-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
LILRB2 Interaction with HLA Class I Correlates with Control of HIV-1 Infection
Leukocyte immunoglobulin-like receptors B1 and B2 (LILRB1 and LILRB2) bind HLA class I allotypes with variable affinities. Here, we show that the binding strength of LILRB2 to HLA class I positively associates with level of viremia in a large cohort of untreated HIV-1-infected patients. This effect appears to be driven by HLA-B polymorphism and demonstrates independence from class I allelic effects on viral load. Our in vitro experiments suggest that strong LILRB2-HLA binding negatively affects antigen-presenting properties of dendritic cells (DCs). Thus, we propose an impact of LILRB2 on HIV-1 immune control through altered regulation of DCs by LILRB2-HLA engagement.
Published in the journal: LILRB2 Interaction with HLA Class I Correlates with Control of HIV-1 Infection. PLoS Genet 10(3): e32767. doi:10.1371/journal.pgen.1004196
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004196Summary
Leukocyte immunoglobulin-like receptors B1 and B2 (LILRB1 and LILRB2) bind HLA class I allotypes with variable affinities. Here, we show that the binding strength of LILRB2 to HLA class I positively associates with level of viremia in a large cohort of untreated HIV-1-infected patients. This effect appears to be driven by HLA-B polymorphism and demonstrates independence from class I allelic effects on viral load. Our in vitro experiments suggest that strong LILRB2-HLA binding negatively affects antigen-presenting properties of dendritic cells (DCs). Thus, we propose an impact of LILRB2 on HIV-1 immune control through altered regulation of DCs by LILRB2-HLA engagement.
Introduction
HIV-1 disease progression is influenced by host genetic factors and varies greatly among infected individuals. Polymorphism in the HLA class I locus has been consistently shown to associate with HIV-1 infection outcomes by both the candidate gene approach [1] and genome-wide association studies [2], [3]. The influence of specific HLA class I alleles on HIV-1 disease is particularly obvious for HLA-B alleles, among which HLA-B*57 and -B*27 exhibit consistent protective effects [4], [5], [6], [7] and an allelic group called HLA-B*35-Px associates with accelerated disease progression [8].
HLA class I involvement in HIV-1 disease is primarily thought to be linked to cytotoxic CD8+ T lymphocyte (CTL) responses, which are restricted by the host's class I allotypes [9], [10]. However, alternative mechanisms may exist, given the fact that the HLA class I molecules represent important ligands for receptors regulating activities of innate immune cells. These include the killer cell immunoglobulin-like receptors (KIRs) and leukocyte immunoglobulin-like receptors (LILRs). Members of both receptor families have been implicated in anti-HIV immunity. For instance, certain combinations of HLA-B and KIR3DL/S1 alleles encoding receptor-ligand pairs associate with slower disease progression, which may be due to increased natural killer cell responsiveness to infected cells [11], [12]. In addition, a strong LILRB2-HLA-B*35-Px interaction is suggested to impair dendritic cell (DC) function during HIV-1 infection, possibly leading to faster disease progression [13]. Down-modulation of DC function was also observed as a result of a stronger interaction between LILRB2 and HLA-B*27 loaded with the viral escape mutant KK10 L6M compared to the wild type peptide loaded complex [14].
LILRB1 and LILRB2 are the most well-studied members of the LILR family [15], [16]. These two receptors share 82% sequence homology and bind both classical and non-classical HLA class I molecules [17], [18]. LILRB2 is exclusively expressed on cells of the myeloid lineage, including conventional DCs, whereas LILRB1 can also be expressed by lymphoid cells. Upon ligand engagement, LILRB1 and LILRB2 induce inhibitory signals via immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in their cytoplasmic tails. Thus, these inhibitory receptors, whose ligands are ubiquitously expressed, might play a role in elevating the activation threshold of the myelomonocytic cells and preventing self-damage. LILRB1/B2 interactions with HLA class I involve β2-microglobulin (β2m) and the α3 domain of the class I molecule, which are relatively conserved across allotypes [19], [20], [21]. A recent study demonstrated variability in binding of LILRB1 - and LILRB2-Fc fusion proteins to individual class I allotypes, which included 31 HLA-A, 47 HLA-B and 16 HLA-C allotypes, indicating that additional regions of HLA class I molecules are involved in the interaction [22]. Compared to LILRB1, LILRB2 showed a greater degree of variability in binding to HLA allotypes. Notably, HLA-B*57 : 01 and -B*27 : 05, which associate with protection in HIV/AIDS, were among the weakest LILRB2 binders. Such a low binding level may reduce inhibitory effects of LILRB2 in DCs and thus contribute to the protective effect of the corresponding alleles.
Based on these findings, we hypothesized that the differential LILRB1/2-HLA binding may impact overall immune response to HIV-1 through modification of DC function and thus influence HIV-1 disease outcomes. Specifically, HLA molecules that bind more strongly to LILRB2 were predicted to blunt DC function, which may ultimately contribute to reduced immune control of viral replication and more rapid disease progression. To test this hypothesis, we used epidemiological and HLA genotyping data from several natural history cohorts of HIV-1-infected persons and analyzed clinical outcomes in these patients in relation to in vitro determined levels of interactions between individual HLA class I allotypes and LILRB1/B2. Our data suggest that the binding strength between LILRB2 and HLA may contribute to HIV-1 control.
Results
Effect of HLA-B on viral control correlates with the LILRB2 binding strength
To evaluate the influence of LILR-HLA interactions on HIV-1 disease, we tested for a potential correlation between LILR-HLA binding level and the strength of HLA allelic associations with viral control. Previously defined binding scores for HLA class I allotypes (Table S1) were used as a measure of LILR-HLA binding strength (Material and Methods, [22]). We compared the distribution of the HLA alleles in HIV-1 controllers and noncontrollers, all in the absence of therapy. Controllers were defined as individuals whose longitudinal mean viral load (mVL) remained below 2,000 copies per ml of plasma in the absence of therapy, whereas noncontrollers were patients whose mVL exceeded 10,000 copies per ml. Odds ratios (ORs) were calculated for each HLA allele using a univariate logistic regression model (Table S1), and significant ORs (p<0.05) were tested for correlations with the LILRB1/B2 binding scores in patients of European and African descent (referred to as whites and blacks, respectively). No relationship was found between the strength of LILRB1-HLA binding scores and the ORs of the corresponding alleles (Table S2). However, LILRB2 binding strength to HLA-B demonstrated a significant positive correlation with the ORs of the respective alleles in white patients (r = 0.64, p = 0.01; Table 1 and Figure 1). The correlation in our smaller cohort of black patients occurred in the same direction, but did not reach significance (r = 0.24, p = 0.6). Permutation analyses indicated that the significant positive correlation in whites is unlikely to have occurred by chance (p = 0.03). This finding suggests that the interaction between HLA-B and LILRB2 may participate in the overall effect of HLA-B alleles on HIV-1 control, where weaker binding of a given HLA-B allotype to LILRB2 correlates with greater protection of the corresponding allele, possibly as a consequence of enhanced DC function.
Fig. 1. LILRB2 binding strength and odds ratios for viral load control for individual HLA-B alleles. 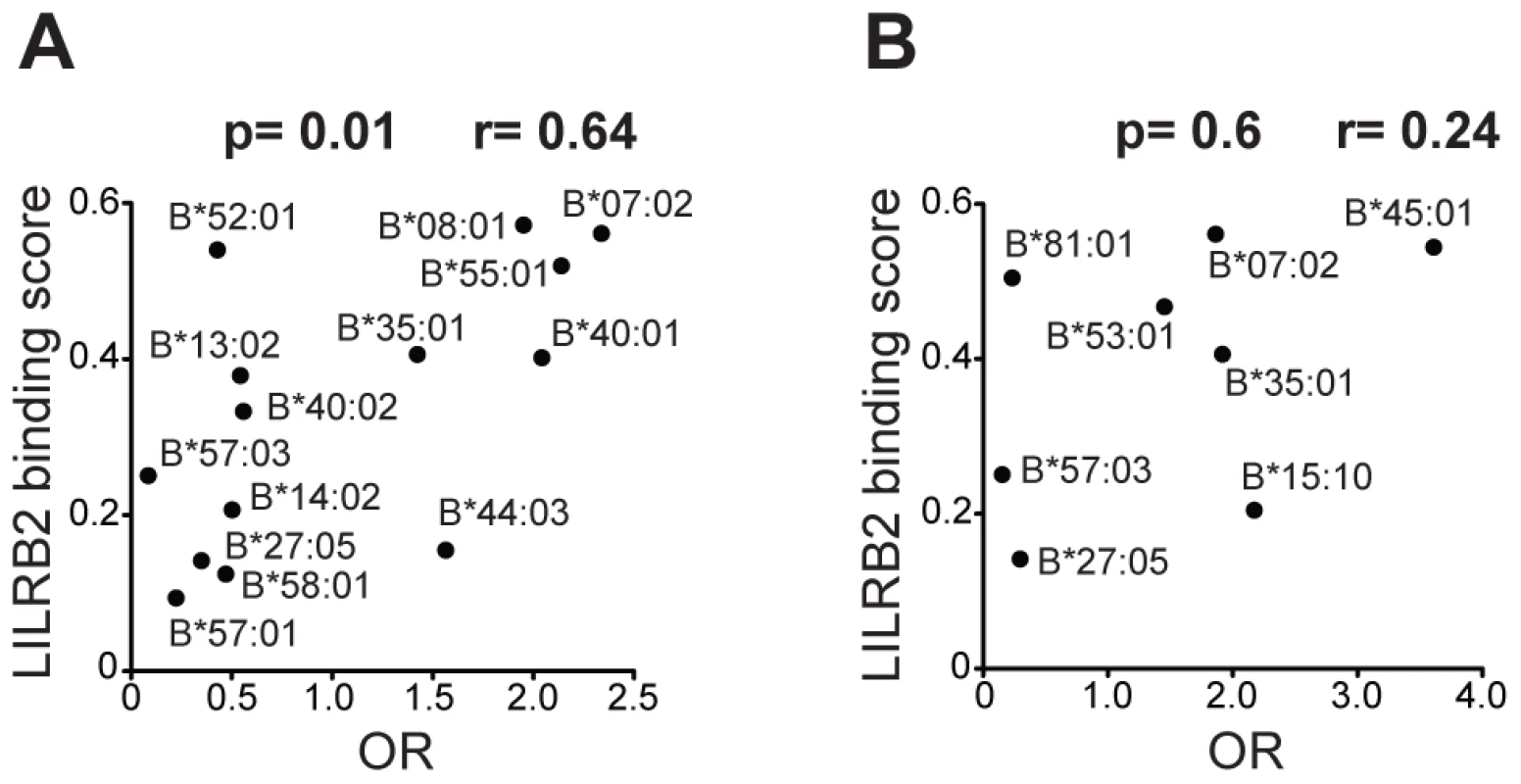
The data plotted includes only alleles with significant association (p<0.05) for white (A) and black (B) patients in a univariate analysis for each HLA-B allele. Spearman correlation coefficient and p values are indicated. Tab. 1. Spearman correlation between LILRB2 binding strength and odds ratios of <i>HLA</i> alleles (p<0.05) for viral load control in HIV-1-infected individuals. 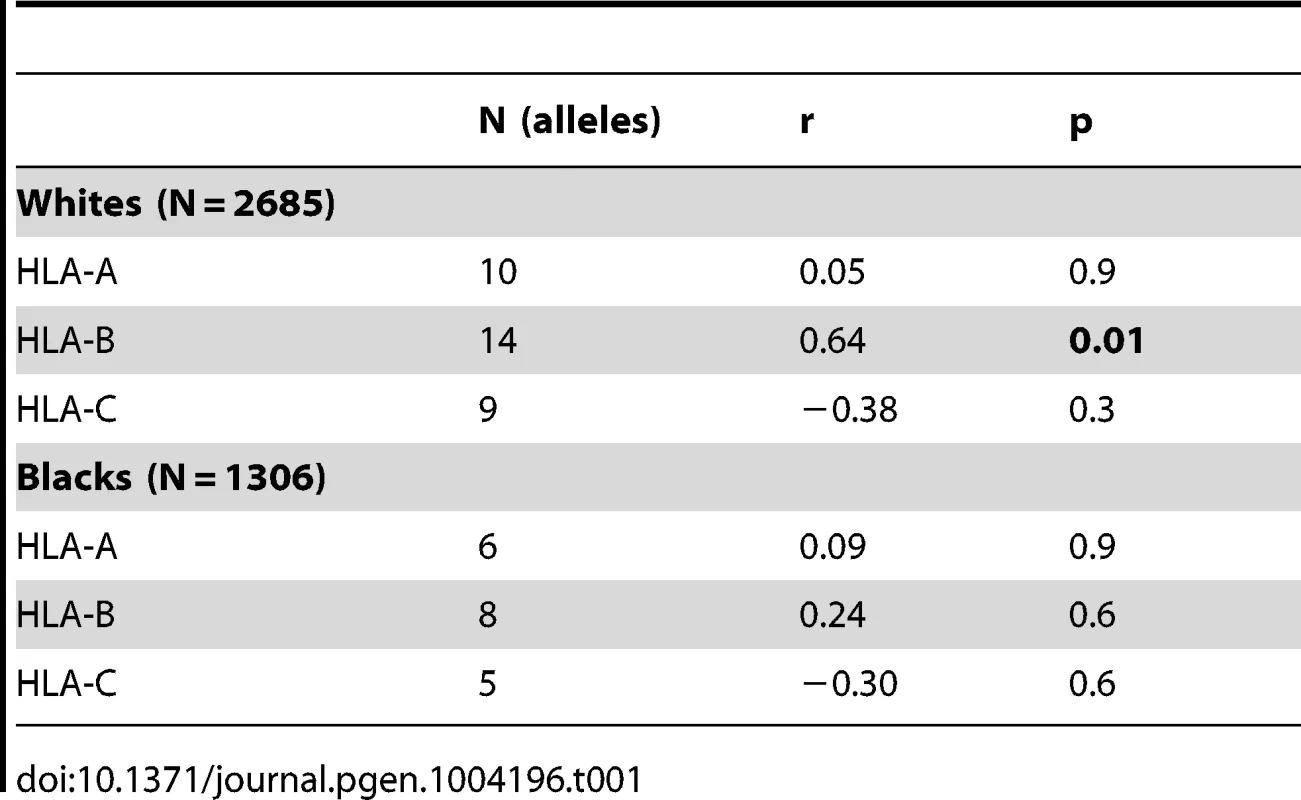
LILRB2 binding strength to HLA class I correlates with viral load in HIV-1 infection
A more rigorous test for an effect of LILRB2 on HIV-1 outcomes was performed by assigning to each patient four LILRB2-related scores, three locus-specific (A, B, C for HLA-A, -B and -C, respectively) scores and one combined (ABC) score, based on each patient's class I genotype, and then correlating these scores with measurements of HIV-1 disease outcomes in each patient. Locus-specific scores were generated as a sum of binding scores corresponding to the two alleles at each locus to reflect average LILRB2 binding. The combined ABC binding score was a sum of A, B and C scores, but for HLA-C, only 1/10 of the sum was incorporated into the final score since HLA-C is known to be expressed on the cell surface at roughly 1/10 the level of HLA-A and -B [23]. This combined score, which was used as a measure of average LILRB2 binding to class I on the cell surface, may be more relevant to the physiological consequences of the LILRB2 ligation than the locus-specific scores, since LILRB2 binds to all HLA-A, -B and -C allotypes [17], [22]. Notably, the variation in the B binding scores appears to be the main contributor to the variation of the ABC scores at the population level (Figure 2), given the relatively small range of the A scores and low 10% contribution of the C scores.
Fig. 2. LILRB2-HLA binding score variations in 2900 white (A) and 1490 black (B) patients. 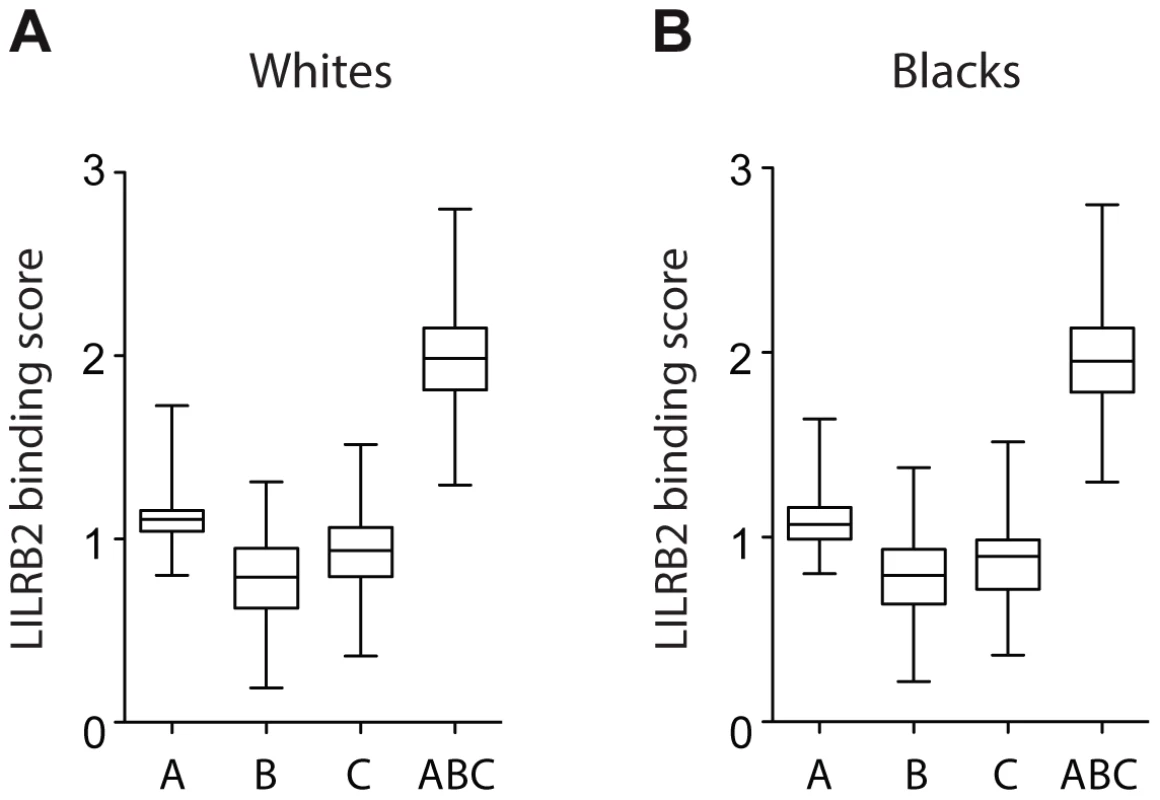
A, B and C binding scores represent the sum of the binding scores for two alleles of the corresponding HLA class I locus. ABC binding score represents the sum of the locus-specific binding scores with the C scores counted at 1/10 level. Alleles with undefined scores were assigned the average of the scores for a given locus. Box and Whisker plots reflect median, the 25% and 75% percentiles and the minimum and maximum of all data. A significant positive correlation of the LILRB2-ABC binding scores with mVL used as a continuous variable was observed in both white and black patients using a univariate model (r = 0.21, p = 3×10−30 and r = 0.14, p = 5×10−8, respectively; Table 2). Locus specific analyses indicated that this correlation is driven mainly by the B scores (r = 0.24, p = 1×10−38 in whites and r = 0.16, p = 6×10−10 in blacks), since A scores show no significant correlation and C scores actually trend in the opposite direction, where higher binding of HLA-C to LILRB2 confers slight protection.
Tab. 2. Spearman correlation between LILRB2 binding strength and mVL in HIV-1-infected individuals. 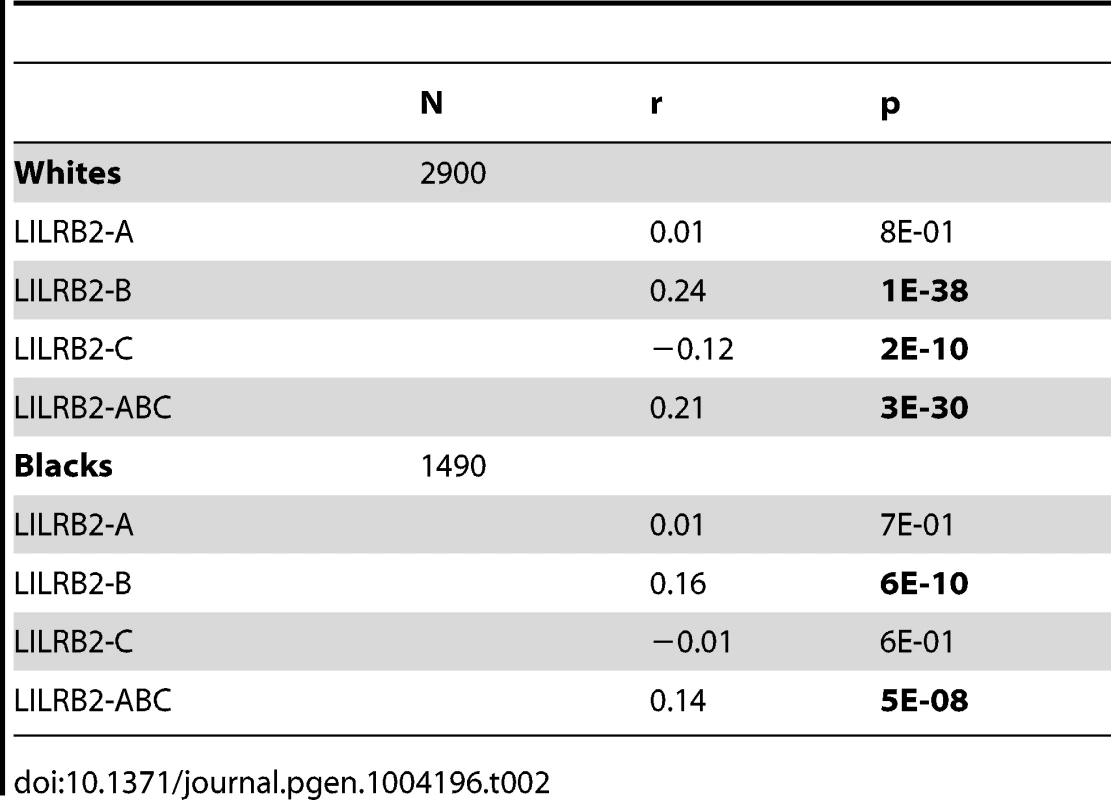
To confirm that the LILRB2-HLA binding effect on HIV-1 disease outcomes is independent of the effects of individual class I alleles that are not related to LILRB2 binding, we used regression models with stepwise selection with p<0.05 as a threshold for inclusion, which included all class I alleles with phenotypic frequency of >2%, and LILRB2-HLA binding scores as continuous variables. The LILRB2-HLA binding effect on viral control was tested first in a categorical analysis comparing controllers to noncontrollers. The A, B and ABC binding scores demonstrated significant independent effects on viral control in white patients (OR = 1.2–1.3 for a change of 0.1 binding unit, p = 10−3–10−18; Table 3), whereas C score did not remain in the model. The B and ABC binding scores predicted viral control independently of all individual class I alleles in blacks as well (OR = 1.1–1.3 for a change of 0.1 unit binding, p = 10−5–10−6; Table 4), whereas the A and C binding scores did not stay in the model. Thus, the inverse correlation between the level of HIV-1 control and LILRB2 binding scores to HLA-B allotypes and to combined ABC allotypes were consistent in the two racial groups.
Tab. 3. Effect of LILRB2-HLA binding strength and individual class I alleles on viral control (controllers vs. non-controllers) in white patients. 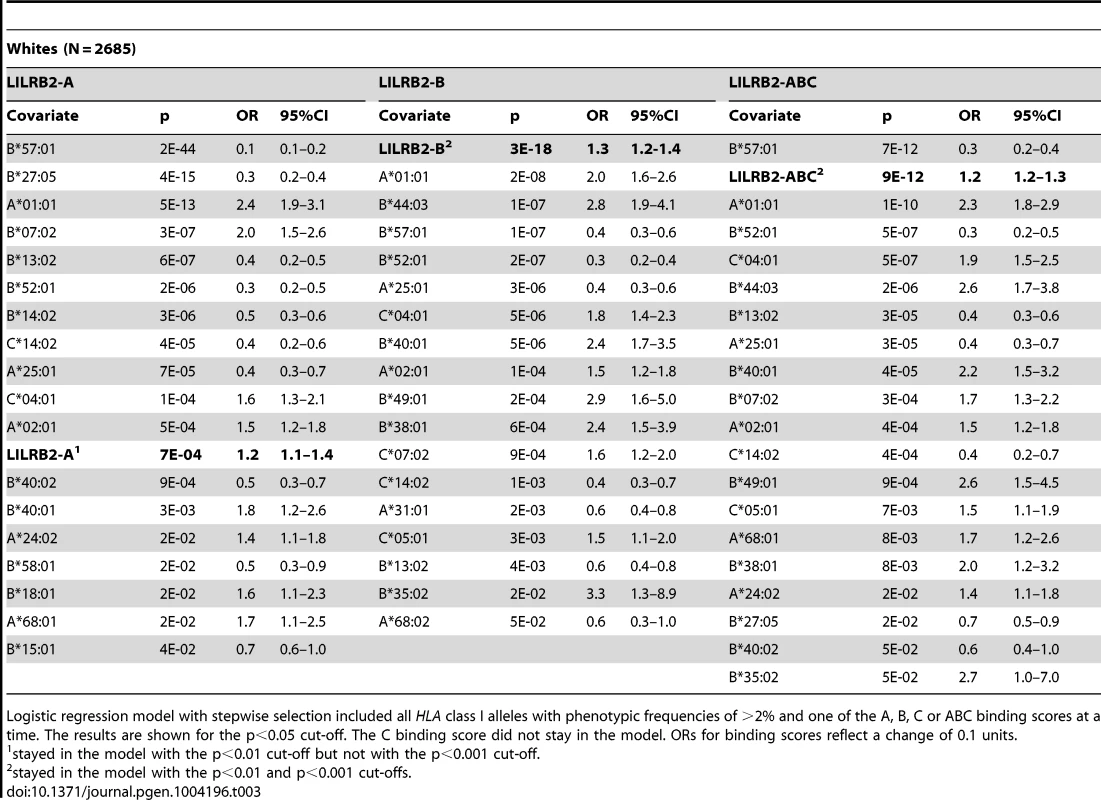
Logistic regression model with stepwise selection included all HLA class I alleles with phenotypic frequencies of >2% and one of the A, B, C or ABC binding scores at a time. The results are shown for the p<0.05 cut-off. The C binding score did not stay in the model. ORs for binding scores reflect a change of 0.1 units. Tab. 4. Effect of the LILRB2-HLA binding strength and individual class I alleles on viral control in black patients. 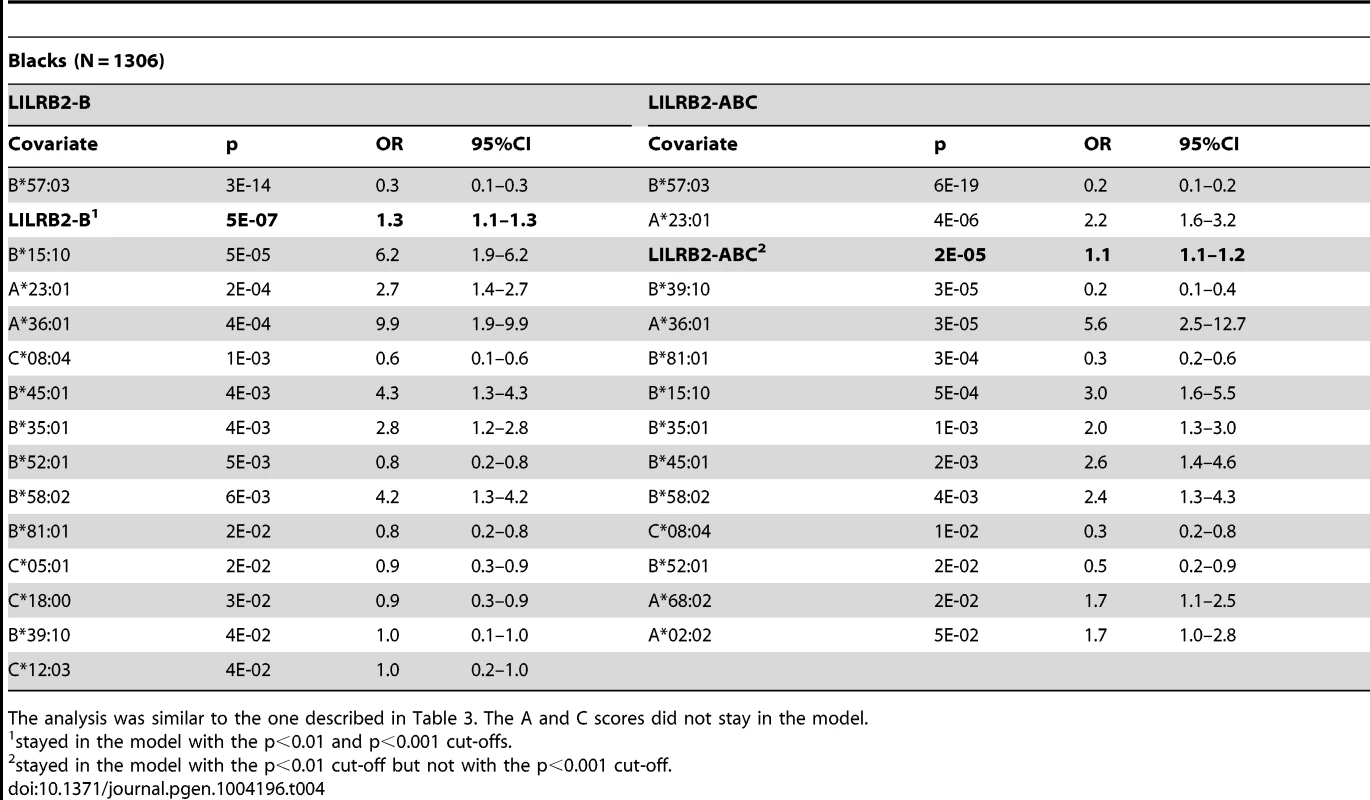
The analysis was similar to the one described in Table 3. The A and C scores did not stay in the model. Next, we applied the linear regression model with stepwise selection to the analyses of mVL in the absence of therapy where mVL was a continuous variable. Among the four binding scores tested, the B and ABC scores showed significant positive correlations with mVL independently of the effects of individual class I alleles in both whites and blacks (Tables S3–S4). This analysis indicates that an increase in 0.1 unit of the ABC binding score would predict 0.08 and 0.03 log10 higher mVL in white and black patients, respectively, independently of individual HLA class I alleles. This translates to an increase of 1.1 and 0.5 log10 mVL in whites and blacks, respectively, when comparing patients with the highest ABC binding score to patients with the lowest score.
To test the stability of the regression models, we applied more stringent conditions in stepwise selection (p<0.01 and p<0.001 cut-offs). The B and ABC scores remained significant in the categorical analysis of viral load control in whites at both cut-offs (Table 3). While similar stability was observed for the B score in blacks, the ABC score remained significant in categorical analyses only at the intermediate cut-off (Table 4). In the continuous analysis of mVL, the binding scores demonstrated variable stability (Tables S3–S4).
Thus, we observed consistent associations for LILRB2-B and -ABC binding scores with HIV-1 control tested in both categorical and continuous analysis of mVL across the two racial groups. The effects were always less pronounced in the black population perhaps due to smaller number of individuals in this group. We also tested for a potential effect of LILRB2-HLA binding level on disease progression using a Cox model in a smaller cohort of seroconverts (780 whites and 287 blacks), but there was no significant effect on time to AIDS outcomes (see Materials and Methods) when individual class I alleles were included as covariables (data not shown). This negative result may be due to low statistical power, or the LILRB2 binding effect on HIV-1 control may be outcome-specific and influence viral load only.
Functional effects of LILRB2-HLA interactions
Functional properties of DCs that result from altered LILRB2-HLA interactions were interrogated using mixed leukocyte reactions, an assay that measures the ability of DCs to stimulate antigen-specific T cell responses. Monocyte-derived dendritic cells (MDDC) were exposed to a panel of different recombinant HLA molecules, followed by cytokine-mediated maturation and incubation with CFSE-labeled allogeneic T cells according to a previously described protocol [24]. We observed divergent effects of different HLA allotypes on proliferative activities of allogeneic T cells, where the highest levels of proliferation were observed after MDDC exposure to HLA class I allotypes that have weakest binding to LILRB2, and the lowest proliferative activities were observed following exposure to HLA class I molecules with strongest binding to LILRB2 (Figures 3A and S2). These data are consistent with an inverse relationship between MDDC function and corresponding LILRB2-HLA binding strength (Figure 3B). siRNA-mediated knockdown of LILRB2 surface expression on MDDC (Figure S1) reversed inhibitory effects of HLA class I allotypes in a reciprocal hierarchical order (Figures 3A and S2), leading to a positive association between fold changes in MDDC function after LILRB2 knockdown and corresponding LILRB2-HLA binding scores (Figure 3C). However, inhibitory effects of two specific HLA class I allotypes (HLA-A*02 : 01 and -C*01 : 02) on DC function were not significantly affected by LILRB2 knockdown, suggesting that these HLA allotypes may interact with additional, as of yet unidentified immunoregulatory receptors on DCs. In contrast to antigen-presentation properties, secretion of TNFα, IL-6 or IL-12p70 by MDDC was not significantly influenced by LILRB2-HLA-B interactions (Figure S3). Together, these results suggest that LILRB2-HLA impact immune control of HIV-1 through alterations of the functional antigen-presenting properties of DCs.
Fig. 3. Impact of LILRB2-HLA interactions on functional properties of dendritic cells. 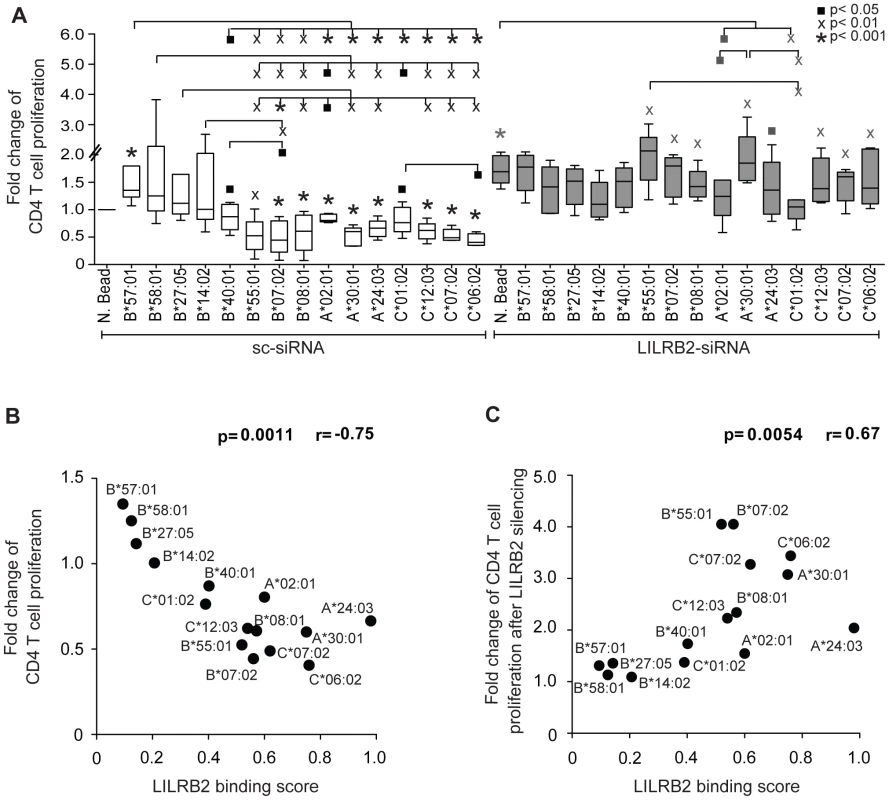
(A) Fold changes in proliferative activities of allogeneic CD4+ T cells after exposure to MDDC treated with indicated HLA-A, -B or -C allotypes normalized to MDDC treated with negative beads (N. Bead), in the absence (white bars, n = 5, 8, 5 for HLA-A, -B, -C allotypes, respectively) or presence (gray bars, n = 5, 6, 5 for HLA-A, -B, -C allotypes, respectively) of siRNA-mediated downregulation of LILRB2 surface expression on MDDC. Significance was tested using one-way ANOVA followed by post-hoc analysis with the Tukey multiple comparison test, or using paired t-tests, as appropriate, (▪p<0.05, Xp<0.01, *p<0.001). (B): Spearman correlation between proliferative activities of allogeneic CD4+ T cells after incubation with MDDC treated with indicated HLA-A, -B and -C allotypes and corresponding LILRB2-HLA binding scores. (C): Spearman correlation between the ratios of MDDC function in the presence or absence of siRNA-mediated LILRB2 downregulation, and corresponding LILRB2-HLA-A, -B, -C binding scores. Discussion
Among all human MHC class I alleles, those encoded at the HLA-B locus have the highest degree of genetic variation and the dominant influence on HIV/AIDS [5]. Association of particular HLA-B alleles with HIV-1 infection outcomes is traditionally linked to the ability of the corresponding allotypes to elicit CTL responses. This concept is supported by numerous studies of HLA-restricted CTL responses and viral sequence evolution in carriers of specific HLA class I alleles [25]. The distinct effect of the HLA-B locus on cellular immune responses to HIV-1 is likely due to its greater level of diversity, which results in the presentation of a broader repertoire of viral peptides that can be presented by HLA-B allotypes as compared to HLA-A or HLA-C. In addition, relative resistance of HLA-B to downregulation by HIV-1 viral protein Nef compared to HLA-A [26] as well as low expression level of HLA-C were suggested to contribute to the principal role of the HLA-B locus in HIV-1 disease. However, the structural polymorphism of HLA-B can also influence its binding to receptors other than the T cell receptor. Based on the work presented herein, we propose that variation in binding properties of HLA-B to the inhibitory myelomonocytic receptor LILRB2 can contribute to the overall HLA effects on HIV-1 infection outcomes.
The ORs of individual HLA-B alleles determined by comparing HIV-1 controllers to noncontrollers correlate significantly with their LILRB2 binding strength in white patients (Figure 1A). A similar trend was observed in blacks, though not significantly so (Figure 1B), perhaps due to a smaller number of alleles considered in blacks (n = 8) as compared to whites (n = 14). B*81 : 01, an allotype present almost exclusively among blacks, appears to be an outlier in that it binds strongly to LILRB2, but associates with robust protection against HIV-1. B*81 : 01 contains an unusual polymorphism in the α3 domain that dramatically decreases CD8 binding (the same domain that is centrally involved in LILRB2 binding [27]), which may explain in part the protective role of the B*81 : 01 in HIV/AIDS [28], [29].
A more powerful and direct analysis of a correlation between LILRB2 binding scores and the level of viremia was conducted by assigning to each patient a LILRB2 binding score based on their class I genotypes and correlating these with the mVL determined from each patient. Our analyses included locus-specific (A, B, C) scores as well as a global ABC score, which was used as a measure of average LILRB2 binding to HLA class I overall. Highly significant correlations between mVL and B and ABC binding scores were observed in both white and black patients (Table 2). Two confounding factors may contribute to this strong correlation, including linkage disequilibrium between the HLA class I loci and the effects of individual HLA alleles on HIV-1 that are not related to LILRB2 function. Therefore, regression models with stepwise selection that included all individual class I alleles and LILRB2 binding scores were employed. The analyses indicated consistent effects for the B and ABC binding scores, both of which associated with viral replication tested in a categorical analysis (controllers vs. noncontrollers, Tables 3–4) and when mVL was used as a continuous variable in white and black patients (Tables S3–S4). Whereas the ABC score demonstrated effects similar to the B score, the B score accounts for all or nearly all of the combined ABC effect. The OR for viral control was 1.1–1.2 per 0.1 unit increase of the ABC binding score when comparing controllers to noncontrollers (Tables 3–4). It is not possible to compare directly the strength of the LILRB2 binding effect to the strength of individual HLA allelic effects since the former is based on a continuous variable (LILRB2 binding score) and the latter on a dichotomous variable (presence vs. absence of each allele). However, a comparison of the two patient groups at the extreme ends of the ABC binding scores (i.e. 10% of patients with the lowest scores vs. 10% of patients with the highest scores) results in an OR of 0.3–0.4, which is close to the strength of the protective effect of B*57 in the same model (OR = 0.2–0.3, Tables 3–4).
Neither the A nor the C binding scores demonstrated consistent effects on viral load when individual class I alleles were included in the model: the A score remained only in the categorical model when the least stringent p-value cut-off (<0.05) was used (Table 3), and the C score was not significant in any of the analyses. Thus, the negative correlation with mVL that was observed for the C scores in the univariate model (Table 2) is likely due to effects of individual alleles that are not related to LILRB2 binding and/or to linkage disequilibrium between HLA-B and -C.
Notably, the effects of B*27 : 05, B*57 : 01 and B*57 : 03 were substantially diminished in models that included LILRB2 binding scores relative to their effects in the absence of this covariable (Tables 3–4 and S3–S6). Alternatively, the effect of B*81 : 01 was not markedly influenced by inclusion of the binding scores in the model. These results indicate that the protective effects of the B*27 and B*57 alleles may be partially due to low LILRB2 binding, but this does not appear to be the case for B*81 : 01.
The correlation between HIV-1 immune control and the binding strength of LILRB2 to HLA-B allotypes specifically (and not HLA-A or -C) is difficult to comprehend, since LILRB2 binds all class I molecules without discrimination [22]. The substantially greater variation in binding scores for HLA-B as compared to HLA-A allotypes (Figure 2) may result in a greater influence of HLA-B on differential immune responses to HIV-1 across infected individuals. While HLA-C allotypes also show fairly broad variation in binding scores similar to HLA-B, their lower expression levels may diminish their effect in regulating myelomonocytic cells in HIV-1 infection. Alternatively, HLA-B expressed on the cell surface may behave in a distinct manner, for example due to the presence of intracellular cysteines as suggested by Gruda et al. [30]. Nevertheless, our model with combined ABC binding scores supports the idea that the average class I binding strength to LILRB2 can influence viral control, and the variation in this binding is mostly due to the allotypic diversity of the HLA-B binding strength to LILRB2.
The effect of LILRB2 binding to HLA class I on immune response to HIV-1 may be mediated by subsets of DCs expressing this receptor. Recent work demonstrated that dermal CD14+ DCs express both LILRB1 and LILRB2 [31]. These cells, along with Langerhans cells (LCs) and CD1a+ dermal DCs, are among the first immune cells encountered by HIV-1 in sexual transmission. Interestingly, CD14+ dermal DCs are less efficient at priming CTL than are LCs, and this difference has been attributed to the lack of LILRB1/2 expression by LCs [31]. The reduced ability of dermal CD14+ DCs to prime CTL was suggested to be due to competition between LILRB1/2 and CD8 in binding HLA class I, which has been demonstrated previously [27]. This competition may happen at the DC-T cell interface where LILRB molecules can interact in cis with HLA class I [32] on the DC surface, masking class I molecules from CD8 expressed by the T cells in a manner that does not necessarily involve inhibitory receptor signaling. Variation in the strength of LILRB2 binding to HLA class I may influence the capacity of dermal CD14+ DCs to prime virus-specific CTL and explain the effect of LILRB2 binding on viral load described herein. An alternative mechanism that is supported by our in vitro data implicates inhibition of DCs after LILRB2 ligation and receptor-mediated signal transduction. Our experiments demonstrate that stronger ligation of LILRB2 on the surface of MDDC by HLA in trans at an immature stage result in decreased capacity of these cells to stimulate T cell proliferation when they mature. This is in line with earlier work suggesting a regulatory role of the LILRB2 ligation in DC function [13], [14], [31], [33], [34]. Taken together, these data suggest that LILRB2-HLA interactions influence HIV-1 disease outcomes by regulating functional properties of DCs and their ability to generate antigen-specific T cell responses. Such effects are likely to be amplified by upregulation of LILRB2 surface expression on DCs in peripheral blood [35], [36] and lymph nodes [37] during progressive HIV-1 infection.
We have recently demonstrated a correlation between HLA-C expression level and HIV-1 control [38]. Analyses of in vivo CTL responses indicated that differential HLA-C expression influences CTL responses to HIV-1 peptides despite its lower overall cell surface expression relative to that of HLA-A and -B. The ability of HLA molecules, even those expressed at low levels, to trigger CTL killing of target cells is supported by in vitro data showing that as few as three HLA/peptide complexes can trigger CTL killing [39]. The mechanism of differential immune responses suggested in the current work is distinct from allotype-restricted CTL killing and involves regulation of DCs through engagement of LILRB2 with all allotypes of HLA-A, -B and -C (i.e. it is not allotype specific, distinguishing it from CTL killing). Due to the relatively low amount of HLA-C on the cell surface, the variation in its expression level would contribute minimally to the diversity of LILRB2 binding to HLA class I as a whole. Thus, differential HLA-C expression level has a significant effect on HLA-C-restricted CTL responses [38], but the overall low expression of HLA-C compared to HLA-A and -B limits its relative importance in mediating a response through LILRB2, which binds (at various levels) to all class I molecules.
The data presented herein underscore the complexity of HLA class I involvement in control of HIV-1 that goes beyond peptide presentation to CD8+ T cells. We propose that the LILRB2-HLA class I interaction may contribute to the effect of class I on HIV/AIDS through regulation of DC function. The relative size of this effect compared to the CTL or NK cell responses requires further investigation.
Materials and Methods
Study subjects
We used data from a total of 5126 HIV-1-infected individuals from eight US and one European cohorts: the AIDS Linked to Intravenous Experience (ALIVE), the U.S. military HIV Natural History Study (DoD HIV NHS), the DC Gay Cohort Study (DCG), the Multicenter AIDS Cohort Study (MACS), the Multicenter Hemophilia Cohort Study (MHCS), the Massachusetts General Hospital Controller Cohort (MGH), the San Francisco City Clinic Cohort (SFCCC), the Study on the Consequences of Protease Inhibitor Era (SCOPE) and the Swiss HIV Cohort Study (SHCS). Patients from MACS, MGH, SCOPE and SHCS, including 2685 white and 1306 black patients, were categorized in controller and noncontroller groups for the analysis of HLA class I impact on HIV-1 immune control. Longitudinal viral load data were available for 2900 white and 1490 black patients from ALIVE, MACS, MGH, DoD HIV NHS, SCOPE and SHCS. Seroconversion time and AIDS progression data were known for 780 white and 287 black patients from ALIVE, DCG, MHCS and SFCCC.
Ethics statement
This study was approved by the protocol review office of the US National Cancer Institute institutional review board, as well as by the institutional review board of Massachusetts General Hospital. Informed consent was obtained at the study sites from all individuals. Patients' ethnicities were defined based on self-report.
HLA genotyping
We performed genotyping of the HLA-A/B/C following the PCR-SSOP (sequence-specific oligonucleotide probing) typing protocol and PCR-SBT (sequence based typing) recommended by the 13th International Histocompatibility Workshop (http://www.ihwg.org). All HLA class I genotypes were defined to 4-digit resolution with the exceptions of A*74 : 01/2, C*17 and C*18, which were determined to 2-digits.
LILRB2-HLA binding scores
LILRB2-HLA binding scores were defined in a previously described experiment [22]. Briefly, a set of LILR-Fc fusion proteins was tested for binding with LABScreen HLA class I SABs at a concentration of 0.5, 1 and 2 µM. The level of binding was assessed by measuring the median fluorescence intensity (MFI) of the LILR-Fc bound to the beads using appropriate normalizations, which included subtraction of the Fc-negative control MFI and division of the result by the MFI of W6/32 (monoclonal anti-HLA class I antibodies recognizing β2m-associated HLA molecules). The normalized values were assigned to each HLA allotype as binding scores. Each binding score is a function of avidity of bivalent LILRB2-Fc for HLA, which in turn depends on the affinity of monomeric LILRB to HLA. Therefore, the binding score can be used as a quantitative characteristic of the strength of LILR-HLA interactions. The relative LILR binding to different HLA allotypes was similar at each of the LILR concentrations tested (Figure S4). This consistency between LILR concentrations assured us that the difference in MFIs between the allotypes is mainly due to difference in binding strength, and is not an experimental artifact. The 1 µM concentration results were chosen as a representative dataset. Among the HIV-1-infected patients used for the analyses, frequencies of the HLA-A/B/C alleles with unknown binding scores were 2/11/24% in white and 8/13/39% in black patients. To avoid power loss, we used mean values for the corresponding locus for each genotype with unknown score. The pairs of alleles A*74 : 01/2 and B*81 : 01 differ only at the signal peptide, therefore, they were treated as individual alleles in the context of LILRB2 binding.
Mixed leukocyte reactions
Monocyte Derived Dendritic Cells (MDDC) were prepared as described previously. Briefly, 2 × 108 PBMCs were plated in 5% pooled human serum medium and incubated during 60 min at 37°C to adhere monocytes. After discarding non-adherent cells, monocytes were differentiated into MDDC in the presence of RPMI 1640 medium supplemented with 50 µg/ml of GM-CSF (Amgen) and 10% fetal bovine serum. On day 5, immature MDDC were gently detached using PBS with 0.5% BSA and 2 mM EDTA, harvested and plated at 4x105 cell/well in a round-bottom 96-well plate (Costar). Next, cells were incubated with beads coated with selected HLA-B allotypes, or uncoated control beads (One Lambda) for 30 min at 37°C, washed, and subsequently matured in the presence of a previously described cytokine cocktail containing 5 ng/ml IL-1β, 5 ng/ml TNFα, 1 µg/ml PGE-2 and 0.15 µg/ml IL-6. After 16 hours, mature MDDC were mixed with negatively-isolated CFSE-labeled allogeneic T cells at a DC:T cell ratio of 1∶100 for mixed lymphocyte reactions. Allogeneic T cell proliferation was determined after 6 days in culture by investigators blinded towards the added HLA class I molecules, using an LSRFortessa flow cytometer (Becton Dickinson).
Cytokine secretion assays
To analyze cytokine secretion, immature MDDC were prepared and treated with HLA class I molecules as described above and then matured using 5 µg/ml CL097 (InvivoGen) in the presence of 5 µg/ml brefeldin A. After 20 hours, cells were fixed and permeabilized, stained with antibodies recognizing intracellular IL-12p70, TNFα and IL-6, and processed to flow cytometric acquisition by investigators blinded towards the added HLA class I allotypes.
siRNA-mediated gene knockdown
106 MDDC were suspended in 300 µl Optimem (Gibco) in the presence of 2 nmol of either LILRB2-specific (LILRB2-siRNA) or scramble control siRNA (sc-siRNA) pools (On-TARGET plus SMARTpool, Dharmacon) and transferred to a 4-mm electroporation cuvette (Bio-Rad Laboratories). Cells were left on ice for 10 min, electroporated (900 V, 0.75 msec square wave; Genepulser Xcell; Bio-Rad Laboratories), and transferred back to culture medium for another 24 to 48 hours. Efficiency of specific siRNA-mediated LILRB2 knockdown was determined by flow cytometry using an anti-LILRB2 antibody (clone 42D1, Biolegend).
Statistical analysis
We used SAS 9.1 (SAS Institute) for data management and statistical analyses. The effect of HLA alleles on viral control was determined by categorical analysis of the allelic frequencies in HIV-1 controllers and noncontrollers. Corresponding ORs were calculated using logistic regression model with SAS procedure PROC LOGISTIC. Relationships between viral loads and LILRB2-HLA binding scores were analyzed by the Spearman correlation test using PROC CORR. Permutation analysis was done by random assignment of binding scores to HLA-B alleles (10,000 times) and testing the probability of significant Spearman correlation of the binding scores with ORs with p<0.05.
LILRB2 binding scores as continuous variables and presence versus absence of all individual HLA class I alleles of frequency ≥2% were included with stepwise selection in all regression models. Results in the tables are for the models using a threshold of a two-sided p value <0.05 for inclusion of a covariate as a significant independent effect. The stability of regression models was tested using more stringent thresholds of p<0.01 and p<0.001 for inclusion in the model. The results for the binding scores are indicated in the footnotes to the tables.
Cox proportional hazards model was applied to perform AIDS progression analysis by using PROC PHREG. For this, we estimated the seroconversion date as the midpoint between the first positive and the last negative HIV-1 antibody test (mean interval, 0.79 years; range, 0.07 to 3.0 years). Four end points reflecting disease progression (AIDS outcomes) were evaluated: time to CD4<200 cells/ml; progression to AIDS according to the 1987 definition by the Centers for Disease Control and Prevention (CDC, [40]); progression to AIDS according to the 1993 definition by CDC; and AIDS-related death [41].
Data of in vitro experiments were presented as Box and Whisker plots, reflecting the median, minimum, maximum and the 25th and 75th percentiles. Significance was tested using one-way ANOVA followed by post-hoc analysis with the Tukey multiple comparison test, or using paired t-tests, as appropriate.
Supporting Information
Zdroje
1. CarringtonM, O′BrienSJ (2003) The influence of HLA genotype on AIDS. Annu Rev Med 54 : 535–551.
2. FellayJ, ShiannaKV, GeD, ColomboS, LedergerberB, et al. (2007) A whole-genome association study of major determinants for host control of HIV-1. Science 317 : 944–947.
3. PereyraF, JiaX, McLarenPJ, TelentiA, de BakkerPI, et al. (2010) The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 330 : 1551–1557.
4. GaoX, BashirovaA, IversenAK, PhairJ, GoedertJJ, et al. (2005) AIDS restriction HLA allotypes target distinct intervals of HIV-1 pathogenesis. Nat Med 11 : 1290–1292.
5. KiepielaP, LeslieAJ, HoneyborneI, RamduthD, ThobakgaleC, et al. (2004) Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature 432 : 769–775.
6. MagierowskaM, TheodorouI, DebreP, SansonF, AutranB, et al. (1999) Combined genotypes of CCR5, CCR2, SDF1, and HLA genes can predict the long-term nonprogressor status in human immunodeficiency virus-1-infected individuals. Blood 93 : 936–941.
7. MiguelesSA, SabbaghianMS, ShupertWL, BettinottiMP, MarincolaFM, et al. (2000) HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci U S A 97 : 2709–2714.
8. GaoX, NelsonGW, KarackiP, MartinMP, PhairJ, et al. (2001) Effect of a single amino acid change in MHC class I molecules on the rate of progression to AIDS. N Engl J Med 344 : 1668–1675.
9. KiepielaP, NgumbelaK, ThobakgaleC, RamduthD, HoneyborneI, et al. (2007) CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med 13 : 46–53.
10. KosmrljA, ReadEL, QiY, AllenTM, AltfeldM, et al. (2010) Effects of thymic selection of the T-cell repertoire on HLA class I-associated control of HIV infection. Nature 465 : 350–354.
11. MartinMP, GaoX, LeeJH, NelsonGW, DetelsR, et al. (2002) Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet 31 : 429–434.
12. MartinMP, QiY, GaoX, YamadaE, MartinJN, et al. (2007) Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet 39 : 733–740.
13. HuangJ, GoedertJJ, SundbergEJ, CungTD, BurkePS, et al. (2009) HLA-B*35-Px-mediated acceleration of HIV-1 infection by increased inhibitory immunoregulatory impulses. J Exp Med 206 : 2959–2966.
14. LichterfeldM, KavanaghDG, WilliamsKL, MozaB, MuiSK, et al. (2007) A viral CTL escape mutation leading to immunoglobulin-like transcript 4-mediated functional inhibition of myelomonocytic cells. J Exp Med 204 : 2813–2824.
15. AndersonKJ, AllenRL (2009) Regulation of T-cell immunity by leucocyte immunoglobulin-like receptors: innate immune receptors for self on antigen-presenting cells. Immunology 127 : 8–17.
16. BarrowAD, TrowsdaleJ (2008) The extended human leukocyte receptor complex: diverse ways of modulating immune responses. Immunol Rev 224 : 98–123.
17. ColonnaM, NavarroF, BellonT, LlanoM, GarciaP, et al. (1997) A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J Exp Med 186 : 1809–1818.
18. ColonnaM, SamaridisJ, CellaM, AngmanL, AllenRL, et al. (1998) Human myelomonocytic cells express an inhibitory receptor for classical and nonclassical MHC class I molecules. J Immunol 160 : 3096–3100.
19. ChapmanTL, HeikemanAP, BjorkmanPJ (1999) The inhibitory receptor LIR-1 uses a common binding interaction to recognize class I MHC molecules and the viral homolog UL18. Immunity 11 : 603–613.
20. ShiroishiM, KurokiK, RasubalaL, TsumotoK, KumagaiI, et al. (2006) Structural basis for recognition of the nonclassical MHC molecule HLA-G by the leukocyte Ig-like receptor B2 (LILRB2/LIR2/ILT4/CD85d). Proc Natl Acad Sci U S A 103 : 16412–16417.
21. WillcoxBE, ThomasLM, BjorkmanPJ (2003) Crystal structure of HLA-A2 bound to LIR-1, a host and viral major histocompatibility complex receptor. Nat Immunol 4 : 913–919.
22. JonesDC, KosmoliaptsisV, AppsR, LapaqueN, SmithI, et al. (2011) HLA class I allelic sequence and conformation regulate leukocyte Ig-like receptor binding. J Immunol 186 : 2990–2997.
23. SnaryD, BarnstableCJ, BodmerWF, CrumptonMJ (1977) Molecular structure of human histocompatibility antigens: the HLA-C series. Eur J Immunol 7 : 580–585.
24. HuangJ, GoedertJJ, SundbergEJ, CungTD, BurkePS, et al. (2009) HLA-B*35-Px-mediated acceleration of HIV-1 infection by increased inhibitory immunoregulatory impulses. J Exp Med 206 : 2959–66.
25. GoulderPJ, WalkerBD (2012) HIV and HLA class I: an evolving relationship. Immunity 37 : 426–440.
26. RajapaksaUS, LiD, PengYC, McMichaelAJ, DongT, et al. (2012) HLA-B may be more protective against HIV-1 than HLA-A because it resists negative regulatory factor (Nef) mediated down-regulation. Proc Natl Acad Sci U S A 109 : 13353–13358.
27. ShiroishiM, TsumotoK, AmanoK, ShirakiharaY, ColonnaM, et al. (2003) Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci U S A 100 : 8856–8861.
28. Martinez-NavesE, BarberLD, MadrigalJA, VulloCM, ClaybergerC, et al. (1997) Interactions of HLA-B*4801 with peptide and CD8. Tissue Antigens 50 : 258–264.
29. McLarenPJ, RipkeS, PelakK, WeintrobAC, PatsopoulosNA, et al. (2012) Fine-mapping classical HLA variation associated with durable host control of HIV-1 infection in African Americans. Hum Mol Genet 21 : 4334–4347.
30. GrudaR, AchdoutH, Stern-GinossarN, GazitR, Betser-CohenG, et al. (2007) Intracellular cysteine residues in the tail of MHC class I proteins are crucial for extracellular recognition by leukocyte Ig-like receptor 1. J Immunol 179 : 3655–3661.
31. BanchereauJ, ZurawskiS, Thompson-SnipesL, BlanckJP, ClaytonS, et al. (2012) Immunoglobulin-like transcript receptors on human dermal CD14+ dendritic cells act as a CD8-antagonist to control cytotoxic T cell priming. Proc Natl Acad Sci U S A 109 : 18885–18890.
32. MasudaA, NakamuraA, MaedaT, SakamotoY, TakaiT (2007) Cis binding between inhibitory receptors and MHC class I can regulate mast cell activation. J Exp Med 204 : 907–920.
33. ChangCC, CiubotariuR, ManavalanJS, YuanJ, ColovaiAI, et al. (2002) Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol 3 : 237–243.
34. RistichV, LiangS, ZhangW, WuJ, HoruzskoA (2005) Tolerization of dendritic cells by HLA-G. Eur J Immunol 35 : 1133–1142.
35. VladG, PiazzaF, ColovaiA, CortesiniR, Della PietraF, et al. (2003) Interleukin-10 induces the upregulation of the inhibitory receptor ILT4 in monocytes from HIV positive individuals. Hum Immunol 64 : 483–489.
36. HuangJ, BurkePS, CungTD, PereyraF, TothI, et al. (2010) Leukocyte immunoglobulin-like receptors maintain unique antigen-presenting properties of circulating myeloid dendritic cells in HIV-1-infected elite controllers. J Virol 84 : 9463–9471.
37. LiQ, SmithAJ, SchackerTW, CarlisJV, DuanL, et al. (2009) Microarray analysis of lymphatic tissue reveals stage-specific, gene expression signatures in HIV-1 infection. J Immunol 183 : 1975–1982.
38. AppsR, QiY, CarlsonJM, ChenH, GaoX, et al. (2013) Influence of HLA-C expression level on HIV control. Science 340 : 87–91.
39. PurbhooMA, IrvineDJ, HuppaJB, DavisMM (2004) T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol 5 : 524–530.
40. Revision of the CDC surveillance case definition for acquired immunodeficiency syndrome. Council of State and Territorial Epidemiologists; AIDS Program, Center for Infectious Diseases. MMWR Morb Mortal Wkly Rep 36 Suppl 11S–15S.
41. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep 41 : 1–19.
Štítky
Genetika Reprodukčná medicína
Článek Sex Chromosome Turnover Contributes to Genomic Divergence between Incipient Stickleback SpeciesČlánek Final Pre-40S Maturation Depends on the Functional Integrity of the 60S Subunit Ribosomal Protein L3
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2014 Číslo 3- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- , a Gene That Influences the Anterior Chamber Depth, Is Associated with Primary Angle Closure Glaucoma
- Genomic View of Bipolar Disorder Revealed by Whole Genome Sequencing in a Genetic Isolate
- The Rate of Nonallelic Homologous Recombination in Males Is Highly Variable, Correlated between Monozygotic Twins and Independent of Age
- Genetic Determinants Influencing Human Serum Metabolome among African Americans
- Heterozygous and Inherited Mutations in the Smooth Muscle Actin () Gene Underlie Megacystis-Microcolon-Intestinal Hypoperistalsis Syndrome
- Genome-Wide Meta-Analysis of Homocysteine and Methionine Metabolism Identifies Five One Carbon Metabolism Loci and a Novel Association of with Ischemic Stroke
- Cancer Evolution Is Associated with Pervasive Positive Selection on Globally Expressed Genes
- Genetic Diversity in the Interference Selection Limit
- Integrating Multiple Genomic Data to Predict Disease-Causing Nonsynonymous Single Nucleotide Variants in Exome Sequencing Studies
- An Evolutionary Analysis of Antigen Processing and Presentation across Different Timescales Reveals Pervasive Selection
- Cleavage Factor I Links Transcription Termination to DNA Damage Response and Genome Integrity Maintenance in
- DNA Dynamics during Early Double-Strand Break Processing Revealed by Non-Intrusive Imaging of Living Cells
- Genetic Basis of Metabolome Variation in Yeast
- Modeling 3D Facial Shape from DNA
- Dysregulated Estrogen Receptor Signaling in the Hypothalamic-Pituitary-Ovarian Axis Leads to Ovarian Epithelial Tumorigenesis in Mice
- Photoactivated UVR8-COP1 Module Determines Photomorphogenic UV-B Signaling Output in
- Local Evolution of Seed Flotation in Arabidopsis
- Chromatin Targeting Signals, Nucleosome Positioning Mechanism and Non-Coding RNA-Mediated Regulation of the Chromatin Remodeling Complex NoRC
- Nucleosome Acidic Patch Promotes RNF168- and RING1B/BMI1-Dependent H2AX and H2A Ubiquitination and DNA Damage Signaling
- The -Induced Arabidopsis Transcription Factor Attenuates ABA Signaling and Renders Seedlings Sugar Insensitive when Present in the Nucleus
- Changes in Colorectal Carcinoma Genomes under Anti-EGFR Therapy Identified by Whole-Genome Plasma DNA Sequencing
- Selection of Orphan Rhs Toxin Expression in Evolved Serovar Typhimurium
- FAK Acts as a Suppressor of RTK-MAP Kinase Signalling in Epithelia and Human Cancer Cells
- Asymmetry in Family History Implicates Nonstandard Genetic Mechanisms: Application to the Genetics of Breast Cancer
- Co-translational Localization of an LTR-Retrotransposon RNA to the Endoplasmic Reticulum Nucleates Virus-Like Particle Assembly Sites
- Sex Chromosome Turnover Contributes to Genomic Divergence between Incipient Stickleback Species
- DWARF TILLER1, a WUSCHEL-Related Homeobox Transcription Factor, Is Required for Tiller Growth in Rice
- Functional Organization of a Multimodular Bacterial Chemosensory Apparatus
- Genome-Wide Analysis of SREBP1 Activity around the Clock Reveals Its Combined Dependency on Nutrient and Circadian Signals
- The Yeast Sks1p Kinase Signaling Network Regulates Pseudohyphal Growth and Glucose Response
- An Interspecific Fungal Hybrid Reveals Cross-Kingdom Rules for Allopolyploid Gene Expression Patterns
- Temperate Phages Acquire DNA from Defective Prophages by Relaxed Homologous Recombination: The Role of Rad52-Like Recombinases
- Dying Cells Protect Survivors from Radiation-Induced Cell Death in
- Determinants beyond Both Complementarity and Cleavage Govern MicroR159 Efficacy in
- The bHLH Factors Extramacrochaetae and Daughterless Control Cell Cycle in Imaginal Discs through the Transcriptional Regulation of the Phosphatase
- The First Steps of Adaptation of to the Gut Are Dominated by Soft Sweeps
- Bacterial Regulon Evolution: Distinct Responses and Roles for the Identical OmpR Proteins of Typhimurium and in the Acid Stress Response
- Final Pre-40S Maturation Depends on the Functional Integrity of the 60S Subunit Ribosomal Protein L3
- Mitogen-Activated Protein Kinase (MAPK) Pathway Regulates Branching by Remodeling Epithelial Cell Adhesion
- CDP-Diacylglycerol Synthetase Coordinates Cell Growth and Fat Storage through Phosphatidylinositol Metabolism and the Insulin Pathway
- Coronary Heart Disease-Associated Variation in Disrupts a miR-224 Binding Site and miRNA-Mediated Regulation
- TBX3 Regulates Splicing : A Novel Molecular Mechanism for Ulnar-Mammary Syndrome
- Identification of Interphase Functions for the NIMA Kinase Involving Microtubules and the ESCRT Pathway
- Is a Cancer-Specific Fusion Gene Recurrent in High-Grade Serous Ovarian Carcinoma
- LILRB2 Interaction with HLA Class I Correlates with Control of HIV-1 Infection
- Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle
- Parent-of-Origin Effects Implicate Epigenetic Regulation of Experimental Autoimmune Encephalomyelitis and Identify Imprinted as a Novel Risk Gene
- The Phospholipid Flippase dATP8B Is Required for Odorant Receptor Function
- Noise Genetics: Inferring Protein Function by Correlating Phenotype with Protein Levels and Localization in Individual Human Cells
- DUF1220 Dosage Is Linearly Associated with Increasing Severity of the Three Primary Symptoms of Autism
- Sugar and Chromosome Stability: Clastogenic Effects of Sugars in Vitamin B6-Deficient Cells
- Pheromone-Sensing Neurons Expressing the Ion Channel Subunit Stimulate Male Courtship and Female Receptivity
- Gene-Based Sequencing Identifies Lipid-Influencing Variants with Ethnicity-Specific Effects in African Americans
- Telomere Shortening Unrelated to Smoking, Body Weight, Physical Activity, and Alcohol Intake: 4,576 General Population Individuals with Repeat Measurements 10 Years Apart
- A Combination of Activation and Repression by a Colinear Hox Code Controls Forelimb-Restricted Expression of and Reveals Hox Protein Specificity
- An ER Complex of ODR-4 and ODR-8/Ufm1 Specific Protease 2 Promotes GPCR Maturation by a Ufm1-Independent Mechanism
- Epigenetic Control of Effector Gene Expression in the Plant Pathogenic Fungus
- Genetic Dissection of Photoreceptor Subtype Specification by the Zinc Finger Proteins Elbow and No ocelli
- Validation and Genotyping of Multiple Human Polymorphic Inversions Mediated by Inverted Repeats Reveals a High Degree of Recurrence
- CYP6 P450 Enzymes and Duplication Produce Extreme and Multiple Insecticide Resistance in the Malaria Mosquito
- GC-Rich DNA Elements Enable Replication Origin Activity in the Methylotrophic Yeast
- An Epigenetic Signature in Peripheral Blood Associated with the Haplotype on 17q21.31, a Risk Factor for Neurodegenerative Tauopathy
- Lsd1 Restricts the Number of Germline Stem Cells by Regulating Multiple Targets in Escort Cells
- RBPJ, the Major Transcriptional Effector of Notch Signaling, Remains Associated with Chromatin throughout Mitosis, Suggesting a Role in Mitotic Bookmarking
- The Membrane-Associated Transcription Factor NAC089 Controls ER-Stress-Induced Programmed Cell Death in Plants
- The Functional Consequences of Variation in Transcription Factor Binding
- Comparative Genomic Analysis of N-Fixing and Non-N-Fixing spp.: Organization, Evolution and Expression of the Nitrogen Fixation Genes
- An Insulin-to-Insulin Regulatory Network Orchestrates Phenotypic Specificity in Development and Physiology
- Suicidal Autointegration of and Transposons in Eukaryotic Cells
- A Multi-Trait, Meta-analysis for Detecting Pleiotropic Polymorphisms for Stature, Fatness and Reproduction in Beef Cattle
- Genome-Wide DNA Methylation Analysis Predicts an Epigenetic Switch for GATA Factor Expression in Endometriosis
- Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion
- The and Hybrid Incompatibility Genes Suppress a Broad Range of Heterochromatic Repeats
- The Kil Peptide of Bacteriophage λ Blocks Cytokinesis via ZipA-Dependent Inhibition of FtsZ Assembly
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle
- Genome-Wide DNA Methylation Analysis of Human Pancreatic Islets from Type 2 Diabetic and Non-Diabetic Donors Identifies Candidate Genes That Influence Insulin Secretion
- Genetic Dissection of Photoreceptor Subtype Specification by the Zinc Finger Proteins Elbow and No ocelli
- GC-Rich DNA Elements Enable Replication Origin Activity in the Methylotrophic Yeast
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



