). Control samples showed clear separation into distinct European countries in plots of the first and second principal components, with good overlay of case samples, and plots of observed versus expected association results showed no inflation of signal (λ = 1.004; Figure 1).
Fig. 1. Manhattan Plot of association statistics. 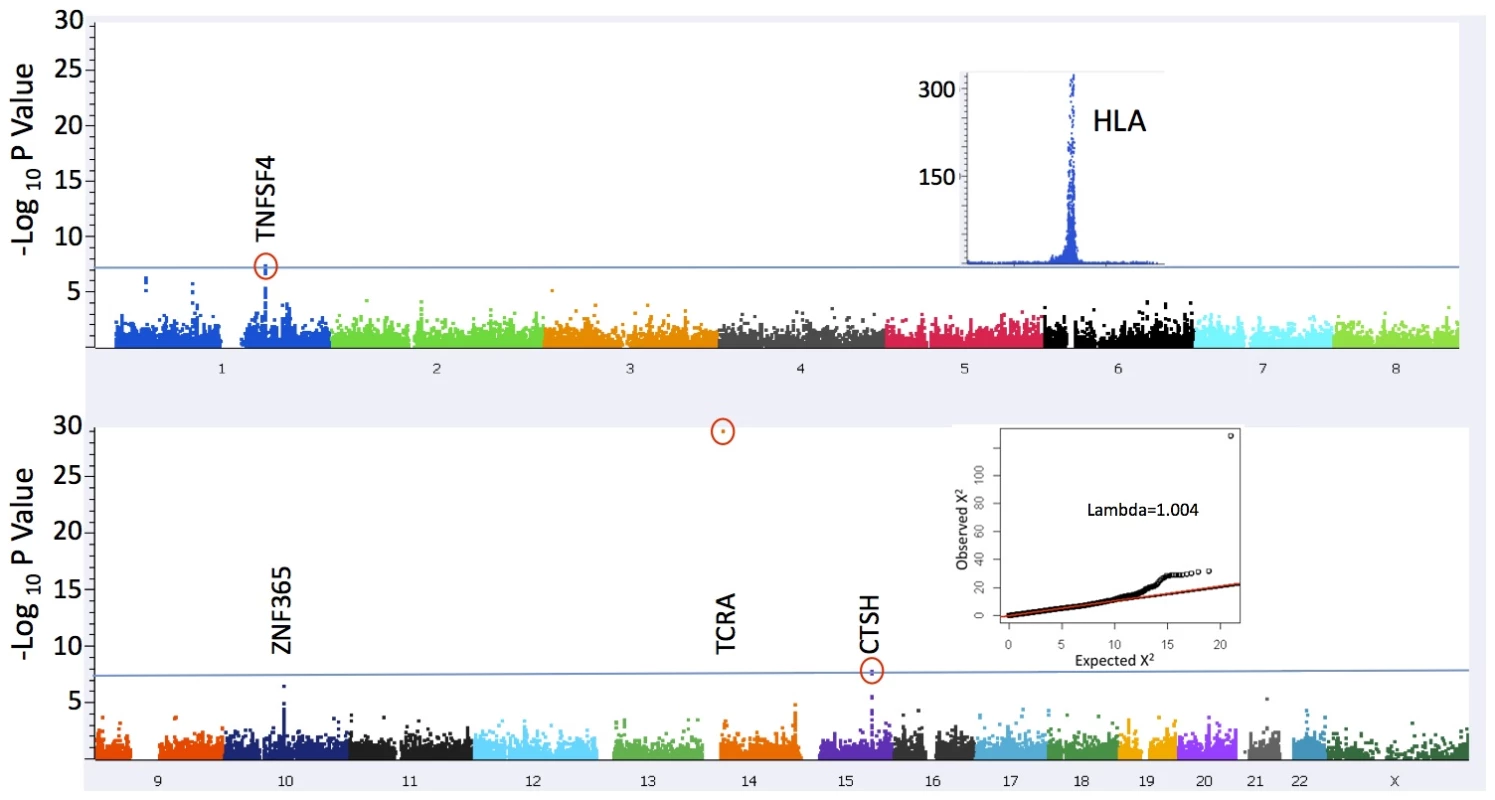
The significance threshold used (blue line) was P = 5×10−8; The insets depict plots of 1) association results in a broad region encompassing the HLA locus (chr 6:24,067–35,474 kb) that were excluded from the present analysis (see Methods) and 2) QQ plot of results for 109,777 markers after excluding a 1 Mb window surrounding the associated loci (λ = 1.004). The inflation statistic for all 111,240 tested markers is 1.04. Tab. 1. Sample collections. 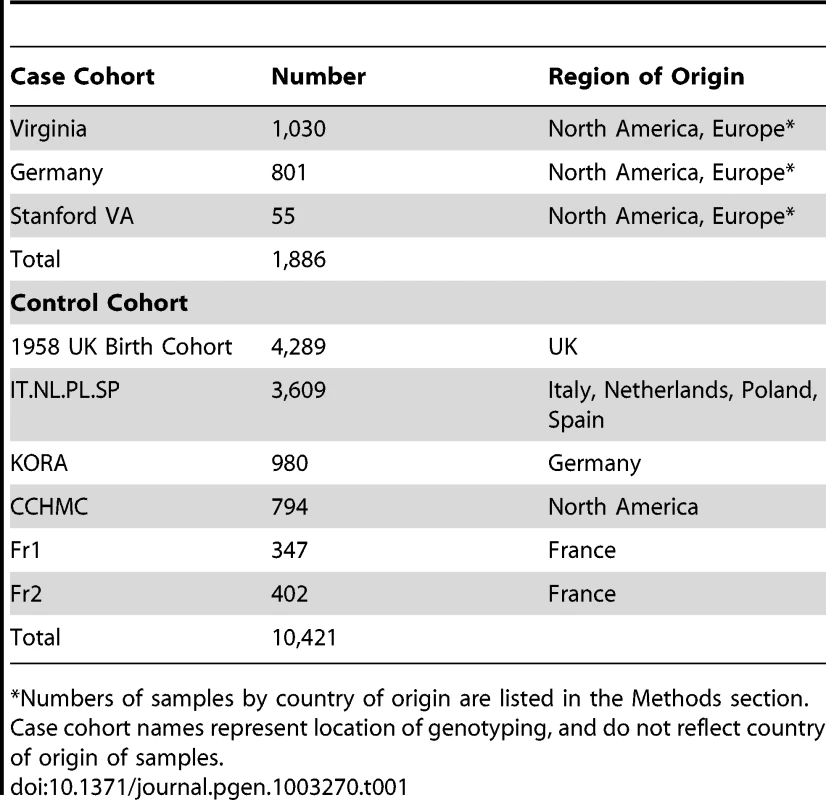
Numbers of samples by country of origin are listed in the Methods section. Results/Discussion
One previously reported, and two novel non-HLA loci surpassed genome wide significance (gws) P<5×10−8 in this study (Figure 1 and Table 2). The strongest association was with rs1154155 (MAF = 0.15, P = 8.87×10−30 OR = 1.72) in the T cell receptor (TCR) alpha (TRA@) locus, on chromosome 14, replicating signal previously reported using smaller samples [7], [12], [13]. The TCR protein is comprised of alpha and beta chains. As for immunoglobulin loci, TCR loci undergo somatic DNA recombination during T cell development, generating a large number of possible proteins specific to individual T-cell clones. T cells bearing specific recombinants are then negatively or positively selected, allowing adaptation of the immune system to past environmental history.
Tab. 2. Non-HLA narcolepsy risk variant loci reaching genome-wide significance. 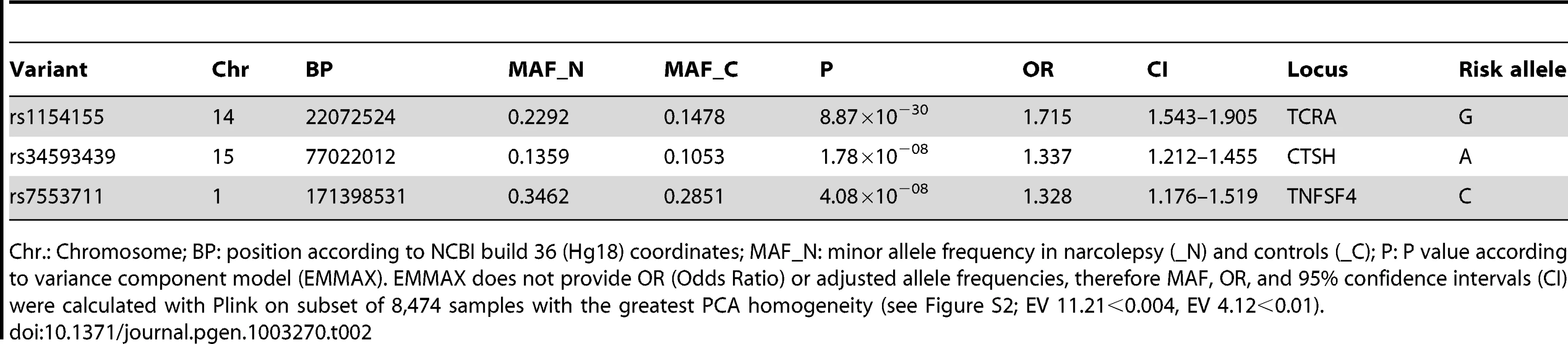
Chr.: Chromosome; BP: position according to NCBI build 36 (Hg18) coordinates; MAF_N: minor allele frequency in narcolepsy (_N) and controls (_C); P: P value according to variance component model (EMMAX). EMMAX does not provide OR (Odds Ratio) or adjusted allele frequencies, therefore MAF, OR, and 95% confidence intervals (CI) were calculated with Plink on subset of 8,474 samples with the greatest PCA homogeneity (see Figure S2; EV 11.21<0.004, EV 4.12<0.01). The T cell receptor binds foreign or self-peptides presented by Class II MHC proteins (such as the DQ alpha/beta heterodimer), allowing initiation and regulation of immune responses. It is thus the natural receptor of DQB0602. The TRA@ locus was sparsely covered on the ImmunoChip (15 SNPs within a 1 Mb window of rs1154155, none with r2 above 0.5) precluding fine mapping or haplotype analysis, although providing robust replication of the previously reported findings. SNP rs1154155 is located close to the J10 segment region of the locus, with linkage disequilibrium (LD) data suggesting the involvement of a specific J segment in the narcolepsy pathophysiology. The association with TRA@ is unique to narcolepsy, as no other autoimmune diseases have been associated with this locus.
Two SNPs rs34593439, and rs34843303, located in intron 1 of Cathepsin H (CTSH), a papain-like cysteine protease, reached gws (MAF = 0.11, P = 1.78×10−8 OR = 1.34 and MAF = 0.11, P< = 2.79×10−8 OR = 1.35, respectively). Another SNP located in intron 1, rs3825932T has been previously reported to be associated with type 1 diabetes [14], [15]. Although in close proximity, this marker is in weak LD with rs34593439 and rs34843303 (r2 = 0.23 and 0.23 respectively) and shows no significant association in the present sample (P = 0.01). The local region of LD surrounding these markers encompasses exon 1, where 4 potentially functional polymorphisms have been identified. One of which, SNP rs2289702T (p.Gly11Arg, MAF = 0.11), is in tight LD with our markers (pairwise r2 = 0.96 and 0.98 respectively, 1000genomes data, phase 1 release V3), and could be the culprit behind this association. Following imputation in a 1 Mb window surrounding CTSH, SNPs rs2289702 and rs34593439 were the two most highly associated variants (respectively) (Figure 2). The Arg allele of rs2289702 also underlies a minor histocompatability antigen restricted by HLA-A*3101 and HLA-A*3303, causing selective lysis of hematopoetic cells by cytotoxic lymphocytes [16]. p.Gly11Arg is located within the signal peptide sequence of CTSH, where the introduction of a highly charged arginine could affect trafficking or cleavage, as predicted by some but not all signal peptide predicting programs (see Methods).
Fig. 2. Association signal at the mapping intervals flanking rs34593439 and rs7553711. 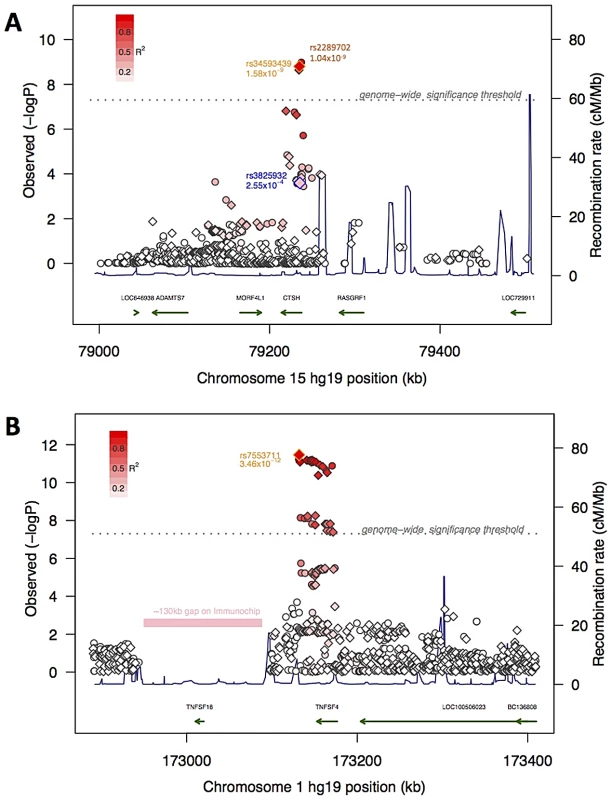
Association scores at 15q25.1 (panel A) and 1q25.1 (panel B). Genotyped (diamonds) and imputed (circles) SNPs are indicated and the top genotyped SNP in the interval is outlined in orange. A SNP in 15q25.1 previously associated with Diabetes is outlined in blue. The degree of red color in each diamond or circle indicates the strength of LD with the top SNP (on a scale shown in the legend at the upper left hand corner of the plot). The X-axis shows the chromosome and physical distance (kb) from the human genome reference sequence (hg19), the left Y-axis shows the negative base ten logarithm of the p-value and the right Y-axis shows recombination rate (cM/Mb) as a navy line. The genome-wide significance threshold (P<5×10−8) is given by the dashed grey line. Genes in the regions are annotated at the bottom as green arrows. Also indicated in 1q25.1 is a ∼130 kb region with no SNPs on the ImmunoChip. Cathepsins are primarily located within the lysosomal/endosomal compartment and typically activated by low pH. These enzymes play diverse and important roles including cellular recycling of proteins, activation of selected preprohormones, antigen processing, and loading of peptides onto MHC class II proteins. Eleven family members are known. Cystatins and other endogenous inhibitors are known to regulate cathepsin activity, and the balance of these activities has been proposed to be the major selector for the repertoire of surface peptide –MHC II complexes [17]. Deficiencies in selected cathepsins impair immune cell development (NKT cells in Cathepsin S or L deficient mice, thymocytes and T cell repertoire in cathepsin L deficient mice), and produce defects in immune cell effector functions (cytotoxic T cell, neutrophil and mast cell defects in cathepsin C deficient mice; see [18]). Cathepsin H is somewhat unique in that it can have both exopeptidase and endopeptidase activities, depending on the presence of a bound mini-chain (a remnant of the pro-enzyme) within the active cleft. Although ubiquitously expressed, CTSH expression is especially high in type II pneumocytes, where it plays a key role in the maturation of lung surfactant protein B [17], [19]. CTSH is also highly expressed in MHC class II positive immune cells such as B cells, monocytes and dendritic cells, but not T cells, notably in the presence of inflammation. For example, CTSH enzyme activity increases in parallel with proinflammatory cytokines during the development of autoimmune inflammation in a NOD mouse model of Sjögren's syndrome [20]. One hypothesis may be that decreased CTSH activity reduces antigen processing resulting in an altered repertoire presented by DQB0602 and resulting in increased risk of narcolepsy.
SNPs located in the Tumor Necrosis Factor (ligand) Superfamily member 4 (TNFSF4; also called OX40L or CD252) are strongly associated with narcolepsy. SNP rs7553711 reached gws (MAF 0.29, P = 4.08×10−8 and OR = 1.33). No other SNP was more strongly associated with narcolepsy following imputation in a 1 Mb window around this locus, although additional strongly associated variants were identified (Figure 2). TNFSF4 is known to be strongly associated with systemic lupus erythematosis (SLE) [21] and systemic sclerosis [22], [23] and SNPs in this region were densely represented on the ImmunoChip. Two distinct haplotypes composed of SNPs upstream of the gene confer susceptibility or resistance to SLE, whereas our most significantly associated SNP markers in narcolepsy are downstream of the gene in a separate haplotype block. The SNPs associated with SLE and narcolepsy are in weak LD, and rs844648, an established marker of SLE, is not strongly associated with narcolepsy (p = 0.016). Interestingly, rs7553711 maps to a potential enhancer site (H3K4Me1 site, UCSC browser, Layered H3K27 Track).
The association of narcolepsy with SNPs in TNFSF4 is consistent with a primary role of antigen presentation to T cells in narcolepsy. Like CTSH, OX40L is primarily expressed in MHC Class II-positive antigen presenting cells (e.g. dendritic and B cells). Optimal activation of T cells following the binding of T cell receptor - MHC class II/antigen complex requires the action of additional costimulatory factors, notably involving receptor/ligand pairs from the tumor necrosis superfamily. The interaction of two of these, OX40 receptor (encoded by TNFRSF4) and OX40L ligand (encoded by TNFSF4), provides an important costimulatory signal supporting Th1 and Th2 responses, promoting expansion and survival of effector T cells and the generation of T memory cells. Although less understood, OX40/OX40L interactions also play a role in the activity and homeostasis of T regulatory cells. Signaling of this pair is tightly controlled, as OX40 is not expressed in resting T cells, only appearing approximately one day following initial activation. Similarly, OX40L is found only at sites of inflammation, first on the surface of antigen presenting cells, but later on diverse cell types including mast cells, suggesting a role distinct from T cell priming or memory cell generation. OX40-OX40L interactions are known to be involved in autoimmune disease, (e.g. SLE) likely acting through a disruption of tolerance. OX40 signaling within responding T cells renders them resistant to Treg - mediated suppression, and acts within the Treg cells to inhibit suppressive functions. In addition, sustained inflammatory response may result from excessive OX40-OX40L signaling and consequent increased survival of effector T-cells (see [24], [25]).
Two other regions showed suggestive associations, including SNPs between MIR-552 and GJB5 on Chromosome 1p34.3 (rs10915020 MAF = 0.84, P = 5.40×10−07, OR = 1.32), and near ZNF365 on chromosome 10q21.2 (rs10995245 MAF = 0.35, P = 3.24×10−07 OR = 1.20). ZNF365 is highly expressed in the brain and has been implicated in susceptibility to breast cancer, Crohn's Disease, and more recently, atopic dermatitis [26]–[28]. None of these reached genome-wide significance levels after correcting with the EMMAX [29] procedure in the current study, although nearly reaching or surpassing Bonferroni significance (P = 4.5×10−07)(Table S1). Increased sample size and replication will be needed to confirm these loci.
Our study, analyzing 1886 narcolepsy-cataplexy cases of European ancestry, is the largest collaborative cohort study of narcolepsy to date, including samples from across the United States, Canada and Europe, and representing the majority of available case samples of European ancestry. To preserve the statistical power afforded by this sample size, we elected not to split our cases into discovery and replication cohorts, and thus our study is limited by the lack of replication in an ethnically similar population. We identified two novel narcolepsy susceptibility genes, CTSH and TNFSF4 (OX40L), and confirmed strong associations with HLA and TRA@. The two new loci identified outline with striking clarity that the key pathology underlying narcolepsy likely resides in the interaction between T cells and antigen presenting cells.
Although a role of antigen presentation to CD4 T cells is likely the primary susceptibility pathway for the disorder, narcolepsy was not associated with all components of this pathway as represented on the array. For example, we found no association at the p<10−4 threshold with the class II invariant chain, AEP and cathepsin B (CTSB) genes or, more surprisingly, with genes encoding other co-stimulatory molecules such as CD28, cytotoxic T-lymphocyte antigen-4 (CTLA4) and their cognate ligands, CD80 and CD86 (these have been involved in many other autoimmune disorders) (see Table S1). The present results also show limited overlap in susceptibility loci between narcolepsy and loci associated with classical autoimmune disorders, a fact that may be unsurprising based on the lack of readily identifiable autoantibodies, or other clear signs of inflammatory damage in the disease. To date, the TCR locus has only been observed in narcolepsy. Notably, we found no associations with loci widely shared among other autoimmune diseases such as interleukin genes and receptors (IL2, IL21, IL12, IL2RA, IL23R) acting in differentiation; PTPN2 and 22, SH2B3 and TAGAP involved in immune-cell activation and signaling; and IRF5, TNFAIP3 involved in TNF signaling and innate immunity (Table S1). Together with findings implicating pandemic H1N1 influenza as a trigger, narcolepsy may offer a unique opportunity, furthering our understanding of how HLA class II presentation of foreign and self-antigens predispose to autoimmunity.
Methods
Ethics statement
Informed consent in accordance with governing institutions was obtained from all subjects. The research protocol at Stanford was approved by the IRB Panel on Medical Human Subjects.
Samples
Cases included in this study all met criteria for narcolepsy/hypocretin deficiency (clear-cut cataplexy and DQB1*06 : 02 positive, or low cerebrospinal fluid hypocretin-1). Samples included 1301 patients sourced from the Stanford Center for Narcolepsy database (North America, and worldwide collaborators), and 585 samples contributed by the European narcolepsy network (EU-NN). ImmunoChip typing was performed at centers in the US and in Germany. Informed consent in accordance with governing institutions was obtained. Countries of origin included: United States (657), France (296), Italy (157), Germany (157), the Netherlands (111), Czech Republic (104), Canada (101), Austria (83), Denmark (74), Spain (51) and Norway (32). A further 63 cases came from Argentina, Australia, Finland, Israel, Poland, Portugal, Slovakia, Switzerland and Turkey, each with fewer than 20 samples. Control genotypes were contributed through multiple immunochip consortium collaborators including 4289 samples from the United Kingdom 1958 Birth Cohort, 3609 samples from selected European countries including Italy (1251), Netherlands (1173), Poland (529) and Spain (656), 980 samples from the German KORA cohort; 794 Samples from Cincinnati through CCHMC [30]; and 749 French samples (2 collaborators).
Data analysis and statistics
Genotyping of cases was performed following Illumina's recommendation at U Virginia, USA, U of Munich, Germany, and Stanford University, Palo Alto, CA USA. NCBI build 36 (hg18) mapping was used as reference. Illumina manifest file Immuno_BeadChip_1149691_B.bpm was used in the majority of cases. In cases where file Immuno_BeadChip_11419691_A was used, map positions were converted to be consistent with 1149691_B, or omitted from the analysis. Genotypes were called using Illumina GeneExpress (Illumina GenomeStudio GenTrain2.0 algorithm), with extensive additional curation. Individuals with call rate under 98% (123 controls, 147 cases), and samples which were related (pi hat>0.2) were excluded from further analysis. Data from all sources were merged in forward-strand format. We identified 142,054 high quality SNPs with call rate above 99% (in both cases and controls separately), and passing HWE filtering in controls (P>1×10−5) using the Plink suite of software [31]. We excluded a broad region around the HLA complex (7,893 markers at Chr 6 : 24,067–35,474 kb) due to the strong LD effects with DQB1*06 : 02. This region contained nearly 3000 SNPs associated with narcolepsy at GWA significant levels. We additionally excluded SNPs with minor allele frequency below 1% (22,921 SNPs). Finally 111,240 high quality SNPs of MAF≥0.01 (including 91,804 MAF≥0.05) were selected for the analysis presented here. Principal components analysis (PCA) was performed to identify 162 outliers (133 controls, 29 cases; Golden Helix SVS, v7), and those were removed. Genome wide association analysis was performed using a variance component model implemented in EMMAX [29]. The EMMAX software does not return odds ratio or adjusted allele frequency data after correction for stratification. We therefore calculated OR and MAF (using Plink) for our tables based on a more homogeneous subsample of 8474 cases/controls based on principal components (EV 11.21<0.004, EV 4.12<0.01, see Figure S1). Linkage disequilibrium (as r2) values and haplotype analysis were calculated using Plink and Haploview [32] using data from our sample and/or from the 1000 genomes dataset [33]. QQ plots were generated using estlambda (http://www.genabel.org/GenABEL/estlambda.html), and Manhattan and PCA plots were made using SVS software.
Imputation
Imputation and phasing of ImmunoChip genotypes were performed using Beagle v3.3 [34] against 4 European populations (286 individuals from CEU, TSI, GBR, IBS) in the 1000 genomes integrated data set (phase 1 release v3) within a 1 Mb window of the top hit at the CTSH and TNFSF4 loci. SNPs with an imputation R2 value≥0.8 (representing reliability of imputation) were considered in the analysis. Pairwise LD was calculated in Plink. Association P values in Figure 2 were calculated with Plink, as EMMAX would be inappropriate in this context, and therefore P values are slightly different than those presented in Table 2.
http://faculty.washington.edu/browning/beagle/beagle.html
http://bochet.gcc.biostat.washington.edu/beagle/1000_Genomes.phase1_release_v3/
Cleavage prediction
Sequence used: MWATLPLLCAGAWLL[G/R]VPVCGAAELCVNSLEKFHFKSWTSKHRKTYSTEEYHHRLQTFAS
SignalIP: http://www.cbs.dtu.dk/services/SignalP/ Both alleles are predicted to have normal cleavage
SigPred: http://bmbpcu36.leeds.ac.uk/prot_analysis/Signal.html Predicts cleavage unlikely for Arg variant.
Supporting Information
Zdroje
1. MignotE (1998) Genetic and familial aspects of narcolepsy. Neurology 50: S16–22.
2. HanF, LinL, WarbySC, FaracoJ, LiJ, et al. (2011) Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Annals of neurology 70 : 410–417.
3. PartinenM, Saarenpaa-HeikkilaO, IlveskoskiI, HublinC, LinnaM, et al. (2012) Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLoS ONE 7: e33723 doi:10.1371/journal.pone.0033723.
4. LongstrethWTJr, TonTG, KoepsellTD (2009) Narcolepsy and streptococcal infections. Sleep 32 : 1548.
5. AranA, EinenM, LinL, PlazziG, NishinoS, et al. (2010) Clinical and therapeutic aspects of childhood narcolepsy-cataplexy: a retrospective study of 51 children. Sleep 33 : 1457–1464.
6. HongSC, LinL, LoB, JeongJH, ShinYK, et al. (2007) DQB1*0301 and DQB1*0601 modulate narcolepsy susceptibility in Koreans. Human immunology 68 : 59–68.
7. HorH, KutalikZ, DauvilliersY, ValsesiaA, LammersGJ, et al. (2010) Genome-wide association study identifies new HLA class II haplotypes strongly protective against narcolepsy. Nature genetics 42 : 786–789.
8. HanF, LinL, LiJ, DongSX, AnP, et al. (2012) HLA-DQ association and allele competition in Chinese narcolepsy. Tissue Antigens 80 : 328–335.
9. WinkelmannJ, LinL, SchormairB, KornumBR, FaracoJ, et al. (2012) Mutations in DNMT1 cause autosomal dominant cerebellar ataxia, deafness and narcolepsy. Human molecular genetics 21 : 2205–2210.
10. RamosPS, CriswellLA, MoserKL, ComeauME, WilliamsAH, et al. (2011) A comprehensive analysis of shared loci between systemic lupus erythematosus (SLE) and sixteen autoimmune diseases reveals limited genetic overlap. PLoS Genet 7: e1002406 doi:10.1371/journal.pgen.1002406.
11. Gutierrez-AchuryJ, Coutinho de AlmeidaR, WijmengaC (2011) Shared genetics in coeliac disease and other immune-mediated diseases. Journal of internal medicine 269 : 591–603.
12. HallmayerJ, FaracoJ, LinL, HesselsonS, WinkelmannJ, et al. (2009) Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nature genetics 41 : 708–711.
13. MiyagawaT, HondaM, KawashimaM, ShimadaM, TanakaS, et al. (2010) Polymorphism located in TCRA locus confers susceptibility to essential hypersomnia with HLA-DRB1*1501-DQB1*0602 haplotype. Journal of human genetics 55 : 63–65.
14. CooperJD, SmythDJ, SmilesAM, PlagnolV, WalkerNM, et al. (2008) Meta-analysis of genome-wide association study data identifies additional type 1 diabetes risk loci. Nature genetics 40 : 1399–1401.
15. BarrettJC, ClaytonDG, ConcannonP, AkolkarB, CooperJD, et al. (2009) Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nature genetics 41 : 703–707.
16. TorikaiH, AkatsukaY, MiyazakiM, TsujimuraA, YatabeY, et al. (2006) The human cathepsin H gene encodes two novel minor histocompatibility antigen epitopes restricted by HLA-A*3101 and -A*3303. British journal of haematology 134 : 406–416.
17. ConusS, SimonHU (2010) Cathepsins and their involvement in immune responses. Swiss medical weekly 140: w13042.
18. ColbertJD, MatthewsSP, MillerG, WattsC (2009) Diverse regulatory roles for lysosomal proteases in the immune response. European journal of immunology 39 : 2955–2965.
19. BuhlingF, KouadioM, ChwieralskiCE, KernU, HohlfeldJM, et al. (2011) Gene targeting of the cysteine peptidase cathepsin H impairs lung surfactant in mice. PLoS ONE 6: e26247 doi:10.1371/journal.pone.0026247.
20. LiX, WuK, EdmanM, Schenke-LaylandK, MacVeigh-AloniM, et al. (2010) Increased expression of cathepsins and obesity-induced proinflammatory cytokines in lacrimal glands of male NOD mouse. Investigative ophthalmology & visual science 51 : 5019–5029.
21. Cunninghame GrahamDS, GrahamRR, MankuH, WongAK, WhittakerJC, et al. (2008) Polymorphism at the TNF superfamily gene TNFSF4 confers susceptibility to systemic lupus erythematosus. Nature genetics 40 : 83–89.
22. GourhP, ArnettFC, TanFK, AssassiS, DivechaD, et al. (2010) Association of TNFSF4 (OX40L) polymorphisms with susceptibility to systemic sclerosis. Annals of the rheumatic diseases 69 : 550–555.
23. Bossini-CastilloL, BroenJC, SimeonCP, BerettaL, VonkMC, et al. (2011) A replication study confirms the association of TNFSF4 (OX40L) polymorphisms with systemic sclerosis in a large European cohort. Annals of the rheumatic diseases 70 : 638–641.
24. IshiiN, TakahashiT, SorooshP, SugamuraK (2010) OX40-OX40 ligand interaction in T-cell-mediated immunity and immunopathology. Advances in immunology 105 : 63–98.
25. GoughMJ, WeinbergAD (2009) OX40 (CD134) and OX40L. Advances in experimental medicine and biology 647 : 94–107.
26. HirotaT, TakahashiA, KuboM, TsunodaT, TomitaK, et al. (2012) Genome-wide association study identifies eight new susceptibility loci for atopic dermatitis in the Japanese population. Nature genetics
27. WatermanM, XuW, StempakJM, MilgromR, BernsteinCN, et al. (2011) Distinct and overlapping genetic loci in Crohn's disease and ulcerative colitis: correlations with pathogenesis. Inflammatory bowel diseases 17 : 1936–1942.
28. LindstromS, VachonCM, LiJ, VargheseJ, ThompsonD, et al. (2011) Common variants in ZNF365 are associated with both mammographic density and breast cancer risk. Nature genetics 43 : 185–187.
29. KangHM, SulJH, ServiceSK, ZaitlenNA, KongSY, et al. (2010) Variance component model to account for sample structure in genome-wide association studies. Nature genetics 42 : 348–354.
30. ThompsonSD, SudmanM, RamosPS, MarionMC, RyanM, et al. (2010) The susceptibility loci juvenile idiopathic arthritis shares with other autoimmune diseases extend to PTPN2, COG6, and ANGPT1. Arthritis and rheumatism 62 : 3265–3276.
31. PurcellS, NealeB, Todd-BrownK, ThomasL, FerreiraMA, et al. (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics 81 : 559–575.
32. BarrettJC, FryB, MallerJ, DalyMJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21 : 263–265.
33. ClarkeL, Zheng-BradleyX, SmithR, KuleshaE, XiaoC, et al. (2012) The 1000 Genomes Project: data management and community access. Nature methods 9 : 459–462.
34. BrowningSR, BrowningBL (2007) Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. American journal of human genetics 81 : 1084–1097.
Štítky
Genetika Reprodukčná medicína
Článek MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease MiceČlánek Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2013 Číslo 2- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- A Meta-Analysis of Thyroid-Related Traits Reveals Novel Loci and Gender-Specific Differences in the Regulation of Thyroid Function
- Genetic Landscape of Open Chromatin in Yeast
- Deleterious Alleles in the Human Genome Are on Average Younger Than Neutral Alleles of the Same Frequency
- Age-Dependent Transition from Cell-Level to Population-Level Control in Murine Intestinal Homeostasis Revealed by Coalescence Analysis
- Next-Generation Sequencing Identifies the Danforth's Short Tail Mouse Mutation as a Retrotransposon Insertion Affecting Expression
- ImmunoChip Study Implicates Antigen Presentation to T Cells in Narcolepsy
- Massive Mitochondrial Gene Transfer in a Parasitic Flowering Plant Clade
- Comment on “Genomic Hypomethylation in the Human Germline Associates with Selective Structural Mutability in the Human Genome”
- The Prefoldin Bud27 Mediates the Assembly of the Eukaryotic RNA Polymerases in an Rpb5-Dependent Manner
- Genetic Determinants of Trabecular and Cortical Volumetric Bone Mineral Densities and Bone Microstructure
- Encodes a Novel and -Genus-Specific Regulator of Photoperiodic Flowering in Rice
- Only One Isoform of CTP Synthase Forms the Cytoophidium
- Mechanisms Involved in the Functional Divergence of Duplicated GroEL Chaperonins in DK1622
- A Genome-Wide RNAi Screen in Identifies the Nicotinic Acetylcholine Receptor Subunit ACR-7 as an Antipsychotic Drug Target
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Ancient DNA Reveals Prehistoric Gene-Flow from Siberia in the Complex Human Population History of North East Europe
- Inflammation-Mediated Genetic and Epigenetic Alterations Drive Cancer Development in the Neighboring Epithelium upon Stromal Abrogation of TGF-β Signaling
- MicroRNA-3148 Modulates Allelic Expression of Toll-Like Receptor 7 Variant Associated with Systemic Lupus Erythematosus
- RNAi–Based Functional Profiling of Loci from Blood Lipid Genome-Wide Association Studies Identifies Genes with Cholesterol-Regulatory Function
- CELF Family RNA–Binding Protein UNC-75 Regulates Two Sets of Mutually Exclusive Exons of the Gene in Neuron-Specific Manners in
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- The Ubiquitin Ligase Subunit Acts in Target Tissue to Restrict Tracheal Terminal Cell Branching and Hypoxic-Induced Gene Expression
- Mitotic Evolution of Shows a Stable Core Genome but Recombination in Antigen Families
- Tysnd1 Deficiency in Mice Interferes with the Peroxisomal Localization of PTS2 Enzymes, Causing Lipid Metabolic Abnormalities and Male Infertility
- A Regulatory Pathway, Ecdysone-Transcription Factor Relish-Cathepsin L, Is Involved in Insect Fat Body Dissociation
- PcG-Mediated Higher-Order Chromatin Structures Modulate Replication Programs at the BX-C
- MSH3 Polymorphisms and Protein Levels Affect CAG Repeat Instability in Huntington's Disease Mice
- JNK-Interacting Protein 3 Mediates the Retrograde Transport of Activated c-Jun N-Terminal Kinase and Lysosomes
- Discovery of a Splicing Regulator Required for Cell Cycle Progression
- Rearrangements of 2.5 Kilobases of Noncoding DNA from the Locus Define Predictive Rules of Genomic -Regulatory Logic
- Admixture Mapping in Lupus Identifies Multiple Functional Variants within IFIH1 Associated with Apoptosis, Inflammation, and Autoantibody Production
- Roles of the Developmental Regulator Homothorax in Limiting Longevity in
- miR-199a-5p Is Upregulated during Fibrogenic Response to Tissue Injury and Mediates TGFbeta-Induced Lung Fibroblast Activation by Targeting Caveolin-1
- A Kinome-Wide RNAi Screen in Glia Reveals That the RIO Kinases Mediate Cell Proliferation and Survival through TORC2-Akt Signaling in Glioblastoma
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
- SOX2 Co-Occupies Distal Enhancer Elements with Distinct POU Factors in ESCs and NPCs to Specify Cell State
- Retrotransposon Activates Ectopic Expression: A Short Tail
- Confounding by Repetitive Elements and CpG Islands Does Not Explain the Association between Hypomethylation and Genomic Instability
- Cell Reprogramming Requires Silencing of a Core Subset of Polycomb Targets
- Properties and Modeling of GWAS when Complex Disease Risk Is Due to Non-Complementing, Deleterious Mutations in Genes of Large Effect
- Essential Developmental, Genomic Stability, and Tumour Suppressor Functions of the Mouse Orthologue of
- Conditional Inactivation of the DNA Damage Response Gene in Mouse Testis Reveals Separable Roles for Components of the RAD9-RAD1-HUS1 Complex in Meiotic Chromosome Maintenance
- Genome-Wide Analysis Points to Roles for Extracellular Matrix Remodeling, the Visual Cycle, and Neuronal Development in Myopia
- Patterning of Leaf Vein Networks by Convergent Auxin Transport Pathways
- An Evolutionary Perspective on Epistasis and the Missing Heritability
- A Retrotransposon Insertion in the 5′ Regulatory Domain of Ptf1a Results in Ectopic Gene Expression and Multiple Congenital Defects in Danforth's Short Tail Mouse
- The Mub1/Ubr2 Ubiquitin Ligase Complex Regulates the Conserved Dsn1 Kinetochore Protein
- Mutations Can Cause Enamel-Renal Syndrome (ERS)
- Yemanuclein and HIRA Cooperate for Assembly of H3.3-Containing Nucleosomes in the Male Pronucleus
- Hepatocyte Growth Factor, a Determinant of Airspace Homeostasis in the Murine Lung
- ISWI and CHD Chromatin Remodelers Bind Promoters but Act in Gene Bodies
- COM-1 Promotes Homologous Recombination during Meiosis by Antagonizing Ku-Mediated Non-Homologous End Joining
- Control of Multicellular Development by the Physically Interacting Deneddylases DEN1/DenA and COP9 Signalosome
- Antagonism Versus Cooperativity with TALE Cofactors at the Base of the Functional Diversification of Hox Protein Function
- Dynamic Association of NUP98 with the Human Genome
- Ectopic Expression of Induces Spinal Defects, Urogenital Defects, and Anorectal Malformations in Mice
- Regulation of Contributes to the Lineage Potential of Neurogenin3+ Endocrine Precursor Cells in the Pancreas
- Gene-Based Testing of Interactions in Association Studies of Quantitative Traits
- The Amidation Step of Diphthamide Biosynthesis in Yeast Requires , a Gene Identified through Mining the - Interaction Network
- Plant-Symbiotic Fungi as Chemical Engineers: Multi-Genome Analysis of the Clavicipitaceae Reveals Dynamics of Alkaloid Loci
- Genome-Wide Diversity in the Levant Reveals Recent Structuring by Culture
- DNA Methylation Mediated Control of Gene Expression Is Critical for Development of Crown Gall Tumors
- Identification of the SlmA Active Site Responsible for Blocking Bacterial Cytokinetic Ring Assembly over the Chromosome
- Expression of a Novel P22 ORFan Gene Reveals the Phage Carrier State in Typhimurium
- Altered Cohesin Gene Dosage Affects Mammalian Meiotic Chromosome Structure and Behavior
- Quantitative Analysis of Histone Modifications: Formaldehyde Is a Source of Pathological N-Formyllysine That Is Refractory to Histone Deacetylases
- Duplicate Abalone Egg Coat Proteins Bind Sperm Lysin Similarly, but Evolve Oppositely, Consistent with Molecular Mimicry at Fertilization
- Lessons from on the Strengths and Weaknesses of Structured Association Mapping
- DNA–Methylome Analysis of Mouse Intestinal Adenoma Identifies a Tumour-Specific Signature That Is Partly Conserved in Human Colon Cancer
- Transposon Variants and Their Effects on Gene Expression in
- Polygenic Modeling with Bayesian Sparse Linear Mixed Models
- Single Transmembrane Peptide DinQ Modulates Membrane-Dependent Activities
- The JNK Signaling Pathway Activates Expression of Stress Response Genes by Derepressing the Fos/HDAC Repressor Complex
- The Interaction of CtIP and Nbs1 Connects CDK and ATM to Regulate HR–Mediated Double-Strand Break Repair
- Regulation of Metamorphosis by Xenobiotic Response Regulators
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Complex Inheritance of Melanoma and Pigmentation of Coat and Skin in Grey Horses
- Coordination of Chromatid Separation and Spindle Elongation by Antagonistic Activities of Mitotic and S-Phase CDKs
- Autophagy Induction Is a Tor- and Tp53-Independent Cell Survival Response in a Zebrafish Model of Disrupted Ribosome Biogenesis
- Assembly of the Auditory Circuitry by a Genetic Network in the Mouse Brainstem
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



