-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Feeding Cells Induced by Phytoparasitic Nematodes Require γ-Tubulin Ring Complex for Microtubule Reorganization
Reorganization of the microtubule network is important for the fast isodiametric expansion of giant-feeding cells induced by root-knot nematodes. The efficiency of microtubule reorganization depends on the nucleation of new microtubules, their elongation rate and activity of microtubule severing factors. New microtubules in plants are nucleated by cytoplasmic or microtubule-bound γ-tubulin ring complexes. Here we investigate the requirement of γ-tubulin complexes for giant feeding cells development using the interaction between Arabidopsis and Meloidogyne spp. as a model system. Immunocytochemical analyses demonstrate that γ-tubulin localizes to both cortical cytoplasm and mitotic microtubule arrays of the giant cells where it can associate with microtubules. The transcripts of two Arabidopsis γ-tubulin (TUBG1 and TUBG2) and two γ-tubulin complex proteins genes (GCP3 and GCP4) are upregulated in galls. Electron microscopy demonstrates association of GCP3 and γ-tubulin as part of a complex in the cytoplasm of giant cells. Knockout of either or both γ-tubulin genes results in the gene dose-dependent alteration of the morphology of feeding site and failure of nematode life cycle completion. We conclude that the γ-tubulin complex is essential for the control of microtubular network remodelling in the course of initiation and development of giant-feeding cells, and for the successful reproduction of nematodes in their plant hosts.
Published in the journal: Feeding Cells Induced by Phytoparasitic Nematodes Require γ-Tubulin Ring Complex for Microtubule Reorganization. PLoS Pathog 7(12): e32767. doi:10.1371/journal.ppat.1002343
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002343Summary
Reorganization of the microtubule network is important for the fast isodiametric expansion of giant-feeding cells induced by root-knot nematodes. The efficiency of microtubule reorganization depends on the nucleation of new microtubules, their elongation rate and activity of microtubule severing factors. New microtubules in plants are nucleated by cytoplasmic or microtubule-bound γ-tubulin ring complexes. Here we investigate the requirement of γ-tubulin complexes for giant feeding cells development using the interaction between Arabidopsis and Meloidogyne spp. as a model system. Immunocytochemical analyses demonstrate that γ-tubulin localizes to both cortical cytoplasm and mitotic microtubule arrays of the giant cells where it can associate with microtubules. The transcripts of two Arabidopsis γ-tubulin (TUBG1 and TUBG2) and two γ-tubulin complex proteins genes (GCP3 and GCP4) are upregulated in galls. Electron microscopy demonstrates association of GCP3 and γ-tubulin as part of a complex in the cytoplasm of giant cells. Knockout of either or both γ-tubulin genes results in the gene dose-dependent alteration of the morphology of feeding site and failure of nematode life cycle completion. We conclude that the γ-tubulin complex is essential for the control of microtubular network remodelling in the course of initiation and development of giant-feeding cells, and for the successful reproduction of nematodes in their plant hosts.
Introduction
Root-knot nematodes (RKN) Meloidogyne spp. are minuscule worms which are widespread in the soil. They are obligate sedentary phyto-endoparasites known to infect above 3000 plant species. In the course of a compatible interaction, the nematodes of the genera Meloidogyne are able to alter the host plant metabolic pathways to their own benefit [1]. The parasitic cycle commences when the motile second-stage juvenile (J2) penetrates a root in the elongation zone [2]. This infective stage nematode migrates via intercellular space of the root cortex towards the root tip and then moves acropetally along a xylem pole to the differentiation zone of the root vascular tissue where it establishes the feeding site by altering the developmental and metabolic program of the vascular parenchymal cells [3]. A typical nematode feeding site (NFS) consists of 6 to 8 hypertrophic cells, named giant cells, with dense cytoplasm containing numerous organelles and characterised by high metabolic activity [4]. These cells serve as the exclusive source of nutrients for the nematode until their reproduction.
In the course of differentiation giant cells undergo karyokinesis followed by incomplete cytokinesis as well as endoreduplication cycles, resulting in the accumulation of multiple enlarged nuclei [5], [6]. This is accompanied by the partial depolymerisation/fragmentation of both main components of plant cytoskeleton: microtubules (MTs) and actin filaments [7]. The reorganization of the cytoskeleton is essential for establishment of the feeding site and successful nematode reproduction [7]–[9].
Microtubules are dynamic filaments formed by polymerization of heterodimeric protein α-/β-tubulin. They are essential for the spatial organization of the cytoplasm, establishment of the cell shape and polarity, cell division, intracellular transport and cell wall deposition. In plants MTs form four functionally specialized arrays: 1) interphase cortical network regulates the cell architecture including the direction of cell expansion; 2) preprophase band (PPB) during G2/M transition predicts site of the forthcoming division [3,10) mitotic spindle separates daughter chromatides; 4) phragmoplast mediates trafficking of components required for the cell-plate synthesis during cytokinesis. The organization of these arrays requires initiation of new MTs, their elongation, shrinking, severing and bundling with other MTs. Initiation of new MTs occurs on structures called MT-organizing centres (MTOCs) [11]. In animals, centrosomes serve as MTOC during both interphase and cell division. Higher plants lack a conspicuous MTOC and new MTs are nucleated from multiple dispersed sites [12]. A key component of MTOCs is γ-tubulin, an evolutionary conserved homologue protein of α - and β-tubulin [13]–[16]. γ-Tubulin localizes to the MT nucleation sites of interphase and dividing plant cells. There are two γ-tubulin genes in the genome of Arabidopsis thaliana and their transcripts were observed in seedlings, roots, flowers and tissue culture cells [17]. Using heterologous expression in fission yeast Horio and Oakley [18] have shown that Arabidopsis γ-tubulin was targeted to MTOCs and was able to nucleate MTs. Downregulation or knockout of both genes causes disorganization of cortical microtubule network, spindle and phragmoplast [19], [20]. Thus, plant γ-tubulin plays an essential role in MT organization at all stages of the plant cell cycle.
In active MTOCs, γ-tubulin associates with five proteins forming the γ-tubulin ring complex, or γTuRC [11]. Collectively, six proteins are called γ-tubulin complex proteins (GCPs), with γ-tubulin itself being GCP1. The γTuRC binds to MT minus ends and prevents it from depolymerisation [21]. The Arabidopsis thaliana genome contains orthologues of all components of mammalian γTuRC: two γ-tubulin genes (TUBG1 and TUBG2) and γ-tubulin complex protein genes GCP2 to GCP6 [22]. GCP2-GCP6 proteins may function as a scaffold for the interaction between 13 γ-tubulin molecules and the MT minus end. Electron microscopy revealed an open ring structure containing γ-tubulin clusters and similar clusters have been found on the minus ends of MTs [23]. In fungal and animal cells, components of the γTuRC preferentially appear at the spindle pole body and the centrosome [24]. In plant cells, γ-tubulin is spatially not restricted to MT ends but also colocalizes along MTs [25], [26] where it nucleates new MTs [27]. Plant GCP proteins are also required for nucleation of MTs. For example two core γTuRC components GCP2 and GCP3 decorate the nuclear envelope of tobacco (Nicotiana tabacum) BY-2 suspension cells and are required for MT nucleation and cell division [28], [22], [29]. Recently it has been shown that GCP4 is associated in vivo with γ-tubulin in Arabidopsis thaliana being an essential component for the function of γ-tubulin in MT nucleation and organization in plant cells [30]. However, the role of other GCP proteins in functional plant γTuRC remains unknown.
The precise coordination of the feeding site establishment and microtubule reorganization suggests that RKN can control the host's cytoskeleton. An example of this control is synchronous assembly of multiple disorganised and enlarged spindles and misaligned phragmoplasts [7]. These phragmoplasts fail to assemble a cell plate and consequently result in the formation of multinucleate cells. Whether these abnormal arrays are simply the remnants of a prematurely aborted cytokinesis or the result of specific rearrangement of MTs and microfilaments in response to parasite factors requires further investigation. However, all data demonstrate that abnormal cytokinesis is principal event for the establishment of the feeding site and successful completion of the nematode life cycle [8].
In order to investigate the role of γ-tubulin in the rearrangements of the cytoskeleton in nematode feeding cells [7] we have carried out nematode infection tests on roots of γ-tubulin mutant lines and show here that knockouts of either gene delays feeding site development. Immunocytochemical analysis show that γ-tubulin protein localizes to the cell cortex and cytoplasm of feeding cells, the nuclear surface and along malformed phragmoplasts. Moreover, γ-tubulin co-localizes and makes a complex with a component of γTuRC, GCP3 [22]. Our data demonstrate that accelerated growth of giant cells and establishment of functional nematode feeding site requires functional γTuRC.
Results
Expression of γTuRC Genes in Galls
To explore the role of γTuRC for gall development, we determined transcription levels of four key members (TUBG1, TUBG2, GCP3 and GCP4) by quantitative reverse transcriptase-mediated real-time PCR (qRT-PCR). The total RNA for the assays was extracted from Arabidopsis roots infected with M. incognita at three stages of gall development: at young stage (7 days after inoculation-DAI), intermediate stage (14 DAI), at a mature stage of gall development (21 DAI) and uninfected roots. Transcription levels augmented in galls for both γ-tubulin genes (TUBG1 and TUBG2) preferentially at early developmental stage (7 DAI) and for two GCPs (GCP3 and GCP4) at intermediate stages (14 DAI) of gall development (Figure 1).
Fig. 1. Analysis of Expression Levels of TUBG1, TUBG2, GCP3 and GCP4 in Galls. 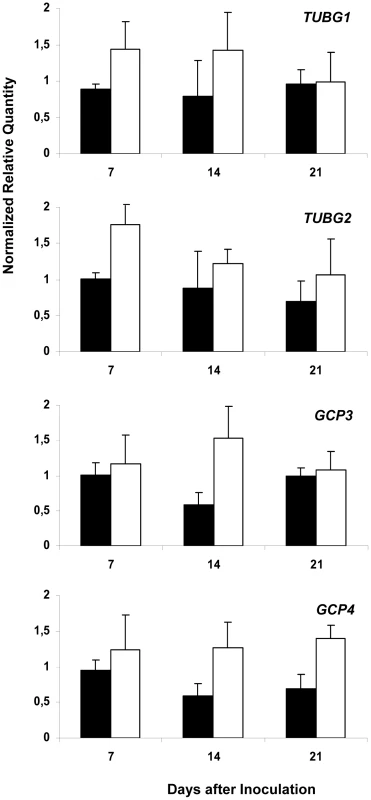
Relative amount of transcripts of TUBG1, TUBG2, GCP3 and GCP4 genes in Arabidopsis galls 7, 14 and 21 DAI (white bars) with Meloidogyne incognita by quantitative RT-PCR in comparison to uninfected condition (black bars). All values were normalized according to qBase with the two reference genes At5g62050 and At5g10790, and expressed as normalized relative transcript quantities. The bars are means ±SD of three independent biological replicates. Nematode Feeding Site Development
To address the functional significance of the upregulation of γTuRC genes during feeding site development, we investigated the morphology of galls at different developmental stages in 4 mutant lines (tubg1-1, tubg2-1, tubg1-1 tubg2-2 and amiR-GCP4-9) (Figure 2). Infection tests were performed to evaluate the competence of nematode development and reproduction in mutant lines (Figure 3). Cortical, epidermal and root hair cells of uninfected roots, of tubg1-1 and tubg2-1 of mature seedlings (40 days after sowing-DAS) were swollen, expanding isotropically (Figure 2A, 2E and 2I) but no perceptible phenotype was seen in the vascular tissue where galls develop. This phenotype was not detected in young roots (14 DAS) used for nematode infection (Figure S1; wild-type root 1A and mutant lines 1B to 1D) and nematode penetration or infection occurred normally (Figure S1E). Lateral root development also appeared similar in both wild-type and mutant lines. A lack of root phenotype in roots of both γ-tubulin mutant lines was also observed by Pastuglia et al. [20]. Based on these observations we predict that the mild phenotype present in mature uninfected roots (40 DAS) should not influence on gall development. The abnormalities of root morphology and cells expansion were more pronounced in the double mutant (Figure 2M). Although, knockdown of γ-tubulin genes had no discernable effect on the ability of nematodes to penetrate, migrate, and induce giant cells, feeding sites development was delayed at 7 DAI in both mutant lines (Figures 2B, 2F and 2J). At 14 DAI giant cells in γ-tubulin mutants were smaller and contained enlarged vacuoles (Figures 2C, 2G and 2K). At this stage nematodes often remained vermiform at stage 2 juvenile (J2) whereas in wild-type parasitic J2 were larger. At 21 DAI, wild-type plants contained typical multinucleated cells with dense cytoplasm (Figure 2D). In contrast, the infected roots of γ-tubulin mutant lines had smaller feeding cells (Figures 2H and 2L) with less nuclei and large vacuoles. The development of a fraction of nematodes was arrested at the parasitic juvenile stages. In the double mutant line (tubg1-1 tubg2-2) infection process resulted in tiny giant cells containing an average of two nuclei (Figures 2N). Uninfected roots of the GCP4 mutant line did not show any evident phenotype (Figure 2O) except for shorter root hairs. Young giant cells in the amiR-GCP4-9 line were smaller than in wild-type, contained a reduced number of nuclei and enlarged vacuoles (Figure 2P). At later stages of development (14 DAI and 21DAI) nematode development was delayed and giant cells remained small (Figure 2Q at 14 DAI). Examination of giant cell morphology correlates with nematode reproduction. Assessment of the nematode life cycle in γ-tubulin mutant lines (tubg1-1 and tubg2-1) showed arrest of half of galls development and delayed nematode maturation. Consequently, production of egg masses was significantly reduced (Figure 3). A similar analysis could not be performed with the double mutant since the plants are viable for only 3 weeks.
Fig. 2. Histological Analysis of Galls and Roots in γ-Tubulin Mutant and Wild-Type Arabidopsis seedlings. 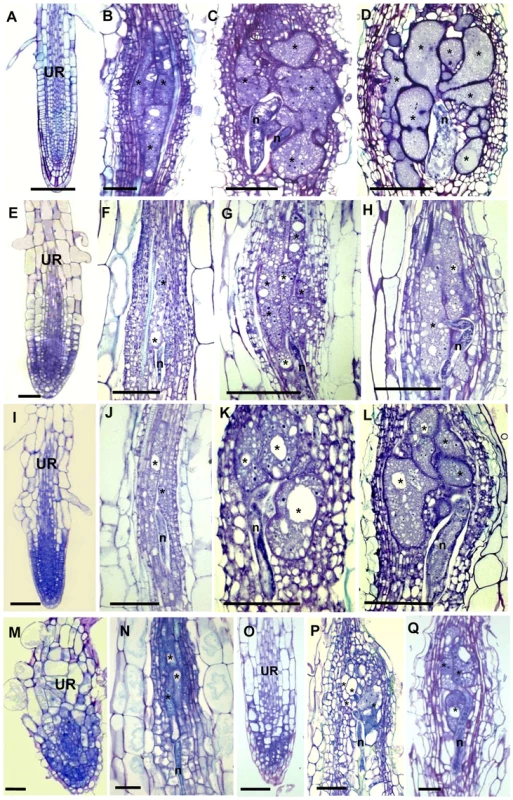
Bright-field images of sections stained with toluidine blue. (A) Uninfected root of wild-type seedlings 40 DAS. (B) Gall in wild type roots 7 DAI. (C) Gall in wild-type roots 14 DAI. (D) Gall in wild-type roots 21 DAI. (E) Uninfected root of the γ-tubulin mutant tubg1-1 seedlings 40 DAS. (F) Gall in tubg1-1 mutant 7 DAI. (G) Gall in tubg1-1 mutant 14 DAI. (H) Gall in tubg1-1 mutant 21 DAI. (I) Uninfected root of the γ-tubulin mutant tubg2-1 seedlings 40 DAS. (J) Gall in tubg2-1 mutant 7 DAI. (K) Gall in tubg2-1 mutant 14 DAI (L) Gall in tubg2-1 mutant 21 DAI. (M) Uninfected root of γ-tubulin double mutant tubg1-1 tubg2-2. (N) Gall in tubg1-1 tubg2-2 3 DAI. (O) Gall in tubg1-1 tubg2-2 7 DAI. (P) Gall in amiR-GCP4-9 7 DAI. (Q) Gall in amiR-GCP4-9 14 DAI. UR, uninfected root; Asterisks, giant cell; G, gall; n, nematode. Bars = 100 µm (A) to (D); 50 µm (E) to (L); 20 µm (M) to (Q). Fig. 3. Nematode Infection Test of γ-Tubulin Mutants tubg1-1 and tubg2-1 Compared to Wild-Type WS or Col-0. 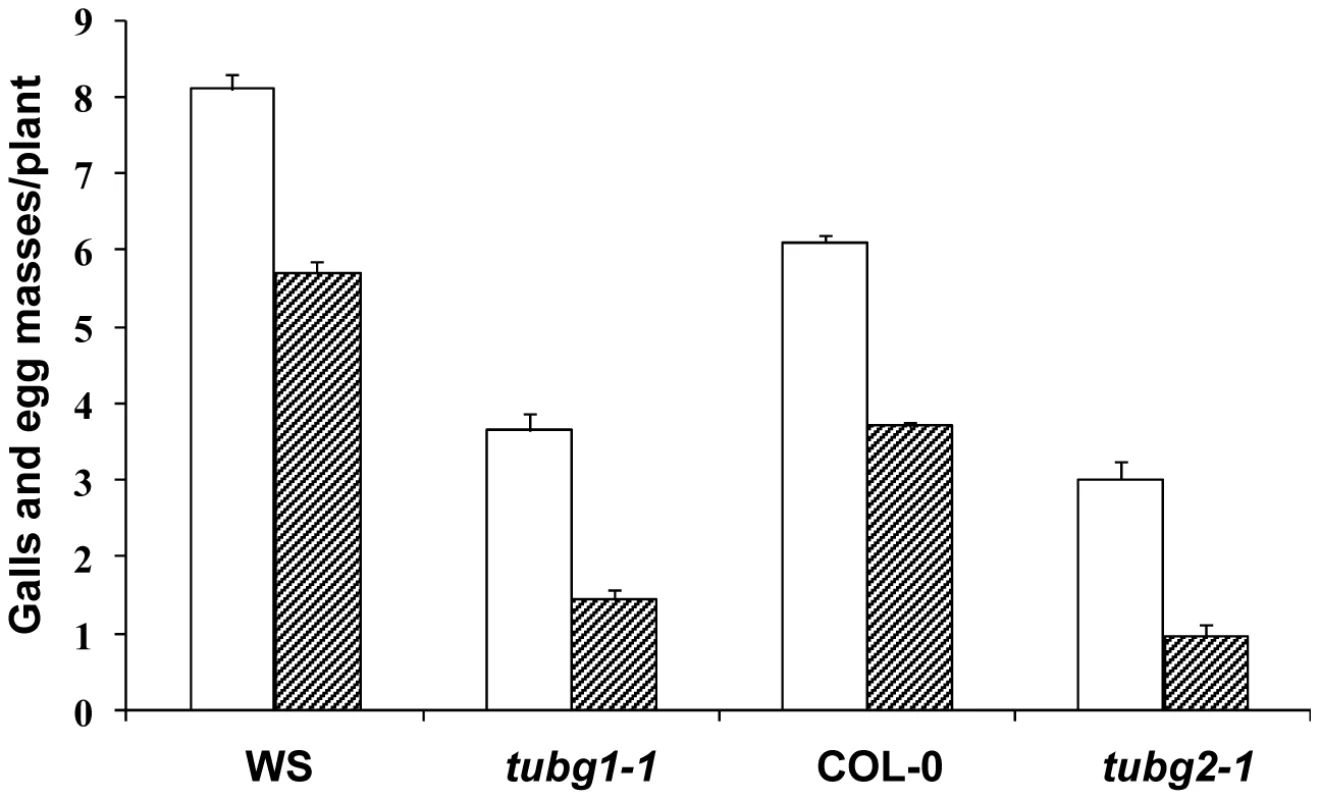
The number of galls (white bars) and egg-masses (lined bars) are significantly decreased in nematode infected roots of both mutant lines compared to wild-type. Data shown represent means±SD from at least two experiments in which a minimum of 60 seedlings of each line were evaluated for nematode infection. Statistically significant differences were determined by the one-way-ANOVA using the SPSS for Windows statistical data analysis package (P≤0.05). Localization of γ-Tubulin in Nematode Feeding Cells
The localization of γ-tubulin in galls of wild-type and mutant lines was analysed by immunocytochemistry (Figure 4) using a polyclonal antiserum [20]. In order to locate both γ-tubulin proteins separately in galls, we used the mutant tubg1-1 for TUBG2 localization and , tubg2-1 for TUBG1 detection. In wild-type and mutant roots (tubg1-1, tubg2-1 and tubg1-1 tubg2-2) γ-tubulin staining was observed in all root cells (Figure 4D). At 14 DAI galls of wild-type seedlings, γ-tubulin protein was localized throughout the giant-feeding cells and fewer label was detected in the neighbouring cells (Figure 4A and Figure S2A and S2D). At the same stage of infection, roots of tubg1-1 revealed γ-tubulin staining in the giant-cell cortex and less in the cytoplasm (Figure 4B and Figure S2B and S2E) while in tubg2-1 mutant γ-tubulin was localized along the giant cell cortex and around the nuclei (Figure 4C and Figure S2C and S2F). Weak γ-tubulin expression was seen in giant cells of tubg1-1 tubg2-2 (Figure 4E).
Fig. 4. Immunofluorescence Detection of γ-Tubulin on Galls and Roots in Mutants and Wild-Type Arabidopsis seedlings. 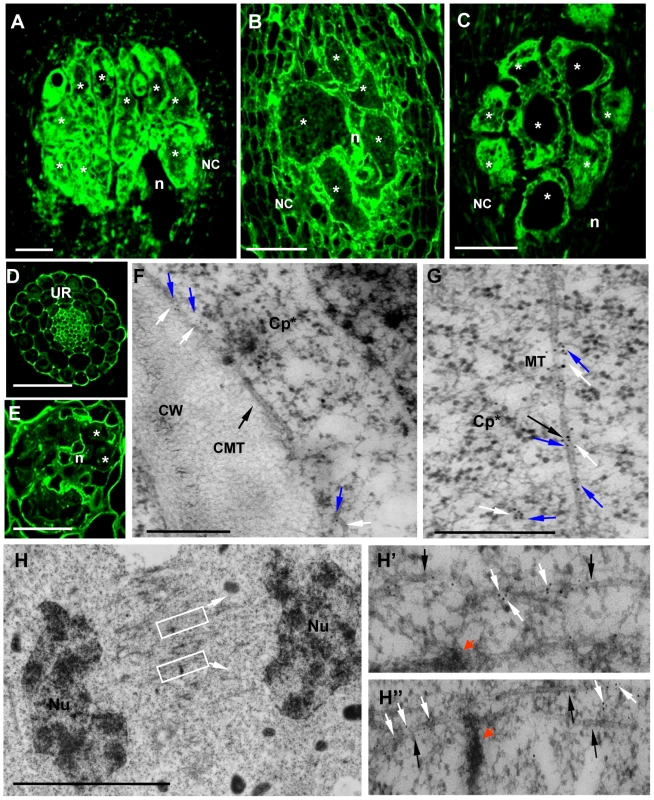
Galls 14 DAI of wild-type (A), of tubg1-1 (B), of tubg2-1 (C). Uninfected root of wild-type seedling (D). Gall 7 DAI of tubg1-1 tubg2-2 (E). Dissected galls (14 DAI) were sectioned and processed for double immunoelectron microscopy with anti-γ- and α-tubulin primary antibodies, followed by secondary 10 and 5 nm gold-conjugated antibody respectively. (F) γ-tubulin (white arrows) is localized with cortical MT (black arrow) and associated with α-tubulin (blue arrows). (G) γ-tubulin binds to cytoplasmic MT. (H), (H') and (H'') γ-tubulin is dispersed throughout the misaligned phragmoplast MTs and co-localizes with α-tubulins. Fragments of cell wall are visible at MT ends as dark patches (red arrows) possibly inducing failure in giant cell cytokinesis. Asterisks, giant cell; n, nematode, UR, uninfected root; NC, neighboring cells; CMT, cortical microtubule; Cp* giant cell cytoplasm; CW, cell wall; MT, microtubule; Nu, nucleus. Bars = 50 µm in (A) to (E), 500 nm in (F) and (G) and 5 µm in (H). Immuno-gold analysis of the infected wild-type plants (14 DAI) by electron microscopy demonstrate that γ-tubulin co-localize with MTs (α-tubulin) in the giant cell cortex (Figure 4F), cytoplasm (Figure 4G), at the nuclear surface (Figure S3B and S3B') and with the phragmoplast during mitosis (Figure 4H to 4H'). Cell wall fragments were visible at the MT ends as dark patches of severely misaligned phragmoplasts (Figure 4H and 4H'). Free cytosolic γ-tubulin is apparent in Figure S3A.
Spindles in Root Cells of γ-Tubulin Mutants of A. thaliana Are Curved
Crosses between γ-tubulin mutant lines (tubg1-1 and tubg2-1) and marker lines expressing Pro35S-MBD:GFP and nuclear histone H2B:YFP were used to study microtubule organization in the mutant background (Figure S4). Mitotic spindles were bowed and chromosomes were often misaligned in both γ-tubulin mutant lines (Figure S4A for tubg1-1 and 4B, 4C for tubg2-1) in comparison with wild-type root cells (Figure 4D).
GCP3 Co-localizes with γ-Tubulins in Nematode Feeding Cells
GCP3 localization was observed in giant cells of wild-type (Figure 5A) as well as tubg1-1 (Figure 5B) and tubg2-1 (Figure 5C) plants concentrating around the nuclei and cell cortex. Co-localization of GCP3 and γ-tubulin was analysed using electron microscopy. Both proteins were found in the cytoplasm (Figure 5D), at the nuclear surface (Figure 5E and 5E'), and at the cell cortex (Figure S5A and S5A'). Commonly, multiple colloidal gold particles corresponding to γ-tubulin and GCP3 grouped together forming compact clusters in the cytoplasm (Figure 5D). We have measured the distance between closest gold particles and found that majority of them were in the proximity of less than 10 nm (Figure 5F). These data provide compelling evidence for the interaction between GCP3 and γ-tubulin in giant cells as part of a single multi-protein complex.
Fig. 5. γ-Tubulin Complex Protein 3 (GCP3) is Present in Giant Cells and Co-localizes with γ-Tubulin. 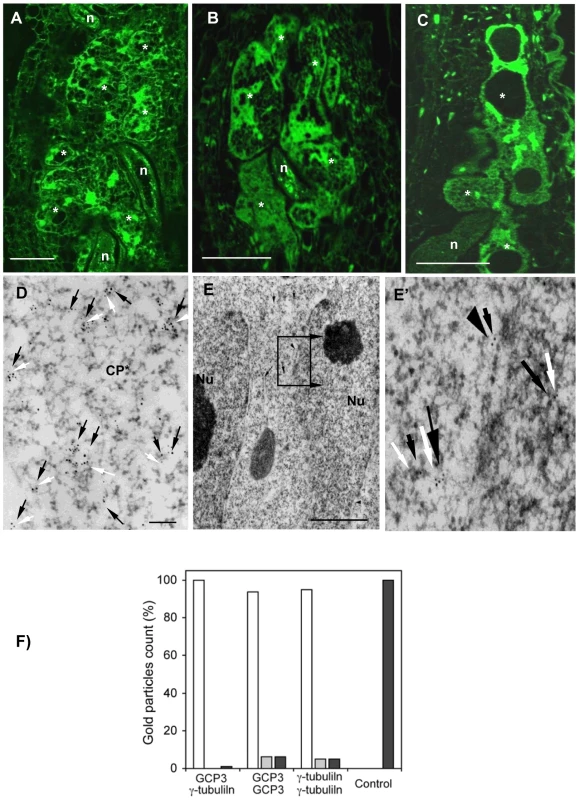
(A) to (C) Immunostaining of GCP3 (green) in galls 14DAI of wild-type and mutant lines. Gall in wild-type roots (A). Galls in tubg1-1 roots (B). Galls in tubg2-1 roots (C). Dissected wild type galls (14 DAI) were sectioned and processed for double immunoelectron microscopy with anti-GCP3 and γ-tubulin primary antibodies, followed by secondary 10 and 5 nm gold-conjugated antibody respectively. GCP3 (black arrows) and γ-tubulin (white arrows) co-localize in the cytoplasm (D) and at the nuclear surface (E, E'). Both proteins are also present as monomers in the cytoplasm. (F) Histogram illustrating distances between gold particles showing that GCP3 and γ-tubulin are often in proximity less than 10 nm suggesting their interaction. White bars are for distances less then 10 nm, grey for distances between 10 and 50 nm, and black bars for distances above 50 nm. Asterisks, giant cell; n, nematode; Nu, nucleus; Cp*, giant cell cytoplasm. Bars = 50 µm (A) to (C); 100 nm (D); 300 nm (E). Analysis of TUBG1-GFP Localization in Uninfected and Nematode Infected Roots
35Spro:TUBG1-GFP and 35Spro:GFP-TUBG1 were transiently expressed in tobacco leaf cells using Agrobacterium infiltration. GFP fluorescence was observed in the Arabidopsis cytoplasm and in the nucleus for both constructs (Figure S6) and discrete fluorescent dots were apparent in the cytoplasm and cell cortex. Arabidopsis seedlings were transformed with 35Spro:TUBG1-GFP and 35Spro:GFP-TUBG1 constructs and the F1 generation was analyzed for GFP expression. Seedlings containing the C-terminal fusion (TUBG1-GFP) showed the best germination efficiency. The roots of seedlings germinated on the vertical plates exhibit skewing to the left if observed from the top side of the plate (Figure S6A) and the leaves were curling (Figure S7C) compared to wild-type (Figure S7B and S7D respectively). Confocal microscopy imaging of roots revealed a left-handed twisting phenotype (Figure 6A and 6B). The twisting is likely to result from the miss-shaping of the root cells and disorganization of the root tissues as shown in a cross section (Figure 6F compared to 6G). Sections of a shoot apex (Figure 6H compared to 6I) showed right-handed displacement of the cells. To verify if twisting was caused by the altered properties of MTs we treated γ-tubulin overexpressing seedlings with MT polymerisation inhibitors propyzamide and oryzalin. Both treatments inhibited root skewing and left-handed twist of the epidermal cell layers implying that ectopic expression of γ-tubulin increase stability of the microtubules leading to the organ twisting phenotype (Figure 6C, 6D compared to a twisted root in 6E). Plants expressing γ-tubulin-GFP lacking a twisted phenotype but still showing GFP-fluorescence were used for localization studies. Transgenic root apical meristem and lateral root meristem displayed a patchy expression pattern (Figure 6J and 6K) that varied between different roots suggesting a post-transcriptional control of γ-tubulin expression. In mitotic cells γ-tubulin was localized to the spindle (Figure 6L) and the phragmoplast (Figure 6M). During the interphase, multiple discrete foci and homogeneous fluorescence were observed in the cytoplasm (Figure 6L inset and 6M).
Fig. 6. Ectopic Expression of γ-Tubulin in Arabidopsis thaliana Seedlings Causes Root Twisting and Leaf Curling. 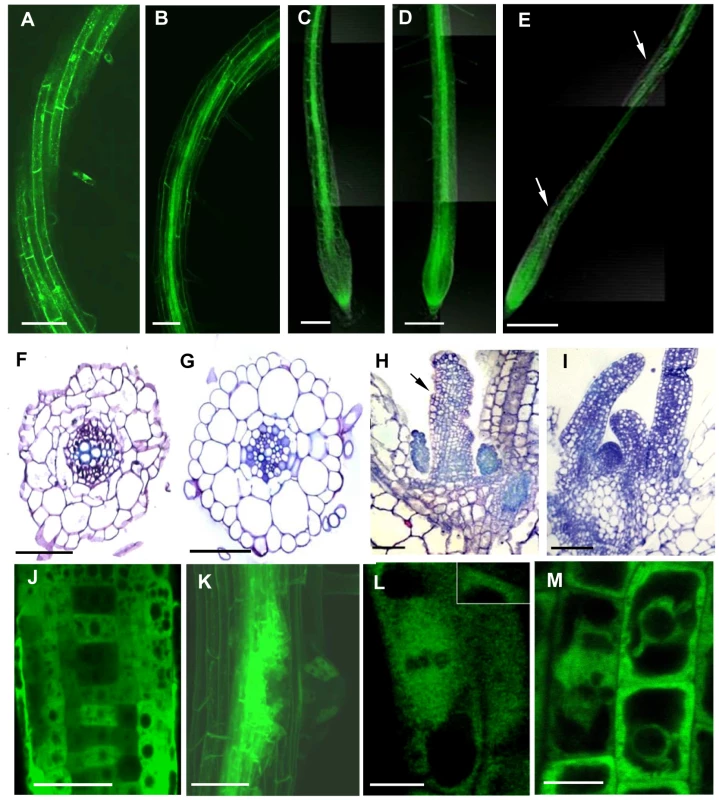
γ-Tubulin overexpressing roots show twisted phenotype (A) and (B). Roots treated with either oryzalin (C) or propyzamide (D) did not show root twisting as observed (arrows) in the γ-tubulin overexpressing untreated roots (E). Cross section of a γ-tubulin overexpressing root stained with toluidine blue showed miss-shaping of the root cells and disorganization of the root tissues (F) compared to the wild-type (G). Longitudinal section of a shoot apical meristem stained with toluidine blue showing a leaf curling phenotype (black arrow) of γ-tubulin overexpressing seedlings (H) compared to the wild-type (I). γ-tubulin localization (green) in the root elongation zone (J) and lateral root meristem showing a patchy expression pattern (K). γ-tubulin localization along a spindle (L) and a phragmoplast (M). Bars = 100 µm in (A) to (E); 50 µm (F) to (I), (J) and (K); 20 µm (L) and (M). We investigated the mobility of γ-tubulin in the cytoplasm using fluorescence loss in photobleaching (FLIP) analysis. Most of the γ-tubulin-GFP fusion protein was found to be highly mobile (Figure S8) suggesting that only a minor fraction of total γ-tubulin is utilised in the nucleation of microtubules.
γ-Tubulin-GFP fluorescence was also observed in the cells of uninfected root vasculature (Figure 7A) as well as throughout all gall tissues (Figure 7B). Young giant cells (3 DAI and 5 DAI) showed concentration of γ-tubulin around the nuclei and diffuse fluorescence in the cytoplasm (Figure 7C and 7D). At the later stages (7 DAI and 10 DAI) γ-tubulin expression was observed in all gall and giant cells (Figure 7E, 7E' and 7F) and a speckled fluorescence was notable around the perinuclear cytoplasm (Figure 7G).
Fig. 7. γ-Tubulin Localization and Overexpression in Giant Cells of Nematode Infected Roots of the TUBG1-GFP line. 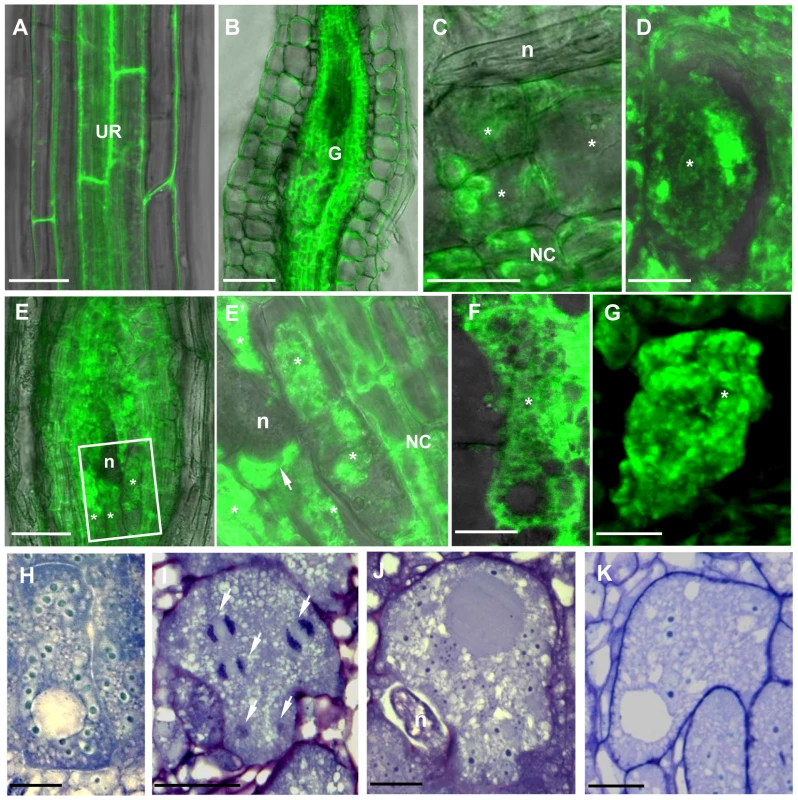
(A) γ-Tubulin localization in an uninfected root. (B) γ-Tubulin localization in a whole gall. (C) γ-Tubulin localization in young giant cells 3 DAI (D) and 5 DAI. (E) γ-Tubulin localization in a whole gall 7 DAI and (E') detail of a giant cell showing accumulation of γ-tubulin protein close to the nematode head (arrow). (F) γ-Tubulin localization in a giant cell 10 DAI. (G) γ-Tubulin localization around the nuclei of a giant cell 7 DAI. (H) Giant cell overexpressing γ-tubulin 7 DAI. (I) Mitotic events in a giant cell (white arrows) overexpressing γ-tubulin 14 DAI. (J) Giant cell overexpressing γ-tubulin 21 DAI. (K) Giant cell in a wild-type gall 14 DAI. UR, uninfected root; Asterisks, giant cell; G, gall; NC, neighbouring cells; n, nematode, Nu, nucleus. Bars = 50 µm in (A) and (B), 20 µm in (C), (E), (H) and (K), 10 µm in (D), (G), (I) and (J), 5 µm (F). Young (7 DAI; Figure 7H) as well as transitional and mature giant cells (14 DAI and 21 DAI; Figure 7I and 7J) remained small and contained more nuclei than wild-type control (Figure 7K). Mitotic chromosomes were detectable in giant cells of γ-tubulin overexpressing lines (Figure 7I), but not in the wild-type (Figure 7K). Area measurements on giant cells confirmed a decreased size compared to wild-type (Figure 8A). Nuclei counts per section at the core of giant cells (7 DAI) validated the observation of a larger number of nuclei per giant cell when under ectopic γ-tubulin expression (Figure 8B). Infection tests have shown a decrease in gall number and egg mass production in γ-tubulin overexpressing lines (Figure 8C).
Fig. 8. Giant Cell Area, Number of Nuclei and Infection Tests of γ-Tubulin Mutants Compared to Wild-Type. 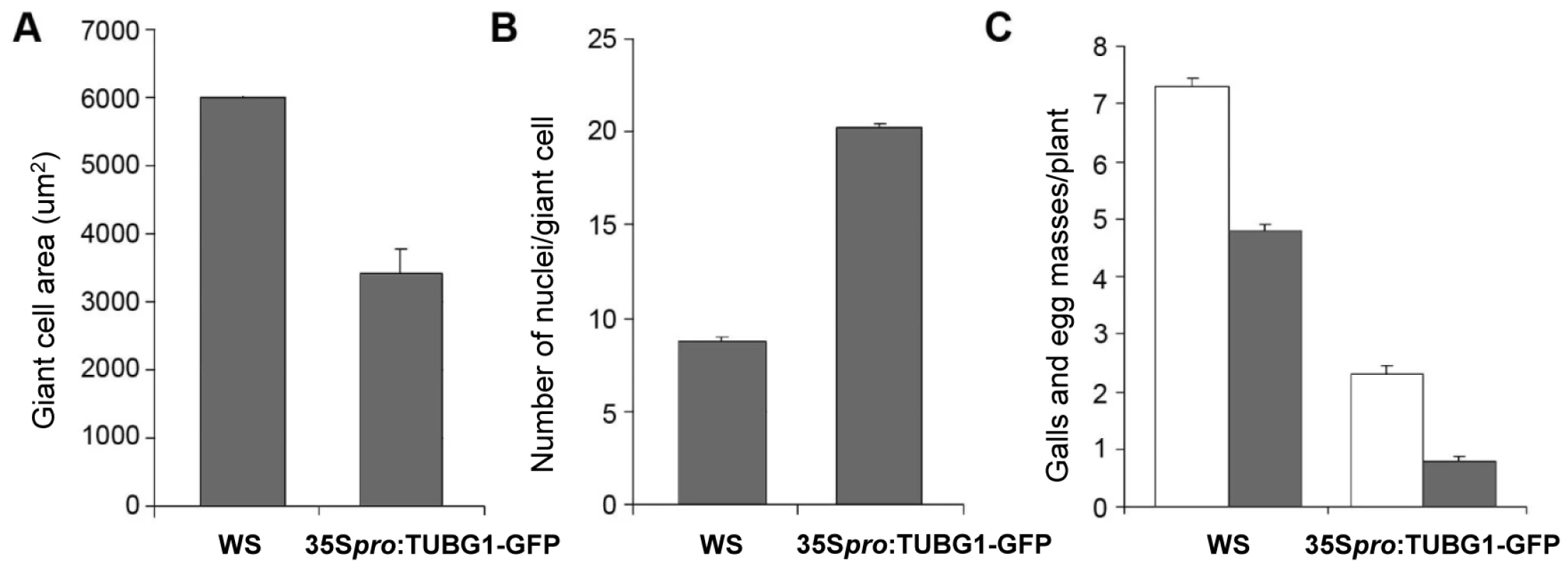
Giant cell area (A) and number of nuclei (B) in roots under ectopic expression of γ-tubulin compared to wild-type and nematode infection test (C) of roots under ectopic expression of γ-tubulin. Area was measured on 60 giant cells. Number of nuclei was counted on 60 giant cells. The number of galls (white bars) and egg-masses (lined bars) are significantly decreased under ectopic expression of γ-tubulin compared to wild-type. Data shown represent means±SD from at least two experiments in which a minimum of 60 seedlings of each line were evaluated for nematode infection. Statistically significant differences were determined by the one-way-ANOVA using the SPSS for Windows statistical data analysis package (P≤0.05). Discussion
Role of γ-Tubulin and GCP3 in Nematode Infection
Transcription analyses demonstrate augmentation of the γ-tubulin (TUBG1 and TUBG2) and two γ-tubulin-complex protein genes (GCP3 and GCP4) in the course of infection with root-knot nematodes in galls. This corroborates with in situ hybridization analysis which has shown the transcriptional activation of the γ-tubulin genes in giant feeding cells as well as in neighbouring tissues that derived from M. incognita targeted vascular root cells [7]. The transcription levels of TUBG1 and TUBG2 were higher at an early stage of gall development (7 DAI) while GCP3 and GCP4 transcription increased at an intermediate stage (14 DAI). The transcription of three genes (TUBG1, TUBG2 and GCP3) declined in mature giant cells (21 DAI) coinciding with the end of the mitotic activity in giant-feeding cells. In addition to γ-tubulin proteins, both GCP3 and GCP4 are indispensable components for the MT nucleating activity of γTuRC in plant cells [22], [30]. This suggests that an optimum level of γ-tubulin and GCP in galls is necessary to provide a sufficient number of new microtubule nucleation sites for the remodelling of the MT network in giant-feeding cells and to support recurrent ongoing mitotic activity in both giant - and neighbouring cells [7].
Analysis of the T-DNA knockout lines demonstrates the importance of γ-tubulin for the establishment of the feeding site and completion of the parasite life cycle. No visible phenotype was observed in the vasculature of single knockout plants before infection by the root-knot nematodes in agreement with the partial functional redundancy of TUBG1 and TUBG2 proposed by Pastuglia et al. [20] and Binarova et al. [19]. Both double mutant tubg1-1-tubg2-2 and RNAi knockdown plants exhibit disruption of anisotropic cell expansion [19], [20] in a similar manner to the drug-induced reduction of the number of nucleation sites and randomization of cortical MTs [31]. Although nematodes were able to penetrate, migrate, and induce gall formation in roots of tubg1-1 and tubg2-2 lines, there was a significant delay in feeding site formation, indicating that both γ-tubulin genes are required for proper nematode feeding site development. Consequently, the number of nematodes that could complete their life cycle and reproduce was significantly lower in both T-DNA lines as compared to wild-type. It has been demonstrated in γ-tubulin knockout lines that reduced MT nucleation can delay chromosome separation and nuclear proliferation, thereby inhibiting cell division and impairing growth polarity [32]. We have observed that residual levels of γ-tubulin in the double knockout line is sufficient for gall induction in agreement with the dose-dependent effect of γ-tubulin on the early feeding site development and underlines the functional significance of a rise of γ-tubulin gene transcription.
Localization of γ-tubulin throughout giant cell differentiation illustrates its role during nematode infection. At the early stages of gall development (3 DAI) γ-tubulin is mainly localized at the nuclear surface in giant-feeding cells as well as neighbouring cells. As giant cells matured, a scattered GFP fluorescence was also detected in the cytoplasm and cell cortex. Accumulation of γ-tubulin close to the nematode head suggests reorganization of the microtubule network in the region proximal to the secretion and/or feeding processes, consistent with the accumulation of ER and other organelles at this site (de Almeida Engler, unpublished data). At this stage, nematodes are alternately injecting secretions and feeding on the cell cytoplasm. It is known that the cytoskeleton acts differently depending on the plant host and the invading pathogen, and that the MT response to infection is variable between different plant-microbe interactions [33], [34]. So far, the molecular mechanisms regulating MT dynamics during host/parasite interactions is not well understood and our data suggest that increased γ-tubulin expression might be part of this apparatus.
γ-Tubulins Co-localize with MT and GCP3
Immunolocalization experiments demonstrate that γ-tubulin and GCP3 co-localize each other and MTs around the nuclei, in the cell cortex and in mitotic MT arrays. Therefore dispersed free or MT associated γTuRCs in giant cells might provide nucleation of new MTs required for fast array reorganization in giant cells. γ-Tubulin was shown to associate with preprophase bands (PPBs), spindle, phragmoplast and cortical cytoplasm of soybean, onion, Arabidopsis and cells of other species [17], [25], [35], [36], [37]. Each MT array appears to have multiple sites for the nucleation of new MTs which can be located along the lattice of extant MTs, resulting in branching of cortical MTs. This activity may result in the array composed of randomly oriented bundles observed in the giant cell cortex. In addition, γ-tubulin cooperates with other known regulators of MT organization including MOR1/GEM1 and MAP65 to regulate MT organization in giant cells [8], [38], [39], [40], [41].
Although γ-TuRC components are conserved in plant genomes, their association in a functional complex has not yet been proven. The pioneering study of Seltzer et al. has provided biochemical evidence for the association of Arabidopsis GCP2, GCP3 and γ-tubulin in the cytoplasmic soluble complex [22]. Recently Nakamura et al. [42] have shown that all six members of the Arabidopsis γ-TuRC immunoprecipitate jointly. Immunogold electron microscopy shows that γ-tubulin and GCP3 are located less then 10 nm apart from each other proving further evidence for the existence of γ-TuRC in vivo and its close association with the MT lattice.
Both GCP2 and GCP3 localize at the nuclear envelope and play a role in MT nucleation [22], [28] and GCP2 can control organization of cortical MTs by positioning the γ-tubulin-containing complex on pre-existing MTs [29], [43]. The presence of abnormal spindles in uninfected Arabidopsis roots of tubg1-1 and tubg2-2 lines agrees with the previous observations of collapsed or defective spindles and chromosome segregation defects in γ-tubulin mutants of several species such as S. pombe, S. cerevisiae and Drosophila melanogaster [14], [44], [45], [46], [47]. In addition, γ-tubulin depletion in A. nidulans abolishes nucleation of spindle MTs [48]. This suggests that γ-tubulins have conserved functions in organizing the spindle in phylogenetically distant organisms. Furthermore, a reduction of the γ-tubulin signal seen on spindle and phragmoplast in amiR-GCP4 cells suggests their interaction with these arrays during mitosis in plant cells [30].
Overexpression of γ-Tubulin Induces Stability of Microtubule Network
The localization of γ-tubulin-GFP in living root cells corroborates with results obtained by immunostaining. Indeed, a strong GFP signal was observed on mitotic spindles and phragmoplasts during mitosis as well as during interphase around nuclei, a known site of MT nucleation. The apparent speckled fluorescence in the cytoplasm indicates the presence of discrete γTuRCs. A similar distribution pattern has been observed in BY-2 cells transiently expressing a GFP-γ-tubulin fusion protein. There, at the end of cell division γ-tubulin was firstly accumulated at the daughter nuclear surfaces and evenly spread along the cell cortex [49], [50]. In animals, γ-tubulin is also important for the coordination of late mitotic events [51] and has a MT-independent function in mitotic checkpoint control [52]. Whether plant γ-tubulin performs similar functions remains unknown, but it can not be ruled out that reduction of nuclei number in the giant cells of the double mutant results from the deficiency in these activities.
Our FRAP analysis indicate that the absolute majority of γ-tubulin-GFP freely distributes in the cytoplasm supporting the idea that microtubule nucleation events in plants are driven by γ-tubulin-containing complexes released from the nucleation sites and redistributed around the cell. Electron microscopy observations confirm the cytoplasmic distribution of γ-tubulin and the presence of a large number of closely associated γ-tubulin and GCP3 proteins pointing towards their interaction.
Analysis of the ectopic γ-tubulin expression in plants shows a curling phenotype in leaves and skewing of roots, suggesting an alteration in dynamics and/or organization of MT arrays. Treatment of seedlings with oryzalin and propyzamide restored the normal pattern indicating that overexpression of γ-tubulin leads to MT stabilization or excessive nucleation. It is also plausible that ectopic expression of γ-tubulin in giant cells provokes higher mitotic activity by accelerating nuclear division. In addition, excessive nucleation of MTs might impede giant cell expansion, nematode feeding and interfere with gall development and eggmass production. Our previous reports suggested that cytoskeleton stabilization by drug treatment (taxol) or ADF2 downregulation can disturb gall development and consequently nematode maturation [7], [9].
Microtubule Dynamics in Giant Cells and Concluding Remarks
Taking into account present and previous data [7] we propose a model for MT dynamics in giant cells (Figure 9). The transcription analysis shows that two components of plant MTOCs (γ-tubulin and GCPs) are highly expressed in galls. Knockout of either TUBG1 or TUBG2 genes results in inhibition of gall and nematode development, demonstrating that both proteins are important for feeding site formation, while in non-infected plants gene redundancy has been observed [20]. Predominant localization of TUBG2 at the giant cell cortex compared to a more cytoplasmic distribution of TUBG1 suggests that they might exert different functions within these large feeding cells. Double knockout completely abolishes gall development. The reduction of γ-tubulin protein level compromises the MT network integrity. Our previous findings demonstrate the significance of MT and actin filaments during interphase as well as mitosis [7]-[9] for successful nematode infection and reproduction. γ-tubulin is crucial for the organization and function of mitotic spindle and phragmoplast.
Fig. 9. Microtubule Organization in Giant Cells. 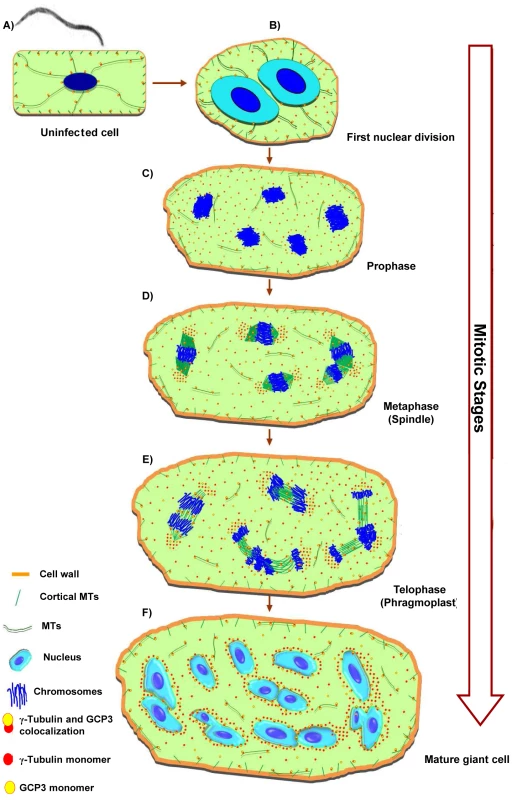
The model of giant cell cytoskeleton reorganisation is based on observations of a large number of gall sections. The illustrations do not precisely reflect the total number of chromosomes or nuclei effectively present per giant-cell. Root-knot nematodes invade root cells and induce vascular cells (A) to become giant-feeding cells (B) to (F). The first visible symptom of nematode infection on the microtubule cytoskeleton of a young giant cell is the increase in density of tubulins in the cytoplasm. At this stage, the first nuclear division results into two enlarged nuclei with outsized nucleoli (B). Giant cells contain a dense cytoplasm and scarce cytoplasmic microtubules which co-localize with GCP3 protein. Young giant cells contain a dense network of randomly distributed cortical microtubules bound to γ-tubulins, and GCP3 (A) to (F). During giant cell expansion the cytoplasm contain scarce microtubules, and γ-tubulins and GCP3 proteins. The cytoplasmic microtubules remain disarrayed throughout giant cell development. Mitotic giant cells harbour nuclei containing a large number of condensed chromosomes often dividing in synchrony (C) to (E). During prophase nuclei are often clearly separated containing their packed chromosomes (C). In the course of metaphase to telophase spindles are large and malformed (D). Accumulation of γ-tubulin and GCP3 occurs mainly around the chromosomes and on the spindles. γ-tubulin and GCP3 localize to giant cell phragmoplasts which are misaligned and fail to expand centrifugally resulting in aborted cytokinesis (E). Some nuclei appear to show incomplete division or to have fused (F). The density of cortical microtubules is reduced and and cytoplasmic microtubules are sparse. Mature giant cells finally present multiple lobed nuclei which recurrently appear connected to each other, are surrounded by γ-tubulin and GCP3 proteins which are often co-localized suggesting the presence of MTOCS at these sites of giant cells. Our data suggests that γ-tubulin and GCP3 recruitment contributes to microtubule nucleation in mitotic and cortical arrays in root-knot nematode feeding cells. The overall effect of one or both γ-tubulin and the GCP4 genes knockout on the MT network of giant cells agrees with the hypothesis that γTuRC proteins including γ-tubulins and GCP4 are required for proper functioning of mitotic arrays and MT nucleation. Bowed spindles observed in root tip cells can be caused by abnormal nucleation of MTs. Since unusually shaped and enlarged spindles are normally observed in giant cells, these anomalies might be caused by the unbalance between γ-tubulin and GCP3 concentration in giant cells, ultimately resulting in aneuploid nuclei that failed to divide [5], [53]. High γ-tubulin and GCP3 concentration may play a role in the formation of disarrayed phragmoplasts observed in giant-feeding cells and might contribute to the misalignment of the cell plate and arrest in giant cell division.
The apparent fragmentation and reduction in density of interphase MTs in giant cells could result from defective nucleation. Nucleation, dynamics, and spatial organization of MTs are tightly coordinated processes in plant cells. Proteins secreted by nematodes may induce MT reorganization by altering MT dynamics. Up-regulation of γTuRC proteins in galls provides an excess of MT nucleation sites, which in combination with other factors might control MT dynamics leading to the reorganization of the entire array by site-specific destabilization/stabilization activity.
Microtubule response to pathogen invasion depends on the type of plant–microbe interactions [33] and nematodes are the only known pathogens capable of inducing the long-term cytoskeleton rearrangements on their host plants. The local reduction of density of the cytoplasmic MTs may facilitate susceptibility of host plants to nematodes in a similar manner to other systems. For example, microtubule depolymerisation at the infection site has been observed in the soybean and parsley cells attacked by the oomycete Phytophthora sojae [54], [55]. Stabilization of MTs by taxol blocks gall development, while breakdown permits nematode reproduction [7]. Although what stimulates cytoskeletal responses in nematode feeding cells is still not known, MT and actin rearrangements might be directly or indirectly induced by effectors secreted by the nematodes still to be identified.
Here we show that a tight balance between MT nucleation and dynamics is required for the successful nematode infection and γTuRC is essential to exercise this balance. The knockout of the individual components of γTuRCs reduces the efficiency of MT nucleation and consequently affects gall development and inhibits nematode reproduction, while the overexpression of γ-tubulin causes overall stabilisation of the MT network and produces a similar effect on the nematode life cycle. Since upregulation of MT nucleation in developing giant-feeding cells is essential for nematode parasitism, the components of γTuRC can be envisaged as potential targets to design alternative strategies to control pathogen invasion and spread.
Materials and Methods
Plant Material, Growth Conditions, Nematode Inoculation and Infection Tests
T-DNA mutagenized lines of the two γ-tubulin genes of Arabidopsis thaliana were obtained from the Versailles and SALK collections as described by Pastuglia et al. [20]. The CVP11 (tubg1-1) and T628 (tubg2-2) lines have been acquired from a T-DNA–mutagenised population of the ecotype Wassilevskija (WS) from Pastuglia et al. [56]. The SALK_004612 (tubg2-1) line was acquired from the ABRC and originates from a T-DNA–mutagenised population of the Columbia-0 (Col-0) ecotype [57]. Surface-sterilized seeds of WS, Col-0 and γ-tubulin knockdown lines were germinated on Murashige and Skoog medium (Duchefa, Haarlem, the Netherlands) containing 1% sucrose and 0.8% plant cell culture–tested agar (Sigma-Aldrich) and 50 mg/mL kanamycin for T-DNA mutagenised lines. After 10 days under growth chamber conditions of 16-h/8-h light/dark cycles at 25°C kanamycin resistant seedlings were transferred to Knop medium which favors root development [58]. Plates were inclined at an angle of 60° to allow the roots to grow along the surface to facilitate harvesting of roots and galls. For nematodes inoculation, around 100 surface-sterilized, freshly hatched second stage juveniles (J2s) of Meloidogyne incognita were adjusted on each 14 day old seedling as previously described [9]. For nematode infection tests seedlings were kept in Knop medium during 60 days to allow nematodes to complete their life cycle. At 14 days after inoculation galls were counted and after 60 days egg mass numbers were scored.
Quantitative RT-PCR
Total RNA of non-meristematic roots and galls of A. thaliana cv. WS dissected at various time points after nematode inoculation (7, 14, 21 DAI) was extracted according to the procedure described by Laroche-Raynal et al. [59]. One microgram of high-quality RNA was reverse-transcribed using the iScript cDNA Synthesis Kit (Bio-Rad, Marnes-la-Coquette, France). Amplification and detection were performed in the Opticon 2 system (MJ research, Bio-Rad). Reaction mixtures were of a final volume of 15 µL, containing 7.5 µL qPCR MasterMix Plus For SYBR Green I No Rox (Eurogentec, Angers, France), 0.13 µM of each primer and 5 µL of 50-fold diluted cDNA templates. PCR conditions were as follows: 95°C for 15 min, followed by 40 cycles of 95°C for 15 s, 58°C for 30 s and 72°C for 30s. At the end of the programme, a melting curve (from 65 to 95°C, read every 0.5°C) was determined to ensure that only single products were generated. At5g10790 and At5g62050 were used for normalization of qRT-PCR data. These two genes were previously identified as showing constant expression in response to different biotic stimuli [60], [61], [62]. Raw data were treated using the MJ Opticon Monitor Analysis software (version 3.1; Bio-Rad). Quantifications were performed with the modified ÄCt method employed by the qBase1.3.5 software, and expressed as normalized relative quantity. The primer list is given in Table S1. Three independent quantitative RT-PCR reactions were carried out per sample and three biological replicates were performed. The bars represent mean values from three independent experiments.
Morphological Analysis
For morphology observation of uninfected roots and galls of γ-tubulin knockout lines compared to control WS and Col-0 (tubg1-1 line was made in WS and tubg2-1 in Col-0; 20), samples grown in vitro were fixed in 2% glutaraldehyde in 50 mM PIPES buffer, pH 6.9, and then dehydrated and embedded in Technovit 7100 (Heraeus Kulzer) as described by the manufacturer. Embedded roots and gall tissues were sectioned (3 µm) and stained in 0.05% toluidine blue and mounted in Depex (Sigma-Aldrich). Microscopic observations were performed using bright-field optics and images were performed with a digital camera (Axiocam, Zeiss).
Immunofluorescence Analyses of γ-Tubulin, α-Tubulin and GCP3
Uninfected roots and feeding sites of roots, inoculated with J2s of M. incognita, of A. thaliana cv. WS, Col-0, tubg1-1, tubg2-1, and tubg1-1 tubg2-2 mutant lines were fixed in 4% formaldehyde in 50mM Pipes buffer (pH 6.9). Dissected galls (7, 14, and 21 DAI) were dehydrated and embedded in butyl-methylmethacrylate as described by Kronenberger et al. [63] with some modifications. The immunolocalization procedure was performed essentially as described by de Almeida-Engler et al. [7]. Slides containing sectioned nematode feeding sites were incubated with acetone absolute for 30 min to remove the plastic. Primary and secondary antibodies were diluted 1∶300 and 1∶500 (v/v) respectively, in blocking solution (1% bovine serum albumin in 50 mM Pipes, pH 6.9). Sections were incubated with blocking solution for 30 min, and overnight at 4°C with the primary antibodies. As controls, sections were incubated with pre-immune serum or without primary antibodies. Anti-γ-tubulin and anti-GCP3 were generated respectively as described by Pastuglia et al. [20] and Seltzer et al. [22]. First, incubation with a polyclonal anti-γ-tubulin or anti-GCP3 has been performed and slides were then washed in Pipes buffer for 15 min. Slides were subsequently incubated for 2 h at 37°C with the secondary antibody anti-rabbit Alexa 488 (green fluorescence) or Alexa 594 (red fluorescence) and washed in Pipes buffer for 15 min. For DNA visualization sections were stained with 4′, 6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich) at 1 µg/ml in Pipes buffer. Slides were mounted in 90% glycerol in ddH2O and were observed with a microscope (Axioplan 2; Zeiss) equipped for epifluorescence microscopy and differential interference contrast optics, and images were collected with a digital Axiocam (Zeiss).
Immunoelectron Microscopy of Galls and Juvenile Nematodes
Root galls of A. thaliana cv. WS and Col-0 were dissected at 14 DAI after inoculation and fixed in a mixture of 1.5% glutaraldehyde, 3% paraformaldehyde in 10 mM PBS containing 150 mM NaCl (pH 7.2) for 3 h at room temperature. After several washes in PBS buffer fixed galls were incubated in 0.5 M NH4Cl for 1 h, dehydrated in graded ethanol series, embedded in acrylic resin LR White (Sigma), and polymerized overnight at 60°C. Ultrathin sections were collected on parlodion-coated nickel grids, treated with 0.1 M HCl for 10 min, and washed at least twice with bidistilled water. The grids were pre-incubated in 1% bovin serum albumin (BSA) in PBS for 15 min, prior to incubation with pre-immune goat serum (Sigma) diluted 1∶10 in BSA-PBS for 1 h. Immuno-labelling was performed with primary antibodies diluted with BSA-PBS. Double labelling was done by treatment of grids either with rabbit polyclonal anti-γ-tubulin (1∶500) and the monoclonal anti-α-tubulin (1∶500), for 90 min at room temperature. The grids were washed 3 times for 5 min in BSA-PBS and incubated for 1 h with secondary antibodies (10 nm gold-goat anti-rabbit and 5 nm gold-goat anti-mouse antisera; British BioCell International) diluted 1∶20 with BSA-PBS. Other grids containing gall sections were incubated in the following immunoreagents: first, grids were incubated with rabbit polyclonal antiserum anti-GCP3 diluted in BSA-PBS. Samples were washed 3 times for 5 min in BSA-PBS and incubated for 1 h with the secondary antibody (10 nm gold-goat anti-rabbit). Secondly, grids were incubated with the rabbit polyclonal antibody against γ-tubulin (1∶300), washed 3 times for 5 min in BSA-PBS and incubated for 1 h with the secondary antibody goat anti-rabbit gold (5 nm). All grids were rinsed 3 times for 5 min in PBS, rinsed in bidistilled water and stained 5 min in 2% aqueous uranyl acetate and 2 min in 1% lead citrate. Samples were observed under a Philips 400 T electron microscope.
In vivo Observations on Whole Roots and Fresh Nematode Infected Root Slices and Confocal Microscopy
Observation of MTs and nuclei in the nematode feeding sites in wild-type and mutant lines was performed in nematode infected A. thaliana seedlings harbouring the MT binding domain of MAP4 fused to -GFP and H2B Histone-YFP (MBD::GFP - His::YFP). More than 50 root meristems and young galls (2 to 7 DAI) were directly observed under the microscope. At least 50 galls at various time points after infection (7 to 15 DAI) were dissected from roots and embedded in 5% agar. Fresh thick sections of 50–100 µm (7 to 10 DAI) or 150–200 µm (10 to 14 DAI) were performed with a HM650V vibrotome Microm (Walldorf, Germany). Whole roots and fresh slices were observed using an inverted confocal microscope (model LSM510 META; Zeiss). YFP and GFP fluorescence were monitored in Lambda mode with a 499 to 550 nm beam path (488 nm excitation line). The fluorescent dye Syto-82 (Molecular Probes) was used at 1 mM final concentration. GFP or YFP and Syto-82 fluorescence were monitored in Lambda mode using the 488 nm argon laser line excitation and spectral detection using 10 nm steps for emission between 499 to 620 nm. All observations were obtained from at least three independent experiments.
Constructing TUBG1::GFP and Plant Transformation
The complete coding sequences of TUBG1 gene was amplified by PCR, using ecotype cDNAs as the template, the primers pairs attB1F [5′-AA AAA GCA GGC TTC-(ACC ATG)-(18/25 nt template-specific seq)-3′] and attB2R [5′-A GAA AGC TGG GTG (TTA)-(template-specific seq)-3′] with adapter primers attB1F (5′-GGGACAAGTTTGTACAAA AAAGCAGGCT-3′) and attB2 R (5′-GGGGACCACTTTGTACAAGAAAGCTGGGT-3′). These sequences were then inserted into plant expression vectors, using Gateway™ Technology (Invitrogen) and the pDONR207 donor vector (Invitrogen). Cloning was carried out in Escherichia coli DH10β cells. Transient expression of γ-tubulin in tobacco leaves was performed in leaves of Nicotiana benthamiana by infiltrating Agrobacterium tumefasciens strain C58C1 harbouring the 35Spro:TUBG1-GFP and 35Spro:GFP-TUBG1 with a syringe and observations were performed 48 hours after infiltration on the confocal microscope (Zeiss, LSM 510). To generate transgenic plants expressing the γ-tubulin protein the 35Spro:TUBG1-GFP (TUBG1-GFP) plasmid was transformed into Agrobacterium tumefasciens strain C58C1 and Arabidospsis thaliana Col-0 were transformed by floral dipping [64]. Plants with γ-tubulin-GFP fluorescence which did not present a visible phenotype and showing less expression were used for localization studies. On the other hand, seedlings with root twisting harbouring γ-tubulin-GFP fluorescence and showing additional γ-tubulin-GFP expression were used for overexpression studies.
Oryzalin and Propyzamide Treatments
To test the effect of MT cytoskeleton inhibitors on the γ-tubulin overexpressing line presenting twisted roots, TUBG1-GFP seeds were germinated on MS medium, transferred to the same medium supplemented with oryzalin (0.5 µM) or propyzamide (5 µM) and incubated overnight at room temperature. Control experiments were performed using the same medium in the absence of inhibitors. Treated and untreated roots were imaged with a confocal laser scanning microscope (LSM510 META; Zeiss) using the tile-scan mode with the 10x/0.3NA objective. GFP fluorescence was recorded using 488-nm laser excitation and the 505–530 nm BP emission filter.
Accession Numbers
Arabidopsis Genome Initiative locus identifiers for the genes mentioned in this article are as follows: At3g61650 (TUBG1), At5g05620 (TUBG2), At5g06680 (GCP3) and At3g53760 (GCP4).
Supporting Information
Zdroje
1. JanskySHSimonRSpoonerDM 2008 A test of taxonomic predictivity: Resistance to early blight in wild relatives of cultivated potato. Phytopathology 98 680 687
2. WyssUGrundlerFMWMunchA 1992 The parasitic behavior of second-stage juveniles of Meloidogyne incognita in roots of Arabidopsis thaliana. Nematologica 38 98 111
3. Von MendeN 1997 Invasion and migration behaviour of sedentary nematodes. FenollCGrundlerFMWOhlSA Cellular and molecular aspects of plant-nematodes interactions 21 Dordrecht, The Netherlands Kluwer Academics Publishers 1129 1140
4. JonesMGK 1981 The development and function of plant cells modified by endoparasitic nematodes. ZuckermanBMRhodeRA Plant Parasitic Nematodes New York Academic Press 225 279
5. WiggersRJStarrJLPriceHJ 1990 DNA content and variation in chromosome number in plant cells affected by Meloidogyne incognita and M. arenaria. Phytopathology 80 1391 1395
6. de Almeida EnglerJDe VleesschauwerVBurssensSCelenzaJLJInzéD 1999 Molecular markers and cell cycle inhibitors show the importance of cell cycle progression in nematode-induced galls and syncytia. Plant Cell 11 793 808
7. de Almeida EnglerJVan PouckeKKarimiMDe GroodtRGheysenG 2004 Dynamic cytoskeleton rearrangements in giant cells and syncytia of nematode-infected roots. Plant J 38 12 26
8. CaillaudMCLecomtePJammesFQuentinMPagnottaS 2008 MAP65-3 microtubule-associated protein is essential for nematode-induced giant cell ontogenesis in Arabidopsis. Plant Cell 20 423 437
9. ClémentMKetelaarTRodiucNBanoraMYSmertenkoA 2009 Actin-depolymerizing factor2-mediated actin dynamics are essential for root-knot nematode infection of Arabidopsis. Plant Cell 21 2963 2979
10. MineyukiYAioiHYamashitaMNagahamaY 1996 A comparative study on stainability of preprophase bands by the PSTAIR antibody. J Plant Res 109 185 192
11. WieseCZhengY 2006 Microtubule nucleation: γ-Tubulin and beyond. J Cell Sci 119 4143 4153
12. PastugliaMBouchezD 2007 Molecular encounters at microtubule ends in the plant cell cortex. Curr Opin Plant Biol 10 557 563
13. OakleyBROakleyCEYoonYSJungMK 1990 γ-Tubulin is a component of the spindle pole body that is essential for microtubule function in Aspergillus nidulans. Cell 61 1289 1301
14. HorioTUzawaSJungMKOakelyBRTanakaK 1991 The fission yeast γ-tubulin is essential for mitosis and is localized at microtubule organizing centers. J Cell Sci 99 693 700
15. StearnsTEvansLKirschnerM 1991 γ-Tubulin is a highly conserved component of the centrosome. Cell 65 825 836
16. ZhengYJungMKOakleyBR 1991 γ-Tubulin is present in Drosophila melanogaster and Homo sapiens and is associated with the centrosome. Cell 65 817 823
17. LiuBJoshiHCWilsonTJSilflowCDPalevitzBA 1994 γ-Tubulin in Arabidopsis: gene sequence, immunoblot, and immunofluorescence studies. Plant Cell 6 303 314
18. HorioTOakleyBR 2003 Expression of Arabidopsis γ-tubulin in fission yeast reveals conserved and novel functions of γ-tubulin. Plant Physiol 133 1926 1934
19. BinarovaPCenklovaVProchazkovaJDoskocilovaAVolcJ 2006 γ-Tubulin is essential for acentrosomal microtubule nucleation and coordination of late mitotic events in Arabidopsis. Plant Cell 18 1199 1212
20. PastugliaMAzimzadehJGoussotMCamilleriCBelcramK 2006 γ-tubulin is essential for microtubule organization and development in Arabidopsis. Plant Cell 18 1412 1425
21. JobDValironOOakleyB 2003 Microtubule nucleation. Cur Opin Cell Biol 15 111 117
22. SeltzerVJanskiNCanadayJHerzogEErhardtM 2007 Arabidopsis GCP2 and GCP3 are part of a soluble γ-tubulin complex and have nuclear envelope targeting domains. Plant J 52 322 331
23. ZhengYWongMLAlbertsBMitchisonT 1995 Nucleation of microtubule assembly by a γ-tubulin-containing ring complex. Nature 378 578 583
24. Raynaud-MessinaBMerdesA 2007 γ-Tubulin complexes and microtubule organization. Curr Opin Cell Biol 19 24 30
25. LiuBMarcJJoshiHCPalevitzBA 1993 A γ-tubulin related protein associated with the microtubule arrays of higher plants in a cell cycle-dependent manner. J Cell Sci 104 1217 1228
26. PanterisEApostolakosPGräfRGalatisB 2000 γ-tubulin colocalizes with microtubule arrays and tubulin paracrystals in dividing vegetative cells of higher plants. Protoplasma 210 179 187
27. MurataTSonobeSBaskinTIHyodoSHasezawaS 2005 Microtubule-dependent microtubule nucleation based on recruitment of γ-tubulin in higher plants. Nat Cell Biol 7 961 968
28. ErhardtMStoppin-MelletVCampagneSCanadayJMuttererJ 2002 The plant Spc98p homologue co-localizes with γ-tubulin at microtubule nucleation sites and is required for microtubule nucleation. J Cell Sci 115 2423 2431
29. NakamuraMHashimotoT 2009 A mutation in the Arabidopsis gamma-tubulin-containing complex causes helical growth and abnormal microtubule branching. J Cell Sci 122 2208 2217
30. KongZHottaTLeeYJHorioTLiuB 2010 The γ-Tubulin complex protein GCP4 is required for organizing functional microtubule arrays in Arabidopsis thaliana. Plant Cell 22 191 204
31. BaskinTIBeemsterGTJudy-MarchJEMargaF 2004 Disorganization of cortical microtubules stimulates tangential expansion and reduces the uniformity of cellulose microfibril alignment among cells in the root of Arabidopsis. Plant Physiol 135 2279 2290
32. WhittingtonATVugrekOWeiKJHasenbeinNGSugimotoK 2001 MOR1 is essential for organizing cortical microtubules in plants. Nature 411 610 613
33. TakemotoDHardhamAR 2004 The cytoskeleton as a regulator and target of biotic interactions in plants. Plant Physiol 136 3864 3876
34. MiklisMConsonniCBhatRALipkaVSchulze-LefertP 2007 Barley MLO modulates actin-dependent and actin-independent antifungal defence pathways at the cell periphery. Plant Physiol 144 1132 1143
35. DibbayawanTPHarperJDIMarcJ 2001 α-Tubulin antibody against a plant peptide sequence localizes to cell division-specific microtubule arrays and organelles in plants. Micron 32 671 678
36. DrykovaDCenklovaVSulimenkoVVolcJDraberP 2003 Plant γ-tubulin interacts with αβ-tubulin dimers and forms membrane-associated complexes. Plant Cell 15 465 480
37. BrownRCLemmonBEHorioT 2004 γ-Tubulin localisation changes from discrete polar organizers to anastral spindles and phragmoplasts in mitosis of Marchantia polymorpha L. Protoplasma 224 187 193
38. SchmitAC 2002 Acentrosomal microtubule nucleation in higher plants. Int Rev Cytol 220 257 289
39. SmertenkoAPChangHYWagnerVKaloritiDFenykS 2004 The Arabidopsis MT-associated protein AtMAP65-1: Molecular analysis of its MT bundling activity. Plant Cell 16 2035 2047
40. FaveryBChelyshevaLALebrisMJammesFMarmagneA 2004 Arabidopsis formin AtFH6 is a plasma membrane-associated protein upregulated in giant cells induced by parasitic nematodes. Plant Cell 16 2529 2540
41. ChangHYSmertenkoAPIgarashiHDixonDPHusseyPJ 2005 Dynamic interaction of NtMAP65-1a with MTs in vivo. J Cell Sci 118 3195 3201
42. NakamuraMEhrhardtDWHashimotoT 2010 Microtubule and katanin-dependent dynamics of microtubule nucleation complexes in the acentrosomal Arabidopsis cortical array. Nat Cell Biol 12 1064 1070
43. ZengCJTLeeYRJLiuB 2009 The WD40 Repeat Protein NEDD1 Functions in Microtubule Organization during Cell Division in Arabidopsis thaliana. The Plant Cell microtubule organization and development in Arabidopsis. Plant Cell 18 1412 1425
44. SobelSGSnyderM 1995 A highly divergent γ-tubulin gene is essential for cell growth and proper microtubule organization in Saccharomyces cerevisiae. J Cell Biol 131 1775 1788
45. SunkelCEGomesRSampaioPPerdigaoJGonzalezC 1995 γ-Tubulin is required for the structure and function of the microtubule organizing centre in Drosophila neuroblasts. EMBO J 14 28 36
46. MarschallLGJengRLMulhollandJStearnsT 1996 Analysis of Tub4p, a yeast γ-tubulin-like protein: Implications for microtubule-organizing center function. J Cell Biol 134 443 454
47. SpangAGeisslerSGreinKSchiebelE 1996 γ-Tubulin-like Tub4p of Saccharomyces cerevisiae is associated with the spindle pole body substructures that organize microtubules and is required for mitotic spindle formation. J Cell Biol 134 429 441
48. MartinOsmani SAMAOakleyBR 1997 The role of γ-tubulin in mitotic spindle formation and cell cycle progression in Aspergillus nidulans. J Cell Sci 110 623 633
49. CanadayJStoppin-MelletVMuttererJLambertAMSchmitAC 2000 Higher plant cells: γ-tubulin and microtubule nucleation in the absence of centrosomes. Microsci Res Technol 49 487 495
50. KumagaiFNagataTYaharaNMoriyamaYHorioT 2003 γ-Tubulin distribution during cortical microtubule reorganization at the M/G1 interface in tobacco BY-2 cells. Eur J Cell Biol 82 43 51
51. HendricksonTWYaoJBhadurySCorbettAHJoshiHC 2001 Conditional mutations in γ-tubulin reveal its involvement in chromosome segregation and cytokinesis. Mol Biol Cell 12 2469 2481
52. PrigozhinaNLOakleyCELewisAMNayakTOsmaniSA 2004 γ-Tubulin plays an essential role in the coordination of mitotic events. Mol Biol Cell 15 1374 1386
53. GrossPJuliusCSchmelzerEHahlbrockK 1993 Translocation of cytoplasm and nucleus to fungal penetration sites is associated with depolymerization of microtubules and defence gene activation ininfected, cultured parsley cells. EMBO J 12 1735 1744
54. CahillDRookesJMichalczykAMcDonaldKDrakeA 2002 Microtubule dynamics in compatible and incompatible interactions of soybean hypocotyl cells with Phytophthora sojae. Plant Pathol 51 629 640
55. HuangCSMaggentiAR 1969 Mitotic aberrations and nuclear changes of developing giant cells in Vicia faba caused by root knot nematode Meloidogyne javanica. Phytopathology 447 455
56. BechtoldNEllisJPelletierG 1993 In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci 316 1194 1199
57. AlonsoJMStepanovaANLeisseTJKimCJChenH 2003 Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653 657
58. SijmonsPCGrundlerFMWvon MendeNBurrowsPRWyssU 1991 Arabidopsis thaliana as a new model host for plantparasitic nematodes. Plant J 1 245 254
59. Laroche-RaynalMAspartLDelsenyMPenonP 1984 Characterization of radish mRNA at three developmental stages. Plant Sci Lett 35 139 146
60. QuentinMAllasiaVPegardA 2009 Imbalanced lignin biosynthesis promotes the sexual reproduction of homothallic oomycete pathogens. PLoS Pathog 5 e1000264
61. AttardAGourguesMCallemeyn-TorreNKellerH 2010 The immediate activation of defense responses in Arabidopsis roots is not sufficient to prevent Phytophthora parasitica infection. New Phytol 187 449 460
62. HokSDanchinEAllasiaVPanabieresFAttardA 2011 An Arabidopsis (malectin-like) leucine-rich repeat receptor-like kinase contributes to downy mildew disease. Plant Cell Environ 34 1944 1957
63. KronenbergerJDesprezTHofteHCabocheMTraasJ 1993 A methacrylate embedding procedure developed for immunolocalization on plant tissues is also compatible with in situ hybridization. Cell Biol Int 17 1013 1201
64. CloughSJBentAF 1998 Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735 743
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Genesis of Mammalian Prions: From Non-infectious Amyloid Fibrils to a Transmissible Prion DiseaseČlánek Role of Permissive Neuraminidase Mutations in Influenza A/Brisbane/59/2007-like (H1N1) VirusesČlánek Allelic Variation on Murine Chromosome 11 Modifies Host Inflammatory Responses and Resistance toČlánek Multifaceted Regulation of Translational Readthrough by RNA Replication Elements in a TombusvirusČlánek Latent KSHV Infection of Endothelial Cells Induces Integrin Beta3 to Activate Angiogenic PhenotypesČlánek Controlling Viral Immuno-Inflammatory Lesions by Modulating Aryl Hydrocarbon Receptor Signaling
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2011 Číslo 12- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Inhibition of Apoptosis and NF-κB Activation by Vaccinia Protein N1 Occur via Distinct Binding Surfaces and Make Different Contributions to Virulence
- Genesis of Mammalian Prions: From Non-infectious Amyloid Fibrils to a Transmissible Prion Disease
- Kaposi's Sarcoma Herpesvirus microRNAs Target Caspase 3 and Regulate Apoptosis
- Nutritional Immunology: A Multi-Dimensional Approach
- Role of Permissive Neuraminidase Mutations in Influenza A/Brisbane/59/2007-like (H1N1) Viruses
- Vaccinomics and Personalized Vaccinology: Is Science Leading Us Toward a New Path of Directed Vaccine Development and Discovery?
- Symbiont Infections Induce Strong Cytoplasmic Incompatibility in the Tsetse Fly
- Allelic Variation on Murine Chromosome 11 Modifies Host Inflammatory Responses and Resistance to
- Computational and Biochemical Analysis of the Effector AvrBs2 and Its Role in the Modulation of Type Three Effector Delivery
- Granzyme B Inhibits Vaccinia Virus Production through Proteolytic Cleavage of Eukaryotic Initiation Factor 4 Gamma 3
- Association of Activating KIR Copy Number Variation of NK Cells with Containment of SIV Replication in Rhesus Monkeys
- Fungal Virulence and Development Is Regulated by Alternative Pre-mRNA 3′End Processing in
- versus the Host: Remodeling of the Bacterial Outer Membrane Is Required for Survival in the Gastric Mucosa
- Follicular Dendritic Cell-Specific Prion Protein (PrP) Expression Alone Is Sufficient to Sustain Prion Infection in the Spleen
- Autophagy Protein Atg3 is Essential for Maintaining Mitochondrial Integrity and for Normal Intracellular Development of Tachyzoites
- Longevity and Composition of Cellular Immune Responses Following Experimental Malaria Infection in Humans
- Sequential Adaptive Mutations Enhance Efficient Vector Switching by Chikungunya Virus and Its Epidemic Emergence
- Acquisition of Pneumococci Specific Effector and Regulatory Cd4 T Cells Localising within Human Upper Respiratory-Tract Mucosal Lymphoid Tissue
- The Meaning of Death: Evolution and Ecology of Apoptosis in Protozoan Parasites
- Deficiency of a Niemann-Pick, Type C1-related Protein in Is Associated with Multiple Lipidoses and Increased Pathogenicity
- Feeding Cells Induced by Phytoparasitic Nematodes Require γ-Tubulin Ring Complex for Microtubule Reorganization
- Eight RGS and RGS-like Proteins Orchestrate Growth, Differentiation, and Pathogenicity of
- Prion Uptake in the Gut: Identification of the First Uptake and Replication Sites
- Nef Decreases HIV-1 Sensitivity to Neutralizing Antibodies that Target the Membrane-proximal External Region of TMgp41
- Multifaceted Regulation of Translational Readthrough by RNA Replication Elements in a Tombusvirus
- A Temporal Role Of Type I Interferon Signaling in CD8 T Cell Maturation during Acute West Nile Virus Infection
- The Membrane Fusion Step of Vaccinia Virus Entry Is Cooperatively Mediated by Multiple Viral Proteins and Host Cell Components
- HIV-1 Capsid-Cyclophilin Interactions Determine Nuclear Import Pathway, Integration Targeting and Replication Efficiency
- Neonatal CD8 T-cell Hierarchy Is Distinct from Adults and Is Influenced by Intrinsic T cell Properties in Respiratory Syncytial Virus Infected Mice
- Two Novel Transcriptional Regulators Are Essential for Infection-related Morphogenesis and Pathogenicity of the Rice Blast Fungus
- Five Questions about Non-Mevalonate Isoprenoid Biosynthesis
- The Human Cytomegalovirus UL11 Protein Interacts with the Receptor Tyrosine Phosphatase CD45, Resulting in Functional Paralysis of T Cells
- Wall Teichoic Acids of Limit Recognition by the Drosophila Peptidoglycan Recognition Protein-SA to Promote Pathogenicity
- A Novel Role for the NLRC4 Inflammasome in Mucosal Defenses against the Fungal Pathogen
- Inflammasome-dependent Pyroptosis and IL-18 Protect against Lung Infection while IL-1β Is Deleterious
- CNS Recruitment of CD8+ T Lymphocytes Specific for a Peripheral Virus Infection Triggers Neuropathogenesis during Polymicrobial Challenge
- Latent KSHV Infection of Endothelial Cells Induces Integrin Beta3 to Activate Angiogenic Phenotypes
- A Receptor-based Switch that Regulates Anthrax Toxin Pore Formation
- Targeting of Heparin-Binding Hemagglutinin to Mitochondria in Macrophages
- Chikungunya Virus Neutralization Antigens and Direct Cell-to-Cell Transmission Are Revealed by Human Antibody-Escape Mutants
- Ce-Duox1/BLI-3 Generated Reactive Oxygen Species Trigger Protective SKN-1 Activity via p38 MAPK Signaling during Infection in
- Structural Elucidation and Functional Characterization of the Effector Protein ATR13
- Controlling Viral Immuno-Inflammatory Lesions by Modulating Aryl Hydrocarbon Receptor Signaling
- SAMHD1-Deficient CD14+ Cells from Individuals with Aicardi-Goutières Syndrome Are Highly Susceptible to HIV-1 Infection
- Acid Stability of the Hemagglutinin Protein Regulates H5N1 Influenza Virus Pathogenicity
- Cryo Electron Tomography of Herpes Simplex Virus during Axonal Transport and Secondary Envelopment in Primary Neurons
- A Novel Human Cytomegalovirus Locus Modulates Cell Type-Specific Outcomes of Infection
- Juxtamembrane Shedding of AMA1 Is Sequence Independent and Essential, and Helps Evade Invasion-Inhibitory Antibodies
- Pathogenesis and Host Response in Syrian Hamsters following Intranasal Infection with Andes Virus
- IRGM Is a Common Target of RNA Viruses that Subvert the Autophagy Network
- Epstein-Barr Virus Evades CD4 T Cell Responses in Lytic Cycle through BZLF1-mediated Downregulation of CD74 and the Cooperation of vBcl-2
- Quantitative Multicolor Super-Resolution Microscopy Reveals Tetherin HIV-1 Interaction
- Late Repression of NF-κB Activity by Invasive but Not Non-Invasive Meningococcal Isolates Is Required to Display Apoptosis of Epithelial Cells
- Polar Flagellar Biosynthesis and a Regulator of Flagellar Number Influence Spatial Parameters of Cell Division in
- Epstein-Barr Virus Nuclear Antigen 3C Stabilizes Gemin3 to Block p53-mediated Apoptosis
- The Enteropathogenic (EPEC) Tir Effector Inhibits NF-κB Activity by Targeting TNFα Receptor-Associated Factors
- Toward an Integrated Model of Capsule Regulation in
- A Systematic Screen to Discover and Analyze Apicoplast Proteins Identifies a Conserved and Essential Protein Import Factor
- A Host Small GTP-binding Protein ARL8 Plays Crucial Roles in Tobamovirus RNA Replication
- Comparative Pathobiology of Fungal Pathogens of Plants and Animals
- Synergistic Roles of Eukaryotic Translation Elongation Factors 1Bγ and 1A in Stimulation of Tombusvirus Minus-Strand Synthesis
- Engineered Immunity to Infection
- Inflammatory Monocytes and Neutrophils Are Licensed to Kill during Memory Responses
- Sialidases Affect the Host Cell Adherence and Epsilon Toxin-Induced Cytotoxicity of Type D Strain CN3718
- Eurasian-Origin Gene Segments Contribute to the Transmissibility, Aerosol Release, and Morphology of the 2009 Pandemic H1N1 Influenza Virus
- SARS Coronavirus nsp1 Protein Induces Template-Dependent Endonucleolytic Cleavage of mRNAs: Viral mRNAs Are Resistant to nsp1-Induced RNA Cleavage
- Identification and Characterization of a Novel Non-Structural Protein of Bluetongue Virus
- Functional Analysis of the Kinome of the Wheat Scab Fungus
- Norovirus Regulation of the Innate Immune Response and Apoptosis Occurs via the Product of the Alternative Open Reading Frame 4
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Controlling Viral Immuno-Inflammatory Lesions by Modulating Aryl Hydrocarbon Receptor Signaling
- Fungal Virulence and Development Is Regulated by Alternative Pre-mRNA 3′End Processing in
- Epstein-Barr Virus Nuclear Antigen 3C Stabilizes Gemin3 to Block p53-mediated Apoptosis
- Engineered Immunity to Infection
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



