-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Development of a Standardized Screening Rule for Tuberculosis in People Living with HIV in Resource-Constrained Settings: Individual Participant Data Meta-analysis of Observational Studies
Background:
The World Health Organization recommends the screening of all people living with HIV for tuberculosis (TB) disease, followed by TB treatment, or isoniazid preventive therapy (IPT) when TB is excluded. However, the difficulty of reliably excluding TB disease has severely limited TB screening and IPT uptake in resource-limited settings. We conducted an individual participant data meta-analysis of primary studies, aiming to identify a sensitive TB screening rule.Methods and Findings:
We identified 12 studies that had systematically collected sputum specimens regardless of signs or symptoms, at least one mycobacterial culture, clinical symptoms, and HIV and TB disease status. Bivariate random-effects meta-analysis and the hierarchical summary relative operating characteristic curves were used to evaluate the screening performance of all combinations of variables of interest. TB disease was diagnosed in 557 (5.8%) of 9,626 people living with HIV. The primary analysis included 8,148 people living with HIV who could be evaluated on five symptoms from nine of the 12 studies. The median age was 34 years. The best performing rule was the presence of any one of: current cough (any duration), fever, night sweats, or weight loss. The overall sensitivity of this rule was 78.9% (95% confidence interval [CI] 58.3%–90.9%) and specificity was 49.6% (95% CI 29.2%–70.1%). Its sensitivity increased to 90.1% (95% CI 76.3%–96.2%) among participants selected from clinical settings and to 88.0% (95% CI 76.1%–94.4%) among those who were not previously screened for TB. Negative predictive value was 97.7% (95% CI 97.4%–98.0%) and 90.0% (95% CI 88.6%–91.3%) at 5% and 20% prevalence of TB among people living with HIV, respectively. Abnormal chest radiographic findings increased the sensitivity of the rule by 11.7% (90.6% versus 78.9%) with a reduction of specificity by 10.7% (49.6% versus 38.9%).Conclusions:
Absence of all of current cough, fever, night sweats, and weight loss can identify a subset of people living with HIV who have a very low probability of having TB disease. A simplified screening rule using any one of these symptoms can be used in resource-constrained settings to identify people living with HIV in need of further diagnostic assessment for TB. Use of this algorithm should result in earlier TB diagnosis and treatment, and should allow for substantial scale-up of IPT.
: Please see later in the article for the Editors' Summary
Published in the journal: Development of a Standardized Screening Rule for Tuberculosis in People Living with HIV in Resource-Constrained Settings: Individual Participant Data Meta-analysis of Observational Studies. PLoS Med 8(1): e32767. doi:10.1371/journal.pmed.1000391
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1000391Summary
Background:
The World Health Organization recommends the screening of all people living with HIV for tuberculosis (TB) disease, followed by TB treatment, or isoniazid preventive therapy (IPT) when TB is excluded. However, the difficulty of reliably excluding TB disease has severely limited TB screening and IPT uptake in resource-limited settings. We conducted an individual participant data meta-analysis of primary studies, aiming to identify a sensitive TB screening rule.Methods and Findings:
We identified 12 studies that had systematically collected sputum specimens regardless of signs or symptoms, at least one mycobacterial culture, clinical symptoms, and HIV and TB disease status. Bivariate random-effects meta-analysis and the hierarchical summary relative operating characteristic curves were used to evaluate the screening performance of all combinations of variables of interest. TB disease was diagnosed in 557 (5.8%) of 9,626 people living with HIV. The primary analysis included 8,148 people living with HIV who could be evaluated on five symptoms from nine of the 12 studies. The median age was 34 years. The best performing rule was the presence of any one of: current cough (any duration), fever, night sweats, or weight loss. The overall sensitivity of this rule was 78.9% (95% confidence interval [CI] 58.3%–90.9%) and specificity was 49.6% (95% CI 29.2%–70.1%). Its sensitivity increased to 90.1% (95% CI 76.3%–96.2%) among participants selected from clinical settings and to 88.0% (95% CI 76.1%–94.4%) among those who were not previously screened for TB. Negative predictive value was 97.7% (95% CI 97.4%–98.0%) and 90.0% (95% CI 88.6%–91.3%) at 5% and 20% prevalence of TB among people living with HIV, respectively. Abnormal chest radiographic findings increased the sensitivity of the rule by 11.7% (90.6% versus 78.9%) with a reduction of specificity by 10.7% (49.6% versus 38.9%).Conclusions:
Absence of all of current cough, fever, night sweats, and weight loss can identify a subset of people living with HIV who have a very low probability of having TB disease. A simplified screening rule using any one of these symptoms can be used in resource-constrained settings to identify people living with HIV in need of further diagnostic assessment for TB. Use of this algorithm should result in earlier TB diagnosis and treatment, and should allow for substantial scale-up of IPT.
: Please see later in the article for the Editors' SummaryIntroduction
By the end of 2009, an estimated 33 million people were living with HIV, the vast majority in sub-Saharan Africa and Asia. Tuberculosis (TB) remains the most common cause of death in people living with HIV. Compared to people without HIV, people living with HIV have a more than 20-fold increased risk of developing TB [1]. TB disease may occur at any stage of HIV disease and is frequently the first recognized presentation of underlying HIV infection [2],[3]. Without antiretroviral treatment (ART), up to 50% of people living with HIV who are diagnosed with TB die during the 6–8 mo of TB treatment [4]–[6]. This risk increases to 72%–98% among those with multi-drug (MDR) or extensively drug-resistant (XDR) TB [7],[8]. Although ART can reduce the incidence of TB both at individual [9] and population [10] levels, people living with HIV on ART still have higher TB incidence rates and a higher risk of dying from TB [11]. Routine TB screening offers the opportunity to diagnose and promptly treat TB disease, and to identify those without TB disease who may be eligible for TB preventive therapy [12]. The use of TB preventive therapy can reduce TB incidence and is therefore of considerable benefit to patients [13].
For these reasons, the World Health Organization (WHO) recommends regular screening for active TB disease of all people living with HIV and providing either treatment for active disease or isoniazid preventive therapy (IPT) to mitigate TB morbidity, mortality, and transmission [14]. However, in 2009, of the estimated 33 million people living with HIV, only 1.7 million (5%) were screened for TB, and about 85,000 (0.2%) were offered IPT [15]. Currently there is no internationally accepted evidence-based tool to screen for TB in people living with HIV. Several studies have shown that the presenting signs and symptoms of TB in people living with HIV are different from those in people without HIV to diagnose TB; for example, many people living with HIV who have culture-confirmed TB do not report having a prolonged cough, which is one of the standard TB screening questions used by national TB control programs globally [16]. Moreover, the most widely available TB diagnostic tests such as smear microscopy and chest radiography perform poorly among people living with HIV; because most people living with HIV and TB have either sputum acid-fast bacilli (AFB) smear negative pulmonary or extrapulmonary TB [17].
We conducted an individual participant data meta-analysis of published and unpublished studies to develop a simple, standardized TB screening rule for resource-constrained settings that will adequately separate patients into two groups: (1) those for whom TB is reliably excluded, and IPT and ART, if indicated, can be initiated; and (2) those who require further investigation for TB disease. We describe the results of this meta-analysis and propose an algorithm for TB screening among people living with HIV in resource-constrained settings.
Methods
We proceeded through several steps. First, we prospectively enumerated criteria for studies to be included in our meta-analysis. Second, we searched for and selected studies that met these criteria. Third, we sought primary data from investigators and mapped individual-level data to common symptoms. Fourth, we identified five symptoms available in most studies and, within each study, computed the sensitivity and specificity of 23 screening rules derived from these five symptoms. Finally, we used meta-analysis methods to estimate the performance of all 23 rules, as well as the association of study-level and individual-level correlates with performance.
Inclusion of Studies
We defined studies as being eligible for inclusion in this analysis if they met the following criteria: (1) collected sputum specimens from people living with HIV regardless of signs or symptoms; (2) used mycobacterial culture of at least one specimen to diagnose TB; and (3) collected data about signs and symptoms.
Search Strategy and Selection of Studies
To identify studies eligible for the meta-analysis, we conducted a systematic literature review of studies related to TB screening among people living with HIV in June 2008 using PubMed and various combinations of the following keywords: “HIV,” “Tuberculosis,” “TB screening,” “Smear-negative TB,” “Sputum negative TB,” “TB case finding,” “Intensified TB case finding,” “Isoniazid prevention treatment, trial or therapy.” We also searched for abstracts presented at conferences organized by the International Union Against TB and Lung Diseases and the International AIDS Society between 2000–2008. No language restriction was placed on the search. We reviewed all retrieved titles and abstracts for relevance to the topic. The reference lists of retrieved studies were also reviewed to identify further studies that meet the eligibility criteria. In addition, recognized experts in the field were contacted to identify studies that were not available (e.g., unpublished) in the initial electronic search. Studies that involve concomitant HIV testing and mycobacterial culture on all patients are resource intensive and challenging to implement in countries with a high burden of TB and HIV. Therefore, we believe it is unlikely that eligible studies would have been completed but missed by our search strategy.
We found 2,119 publications and reviewed all their abstracts. Using the criteria above, we selected 53 for review of the full text. Twenty-one articles included information on signs and symptoms for TB screening in people living with HIV. A total of 14 studies (six published and eight unpublished at the time of the search) met the inclusion criteria of our meta-analysis (Figure 1). The corresponding authors or principal investigators were contacted for all 14 studies to confirm that their studies met all the eligibility criteria. One unpublished dataset was excluded for not meeting the inclusion criteria after verification with the principal investigator, and another one was excluded because the investigators could not submit the data within the agreed timeframe. A total of 12 investigators (representing six published and six unpublished studies at the time of the search) provided de-identified individual patient data for inclusion in the primary meta-analysis within an agreed time framework (Table 1) [18]–[29]. In November 2010, immediately preceding manuscript publication, we re-ran the search strategy again to look for additional studies that were reported since the initial search and should have been included in the meta-analysis. The search found seven eligible studies, of which all except one [30] were included in our meta-analysis as unpublished datasets.
Fig. 1. Search strategy and studies included in the meta-analysis (PRISMA flow diagram). 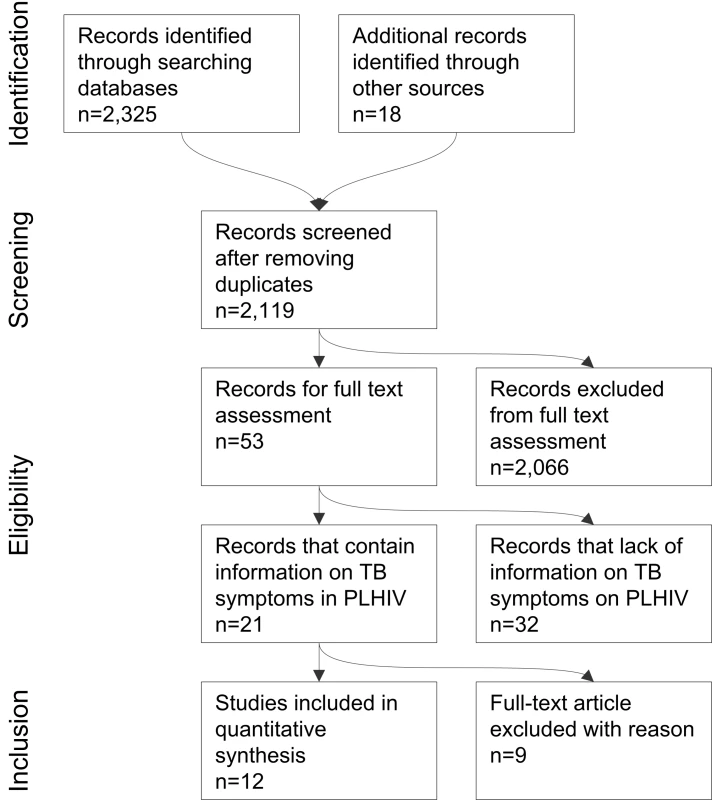
Tab. 1. Summary of studies included in the meta-analysis. 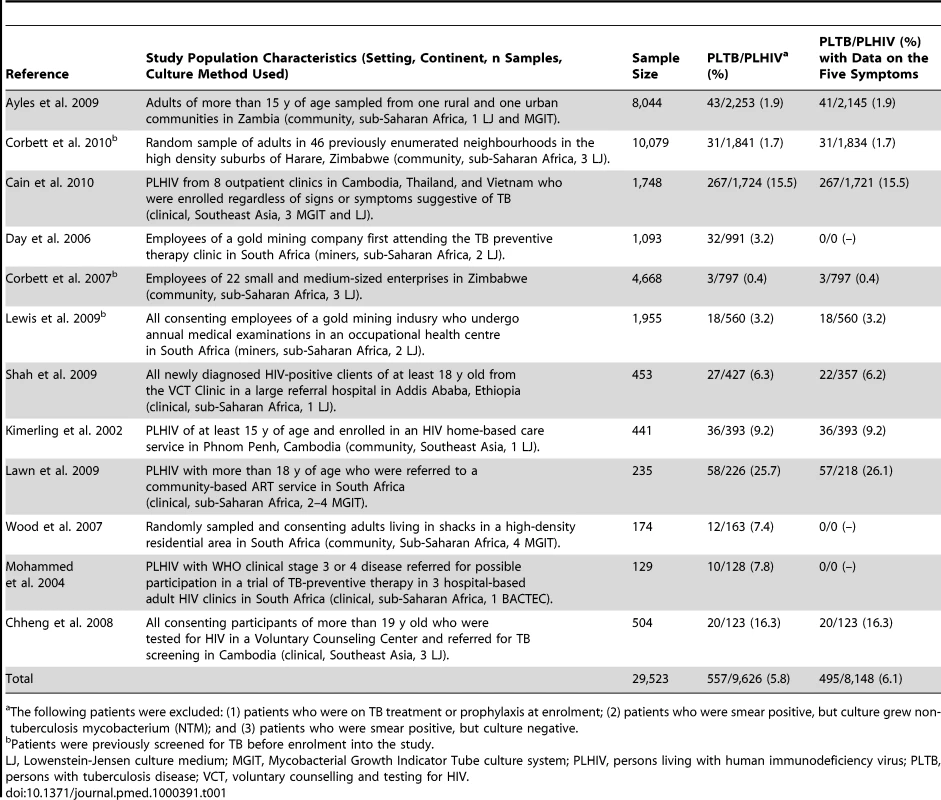
The following patients were excluded: (1) patients who were on TB treatment or prophylaxis at enrolment; (2) patients who were smear positive, but culture grew non- tuberculosis mycobacterium (NTM); and (3) patients who were smear positive, but culture negative. Investigators for all included studies signed a data sharing and confidentiality agreement, and agreed to a data management, analysis, and publication plan. During design and analysis phases of the meta-analysis, the investigators of the studies and data managers of the included studies held multiple discussions by email, by teleconference, and in person in Geneva, Switzerland and Atlanta, Georgia, United States.
Data Abstraction and Management
The list of variables from the most comprehensive dataset [20] was used to construct an initial master list of variables. All the variables from each study included in the meta-analysis were mapped to this master list. Principal investigators and data managers for the 12 studies worked with the meta-analysis investigators to ensure accurate mapping of data from the primary studies to the master variable list. In the end, the final dataset of the meta-analysis included 159 variables that appeared in at least two of the studies. We identified five symptoms common to most studies and limited the meta-analysis to the nine studies with substantially complete information for those five symptoms.
Case Definitions
We defined a TB patient as any person living with HIV and at least one specimen culture positive for Mycobacterium tuberculosis (MTB). We defined participants as having no TB if cultures were negative for MTB and participants were judged not to have TB on the basis of the original study definition of the investigators. We excluded from the analysis: (1) patients who were receiving treatment for TB (infection or disease) at enrolment; (2) patients who were AFB smear positive, but whose culture grew non-tuberculous mycobacteria; and (3) patients who were AFB smear positive or scanty, but sputum culture negative.
Sources of Study Heterogeneity
All studies were reviewed to identify study-level characteristics that could substantially impact the findings of the meta-analysis. Two studies were conducted exclusively among gold miners living in South Africa [21],[23], a population that may not be broadly generalizable, because of its demographics, its high prevalence of non-TB illnesses (e.g., silicosis) that can produce cough, and the practice of annual TB radiological screening. Five studies [18],[19],[22],[25],[27] were conducted among individuals drawn from a community setting through prevalence surveys, which may lead to enrolment of patients with a different spectrum of TB and HIV disease than would be found among patients seeking care. Three studies [19],[22],[23] involved participants who were previously screened for TB or who had had access to routine TB screening before being enrolled into the studies. Finally, three studies exclusively used liquid media to culture specimens [26]–[28], two studies used both solid and liquid media [18],[20], and seven studies exclusively used solid media (Table 1). Liquid media have substantially increased sensitivity for growing MTB, particularly in patients with low levels of TB bacilli, as would be expected in a population of people living with HIV being screened for TB [31]. Studies that used liquid media, therefore, would have improved ability to classify patients correctly into those who have TB and those who do not. We explored the impact of these factors on the performance of the screening algorithms and analyzed subsets of the final dataset grouped according to these characteristics.
Data Analysis
We compared characteristics of patients with TB to those of patients without TB to derive a standardized rule for TB screening among people living with HIV. The goal of TB screening is to divide the population of people living with HIV into two groups: (1) those who do not have TB; and (2) those who need further evaluation for the diagnosis of TB (i.e., TB suspects). We restricted our analysis to clinical symptoms that could be readily assessed at any level of the health system and that were asked about in all studies: current cough (C), haemoptysis (H), fever (F), night sweats (S), and weight loss (W). Using the four studies that included chest radiography data [20],[23],[24],[26], we also evaluated the impact of adding abnormal chest radiography findings to the TB screening rule. Only observations with no missing data for the symptoms of interest were included in the analysis.
We considered “1-of-n” rules as candidates for screening for TB that could best classify patients into two groups (not TB and suspected TB) with high sensitivity [20]. The “1” represents the minimum number of symptoms that must be present in an individual to be classified as a suspected TB patient and the “n” represents the number of symptom(s) in a given rule. For example, a “1-of-3” rule could be a set of symptoms such as current cough, fever, and weight loss, abbreviated here as CFW. An individual with at least one symptom specified in this particular rule would be classified as a TB suspect; an individual without any of these symptoms would be classified as not having TB. We considered all combinations of the five candidate symptoms except for combinations that include both current cough and haemoptysis, yielding a total of 23 candidate rules: two 1-of-4 rules (CFSW, HFSW), seven 1-of-3 rules (CFS, CFW, CSW, HFS, HFW, HSW, FSW), nine 1-of-2 rules (CF, CS, CW, HF, HS, HW, FS, FW, SW), and five 1-of-1 rules (C, H, F, S, W) (see also Table 3.) The analysis with abnormal chest radiographic findings (X) considered these 23 rules, each augmented with this additional sign (e.g., CFSWX).
Other analyses have considered m-of-n rules with m>1 [20]. These rules cannot exceed the sensitivity of 1-of-n rules. Suppose that a positive screen requires the presence of at least two symptoms out of current cough, fever, and night sweats, the number of true positives for this 2-of-3 rule will not be greater than the number of true positives from the corresponding 1-of-3 rules. Because our aim in this analysis was to identify the most sensitive rule, we did not include rules of this kind in our analysis.
We applied two closely related methods for cross-study analysis of sensitivity and specificity of these 23 candidate screening rules: bivariate random-effects meta-analysis (BREMA) and the hierarchical summary relative operating characteristic (HSROC) curve [32],[33]. BREMA jointly models sensitivity and specificity while accommodating between-study heterogeneity, and HSROC models tradeoffs between sensitivity and specificity across study populations. Both methods can be unified in the same model. In addition to sensitivity and specificity, we calculated predictive value negative and likelihood ratio negative of each candidate rule [34]. Our goal was to identify a combination of symptoms that achieved the highest possible sensitivity and the lowest possible negative likelihood ratio for ruling out TB disease (without any predetermined cut-off points).
To further understand between-study heterogeneity and other factors associated with the diagnostic performance of the most sensitive rule, we analyzed several study-level predictors (setting, prior screening of study participants, culture medium used, and geographic region) and participant-level predictors (age, gender, CD4 T-lymphocyte cell count [CD4], and abnormal chest radiographic findings). Our analytic methods produced odds ratios that reflect the magnitude of association between each factor and the probability of correctly identifying persons with TB (sensitivity) or without TB (specificity). For a range of TB prevalence values, we calculated the negative predictive values at levels of each covariate. We calculated the ratio of the number of patients that screen positive but who actually have no TB (false positives) and hence unnecessarily require additional TB diagnostic evaluation (e.g., culture) to the number of patients that screen positive and actually have TB (true positives), which is referred to as the number needed to screen. We calculated this ratio for different rules using a theoretical population of 1,000 people living with HIV with different levels of TB prevalence. This ratio provides proxy information similar to a marginal cost-effectiveness analysis for different screening rules and it helps quantify how much a health program would need to invest (as measured by additional diagnostic tests) for every patient with TB identified through the screening rule [35].
Each observation with a missing covariate value was omitted from analysis of that covariate. BREMA models were fitted using SAS procedure Glimmix (SAS 9.22, SAS Institute), and further calculations were performed in R (R 2.10.1, R Development Core Team).
Ethical Review
All data collection included in the meta-analysis was approved by institutional ethical review boards at the respective institutions during the original study; if necessary, principal investigators requested additional approval from institutional review boards for the inclusion of the primary dataset in the meta-analysis. All data for the meta-analysis were provided completely de-identified. In the meta-analysis dataset, investigators were not able to link case records to individuals.
Results
Investigators provided data about 29,523 participants, of whom 10,057 were people living with HIV. The dataset included 9,626 people living with HIV who had TB screening and sputum culture performed, of whom 8,148 could be evaluated on the five symptoms of interest from nine of 12 studies (Figure 2).
Fig. 2. Flow chart of study participants included in the individual patient data meta-analysis. 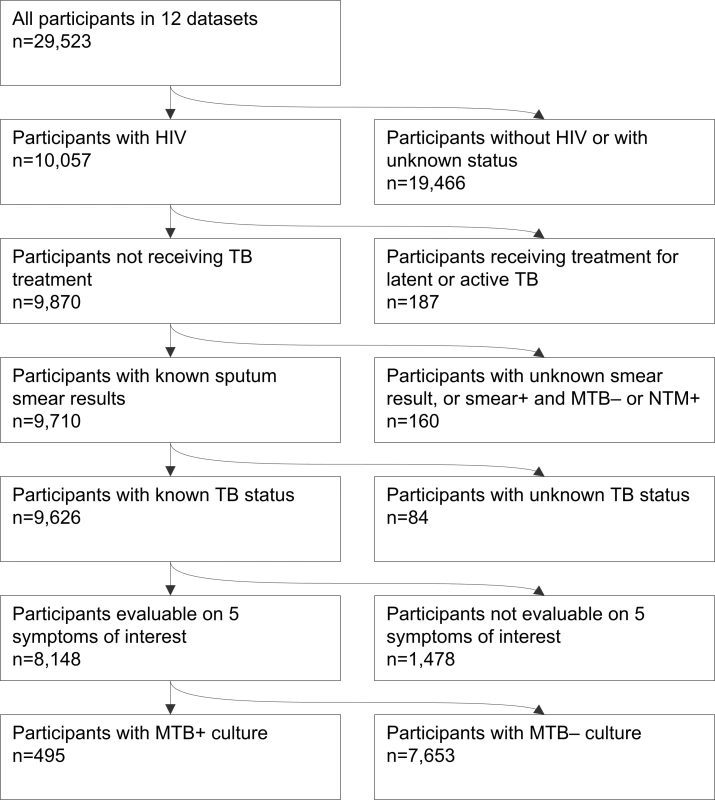
Most patients (77% [7,386/9,626]) were from sub-Saharan Africa; the rest were from Southeast Asian countries. The median age was 34 y (interquartile range [IQR], 27–41 y). Of the 9,626 patients with HIV in the 12 studies, CD4 cell count information was available for 3,489 (36%) and chest radiography information for 3,903 (41%). The median CD4 count was 248 cells/µl (IQR, 107–409).
The overall prevalence of TB disease was 5.8% (557/9,626), ranging across studies from 0.4% to 25.7% (Table 1). More than half of TB patients (52% [288/557]) had sputum smear negative pulmonary TB, whereas 39% (218/557) had sputum smear positive pulmonary, and 5% (28/557) had exclusively extrapulmonary TB. The anatomic site of TB was not specified in 4% (23/557) of patients.
Table 2 summarizes the distribution of common variables, and Table S1 summarizes how each question was actually asked in each study. Because duration of cough was included in many studies but was asked about in different ways, we were able to analyze data using three different cough variables: cough in the past 4 wk (information available for 39.3% of participants); cough lasting for 2 wk or more (information available for 47.1%); and cough present in the last 24 h, which is referred to as “current cough” (information available for 89.6%).
Tab. 2. Characteristics of participants with and without TB for variables included in the analysis. 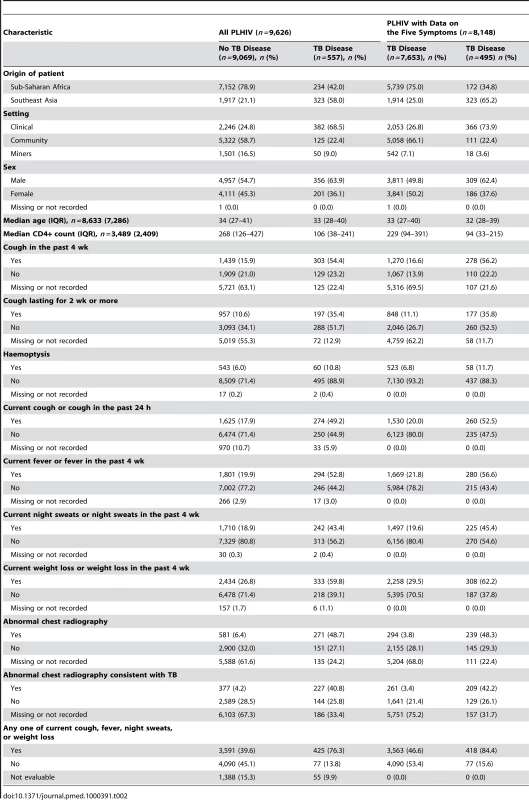
We analyzed the performance of individual and combinations of symptoms as screening rules using data from the 8,148 participants who could be evaluated based on the five candidate symptoms. Table 3 shows the diagnostic performance characteristics for the 23 candidate combinations of symptoms, sorted from highest sensitivity to lowest. The most sensitive rule was the presence of any one of the following symptoms: current cough, fever, night sweats, and weight loss (CFSW). The population-average sensitivity of this symptom combination was 78.9% (95% confidence interval [CI] 58.3%–90.9%) with the negative likelihood ratio of 0.426 (95% CI 0.349–0.520), which corresponds to a postscreening reduction in the probability of TB by 15%–20% [34].
Tab. 3. Diagnostic performance of 23 candidate 1-of-n rules. 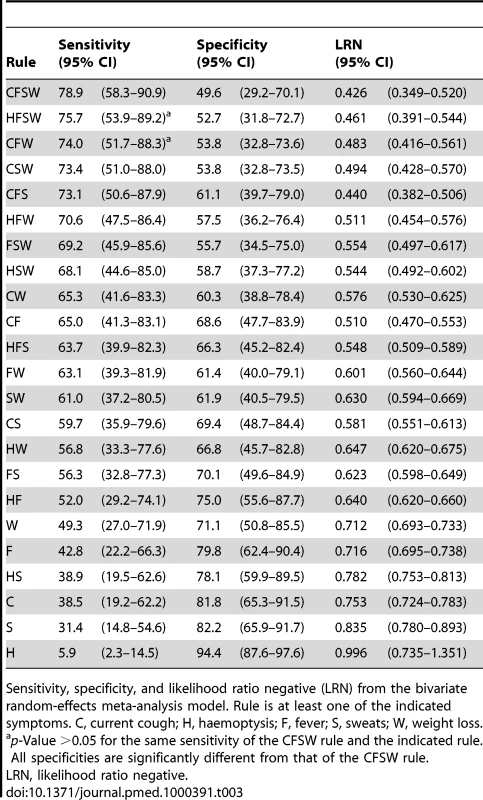
Sensitivity, specificity, and likelihood ratio negative (LRN) from the bivariate random-effects meta-analysis model. Rule is at least one of the indicated symptoms. C, current cough; H, haemoptysis; F, fever; S, sweats; W, weight loss. The nine included studies demonstrated significant between-study heterogeneity on both sensitivity (p<0.001) and specificity (p<0.001) of the rule CFSW (see also Figure 3). The bivariate graphic shows that six studies have study-level specificities below and three above the population average specificity. Furthermore, this rule has the highest-ranking sensitivity in eight of the nine included studies (Table S2). The hierarchical summary relative operating characteristic curves (Figure S1) show slightly better overall diagnostic performance of the rules CFS and CF, but our application requires the highest sensitivity possible, allowing for some tradeoffs with lower specificity. Figure 3 shows that three studies are outliers, and they represent studies of patients who were previously screened for TB or studies in which much of the population likely had previous TB screening (e.g., miners); this can modify the performance characteristics of the screening rule.
Fig. 3. Diagnostic performance of CFSW rule in the included studies. 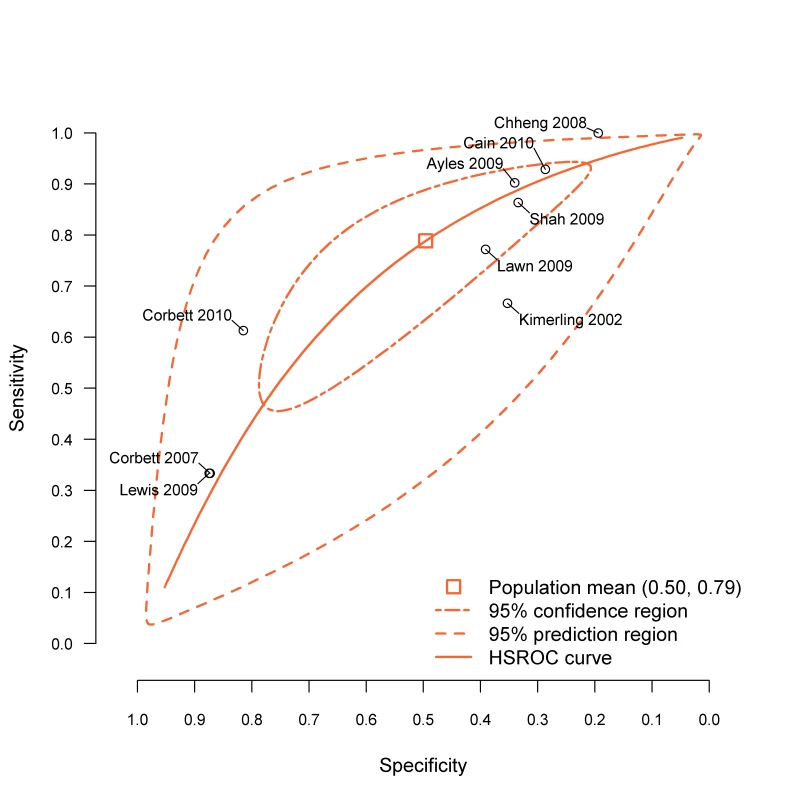
BREMA, bivariate random-effects meta-analysis; HSROC, hierarchical summary relative operating characteristic. The CFSW rule has sensitivity of 90.1% (95% CI 76.3%–96.2%) and 67.1% (95% CI 41.7%–85.3%) among participants selected from clinical and community settings, respectively. Similarly the sensitivity of the rule among those who had not been previously screened for TB was higher at 88.0% (95% CI 76.1%–94.4%) compared to those who had been screened for TB at 40.5% (95% CI 16.6%–69.9%). At the 95% confidence level, the sensitivity of this rule could not be statistically distinguished from the sensitivity of the rule that substitutes haemoptysis for current cough (HFSW, 75.7% sensitive [95% CI 53.9–89.2%]) or the rule that drops night sweats (CFW, 74.0% sensitive [51.7–88.3%]). All other rules had lower sensitivity.
Regression analysis of study-level predictors revealed that studies in which TB screening was performed in clinical settings had 4.5 times the odds for a true-positive screening result compared to studies in which TB screening was performed in a community setting (95% CI 1.0–19.5). Studies of participants who had not previously been screened for TB had 10.8 times the odds for a true-positive screen (95% CI 2.4–47.8) compared with studies in which participants had previously been screened for TB. Participants with CD4 cell count <200 cells/µl had 6.4 times the odds of a true-positive screen (95% CI 2.9–14.2). Statistically significant predictors of true-negative results include prescreening, geographic region, participant age ≥33 y, CD4 cell count <200 cells/ml, and abnormal result on chest radiograph (Table 4).
Tab. 4. Association of study-level and individual-level predictors with the diagnostic performance of CFSW rule. 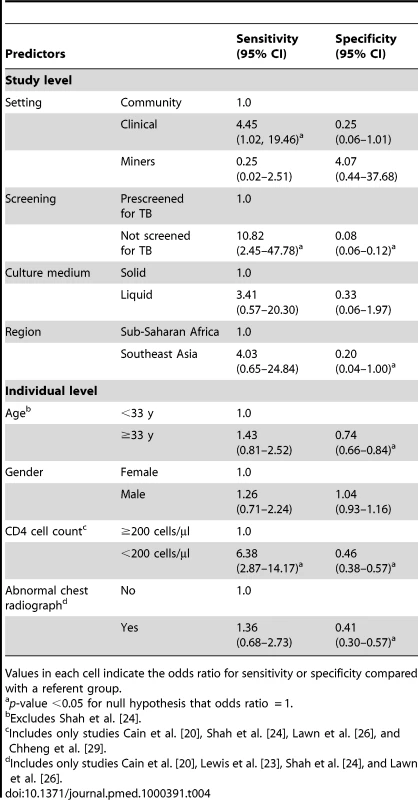
Values in each cell indicate the odds ratio for sensitivity or specificity compared with a referent group. Table 5 shows the negative predictive value and the numbers needed to screen for the CFSW rule adjusted for individual - and study-level covariates. In a setting with 5% TB prevalence among people living with HIV, the rule has a negative predictive value of 98.3% (95% CI 97.5%–98.8%) for patients screened in a clinical setting and 97.3% (95% CI 96.9%–97.7%) for patients screened in a community setting. The numbers needed to screen at the same prevalence of TB are 15 and 11 for clinical and community setting, respectively. The negative predictive value was similar in those having high (≥200) and low (<200) CD4 count at 96.9% (95% CI 95.1%–98.0%) and 98.9% (95% CI 97.5%–99.5%), respectively (see also Table S3).
Tab. 5. Negative predictive value (NPV) and number needed to screen (NNS) using rule CFSW in a hypothetical population of 1,000 people living with HIV stratified by study and individual level predictors. 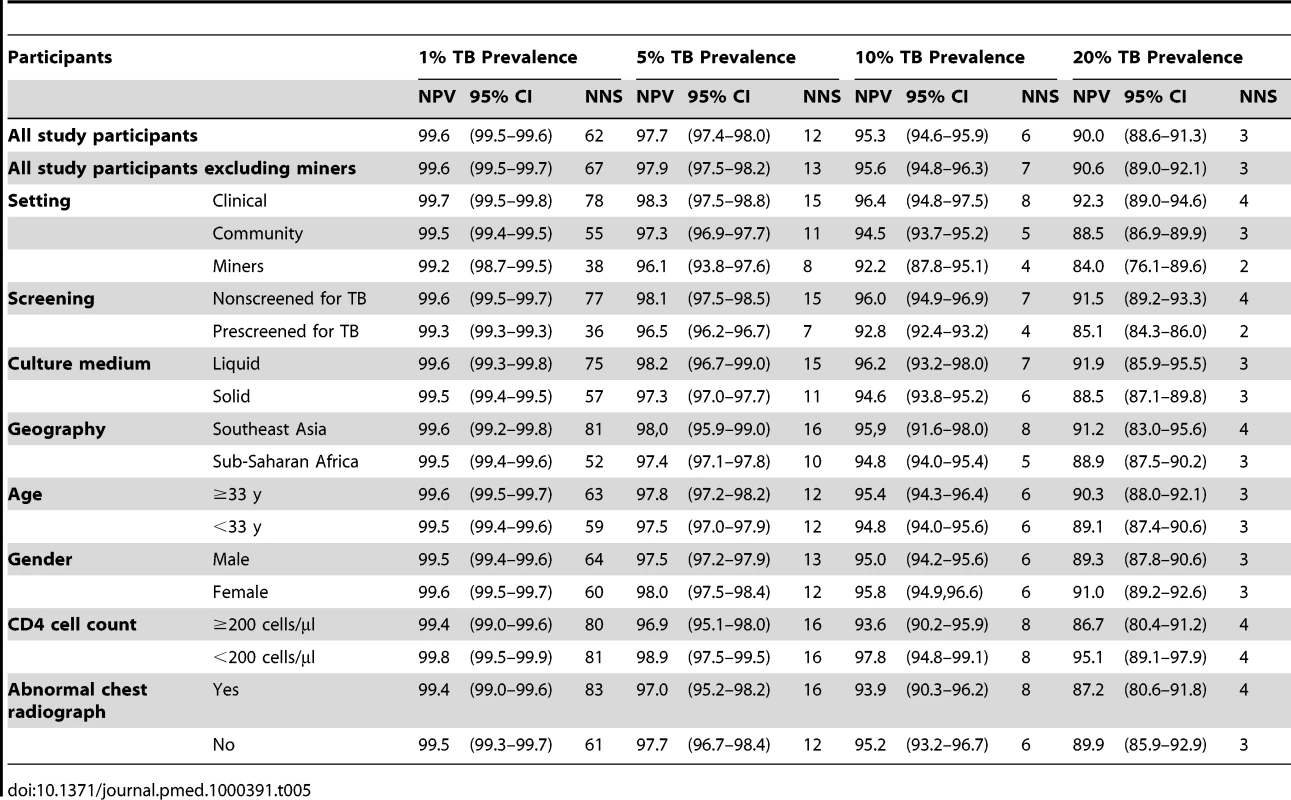
Four studies [20],[23],[24],[26] consistently recorded information on chest radiograph, allowing screening rules with this sign to be evaluated using data from 2,805 participants The addition of abnormal chest radiographic findings into the CFSW rule increases the sensitivity to 90.6% (95% CI 66.7%–97.9%) with a specificity of 38.9% (95% CI 12.8%–73.3%), and a likelihood ratio negative of 0.242 (95% CI 0.102–0.571). Fifteen of the 23 rules included in our analysis outperform the symptom-based CFSW rule when abnormal chest radiographic findings are added (Table S4).
On the basis of our meta-analysis findings and incorporating current WHO recommendations on provision of IPT, we developed a simple TB screening algorithm for public health programmes to screen people living with HIV, and, depending on the outcome of screening, to either provide IPT or evaluate patients further for TB or other diseases (Figure 4).
Fig. 4. Algorithm for TB screening in person living with HIV in HIV prevalent and resource-constrained settings. 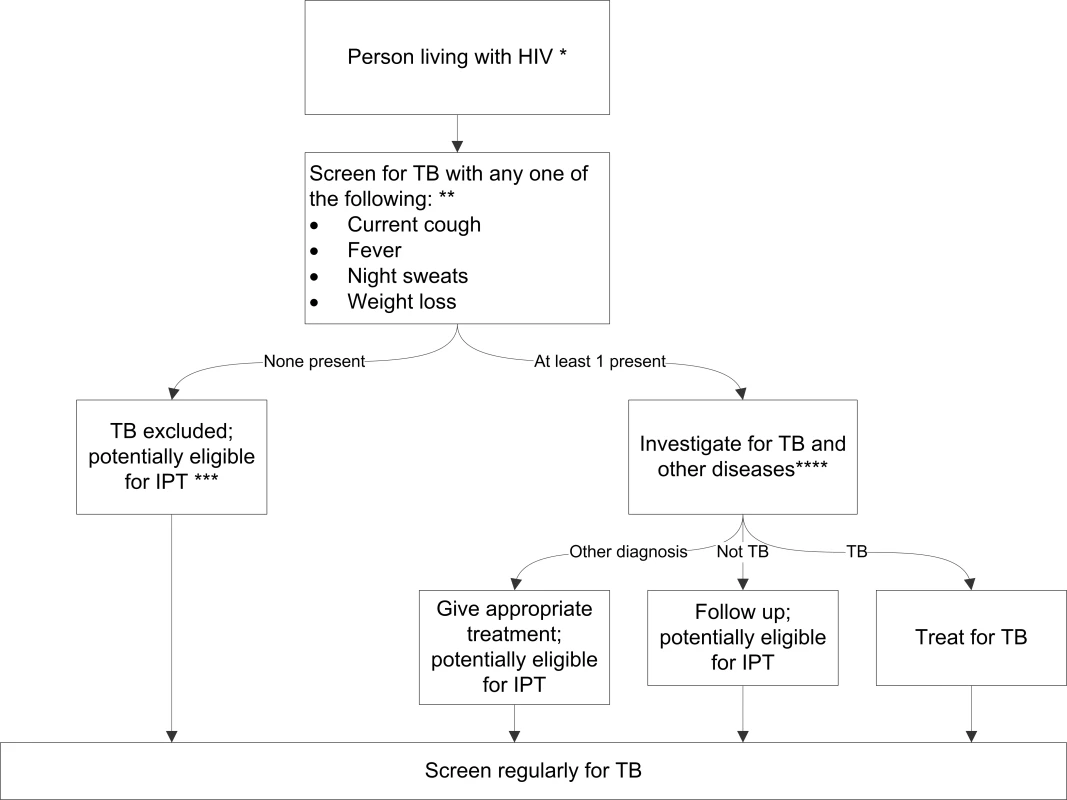
* Every person living with HIV needs to be evaluated for ART eligibility, and all settings providing care should reduce TB transmission through proper measures. ** Chest radiography is not required to classify patients into the TB and not-TB groups, but can be done, if available, to increase the sensitivity of screening. *** Assess for contraindications, including active hepatitis (acute or chronic), regular and heavy alcohol consumption, and symptoms of peripheral neuropathy, is required prior to initiating IPT. Past history of TB is not a contraindication for starting IPT. Tuberculin skin test may be performed as part of eligibility screening in some settings. **** Investigations for TB should be done in accordance with existing national guidelines. Discussion
We found that the absence of all of current cough, fever, night sweats, and weight loss can identify a subset of people living with HIV who have low probability of having TB disease. This screening rule was superior over other candidate rules in eight of the nine studies included and had an overall favourable performance over competing rules in the hierarchical summary relative operating characteristic (HSROC) analysis. We also demonstrated that the negative predictive value of the rule was high across a range of TB disease prevalence estimates and across different population subsets, including those with low and high CD4 count, and those drawn from clinical and community settings and South African miners. We believe that these screening questions are likely to be acceptable to practitioners, because they are symptoms classically associated with TB disease.
Underdiagnosis and delayed diagnosis of TB contribute to excess mortality among people living with HIV [17]. Similarly, concerns about the ability to reliably rule out active TB before initiating IPT have been a major barrier for wider use of this intervention. In the absence of a rapid and effective TB diagnostic tool available at the point-of-care, simple clinical algorithms must be used to screen people living with HIV for TB, dividing them into those in whom active TB is excluded and those who require further evaluation. This meta-analysis synthesizes the best available evidence for how to do this by relying on individual patient data of culture-confirmed TB cases from people living with HIV in the two regions of the world with the most severe burden of the TB and HIV dual epidemic.
The major change to existing practice would be the replacement of chronic cough with current cough as a screening question and the addition of other symptoms to standard screening. National TB programs have traditionally defined a TB “suspect” as someone with cough lasting greater than 2 or 3 wk, and designed case-finding activities to investigate up to ten TB suspects for every TB case detected [36]. However, studies included in this analysis have shown that chronic cough is highly insensitive for TB disease in people living with HIV; using this symptom as a screening rule would miss cases and contribute to diagnostic delays [16],[20]. Using the combination of symptoms that we propose, in a population of people living with HIV with a 5% TB prevalence (excluding miners), requires the investigation of 13 extra patients for every TB case detected, a ratio of TB suspects to a TB case not much different from what national TB control programmes target in the general population.
There has been ongoing debate about the importance of chest radiography in screening people living with HIV for IPT eligibility [37],[38]. Our analysis showed that the addition of abnormal chest radiography findings into the screening rule of CFSW increases the sensitivity of the rule by 11.7% (90.6% versus 78.9%) with a reduction of specificity by 10.7% (49.6% versus 38.9%). However, for example at a 5% TB prevalence rate among people living with HIV, augmenting the CFSW rule with abnormal chest radiographic findings increases the negative predictive value by a margin of only 1% (98.7% versus 97.8%), albeit with the same number of cases needed to be screened. On the other hand, the addition of abnormal chest radiographic findings to the rule at TB prevalence of 20% among people living with HIV increases the negative predictive value by almost 4% (94.3% versus 90.4%) without additional cases needed to be screened. It is also worth noting that the CFSW screening rule has higher sensitivity among those who presented into a clinical setting (90%) and among those who have not been previously screened for TB (88%).
Our findings show that the utility of the proposed symptom-based screening rule have excellent performance in most settings with TB and HIV burden. However, the negative predictive value will fall in those settings with higher TB prevalence when symptom screening alone is used, as it depends on prevalence of disease. In particular settings (e.g., antiretroviral clinics with a very high TB burden [39]), consideration must be given to use of an algorithm that contains chest radiography, or even adding additional sensitive investigations (e.g., culture) while screening people living with HIV for TB [30],[40]. People living with HIV and receiving IPT should also be regularly screened for TB during their visit to a health facility or contact with health care provider so as to promptly detect active TB, if it develops. Programme managers need to weigh the financial, technical, and logistic difficulties, and patient cost and inconvenience associated with performing chest radiography or other additional sensitive investigations on all people living with HIV as part of a screening program compared with an approach that relies only on symptomatic screening.
When interpreting our results, one must bear in mind that only a few variables were common to all studies included in the meta-analysis. It is possible that the addition of one or more symptoms not included in our list of common symptoms could have improved the performance of our proposed screening rule. However, at least one study included in our meta-analysis explored over 80 million combinations of about 100 signs and symptoms and found a symptom combination (cough and fever of any duration and night sweats for 3 weeks or longer), which was similar to the one we propose as the best performing one [20]. Furthermore, questions were not asked in a uniform manner across all studies, and the reporting of symptoms can be highly dependent on factors such as the quality of the interview and interviewer, the circumstances under which questions are asked, and the social and cultural factors that shape individual perceptions of symptoms and disease [41]. We reviewed all questions carefully with principal investigators and data managers to ensure accurate mapping of differently phrased questions to common variables. Our study relied on patients drawn from multiple countries and multiple settings, and the variation in the performance of the proposed screening rule across these different settings suggests that variation in patient self-report of symptoms is unlikely to have major impact, at least at the population level. In some studies, only one sputum specimen was collected for culture, while multiple cultures are required to maximize sensitivity. Some patients with TB may have been incorrectly classified as not having TB. Extrapulmonary TB is an important cause of morbidity and mortality in people living with HIV, but most studies included in the meta-analysis focused on screening for pulmonary TB. Young children were not included in the studies. We did not specifically look into the role of tuberculin skin test in the proposed screening rule. Ideally, the utility of the algorithm we propose, based on the screening rule from our meta-analysis, should be studied prospectively using a standardized protocol in multiple diverse sites; this is particularly important as the studies included in our analysis came from only two geographical regions of the world. Similarly, because of the time required for the data aggregation, statistical analysis, manuscript preparation, and publication, there was one potentially eligible study that was not included in our analysis [30]. We believe that the exclusion of this single study from South Africa, a country from which we have included similar studies already, will not affect the interpretation of our data and conclusions. In the future, as more studies are reported, particularly from other regions, it will be important to repeat the meta-analysis.
Greatly improving TB screening, diagnosis, and treatment in people living with HIV will require deployment of a rapid, accurate, point-of-care TB diagnostic test. In the absence of such a test, we believe that a standardized algorithm employing symptoms, as we propose here, can improve the diagnosis and treatment of TB for people living with HIV, and by doing so would save many lives. Reliable exclusion of TB disease will facilitate safer initiation of antiretroviral therapy and will allow for broader use of IPT, which can substantially reduce TB incidence. Earlier and accurate HIV and TB screening and treatment may also help identify infectious cases earlier, thereby reducing both HIV and TB transmission. Evidence-based and internationally recommended guidelines should be used to expedite the diagnosis and treatment of TB in people living with HIV [42].
Supporting Information
Zdroje
1. GetahunH
GunnebergC
GranichR
NunnP
2010 HIV infection-associated tuberculosis: the epidemiology and the response. Clin Infect Dis 50 S201 207
2. SonnenbergP
GlynnJR
FieldingK
MurrayJ
Godfrey-FaussettP
2005 How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis 191 150 158
3. HavlirDV
GetahunH
SanneI
NunnP
2008 Opportunities and challenges for HIV care in overlapping HIV and TB epidemics. JAMA 300 423 430
4. MukadiYD
WiktorSZ
CoulibalyIM
CoulibalyD
MbengueA
1997 Impact of HIV infection on the development, clinical presentation, and outcome of tuberculosis among children in Abidjan, Cote d'Ivoire. Aids 11 1151 1158
5. ManosuthiW
ChottanapandS
ThongyenS
ChaovavanichA
SungkanuparphS
2006 Survival rate and risk factors of mortality among HIV/tuberculosis-coinfected patients with and without antiretroviral therapy. J Acquir Immune Defic Syndr 43 42 46
6. LawnSD
MyerL
OrrellC
BekkerLG
WoodR
2005 Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. Aids 19 2141 2148
7. GandhiNR
MollA
SturmAW
PawinskiR
GovenderT
2006 Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 368 1575 1580
8. WellsCD
CegielskiJP
NelsonLJ
LasersonKF
HoltzTH
2007 HIV infection and multidrug-resistant tuberculosis: the perfect storm. J Infect Dis 196 Suppl 1 S86 107
9. BadriM
WilsonD
WoodR
2002 Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet 359 2059 2064
10. MirandaA
MorganM
JamalL
LasersonK
BarreiraD
2007 Impact of antiretroviral therapy on the incidence of tuberculosis: the Brazilian experience, 1995-2001. PLoS ONE 2 e826 doi:10.1371/journal.pone.0000826
11. LawnSD
MyerL
BekkerLG
WoodR
2006 Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. Aids 20 1605 1612
12. BurgessAL
FitzgeraldDW
SevereP
JosephP
NoelE
2001 Integration of tuberculosis screening at an HIV voluntary counselling and testing centre in Haiti. Aids 15 1875 1879
13. AkoloC
AdetifaI
ShepperdS
VolminkJ
2010 Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev CD000171
14. WHO 2004 Interim policy on collaborative TB/HIV activities, World Health Organization. Geneva: WHO. WHO/HTM/TB/2004.330 WHO/HTM/HIV/2004.1. 2004
15. WHO 2010 Global tuberculosis control report: World Health Organization. Geneva: WHO. WHO/HTM/TB/ 2010.7
16. ReidMJ
ShahNS
2009 Approaches to tuberculosis screening and diagnosis in people with HIV in resource-limited settings. Lancet Infect Dis 9 173 184
17. GetahunH
HarringtonM
O'BrienR
NunnP
2007 Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet 369 2042 2049
18. AylesH
SchaapA
NotaA
SismanidisC
TembweR
2009 Prevalence of tuberculosis, HIV and respiratory symptoms in two Zambian communities: implications for tuberculosis control in the era of HIV. PLoS One 4 e5602 doi:10.1371/journal.pone.0005602
19. CorbettEL
ZezaiA
CheungYB
BandasonT
DauyaE
2010 Provider-initiated symptom screening for tuberculosis in Zimbabwe: diagnostic value and the effect of HIV status. Bull World Health Organ 88 13 21
20. CainKP
McCarthyKD
HeiligCM
MonkongdeeP
TasaneeyapanT
2010 An algorithm for tuberculosis screening and diagnosis in people with HIV. N Engl J Med 362 707 716
21. DayJH
CharalambousS
FieldingKL
HayesRJ
ChurchyardGJ
2006 Screening for tuberculosis prior to isoniazid preventive therapy among HIV-infected gold miners in South Africa. Int J Tuberc Lung Dis 10 523 529
22. CorbettEL
BandasonT
CheungYB
MunyatiS
Godfrey-FaussettP
2007 Epidemiology of tuberculosis in a high HIV prevalence population provided with enhanced diagnosis of symptomatic disease. PLoS Med 4 e22 doi:10.1371/journal.pmed.0040022
23. LewisJJ
CharalambousS
DayJH
FieldingKL
GrantAD
2009 HIV infection does not affect active case finding of tuberculosis in South African gold miners. Am J Respir Crit Care Med 180 1271 1278
24. ShahS
DemissieM
LambertL
AhmedJ
LeulsegedS
2009 Intensified tuberculosis case finding among HIV-Infected persons from a voluntary counseling and testing center in Addis Ababa, Ethiopia. J Acquir Immune Defic Syndr 50 537 545
25. KimerlingME
SchuchterJ
ChantholE
KunthyT
StuerF
2002 Prevalence of pulmonary tuberculosis among HIV-infected persons in a home care program in Phnom Penh, Cambodia. Int J Tuberc Lung Dis 6 988 994
26. LawnS
EdwardsD
KranzerK
VogtM
BekkerL
2009 Urine lipoarabinomannan assay for tuberculosis screening prior to antiretroviral therapy: diagnostic yield and association with immune reconstitution disease. AIDS 23 1875 1880
27. WoodR
MiddelkoopK
MyerL
GrantAD
WhitelawA
2007 Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med 175 87 93
28. MohammedA
EhrlichR
WoodR
CilliersF
MaartensG
2004 Screening for tuberculosis in adults with advanced HIV infection prior to preventive therapy. Int J Tuberc Lung Dis 8 792 795
29. ChhengP
TamhaneA
NatpratanC
TanV
LayV
2008 Pulmonary tuberculosis among patients visiting a voluntary confidential counseling and testing center, Cambodia. Int J Tuberc Lung Dis 12 54 62
30. BassettIV
WangB
ChettyS
GiddyJ
LosinaE
2010 Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa. Clin Infect Dis 51 823 829
31. CrucianiM
ScarparoC
MalenaM
BoscoO
SerpelloniG
2004 Meta-analysis of BACTEC MGIT 960 and BACTEC 460 TB, with or without solid media, for detection of mycobacteria. J Clin Microbiol 42 2321 2325
32. HarbordRM
DeeksJJ
EggerM
WhitingP
SterneJA
2007 A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 8 239 251
33. ReitsmaJB
GlasAS
RutjesAW
ScholtenRJ
BossuytPM
2005 Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 58 982 990
34. GrimesDA
SchulzKF
2005 Refining clinical diagnosis with likelihood ratios. Lancet 365 1500 1505
35. ReillyB
EvansA
2006 Translating clinical research into clinical practice: impact of using prediction rules to make decisions. An Intern Med 144 210 212
36. WHO 2004 Compendium of indicators for monitoring and evaluating national tuberculosis programs. WHO/HTM/TB/2004.344. Geneva: WHO
37. MosimaneotsileB
TalbotEA
MoetiTL
HoneNM
MoalosiG
2003 Value of chest radiography in a tuberculosis prevention programme for HIV-infected people, Botswana. Lancet 362 1551 1552
38. SamandariTAT
ArwadyA
YoonJ
NyirendaS
2007 Asymptomatic pulmonary TB among HIV-infected adults screened for the Botswana isoniazid preventive therapy clinical trial, 2004-2006 [Abstract 862]. In: Proceedings of the 14th Conference on Retroviruses and Opportunistic Infections; 25-28 February 2007; Los Angeles, California, United States
39. KranzerK
HoubenRM
GlynnJR
BekkerLG
WoodR
2010 Yield of HIV-associated tuberculosis during intensified case finding in resource-limited settings: a systematic review and meta-analysis. Lancet Infect Dis 10 93 102
40. LawnSD
WoodR
De CockKM
KranzerK
LewisJJ
2010 Antiretrovirals and isoniazid preventive therapy in the prevention of HIV-associated tuberculosis in settings with limited health-care resources. Lancet Infect Dis 10 489 498
41. BanerjiD
AndersenS
1963 A sociological study of awareness of symptoms among persons with pulmonary tuberculosis. Bull World Health Organ 29 665 683
42. WHO 2007 Improving the diagnosis and treatment of smear-negative pulmonary and extrapulmonary tuberculosis among adults and adolescents: recommendations for HIV-prevalent and resource-constrained settings. WHO/HTM/TB/2007.376 WHO/HIV/2007.01. Geneva: WHO
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2011 Číslo 1- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- A Simple Novel Method for Determining Mortality Rates in HIV Treatment Programs Worldwide
- Setting Implementation Research Priorities to Reduce Preterm Births and Stillbirths at the Community Level
- A Research Agenda for Malaria Eradication: Monitoring, Evaluation, and Surveillance
- A Research Agenda for Malaria Eradication: Cross-Cutting Issues for Eradication
- A Research Agenda to Underpin Malaria Eradication
- Correcting Mortality for Loss to Follow-Up: A Nomogram Applied to Antiretroviral Treatment Programmes in Sub-Saharan Africa
- The Impact of eHealth on the Quality and Safety of Health Care: A Systematic Overview
- Setting Research Priorities to Reduce Almost One Million Deaths from Birth Asphyxia by 2015
- Predicting Live Birth, Preterm Delivery, and Low Birth Weight in Infants Born from In Vitro Fertilisation: A Prospective Study of 144,018 Treatment Cycles
- Some Lessons for the Future from the Global Malaria Eradication Programme (1955–1969)
- A Research Agenda for Malaria Eradication: Basic Science and Enabling Technologies
- A Research Agenda for Malaria Eradication: Vector Control
- The Role of Research in Viral Disease Eradication and Elimination Programs: Lessons for Malaria Eradication
- The Influence of Distance and Level of Care on Delivery Place in Rural Zambia: A Study of Linked National Data in a Geographic Information System
- Using the Delphi Technique to Determine Which Outcomes to Measure in Clinical Trials: Recommendations for the Future Based on a Systematic Review of Existing Studies
- Development of a Standardized Screening Rule for Tuberculosis in People Living with HIV in Resource-Constrained Settings: Individual Participant Data Meta-analysis of Observational Studies
- WHO/PLoS Collection “No Health Without Research”: A Call for Papers
- Estimates of Pandemic Influenza Vaccine Effectiveness in Europe, 2009–2010: Results of Influenza Monitoring Vaccine Effectiveness in Europe (I-MOVE) Multicentre Case-Control Study
- A Research Agenda for Malaria Eradication: Vaccines
- A Research Agenda for Malaria Eradication: Health Systems and Operational Research
- A Research Agenda for Malaria Eradication: Diagnoses and Diagnostics
- A Research Agenda for Malaria Eradication: Drugs
- A Research Agenda for Malaria Eradication: Modeling
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- The Impact of eHealth on the Quality and Safety of Health Care: A Systematic Overview
- A Research Agenda for Malaria Eradication: Cross-Cutting Issues for Eradication
- Estimates of Pandemic Influenza Vaccine Effectiveness in Europe, 2009–2010: Results of Influenza Monitoring Vaccine Effectiveness in Europe (I-MOVE) Multicentre Case-Control Study
- Using the Delphi Technique to Determine Which Outcomes to Measure in Clinical Trials: Recommendations for the Future Based on a Systematic Review of Existing Studies
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



