-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Balanced Nuclear and Cytoplasmic Activities of EDS1 Are Required for a Complete Plant Innate Immune Response
An important layer of plant innate immunity to host-adapted pathogens is conferred by intracellular nucleotide-binding/oligomerization domain-leucine rich repeat (NB-LRR) receptors recognizing specific microbial effectors. Signaling from activated receptors of the TIR (Toll/Interleukin-1 Receptor)-NB-LRR class converges on the nucleo-cytoplasmic immune regulator EDS1 (Enhanced Disease Susceptibility1). In this report we show that a receptor-stimulated increase in accumulation of nuclear EDS1 precedes or coincides with the EDS1-dependent induction and repression of defense-related genes. EDS1 is capable of nuclear transport receptor-mediated shuttling between the cytoplasm and nucleus. By enhancing EDS1 export from inside nuclei (through attachment of an additional nuclear export sequence (NES)) or conditionally releasing EDS1 to the nucleus (by fusion to a glucocorticoid receptor (GR)) in transgenic Arabidopsis we establish that the EDS1 nuclear pool is essential for resistance to biotrophic and hemi-biotrophic pathogens and for transcriptional reprogramming. Evidence points to post-transcriptional processes regulating receptor-triggered accumulation of EDS1 in nuclei. Changes in nuclear EDS1 levels become equilibrated with the cytoplasmic EDS1 pool and cytoplasmic EDS1 is needed for complete resistance and restriction of host cell death at infection sites. We propose that coordinated nuclear and cytoplasmic activities of EDS1 enable the plant to mount an appropriately balanced immune response to pathogen attack.
Published in the journal: Balanced Nuclear and Cytoplasmic Activities of EDS1 Are Required for a Complete Plant Innate Immune Response. PLoS Pathog 6(7): e32767. doi:10.1371/journal.ppat.1000970
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000970Summary
An important layer of plant innate immunity to host-adapted pathogens is conferred by intracellular nucleotide-binding/oligomerization domain-leucine rich repeat (NB-LRR) receptors recognizing specific microbial effectors. Signaling from activated receptors of the TIR (Toll/Interleukin-1 Receptor)-NB-LRR class converges on the nucleo-cytoplasmic immune regulator EDS1 (Enhanced Disease Susceptibility1). In this report we show that a receptor-stimulated increase in accumulation of nuclear EDS1 precedes or coincides with the EDS1-dependent induction and repression of defense-related genes. EDS1 is capable of nuclear transport receptor-mediated shuttling between the cytoplasm and nucleus. By enhancing EDS1 export from inside nuclei (through attachment of an additional nuclear export sequence (NES)) or conditionally releasing EDS1 to the nucleus (by fusion to a glucocorticoid receptor (GR)) in transgenic Arabidopsis we establish that the EDS1 nuclear pool is essential for resistance to biotrophic and hemi-biotrophic pathogens and for transcriptional reprogramming. Evidence points to post-transcriptional processes regulating receptor-triggered accumulation of EDS1 in nuclei. Changes in nuclear EDS1 levels become equilibrated with the cytoplasmic EDS1 pool and cytoplasmic EDS1 is needed for complete resistance and restriction of host cell death at infection sites. We propose that coordinated nuclear and cytoplasmic activities of EDS1 enable the plant to mount an appropriately balanced immune response to pathogen attack.
Introduction
In animals and plants, innate immune responses of individual cells constitute a major barrier to pathogen infection. Recognition of microbe - or damage-associated molecules is mediated by germ line encoded receptors whose activation is transduced by intracellular signaling systems to an anti-microbial response. Plant innate immunity is expressed as several layers [1]. Membrane pattern recognition receptors (PRRs) with external ligand recognition domains and intracellular kinase domains detect conserved pathogen molecules (Microbe Associated Molecular Patterns or MAMPs) in a similar manner to non-self recognition in animals [2]. PRR activation triggers a resistance response known as MAMP-triggered immunity (MTI) that is normally sufficient to resist colonization by non-adapted microbes. Successful pathogens have evolved effectors that dampen MTI and raise thresholds for activation of defense thereby allowing host invasion [1], [2]. During infection, many pathogen effectors are delivered inside host cells and plants possess intracellular recognition systems mediated by nucleotide-binding and oligomerisation domain (NB or NOD)-leucine rich repeat (LRR) immune receptors [1]. Structurally related NOD-LRR proteins, known also as CATERPILLER, NACHT-LRR or NOD-like receptors (NLRs), serve as pathogen and damage sensors in innate immune responses and cell death control in mammals [3]. Plant NB-LRR genes are often located within polymorphic gene clusters [4]. Consistent with a high genetic diversity, particular plant NB-LRRs recognize specific pathogen effectors or their actions on host molecular targets in a process known as effector-triggered immunity (ETI) [1]. The activation of NB-LRRs potentiates host defenses, accelerates defense-associated transcriptional reprogramming and often leads to programmed cell death at attempted infection sites as part of a hypersensitive response (HR) [5], [6], [7]. NB-LRR proteins and host cell death are necessarily under strict control. The biochemical mechanisms underlying receptor activation reveal that NB-LRR proteins behave as molecular switches which are structurally constrained in their inactive forms and activated by release from repression [3], [8]. However, little is known about events between NB-LRR activation and defense induction or the mechanisms which limit resistance signaling to prevent auto-immune reactions.
Recent studies of several plant NB-LRR receptors reveal that they partially localize to and function inside nuclei to trigger innate immune responses [9], [10], [11]. A powdery mildew effector-triggered interaction was observed between barley MLA10 receptor and members of the WRKY family of plant transcription factors [11]. Also, the tobacco N receptor recognizing tobacco mosaic virus (TMV) interacts with certain plant Squamosa Promoter-like (SPL) transcription factors [12], suggesting a close association between some NB-LRRs and the transcription machinery. N resistance requires a host chloroplastic sulfurtransferase that becomes partially relocalized to the cytosol and nucleus by the TMV p50 effector [13]. Thus, dynamic signaling between the cytoplasm and nucleus is likely to be important for innate immune responses [14]. In line with this, Arabidopsis plants carrying mutations in genes encoding components of the nucleocytoplasmic trafficking machinery such as the nucleoporins MOS3 (Modifier of snc1,3)/SAR3/Nup96 and MOS7/Nup88, and importins MOS6/AtImpα3 and AtImpα4, display defects in resistance to pathogens [15], [16], [17], [18].
The plant immune regulator EDS1 (Enhanced Disease Susceptibility1) is an essential component of basal resistance to virulent (host-adapted) biotrophic and hemi-biotrophic pathogens [19], [20], [21], forming complexes in the cytoplasm and nucleus with its defense co-regulators PAD4 (Phytoalexin Deficient4) and SAG101 (Senescence Associated Gene101) [20], [22]. EDS1 is also required for resistance conditioned by NB-LRRs that possess an N-terminal Toll/Interleukin-1 Receptor (TIR) domain (known as TIR-NB-LRR receptors) [20], [22], [23], [24]. While Arabidopsis TIR-NB-LRR receptor RPS4 functions inside nuclei, EDS1 is not necessary for its steady state accumulation or nuclear accessibility [10]. Instead, EDS1 was found to signal after TIR-NB-LRR immune receptor activation and upstream of the transcriptional reprogramming of defense genes, production of resistance hormone salicylic acid (SA) and host cell death [10], [20]. These data are consistent with EDS1 transducing signals generated by activated immune receptors to trigger defense and cell death programs.
Convergence of numerous TIR-NB-LRR receptor activities on EDS1 raises the question of how diverse stimuli are coordinated inside cells to produce a measured immune response. To address this, we have examined where in the cell EDS1 signals in Arabidopsis TIR-NB-LRR-conditioned resistance and cell death. We report that there is an increase in the EDS1 nuclear pool during RPS4-triggered resistance to avirulent Pseudomonas syringae bacteria which precedes or coincides with EDS1-dependent transcriptional induction and repression of host genes. We also provide evidence for an essential role of nuclear EDS1 in basal and TIR-NB-LRR-conditioned immunity and in reprogramming defense gene expression. While nuclear EDS1 directs transcriptional changes, our data highlight the need also for cytoplasmic EDS1 to induce a complete immune response.
Results
Triggering of TIR-NB-LRR resistance to bacteria leads to an early rise in EDS1 nuclear accumulation
The Arabidopsis snc1 (suppressor of npr1-1, constitutive1) mutant displays EDS1-dependent constitutive resistance and dwarfism due to auto-activation of a TIR-NB-LRR protein [25], [26]. We assessed whether this deregulated immune response is associated with a change in relative amounts of cytoplasmic and nuclear EDS1 that might reflect EDS1 activity in one compartment. As expected, combining Col eds1-2 [5] with snc1 (in accession Col-0) to produce a snc1/eds1-2 double mutant led to full suppression of snc1 dwarfism (Figure S1A) and resistance (data not shown). Western blot analysis of EDS1 protein revealed that total EDS1 amounts were higher in snc1 compared to wild type (wt) (Figure 1A). However, a proportionate increase in EDS1 accumulation was observed in both nuclei-depleted and nuclear-enriched snc1 fractions. A stable eds1-2 transgenic line expressing EDS1 driven by its native promoter and fused at the C-terminus to yellow fluorescent protein (YFP) was selected (EDS1-YFP). It complemented the eds1 defect in basal resistance (Figure S1E) and was detectable by fluorescence in the cytoplasm and nucleus using a confocal laser-scanning microscope (Figure 1B). Fractionation of EDS1-YFP leaf tissues showed integrity of the EDS1-YFP fusion protein and a similar nucleo-cytoplasmic distribution on Western blots of the same samples probed with anti-EDS1 or anti-GFP antibodies (Figure S1B). Since the native EDS1 (Figure 1A) and EDS1-YFP proteins displayed a similar distribution, we reasoned that EDS1-YFP fluorescence imaging could be used as a reliable indicator of EDS1 localization in leaf cells. The EDS1-YFP line was crossed into snc1/eds1-2 and the subcellular localization of EDS1-YFP protein examined in vivo by confocal imaging. Consistent with the distribution of native EDS1 protein (Figure 1A), EDS1-YFP fluorescence was higher in both the cytoplasm and nuclei of snc1/eds1-2 leaf cells compared to eds1-2 (Figure 1B). These results show that constitutive TIR-NB-LRR resistance increases accumulation of EDS1 protein in both cell compartments. We tested whether the constitutive resistance of snc1 plants could be explained by increased levels of EDS1. As in snc1, the EDS1-YFP line (Figure 1B) and a selected Col eds1-2 line expressing a functional EDS1-HA fusion under control of the constitutive CaMV 35S promoter, accumulated higher amounts of EDS1 in both nuclei-depleted and nuclei-enriched fractions compared to wt (Figure S1C). However, neither of the two lines displayed dwarfism (Figure S1D) or enhanced basal resistance to virulent Pseudomonas syringae pv tomato strain DC3000 (Pst DC3000) bacteria (Figure S1E). Therefore, raising EDS1 steady state levels does not per se produce an auto-immune phenotype. We concluded that the constitutive resistance of snc1 depends on additional signals generated by the activated TIR-NB-LRR protein.
Fig. 1. Deregulated resistance in snc1 leads to increased accumulation of EDS1 in the nucleus and cytoplasm of Arabidopsis leaves. 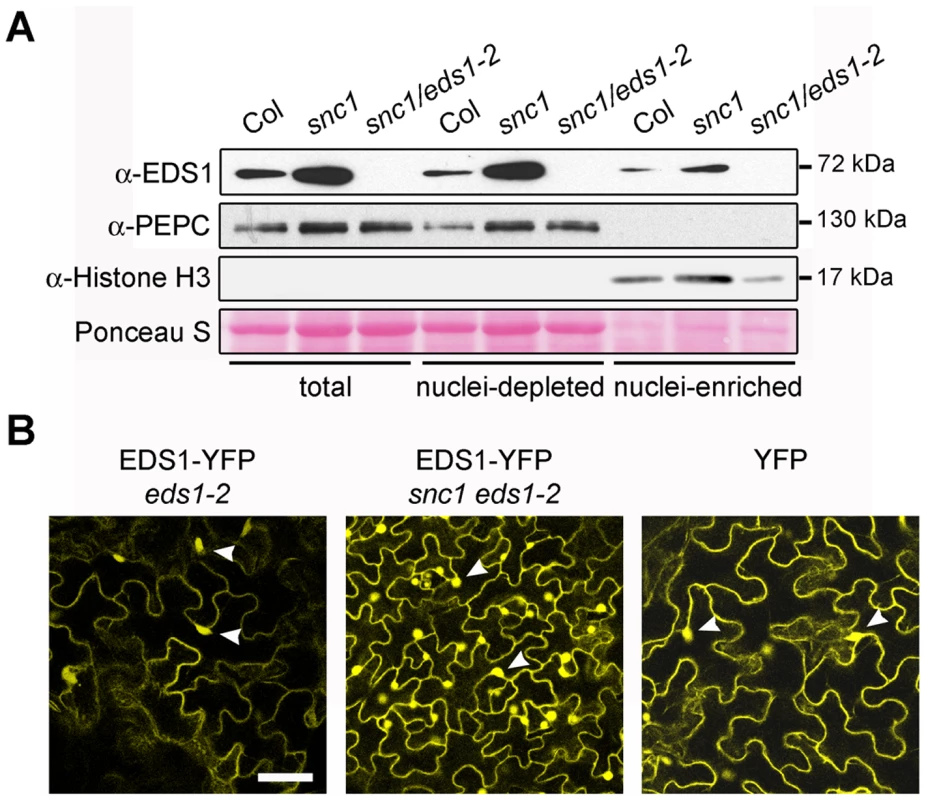
(A) Western blot showing EDS1 protein levels in total, nuclei-depleted and nuclei-enriched fractions prepared from 4-week-old soil grown plants. PEPC was used as cytosolic marker and Histone H3 as nuclear marker. Molecular weight of protein markers are shown on the right. Nuclei-enriched fractions are 30× concentrated (v/v) compared to nuclei-depleted fractions. (B) Confocal images of leaves from snc1 and snc1/eds1-2 transgenic plants expressing EDS1-YFP fusion under the control of the EDS1 native promoter. A confocal image from a stable transgenic line expressing free YFP protein from 35S promoter is provided for comparison. Images were taken at identical microscope settings. Scale bar is 40 µm and white arrowheads depict nuclei. We examined whether there is a change in EDS1 subcellular distribution at an early time point after triggering TIR-NB-LRR resistance that may be short-lived or masked by constitutive activation of the immune pathway. Leaves of wt plants were spray-inoculated with 10 mM MgCl2 (mock treatment), virulent Pst DC3000 or avirulent Pst DC3000 expressing the effector AvrRps4 (Pst DC3000 AvrRps4) recognized by TIR-NB-LRR receptor RPS4 [27]. As expected, Pst DC3000 AvrRps4 infection produced leaf-spot disease symptoms on eds1-2 but not on wt leaves after 2–3 days. Total protein, nuclei-enriched and nuclei-depleted fractions were prepared at 0, 1, 3 and 8 h after inoculation. Western blot analysis revealed an increase in EDS1 nuclear amounts 3 h and 8 h after inoculation with Pst DC3000 AvrRps4 that was not reflected in changes of EDS1 levels in total or nuclei-depleted fractions (Figure 2). No nuclear enrichment of EDS1 was observed at these time points in response to virulent Pst DC3000 or mock treatment (Figure 2). We concluded that an early and potentially important host response to avirulent bacteria involves a change in EDS1 leading to its increase in the nuclear compartment.
Fig. 2. EDS1 nucleo-cytoplasmic distribution after inoculation with virulent and avirulent Pst DC3000 bacteria. 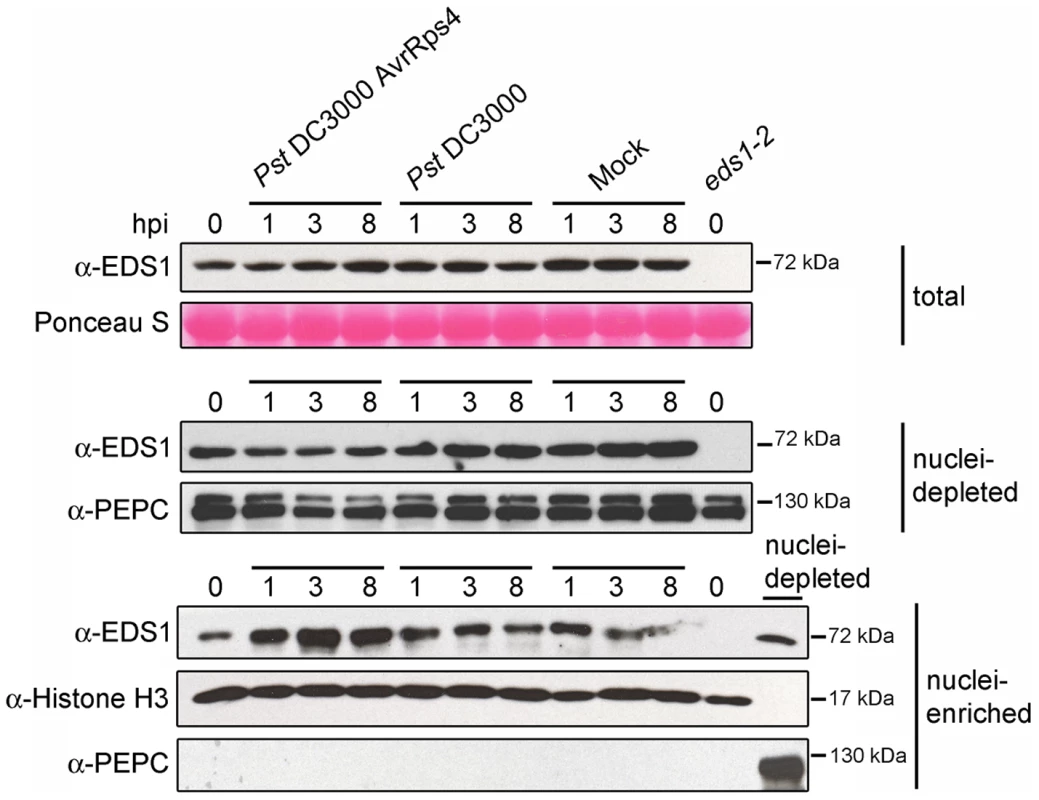
Western blot showing EDS1 protein levels in total, nuclei-depleted and nuclei-enriched fractions prepared from 4-week-old soil grown plants. Samples were collected at 0, 1, 3 and 8 h post inoculation (hpi) with bacterial suspensions or 10 mM MgCl2 (Mock). Ponceau staining of the membrane with total protein extracts shows equal loading. PEPC and Histone H3 were used as cytosolic and nuclear markers, respectively. Nuclei-enriched fractions are 30× concentrated compared to nuclei-depleted fractions. The nuclei-depleted fraction from wt (Col-0) untreated plants was loaded together with nuclei-enriched fractions to check for potential cytosolic contamination with anti-PEPC. Molecular weights of protein markers are shown on the right. EDS1 nuclear accumulation precedes EDS1-dependent transcriptional reprogramming
Transcriptional profiles of wt and eds1 responses to Pst DC3000 AvrRps4 bacteria infiltrated into leaves [5] were analyzed and candidate genes selected whose expression (at 6 h after infection) was induced or repressed in an EDS1-dependent manner (Tables S1 and S2). Prominent among the EDS1-dependent upregulated genes are components of SA biosynthesis and signaling (ICS1, PBS3 and CBP60g) [28], [29], [30] and FMO1 which positively regulates an SA-independent branch of EDS1 defense [5], [31]. PR1, a commonly used SA response marker [32], was also identified in this group. Among the genes showing EDS1-dependent repression was DND1, a negative component of plant innate immunity encoding a cyclic nucleotide-gated channel [33] and ERECTA, a receptor-like kinase required for resistance to the bacteria Ralstonia solanacearum and necrotrophic fungi [34], [35]. A transcription factor (MYB48) and predicted nucleic acid binding protein gene (At1g66140), both with unknown functions, were also selected for analysis. In order to validate the expression trends and measure transcriptional changes in relation to EDS1 nuclear accumulation, we quantified transcript levels of the chosen EDS1-dependent up - and down-regulated genes in the same leaf tissue extracts that were used for analysis of EDS1 protein (Figure 2). The genes displayed significant EDS1-dependent induction (Figure 3A) or repression (Figure 3B) at 8 hpi with Pst DC3000 AvrRps4. Induction of the EDS1-dependent genes in response to virulent Pst DC3000 was not observed at 8 hpi but was seen at 24 hpi, consistent with Pst DC3000 triggering a slower transcriptional response [6]. We concluded from these data that Pst DC3000 AvrRps4-triggered EDS1 nuclear accumulation precedes or coincides with EDS1-dependent transcriptional reprogramming of defense-related genes. An EDS1-dependent increase in PAD4 transcripts and EDS1 induction also occurred at 8 hpi with Pst DC3000 AvrRps4 (Figure 3A). Thus, EDS1 nuclear enrichment observed 3 h after pathogen challenge (Figure 2) is unlikely to be due to increased EDS1 gene expression but rather to a post-transcriptional mechanism.
Fig. 3. Quantitative transcript profiling of EDS1-dependent genes in response to virulent and avirulent Pst DC3000 bacteria. 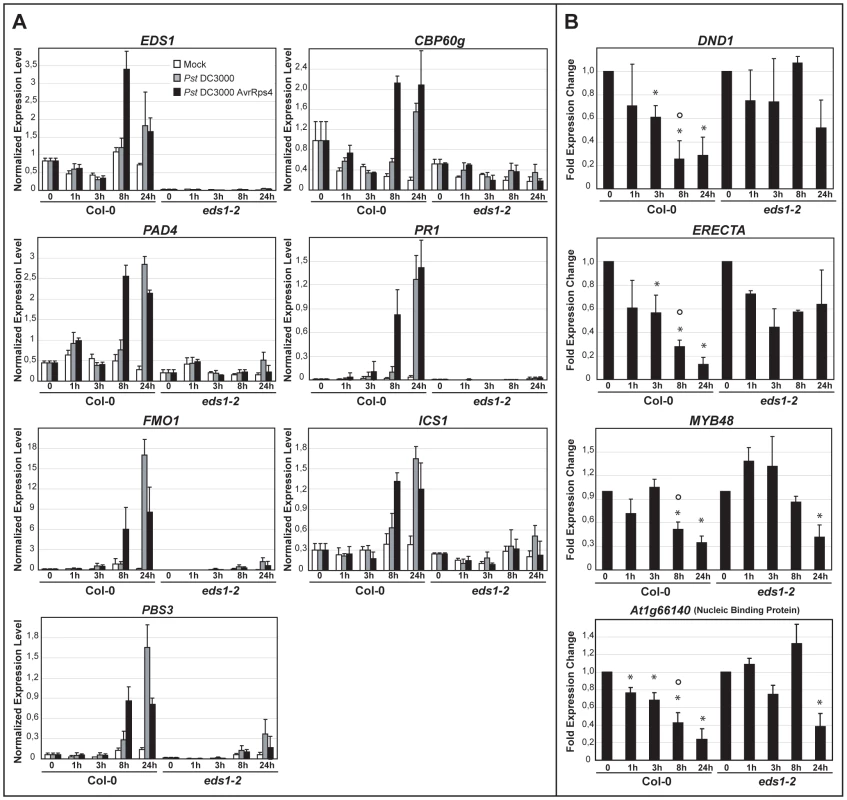
Four-week-old plants were spray-inoculated with 10 mM MgCl2 (Mock, white bars), Pst DC3000 (grey bars) or Pst DC3000 AvrRps4 (black bars) and leaf samples collected at 0, 1, 3, 8 and 24 h post inoculation. Bars represent means and standard deviation from three biological replicates. Transcript levels were determined by qRT-PCR and normalized using the internal control UBIQUITIN. (A) Relative transcript levels of genes displaying EDS1-dependent induction after infection with either bacterial strain. (B) Relative transcript levels of genes displaying EDS1-dependent repression during RPS4-mediated resistance with bars representing the fold change in Pst DC3000 AvrRps4-treated samples compared to mock-treated samples. * indicates significant differences between expression at the indicated h post inoculation and 0 h in each genotype and ° indicates significant differences between Col-0 and eds1-2 at the indicated time point (t-test, p-value <0.01). Slower transcriptional changes in response to Pst DC3000 AvrRps4 compared with previous analysis (Table S2, [5]) are probably due to the use of spray inoculation instead of leaf infiltration for bacterial challenge. Nuclear EDS1 is shuttled to the cytoplasm via the CRM1/XPO1 export machinery
We reasoned that EDS1 may need to attain a certain concentration in nuclei to fulfill its defense signaling function downstream of TIR-NB-LRR receptor activation. This is supported by the higher nuclear EDS1 amounts observed in snc1 immune-activated tissues (Figure 1) and lower levels of nuclear EDS1 in an Arabidopsis mos7 mutant which has a defective Nucleoporin 88 and displays compromised immune responses [16]. In animals, Nup88 modulates CRM1-mediated nuclear export of proteins containing a leucine-rich-type nuclear export sequence (NES) [36]. Thus, EDS1 might possess a functional leucine-rich-type NES and be exported from the nucleus via the Arabidopsis CRM1 homolog XPO1 [37] as a mechanism to control nuclear accumulation. The EDS1 amino acid sequence contains two predicted bipartite nuclear localization signals (NLS) at positions 366 and 440 [19] and a putative leucine-rich nuclear export sequence (NES) around amino acid 530 (Figure S2A) that might enable nucleo-cytoplasmic movement. However, mutation of core residues in the EDS1 NLS or NES (Figure S2B) did not lead to obvious relocalization of YFP-tagged EDS1 protein in transient plant expression assays (data not shown). The functionality of an NES can also be determined by assessing protein localization after treatment with the nuclear export inhibitor Ratjadone A (RatA) which inhibits plant and animal XPO1/CRM1 exportins [37], [38]. We examined mesophyll protoplasts generated from the EDS1-YFP line for EDS1-YFP nuclear and cytoplasmic accumulation in the presence or absence of RatA. There was a marked shift in EDS1-YFP fluorescence to nuclei in RatA-treated compared to mock-treated protoplasts (Figure 4), consistent with EDS1 normally being shuttled out of the nucleus via NES-driven nuclear export. As a control, protoplasts generated from a transgenic line expressing YFP under the CaMV 35S promoter did not respond to RatA treatment (Figure 4) because this protein is able to diffuse between the cytoplasm and nucleus [9].
Fig. 4. EDS1-YFP shuttles from the nucleus to the cytoplasm in Arabidopsis protoplasts. 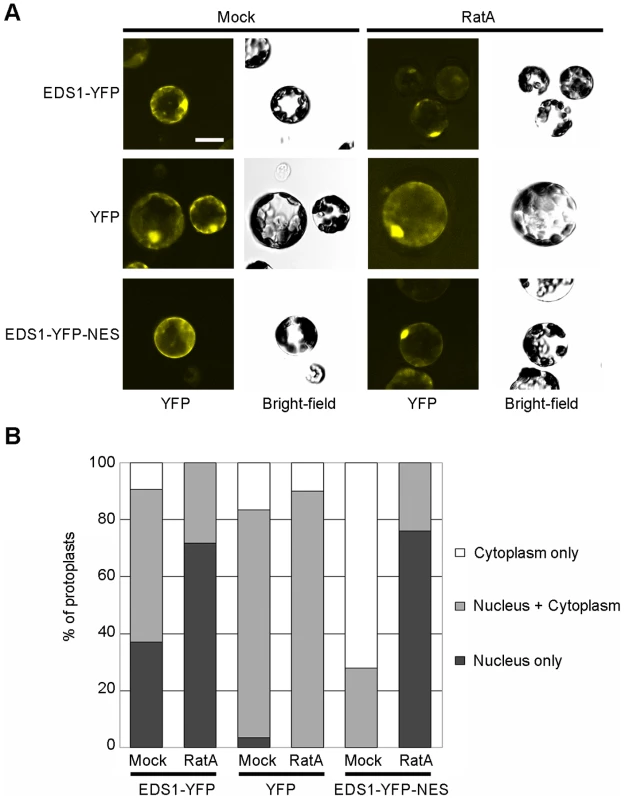
(A) YFP fluorescence and bright-field images of Arabidopsis protoplasts from stable transgenic lines, as indicated, 4 h after treatment with 15 ng/ml of Ratjadone A (RatA) or methanol (Mock). Bar is 10 µm. (B) Percentage of protoplasts showing fluorescence only in the nucleus, only in the cytoplasm or in both compartments determined by counting at least 50 protoplasts per treatment. The experiment was performed three times with similar results. Enhanced EDS1 export from inside nuclei compromises resistance
To establish whether EDS1 operates in the nucleus or cytoplasm or both compartments in transducing resistance signals, we attempted first to reduce EDS1 nuclear accumulation by increasing the rate of protein nuclear export through fusion of an additional functional NES sequence [11], [39]. An NES (LALKLAGLDI) or mutated ‘nes’ (LALKAAGADA) [39] was attached to the C-terminus of EDS1-YFP and this fusion protein expressed stably in Col eds1-2 under the control of the EDS1 native promoter. Multiple independent transgenic lines were selected that expressed the EDS1-YFP-NES/nes fusions at levels similar to EDS1 in wt, as monitored on a Western blot (Figure 5A). EDS1-YFP-NES fluorescence was detected in the cytoplasm and in a low proportion of nuclei whereas EDS1-YFP-nes accumulated in both compartments of leaf epidermal cells (Figure 5B and S3A). Protoplasts derived from an EDS1-YFP-NES line exhibited fluorescence in the cytoplasm and at the nuclear rim (Figure 4) consistent with the NES fusion increasing the rate of EDS1 export from nuclei. There was a strong increase in EDS1-YFP-NES nuclear accumulation in this line after RatA treatment (Figure 4), indicating that the NES-tagged protein has the capacity to enter nuclei and that RatA treatment inhibits NES-driven nuclear export. We monitored the distribution of EDS1-YFP-NES (in lines #2–10 and #2–11) and EDS1-YFP-nes (line #1–2) compared to EDS1-YFP in nuclei-depleted and nuclei-enriched fractions on a Western blot probed with anti-EDS1 antibody. This did not reveal obvious differences in nuclear accumulation between the EDS1-YFP-NES and EDS1-YFP-nes extracts (Figure S3B), contrasting with the distinct in vivo EDS1-YFP-NES/nes fluorescence patterns (Figure 5B). To investigate reasons for this discrepancy, we imaged fluorescence in sections through individual nuclei in EDS1-YFP-NES line #2–11 and EDS1-YFP-nes line #1–2 on a confocal microscope and compared with the EDS1-YFP line. EDS1-YFP-NES protein fluorescence was detected mostly in the cytoplasm, at the nuclear rim (as seen before in the protoplasts; Figure 4) and inside nuclei in ∼5% of epidermal cells (Figure S3C). EDS1-YFP-nes and EDS1-YFP fluorescence was observed inside the nuclear compartment in most imaged cells (Figure S3C). A similar distribution was found in eds1-2 epidermal cells transiently expressing EDS1-YFP-NES/nes constructs after particle bombardment (Figure S3D). Together, the data suggest that addition of a functional NES to EDS1-YFP reduces its accumulation inside nuclei but the NES does not allow complete release of EDS1-YFP from the nuclear envelope and associated structures. This may account for its fractionation with nuclei during biochemical separation.
Fig. 5. Enhanced export of EDS1 from nuclei leads to reduced resistance. 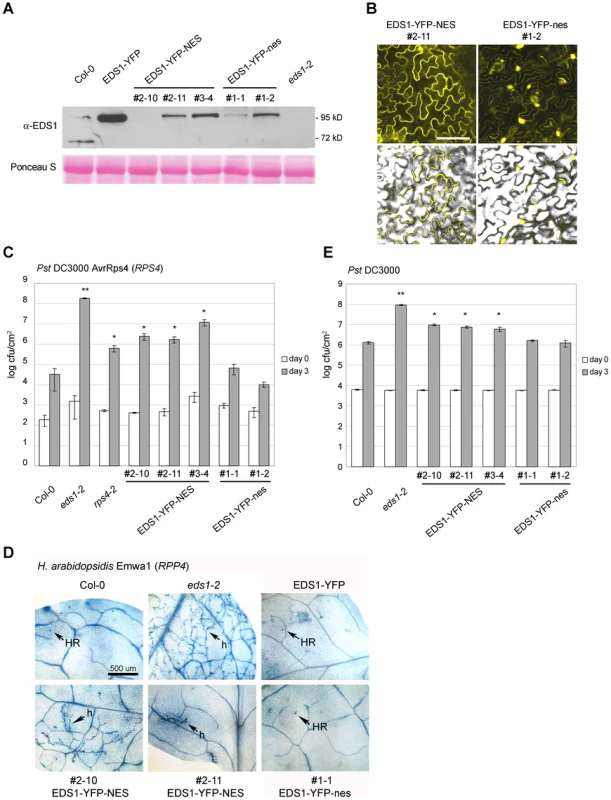
(A) Western blot showing total EDS1 protein levels in wt plants and in selected independent transgenic lines expressing the indicated EDS1 fusions. NES and nes denote functional and non-functional nuclear export signals, respectively. Ponceau S staining of the membrane shows equal loading. (B) Confocal images showing the subcellular distribution of the EDS1-YFP-NES and EDS1-YFP-nes fusions in one representative transgenic line 5 d post inoculation with virulent H. arabidopsidis Noco2. Bar is 15 µm. (C) The indicated genotypes were spray-inoculated with avirulent Pst DC3000 AvrRps4 bacterial suspension and bacterial titers determined 3 h (day 0, white bars) and 3 d (grey bars) post inoculation. Bars represent means of 5 replicates ± standard error. ** p value<0.001; * p value<0.05. (D) Infection phenotypes of leaves inoculated with a spore suspension of avirulent H. arabidopsidis isolate Emwa1. Leaves were stained with lactophenol trypan blue 6 d post inoculation to visualize pathogen growth and host cell death. h: hyphae; HR: hypersensitive response. Bar is 500 µm. (E) The indicated genotypes were spray- inoculated with a virulent Pst DC3000 suspension and bacterial titers determined 3 h (day 0, white bars) and 3 d (grey bars) post inoculation. Bars represent means of 4 replicates ± standard error. ** p value<0.001; * p value<0.05. Similar results were obtained in at least three independent experiments. We examined whether resistance mediated by the TIR-NB-LRR genes RPS4 [27] or RPP4 [40] is affected by increased removal of EDS1 from inside nuclei. The EDS1-YFP-NES lines displayed reduced RPS4 resistance to Pst DC3000 AvrRps4, measured by bacterial growth (Figure 5C), and RPP4 resistance to H. arabidopsidis isolate Emwa1, monitored by trypan blue staining of leaves for pathogen structures and plant cell death (Figure 5D). By contrast, the EDS1-YFP-nes lines were fully resistant (Figure 5C and D). Basal resistance to virulent Pst DC3000 (Figure 5E) and H. arabidopsidis Noco2 (data not shown) was also compromised in the EDS1-YFP-NES transgenics but was unaffected in EDS1-YFP-nes lines. These data suggest that EDS1 needs to accumulate to a sufficient level inside nuclei to signal a full innate immune response against virulent and avirulent pathogens. With both pathogens, a substantial degree of resistance remained in the EDS1-YFP-NES lines compared to the complete susceptibility of eds1 mutants.
EDS1 release to the nucleus drives TIR-NB-LRR and basal resistance responses
We reasoned that the residual pathogen resistance in the EDS1-YFP-NES lines (Figure 5) might be conferred by a low amount of EDS1-YFP-NES protein initially entering nuclei before it is exported or partially trapped at the nuclear envelope (Figure 4 and S3), arguing for an entirely nuclear function of EDS1. Alternatively, the intermediate resistance could reflect a contribution of cytoplasmic EDS1 to the immune response. We therefore used a different strategy to control EDS1 localization inside cells by fusing the C-terminus of EDS1-YFP or YFP alone (as control) to the steroid binding domain of the mammalian glucocorticoid receptor (GR) in a cassette driven by the CaMV 35S promoter [41]. Proteins fused to GR are normally retained in the cytoplasm through association with an Hsp90 chaperone complex [42] and treatment of plant cells with the steroid hormone Dexamethasone (Dex) drives nuclear localization of the GR fusion protein [41], [43].
From multiple independent transgenic lines expressing EDS1-YFP-GR or YFP-GR in an eds1-2 background (in Arabidopsis accession Ler) two lines (#1 and #4) were selected that had detectable EDS1-YFP-GR protein on a Western blot of leaf extracts probed with anti-GFP antibody (Figure 6A). Extracts were also probed with anti-EDS1 antibody which gave weaker signals than with anti-GFP and showed that EDS1-YFP-GR protein accumulated at a rather low level (Figure S4A) despite being driven by the 35S promoter (Figure S4B). A single line expressing YFP-GR (Figure S4C) was taken as control. Before Dex treatment, weak YFP fluorescence was observed in the cytoplasm of the EDS1-YFP-GR lines, as shown for line #4 (Figure 6B). YFP fluorescence was also detected in nuclei at 5 h (not shown) and 13 h (Figure 6B) after spraying leaves with 30 µM Dex. At 13 h (after Dex) this was associated with an increase in EDS1-YFP-GR protein in total, nuclei-depleted and nuclei-enriched fractions (Figure 6A) but not with a change in EDS1-YFP-GR transcript levels (Figure S4B). The EDS1-YFP-GR transgenic lines developed normally before or after treatment with the steroid hormone. We therefore used them to investigate the roles of cytoplasmic and nuclear EDS1 in the plant immune response.
Fig. 6. Nuclear release of EDS1-YFP-GR by Dexamethasone (Dex) treatment leads to protein accumulation in nuclei and enhanced disease resistance. 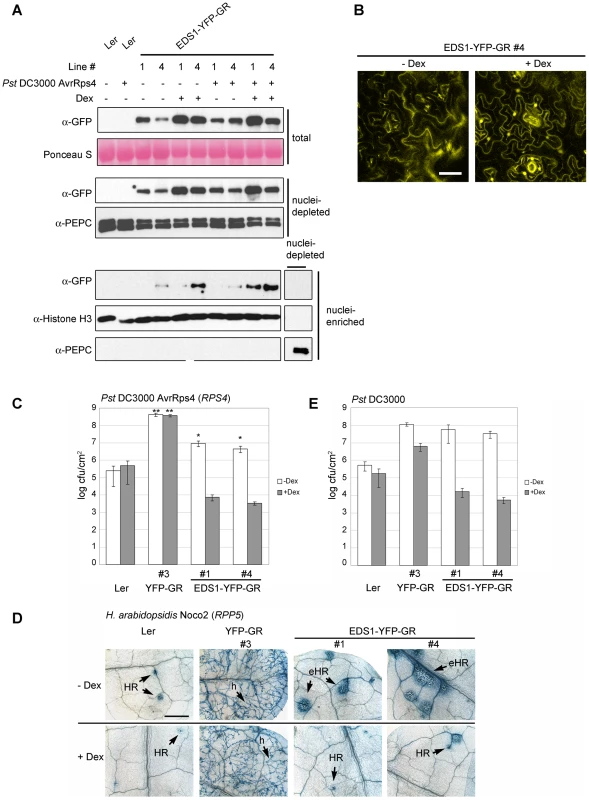
(A) Leaves of 4-week-old plants were pre-treated with Dex (+) or mock solution (−) and spray-inoculated with buffer (−) or Pst DC3000 AvrRps4 (+) and protein samples prepared 8 h post inoculation, as indicated. Western blot shows EDS1 protein levels in total, nuclei-depleted and nuclei-enriched fractions in transgenic lines #1 and #4 expressing the EDS1-YFP-GR fusion protein. Ponceau S staining of the membrane shows equal loading. PEPC and Histone H3 signals were used, respectively, as cytosolic and nuclear marker. (B) Confocal images showing EDS1-YFP-GR subcellular distribution in a representative transgenic line with or without Dex treatment 13 h after Dex treatment. Bar is 30 µm. (C) Untreated (white bars) or Dex-pretreated (5 h, grey bars) 4-week-old plants of the indicated genotypes were spray-inoculated with avirulent Pst DC3000 AvrRps4 and bacterial titers determined 4 d post inoculation. Bars represent means of 4 replicates ± standard error. ** p value <0.001; * p value <0.05. (D) Untreated or Dex-pretreated (5 h) 2-week-old plants were spray-inoculated with a spore suspension of avirulent H. arabidopsidis isolate Noco2. Leaves were stained with lactophenol trypan blue 7 d post inoculation to visualize pathogen growth and host cell death. h: hyphae; HR: hypersensitive response; eHR: expanded HR. Bar is 500 µm. (E) Untreated (white bars) or Dex-pretreated (5 h, grey bars) 4-week-old plants were spray-inoculated with virulent Pst DC3000 bacterial suspension and bacterial titers determined 4 d post inoculation. Bars represent means of 4 replicates ± standard error. ** p value <0.001; * p value <0.05. Results of the bacterial and oomycete infection assays are representative of three independent experiments. EDS1-YFP-GR plants were first analyzed for their response to avirulent Pst DC3000 AvrRps4. Leaves were pre-treated with 30 µM Dex for 5 h before bacterial inoculation and bacterial titers were counted at day 4. In the absence of Dex, the EDS1-YFP-GR lines displayed resistance to Pst DC3000 AvrRps4 that was intermediate between wt and eds1-2 (Figure 6C). Treatment with Dex reduced bacterial numbers by ∼1000-fold to levels below those seen in wt (Figure 6C). Wt (Ler) and control YFP-GR (Ler eds1-2) backgrounds exhibited, respectively, similar levels of resistance and susceptibility with or without Dex treatment (Figure 6C). Also, Dex application to wt plants did not alter levels of native EDS1 protein (Figure S4D), indicating that the resistance of the EDS1-YFP-GR lines is conditioned by Dex-induced release of EDS1. Resistance mediated by a different EDS1-dependent TIR-NB-LRR gene (RPP5) [44] to H. arabidopsidis isolate Noco2 was also conditional on Dex treatment, with pathogen growth being efficiently contained by a plant hypersensitive response at infection sites (HR; Figure 6D). As with Pst DC3000 AvrRps4 bacteria, the EDS1-YFP-GR lines displayed partial resistance to H. arabidopsidis in the absence of Dex. Notably, this was associated with spreading cell death at infection foci (Figure 6D). Without Dex treatment, basal resistance in these lines to virulent Pst DC3000 was suppressed to similar levels as in eds1-2 and was equivalent to resistance in wt after Dex application (Figure 6E).
The EDS-YFP-GR signal in nuclei-enriched fractions was strongest when Dex treatment was followed by inoculation with Pst DC3000 AvrRps4 (Figure 6A and S4A). Since EDS1-YFP-GR is expressed under the control of a constitutive promoter, it is unlikely that the increase is due to transcriptional up-regulation. Indeed, no significant change in expression of the EDS1-YFP-GR transgenes was observed in leaves 8 h after challenge with Pst DC3000 AvrRps4, with or without Dex pretreatment (Figure S4B). These data suggest that recognition of AvrRps4 or another pathogen stimulus enhances nuclear accumulation of Dex-released EDS1-YFP-GR.
We monitored expression of genes displaying EDS1-dependent transcriptional changes 8 h after Pst DC3000 AvrRps4 inoculation (Figure 3) in the EDS1-YFP-GR transgenic lines with or without 5 h Dex pre-treatment. The 13 h time point after Dex application is when EDS1-YFP-GR protein signals were monitored on Western blots and by fluorescence imaging (Figure 6A and B). The results show Dex-dependent reprogramming of EDS1-induced and EDS1-repressed genes in response to DC3000 AvrRps4 (Figure 7). Whereas cytosolic retention of EDS1-YFP-GR substantially reduced pathogen-induced expression changes, Dex-induced nuclear accumulation of EDS1-YFP-GR allowed similar or larger transcriptional changes than in DC3000 AvrRps4-challenged wt plants (Figure 7). We concluded that nuclear EDS1 is needed to drive pathogen-induced reprogramming of transcription.
Fig. 7. Quantitative transcript profiling of EDS1-dependent genes in EDS1-YFP-GR transgenic lines after triggering RPS4 resistance. 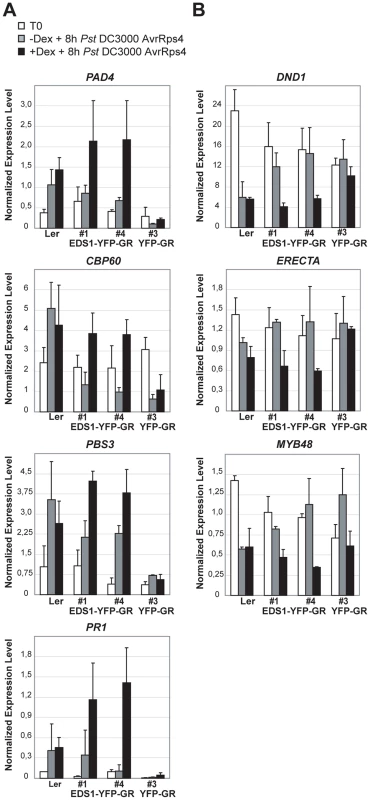
Four-week-old plants untreated (grey bars) or pre-treated with Dexamethasone (Dex) for 5 h (black bars) were inoculated with Pst DC3000 AvrRps4. Leaf samples were collected from untreated plants (T0) and 8 h after pathogen inoculation. Bars represent means and standard deviation of two or three biological replicates. Relative transcript levels were determined by qRT-PCR and normalized using the internal control UBIQUITIN. Expression was normalized in different samples using the endogenous control gene UBIQUITIN. (A) Up-regulated genes. (B) Down-regulated genes. Discussion
Plant innate immunity to invasive biotrophic or hemi-biotrophic pathogens involves the rapid mobilization of defense and cell death programs. However, these are energetically costly and disturb normal development and fitness and are therefore under strict genetic control [45], [46]. We provide evidence that Arabidopsis EDS1 coordinates immune responses by functioning in both the nucleus and the cytoplasm. Although nuclear EDS1 is required for TIR-NB-LRR-induced reprogramming of defense gene expression and resistance, a cytoplasmic EDS1 pool is maintained during infection and development, and is needed for complete resistance to bacterial and oomycete pathogens.
Maintaining EDS1 nucleo-cytoplasmic balance
We used a combination of biochemical fractionation of leaf tissue extracts and in vivo imaging of fluorescent-tagged proteins in leaf cells to assess EDS1 accumulation in the cytoplasmic and nuclear compartments. Enhanced EDS1 expression in snc1 mutants (with constitutive resistance due to an auto-activate TIR-NB-LRR protein (Figure 1)) [26] or reduced EDS1 in mos7 mutants (with compromised resistance because of a defect in Nup88-mediated nuclear retention [16]) resulted in similar EDS1 nuclear and cytoplasmic ratios to those found in healthy wt plants. We also observed higher EDS1 accumulation in both cell compartments at late stages of infection with virulent H. arabidopsidis (data not shown), suggesting that nuclear and cytoplasmic EDS1 pools need to be equilibrated during prolonged activation of defense and development. These EDS1 accumulation patterns, together with our earlier finding that EDS1 forms different complexes with its signaling partners PAD4 and SAG101 in the cytoplasm and nuclei of healthy leaf cells [22], suggest distinct but cooperative roles of EDS1 cytoplasmic and nuclear pools. Analysis of mesophyll protoplasts derived from the EDS1-YFP and EDS1-YFP-NES transgenic lines (Figure 4) showed that EDS1 is capable of nuclear transport receptor (CRM1/XPO1)-mediated shuttling between the cytoplasm and nucleus through the nuclear pores. It is therefore likely that cytoplasmic and nuclear EDS1 pools communicate through the nuclear pores to coordinate resistance and cell death programs. There may be a requirement for optimal cycling of EDS1 between the nuclear and cytoplasmic compartments, consistent with dynamic signaling across the nuclear envelope being critical for animal and plant immune responses [14].
Nuclear EDS1 directs transcriptional reprogramming
An increase in nuclear EDS1 accumulation either by pathogen infection triggering TIR-NB-LRR activation (Figure 2) or by Dex-induced release of a EDS1-YFP-GR fusion to nuclei accompanying a pathogen stimulus (Figure 6) correlated with the induction or repression of particular host genes (Figures 3 and 7), supporting a role for nuclear EDS1 in driving transcriptional reprogramming during plant defense. Among the EDS1-induced genes are components of SA biosynthesis and signaling (e.g ICS1, PAD4, PBS3 and CBP60g) [28], [29], [30], [47] (Table S2). Hence, a key step of EDS1 nuclear action is to stimulate the SA pathway. Although SA has a minor role in local TIR-NB-LRR triggered resistance and cell death [20], [40], [48] it is a central component of plant systemic resistance to biotrophic pathogens [49]. Accordingly, EDS1 is required for systemic signaling beyond pathogen infection sites [50], [51]. EDS1-directed repression of genes such as DND1 [33] and ERECTA [34], [35] which have a negative impact on resistance to biotrophic pathogens but contribute to resistance to some necrotrophic pathogens, suggests a ‘master’ role of EDS1 complexes in coordinating gene expression outputs. Control of ERECTA expression may be of particular significance since this gene affects plant growth and development as well as responses to environmental stimuli through a network of cis - and trans-regulation [52]. The precise mode of action of EDS1 inside nuclei remains unclear. The primary EDS1 amino acid sequence does not have obvious DNA-binding domains [19] and analysis of nuclear EDS1 has not so far revealed specific association with chromatin (S. Blanvillain-Baufumé, R.P. Huibers and J.E. Parker, unpublished). However, interactions have been found between EDS1 and a number of transcription factors in yeast 2-hybrid assays (S. Blanvillain-Baufumé, R.P. Huibers and J.E. Parker, unpublished), suggesting a mechanism by which EDS1 could modulate transcription. Nuclear EDS1 complexes may work by binding transcription factors and/or repressors in the nucleoplasm to guide activities and associations with the DNA. Restraining proteins away from their site of action has emerged as an important mechanism for controlling transcriptional activators such as NF-KB in mammalian cells [53] and a number of transcriptional regulators in plants (such as bZIP10, WRKY33, NPR1), and emphasizes the extent of intracellular protein dynamics during stress signaling [14]. The role of the EDS1 signaling partners PAD4 and SAG101 in this process is also not clear. They form transient complexes with EDS1 that distribute differently between the cytoplasm and nucleus and, together, are essential for immune response and cell death activation [20], [22]. One model is that PAD4 and SAG101 help EDS1 to circulate between the cytoplasm and nucleus to coordinate the binding and release of transcription factors. It will be important to test whether EDS1 mislocalization affects association with PAD4 or SAG101 and if these components participate in EDS1 interactions with transcription factors in the cell.
Evidence points to EDS1 operating downstream of activated RPS4 [10]. We therefore postulate that a change in EDS1 nucleo-cytoplasmic status leading to transcriptional reprogramming is triggered by RPS4 recognition of AvrRps4. However, we cannot exclude the possibility that an alteration in EDS1 occurs as part of the plant response to bacterial MAMPs. An early MAMP-induced change might be effectively dampened by virulent DC3000 bacteria and reinstated (or amplified) by RPS4 responding to AvrRps4 [1]. Whatever the stimulus, a rise in EDS1 alone seems insufficient to trigger resistance because a mild increase in EDS1 levels (in the pEDS1:EDS1-YFP transgenic line) or strong over expression of EDS1 (in the p35S:EDS1-HA line) resulting in over-accumulation of EDS1 in the nuclear and cytoplasmic compartments did not produce an auto-immune response (Figure S1C–E). These data suggest that R protein activation is a necessary step for EDS1 inside nuclei to reprogram transcription. In this regard, nuclear EDS1 levels appear to be controlled post-transcriptionally. This holds for the early nuclear accumulation of EDS1 after RPS4 activation because it occurs before changes in EDS1 transcript levels are observed (Figures 2 and 3). It is therefore distinct from pathogen-induced increases in EDS1 and PAD4 mRNAs and total protein at later time points which are attributed to a positive feedback loop involving SA to amplify host resistance [5], [20], [47]. Post-transcriptional regulation likely also accounts for the rise in EDS1-YFP-GR steady state levels after its Dex-induced release to the nucleus because bacterial and Dex treatments did not alter EDS1-YFP-GR mRNA abundance (Figure S4B). The low level of EDS1-YFP-GR protein accumulating in the cytoplasm before Dex treatment (Figure 6 and S4) may be a consequence of impeding nuclear access or nucleo-cytoplasmic cycling of EDS1. In line with the tendency for nuclear and cytoplasmic EDS1 to equilibrate, we postulate that an initial rise in nuclear EDS1-YFP-GR causes a rapid adjustment of the cytoplasmic EDS1 pool, thereby permitting a complete immune response to be activated (Figure 6). Modification of EDS1 leading to increased protein stabilization likely alters its functions inside nuclei to permit reprogramming of transcription. A post-transcriptional process was also proposed to account for lowered total cellular accumulation of EDS1 and the SA response regulator NPR1 in the Arabidopsis mos7 (nup88) nucleoporin mutant since their corresponding transcript levels were not reduced [16]. A growing body of evidence shows that targeted degradation by the proteasome coordinates the exchange of transcription components on and off the chromatin, allowing dynamic shifts from repression to activation of genes [54], [55]. A recent analysis indeed shows that proteasome-mediated turnover of NPR1 in the nucleus is necessary for its function as a transcriptional co-activator in systemic resistance [56].
Role for cytoplasmic EDS1 in modulating pathogen-induced cell death
Partial resistance to bacteria and oomycete pathogens exhibited by the EDS1-YFP-NES (Figure 5) and EDS1-YFP-GR lines (in the absence of the Dex release stimulus; Figure 6) points to a role of cytoplasmic EDS1 in promoting an efficient immune response. It is possible that transient EDS1 nuclear pools in the EDS1-YFP-NES lines (Figure 4) or leakiness in EDS1-YFP-GR cytoplasmic retention (though undetectable in the microscope – Figures 6B) could account for the residual resistance. Also, fusion of an additional NES to EDS1 may have unexpected consequences since, although it reduces levels of EDS1-YFP inside nuclei, it appears to impede efficient release of EDS1 from the nuclear rim (Figure 4 and S3). Nevertheless, the fact that EDS1 is actively shuttled from the nucleus to the cytoplasm where it forms different complexes [22] and a cytoplasmic EDS1 pool is maintained throughout infection and development, argue for EDS1 functions in the cytoplasm. A role for cytoplasmic EDS1 is most evident in TIR-NB-LRR resistance to Pst DC3000 AvrRps4 (Figures 5C and 6C) and H. arabidopsidis (Figures 5D and 6D) but less clear in EDS1-dependent basal resistance (Figures 5E and 6E). The cytoplasmic EDS1 pool may counter-balance activities of nuclear EDS1 by, for example, sequestering transcription factors outside the nuclei. Alternatively, but not exclusively, EDS1 cytosolic complexes might have a unique signaling function needed for a complete immune response. Particularly noticeable was the expansion of cell death lesions at sites of infection by the obligate biotrophic pathogen H. arabidopsidis in EDS1-YFP-GR lines in the absence of Dex (Figure 6D). These lesions extended beyond obvious pathogen structures and were therefore different from the characteristic trailing necrotic phenotype observed in Arabidopsis mutants with relaxed TIR-NB-LRR resistance [20]. No cell death was observed in the EDS1-YFP-GR lines before pathogen inoculation indicating that the cell death requires a pathogen stimulus. One scenario is that failure to restrict host cell death in these lines is due to diminished EDS1 nuclear function in regulating genes that suppress or contain cell death. Supporting this, a recent study showed that SA antagonism of EDS1-driven cell death initiation is needed for a complete immune response to biotrophic pathogens [57]. It is possible that cytosolic EDS1 actively promotes cell death in response to oxidative stress signals emanating from the chloroplasts [57], [58], [59] and this is counter-balanced by transcriptional reprogramming in the nucleus to moderate potentially destructive cellular events. Future work aims to characterize the cytoplasmic and nuclear activities of EDS1 complexes which seem to be carefully poised in the cell for optimal responsiveness to biotic stress.
Materials and Methods
Plant growth and pathology assays
Arabidopsis wild type accessions, eds1-2 [5] and rps4-2 [10] mutants have been described. Plants were grown in soil in controlled environment chambers under a 10 h light regime (150–200 µE/m2s) at 22°C and 65% relative humidity. Pst DC3000 and Pst DC3000 AvrRps4 strains were grown for 24 h at 28°C on NYGA solid medium supplemented with the corresponding antibiotics. For bacterial growth assays and expression analyses, 4-week-old plants were spray-inoculated with bacterial suspensions at 4×108 cfu/ml in 10 mM MgCl2 with 0,04% (v/v) Silwet L-77 (Lehle Seeds) or mock-treated with 10 mM MgCl2 containing 0,04% (v/v) Silwet L-77. In planta bacterial titers were determined at the indicated time points after inoculation by shaking leaf discs in 10 mM MgCl2 with 0,01% (v/v) Silwet L-77 at 28°C for 1 h [60]. At least five plants per genotype were used for each sampling. Bacterial numbers were compared between lines using a two-tailed Student's t-test. H. arabidopsidis isolates Emwa1 and Noco2 were maintained and inoculated onto 2-week-old plants at 4×104 spores/ml as described [22]. Plant cell death and H. arabidopsidis infection structures were visualized under a light microscope after staining leaves with lactophenol trypan blue [23].
Arabidopsis stable transgenic lines
Binary vectors suitable for Gateway cloning (Invitrogen) and protein localization studies were generated. Monomeric yellow fluorescent protein (YFP) was PCR-amplified from vector pcDNA3-mYFP (obtained from Dr. Irine Prastio, Howard Hughes Medical Institute, UC San Diego, CA) and ligated into the binary vector pXCS-HisHA containing a CaMV 35S promoter [61], generating pXCS-YFP. A Gateway recombination cassette (reading frame B as EcoRV-fragment, Invitrogen) was ligated and the CaMV 35S promoter removed, resulting in the Gateway destination vector pXCG-YFP. Genomic Ler EDS1 sequence including 1.4 kb of endogenous promoter and 2.1 kb of coding sequence without stop codon was cloned in pENTR/D-TOPO (Invitrogen) and an LR reaction was performed to generate the vector pXCG-pEDS1-EDS1-YFP. Constructs were used to transform Col eds1-2 plants [5] using the floral-dip method [62]. Several independent EDS1-YFP transgenic lines were generated that fully complemented the eds1-2 mutation and a representative line used for further analysis. Functional NES from PKI (LALKLAGLDI) and the non-functional nes (LALKAAGADA) [39] were attached to the C-terminus of mYFP through PCR amplification of vector pcDNA3-mYFP. The same strategy as described above was followed to generate construct pXCG-pEDS1-EDS1-YFP-NES/nes and stable Arabidopsis Col eds1-2 stable transgenic lines. To generate transgenic plants expressing EDS1-HA under the CaMV 35S promoter, an LR reaction was made between the pENTR/D-TOPO (Invitrogen) vector containing genomic Ler EDS1 coding sequence [22] and the pXCS-3xHA vector [61]. Constructs were transferred to A. tumefaciens strain GV3101 (pMP90RK) and transformed into Arabidopsis Col eds1-2 plants. To generate transgenic plants expressing StrepII-3xHA-YFP driven by the CaMV 35S promoter, the plasmid pENS-StrepII-3xHA-GW was made using the vector pXCS-3xHA [61]. YFP was PCR-amplified from vector pcDNA3-mYFP and cloned in pENTR/D-TOPO (Invitrogen) and an LR reaction performed to obtain the pENS-StrepII-3xHA-YFP plasmid. Constructs were transformed into Arabidopsis Col plants, as described above. GR fusions were generated using the vector pBI-ΔGR [41] and cEDS1-YFP amplified from the pEXG-cEDS1-YFP vector. Constructs were transferred to A. tumefaciens strain GV3101 (pMP90) and used to transform Ler eds1-2 plants [19].
Protein expression analysis
Total protein extracts were prepared by grinding leaf material in liquid nitrogen. Samples were resuspended in equal volumes of 2× Laemmli loading buffer, boiled for 5 min and centrifuged to remove cell debris. Proteins were separated by SDS-PAGE and electroblotted to nitrocellulose membranes for protein gel blot analysis. Equal loading was monitored by staining membranes with Ponceau S (Sigma-Aldrich). Nuclear fractionation of Arabidopsis tissue was performed as previously described [22] using 4-week-old plants. Nuclei-enriched fractions were 30× more concentrated than nuclei-depleted fractions based on the final volume of each fraction. Antibodies used for immunoblot analysis were as described: anti-EDS1 [22], anti-PEPC (Rockland; [63]), anti-Histone H3 (Abcam; [22]), anti-PICKLE [64] and anti-CSN4 (BIOMOL International).
Generation of Arabidopsis protoplasts and shuttling assay
Arabidopsis leaf mesophyll protoplasts were prepared from 4-week-old plants grown in a normal light/dark regime, according to Asai et al [65] with some modifications. Leaf strips were digested in enzyme solution (0.4 M Mannitol; 20 mM KCl; 20 mM MES pH 5.7) with 1.5% cellulase (Onozuka R-10, Merck, Darmstadt, Germany) and 0.4% Macerozyme (R-10, Serva, Heidelberg, Germany). The solution was vacuum infiltrated for 3 min, incubated for 30 min with vacuum pressure and then for 2 h with gentle shaking. The protoplast solution was filtered through a 62 µm nylon mesh and washed with W5 solution (154 mM NaCl, 25 mM CaCl2·2H2O, 5 mM KCl, 2 mM MES pH 5.7). Isolated protoplasts were resuspended in Mannitol solution (0.4 M Mannitol, 15 mM MgCl2·6H2O, 4 mM MES, pH 5.8). The nuclear export inhibitor Ratjadone A (Alexis Biochemicals) was dissolved in methanol (10 ng/µl) and added to protoplasts to a final concentration of 15 ng/ml. Control samples were mock treated-with an equal concentration of methanol.
Confocal imaging
Arabidopsis leaves or protoplast solutions were examined with a confocal laser-scanning microscope Leica TCS 4D.
Gene expression analyses
Total RNA was extracted from plant leaves using TRI-reagent (SIGMA) and RNA was reverse transcribed into cDNA using SuperScriptII (Invitrogen) following the manufacturer's instructions. Quantitative RT-PCR experiments were performed in an iQ5 Real-Time PCR Detection System (Bio-Rad) using Brilliant SYBR Green QPCR Core Reagent (Stratagene) as dye. Experiments were performed using three independent biological samples. Relative transcript levels were calculated using the iQ5 Optical System Software (Version 2.0). Ubiquitin UBQ10 (At4g05320) transcript levels were used as internal reference. Primers used in these experiments are available on request.
Supporting Information
Zdroje
1. JonesJD
DanglJL
2006 The plant immune system. Nature 444 323 329
2. ZipfelC
2009 Early molecular events in PAMP-triggered immunity. Curr Opin Plant Biol 12 414 420
3. ChenG
ShawMH
KimYG
NunezG
2009 NOD-like receptors: role in innate immunity and inflammatory disease. Annu Rev Pathol 4 365 398
4. ClarkRM
SchweikertG
ToomajianC
OssowskiS
ZellerG
2007 Common sequence polymorphisms shaping genetic diversity in Arabidopsis thaliana. Science 317 338 342
5. BartschM
GobbatoE
BednarekP
DebeyS
SchultzeJL
2006 Salicylic acid-independent ENHANCED DISEASE SUSCEPTIBILITY1 signaling in Arabidopsis immunity and cell death is regulated by the monooxygenase FMO1 and the Nudix hydrolase NUDT7. Plant Cell 18 1038 1051
6. TaoY
XieZ
ChenW
GlazebrookJ
ChangHS
2003 Quantitative nature of Arabidopsis responses during compatible and incompatible interactions with the bacterial pathogen Pseudomonas syringae. Plant Cell 15 317 330
7. GreenbergJT
YaoN
2004 The role and regulation of programmed cell death in plant-pathogen interactions. Cell Microbiol 6 201 211
8. LukasikE
TakkenFL
2009 STANDing strong, resistance proteins instigators of plant defence. Curr Opin Plant Biol 12 427 436
9. Burch-SmithTM
SchiffM
CaplanJL
TsaoJ
CzymmekK
2007 A novel role for the TIR domain in association with pathogen-derived elicitors. PLoS Biol 5 e68
10. WirthmuellerL
ZhangY
JonesJD
ParkerJE
2007 Nuclear accumulation of the Arabidopsis immune receptor RPS4 is necessary for triggering EDS1-dependent defense. Curr Biol 17 2023 2029
11. ShenQH
SaijoY
MauchS
BiskupC
BieriS
2007 Nuclear activity of MLA immune receptors links isolate-specific and basal disease-resistance responses. Science 315 1098 1103
12. CaplanJ
PadmanabhanM
Dinesh-KumarSP
2008 Plant NB-LRR immune receptors: from recognition to transcriptional reprogramming. Cell Host Microbe 3 126 135
13. CaplanJL
MamillapalliP
Burch-SmithTM
CzymmekK
Dinesh-KumarSP
2008 Chloroplastic protein NRIP1 mediates innate immune receptor recognition of a viral effector. Cell 132 449 462
14. GarciaAV
ParkerJE
2009 Heaven's Gate: nuclear accessibility and activities of plant immune regulators. Trends Plant Sci 14 479 487
15. BhattacharjeeS
LeeLY
OltmannsH
CaoH
Veena
2008 IMPa-4, an Arabidopsis importin alpha isoform, is preferentially involved in agrobacterium-mediated plant transformation. Plant Cell 20 2661 2680
16. ChengYT
GermainH
WiermerM
BiD
XuF
2009 Nuclear Pore Complex Component MOS7/Nup88 Is Required for Innate Immunity and Nuclear Accumulation of Defense Regulators in Arabidopsis. Plant Cell 21 2503 2516
17. PalmaK
ZhangY
LiX
2005 An importin alpha homolog, MOS6, plays an important role in plant innate immunity. Curr Biol 15 1129 1135
18. ZhangY
LiX
2005 A putative nucleoporin 96 Is required for both basal defense and constitutive resistance responses mediated by suppressor of npr1-1,constitutive 1. Plant Cell 17 1306 1316
19. FalkA
FeysBJ
FrostLN
JonesJD
DanielsMJ
1999 EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc Natl Acad Sci U S A 96 3292 3297
20. FeysBJ
MoisanLJ
NewmanMA
ParkerJE
2001 Direct interaction between the Arabidopsis disease resistance signaling proteins, EDS1 and PAD4. Embo J 20 5400 5411
21. LipkaV
DittgenJ
BednarekP
BhatR
WiermerM
2005 Pre - and postinvasion defenses both contribute to nonhost resistance in Arabidopsis. Science 310 1180 1183
22. FeysBJ
WiermerM
BhatRA
MoisanLJ
Medina-EscobarN
2005 Arabidopsis SENESCENCE-ASSOCIATED GENE101 stabilizes and signals within an ENHANCED DISEASE SUSCEPTIBILITY1 complex in plant innate immunity. Plant Cell 17 2601 2613
23. AartsN
MetzM
HolubE
StaskawiczBJ
DanielsMJ
1998 Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc Natl Acad Sci U S A 95 10306 10311
24. VenugopalSC
JeongRD
MandalMK
ZhuS
Chandra-ShekaraAC
2009 Enhanced disease susceptibility 1 and salicylic acid act redundantly to regulate resistance gene-mediated signaling. PLoS Genet 5 e1000545
25. LiX
ClarkeJD
ZhangY
DongX
2001 Activation of an EDS1-mediated R-gene pathway in the snc1 mutant leads to constitutive, NPR1-independent pathogen resistance. Mol Plant Microbe Interact 14 1131 1139
26. ZhangY
GoritschnigS
DongX
LiX
2003 A gain-of-function mutation in a plant disease resistance gene leads to constitutive activation of downstream signal transduction pathways in suppressor of npr1-1, constitutive 1. Plant Cell 15 2636 2646
27. GassmannW
HinschME
StaskawiczBJ
1999 The Arabidopsis RPS4 bacterial-resistance gene is a member of the TIR-NBS-LRR family of disease-resistance genes. Plant J 20 265 277
28. WangL
TsudaK
SatoM
CohenJD
KatagiriF
2009 Arabidopsis CaM binding protein CBP60g contributes to MAMP-induced SA accumulation and is involved in disease resistance against Pseudomonas syringae. PLoS Pathog 5 e1000301
29. OkrentRA
BrooksMD
WildermuthMC
2009 Arabidopsis GH3.12 (PBS3) conjugates amino acids to 4-substituted benzoates and is inhibited by salicylate. J Biol Chem 284 9742 9754
30. WildermuthMC
DewdneyJ
WuG
AusubelFM
2001 Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414 562 565
31. MishinaTE
ZeierJ
2006 The Arabidopsis flavin-dependent monooxygenase FMO1 is an essential component of biologically induced systemic acquired resistance. Plant Physiol 141 1666 1675
32. LairdJ
ArmengaudP
GiuntiniP
LavalV
MilnerJJ
2004 Inappropriate annotation of a key defence marker in Arabidopsis: will the real PR-1 please stand up? Planta 219 1089 1092
33. CloughSJ
FenglerKA
YuIC
LippokB
SmithRKJr
2000 The Arabidopsis dnd1 “defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel. Proc Natl Acad Sci U S A 97 9323 9328
34. GodiardL
SauviacL
ToriiKU
GrenonO
ManginB
2003 ERECTA, an LRR receptor-like kinase protein controlling development pleiotropically affects resistance to bacterial wilt. Plant J 36 353 365
35. LlorenteF
Alonso-BlancoC
Sanchez-RodriguezC
JordaL
MolinaA
2005 ERECTA receptor-like kinase and heterotrimeric G protein from Arabidopsis are required for resistance to the necrotrophic fungus Plectosphaerella cucumerina. Plant J 43 165 180
36. RothP
XylourgidisN
SabriN
UvA
FornerodM
2003 The Drosophila nucleoporin DNup88 localizes DNup214 and CRM1 on the nuclear envelope and attenuates NES-mediated nuclear export. J Cell Biol 163 701 706
37. HaasenD
KohlerC
NeuhausG
MerkleT
1999 Nuclear export of proteins in plants: AtXPO1 is the export receptor for leucine-rich nuclear export signals in Arabidopsis thaliana. Plant J 20 695 705
38. MeissnerT
KrauseE
VinkemeierU
2004 Ratjadone and leptomycin B block CRM1-dependent nuclear export by identical mechanisms. FEBS Lett 576 27 30
39. WenW
MeinkothJL
TsienRY
TaylorSS
1995 Identification of a signal for rapid export of proteins from the nucleus. Cell 82 463 473
40. van der BiezenEA
FreddieCT
KahnK
ParkerJE
JonesJD
2002 Arabidopsis RPP4 is a member of the RPP5 multigene family of TIR-NB-LRR genes and confers downy mildew resistance through multiple signalling components. Plant J 29 439 451
41. SimonR
IgenoMI
CouplandG
1996 Activation of floral meristem identity genes in Arabidopsis. Nature 384 59 62
42. DittmarKD
DemadyDR
StancatoLF
KrishnaP
PrattWB
1997 Folding of the glucocorticoid receptor by the heat shock protein (hsp) 90-based chaperone machinery. The role of p23 is to stabilize receptor.hsp90 heterocomplexes formed by hsp90.p60.hsp70. J Biol Chem 272 21213 21220
43. GalloisJL
WoodwardC
ReddyGV
SablowskiR
2002 Combined SHOOT MERISTEMLESS and WUSCHEL trigger ectopic organogenesis in Arabidopsis. Development 129 3207 3217
44. ParkerJE
ColemanMJ
SzaboV
FrostLN
SchmidtR
1997 The Arabidopsis downy mildew resistance gene RPP5 shares similarity to the toll and interleukin-1 receptors with N and L6. Plant Cell 9 879 894
45. AlcazarR
GarciaAV
ParkerJE
ReymondM
2009 Incremental steps toward incompatibility revealed by Arabidopsis epistatic interactions modulating salicylic acid pathway activation. Proc Natl Acad Sci U S A 106 334 339
46. TianD
TrawMB
ChenJQ
KreitmanM
BergelsonJ
2003 Fitness costs of R-gene-mediated resistance in Arabidopsis thaliana. Nature 423 74 77
47. ZhouN
TootleTL
TsuiF
KlessigDF
GlazebrookJ
1998 PAD4 functions upstream from salicylic acid to control defense responses in Arabidopsis. Plant Cell 10 1021 1030
48. NawrathC
MetrauxJP
1999 Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11 1393 1404
49. VlotAC
KlessigDF
ParkSW
2008 Systemic acquired resistance: the elusive signal(s). Curr Opin Plant Biol 11 436 442
50. RusterucciC
AvivDH
HoltBF3rd
DanglJL
ParkerJE
2001 The disease resistance signaling components EDS1 and PAD4 are essential regulators of the cell death pathway controlled by LSD1 in Arabidopsis. Plant Cell 13 2211 2224
51. TrumanW
BennettMH
KubigsteltigI
TurnbullC
GrantM
2007 Arabidopsis systemic immunity uses conserved defense signaling pathways and is mediated by jasmonates. Proc Natl Acad Sci U S A 104 1075 1080
52. KeurentjesJJ
FuJ
TerpstraIR
GarciaJM
van den AckervekenG
2007 Regulatory network construction in Arabidopsis by using genome-wide gene expression quantitative trait loci. Proc Natl Acad Sci U S A 104 1708 1713
53. HuangTT
KudoN
YoshidaM
MiyamotoS
2000 A nuclear export signal in the N-terminal regulatory domain of IkappaBalpha controls cytoplasmic localization of inactive NF-kappaB/IkappaBalpha complexes. Proc Natl Acad Sci U S A 97 1014 1019
54. LipfordJR
SmithGT
ChiY
DeshaiesRJ
2005 A putative stimulatory role for activator turnover in gene expression. Nature 438 113 116
55. MurataniM
KungC
ShokatKM
TanseyWP
2005 The F box protein Dsg1/Mdm30 is a transcriptional coactivator that stimulates Gal4 turnover and cotranscriptional mRNA processing. Cell 120 887 899
56. SpoelSH
MouZ
TadaY
SpiveyNW
GenschikP
2009 Proteasome-mediated turnover of the transcription coactivator NPR1 plays dual roles in regulating plant immunity. Cell 137 860 872
57. StrausMR
RietzS
Ver Loren van ThemaatE
BartschM
ParkerJE
2010 Salicylic acid antagonism of EDS1-driven cell death is important for Arabidopsis immune and oxidative stress responses. Plant J In press
58. MateoA
MuhlenbockP
RusterucciC
ChangCC
MiszalskiZ
2004 LESION SIMULATING DISEASE 1 is required for acclimation to conditions that promote excess excitation energy. Plant Physiol 136 2818 2830
59. MuhlenbockP
Szechynska-HebdaM
PlaszczycaM
BaudoM
MullineauxPM
2008 Chloroplast signaling and LESION SIMULATING DISEASE1 regulate crosstalk between light acclimation and immunity in Arabidopsis. Plant Cell 20 2339 2356
60. TorneroP
DanglJL
2001 A high-throughput method for quantifying growth of phytopathogenic bacteria in Arabidopsis thaliana. Plant J 28 475 481
61. WitteCP
NoelLD
GielbertJ
ParkerJE
RomeisT
2004 Rapid one-step protein purification from plant material using the eight-amino acid StrepII epitope. Plant Mol Biol 55 135 147
62. CloughSJ
BentAF
1998 Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735 743
63. NoelLD
CagnaG
StuttmannJ
WirthmullerL
BetsuyakuS
2007 Interaction between SGT1 and cytosolic/nuclear HSC70 chaperones regulates Arabidopsis immune responses. Plant Cell 19 4061 4076
64. AichingerE
VillarCB
FarronaS
ReyesJC
HennigL
2009 CHD3 proteins and polycomb group proteins antagonistically determine cell identity in Arabidopsis. PLoS Genet 5 e1000605
65. AsaiT
StoneJM
HeardJE
KovtunY
YorgeyP
2000 Fumonisin B1-induced cell death in arabidopsis protoplasts requires jasmonate-, ethylene-, and salicylate-dependent signaling pathways. Plant Cell 12 1823 1836
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System inČlánek A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 7- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- The Mouse Resistance Protein Irgm1 (LRG-47): A Regulator or an Effector of Pathogen Defense?
- Leprosy and the Adaptation of Human Toll-Like Receptor 1
- Intergenomic Arms Races: Detection of a Nuclear Rescue Gene of Male-Killing in a Ladybird
- The Role of Chemokines during Viral Infection of the CNS
- Bottlenecks and the Maintenance of Minor Genotypes during the Life Cycle of
- DNA Damage Triggers Genetic Exchange in
- The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System in
- Uropathogenic Modulates Immune Responses and Its Curli Fimbriae Interact with the Antimicrobial Peptide LL-37
- Biogenesis of the Inner Membrane Complex Is Dependent on Vesicular Transport by the Alveolate Specific GTPase Rab11B
- A Spatio-Temporal Analysis of Matrix Protein and Nucleocapsid Trafficking during Vesicular Stomatitis Virus Uncoating
- Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion
- Quasispecies Theory and the Behavior of RNA Viruses
- Bid Regulates the Pathogenesis of Neurotropic Reovirus
- Distinct Roles for Dectin-1 and TLR4 in the Pathogenesis of Keratitis
- Unexpected Inheritance: Multiple Integrations of Ancient Bornavirus and Ebolavirus/Marburgvirus Sequences in Vertebrate Genomes
- Balanced Nuclear and Cytoplasmic Activities of EDS1 Are Required for a Complete Plant Innate Immune Response
- Adaptation of Hepatitis C Virus to Mouse CD81 Permits Infection of Mouse Cells in the Absence of Human Entry Factors
- An Outer Membrane Receptor of Involved in Zinc Acquisition with Vaccine Potential
- Inositol Hexakisphosphate-Induced Autoprocessing of Large Bacterial Protein Toxins
- Plus- and Minus-End Directed Microtubule Motors Bind Simultaneously to Herpes Simplex Virus Capsids Using Different Inner Tegument Structures
- Distinct Pathogenesis and Host Responses during Infection of by and
- Can Bacteria Evolve Resistance to Quorum Sensing Disruption?
- RNA Virus Replication Complexes
- PPARγ and LXR Signaling Inhibit Dendritic Cell-Mediated HIV-1 Capture and -Infection
- The Virulence Protein SopD2 Regulates Membrane Dynamics of -Containing Vacuoles
- Genome-Wide Mutagenesis Reveals That ORF7 Is a Novel VZV Skin-Tropic Factor
- Adaptive Evolution of Includes Retroviral Insertion and Positive Selection at Two Clusters of Residues Flanking the Substrate Groove
- A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
- Transduction of Human T Cells with a Novel T-Cell Receptor Confers Anti-HCV Reactivity
- Identification of GBV-D, a Novel GB-like Flavivirus from Old World Frugivorous Bats () in Bangladesh
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- HIV gp41 Engages gC1qR on CD4+ T Cells to Induce the Expression of an NK Ligand through the PIP3/H2O2 Pathway
- Oxidation of Helix-3 Methionines Precedes the Formation of PK Resistant PrP
- Protection from the 2009 H1N1 Pandemic Influenza by an Antibody from Combinatorial Survivor-Based Libraries
- Murine Gamma-Herpesvirus 68 Hijacks MAVS and IKKβ to Initiate Lytic Replication
- Viral Protein Inhibits RISC Activity by Argonaute Binding through Conserved WG/GW Motifs
- TOPO3α Influences Antigenic Variation by Monitoring Expression-Site-Associated Switching in
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- The Meningococcal Vaccine Candidate Neisserial Surface Protein A (NspA) Binds to Factor H and Enhances Meningococcal Resistance to Complement
- Network Modeling Reveals Prevalent Negative Regulatory Relationships between Signaling Sectors in Arabidopsis Immune Signaling
- Endothelial Galectin-1 Binds to Specific Glycans on Nipah Virus Fusion Protein and Inhibits Maturation, Mobility, and Function to Block Syncytia Formation
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
- Integration Preferences of Wildtype AAV-2 for Consensus Rep-Binding Sites at Numerous Loci in the Human Genome
- Epigenetic Analysis of KSHV Latent and Lytic Genomes
- Vaccinia Virus–Encoded Ribonucleotide Reductase Subunits Are Differentially Required for Replication and Pathogenesis
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- RNA Virus Replication Complexes
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



