-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
CO Acts as a Signalling Molecule in Populations of the Fungal Pathogen
When colonising host-niches or non-animated medical devices, individual cells of the fungal pathogen Candida albicans expand into significant biomasses. Here we show that within such biomasses, fungal metabolically generated CO2 acts as a communication molecule promoting the switch from yeast to filamentous growth essential for C. albicans pathology. We find that CO2-mediated intra-colony signalling involves the adenylyl cyclase protein (Cyr1p), a multi-sensor recently found to coordinate fungal responses to serum and bacterial peptidoglycan. We further identify Lys 1373 as essential for CO2/bicarbonate regulation of Cyr1p. Disruption of the CO2/bicarbonate receptor-site interferes selectively with C. albicans filamentation within fungal biomasses. Comparisons between the Drosophila melanogaster infection model and the mouse model of disseminated candidiasis, suggest that metabolic CO2 sensing may be important for initial colonisation and epithelial invasion. Our results reveal the existence of a gaseous Candida signalling pathway and its molecular mechanism and provide insights into an evolutionary conserved CO2-signalling system.
Published in the journal: CO Acts as a Signalling Molecule in Populations of the Fungal Pathogen. PLoS Pathog 6(11): e32767. doi:10.1371/journal.ppat.1001193
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001193Summary
When colonising host-niches or non-animated medical devices, individual cells of the fungal pathogen Candida albicans expand into significant biomasses. Here we show that within such biomasses, fungal metabolically generated CO2 acts as a communication molecule promoting the switch from yeast to filamentous growth essential for C. albicans pathology. We find that CO2-mediated intra-colony signalling involves the adenylyl cyclase protein (Cyr1p), a multi-sensor recently found to coordinate fungal responses to serum and bacterial peptidoglycan. We further identify Lys 1373 as essential for CO2/bicarbonate regulation of Cyr1p. Disruption of the CO2/bicarbonate receptor-site interferes selectively with C. albicans filamentation within fungal biomasses. Comparisons between the Drosophila melanogaster infection model and the mouse model of disseminated candidiasis, suggest that metabolic CO2 sensing may be important for initial colonisation and epithelial invasion. Our results reveal the existence of a gaseous Candida signalling pathway and its molecular mechanism and provide insights into an evolutionary conserved CO2-signalling system.
Introduction
Candida albicans is the predominant fungal pathogen of humans. In healthy individuals C. albicans resides as a commensal of the gastrointestinal, oral and vaginal tracts. C. albicans can cause superficial infections which, although not life threatening, provide discomfort to the individual and require treatment with antifungals which is a constant drain on hospitals resources. However, C. albicans infections are life threatening when the individual's immune system becomes compromised as a result of age, cancer, chemotherapy hospitalisation and AIDS. Under these circumstances superficial infections may readily develop into systemic disease where mortality rates are reported to be up to 40%, which is higher than those for most bacterial infections [1], [2], [3]. For example, oropharyngeal candidiasis is common in patients with haematological malignancies (up to 60%) and those undergoing radiotherapy [4], [5], [6]. Here, a few fungal cells develop into biomasses measuring several millimetres in diameter that penetrate and invade the underlying tissue, eventually leading to dissemination of Candida into the blood stream and subsequently systemic infection [7].
Development from superficial infection to invasive disease is mediated by many well characterised virulence factors including morphological transition. C. albicans can exist in yeast, pseudohyphal and true hyphal growth forms, all of which are important for the virulence of the organism [8]. Yeast cells are thought to be essential for growth and dissemination [9], while the hyphal forms are essential for invading mucosal membranes [9]. This morphological transition is mediated by host environmental cues including temperature, pH, serum, O2, and CO2, which the pathogen encounters during disease progression [5], [10], [11].
The virulence-associated morphological transitions of C. albicans are largely controlled through the secondary messenger cAMP. In C. albicans, cAMP is synthesised by the fungal adenylyl cyclase (AC), Cyr1p [12], a member of the Class III nucleotidyl cyclase family [13]. Activity of Cyr1p governs most processes essential to C. albicans virulence including tissue adhesion followed by the invasion of the underlying host-barriers, and biofilm formation [14]. C. albicans AC activity is subject to both positive and negative regulation, with an increasing number of molecules directly interacting with specific domains of the protein [10], [15], [16]. For example, bacterial peptidoglycan stimulates Cyr1p via the enzyme's leucine-rich-region [16], and CO2/HCO3− directly activates the Cyr1p C-terminal catalytic domain [10]. These forms of regulation enable C. albicans to recognise and respond (via filamentation) to specific host environmental conditions during disease progression.
In addition to host environmental cues, the morphological transition of C. albicans is also regulated by soluble chemical mediators, termed quorum sensing molecules (QSMs). QSMs are secreted into the environment by a variety of microorganisms [for recent reviews see 17], [18], and upon reaching threshold concentrations, impact on microbial behaviour by influencing expression of virulence determinants [19]. QSMs including the self-generated sesquiterpene farnesol [20] and 3-oxo-C12 homoserine lactone (HSL) secreted by Pseudomonas aeruginosa [21] inhibit C. albicans filamentation though cAMP dependent signalling cascades [22].
Further to soluble chemical mediators, volatile compounds can also act as signalling molecules. For example, in Saccharomyces cerevisiae, nutrient limited yeast cells release volatile ammonia, which when sensed by another colony inhibits its growth in the direction of the signal [23]. CO2 is a volatile gas that has recently been described as a predominant regulator of C. albicans virulence factors and has been shown to effect the virulence of other microbial species [24], [25]. In C. albicans CO2 functions in two processes key to pathogenicity, one metabolic and the other cell signalling to promote filamentation [10]. In biological systems CO2 is maintained in equilibrium with its hydrated form, HCO3−, via the actions of carbonic anhydrase. HCO3− is required for metabolism, but when at high concentrations HCO3− directly activates adenylyl cyclase increasing cytosolic cAMP and promoting filamentation [10]. To date, only the effects of high (5%) exogenous CO2 concentrations have been investigated in microbial species. However, microbes continuously secrete metabolically generated CO2 into their immediate microenvironment at levels perceived to be lower than 5%. Here, we investigate the effects of self generated CO2 on pathogenicity associated traits of C. albicans. Previously we identified the carbonic anhydrase, Nce103p, as being essential for growth under CO2 limiting conditions [10]. Now we explore a new application of the mutant strain Δnce103 as a CO2 biosensor to report on CO2 concentrations within fungal biomasses. Using our CO2-dependent bio-sensing strain, we demonstrate that build-up of self-generated, metabolic CO2 occurs in a fungal population. Furthermore, we show that CO2 mediates its effects as a hierarchy, with low concentrations of CO2 functioning to fill metabolic demand, then once CO2 exceeds a critical threshold, it promotes filamentation and subsequent surface invasion of the pathogen. We show that microbial CO2, like environmental CO2, is sensed by the AC catalytic domain and identify a bicarbonate receptor site in Cyr1p.
Results
C. albicans-generated CO2 accumulates under diffusion-limiting conditions
CO2 is generated during metabolism and acts as an important cellular signalling molecule in many organisms. CO2 influences microbial virulence and organisms behaviours such as mating, feeding or ventilation [26]. We confirmed that, when grown under diffusion-limiting conditions (i.e., closed systems), C. albicans accumulated self-generated CO2 (Figure 1 A, B). Next we asked whether self-generated CO2 could be utilized by C. albicans to meet the organism's growth requirements. In normal atmospheres, (0.03% CO2) the C. albicans carbonic anhydrase (CA), Nce103p, is essential for catalyzing the hydration of CO2 to bicarbonate to meet metabolic demands. Therefore, in ‘open’ systems (i.e., under the 0.03% CO2 in air), deletion of NCE103 results in a depletion of bicarbonate levels which inhibits growth. However, at elevated CO2 concentrations (such as 5% CO2 experienced by C. albicans when inside an infected mammalian host) there is sufficient CO2 spontaneously hydrated to bicarbonate to meet the metabolic requirements restoring growth (Figure 2A). Therefore, the carbonic anhydrase mutant (TK1; Δnce103) can only grow in environments with elevated concentrations of CO2 [10], and as a result, functions as a CO2 bio-indicator. The Δnce103 bio-indicator strain failed to grow when co-incubated with wild type (SC5314; WT) cells in an open system, but grew in the presence of WT C. albicans in a closed system without exogenously supplied CO2 (Figure 2B). Furthermore, incubation of surplus (10,000 CFUs/plate) Δnce103, on its own, in closed but not open systems also restored the growth of Δnce103 (Figure S1), suggesting that in closed systems the elevated CO2 levels are sufficient to complement the growth of Δnce103.
Fig. 1. Closed systems enable CO2 accumulation. 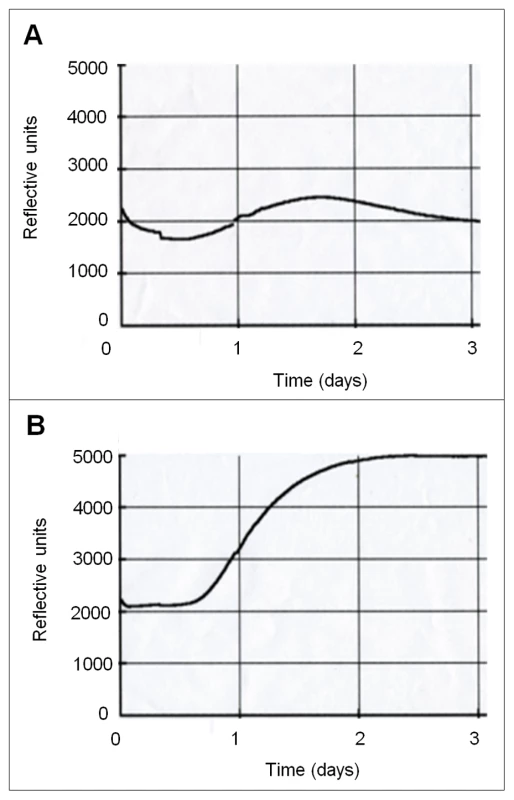
10,000 Wild type cells were inoculated onto 10 ml DMEM pH7 agar in BacT/ALERT bottles. Bottles were either incubated as an open system (A) where free diffusion of metabolically generated gases was permitted or as a closed system (B) where diffusion was inhibited. CO2 accumulation was measured for 48 hr at 37°C in BacT/ALERT 3D automated microbial detection system (bioMerieux). Reflective units depicted on the X-axis are a direct measurement of the CO2 concentration in the system (see material and methods for details). Fig. 2. CO2 as a self generated volatile communication molecule. 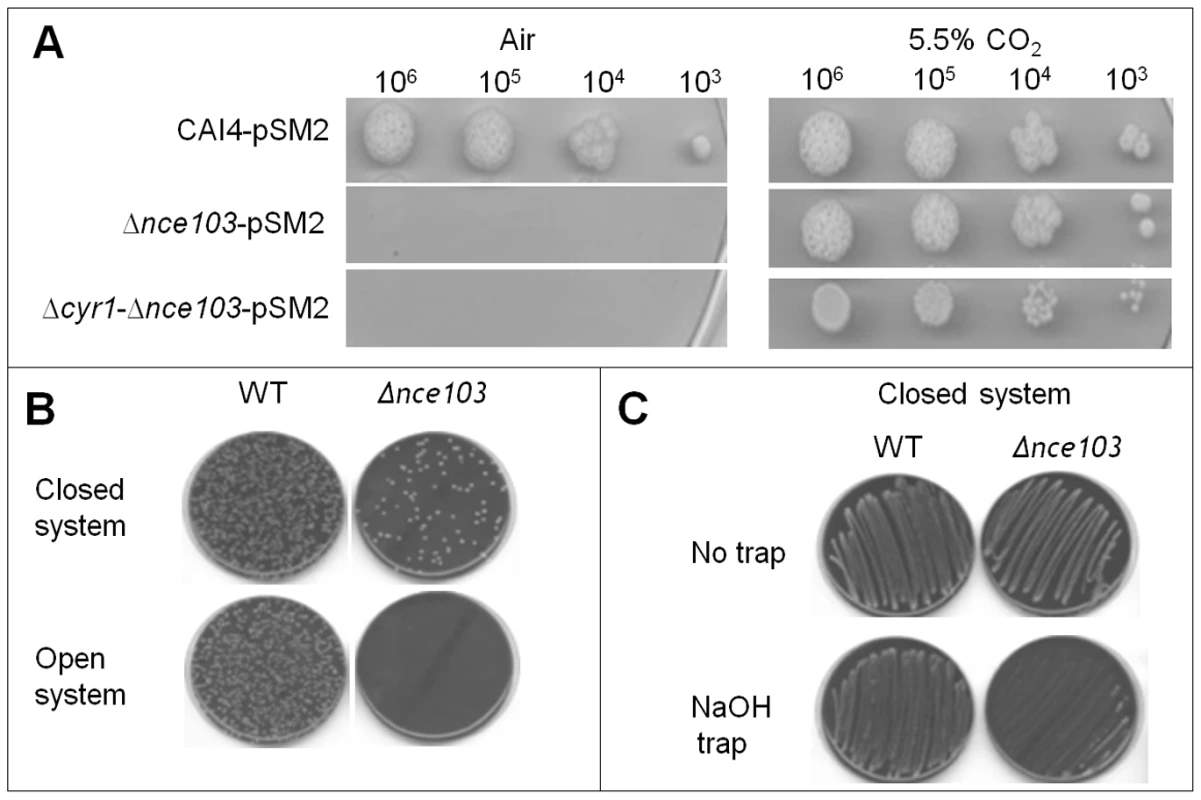
A) Cell dilutions (5 µl) of CAI4-pSM2, Δnce103-pSM2 and Δcyr1-Δnce103-pSM2 were spotted onto YNB plates and incubated in the presence of 0.03% (air) or 5% CO2 for 48 hours. B) 1000 wild type cells and 200 Δnce103 cells were inoculated onto separate CBA plates and both plates placed together into zip-locked polyvinyl chloride bags, which were sealed (closed system; top) or left open (open system; bottom) and incubated at 37°C for 48 hours. C) Wild type and Δnce103 cells were incubated in a closed system, as described in B), in the absence or presence of a NaOH trap. To confirm that it was volatile CO2 generated by the WT strain which restored growth to the Δnce103 CO2 bio-indicator strain, we included hydroxide into the closed system, which specifically traps CO2 in the form of carbonate [27]. Solid sodium hydroxide interfered with the growth of the Δnce103 CO2 bio-indicator strain, but not WT (Figure 2C). The diminished growth in the presence of sodium hydroxide is most likely caused by CO2 trapping and not oxygen depletion, as oxygen levels are not influenced by the CO2 trap. Taken together, these results reveal that metabolically generated CO2 can provide sufficient HCO3− to meet the metabolic demands of C. albicans, and that this CO2 can be provided in the form of a volatile signal from neighbouring colonies.
Consistent with the idea that the CO2 generated by WT C. albicans is supplying CO2/HCO3− to meet the metabolic demand of the Δnce103 bio-indicator strain, rescue was independent from the cAMP signalling system, as the Δcyr1-Δnce103 strain (RH12) was also complemented when incubated at elevated CO2 (Figure 2A). These data suggest that there is sufficient CO2 generated during normal metabolism of WT C. albicans to support the growth of the Δnce103 bio-indicator strain, as long as diffusion of the generated CO2 is limited.
CO2 accumulates inside a fungal biomass
We next asked whether CO2 levels sufficient for signalling would build-up within a fungal biomass. To address this question, we grew the Δnce103 CO2 bio-indicator strain on its own, or mixed with equal numbers of DAY286, a Δhis1 strain which is wild type for carbonic anhydrase and adenylyl cyclase, in an open system to specifically test whether CO2 accumulation could occur between cells growing in the same biomass. Using the different auxotrophic tags (HIS+ and HIS−) to distinguish the two strains after incubation within mixed biomasses, we were able to directly test whether metabolically generated CO2 from DAY286 could complement the growth of Δnce103, while Δnce103 on its own would be restricted in growth. Co-incubation of Δnce103 with DAY286 enhanced the recovery of Δnce103 600-fold (p = >0.0001) when compared to incubation of the CO2 bio-indicator strain on its own (Figure 3). To exclude that DAY286 was able to fill the metabolic demands of Δnce103 by providing other metabolic intermediates other than CO2, the Δnce103 strain was also co-incubated with a surplus (1×106 cells) of heat-killed DAY286 cells. However, co-incubation of Δnce103 and heat-killed DAY286 did not enhance the recovery of Δnce103 compared to incubation of the CO2 bio-indicator strain alone (Figure 3, p = >0.0001), suggesting that within a fungal biomass, even in an open system, there is an accumulation of metabolic CO2 sufficient to promote the growth of Δnce103. These data also prove that the carbonic anhydrase is essential because it ‘captures’ metabolically generated CO2 as HCO3− which is needed to meet metabolic requirements of cells deep within the colony.
Fig. 3. CO2 signal build-up occurs within fungal populations. 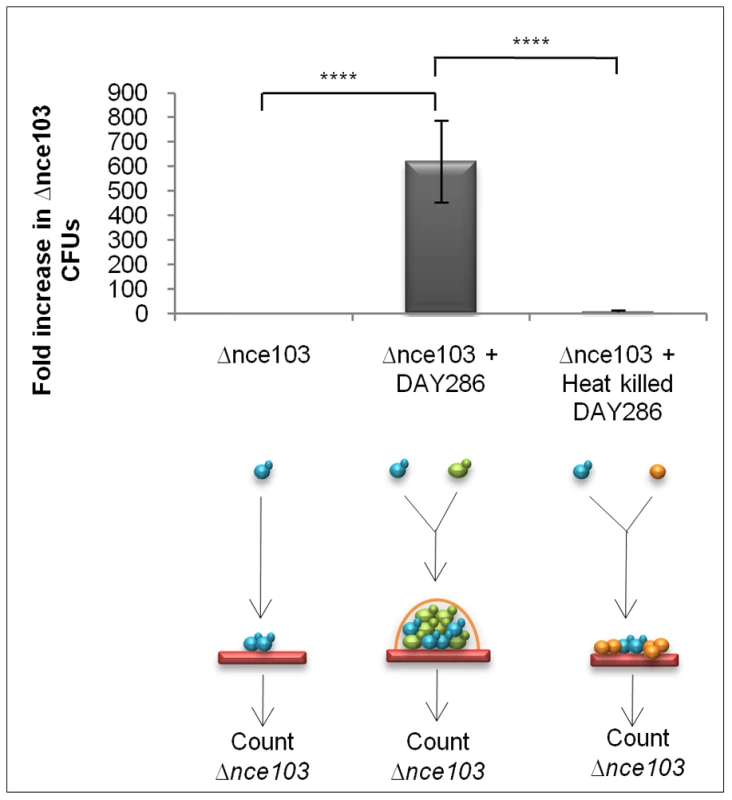
Equal cell numbers (500 CFUs) of DAY286 (represented as green cells in the diagram) and Δnce103 (represented as blue cells in the diagram), 1000 Δnce103 alone and 1000 Δnce103 with 1×106 heat-killed DAY286 (represented as orange cells in the diagram) were spotted onto YPD agar and incubated for 48 hr at 37°C. Cells were recovered, populations separated by plating onto selective media and Δnce103 CFUs counted. Values are the mean and standard deviation from 8 independent experiments (**** indicates that the P statistic for the represented data was greater than 0.0001). The volatile messenger CO2 affects C. albicans colony morphology
CO2 is not only required for metabolism, but it also acts as a signal for cAMP-dependent filamentation of C. albicans. Therefore, we sought to determine whether metabolically generated CO2 could act within a biomass to modulate morphology. To test whether C. albicans produces sufficient CO2 to affect filamentation, we incubated wild type cells under open and closed conditions. In open systems, no colonies filamented, while under closed conditions, we observed changes in colony morphology after 48 hours. Plating 500 CFUs produced extensive filamentous colonies (Figure 4A). Microscopic analysis of resuspended colonies confirmed that the majority of the cells filamented in the closed system (forming a highly interwoven mass of cells resistant to mechanical stress; Figure 4A). As C. albicans hyphal induction is critically dependent on cAMP signalling cascades [12], we tested a strain deficient for adenylyl cyclase CR276-CTRL (RH20; Δcyr1) in our open and closed systems. The Δcyr1 strain did not show any changes in colony morphology, even when incubation periods were extended to 72 hours to account for its known reduced growth rate (Figure 4B). Additionally, altered morphology was independent of carbonic anhydrase, as Δnce103, but not Δcyr1-Δnce103, formed filaments in the presence of 5% CO2 (Figure 4C). Interestingly, the extent of filamentation of the WT strain was biomass dependent. At 48 hours, plating in the presence of 50 CFUs generated wrinkled colonies (Figure 4D), which were not fully filamentous.
Fig. 4. CO2 affects C. albicans colony morphology. 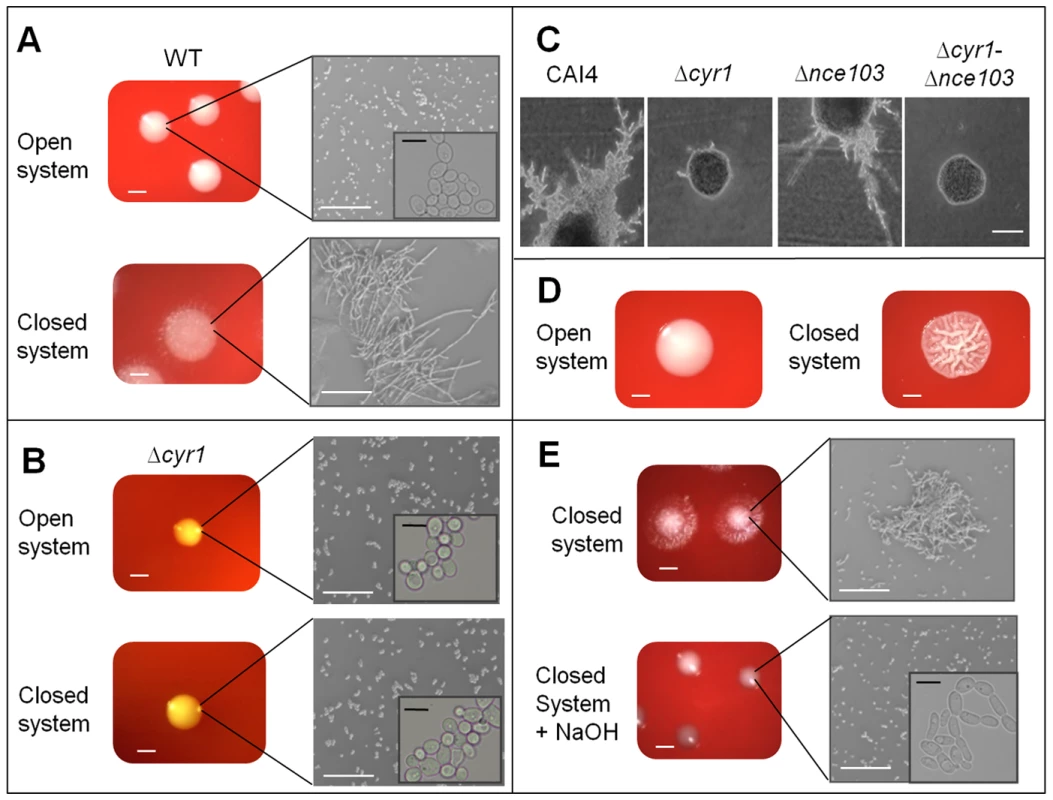
A) 500 CFU of wild type SC5314 were seeded onto CBA and incubated under closed and open conditions at 37°C for 48 hr (cells were resuspended at the required cell density, i.e. 5×103 cells/ml, and then 100µl spread plated onto the plates to obtain single colonies (i.e. 500 colonies/plate; scale bar represents 1 mm). Cells were then resuspended in water and viewed at 200× magnification (scale bar represents 100 µm). Insert shows yeast cells at 2000× magnification (scale bar represents 10 µm). B) 500 CFU of CR276-CTRL (Δcyr1) were seeded onto CBA and incubated in both closed and open systems at 37°C for 72 hr (scale bar represents 1 mm). Cells were then resuspended in water and viewed at 200× magnification (scale bar represents 100 µm). Insert shows yeast cells at 2000× magnification (scale bar represents 10 µm). C) CAI4-pSM2, Δcyr1-Δnce103-pSM2 and Δnce103-pSM2 cells were streaked onto DMEM pH7 media and incubated at 37°C for 24 hours supplemented with 5% CO2. Images were taken at 100× magnification (scale bar represents 100 µm). D) SC5314 cells were suspended in sterile water and seeded onto CBA at an initial cell concentration of 50 CFUs and incubated in both closed and open conditions for 48 hrs 37°C (scale bar represents 1 mm). E) 500 WT (SC5314) CFUs were incubated on CBA for 48 hr in closed systems either with or without 0.5g of solid NaOH (scale bar represents 1 mm). Single colonies were resuspended in sterile water and images taken at 200× magnification (scale bar represents 100 µm). Insert shows yeast cells at 2000× magnification (scale bar represents 10 µm). Addition of the hydroxide trap into the closed system inhibited the morphological transition observed previously (Figure 4E). Furthermore, microscopic inspection of WT colonies, after co-incubation with Δnce103 in closed environments, confirmed that the colonies were smooth, round, yeast colonies, similar to those observed in the open system (data not shown). These observations suggest that the Δnce103 strain acts as a CO2 sink removing the majority of the gas from the system. Therefore, C. albicans produces CO2 which affects morphology, and cAMP is essential for the observed morphological effects.
Fungal CO2/HCO3− sensing is mediated by lysine 1373 of the Cyr1p catalytic domain
Directly testing the in vivo relevance of CO2 chemosensing would be greatly facilitated by an adenylyl cyclase variant with specifically diminished CO2 sensitivity. Previously we have shown that CO2/HCO3− activates the catalytic domain of the fungal adenylyl cyclase, Cyr1p [10], confirming that this Class III AC belongs to the bicarbonate-responsive soluble AC (sAC) subfamily [13], [28]. Structural studies and in vitro work on mutated bacterial sAC-family enzymes indicated a mechanism for bicarbonate regulation, along with a potential bicarbonate binding site [29], [30]. However, it remains to be shown whether the mechanism of activation and potential binding site generally apply to sAC-like enzymes, in particular from eukaryotes, and whether they are responsible for the in vivo effects of CO2 on AC activity.
Using sequence alignments of Class III ACs, we generated a homology model of Cyr1p and identified the Cyr1p site corresponding to the proposed bacterial CO2 receptor site (Figure 5A) [13], [29]. A lysine residue [29], Lys1373 in C. albicans Cyr1p, would be a key interaction partner for bicarbonate in this receptor site. Class III ACs are dimers with shared active sites – i.e. residues from both monomers contribute to each active site – so that only the dimer can display activity. In contrast to ‘heterodimeric’ Class III ACs, which have one active site and a second, related-but-degenerated, ‘regulatory’ site in their dimer interface, homodimeric Class III ACs, like Cyr1p, have two identical catalytic sites in their dimer interface. In these ACs, it is believed that both sites can act as active or as regulatory sites. The putative bicarbonate-interacting lysine residue is strictly conserved in both “active’ and ‘regulatory’ sites (for example, in mammalian sAC, Lys334, would be the corresponding residue in the active site). In active sites, the conserved lysine at this position is essential for substrate binding [13]. Because Lys1373 of Cyr1p should be essential for substrate binding in at least one of the two sites formed at the homodimer interface, we predicted CYR11373 would be inactive on its own. We integrated full-length Cyr1p with Lys1373 point mutated to alanine, under the control of the TEF2 promoter, into an adenylyl cyclase null, generating strain CR276-CYR11373 (RH22; cyr1/cyr1:pTEF2 CYR11373). CR276-CYR11373 was refractory to both CO2 and serum induction of filamentation, behaving similarly to the vector-control strain CR276-CTRL (RH20; Figure S2). Thus, CYR11373 homodimers encode a non-functional AC.
Fig. 5. Mechanism of CO2 sensing in C. albicans. 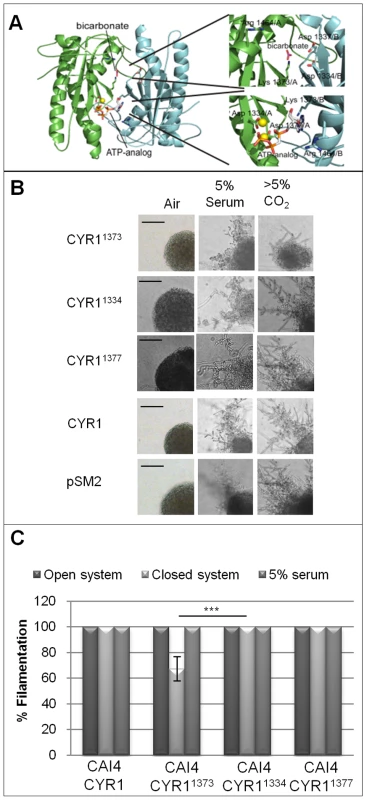
A) Homology model of the Cyr1p homodimer with bound substrate analogue and the activator bicarbonate. The protein chains are shown in green and cyan, respectively, and ligands and functional residues mutated in our study are shown in stick representation and labelled with residue number and chain A or B. Lys1373 can either bind the substrate base (active site) or bicarbonate (regulatory site). B) Point-mutated CYR1 (1373, 1334 and 1377 aa), and control plasmids (wt CYR1 and the vector control pSM2) were integrated into in URA3 locus of CAI4. Colonies were grown on DMEM pH7 itself, or supplemented with either 5% serum, or 5% CO2. The colony depicted for CAI4-CYR11373 displaying an attenuated response to CO2 is representative of approximately 30% of the population. The differential response to CO2 is hypothesised to result from the variations in levels of CO2-responsive Cyr1p homodimers, Cyr1-Cyr11373 heterodimers and non-functional Cyr1p1373 homodimers. All other colonies are representative of the entire population (scale bar represents 100 µm). C) Cell suspensions of the desired strains were inoculated onto DMEM pH7 supplemented with 10% serum or CBA agar (CBA plates were either incubated in open or closed systems) and after 48 hours colony morphology assessed. Results are the mean and standard deviations from 4 replicate experiments (*** indicates that the P statistic for the represented data is greater than 0.001). To specifically test the role of this lysine in the bicarbonate activation of Cyr1p and to generate an AC with a selective defect in its bicarbonate responsiveness, we generated strains containing mutant/WT heterodimers. Due to the dimeric architecture of Class III ACs, one wild type Cyr1p monomer could interact with one Cyr1p1373 monomer, allowing basal AC activity, but preventing bicarbonate stimulation due to disruption of the bicarbonate interacting site in the second ‘regulatory’ centre (as described above). The point mutated Cyr1p was integrated into a strain expressing wild type adenylyl cyclase, generating strain CAI4-CYR11373 (RH25; CYR/CYR1/pTEF2 CYR11373). Consistent with the expected heterodimer formation and with specific interruption of CO2-induced cAMP formation, CAI4-CYR11373, but not the control strain CAI4-CYR1 (RH24; CYR1/CYR1/pTEF2 CYR1), displayed a signal-specific defect to the filamentation inducing cues (Figure 5B, C), despite the two strains expressing comparable levels of CYR1, (Figure S3). CAI4-CYR11373 filamented in response to serum, but much less in response to CO2, while the control strain expressing wild type AC, CAI4-CYR1, filamented equally in response to both serum and CO2. The incomplete suppression of CO2-induced filamentation in CAI4-CYR11373 is consistent with the statistical formation of homodimers and heterodimers between the co-expressed wild type and variant protein, which will also yield fully CO2-sensitive wild type homodimers.
To confirm that the observed phenotype is specific to the destruction of the bicarbonate binding site in the CYR11373 heterodimers, rather than a general influence on AC activity, two additional point mutations, that inactivate Cyr1p stimulus-independent, were constructed. Asp1334 and Asp1377 (involved in active site Mg2+ binding) were mutated to Ala and also expressed under the control of the TEF2 promoter. Integration of these constructs into the adenylyl cyclase mutant (CR272-CYR11334; RH26, and CR276-CYR11377; RH27) confirmed that the proteins were catalytically inactive (Figure S2). However, expression of these inactive proteins in CAI4 (containing two genomic copies of CYR1; CAI4-CYR11334; RH28, and CAI4-CYR11377; RH29) did not perturb hyphal induction in response to 5% serum or elevated CO2, as Lys1373 did (Figure 5B, C). Thus, CAI4-CYR11373 shows a specific disruption of CO2-induced filamentation and therefore, the identified lysine, which likely acts as bicarbonate binding site, serves as physiological CO2 “switch” in Cyr1p and perhaps in all related sAC-type enzymes.
Self-generated CO2 is the volatile signal that accumulates under diffusion-limiting conditions and induces filamentation
We next directly tested whether CO2 is the volatile messenger inducing Candida filamentation by taking advantage of the CO2 insensitive mutant strain CAI4-CYR11373. CAI4-CYR11373 showed an attenuated response in the closed system, with 30% (±9%, P = 0.001) of cells producing smooth colonies indicative of reduced filamentation, while CAI4-CYR1, CAI4-CYR11334 and CAI4-CYR11377 were 100% filamentous (Figure 5C). However, CAI4-CYR11373 cells that were inoculated onto DMEM agar supplemented with 5% serum always produced 100% filamentous colonies, confirming that the reduced filamentation was signal-specific (Figure 5B, C); i.e., CO2-induced differentiation was diminished while serum-induced differentiation was unaffected.
CO2 activation of Cyr1p may have a role in pathogenicity
The morphological transition of C. albicans is essential to the organism's virulence. As CO2 is a potent inducer of hyphal development we tested whether the identified CO2-recognition-mechanism regulates C. albicans pathogenicity in an in vivo model. Initially to test this hypothesis, we selected the Toll-deficient Drosophila melanogaster infection model to provide a controlled yet reduced (in respects to filament inducing cues) environment, as only a subpopulation of the cells were CO2 insensitive. D. melanogaster was infected with CAI4-CYR11373 and CAI4-CYR1 and survival assessed over 48 hours. Although the percentage mortalities of Toll-deficient D. melanogaster infected with either strain were similar at the end-point of the time-course experiment, CAI4-CYR11373 killed D. melanogaster at a significantly slower rate (p = 0.005) compared to CAI4-CYR1 (Figure 6A). The reduced virulence of CAI4-CYR11373 over CAI4-CYR1 was not attributed to differences in growth rates or fungal burden, as these were comparable between the two strains (Figure S4 and Table S1).
Fig. 6. CAI4-CYR11373 may have implications for virulence. 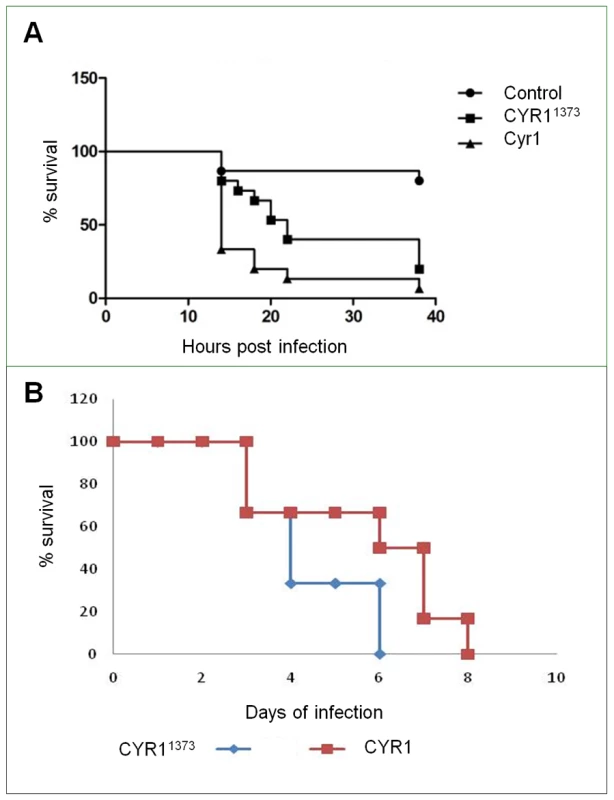
A) Toll deficient D. melanogaster were infected by injection into the thorax with C. albicans strains CAI4-CYR1, CAI4-CYR11373 or sterile YDP (CTRL). Experiments were performed with groups of 15 adult files and incubated at 30°C for 38 hours. Values represent the mean and standard deviation from 5 independent experiments. B) CAI4-CYR1, CAI4-CYR11373 were intravenously injected (2.4–2.5×104 CFU/g) into 6 female BALB/c mice (6–8 weeks old) and survival monitored. Mice were culled when they showed signs of severe illness or their weight had decreased by more than 20%. To investigate how the point-mutated adenylyl cyclase would affect the virulence of C. albicans in the mammalian host, the mouse model of disseminated candidiasis was utilised. CAI4-CYR1 and CAI4-CYR11373 displayed no significant difference in their ability to cause system infection after intravenous injection (Figure 6B). There was, however, a greater degree of variation in fungal burdens, weight loss and outcome scores compared with control strain (Table S2), which may be reflective of the different populations obtained in the CAI4-CYR11373 strain (i.e. 70% CO2 responsive and 30% CO2 non responsive).
As the fly model identified that CYR11373 was delayed in its ability to cause infection, we also sampled mice at days 1, 2 and 3 days post-infection to determine whether the delayed ability of CYR11373 to cause infection was also present in the mouse model. However, there were no statistically significant differences in kidney burdens, weight changes or outcome scores, but again there was greater variability in the CAI4-CYR11373 data, which was not observed for the CAI4-CYR1 strain (Table S2). The differences in outcome between the two infection models may be expected. Although the CAI4-CYR11373 strain is reduced in its ability to filament in response to elevated CO2 it is responsive to serum or elevated temperature, cues absent in the fly model.
Discussion
CO2 is a biologically important molecule and has major implications for disease progression. As well as host derived CO2, microorganisms themselves generate and secrete metabolic CO2 into their microenvironment which has the potential to impact on the organism's virulence. We observed that fungal derived, metabolic CO2 accumulated in C. albicans biomasses to sufficient levels to first provide HCO3− as a metabolic intermediate to promote growth and then subsequently to induce the morphological transition crucial for C. albicans pathogenicity through activation of Cyr1p via lysine residue 1373.
CO2 is produced by multiple metabolic processes and the data presented here suggest that nutrient availability affects production rates. For instance, we found that fungal biomasses grown on nutrient rich media (YPD) were able to support the growth of over ten times the amount of our bio-indicator strain (Δnce103) compared to those grown on nutrient limiting media (YNB; data not shown). This result may reflect the increased flux through metabolic pathways as the organism utilises the available nutrients. In accordance with this Ghosh et al. recently proposed that the catalysis of arginine to urea and urea's subsequent breakdown to CO2 produces sufficient CO2 to induce C. albicans germ tube formation when engulfed by macrophages [31]. Therefore, arginine biosynthesis maybe a key contributor to CO2 production in C. albicans.
Accumulation of metabolically generated CO2 in race tubes has been shown to impact on asexual spore development in Neurospora crassa [32], [33]. Here, simple displacement of the accumulated CO2 (by inverting the tubes) restores conidial banding. These results suggest that the heavier density of CO2 compared to O2 and N2 allow it to accumulate in a system more freely rather than diffusing away. In accordance with this, we found that growth of the Δnce103 strain was enhanced at the bottom of the colony (17-fold, P = 0.001) where agar invasion was observed to stem from the centre of the colony, suggesting that the concentration of CO2 is highest at the lower extremities of the biomass (data not shown).
The ability to accumulate in a system is essential for communication molecules, with many molecules only having an impact once a threshold concentration is reached. However, unlike conventional QSMs, CO2 may not be specifically generated for the purpose of communication. This is mainly due to the lack of evidence for a single pathway controlling CO2 output, although the work of Ghosh et al suggest that arginine biosynthesis may play a significant role in the production of CO2 in C. albicans [31]. Therefore, it is more likely that the organisms have evolved to sense and respond to CO2 gradients as a form of diffusion sensing rather than CO2 being a true quorum sensing molecule.
However, the interplay between CO2 production and other microbial species maybe relevant. When colonising mucosal membranes and epithelia C. albicans will be in contact with other microbes residing in the same niche. For example we found that under diffusion limiting conditions significantly fewer colony forming units (10-fold less) of Escherichia coli or Pseudomonas aeruginosa were required to restore growth of the CO2 bio-indicator strain, Δnce103, compared to wild type C. albicans (data not shown). Given that C. albicans is found in mixed microbial biofilms on medical devices it is interesting to speculate about the role the metabolically generated CO2 in biofilm establishment and maintenance.
Signalling molecules normally interact with membrane associated receptors to initiate intracellular signalling cascades terminating in a transcriptional response which subsequently induces the desired effect. Unlike most signalling molecules, CO2 enters the cell by simple diffusion and is maintained in the cell through hydration to HCO3− via the actions of carbonic anhydrase. Although HCO3− is a metabolic intermediate and will feed into various metabolic processes, a conserved HCO3− binding site was identified in the adenylyl cyclase, Cyr1p, involving lysine residue 1373, which enables CO2/HCO3− to bind and directly stimulate Cyr1p and hence activate cAMP dependent signalling cascades. Mutation of the HCO3− binding site resulted in a subpopulation of cells that were CO2 non responsive.
Introduction of the CO2 sensing deficient strain (CAI4-CYR11373) into the Toll-deficient D. melanogaster infection model highlighted its reduced ability to kill the host. In the mouse model for disseminated candidiasis this attenuated virulence was not observed. However, this was hypothesised as the mutated strain remained fully responsive to other host environmental cues, including the elevated temperature and presence of serum in mammals, which are absent in the fly infection model. Taking this into consideration we hypothesise that the ability to sense and respond to metabolically generated CO2 gradients is important during colonisation and initial invasion of mucosal membranes lining the oral and vaginal tracts during superficial infections where environmental CO2 conditions are low and not as important during systemic infection (Figure 7). Here, in an expanding fungal biomass self produced metabolic CO2 gradually accumulates and once reaching threshold concentrations directly activates the soluble adenylyl cyclase, Cyr1p via the catalytic, bicarbonate receptor site. The resulting increase in cytosolic cAMP, in conjunction with other epithelial adhesion mechanisms, functions to induce the morphological switch in C. albicans. Hyphal formation results in the penetration and invasion of the underlying epithelial cells, which subsequently enhances the dissemination of the fungal pathogen. Our data supports this as we routinely found enhanced levels of Δnce103 cells in the biomass sections that were invading into the agar, similar to what is observed in oropharyngeal candidiasis, suggesting that cells towards the bottom of the biomass are exposed to higher concentrations of CO2 than cells on the surface, which would support hyphal development. Therefore, we hypothesise that during superficial infections that occur in niches where environmental CO2 concentrations are low (for example, on the skin and mucosal membranes lining the oral cavity) C. albicans can use self generated, metabolic CO2 to enhance adhesion and promote filamentation of the underlining cells increasing the opportunity for dissemination into the bloodstream.
Fig. 7. Model for metabolic CO2 signalling in fungal pathogenicity. 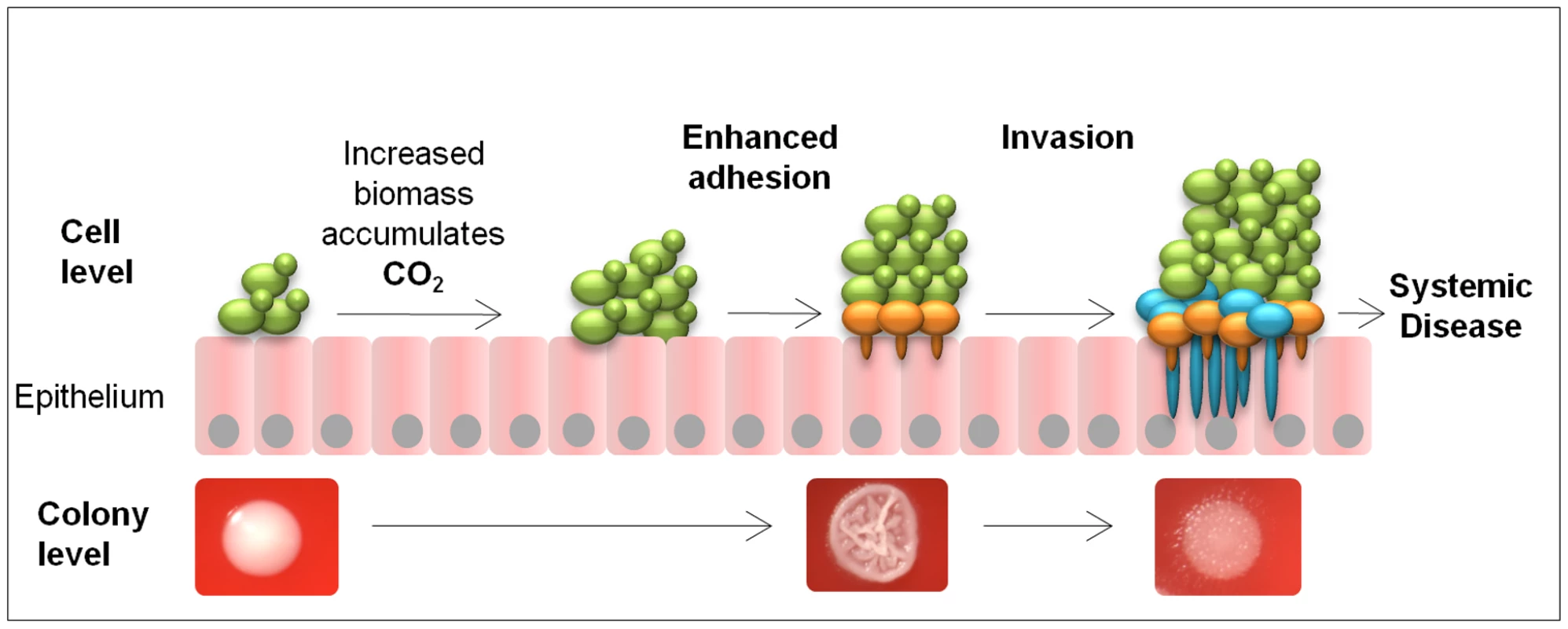
As the cells proliferate on the epithelial surface, increasing fungal growth generates pockets of elevated CO2 located at the bottom of the biomass. Cells exposed to the elevated CO2 undergo morphological switching, promoting hyphal development and hence increasing the adherence of the organism. At the same time, the protruding hyphae would expose the pathogen to host environmental signals like serum, pH and further increases in CO2 levels, which would further enhance hyphal development, increasing the opportunity for tissue invasion. In line with CO2 playing an enhancing role in microbial virulence, hypercapnia (elevated CO2) has recently been shown to inhibit the production of anti-microbial peptides in Drosophila [34]. Furthermore, elevated CO2 levels suppress the mammalian inflammatory response [35], [36], [37]. Therefore, pathogen associated, metabolically generated CO2 may play multiple roles in the infection process. One would operate at a local level, suppressing the host's immune system in the underlining epithelia and rendering the host susceptible to infection. Secondly, high CO2 would enhance the microbe's pathogenicity, providing more opportunity for host cell invasion.
In conclusion, Cyr1p is a multifunctional sensor that is essential to fungal pathology. It contains multiple domains that mediate signal-specific enzyme activation in C. albicans in response to diverse filamentation-inducing molecules. We have now identified the mechanism by which this AC is stimulated in vitro and in vivo by CO2, supplied by the environment or the fungal biomass itself. Our results give novel molecular insights into this pathogenicity mechanism, as well as an evolutionary conserved CO2-chemoreception system. Interfering with fungal CO2-sensing may reveal novel approaches for therapeutic intervention.
Methods
Ethics statement
All animal experimentation was done in accordance with United Kingdom Home Office regulations and was approved by both the Home Office and the University of Aberdeen ethical review committee. All mice were checked and weighed at least once daily, and if they showed any signs of severe disease and/or had lost 20% of their original body weight mice were humanely terminated immediately. Mice sampled at defined time points were also humanely terminated prior to aseptic removal of kidneys for burden determination.
Strains and media
C. albicans strains and transforming plasmids used in this study are listed in Table S3. Columbia blood agar plates (CBA), a quality-controlled growth medium routinely used in diagnostic microbiology laboratories, supplemented with 5% defibrinated horse blood were either purchased premade, or were made from Columbia blood agar base [38] from Oxoid (2.3% peptone, 0.1% starch, 0.5% NaCl, 1% agar, pH 7.3). Dulbecco's Modified Eagle Medium (DMEM) without bicarbonate and pyruvate was obtained from GIBCO and used at pH7, (1.34% DMEM, 3.57% HEPES supplemented to a final concentration of 2% glucose). YNB and YPD were made as described previously [10]. Where supplementation with 5% CO2 was required, plates were incubated in a CO2 incubator (Infors HT Minitron) enriched with 5% (vol/vol) CO2. Solidified or serum supplemented media contained 2% agar and 5% horse serum.
Toll transheterozygotes flies were generated by crossing flies carrying a loss of function allele of Toll (Tl1-RXA; obtained from the Tübingen Drosophila Stock Collection) and flies carrying a thermo-sensitive allele of Toll, with a strong phenotype at 29°C (Tl3; obtained from the Bloomington Stock Center). All stocks were maintained on standard fly medium at 25°C, except during infection experiments where flies were incubated at 30°C.
Open and closed systems
For diffusion-permitting (open) systems, plates were incubated in the standard way with no additional sealing mechanism. To generate a diffusion-limiting (closed) environment, standard 10 cm petri dishes containing CBA (20 ml) were sealed with two layers of laboratory sealing film (Parafilm) followed by three layers of standard cling-film (low density polyvinyl chloride). To minimise diffusion the sealing process was repeated twice. When plates were to be incubated in parallel, standard petri dishes were placed into, zip-locked polyvinyl chloride bags (15.5×23 cm). To ensure that the bags were air tight they were sealed mechanically with an additional polyethylene bar making the bags both air and water tight.
Measurement of CO2 accumulation in diffusion-limiting conditions
Sodium hydroxide was used as a CO2-trap as described by the equation below. Plates were incubated in air tight plastic bags containing a separate vial of 4M NaOH, or 0.5g of solid NaOH crystals for 48 hrs. To measure CO2 accumulation the BacT/ALERT system [39] was used with some modifications. The prefilled bottles were emptied in a sterile environment, media replaced with 20 ml of solidified DMEM pH7 and the agar surface seeded with 10,000 SC5314 cells. Bottles for incubation in closed systems were sealed as described for agar plates. CO2 accumulation was directly measured using a BacT/ALERT 3D automated microbial detection system (bioMerieux) where microbial CO2 production is assessed by a colorimetric sensor and detection system (red L.E.D and red-light-absorbing photodiode). Emitted light is recorded as a voltage signal that is directly proportional to the reflective light and hence the concentration of CO2 in the bottle.
Heterogeneous populations of CAI4 and Δnce103
Heterogeneous cell suspensions containing equal proportions (500 cells/µl) of DAY286 and Δnce103 were spotted (1 µl total) onto individual YPD or YNB plates and incubated at 37°C for 48 hrs. Initially 1 ml of sterile water was used to wash the single colony from the plate with light agitation of the agar to remove adhered cells. From the recovered 800 µl, 200 µl was plated onto YNB, 5% CO2 to promote growth of the strictly CO2-requiring strain Δnce103 strain only (DAY286 will not grow under these conditions as it is Δhis1/Δhis1). Stability of the different phenotypic markers was verified upon replica-plating of colonies. The number of colonies was counted, and after taking into account the dilution factor, related back to the initial number of colonies in the cell suspension. Initial cell suspensions were always replica plated onto YNB and YPD to obtain the average starting cell concentration for each strain.
Molecular modelling of a nucleotide and bicarbonate complex of homodimeric Cyr1p catalytic domain
The amino acid sequence of Cyr1p was duplicated and aligned with the sequences of two chains of a homodimeric substrate analogue complex of CyaC from Spirulina platensis (PDB ID 1WC0; [30]) by using Genedoc (http://www.psc.edu/biomed/genedoc). A homology model for Cyr1p was generated with this alignment using Modeller [40], and nucleotide and divalent ions positioned by superposition with the experimentally determined CyaC complex structure. Bicarbonate was then positioned manually at the site proposed for binding in bacterial sAC-like enzymes [13]. The model was visualized using Pymol (DeLano Scientific; http://www.pymol.org).
Site directed mutagenesis of CYR1
Lys 1373, Asp 1334 and Asp 1377 were point mutated to Ala by site directed mutagenesis using the following sets of primers (mutations underlined) 1373F-tggatatgaagtggcgactgaaggtgatg and Primer 7-ctatttaagttcattaactgttttcatgat, Primer 8-aacttgtttcactcccagca and 1373R-atcaccttcagtcgccacttcatatccac, 1334F-ggttttcactgcgatcaaaaactcaac and Primer 7, 1334R-gttgagtttttgatcgcagtgaaaacc and Primer 8, 1377F-gactgaaggtgcggcgttcatgg and Primer 7, 1377R-ccatgaacgccgcaccttcagtc and Primer 8. The resulting PCR fragments were ligated into the SpeI and BamHI restriction sites of pSM2. The 5′ domain of CYR1 together with the TEF2 promoter were subsequently ligated into the pSM2 plasmid using XbaI and HindIII (site located with C-terminal domain of CYR1) restriction sites forming pACL1, pACL2, and pACL3. Full-length, native CYR1 cloned into pFM2 under the control of the TEF2 promoter was subsequently restricted using SacI and BamHI restriction sites and ligated into pSM2 forming plasmid pSMTC. Plasmids pACL1, pACL2, pACL3, pSMTC and pSM2 (vector control) were integrated into the URA3 locus of CR276 and CAI4, generating strains RH20-25 (Figure 7A) using standard heat-shock procedures as previously described [41].
Southern blot analysis
Single copy integration of pACL1 was confirmed for five resulting CAI4 transformants by southern analysis using DIG High primer DNA labelling and detection (Roche) as per the manufacturer's recommendations. DNA probe (1 kb) was PCR amplified using primer-44 5′TTGGTGACATTGAGGCGTTA and primer-47 5′GTTCAATTGTCATTCCGGCAT.
Semi-quantitative RT-PCR
To assess transcript levels of CYR1 total RNA was extracted from cultures (50 ml YPD) grown to OD600 0.5. Cells were harvested through centrifugation and immediately frozen in liquid nitrogen. Samples were disrupted using a Mikro-dismembrator S (Sartouis) at 2000 rpm, 2 minutes and RNA immediately extracted using the Qiagen RNeasy Kit according to the manufacturer's recommendations. CYR1 expression levels (native CYR1 and CYR11373) were analysed by semi quantitative RT-PCR using the BioRad one-step RT-PCR Kit with Syber Green (primers CYR1-F 5′GACGACAACAAACGTGCCAGAACA and CYR1-R 5′ AATCACGTGCTGAAACATGGTCCC). CYR1 levels were normalised to ACT1.
Disruption of NCE103
The strain, RH12 (Δnce103 Δcyr1), was constructed in the Δcyr1 background strain, CR276, using the HisG-URA3-HisG cassette to disrupt the 847 bp NCE103 open reading frame (GenBank association number EAL03010) from positions +153 to +807 as described previously [10]. Correct integration of the HisG-URA3-HisG cassette into the NCE103 locus was confirmed by PCR.
Mouse infection models
Survival experiments
CAI4-CYR1 and CAI4-CYR11373 were grown on YPD plates at 30°C for 16 hrs and cells were washed off plates with saline, the resulting cell suspensions washed twice with sterile saline and then resuspended in saline to provide inocula for infection. For each C. albicans strain 6 female (6–8 weeks old) BALB/c mice (Harlan, UK) were intravenously challenged with 2.4–2.5×104 CFU/g of each strain. Mice were monitored and weighed at least once daily, with mice culled when they displayed signs of severe illness, or when their weight decreased by 20%. When culled, the kidneys were aseptically removed and burdens determined. Survival data were compared by the Kaplan-Meier Log-rank statistic.
Outcome scores
For each C. albicans strain 9 mice were challenged intravenously, as described above, with three mice sampled on days 1, 2 and 3 post-infection. For each mouse kidney burdens and percentage weight change were determined, with the two parameters used to determine infection outcome scores [42]. Kidney burdens, weight change and outcome scores were compared by the Mann-Whitney U statistic.
Supporting Information
Zdroje
1. AlmiranteB
RodríguezD
ParkBJ
Cuenca-EstrellaM
PlanesAM
2005 Epidemiology and Predictors of Mortality in Cases of Candida Bloodstream Infection: Results from Population-Based Surveillance, Barcelona, Spain, from 2002 to 2003. J Clin Microbiol 43 1829 1835
2. KlevayMJ
ErnstEJ
HollanbaughJL
MillerJG
PfallerMA
2008 Therapy and outcome of Candida glabrata versus Candida albicans bloodstream infection. Diag Microbiol Infect Dis 60 273 277
3. LeroyO
GangneuxJ-P
MontraversP
MiraJ-P
GouinF
2009 Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: A multicenter, prospective, observational study in France (2005–2006). Crit Care Med 37 1612 1618
4. BodeyGP
1986 Candidiasis in cancer patients. Am J Med 77 13 19
5. OddsFC
1988 Candida and candidosis A review and bibliography
6. ScullyC
el-KabirM
SamaranayakeL
1994 Candida and oral candidosis: a review. Crit Rev Oral Biol Med 5 125 157
7. FarahC
AshmanR
ChallacombeS
2000 Oral Candidosis. Clin Dermatol 18 553 562
8. LoH-J
KöhlerJR
DiDomenicoB
LoebenbergD
CacciapuotiA
1997 Nonfilamentous C. albicans Mutants Are Avirulent. Cell 90 939 949
9. SavilleSP
LazzellAL
MonteagudoC
Lopez-RibotJL
2003 Engineered Control of Cell Morphology In Vivo Reveals Distinct Roles for Yeast and Filamentous Forms of Candida albicans during Infection. Eukaryotic Cell 2 1053 1060
10. KlengelT
LiangW-J
ChaloupkaJ
RuoffC
SchroppelK
2005 Fungal Adenylyl Cyclase Integrates CO2 Sensing with cAMP Signaling and Virulence. Curr Biol 15 2021 2026
11. BuffoJ
HermanM
SollD
1984 A characterization of pH regulated dimorphism in Candida albicans. Mycopathologia 85 21 30
12. RochaCRC
SchroppelK
HarcusD
MarcilA
DignardD
2001 Signaling through Adenylyl Cyclase Is Essential for Hyphal Growth and Virulence in the Pathogenic Fungus Candida albicans. Mol Biol Cell 12 3631 3643
13. KamenetskyM
MiddelhaufeS
BankEM
LevinLR
BuckJ
2006 Molecular Details of cAMP Generation in Mammalian Cells: A Tale of Two Systems. J Mol Biol 362 623 639
14. VerstrepenKJ
KlisFM
2006 Flocculation, adhesion and biofilm formation in yeasts. Mol Microbiol 60 5 15
15. FangH-M
WangY
2006 RA domain-mediated interaction of Cdc35 with Ras1 is essential for increasing cellular cAMP level for Candida albicans hyphal development. Mol Microbiol 61 484 496
16. XuX-L
LeeRTH
FangH-M
WangY-M
LiR
2008 Bacterial Peptidoglycan Triggers Candida albicans Hyphal Growth by Directly Activating the Adenylyl Cyclase Cyr1p. Cell Host & Microbe 4 28 39
17. HoganDA
2006 Talking to Themselves: Autoregulation and Quorum Sensing in Fungi. Eukaryot Cell 5 613 619
18. ShankE
KolterR
2009 New developments in microbial interspecies signaling. Curr Opin Microbiol 12 1 10
19. HughesDT
SperandioV
2008 Inter-kingdom signalling: communication between bacteria and their hosts. Nat Rev Micro 6 111 120
20. HornbyJM
JensenEC
LisecAD
TastoJJ
JahnkeB
2001 Quorum Sensing in the Dimorphic Fungus Candida albicans Is Mediated by Farnesol. Appl Environ Microbiol 67 2982 2992
21. HoganDA
VikA
KolterR
2004 A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol Microbiol 54 1212 1223
22. Davis-HannaA
PiispanenAE
StatevaLI
HoganDA
2008 Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis. Mol Microbiol 67 47 62
23. PalkovaZ
JanderovaB
GabrielJ
ZikanovaB
PospisekM
1997 Ammonia mediates communication between yeast colonies. Nature 390 532 536
24. BahnY-S
CoxGM
PerfectJR
HeitmanJ
2005 Carbonic Anhydrase and CO2 Sensing during Cryptococcus neoformans Growth, Differentiation, and Virulence. Curr Biol 15 2013 2020
25. Gewiss MogensenE
JanbonGuilhem
ChaloupkaJames
SteegbornClemens
FuMan Shun
2006 Cryptococcus neoformans Senses CO2 through the Carbonic Anhydrase Can2 and the Adenylyl Cyclase Cac1. Eukaryotic Cell 5 103 111
26. SharabiK
LecuonaE
HeleniusIT
BeitelG
SznajderJI
2009 Sensing, physiological effects and molecular response to elevated CO2 levels in eukaryotes. Journal of Cellular and Molecular Medicine 9999
27. ChristensenBE
FacerJF
1939 Simple wet combustion method for the determination of carbon, oxygen equivalence and empirical formula by iodic acid oxidation. J Am Chem Soc 61 3001 3005
28. ChenY
CannMJ
LitvinTN
IourgenkoV
SinclairML
2000 Soluble Adenylyl Cyclase as an Evolutionarily Conserved Bicarbonate Sensor. Science 289 625 628
29. CannMJ
HammerA
ZhouJ
KanacherT
2003 A Defined Subset of Adenylyl Cyclases Is Regulated by Bicarbonate Ion. J Biol Chem 278 35033 35038
30. SteegbornC
LitvinTN
LevinLR
BuckJ
WuH
2005 Bicarbonate activation of adenylyl cyclase via promotion of catalytic active site closure and metal recruitment. Nat Struct Mol Biol 12 32 37
31. GhoshS
NavarathnaDHMLP
RobertsDD
CooperJT
AtkinAL
2009 Arginine-Induced Germ Tube Formation in Candida albicans Is Essential for Escape from Murine Macrophage Line RAW 264.7. Infect Immun 77 1596 1605
32. BeldenWJ
LarrondoLF
FroehlichAC
ShiM
ChenC-H
2007 The band mutation in Neurospora crassa is a dominant allele of ras-1 implicating RAS signaling in circadian output. Genes & Dev 21 1494 1505
33. ParkS
LeeK
2004 Inverted race tube assay for circadian clock studies of the Neurospora accessions. Fungal Genet Newslett 51 12 14
34. HeleniusIT
KrupinskiT
TurnbullDW
GruenbaumY
SilvermanN
2009 Elevated CO2 suppresses specific Drosophila innate immune responses and resistance to bacterial infection. Proceedings of the National Academy of Sciences 106 18710 18715
35. De Smet
HildeR
BerstenAD
BarrHA
DoyleIR
2007 Hypercapnic acidosis modulates inflammation, lung mechanics, and edema in the isolated perfused lung. Journal of Critical Care 22 305 313
36. HalbertsmaFJJ
VanekerM
PickkersP
SnijdelaarDG
van EgmondJ
2008 Hypercapnic acidosis attenuates the pulmonary innate immune response in ventilated healthy mice. Critical Care Medicine 36 2403 2406
37. O'CroininDF
NicholAD
HopkinsN
BoylanJ
O'BrienS
2008 Sustained hypercapnic acidosis during pulmonary infection increases bacterial load and worsens lung injury. Critical Care Medicine 36 2128 2135
38. EllnerPD
StoesselCJ
DrakefordE
VasiF
1966 A new culture medium for clinical bacteriology. American Journal of Clinical Pathology 45 502 504
39. ThorpeTC
WilsonML
TurnerJE
DiGuiseppiJL
WillertM
1990 BacT/Alert: an automated colorimetric microbial detection system. J Clin Microbiol 28 1608 1612
40. SaliA
BlundellTL
1993 Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234 779 815
41. WaltherA
WendlandJ
2003 An improved transformation protocol for the human fungal pathogen Candida albicans. Curr Genetics 42 339 343
42. MacCallumDM
CosteA
IscherF
JacobsenMD
OddsFC
2010 Genetic Dissection of Azole Resistance Mechanisms in Candida albicans and Their Validation in a Mouse Model of Disseminated Infection. Antimicrob Agents Chemother 54 1476 1483
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease AgentČlánek TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease SusceptibilityČlánek The Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive ParasiteČlánek Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 11- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Patients with Discordant Responses to Antiretroviral Therapy Have Impaired Killing of HIV-Infected T Cells
- A Molecular Mechanism for Eflornithine Resistance in African Trypanosomes
- Tyrosine Sulfation of the Amino Terminus of PSGL-1 Is Critical for Enterovirus 71 Infection
- Autoimmunity as a Predisposition for Infectious Diseases
- The Subtilisin-Like Protease AprV2 Is Required for Virulence and Uses a Novel Disulphide-Tethered Exosite to Bind Substrates
- Structural Analysis of HIV-1 Maturation Using Cryo-Electron Tomography
- Tick Histamine Release Factor Is Critical for Engorgement and Transmission of the Lyme Disease Agent
- Interferon-Inducible CXC Chemokines Directly Contribute to Host Defense against Inhalational Anthrax in a Murine Model of Infection
- TGF-b2 Induction Regulates Invasiveness of -Transformed Leukocytes and Disease Susceptibility
- The Origin of Intraspecific Variation of Virulence in an Eukaryotic Immune Suppressive Parasite
- CO Acts as a Signalling Molecule in Populations of the Fungal Pathogen
- SV2 Mediates Entry of Tetanus Neurotoxin into Central Neurons
- MAP Kinase Phosphatase-2 Plays a Critical Role in Response to Infection by
- Glycosylation Focuses Sequence Variation in the Influenza A Virus H1 Hemagglutinin Globular Domain
- Potentiation of Epithelial Innate Host Responses by Intercellular Communication
- Fcγ Receptor I Alpha Chain (CD64) Expression in Macrophages Is Critical for the Onset of Meningitis by K1
- ANK, a Host Cytoplasmic Receptor for the Cell-to-Cell Movement Protein, Facilitates Intercellular Transport through Plasmodesmata
- Analysis of the Initiating Events in HIV-1 Particle Assembly and Genome Packaging
- Evolution of Linked Avirulence Effectors in Is Affected by Genomic Environment and Exposure to Resistance Genes in Host Plants
- Structural Basis of HIV-1 Neutralization by Affinity Matured Fabs Directed against the Internal Trimeric Coiled-Coil of gp41
- Hepatitis C Virus (HCV) Evades NKG2D-Dependent NK Cell Responses through NS5A-Mediated Imbalance of Inflammatory Cytokines
- Host Cell Invasion and Virulence Mediated by Ssa1
- Global Gene Expression in Urine from Women with Urinary Tract Infection
- Should the Human Microbiome Be Considered When Developing Vaccines?
- HapX Positively and Negatively Regulates the Transcriptional Response to Iron Deprivation in
- Enhancing Oral Vaccine Potency by Targeting Intestinal M Cells
- Herpes Simplex Virus Reorganizes the Cellular DNA Repair and Protein Quality Control Machinery
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- Cryo Electron Tomography of Native HIV-1 Budding Sites
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- Modelling the Evolution and Spread of HIV Immune Escape Mutants
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
- Platelet-Activating Factor Receptor Plays a Role in Lung Injury and Death Caused by Influenza A in Mice
- Genetic and Structural Basis for Selection of a Ubiquitous T Cell Receptor Deployed in Epstein-Barr Virus Infection
- Ubiquitin-Regulated Nuclear-Cytoplasmic Trafficking of the Nipah Virus Matrix Protein Is Important for Viral Budding
- Pneumolysin Activates the NLRP3 Inflammasome and Promotes Proinflammatory Cytokines Independently of TLR4
- Immune Evasion by : Differential Targeting of Dendritic Cell Subpopulations
- Survival in Selective Sand Fly Vector Requires a Specific -Encoded Lipophosphoglycan Galactosylation Pattern
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Zn Inhibits Coronavirus and Arterivirus RNA Polymerase Activity and Zinc Ionophores Block the Replication of These Viruses in Cell Culture
- The Female Lower Genital Tract Is a Privileged Compartment with IL-10 Producing Dendritic Cells and Poor Th1 Immunity following Infection
- Crystal Structure and Size-Dependent Neutralization Properties of HK20, a Human Monoclonal Antibody Binding to the Highly Conserved Heptad Repeat 1 of gp41
- The Arabidopsis Resistance-Like Gene Is Activated by Mutations in and Contributes to Resistance to the Bacterial Effector AvrRps4
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



