-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Clinical and Economic Impact of Point-of-Care CD4 Testing in Mozambique and Other Resource-Limited Settings: A Cost-Effectiveness Analysis
Background:
Point-of-care CD4 tests at HIV diagnosis could improve linkage to care in resource-limited settings. Our objective is to evaluate the clinical and economic impact of point-of-care CD4 tests compared to laboratory-based tests in Mozambique.Methods and Findings:
We use a validated model of HIV testing, linkage, and treatment (CEPAC-International) to examine two strategies of immunological staging in Mozambique: (1) laboratory-based CD4 testing (LAB-CD4) and (2) point-of-care CD4 testing (POC-CD4). Model outcomes include 5-y survival, life expectancy, lifetime costs, and incremental cost-effectiveness ratios (ICERs). Input parameters include linkage to care (LAB-CD4, 34%; POC-CD4, 61%), probability of correctly detecting antiretroviral therapy (ART) eligibility (sensitivity: LAB-CD4, 100%; POC-CD4, 90%) or ART ineligibility (specificity: LAB-CD4, 100%; POC-CD4, 85%), and test cost (LAB-CD4, US$10; POC-CD4, US$24). In sensitivity analyses, we vary POC-CD4-specific parameters, as well as cohort and setting parameters to reflect a range of scenarios in sub-Saharan Africa. We consider ICERs less than three times the per capita gross domestic product in Mozambique (US$570) to be cost-effective, and ICERs less than one times the per capita gross domestic product in Mozambique to be very cost-effective. Projected 5-y survival in HIV-infected persons with LAB-CD4 is 60.9% (95% CI, 60.9%–61.0%), increasing to 65.0% (95% CI, 64.9%–65.1%) with POC-CD4. Discounted life expectancy and per person lifetime costs with LAB-CD4 are 9.6 y (95% CI, 9.6–9.6 y) and US$2,440 (95% CI, US$2,440–US$2,450) and increase with POC-CD4 to 10.3 y (95% CI, 10.3–10.3 y) and US$2,800 (95% CI, US$2,790–US$2,800); the ICER of POC-CD4 compared to LAB-CD4 is US$500/year of life saved (YLS) (95% CI, US$480–US$520/YLS). POC-CD4 improves clinical outcomes and remains near the very cost-effective threshold in sensitivity analyses, even if point-of-care CD4 tests have lower sensitivity/specificity and higher cost than published values. In other resource-limited settings with fewer opportunities to access care, POC-CD4 has a greater impact on clinical outcomes and remains cost-effective compared to LAB-CD4. Limitations of the analysis include the uncertainty around input parameters, which is examined in sensitivity analyses. The potential added benefits due to decreased transmission are excluded; their inclusion would likely further increase the value of POC-CD4 compared to LAB-CD4.Conclusions:
POC-CD4 at the time of HIV diagnosis could improve survival and be cost-effective compared to LAB-CD4 in Mozambique, if it improves linkage to care. POC-CD4 could have the greatest impact on mortality in settings where resources for HIV testing and linkage are most limited.
Please see later in the article for the Editors' Summary
Published in the journal: The Clinical and Economic Impact of Point-of-Care CD4 Testing in Mozambique and Other Resource-Limited Settings: A Cost-Effectiveness Analysis. PLoS Med 11(9): e32767. doi:10.1371/journal.pmed.1001725
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001725Summary
Background:
Point-of-care CD4 tests at HIV diagnosis could improve linkage to care in resource-limited settings. Our objective is to evaluate the clinical and economic impact of point-of-care CD4 tests compared to laboratory-based tests in Mozambique.Methods and Findings:
We use a validated model of HIV testing, linkage, and treatment (CEPAC-International) to examine two strategies of immunological staging in Mozambique: (1) laboratory-based CD4 testing (LAB-CD4) and (2) point-of-care CD4 testing (POC-CD4). Model outcomes include 5-y survival, life expectancy, lifetime costs, and incremental cost-effectiveness ratios (ICERs). Input parameters include linkage to care (LAB-CD4, 34%; POC-CD4, 61%), probability of correctly detecting antiretroviral therapy (ART) eligibility (sensitivity: LAB-CD4, 100%; POC-CD4, 90%) or ART ineligibility (specificity: LAB-CD4, 100%; POC-CD4, 85%), and test cost (LAB-CD4, US$10; POC-CD4, US$24). In sensitivity analyses, we vary POC-CD4-specific parameters, as well as cohort and setting parameters to reflect a range of scenarios in sub-Saharan Africa. We consider ICERs less than three times the per capita gross domestic product in Mozambique (US$570) to be cost-effective, and ICERs less than one times the per capita gross domestic product in Mozambique to be very cost-effective. Projected 5-y survival in HIV-infected persons with LAB-CD4 is 60.9% (95% CI, 60.9%–61.0%), increasing to 65.0% (95% CI, 64.9%–65.1%) with POC-CD4. Discounted life expectancy and per person lifetime costs with LAB-CD4 are 9.6 y (95% CI, 9.6–9.6 y) and US$2,440 (95% CI, US$2,440–US$2,450) and increase with POC-CD4 to 10.3 y (95% CI, 10.3–10.3 y) and US$2,800 (95% CI, US$2,790–US$2,800); the ICER of POC-CD4 compared to LAB-CD4 is US$500/year of life saved (YLS) (95% CI, US$480–US$520/YLS). POC-CD4 improves clinical outcomes and remains near the very cost-effective threshold in sensitivity analyses, even if point-of-care CD4 tests have lower sensitivity/specificity and higher cost than published values. In other resource-limited settings with fewer opportunities to access care, POC-CD4 has a greater impact on clinical outcomes and remains cost-effective compared to LAB-CD4. Limitations of the analysis include the uncertainty around input parameters, which is examined in sensitivity analyses. The potential added benefits due to decreased transmission are excluded; their inclusion would likely further increase the value of POC-CD4 compared to LAB-CD4.Conclusions:
POC-CD4 at the time of HIV diagnosis could improve survival and be cost-effective compared to LAB-CD4 in Mozambique, if it improves linkage to care. POC-CD4 could have the greatest impact on mortality in settings where resources for HIV testing and linkage are most limited.
Please see later in the article for the Editors' SummaryIntroduction
In sub-Saharan Africa, over 50% of HIV-infected patients remain unlinked to clinical care, despite the dramatic scale-up of HIV treatment over the past decade [1]. Point-of-care technologies have been widely promoted as a mechanism to improve triage and linkage of newly diagnosed HIV-infected patients to care [2]–[5].
After initial HIV diagnosis, patients undergo immunological staging, in which the severity of immunosuppression, as quantified by CD4 count, determines eligibility for antiretroviral therapy (ART) initiation. Current standard of care throughout sub-Saharan Africa at the time of HIV diagnosis is a laboratory-based CD4 test, when available [5]. Patients receive the results of the CD4 test at a return visit and are triaged to clinical care depending on their ART eligibility as determined by national policy guidelines [6]. Patient attrition at each of these steps after HIV diagnosis is high, ranging from 17% to 80% in resource-limited settings [7]–[13]. The World Health Organization (WHO) has targeted these steps in linkage to care as opportunities for improvement and has underscored the potential offered by point-of-care CD4 tests to expedite immunologic staging [5].
Data from multiple regions throughout sub-Saharan Africa demonstrate that point-of-care CD4 testing (POC-CD4) can improve overall linkage to care [14]–[18]. Our objective is to assess the clinical outcomes and cost-effectiveness of POC-CD4 compared to laboratory-based CD4 testing (LAB-CD4) for immunologic staging in Mozambique and to examine the generalizability of these results to settings throughout sub-Saharan Africa with a diversity of opportunities to access care.
Methods
Ethics Statement
This work was approved by the Partners Human Research Committee, Boston, Massachusetts, US (Protocol #2003 P001019).
Analytic Overview
We use the Cost-Effectiveness of Preventing AIDS Complications–International (CEPAC-I) model to project the clinical impact, costs, and cost-effectiveness of POC-CD4 at the time of HIV diagnosis at outpatient voluntary testing and counseling (VCT) clinics [19]–[22]. In a simulated cohort of newly diagnosed HIV-infected patients in Mozambique, we investigate two strategies for immunologic staging: LAB-CD4 versus POC-CD4. The two strategies differ in terms of (1) the probability of linkage to care, (2) CD4 test sensitivity and specificity, and (3) CD4 test cost. For each strategy, the model simulates 2 million patients to produce stable outputs. We use deterministic and probabilistic sensitivity analyses to examine the generalizability of our results to sub-Saharan settings with different populations, infrastructures, and available resources for HIV testing, clinical care, and retention in care.
We use the model to project 5-y survival, life expectancy (LE), and per person lifetime direct medical costs of HIV care (in 2011 US dollars). Future benefits and costs are discounted at 3% per year [23]. We calculate the incremental cost-effectiveness ratio (ICER) as the ratio of the difference in outcomes between the two strategies of care (Δcosts/ΔLE) after immunologic staging [23]. Guided by recommendations from the WHO Commission on Macroeconomics and Health [24] and WHO-CHOICE [25], we consider a strategy to be “cost-effective” if its ICER is less than three times the country-specific per capita gross domestic product (GDP) (Mozambique 2011 GDP, US$570) and “very cost-effective” if its ICER is less than one times the per capita GDP [26].
Model Structure
The CEPAC-I model is a previously published Monte Carlo state-transition model of HIV natural history, case detection, linkage, and treatment [19]–[22].
Cohort characteristics
At the simulation's start, patients have just been diagnosed with HIV at a VCT clinic. Their baseline characteristics are drawn randomly from distributions of age, gender, CD4 count, and HIV RNA, populated from region-specific clinical trials and cohort data [14],[27]. In each month, simulated patients move between health states, specified broadly as chronic HIV infection, acute clinical events, and death. The simulated cohort excludes incident and acute HIV cases.
Linkage to care
To link to care after HIV diagnosis, simulated patients must complete a CD4 test for immunological staging, which determines ART eligibility. Linkage to care requires completion of three sequential steps: (1) obtaining the patient sample and completing the CD4 test (“test completion”), (2) alerting the patient to the test result (“result receipt”), and (3) initiating HIV clinical care after receipt of the immunologic staging result (“initiating care”). Based on the “true CD4 count” (in vivo), patients are either “ART eligible” (i.e., true CD4 count ≤ threshold for ART eligibility) or “ART ineligible” (i.e., true CD4 count> threshold). The “observed CD4 count” is the test result given to the patient, which can differ from the true CD4 count depending on CD4 test characteristics. Patients can receive observed CD4 count results 1 wk or more after HIV diagnosis (LAB-CD4) or sooner (POC-CD4). For patients who receive their CD4 test result, the probability of initiating care depends on the observed CD4 count (Figure 1). Patients who do not initiate care after immunologic staging (i.e., “unlinked”) can link in subsequent months following an acute opportunistic infection (OI), tuberculosis (TB), or if they undergo repeat HIV testing or immunologic staging. Unlinked patients progress with natural history of HIV.
Fig. 1. Schematic comparing two strategies for immunological staging after HIV diagnosis: LAB-CD4 and POC-CD4. 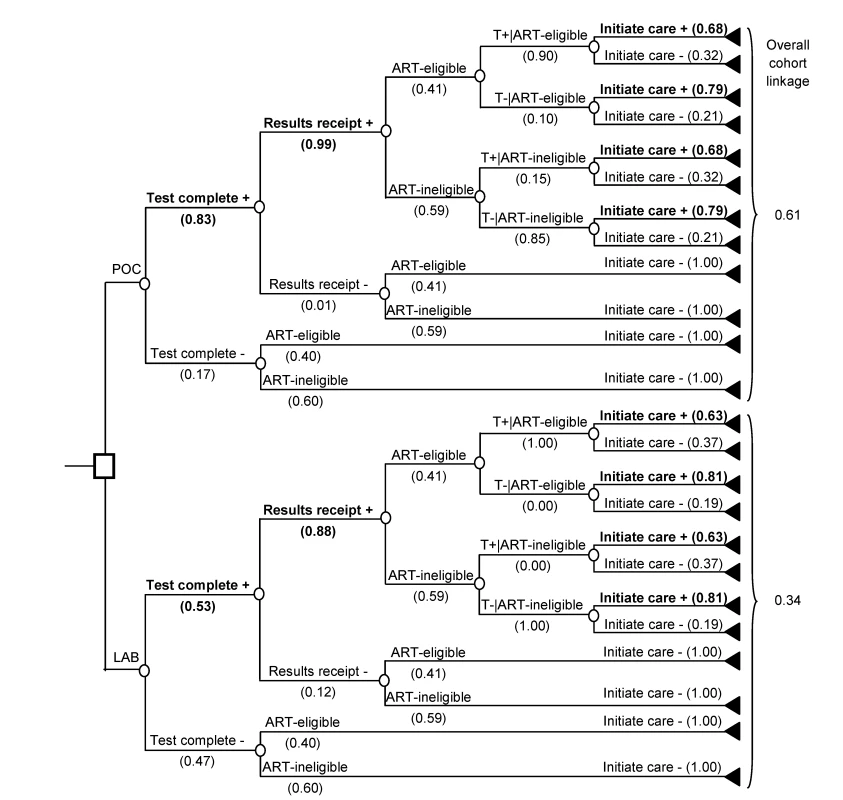
In both LAB-CD4 and POC-CD4, literature-based probabilities are shown for: completing a laboratory CD4 test, receiving the test result, and successful initiation of care (Table 1). Probability of linkage depends on ART eligibility as determined by the “true CD4 count,” as well as the “observed CD4 count” test result (T) [14]. Bolded branches denote where the outcomes result in linkage to care. T+, observed CD4 count ≤250/µl; T−, observed CD4 count>250/µl. CD4 count for immunologic staging
We use two parameters to calculate the observed CD4 count compared to the true CD4 count: precision and bias. The coefficient of variation (CV) characterizes precision (i.e., the variability of observed CD4 count from repeat tests on a single specimen); we calculate the observed CD4 count as the true CD4 count plus a random draw from a normal distribution with zero mean and standard deviation (SD) equal to the product of the CV and the true CD4 count. To represent any systematic bias of the CD4 test (i.e., if the observed CD4 count is consistently greater or less than the true CD4 count), we also vary the mean of the observed CD4 count from the true CD4 count. For both precision and bias, we use a percentage of the true CD4 count because variations in the observed CD4 count will range more widely for higher true CD4 count values [28].
CD4 test sensitivity and specificity depend on three parameters: the test's precision and bias, the true CD4 count of the population, and the ART-eligibility threshold. We use model output for true and observed CD4 counts to calculate the sensitivity and specificity associated with an ART-eligibility threshold.
Patients whose ART eligibility is misclassified by the point-of-care CD4 test are triaged and treated accordingly. If misclassified as ART eligible (i.e., truly ART ineligible, with true CD4 count>250/µl but observed CD4 count ≤250/µl), linked patients initiate ART, which improves their projected clinical outcomes and increases costs. Patients misclassified as ART ineligible (i.e., truly ART eligible, with true CD4 count ≤250/µl but observed CD4 count>250/µl) do not start ART after linkage and have worse clinical outcomes. To remain conservative towards potential benefits of POC-CD4, patients initially misclassified as ART ineligible are never successfully transitioned to ART.
HIV clinical care
Linked patients are treated with care concordant with national guidelines in Mozambique according to the observed CD4 count, including clinical visits, laboratory monitoring, and ART for eligible patients. ART efficacy depends on patient adherence; those with better adherence have a greater probability of virologic suppression and associated immunologic recovery [29]. To account for interruptions in care after linkage [30]–[32], simulated patients have a monthly probability of becoming lost to follow-up (LTFU), with a probability of returning to care. When LTFU, patients discontinue ART and co-trimoxazole prophylaxis and experience HIV natural history.
Input Parameters
Cohort characteristics
We derive cohort characteristics from a published study of immunologic staging in Mozambique: mean CD4 count 300/µl (SD, 230/µl), mean age 32.7 y (SD, 10.1 y), and 65% female (Table 1) [14].
Tab. 1. Model input parameters for analysis of immunological staging by POC-CD4 versus LAB-CD4 in Mozambique. 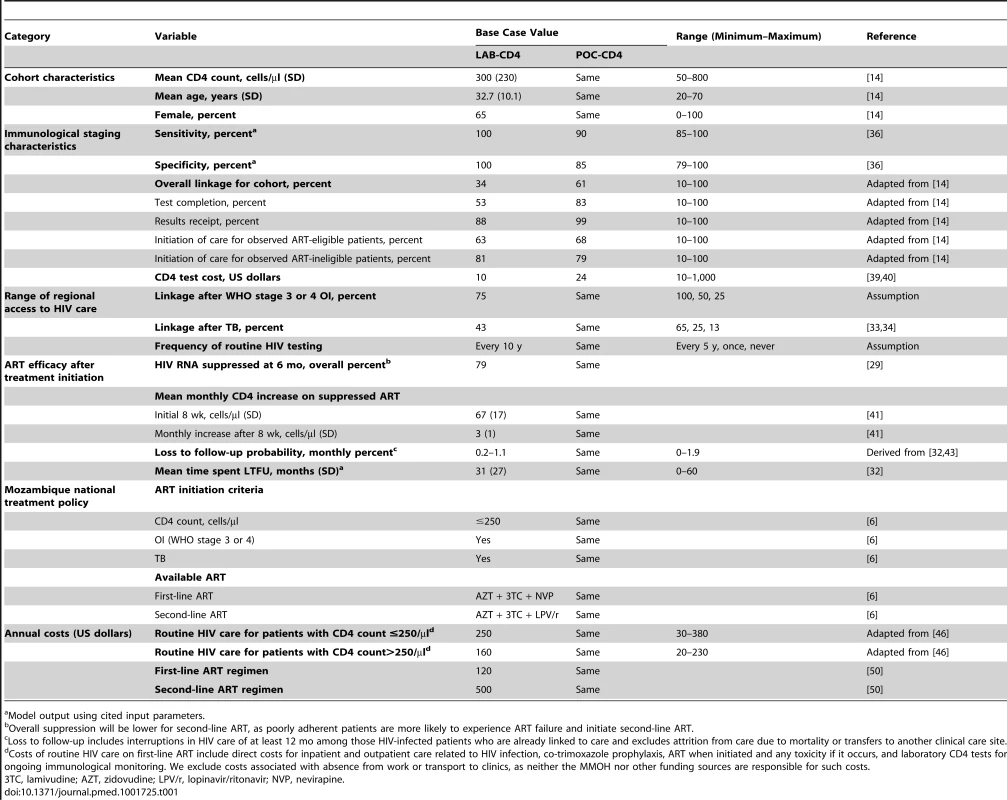
Model output using cited input parameters. Linkage to care
The overall cohort linkage for LAB-CD4 is 34%. This is equivalent to the product of CD4 test completion (53%), CD4 test result receipt conditional on test completion (88%), and initiation of care conditional on receiving results (74%). Because the probability of initiation of care is different for patients whose observed CD4 count makes them ART eligible (63%) or ineligible (81%), it is weighted by the proportion of the linked population that is ART eligible (42%) or ineligible (58%) (Figure 1; Table 1). In POC-CD4, 61% of the cohort links to care, with improved CD4 test completion (83%) and result receipt (99%). Initiation of care occurs in 74%; 68% from the observed ART-eligible patients and 79% from the ART-ineligible patients, weighted by the 46% of the population who are ART eligible and the 54% who are ART ineligible. For rates of initiating care after receipt of an ART-ineligible CD4 test result, we use unpublished data collected in the same study protocol as the ART-eligible linkage rates [14].
For unlinked patients, we estimate that repeat testing occurs at a monthly rate of once every 10 y, as only 39% of HIV-infected individuals are estimated to know their HIV status in South Africa, where HIV testing services are more robust than in Mozambique [11]. Unlinked patients will link to HIV care 43% of the time if ill with TB (reported range, 13%–62%) [10],[33]–[35] and 75% of the time with WHO stage 3/4 OI (Table 1).
CD4 count for immunologic staging
LAB-CD4 uses the gold standard laboratory CD4 test, assuming perfect performance characteristics (sensitivity/specificity, 100%/100%) to ensure the analysis is not biased towards POC-CD4.
POC-CD4 uses an Alere Pima point-of-care CD4 test (Alere, Waltham, Massachusetts, US). We estimate the point-of-care CD4 test's precision at 32.6% CV, as reported from a rural clinical care setting operated by non-research staff [36], and the test's bias at 0% (reported range, −12.3% to +16.5%) [36]–[38]. The point-of-care CD4 test has a sensitivity of 90% and a specificity of 85%, when ART eligibility is at CD4 count ≤250/µl (Figure 2A).
Fig. 2. Test characteristics of the point-of-care CD4 test as determined by model output. 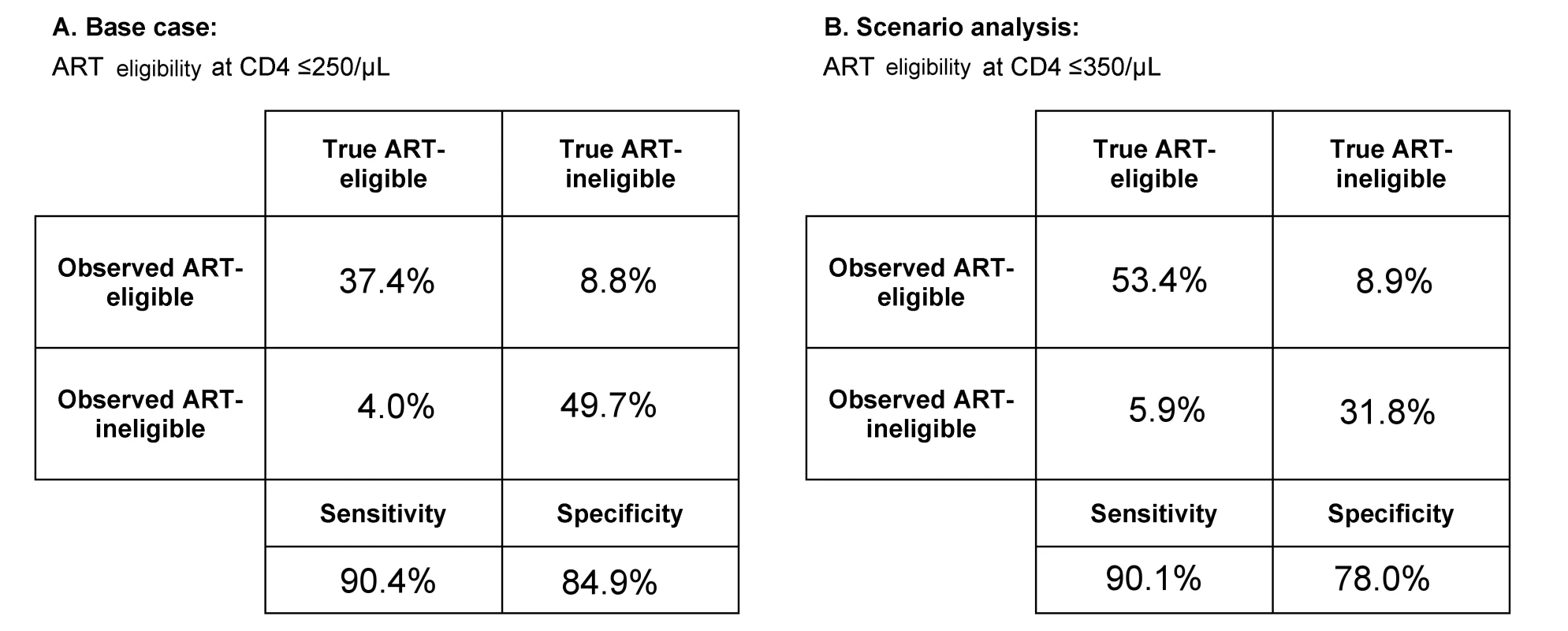
At the time of HIV diagnosis and immunologic staging, the model captures both the “true CD4 count” of the patient and the “observed CD4 count,” or CD4 test result. The observed CD4 test result has variability around the true CD4 count, depending on the test itself (e.g., point-of-care CD4 test with precision of 32.6% CV). (A) Using model output, we calculate the sensitivity (i.e., observed CD4 count ≤250/µl, true CD4 count ≤250/µl) and specificity (i.e., observed CD4 count >250/µl, true CD4 count >250/µl) for point-of-care CD4 tests when policy sets ART eligibility at CD4 count ≤250/µl. (B) In a scenario in which ART eligibility is at CD4 count ≤350/µl, we use model output to calculate the sensitivity (i.e., observed CD4 count ≤350/µl, true CD4 count ≤350/µl) and specificity (i.e., observed CD4 count >350/µl, true CD4 count>350/µl) for point-of-care CD4 tests. The laboratory CD4 test is estimated to cost US$10/test in Mozambique [39], whereas each point-of-care CD4 test costs US$24 [40]. Estimated test cost includes investments in equipment amortized over its usable lifetime, costs of materials to complete each test (including quality controls), and labor. We use the highest reported test cost from a microcosting approach [39],[40] to ensure that the analysis is not biased towards POC-CD4.
HIV clinical care
The Mozambique national guidelines inform inputs regarding patient monitoring, prophylaxis, and ART initiation at CD4 count ≤250/µl or a WHO stage 3 or 4 OI, including TB. Biannual laboratory CD4 tests monitor patient responses to ART; HIV RNA is not available [6]. Following ART initiation, first-line ART leads to virologic suppression in 79% of patients at 6 mo [29], resulting in rising CD4 counts [41]. Monitoring for ART failure uses immunologic criteria (e.g., ≥50% decrease in CD4 count or a CD4 count below nadir after ≥1 y of ART) [42] and prompts a switch to protease inhibitor–based second-line ART.
Estimates of loss to follow-up are from a systematic review of sub-Saharan African studies that excludes mortality [32]; we also correct for those patients who transfer care (i.e., not truly LTFU) [43]. The probability of LTFU is inversely related to adherence; patients with better ART adherence have lower LTFU rates (monthly probability, 0.2%) compared to patients with poorer adherence (monthly LTFU probability, 1.1%) [44]. Patients who are LTFU have a monthly probability of returning to care (1.0%) after being lost for 1 y [45] and return to care 50% of the time with a WHO stage 3/4 OI or TB. These input parameters result in 30.0%–34.8% of the cohort experiencing at least one interruption in HIV care that lasts 2.6 y (SD, 2.3 y) (Table 1). To assess the model's internal validity, we compare model output for LTFU at 36 mo with reported values from the published literature.
HIV care costs
Linked patients experience routine HIV care costs independent of initial immunologic staging strategy. We use costs of routine comprehensive HIV care for patients on ART ≤6 mo or >6 mo derived from 11 HIV treatment facilities in Mozambique, including the costs of clinical care, treatment and prophylaxis for OIs, and laboratory monitoring [46]. Given the model's structure, we use current CD4 count as a proxy for duration of ART (i.e., CD4 count ≤250/µl for ART ≤6 mo; CD4 count>250/µl for ART>6 mo). We assume that unlinked or LTFU patients incur only 20% of the costs of comprehensive HIV care. We evaluate the approach by comparing these estimated annual HIV care costs (US$160–US$250/y) with published primary data from countries with GDP and available HIV clinical care similar to those of Mozambique (reported range, US$115–US$338/y) [47]–[49]. Antiretroviral medication costs are from the Clinton Health Access Initiative (Table 1) [50]. All costs are converted to 2011 US dollars [26].
Validation of the Model
We simulate HIV-uninfected individuals in the model to demonstrate its internal consistency.
Deterministic Sensitivity Analyses
Guided by published literature, we perform one-way sensitivity analyses for overall linkage by individually varying the probability of test completion, receipt of CD4 test results, and initiation of care [15]–[17],[51], and point-of-care and laboratory test characteristics (see Tables S1–S3) [36]–[38],[52]–[56]. We vary CD4 test costs to capture the economies of scale associated with using one machine to complete more tests per day, as well as the lower labor costs in Mozambique and other sub-Saharan African countries [57]. We investigate cohort characteristics (e.g., age, gender, and mean CD4 count at the time of diagnosis) and features of clinical care (e.g., laboratory monitoring strategies, LTFU rates, and routine care costs) (Table 1).
Scenario Analyses
Regional access to care
We consider the clinical outcomes and cost-effectiveness of POC-CD4 compared to LAB-CD4 in four different settings within sub-Saharan Africa to represent a range of access to repeat HIV testing, staging, and linkage to HIV care when ill with an acute OI or TB (Table 1).
ART eligibility at CD4 count ≤350/µl
To examine how expanded access to ART in Mozambique might affect the clinical and economic benefits of POC-CD4, we perform a scenario analysis in which national policy recommends initiation of ART at CD4 count ≤350/µl, such as in other sub-Saharan African countries. Using a CV of 32.6% and 0% bias [36], point-of-care CD4 tests have a sensitivity of 90% and specificity of 78% when ART eligibility is at CD4 count ≤350/µl (Figure 2B).
Probabilistic Sensitivity Analysis
We perform a multi-way probabilistic sensitivity analysis to evaluate the effect of uncertainty around input parameters in the model and include all nine parameters for which one-way sensitivity analyses result in appreciable changes in ICERs. The model randomly selects a value for each parameter from a prespecified probability distribution (Table 2) and uses this combination of parameter values to calculate the expected clinical outcomes and costs for each strategy. The same process repeats 10,000 times for each strategy to obtain a distribution of outcomes and costs for each strategy. We first identify the more cost-effective strategy for each of the iterations and then assess the proportion of runs in which each strategy is identified as more cost-effective within a range of willingness-to-pay thresholds.
Tab. 2. Input parameters for probabilistic sensitivity analysis of immunological staging by POC-CD4 versus LAB-CD4 in Mozambique. 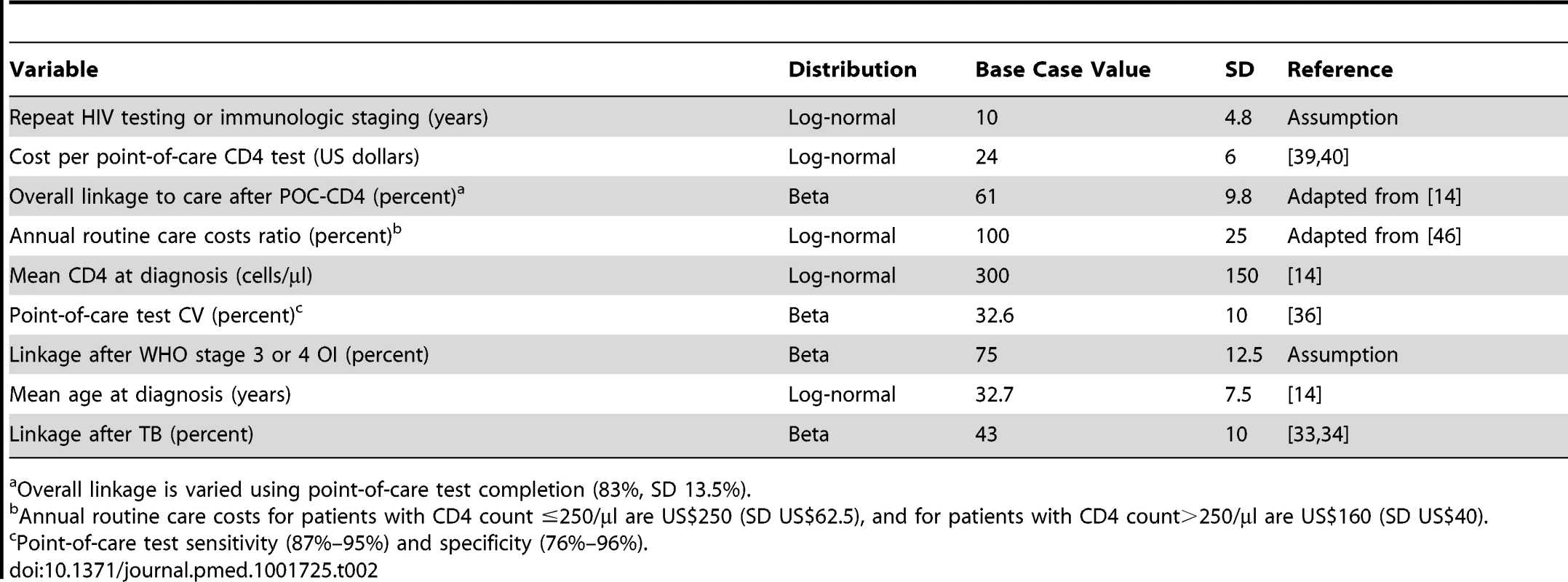
Overall linkage is varied using point-of-care test completion (83%, SD 13.5%). Estimates of Uncertainty
We calculate the 95% confidence interval using model output for 5-y survival, costs, and life expectancy (undiscounted and discounted). We use Fieller's theorem to calculate the 95% confidence interval for the ICER [58],[59].
Programmatic Considerations and Affordability
To investigate the affordability of POC-CD4, we assess the annual financial outlay associated with POC-CD4 compared to LAB-CD4 from the perspective of the Mozambique Ministry of Health (MMOH) and the donors who together provide funding for Mozambique's national response to the HIV/AIDS epidemic [60]. We consider the undiscounted direct costs of the two strategies for immunologic staging, as well as the costs of guideline-concordant HIV care incurred by those who link to care (Table 1). We include all equipment costs for immunologic staging in year one. We estimate that 120,000 people are newly diagnosed with HIV infection in the first year of the rollout of this strategy in Mozambique [61].
Results
Validation of the Model, Including Loss to Follow-Up
When the model simulates HIV-uninfected patients in Mozambique, life expectancy from birth is 54.7 and 58.3 y for males and females, respectively. This is consistent with WHO-reported overall life expectancy for Mozambique (i.e., 52.0 and 53.0 y, respectively), since the WHO estimates include HIV-infected people [62]. Of the simulated cohort, 10.6% are LTFU at 36 mo, which is consistent with estimates of 12.0% derived from published data [32],[43].
Base Case
Five-year survival with LAB-CD4 is 60.9% (95% CI, 60.9%–61.0%), which increases to 65.0% (95% CI, 64.9%–65.0%) with POC-CD4 (Table 3). Discounted life expectancy is 9.6 y (95% CI, 9.6–9.6 y) with LAB-CD4 and increases to 10.3 y (95% CI, 10.3–10.3 y) with POC-CD4. Per person discounted lifetime costs are US$2,440 (95% CI, US$2,440–US$2,450) with use of LAB-CD4 and increase to US$2,800 (95% CI, US$2,790–US$2,800) with POC-CD4, which results in an ICER of US$500/year of life saved (YLS) in Mozambique. The 95% confidence interval surrounding the ICER is US$480–US$520/YLS, a value that has interpretable meaning in this situation because it reflects an unambiguous trade-off between costs and health outcomes [59],[63].
Tab. 3. Base case results of POC-CD4 versus LAB-CD4 for immunologic staging for HIV-infected persons in Mozambique. 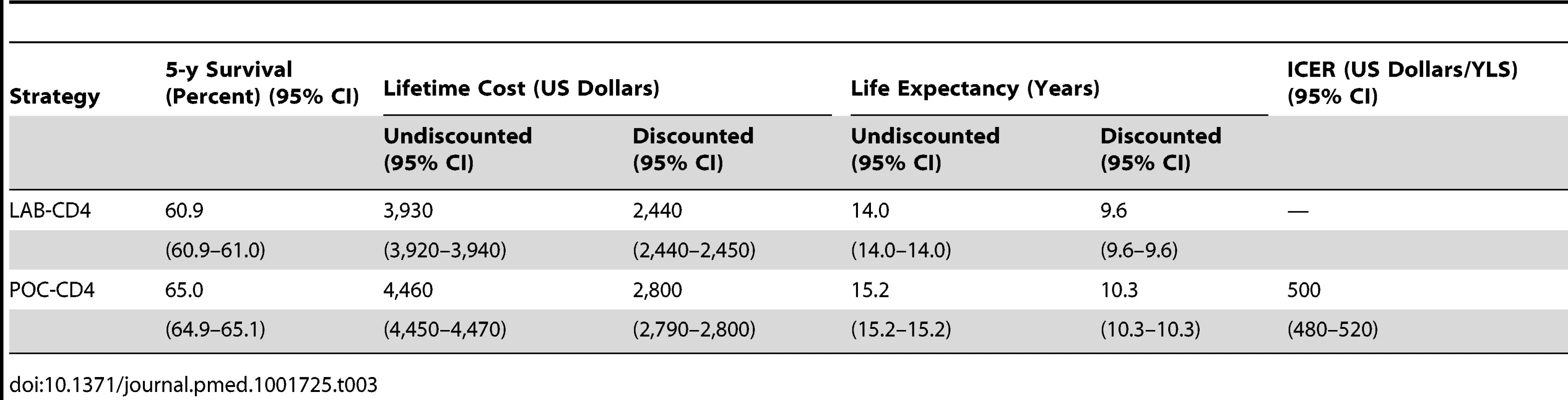
One-Way Sensitivity Analyses
POC-CD4 results in improved clinical outcomes and remains cost-effective when compared to LAB-CD4 under a wide range of conditions in one-way sensitivity analyses (Figure 3). Clinical outcomes improve compared to LAB-CD4 as long as overall linkage increases with POC-CD4, which could result if test completion is ≥50%, receipt of results is ≥60%, or initiation of clinical care is ≥49% as a weighted average of ART-eligible and -ineligible patients. POC-CD4 remains cost-effective compared to LAB-CD4, even when the cost of the point-of-care test is far greater than currently reported. POC-CD4 is no longer cost-effective only when repeat HIV testing or immunologic staging for those patients who remain unlinked occurs every 9 mo or more frequently.
Fig. 3. Tornado diagram of one-way sensitivity analyses when ART-eligibility threshold is at CD4 count ≤250/µl. 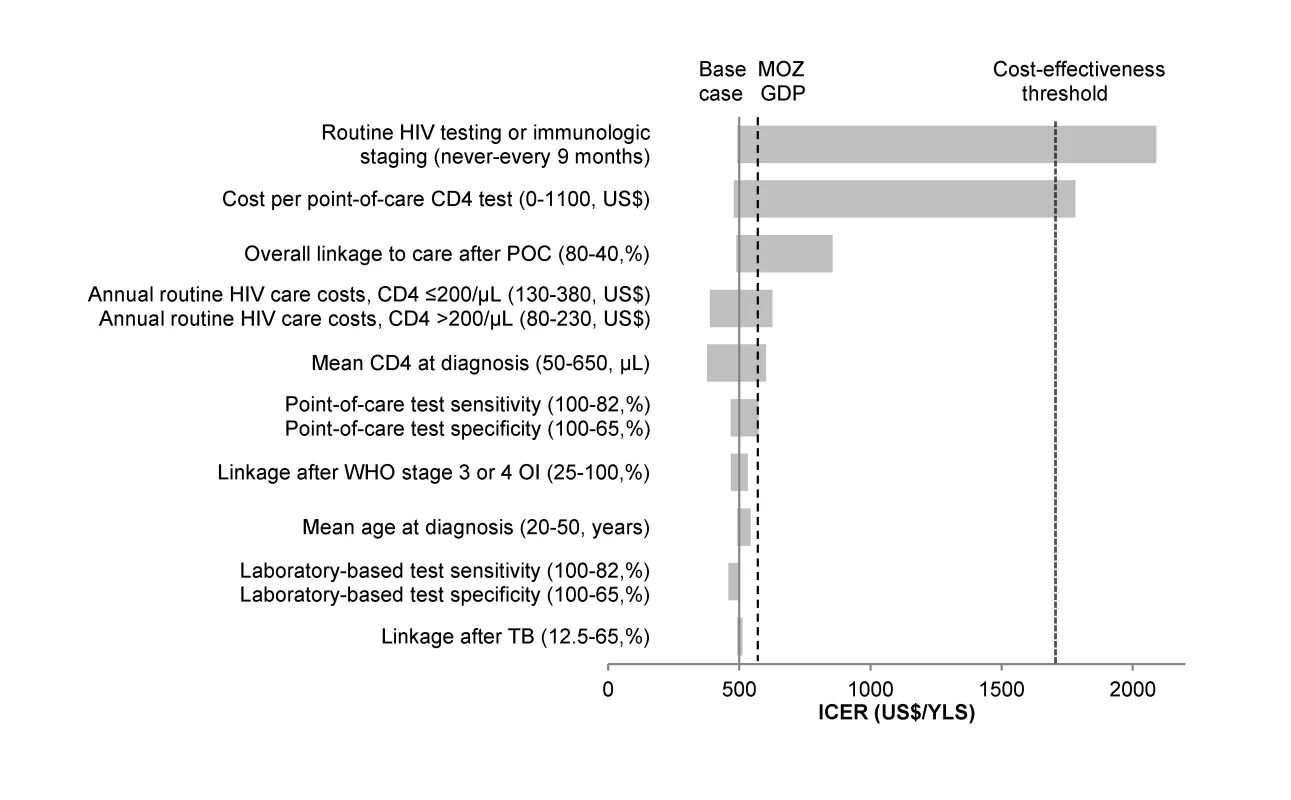
A range of parameters varied in one-way sensitivity analyses are displayed on the vertical axis. The ICER (US dollars/YLS) of POC-CD4 compared to LAB-CD4 is represented on the x-axis. The solid vertical line indicates the ICER of the base case (US0/YLS). The dashed vertical line represents the per capita GDP of Mozambique (MOZ GDP), i.e., the “very cost-effective” threshold; the dotted vertical line represents three times GDP, i.e., the “cost-effective” threshold. For each parameter, the horizontal bar represents the range of ICERs that result from varying that parameter along the range of values indicated in the parentheses; the first value listed in the parentheses is the one that results in the lowest ICER. Of all one-way sensitivity analyses, the ICER of POC-CD4 compared to LAB-CD4 crosses the cost-effectiveness threshold (into the area of not being cost-effective) (US,710/YLS) only when point-of-care CD4 test cost is>US,100/test or when repeat HIV testing or immunologic staging are completed every 9 mo or more frequently. The benefits of earlier linkage and ART initiation with POC-CD4 might be attenuated if higher rates of loss to follow-up result after linkage with POC-CD4 compared with LAB-CD4. When the monthly probability of loss to follow-up is higher among patients who link to care via POC-CD4 (monthly probability, 0.02–0.003) than among those who link to care via LAB-CD4 (monthly probability, 0.01–0.002), then the percentage of the cohort who experiences one or more interruptions in care increases from 30.0% (LAB-CD4) to 45.5% (POC-CD4), and clinical outcomes are equivalent (discounted life expectancy, 9.6 y). When POC-CD4 includes an increased rate of loss to follow-up among ART-ineligible patients (monthly probability, 0.02 for all ART-ineligible patients with POC-CD4; therefore, 49.8% of patients experience LTFU), clinical outcomes are worse with POC-CD4 (discounted life expectancy, 9.4 y) compared to LAB-CD4 (discounted life expectancy, 9.6 y).
Sensitivity Analysis for CD4 Test Characteristics
POC-CD4 results in better clinical outcomes and is cost-effective compared with LAB-CD4 even at reduced point-of-care test sensitivity (82%) and specificity (65%), which can result from either poor precision or extreme bias (Table 4). Such values are far below those published [36],[38],[52]. If the point-of-care CD4 test operates with perfect sensitivity and specificity, the ICER of POC-CD4 compared to LAB-CD4 is US$470/YLS, which reflects the increased rates of linkage with POC-CD4 and represents the cost-effectiveness ratio for ART in Mozambique. When laboratory CD4 tests operate with less precision or more bias (i.e., no longer a “perfect test”), then POC-CD4 becomes even more cost-effective compared to LAB-CD4 (ICER, US$460–US$500/YLS) (Figure 3).
Tab. 4. One-way and two-way sensitivity analyses of point-of-care CD4 test characteristics on misclassification of patients, clinical outcomes, costs, and cost-effectiveness compared to laboratory-based CD4 tests. 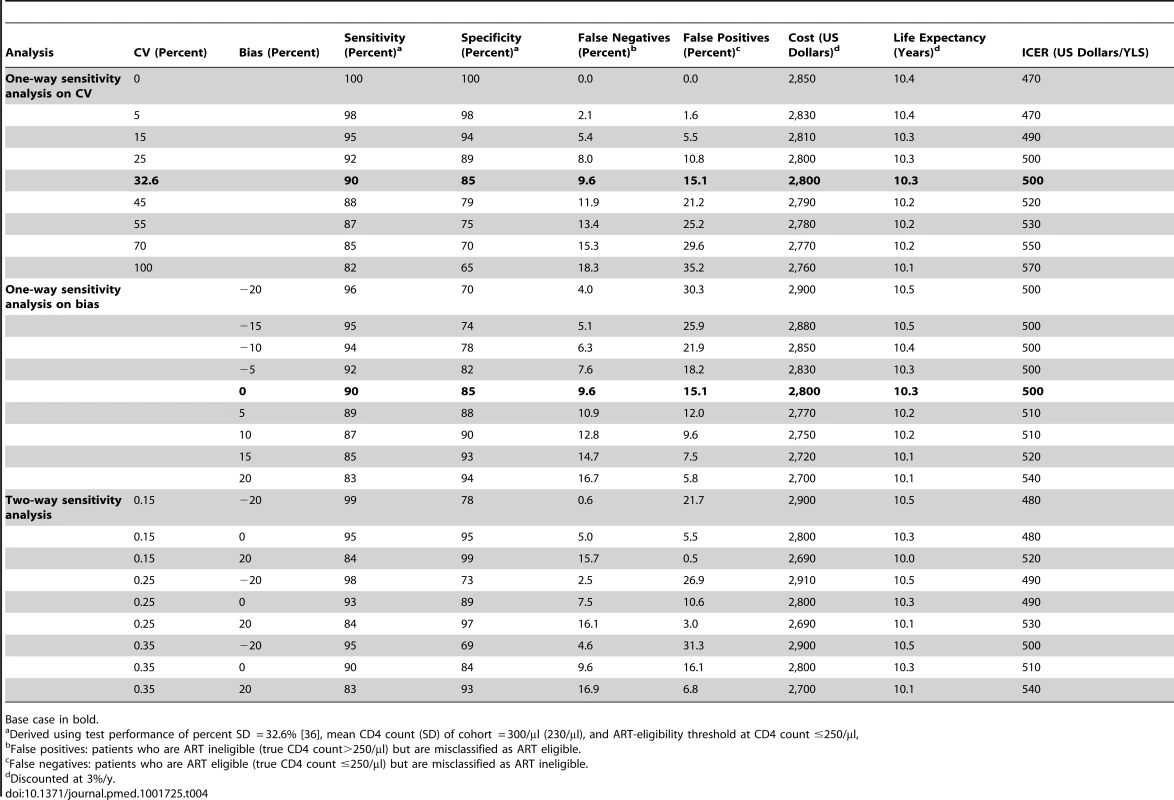
Base case in bold. Probabilistic Sensitivity Analysis
When varying nine parameters based on their prespecified probability distributions (Table 2), POC-CD4 is more cost-effective than LAB-CD4 92% of the time at the willingness-to-pay threshold of US$570/YLS, or the Mozambique 2011 per capita GDP. The probability of POC-CD4 being more cost-effective than LAB-CD4 is even greater at higher willingness-to-pay thresholds.
Scenario Analyses
Regional access to care
In settings where access to care provides fewer opportunities to test and link to care, POC-CD4 leads to a greater increase in 5-y survival than LAB-CD4. For instance, as shown in Figure 4A, 2.3% of deaths (red) are averted at 5 y with POC-CD4 compared to LAB-CD4 in a setting with greater access (Figure 4A, far left column), but 8.1% of deaths (yellow) are averted if POC-CD4 is used in a setting with less access to care (Figure 4A, far right column). The clinical benefits of POC-CD4 increase as POC-CD4 linkage improves, reflected in an increased percentage of deaths averted (Figure 4A, ascending the vertical axis). Where regional access to care is less robust, POC-CD4 becomes more cost-effective compared to LAB-CD4, as long as linkage to care with POC-CD4 is better than with LAB-CD4 (Figure 4C). POC-CD4 ceases to be clinically preferred or cost-effective compared to LAB-CD4 only when POC-CD4 improves linkage by <5% in settings where repeat HIV testing occurs at least every 5 y and diagnosis with an OI always leads to linkage to HIV care (Figure 4A and 4C, far left column).
Fig. 4. Multi-way sensitivity analysis on regional access to care and linkage to care with POC-CD4 compared to LAB-CD4. 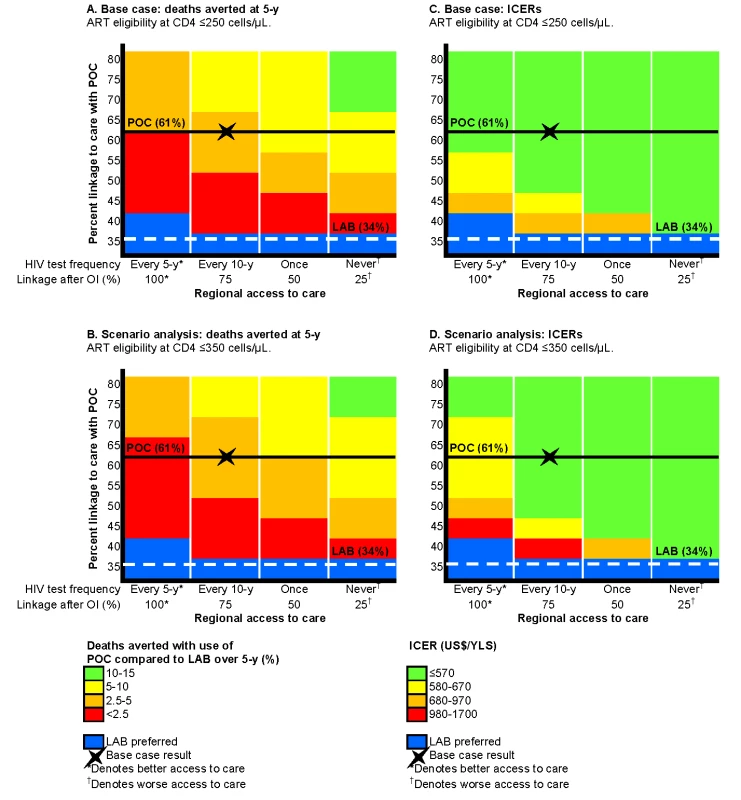
Projected decrease in 5-y mortality and ICERs with use of POC-CD4 compared to LAB-CD4 in four settings that represent a range of access to care (decreasing access to care from left to right) and with different probabilities of linkage with POC-CD4 (increasing up the vertical axis). The base case is indicated by the X in each figure; the horizontal lines represent the base case overall linkage rates (POC-CD4, solid black; LAB-CD4, dashed white). Decreased mortality at 5 y with POC-CD4 is projected in settings that use current Mozambique guidelines for ART eligibility (CD4 count ≤250/µl) (A) or earlier ART eligibility (CD4 count ≤350/µl) (B). More deaths could be averted (noted by changes in color towards green) in settings with fewer opportunities to access care or by improving POC-CD4 linkage rates compared to LAB-CD4. Blue denotes the few situations in which LAB-CD4 results in better clinical outcomes than POC-CD4 at 5 y. (C) displays the ICERs of POC-CD4 compared with LAB-CD4 given current Mozambique guidelines (ART eligibility at CD4 count ≤250/µl); (D) displays ICERs in settings with earlier ART eligibility (CD4 count ≤350/µl). POC-CD4 is at the very cost-effective threshold (i.e., US0–US0/YLS in [C] and US0–US,030/YLS in [D]) compared to LAB-CD4 except when linkage with POC-CD4 is <5% better than LAB-CD4 in settings with repeat HIV testing every 5 y. ART eligibility at CD4 count ≤350/µl
At an alternative ART-eligibility threshold of CD4 count ≤350/µl, 5-y survival increases to 61.5% with use of LAB-CD4 and 65.1% with POC-CD4. The discounted life expectancy increases to 9.8 y with LAB-CD4; POC-CD4 further improves life expectancy to 10.4 y. Per person discounted lifetime costs are also greater with the alternative ART-eligibility threshold (LAB, US$2,570; POC, US$2,900), resulting in an ICER of US$530/YLS for POC-CD4 compared to LAB-CD4. As linkage after point-of-care CD4 tests improves, POC-CD4 is clinically preferred and more cost-effective compared to LAB-CD4 in settings where access to repeat testing and linkage to care is less available (Figure 4B and 4D).
Programmatic Considerations and Affordability
For the 120,000 newly diagnosed patients in Mozambique in 1 y, we estimate that the MMOH and other donors would pay US$600,000 for immunologic staging with LAB-CD4, which would increase to US$2,400,000 with POC-CD4. Taking into account the undiscounted costs of follow-up HIV care for those patients who successfully link, the MMOH and other donors would fund US$78.2 million for LAB-CD4 compared to US$94.1 million for POC-CD4 over 5 y. The costs associated with immunological staging itself are a very small proportion of the overall 5-y costs (LAB-CD4 0.8%; POC-CD4 2.5%); most of the increased costs are related to clinical care and ART for the patients who link to care.
Discussion
Using a simulation model of HIV disease, we find that a POC-CD4 strategy of immunological staging results in nearly one full year of additional life expectancy compared to LAB-CD4 and is near the very cost-effective threshold in Mozambique. To remain conservative regarding any benefit of POC-CD4, we use the published estimates of point-of-care CD4 test characteristics and costs that are among the least favorable. The value of POC-CD4 compared to LAB-CD4 will likely be even greater if point-of-care CD4 tests operate with improved test characteristics [53] or lower cost [39], or if laboratory-based CD4 tests operate with less than perfect test characteristics. The majority of costs are due to the clinical care provided after linkage to care; as the costs of care decrease, the relative value of POC-CD4 increases compared to LAB-CD4.
POC-CD4 results in better clinical outcomes and is at the very cost-effective threshold compared to LAB-CD4 if POC-CD4 leads to enhanced linkage; much of this improvement is due to increased test completion and receipt of results. Attention must therefore remain focused on sustaining improvements in any of the sequential steps of linkage after POC-CD4 [15],[64]. Settings in sub-Saharan Africa with robust transport and centralized laboratory systems already in place might invest to improve LAB-CD4 as an alternative approach for improving patient outcomes. However, the costs of improving transport and infrastructure could well outpace the costs associated with POC-CD4, which could also be implemented more quickly to assist in rapid scale-up.
Although performance characteristics should be a high priority with any new diagnostic test, our analysis suggests that the impact of point-of-care CD4 tests on linkage outweighs the effect of the tests' performance characteristics within reasonable ranges. Because the goal of immunological staging is to expedite linkage to care for those most in need, a POC-CD4 strategy with some misclassified test results can still lead to improved clinical outcomes, if overall linkage is better than with LAB-CD4 and if ART programs can incorporate new ART-eligible patients promptly.
Our results hold in a diversity of conditions. The more expensive POC-CD4 strategy still offers excellent value under a wide range of plausible scenarios that represent a variety of settings in sub-Saharan Africa, including a range of linkage rates, opportunities for subsequent access to care, and loss to follow-up. However, this value is realized only if ART is available for those patients who link to care, if patients remain in care, and if sufficient and sustainable funds are available for a lifetime of clinical care.
As with many cost-effectiveness models, there is a fundamental assumption about what constitutes cost-effective care. We apply the WHO-CHOICE standard that uses per capita GDP as a threshold. Mozambique stands in the lowest tertile of per capita GDP reported in sub-Saharan Africa (US$570; range, US$220–US$12,400). In considering the generalizability of these results to other sub-Saharan countries with greater capacity to pay for lifesaving care (e.g., Angola or Botswana, with per capita GDPs of US$5,300 and US$9,500, respectively), POC-CD4 will likely remain an attractive option over an even wider range of input value assumptions.
Despite its good value, POC-CD4 is not without cost. We estimate an additional US$1,800,000 due to point-of-care CD4 tests in the first year of use, if 120,000 patients are eligible for immunological staging. An overwhelming majority of the increased costs associated with POC-CD4 over 5 y (88.7% of the US$15.9 million) are due not to the test itself, but rather to the downstream costs (e.g., ART and clinical visits) associated with an increased number of people engaged in HIV clinical care. Such costs would be incurred for any patients who link to care, regardless of method of linkage (e.g., POC-CD4). Given the annual budget of more than US$146 million for HIV care funded by Mozambique and donors [60], our analysis suggests that POC-CD4 is a feasible option because the increased associated costs represent 2.2% of what is currently spent on the AIDS response in Mozambique.
While some argue that the most resource-constrained settings cannot afford the cost of POC-CD4, our results indicate that this is likely where the greatest value lies. Our findings support the use of POC-CD4 particularly in settings where alternative opportunities for linkage to care are limited or other interventions have failed. The comparative value of POC-CD4 compared to LAB-CD4 at VCT clinics may be less if other strategies are also used to enhance subsequent linkage to HIV care after an initial failure to link, including home-based testing and linkage to care [65], mobile clinics [66], mHealth technologies [67], peer navigators [68]–[70], or decentralization of HIV clinical care [71]. Our results underscore that the opportunity to access care after HIV diagnosis is an important indicator of resource limitation and could guide where POC-CD4 implementation would be of greatest value. According to the nationwide registry of CD4 testing in the national health service (I. V. J.), 22% of CD4 tests in the public sector are now being performed using POC-CD4 in Mozambique, with site selection focused on areas with poor access to existing laboratories. Because each country in sub-Saharan Africa includes a diversity of settings for access to care, the value of POC-CD4 implementation could be maximized by targeting specific settings where opportunities for subsequent linkage are least available.
This analysis has several limitations. We do not address the use of point-of-care CD4 tests for routine monitoring [72]. In cases where point-of-care testing will repeatedly influence clinical management, poor test characteristics and increased cost of a point-of-care test could have a greater impact on clinical outcomes and lifetime costs. Although POC-CD4 costs include estimates for labor, quality control, etc., a more comprehensive rollout of POC-CD4 could reveal additional operational challenges such as instrument or operator failure, which could further reduce the efficacy or increase the costs of POC-CD4 compared to LAB-CD4. In our simulation, we do not directly assess the POC-CD4 impact of increasing ART coverage on reducing HIV transmissions [73]. However, the incorporation of any decreased transmissions due to earlier ART initiation resulting from POC-CD4 into this model-based analysis would further increase the value of POC-CD4 compared with LAB-CD4.
Too many eligible patients still await ART initiation. It is important to identify cost-effective methods for immunologic staging that will expedite access to care for the high-priority cases of the most immunosuppressed individuals throughout sub-Saharan Africa. Point-of-care CD4 tests are now available, and a growing body of evidence supports improved overall linkage to care with their use. We find that a POC-CD4 strategy can avert deaths and offers excellent value for immunologic staging compared to LAB-CD4 across a wide range of parameters in Mozambique, as well as in a diversity of resource-limited settings. Despite a modest increase in costs, POC-CD4 could remain economically efficient and have the greatest impact on mortality in settings throughout sub-Saharan Africa, where health care resources and systems are the most limited.
Supporting Information
Zdroje
1. Joint United Nations Programme on HIV/AIDS (2012) Together we will end AIDS. Available: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/jc2296_unaids_togetherreport_2012_en.pdf. Accessed 10 June 2014.
2. ScanlonML, VreemanRC (2013) Current strategies for improving access and adherence to antiretroviral therapies in resource-limited settings. HIV AIDS (Auckl) 5 : 1–17.
3. ZachariahR, ReidSD, ChailletP, MassaquoiM, SchoutenEJ, et al. (2011) Viewpoint: why do we need a point-of-care CD4 test for low-income countries? Trop Med Intl Health 16 : 37–41.
4. JaniIV, PeterTF (2013) How point-of-care testing could drive innovation in global health. N Engl J Med 368 : 2319–2324.
5. World Health Organization (2013) Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva: World Health Organization. Available: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1. Accessed 11 June 2014.
6. Mozambique Ministry of Health (2009) Guia de tratmento antiretrovial e infecções oportunistas no adulto, adolescente e grávida. Maputo: Ministry of Health. Available: http://www.who.int/hiv/pub/guidelines/mozambique_art.pdf. Accessed 11 June 2014.
7. BassettIV, ReganS, ChettyS, GiddyJ, UhlerLM, et al. (2010) Who starts antiretroviral therapy in Durban, South Africa? Not everyone who should. AIDS 24 (Suppl 1)S37–S44.
8. MicekMA, Gimbel-SherrK, BaptistaAJ, MatedianaE, MontoyaP, et al. (2009) Loss to follow-up of adults in public HIV care systems in central Mozambique: identifying obstacles to treatment. J Acquir Immune Defic Syndr 52 : 397–405.
9. LarsonBA, BrennanA, McNamaraL, LongL, RosenS, et al. (2010) Early loss to follow up after enrolment in pre-ART care at a large public clinic in Johannesburg, South Africa. Trop Med Int Health 15 (Suppl 1)43–47.
10. KranzerK, ZeineckerJ, GinsbergP, OrrellC, KalaweNN, et al. (2010) Linkage to HIV care and antiretroviral therapy in Cape Town, South Africa. PLoS ONE 5: e13801.
11. KranzerK, GovindasamyD, FordN, JohnstonV, LawnSD (2012) Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc 15 : 17383.
12. KayigambaFR, BakkerMI, FikseH, MugishaV, AsiimweA, et al. (2012) Patient enrolment into HIV care and treatment within 90 days of HIV diagnosis in eight Rwandan health facilities: a review of facility-based registers. PLoS ONE 7: e36792.
13. RosenS, FoxMP (2011) Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med 8: e1001056.
14. JaniIV, SitoeNE, AlfaiER, ChongoPL, QuevedoJI, et al. (2011) Effect of point-of-care CD4 cell count tests on retention of patients and rates of antiretroviral therapy initiation in primary health clinics: an observational cohort study. Lancet 378 : 1572–1579.
15. PattenGE, WilkinsonL, ConradieK, IsaakidisP, HarriesAD, et al. (2013) Impact on ART initiation of point-of-care CD4 testing at HIV diagnosis among HIV-positive youth in Khayelitsha, South Africa. J Int AIDS Soc 16 : 18518.
16. LarsonBA, SchnippelK, NdibongoB, XuluT, BrennanA, et al. (2012) Rapid point-of-care CD4 testing at mobile HIV testing sites to increase linkage to care: an evaluation of a pilot program in South Africa. J Acquir Immune Defic Syndr 61: e13–e17.
17. FaalM, NaidooN, GlencrossDK, VenterWD, OsihR (2011) Providing immediate CD4 count results at HIV testing improves ART initiation. J Acquir Immune Defic Syndr 58: e54–e59.
18. WynbergE, CookeG, ShroufiA, StevenDR, FordN (2014) Impact of point-of-care CD4 testing on linkage to HIV care: a systematic review. J Int AIDS Soc 17 : 18809.
19. GoldieSJ, YazdanpanahY, LosinaE, WeinsteinMC, AnglaretX, et al. (2006) Cost-effectiveness of HIV treatment in resource-poor settings—the case of Côte d'Ivoire. N Engl J Med 355 : 1141–1153.
20. WalenskyRP, WolfLL, WoodR, FofanaMO, FreedbergKA, et al. (2009) When to start antiretroviral therapy in resource-limited settings. Ann Intern Med 151 : 157–166.
21. WalenskyRP, WoodR, CiaranelloAL, PaltielAD, LorenzanaSB, et al. (2010) Scaling up the 2010 World Health Organization HIV treatment guidelines in resource-limited settings: a model-based analysis. PLoS Med 7: e1000382.
22. BassettIV, GovindasamyD, ErlwangerAS, HyleEP, KranzerK, et al. (2014) Mobile HIV screening in Cape Town, South Africa: clinical impact, cost and cost-effectiveness. PLoS ONE 9: e85197.
23. Gold MR, Siegel JE, Russell LB, Weinstein MC, editors (1996) Cost effectiveness in health and medicine. New York: Oxford University Press.
24. World Health Organization (2001) Macroeconomics and health: investing in health for economic development. Report of the Commission on Macroeconomics and Health. Available: http://whqlibdoc.who.int/publications/2001/924154550x.pdf. Accessed 10 June 2014.
25. World Health Organization (2005) Cost effectiveness and strategic planning (WHO-CHOICE): cost-effectiveness thresholds. Available: http://www.who.int/choice/costs/CER_thresholds/en/index.html. Accessed June 3, 2014.
26. International Monetary Fund (2012) World economic financial surveys: World Economic Outlook Database [database]. Available: http://www.imf.org/external/pubs/ft/weo/2012/02/weodata/index.aspx. Accessed 5 June 2014.
27. HolmesCB, WoodR, BadriM, ZilberS, WangB, et al. (2006) CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr 42 : 464–469.
28. ScottLE, GalpinJS, GlencrossDK (2003) Multiple method comparison: statistical model using percentage similarity. Cytometry B Clin Cytom 54 : 46–53.
29. MessouE, ChaixML, GabillardD, MingaA, LosinaE, et al. (2011) Association between medication possession ratio, virologic failure and drug resistance in HIV-1-infected adults on antiretroviral therapy in Côte d'Ivoire. J Acquir Immune Defic Syndr 56 : 356–364.
30. AhonkhaiAA, NoubaryF, MunroA, StarkR, WilkeM, et al. (2012) Not all are lost: interrupted laboratory monitoring, early death, and loss to follow-up (LTFU) in a large South African treatment program. PLoS ONE 7: e32993.
31. MillsEJ, FunkA, KantersS, KawumaE, CooperC, et al. (2013) Long-term healthcare interruptions among HIV-positive patients in Uganda. J Acquir Immune Defic Syndr 12: e23–e27.
32. FoxMP, RosenS (2010) Patient retention in antiretroviral therapy programs up to three years on treatment in sub-Saharan Africa, 2007–2009: systematic review. Trop Med Int Health 15 (Suppl 1)1–15.
33. ZachariahR, HarriesAD, ManziM, GomaniP, TeckR, et al. (2006) Acceptance of anti-retroviral therapy among patients infected with HIV and tuberculosis in rural Malawi is low and associated with cost of transport. PLoS ONE 1: e121.
34. ZachariahR, Tayler-SmithK, ManziM, MassaquoiM, MwagombaB, et al. (2011) Retention and attrition during the preparation phase and after start of antiretroviral treatment in Thyolo, Malawi, and Kibera, Kenya: implications for programmes? Trans R Soc Trop Med Hyg 105 : 421–430.
35. Voss De LimaY, EvansD, Page-ShippL, BarnardA, SanneI, et al. (2013) Linkage to care and treatment for TB and HIV among people newly diagnosed with TB or HIV-associated TB at a large, inner city South African hospital. PLoS ONE 8: e49140.
36. GlencrossDK, CoetzeeLM, FaalM, MasangoM, StevensWS, et al. (2012) Performance evaluation of the Pima point-of-care CD4 analyser using capillary blood sampling in field tests in South Africa. J Int AIDS Soc 15 : 3.
37. SukapiromK, OnlamoonN, ThepthaiC, PolsrilaK, TassaneetrithepB, et al. (2011) Performance evaluation of the Alere PIMA CD4 test for monitoring HIV-infected individuals in resource-constrained settings. J Acquir Immune Defic Syndr 58 : 141–147.
38. JaniIV, SitoeNE, ChongoPL, AlfaiER, QuevedoJI, et al. (2011) Accurate CD4 T-cell enumeration and antiretroviral drug toxicity monitoring in primary healthcare clinics using point-of-care testing. AIDS 25 : 807–812.
39. Jani IV, Sitoe N, Quevedo J, Lehe J, Peter T (2011) Cost-comparison of point-of-care and laboratory CD4 testing in resource-limited settings [abstract]. 6th IAS Conference on HIV Pathogenesis and Treatment; 17–20 July 2011; Rome, Italy. Available: http://www.iasociety.org/Abstracts/A200743694.aspx. Accessed 2 June 2014.
40. LarsonB, SchnippelK, NdibongoB, LongL, FoxMP, et al. (2012) How to estimate the cost of point-of-care CD4 testing in program settings: an example using the Alere Pima Analyzer in South Africa. PLoS ONE 7: e35444.
41. TuboiSH, BrinkhofMW, EggerM, StoneRA, BraitsteinP, et al. (2007) Discordant responses to potent antiretroviral treatment in previously naive HIV-1-infected adults initiating treatment in resource-constrained countries: the Antiretroviral Therapy in Low-Income Countries (ART-LINC) collaboration. J Acquir Immune Defic Syndr 45 : 52–59.
42. MessouE, AnglaretX, DuvignacJ, Konan-N'driE, KomenaE, et al. (2010) Antiretroviral treatment changes in adults from Côte d'Ivoire: the roles of tuberculosis and pregnancy. AIDS 24 : 93–99.
43. BrinkhofMW, Pujades-RodriguezM, EggerM (2009) Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS ONE 4: e5790.
44. MessouE, KouakouM, GabillardD, GouesseP, KoneM, et al. (2011) Medication possession ratio: predicting and decreasing loss to follow-up in antiretroviral treatment programs in Côte d'Ivoire. J Acquir Immune Defic Syndr 57 (Suppl 1)S34–S39.
45. ChiBH, YiannoutsosCT, WestfallAO, NewmanJE, ZhouJ, et al. (2011) Universal definition of loss to follow-up in HIV treatment programs: a statistical analysis of 111 facilities in Africa, Asia, and Latin America. PLoS Med 8: e1001111.
46. Centers for Diseases Control and Prevention, ICF Macro (2011) The cost of comprehensive HIV treatment in Mozambique. Report of a cost study of HIV treatment programs in Mozambique. Atlanta (Georgia): Centers for Diseases Control and Prevention.
47. LarsonBA, BiiM, Henly-ThomasS, McCoyK, SaweF, et al. (2013) ART treatment costs and retention in care in Kenya: a cohort study in three rural outpatient clinics. J Int AIDS Soc 16 : 18026.
48. MenziesNA, BerrutiAA, BerzonR, FillerS, FerrisR, et al. (2011) The cost of providing comprehensive HIV treatment in PEPFAR-supported programs. AIDS 25 : 1753–1760.
49. AliyuHB, ChukuNN, Kola-JebutuA, AbubakarZ, TorpeyK, et al. (2012) What is the cost of providing outpatient HIV counseling and testing and antiretroviral therapy services in selected public health facilities in Nigeria? J Acquir Immune Defic Syndr 61 : 221–225.
50. Clinton Health Access Initiative (2011) Antiretroviral (ARV) ceiling price list. Available: http://www.clintonhealthaccess.org/files/chai_arv_ceilingPriceList_201105_english.pdf. Accessed 30 May 2014.
51. RosenS, FoxMP, GillCJ (2007) Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med 4: e298.
52. Mtapuri-ZinyoweraS, ChidemeM, MangwanyaD, MugurungiO, GudukeyaS, et al. (2010) Evaluation of the PIMA point-of-care CD4 analyzer in VCT clinics in Zimbabwe. J Acquir Immune Defic Syndr 55 : 1–7.
53. DiawPA, DaneauG, ColyAA, NdiayeBP, WadeD, et al. (2011) Multisite evaluation of a point-of-care instrument for CD4(+) T-cell enumeration using venous and finger-prick blood: the PIMA CD4. J Acquir Immune Defic Syndr 58: e103–e111.
54. van Schaik N, Kranzer K, Myer L, Radithlhalo E, Thebus E, et al.. (2011) Field validation of the PIMA™ Analyzer in a mobile clinic setting in South Africa [abstract]. 18th Conference on Retroviruses and Opportunistic Infections; 27 February–2 March 2011; Boston, Massachusetts, US.
55. ManabeYC, WangY, ElbireerA, AuerbachB, CastelnuovoB (2012) Evaluation of portable point-of-care CD4 counter with high sensitivity for detecting patients eligible for antiretroviral therapy. PLoS ONE 7: e34319.
56. MnyaniCN, McIntyreJA, MyerL (2012) The reliability of point-of-care CD4 testing in identifying HIV-infected pregnant women eligible for antiretroviral therapy. J Acquir Immune Defic Syndr 60 : 260–264.
57. World Health Organization (2008) Choosing interventions that are cost effective (WHO-CHOICE): country-specific unit costs. Available: http://www.who.int/choice/country/country_specific/en/index.html. Accessed 29 May 2014.
58. Finney DJ (1971) Statistical method in biological assay. London: Griffin.
59. PolskyD, GlickHA, WillkeR, SchulmanK (1997) Confidence intervals for cost-effectiveness ratios: a comparison of four methods. Health Econ 6 : 243–252.
60. Republic of Mozambique National AIDS Council, Conselho Nacional de Combate ao HIV/SIDA (2012) GARPR (global AIDS response progress report): for the period 2010–2011. Available: http://www.unaids.org/en/dataanalysis/knowyourresponse/countryprogressreports/2012countries/ce_MZ_Narrative_Report[1].pdf. Accessed 13 May 2014.
61. Joint United Nations Programme on HIV/AIDS (2013) Mozambique. Available: http://www.unaids.org/en/Regionscountries/Countries/Mozambique/. Accessed 2 August 2013.
62. World Health Organization (2011) Countries: Mozambique. Available: http://www.who.int/countries/moz/en/. Accessed 20 January 2014.
63. StinnettAA, MullahyJ (1998) Net health benefits: a new framework for the analysis of uncertainty in cost-effectiveness analysis. Med Decis Making 18: S68–S80.
64. LarsonBA, SchnippelK, BrennanA, LongL, XuluT, et al. (2013) Same-day CD4 testing to improve uptake of HIV care and treatment in South Africa: point-of-care is not enough. AIDS Res Treat 2013 : 941493.
65. van RooyenH, BarnabasRV, BaetenJM, PhakathiZ, JosephP, et al. (2013) High HIV testing uptake and linkage to care in a novel program of home-based HIV counseling and testing with facilitated referral in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr 64: e1–e8.
66. GovindasamyD, KranzerK, van SchaikN, NoubaryF, WoodR, et al. (2013) Linkage to HIV, TB and non-communicable disease care from a mobile testing unit in Cape Town, South Africa. PLoS ONE 8: e80017.
67. SiednerMJ, HabererJE, BwanaMB, WareNC, BangsbergDR (2012) High acceptability for cell phone text messages to improve communication of laboratory results with HIV-infected patients in rural Uganda: a cross-sectional survey study. BMC Med Inform Decis Mak 12 : 56.
68. BassettIV, GiddyJ, ChaissonCE, RossD, BogartLM, et al. (2013) A randomized trial to optimize HIV/TB care in South Africa: design of the Sizanani trial. BMC Infect Dis 13 : 390.
69. NsigayeR, WringeA, RouraM, KalluvyaS, UrassaM, et al. (2009) From HIV diagnosis to treatment: evaluation of a referral system to promote and monitor access to antiretroviral therapy in rural Tanzania. J Int AIDS Soc 12 : 31.
70. HatcherAM, TuranJM, LeslieHH, KanyaLW, KwenaZ, et al. (2012) Predictors of linkage to care following community-based HIV counseling and testing in rural Kenya. AIDS Behav 16 : 1295–1307.
71. BemelmansM, van den AkkerT, FordN, PhilipsM, ZachariahR, et al. (2010) Providing universal access to antiretroviral therapy in Thyolo, Malawi through task shifting and decentralization of HIV/AIDS care. Trop Med Int Health 15 : 1413–1420.
72. EstillJ, EggerM, BlaserN, VizcayaLS, GaroneD, et al. (2013) Cost-effectiveness of point-of-care viral load monitoring of ART in resource-limited settings: mathematical modelling study. AIDS 27 : 1483–1492.
73. CohenMS, ChenYQ, McCauleyM, GambleT, HosseinipourMC, et al. (2011) Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 365 : 493–505.
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2014 Číslo 9- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Monitoring and Evaluating Progress towards Universal Health Coverage in Ghana
- Sorting Reality from What We Think We Know About Breast Cancer in Africa
- The PLOS “Monitoring Universal Health Coverage” Collection: Managing Expectations
- Monitoring and Evaluating Progress towards Universal Health Coverage in India
- Monitoring and Evaluating Progress towards Universal Health Coverage in Tanzania
- Monitoring and Evaluating Progress towards Universal Health Coverage in Brazil
- Monitoring and Evaluating Progress towards Universal Health Coverage in Thailand
- Monitoring and Evaluating Progress towards Universal Health Coverage in Estonia
- Monitoring and Evaluating Progress towards Universal Health Coverage in Chile
- Prioritizing Pregnant Women for Long-Lasting Insecticide Treated Nets through Antenatal Care Clinics
- Financial Risk Protection and Universal Health Coverage: Evidence and Measurement Challenges
- Convergence of Mortality Rates among Patients on Antiretroviral Therapy in South Africa and North America
- Malaria Prevention during Pregnancy—Is There a Next Step Forward?
- Beyond UHC: Monitoring Health and Social Protection Coverage in the Context of Tuberculosis Care and Prevention
- Intimate Partner Violence and Reproductive Coercion: Global Barriers to Women's Reproductive Control
- Genetic Predisposition to Increased Blood Cholesterol and Triglyceride Lipid Levels and Risk of Alzheimer Disease: A Mendelian Randomization Analysis
- Readmissions after Hospitalization for Heart Failure, Acute Myocardial Infarction, or Pneumonia among Young and Middle-Aged Adults: A Retrospective Observational Cohort Study
- Intermittent Preventive Treatment of Malaria in Pregnancy with Mefloquine in HIV-Infected Women Receiving Cotrimoxazole Prophylaxis: A Multicenter Randomized Placebo-Controlled Trial
- Equity-Oriented Monitoring in the Context of Universal Health Coverage
- The Clinical and Economic Impact of Point-of-Care CD4 Testing in Mozambique and Other Resource-Limited Settings: A Cost-Effectiveness Analysis
- Effective Coverage: A Metric for Monitoring Universal Health Coverage
- Mortality in Patients with HIV-1 Infection Starting Antiretroviral Therapy in South Africa, Europe, or North America: A Collaborative Analysis of Prospective Studies
- Global Financing and Long-Term Technical Assistance for Multidrug-Resistant Tuberculosis: Scaling Up Access to Treatment
- Oral Cholera Vaccine Development and Use in Vietnam
- Monitoring and Evaluating Progress towards Universal Health Coverage in Bangladesh
- Monitoring and Evaluating Progress towards Universal Health Coverage in South Africa
- Monitoring and Evaluating Progress towards Universal Health Coverage in Ethiopia
- Preventing Acute Malnutrition in Young Children: Improving the Evidence for Current and Future Practice
- Monitoring and Evaluating Progress towards Universal Health Coverage in Singapore
- Monitoring and Evaluating Progress towards Universal Health Coverage in China
- Monitoring and Evaluating Progress towards Universal Health Coverage in Tunisia
- Receptor-Defined Subtypes of Breast Cancer in Indigenous Populations in Africa: A Systematic Review and Meta-Analysis
- WHO Essential Medicines Policies and Use in Developing and Transitional Countries: An Analysis of Reported Policy Implementation and Medicines Use Surveys
- Intermittent Preventive Treatment of Malaria in Pregnancy with Mefloquine in HIV-Negative Women: A Multicentre Randomized Controlled Trial
- Preventing Acute Malnutrition among Young Children in Crises: A Prospective Intervention Study in Niger
- Monitoring Progress towards Universal Health Coverage at Country and Global Levels
- Monitoring Intervention Coverage in the Context of Universal Health Coverage
- Regular Breakfast Consumption and Type 2 Diabetes Risk Markers in 9- to 10-Year-Old Children in the Child Heart and Health Study in England (CHASE): A Cross-Sectional Analysis
- Proton Pump Inhibitors and Hospitalization with Hypomagnesemia: A Population-Based Case-Control Study
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Proton Pump Inhibitors and Hospitalization with Hypomagnesemia: A Population-Based Case-Control Study
- Monitoring and Evaluating Progress towards Universal Health Coverage in Chile
- Malaria Prevention during Pregnancy—Is There a Next Step Forward?
- Financial Risk Protection and Universal Health Coverage: Evidence and Measurement Challenges
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



