-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion
Viral infection leads to induction of pattern-recognition receptor signaling, which leads to interferon regulatory factor (IRF) activation and ultimately interferon (IFN) production. To establish infection, many viruses have strategies to evade the innate immunity. For the hepatitis B virus (HBV), which causes chronic infection in the liver, the evasion strategy remains uncertain. We now show that HBV polymerase (Pol) blocks IRF signaling, indicating that HBV Pol is the viral molecule that effectively counteracts host innate immune response. In particular, HBV Pol inhibits TANK-binding kinase 1 (TBK1)/IκB kinase-ε (IKKε), the effector kinases of IRF signaling. Intriguingly, HBV Pol inhibits TBK1/IKKε activity by disrupting the interaction between IKKε and DDX3 DEAD box RNA helicase, which was recently shown to augment TBK1/IKKε activity. This unexpected role of HBV Pol may explain how HBV evades innate immune response in the early phase of the infection. A therapeutic implication of this work is that a strategy to interfere with the HBV Pol-DDX3 interaction might lead to the resolution of life-long persistent infection.
Published in the journal: Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion. PLoS Pathog 6(7): e32767. doi:10.1371/journal.ppat.1000986
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000986Summary
Viral infection leads to induction of pattern-recognition receptor signaling, which leads to interferon regulatory factor (IRF) activation and ultimately interferon (IFN) production. To establish infection, many viruses have strategies to evade the innate immunity. For the hepatitis B virus (HBV), which causes chronic infection in the liver, the evasion strategy remains uncertain. We now show that HBV polymerase (Pol) blocks IRF signaling, indicating that HBV Pol is the viral molecule that effectively counteracts host innate immune response. In particular, HBV Pol inhibits TANK-binding kinase 1 (TBK1)/IκB kinase-ε (IKKε), the effector kinases of IRF signaling. Intriguingly, HBV Pol inhibits TBK1/IKKε activity by disrupting the interaction between IKKε and DDX3 DEAD box RNA helicase, which was recently shown to augment TBK1/IKKε activity. This unexpected role of HBV Pol may explain how HBV evades innate immune response in the early phase of the infection. A therapeutic implication of this work is that a strategy to interfere with the HBV Pol-DDX3 interaction might lead to the resolution of life-long persistent infection.
Introduction
Hepatitis B virus (HBV) is the prototypic member of the hepadnavirus family and a major cause of liver diseases. An estimated 400 million people are persistently infected with HBV worldwide. A significant subset of these HBV carriers progresses to severe liver disease, such as hepatocellular carcinoma, which may cause up to one million deaths per year. Interferon and nucleoside analogs such as lamivudine and adefovir are used to treat chronic hepatitis B patients but have limited utility due to the adverse effect and the emergence of drug-resistant variants, respectively [1]. Thus, there is a clear medical need for new therapeutic strategies.
Viral infection leads to the initiation of antiviral innate immune responses resulting in the expression of type I interferons (IFNs), IFNα and IFNβ, and pro-inflammatory cytokines [2]. Recently, the cellular mechanisms used to detect viruses and elicit production of IFNs and pro-inflammatory cytokines have been described in detail. It is now well-established that viruses, similar to bacteria and fungi, are initially recognized by host pattern-recognition receptors (PRRs) [2], [3]. Viral nucleic acids (both RNA and DNA) are the most important pathogen-associated molecular patterns (PAMPs) recognized by PRRs [3]. Two families of PRRs have been defined. The first is a subfamily of Toll-like receptors (TLRs) that include TLR3, TLR7, TLR8, and TLR9, which are mainly expressed in the endosomes of some cell types, especially plasmacytoid dendritic cells. Recognition by TLRs of viral PAMPs initiates TLR-mediated signaling pathways that culminate in the activation of transcription factors NFκB, IRF3, and IRF7. Specifically, TLRs recruit signaling adaptors, including TIR-domain-containing adaptor protein inducing IFNβ (TRIF). This activates TANK-binding kinase 1 (TBK1)/IκB kinase-ε (IKKε) to phosphorylate and activate the transcription factors IFN-regulatory factors (IRF) 3 and 7 [2], [4]. The second family of PRRs are comprised of the retinoic-acid inducible gene I (RIG-I)-like receptors (RLRs), including RIG-I and melanoma differentiation-associated gene 5 (MDA-5) [3]. Similar to TLRs, the recognition of viral nucleic acids by RLRs leads to a cascade of signaling events that result in activation of NF-κB, IRF3, and IRF7. Specifically, RLRs recruit the signaling adaptor protein IFNβ-promoter stimulator 1(IPS-1, also known as MAVS, VISA, or Cardif), activating the downstream TBK1/IKKε kinases, which then phosphorylate and activate IRF3 and IRF7 [2]. The capacity of both signaling pathways to restrict viral replication is consistent with their downstream convergence at the TBK1/IKKε kinases responsible for activation of IRF-3.
As stated above, viral infection leads to activation of cellular signaling such as IRF signaling, which culminates in IFN production. Infection by HBV appeared to be an exception. In an acute HBV-infected chimpanzee model, Chisari and colleagues have reported that HBV fails to induce transcription of any cellular genes that relate to the entry and expansion of the virus, implicating the lack of innate immune response upon HBV infection [5], [6]. By contrast to the earlier report, evidence was accumulating, which indicated that the innate immune system is, in fact, able to sense HBV infection. An early induction of innate immune response, as shown by the early development of natural killer cell and natural killer T cells response, was observed in two patients with acute viral infection [7]. More recently, by using a HepaRG cells, a permissive hepatocyte cell line for HBV infection, Zoulim and colleagues found that HBV infection elicits a strong innate antiviral response that leads to a significant reduction of HBV DNA synthesis [8]. Taken together, it is conceivable that one of viral proteins could impair innate immune response early in infection.
Two recent reports have shown that DDX3 DEAD box RNA helicase, which is known to be involved in diverse steps of RNA metabolism, could augment IRF signaling via its interaction with IKKε or TBK [9], [10]. In other words, DDX3 augments TBK/IKKε activity, which phosphorylates IRF3 and IRF7. Interestingly, our group has shown that DDX3 binds to HBV Pol (P protein) and inhibits viral reverse transcription [11]. Since DDX3 is essential for augmentation of IRF signaling, we postulated that HBV Pol impairs antiviral innate immune responses by inhibiting IRF signaling via its interaction with DDX3. Consistent with the notion, our results demonstrate that IRF signaling is significantly inhibited by HBV Pol, a finding that defines HBV Pol as a viral protein that counteracts antiviral pattern recognition receptor signaling.
Results
HBV Pol inhibits IFN promoter activity
To determine if HBV proteins could impair IRF signaling and counteract host innate immune responses to HBV infection, we tested the ability of HBV proteins to inhibit interferon (IFN) β promoter activity. Synthetic dsRNA mimic polyinosine-polycytidylic acid (poly I∶C) is recognized by TLR3 in endosomes when added to medium [12]. To induce IRF signaling, human hepatoma HepG2 cells, which support viral genome replication, were transfected with one of three viral protein expression constructs — core, polymerase (Pol), or HBx —, a TLR3 expression construct , and a reporter construct expressing luciferase under control of the IFNβ promoter (Fig. 1A). Cells were complemented with the TLR3 expression construct because HepG2 cells are deficient in TLR3 expression [13]. To ensure that the physiological levels of viral proteins were attained, the amount of the three viral protein expression constructs for transfection were determined by cotransfection with the corresponding gene-null HBV replicon: for HBV Pol (see Fig. S2), for core protein (data not shown), and for HBx [14]. Two days following transfection, cells were treated with poly I∶C. Eight hours post-poly I∶C treatment, cells were harvested and luciferase activity was measured. As anticipated, the luciferase activity was significantly induced when cells were complemented with TLR3, compared to that induced by poly I∶C only (Fig. 1A). The data indicated that HepG2 cells were able to recognize extracellular dsRNA and induce IFN production when complemented with TLR3. More importantly, the data showed that HBV Pol, but not other viral proteins including HBx, significantly suppressed IFN promoter activity (Fig. 1A). The inhibition of IFN promoter activity by HBV Pol was somewhat unexpected, since current literature suggests that almost all of its known function is confined to the functions – encapsidation and viral reverse transcription – occurring inside of nucleocapsids [15]. Further, reverse transcriptase activity of HBV Pol appeared not to be involved in the inhibition of IFN promoter activity, as the YMHD mutant of HBV Pol, reverse transcriptase activity deficient mutant, remained to inhibit IFN promoter activity (Fig. 1A). In addition, we found that three viral envelope glycoproteins – L-HBsAg, and M-HBsAg, and S-HBsAg – had no impact on IFN promoter activity (Fig. 1B), indicating that HBV Pol is the only viral protein that has an inhibitory effect on IFNβ production.
Fig. 1. HBV Pol inhibits dsRNA-induced IFNβ promoter activation. 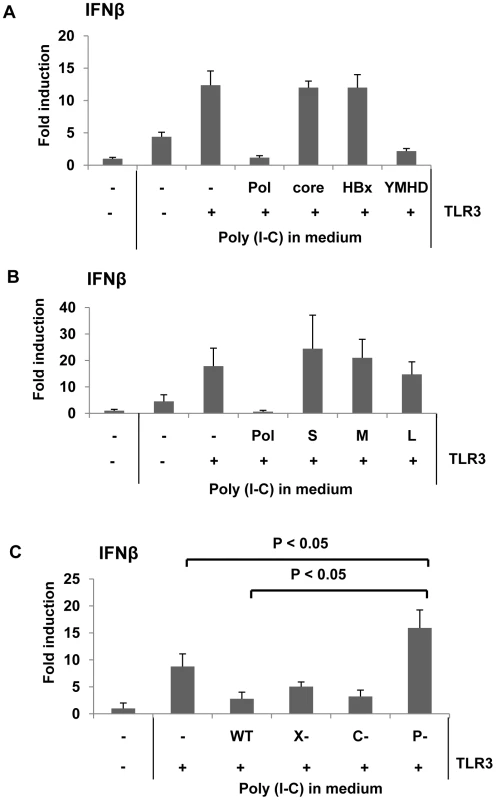
(A) HepG2 cells in 6-well plate were transfected with HBV Pol, core, or HBx, and the YMHD mutant of HBV Pol expression constructs together with an IFNβ luciferase reporter and TLR3 expression constructs. Cells were stimulated with 25 µg/mL poly I∶C directly added to culture medium for 8 h. Data are expressed as the mean fold induction ± s.d. relative to mock-treated cells. The results are representative of at least four independent experiments. (B) An experiment was performed as shown in panel (A), with an exception of transfecting three viral surface antigen expression constructs: L-HBsAg (L), and M-HBsAg (M), and S-HBsAg (S). (C) HepG2 cells were transfected with empty vector, WT, X-null, C-null, and P-null HBV replicon together with the IFNβ reporter construct. Student's t test was used for statistical analysis, and a P value of <0.05 was considered statistically significant. Poly I∶C treatment was done as in panel (A). (See also Fig. S1). To substantiate the above results in the context of viral life cycle, the impact of viral proteins on IFNβ production was examined by using a viral replicon, which could lead to viral genome replication when transfected [14]. Three mutants were made in which one viral gene was inactivated per construct: (i) P (Pol)-null, (ii) C (core)-null, and (iii) X-null (Fig. S1). An increase in IFNβ production by one of the HBV mutant constructs would point out that particular gene in the inhibition of IFN production. Luciferase activity was monitored following transfection as described above. The data revealed that only cells transfected with the HBV P-null replicon construct induced a higher level of IFN production, whereas the other three replicons, including the wild-type, induced modest level of IFN production (Fig. 1C). These results suggested that HBV Pol derived from the HBV replicon – wild-type or X-null, or C-null replicon – decreased IFN production, implicating the physiological relevance of the findings in the viral life cycle. Intriguingly, IFN promoter activity induced by HBV P-null replicon was significantly higher than what was achieved by poly I∶C (Fig. 1C). The implication is that some yet-to-be known viral PAMPs derived from HBV P-null replicon could contribute to the augmented IFN promoter activity (see Discussion).
HBV Pol inhibits IFNβ promoter triggered by RIG-I/MDA5 as well as TLR3 receptor signaling
To examine whether HBV Pol could suppress diverse PAMP-mediated signaling, poly I∶C was given to cells by two distinct routes: (i) addition to medium, and (ii) transfection via lipofectin. The poly I∶C is recognized by TLR3 in endosomes by adding it to the cell medium, whereas it is recognized by MDA5 when it is delivered to the cytoplasm by transfection via lipofectin [16], [17]. Cells were transfected with incremental dose of the HBV Pol and TLR3 expression constructs along with the reporter construct. Following treatment with poly I∶C in the medium, the reporter assay result showed a dose-related decrease of IFN promoter activity by HBV Pol expression, corroborating the above conclusions (Fig. 2A). Likewise, when poly I∶C was given by lipofectin transfection, IFN promoter activity was similarly decreased by HBV Pol (Fig. 2B).
Fig. 2. HBV Pol inhibits both dsRNA- and Sendai virus-induced IFNβ promoter activation. 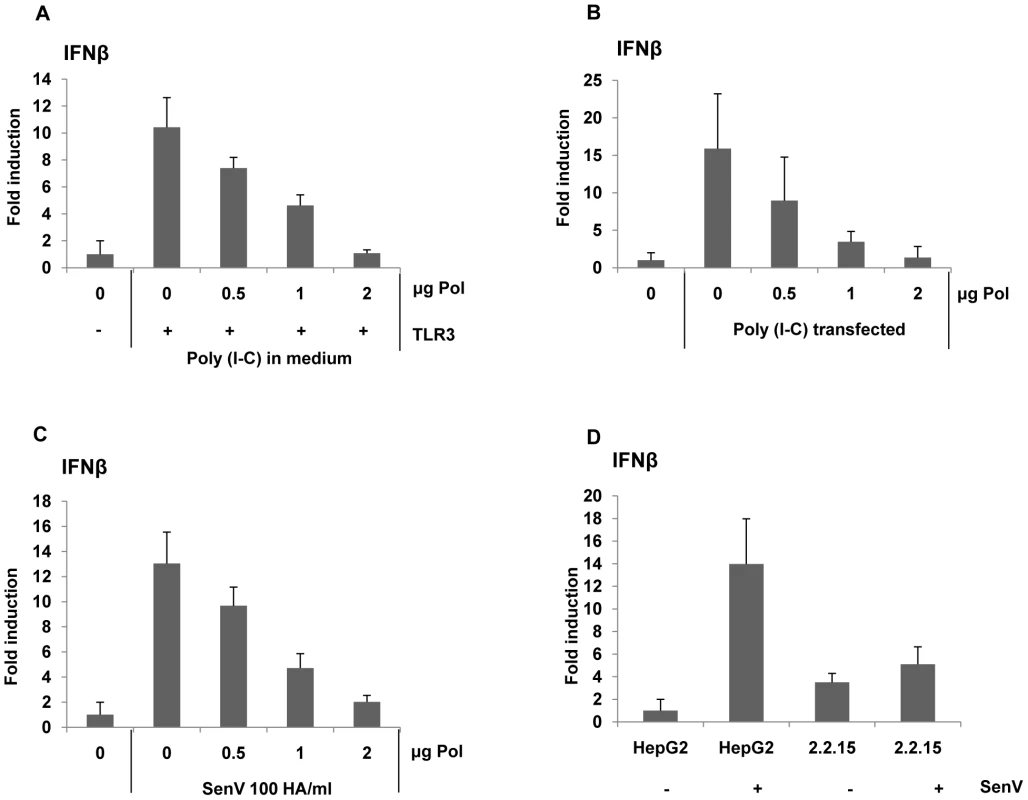
HepG2 cells were transfected with increasing doses of the HBV Pol expression construct, along with the IFNβ luciferase reporter construct and TLR3 expression construct. Cells were stimulated with 25 µg/mL poly I∶C directly added to the medium for 8 h (A), with 25 µg/mL poly I∶C transfected with lipofectin for 8 h (B), or with Sendai virus (SenV) 100 HA/mL for 6 h (C). Data are expressed as the mean fold induction ± s.d. relative to control levels. The results are representative of three independent experiments, each performed in triplicate. (D) Cells (HepG2 or HepG2.2.15) were transfected with an IFNβ luciferase reporter and then, infected with Sendai virus (SenV) 100 HA/mL for 6 h before harvest. Next, innate immune response was triggered by Sendai virus (SenV), a potent stimulus of the RIG-I pathway [17], [18]. Cells were transfected with the HBV Pol expression construct and the IFN-luciferase construct. Two days after transfection, cells were treated with 100 HA U/mL of SenV for 6 h before harvest. The data indicated that HBV Pol diminished IFN promoter activity in a dose-related manner (Fig. 2C). Overall, the data presented suggest that HBV Pol suppresses RIG-I mediated IFN production as well as TLR3-mediated IFN production.
To substantiate the above findings, we wanted to eliminate a possibility that the reduction of the IFN promoter activities by HBV Pol is ascribed to the over-expression of HBV Pol. To assess whether the HBV Pol we expressed ranges the physiological level, we sought to show that the amount of viral DNAs synthesized via complementation by the HBV Pol in the HBV P-null replicon transfected cells is comparable to that of WT HBV replicon transfected cells. To this end, HepG2 cells were transfected with the same four increasing amount of the HBV Pol expression construct as above to complement the P-null construct (i.e., P-) for the viral genome replication. Viral DNAs extracted from cytoplasmic capsids were measured by Southern blot analysis (Fig. S2). The data showed that the amount of viral DNA synthesized by complementation was less than that of WT HBV replicon (Fig. S2, lane 1 versus lanes 3 to 5), suggesting that the HBV Pol expressed in the Fig. 2A to Fig. 2C was not exceeding physiological level. In addition, by using HepG2.2.15 cell line that stably expresses viral proteins and support HBV replication [19], we consistently found that IFN promoter activity induced upon SenV (Sendai virus) infection was pronouncedly diminished in HepG2.2.15 cells (Fig. 2D), validating the impact of HBV Pol in a more physiological setting.
HBV Pol abrogates nuclear translocation and phosphorylation of IRF3
Phosphorylation and nuclear translocation of IRF3 represent hallmarks of antiviral innate immunity. To examine whether HBV Pol inhibits the phosphorylation of IRF3, cells were transfected with TLR3 and HBV Pol construct as indicated. Eight hours before harvest, cells were then treated with poly I∶C. Endogenous IRF3 and its phosphorylated counterparts were detected by Western blot analysis with anti-IRF3 and anti-phosphorylated IRF3 (Ser396) antibodies, respectively (Fig. 3A). As anticipated, Western blot analysis indicated that the higher molecular weight bands of endogenous IRF3, which represent phosphorylated IRF3, appeared when cells were treated with poly I∶C (Fig. 3A, lane 2). However, the phosphorylated IRF3 was undetectable following HBV Pol expression (Fig. 3A, lane 3), suggesting that HBV Pol inhibits phosphorylation of IRF3. (see below for the explanation for lane 4).
Fig. 3. HBV Pol inhibits both phosphorylation and nuclear translocation of IRF3. 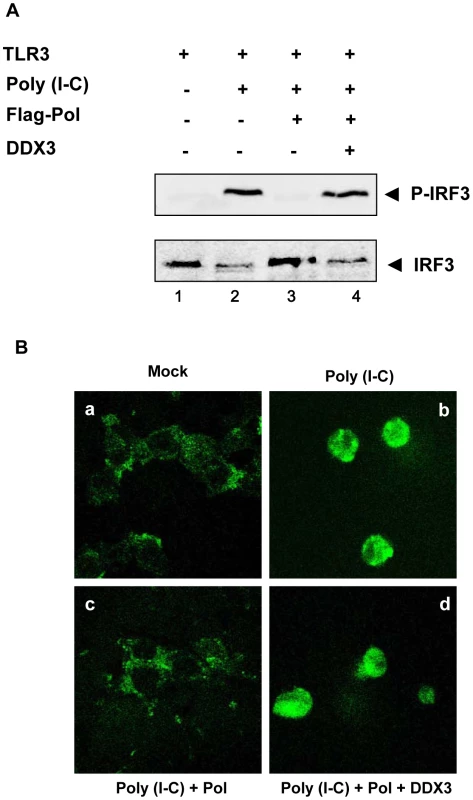
(A) HEK293 cells were transfected with TLR3, HBV Pol, and DDX3 expression construct as indicated. Two days posttransfection, cells were stimulated with poly I∶C for 8 h. Western blot analysis was performed as described in Materials and Methods. (B) HEK293 cells were transfected with GFP-IRF3, TLR3, HBV Pol, and DDX3 expression constructs, as labeled either above or below each panel by a, b, c, and d. Two days posttransfection, cells were treated with poly I∶C for 8 h. Cells were examined by confocal microscopy. Nuclear translocation of IRF3 was examined by fluorescence microscopy. Cells were transfected with the IRF3-GFP fusion protein construct to monitor IRF3 nuclear localization (Fig. 3B). Cells were also transfected with HBV Pol and TLR3 constructs and then treated with poly I∶C. As previously demonstrated [20], IRF3-GFP predominantly found in the cytoplasm was induced to undergo nuclear localization only when cells were treated with poly I∶C (Fig. 3B, panel a versus panel b). By contrast, IRF3-GFP remained localized in cytoplasm when cells were transfected by HBV Pol and treated with poly I∶C (Fig. 3B, panel c), suggesting that HBV Pol prevents the nuclear translocation of IRF3-GFP. Overall, these data are consistent with the conclusion that HBV Pol inhibits the IRF signaling. (see below for the explanation for panel d).
HBV Pol suppressed both TRIF and RIG-I triggered IRF signaling
To further elucidate the specific mechanism by which viral HBV Pol interferes with IRF activation, the impact of HBV Pol on the signaling pathway leading to IRF activation was investigated. To trigger RIG-I mediated IRF3 signaling, RIG-I was over-expressed. On the other hand, TRIF is an adaptor for the TLR3 receptor and mimics TLR3 signaling when over-expressed. To trigger TLR-mediated IRF3-signaling, TRIF over-expression was employed. Cells were transfected with the IRF3 reporter construct and an incremental dose of HBV Pol expression construct, along with either RIG-I or TRIF (Fig. 4A and Fig. 4B). The data indicated that the luciferase activity was decreased by HBV Pol in a dose-dependent manner (Fig. 4A and Fig. 4B). Thus, we concluded that HBV Pol inhibited both TLR-mediated and RIG-I-mediated IRF3 signaling.
Fig. 4. HBV Pol inhibits both RIG-I- and TRIF-induced IRF3 activation. 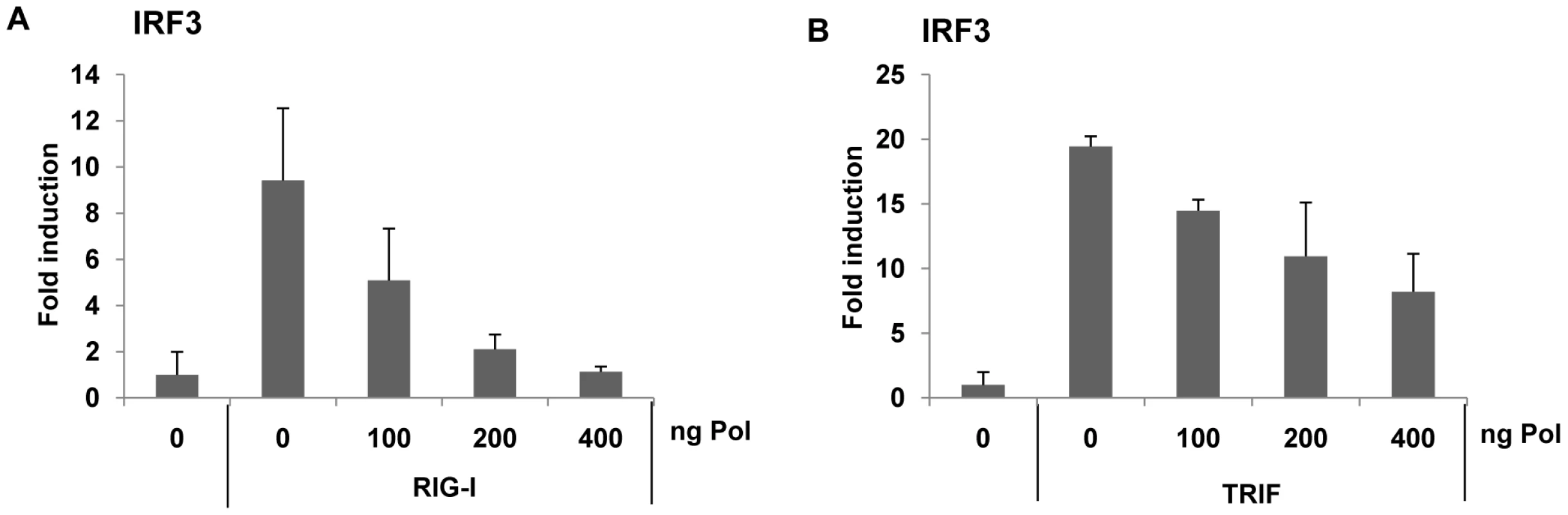
HepG2 cells were cotransfected with increasing doses of the HBV Pol expression construct to determine the effect of HBV Pol on IRF3 signaling. To monitor IRF3 activation, cells were transfected with IRF3-GAL4 expression plasmid together with the GAL4-dependent pFR luciferase reporter construct. Note that 5-fold less amount of HBV Pol construct was used, because 24-well plate, instead of 6-well, was used. To induce IRF3 signaling, RIG-I (A) or TRIF (B) expression constructs were transfected. Data are expressed as the mean fold induction ± s .d. relative to control levels. The results are representative of at least two independent experiments, each performed in triplicate. HBV Pol suppressed TBK1/IKKε triggered IRF 3/7 signaling
The TBK1/IKKε complex represents the effecter protein kinase of IRF signaling, phosphorylating IRF3/7, when activated by appropriate recruitment of IPS/MAVS or TRIF (Fig. 5A). The results shown above indicated that both TLR3 - or RIG-I-mediated IRF signaling was inhibited by HBV Pol, and the phosphorylation of IRF3/7 was blocked by HBV Pol. Thus, it is conceivable that HBV Pol inhibits TBK1/IKKε activity. To gain further insight into the mechanism by which HBV Pol interferes with TLR3-mediated and RIG-I-mediated IRF signaling, we determined if TBK1 or IKKε triggered IRF signaling could also be blocked by HBV Pol. Cells were transfected with either TBK1 or IKKε, and IRF3 reporter construct along with incremental doses of the HBV Pol construct. Results indicated that IRF3 signaling was triggered either by TBK1 or IKKε over-expression and was blocked by HBV Pol in a dose-dependent manner (Fig. 5B and 5C). Essentially identical data were obtained with an IRF7 reporter assay performed in parallel (data not shown). Thus, the data suggested that HBV Pol exerts its inhibitory effect on TBK1/IKKε or another downstream point in this pathway. An ISRE reporter assay was then carried out, which indicated that HBV Pol inhibited IKKε-triggered ISRE activation, but not IRF3-triggered ISRE activation (Fig. 5D and 5E). These results pointed to TBK1/IKKε as the molecular target of HBV Pol for the inhibition of IRF signaling.
Fig. 5. HBV Pol blocks IRF activation by inhibiting TBK1/IKKε activity. 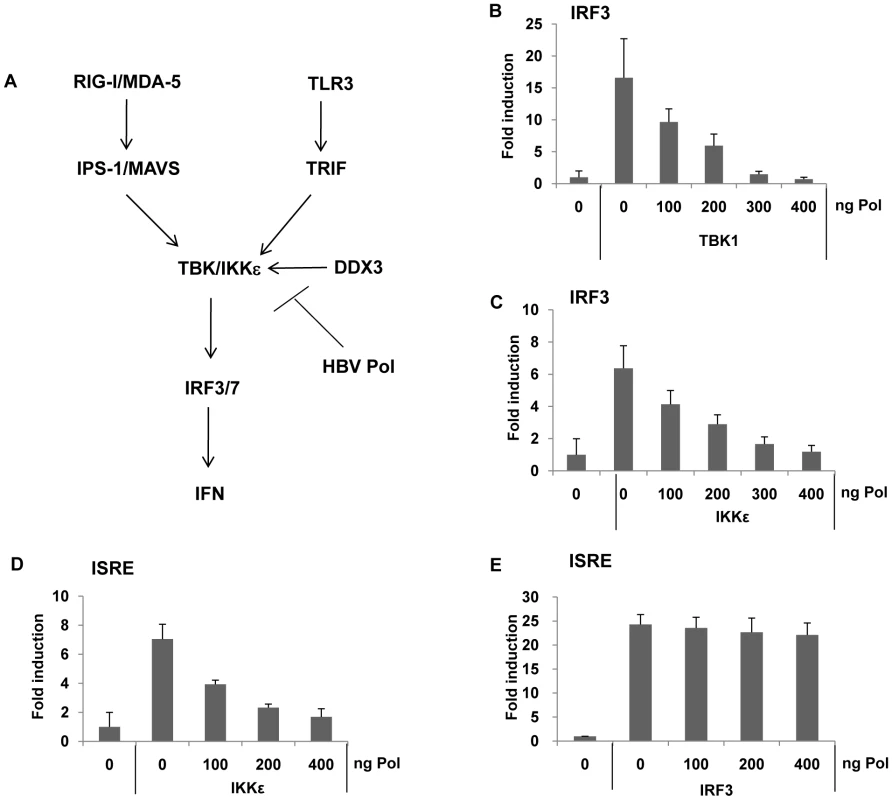
(A) Schematic of IRF signaling pathway leading to IFN production. TBK1 serves as a central effector kinase, but TBK1 function is also shared by the highly related IKKε kinase in some cells. DDX3 is described in an experiment shown in Fig. 6 (see text). (B and C) HepG2 cells were cotransfected with an incremental amount of HBV Pol expression construct to determine the effect of HBV Pol on IRF3 signaling. To induce IRF3 signaling, or TBK1 (B) or IKKε (C) expression constructs were transfected. IRF3 activation was monitored as shown in Fig. 4. (D and E) Cells are transfected as in (B and C), respectively, except that ISRE-luciferase reporter construct was transfected, instead of the IRF reporter construct. HBV Pol inhibits TBK1/IKKε activity by disrupting IKKε-DDX3 interaction
Next, we examined whether HBV Pol inhibits TBK1/IKKε activity directly or indirectly. Recently, two independent groups demonstrated that DDX3 enhanced TBK1/IKKε activity via its interaction with TBK1 or IKKε [9], [10]. Additionally, we have shown that DDX3 binds to HBV Pol [11]. Thus, we hypothesized that HBV Pol would inhibit TBK1/IKKε activity via interaction with DDX3. One prediction of the hypothesis is that over-expression of DDX3 would restore IRF signaling that has been inhibited by HBV Pol. Previous studies were limited to HEK293 and RAW264.7 macrophages; therefore, we confirmed the importance of DDX3 for the activation of IRF signaling in HepG2 cells following downregulation of DDX3 (Fig. S3A and Fig. S3B). To determine if DDX3 could restore IRF3 signaling suppressed by HBV Pol, cells were transfected by either TBK1 or IKKε constructs along with a maximal level of HBV Pol to obtain the highest level of IRF signaling inhibition. Cells were also cotransfected with increasing doses of the DDX3 construct to determine if DDX3 could restore the diminished IRF signaling. The luciferase data revealed that the ectopic expression of DDX3 rescued the IRF signaling in a dose-dependent manner (Fig. 6A and 6B), indicating that DDX3 antagonizes the inhibitory effect of HBV Pol on IRF signaling. Consistently, ectopic expression of DDX3 also rescued the phosphorylation and nuclear translocation of IRF3 (Fig. 3A, lane 4; Fig. 3B, panel d).
Fig. 6. HBV Pol inhibits TBK1/IKKε via an interaction with DDX3. 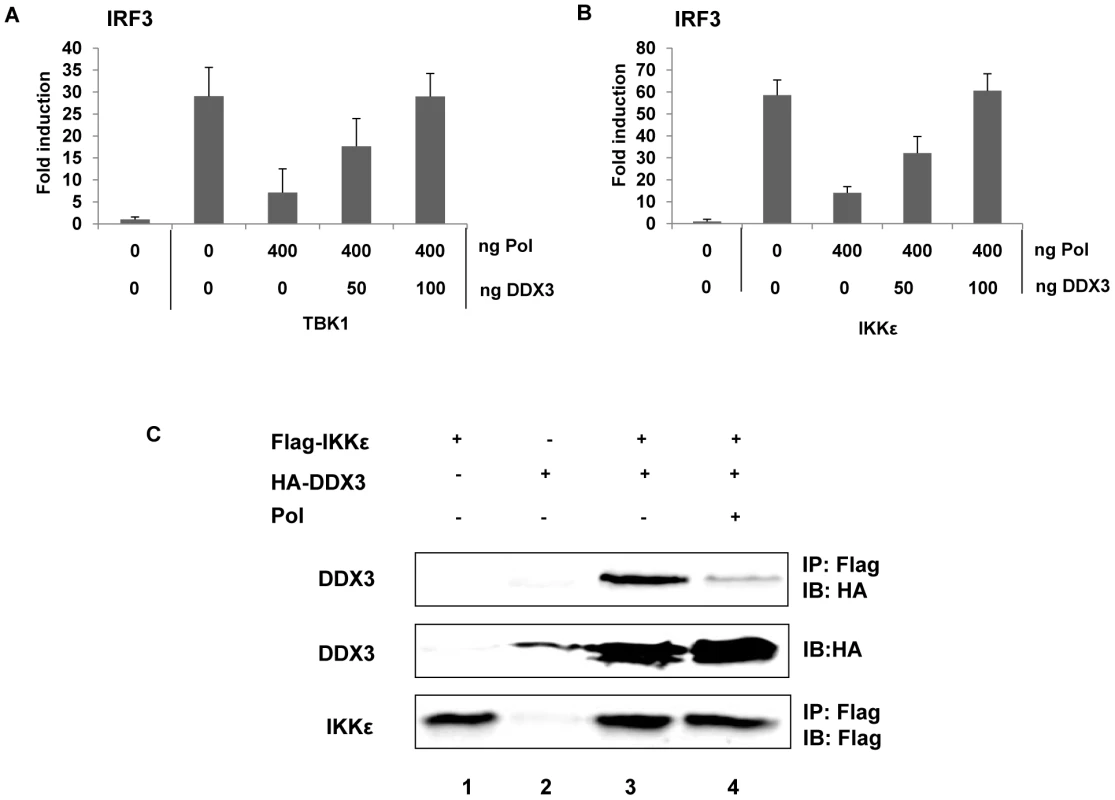
(A and B) DDX3 rescued IRF signaling, which was inhibited by HBV Pol. HepG2 cells were transfected with indicated amounts of HBV Pol and DDX3 expression constructs. To induce IRF3 signaling, TBK1 (A) or IKKε (B) expression constructs (100 ng) were transfected. IRF3 activation was monitored as shown in Fig. 4. Data are expressed as the mean fold induction ± s.d. relative to control levels. The results are representative of at least two independent experiments each performed in triplicate. (C) HBV Pol disrupted the interaction between DDX3 and IKKε. HEK 293 cells were transfected with Flag-IKKε, HA-DDX3, and HBV Pol expression constructs. Cell lysates were analyzed by IP with the indicated antibodies to assess the interaction between DDX3 and IKKε. For the detection of the Flag-tagged IKKε, mouse anti-FLAG M2 antibody (Sigma, 1∶5,000) was used; anti-HA antibody (Amersham) was used to detect DDX3. The results are representative of three independent experiments. Given the functional interaction between HBV Pol and DDX3 [11], it is possible that HBV Pol disrupts the IKKε-DDX3 interaction. To assess this possibility, a co-immunoprecipitation (co-IP) following transfection of Flag-IKKε, HA-DDX3, and HBV Pol expression constructs was performed (Fig. 6C). Similar to previous reports [9], an interaction between IKKε and DDX3 was observed (Fig. 6C, lane 3). Importantly, when the HBV Pol construct was cotransfected, the IKKε-DDX3 interaction was significantly diminished, consistent with an interpretation that HBV Pol disrupts the IKKε-DDX3 interaction (Fig. 6C, lane 4). It was also noted that DDX3 level was enhanced and a higher molecular weight form of DDX3 was evident when IKKε was ectopically expressed, which might represent a phosphorylated form (Fig. 6C, lanes 3 and 4); intriguingly, the similar changes of DDX3 were also observed in the previous report [9]. It is conceivable that the phosphorylation of DDX3 by IKKε stabilizes DDX3. Based on the antagonistic activity of DDX3 on the inhibitory effect of HBV Pol on IRF signaling and the disruption of IKKε-DDX3 interaction by HBV Pol, we concluded that HBV Pol suppresses IRF signaling by disrupting the IKKε-DDX3 interaction.
Discussion
HBV, that has been dubbed a “stealth virus”, efficiently evades antiviral innate immune responses early in infection [6]. However, the underlying immune evasion mechanism remained enigmatic. The data presented here revealed that HBV evades host innate immune response via the inhibition of pattern recognition receptor signaling by one of the viral proteins. Results presented here clearly show that HBV Pol is the viral protein that blocks the TLR3-and RIG-I-induced pattern recognition receptor signaling in a physiologically relevant setting (Fig. 2). Further, evidence suggests that HBV Pol suppresses IRF signaling by inhibiting TBK1/IKKε activity, the effector protein kinase of IRF3/7 signaling (Fig. 3). Importantly, we also demonstrated that HBV Pol inhibits TBK1/IKKε activity by disrupting the interaction between IKKε and DDX3 (Fig. 6C). Overall, besides its inherent catalytic role in viral reverse transcription, our results here confer a novel immune-regulatory role to HBV Pol.
Recent evidence obtained in cultured cells showed that HBV is capable of inducing innate host response [8], [21]. However, it remained to be learned how HBV abrogates innate immune response. The data presented here indicate that HBV Pol blocks both TLR-mediated and RIG-I-mediated IRF3 signaling (Fig. 1 and Fig. 2). Importantly, two experiments presented here supported the physiological relevance of the impact of HBV Pol on innate immune response. First, the data in Fig. 1C showed that the inhibition of IRF signaling by HBV Pol is physiologically relevant, since the inhibitory effect by HBV Pol was observed under the condition, where the physiological level of viral proteins was attained by using either wild-type or mutant HBV replicon in HepG2 cells (Fig. 1C). This finding is further strengthened by the evidence that IFN production was only marginally induced upon SenV infection in HepG2.2.15 cell line compared to a parental HepG2 cells (Fig. 2D).
Further, the data shown in Fig. 1C is significant in a few respects. It was not unexpected that the extent of inhibition attained by the X-null and C-null construct was comparable to that by the wild-type replicon (Fig. 1C), since similar level of HBV Pol would be expressed in transient transfection setting, regardless of whether viral genome replication is induced. Secondly, the data clearly ruled out a possibility that either X protein (i.e., HBx) or core protein is related to the inhibition, since IFNβ production in either X-null or C-null transfected cells was comparable to that of WT transfected cells (Fig. 1C). Thirdly, the cells transfected by the P-null replicon mounted significantly and reproducibly higher IFN production than that by mock DNA, suggesting that a viral molecule derived from the P-null replicon caused augmentation of IFNβ production, in addition to what has already been induced by poly(I∶C) (Fig. 1C). It is tempting to speculate that the viral molecule, perhaps viral RNAs, may represent viral PAMPs that caused enhanced IFNβ production.
Although the liver is an important site for chronic viral infection, little is known about how innate immune response is initiated in hepatocytes [13], [22]. We chosen HepG2 cell for the most experiments, a hepatoma cell line, that supports HBV genome replication in a manner depending on HBx expression [14]. Since TLR3 expression is deficient in HepG2 cells, IFNβ reporter assays were carried out following complementation of TLR3; note that HepG2 cell is proficient in RIG-I mediated signaling, which senses SenV [13]. On the other hand, unlike to most other viruses, the PAMP signature of HBV, which are sensed by pattern recognition receptors such as TLRs or RIG-I, has not been defined [2]. Consequently, our analysis was limited to use of poly I∶C or SenV to trigger pattern recognition receptor signaling. However, it should be noted that the utilization of heterologous inducers such as poly I∶C or SenV in this work does not invalidate our conclusions, since the HBV Pol inhibits TBK/IKKε kinase, which lies downstream of PAMPs in IRF signaling (Fig. 5A). Nonetheless, natural viral infection, rather than transfection, involving susceptible human hepatocytes, merit further investigation, as recently demonstrated in HCV infection [23].
In terms of the impact of HBV Pol on IFN signaling, three observations are worthy of mentioning. First, our preliminary data indicated that in addition to its inhibitory effect on IRF signaling, HBV Pol inhibits, to a lesser extent, NF-kB signaling, which also contributes to IFNβ production (data not shown). If it holds, it would suggest an intriguingly possibility in that HBV Pol blocks IFNβ production by interfering two distinct signaling pathways, leading to IFNβ production. Work is in progress to obtain the mechanistic details. Second, relevantly, abundant detection of HBV Pol in nonencapsidated state has implicated that HBV Pol could contributes to viral pathogenesis or immune evasion [24], [25]. Lastly, in fact, HBV Pol has been previously identified as one of viral proteins that confer the resistance to IFN treatment [26], [27]. It should be noted, however, that we found its inhibitory role in IFN induction (i.e., IRF signaling), whereas the published work found its inhibitory effect in IFN action (i.e., JAK/Stat signaling).
DEAD-box RNA helicases constitute a large family of proteins that comprises at least 38 members in human genome [28]. DEAD-box RNA helicases exhibit multiple roles in diverse aspects of RNA metabolism such as transcription, pre-mRNA splicing, RNA export, translation, and RNA decay [29]. Not surprisingly, DDX3 has also been implicated in multiple distinct cellular processes as well. First, DDX3 has been reported to act as a transcriptional factor in the nucleus [30]. Secondly, DDX3 was shown to bind to eIF4E, a translation initiation factor with cap-binding properties, effectively suppressing translation [31]. Finally, DDX3 has been implicated in various viral life cycles. For instance, DDX3 was shown to be essential for nuclear export of human immunodeficiency virus-1 (HIV-1) RNA through the Rev/RRE pathway [32]. In addition, DDX3 supports viral replication of hepatitis C virus (HCV) genome via its interaction with the HCV core protein [33], [34]. In contrast, DDX3 was shown to inhibit HBV genome replication via its interaction with HBV Pol [11]. Besides its roles in RNA metabolism, a novel function relevant to innate immunity was recently reported by two groups [9], [10]. Although the augmentation of IRF signaling by DDX3 was found independently by two groups, discrepancies have been noted regarding the specific mechanism for DDX3-mediated IRF3 activation. Specifically, Bowie and colleagues [9] demonstrated that the IKKε-DDX3 interaction is significantly enhanced upon SenV infection with concomitant IRF3 phosphorylation, indicating that DDX3 stimulates the protein kinase activity of IKKε. In contrast, Decker and colleagues [10] concluded that DDX3 acts as a transcription factor of the IFNβ promoter, which is in agreement with the transcriptional role of DDX3 reported by another study [30], [35]. Interestingly, we have demonstrated that HBV Pol disrupts the IKKε-DDX3 interaction (Fig. 6C), which is consistent with the conclusions of Bowie and colleagues (7). However, in-depth analyses are needed to clarify this mechanistic issue.
Throughout evolution, viruses have developed strategies to evade host immune response [36]. Bowie and colleagues [9] revealed that the vaccinia virus K7 protein interferes with IRF signaling by inhibiting TBK1/IKKε activity via a mechanism involving its interaction with DDX3. Here, we demonstrated that HBV Pol impairs IRF signaling by inhibiting TBK1/IKKε activity via the HBV Pol-DDX3 interaction. Although diverse viral proteins including structural proteins and nonstructural proteins have evolved to evade immune response [36], it is intriguing that viral polymerase, besides its inherent catalytic contribution, has evolved to interfere innate immune response. More importantly, it is interesting that vaccinia virus and HBV, two unrelated viruses, acquired the ability to evade the immune response by subverting DDX3 during evolution.
Administration of TLR ligands was shown to inhibit HBV replication in a transgenic mouse model, implying that pattern recognition receptor signaling could be exploited for the treatment of chronic HBV infections [37]. Subsequent studies corroborated the above findings either by transfection studies using hepatoma cell lines [38] or by using nonparenchymal liver cells from mice [39]. Along this line, we speculate that disruption of the HBV Pol-DDX3 interaction by therapeutic intervention could invoke sustained antiviral immune responses leading to resolution of chronic viral infections. In this regard, structural elucidation of the HBV Pol-DDX3 interaction merits further investigation.
This work was presented at the International Meeting on Molecular Biology of Hepatitis B Viruses, which was held in Tours, France from August 30 to September 3 of 2009 [40] and the result similar to ours will be reported by others [41].
Materials and Methods
Cell culture, transfection
HepG2, HepG2.2.15, and HEK293 cells were grown in Dulbecco's Modified Eagle's medium supplemented with 10% fetal bovine serum (GIBCO-BRL) and 10 µg/mL gentamycin at 37°C in 5% CO2 and were passaged every third day. Cells were transfected using polyethylenimine (25 kDa, Aldrich, St. Louis, MO) as described [42]. The amount of plasmid DNA transfected (12 µg per 60-mm plate and 30 µg per 100-mm plate) was kept constant by inclusion of vector DNA, pcDNA3. HEK293 cells were employed for the experiment shown in Fig. 3 and Fig. 6, because of its higher transfection efficiency (>80%) compared to that of HepG2 cells (<10%) (data not shown).
Reagents
Polyinosine-polycytidylic acid (poly I∶C) was purchased from Amersham. Sendai virus (SenV) was kindly provided by Prof. Moon-Jung Song (Korea University).
Plasmids
All DNA constructs were generated by overlap extension PCR protocols as previously described [43]. HBV Pol expression construct encoding HBV Pol with three copies of Flag tag at its N-terminus has been previously described [42]. The HBV replicon construct (i.e., WT) and its X-null version has been described previously [14]. The HBV C-null and P-null replicons have been described [42]. YMHD mutant of HBV Pol was made by substitution of the aspartic acid residue (i.e., 540D), a constituent of YMDD motif critical for catalysis, to histidine (H), as previously described [44]. Three constructs that was used to express the viral envelope glycoproteins – L-HBsAg, and M-HBsAg, and S-HBsAg, was made by inserting the respective ORFs into pcDNA3 (Invitrogen). The sources of the remaining plasmids are as follows: IRF3-GAL4, IRF7-GAL4, pFR luciferase reporter, IFNβ-Luc reporter (Bowie, Trinity College Dublin, Ireland), TBK1-Flag, IKKε-Flag (Dr. Fitzgerald, University of Massachusetts Medical School, Worcester, MA), Flag-RIG-I (Dr. Fujita, Kyoto University), Flag-TRIF (Akira, Osaka University), HA-TLR3 (InvivoGen), pISRE-Luc reporter (Stratagene), HA-DDX3, AS-DDX3 (K. T. Jeang, N.I.H.), and IRF3-GFP (Garcia-Sastre, Mt. Sinai School of Medicine).
Western blot analysis
Western blot analysis was performed as described [42]. For the detection of the Flag tagged Pol protein, mouse anti-FLAG M2 antibody (Sigma, 1∶5000) was used. Anti-IRF antibody (Invitrogen) and phospho-specific IRF3 (Ser396) antibody (Cell Signaling) were used to detect IRF3 and phosphorylated IRF3, respectively.
Reporter gene assays
Promoter induction and transcription factor activation were measured using HepG2 cells seeded onto 24-well or 6-well plates and transfected after 24 h with expression vectors and luciferase reporter gene. For the IRF3/7 assay, an IRF3/7-GAL4 fusion vector was used in combination with the pFR luciferase reporter, as previously described [45].
Southern blot analysis
Southern blot analysis was performed as previously described [46]. Briefly, the extracted viral DNA was separated by electrophoresis through a 1.3% agarose gel in a 0.5× Tris-acetate-EDTA buffer and then transferred onto a nylon membrane. The nylon membrane was prehybridized and hybridized with a 32P-labeled full-length HBV DNA probe in a hybridization solution for 16 h at 65°C. Images were obtained using the phosphoimager (BAS-2500; Fujifilm).
Co-immunoprecipitation
Immunoprecipitation was performed as described with modifications [47]. Briefly, after transient transfection, the medium was removed and the cells were rinsed twice in cold PBS, incubated for 30 min at 4°C in lysis buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM DTT, 0.2 mM PMSF, and 1% NP-40], and collected by scraping. Cell debris was removed through centrifugation at 10,000×g for 10 min at 4°C. Extracts were pre-cleared with protein G–agarose beads for 1 h at 4°C. The primary antibody was added for 1 h at 4°C, and immunoglobulin complexes were collected on protein G–agarose beads for 1 h at 4°C. The beads were washed five times with 1 ml of lysis buffer. Protein complexes were recovered by boiling in Laemmli sample buffer and analyzed by SDS–PAGE.
Confocal imaging
HEK293 cells were grown on 18-mm coverslips in 12-well plates and transfected with 2.5 µg of total DNA. At 48 h after transfection, cells were fixed with 3.7% formaldehyde. Slides were mounted onto glass slides with ProLong Antifade Kit (Molecular Probes) and examined by confocal microscopy (LSM 510 Meta; Carl Zeiss, Germany).
Accession numbers
HBV ayw subtype: V01460 J02203
DDX3: accession NM_001356.3
Supporting Information
Zdroje
1. ZoulimF
2006 Antiviral therapy of chronic hepatitis B. Antiviral Res 71 206 215
2. AkiraS
UematsuS
TakeuchiO
2006 Pathogen recognition and innate immunity. Cell 124 783 801
3. PichlmairA
Reis e SousaC
2007 Innate recognition of viruses. Immunity 27 370 383
4. HondaK
TaniguchiT
2006 IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol 6 644 658
5. WielandS
ThimmeR
PurcellRH
ChisariFV
2004 Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci U S A 101 6669 6674
6. WielandSF
ChisariFV
2005 Stealth and cunning: hepatitis B and hepatitis C viruses. J Virol 79 9369 9380
7. FisicaroP
ValdattaC
BoniC
MassariM
MoriC
2009 Early kinetics of innate and adaptive immune responses during hepatitis B virus infection. Gut 58 974 982
8. LuciforaJ
DurantelD
TestoniB
HantzO
LevreroM
2010 Control of hepatitis B virus replication by innate response of HepaRG cells. Hepatology 51 63 72
9. SchroderM
BaranM
BowieAG
2008 Viral targeting of DEAD box protein 3 reveals its role in TBK1/IKKepsilon-mediated IRF activation. EMBO J 27 2147 2157
10. SoulatD
BurckstummerT
WestermayerS
GoncalvesA
BauchA
2008 The DEAD-box helicase DDX3X is a critical component of the TANK-binding kinase 1-dependent innate immune response. EMBO J 27 2135 2146
11. WangH
KimS
RyuWS
2009 DDX3 DEAD-Box RNA helicase inhibits hepatitis B virus reverse transcription by incorporation into nucleocapsids. J Virol 83 5815 5824
12. AlexopoulouL
HoltAC
MedzhitovR
FlavellRA
2001 Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413 732 738
13. LiK
ChenZ
KatoN
GaleMJr
LemonSM
2005 Distinct poly(I-C) and virus-activated signaling pathways leading to interferon-beta production in hepatocytes. J Biol Chem 280 16739 16747
14. ChaMY
RyuDK
JungHS
ChangHE
RyuWS
2009 Stimulation of hepatitis B virus genome replication by HBx is linked to both nuclear and cytoplasmic HBx expression. J Gen Virol 90 978 986
15. NassalM
2008 Hepatitis B viruses: reverse transcription a different way. Virus Res 134 235 249
16. SaitoT
GaleMJr
2008 Differential recognition of double-stranded RNA by RIG-I-like receptors in antiviral immunity. J Exp Med 205 1523 1527
17. KatoH
TakeuchiO
SatoS
YoneyamaM
YamamotoM
2006 Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441 101 105
18. YoneyamaM
KikuchiM
NatsukawaT
ShinobuN
ImaizumiT
2004 The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol 5 730 737
19. SellsMA
ChenML
AcsG
1987 Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc Natl Acad Sci U S A 84 1005 1009
20. MibayashiM
Martinez-SobridoL
LooYM
CardenasWB
GaleMJr
2007 Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J Virol 81 514 524
21. WuJ
MengZ
JiangM
PeiR
TripplerM
2009 Hepatitis B virus suppresses toll-like receptor-mediated innate immune responses in murine parenchymal and nonparenchymal liver cells. Hepatology 49 1132 1140
22. PreissS
ThompsonA
ChenX
RodgersS
MarkovskaV
2008 Characterization of the innate immune signalling pathways in hepatocyte cell lines. J Viral Hepat 15 888 900
23. WangN
LiangY
DevarajS
WangJ
LemonSM
2009 Toll-like Receptor 3 Mediates Establishment of an Antiviral State Against Hepatitis C Virus in Hepatoma Cells. J Virol
24. YaoE
GongY
ChenN
TavisJE
2000 The Majority of Duck Hepatitis B Virus Reverse Transcriptase in Cells Is Nonencapsidated and Is Bound to a Cytoplasmic Structure. J Virol 74 8648 8657
25. CaoF
TavisJE
2004 Detection and characterization of cytoplasmic hepatitis B virus reverse transcriptase. J Gen Virol 85 3353 3360
26. FosterGR
AckrillAM
GoldinRD
KerrIM
ThomasHC
1991 Expression of the terminal protein region of hepatitis B virus inhibits cellular responses to interferons alpha and gamma and double-stranded RNA [published erratum appears in Proc Natl Acad Sci U S A 1995 Apr 11;92(8):3632]. Proc Natl Acad Sci U S A 88 2888 2892
27. WuM
XuY
LinS
ZhangX
XiangL
2007 Hepatitis B virus polymerase inhibits the interferon-inducible MyD88 promoter by blocking nuclear translocation of Stat1. J Gen Virol 88 3260 3269
28. LinderP
2006 Dead-box proteins: a family affair–active and passive players in RNP-remodeling. Nucleic Acids Res 34 4168 4180
29. RocakS
LinderP
2004 DEAD-box proteins: the driving forces behind RNA metabolism. Nat Rev Mol Cell Biol 5 232 241
30. ChaoCH
ChenCM
ChengPL
ShihJW
TsouAP
2006 DDX3, a DEAD box RNA helicase with tumor growth-suppressive property and transcriptional regulation activity of the p21waf1/cip1 promoter, is a candidate tumor suppressor. Cancer Res 66 6579 6588
31. ShihJW
TsaiTY
ChaoCH
Wu LeeYH
2008 Candidate tumor suppressor DDX3 RNA helicase specifically represses cap-dependent translation by acting as an eIF4E inhibitory protein. Oncogene 27 700 714
32. YedavalliVS
NeuveutC
ChiYH
KleimanL
JeangKT
2004 Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell 119 381 392
33. AriumiY
KurokiM
AbeK
DansakoH
IkedaM
2007 DDX3 DEAD-box RNA helicase is required for hepatitis C virus RNA replication. J Virol 81 13922 13926
34. OwsiankaAM
PatelAH
1999 Hepatitis C virus core protein interacts with a human DEAD box protein DDX3. Virology 257 330 340
35. BotlaguntaM
VesunaF
MironchikY
RamanA
LisokA
2008 Oncogenic role of DDX3 in breast cancer biogenesis. Oncogene 27 3912 3922
36. BowieAG
UnterholznerL
2008 Viral evasion and subversion of pattern-recognition receptor signalling. Nat Rev Immunol 8 911 922
37. IsogawaM
RobekMD
FuruichiY
ChisariFV
2005 Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J Virol 79 7269 7272
38. GuoH
JiangD
MaD
ChangJ
DoughertyAM
2009 Activation of pattern recognition receptor-mediated innate immunity inhibits the replication of hepatitis B virus in human hepatocyte-derived cells. J Virol 83 847 858
39. WuJ
LuM
MengZ
TripplerM
BroeringR
2007 Toll-like receptor-mediated control of HBV replication by nonparenchymal liver cells in mice. Hepatology 46 1769 1778
40. WangH
RyuW.-S
2009 HBV Polymerase inhibits pattern recognition receptor-mediated innate immune response via its interaction with DDX3 DEAD-BOX RNA helicase. 109 2009 International meeting The Molecular Biology of Hepatitis B Viruses
41. YuS
ChenJ
WuM
ChenH
KatoN
2010 Hepatitis B virus polymerase inhibits RIG-I - and Toll-like receptor 3-mediated beta interferon induction in human hepatocytes through interference with interferon regulatory factor 3 activation and dampening of the interaction between TBK1/IKK and DDX3. J Gen Virol 91(8) 2080 2090
42. RyuDK
KimS
RyuWS
2008 Hepatitis B virus polymerase suppresses translation of pregenomic RNA via a mechanism involving its interaction with 5′ stem-loop structure. Virology 373 112 123
43. LeeJ
LeeH-J
ShinM-K
RyuW-S
2004 Versatile PCR-mediated insertion or deletion mutagenesis. BioTechniques 36 398 400
44. RadziwillG
TuckerW
SchallerH
1990 Mutational analysis of the hepatitis B virus P gene product: domain structure and RNase H activity. J Virol 64 613 620
45. StackJ
HagaIR
SchroderM
BartlettNW
MaloneyG
2005 Vaccinia virus protein A46R targets multiple Toll-like-interleukin-1 receptor adaptors and contributes to virulence. J Exp Med 201 1007 1018
46. ShinM-K
LeeJ
RyuW-S
2004 A novel cis-acting element facilitates minus-strand DNA synthesis during reverse transcription of the hepatitis B viruses genome. J Virol 78 6252 6262
47. PyronnetS
ImatakaH
GingrasAC
FukunagaR
HunterT
1999 Human eukaryotic translation initiation factor 4G (eIF4G) recruits mnk1 to phosphorylate eIF4E. Embo J 18 270 279
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System inČlánek A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 7- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- The Mouse Resistance Protein Irgm1 (LRG-47): A Regulator or an Effector of Pathogen Defense?
- Leprosy and the Adaptation of Human Toll-Like Receptor 1
- Intergenomic Arms Races: Detection of a Nuclear Rescue Gene of Male-Killing in a Ladybird
- The Role of Chemokines during Viral Infection of the CNS
- Bottlenecks and the Maintenance of Minor Genotypes during the Life Cycle of
- DNA Damage Triggers Genetic Exchange in
- The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System in
- Uropathogenic Modulates Immune Responses and Its Curli Fimbriae Interact with the Antimicrobial Peptide LL-37
- Biogenesis of the Inner Membrane Complex Is Dependent on Vesicular Transport by the Alveolate Specific GTPase Rab11B
- A Spatio-Temporal Analysis of Matrix Protein and Nucleocapsid Trafficking during Vesicular Stomatitis Virus Uncoating
- Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion
- Quasispecies Theory and the Behavior of RNA Viruses
- Bid Regulates the Pathogenesis of Neurotropic Reovirus
- Distinct Roles for Dectin-1 and TLR4 in the Pathogenesis of Keratitis
- Unexpected Inheritance: Multiple Integrations of Ancient Bornavirus and Ebolavirus/Marburgvirus Sequences in Vertebrate Genomes
- Balanced Nuclear and Cytoplasmic Activities of EDS1 Are Required for a Complete Plant Innate Immune Response
- Adaptation of Hepatitis C Virus to Mouse CD81 Permits Infection of Mouse Cells in the Absence of Human Entry Factors
- An Outer Membrane Receptor of Involved in Zinc Acquisition with Vaccine Potential
- Inositol Hexakisphosphate-Induced Autoprocessing of Large Bacterial Protein Toxins
- Plus- and Minus-End Directed Microtubule Motors Bind Simultaneously to Herpes Simplex Virus Capsids Using Different Inner Tegument Structures
- Distinct Pathogenesis and Host Responses during Infection of by and
- Can Bacteria Evolve Resistance to Quorum Sensing Disruption?
- RNA Virus Replication Complexes
- PPARγ and LXR Signaling Inhibit Dendritic Cell-Mediated HIV-1 Capture and -Infection
- The Virulence Protein SopD2 Regulates Membrane Dynamics of -Containing Vacuoles
- Genome-Wide Mutagenesis Reveals That ORF7 Is a Novel VZV Skin-Tropic Factor
- Adaptive Evolution of Includes Retroviral Insertion and Positive Selection at Two Clusters of Residues Flanking the Substrate Groove
- A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
- Transduction of Human T Cells with a Novel T-Cell Receptor Confers Anti-HCV Reactivity
- Identification of GBV-D, a Novel GB-like Flavivirus from Old World Frugivorous Bats () in Bangladesh
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- HIV gp41 Engages gC1qR on CD4+ T Cells to Induce the Expression of an NK Ligand through the PIP3/H2O2 Pathway
- Oxidation of Helix-3 Methionines Precedes the Formation of PK Resistant PrP
- Protection from the 2009 H1N1 Pandemic Influenza by an Antibody from Combinatorial Survivor-Based Libraries
- Murine Gamma-Herpesvirus 68 Hijacks MAVS and IKKβ to Initiate Lytic Replication
- Viral Protein Inhibits RISC Activity by Argonaute Binding through Conserved WG/GW Motifs
- TOPO3α Influences Antigenic Variation by Monitoring Expression-Site-Associated Switching in
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- The Meningococcal Vaccine Candidate Neisserial Surface Protein A (NspA) Binds to Factor H and Enhances Meningococcal Resistance to Complement
- Network Modeling Reveals Prevalent Negative Regulatory Relationships between Signaling Sectors in Arabidopsis Immune Signaling
- Endothelial Galectin-1 Binds to Specific Glycans on Nipah Virus Fusion Protein and Inhibits Maturation, Mobility, and Function to Block Syncytia Formation
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
- Integration Preferences of Wildtype AAV-2 for Consensus Rep-Binding Sites at Numerous Loci in the Human Genome
- Epigenetic Analysis of KSHV Latent and Lytic Genomes
- Vaccinia Virus–Encoded Ribonucleotide Reductase Subunits Are Differentially Required for Replication and Pathogenesis
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- RNA Virus Replication Complexes
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



