-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Role of Chemokines during Viral Infection of the CNS
article has not abstract
Published in the journal: The Role of Chemokines during Viral Infection of the CNS. PLoS Pathog 6(7): e32767. doi:10.1371/journal.ppat.1000937
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1000937Summary
article has not abstract
Viral infection of the central nervous system (CNS) poses unique challenges to the immune system with regards to controlling and eliminating the invading pathogen. These obstacles include the presence of a blood–brain barrier (BBB) that provides a physical and physiological barrier that is difficult for cells and molecules to cross, the absence of classic lymphatic drainage that may impair the generation of an adaptive immune response, and limited MHC class I or II expression on resident cells of the CNS, even during periods of neuroinflammation. In addition, the CNS is composed of a variety of highly specialized cells, many of which have limited renewal capacity, that represent potential targets of infection by numerous different viruses. Nonetheless, antigen-specific lymphocytes are ultimately able to accumulate within the CNS and contribute to defense by reducing or eliminating the invading viral pathogen. Alternatively, infiltration of activated cells of the immune system may be detrimental, as these cells can contribute to neuropathology that may result in long-term cellular damage or death. Understanding the mechanisms that govern leukocyte trafficking from the microvasculature into the CNS parenchyma is therefore critical for comprehending the molecular and cellular events that control neuroinflammation following infection by neurotropic viruses. Chemokines, small (8–10 kDa) proteins expressed by almost all nucleated cell types, are divided into four subfamilies based upon the number and spacing of conserved cysteine residues present within the amino terminus of the protein. Chemokine function is controlled through often promiscuous signaling via seven transmembrane G-protein-coupled receptors. While initially characterized as important in inflammation by targeting distinct leukocyte populations, chemokines are now considered critical mediators of a variety of biological processes, including development, tissue homeostasis, and coordinated immune responses during viral infection.
Resident Cells of the CNS Secrete Chemokines in Response to Viral Infection
Chemokines are now recognized as critical regulators of leukocyte trafficking into the CNS. This leads to the inevitable questions, which cells are producing chemokines and how is this controlled? Numerous studies have revealed that resident cell populations of the CNS are able to synthesize and secrete a variety of chemokines. Astrocytes and microglia are the primary source of chemokines following infection with a wide range of neurotropic viruses, including the JHM strain of mouse hepatitis virus (JHMV), lymphocytic choriomeningitis virus (LCMV), Theiler's murine encephalitis virus (TMEV), herpes simplex virus 1 (HSV1), and human immunodeficiency virus (HIV) [1]–[4]. Neurons are also capable of secreting chemokines during HIV and West Nile virus (WNV) infection [5], [6], while endothelial cells express chemokines during simian immunodeficiency virus-induced encephalitis [7]. Both in vitro and in vivo studies have highlighted that CNS viral infection often results in distinct chemokine signature patterns. For example, Prehaud and colleagues have demonstrated that in vitro infection of neurons with rabies virus (RABV) results in robust production of chemokines, whereas HSV-1-infected neurons do not [8]. However, specific chemokines, e.g., CXCL10 and CCL5, are often expressed independently of either cellular tropism or viral genetics, suggesting that factor(s) either secreted in response to infection (such as type I interferon [IFN]) or utilized for viral recognition are shared between many neurotropic viruses. Toll-like receptors (TLRs) recognize both DNA and RNA and they are able to rapidly respond to viral infection, in part, by promoting chemokine gene expression. During TMEV infection, TLR2 and TLR3 cooperation leads to the expression of the macrophage chemoattractants CCL2 and CCL5 [3], while TLR2 and TLR9 mediate chemokine expression during HSV-1 infection [4], [9]. Type I IFNs regulate glial-derived chemokine expression in response to CNS infection with LCMV (Traub strain) and HSV-1 [2], [9]; however, this pathway is dispensable for expression of other chemokines, e.g., CCL2 following infection with JMHV [10]. Rather, JHMV viral proteins influence chemokine secretion through as yet undefined mechanisms [11], while the HIV-1 protein Nef influences neuronal chemokine secretion [5]. Moreover, WNV-infected cerebellar granule cell neurons readily secrete CXCL10 in vitro, while CXCL10 expression by WNV-infected cortical neurons is muted [12]. The consequence of this differential expression of CXCL10 is reflected in altered migration of defined inflammatory cells into the cerebellum at the expense of other WNV-infected CNS regions [12]. Collectively, these data illustrate that viral infection of the CNS by a wide variety of neurotropic viruses induces highly orchestrated and individual patterns of chemokine secretion by resident cells of the CNS, evoked by disparate pathways that converge into often overlapping profiles of inflammatory cell infiltration.
Chemokines Regulate Immune Cell Access into the CNS
Signaling events that occur early following viral infection are often critical in dictating outcome. Recent studies have highlighted the importance of innate immune cells in contributing to a protective response, and we are just now learning how chemokines are involved in attracting these cells to the CNS. Infection of mice with neurotropic virus such as HSV-1 and JHMV results in the rapid accumulation of neutrophils to the CNS [13], [14]. Studies using the JHMV model system have provided insight into the functional relevance of neutrophil migration to the CNS, as these cells are required to contribute to the permeabilization of the BBB [14]. During JHMV infection, astrocyte - and endothelial-derived expression of ELR+ (glutamic acid-leucine-arginine) CXC chemokines, including CXCL1, attracts CXCR2-reactive neutrophils to the CNS [14]. Neutralization of this signaling axis specifically abrogates neutrophil infiltration, thereby preventing BBB degradation and the ensuing entry of protective JHMV-specific T lymphocytes [14]. In the absence of CXCR2 signaling, JHMV-infected mice experience higher viral loads and quickly succumb to infection, indicating that neutrophil targeting of the CNS is critical in host defense [14]. Conversely, McGavern and colleagues have suggested that during acute LCMV infection (Armstrong strain), cytotoxic T lymphocyte (CTL)-mediated chemokine gene expression contributes to fatal meningoencephalitis, in part, by attracting neutrophils and monocytes into the CNS, and this is associated with fatal vascular permeability and seizures, thus highlighting a detrimental role for neutrophils in response to viral infection [15]. In addition to enhancing the permeabilization of the BBB by recruiting neutrophils and monocytes, chemokines can also function as gatekeepers regulating leukocyte penetration into the parenchyma. Following WNV infection of the CNS, CXCL12 retains antigen-sensitized lymphocytes within the perivascular space. Antagonism of CXCR4, the receptor for CXCL12, enhances T lymphocyte entry into the CNS parenchyma, and this correlates with reduced WNV burden, enhanced survival, and limited neuropathology [16]. Thus, expression of chemokines early in response to infection with neurotropic viruses aids in effective host defense by promoting vascular permeability and regulating parenchymal lymphocyte infiltration (Figure 1A). However, it should be emphasized that the consequences of BBB degradation can vary from efficient viral clearance to fatal encephalitis and seizures, depending upon the virus and the route of infection.
Fig. 1. Functional roles of chemokines in response to viral infection of the CNS. 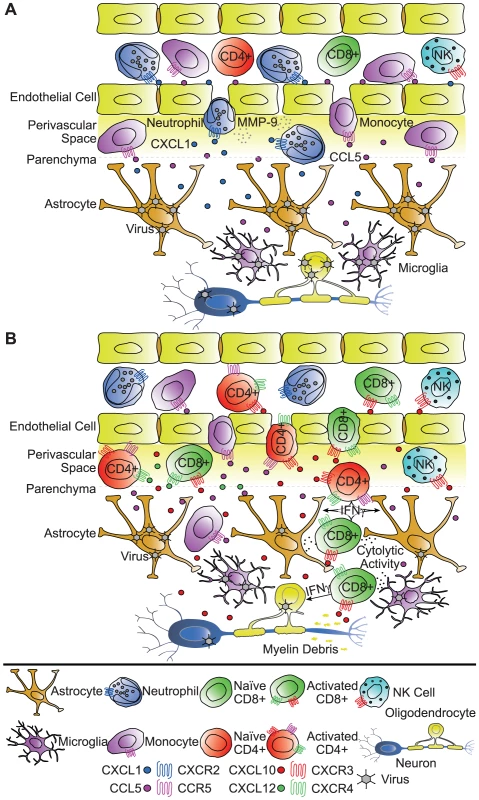
Potential roles of chemokines in attracting innate immune cells (A) and lymphocytes (B) into the CNS during acute viral infection. The cartoons emphasize several key points derived from recent studies focusing on experimental infection with neurotropic viruses. (A) Early (days 1–3) after viral infection, activated and/or virally infected astrocytes, microglia, and endothelial cells secrete chemokines that serve to attract myeloid cells to the CNS. Among the earliest cells to respond to viral infection, neutrophils are recruited into the CNS by virtue of CXCR2 responding to ligands expressed within the CNS (e.g., CXCL1). Monocytes are also attracted into the CNS via the chemokine CCL5 and its receptor CCR5. Neutrophils and monocytes participate in the degradation of the blood–brain barrier (BBB), in part through the release of the matrix metalloproteinase MMP-9, and therefore ensure successive infiltration of virus-specific lymphocytes into the CNS. (B) During the acute stage of disease, astrocytes, microglia, neurons, and endothelial cells continue to secrete chemokines, serving to attract activated T lymphocytes, NK cells, and monocytes into the CNS. CD8+ and CD4+ T lymphocytes bearing the receptor CXCR3 and/or CCR5 are attracted by the chemokines CXCL10 and CCL5, respectively, and mediate viral control through direct cytolytic activity and/or cytokine secretion. CXCL12, which signals through CXCR4, may, however, sequester T lymphocytes within the perivascular space and regulate penetration of the parenchyma, thus inhibiting efficient viral clearance. Chemokines and Neuroprotection during Viral Infection
Although early signaling events are clearly important for host defense during viral infection, the infiltration and anti-viral activity of T lymphocytes are requisite for viral clearance and survival. CXCL10, which is prominently expressed within the CNS during many viral infections [1], [17], functions to attract activated T lymphocytes bearing the receptor CXCR3. Neutralization or genetic silencing of CXCL10 following infection with HSV, JHMV, and WNV dramatically reduces T cell trafficking into the CNS, thus preventing efficient viral control and often resulting in poor resolution [6], [18], [19]. In addition to attracting T lymphocytes, the CXCR3 ligands CXCL10 and CXCL9 also attract natural killer (NK) cells during JHMV infection [20], [21]; however, their role in viral clearance remains unclear. The macrophage and T lymphocyte chemokine CCL5, or one of its receptors, CCR5, also promotes leukocyte trafficking into the CNS and subsequent viral control during JHMV infection and WNV-induced encephalitis [22], [23]. The clinical relevance of this observation was revealed when homozygosity for the defective human CCR5 allele (CCR5Δ32) was associated with an increased risk for symptomatic WNV infection [24]. Collectively, these data demonstrated that chemokine expression during viral infection promotes the generation and infiltration of immune effector cells necessary for quelling viral replication (Figure 1B).
Chemokines and Neuropathology following Viral Infection
A potential consequence of chemokine secretion and the subsequent accumulation of leukocytes within the CNS, while important for viral control in many instances, is the development of neuropathology. For example, the fatal meningoencephalitis induced by LCMV infection is mediated by infiltration of virus-specific CTLs that promote subsequent myeloid cell and leukocyte entry [15], [25]. During infection with LCMV (Traub), genetic silencing of CXCL10 or its receptor CXCR3 reduces the infiltration of CD8+ T cells, conferring either partial or near complete protection from immunopathology and death [26], [27]. However, CXCL10 remains dispensable for T cell infiltration or the development of fatal inflammation during infection with LCMV (Armstrong) [28], further highlighting underlying differences in viral strains and chemokine utilization with regards to disease outcome. During JHMV infection, sustained CXCL10 and CCL5 expression leads to continuing immune cell infiltration that manifests an immune-mediated demyelinating disease. Neutralization of either chemokine during persistent JHMV infection abrogates the immune infiltration and greatly reduces both disease severity and demyelination [29], [30]. In addition to attracting inflammatory cells that contribute to neuropathology, CXCL10, which is chronically expressed within the brains of patients suffering from HIV-associated neurological disorders, can directly induce neuronal cell death [5]. In addition, proteolytically cleaved CXCL12, which is also detectable within the brains of HIV-1-infected patients, is capable of inducing neurotoxicity and apoptosis [31]. Although beyond the scope of this review, extensive work has focused upon the direct and indirect roles of the chemokine receptors CXCR4 and CCR5 (and their associated ligands) in contributing to HIV-associated dementia (reviewed in [32], [33]). Therefore, chemokines are critical mediators of neuropathology during viral infections of the CNS, either by attracting pathogenic inflammatory cells or directly mediating neurotoxicity and cell death.
Conclusions
From this brief review, it is evident that the biological roles of chemokines in host defense and/or disease in response to viral infection of the CNS are constantly evolving. An emerging picture has developed that indicates that chemokines and their receptors are intimately involved in generation of effective host responses to viral infections within the CNS. Paradoxically, chemokine expression has also been associated with neuropathology. Thus, chemokines and/or chemokine receptors are potentially relevant targets for treating various viral-induced neuropathies by dampening specific biological functions associated with disease. Recent evidence has emerged implicating chemokines, specifically CXCR4 and CXCL12, as important mediators of neurogenesis [34]; thus, chemokines produced during viral infections may influence neural precursor cell function and therefore influence recovery and repair. We can only look forward to future research that will undoubtedly uncover new and exciting roles for the chemokines in host defense, disease, and recovery within the context of the virally infected CNS.
Zdroje
1. LaneTE
AsensioVC
YuN
PaolettiAD
CampbellIL
1998 Dynamic regulation of alpha - and beta-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J Immunol 160 970 978
2. ChristensenJE
SimonsenS
FengerC
SorensenMR
MoosT
2009 Fulminant lymphocytic choriomeningitis virus-induced inflammation of the CNS involves a cytokine-chemokine-cytokine-chemokine cascade. J Immunol 182 1079 1087
3. SoEY
KimBS
2009 Theiler's virus infection induces TLR3-dependent upregulation of TLR2 critical for proinflammatory cytokine production. Glia 57 1216 1226
4. AravalliRN
HuS
RowenTN
PalmquistJM
LokensgardJR
2005 Cutting edge: TLR2-mediated proinflammatory cytokine and chemokine production by microglial cells in response to herpes simplex virus. J Immunol 175 4189 4193
5. van MarleG
HenryS
TodorukT
SullivanA
SilvaC
2004 Human immunodeficiency virus type 1 Nef protein mediates neural cell death: a neurotoxic role for IP-10. Virology 329 302 318
6. KleinRS
LinE
ZhangB
LusterAD
TollettJ
2005 Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J Virol 79 11457 11466
7. SassevilleVG
SmithMM
MackayCR
PauleyDR
MansfieldKG
1996 Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am J Pathol 149 1459 1467
8. PrehaudC
MegretF
LafageM
LafonM
2005 Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J Virol 79 12893 12904
9. WuestT
AustinBA
UematsuS
ThapaM
AkiraS
2006 Intact TRL 9 and type I interferon signaling pathways are required to augment HSV-1 induced corneal CXCL9 and CXCL10. J Neuroimmunol 179 46 52
10. IrelandDD
StohlmanSA
HintonDR
AtkinsonR
BergmannCC
2008 Type I interferons are essential in controlling neurotropic coronavirus infection irrespective of functional CD8 T cells. J Virol 82 300 310
11. ScottEP
BraniganPJ
Del VecchioAM
WeissSR
2008 Chemokine expression during mouse-hepatitis-virus-induced encephalitis: contributions of the spike and background genes. J Neurovirol 14 5 16
12. ZhangB
ChanYK
LuB
DiamondMS
KleinRS
2008 CXCR3 mediates region-specific antiviral T cell trafficking within the central nervous system during West Nile virus encephalitis. J Immunol 180 2641 2649
13. YanXT
TumpeyTM
KunkelSL
OakesJE
LauschRN
1998 Role of MIP-2 in neutrophil migration and tissue injury in the herpes simplex virus-1-infected cornea. Invest Ophthalmol Vis Sci 39 1854 1862
14. HoskingMP
LiuL
RansohoffRM
LaneTE
2009 A protective role for ELR+ chemokines during acute viral encephalomyelitis. PLoS Pathog 5 e1000648 doi:10.1371/journal.ppat.1000648
15. KimJV
KangSS
DustinML
McGavernDB
2009 Myelomonocytic cell recruitment causes fatal CNS vascular injury during acute viral meningitis. Nature 457 191 195
16. McCandlessEE
ZhangB
DiamondMS
KleinRS
2008 CXCR4 antagonism increases T cell trafficking in the central nervous system and improves survival from West Nile virus encephalitis. Proc Natl Acad Sci U S A 105 11270 11275
17. AsensioVC
CampbellIL
1997 Chemokine gene expression in the brains of mice with lymphocytic choriomeningitis. J Virol 71 7832 7840
18. LiuMT
ChenBP
OertelP
BuchmeierMJ
ArmstrongD
2000 The T cell chemoattractant IFN-inducible protein 10 is essential in host defense against viral-induced neurologic disease. J Immunol 165 2327 2330
19. WuestTR
CarrDJ
2008 Dysregulation of CXCR3 signaling due to CXCL10 deficiency impairs the antiviral response to herpes simplex virus 1 infection. J Immunol 181 7985 7993
20. TrifiloMJ
Montalto-MorrisonC
StilesLN
HurstKR
HardisonJL
2004 CXC chemokine ligand 10 controls viral infection in the central nervous system: evidence for a role in innate immune response through recruitment and activation of natural killer cells. J Virol 78 585 594
21. MuseM
KaneJA
CarrDJ
FarberJM
LaneTE
2008 Insertion of the CXC chemokine ligand 9 (CXCL9) into the mouse hepatitis virus genome results in protection from viral-induced encephalitis and hepatitis. Virology 382 132 144
22. GlassWG
LimJK
CholeraR
PletnevAG
GaoJL
2005 Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J Exp Med 202 1087 1098
23. GlassWG
LiuMT
KuzielWA
LaneTE
2001 Reduced macrophage infiltration and demyelination in mice lacking the chemokine receptor CCR5 following infection with a neurotropic coronavirus. Virology 288 8 17
24. LimJK
LouieCY
GlaserC
JeanC
JohnsonB
2008 Genetic deficiency of chemokine receptor CCR5 is a strong risk factor for symptomatic West Nile virus infection: a meta-analysis of 4 cohorts in the US epidemic. J Infect Dis 197 262 265
25. Fung-LeungWP
KundigTM
ZinkernagelRM
MakTW
1991 Immune response against lymphocytic choriomeningitis virus infection in mice without CD8 expression. J Exp Med 174 1425 1429
26. ChristensenJE
de LemosC
MoosT
ChristensenJP
ThomsenAR
2006 CXCL10 is the key ligand for CXCR3 on CD8+ effector T cells involved in immune surveillance of the lymphocytic choriomeningitis virus-infected central nervous system. J Immunol 176 4235 4243
27. ChristensenJE
NansenA
MoosT
LuB
GerardC
2004 Efficient T-cell surveillance of the CNS requires expression of the CXC chemokine receptor 3. J Neurosci 24 4849 4858
28. HoferMJ
CarterSL
MullerM
CampbellIL
2008 Unaltered neurological disease and mortality in CXCR3-deficient mice infected intracranially with lymphocytic choriomeningitis virus-Armstrong. Viral Immunol 21 425 433
29. LiuMT
KeirsteadHS
LaneTE
2001 Neutralization of the chemokine CXCL10 reduces inflammatory cell invasion and demyelination and improves neurological function in a viral model of multiple sclerosis. J Immunol 167 4091 4097
30. GlassWG
HickeyMJ
HardisonJL
LiuMT
ManningJE
2004 Antibody targeting of the CC chemokine ligand 5 results in diminished leukocyte infiltration into the central nervous system and reduced neurologic disease in a viral model of multiple sclerosis. J Immunol 172 4018 4025
31. VergoteD
ButlerGS
OomsM
CoxJH
SilvaC
2006 Proteolytic processing of SDF-1alpha reveals a change in receptor specificity mediating HIV-associated neurodegeneration. Proc Natl Acad Sci U S A 103 19182 19187
32. KaulM
ZhengJ
OkamotoS
GendelmanHE
LiptonSA
2005 HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ 12 Suppl 1 878 892
33. Gonzalez-ScaranoF
Martin-GarciaJ
2005 The neuropathogenesis of AIDS. Nat Rev Immunol 5 69 81
34. KolodziejA
SchulzS
GuyonA
WuDF
PfeifferM
2008 Tonic activation of CXC chemokine receptor 4 in immature granule cells supports neurogenesis in the adult dentate gyrus. J Neurosci 28 4488 4500
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System inČlánek A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2010 Číslo 7- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- The Mouse Resistance Protein Irgm1 (LRG-47): A Regulator or an Effector of Pathogen Defense?
- Leprosy and the Adaptation of Human Toll-Like Receptor 1
- Intergenomic Arms Races: Detection of a Nuclear Rescue Gene of Male-Killing in a Ladybird
- The Role of Chemokines during Viral Infection of the CNS
- Bottlenecks and the Maintenance of Minor Genotypes during the Life Cycle of
- DNA Damage Triggers Genetic Exchange in
- The Role of Coupled Positive Feedback in the Expression of the SPI1 Type Three Secretion System in
- Uropathogenic Modulates Immune Responses and Its Curli Fimbriae Interact with the Antimicrobial Peptide LL-37
- Biogenesis of the Inner Membrane Complex Is Dependent on Vesicular Transport by the Alveolate Specific GTPase Rab11B
- A Spatio-Temporal Analysis of Matrix Protein and Nucleocapsid Trafficking during Vesicular Stomatitis Virus Uncoating
- Hepatitis B Virus Polymerase Blocks Pattern Recognition Receptor Signaling via Interaction with DDX3: Implications for Immune Evasion
- Quasispecies Theory and the Behavior of RNA Viruses
- Bid Regulates the Pathogenesis of Neurotropic Reovirus
- Distinct Roles for Dectin-1 and TLR4 in the Pathogenesis of Keratitis
- Unexpected Inheritance: Multiple Integrations of Ancient Bornavirus and Ebolavirus/Marburgvirus Sequences in Vertebrate Genomes
- Balanced Nuclear and Cytoplasmic Activities of EDS1 Are Required for a Complete Plant Innate Immune Response
- Adaptation of Hepatitis C Virus to Mouse CD81 Permits Infection of Mouse Cells in the Absence of Human Entry Factors
- An Outer Membrane Receptor of Involved in Zinc Acquisition with Vaccine Potential
- Inositol Hexakisphosphate-Induced Autoprocessing of Large Bacterial Protein Toxins
- Plus- and Minus-End Directed Microtubule Motors Bind Simultaneously to Herpes Simplex Virus Capsids Using Different Inner Tegument Structures
- Distinct Pathogenesis and Host Responses during Infection of by and
- Can Bacteria Evolve Resistance to Quorum Sensing Disruption?
- RNA Virus Replication Complexes
- PPARγ and LXR Signaling Inhibit Dendritic Cell-Mediated HIV-1 Capture and -Infection
- The Virulence Protein SopD2 Regulates Membrane Dynamics of -Containing Vacuoles
- Genome-Wide Mutagenesis Reveals That ORF7 Is a Novel VZV Skin-Tropic Factor
- Adaptive Evolution of Includes Retroviral Insertion and Positive Selection at Two Clusters of Residues Flanking the Substrate Groove
- A Systems Immunology Approach to Plasmacytoid Dendritic Cell Function in Cytopathic Virus Infections
- Transduction of Human T Cells with a Novel T-Cell Receptor Confers Anti-HCV Reactivity
- Identification of GBV-D, a Novel GB-like Flavivirus from Old World Frugivorous Bats () in Bangladesh
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- HIV gp41 Engages gC1qR on CD4+ T Cells to Induce the Expression of an NK Ligand through the PIP3/H2O2 Pathway
- Oxidation of Helix-3 Methionines Precedes the Formation of PK Resistant PrP
- Protection from the 2009 H1N1 Pandemic Influenza by an Antibody from Combinatorial Survivor-Based Libraries
- Murine Gamma-Herpesvirus 68 Hijacks MAVS and IKKβ to Initiate Lytic Replication
- Viral Protein Inhibits RISC Activity by Argonaute Binding through Conserved WG/GW Motifs
- TOPO3α Influences Antigenic Variation by Monitoring Expression-Site-Associated Switching in
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- The Meningococcal Vaccine Candidate Neisserial Surface Protein A (NspA) Binds to Factor H and Enhances Meningococcal Resistance to Complement
- Network Modeling Reveals Prevalent Negative Regulatory Relationships between Signaling Sectors in Arabidopsis Immune Signaling
- Endothelial Galectin-1 Binds to Specific Glycans on Nipah Virus Fusion Protein and Inhibits Maturation, Mobility, and Function to Block Syncytia Formation
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
- Integration Preferences of Wildtype AAV-2 for Consensus Rep-Binding Sites at Numerous Loci in the Human Genome
- Epigenetic Analysis of KSHV Latent and Lytic Genomes
- Vaccinia Virus–Encoded Ribonucleotide Reductase Subunits Are Differentially Required for Replication and Pathogenesis
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- RNA Virus Replication Complexes
- Virus-Infection or 5′ppp-RNA Activates Antiviral Signal through Redistribution of IPS-1 Mediated by MFN1
- Functional Genetic Diversity among Complex Clinical Isolates: Delineation of Conserved Core and Lineage-Specific Transcriptomes during Intracellular Survival
- Extreme CD8 T Cell Requirements for Anti-Malarial Liver-Stage Immunity following Immunization with Radiation Attenuated Sporozoites
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



