-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Orthostatic Hypotension and the Long-Term Risk of Dementia: A Population-Based Study
Using data from the Rotterdam study, Mohammad Ikram and colleagues examine the association between orthostatic hypotension and the risk of developing dementia over 25 years.
Published in the journal: Orthostatic Hypotension and the Long-Term Risk of Dementia: A Population-Based Study. PLoS Med 13(10): e32767. doi:10.1371/journal.pmed.1002143
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1002143Summary
Using data from the Rotterdam study, Mohammad Ikram and colleagues examine the association between orthostatic hypotension and the risk of developing dementia over 25 years.
Introduction
Cardiovascular health is now well-established as a key determinant in the prevention of dementia, including Alzheimer disease [1,2], but the mechanisms by which vascular damage leads to cognitive decline remain largely unknown. As cerebral hypoperfusion is widely implicated in dementia [3,4], cerebral haemodynamics have been suggested as a potential link between vascular risk factors and dementia [5]. Two important mechanisms for maintenance of proper and continuous cerebral perfusion are local vasoreactivity and autonomous nervous system function. Cerebral vasoreactivity has indeed been found to be associated with the risk of developing dementia in the general population [6], but the role of autonomous nervous system function in the onset of dementia has been less well studied.
Autonomic dysfunction may result in orthostatic hypotension (OH), which affects 20%–30% of the elderly population [7,8]. OH is characterised by a marked drop in blood pressure following postural change, insufficiently compensated for by sympathetic and parasympathetic mechanisms. This drop in blood pressure may elicit transient cerebral hypoperfusion, especially in the absence of a compensatory increase in heart rate. OH is associated with an increased risk of cardiovascular events, stroke, and mortality [9]. Moreover, OH is highly prevalent among patients with dementia and mild cognitive impairment, compared to healthy controls [10–13], but only one study has assessed the longitudinal relation between OH and the risk of dementia in initially healthy participants. In this Swedish population, OH was associated with an increased risk of dementia at re-examination after 6 y, but the investigators were unable to adjust for cardiovascular risk factors aside from hypertension, and attrition was substantial, with 37.5% of participants lost to follow-up between examination rounds [14]. These limited data regarding OH and cognition prompted a recent review and meta-analysis to conclude that longitudinal studies using standardised criteria are needed to elucidate whether OH is an independent risk factor for developing dementia [9,15]. We therefore aimed to determine the association between OH and the risk of dementia, in a long-term, ongoing population-based study.
Methods
Ethics Statement
The Rotterdam Study has medical ethics committee approval per the Population Study Act Rotterdam Study, executed by the Ministry of Health, Welfare and Sports of the Netherlands. Written informed consent was obtained from all participants. For the current study, the analysis plan was drafted in June 2015.
Study Population
This study is embedded within the Rotterdam Study, a large ongoing population-based cohort study in the Netherlands, with an initial study population of 7,983 participants (78% of invitees) aged ≥55 y from the Ommoord area, a suburb of Rotterdam. The Rotterdam Study methods have been described in detail previously [16]. In brief, participants were interviewed at home and subsequently examined at the research centre for baseline assessment from October 1989 to July 1993. OH was determined during baseline assessment. Of 7,983 interviewed participants, 7,157 (89.7%) visited the research centre for the baseline physical examination. As of 2015, five follow-up examinations have been carried out.
Orthostatic Hypotension Assessment
Blood pressure and heart rate were measured using an automatic recorder (Dinamap, Critikon). The baseline blood pressure reading was the mean of two measurements on the right upper arm with the participant in supine position, after 5 min of rest. Measurements were repeated in the standing position after 1, 2, and 3 min. OH was defined as ≥20 mm Hg decrease in systolic blood pressure (SBP) or ≥10 mm Hg decrease in diastolic blood pressure (DBP) after postural change at any of the three measurements, in accordance with the Consensus Committee of the American Autonomic Society and the American Academy of Neurology [17,18]. We defined severity of OH by degree of blood pressure drop, i.e., ≥20/10 but <30/15, ≥30/15 but <40/20, and ≥40/20 mm Hg. We calculated continuous measures of blood pressure change in response to postural change, expressed as the coefficient of variation of within participant variability, defined as the ratio of the standard deviation (SD) to the mean of all measurements (i.e., measurements in supine and upright position combined). Furthermore, we determined the maximum increase in heart rate within 3 min after postural change. Directly afterwards, participants were asked whether they had felt unwell within the minutes following postural change.
Dementia Screening and Cognitive Function Assessment
Participants were screened for dementia at baseline and follow-up examinations using a three-step protocol [19]. Screening was done using the Mini-Mental State Examination (MMSE) and the Geriatric Mental State Schedule (GMS) organic level. Those with MMSE < 26 or GMS > 0 subsequently underwent examination and informant interview using the Cambridge Examination for Mental Disorders of the Elderly (CAMDEX). Additionally, the total cohort was continuously monitored for dementia through computerised linkage of medical records from general practitioners and the regional institute for outpatient mental healthcare with the study database. Available neuroimaging data were used when required for establishing a diagnosis. For all suspected cases of dementia, a consensus panel led by a consultant neurologist (P. J. K.) decided on the final diagnosis in accordance with standard criteria for dementia (DSM-III-R), Alzheimer disease (NINCDS-ADRDA), and vascular dementia (NINDS-AIREN). Follow-up until 1 January 2014 was near complete (94.0% of potential person years), and participants were censored within this follow-up period at date of dementia diagnosis, date of death, date of loss to follow-up, or 1 January 2014, whichever came first.
Other Measurements
We assessed smoking status (i.e., current, former, never), alcohol intake, use of antihypertensive medication, use of lipid-lowering medication, and use of anticholinergic medication at baseline by interview. Anticholinergic medication included antipsychotic and antidepressant medication, but also drugs prescribed against parkinsonism, urinary incontinence, or obstructive pulmonary disease that can have anticholinergic side effects. Fasting serum lipid levels were measured at baseline. Hypertension was defined as the use of antihypertensive medication and/or elevated systolic or DBP (>140/90 mm Hg). Body mass index (kg/m2) was computed from measurements of height and weight. Diabetes mellitus was defined as the use of blood-glucose-lowering medication at baseline or a random serum glucose level ≥11.1 mmol/l [20]. Myocardial infarction and atrial fibrillation were assessed by direct questioning and presence of abnormalities on a 12-lead electrocardiogram as determined by study physicians and reviewed by a cardiologist. Heart failure was determined using a validated score, similar to the definition of heart failure of the European Society of Cardiology [21]. APOE genotype was determined using polymerase chain reaction on coded DNA samples.
Analysis
Analyses included all non-demented, stroke-free participants attending the study centre for examination. Of 7,157 participants attending the study centre, 531 were ineligible due to prevalent dementia (n = 312), stroke (n = 168), or both (n = 51). Missing covariate data (maximum 17.6%), except for APOE genotype, were imputed using 5-fold multiple imputation, based on determinant (presence of OH and postural SBP variability), outcome, and included covariates. Distribution of covariates was similar in the imputed versus non-imputed dataset.
We determined the association between presence of OH and incident dementia, using Cox proportional hazard models. We repeated the analysis with dementia or death as a joint outcome measure, to reduce selection due to competing risk (upon referee’s request). Subsequently, we analysed categories of increasing severity of orthostatic blood pressure drop, and OH with and without feeling unwell. Because of right-skewedness, we performed a natural logarithmic transformation of SBP variability to obtain a roughly normal distribution (mean −2.52, SD 0.58). Z-scores were computed by dividing the difference between the individual value and the population mean by the population SD. We determined the association between SBP variability related to postural change and incident dementia, per quartile and continuously per SD increase, using a Cox model. To eliminate a paradoxical impact of high blood pressure variability in those with excessive increases, we repeated analyses after excluding those with a ≥20 mm Hg increase in SBP or ≥10 mm Hg increase in DBP within 3 min (upon referee’s request). Furthermore, we determined whether associations extended to those without a formal diagnosis of OH. We then assessed whether the risk of dementia in relation to orthostatic blood pressure drops was modified by response in heart rate after postural change, by testing for multiplicative interaction in the above Cox model and providing risk estimates of OH for dementia per quartile of response in heart rate. We verified that the proportional hazard assumption was not violated in these models by plotting the partial (Schoenfeld) residuals against follow-up time.
All analyses were adjusted for age and sex (model I), and additionally in a second model for smoking status, alcohol intake, systolic and diastolic blood pressure, use of antihypertensive medication, ratio of serum total cholesterol to high-density lipoprotein, use of lipid-lowering medication, diabetes mellitus, body mass index, use of anticholinergic medication, and APOE genotype (model II).
We repeated the analyses for Alzheimer disease and vascular dementia separately, after censoring participants at time of incident stroke, after excluding those with Parkinson disease at baseline, after excluding those with heart disease (i.e., coronary heart disease, heart failure, atrial fibrillation; upon referee’s request), and after excluding those with possible postural tachycardia syndrome (defined as a ≥30 beats per minute increase in heart rate or any heart rate of ≥120 beats per minute). Finally, we performed several sensitivity analyses: (1) for men and women separately, (2) for persons above and below the median age (68.5 y), (3) excluding the first 5 y of follow-up to assess for reverse causality, (4) for those with and without heart failure at baseline, (5) for those with and without a history of hypertension, (6) distinguishing use of antihypertensive drugs, and (7) for those with and without diabetes (upon referee’s request). Finally, we repeated analyses for OH, SBP variability, and dementia risk in a subset of participants without significant comorbidity (i.e., excluding those with heart disease, Parkinson disease, and diabetes; upon referee’s request).
All analyses were done using IBM SPSS Statistics version 23.0. Alpha level (type 1 error) was set at 0.05.
Results
Of 6,626 eligible participants, 6,303 (95.1%) underwent examination for OH. No baseline blood pressure measurement was obtained in eight individuals, and no measurement after postural change in 91 individuals, leaving a total of 6,204 (93.6%) cases for analysis. Baseline characteristics of participants are shown in Table 1.
Tab. 1. Baseline characteristics (n = 6,204). 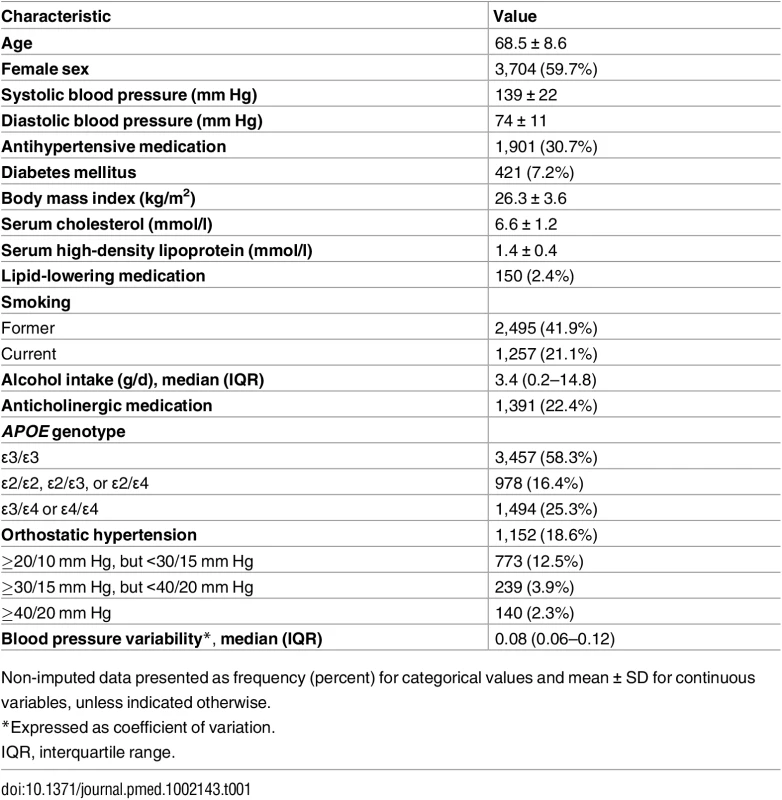
Non-imputed data presented as frequency (percent) for categorical values and mean ± SD for continuous variables, unless indicated otherwise. Overall, 1,152/6,204 (18.6%) participants had OH. The prevalence of OH steeply increased with age, to 30.6% of those aged ≥75 y. Although prevalence in the elderly was similar for men and women, there was a slightly higher prevalence in women at younger ages (Fig 1). Of all patients with OH, 160 (13.9%) reported feeling unwell along with their blood pressure drop.
Fig. 1. Age-specific prevalence of orthostatic hypotension in men and women. 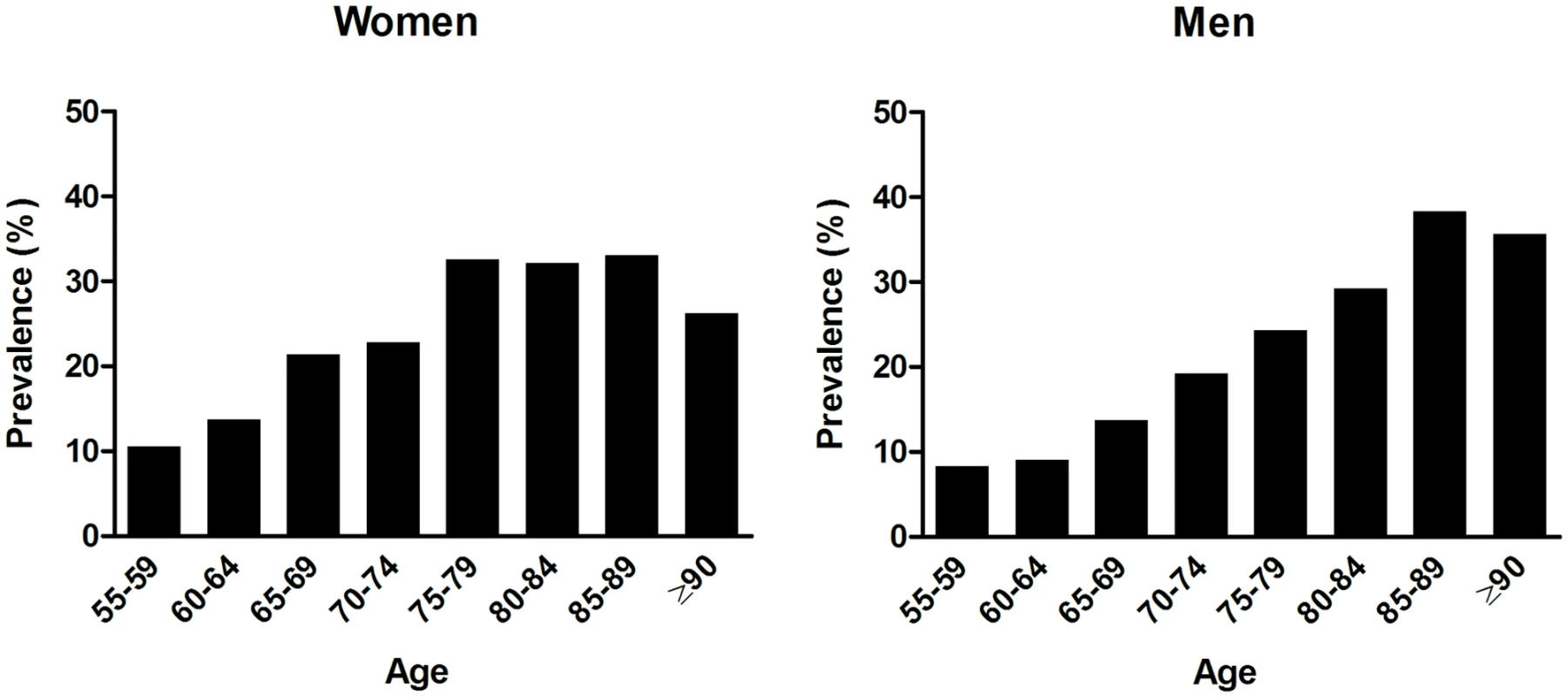
During a median follow-up time of 15.3 y (interquartile range 8.3–20.8), 1,176 individuals developed dementia, of whom 935 (79.5%) were diagnosed with Alzheimer disease, 95 (8.1%) with vascular dementia, 43 (3.7%) with Parkinson dementia, and 30 (2.6%) with another type of dementia, and in 73 (6.2%) no definite subdiagnosis could be made. Of all incident dementia cases, 129 were preceded by a stroke a median 3.7 y (interquartile range 1.2–7.2) before diagnosis of dementia.
OH at baseline was associated with an increased risk of dementia during follow-up (adjusted hazard ratio [aHR] 1.15, 95% CI 1.00–1.34, p = 0.05; Table 2). Similarly on a continuous scale, variability in SBP related to postural change was associated with an increased risk of dementia (aHR per SD increase: 1.08, 95% CI 1.01–1.16, p = 0.02). This association was similar when excluding those who fulfilled the formal criteria for OH (aHR 1.08, 95% CI 1.00–1.17) and unaltered by excluding those with a marked increase in blood pressure following postural change (see S1 Table). Results were similar for Alzheimer disease only. For vascular dementia, we observed higher risk estimates with OH than for Alzheimer disease in the age - and sex-adjusted model (aHR 1.53, 95% CI 0.97–2.43), but these were largely explained by cardiovascular risk factors, so that fully adjusted estimates were similar to those for Alzheimer disease (aHR 1.20, 95% CI 0.73–1.96; Table 2). We did not observe a clear exposure-response relation for severity of OH, because of lower effect estimates for participants with the most severe blood pressure drops (Fig 2). In contrast, the risk of dementia strongly increased per quartile of blood pressure variability (Fig 2; for a full table with risk estimates per quartile and per SD, see S2 Table). Risk estimates were similar when modelling dementia or death as a joint outcome (OH: aHR 1.17, 95% CI 1.08–1.27; blood pressure variability: aHR 1.08, 95% CI 1.04–1.12). Estimates for both OH and SBP variability were attenuated when incorporating these simultaneously in a model (OH: aHR 1.07, 95% CI 0.90–1.27; blood pressure variability: aHR 1.06, 95% CI 0.99–1.14).
Tab. 2. Orthostatic hypotension and the risk of dementia. 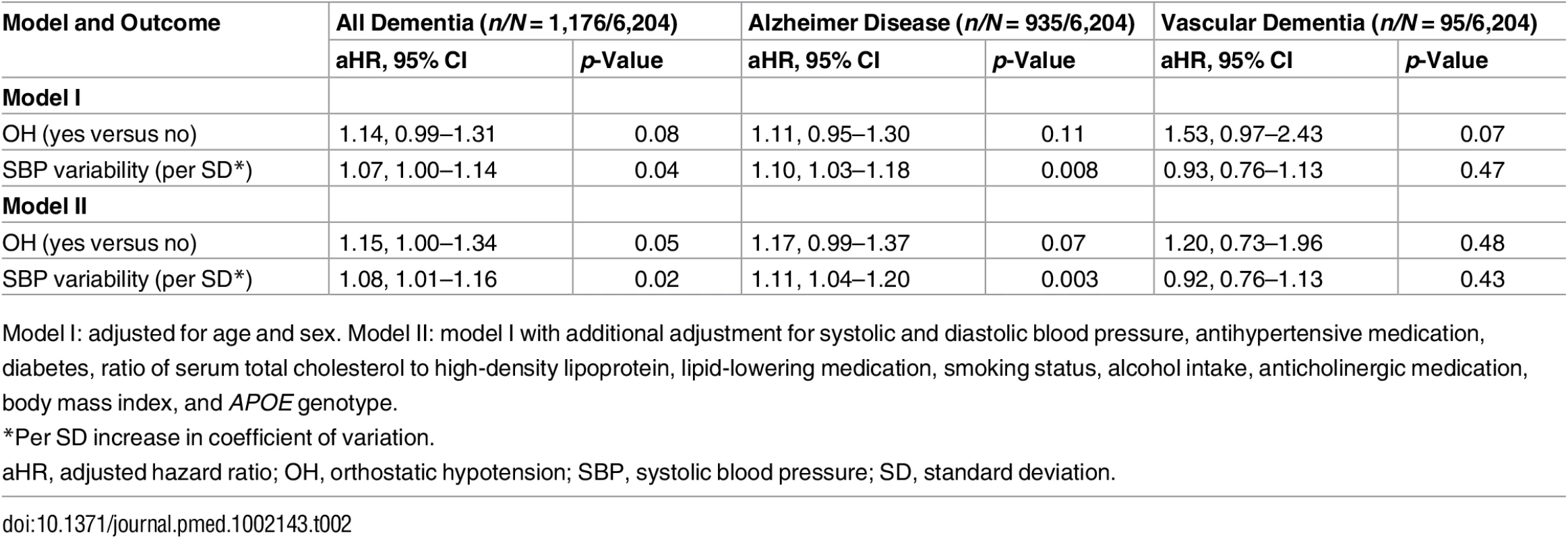
Model I: adjusted for age and sex. Model II: model I with additional adjustment for systolic and diastolic blood pressure, antihypertensive medication, diabetes, ratio of serum total cholesterol to high-density lipoprotein, lipid-lowering medication, smoking status, alcohol intake, anticholinergic medication, body mass index, and APOE genotype. Fig. 2. Risk of dementia in relation to severity of orthostatic blood pressure drop (in mm Hg) and quartiles of systolic blood pressure variability. 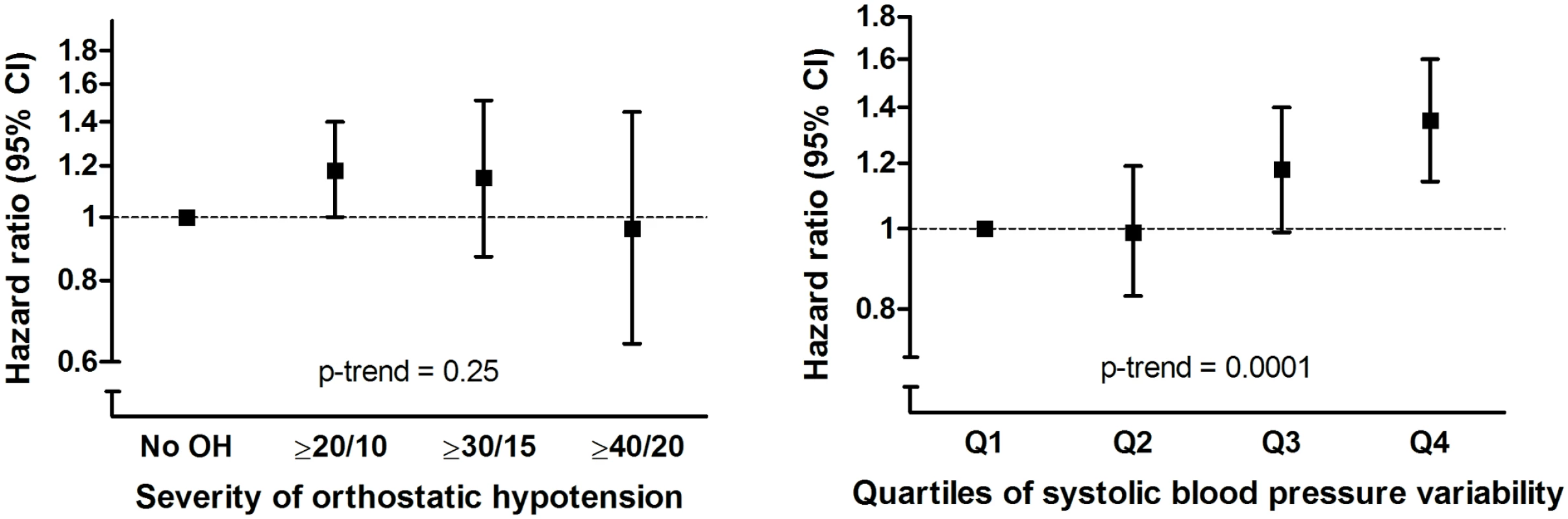
OH, orthostatic hypotension. Results for OH where individuals reported feeling unwell along with the blood pressure drop were similar to results for OH without feeling unwell (aHR 1.20, 95% CI 0.86–1.66, versus aHR 1.15, 95% CI 0.98–1.34, respectively). The risk of dementia related to OH was most profound in participants who lacked a compensatory increase in heart rate (aHR for lowest quartile of heart rate response 1.39, 95% CI 1.04–1.85, p-value for interaction = 0.05; Fig 3). This risk was similar after excluding all participants taking beta-blockers (see S2 Table).
Fig. 3. Risk of dementia in relation to orthostatic hypotension, stratified per quartile of response in heart rate. 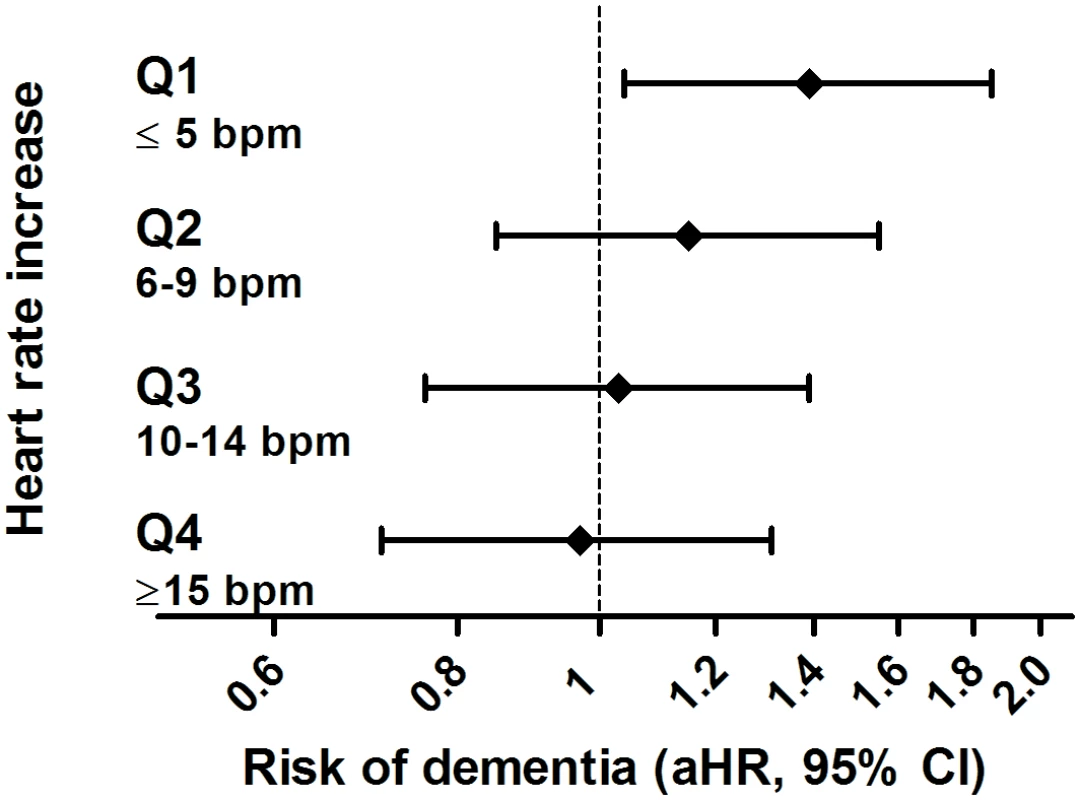
aHR, adjusted hazard ratio; bpm, beats per minute. Sensitivity analyses showed similar results after censoring for incident stroke, excluding participants with prevalent Parkinson disease, excluding those with possible postural tachycardia syndrome, or omitting the first 5 y of follow-up (Table 3). A history of hypertension or use of any antihypertensive medication did not modify the risk of dementia associated with OH (Table 3). Amongst 177 participants with heart failure at baseline, risk estimates for OH were higher than in those without heart failure, albeit not statistically significantly (aHR 1.52, 95% CI 0.63–3.66, p-value for interaction = 0.07). Risk estimates for both OH and SBP variability were slightly stronger when excluding participants with cardiac disease, neurodegenerative comorbidity, or diabetes all together (see S3 Table).
Tab. 3. Sensitivity analyses for the association between orthostatic hypotension and incident dementia. 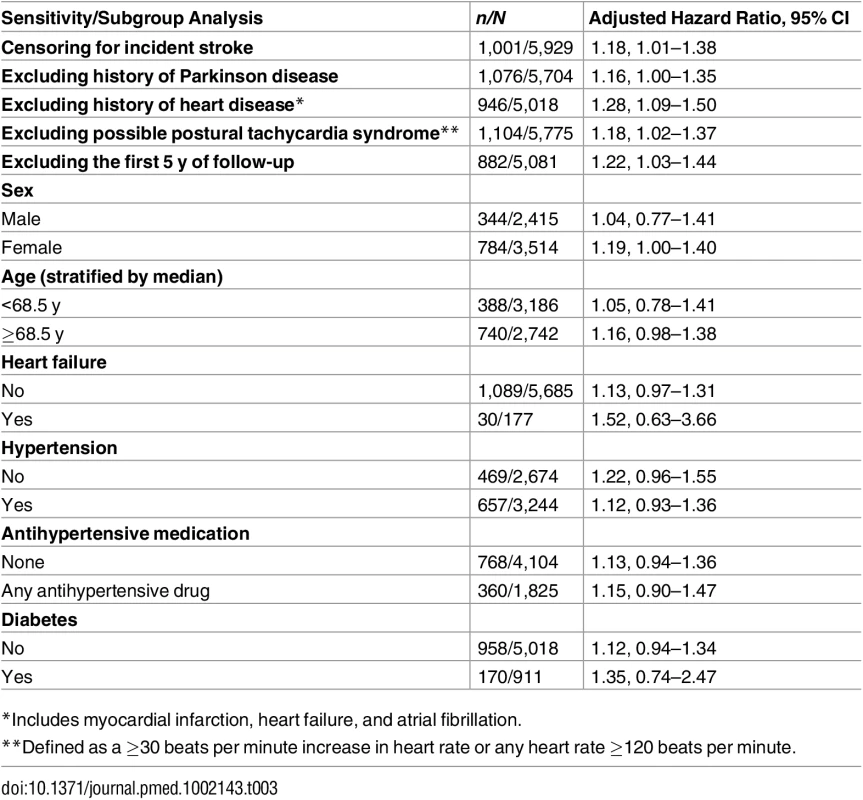
*Includes myocardial infarction, heart failure, and atrial fibrillation. Discussion
In this large population-based study, OH was present in nearly one in five participants and was associated with a 15% increase in long-term risk of dementia. The risk of developing dementia was highest in those with OH lacking a compensatory increase in heart rate. Similarly, higher variability in blood pressure related to postural change was associated with a higher risk of dementia, even in individuals without a formal diagnosis of OH.
Prevalence of OH in our study was high and increased steeply with age, in line with previous studies among individuals within a similar age range in the community [7,8]. A few studies have investigated OH in relation to cognitive test performance. In the ARIC study, OH was associated with decline on two cognitive tests, but this decline was largely explained by cardiovascular risk factors [22]. Two smaller studies found no overall association between OH and decline on the MMSE after 2 y [7,23]. Conversely, OH was found to increase the risk of conversion from mild cognitive impairment to dementia after 3 y [24], as well as the risk of dementia in patients with Parkinson disease [25]. Only one other study has assessed the relation between OH and the risk of dementia in initially healthy individuals. In a sample of 1,480 individuals in the Swedish general population, OH was associated with the risk of having dementia at re-examination after 6 y [14]. However, the investigators were unable to fit survival models or adjust for cardiovascular risk factors aside from hypertension, and attrition was substantial, with 37.5% of participants lost to follow-up [14]. We found OH to be associated with long-term risk of dementia on continuous follow-up, independent of various other risk factors.
The most apparent explanation for our findings is that OH causes brain damage due to recurrent transient cerebral hypoperfusion. Autonomic nervous system function is responsible for maintaining continuous cerebral perfusion, together with local vasoreactivity, which has previously been associated with the risk of dementia in the general population [6]. Brief episodes of hypoperfusion elicited by sudden blood pressure drops may lead to hypoxia, with detrimental effects on brain tissue via, for instance, neuroinflammation and oxidative stress [26]. These mechanisms have been suggested to be of particular relevance in the pathogenesis of small vessel disease [27], and orthostatic blood pressure drops in patients with dementia have been associated with deep white matter and basal ganglia hyperintensities [28], albeit not with overall white matter lesion volume [29]. The reduction in cerebral blood flow with autonomic failure has also been reported to predominantly affect the hippocampus [30], possibly linking hypoperfusion to early Alzheimer pathology. Another potential explanation for our findings might be that OH serves as a marker of other detrimental consequences of autonomic dysfunction, such as blood pressure variability [31,32], response to Valsalva manoeuvre [13,33], cardiovascular reflex and heart rate variability [34,35], and 30/15 ratio [35]. Several of these measures may be linked to dementia via hypoperfusion, but other pathways could be involved also. For instance, decreased arterial wall compliance with hypertension likely contributes to OH by diminishing baroreceptor sensitivity [36]. Arterial stiffness and OH are both associated with increased burden of cerebral white matter lesions and vascular disease including stroke [9,28,37,38]. As these are established risk factors for dementia, these conditions may act as mediators or indicate shared aetiology between diseases. Nevertheless, we did not see risk estimates for all-cause dementia attenuated after adjustment for cardiovascular risk factors, censoring for clinical stroke, or restricting the population to those without cardiac or neurodegenerative comorbidities. Alternatively, sympathetic failure can occur with diabetic neuropathy, and although we adjusted for clinical history of diabetes, some patients with impaired fasting glucose might already have had autonomic derailment together with subclinical small vessel disease. We had no other direct measures of autonomic dysfunction to ascertain whether OH is the main driving force behind our findings. Blood pressure variability in our study was measured following postural change and therefore reflects a distinct orthostatic response, although it might in part occur as a manifestation of wider autonomic failure, unrelated to postural change. Accordingly, risk estimates for both OH and blood pressure variability attenuated after incorporating both in the same model, but it remains unclear whether this is because they are both computed from postural-change-related measurements or because they reflect the same underlying autonomic dysfunction. Given the interaction we found between OH and the lack of a compensatory increase in heart rate, the impact of OH may well vary with the degree of overall autonomic failure, and future studies are encouraged to incorporate various measures of autonomic dysfunction collected in the same individuals simultaneously (for which reported risks and effect estimates may serve as a guidance). Although autonomic dysfunction may even reflect early signs of neurodegeneration, the long follow-up duration of our study renders reverse causality less likely.
The risk of dementia associated with OH in our study was independent of whether participants reported feeling unwell along with the blood pressure drop, and the vast majority of patients with OH did not have symptoms during testing. Although for blood pressure variability we observed an exposure-response association, we did not find this for severity of OH itself. As OH is also associated with mortality [9], this finding may be attributable to competing risk, causing the most severely affected participants to die at a younger age, prior to developing dementia. Alternatively, rather than the degree of blood pressure drop, the lack of compensatory increase in heart rate may better reflect the severity of consequences of orthostatic drops in blood pressure. Taken together, our findings suggest that formal assessment of OH is necessary to have sufficient test sensitivity, and incorporation of heart rate response in the definition of OH may contribute to evaluating aetiology and clinical severity. Hypotension might be harmful even without accompanying clinical symptoms such as light-headedness. This lack of symptoms with orthostasis was previously observed in patients with dementia [39] and may warrant caution in view of studies linking low blood pressure in late life to cognitive decline and dementia [40].
OH most commonly arises due to autonomic dysfunction in the absence of neurological disease, but may be provoked by synucleinopathies (e.g., Parkinson disease), small fibre peripheral neuropathy, volume depletion (e.g., due to diuretics), and diminished cardiac pump function. In addition, several drugs can cause or aggravate OH, including antihypertensive agents and antidepressants. Participants in our study with heart failure at baseline seemed particularly affected by OH, possibly due to the lack of a compensatory increase in stroke volume. OH has been associated with the development of structural cardiac changes, including left ventricular hypertrophy [41], which may function as a mediator towards dementia [42,43]. However, the subgroup of participants with heart failure in our study was too small to draw any firm conclusions. We found similar associations between OH and dementia after excluding those with Parkinson disease, and in users versus non-users of antihypertensive medication.
Although we believe our findings are valid, there are certain limitations to our study to take into account. First, measures of OH were not available for all eligible participants. Although this was largely due to logistic reasons, we cannot completely rule out selection bias. Second, despite adjustment for many potentially confounding factors, residual confounding may still occur. However, given the lack of attenuation of our results in our second, more fully adjusted model, residual confounding is unlikely to result from the most relevant, included covariates. Third, we continued blood pressure measurements for up to 3 min after postural change, and while this approach is in line with international guidelines, it may have resulted in missed orthostatic blood pressure drops beyond this time window [44]. However, any misclassification (i.e., missed diagnosis of OH) would likely have led to underestimation of the true effect. Fourth, subtypes of dementia were based on clinical diagnosis, and mixed pathology (e.g., Lewy bodies) in patients with clinical Alzheimer disease may contribute to the observed associations. Fifth, we were unable to adjust for the fact that OH predisposes for falls, which may contribute to cognitive decline due to traumatic brain injury. Finally, the majority of our study population was of European descent, and findings may not be applicable to other ethnicities.
In conclusion, OH is associated with an increased risk of dementia in the general population. This finding supports an important role for maintaining continuous cerebral perfusion in the prevention of dementia.
Supporting Information
Zdroje
1. Andrieu S, Coley N, Lovestone S, Aisen PS, Vellas B. Prevention of sporadic Alzheimer’s disease: lessons learned from clinical trials and future directions. Lancet Neurol. 2015;14 : 926–944. doi: 10.1016/S1474-4422(15)00153-2 26213339
2. Gardener H, Wright CB, Rundek T, Sacco RL. Brain health and shared risk factors for dementia and stroke. Nat Rev Neurol. 2015;11 : 651–657. doi: 10.1038/nrneurol.2015.195 26481296
3. Ruitenberg A, Heijer den T, Bakker SLM, van Swieten JC, Koudstaal PJ, Hofman A, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57 : 789–794. 15929050
4. Binnewijzend MAA, Kuijer JPA, Benedictus MR, van der Flier WM, Wink AM, Wattjes MP, et al. Cerebral blood flow measured with 3D pseudocontinuous arterial spin-labeling MR imaging in Alzheimer disease and mild cognitive impairment: a marker for disease severity. Radiology. 2013;267 : 221–230. doi: 10.1148/radiol.12120928 23238159
5. la Torre de JC. Cerebral hemodynamics and vascular risk factors: setting the stage for Alzheimer’s disease. J Alzheimers Dis. 2012;32 : 553–567. doi: 10.3233/JAD-2012-120793 22842871
6. Wolters FJ, De Bruijn RF, Hofman A, Koudstaal PJ, Ikram MA, Heart Brain Connection Collaborative Research Group. Cerebral vasoreactivity, apolipoprotein E, and the risk of dementia: a population-based study. Arterioscler Thromb Vasc Biol. 2016;36 : 204–210. doi: 10.1161/ATVBAHA.115.306768 26586657
7. Viramo P, Luukinen H, Koski K, Laippala P, Sulkava R, Kivelä SL. Orthostatic hypotension and cognitive decline in older people. J Am Geriatr Soc. 1999;47 : 600–604. 10323655
8. Alagiakrishnan K, Patel K, Desai RV, Ahmed MB, Fonarow GC, Forman DE, et al. Orthostatic hypotension and incident heart failure in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2014;69 : 223–230. doi: 10.1093/gerona/glt086 23846416
9. Angelousi A, Girerd N, Benetos A, Frimat L, Gautier S, Weryha G, et al. Association between orthostatic hypotension and cardiovascular risk, cerebrovascular risk, cognitive decline and falls as well as overall mortality: a systematic review and meta-analysis. J Hypertens. 2014;32 : 1562–1571. doi: 10.1097/HJH.0000000000000235 24879490
10. Mehrabian S, Duron E, Labouree F, Rollot F, Bune A, Traykov L, et al. Relationship between orthostatic hypotension and cognitive impairment in the elderly. J Neurol Sci. 2010;299 : 45–48. doi: 10.1016/j.jns.2010.08.056 20855089
11. Sonnesyn H, Nilsen DW, Rongve A, Nore S, Ballard C, Tysnes OB, et al. High prevalence of orthostatic hypotension in mild dementia. Dement Geriatr Cogn Disord. 2009;28 : 307–313. doi: 10.1159/000247586 19828952
12. Nicolini P, Ciulla MM, Malfatto G, Abbate C, Mari D, Rossi PD, et al. Autonomic dysfunction in mild cognitive impairment: evidence from power spectral analysis of heart rate variability in a cross-sectional case-control study. PLoS ONE. 2014;9:e96656. doi: 10.1371/journal.pone.0096656 24801520
13. Jensen-Dahm C, Waldemar G, Staehelin Jensen T, Malmqvist L, Moeller MM, Andersen BB, et al. Autonomic dysfunction in patients with mild to moderate Alzheimer’s disease. J Alzheimers Dis. 2015;47 : 681–689. doi: 10.3233/JAD-150169 26401703
14. Elmståhl S, Widerström E. Orthostatic intolerance predicts mild cognitive impairment: incidence of mild cognitive impairment and dementia from the Swedish general population cohort Good Aging in Skåne. Clin Interv Aging. 2014;9 : 1993–2002. doi: 10.2147/CIA.S72316 25429211
15. Sambati L, Calandra-Buonaura G, Poda R, Guaraldi P, Cortelli P. Orthostatic hypotension and cognitive impairment: a dangerous association? Neurol Sci. 2014;35 : 951–957. doi: 10.1007/s10072-014-1686-8 24590841
16. Hofman A, Brusselle GGO, Darwish Murad S, van Duijn CM, Franco OH, Goedegebure A, et al. The Rotterdam Study: 2016 objectives and design update. Eur J Epidemiol. 2015;30 : 661–708. doi: 10.1007/s10654-015-0082-x 26386597
17. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Neurology. 1996;46 : 1470. 8628505
18. Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21 : 69–72. doi: 10.1007/s10286-011-0119-5 21431947
19. Schrijvers EMC, Verhaaren BFJ, Koudstaal PJ, Hofman A, Ikram MA, Breteler MMB. Is dementia incidence declining?: trends in dementia incidence since 1990 in the Rotterdam Study. Neurology. 2012;78 : 1456–1463. doi: 10.1212/WNL.0b013e3182553be6 22551732
20. Diabetes mellitus. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1985;727 : 1–113. 3934850
21. Mosterd A, Hoes AW, de Bruyne MC, Deckers JW, Linker DT, Hofman A, et al. Prevalence of heart failure and left ventricular dysfunction in the general population; the Rotterdam Study. Eur Heart J. 1999;20 : 447–455. 10213348
22. Rose KM, Couper D, Eigenbrodt ML, Mosley TH, Sharrett AR, Gottesman RF. Orthostatic hypotension and cognitive function: the Atherosclerosis Risk in Communities Study. Neuroepidemiology. 2010;34 : 1–7. doi: 10.1159/000255459 19893322
23. Yap PLK, Niti M, Yap KB, Ng TP. Orthostatic hypotension, hypotension and cognitive status: early comorbid markers of primary dementia? Dement Geriatr Cogn Disord. 2008;26 : 239–246. doi: 10.1159/000160955 18841007
24. Hayakawa T, McGarrigle CA, Coen RF, Soraghan CJ, Foran T, Lawlor BA, et al. Orthostatic blood pressure behavior in people with mild cognitive impairment predicts conversion to dementia. J Am Geriatr Soc. 2015;63 : 1868–1873. doi: 10.1111/jgs.13596 26313614
25. Anang JBM, Gagnon J-F, Bertrand J-A, Romenets SR, Latreille V, Panisset M, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology. 2014 Sep 30;83 : 1253–1260. doi: 10.1212/WNL.0000000000000842 25171928
26. Raz L, Knoefel J, Bhaskar K. The neuropathology and cerebrovascular mechanisms of dementia. J Cereb Blood Flow Metab. 2016;36 : 172–186. 26174330
27. Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 2013;12 : 483–497. doi: 10.1016/S1474-4422(13)70060-7 23602162
28. Ballard C, O’Brien J, Barber B, Scheltens P, Shaw F, McKeith I, et al. Neurocardiovascular instability, hypotensive episodes, and MRI lesions in neurodegenerative dementia. Ann N Y Acad Sci. 2000;903 : 442–445. 10818535
29. Soennesyn H, Nilsen DW, Oppedal K, Greve OJ, Beyer MK, Aarsland D. Relationship between orthostatic hypotension and white matter hyperintensity load in older patients with mild dementia. PLoS ONE. 2012;7:e52196. doi: 10.1371/journal.pone.0052196 23284932
30. Laosiripisan J, Tarumi T, Gonzales MM, Haley AP, Tanaka H. Association between cardiovagal baroreflex sensitivity and baseline cerebral perfusion of the hippocampus. Clin Auton Res. 2015;25 : 213–218. doi: 10.1007/s10286-015-0296-8 26280218
31. Alpérovitch A, Blachier M, Soumaré A, Ritchie K, Dartigues J-F, Richard-Harston S, et al. Blood pressure variability and risk of dementia in an elderly cohort, the Three-City Study. Alzheimers Dement. 2014;10(5 Suppl):S330–S337. doi: 10.1016/j.jalz.2013.05.1777 23954028
32. Yamaguchi Y, Wada M, Sato H, Nagasawa H, Koyama S, Takahashi Y, et al. Impact of ambulatory blood pressure variability on cerebral small vessel disease progression and cognitive decline in community-based elderly Japanese. Am J Hypertens. 2014;27 : 1257–1267. doi: 10.1093/ajh/hpu045 24651635
33. Algotsson A, Viitanen M, Winblad B, Solders G. Autonomic dysfunction in Alzheimer’s disease. Acta Neurol Scand. 1995;91 : 14–18. 7732768
34. Collins O, Dillon S, Finucane C, Lawlor B, Kenny RA. Parasympathetic autonomic dysfunction is common in mild cognitive impairment. Neurobiol Aging. 2012;33 : 2324–2333. doi: 10.1016/j.neurobiolaging.2011.11.017 22188719
35. Idiaquez J, Sandoval E, Seguel A. Association between neuropsychiatric and autonomic dysfunction in Alzheimer’s disease. Clin Auton Res. 2002;12 : 43–46. 12102448
36. Mattace-Raso FU, Van den Meiracker AH, Bos WJ, Van der Cammen TJ, Westerhof BE, Elias-Smale S, et al. Arterial stiffness, cardiovagal baroreflex sensitivity and postural blood pressure changes in older adults: the Rotterdam Study. J Hypertens. 2007;25 : 1421–1426. 17563564
37. Van Sloten TT, Protogerou AD, Henry RM, Schram MT, Launer LJ, Stehouwer CD. Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2015;53 : 121–130. doi: 10.1016/j.neubiorev.2015.03.011 25827412
38. Van Sloten TT, Sedaghat S, Laurent S, London GM, Pannier B, Ikram MA, et al. Carotid stiffness is associated with incident stroke: a systematic review and individual participant meta-analysis. J Am Coll Cardiol. 2015;66 : 2116–2125. doi: 10.1016/j.jacc.2015.08.888 26541923
39. Bengtsson-Lindberg M, Larsson V, Minthon L, Wattmo C, Londos E. Lack of orthostatic symptoms in dementia patients with orthostatic hypotension. Clin Auton Res. 2015;25 : 87–94. doi: 10.1007/s10286-014-0244-z 24743866
40. Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4 : 487–499. 16033691
41. Magnusson M, Holm H, Bachus E, Nilsson P, Leosdottir M, Melander O, et al. Orthostatic hypotension and cardiac changes after long-term follow-up. Am J Hypertens. 2016;29 : 847–852. doi: 10.1093/ajh/hpv187 26643688
42. de Bruijn RFAG Portegies MLP, Leening MJG, Bos MJ, Hofman A, van der Lugt A, et al. Subclinical cardiac dysfunction increases the risk of stroke and dementia: the Rotterdam Study. Neurology. 2015;84 : 833–840. doi: 10.1212/WNL.0000000000001289 25632093
43. Jefferson AL, Beiser AS, Himali JJ, Seshadri S, O’Donnell CJ, Manning WJ, et al. Low cardiac index is associated with incident dementia and Alzheimer’s disease: the Framingham Heart Study. Circulation. 2015;131 : 1333–1339. doi: 10.1161/CIRCULATIONAHA.114.012438 25700178
44. Gibbons CH, Freeman R. Delayed orthostatic hypotension: a frequent cause of orthostatic intolerance. Neurology. 2006;67 : 28–32. 16832073
Štítky
Interné lekárstvo
Článok vyšiel v časopisePLOS Medicine
Najčítanejšie tento týždeň
2016 Číslo 10- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Intermitentní hladovění v prevenci a léčbě chorob
- Statinová intolerance
- Co dělat při intoleranci statinů?
- Monoklonální protilátky v léčbě hyperlipidemií
-
Všetky články tohto čísla
- Sailing in Uncharted Waters: Carefully Navigating the Polio Endgame
- Core Outcome Set–STAndards for Reporting: The COS-STAR Statement
- Improving the Science of Measles Prevention—Will It Make for a Better Immunization Program?
- Burden of Six Healthcare-Associated Infections on European Population Health: Estimating Incidence-Based Disability-Adjusted Life Years through a Population Prevalence-Based Modelling Study
- Population Immunity against Serotype-2 Poliomyelitis Leading up to the Global Withdrawal of the Oral Poliovirus Vaccine: Spatio-temporal Modelling of Surveillance Data
- Conveying Equipoise during Recruitment for Clinical Trials: Qualitative Synthesis of Clinicians’ Practices across Six Randomised Controlled Trials
- How Relevant Is Sexual Transmission of Zika Virus?
- A Holistic, Person-Centred Care Model for Victims of Sexual Violence in Democratic Republic of Congo: The Panzi Hospital One-Stop Centre Model of Care
- Impact on Epidemic Measles of Vaccination Campaigns Triggered by Disease Outbreaks or Serosurveys: A Modeling Study
- The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling
- Circulating Apolipoprotein E Concentration and Cardiovascular Disease Risk: Meta-analysis of Results from Three Studies
- Towards Equity in Service Provision for Gay Men and Other Men Who Have Sex with Men in Repressive Contexts
- Obstetric Facility Quality and Newborn Mortality in Malawi: A Cross-Sectional Study
- Prophylactic Oral Dextrose Gel for Newborn Babies at Risk of Neonatal Hypoglycaemia: A Randomised Controlled Dose-Finding Trial (the Pre-hPOD Study)
- Characterization of Novel Antimalarial Compound ACT-451840: Preclinical Assessment of Activity and Dose–Efficacy Modeling
- Regulatory T Cell Responses in Participants with Type 1 Diabetes after a Single Dose of Interleukin-2: A Non-Randomised, Open Label, Adaptive Dose-Finding Trial
- Orthostatic Hypotension and the Long-Term Risk of Dementia: A Population-Based Study
- Tradeoffs in Introduction Policies for the Anti-Tuberculosis Drug Bedaquiline: A Model-Based Analysis
- Whole Genome Sequence Analysis of a Large Isoniazid-Resistant Tuberculosis Outbreak in London: A Retrospective Observational Study
- Texas and Its Measles Epidemics
- Impacts on Breastfeeding Practices of At-Scale Strategies That Combine Intensive Interpersonal Counseling, Mass Media, and Community Mobilization: Results of Cluster-Randomized Program Evaluations in Bangladesh and Viet Nam
- The Tuberculosis Cascade of Care in India’s Public Sector: A Systematic Review and Meta-analysis
- PLOS Medicine
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Prophylactic Oral Dextrose Gel for Newborn Babies at Risk of Neonatal Hypoglycaemia: A Randomised Controlled Dose-Finding Trial (the Pre-hPOD Study)
- Regulatory T Cell Responses in Participants with Type 1 Diabetes after a Single Dose of Interleukin-2: A Non-Randomised, Open Label, Adaptive Dose-Finding Trial
- Orthostatic Hypotension and the Long-Term Risk of Dementia: A Population-Based Study
- Improving the Science of Measles Prevention—Will It Make for a Better Immunization Program?
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



