-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions
Policy discussions about the feasibility of massively scaling up antiretroviral therapy (ART) to reduce HIV transmission and incidence hinge on accurately projecting the cost of such scale-up in comparison to the benefits from reduced HIV incidence and mortality. We review the available literature on modelled estimates of the cost of providing ART to different populations around the world, and suggest alternative methods of characterising cost when modelling several decades into the future. In past economic analyses of ART provision, costs were often assumed to vary by disease stage and treatment regimen, but for treatment as prevention, in particular, most analyses assume a uniform cost per patient. This approach disregards variables that can affect unit cost, such as differences in factor prices (i.e., the prices of supplies and services) and the scale and scope of operations (i.e., the sizes and types of facilities providing ART). We discuss several of these variables, and then present a worked example of a flexible cost function used to determine the effect of scale on the cost of a proposed scale-up of treatment as prevention in South Africa. Adjusting previously estimated costs of universal testing and treatment in South Africa for diseconomies of small scale, i.e., more patients being treated in smaller facilities, adds 42% to the expected future cost of the intervention.
Published in the journal: HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions. PLoS Med 9(7): e32767. doi:10.1371/journal.pmed.1001247
Category: Review
doi: https://doi.org/10.1371/journal.pmed.1001247Summary
Policy discussions about the feasibility of massively scaling up antiretroviral therapy (ART) to reduce HIV transmission and incidence hinge on accurately projecting the cost of such scale-up in comparison to the benefits from reduced HIV incidence and mortality. We review the available literature on modelled estimates of the cost of providing ART to different populations around the world, and suggest alternative methods of characterising cost when modelling several decades into the future. In past economic analyses of ART provision, costs were often assumed to vary by disease stage and treatment regimen, but for treatment as prevention, in particular, most analyses assume a uniform cost per patient. This approach disregards variables that can affect unit cost, such as differences in factor prices (i.e., the prices of supplies and services) and the scale and scope of operations (i.e., the sizes and types of facilities providing ART). We discuss several of these variables, and then present a worked example of a flexible cost function used to determine the effect of scale on the cost of a proposed scale-up of treatment as prevention in South Africa. Adjusting previously estimated costs of universal testing and treatment in South Africa for diseconomies of small scale, i.e., more patients being treated in smaller facilities, adds 42% to the expected future cost of the intervention.
Introduction
Informed by biological plausibility [1], observational studies [2], and a trial [3] showing that ART reduces transmission of HIV within heterosexual serodiscordant couples, recent modelling papers [4]–[6] have projected the reduction in HIV incidence and the impact on health care costs that would follow from achieving close-to-universal coverage with HIV testing and ART. These papers argue that sufficiently universal ART coverage would eventually pay for itself by suppressing HIV incidence and therefore averting the future need for HIV care, including ART. Other papers in the July 2012 PLoS Medicine Collection, “Investigating the Impact of Treatment on New HIV Infections” analyse the sensitivity of the projected population-level incidence reductions to the structure and assumptions of an epidemiological projection model [7]–[9]. This paper focuses on the cost side of such projection models. We begin with a general discussion of cost accounting identities versus flexible cost functions. Then we review the available literature on modelled estimates of the projected cost of ART provision, including ART for prevention, with a focus on identifying determinants authors have included, implicitly or explicitly, in their assumed cost function for ART service delivery. We then discuss the evidence for a number of such cost determinants. Finally, we present an example of a flexible cost function used to explore how economies of scale might affect the costs of scaling up ART in South Africa. A second paper focussing on economic evaluation in this collection further discusses how operational and effectiveness issues in scaling up ART for prevention will affect its cost-effectiveness [10].
Cost Accounting Identities versus Flexible Cost Functions
Just as most epidemiological projection models include a functional representation of epidemiological concepts such as the force of infection, cost projection models include a function or a set of functions to characterise the relationship between the total cost of ART service delivery and various determinants of cost, such as the number of patients on treatment, the stage in their disease at which they were recruited, and the ART regimen they receive. Most existing cost projections assume a single constant unit cost per patient-year, or per patient-year on a certain regimen, across large populations and often extended projection periods. A somewhat more complex approach is to assume a single unit cost for each of a set of services received by an HIV-positive patient, such as a unit cost for each type of laboratory test or outpatient visit or inpatient day, and then multiply these unit costs by an estimate of the number of each of these services per patient-year and by the number of patient-years delivered in a year. We call such an equation an accounting identity and designate a total annual cost so defined as an accounting identity cost function, TCAI. In its simplest form such a cost function can be written as(1)where k indexes the facilities delivering ART, qk represents the output of facility k in a single time period, typically a year, and A is the average variable cost per patient-year. Cost accounting identities impose the discipline of arithmetical consistency on discussions of budgets, costs, expenditures, and efficiency, and predict future expenditures over the short run. They are a natural approach when estimating how much delivery of a service “should” cost. (See the discussion of the distinction between “normative” and “positive” cost functions in Text S2.) As such, they are often sufficient for capturing the impact of incremental policies, e.g., an extension of a health care intervention to a slightly larger proportion of the same population by increasing coverage.
However, cost accounting identities cannot be used to predict how costs will change when civil servants, managers, providers, and patients have an opportunity to adjust service delivery by, for example, substituting one input for another, or changing the scale and scope of operations, eligibility criteria, task shifting, or the deployment of supply - or demand-side incentives. We argue that, as a result, cost accounting identities are too rigid to model large-scale changes over periods of more than a few years—such as those required to achieve the HIV prevention benefits of ART. For these purposes, a more flexible cost function such as(2)can provide a more plausible characterisation and projection of total annual costs. In Equation 2, p and Z are vectors representing, respectively, the set of relevant input prices and all other policy and environmental determinants of cost, many of which we discuss in this paper. The notation f(…) stands in for a flexible functional form chosen either to fit the data or, when data is lacking, to fit the analysts' assumptions (see Text S2 for more details). For simplicity, in both Equation 1 and 2 we have suppressed the time subscripts, but in a more formal development, time might itself influence price, output, or other policy determinants.
The Use of Cost Functions in Published Modelled Economic Analyses of ART
In order to determine the current state of the art, we reviewed the available literature on modelled estimates of the projected cost of ART provision to a variety of eligible populations, including ART for prevention. We searched eight databases (PubMed, HealthSTAR, POPLINE, EconLit, HEED, Web of Knowledge [Science and Social Sciences], Embase and CAB Health) for the years 1988–2011 using any combination of the terms cost*, econ*, and HIV or AIDS. We supplemented the identified articles by reviewing the reference lists of identified articles, additional review articles, and grey literature (slides, conference proceedings, books, and manuals). We included all articles in any language that contained modelled cost data of any kind as well as ART as an intervention, except where it was used for the prevention of mother-to-child transmission only. Abstracts and articles in all languages (English, Italian, Spanish, French, and German) were read in full by the first author, who made the decision whether to include the article in the review. We excluded editorials and letters, articles without quantitative data, and articles that did not include a modelled estimate, such as papers reporting cost data from a single site. The last have been reviewed repeatedly in the past [11]–[15]. We reviewed the included articles with regards to their economic evaluation method, the type of model used, their time horizon, the outcome metric and result, and whether the input cost (often in the form of average per patient cost per unit time) was constant or had been varied by determinants such as types of regimens used, health state, time on treatment, and mode of delivery, in either the main or the sensitivity analysis.
We identified 45 published articles, one conference abstract, and four reports on modelled economic analyses of ART provision worldwide (Table 1; Text S1). Thirty-eight analyses were for single countries, four were for wider regions, and eight were global. Five analyses, all for single countries, specifically considered the impact of ART on HIV transmission; we discuss these separately.
Tab. 1. Overview of the methods and results of previously published modelled economic analyses of antiretroviral treatment. 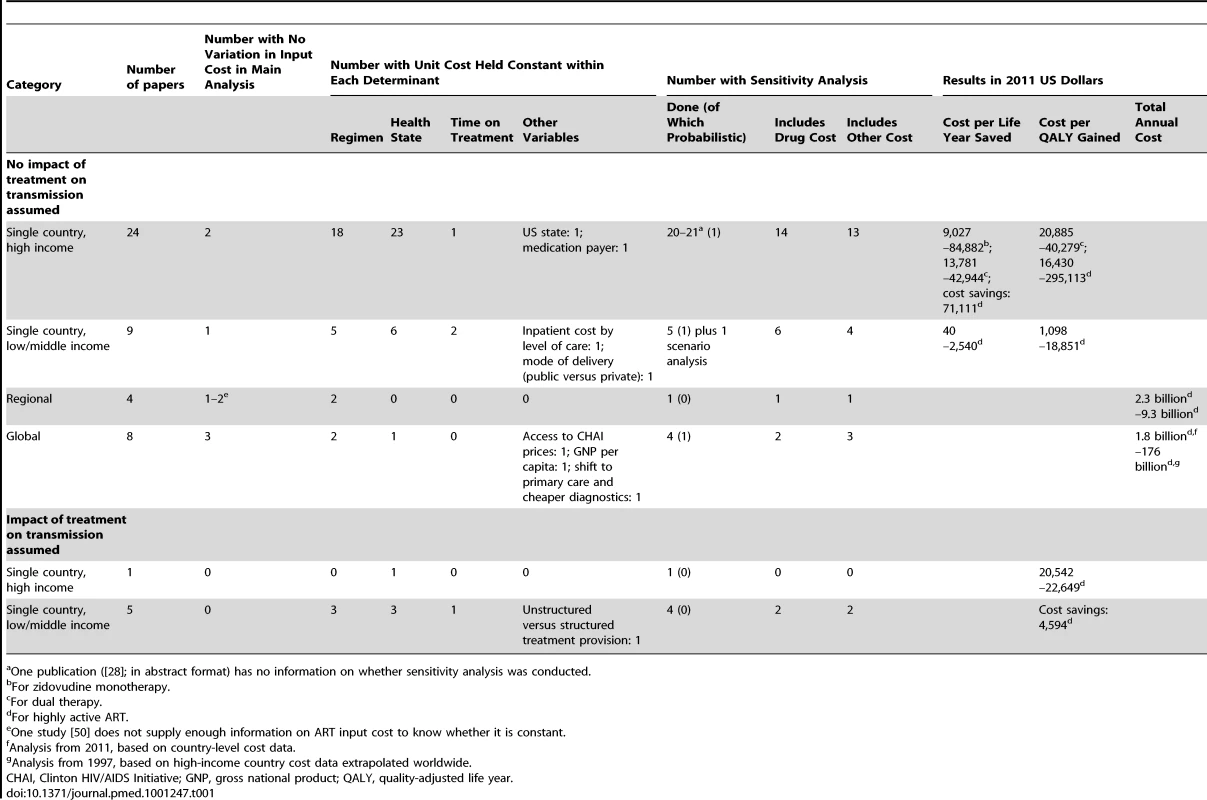
One publication ([28]; in abstract format) has no information on whether sensitivity analysis was conducted. Thirty-three analyses modelled ART programmes within a single country, without considering the transmission impact of ART [16]–[48]. Most of the 24 high-income-country analyses compared the incremental cost and effectiveness of a new drug regimen with that of an older one [22],[24]–[26],[33],[36]–[39]. Amongst the nine low - and middle-income-country (LMIC) analyses, six analyses focussed on the choice of eligibility criteria [40]–[45],[47],[48]. One analysis compared ART with no ART [43], one, first-line treatment with first - and second-line treatment [40], and one, different regimens for women previously exposed to single-dose nevirapine as part of prevention of mother-to-child transmission [47].
In terms of the use of cost functions, most of these single-country papers varied input cost (i.e., the cost per patient per unit of time) by protocol-related variables such as treatment regimen, health state (defined by the absence or presence of symptoms, opportunistic infections, AIDS-defining diseases, and/or CD4 cell count levels), and/or time on treatment (see Table 1). Only two papers, both of them on LMICs, varied cost by level of care (secondary versus tertiary) [43] or mode of health care provision (public versus private) [44]; none of the papers varied per patient cost by scale or other programmatic variables.
The four regional studies [49]–[52] all focussed on sub-Saharan Africa (with one study [52] additionally including Southeast Asia). These studies modelled the cost of defined increases in ART coverage from a low baseline [49],[50] and the cost effectiveness of ART provision through the specific setting of an antenatal care clinic [51]. One paper used the same constant input cost for all patients [52]; two papers varied input cost by regimen [49],[52]. None of the papers varied per patient cost by any other variables.
The eight global studies, published between 1997 and 2011, describe a clear evolution in both data availability and modelling technique [53]–[60]. The older analyses estimate cost based only on the number of HIV-positive people from a number of sources, varying assumptions of ART coverage at baseline, with costs based on guidelines and prices from high-income countries [53],[54]. Later analyses model global cost under concrete programmes, such as the World Health Organization's 3 by 5 initiative [57] and the Global Fund to Fight AIDS, Tuberculosis and Malaria [56],[58], based on per patient cost estimates from relevant LMICs and more advanced epidemiological models of the number of patients in need of ART, such as the Spectrum model [58],[59] and the Resource Needs Model [60]. Three of the eight global analyses used constant input costs for all patients [53]–[55]; two varied input cost by regimen [57],[58], and one additionally by health state [58]. One study included the impact of access to pool procurement prices negotiated by the Clinton HIV/AIDS Initiative on per patient cost [57], one varied drug prices by per capita gross national product [56], and one assumed a reduction of per patient cost of 65% by 2020 as a result of task shifting and cheaper point-of-care diagnostics [60]. No other cost determinants were considered.
Five studies between 2006 and 2011 that analysed the cost of ART for a single country included an impact of treatment on HIV transmission and, hence, on the number of future infections and future cost [4],[5],[61]–[63]. Three of these analyses were cost-effectiveness analyses of different strategies of eligibility and coverage [61]–[63]; two were analyses of the cost impact and cost benefit of earlier treatment initiation, including universal testing and treatment [4],[5]. With respect to cost functions, three of the analyses varied input cost by regimen [4],[5],[63], three by health state [61]–[63], and one by time on treatment [62]; additionally, one analysis varied input cost by whether treatment was administered in a structured way in the public sector or an unstructured way in the private sector [62]. No other variation in cost was considered.
Potential Determinants of a Flexible Cost Function
As summarised above, most modelled estimates of the projected cost of ART provision to date have used cost accounting identities, with minimal use of cost functions. If a more flexible cost function is chosen for modelling the future cost of ART over several decades, which variables should be included in this function? Here and in Table 2, we review the evidence for some possible determinants of the cost of ART provision.
Tab. 2. Schematic summary of determinants of the cost of ART provision. 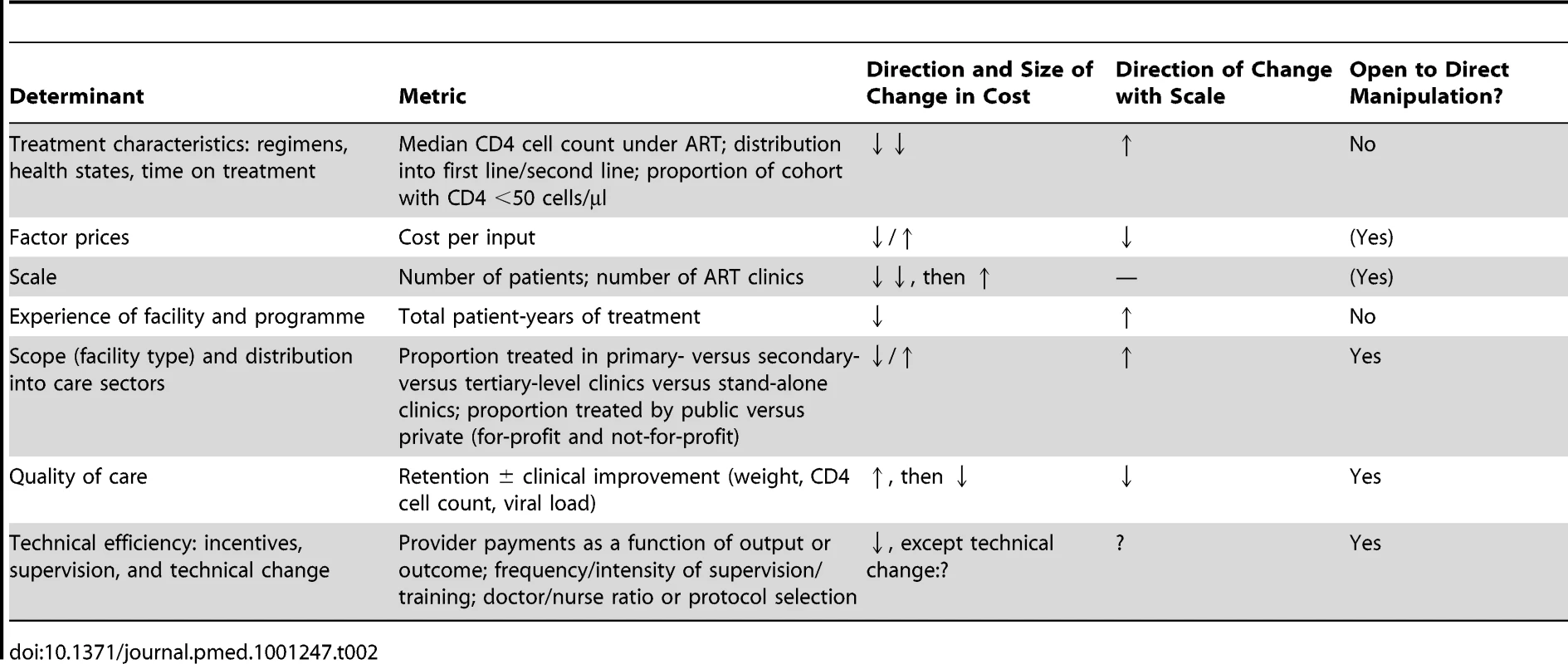
Treatment Characteristics: Regimens, Health States, Time on Treatment
Most reviewed papers recognised that more complex cases of any disease engender higher treatment costs. Modellers addressed this by assuming a unit cost that varied by treatment regimen, health state, or time on treatment. These are important cost determinants, since the cost of a national programme will be largely defined by the distribution of the national treatment cohort into first - and second-line regimens (with second-line regimens being much more expensive in most countries) [64] and into CD4 cell count strata associated with different disease burden and cost. Likewise, an analysis of hospitalisation frequency and cost in the same patients before and after ART initiation found the cost of hospitalisation per patient-year in patients with CD4 cell count <100 cells/µl to be ten times higher than in patients with CD4 cell count >350 cells/µl [65] (see also [66]–[68]). However, we argue that these characteristics are not the only ones that input cost should vary by, and their relevance for total cost might be overwhelmed in situations of rapid scale-up or large-scale changes to programme delivery such as task shifting to lower levels of facilities and health care cadres.
Factor Prices
The prices of factors of production, including labour, supplies, utilities, transportation, equipment, and buildings, clearly affect the cost of health services. By varying input cost by treatment regimen and, in some cases, also changing the cost of laboratory tests over time, most of the reviewed analyses have taken factor prices into account. And for good reason: the cost of antiretroviral drugs—in many countries the largest component of the cost of ART provision—has changed dramatically over the last ten years, especially for LMICs. By October 2000, the prices of antiretroviral drugs in resource-constrained settings had fallen by 90% on average [69], owing largely to the increased availability of generically manufactured drugs from three Indian companies and the possibility of importing these drugs in parallel with patent-protected drugs under the World Trade Organization Agreement on Trade-Related Aspects of Intellectual Property Rights [70]. The price of the non-generic version of the most common first-line drug combination (stavudine+lamivudine+nevirapine) dropped by 93% from US$10,439 to US$727 between June 2000 and September 2001 [71]. Even though the price of the regimen fell by another 54% between 2001 and 2008, the scope for further reductions in the price of antiretrovirals is assumed to be limited, shifting the focus to the cost of other factor prices such as service delivery, laboratory tests, and overheads.Reductions in all of these are targeted by UNAIDS's Treatment 2.0 initiative [72].
Scale
As mentioned, none of the reviewed papers considered an impact of scale, i.e., the size or coverage of the programme, on cost, despite the dramatic increases in scale modelled by some of the papers—especially those analysing the cost of treatment for prevention [4],[5]. This stands in contrast to much of economic theory, which assumes a U-shaped relationship between scale and average cost, with cost per unit of output at first decreasing as quantities of output increase, because inputs (e.g., staff) are shared to produce an increasing number of outputs (e.g., patients seen). When scaling up further, beyond a certain number of outputs, new inputs will be required, leading to increasing average cost for large facilities or broadly expanded programmes. Scale economies seem plausible in ART service delivery because the cost of some functions of an ART treatment site, such as building maintenance, personnel management, and the transportation of supplies, will increase in more direct proportion to the number of sites than to the number of patients each one serves. This means that at the site level, increasing the number of patients generates a less than proportionate increase in cost.
Only a few programmes have produced data that have allowed this relationship to be examined empirically. Economies of scale have been found in HIV prevention programmes [73]–[75] and in the modelled cost of hygiene outreach interventions, the latter showing a U-shaped relationship between coverage and average or marginal cost [76]. The worked example below and Text S2 provide more discussion of the concept and application of scale economies.
Experience of Facility and Programme
The implementation of most interventions is traditionally assumed to benefit from “learning by doing”, which results in reductions in average cost. Since this learning often coincides with scale-up, this relationship is not always easy to distinguish from the reduction of average cost with scale mentioned above. In an analysis of data from ART clinics supported by the US President's Emergency Plan for AIDS Relief, Menzies et al. found that median per patient cost across a number of sites in different countries decreased with each successive six-month period from the start of the ART programme at each site [77], with the biggest decrease between the first and the second six-month periods. The potential effect on cost of increased facility and programme experience over time was not considered in any of the reviewed papers.
Scope (Facility Type) and Distribution into Care Sectors (Private versus Public)
As with scale, the cost of a national ART programme will also be affected by a change in the scope of ART provision, i.e., the type of facilities (e.g., primary health care clinics versus specialised ART clinics at secondary - or tertiary-level hospitals) and whether or not they are in the public or the private sector, with the private sector further divided into for-profit and not-for-profit (e.g., non-governmental organisations [NGOs]). Generally, larger health care facilities, such as hospitals, can achieve economies of scope by spreading the cost of infrastructure over the production of multiple health services. Rosen et al. compared the cost of ART provision per patient-year for the first 12 months of treatment across a clinic in a public hospital, a group of private general practitioners, a private NGO-run HIV clinic, and a private NGO-run primary health care clinic in South Africa [78]. They found costs to vary significantly between sites as a result of differences in service delivery (see Figure 1). Since patient mix was comparable across three of the four sites, only a small portion of the difference in cost could be ascribed to differences in disease severity. Amongst the reviewed papers, only three included level of care as a variable determining input cost (in South Africa [43], India [44], and Thailand [63]). Future cost projections should include information on the variation of cost by level of care and mode of delivery, as well as the expected distribution of the treatment cohort between different levels and modes, especially where these are likely to change as a result of planned dramatic increases in the size of the programme.
Fig. 1. Annual per patient cost of ART provision in four different settings in South Africa. 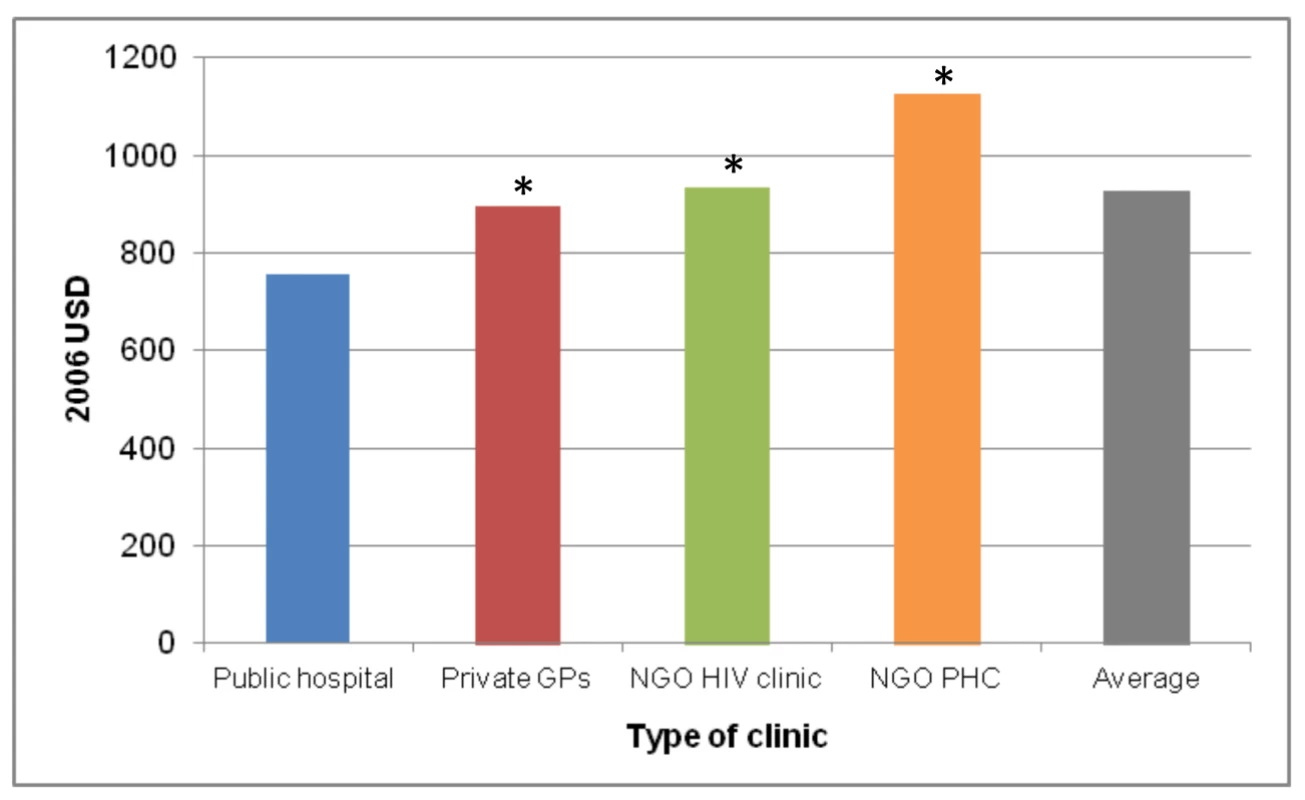
Based on [78]. *, difference from public hospital significant at p<0.05. GPs, general practitioners; PHC, primary health care clinic; USD, US dollars. Quality of Care
Quality of health care is notoriously difficult to measure, but in ART service delivery, a facility's success at retaining patients in treatment, and improving the patient cohort's health on average, is a reasonable proxy. The same analysis by Rosen et al. compared the cost per quality-adjusted output between the four settings, using routinely collected data (such as patient status, CD4 cell counts, viral loads, and the absence or presence of new World Health Organization stage 3 or 4 conditions) to calculate patient retention in care and response to treatment [78]. While the cost of patients who were no longer in care (i.e., had died or been lost to follow-up during the first 12 months after treatment initiation) was comparable across settings, the cost per patient in care and responding to treatment, and the cost per patient in care and not responding to treatment, was significantly different between the four clinics (Figure 2). Depending on the quality of care in each clinic, and the resulting levels of loss to follow-up and treatment failure, the additional cost per patient in care and responding was 22% and 48% of the average annual cost per patient at two sites because of resources spent on patients either leaving care or not responding to care.
Fig. 2. Annual per patient cost of ART provision per type of outcome in four different settings in South Africa. 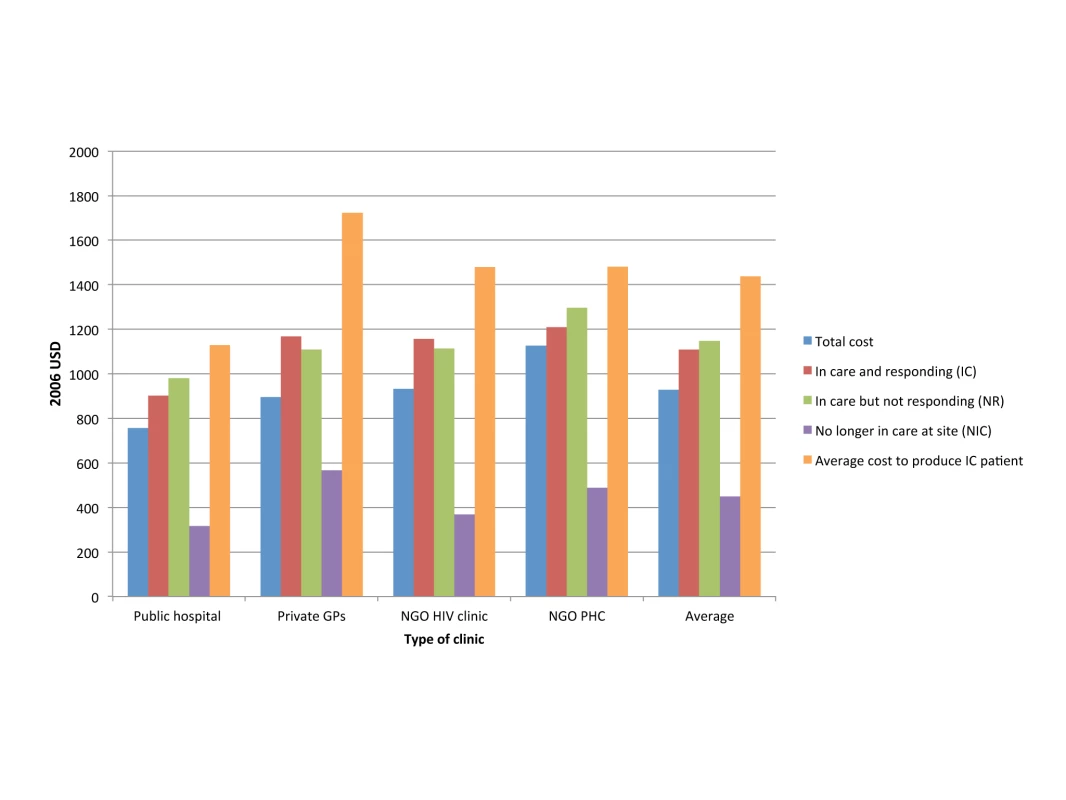
Based on [78]. GPs, general practitioners; PHC, primary health care clinic; USD, US dollars. Technical Efficiency: Incentives, Supervision, and Technical Change
Technical efficiency is defined as the production of a good or service without waste, and is thus another important determinant of cost. Both public and private sector providers face constraints in the availability and quality of staff, which will affect the cost of rolling out an intervention differently at a different scale. Staffing in the public sector faces constraints such as lower wages, low work morale, and staff absenteeism, which result in low quality of care. Staffing in the private sector may not be subject to those issues to the same extent because of fee-for-service financing mechanisms, but fee-for-service mechanisms have the undesirable effect of deterring patients, especially uninsured patients, from seeking treatment [79]. Leonard and colleagues have shown that non-financial incentives such as encouragement and supervision by a peer can improve the quality of care provided by health care workers [80],[81]. As donor programmes such as the US President's Emergency Plan for AIDS Relief and its contractors relinquish direct control of patient treatment in favour of subsidies to NGOs or technical support for local government provision, the issue of management will become increasingly important as a determinant of technical efficiency and therefore costs.
Our view that programme characteristics such as scale/coverage, scope, managerial incentives, and quality/effectiveness can have important effects on the costs of ART delivery is endorsed by a second paper in this PLoS Medicine collection [10], which also points to the difficulty of projecting the future costs of technologies that are not yet widely used or have not even been invented. The solution to the former problem is to collect cost data on a wide range of current practices, and project future costs under the hypothesis that the technology mix will shift, e.g., towards smaller scale treatment programmes, as in the example in the next section. Projecting the costs of unknown future innovations is a less tractable problem, but arguably could best be approached by using simple flexible functions of a few fundamental variables like input prices, and allowing technical efficiency to improve according to a time trend.
A Worked Example of a Flexible Cost Function: The Impact of Scale on the Cost of Universal Testing and Treatment
For achieving the target coverage for universal testing and treatment in South Africa, Granich et al. [4] proposed a scale-up from 1.5 million patients on ART in June 2011 [82] to 4.1 million patients by mid-2016. While a flexible cost model of this scale-up proposal could incorporate any of the cost determinants described above, we have data on only one of these: the current size distribution of treatment facilities, i.e., scale. Since economies of scale seem likely to be a persistent feature of ART service delivery, we use this cost determinant in this example, with the hope that more of the data needed to model other potentially important cost determinants will become available in the future. We reviewed the actual size distribution of accredited ART treatment sites in South Africa in June 2010, using government and other sources (Figure 3). When the logarithm of size is charted against the logarithm of size rank, many size distributions in nature are approximately linear, following Zipf's law [83]. We hypothesize that the marked nonlinearity of the size distribution of South Africa's ART sites in 2010 was due to the recent scale-up occurring in larger sites and was temporary. If that is true, and if the largest 50 sites are assumed to retain their current patient loads during programme expansion, then expansion from 1 million patients at the beginning of 2010 to 4.1 million in 2016 would require that more sites be opened and that the scale of smaller sites be increased sufficiently to accommodate the additional patients. As a result, the size distribution of ART sites would straighten out over time. Then, as patient load subsequently contracts over time due to the hypothesized prevention success of the universal test-and-treat policy, we expect the size distribution to mature into a power law that is linear in logarithms, which first steepens, as smaller sites contract first and, once the number of enrolled patients contracts to below 1 million, contracts proportionally at all sites (see Text S2 for details).
Fig. 3. Size-rank distribution of ART facilities in 2010 and projected to future years in order to implement a universal test-and-treat strategy in South Africa. 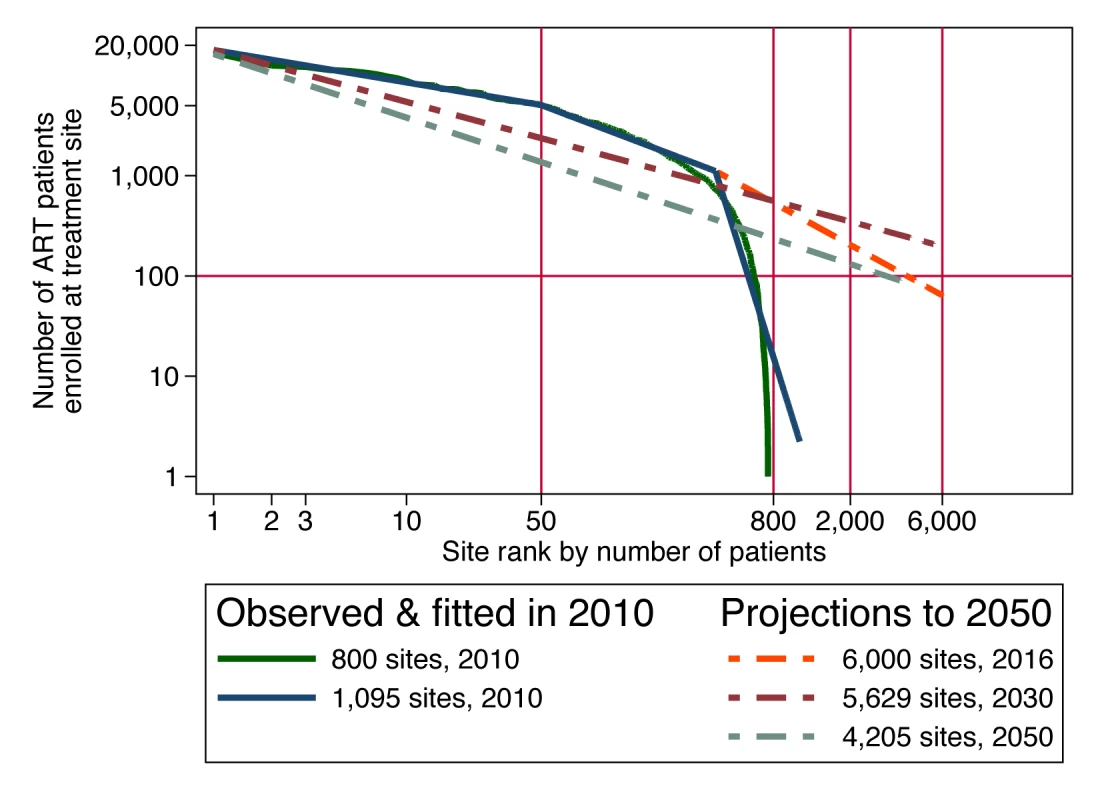
Assuming a plausible size distribution of the patient load at ART sites allows us to estimate the effect that a cost function incorporating scale economies would have on the projection of total cost. Suppose that the production technology of ART services exhibits a scale elasticity of 0.7, meaning that every 10% increase in scale is associated with only a 7% increase in total cost, because of scale efficiencies. Assuming for simplicity that all economies of scale occur at the facility level, total cost (tc) for the country would be the sum of(3)over all the sites in the country, where Ak = f(pk, Zk), held constant at in the present analysis (US$7,600, calibrated from the known size distribution of patients and total cost per patient in 2010; Text S2 gives results for other elasticities of scale between 1.0 and 0.5). Since average cost at a site is defined as total cost at that site divided by quantity of patients at that site, the facility-specific average cost function (atck) consistent with Equation 3 is(4)Applying this cost function to the current and projected facility size distribution yields estimates of the total national cost of ART in each year of the simulation, which we compare to the Granich et al. estimates for the same scale-up scenario (Figure 4). Our assumption that the number of clinics must expand substantially to serve the estimated 4.1 million patients means that an increasing proportion of patients will be served in smaller clinics, which suffer from diseconomies of operating at small scale. In comparison to Granich's estimate of a peak annual cost of US$3.5 billion in 2016, the scale-adjusted estimate is US$4.4 billion, or 26% higher. As the number of patients moderates over time (due to Granich et al.'s assumptions of a strong population-level reduction in HIV transmission and of a concomitant 40% reduction in risky behaviour), the excess of scale-adjusted costs over accounting identity costs declines to below 20% and then rises again to 50% by the year 2050. Total accumulated cost over the 40-year period of the projection rises from US$75 billion to US$106 billion, an increase of 42%.
Fig. 4. Impact of scale elasticity on future cost of a universal test-and-treat strategy in South Africa. 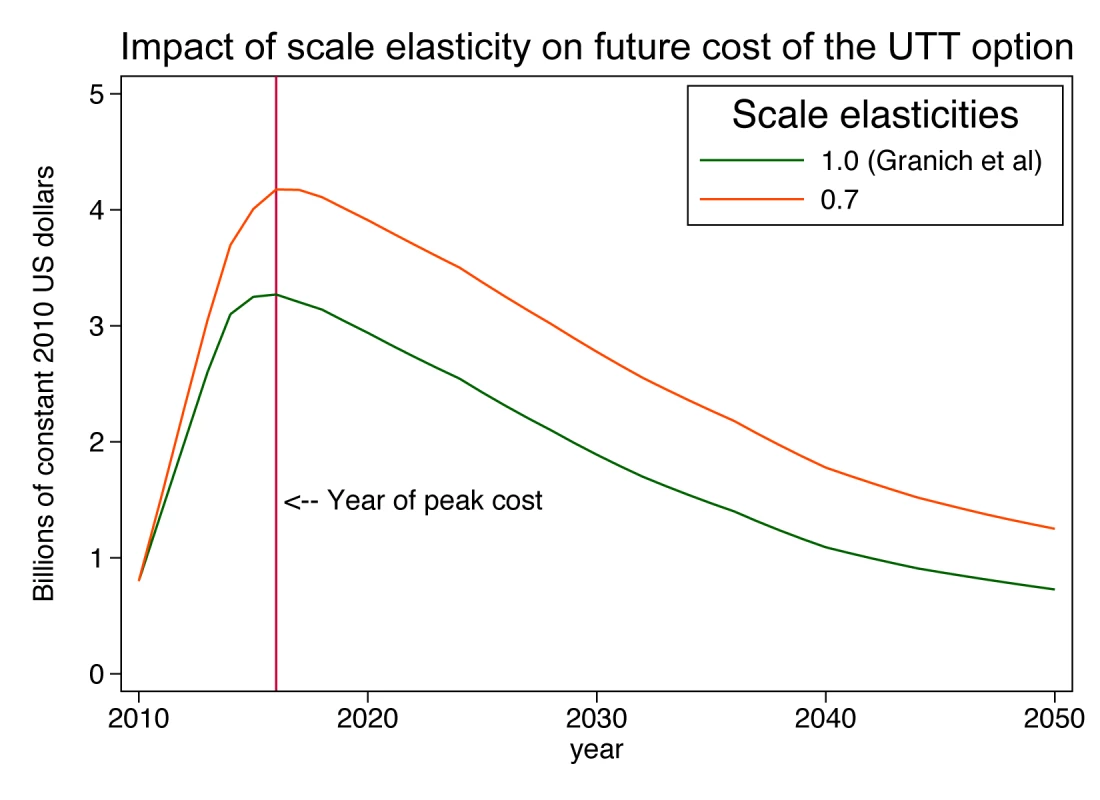
UTT, universal testing and treatment. This example shows that the simple adjustment of the cost per patient-year for scale and a plausible pattern of distribution of patients into clinics can have a major impact on projected costs over future decades and can highlight the challenge of scaling up a treatment programme to full coverage of people outside urban areas.
Conclusions and Recommendations for Improved Cost Projections
For modellers' projections of alternative ART scale-up scenarios to attract serious policy attention, the assumptions and structure of the cost side of these models, like those on the epidemiological side, should be plausible, supported by observational studies, and, where available, based on results from trials of the costs of alternative service delivery methods. The envisaged cost-effectiveness analyses alongside the planned large-scale trials of treatment as prevention that will be rolled out over the next years provide a historic opportunity to collect such data and allow more precise projections of the future cost of ART programmes using flexible cost functions. Text S2 provides a summary of the differences in data and algebra needed for an accounting identity versus a flexible cost function for estimating cost for an individual facility's or a country's national ART programme. Data collection on large samples of facilities should go beyond measuring the quantity and quality of ART services, to capturing the actual cost of services delivered in a sample of facilities at different levels of care and details about all of the above-listed determinants of cost. With such data on a sample of ART facilities within its own borders, a country's government and any donors supporting its HIV care programme can not only improve their projections of the long-term implications of any given commitment to antiretroviral treatment, but also model the benefits of policies to improve the cost-effectiveness of their efforts.
Key Points
-
In modelling the projected costs of a health programme, flexible cost functions, in which costs vary with certain known or assumed determinants, provide a more plausible characterisation and projection of total annual costs than simple accounting identities.
-
A review of previous models estimating the cost of ART provision indicates that while most models accounted for how costs vary with patient health status and treatment regimen, variability in other determinants of cost was rarely included.
-
Potential determinants of cost that could be included in flexible cost functions for ART provision when modelling over several decades into the future include patient health status and treatment regimen, factor prices, programme/facility scale, facility experience, facility type, quality of care, and the technical efficiency of staff.
-
A worked example of a flexible cost function modelling the impact of one of these determinants, programme scale, on the costs of a proposed universal testing and treatment programme in South Africa found that the inefficiencies of small scale could add up to 42% to the total future cost of the programme.
-
Another article in this PLoS Medicine collection [10] discusses additional operational and effectiveness issues relevant for the economic evaluation of scaling up ART for prevention.
Supporting Information
Zdroje
1. GilliamBLDyerJRFiscusSAMarcusCZhouS 1997 Effects of reverse transcriptase inhibitor therapy on HIV-1 viral burden in semen. J Acquir Immune Defic Syndr 15 54 60
2. MontanerJSGLimaVDBarriosRYipBWoodE 2010 Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet 376 532 539
3. CohenMChenYQMcCauleyMGambleTHosseinipourMC 2011 Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 365 493 505
4. GranichRMGilksCFDyeCDe CockKMWilliamsBG 2009 Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet 373 48 57
5. HontelezJACde VlasSJTanserFBakkerRBärnighausenT 2011 The impact of the new WHO antiretroviral treatment guidelines on HIV epidemic dynamics and cost in South Africa. PLoS ONE 6 e21919 doi:10.1371/journal.pone.0021919
6. WagnerBBlowerS 2010 Costs of eliminating HIV in South Africa have been underestimated. Lancet 376 953
7. EatonJWJohnsonLFSalomonJABärnighausenTBendavidE 2012 HIV treatment as prevention: systematic comparison of mathematical models of the potential impact of antiretroviral therapy on HIV incidence in South Africa. PLoS Med 9 e1001245 doi:10.1371/journal.pmed.1001245
8. WilsonDP 2012 HIV treatment as prevention: natural experiments highlight limits of antiretroviral treatment as HIV prevention. PLoS Med 9 e1001231 doi:10.1371/journal.pmed.1001231
9. DelvaWEatonJWMengFFraserCWhiteRG 2012 HIV treatment as prevention: optimising the impact of expanded HIV treatment programmes. PLoS Med 9 e1001258 doi:10.1371/journal.pmed.1001258
10. BärnighausenTSalomonJASangrujeeN 2012 HIV treatment as prevention: issues in economic evaluation. PLoS Med 9 e1001263 doi:10.1371/journal.pmed.1001263
11. YouleMTruemanPSimpsonK 1999 Health economics in HIV disease. A review of the European literature. Phamacoeconomics 15 Suppl 1 1 12
12. BeckEJMinersAHYolleyK 2001 The cost of HIV treatment and care: a global review. Phamacoeconomics 19 13 39
13. BeckEJHarlingGGerbaseSDeLayP 2010 The cost of treatment and care for people living with HIV infection: implications of published studies, 1999–2008. Curr Opin HIV AIDS 5 215 224
14. BollingerLAdesinaA 2011 Review of available ART costs, major cost drivers and potential efficiency gains [presentation]. UNAIDS Economics Reference Group; 7–8 March 2011; Geneva, Switzerland
15. GalárragaOWirtzVJFiguero-LaraASanta-Ana-TellezYCoulibalyI 2011 Unit costs for delivery of antiretroviral treatment and prevention of mother-to-child transmission of HIV. A systematic review for low - and middle-income countries. Pharmacoeconomics 29 579 599
16. OddoneECowperPHamiltonJMatcharDBHartiganP 1993 Cost effectiveness analysis of early zidovudine treatment of HIV infected patients. BMJ 307 1322 1325
17. SchulmanKLynneLGlickHEisenbergJ 1991 Cost-effectiveness of low-dose zidovudine therapy for asymptomatic patients with human immunodeficiency virus (HIV) infection. Ann Intern Med 114 798 801
18. DaviesDCarneCCamilleri-FerranteC 1999 Combined antiviral treatment in HIV infection. Is it value for money? Public Health 113 315 317
19. ChancellorJVHillAMSabinCASimpsonKNYouleM 1997 Modelling the cost effectiveness of lamivudine/zidovudine combination therapy in HIV infection. Pharmacoeconomics 12 54 66
20. MauskopfJLaceyLKempelASimpsonK 1998 The cost-effectiveness of treatment with lamivudine and zidovudine compared with zidovudine alone: a comparison of Markov model and trial data estimates. Am J Manag Care 4 1004 1012
21. SimpsonKHatziandreuEJAnderssonFShakespeareAOleksyI 1994 Cost effectiveness of antiviral treatment with zalcitabine plus zidovudine for AIDS patients with CD4+ counts less than 300/microliters in 5 European countries. Pharmacoeconomics 6 553 562
22. BiddleAKSimpsonKN 2000 Modeling the use of triple combination therapy in five countries: nevirapine, zidovudine, and didanosine. Value Health 3 186 201
23. SendiPPBucherHHarrTCraigBASchwietertM 1999 Cost effectiveness of highly active antiretroviral therapy in HIV-infected patients. AIDS 13 1115 1122
24. CookJDasbachECoplanPMarksonLYinD 1999 Modeling the long-term outcomes and costs of HIV antiretroviral therapy using HIV RNA levels: applications to a clinical trial. AIDS Res Hum Retroviruses 15 499 508
25. TruemanPYouleMSabinCAMinersAHBeckEJ 2000 The cost-effectiveness of triple nucleoside analogue therapy antiretroviral regimens in the treatment of HIV in the United Kingdom. HIV Clin Trials 1 27 35
26. MinersASabinCTruemanPYouleMMocroftA 2001 Assessing the cost-effectiveness of highly active antiretroviral therapy for adults with HIV in England. HIV Med 2 52 58
27. KahnJGHaileBKatesJChangS 2001 Health and federal budgetary effects of increasing access to antiretroviral medications for HIV by expanding Medicaid. Am J Public Health 91 1464 1473
28. RisebroughNOhPRachlisAMcMurchyDBastM 1999 Economic evaluation of triple ART with indinavir or abacavir and ZDV+3TC compared to dual therapy ZDV+3TC [abstract]. 6th Conference on Retroviruses and Opportunistic Infections; 31 Jan–4 Feb 1999; Chicago, Illinois, US
29. CaroJO'BrienJMiglaccio-WalleKRaggioG 2001 Economic analysis of initial HIV treatment: efavirenz - versus indinavir-containing triple therapy. Pharmacoeconomics 19 95 104
30. SchackmanBRFreedbergKAWeinsteinMCSaxPELosinaE 2002 Cost-effectiveness implications of the timing of antiretroviral therapy in HIV-infected adults. Arch Intern Med 162 2478 2486
31. SchackmanBRGoldieSJWeinsteinMCLosinaEZhangH 2001 Cost-effectiveness of earlier initiation of antiretroviral therapy for uninsured HIV-infected adults. Am J Public Health 91 1456 1463
32. YazdanpanahYGoldieSLosinaEWeinsteinMCLebrunT 2002 Lifetime cost of HIV care in France during the era of highly active antiretroviral therapy. Antivir Ther 7 257 266
33. FreedbergKALosinaEWeinsteinMCPaltielDCohenC 2001 The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med 344 824 831
34. MauskopfJATolsonJMSimpsonKNPhamSVAlbrightJ 2000 Impact of zidovudine-based triple combination therapy on an AIDS drug assistance program. J Acquir Immune Defic Syndr 23 302 313
35. MooreRDBartlettJ 1996 Combination antiretroviral therapy in HIV infection: an economic perspective. Pharmacoeconomics 10 109 113
36. SimpsonKNLuoMPChumneyESunEBrunS 2004 Cost-effectiveness of lopinavir/ritonavir versus nelfinavir as the first-line highly active antiretroviral therapy regimen for HIV infection. HIV Clin Trials 5 294 304
37. MunakataJSandersGOwensDBayoumiA 2003 Cost effectiveness of enfuvirtide in the treatment of drug-resistant HIV infection. Med Decis Making 23 569
38. SnedecorSHartzemaASchillerK 2005 Cost effectiveness of HIV treatment innovations of greater efficacy than highly active antiretroviral therapy (HAART). Value Health 8 244
39. SaxPLosinaEWeinsteinMPaltielAGoldieS 2005 Cost-effectiveness of enfuvirtide in treatment-experienced patients with advanced HIV disease. J Acquir Immune Defic Syndr 39 69 77
40. LongEBrandeauMGalvinCVinichenkoTToleS 2006 Effectiveness and cost-effectiveness of strategies to expand antiretroviral therapy in St. Petersburg, Russia. AIDS 20 2207 2215
41. GoldieSYazdanpanahYLosinaEWeinsteinMAnglaretX 2006 Cost-effectiveness of HIV treatment in resource-poor settings—the case of Cote d'Ivoire. N Engl J Med 355 1141 1153
42. PatonNChapmanCSangeethaSMandaliaSBellamyR 2006 Cost and cost-effectiveness of antiretroviral therapy for HIV infection in Singapore. Int J STD AIDS 17 699 705
43. ClearySMcIntyreDBoulleA 2006 The cost-effectiveness of antiretroviral treatment. Cost Eff Resour Alloc 4 20
44. OverMRevengaAMsasakiEPeerapatanapokinWGoldJ 2007 The economics of effective AIDS treatment in Thailand. AIDS 21 Suppl 4 S105 S116
45. WalenskyRPWoodRCiaranelloALPaltielADLorenzanaSB 2010 Scaling up the 2010 World Health Organization HIV treatment guidelines in resource-limited settings: a model-based analysis. PLoS Med 7 e1000382 doi:10.1371/journal.pmed.1000382
46. BendavidEGrantPTalbotAOwensDKZolopaA 2011 Cost-effectiveness of antiretroviral regimens in the World Health Organization's treatment guidelines: a South African analysis. AIDS 25 211 220
47. CiaranelloALLockmanSFreedbergKAHughesMChuJ 2011 First-line antiretroviral therapy after single-dose nevirapine exposure in South Africa: a cost-effectiveness analysis of the OCTANE trial. AIDS 25 479 492
48. BachmannMO 2006 Effectiveness and cost effectiveness of early and late prevention of HIV/AIDS progression with antiretrovirals or antibiotics in Southern African adults. AIDS Care 18 109 120
49. BonnelR 2000 Costs of scaling HIV program activities to a national level in sub-Saharan Africa: methods and estimates Washington (District of Columbia) World Bank
50. KumaranayakeLContehLKurowskiCWattsC 2001 Preliminary estimates of the cost of expanding TB, malaria and HIV/AIDS activities for sub-Saharan Africa Geneva Working Group 5, WHO Commission on Macroeconomics and Health
51. CreeseAFloydKAlbanAGuinnessL 2002 Cost-effectiveness of HIV/AIDS interventions in Africa: a systematic review of the evidence. Lancet 359 1635 1642
52. HoganDRBaltussenRHayashiCLauerJASalomonJA 2005 Achieving the millennium development goals for health: cost effectiveness analysis of strategies to combat HIV/AIDS in developing countries. BMJ 331 1431 1437 doi:10.1136/bmj.38643.368692.68
53. FloydKGilksC 1997 Cost and financing aspects of providing antiretroviral therapy. Van PraagEFernyakSMartin KatzA The implications of antiretroviral treatments. Informal consultation. World Health Organization, Office of HIV/AIDS and Sexually Transmitted Diseases Geneva World Health Organization
54. HoggRWeberACraibKAslamAO'ShaughnessyM 1998 One world, one hope: the cost of providing antiretroviral therapy to all nations. AIDS 12 2203 2209
55. AttaranASachsJ 2001 Defining and refining international donor support for combating the AIDS epidemic. Lancet 357 57 61
56. SchwartländerBStoverJWalkerNBollingerLMcGreeveyW 2001 Resource needs for HIV/AIDS. Science 292 2434 2436
57. GutierrezJJohnsBAdamTBertozziSMEdejerTT 2004 Achieving the WHO/UNAIDS antiretroviral treatment 3 by 5 goal: what will it cost? Lancet 364 63 64
58. StoverJKorenrompEBlakleyMKomatsuRViisainenK 2011 Long-term costs and health impact of continued global fund support for antiretroviral therapy. PLoS ONE 6 e21048 doi:10.1371/journal.pone.0021048
59. ReschSKorenrompEStoverJBlakleyMKrubinerC 2011 Economic returns to investment in AIDS treatment in low and middle income countries. PLoS ONE 6 e25310 doi:10.1371/journal.pone.0025310
60. SchwartländerBStoverJHallettTAtunRAvilaC 2011 Towards an improved investment approach for an effective response to HIV/AIDS. Lancet 377 2031 2041
61. LongEFBrandeauMLOwensDK 2010 The cost-effectiveness and population outcomes of expanded HIV screening and antiretroviral treatment in the United States. Ann Intern Med 153 778 789
62. OverMHeywoodPGoldJGuptaIHiraS 2004 HIV/AIDS treatment and prevention in India: modeling the costs and consequences Washington (District of Columbia) The International Bank for Reconstruction and Development/The World Bank
63. VijayaraghavanAEfrusyMBMazonsonPDEbrahimOSanneIM 2007 Cost effectiveness of alternative strategies for initiating and monitoring highly active antiretroviral therapy in the developing world. J Acquir Immune Defic Syndr 46 91 100
64. LongLFoxMSanneIRosenS 2010 The high cost of second-line antiretroviral therapy for HIV/AIDS in South Africa. AIDS 24 915 919
65. BrennanAMeyer-RathGFoxMModisenyaneTMartisonN 2011 Rates and cost of hospitalization before and after initiation of antiretroviral therapy in South Africa [abstract]. International Workshop on HIV Observational Databases; 24–26 March 2011; Prague, Czech Republic
66. KrentzHBAuldMCGillMJ 2004 The high cost of medical care for patients who present late (CD4 <200 cells/microL) with HIV infection. HIV Med 5 93 98
67. NykampDBarnettCWLagoMParhamDLFernandezES 1997 Cost of medication therapy in ambulatory HIV-infected patients. Ann Pharmacother 31 303 307
68. LeisegangRClearySHislopMDavidseARegensbergL 2009 Early and late direct costs in a Southern African antiretroviral treatment programme: a retrospective cohort analysis. PLoS Med 6 e1000189 doi:10.1371/journal.pmed.1000189
69. Perez-CasasCHerranzEFordN 2001 Pricing of drugs and donations: options for sustainable equity pricing. Trop Med Int Health 6 960 964
70. World Trade Organization 2001 Declaration on the TRIPS agreement and public health. WT/MIN(01)/DEC/2
71. Médecins Sans Frontières Campaign for Access to Essential Medicines 2008 Untangling the web of antiretroviral price reductions, 11th edition Geneva Médecins Sans Frontières
72. Joint United Nations Programme on HIV/AIDS 2011 Toward an improved framework for HIV investments Geneva Joint United Nations Programme on HIV/AIDS
73. MarseilleEDandonaLMarshallNGaistPBautista-ArradondoS 2007 HIV prevention cost and program scale: data from the PANCEA project in five low and middle-income countries. BMC Health Serv Res 7 108
74. GuinnessLKumaranayakeLRajaramanBSankaranarayananGVannelaG 2005 Does scale matter? The costs of HIV-prevention interventions for commercial sex workers in India. Bull World Health Organ 83 747 755
75. GuinnessLKumaranayakeLHansonK 2007 A cost function for HIV prevention services: is there a ‘u’-shape? Cost Eff Resour Alloc 5 13
76. JohnsBBaltussenR 2004 Accounting for the cost of scaling-up health interventions. Health Econ 13 1117 1124
77. MenziesNABerrutiAABerzonRFillerSFerrisR 2011 The cost of providing comprehensive HIV treatment in PEPFAR-supported programs. AIDS 25 1753 1760
78. RosenSLongLSanneI 2008 The outcomes and outpatient costs of different models of antiretroviral treatment delivery in South Africa. Trop Med Int Health 13 1005 1015
79. KabugoCBahendekaSMwebazeRMalambaSKatuntuD 2005 Long-term experience providing antiretroviral drugs in a fee-for-service HIV clinic in Uganda. Evidence of extended virologic and CD4+ cell count responses. J Acquir Immune Defic Syndr 38 578 583
80. LeonardKLMasatuMC 2010 Professionalism and the know-do gap: exploring intrinsic motivation among health workers in Tanzania. Health Econ 19 1461 1477
81. DasJHammerJLeonardK 2008 The quality of medical advice in low-income countries. J Econ Perspect 22 93 114
82. Republic of South Africa 2010 Country progress report on the declaration of commitment on HIV/AIDS. 2010 Report Pretoria Republic of South Africa
83. ZipfGK 1935 The psychobiology of language Boston Houghton-Mifflin
Štítky
Interné lekárstvo
Článek Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-AnalysisČlánek The Co-Management of Tuberculosis and Diabetes: Challenges and Opportunities in the Developing WorldČlánek Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsiaČlánek HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-MakingČlánek United States Private-Sector Physicians and Pharmaceutical Contract Research: A Qualitative Study
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2012 Číslo 7- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- HIV Treatment as Prevention: Issues in Economic Evaluation
- Risk of Venous Thromboembolism in Patients with Cancer: A Systematic Review and Meta-Analysis
- HIV Treatment as Prevention: Natural Experiments Highlight Limits of Antiretroviral Treatment as HIV Prevention
- HIV Treatment as Prevention: Optimising the Impact of Expanded HIV Treatment Programmes
- Reduction in Infection Rates after Mandatory Hospital Public Reporting: Findings from a Longitudinal Cohort Study in Canada
- Medical Device Regulation: Time to Improve Performance
- Averting an Impending Storm: Can We Reengineer Health Systems to Meet the Needs of Aging Populations?
- Thinking Forward: The Quicksand of Appeasing the Food Industry
- The Co-Management of Tuberculosis and Diabetes: Challenges and Opportunities in the Developing World
- Community Mobilization in Mumbai Slums to Improve Perinatal Care and Outcomes: A Cluster Randomized Controlled Trial
- Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsia
- Social Entrepreneurship for Sexual Health (SESH): A New Approach for Enabling Delivery of Sexual Health Services among Most-at-Risk Populations
- Lessons from Agriculture for the Sustainable Management of Malaria Vectors
- HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions
- HIV Treatment as Prevention: Considerations in the Design, Conduct, and Analysis of Cluster Randomized Controlled Trials of Combination HIV Prevention
- Antiretroviral Therapy for Prevention of Tuberculosis in Adults with HIV: A Systematic Review and Meta-Analysis
- The Effectiveness of Emergency Obstetric Referral Interventions in Developing Country Settings: A Systematic Review
- Digital Humanitarianism: Collective Intelligence Emerging
- The Ethics of Switch/Simplify in Antiretroviral Trials: Non-Inferior or Just Inferior?
- “Big Food,” the Consumer Food Environment, Health, and the Policy Response in South Africa
- Plasma Phospholipid Fatty Acid Concentration and Incident Coronary Heart Disease in Men and Women: The EPIC-Norfolk Prospective Study
- HIV Treatment as Prevention: The Utility and Limitations of Ecological Observation
- How Does Medical Device Regulation Perform in the United States and the European Union? A Systematic Review
- HIV Treatment as Prevention: Models, Data, and Questions—Towards Evidence-Based Decision-Making
- Risk Factors for Death among Children Less than 5 Years Old Hospitalized with Diarrhea in Rural Western Kenya, 2005–2007: A Cohort Study
- United States Private-Sector Physicians and Pharmaceutical Contract Research: A Qualitative Study
- HIV Treatment as Prevention: Debate and Commentary—Will Early Infection Compromise Treatment-as-Prevention Strategies?
- HIV Treatment as Prevention: Principles of Good HIV Epidemiology Modelling for Public Health Decision-Making in All Modes of Prevention and Evaluation
- Effect of a Community-Based Nursing Intervention on Mortality in Chronically Ill Older Adults: A Randomized Controlled Trial
- Surveillance of Infection Severity: A Registry Study of Laboratory Diagnosed
- Consequences of Gestational Diabetes in an Urban Hospital in Viet Nam: A Prospective Cohort Study
- Integrating Mental Health and Development: A Case Study of the BasicNeeds Model in Nepal
- Treatment of Young Children with HIV Infection: Using Evidence to Inform Policymakers
- The Impact of Transnational “Big Food” Companies on the South: A View from Brazil
- HIV Treatment as Prevention: Systematic Comparison of Mathematical Models of the Potential Impact of Antiretroviral Therapy on HIV Incidence in South Africa
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- HIV Treatment as Prevention: Issues in Economic Evaluation
- HIV Treatment as Prevention: Modelling the Cost of Antiretroviral Treatment—State of the Art and Future Directions
- HIV Treatment as Prevention: The Utility and Limitations of Ecological Observation
- Consequences of Gestational Diabetes in an Urban Hospital in Viet Nam: A Prospective Cohort Study
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



