Major Burden of Severe Anemia from Non-Falciparum Malaria Species in Southern Papua: A Hospital-Based Surveillance Study
Background:
The burden of anemia attributable to non-falciparum malarias in regions with Plasmodium co-endemicity is poorly documented. We compared the hematological profile of patients with and without malaria in southern Papua, Indonesia.
Methods and Findings:
Clinical and laboratory data were linked for all patients presenting to a referral hospital between April 2004 and December 2012. Data were available on patient demographics, malaria diagnosis, hemoglobin concentration, and clinical outcome, but other potential causes of anemia could not be identified reliably. Of 922,120 patient episodes (837,989 as outpatients and 84,131 as inpatients), a total of 219,845 (23.8%) were associated with a hemoglobin measurement, of whom 67,696 (30.8%) had malaria. Patients with P. malariae infection had the lowest hemoglobin concentration (n = 1,608, mean = 8.93 [95% CI 8.81–9.06]), followed by those with mixed species infections (n = 8,645, mean = 9.22 [95% CI 9.16–9.28]), P. falciparum (n = 37,554, mean = 9.47 [95% CI 9.44–9.50]), and P. vivax (n = 19,858, mean = 9.53 [95% CI 9.49–9.57]); p-value for all comparisons <0.001. Severe anemia (hemoglobin <5 g/dl) was present in 8,151 (3.7%) patients. Compared to patients without malaria, those with mixed Plasmodium infection were at greatest risk of severe anemia (adjusted odds ratio [AOR] 3.25 [95% CI 2.99–3.54]); AORs for severe anaemia associated with P. falciparum, P. vivax, and P. malariae were 2.11 (95% CI 2.00–2.23), 1.87 (95% CI 1.74–2.01), and 2.18 (95% CI 1.76–2.67), respectively, p<0.001. Overall, 12.2% (95% CI 11.2%–13.3%) of severe anemia was attributable to non-falciparum infections compared with 15.1% (95% CI 13.9%–16.3%) for P. falciparum monoinfections. Patients with severe anemia had an increased risk of death (AOR = 5.80 [95% CI 5.17–6.50]; p<0.001). Not all patients had a hemoglobin measurement, thus limitations of the study include the potential for selection bias, and possible residual confounding in multivariable analyses.
Conclusions:
In Papua P. vivax is the dominant cause of severe anemia in early infancy, mixed P. vivax/P. falciparum infections are associated with a greater hematological impairment than either species alone, and in adulthood P. malariae, although rare, is associated with the lowest hemoglobin concentration. These findings highlight the public health importance of integrated genus-wide malaria control strategies in areas of Plasmodium co-endemicity.
Please see later in the article for the Editors' Summary
Published in the journal:
Major Burden of Severe Anemia from Non-Falciparum Malaria Species in Southern Papua: A Hospital-Based Surveillance Study. PLoS Med 10(12): e32767. doi:10.1371/journal.pmed.1001575
Category:
Research Article
doi:
https://doi.org/10.1371/journal.pmed.1001575
Summary
Background:
The burden of anemia attributable to non-falciparum malarias in regions with Plasmodium co-endemicity is poorly documented. We compared the hematological profile of patients with and without malaria in southern Papua, Indonesia.
Methods and Findings:
Clinical and laboratory data were linked for all patients presenting to a referral hospital between April 2004 and December 2012. Data were available on patient demographics, malaria diagnosis, hemoglobin concentration, and clinical outcome, but other potential causes of anemia could not be identified reliably. Of 922,120 patient episodes (837,989 as outpatients and 84,131 as inpatients), a total of 219,845 (23.8%) were associated with a hemoglobin measurement, of whom 67,696 (30.8%) had malaria. Patients with P. malariae infection had the lowest hemoglobin concentration (n = 1,608, mean = 8.93 [95% CI 8.81–9.06]), followed by those with mixed species infections (n = 8,645, mean = 9.22 [95% CI 9.16–9.28]), P. falciparum (n = 37,554, mean = 9.47 [95% CI 9.44–9.50]), and P. vivax (n = 19,858, mean = 9.53 [95% CI 9.49–9.57]); p-value for all comparisons <0.001. Severe anemia (hemoglobin <5 g/dl) was present in 8,151 (3.7%) patients. Compared to patients without malaria, those with mixed Plasmodium infection were at greatest risk of severe anemia (adjusted odds ratio [AOR] 3.25 [95% CI 2.99–3.54]); AORs for severe anaemia associated with P. falciparum, P. vivax, and P. malariae were 2.11 (95% CI 2.00–2.23), 1.87 (95% CI 1.74–2.01), and 2.18 (95% CI 1.76–2.67), respectively, p<0.001. Overall, 12.2% (95% CI 11.2%–13.3%) of severe anemia was attributable to non-falciparum infections compared with 15.1% (95% CI 13.9%–16.3%) for P. falciparum monoinfections. Patients with severe anemia had an increased risk of death (AOR = 5.80 [95% CI 5.17–6.50]; p<0.001). Not all patients had a hemoglobin measurement, thus limitations of the study include the potential for selection bias, and possible residual confounding in multivariable analyses.
Conclusions:
In Papua P. vivax is the dominant cause of severe anemia in early infancy, mixed P. vivax/P. falciparum infections are associated with a greater hematological impairment than either species alone, and in adulthood P. malariae, although rare, is associated with the lowest hemoglobin concentration. These findings highlight the public health importance of integrated genus-wide malaria control strategies in areas of Plasmodium co-endemicity.
Please see later in the article for the Editors' Summary
Introduction
Anemia is a common manifestation of Plasmodium infection and is responsible for substantial morbidity [1]–[4] as well as direct [5]–[7] and indirect mortality [8]–[11]. Its pathogenesis is incompletely understood. Acute falciparum malaria results in increased removal from the circulation of parasitized and, to a greater extent, non-parasitized red blood cells through a combination of splenic filtration [12], schizont rupture, macrophage phagocytosis [13], complement-mediated hemolysis [14], and increased free radical damage [15],[16]. In more chronic infections, decreased marrow production of functional red blood cells due to the direct inhibitory effects of parasites [17] and cytokines [13],[18] along with dysregulation of erythropoietin and iron metabolism [19] adds to the anemia of ongoing red blood cell loss [16]. Although some of these processes have been described in the non-falciparum human malarias [20]–[23], less is known about the epidemiology and pathogenesis of anemia due to these Plasmodium species [24]–[26].
In regions of high P. falciparum endemicity, repeated infections from an early age induce robust immunity to clinical disease and a low risk of severe anemia beyond childhood [27]. However, much of the malarious world has low or moderate malarial endemicity. Outside of Africa, P. falciparum invariably co-exists with other Plasmodium species—the most important of which is P. vivax. In these regions, less intense parasite exposure during early life delays the development of immunity and gives rise to the potential for symptomatic and complicated infections at all ages.
Biological differences and interactions between P. falciparum and the other Plasmodium species complicate analyses of the pattern and public health impact of malarial anemia in co-endemic areas. Plasmodium vivax infection has been shown to reduce the risk of anemia secondary to P. falciparum malaria, possibly by providing a degree of cross-species immunity [28]–[31]. However, recent population-based studies have revealed a high risk of severe anemia associated with P. vivax infection and mixed-species infections suggesting that the hematological impact of the non-falciparum malarias may have been underestimated [30],[32]–[35]. The age-associated changes in risk of anemia in non-falciparum and mixed-species infections and the consequences of anemia caused by these species are largely unknown [26],[33],[34].
In the present study we used clinical and laboratory data from Mitra Masyarakat Hospital in southern Papua, Indonesia, to establish the comparative hematological profiles of patients infected by the different Plasmodium species in a co-endemic setting.
Methods
Ethical Approval
Ethical approval for this study was obtained from the Health Research Ethics Committees of the University of Gadjah Mada, Indonesia, Menzies School of Health Research, Darwin, Australia, and the Oxford Tropical Centre, Oxford, UK.
Study Site
Mimika District lies in south-central Papua, the easternmost province of Indonesia. Its geography, climate, and demographics have been described elsewhere [33],[36],[37]. In brief, censuses in 2004 and 2007 estimated the local population to be 130,000 and 170,000 people, respectively, approximately 50% of whom were indigenous Papuans; the remaining 50% being Indonesians from elsewhere in the archipelago [37]. Malaria transmission is limited to lowland areas where it is associated with three mosquito vectors: Anopheles koliensis, An. farauti, and An. punctulatus. The estimated average annual incidence of parasitemia is 876 episodes per 1,000 people, 58% due to P. falciparum, 37% due to P. vivax, 3% due to mixed infection, and 1.8% due to P. malariae [37]. The point prevalence of asexual parasitemia in 2005 was estimated to be 7.5% for P. falciparum, 6.4% for P. vivax, 1.9% for mixed infection, and 0.6% for P. malariae [37].
Until November 2008, Rumah Sakit Mitra Masyarakat (RSMM) was the only referral hospital in the district; since 2008 RSMM treated approximately 80% of patients with malaria attending an inpatient facility in the district. RSMM has 110 beds, a high dependency unit, a 24-hour emergency department, and a busy outpatients department that reviews approximately 300 patients per day, 6 days per week. Treatment is provided free of charge to Papuans and at a cost for non-Papuans.
Laboratory and Data Collection Procedures
Protocols dictate that all patients presenting to the outpatients department with a fever or symptoms consistent with malaria and all inpatients, regardless of diagnosis, should have a malaria blood film. As a result, reliance on clinical symptoms alone to make the diagnosis of malaria is discouraged and rare. Microbiological diagnosis of malaria is usually based on a thick film examination, though confirmatory thin films and histidine rich protein (HRP2)-based rapid diagnostic tests for P. falciparum (Paracheck) are also performed in some cases. In 2004, a random selection of 1,083 positive slides was re-read by an independent expert microscopist with more than 10 years of experience. Concordance was 90% with 1.7% of cases reported as negative on the second reading and 4% of monoinfections reclassified as mixed infections. Complete blood counts are ordered according to clinical indication and are performed using a Coulter Counter (JT Coulter).
Every patient presenting to RSMM for the first time (regardless of department) is assigned a unique hospital record number (HRN). All clinical, laboratory, and pharmacy data from the first and subsequent presentations are linked to this unique HRN. Hospital clerks record basic demographic and administrative information, mortality data, and the diagnoses given by the attending doctor (classified according to the International Classification of Diseases) for each patient presentation to hospital. Ethnicity is recorded as the patient's “Suku” (clan), which is self-reported to the admission clerk on presentation to the hospital. For the purposes of analyses, ethnicity was categorized as Highland Papuan, Lowland Papuan, or non-Papuan on the basis of the location of the clans' village(s). Hematological results are generated by coulter counter (JT Coulter) and collated in a separate laboratory database. Pharmacy data are entered manually into an electronic database by the pharmacist fulfilling the prescription. Since data were gathered from routine hospital surveillance informed consent was not requested from participants, however all records were anonymized to ensure patient confidentiality.
Data Merging and Statistical Analyses
Clinical data were merged with hematology data by creating all possible pairwise combinations for each HRN. Pairs in which the laboratory record fell between the date of presentation and discharge (the same day for outpatient visitations) were kept and if more than one hemoglobin measurement was available for a single event, the lowest was taken (Figure 1). For the purposes of these analyses, mixed infection was defined as concomitant infection with any combination of Plasmodium species.
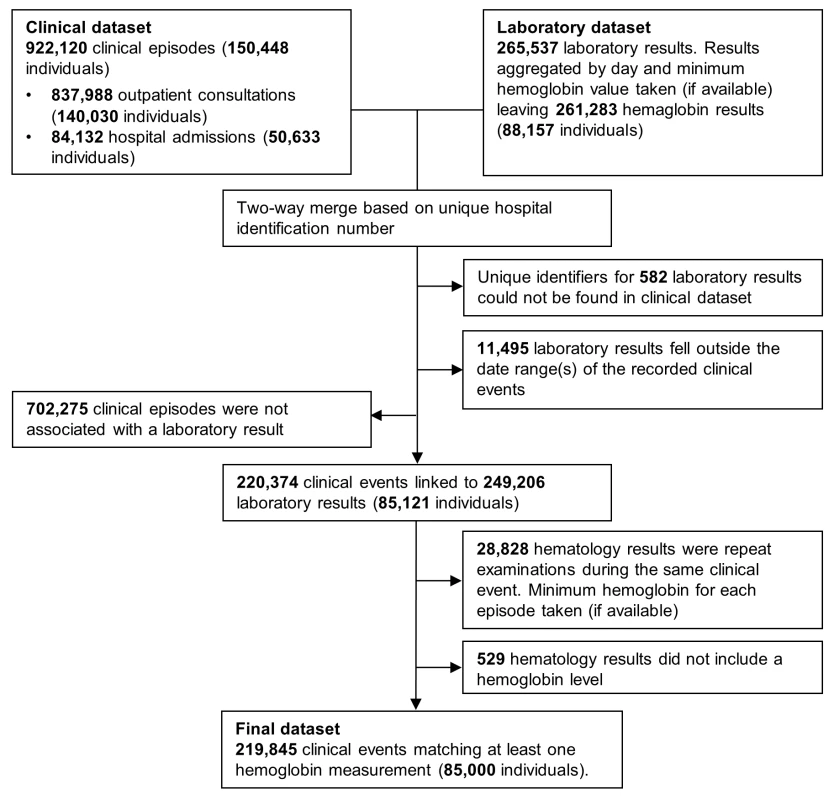
The primary outcomes in this study were the mean hemoglobin concentration (g/dl) associated with infection by the different Plasmodium species with comparison to patients without malaria, presence of severe anemia (hemoglobin less than 5 g/dl), the population attributable fraction (PAF) of severe anemia associated with infection by the different Plasmodium species at all ages from infancy through to adulthood, and all-cause mortality. Continuous hemoglobin data were analysed using linear regression and binary anemia data (such as severe anemia and death) were analysed using logistic regression. Since some patients appeared in the database multiple times, robust standard errors were calculated using the Huber-White sandwich estimator.
Univariable analyses were performed for each of the following variables: Plasmodium species (negative, P. falciparum, P. vivax, P. malariae, P. ovale, or mixed species), sex, self-reported ethnicity (non-Papuan, Highland Papuan, Lowland Papuan), age group (<1 year, 1 to <5 years, 5 to <15 years, ≥15 years), and year of presentation (2004 through to 2012). All of these factors, as well as the interaction between age and Plasmodium species, were associated with clinically important differences in hematological status and included in multivariable models. Department (outpatient versus inpatient) and the number of presentations with malaria in the preceding 2 months (0, 1, or 2 or more) were also included in univariable analyses; however, both factors were omitted from multivariable analyses since department was deemed to be a consequence of severe disease rather than a confounder, and the number of preceding malaria episodes would potentially obscure the net hematological effect of Plasmodium infection. Fractional polynomials were used to allow for the non-linear relationship between age (as a continuous exposure) and the mean hemoglobin and risk of severe anemia, because they make no assumption regarding the shape of this association [38]. To maintain stability of the fractional polynomial models infants under 1 week of age (n = 1114, 0.51%) were excluded since they would not have had ex utero exposure to malaria. Adults over 63 years (the 99th percentile) of age (n = 2,405, 1.1%) were also excluded.
Adjusted PAFs of severe anemia associated with Plasmodium infection were calculated for multiple age groups from infancy through to adulthood from multivariable logistic regression models using the punaf module for STATA, which derives PAFs using the formulae provided in Greenland and Drescher [39]. No allowances were made for within-patient correlation in these analyses since the primary interest was the total hospital workload rather than the hematological status of individuals. Due to very small numbers, patients with P. ovale infections were excluded (n = 31, 0.01%) from the multivariable models. All statistical analyses were done in STATA version 12.1 (StataCorp).
Results
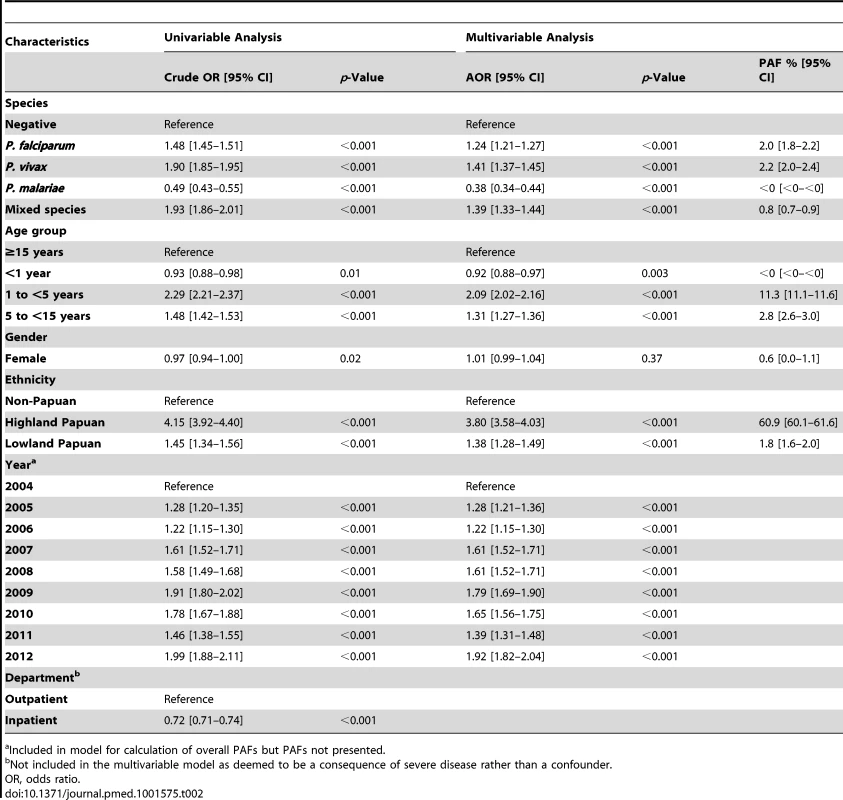
Between April 2004 and December 2012, there were 922,120 patient presentations to RSMM Hospital constituting 837,989 (90.9%) outpatient visitations and 84,131 (9.1%) inpatient admissions (Table 1). The age distribution of all patient presentations combined showed a peak in infancy and a second peak during the late 20 s (Figure 2). Microscopically confirmed malaria was diagnosed in 18.3% (168,525) of patient presentations, with P. falciparum accounting for 53.3% of monoinfections, P. vivax for 32.3%, P. malariae 2.7%, and P. ovale 0.06%. Mixed species infections were detected in 19,569 (11.6%) presentations, which in 18,489 (94.5%) cases were mixed P. falciparum and P. vivax infections.
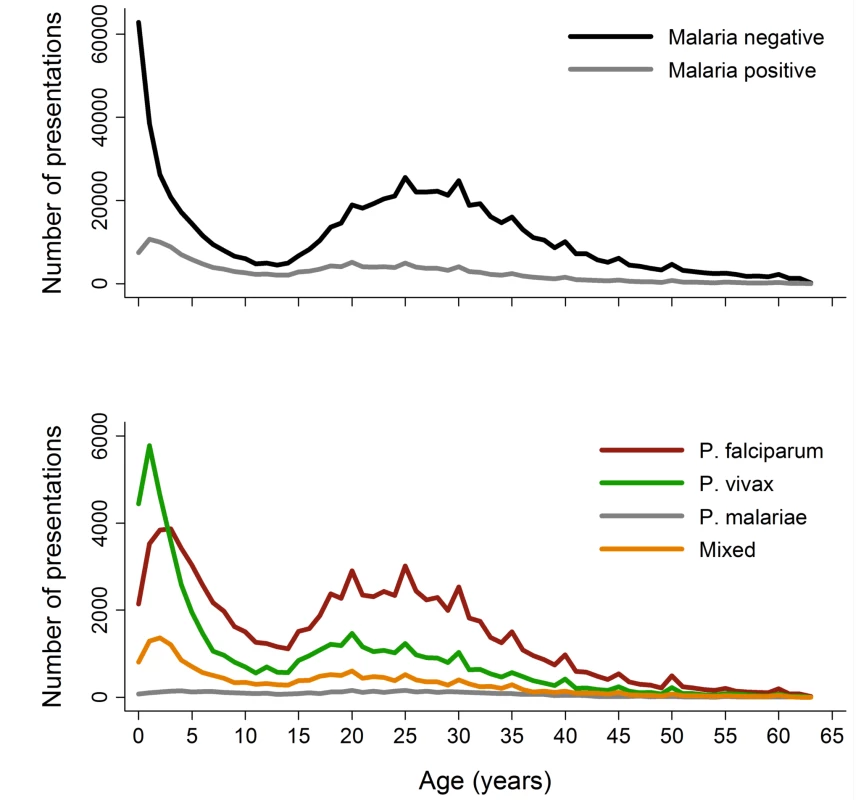
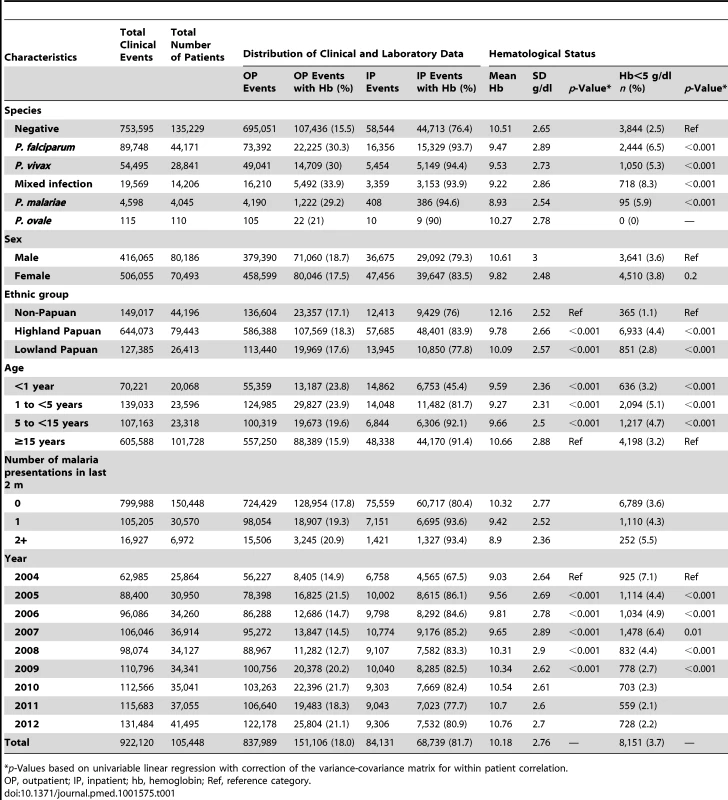
The absolute number of patient presentations with malaria over the study period peaked during the second year of life but as a proportion of all patient presentations was highest during the early teens (Figure 2). Plasmodium vivax infection was the dominant cause of malaria in patients under 3 years of age in both the outpatient and inpatient setting. Thereafter, P. falciparum was the most common malaria parasite.
Availability of Hemoglobin Data
Overall, 219,845 (23.8%) patient presentations (made by 85,000 individuals) were matched with at least one hemoglobin measurement. Of these 85,000 individuals, 40,427 (47.6%) had more than one presentation to hospital; the number of presentations ranging from 1 to 56 (median = 2). Excluding P. ovale (which was rare), 30.6% of malaria outpatient presentations were matched with a hemoglobin measurement compared to 15.5% of non-malaria presentations. The corresponding figures for patients admitted to the wards were 93.9% and 76.4%, respectively. Patients who had a hemoglobin measurement were slightly younger than those who did not have a measurement, the median age being 23.0 years versus 24.5 years in those without malaria and 15.9 years versus 18.2 years for those with malaria; p<0.001 for both comparisons. Measurement of hemoglobin was also more common in males compared to females (odds ratio = 1.02 [95% CI 1.01–1.03]) and Papuans compared to non-Papuans (odds ratio = 1.13 [95% CI 1.12–1.15]); p<0.001 for both comparisons.
The mean of the minimum hemoglobin concentration recorded for each patient presentation was 10.18 g/dl and the maximum 10.34 g/dl. The mean difference between minimum and maximum hemoglobin was greatest for patients with P. falciparum (either alone 0.21 g/dl [standard deviation (SD) = 0.82] or mixed 0.24 g/dl [SD = 0.88]) compared to 0.17 g/dl (SD = 0.80) in patients with P. vivax and 0.14 g/dl (SD = 0.72) in those with P. malariae; p<0.001.
In total 13.7% (30,174/219,845) of the patients with a hemoglobin measurement had presented to hospital with malaria within the previous 2 months, the number of malaria episodes ranging from 1 to 6. The risk of recent malaria presentation was greatest in patients presenting with P. vivax either alone or mixed (20.5%, 5,840/28,503) compared to those with P. falciparum alone (14.6%, 5,493/37,554), P. malariae (6%, 97/1,608), or those without malaria (12.3% 18,742/152,149); p<0.0001. Recent malaria caused by any species was most common in young children aged 1 to 5 years old with an odds ratio of 2.29 (95% CI 2.21–2.37; p<0.0001) compared to adults (see Table 2).
Reduction in Hemoglobin Concentration Associated with Malaria
The mean hemoglobin concentration and prevalence of severe anemia varied significantly by Plasmodium species (Table 1). Mixed infection and P. malariae infection were associated with particularly poor hematological status (mean hemoglobin 9.22 g/dl and 8.93 g/dl, respectively; prevalence of severe anemia 8.3% and 5.9%, respectively) whilst the hematological statuses of patients infected with P. vivax and P. falciparum monoinfection were less severely impaired and broadly similar (Table 1). Highland Papuans had a mean hemoglobin of 9.78 g/dl compared to a mean of 10.09 g/dl in Lowland Papuans and 12.2 g/dl in non-Papuans; p for comparisons with non-Papuans <0.001 (Figure 3). Adult females had significantly lower mean hemoglobin concentrations compared to adult males (mean difference = −1.62 g/dl), and this remained apparent after excluding women known to be pregnant (mean difference = −1.46 g/dl); p<0.001.
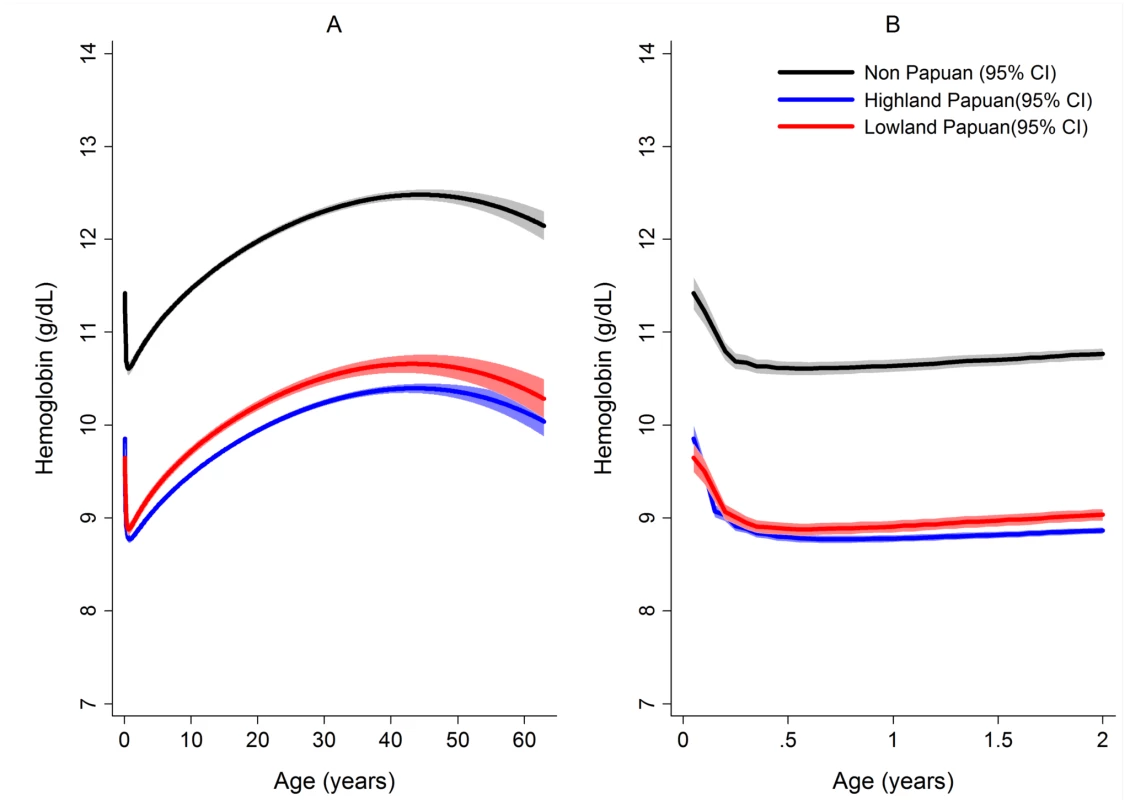
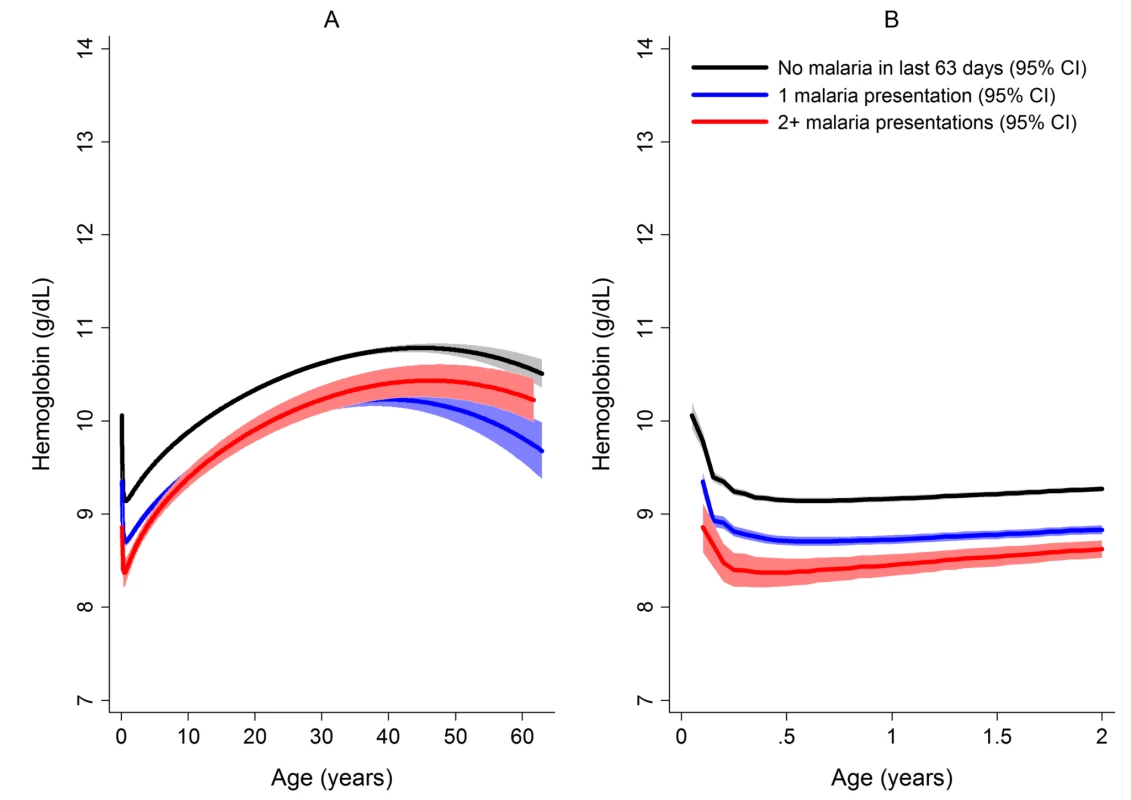
After correcting for confounding factors, patients presenting to hospital with malaria had lower mean hemoglobin concentrations and higher odds of severe anemia than patients without malaria at all ages, but most noticeably during childhood (Figure 4). Overall, P. malariae was associated with the greatest difference in mean hemoglobin compared to those without malaria (−1.40 g/dl [95% CI −1.52 to −1.29 g/dl]) followed by mixed infection (−1.01 g/dl [95% CI −1.07 to −0.95 g/dl]), P. falciparum (−0.70 g/dl [95% CI −0.73 to −0.66 g/dl]), and P. vivax (−0.58 g/dl [95% CI −0.62 to −0.54 g/dl]), p for all comparisons <0.001. Patients without a presentation to hospital with malaria within the preceding 2 months had a mean hemoglobin of 10.32 g/dl (95% CI 10.30–10.33 g/dl) compared to 9.42 g/dl (95% CI 9.39–9.45 g/dl) in patients with a single recent episode of malaria and 8.90 g/dl (95% CI 8.83–8.97 g/dl) in those with two or more episodes of malaria; p<0.001 (Figure 5; Table 1).
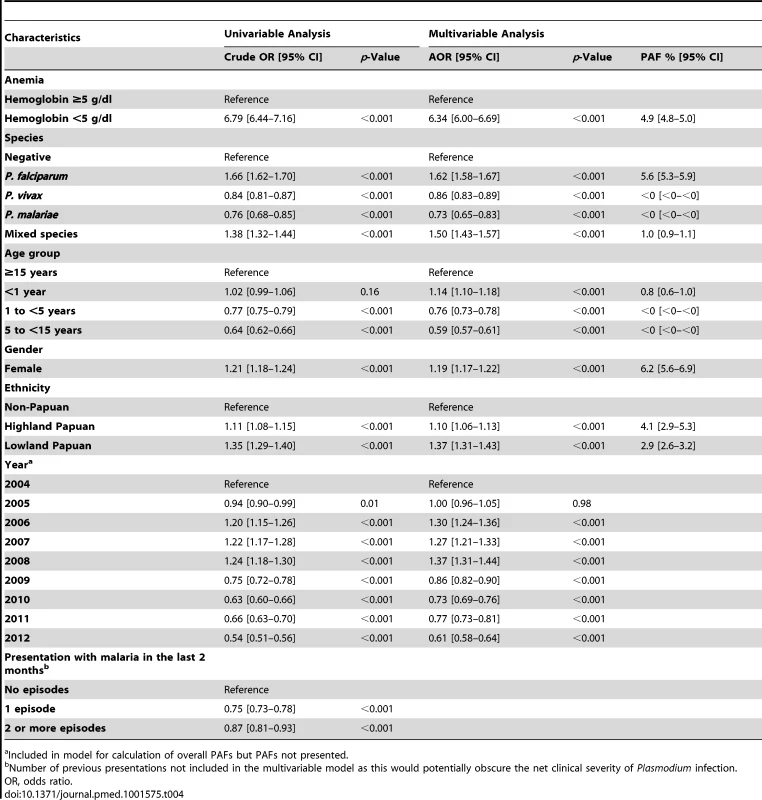
The Risk of Severe Anemia
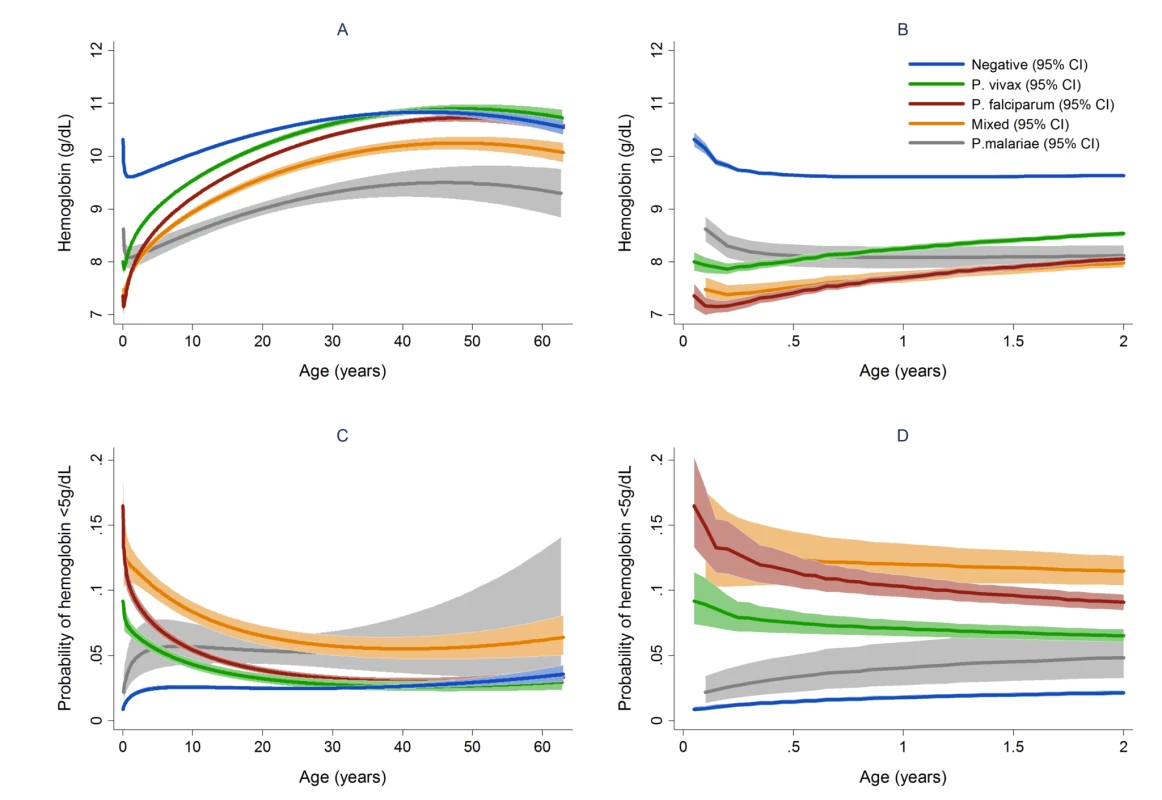
In total 97,665 (44.4%) of the 219,845 patient presentations were associated with a hemoglobin concentration less than 10 g/dl, 27,106 (12.3%) less than 7 g/dl, and 8,151 (3.7%) less than 5 g/dl. Compared to patients without malaria, those with mixed Plasmodium species infections were at the greatest risk of severe anemia (adjusted odds ratio [AOR] 3.25 [95% CI 2.99–3.54]) followed by those with P. malariae (AOR 2.18 [95% CI 1.76–2.67]), P. falciparum (AOR 2.11 [95% CI 2.00–2.23]), and P. vivax (AOR 1.87 [95% CI 1.74–2.01]), p for all comparisons <0.001 (Table 3). Patients with a recent presentation to hospital with P. falciparum malaria were at greater risk of severe anemia compared to those with recent P. vivax infection (5.3% [783/14,743] versus to 3.7% [459/12,507]; AOR = 1.51 [95% CI 1.32–1.73]; p<0.001). The comparative reduction in hemoglobin associated with P. malariae was greatest in adulthood whereas for P. falciparum, P. vivax, and mixed infections, it was worst during infancy (Figure 4).
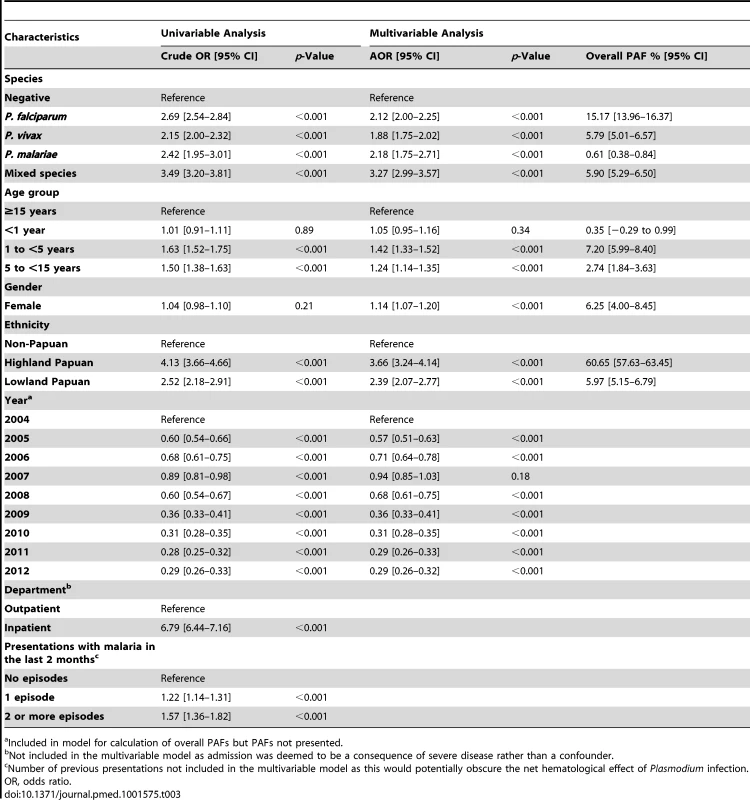
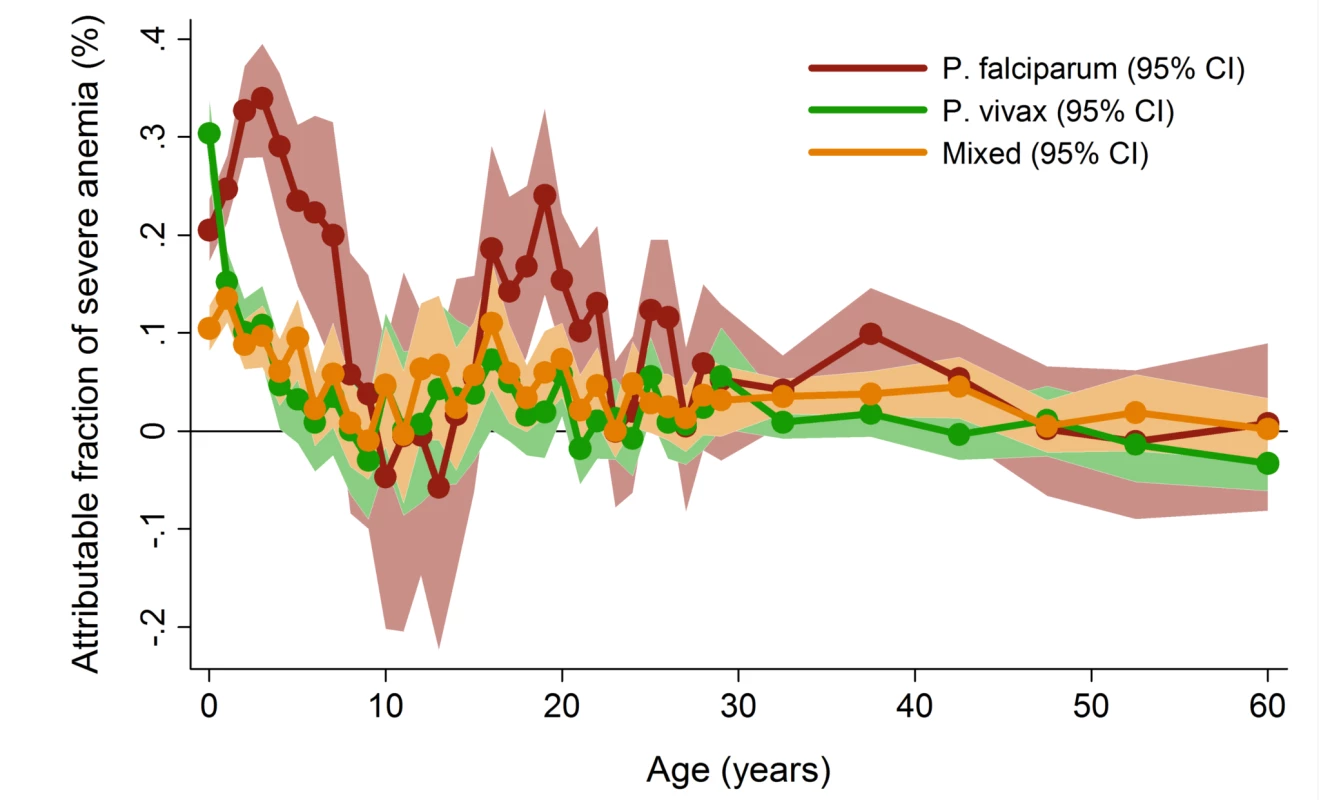
In patients presenting to hospital the overall adjusted population fraction of severe anemia attributable to P. falciparum was 15.1% (95% CI 13.9–16.3%), compared to 5.75% (4.97%–6.53%) for P. vivax, 0.61% (0.38%–0.84%) for P. malariae, and 5.89% (5.28%–6.50%) for mixed species infections. These attributable fractions were unchanged when pregnant women were excluded. The fraction of severe anemia attributable to P. vivax, P. malariae, or mixed species infections was 12.2% (95% CI 11.2%–13.3%). The fraction of severe anemia attributable to P. vivax was highest in infancy, when it rose to 30.4% (95% CI 26.4%–34.2%) compared to 20.5% (95% CI 17.2%–23.7%) for P. falciparum. Following childhood, a second peak in the fraction of anemia attributable to malaria was apparent for all species between the ages of approximately 15 and 25 years (Figure 6).
The Consequences of Severe Anemia
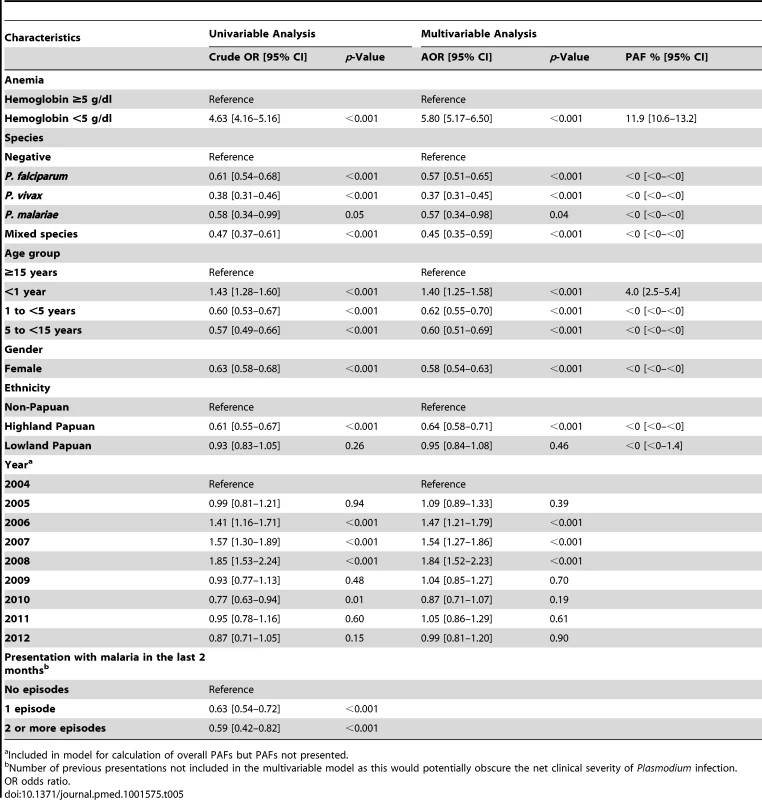
Admission for inpatient care was required in 63.6% (3,166/4,975) of the patients presenting initially to the outpatient department with severe anemia compared to 14% (21,481/151,454) of those without severe anemia (AOR = 6.34 [95% CI 6.00–6.69], p<0.001) (Table 4). Inpatients with severe anemia had a longer hospital stay (median 4 days [interquartile range (IQR) 2–7 days]) compared to 3 days (IQR = 2–4 days) in inpatients without severe anemia (p<0.001).
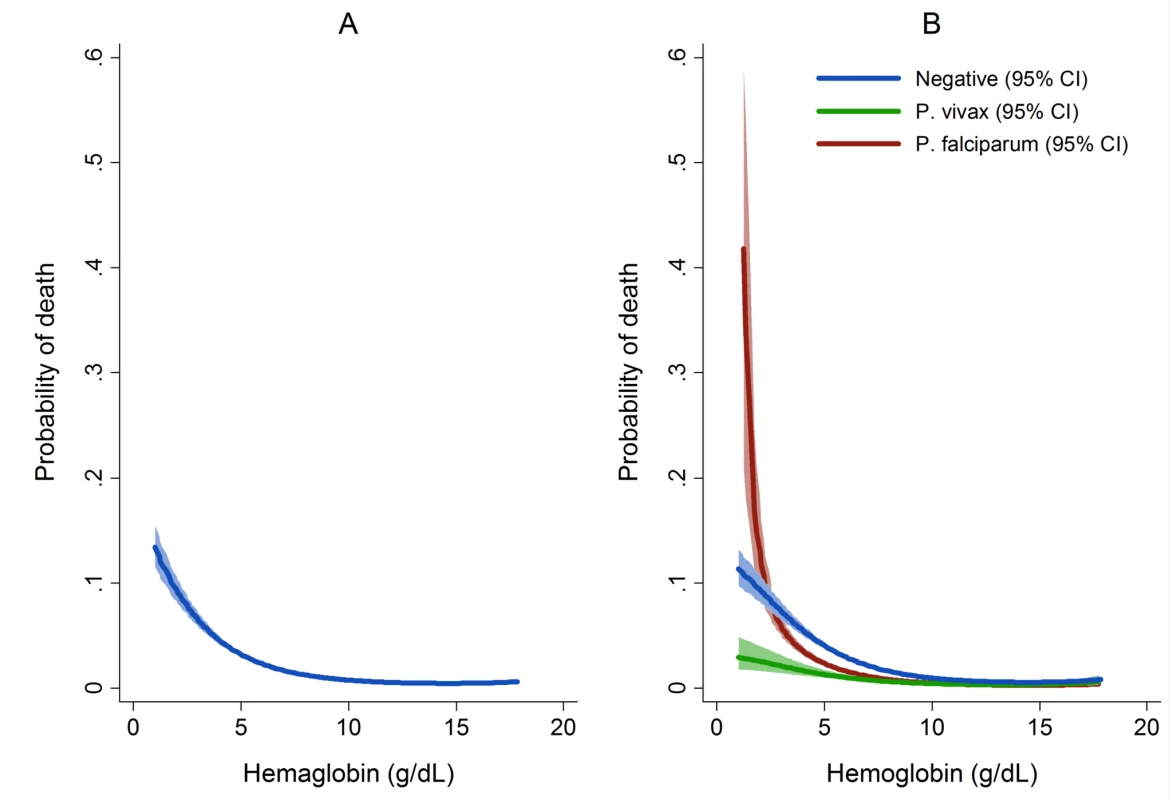
The overall risk of mortality in those admitted to hospital with a hemoglobin measurement was 1.3% (2,781/219,845), the risk increasing as the hemoglobin concentration fell below 7 g/dl (Figure 7). In total 1.5% (2,254/152,149) of patients without malaria died compared to 0.9% (340/37,554) with P. falciparum infection, 0.6% (112/19,858) with P. vivax infection, 0.9% (0.9% 14/1,608) with P. malariae infection, and 0.7% (61/8,645) with mixed infection. The odds ratio for death in patients with a hemoglobin concentration lower than 5 g/dl was 4.63 (95% CI 4.16–5.16), compared to those with a higher hemoglobin concentration, p<0.001. After controlling for age, the AOR of death associated with severe anemia was 5.91 (95% CI 5.17–6.76) for patients without concurrent malaria, 5.93 (95% CI 4.63–7.58) for those with P. falciparum infection, 5.33 (95% CI 3.10–9.17) for mixed infections, 4.43 (95% CI 2.76–7.11) for P. vivax, and 6.58 (95% CI 2.02–21.47) for those with P. malariae. The PAF of death associated with severe anaemia was 11.9% (95% CI 10.6%–13.2%). The full multivariable model for mortality is presented in Table 5.
Discussion
Anemia is highly prevalent throughout the tropics and has major consequences for human health and socioeconomic prosperity [40]. Our large hospital-based study in southern Papua, Indonesia provides a comparative assessment of the pattern of hematological changes associated with the non-falciparum malarias as well as an estimate of their public health importance. We have shown that P. vivax is associated with a high burden of severe anemia in infancy (almost one-quarter of all cases at the hospital), that mixed P. vivax/P. falciparum infection causes significantly more hematological impairment than monoinfection with either species alone, and that in adulthood P. malariae infection is associated with the lowest mean hemoglobin concentration. Approximately 12% of severe anemia at the hospital can be attributed to non-falciparum Plasmodium infection, the consequences of which include a 6-fold greater risk of admission to hospital and an increased duration of inpatient stay. More importantly, lower hemoglobin concentrations were associated with a 5-fold increased risk of death, the worst prognosis being in patients with severe anemia associated with falciparum malaria and those without concurrent malaria.
Infancy is a time of rapid physical and cognitive development as well as increased vulnerability to infectious diseases. Our study shows that the risk of symptomatic vivax malaria is more heavily skewed towards infancy compared with other Plasmodium species, probably reflecting more efficient transmission and faster acquisition of immunity. For a given parasite density, P. vivax evokes a greater immune response [41],[42] than other species. Subsequent parasite exposure is also higher due to its propensity to relapse multiple times. In our study, patients with P. vivax infection were significantly more likely to have had a prior presentation with malaria in the preceding 2 months compared with the other species. Infants are particularly vulnerable to recurrent episodes of vivax malaria, a likely consequence of lower immunity and greater inherent susceptibility to infection; P. vivax has a strong predilection for reticulocytes, which are most abundant in the first 3 months of life [43].
Our study confirms P. vivax as the predominant malaria infection in infancy and highlights its frequent association with severe anemia in this age group. Young children with severe falciparum malarial anemia have previously been shown to have an increased risk of mortality [5],[11],[40] and blood transfusion (with attendant risk of blood borne disease transmission) [5] and they may also have reduced resilience to other infectious and non-infectious diseases. In our study, patients with vivax malaria and severe anemia had a 4.4-fold increase in the probability of death from any cause compared to those without severe anaemia. However the risk of death in those with severe anemia without concomitant malaria and, in particular, severe anemia caused by P. falciparum infection was much higher (Figure 5). It is possible that this is due to P. falciparum being associated with higher parasitemia and potentially a faster and larger decrement in hemoglobin; a rapid drop in hemoglobin permitting less time for the body to make physiological adjustments to ameliorate deleterious effects of severe anemia. In contrast severe anemia caused by P. vivax infection is more likely to be due to a chronic process, resulting from the accumulation of smaller hemoglobin decrements with each recurrent bout of infection. This hypothesis is supported by the greater reduction within a single clinical encounter (maximum to minimum hemoglobin concentration) in patients with P. falciparum (0.21 g/dl) and mixed infections (0.24 g/dl) compared to those with P. vivax monoinfection (0.17 g/dl), and the 1.5-fold greater risk of severe anemia observed following recent falciparum malaria compared to those with recent vivax malaria. In non-malarial severe anemia the greater mortality may reflect a more acute onset compounded by a greater number or severity of comorbidities. Our study was unable to assess the long term effects of malarial anemia, which in other studies has been associated with adverse developmental and socioeconomic sequelae, although defining these is challenging due to the multifactorial aetiology of anemia in malaria-endemic areas [4],[11],[40],[44]. Malaria has been linked with impaired cognitive development, but it is unclear if this is due to the hematological effects of infection or the infection itself [44].
Previous studies in Thailand have shown an association between concomitant P. vivax infection and reduced severity of P. falciparum infection [30],[31]. However this was not apparent in southern Papua where mixed infections after 3 years of age were associated with both a lower mean hemoglobin concentration and greater odds of severe anemia than monoinfection with either species. This could reflect greater antigenic diversity of local parasite strains and thus less cross-reactive immunity, though empiric evidence for this is lacking. Alternatively, it may relate to greater transmission intensity in southern Papua. In this region the majority of severe malarial anemia beyond childhood is likely to be the result of repetitive infections in individuals who have already developed partial immunity. In this situation, the hematological effects of the two species are likely to be additive and any immunomodulatory effect of P. vivax on P. falciparum is likely to be negligible. In Thailand, where transmission is less intense, severe falciparum anemia is more likely to result from a single fulminant infection in a non-immune individual and therefore any immunomodulatory effect of P. vivax would be proportionately more important.
Somewhat unexpectedly, P. malariae was found to be associated with the lowest mean hemoglobin of all locally prevalent Plasmodium species, and a high frequency of severe anemia. Its hematological effects were most apparent in adulthood whereas in childhood it was associated with minimal impairment. Plasmodium. malariae is a slow-growing parasite that usually causes a low parasitemia [45]. It is believed to have a predilection for old red blood cells [45] and hence should have a minimal effect on the average lifespan of circulating red blood cells [46]. A high proportion of P. malariae infections is asymptomatic and therefore unlikely to prompt presentation to a health care service [37]. We suspect that the severity of the hematological impairment seen in this study is related to prolonged red cell destruction and bone marrow suppression as a result of chronic, asymptomatic, or minimally symptomatic parasitemia. Plasmodium malariae is prevalent in much of sub-Saharan Africa [47] and according to the results of this study may be associated with an underappreciated burden of anemia in adults.
Our study has several strengths. It includes patients with uncomplicated malaria presenting to the outpatients department through to those with complicated disease who were admitted to hospital. The analysis was focused on clinical events, with each patient presenting in some cases multiple times over the 8 years of the study period. All children greater than 1 week of age were included whereas other studies have excluded those under 6 months of age [26]. Cases of malaria were microscopically confirmed by quality-assured microscopists and data entry was carried out in a consistent fashion. Because of strict hospital policy, the vast majority of symptomatic malaria infections were likely to have been ascertained. There are also a number of limitations to our study. Hemoglobin measurement was done on an as-needed basis and therefore a degree of selection bias has almost certainly occurred. Although the majority of patients with malaria did not have a hemoglobin measurement, the proportion of patients who did have a measurement was the same for all species (P. ovale excluded) and hence, comparisons of the hematological effects of the different species are likely to be valid. Those without malaria were less likely to have a full blood count, both in the outpatient and inpatient setting. However, the low threshold for hematological investigation at the hospital suggests that patients who did not have a blood test would be unlikely to have severe anemia. Furthermore the absence of malaria at the time of the clinical encounter does not preclude the contribution to anemia of prior, recently treated, asymptomatic, or submicroscopic parasitemia. Our study was also limited to observations documented during the hospital encounter and could not address out-of-hospital deaths associated with severe anaemia. However these biases are likely to have resulted in the derived malaria-attributable fractions of severe anemia and their associated morbidity and mortality being conservative, if anything underestimating the true burden of disease.
Our analyses may have also been subject to residual confounding. Hemoglobin and red cell polymorphisms are known to influence the likelihood of severe malarial anemia [48]–[50] and in some cases may also modulate the risk of uncomplicated parasitemia [51]–[56]. We adjusted for ethnicity, which, in Papua, is strongly indicative of the geographic location and altitude of tribal lands and thus should have accounted for at least some of the potential variation in the prevalence of these disorders by malaria status.
Other potential confounders include nutritional status, geohelminth infestation, bacteremia, chronic disease, and pregnancy status. Apart from pregnancy status, information on these confounders was not available. Malaria and malnutrition may cluster in the same geographic areas, but the mechanisms and direction of any biological link have not been elucidated conclusively. Chronic vivax malaria has been implicated in a state of malnourishment akin to kwashiorkor [57], whereas iron deficiency may provide some protection against malaria infection [58]. Geohelminth infection causes gastrointestinal blood loss, which may exacerbate anemia caused by P. falciparum malaria [59]. Two oft-cited studies by Nacher and Spiegel, respectively, indicate that intestinal helminthiasis increases the risk of falciparum malaria by a factor of 1.5 and 2.2 [60],[61]. The relationship between geohelminth infection and P. vivax malaria is unclear [62],[63]. There is a potential that the severity of anemia, and hence the attributable fraction of anemia, associated with Plasmodium infection in our study may have been overestimated by our inability to control for geohelminth infection. This is unlikely to have been important for children under 3 years old since helminth density typically peaks in adulthood and previous work has shown minimal impact on hemoglobin concentrations prior to 30 months of age [64].
Pregnancy may have also confounded the association of anaemia and malaria in adults. Pregnant women are at comparatively high risk of P. falciparum, and possibly P. vivax, parasitemia in malaria endemic regions [65],[66]. Our previous studies at the same hospital in southern Papua have demonstrated that primigravidae women are at increased risk of severe malarial anemia [36]. Reassuringly in a subgroup analysis the exclusion of women known to be pregnant did not alter the PAF of anemia associated with malaria.
Finally the results of this study cannot necessarily be generalised to individuals with asymptomatic disease. Although cross-sectional survey data could be used to compare the hematological impact of symptomatic and asymptomatic Plasmodium infection this approach would tend to miss patients at the severe end of the spectrum.
In conclusion our very large hospital-based study demonstrates that the non-falciparum malarias make a major contribution to the burden of anemia in southern Papua, with 12% of all severe anemia at the hospital attributable to P. vivax, P. malariae, or mixed species infections. Plasmodium vivax was associated with the greatest attributable fraction of severe anemia in infancy and is likely to be an important cause of indirect morbidity. Conversely, the hematological impact of P. malariae was most apparent in adults. In contrast to earlier reports from regions of low mixed-species endemicity, mixed P. falciparum/P. vivax infections are associated with a significantly greater absolute reduction in hemoglobin and odds of severe anemia than monoinfection with either species alone. Severe malarial anemia, including that caused by P. vivax infection, is associated with an increased likelihood of admission, a prolonged hospital stay, and, particularly with P. falciparum infections, dramatically increased mortality. These findings highlight the public health importance of integrated genus-wide rather than species-specific malaria control strategies in areas of Plasmodium co-endemicity.
Zdroje
1. Roca-FeltrerA, CarneiroI, Armstrong SchellenbergJR (2008) Estimates of the burden of malaria morbidity in Africa in children under the age of 5 years. Trop Med Int Health 13: 771–783.
2. Snow RW, Craig MH, Newton CRJC, Steketee RW (2003) The public health burden of Plasmodium falciparum malaria in Africa: deriving the numbers. BethesdaMaryland: Fogarty International Center, National Institutes of Health.
3. MurphySC, BremanJG (2001) Gaps in the childhood malaria burden in Africa: cerebral malaria, neurological sequelae, anemia, respiratory distress, hypoglycemia, and complications of pregnancy. Am J Trop Med Hyg 64: 57–67.
4. CalisJCJ, PhiriKS, FaragherEB, BrabinBJ, BatesI, et al. (2008) Severe anemia in Malawian children. N Engl J Med 358: 888–899.
5. MarshK, ForsterD, WaruiruC, MwangiI, WinstanleyP, et al. (1995) Indicators of life-threatening malaria in African children. N Engl J Med 332: 1399–1404.
6. ObonyoCO, VululeJ, AkhwaleWS, GrobbeeDE (2007) In-hospital morbidity and mortality due to severe malarial anemia in Western Kenya. Am J Trop Med Hyg 77: 23–28.
7. SlutskerL, TaylorTE, WirimaJJ, SteketeeRW (1994) In-hospital morbidity and mortality due to malaria-associated severe anaemia in two areas of Malawi with different patterns of malaria infection. Trans R Soc Trop Med Hyg 88: 548–551.
8. NevillCG, SomeES, Mung'alaVO, MutemiW, NewL, et al. (1996) Insecticide-treated bednets reduce mortality and severe morbidity from malaria among children on the Kenyan coast. Trop Med Int Health 1: 139–146.
9. AlonsoPL, LindsaySW, Armstrong SchellenbergJR, KeitaK, GomezP, et al. (1993) A malaria control trial using insecticide-treated bed nets and targeted chemoprophylaxis in a rural area of The Gambia, West Africa. 6. The impact of the interventions on mortality and morbidity from malaria. Trans R Soc Trop Med Hyg 87: 37–44.
10. KorenrompEL, Armstrong-SchellenbergJRM, WilliamsBG, NahlenBL, SnowRW (2004) Impact of malaria control on childhood anaemia in Africa - a quantitative review. Trop Med Int Health 9: 1050–1065.
11. BrabinBJ, PremjiZ, VerhoeffF (2001) An analysis of anemia and child mortality. J Nutr 131: 636S–645S; discussion 646S–648S.
12. BuffetPA, SafeukuiI, DeplaineG, BrousseV, PrendkiV, et al. (2011) The pathogenesis of Plasmodium falciparum malaria in humans: insights from splenic physiology. Blood 117: 381–392.
13. WickramasingheSN, AbdallaSH (2000) Blood and bone marrow changes in malaria. Baillieres Best Pract Res Clin Haematol 13: 277–299.
14. WoodruffAW, AnsdellVE, PettittLE (1979) Cause of anaemia in malaria. Lancet 1: 1055–1057.
15. DasBS, NandaNK (1999) Evidence for erythrocyte lipid peroxidation in acute falciparum malaria. Trans R Soc Trop Med Hyg 93: 58–62.
16. HaldarK, MohandasN (2009) Malaria, erythrocytic infection, and anemia. Hematology Am Soc Hematol Educ Program 87–93.
17. AbdallaS, WeatherallDJ, WickramasingheSN, HughesM (1980) The anaemia of P. falciparum malaria. Br J Haematol 46: 171–183.
18. NussenblattV, MukasaG, MetzgerA, NdeeziG, GarrettE, et al. (2001) Anemia and interleukin-10, tumor necrosis factor alpha, and erythropoietin levels among children with acute uncomplicated Plasmodium falciparum malaria. Clin Diagn Lab Immun 8: 1164–1170.
19. ChangK-H, StevensonMM (2004) Malarial anaemia: mechanisms and implications of insufficient erythropoiesis during blood-stage malaria. Int J Parasitol 34: 1501–1516.
20. BhattacharyaJ, Swarup-MitraS (1987) Reduction in erythrocytic GSH level and stability in Plasmodium vivax malaria. Trans R Soc Trop Med Hyg 81: 64–66.
21. SelvamR, BaskaranG (1996) Hematological impairments in recurrent Plasmodium vivax infected patients. Jpn J Med Sci Biol 49: 151–165.
22. WickramasingheSN, LooareesuwanS, NagachintaB, WhiteNJ (1989) Dyserythropoiesis and ineffective erythropoiesis in Plasmodium vivax malaria. Br J Haematol 72: 91–99.
23. HandayaniS, ChiuDT, TjitraE, KuoJS, LampahD, et al. (2009) High deformability of Plasmodium vivax-infected red blood cells under microfluidic conditions. J Infect Dis 199: 445–450.
24. DouglasNM, AnsteyNM, BuffetPA, PoespoprodjoJR, YeoTW, et al. (2012) The anaemia of Plasmodium vivax malaria. Malar J 11: 135.
25. AnsteyNM, DouglasNM, PoespoprodjoJR, PriceRN (2012) Plasmodium vivax: clinical spectrum, risk factors and pathogenesis. Adv Parasitol 80: 151–201.
26. ManningL, LamanM, Rosanas-UrgellA, MichonP, AipitS, et al. (2012) Severe anemia in papua new guinean children from a malaria-endemic area: a case-control etiologic study. PloS Negl Trop Dis 6: e1972 doi:10.1371/journal.pntd.0001972
27. BlolandPB, BorigaDA, RuebushTK, McCormickJB, RobertsJM, et al. (1999) Longitudinal cohort study of the epidemiology of malaria infections in an area of intense malaria transmission II. Descriptive epidemiology of malaria infection and disease among children. Am J Trop Med Hyg 60: 641–648.
28. MaitlandK, WilliamsTN, NewboldCI (1997) Plasmodium vivax and P. falciparum: biological interactions and the possibility of cross-species immunity. Parasitol Today 13: 227–231.
29. MaitlandK, WilliamsTN, BennettS, NewboldCI, PetoTEA, et al. (1996) The interaction between Plasmodium falciparum and P. vivax in children on Espiritu Santo Island, Vanuatu. Trans R Soc Trop Med Hyg 90: 614–620.
30. PriceRN, SimpsonJA, NostenF, LuxemburgerC, HkirijaroenL, et al. (2001) Factors contributing to anemia after uncomplicated falciparum malaria. Am J Trop Med Hyg 65: 614–622.
31. MayxayM, PukrittayakameeS, NewtonPN, WhiteNJ (2004) Mixed-species malaria infections in humans. Trends Parasitol 20: 233–240.
32. LancaEF, MagalhaesBM, Vitor-SilvaS, SiqueiraAM, BenzecrySG, et al. (2012) Risk factors and characterization of Plasmodium vivax-associated admissions to pediatric intensive care units in the Brazilian Amazon. PLoS ONE 7: e35406 doi:10.1371/journal.pone.0035406
33. TjitraE, AnsteyNM, SugiartoP, WarikarN, KenangalemE, et al. (2008) Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med 5: e128 doi:10.1371/journal.pmed.0050128
34. GentonB, D'AcremontV, RareL, BaeaK, ReederJC, et al. (2008) Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med 5: e127 doi:10.1371/journal.pmed.0050127
35. BarcusMJ, BasriH, PicarimaH, ManyakoriC, Sekartuti, et al. (2007) Demographic risk factors for severe and fatal vivax and falciparum malaria among hospital admissions in Northeastern Indonesian Papua. Am J Trop Med Hyg 77: 984–991.
36. PoespoprodjoJR, FobiaW, KenangalemE, LampahDA, WarikarN, et al. (2008) Adverse pregnancy outcomes in an area where multidrug-resistant Plasmodium vivax and Plasmodium falciparum infections are endemic. Clin Infect Dis 46: 1374–1381.
37. KaryanaM, BurdarmL, YeungS, KenangalemE, WarikerN, et al. (2008) Epidemiology of multidrug resistant P. vivax and P. falciparum infection in Southern Papua, Indonesia. Malar J 7: 148.
38. RoystonP, AltmanD (1994) Regression using fractional polynomials of continuous covariates: parsimonious parametric modelling. Appl Statist 43: 429–467.
39. GreenlandS, DrescherK (1993) Maximum likelihood estimation of the attributable fraction from logistic models. Biometrics 49: 865–872.
40. World Health Organization, United Nations Children's Fund (2004) Focusing on anaemia: towards an integrated approach for effective anaemia control. Joint statement by the World Health Organization and the United Nations Children's Fund. Geneva: World Health Organization.
41. HemmerCJ, HolstFGE, KernP, ChiwakataCB, DietrichM, et al. (2006) Stronger host response per parasitized erythrocyte in Plasmodium vivax or ovale then in Plasmodium falciparum malaria. Trop Med Int Health 11: 817–823.
42. YeoTW, LampahDA, TjitraE, PieraK, GitawatiR, et al. (2010) Greater endothelial activation, Weibel-Palade body release and host inflammatory response to Plasmodium vivax, compared with Plasmodium falciparum: a prospective study in Papua, Indonesia. J Infect Dis 202: 109–112.
43. KlingPJ, SchmidtRL, RobertsRA, WidnessJA (1996) Serum erythropoietin levels during infancy: associations with erythropoiesis. J Pediatr 128: 791–796.
44. SachsJ, MalaneyP (2002) The economic and social burden of malaria. Nature 415: 680–685.
45. McKenzieFE, JefferyGM, CollinsWE (2001) Plasmodium malariae blood-stage dynamics. J Parasitol 87: 626–637.
46. McQueenPG (2010) Population dynamics of a pathogen: the conundrum of vivax malaria. Biophys Rev 2: 111–120.
47. MuellerI, ZimmermanPA, ReederJC (2007) Plasmodium malariae and Plasmodium ovale–the “bashful” malaria parasites. Trends Parasitol 23: 278–283.
48. VeenemansJ, Andang'oPEA, MbugiEV, KraaijenhagenRJ, MwanikiDL, et al. (2008) Alpha+ -thalassemia protects against anemia associated with asymptomatic malaria: evidence from community-based surveys in Tanzania and Kenya. J Infect Dis 198: 401–408.
49. DanquahI, MockenhauptFP (2008) Alpha+-thalassaemia and malarial anaemia. Trends Parasitol 24: 479–481.
50. FowkesFJI, AllenS, AllenA, AlpersMP, WeatherallDJ, et al. (2008) Increased microerythrocyte count in homozygous alpha+-thalassaemia contributes to protection against severe malarial anaemia. PLoS Med 5: e56 doi:10.1371/journal.pmed.0050056
51. WilliamsTN, MaitlandK, BennettS, GanczakowskiM, PetoTE, et al. (1996) High incidence of malaria in alpha-thalassaemic children. Nature 383: 522–525.
52. KruatrachueM, CharoenlarpP, ChongsuphajaisiddhiT, HarinasutaC (1962) Erythrocyte glucose-6-phosphate dehydrogenase and malaria in Thailand. Lancet 2: 1183–1186.
53. KimuraM, SoemantriA, IshidaT (2002) Malaria species and Southeast Asian ovalocytosis defined by a 27-bp deletion in the erythrocyte band 3 gene. Southeast Asian J Trop Med Public Health 33: 4–6.
54. FooLC, RekhrajV, ChiangGL, MakJW (1992) Ovalocytosis protects against severe malaria parasitemia in the Malayan aborigines. Am J Trop Med Hyg 47: 271–275.
55. ShimizuH, TamamM, SoemantriA, IshidaT (2005) Glucose-6-phosphate dehydrogenase deficiency and Southeast Asian ovalocytosis in asymptomatic Plasmodium carriers in Sumba Island, Indonesia. J Hum Genet 50: 420–424.
56. SerjeantsonS, BrysonK, AmatoD, BabonaD (1977) Malaria and hereditary ovalocytosis. Hum Genet 37: 161–167.
57. Kitchen SF (1939) Malariology. London: W.B. Saunders.
58. KabyemelaER, FriedM, KurtisJD, MutabingwaTK, DuffyPE (2008) Decreased susceptibility to Plasmodium falciparum infection in pregnant women with iron deficiency. J Infect Dis 198: 163–166.
59. BrookerS, AkhwaleW, PullanR, EstambaleB, ClarkeSE, et al. (2007) Epidemiology of Plasmodium-helminth co-infection in Africa: populations at risk, potential impact on anemia, and prospects for combining control. Am J Trop Med Hyg 77: 88–98.
60. NacherM, SinghasivanonP, YimsamranS, ManibunyongW, ThanyavanichN, et al. (2002) Intestinal helminth infections are associated with increased incidence of Plasmodium falciparum malaria in Thailand. J Parasitol 88: 55–58.
61. SpiegelA, TallA, RaphenonG, TrapeJF, DruilheP (2003) Increased frequency of malaria attacks in subjects co-infected by intestinal worms and Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg 97: 198–199.
62. BoelM, CarraraVI, RijkenM, ProuxS, NacherM, et al. (2010) Complex interactions between soil-transmitted helminths and malaria in pregnant women on the Thai-Burmese border. PloS Negl Trop Dis 4: e887 doi:10.1371/journal.pntd.0000887
63. MeloGC, Reyes-LeccaRC, Vitor-SilvaS, MonteiroWM, MartinsM, et al. (2010) Concurrent helminthic infection protects schoolchildren with Plasmodium vivax from anemia. PLoS ONE 5: e11206 doi:10.1371/journal.pone.0011206
64. StoltzfusRJ, ChwayaHM, MontresorA, AlbonicoM, SavioliL, et al. (2000) Malaria, hookworms and recent fever are related to anemia and iron status indicators in 0- to 5-y old Zanzibari children and these relationships change with age. J Nutr 130: 1724–1733.
65. Brabin BJ (1991) The risks and severity of malaria in pregnant women. Geneva: World Health Organization.
66. NostenF, ter KuileF, MaelankirriL, DecludtB, WhiteNJ (1991) Malaria during pregnancy in an area of unstable endemicity. Trans R Soc Trop Med Hyg 85: 424–429.
Štítky
Interné lekárstvoČlánok vyšiel v časopise
PLOS Medicine
2013 Číslo 12
- MUDr. Lenka Klimešová: Multiodborová vizita je kľúč k efektívnejšej perioperačnej liečbe chronickej bolesti
- Realita liečby bolesti v paliatívnej starostlivosti v Nemecku
- Intermitentní hladovění v prevenci a léčbě chorob
- Statiny indukovaná myopatie: Jak na diferenciální diagnostiku?
- Nech brouka žít… Ať žije astma!
Najčítanejšie v tomto čísle
- Artemisinin Combination Therapy: A Good Antimalarial, but Is the Dose Right?
- Circulating Mitochondrial DNA in Patients in the ICU as a Marker of Mortality: Derivation and Validation
- Timing and Completeness of Trial Results Posted at ClinicalTrials.gov and Published in Journals
- Malaria and Severe Anemia: Thinking beyond
