-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Uterine Rupture by Intended Mode of Delivery in the UK: A National Case-Control Study
Background:
Recent reports of the risk of morbidity due to uterine rupture are thought to have contributed in some countries to a decrease in the number of women attempting a vaginal birth after caesarean section. The aims of this study were to estimate the incidence of true uterine rupture in the UK and to investigate and quantify the associated risk factors and outcomes, on the basis of intended mode of delivery.Methods and Findings:
A UK national case-control study was undertaken between April 2009 and April 2010. The participants comprised 159 women with uterine rupture and 448 control women with a previous caesarean delivery. The estimated incidence of uterine rupture was 0.2 per 1,000 maternities overall; 2.1 and 0.3 per 1,000 maternities in women with a previous caesarean delivery planning vaginal or elective caesarean delivery, respectively. Amongst women with a previous caesarean delivery, odds of rupture were also increased in women who had ≥ two previous caesarean deliveries (adjusted odds ratio [aOR] 3.02, 95% CI 1.16–7.85) and <12 months since their last caesarean delivery (aOR 3.12, 95% CI 1.62–6.02). A higher risk of rupture with labour induction and oxytocin use was apparent (aOR 3.92, 95% CI 1.00–15.33). Two women with uterine rupture died (case fatality 1.3%, 95% CI 0.2–4.5%). There were 18 perinatal deaths associated with uterine rupture among 145 infants (perinatal mortality 124 per 1,000 total births, 95% CI 75–189).Conclusions:
Although uterine rupture is associated with significant mortality and morbidity, even amongst women with a previous caesarean section planning a vaginal delivery, it is a rare occurrence. For women with a previous caesarean section, risk of uterine rupture increases with number of previous caesarean deliveries, a short interval since the last caesarean section, and labour induction and/or augmentation. These factors should be considered when counselling and managing the labour of women with a previous caesarean section.
: Please see later in the article for the Editors' Summary
Published in the journal: Uterine Rupture by Intended Mode of Delivery in the UK: A National Case-Control Study. PLoS Med 9(3): e32767. doi:10.1371/journal.pmed.1001184
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001184Summary
Background:
Recent reports of the risk of morbidity due to uterine rupture are thought to have contributed in some countries to a decrease in the number of women attempting a vaginal birth after caesarean section. The aims of this study were to estimate the incidence of true uterine rupture in the UK and to investigate and quantify the associated risk factors and outcomes, on the basis of intended mode of delivery.Methods and Findings:
A UK national case-control study was undertaken between April 2009 and April 2010. The participants comprised 159 women with uterine rupture and 448 control women with a previous caesarean delivery. The estimated incidence of uterine rupture was 0.2 per 1,000 maternities overall; 2.1 and 0.3 per 1,000 maternities in women with a previous caesarean delivery planning vaginal or elective caesarean delivery, respectively. Amongst women with a previous caesarean delivery, odds of rupture were also increased in women who had ≥ two previous caesarean deliveries (adjusted odds ratio [aOR] 3.02, 95% CI 1.16–7.85) and <12 months since their last caesarean delivery (aOR 3.12, 95% CI 1.62–6.02). A higher risk of rupture with labour induction and oxytocin use was apparent (aOR 3.92, 95% CI 1.00–15.33). Two women with uterine rupture died (case fatality 1.3%, 95% CI 0.2–4.5%). There were 18 perinatal deaths associated with uterine rupture among 145 infants (perinatal mortality 124 per 1,000 total births, 95% CI 75–189).Conclusions:
Although uterine rupture is associated with significant mortality and morbidity, even amongst women with a previous caesarean section planning a vaginal delivery, it is a rare occurrence. For women with a previous caesarean section, risk of uterine rupture increases with number of previous caesarean deliveries, a short interval since the last caesarean section, and labour induction and/or augmentation. These factors should be considered when counselling and managing the labour of women with a previous caesarean section.
: Please see later in the article for the Editors' SummaryIntroduction
Uterine rupture is a complication of pregnancy associated with severe maternal and fetal morbidity and mortality. In high income countries it most commonly occurs in women who have previously delivered by caesarean section [1]. This observation has led to debate about the optimal management of labour and delivery in women who have delivered by caesarean section in previous pregnancies. Women with a previous caesarean delivery have generally been encouraged to attempt a trial of labour in subsequent pregnancies [2], but recent reports of an increased risk of morbidity, particularly due to uterine rupture, are thought to have contributed to a marked decrease in some countries in the number of women attempting vaginal birth after caesarean section [3]. Indeed, the rate of caesarean section delivery in the UK is increasing, with previous caesarean section being the most common primary obstetric indication for repeat section [4].
Three systematic reviews have identified a number of deficiencies with the existing studies of uterine rupture in high income countries, hampering the accurate assessment of the incidence and risk factors for this complication [1],[5],[6]. One of the reviews suggested that a multicentre prospective cohort study or national registry would offer the best opportunity to inform preventive strategies [5]. The aim of this study, therefore, was to carry out a national population-based case-control study using the UK Obstetric Surveillance System (UKOSS) to estimate the incidence of uterine rupture in the UK and to investigate and quantify the associated risk factors and outcomes.
Methods
Ethics Statement
This study was approved by the London Research Ethics Committee (ref 09/H0718/8).
Study Power
A national population-based case-control study was undertaken. Over the 13-mo study period, we anticipated identifying 200 cases (on the basis of an estimated incidence of 1 in 4,000 maternities [7]) and 600 controls. A ratio of three controls per case was planned in the study proposal to maximise the power of the study, given that uterine rupture is a rare condition and the number of cases would be limited by disease incidence. Assuming 10% of women with a previous caesarean section delivering in the UK are induced with prostaglandin and/or receive oxytocin in their labour, and with a 3 to 1 ratio of controls to cases, 106 cases and 316 controls would give an estimated power of 80% at the 5% level of statistical significance to detect a 2.5-fold increase in the odds of uterine rupture in women with a previous caesarean section who have prostaglandin labour induction and/or oxytocin used in labour.
Case Definition
Cases were all women in the UK identified as having a uterine rupture defined as a complete separation of the wall of the pregnant uterus, with or without expulsion of the fetus, involving rupture of membranes at the site of the uterine rupture or extension of the complete separation of the wall of the uterus into uterine muscle separate from any previous scar, and endangering the life of the mother or fetus. Any asymptomatic palpable or visualised defect, noted incidentally at caesarean delivery, for example, was excluded.
Control Definition
Controls were defined as any woman delivering a fetus or infant who had not suffered from a uterine rupture and who had delivered by caesarean section in any previous pregnancy regardless of the mode of delivery of the current pregnancy.
Data Collection
Cases were identified through the monthly mailing of the UKOSS [8] between 1st April 2009 and 30th April 2010. Nominated clinicians in each obstetrician-led maternity unit in the UK were sent a card each month and asked to report all cases of uterine rupture, thus covering the entire cohort of UK births in the study period. Clinicians who reported a case were then asked to complete a data collection form for the case, detailing demographic and other potential risk factors, management, and outcomes. Previous studies using this methodology have suggested good case ascertainment [9],[10]. Controls were obtained from a random sample of obstetrician-led maternity units in the UK in month 4 and month 12 of the study, weighted by the total number of births. The time and day on which reporting clinicians were asked to select controls were randomly identified using data on birth date and time from one region of the UK (Leicestershire), to try and provide a representative sample of women delivering during each 24-h period and on different days of the week. Clinicians were asked to complete a data collection form for the controls that was identical to those used for cases with the exception of the details of the uterine rupture. Up to five reminders were sent if completed forms were not returned. All data requested were anonymous. On receipt of data collection forms, cases were checked to confirm that they met the case definition. Duplicate reports were identified by comparing the woman's year of birth and expected date of delivery.
Statistical Analysis
The overall incidence with 95% CIs of uterine rupture was calculated using the most recently available national birth data (2009 and 2010 for England and Wales [11] and 2009 for Scotland [12] and Northern Ireland [13]) as a proxy denominator for the number of maternities during the study period. To calculate the incidence with 95% CIs of uterine rupture in women with and without a previous caesarean section, the most recently available birth data were used together with an estimate of the proportion of women in the UK who had previously delivered by caesarean section (15%), derived from the rate in a group of population-based controls comprised of women giving birth in the UK in 2005–2006 [14]. This group of population-based controls were identified as the two women delivering immediately before a woman who had a peripartum hysterectomy in the UK during the period from February 2005 to February 2006, and are comparable in characteristic to the available national data on women giving birth in the UK. Information on the proportion of women with a previous caesarean delivery planning a vaginal or caesarean section delivery in their current pregnancy, estimated from that observed in the control women, was used to estimate the denominator for calculation of the incidence and 95% CI of uterine rupture according to planned mode of delivery in women with a previous caesarean section. Denominator data to allow calculation of the incidence and 95% CI of uterine rupture in women with a prior caesarean delivery planning a vaginal delivery according to whether labour was induced with or without prostaglandin and/or oxytocin were also estimated using the proportions observed in the control women.
Potential risk factors for uterine rupture after prior delivery by caesarean section were evaluated by comparing the women with a previous caesarean delivery who had a uterine rupture to the control group of women. Odds ratios (ORs) with 95% CIs were estimated using unconditional logistic regression. A full regression model was developed by including both explanatory and potential confounding factors in a core model if there was a preexisting hypothesis or evidence to suggest they were causally related to uterine rupture, for example, number of previous caesarean section deliveries. Factors with a high proportion of missing data (>20%) were omitted from the full model where there was no evidence (p>0.20) in the unvariate analysis that they were associated with uterine rupture. Continuous variables were tested for departure from linearity by the addition of first-order fractional polynomials to the model and subsequent likelihood ratio testing. Where there was evidence for nonlinearity, continuous variables were presented and treated as categorical in the analysis. Where there was no evidence of departure from linearity, continuous variables are presented as categorical for ease of interpretation, but have been treated as continuous linear terms when adjusting for them in the analysis. Plausible interactions were tested in the full regression model by the addition of interaction terms and subsequent likelihood ratio testing on removal, with a p-value of 0.01 considered evidence of significant interaction to account for multiple testing. Unconditional logistic regression was also used to compare the sociodemographic, parity, previous uterine surgery, and infant birthweight characteristics of women who had a uterine rupture in the absence of a previous caesarean delivery to the group of population-based controls comprised of women giving birth in the UK in 2005–2006 [14]. The Wilcoxon rank-sum test was used to compare medians and Fisher's exact test was used to compare proportions where appropriate. All analyses were carried out using STATA v11 software.
Results
All 223 UK hospitals with obstetrician-led maternity units contributed data to UKOSS during the study period, representing 100% participation. Of the 216 notified cases of uterine rupture, data collection was complete for 90% (Figure 1). There were 159 confirmed cases of uterine rupture in an estimated 852,206 maternities [11]–[13], representing an estimated incidence of 1.9 per 10,000 maternities (95% CI 1.6–2.2). Table 1 shows the estimated incidence of uterine rupture in different categories of women. Data collection forms were received for 448 controls (75% of those requested).
Fig. 1. Case reporting and completeness of data collection. 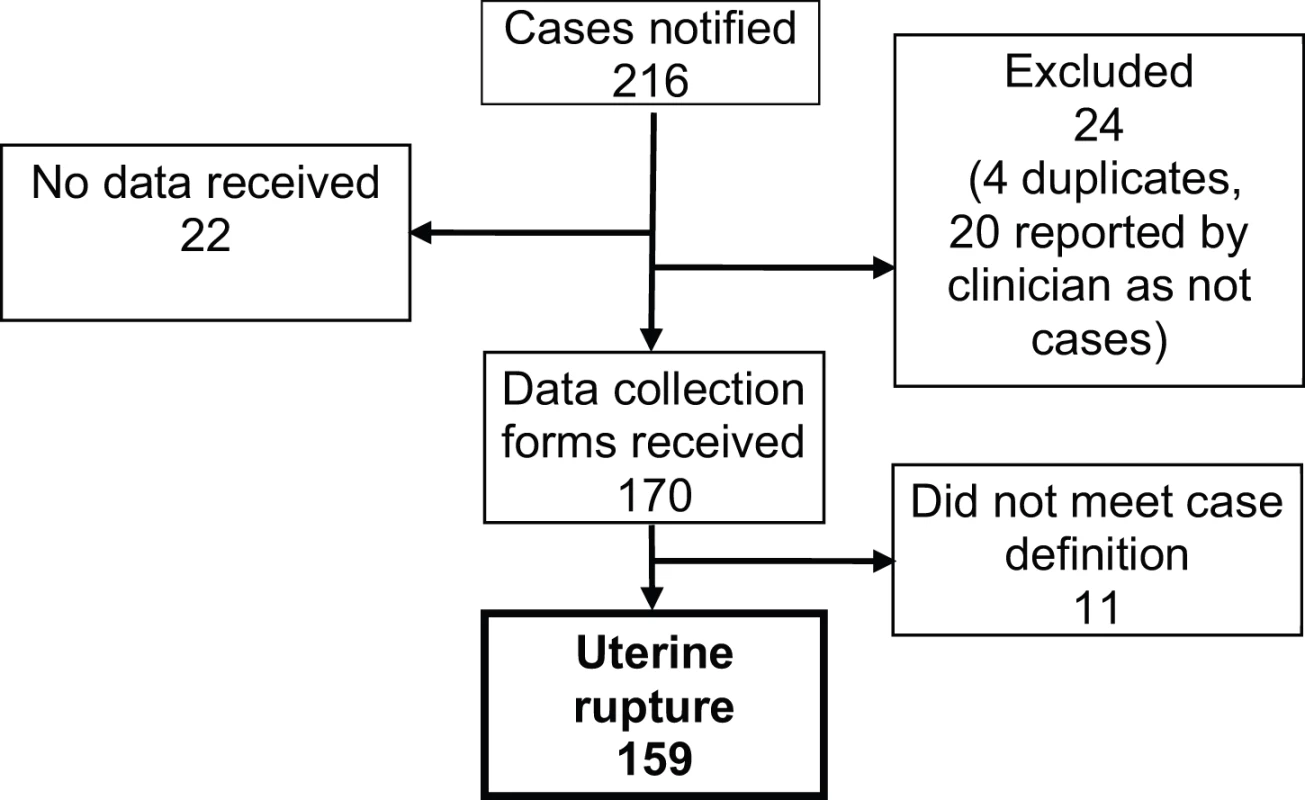
Tab. 1. Estimated incidence of uterine rupture in different categories of women. 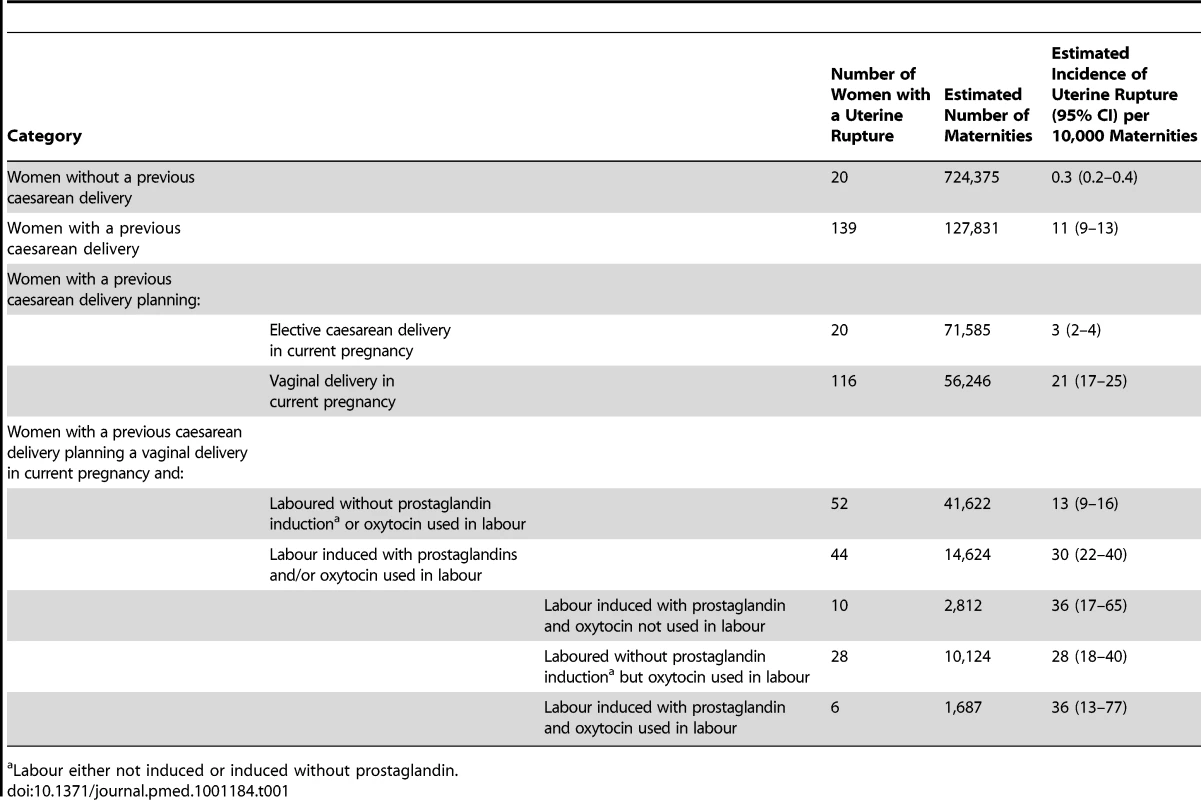
Labour either not induced or induced without prostaglandin. Presentation of Uterine Rupture
The median gestational age at diagnosis of uterine rupture was 39 wk (range 8–42 wk) (Figure 2). All seven of the women who had their rupture diagnosed before 24 wk gestation had a previous delivery by caesarean section and five occurred in association with medical termination of pregnancy. Of the 152 ruptures diagnosed at ≥24 wk gestation, the majority (120/152, 79%) occurred in women who laboured, the median time between diagnosis of labour and diagnosis of rupture being 6.6 h. 21 ruptures diagnosed at or after 24 wk gestation occurred in women with a previous caesarean section who did not labour or have an attempt made at inducing their labour (Figure 2). Compared to the control women, these women were more likely to have placenta praevia (14% versus 1% in control women, unadjusted OR [uOR] 24.72, 95% CI 4.66–131.10). No other significant differences were found, but note the limited power of this analysis due to small numbers.
Fig. 2. Uterine rupture cases by gestational age at rupture, labour, and previous caesarean section status. 
Table 2 shows the clinical symptoms or signs noted prior to the diagnosis of rupture. Fetal heart rate abnormality was the commonest symptom noted, affecting the fetuses of 118 of the women (76%). Bradycardia was the most frequent abnormality, occurring in the fetuses of 40 women. 97 women (62%) presented with a combination of symptoms, the most frequent being abdominal pain and fetal heart rate abnormality, occurring in 28 women.
Tab. 2. Symptoms and signs noted prior to diagnosis of uterine rupture. 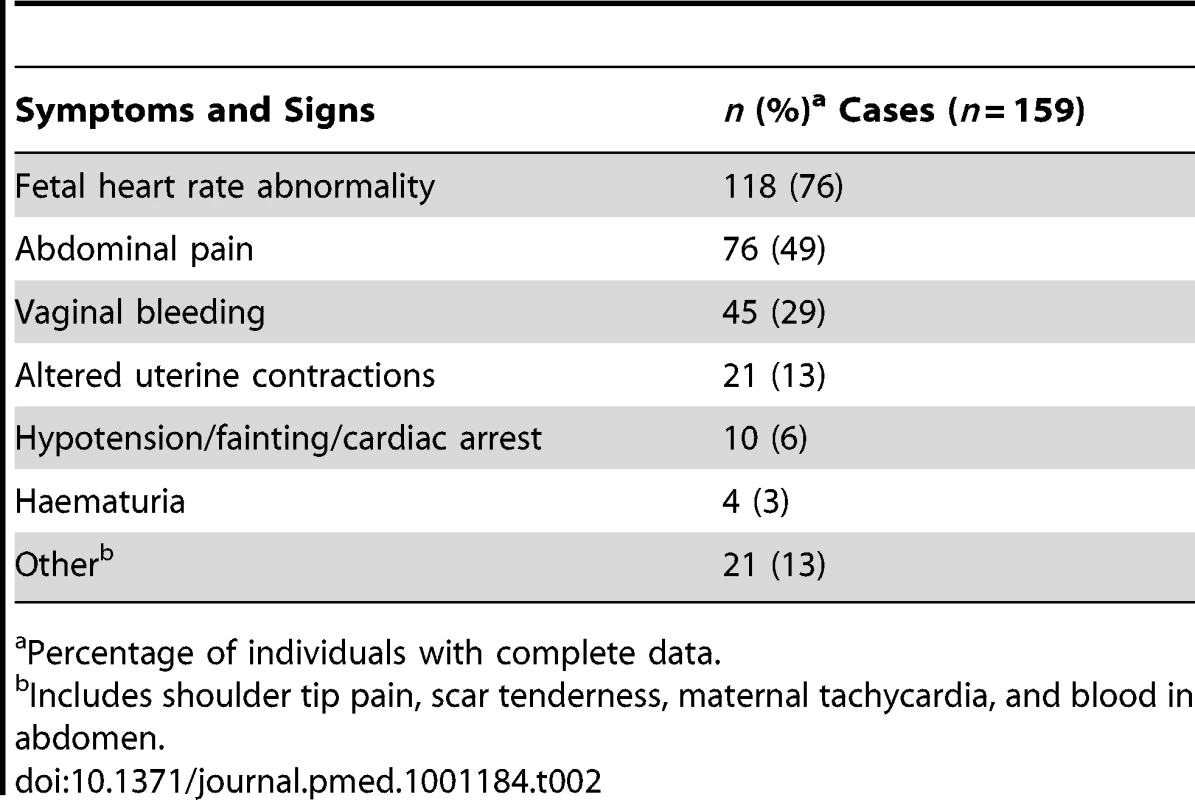
Percentage of individuals with complete data. The 21 women with a previous caesarean delivery who had their rupture diagnosed at or after 24 wk gestation in the absence of labour or attempt at inducing labour, presented as follows: 15 presented with abdominal pain and/or vaginal bleeding (eight before and seven at or after 37 wk), with a fetal heart rate abnormality noted in eight of these; three had rupture before 37 wk in association with placenta praevia; one presented at 37 wk with maternal collapse; and two had fetal heart rate abnormalities noted following membrane rupture (one before and one at 37 wk).
Risk Factors for Uterine Rupture after Prior Delivery by Caesarean Section
A total of 139 (87%) of the uterine ruptures occurred in women who had previously delivered by caesarean section. Table 3 shows the characteristics of these women compared to control women. Women who had two or more previous caesarean deliveries had a raised odds of having a uterine rupture compared to women with only one previous caesarean delivery (adjusted OR [aOR] 3.02, 95% CI 1.16–7.85), as did women who had an interval of less than 12 mo compared to ≥24 mo between their last caesarean section and their last menstrual period in their current pregnancy (aOR 3.12, 95% CI 1.62–6.02). There was no evidence to suggest a departure from linearity in the relationship between odds of rupture and number of caesarean deliveries, with the odds of rupture increasing by 3.02 (95% CI 1.62–5.63) for every one additional caesarean delivery. However, there was evidence of a departure in linearity in the association between uterine rupture and caesarean section-pregnancy interval, with the odds of rupture appearing to plateau for intervals beyond 12 mo (Figure 3).
Fig. 3. Risk of uterine rupture according to the interval between the last caesarean section and start of current pregnancy. 
Adjusted for woman's age as a continuous linear term, ethnicity, body mass index as a continuous linear term, parity as a continuous linear term, number of previous caesarean deliveries as a continuous linear term, previous uterine surgery, placenta praevia, macrosomia, and planned mode of delivery. Tab. 3. Risk factors for uterine rupture in women with prior delivery by caesarean section. 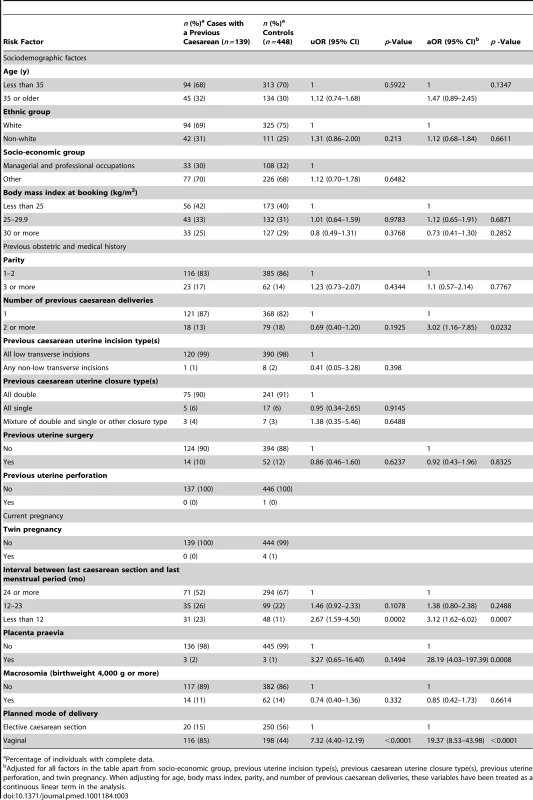
Percentage of individuals with complete data. The presence of placenta praevia also increased the odds of rupture (aOR 28.19, 95% CI 4.03–197.39), although note that this finding is based on a very small number of women and should be interpreted with caution. The odds of rupture was also raised in women who planned to have a vaginal delivery in their current pregnancy compared to women who planned to deliver by elective caesarean section (aOR 19.37, 95% CI 8.53–43.98). This finding was irrespective of whether the women who planned to have a vaginal delivery had their labour induced and/or received oxytocin in labour (Table 4). However, the women who had prostaglandin labour induction and/or oxytocin used in labour appeared to have raised odds of rupture compared to the women who laboured without prostaglandin induction or oxytocin in labour (Table 5). No significant interactions were found.
Tab. 4. Risk factors for uterine rupture in women with prior delivery by caesarean section. 
Percentage of individuals with complete data. Tab. 5. Risk factors for uterine rupture in women with prior delivery by caesarean section who planned to have a vaginal delivery in current pregnancy. 
Percentage of individuals with complete data. Of the 198 controls (44%) who planned to have a vaginal delivery in their current pregnancy, 40% delivered by caesarean section. The proportion ultimately delivering by caesarean section was similar between those who did and did not have their labour induced and/or received oxytocin in labour (33% [44/132] of control women who laboured without prostaglandin induction or oxytocin in labour; 44% [4/9] of control women who had their labour induced with prostaglandin but did not have oxytocin used in labour; 33% [11/33] of control women who laboured without prostaglandin induction but had oxytocin used in labour; and 20% [1/5] of control women who had their labour induced with prostaglandin and had oxytocin used in labour, p = 0.853).
Women with uterine rupture were similar to control women in terms of their Bishop score prior to induction, a measure of the readiness of the cervix for induction (median of 3.5, range 0–8 in women with rupture versus median 4, range 1–7 in control women, p = 0.5879). The proportion of women delivering/rupturing before 37 wk amongst those who had prostaglandin induction and/or oxytocin in labour was slightly higher, although not statistically significantly so, in the women with uterine rupture compared to the control women (14% of the women with rupture who had prostaglandin induction and/or oxytocin, 9% of the control women who had prostaglandin induction and/or oxytocin, p = 0.438). Women with uterine rupture were also similar to control women in terms of the duration of oxytocin use (median 4.8 h, range 0.8–19 in women with rupture versus median 4.8 h, range 0.8–22.5 in control women, p = 0.7908). While dinoprostone (prostin, propess, prostaglandin E) was the agent used for all the control women induced with prostaglandin, dinoprostone was used in 82% and misoprostol in 18% of the women induced with prostaglandin who had a uterine rupture. Intrauterine death was the indication for induction for all of the women who received misoprostol.
Characteristics of Uterine Rupture Cases without a Previous Caesarean Delivery
The characteristics of the 20 women who experienced a uterine rupture in the absence of a previous caesarean delivery are shown in Table 6. Compared to a group of population-based control women [14], these women were more likely to be aged 35 y or older (40% versus 20% in control women, uOR 2.74, 95% CI 1.10–6.85) and were more likely to have a parity of three or more (30% versus 9% in control women, uOR 7.62, 95% CI 2.08–27.93). There was also a suggestion that the women who did not have a previous delivery by caesarean section and experienced a uterine rupture were more likely to have an infant with a birthweight of 4,000 g or more (26% versus 12%, uOR 2.69, 95% CI 0.94–7.70), although this was not statistically significant.
Tab. 6. Characteristics of women who had a uterine rupture in the absence of a prior delivery by caesarean section. 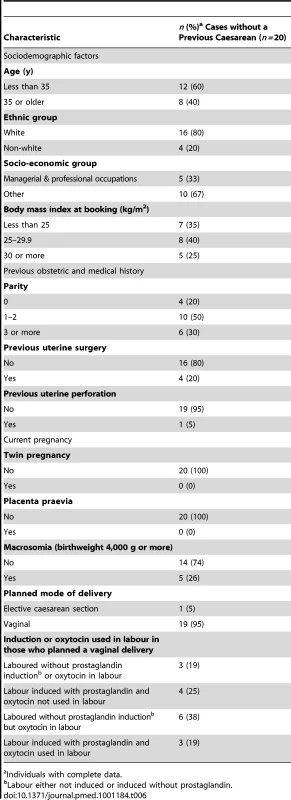
Individuals with complete data. Outcomes following Uterine Rupture
Two of the 159 women with a uterine rupture died, a case fatality of 1.3%, 95% CI 0.2%–4.5%. 15 (9%) women had a hysterectomy following uterine rupture, ten (6%) women had one or more other organs damaged at rupture or removed during surgery, and 69 (43%) women had other or additional morbidity following their uterine rupture. This group included four women who required ventilation and 62 who received a blood transfusion. 50 (31%) of the woman with a uterine rupture were admitted to critical or high dependency care for a median duration of 2 d (range 1–12).
Outcomes were known for 152 of the infants born to women with a uterine rupture. There were 15 stillbirths (12 antepartum, seven of which occurred prior to uterine rupture in women who were induced following intra-uterine death, and three intrapartum) and ten early neonatal deaths. Excluding the stillbirths that occurred prior to uterine rupture, the perinatal mortality rate was 124 per 1,000 (95% CI 75–189), significantly higher than the national rate of 7.5 per 1,000 (risk ratio [RR] 16.46, 95% CI 10.68–25.39) [15]. Major complications were reported in an additional 19 infants, including nine infants diagnosed with neonatal encephalopathy and six diagnosed with respiratory distress syndrome. A total of 56/137 (41%) of the infants were admitted to a neonatal unit for a median duration of 3 d (range 1–48).
Discussion
The incidence of complete uterine rupture in the UK as estimated by this study is 1.9 per 10,000 maternities. To our knowledge, our national prospective study using a robust case definition gives one of the first reliable estimates worldwide of the incidence of clinically significant uterine rupture to guide clinical practice, policy, and guidelines. The incidence estimate demonstrates the rarity of clinically significant rupture, and is lower than frequently quoted rupture rates [6].
Consistent with previous caesarean section being the main risk factor in high income countries for uterine rupture [1], our study estimated the incidence to be 11 and 0.3 per 10,000 maternities in women with and without a previous caesarean delivery, respectively.
Amongst women with a previous caesarean section, our study found the risk of uterine rupture was independently increased with increasing number of previous caesarean deliveries; in women with less than a 12-mo interval between their last caesarean section and the start of their current pregnancy; in women with placenta praevia; and in women who planned to have a vaginal delivery compared to those who planned to deliver by elective caesarean section. A higher risk of uterine rupture with labour induction and/or oxytocin use was also apparent. The inclusion of planned mode of delivery in our analysis is the equivalent of intention to treat in randomised controlled trials and fills an important gap in information required to counsel women with a previous caesarean delivery concerning mode of delivery in their next pregnancy [6]. We estimate the incidence of uterine rupture to be 21 per 10,000 maternities in women with a previous caesarean section who planned to have a vaginal delivery in their current pregnancy compared to 3 per 10,000 maternities in women with a previous caesarean section who planned to deliver by elective caesarean section.
Comparison with Other Studies
A World Health Organization (WHO) systematic review of uterine rupture worldwide, published in 2005, reported an overall median incidence of uterine rupture of 5.3 per 10,000 deliveries based on eight population-based studies identified [1]. Considering only the five population-based studies conducted in a high income country, the incidence was approximately three per 10,000, similar to the overall incidence estimated from our study. One of these five population-based studies was conducted in the UK, and this reported an incidence of two ruptures per 10,000 deliveries (95% CI 1–4, 12 cases) [7], compatible with our overall incidence. However, a more recent larger prospective population-based study in The Netherlands that used very similar methods to our study, reported a higher overall incidence of uterine rupture of 5.9 per 10,000 deliveries (p<0.0001), based on 210 cases [16]. This difference may reflect differing rates and patterns of risk factors in the populations. For example, although The Netherlands has a lower caesarean section rate than the UK [17], it appears to have a higher rate of trial of labour after previous caesarean delivery [18]. It also has, for example, unlike the UK [19], a common practice of single rather than double-layer closure of the uterus at caesarean section [16], which has been reported as a risk factor for uterine rupture [20]. We cannot exclude the possibility that the difference is associated with differential case ascertainment. We had no additional sources of data to check our case ascertainment. However, previous studies using UKOSS have suggested high rates of ascertainment. For example, no additional cases were identified through several alternative data sources checked during UKOSS studies of peripartum hysterectomy [14] and acute fatty liver of pregnancy [21]. It is also possible that this observed difference is due to differential reporting of cases according to severity. We specifically excluded women in whom an incidental asymptomatic uterine dehiscence was noted at caesarean section; women with dehiscence were also excluded in the Dutch study, although this may be open to clinical interpretation.
The recent detailed systematic review from the Agency for Healthcare Research and Quality, US Department of Health and Human Services [6] highlights the importance of using an anatomical definition of uterine rupture that specifically excludes asymptomatic dehiscence and notes only four studies using such a definition. Only one of these studies, including a total of just over 6,000 deliveries and 11 uterine ruptures, was population-based. The studies in total included fewer than 48,000 deliveries, compared to the almost 128,000 with a prior caesarean delivery in our study cohort. The review also noted importantly that none of the four studies included information on induction of labour, a factor that could have the potential to considerably influence the incidence rates, as our study shows. This factor is particularly relevant, as the summary results of the review are driven by a single study [22], based in 19 tertiary centres, including 118 ruptures in 33,037 deliveries, which represents a much higher incidence rate than reported in the other included studies.
The WHO systematic review [1] identified only one study giving a rate of uterine rupture in women without a previous caesarean section; a study conducted in a high income country, where the incidence was 0.6 per 10,000 deliveries in women without a previous caesarean section [23], similar to the rate of 0.3 per 10,000 found in our study. For women with a previous caesarean delivery, the WHO review reported a rate of uterine rupture of around 100 per 10,000 based on the available studies in high income countries, much higher than our estimate of 11 per 10,000. This difference is perhaps a reflection of the fact that the WHO estimate was derived predominantly from facility-based studies; the denominator used to estimate the incidence in such studies is likely to be an underestimate of the true denominator of births due to the referral of high risk and emergency cases into the facility from surrounding areas, leading to an overestimate of the incidence. Although we estimated our denominator data, we are confident it is likely to be an accurate reflection of the true denominator: the most recently available national birth data (2009 and 2010 for England and Wales [11] and 2009 for Scotland [12] and Northern Ireland [13]) covering much of the same time period as our study were used to estimate the total number of maternities in the UK over the study period. Also, whilst the proportion of women in the UK with a previous caesarean delivery was estimated from that observed in a study in 2005–2006 [14], this proportion is unlikely to have altered markedly since, as the caesarean section rate in England has not changed substantially between this time and the study period (24.1% in 2005–2006 [24] compared to 24.8% in 2009–2010 [25]).
Other methodological differences may affect observed estimates of the incidence of uterine rupture. A recent population-based study in Australia conducted by retrospective database review of routinely coded data validated by hospital case records reported the incidence of uterine rupture in women with a previous caesarean section delivery as 13 per 10,000 (95% CI 9–18, 37 cases) [26], similar to that found in our study. However, another recent population-based retrospective study in Norway, which also used coded data for case-ascertainment but with limited validation, reported a higher incidence of uterine rupture in women with a previous caesarean section of 50 per 10,000 (94 cases) [27]. This same study reported incidences of 67 and 20 per 10,000 in women with a previous caesarean delivery undergoing a trial of labour or prelabour caesarean section, respectively, higher than that found in our study. An even higher rate of 90 per 10,000 (224 cases) in women attempting a vaginal delivery after a previous caesarean delivery was reported by a recent population-based Swedish study that used unvalidated coded data for case-ascertainment [28]. The use of coded data from routine hospital administrative systems to identify cases of uterine rupture without concurrent chart review can lead to inaccurate case ascertainment [29], which may explain these differences. We therefore suggest that any future studies of this topic should include case validation to ensure robust and comparable results.
Amongst women undergoing a trial of vaginal birth, a recent meta-analysis reported a lower risk of uterine rupture in women with one compared to two prior caesarean sections (pooled OR from five observational studies 0.42, 95% CI 0.29–0.60, p<0.0001) [30], compatible with our study findings. A number of studies have also shown a short interdelivery (<18 mo) [31],[32] or interpregnancy (<6 mo) [33],[34] interval is associated with an increased risk of uterine rupture amongst women undergoing a trial of vaginal birth after a previous caesarean delivery. One hypothesis to explain this association is that a short interval leads to incomplete fibrosis of the uterine scar from the previous caesarean delivery, thus increasing the risk of rupture. A study that evaluated incision healing after caesarean section using magnetic resonance imaging reported that at least 6 mo were needed for the zonal anatomy of the uterus to recover [35]. Our findings suggest that women with a previous caesarean section should be advised to wait at least 12 mo before conceiving again.
Agents used to prime the cervix and/or increase uterine contractions such as prostaglandins and oxytocin, can lead to hyperstimulation of the uterus [36],[37] which may weaken scars from previous caesarean sections, increasing the risk of rupture. The recent study by Dekker et al. in Australia [26] is one of the few to have stratified their data by labour induction with or without prostaglandin or oxytocin. Amongst women with one previous caesarean section, compared to those who were not induced and had no oxytocin augmentation, elective caesarean was reported to reduce the odds of rupture while the odds of rupture were increased three - to five-fold in women who had labour induction with or without prostaglandin or oxytocin, six-fold in women who had induction with prostaglandin combined with oxytocin, and 14-fold in women who had augmentation with oxytocin after spontaneous onset of labour. However, these estimates were associated with wide CIs owing to the small number of women with uterine rupture in the study (37 cases), which overlap most of our estimates. A systematic review of labour induction in patients with prior caesarean delivery published in 2005 reported that the use of oxytocin or prostaglandin were associated with a nonsignificant increase in uterine rupture compared to spontaneous labour on the basis of the small number of only fair-quality studies they identified [38]. More recently, the study by Zwart et al. in The Netherlands reported a relative risk of around two in women who had augmentation after spontaneous onset of labour or induction of labour with oxytocin alone or prostaglandin alone compared to spontaneous labour, although they were unable to adjust for any potential confounding factors [16].
Strengths and Weaknesses of Our Study
A major strength of our study is its population-based national design that reduces the risk of bias associated with facility-based studies. We also used a robust definition of uterine rupture. We were, however, only able to investigate factors that were adequately recorded in the hospital case notes, although this still allowed a comprehensive assessment of the potential risk factors and confounders for uterine rupture and had the advantage that this information was documented prospectively before uterine rupture or delivery, reducing the potential for information bias. We included the phrase “and endangering the life of mother or fetus” in the case definition and our guidance to clinicians, to aid exclusion of women with asymptomatic dehiscence. However, in our analysis we excluded from the cases only women with dehiscence and did not include or exclude any women on the basis of the subjective characteristic of endangered life. We did not exclude women with dehiscence from the control group as these women formed part of the population at risk.
We had no additional sources of data with which to check case ascertainment, so there remains a possibility that we may have under-ascertained cases, or ascertained only more severe cases, although previous studies using this methodology have suggested good case ascertainment. Although we only received data for 75% of the controls requested, we have no evidence of a systematic bias that may affect the validity of our results.
Conclusions and Policy Implications
Although uterine rupture is associated with significant maternal and perinatal mortality and morbidity, even amongst women with a previous caesarean section planning a vaginal delivery in their current pregnancy, it is rare, occurring in only one of every 500 women. For women with a previous caesarean section, the risk of uterine rupture increases not only with trial of labour but also with the number of previous caesarean deliveries, a short interval since the last caesarean section, and labour induction and/or augmentation. These factors should be considered when counselling and managing the labour of women with a previous caesarean section.
Zdroje
1. HofmeyrGJSayLlmezogluAM 2005 WHO systematic review of maternal mortality and morbidity: the prevalence of uterine rupture. BJOG 112 1221 1228
2. National Collaborating Centre for Women's and Children's Health 2004 Caesarean Section London RCOG
3. DoddJMCrowtherCAHuertasEGuiseJMHoreyD 2004 Planned elective repeat caesarean section versus planned vaginal birth for women with a previous caesarean birth. Cochrane Database Syst Rev CD004224
4. RCOG Clinical Effectiveness Support Unit 2001 The National Sentinel Caesarean Section Audit Report London RCOG
5. GuiseJ-MMcDonaghMSOsterweilPNygrenPChanBKS 2004 Systematic review of the incidence and consequences of uterine rupture in women with previous caesarean section. BMJ 329 19 25
6. GuiseJ-MEdenKEmeisCDenmanMMarshallN 2010 Vaginal birth after cesarean: new insights. evidence report/technology assessment no. 191. (Prepared by the Oregon Health & Science University Evidence-based Practice Center under Contract No. 290-2007-10057-I). AHRQ Publication No. 10-E003 Rockville (Maryland) Agency for Healthcare Research and Quality
7. WaterstoneMBewleySWolfeC 2001 Incidence and predictors of severe obstetric morbidity: case-control study. BMJ 322 1089 1093; discussion 1093–1084
8. KnightMKurinczukJJTuffnellDBrocklehurstP 2005 The UK Obstetric Surveillance System for rare disorders of pregnancy. BJOG 112 263 265
9. KnightM 2008 Antenatal pulmonary embolism: risk factors, management and outcomes. BJOG 115 453 461
10. KnightMKurinczukJJSparkPBrocklehurstP 2008 Cesarean delivery and peripartum hysterectomy. Obstet Gynecol 111 97 105
11. Office for National Statistics 2011 Birth summary tables, England and Wales 2010 Newport Office for National Statistics
12. General Register Office for Scotland 2010 Vital Events Reference Tables 2009 Edinburgh General Register Office for Scotland
13. Northern Ireland Statistics and Research Agency 2010 Registrar General Annual Report 2009 Belfast Northern Ireland Statistics and Research Agency
14. KnightMKurinczukJJSparkPBrocklehurstP 2008 Cesarean delivery and peripartum hysterectomy. Obstet Gynecol 111 97 105
15. Centre for Maternal and Child Enquiries 2010 Perinatal mortality 2008 London Centre for Maternal and Child Enquiries
16. ZwartJJRichtersJMOryFde VriesJIPBloemenkampKWM 2009 Uterine rupture in The Netherlands: a nationwide population-based cohort study. BJOG 116 1069 1078
17. EURO-PERISTAT Project 2008 European PERINATAL HEALTH REPort. Accessed November 2011. Available: www.europeristat.com
18. KweeABotsMLVisserGHABruinseHW 2007 Obstetric management and outcome of pregnancy in women with a history of caesarean section in the Netherlands. Eur J Obstet Gynecol Reprod Biol 132 171 176
19. TullyLGatesSBrocklehurstPMcKenzie-McHargKAyersS 2002 Surgical techniques used during caesarean section operations: results of a national survey of practice in the UK. Eur J Obstet Gynecol Reprod Biol 102 120 126
20. BujoldEGoyetMMarcouxSBrassardNCormierB 2010 The role of uterine closure in the risk of uterine rupture. Obstet Gynecol 116 43 50
21. KnightMNelson-PiercyCKurinczukJJSparkPBrocklehurstP 2008 A prospective national study of acute fatty liver of pregnancy in the UK. Gut 57 951 956
22. SpongCYLandonMBGilbertSRouseDJLevenoKJ 2007 Risk of uterine rupture and adverse perinatal outcome at term after cesarean delivery. Obstet Gynecol 110 801 807
23. MillerDAGoodwinTMGhermanRBPaulRH 1997 Intrapartum rupture of the unscarred uterus. Obstet Gynecol 89 671 673
24. The NHS Information Centre 2007 NHS maternity statistics, England - 2005-06 Leeds The NHS Information Centre
25. The NHS Information Centre 2010 NHS maternity statistics, England - 2009-10 Leeds The NHS Information Centre
26. DekkerGAChanALukeCGPriestKRileyM 2010 Risk of uterine rupture in Australian women attempting vaginal birth after one prior caesarean section: a retrospective population-based cohort study. BJOG 117 1358 1365
27. Al-ZirqiIStray-PedersenBForsénLVangenS 2010 Uterine rupture after previous caesarean section. BJOG 117 809 820
28. KaczmarczykMSparenPTerryPCnattingiusS 2007 Risk factors for uterine rupture and neonatal consequences of uterine rupture: a population-based study of successive pregnancies in Sweden. BJOG 114 1208 1214
29. PorrecoRPClarkSLBelfortMADildyGAMeyersJA 2009 The changing specter of uterine rupture. Am J Obstet Gynecol 200 269 e261–264
30. TahseenSGriffithsM 2010 Vaginal birth after two caesarean sections (VBAC-2)-a systematic review with meta-analysis of success rate and adverse outcomes of VBAC-2 versus VBAC-1 and repeat (third) caesarean sections. BJOG 117 5 19
31. BujoldEGauthierRJ 2010 Risk of uterine rupture associated with an interdelivery interval between 18 and 24 months. Obstet Gynecol 115 1003 1006
32. ShippTDZelopCMRepkeJTCohenALiebermanE 2001 Interdelivery interval and risk of symptomatic uterine rupture. Obstet Gynecol 97 175 177
33. StamilioDMDeFrancoEEmmanuellePOdiboAOPeipertJF 2007 Short interpregnancy interval: risk of uterine rupture and complications of vaginal birth after cesarean delivery. Obstet Gynecol 110 1075 1082
34. EspositoMAMenihanCAMaleeMP 2000 Association of interpregnancy interval with uterine scar failure in labor: a case-control study. Am J Obstet Gynecol 183 1180 1183
35. DicleOKucuklerCPirnarTErataYPosaciC 1997 Magnetic resonance imaging evaluation of incision healing after cesarean sections. Eur Radiol 7 31 34
36. EgarterCHHussleinPWRayburnWF 1990 Uterine hyperstimulation after low-dose prostaglandin E2 therapy: tocolytic treatment in 181 cases. Am J Obstet Gynecol 163 794 796
37. SmithJGMerrillDC 2006 Oxytocin for induction of labor. Clin Obstet Gynecol 49 594 608
38. McDonaghMSOsterweilPGuiseJM 2005 The benefits and risks of inducing labour in patients with prior caesarean delivery: a systematic review. BJOG 112 1007 1015
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2012 Číslo 3- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- No Treatment versus 24 or 60 Weeks of Antiretroviral Treatment during Primary HIV Infection: The Randomized Primo-SHM Trial
- Care Seeking for Neonatal Illness in Low- and Middle-Income Countries: A Systematic Review
- Guidance for Evidence-Informed Policies about Health Systems: Rationale for and Challenges of Guidance Development
- Improving Ethical Review of Research Involving Incentives for Health Promotion
- To VBAC or Not to VBAC
- Impact of Scotland's Smoke-Free Legislation on Pregnancy Complications: Retrospective Cohort Study
- Injectable and Oral Contraceptive Use and Cancers of the Breast, Cervix, Ovary, and Endometrium in Black South African Women: Case–Control Study
- Intermittent Preventive Treatment for Malaria in Papua New Guinean Infants Exposed to and : A Randomized Controlled Trial
- CD4 Cell Count and the Risk of AIDS or Death in HIV-Infected Adults on Combination Antiretroviral Therapy with a Suppressed Viral Load: A Longitudinal Cohort Study from COHERE
- Publication Bias in Antipsychotic Trials: An Analysis of Efficacy Comparing the Published Literature to the US Food and Drug Administration Database
- Better Guidance Is Welcome, but without Blinders
- New Research on Childbirth Has the Potential to Empower Women's Decision Making, but More Is Needed
- Planned Vaginal Birth or Elective Repeat Caesarean: Patient Preference Restricted Cohort with Nested Randomised Trial
- A Comparison of -IV and -5 Panel Members' Financial Associations with Industry: A Pernicious Problem Persists
- Guidance for Evidence-Informed Policies about Health Systems: Linking Guidance Development to Policy Development
- Guidance for Evidence-Informed Policies about Health Systems: Assessing How Much Confidence to Place in the Research Evidence
- Uterine Rupture by Intended Mode of Delivery in the UK: A National Case-Control Study
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Guidance for Evidence-Informed Policies about Health Systems: Assessing How Much Confidence to Place in the Research Evidence
- Uterine Rupture by Intended Mode of Delivery in the UK: A National Case-Control Study
- Guidance for Evidence-Informed Policies about Health Systems: Linking Guidance Development to Policy Development
- Improving Ethical Review of Research Involving Incentives for Health Promotion
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



