-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
An Outer Membrane Receptor of Involved in Zinc Acquisition with Vaccine Potential
Since the concentration of free iron in the human host is low, efficient iron-acquisition mechanisms constitute important virulence factors for pathogenic bacteria. In Gram-negative bacteria, TonB-dependent outer membrane receptors are implicated in iron acquisition. It is far less clear how other metals that are also scarce in the human host are transported across the bacterial outer membrane. With the aim of identifying novel vaccine candidates, we characterized in this study a hitherto unknown receptor in Neisseria meningitidis. We demonstrate that this receptor, designated ZnuD, is produced under zinc limitation and that it is involved in the uptake of zinc. Upon immunization of mice, it was capable of inducing bactericidal antibodies and we could detect ZnuD-specific antibodies in human convalescent patient sera. ZnuD is highly conserved among N. meningitidis isolates and homologues of the protein are found in many other Gram-negative pathogens, particularly in those residing in the respiratory tract. We conclude that ZnuD constitutes a promising candidate for the development of a vaccine against meningococcal disease for which no effective universal vaccine is available. Furthermore, the results suggest that receptor-mediated zinc uptake represents a novel virulence mechanism that is particularly important for bacterial survival in the respiratory tract.
Published in the journal: An Outer Membrane Receptor of Involved in Zinc Acquisition with Vaccine Potential. PLoS Pathog 6(7): e32767. doi:10.1371/journal.ppat.1000969
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000969Summary
Since the concentration of free iron in the human host is low, efficient iron-acquisition mechanisms constitute important virulence factors for pathogenic bacteria. In Gram-negative bacteria, TonB-dependent outer membrane receptors are implicated in iron acquisition. It is far less clear how other metals that are also scarce in the human host are transported across the bacterial outer membrane. With the aim of identifying novel vaccine candidates, we characterized in this study a hitherto unknown receptor in Neisseria meningitidis. We demonstrate that this receptor, designated ZnuD, is produced under zinc limitation and that it is involved in the uptake of zinc. Upon immunization of mice, it was capable of inducing bactericidal antibodies and we could detect ZnuD-specific antibodies in human convalescent patient sera. ZnuD is highly conserved among N. meningitidis isolates and homologues of the protein are found in many other Gram-negative pathogens, particularly in those residing in the respiratory tract. We conclude that ZnuD constitutes a promising candidate for the development of a vaccine against meningococcal disease for which no effective universal vaccine is available. Furthermore, the results suggest that receptor-mediated zinc uptake represents a novel virulence mechanism that is particularly important for bacterial survival in the respiratory tract.
Introduction
The cell envelope of Gram-negative bacteria consists of two membranes, the inner and the outer membrane, which are separated by the periplasm containing the peptidoglycan layer. The outer membrane forms a barrier for harmful compounds from the environment. Most nutrients can pass the outer membrane by passive diffusion via abundant channel-forming outer membrane proteins, collectively called porins. However, diffusion is not an option when the extracellular concentration of a nutrient is low. This is usually the case, for example, with iron. Pathogens are confronted with low concentrations of free iron within the human host, where iron is bound by iron-transport and -storage proteins, such as lactoferrin and transferrin. Hence, efficient iron acquisition mechanisms constitute important virulence factors and have been studied extensively in many pathogens [1], [2].
When grown under iron-limiting conditions, Gram-negative bacteria induce the synthesis of outer membrane proteins that function as receptors for the iron-binding proteins of the host, for heme, or for siderophores, which are small iron-chelating compounds produced and secreted by the bacteria under iron limitation. The resolved crystal structures of such receptors revealed 22-stranded β-barrels, which do not form open channels but are closed by an N-terminal plug domain [3]. After binding of the ligand to the receptor, the subsequent uptake of the nutrient is an active process that requires the energy of the proton gradient across the inner membrane, which is coupled to the receptors in the outer membrane via a complex of three proteins, the TonB complex [4], [5].
While iron-acquisition mechanisms have been studied extensively in many Gram-negative bacteria, little is known yet about the transport of other essential heavy metals, such as zinc and manganese, across the bacterial outer membrane. The concentrations of also these trace elements are low in the human host, which responds to infections, amongst others, by the production of metallothioneins and calprotectin, thereby reducing the availability of metals to the invading pathogens [6], [7]. Therefore, Gram-negative pathogens likely possess effective mechanisms for the acquisition of these metals, which may or may not resemble the iron-acquisition systems.
Neisseria meningitidis is an obligate human pathogen that can colonize the nasopharyngeal mucosa asymptomatically. Occasionally the bacterium enters the bloodstream and can cause sepsis and meningitis with a high mortality rate [8]. While vaccines based on the capsular polysaccharides are available for most pathogenic serogroups of N. meningitidis, a vaccine against serogroup B meningococci is lacking. The polysaccharide capsule of the serogroup B strains is poorly immunogenic due to its resemblance to human glycoproteins [9]. Thus, subcapsular antigens are being studied as alternative vaccine components; however, these studies are frustrated by the high antigenic variability of the major outer membrane proteins. Therefore, attention has shifted to minor antigens, including the TonB-dependent receptors.
When grown under iron limitation, N. meningitidis produces TonB-dependent receptors for lactoferrin [10], transferrin [11], hemoglobin [12], [13] and enterobactin [14], all involved in the uptake of iron. Based on homology searches, Turner et al. identified seven additional genes for putative TonB-dependent family (Tdf) members in the available genome sequences of three Neisserial strains [15]. Interestingly, the expression of some of these tdf genes appeared unaffected by iron availability in various microarray studies [16], [17], indicating that their products might be implicated in the transport of metals other than iron. Here we studied the regulation of the synthesis, the function, and the vaccine potential of one of these receptors and show that this receptor is involved in the uptake of zinc. We therefore named this protein, encoded by locus NMB0964 in the genome sequence of strain MC58 [18], ZnuD for zinc uptake component D.
Results
Regulation of znuD expression by zinc
To study the expression of znuD in N. meningitidis, we raised a polyclonal antiserum against the protein produced in Escherichia coli in inclusion bodies. While the antiserum did recognize the protein produced in E. coli, we could never detect ZnuD when whole cell lysates of N. meningitidis strain HB-1, an unencapsulated derivate of serogroup B strain H44/76, were analyzed on Western blots after growth in tryptic soy broth (TSB) (Figure 1A, lane 1). However, when the bacteria were grown in chemically defined RPMI medium, ZnuD was detectable in the lysates (Figure 1A, lane 2). The specificity of the signal detected was demonstrated by its absence in a constructed znuD knockout strain (Figure 1A, lane 3). We noticed that the addition of even small amounts of TSB to RPMI negatively affected ZnuD synthesis (Figure 1B), suggesting that TSB contains a compound that represses the transcription of znuD. RPMI does not contain a source of trace metals. Since Tdf members are usually regulated by iron availability, we first tested whether znuD expression could be repressed by adding an iron source; however, addition of even up to 10 µM FeCl3 to the medium did not affect ZnuD production (Figure 1C). Next, we decided to test whether a cocktail of trace metals, consisting of 340 nM ZnSO4, 160 nM Na2MoO4, 800 nM MnCl2, 80 nM CoCl2 and 80 nM CuSO4 (final concentrations), could repress znuD expression, which indeed appeared to be the case. Then, all these metal salts were tested separately, and specifically zinc, even at sub-µM concentrations, appeared to repress znuD expression (Figure 1D). Since standard RPMI is not supplemented with a specific zinc source, the available zinc required for bacterial growth is presumably derived from the water or the salts used to constitute the medium. The zinc concentration in the standard RPMI medium measured by inductively coupled plasma mass spectrometry (ICP-MS) was found to be ∼110 parts per billion (∼1.69 µM), which is apparently sufficient for growth of the bacteria but insufficient for repression of znuD expression.
Fig. 1. Regulation of znuD gene expression. 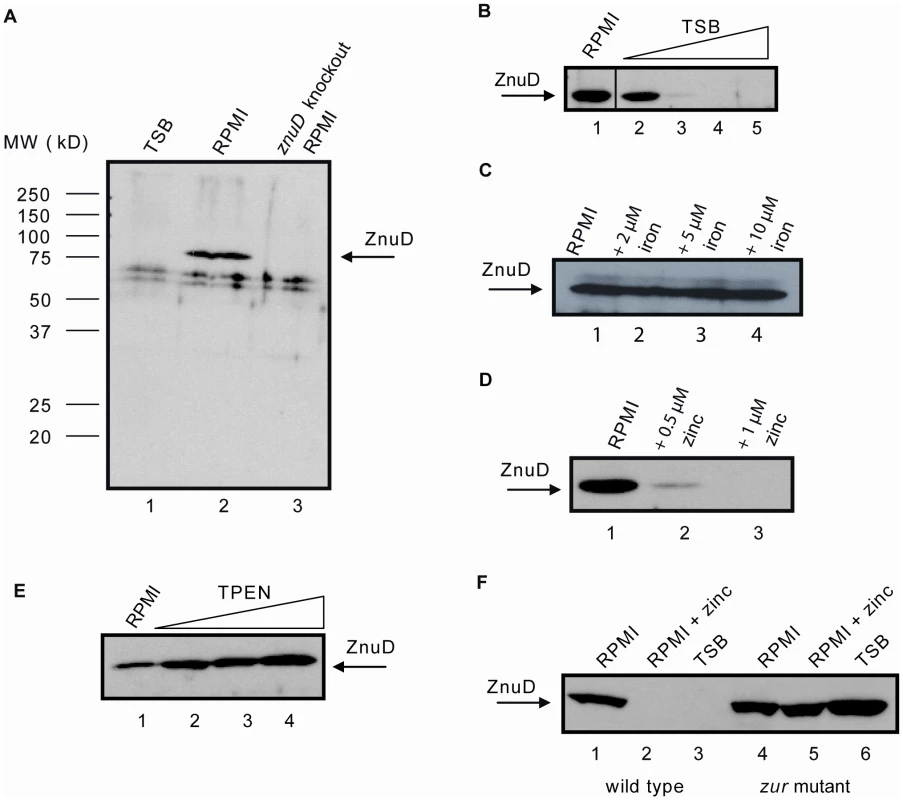
Western blots of cell lysates using rabbit antiserum against ZnuD. (A) HB-1 grown in TSB (lane 1), RPMI (lane 2) and the znuD knockout strain grown in RPMI (lane 3). (B) HB-1 grown in RPMI supplemented with 0, 2, 4, 6 and 8% TSB (lanes 1–5, respectively). (C) HB-1 grown in RPMI supplemented with 0, 2, 5, and 10 µM FeCl3 (lanes 1–4, respectively). (D) HB-1 grown in RPMI supplemented with 0, 0.5 or 1 µM ZnSO4 (lanes 1–3, respectively). (E) HB-1 grown in RPMI supplemented with 0, 0.1, 0.5 or 1 µM TPEN (lanes 1–4, respectively). (F) HB-1 (lanes 1–3) and the zur mutant (lanes 4–6) grown in RPMI (lanes 1 and 4), RPMI with 0.6 µM ZnSO4 (lanes 2 and 5) or TSB (lanes 3 and 6). The zinc regulation of znuD expression was further evaluated by supplementing the RPMI medium with the specific zinc chelator N,N,N′,N′-Tetrakis-(2-pyridylmethyl)-ethylenediamine (TPEN), which resulted in a dose-dependent increase in ZnuD synthesis (Figure 1E). Concentrations of TPEN above 1 µM totally inhibited bacterial growth presumably due to total depletion of zinc from the medium. The growth defect induced by TPEN could be restored by the addition of zinc (not shown). The zinc-dependent regulation of znuD expression was further confirmed by real-time quantitative PCR (RT-qPCR) using total RNA obtained from cultures grown in RPMI supplemented or not with either 0.5 µM ZnSO4 or 0.5 µM TPEN. The data showed a 13.8±1.3-fold repression in the presence of zinc and a 3.8±1.2-fold induction in the presence of TPEN. The fold difference between added TPEN and zinc was 52.6.
Role of the transcriptional regulator Zur in znuD expression
In E. coli, the zinc uptake regulator Zur has been shown to regulate the expression of the znuACB operon. The genes of this operon encode the periplasmic substrate-binding protein, the ATPase and the integral inner membrane component, respectively, of an ABC transporter required for the transport of zinc from the periplasm into the cytoplasm [19]. In the presence of zinc, Zur binds a Zur-binding element (consensus sequence GAAATGTTATANTATAACATTTC) in the promoter of the znuACB operon and thereby blocks transcription [20].
In the genome sequence of N. meningitidis strain MC58, we identified homologues of the E. coli zur gene, i.e. NMB1266, and of a putative znuCBA operon, i.e. NMB0588, NMB0587, and NMB0586. In addition, we found sequences resembling the E. coli Zur binding sequence in the regions upstream of the znuD (GtAATGTTATATaATAACAaact) and znuC (cAAAcGTTATACagTAtCATaTC) (identical nucleotides to the E. coli consensus are in capital case). To confirm the involvement of Zur in the regulation of znuD expression, we generated a zur mutant of strain HB-1, which, indeed, produced ZnuD constitutively (Figure 1F). Also, RT-qPCR demonstrated the involvement of Zur in the expression of znuA and znuD, as znuA and znuD expression levels increased 5.0±0.8-fold and 34.0±0.8-fold, respectively, in the zur mutant compared to its parent strain both grown in RPMI supplemented with 0.5 µM ZnSO4.
ZnuD facilitates zinc acquisition
Since the expression of znuD is regulated by the availability of zinc, it seemed likely that ZnuD acts as a receptor for zinc or a zinc-containing compound. We first analyzed the amino acid sequence and constructed a topology model of the barrel domain of ZnuD using the PROFtmb program at www.rostlab.org [21] and a 3D structural model of the plug domain based on known structures of TonB-dependent receptors using 3D-jigsaw [22] (Figure 2). ZnuD contains two cysteine residues in the putative extracellular loop L3. When these cysteines form a disulfide bond, they bring two stretches of amino acid residues, both rich in histidine and aspartic acid residues, in close proximity (Figure 2). The resulting His - and Asp-rich domain could be of functional importance, since also in the periplasmic zinc-binding protein ZnuA of E. coli, a stretch of His and Asp residues is involved in binding its ligand [23]. Thus, we considered the possibility that ZnuD binds free zinc and transports it into the periplasm. To test this hypothesis, we first determined whether ZnuD could bind zinc. To this end, N. meningitidis strain CE1523, an H44/76 derivative that lacks porin PorA and the polysaccharide capsule, was transformed with a plasmid carrying znuD under the control of an isopropyl-β-D-1-thiogalactopyranoside (IPTG)-inducible promoter. The resultant strain was grown with and without IPTG, and outer membrane vesicles (OMVs) were isolated (Figure 3A, left panel) and compared for their capacity to compete with 4-(2-pyridylazo)resorcinol (PAR) for binding zinc. In the presence of OMVs containing ZnuD, ∼40% more free PAR was measured than in the presence of OMVs lacking ZnuD, indicating that ZnuD is capable of binding zinc (Figure 3B). To demonstrate the involvement of ZnuD in binding zinc directly, ZnuD was produced in E. coli in inclusion bodies, which were isolated, and the protein was folded in vitro into its native conformation. Like many other outer membrane proteins [24], ZnuD displays heat modifiability, i.e. the denatured form has a lower electrophoretic mobility than the correctly folded form, a property that was used to monitor proper folding (Figure 3A, right panel). The native protein was then tested alongside the unfolded protein in the PAR competition assay. The folded protein indeed competed with PAR for zinc while the unfolded protein did not (Figure 3B) showing the specificity of the reaction.
Fig. 2. Topology model of ZnuD. 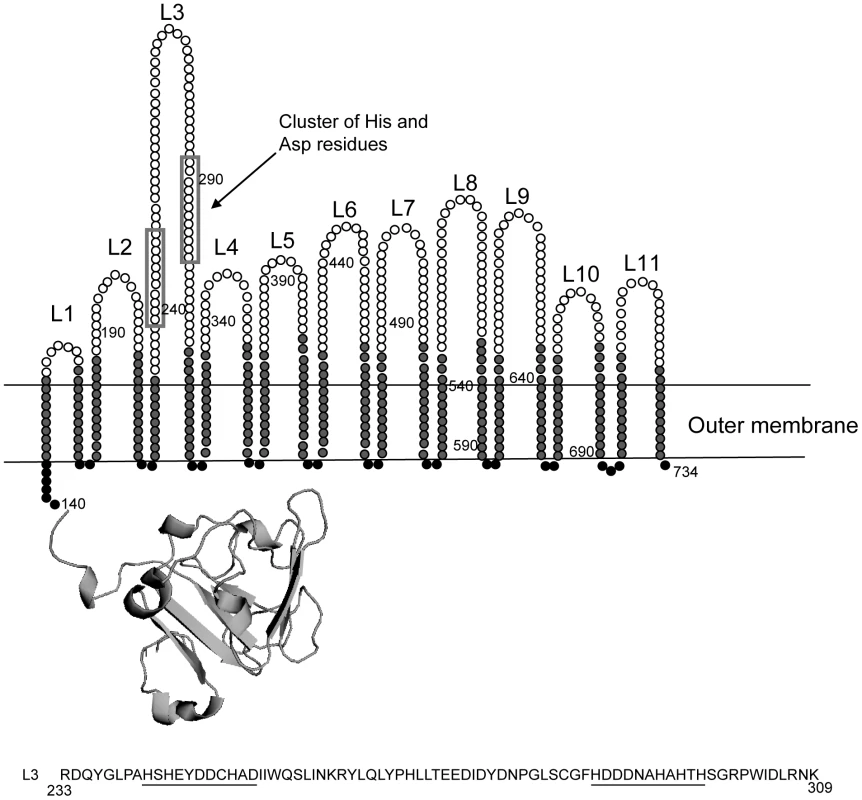
The 22 β-strands are colored light grey, the 11 extracellular loops are white and the periplasmic turns are black. The histidine/aspartic acid stretches are boxed. The plug domain was modeled based on known Tdf structures. The amino acid sequence of loop 3 is shown with the cysteines in bold and the His/Asp-rich stretches underlined. Numbers indicate amino acid positions in the sequence of mature ZnuD. Fig. 3. Zinc binding and transport by ZnuD. 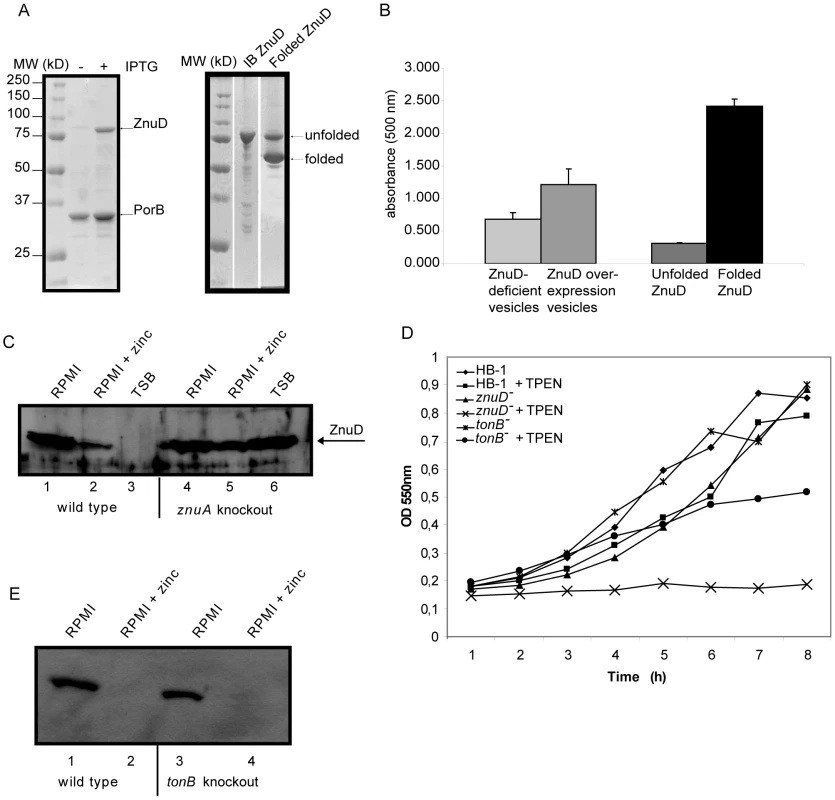
(A) Coomassie-stained SDS-PAGE gels showing the protein content of the OMVs (left) and purified ZnuD preparation (right) used in the PAR-binding assay. OMVs were isolated from strain CE1523 carrying pEN11-znuD that was either induced or not with IPTG for znuD expression as indicated. Purified ZnuD from inclusion bodies (IB ZnuD) was refolded in vitro (Folded ZnuD). The folded ZnuD sample was not heated before SDS-PAGE and the positions of folded and unfolded ZnuD are indicated. (B) Binding of zinc to OMVs either or not containing ZnuD and to purified ZnuD that was either folded or not was measured in a PAR competition assay. Shown are the normalized values of the absorption at 500 nm of five independent measurements. (C) Western blot of whole cell lysates of strain HB-1 (lanes 1–3) and the znuA mutant (lanes 4–6) grown in RPMI (lanes 1 and 4), RPMI with 500 nM ZnSO4 (lanes 2 and 5) or TSB (lanes 3 and 6). (D) Representative growth curves (n = 5) of the znuD and tonB knockout strains and their parent strain in response to zinc limitation. Cultures contained either 0 or 0.3 µM TPEN. (E) Western blot of whole cell lysates of strain HB-1 (lanes 1–2) and the tonB mutant (lanes 3–4) grown in RPMI (lanes 1 and 3) or RPMI with 500 nM ZnSO4 (lanes 2 and 4). If ZnuD is indeed involved in the uptake of free zinc, one would expect a higher external zinc concentration to be required to repress expression of znu genes in the znuD mutant than in the wild-type strain. To test this idea, the znuD mutant and its parent strain were grown in RPMI medium supplemented with 0.5 µM ZnSO4, which largely, but not completely represses znuD expression in the wild-type strain (Figure 1D). The relative levels of znuD and znuA mRNA were then measured by RT-qPCR. Of note, the znuD mutant still contains the first 437 nucleotides of the znuD gene and 100 nucleotides thereof were used for the detection of znuD gene expression. In the znuD mutant, there was 18.6±1.1-fold more znuD and 7.4±1.1-fold more znuA expressed compared to the parent strain, showing that indeed the intracellular zinc concentration in the znuD mutant is lower than that in the parent strain under the applied growth conditions. Also, Western-blot analysis showed that a znuA knockout strain produced high levels of ZnuD in the presence of zinc, confirming that ZnuA is required to sustain sufficient zinc levels in the cell (Figure 3C).
Finally, we tested whether expression of znuD offers any growth advantage under zinc limitation. To deplete the internal zinc stores, the bacteria were first grown overnight on RPMI plates, supplemented with 100 µM ferric chloride to prevent iron depletion. Subsequently, they were inoculated in RPMI supplemented or not with TPEN. In the absence of TPEN, growth of the bacteria was only marginally affected by the absence of ZnuD, but, in contrast to the parental strain, the znuD mutant failed to grow when 0.3 µM TPEN was added to the medium (Figure 3D). These results indicate that znuD expression is indeed beneficial to the cells when the available zinc is limiting.
We also assessed whether TonB is required for ZnuD-mediated zinc acquisition. In growth experiments, a tonB knockout strain grew less well than the parent but better than the znuD mutant in the presence of TPEN (Figure 3D). However, in RT-qPCR experiments, we did not observe increased expression of znuA or znuD after growth of the tonB mutant in RPMI supplemented with 0.5 µM ZnSO4 and, consistently, we could not detect more ZnuD under these conditions by Western-blot analysis (Figure 3E). Together, these results suggest that TonB facilitates the uptake of free zinc through ZnuD, but that uptake can take place also independently of TonB.
Vaccine potential of ZnuD
To investigate whether ZnuD could be a candidate component for a universal N. meningitidis vaccine, we first studied its distribution among various N. meningitidis isolates. ZnuD homologs were found in all the available N. meningitidis genome sequences and they showed a strikingly high 97–99% amino acid identity in the mature part of the protein (Figure S1, Supporting Information). The sequence differences are scattered throughout the protein and are not clustered in predicted extracellular loop regions, which are often antigenically variable in Neisseria outer membrane proteins. We subsequently analyzed the presence of ZnuD in a panel of 32 different N. meningitidis isolates from different serogroups and different clonal lineages. The strains were grown in RPMI medium supplemented or not with 0.5 µM ZnSO4, and whole cell lysates were analyzed by Western blotting with the antiserum raised against ZnuD of strain H44/76. ZnuD was detected in all strains tested and in all cases znuD expression was repressed in the presence of zinc, albeit to different extents (Figure 4A).
Fig. 4. ZnuD synthesis in meningococcal isolates and in vivo. 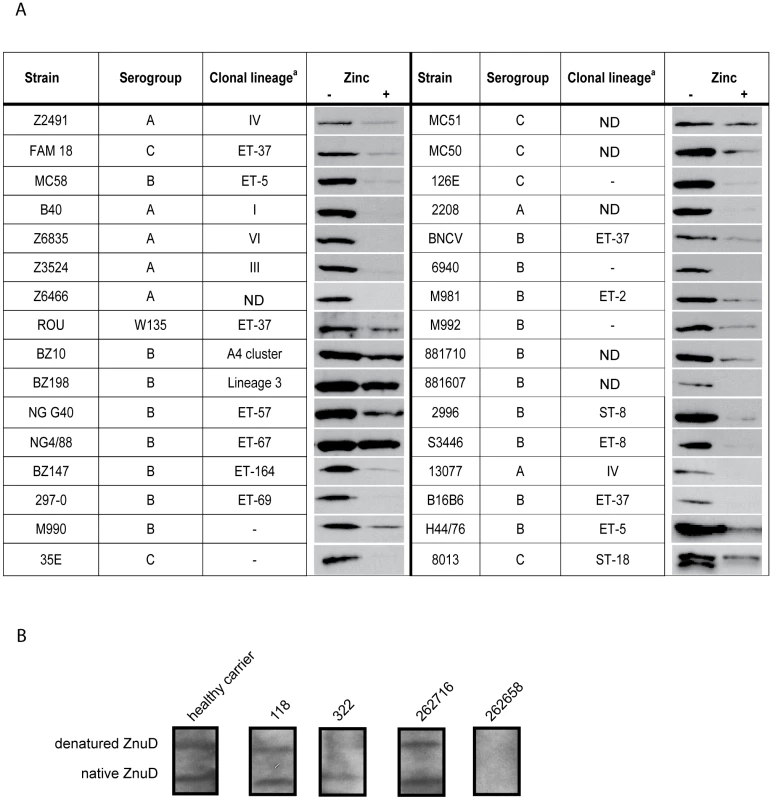
(A) Western blots of cell lysates of the indicated strains grown in RPMI with or without added zinc. a Clonal group designations taken from [40]; –, the strain was typed by Multi-Locus Enzyme Electrophoresis but could not be assigned to a specific clone; ND, not determined. (B) Reaction of human sera with ZnuD on Western blots. The blots contained both native and denatured ZnuD. The specific patient sera used are indicated above the lanes and have been described previously [10], [25]. Also an example of a non-reactive patient serum is shown at the right. To investigate whether ZnuD is expressed in the human host, we tested sera from convalescent patients, healthy carriers and non-carriers [10], [25] for the presence of antibodies that recognize ZnuD on Western blots. The reactivity of the sera was tested against both the denatured and the refolded form of the ZnuD protein (Figure 3A, right panel). We could detect ZnuD-specific antibodies in sera from most convalescent patients and healthy carriers tested (examples are shown in Figure 4B), although also some sera were negative (e.g. serum 262658 in Figure 4B). The positive sera reacted with both the denatured protein and the protein folded in vitro into its native conformation although the relative intensity of the reaction with the two forms of the protein varied (Figure 4B). No ZnuD-specific antibodies were detected in the sera from non-carriers (results not shown).
Next, we immunized 10 mice with OMVs from a strain overexpressing this protein (Figure 3A) and tested the resultant pooled sera for the presence of bactericidal antibodies. Routinely, we perform serum bactericidal assays on bacteria grown in TSB medium; however, under these conditions znuD is not expressed. Therefore, we tested the sera for bactericidal activity on a derivative of strain H44/76 that expressed znuD from an IPTG-inducible promoter and compared cultures grown with and without 1 mM IPTG. The bactericidal titers of the pooled sera were <1∶100 against bacteria not producing ZnuD, but 1∶1042 against bacteria producing ZnuD. Titers in pre-immune sera were <1∶100 independent of whether ZnuD was produced or not. These data clearly show that ZnuD is able to elicit bactericidal antibodies. Thus, since ZnuD is highly conserved among N. meningitidis isolates and elicits bactericidal antibodies, it might be an attractive vaccine component.
ZnuD homologs in other bacteria
Genes encoding the high-affinity ZnuABC uptake system for zinc have been identified in many bacteria, but the involvement of an outer membrane receptor in zinc acquisition has not been described so far. To investigate whether an outer membrane receptor might be more generally associated with zinc acquisition, we searched for ZnuD homologs in other pathogenic bacteria by performing BLAST searches at NCBI. ZnuD homologs with high sequence similarity (∼96% identity) were found in Neisseria gonorrhoeae strain FA1090 (locus NGO_1205) and other N. gonorrhoeae strains. Homologs of ZnuD were also found in other pathogenic bacteria, including Moraxella catarrhalis, Haemophilus parasuis, Mannheimia haemolytica, Acinetobacter baumannii, Pasteurella multocida, Bordetella pertussis, and Actinobacillus pleuropneumoniae (Table 1). All these ZnuD homologs contain the His - and Asp-rich regions suspected to be involved in zinc binding (Figure S2, Supporting Information). Interestingly, in B. pertussis the znuD homolog is located adjacent to a gene cluster containing homologs of the znuABC and zur genes, again indicating a functional relationship between these genes. Thus, it appears that outer membrane receptor-mediated zinc acquisition is not specific for N. meningitidis, but is more common among pathogenic Gram-negative bacteria.
Tab. 1. Identity and similarity of ZnuD with its homologs. 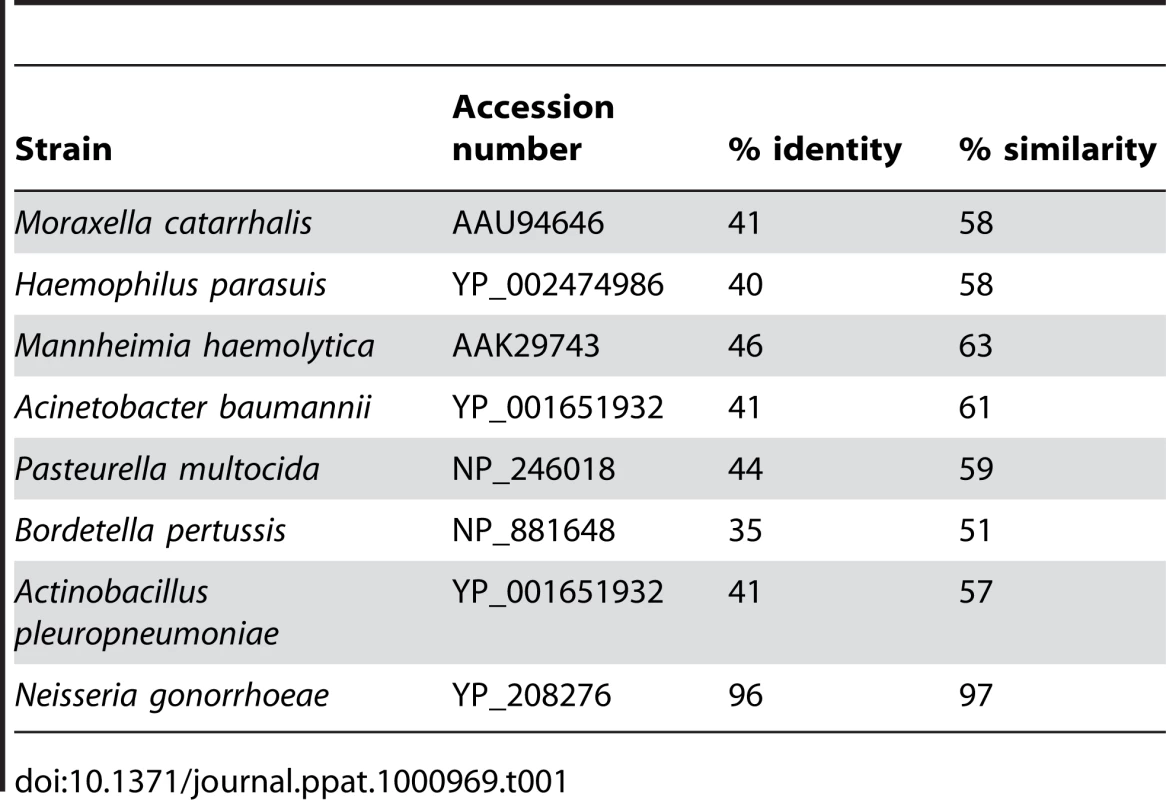
Discussion
The Gram-negative bacterial outer membrane forms a protective barrier that protects the bacteria against harmful compounds from the environment, including antibiotics, detergents and digestive enzymes. By the presence of porins, it functions as a molecular sieve that allows for the passage of hydrophilic solutes with molecular weights up to ∼600 Da by passive diffusion. Most nutrients can pass the outer membrane via the porins. However, passive diffusion is effective only when the external solute concentration is high. The bacteria may also require nutrients that are present in the environment in low concentrations or that are too large to pass through the porins. For such cases, the bacteria have developed active transport systems in the outer membrane that depend on a Tdf receptor with high affinity for its ligand and on the TonB complex that delivers the electrochemical energy of the proton gradient across the inner membrane to the transport process across the outer membrane [3]–[5].
Most Tdf members studied to date are involved in iron acquisition [1], [2], a notable exception being BtuB protein of E. coli, which mediates the uptake of vitamin B12 [26]. Since the free iron concentration in the human host is too low to support bacterial growth, efficient iron-acquisition systems constitute important virulence factors of pathogenic bacteria. These bacteria usually can use multiple iron sources including siderophores produced by themselves or by other micro-organisms, heme, and iron-transport and -storage proteins of the host. For each iron source, the bacteria require a specific receptor. However, based on the available genome sequences, the number of Tdf receptors in a bacterium can be very high. For example, Pseudomonas aeruginosa may contain up to 37 different Tdf receptors [27] suggesting that at least some of these receptors might have functions other than iron or vitamin B12 transport. Indeed, in Caulobacter crescentus, Tdf receptors have been described that are involved in the uptake of carbon sources [28], [29]. C. crescentus thrives in nutrient-poor fresh-water lakes where receptor-mediated active transport across the outer membrane offers a solution to acquire sufficient nutrients. Also, very recently, a receptor for nickel has been described in Helicobacter mustelae [30].
Here, we describe for the first time a Tdf receptor involved in the acquisition of zinc. Like the levels of free iron in the human host, those of free zinc are most likely too low to sustain bacterial growth. For example, although the total concentration of zinc in human serum is approximately 13 µM, the vast majority of it is bound by serum proteins such as albumin [31]. In addition, the human host responds to infections by the production of metallothioneins and calprotectin, which reduce the availability of zinc and other metals to the invading pathogens [6], [7]. The high-affinity ZnuABC system for the uptake of zinc across the inner membrane has been identified in many bacteria, including Neisseria gonorrhoeae [32] and Salmonella enterica [33] where it was shown to be associated with virulence [33], [34]. Likewise, the outer membrane receptor for zinc acquisition identified here, ZnuD, is presumably important for virulence, which, however, is difficult to establish in N. meningitidis, for which a suitable animal model is lacking.
Our results show that ZnuD can bind zinc and that znuD expression facilitates the uptake of free zinc at low zinc concentrations. Although ZnuD is a Tdf member, TonB appeared not to be required for ZnuD-mediated zinc uptake. However, it is entirely possible that ZnuD, besides its function as a receptor for free zinc, could additionally recognize a complexed form of zinc, which may be available in the respiratory tract, in serum and/or in cerebral fluid. If that is the case, we expect that the TonB system will be needed for the acquisition of zinc from such ligand.
Previously, znuD expression was reported to be induced in the presence of active complement [35]. In that microarray study, the expression profiles were compared of N. meningitidis strain Z5463 grown in the presence of serum that was either inactivated or not by heat. Expression of znuD (locus tag NMA1161) was found 23-fold de-repressed in the presence of the untreated serum. A possible explanation for this observation is the presence in serum of albumin, which is known to chelate zinc [31]. Heat treatment of serum will probably release zinc from albumin, which could repress znuD expression, while znuD would be expressed in bacteria exposed to untreated serum where zinc is chelated. Consistent with this explanation is the observation in the same microarray study that also the genes with locus tags NMA1137 and NMA1138, which encode paralogs of the ribosomal proteins L36 and L31 that are induced in many bacterial species under zinc limitation [36], were strongly induced in the presence of the untreated serum [35].
N. meningitidis normally lives as a commensal on the mucosal surfaces in the upper respiratory tract. Zinc is found on mucosal surfaces, but the total zinc concentrations or the amount of free zinc present are not known. However, it is intriguing that ZnuD homologs were particularly found in bacterial species residing in the respiratory tract of humans and animals. Probably, the unbound zinc concentration in the mucosal layers of the respiratory tract is too low to allow sufficient passive diffusion through the porins and, therefore, ZnuD may become essential for bacterial growth and survival particularly in this niche. This hypothesis is strengthened by the detection of specific antibodies against ZnuD in the serum from healthy carriers, which indicates that ZnuD is expressed where the bacteria normally reside, the nasopharynx.
As a pathogen, N. meningitidis can enter the bloodstream and cause sepsis and meningitis with a high mortality rate. A vaccine against serogroup B meningococci is not available, because the corresponding capsular polysaccharide is poorly immunogenic. Outer membrane proteins are being studied as alternative vaccine components, but these studies are frustrated by the high antigenic variability of the major outer membrane proteins. Since ZnuD is highly conserved among N. meningitidis isolates and elicits bactericidal antibodies, it might be an attractive vaccine component, particularly in combination with other minor outer membrane proteins due to the synergistic bactericidal activity of antibodies against such antigens [37].
Materials and Methods
Bacterial strains and growth conditions
Except when indicated otherwise, experiments were performed with the unencapsulated N. meningitidis strain HB-1 [38] and mutants thereof. Strain CE1532 was constructed from strain CE2001, a porA-deficient derivative of H44/76 [39], by inactivating the capsule locus similarly as described for HB-1 [38]. N. meningitidis was grown on GC agar (Oxoid) plates containing Vitox (Oxoid) and antibiotics when appropriate (kanamycin, 100 µg/ml; chloramphenicol, 10 µg/ml) in candle jars at 37°C. Liquid cultures were grown in TSB (Difco) or in RPMI (Sigma) at 37°C with shaking. E. coli strains DH5α and TOP10F′ (Invitrogen) were used for routine cloning. E. coli was propagated on Luria-Bertani (LB) medium supplemented when appropriate with 100 µg/ml ampicillin, 50 µg/ml kanamycin, or 25 µg/ml chloramphenicol.
Construction of plasmids and mutants
Primers used are listed in Table S1 (Supporting Information). The znuD gene without the signal sequence-encoding part was amplified from chromosomal DNA of strain H44/76 by PCR using the primers 0964-F and 0964-R and cloned into pCRII-TOPO (Invitrogen), generating pCRII-znuD. From there, it was subcloned into pET11a (Novagen) using NdeI/BamHI restriction, resulting in plasmid pET11a-znuD.
To obtain a znuD deletion construct, a kanamycin-resistance gene cassette was amplified by PCR with the primers Kan-R and Kan-F from pCR2.1-Kan/Dus [40] and cloned after MluI and BsrGI digestion into pCRII-znuD digested with the same enzymes. In the resulting construct, pCRII-znuD::kan, the kanamycin-resistance cassette substitutes for the region between base pairs 437 and 1344 of znuD. The znuD::kan construct was amplified with primers 0964-R and 0964-F and used to transform strain HB-1 to generate a znuD mutant.
For regulated expression of znuD, the entire znuD gene from H44/76 was amplified with primers ZnuD-F and ZnuD-R. The PCR product was cloned via pCRII-TOPO into the neisserial replicative plasmid pEN11-pldA [40] using NdeI and AatII restriction. In the resulting plasmid, pEN11-znuD, the znuD gene is under control of an IPTG-inducible tandem lac/tac promoter.
To obtain a tonB knockout construct, DNA fragments upstream and downstream of tonB (NMB1730) were amplified using primer couples tonB-1/tonB-2 and tonB-3/tonB-4. The two fragments were each cloned into pCRII-TOPO and then ligated together using the AccI restriction site introduced via the primers and the SpeI site present in the vector. The AccI site was used to insert the chloramphenicol transacetylase gene obtained from pKD3 [41] with primers P1 and P2. The resulting construct was amplified using primers tonB-1 and tonB-4 and the PCR product was used to transform N. meningitidis HB-1 to generate a tonB mutant. The zur and znuA genes were knocked out following the same strategy. The primer couples used to obtain the upstream and downstream fragments were zur-1/zur-2 and zur-3/zur-4, and znuA-1/znuA-2 and znuA-3/znuA-4.
SDS-PAGE and Western blot analysis
Whole cell lysates were prepared from liquid cultures by resuspending cell pellets in sample buffer. Proteins were separated by routine SDS-PAGE or by semi-native SDS-PAGE, which allows for analysis of outer membrane proteins in their native β-barrel conformation [40]. After electrophoresis, the gels were either stained with Coomassie brilliant blue or the proteins were transferred to nitrocellulose membranes (Protran) using a wet transfer system (Biorad) in 25 mM Tris-HCl, 192 mM glycine, 20% methanol. Membranes were blocked for 1 h in PBS containing 0.1% Tween 20 and 0.5% Protifar (Nutricia). Blots were incubated with antibodies in blocking buffer. Antibody binding was detected by using peroxidase-conjugated goat anti-rabbit or anti-human IgG secondary antibodies (Biosource) and enhanced chemiluminescence detection (Pierce).
Isolation and refolding of recombinant ZnuD
E. coli BL21(DE3) (Invitrogen) containing pET11a-znuD was grown in LB to an optical density at 600 nm of 0.6 after which 1 mM IPTG was added and growth was continued for 2 h. ZnuD accumulated in inclusion bodies, which were isolated as described [24]. The inclusion bodies were dissolved in 20 mM Tris-HCl, 100 mM glycine, 6 M urea (pH 8.3), and residual membranes were removed by centrifugation for 1 h at 200,000 g. The protein was then refolded into its native conformation by diluting this stock solution 20-fold in refolding buffer containing 55 mM Tris-HCl, 0.21 mM sodium chloride, 0.88 mM potassium chloride, 880 mM L-arginine and 0.5% 3-dimethyldodecylammoniopropane-sulfonate (SB-12) (Fluka), pH 7.0. After refolding overnight, the sample was dialyzed to 55 mM Tris-HCl, 0.21 mM sodium chloride, 10 mM L-arginine, and 0.5% SB-12, pH 6.5. The protein solution was filtered and stored at 4°C. Proper folding was monitored by semi-native SDS-PAGE where the folded protein has a higher electrophoretic mobility than the denatured protein.
Immunizations
Preparative SDS-PAGE was used to purify ZnuD from inclusion body preparations. After staining the gel with Coomassie brilliant blue, the band corresponding to ZnuD was electro-eluted (Biorad) and used to immunize rabbits at Eurogentec.
To generate OMVs, strain CE1523 containing pEN11-znuD was grown in the presence or absence of 1 mM IPTG. OMVs were prepared by deoxycholate extraction and used to immunize mice as described [10]. Sera from ten mice per group were collected after 42 days and pooled.
Ethics statement
All animal experimentations were performed in compliance with the current Belgian legislation related to the protection and well-being of animals and the European directive 86/609/CEE related to the protection of vertebrate animals used for experimental or other scientific purposes. The experimental protocols have been approved by GSK Biologicals Ethical Commission for Animal Experimentation. All experimental procedures were conducted at the GSK Belgium facilities, which has a full AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care) accreditation since October 25th 2004.
RT-qPCR
RT-qPCR was performed using a 7900HT Fast Real-Time PCR System and SYBR green master mix (Applied Biosystems). Total RNA was isolated using Trizol (Invitrogen) and further purified with nucleospin RNA II columns (Macherey-Nagel) and treated with Turbo DNA-Free (Ambion) to yield DNA-free RNA. cDNA was generated from 1 µg RNA using transcriptor High fidelity cDNA synthesis kit (Roche). As a control, samples without the reverse transcriptase were tested in parallel. PCRs were performed in triplicate. The primers used are listed in Table S1. A melting plot was performed to ensure that the signal originated from the specific amplicon. Data analysis was performed using the comparative cycle threshold method (Applied Biosystems) to determine relative expression levels. The rmpM transcript was used to normalize all data.
ICP-MS
Total zinc concentrations in RPMI medium were measured by ICP-MS using an X Series 2 ICPMS (Thermo Scientific). Filtered (0.22-µm) medium was acidified with HNO3 (suprapure, Merck) prior to the measurements.
Zinc binding assay
In the PAR (Fluka) competition assay, the orange color of a PAR/zinc complex changes towards yellow in the presence of a protein that releases zinc from PAR. The assay was performed as described [42] using 30 µM ZnSO4 and 20 µg OMVs. The assay with the refolded protein as competitor was carried out in 55 mM Tris-HCl, 0.21 mM sodium chloride, 10 mM L-arginine, and 0.5% SB-12, pH 6.5; in this assay, 50 µM PAR, 20 µM ZnSO4, and 16 µg ZnuD were used.
Serum bactericidal assay
Wild type H44/76 carrying pEN11-znuD was inoculated from overnight grown plates in TSB with 125 µM FeCl3 with or without 1 mM IPTG in shaking flasks for 3 h at 37°C until an optical density at 550 nm of 0.5 was reached. Serum bactericidal assays were performed as described [10]. Bactericidal titers are defined as the highest serum dilution yielding >50% killing.
Accession numbers
Accession numbers for the meningococcal proteins described in this study in Genbank are: ZnuD (NMB0964), AAF62323; ZnuA (NMB0586), AAF41014; ZnuB (NMB0587), AAF41015; ZnuC (NMB0588), AAF41016; Zur (NMB1266), AAF41643; TonB (NMB1730), AAF42075. Other accession numbers are provided in Table 1.
Supporting Information
Zdroje
1. RatledgeC
2007 Iron metabolism and infection. Food Nutr Bull 28 S515 523
2. WandersmanC
DelepelaireP
2004 Bacterial iron sources: from siderophores to hemophores. Annu Rev Microbiol 58 611 647
3. WienerMC
2005 TonB-dependent outer membrane transport: going for Baroque? Curr Opin Struct Biol 15 394 400
4. BraunV
2006 Energy transfer between biological membranes. ACS Chem Biol 1 352 354
5. PostleK
1993 TonB protein and energy transduction between membranes. J Bioenerg Biomembr 25 591 601
6. CorbinBD
SeeleyEH
RaabA
FeldmannJ
MillerMR
2008 Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319 962 965
7. DeSK
McMasterMT
AndrewsGK
1990 Endotoxin induction of murine metallothionein gene expression. J Biol Chem 265 15267 15274
8. StephensDS
ZimmerSM
2002 Pathogenesis, therapy, and prevention of meningococcal sepsis. Curr Infect Dis Rep 4 377 386
9. FinneJ
LeinonenM
MäkeläPH
1983 Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet 2 355 357
10. PetterssonA
KortekaasJ
WeynantsVE
VoetP
PoolmanJT
2006 Vaccine potential of the Neisseria meningitidis lactoferrin-binding proteins LbpA and LbpB. Vaccine 24 3545 3557
11. LegrainM
MazarinV
IrwinSW
BouchonB
Quentin-MilletMJ
1993 Cloning and characterization of Neisseria meningitidis genes encoding the transferrin-binding proteins Tbp1 and Tbp2. Gene 130 73 80
12. LewisLA
GrayE
WangYP
RoeBA
DyerDW
1997 Molecular characterization of hpuAB, the haemoglobin-haptoglobin-utilization operon of Neisseria meningitidis. Mol Microbiol 23 737 749
13. StojiljkovicI
HwaV
de Saint MartinL
O'GaoraP
NassifX
1995 The Neisseria meningitidis haemoglobin receptor: its role in iron utilization and virulence. Mol Microbiol 15 531 541
14. CarsonSD
KlebbaPE
NewtonSM
SparlingPF
1999 Ferric enterobactin binding and utilization by Neisseria gonorrhoeae. J Bacteriol 181 2895 2901
15. TurnerPC
ThomasCE
StojiljkovicI
ElkinsC
KizelG
2001 Neisserial TonB-dependent outer-membrane proteins: detection, regulation and distribution of three putative candidates identified from the genome sequences. Microbiology 147 1277 1290
16. DuceyTF
CarsonMB
OrvisJ
StintziAP
DyerDW
2005 Identification of the iron-responsive genes of Neisseria gonorrhoeae by microarray analysis in defined medium. J Bacteriol 187 4865 4874
17. GrifantiniR
FrigimelicaE
DelanyI
BartoliniE
GiovinazziS
2004 Characterization of a novel Neisseria meningitidis Fur and iron-regulated operon required for protection from oxidative stress: utility of DNA microarray in the assignment of the biological role of hypothetical genes. Mol Microbiol 54 962 979
18. TettelinH
SaundersNJ
HeidelbergJ
JeffriesAC
NelsonKE
2000 Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287 1809 1815
19. PatzerSI
HantkeK
1998 The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol Microbiol 28 1199 1210
20. PatzerSI
HantkeK
2000 The zinc-responsive regulator Zur and its control of the znu gene cluster encoding the ZnuABC zinc uptake system in Escherichia coli. J Biol Chem 275 24321 24332
21. BigelowHR
PetreyDS
LiuJ
PrzybylskiD
RostB
2004 Predicting transmembrane beta-barrels in proteomes. Nucleic Acids Res 32 2566 2577
22. BatesPA
KelleyLA
MacCallumRM
SternbergMJ
2001 Enhancement of protein modeling by human intervention in applying the automatic programs 3D-JIGSAW and 3D-PSSM. Proteins Suppl 539 46
23. YatsunykLA
EastonJA
KimLR
SugarbakerSA
BennettB
2008 Structure and metal binding properties of ZnuA, a periplasmic zinc transporter from Escherichia coli. J Biol Inorg Chem 13 271 288
24. DekkerN
MerckK
TommassenJ
VerheijHM
1995 In vitro folding of Escherichia coli outer-membrane phospholipase A. Eur J Biochem 232 214 219
25. van UlsenP
van AlphenL
HopmanCThP
van der EndeA
TommassenJ
2001 In vivo expression of the Neisseria meningitidis proteins homologous to the Haemophilus influenzae Hap and Hia autotransporters. FEMS Immunol Med Microbiol 32 53 64
26. KadnerRJ
LigginsGI
1973 Transport of vitamin B12 in Escherichia coli: genetic studies. J Bacteriol 115 514 521
27. CornelisP
BodilisJ
2009 A survey of TonB-dependent receptors in fluorescent pseudomonads. Environ Microbiol Rep 1 256 262
28. NeugebauerH
HerrmannC
KammerW
SchwarzG
NordheimA
2005 ExbBD-dependent transport of maltodextrins through the novel MalA protein across the outer membrane of Caulobacter crescentus. J Bacteriol 187 8300 8311
29. EisenbeisS
LohmillerS
ValdebenitoM
LeichtS
BraunV
2008 NagA-dependent uptake of N-acetyl-glucosamine and N-acetyl-chitin oligomers across the outer membrane of Caulobacter crescentus. J Bacteriol 190 5230 5238
30. StoofJ
KuipersEJ
van VlietAHM
2010 Characterization of NikR-responsive promoters of urease and metal transport genes of Helicobacter mustelae. Biometals 23 145 159
31. StewartAJ
BlindauerCA
BerezenkoS
SleepD
SadlerPJ
2003 Interdomain zinc site on human albumin. Proc Natl Acad Sci U S A 100 3701 3706
32. ChenCY
MorseSA
2001 Identification and characterization of a high-affinity zinc uptake system in Neisseria gonorrhoeae. FEMS Microbiol Lett 202 67 71
33. AmmendolaS
PasqualiP
PistoiaC
PetrucciP
PetrarcaP
2007 The high affinity Zn2+ uptake system ZnuABC is required for bacterial zinc homeostasis in intracellular environments and contributes to virulence of Salmonella enterica. Infect Immun 75 5867 5876
34. WuH
Soler-GarcíaAA
JerseAE
2009 A strain-specific catalase mutation and mutation of the metal-binding transporter gene mntC attenuate Neisseria gonorrhoeae in vivo but not by increasing susceptibility to oxidative killing by phagocytes. Infect Immun 77 1091 1102
35. DoveJE
YasukawaK
TinsleyCR
NassifX
2003 Production of the signalling molecule, autoinducer-2, by Neisseria meningitidis: lack of evidence for a concerted transcriptional response. Microbiology 149 1859 1869
36. PaninaEM
MironovAA
GeflandMS
2003 Comparative genomics of bacterial zinc regulons: enhanced ion transport, pathogenesis, and rearrangement of ribosomal proteins. Proc Natl Acad Sci U S A 100 9912 9917
37. WeynantsVE
FeronCM
GorajKK
BosMP
DenoëlPA
2007 Additive and synergistic bactericidal activity of antibodies directed against minor outer membrane proteins of Neisseria meningitidis. Infect Immun 75 5434 5442
38. BosMP
TommassenJ
2005 Viability of a capsule - and lipopolysaccharide-deficient mutant of Neisseria meningitidis. Infect Immun 73 6194 6197
39. TommassenJ
VermeijP
StruyvéM
BenzR
PoolmanJT
1990 Isolation of Neisseria meningitidis mutants deficient in class 1 (PorA) and class 3 (PorB) outer membrane proteins. Infect Immun 58 1355 1359
40. BosMP
TefsenB
VoetP
WeynantsVE
van PuttenJPM
2005 Function of neisserial outer membrane phospholipase A in autolysis and assessment of its vaccine potential. Infect Immun 73 2222 2231
41. DatsenkoKA
WannerBL
2000 One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97 6640 6645
42. LimKH
JonesCE
vanden HovenRN
EdwardsJL
FalsettaML
2008 Metal binding specificity of the MntABC permease of Neisseria gonorrhoeae and its influence on bacterial growth and interaction with cervical epithelial cells. Infect Immun 76 3569 3576
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System inČlánek A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 7- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- The Mouse Resistance Protein Irgm1 (LRG-47): A Regulator or an Effector of Pathogen Defense?
- Leprosy and the Adaptation of Human Toll-Like Receptor 1
- Intergenomic Arms Races: Detection of a Nuclear Rescue Gene of Male-Killing in a Ladybird
- The Role of Chemokines during Viral Infection of the CNS
- Bottlenecks and the Maintenance of Minor Genotypes during the Life Cycle of
- DNA Damage Triggers Genetic Exchange in
- The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System in
- Uropathogenic Modulates Immune Responses and Its Curli Fimbriae Interact with the Antimicrobial Peptide LL-37
- Biogenesis of the Inner Membrane Complex Is Dependent on Vesicular Transport by the Alveolate Specific GTPase Rab11B
- A Spatio-Temporal Analysis of Matrix Protein and Nucleocapsid Trafficking during Vesicular Stomatitis Virus Uncoating
- Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion
- Quasispecies Theory and the Behavior of RNA Viruses
- Bid Regulates the Pathogenesis of Neurotropic Reovirus
- Distinct Roles for Dectin-1 and TLR4 in the Pathogenesis of Keratitis
- Unexpected Inheritance: Multiple Integrations of Ancient Bornavirus and Ebolavirus/Marburgvirus Sequences in Vertebrate Genomes
- Balanced Nuclear and Cytoplasmic Activities of EDS1 Are Required for a Complete Plant Innate Immune Response
- Adaptation of Hepatitis C Virus to Mouse CD81 Permits Infection of Mouse Cells in the Absence of Human Entry Factors
- An Outer Membrane Receptor of Involved in Zinc Acquisition with Vaccine Potential
- Inositol Hexakisphosphate-Induced Autoprocessing of Large Bacterial Protein Toxins
- Plus- and Minus-End Directed Microtubule Motors Bind Simultaneously to Herpes Simplex Virus Capsids Using Different Inner Tegument Structures
- Distinct Pathogenesis and Host Responses during Infection of by and
- Can Bacteria Evolve Resistance to Quorum Sensing Disruption?
- RNA Virus Replication Complexes
- PPARγ and LXR Signaling Inhibit Dendritic Cell-Mediated HIV-1 Capture and -Infection
- The Virulence Protein SopD2 Regulates Membrane Dynamics of -Containing Vacuoles
- Genome-Wide Mutagenesis Reveals That ORF7 Is a Novel VZV Skin-Tropic Factor
- Adaptive Evolution of Includes Retroviral Insertion and Positive Selection at Two Clusters of Residues Flanking the Substrate Groove
- A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
- Transduction of Human T Cells with a Novel T-Cell Receptor Confers Anti-HCV Reactivity
- Identification of GBV-D, a Novel GB-like Flavivirus from Old World Frugivorous Bats () in Bangladesh
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- HIV gp41 Engages gC1qR on CD4+ T Cells to Induce the Expression of an NK Ligand through the PIP3/H2O2 Pathway
- Oxidation of Helix-3 Methionines Precedes the Formation of PK Resistant PrP
- Protection from the 2009 H1N1 Pandemic Influenza by an Antibody from Combinatorial Survivor-Based Libraries
- Murine Gamma-Herpesvirus 68 Hijacks MAVS and IKKβ to Initiate Lytic Replication
- Viral Protein Inhibits RISC Activity by Argonaute Binding through Conserved WG/GW Motifs
- TOPO3α Influences Antigenic Variation by Monitoring Expression-Site-Associated Switching in
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- The Meningococcal Vaccine Candidate Neisserial Surface Protein A (NspA) Binds to Factor H and Enhances Meningococcal Resistance to Complement
- Network Modeling Reveals Prevalent Negative Regulatory Relationships between Signaling Sectors in Arabidopsis Immune Signaling
- Endothelial Galectin-1 Binds to Specific Glycans on Nipah Virus Fusion Protein and Inhibits Maturation, Mobility, and Function to Block Syncytia Formation
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
- Integration Preferences of Wildtype AAV-2 for Consensus Rep-Binding Sites at Numerous Loci in the Human Genome
- Epigenetic Analysis of KSHV Latent and Lytic Genomes
- Vaccinia Virus–Encoded Ribonucleotide Reductase Subunits Are Differentially Required for Replication and Pathogenesis
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- RNA Virus Replication Complexes
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



