-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression
Mycoplasma pneumoniae is a causative agent of atypical pneumonia. The formation of hydrogen peroxide, a product of glycerol metabolism, is essential for host cell cytotoxicity. Phosphatidylcholine is the major carbon source available on lung epithelia, and its utilization requires the cleavage of deacylated phospholipids to glycerol-3-phosphate and choline. M. pneumoniae possesses two potential glycerophosphodiesterases, MPN420 (GlpQ) and MPN566. In this work, the function of these proteins was analyzed by biochemical, genetic, and physiological studies. The results indicate that only GlpQ is an active glycerophosphodiesterase. MPN566 has no enzymatic activity as glycerophosphodiesterase and the inactivation of the gene did not result in any detectable phenotype. Inactivation of the glpQ gene resulted in reduced growth in medium with glucose as the carbon source, in loss of hydrogen peroxide production when phosphatidylcholine was present, and in a complete loss of cytotoxicity towards HeLa cells. All these phenotypes were reverted upon complementation of the mutant. Moreover, the glpQ mutant strain exhibited a reduced gliding velocity. A comparison of the proteomes of the wild type strain and the glpQ mutant revealed that this enzyme is also implicated in the control of gene expression. Several proteins were present in higher or lower amounts in the mutant. This apparent regulation by GlpQ is exerted at the level of transcription as determined by mRNA slot blot analyses. All genes subject to GlpQ-dependent control have a conserved potential cis-acting element upstream of the coding region. This element overlaps the promoter in the case of the genes that are repressed in a GlpQ-dependent manner and it is located upstream of the promoter for GlpQ-activated genes. We may suggest that GlpQ acts as a trigger enzyme that measures the availability of its product glycerol-3-phosphate and uses this information to differentially control gene expression.
Published in the journal: A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression. PLoS Pathog 7(9): e32767. doi:10.1371/journal.ppat.1002263
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002263Summary
Mycoplasma pneumoniae is a causative agent of atypical pneumonia. The formation of hydrogen peroxide, a product of glycerol metabolism, is essential for host cell cytotoxicity. Phosphatidylcholine is the major carbon source available on lung epithelia, and its utilization requires the cleavage of deacylated phospholipids to glycerol-3-phosphate and choline. M. pneumoniae possesses two potential glycerophosphodiesterases, MPN420 (GlpQ) and MPN566. In this work, the function of these proteins was analyzed by biochemical, genetic, and physiological studies. The results indicate that only GlpQ is an active glycerophosphodiesterase. MPN566 has no enzymatic activity as glycerophosphodiesterase and the inactivation of the gene did not result in any detectable phenotype. Inactivation of the glpQ gene resulted in reduced growth in medium with glucose as the carbon source, in loss of hydrogen peroxide production when phosphatidylcholine was present, and in a complete loss of cytotoxicity towards HeLa cells. All these phenotypes were reverted upon complementation of the mutant. Moreover, the glpQ mutant strain exhibited a reduced gliding velocity. A comparison of the proteomes of the wild type strain and the glpQ mutant revealed that this enzyme is also implicated in the control of gene expression. Several proteins were present in higher or lower amounts in the mutant. This apparent regulation by GlpQ is exerted at the level of transcription as determined by mRNA slot blot analyses. All genes subject to GlpQ-dependent control have a conserved potential cis-acting element upstream of the coding region. This element overlaps the promoter in the case of the genes that are repressed in a GlpQ-dependent manner and it is located upstream of the promoter for GlpQ-activated genes. We may suggest that GlpQ acts as a trigger enzyme that measures the availability of its product glycerol-3-phosphate and uses this information to differentially control gene expression.
Introduction
Pathogenic bacteria have developed a large battery of enzymes and mechanisms for extracting nutrients from their hosts, and the requirement for nutrient acquisition can be regarded as one of the driving forces for virulence [1]–[3]. In consequence, the metabolic capabilities of a pathogen reflect its adaptation to a particular niche in a particular host.
Mycoplasma pneumoniae is a causative agent of atypical pneumonia, however implication of this bacterium in several additional infections including encephalitis, aseptic meningitis, acute transverses myelitis, stroke, and polyradiculopathy has been reported [4]–[7]. These bacteria are members of the taxonomic class Mollicutes that are characterized by an extreme reductive evolution that results in the smallest genomes that allow independent life. Moreover, the mollicutes have lost the cell wall and most metabolic pathways, since they obtain the building blocks for their cellular macromolecules from the host tissue. However, even in these minimal pathogens, there is a close relation between metabolism and virulence (for a review, see [8]). M. pneumoniae thrives at the apical surface of lung epithelia. Thus, these bacteria must have evolved to utilize the carbon sources present in this niche. The pulmonary surfactant is composed of about 90% phospholipids and 10% proteins [9]. This suggests that phospholipids play a major role in the nutrition of M. pneumoniae.
Glycerophospholipids, the major building blocks of the cell membrane in bacteria and eukaryotes, are degraded in several steps. First, the fatty acids are cleaved from the phospholipids resulting in the formation of glycerophosphodiesters. In these molecules, the phosphate group of glycerol-3-phosphate is linked to another compound, called the head group. In eukaryotes, choline is by far the most abundant head group, and lecithin, the choline-containing phospholipid accounts for about 80% of all phospholipids in human lung cells [9]. In the second step, the choline head group is cleaved due to the activity of a glycerophosphodiesterase resulting in the formation of glycerol-3-phosphate that can feed into glycolysis after oxidation to dihydroxyacetone phosphate (see Figure 1). In M. pneumoniae, the latter reaction is catalyzed by the glycerol-3-phosphate oxidase GlpD [10]. GlpD transfers the electrons to water resulting in the formation of hydrogen peroxide, the major virulence factor of M. pneumoniae [11]. In consequence, the virulence of M. pneumoniae glpD mutant cells is severely attenuated [10].
Fig. 1. Schematic illustration of the machinery for uptake and conversion of carbohydrates leading to the formation of glycerol-3-phosphate in M. pneumoniae. 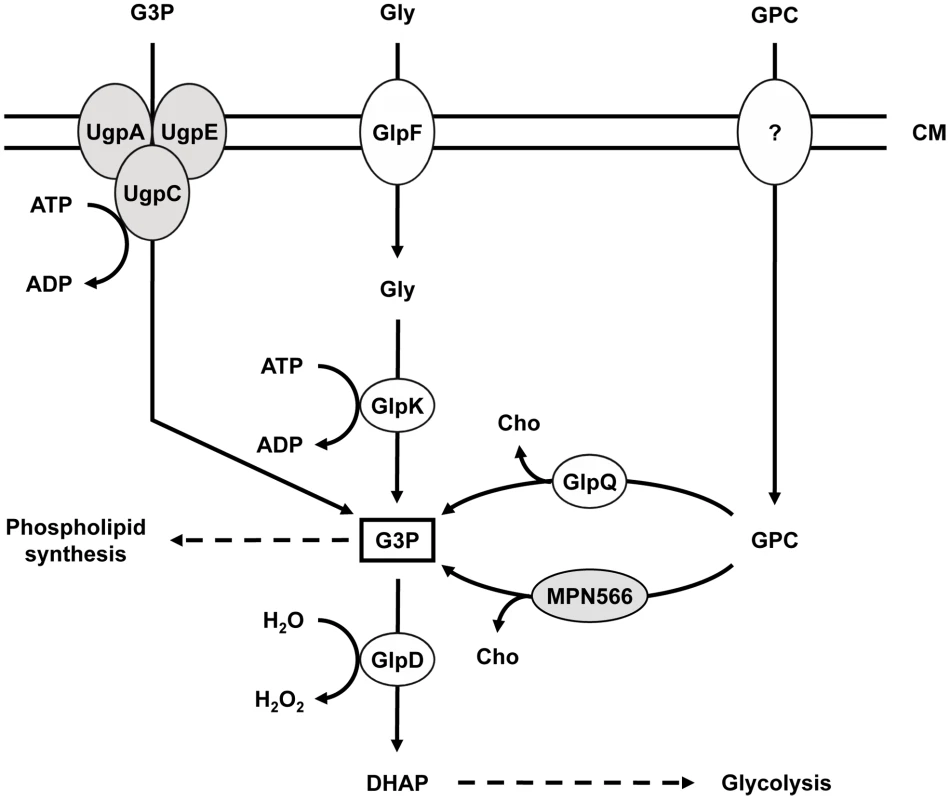
UgpC (MPN134), UgpA (MPN135), and UgpE (MPN136) form a putative ABC transport system for glycerol-3-phosphate, whereas GlpF (MPN043) is the glycerol uptake facilitator. The glycerol kinase GlpK (MPN050) and the glycerol-3-phosphate oxidase GlpD (MPN051) metabolize glycerol to the glycolytic intermediate dihydroxyacetone phosphate. Hydrogen peroxide formation by GlpD is crucial for the cytotoxic effects of M. pneumoniae. GlpQ (MPN420) and MPN566 encode two paralogous glycerophosphodiesterases that may be able to metabolize glycerophosphocholine to glycerol-3-phosphate and choline. The uptake system for glycerophosphocholine is so far unknown. Proteins highlighted in grey seem not to fulfill the predicted function (this work). CM, cell membrane; DHAP, dihydroxyacetone phosphate; G3P, glycerol-3-phosphate; GPC, glycerophosphocholine; Gly, glycerol; Cho, choline. While the metabolism of glycerol has been well studied in M. pneumoniae and other mollicutes such as M. mycoides [10], [12], [13], only little is known about the glycerophosphodiesterases required for lipid utilization. Many bacteria encode multiple glycerophosphodiesterases. In E. coli, both enzymes are enzymatically active in lipid degradation; however, they are differentially regulated, with GlpQ and UgpQ being induced in the presence of glycerol-3-phosphate and under conditions of phosphate starvation, respectively [14], [15]. In B. subtilis, out of three putative glycerophosphodiesterases, only GlpQ has been studied. The corresponding gene is under dual control and its expression is induced when phosphate becomes limiting and glycerol is available [16], [17]. Moreover, glpQ expression is repressed if more favourable carbon sources such as glucose are present [18]. In Haemophilus influenzae, another bacterium thriving in the respiratory tract, the glycerophosphodiesterase is involved in pathogenicity. The enzyme generates choline, which in turn is used for the biosynthesis of the bacterial lipopolysaccharide layer - a major virulence determinant of Gram-negative bacteria [19], [20]. Similarly, glycerophosphodiesterase activity is implicated in virulence of different Borrelia species. The enzyme is only present in the relapsing fever group and may help the bacteria to reach a higher cell density in the blood of the host as compared to Lyme disease spirochaetes [21].
In many bacteria, central enzymes of metabolism do not only fulfil their catalytic function, but in addition, they are also involved in signal transduction. In this way, the information on the availability of important metabolites can be directly determined by the enzyme in charge of their conversion, and this information is then often transferred to the transcription machinery. Collectively, such enzymes have been termed trigger enzymes [22]. They can control gene expression by directly acting as DNA - or RNA-binding transcription factors as the E. coli proline dehydrogenase and the aconitase or by controlling the activity of transcription factors by covalent modification or a regulatory protein-protein interaction as observed for several sugar permeases of the bacterial phosphotransferase system and the B. subtilis glutamate dehydrogenase, respectively [23]–[26].
In this work, we have analyzed the role of the two potential glycerophosphodiesterases encoded in the genome of M. pneumoniae. Biochemical and physiological studies demonstrate that one of the two proteins, GlpQ, is a functional glycerophosphodiesterase. GlpQ is essential for hydrogen peroxide formation in the presence of deacylated phospholipids as the carbon source and, in consequence, for cytotoxicity. Moreover, GlpQ may act as a trigger enzyme by controlling the expression of a set of genes encoding lipoproteins, the glycerol facilitator, and a metal ion ABC transporter.
Results
Identification of M. pneumoniae genes encoding potential glycerophosphodiesterases
Since phospholipids are the most abundant potential carbon sources for M. pneumoniae living at lung epithelial surfaces, we considered the possibility that these bacteria synthesize enzymes that cleave the polar head groups from the glycerophosphodiesters to produce glycerol-3-phosphate that can be utilized by the enzymes of glycerol metabolism [10] (Figure 1). Two genes that potentially encode such enzymes are present in the genome of M. pneumoniae, i.e. mpn420 (renamed to glpQ) and mpn566. An alignment of the corresponding proteins to glycerophosphodiesterases from other bacteria is shown in the supporting information (Figure S1).
Enzymatic activities of the potential glycerophosphodiesterases
In order to assess the biochemical properties and physiological relevance of the putative glycerophosphodiesterases, their corresponding genes, glpQ and mpn566, were cloned into the expression vector pGP172, thus allowing a fusion of the proteins to an N-terminal Strep-tag facilitating purification. The recombinant proteins were purified and the activities were first determined using glycerophosphocholine (GPC) as the substrate and a set of divalent cations. As shown in Figure 2, purified GlpQ was active against GPC, and the activity was highest in the presence of magnesium ions (10 mM). Manganese and zinc ions did also support activity, although to a lesser extent (Figure 2). In contrast, the enzyme was inactive in the presence of calcium and cobalt ions (data not shown). The activity assay with purified MPN566 revealed no activity with GPC, irrespective of the cation present in the assay (data not shown). We did also test the activity of both proteins with glycerophosphoethanolamine and glycerophosphoglycerol. However, neither protein was active with any of these substrates. Thus, our data demonstrate that GlpQ is active as a glycerophosphodiesterase, whereas MPN566 does not exhibit such an activity.
Fig. 2. Effect of divalent cations on GlpQ activity. 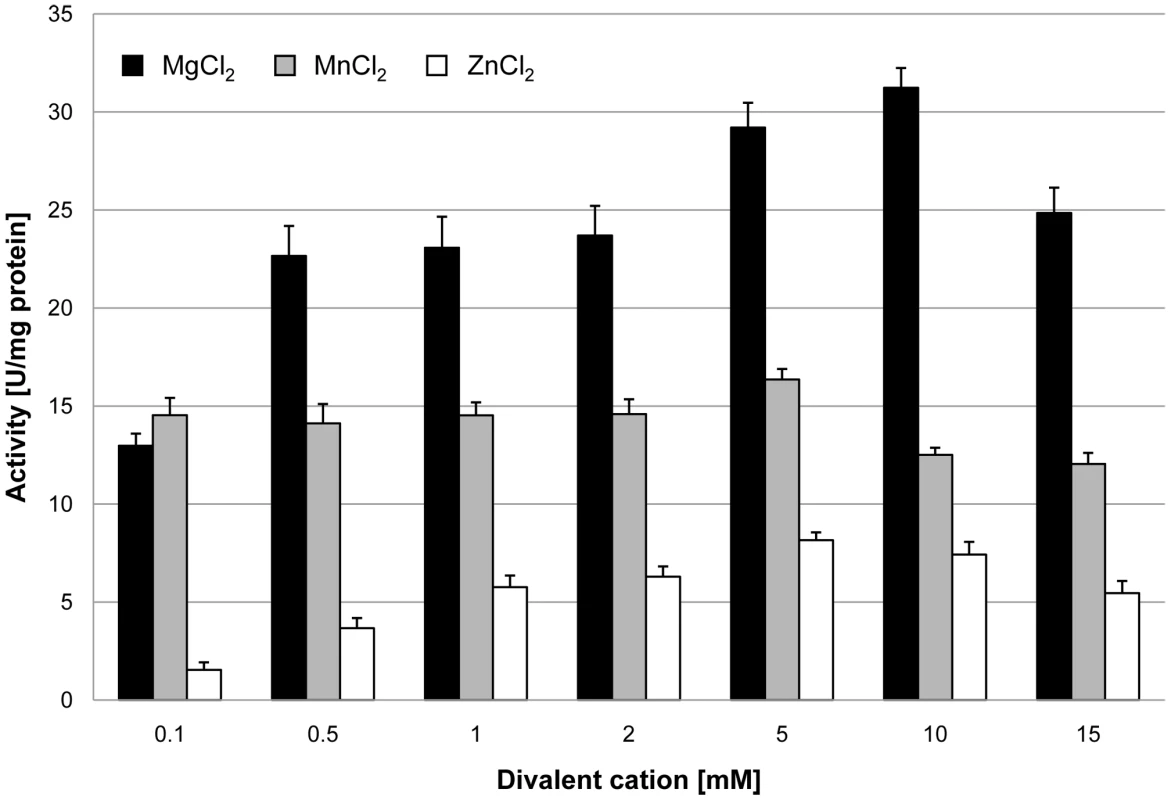
GlpQ activity was measured in the presence of 0.5 mM glycerophosphocholine and various concentrations of divalent cations. Error bars indicate the standard deviation (based on three independent experiments). The two proteins GlpQ and MPN566 share ∼58% identical residues. Thus, it seems surprising that MPN566 was inactive in the enzymatic assay. However, several residues that are known to be important for the activity of glycerophosphodiesterases are conserved in GlpQ but not in MPN566 (Figure S1). These residues include Trp-36 and Glu-38 as well as the conserved HD motif (Asn-51 and Leu-52 in MPN566) and Phe-110. Interestingly, a similar arrangement with two GlpQ-like proteins is also observed in M. genitalium, and as in M. pneumoniae, one protein has all the conserved residues characteristic for glycerophosphodiesterases, whereas the second protein has similar deviations from the consensus as MPN566 (Figure S1). In order to test whether a restoration of the conserved residues would also convert MPN566 to a biologically active glycerophosphodiesterase, we replaced the five amino acids that differ from the consensus by those residues present in GlpQ. The resulting mutant allele was cloned into pGP172, and purification attempted. Unfortunately, this protein was highly unstable and purification was impossible.
Construction of glpQ and mpn566 mutants
The analysis of mutants is one of the most powerful tools for studying gene functions and bacterial physiology. The isolation of desired M. pneumoniae mutants became possible only recently by the introduction of the “Haystack mutagenesis” [27]. To get more insights into the physiological role of the glycerophosphodiesterases GlpQ and its paralog MPN566, we attempted to isolate mutants affected in the corresponding genes.
The strategy of “Haystack mutagenesis” is based on an ordered collection of pooled random transposon insertion mutants that can be screened for junctions between the transposon and the gene of interest due to transposon insertion. The 64 pools were used in a PCR to detect junctions between the glpQ or mpn566 genes and the mini-transposon using the oligonucleotides SS35 and SS40 (for the respective genes) and SH30 (for the mini-transposon) [28] (Figure 3). Positive signals were obtained for both genes. From pools that gave a positive signal, colony PCR with the 50 individual mutants resulted in the identification of the desired glpQ and mpn566 mutants. The presence of the transposon insertion in both genes was verified by Southern blot analysis (Figure 3). To test whether these strains contained only unique transposon insertions, we did another Southern blot using a probe specific for the aac-aphD resistance gene present on the mini-transposon. As shown in Figure 3, only one band hybridizing with this probe was detected in each strain, moreover, this fragment had the same size as the AgeI or PstI/SacI fragment hybridizing to the glpQ and mpn566 probe, respectively (Figure 3). The isolated glpQ and mpn566 mutant strains were designated GPM81 and GPM82. The position of the transposon insertion in the two genes was determined by DNA sequencing. The glpQ gene was disrupted between nucleotides 517 and 518, resulting in a truncated protein of 172 amino acids with one additional amino acid and the following stop codon encoded by the inserted mini-transposon. The disruption of the mpn566 gene was located between nucleotides 157 and 158, resulting in a truncated protein of 52 amino acids with one additional amino acid and the following stop codon.
Fig. 3. Isolation of M. pneumoniae glycerophosphodiesterases transposon insertion mutants. 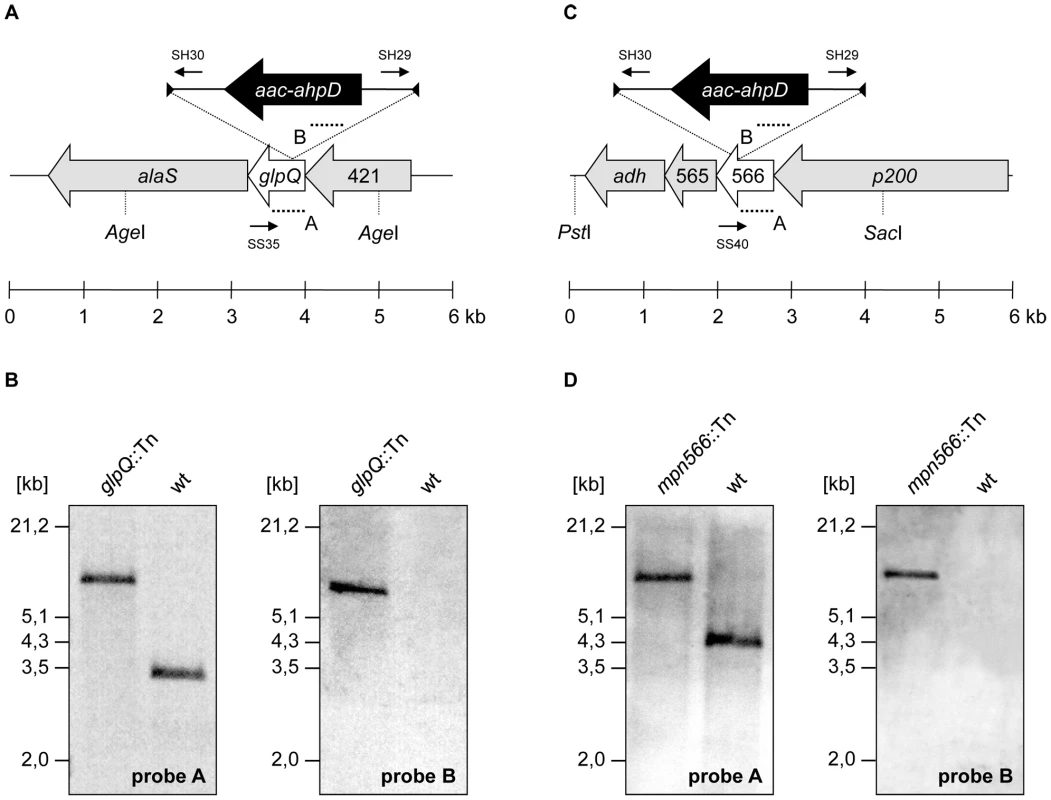
(A, C) Schematic representation of the genomic region surrounding the glpQ and mpn566 gene (both designated as glycerophosphodiesterases) in M. pneumoniae and site of the transposon insertion in the knockout strains GPM81 and GPM82, respectively. The annealing sites of oligonucleotides used for the determination of the transposon insertion site are indicated by small arrows. Probes A and B hybridizing to internal fragments of the glycerophosphodiesterases and the aac-ahpD genes are depicted as dotted lines. (B, D) Southern blot analysis to confirm the single insertion of the mini-transposon into the glpQ and mpn566 gene of the strains GPM81 and GPM82, respectively. Chromosomal DNAs of the wild type and both glycerophosphodiesterase mutants were digested as indicated. Blots were hybridized with the respective glycerophosphodiesterase-specific probe (left) and a probe hybridizing to the aac-ahpD gene of the mini-transposon (right). Contributions of GlpQ and MPN566 to growth and motility
First, we compared the ability of the wild type strain and the two mutant strains to utilize glucose and glycerol as the single carbon sources (Figure 4). As an additional control, we used the glpD mutant strain GPM52. This strain is defective in glycerol-3-phosphate oxidase and therefore unable to utilize glycerol as the only carbon source [10]. As shown in Figure 4A, the wild type and the glpD and mpn566 mutant strains grew well with glucose. In contrast, the glpQ mutant GPM81 grew more slowly and did not reach the final biomass as compared to the other strains. As reported previously, the wild type strain exhibited very slow growth with glycerol as the only carbon source [29]. In this respect, the glpQ and mpn566 mutants were indistinguishable from the wild type. As reported previously, the glpD mutant strain did not grow at all in glycerol-containing medium [10]. In conclusion, the active glycerophosphodiesterase GlpQ is required for maximal growth in the presence of glucose, whereas its absence does not interfere with the slow growth in the presence of glycerol.
Fig. 4. Growth of M. pneumoniae in modified Hayflick medium containing different carbon sources. 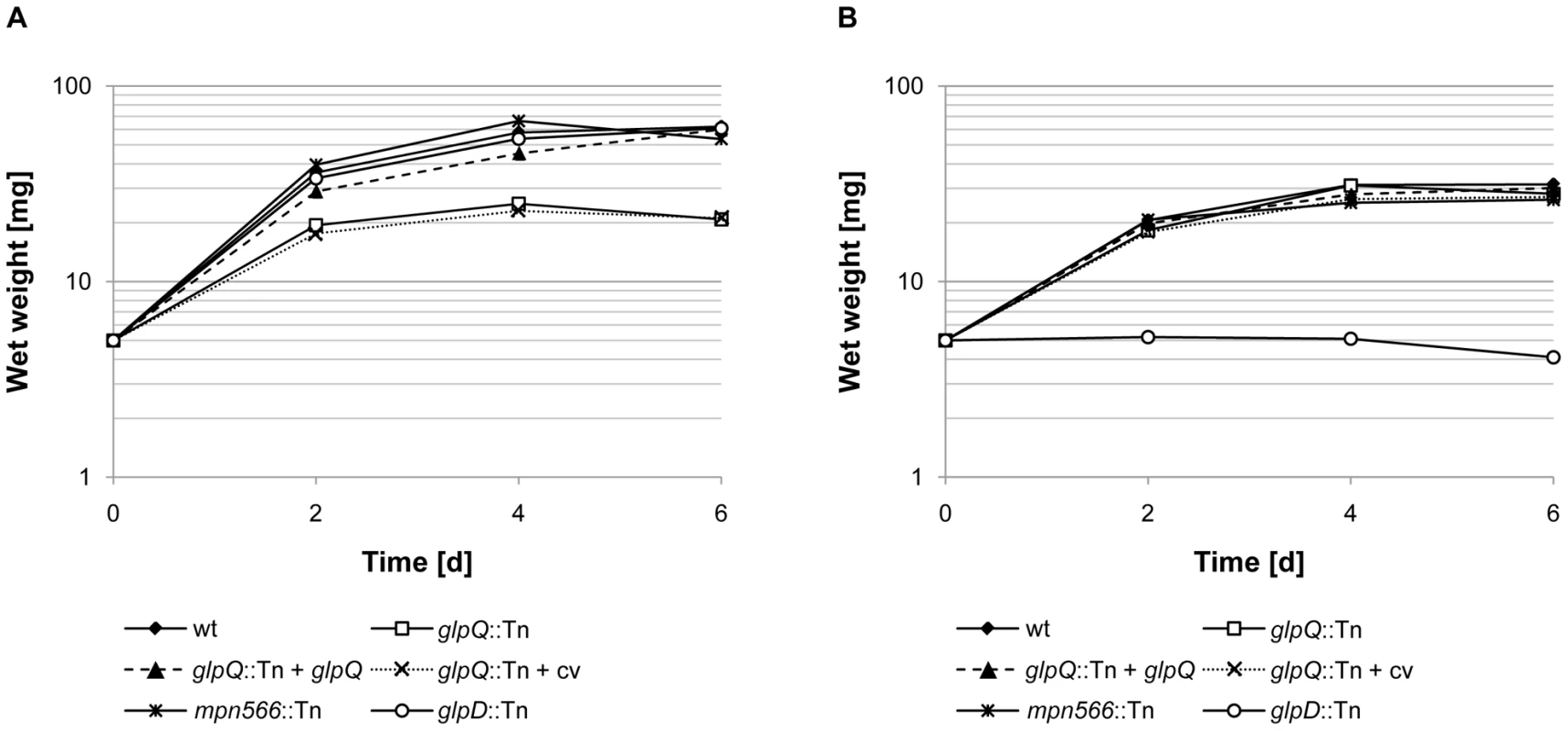
One hundred milliliters of medium were inoculated with 5 mg of the relevant M. pneumoniae cells and incubated for up to six days at 37°C in 150-cm2 cell culture flasks. The following strains were used: wild type (wt), glpQ::Tn, mpn566::Tn, and glpD::Tn, glpQ::Tn + glpQ (complemented mutant) and glpQ::Tn + cv (control strain carrying the empty vector used for complementation). Glucose (A) and glycerol (B) were added to a final concentration of 1% (wt/vol). Attached cells were collected by scraping and growth was monitored by determination of the wet weight of the cell pellets. All measurements were done three times. Results are from a representative experiment. Since the disruption of glpQ affected the growth properties of the bacteria, we wondered whether this might reflect changes in cell morphology and in the movement of the bacteria. The morphology of the wild type and mutant bacteria was analyzed by scanning electron microscopy, and no differences were detected (Figure S2). An analysis of the gliding velocities of the three strains revealed that the wild type strain glided with a velocity of 0.32±0.09 µm/s, whereas the glpQ and mpn566 mutants exhibited velocities of 0.2±0.08 µm/s and 0.3±0.1 µm/s, respectively (Videos S1, S2, and S3). Thus, the active glycerophosphodiesterase GlpQ is required for full gliding velocity of the bacteria.
Implication of GlpQ and MPN566 in hydrogen peroxide production and cytotoxicity
The utilization of glycerol or glycerophosphodiesters results in the generation of hydrogen peroxide, the major cytotoxic product of M. pneumoniae. We asked therefore whether the glpQ and mpn566 disruptions would affect hydrogen peroxide formation and if so, whether it also affects cytotoxicity. Hydrogen peroxide formation was assayed in M. pneumoniae cultures that contained glucose, glycerol, GPC, glycerol-3-phosphate or no carbon source. In the absence of an added carbon source, neither the wild type strain nor the mutants formed substantial amounts of hydrogen peroxide (Figure 5). It is interesting to note that the wild type and the mpn566 mutant formed some hydrogen peroxide even in the absence of any added carbon source. This might result from the presence of low concentrations of phospholipids in the medium. Similarly, essentially no hydrogen peroxide was produced in the presence of glucose. If glycerol was available, maximal hydrogen peroxide formation (9.5 mg/l) was observed in the wild type strain. In the glpD mutant that served as a control, no hydrogen peroxide was formed. This is in good agreement with previous reports on the increase of hydrogen peroxide generation in the presence of glycerol and its dependence on a functional glycerol-3-phosphate oxidase [10]. The hydrogen peroxide production in the glpQ and mpn566 mutants was similar to that observed in the wild type strain. This result reflects that the metabolite glycerol is downstream from the glycerophosphodiesterase activity. In the presence of GPC, the wild type strain produced similar amounts of hydrogen peroxide (9 mg/l) as in the presence of glycerol. In contrast, no hydrogen peroxide formation was detected for the glpQ mutant GPM81, whereas the disruption of mpn566 did not have any effect on the production of hydrogen peroxide (Figure 5). This result is in good agreement with the enzymatic activities of the two proteins: GlpQ is the only active glycerophosphodiesterase in M. pneumoniae, and no glycerol-3-phosphate, the substrate of GlpD, can be formed in its absence, whereas MPN566 is dispensable for the utilization of GPC. We also tested the ability of the M. pneumoniae strains to form hydrogen peroxide in the presence of glycerophosphoethanolamine and glycerophosphoglycerol. These compounds did not stimulate hydrogen peroxide in any of the strains tested (data not shown). This is in excellent agreement with the result of the enzyme assay that suggested that neither GlpQ nor MPN566 is able to degrade these substances. Finally, we tested whether hydrogen peroxide was formed in the presence of glycerol-3-phosphate. As shown in Figure 5, no significant formation of hydrogen peroxide was observed in any of the strains tested. This suggests that the uptake of glycerol-3-phosphate is rather inefficient.
Fig. 5. Examination of M. pneumoniae hydrogen peroxide release. 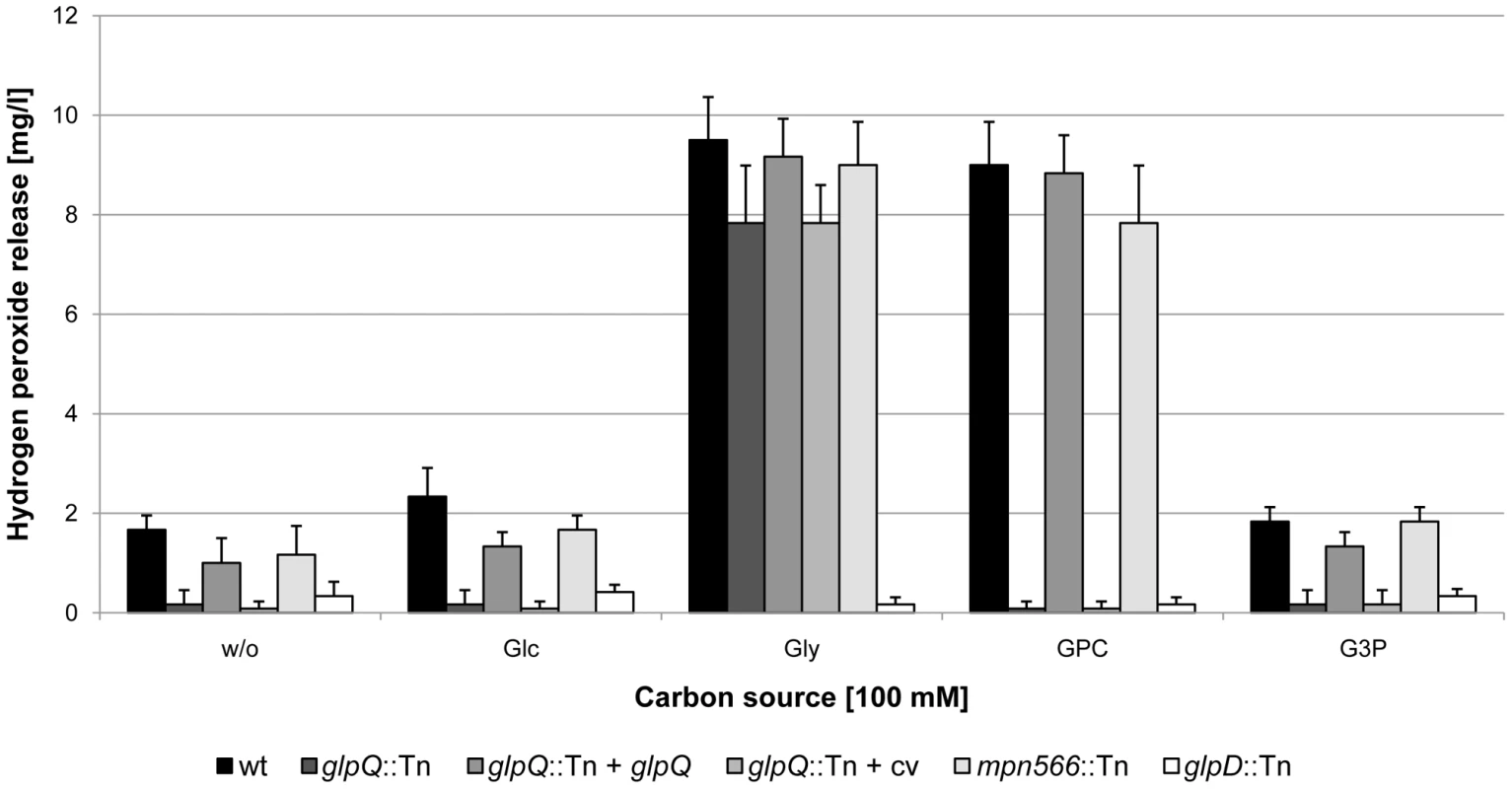
Hydrogen peroxide production of M. pneumoniae mutant strains was measured in the presence of different carbon sources (100 µM) after 2 h. The following strains were used: wild type (wt), glpQ::Tn, mpn566::Tn, and glpD::Tn, glpQ::Tn + glpQ (complemented mutant) and glpQ::Tn + cv (control strain carrying the empty vector used for complementation). Error bars indicate standard deviation (based on three independent experiments). G3P, glycerol-3-phosphate; GPC, glycerophosphocholine; Glc, glucose; Gly, glycerol; w/o, without addition of any carbon source. To assess the cytotoxicity of the different M. pneumoniae strains, we infected confluently grown HeLa cell cultures with M. pneumoniae cells (multiplicity of infection: 2). The cytotoxicity of the mutants was compared to that of the wild type strain and M. pneumoniae GPM52 that is affected in glpD. As shown in Figure 6, the HeLa cells had undergone nearly complete lysis after four days upon infection with wild type M. pneumoniae (cytotoxicity of 89%). As observed previously, the glpD mutant GPM52 has a reduced cytotoxicity (51%) resulting in a large portion of viable cells after infection [10]. For the glpQ mutant GPM81, nearly all HeLa cells had survived the infection suggesting that GlpQ is essential for cytotoxicity. In contrast, cytotoxicity induced by the mpn566 mutant strain GPM82 was equivalent to that of the wild type strain (Figure 6). These data clearly demonstrate that the active glycerophosphodiesterase GlpQ is required for host cell damage, whereas MPN566 is not. Moreover, they support the assumption that hydrogen peroxide formation is the major factor that contributes to host cell damage.
Fig. 6. Cytotoxicity of M. pneumoniae toward HeLa cell cultures. 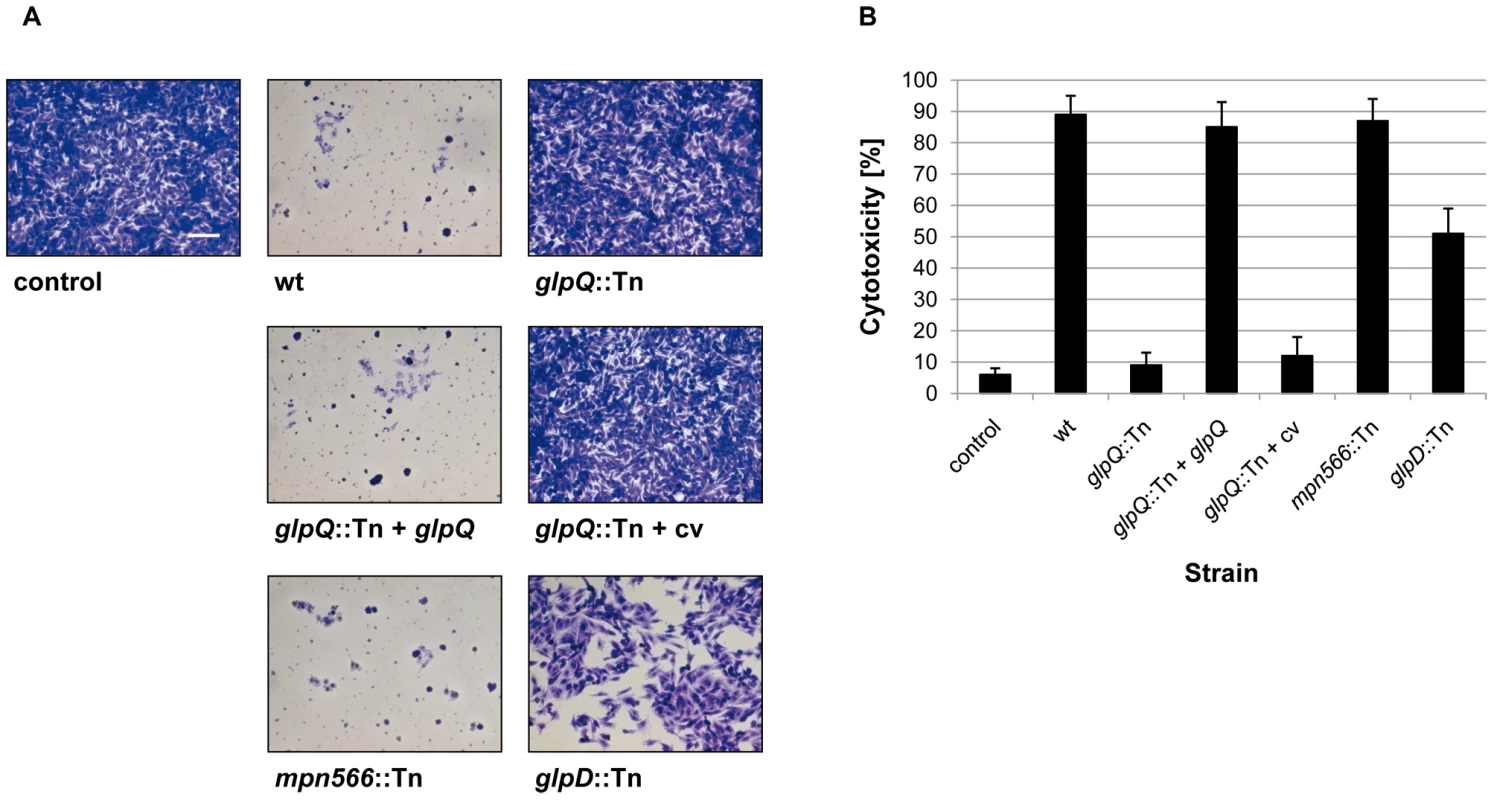
(A) Infection assay to verify cytotoxic effects of M. pneumoniae glpQ::Tn and mpn566::Tn mutant strains. HeLa cells were infected with M. pneumoniae wild type (wt), glpQ::Tn, and mpn566::Tn mutant cells. As control served two HeLa cell cultures: One without addition of any M. pneumoniae cells and another after infection with glpD::Tn mutant strain [10]. Moreover, cytotoxicity of the complemented mutant (glpQ::Tn + glpQ) and of the mutant carrying the empty vector (glpQ::Tn + cv) was tested. After four days, HeLa cell cultures were stained with crystal violet and photographed. All pictures are shown at the same magnification. Scale bar, 0.1 mm. (B) Quantification of cytotoxicity caused by M. pneumoniae in HeLa cell cultures. HeLa cells were infected with different M. pneumoniae strains or left uninfected (negative control). LDH release of HeLa cell cultures after 2 h of infection was used as an index of cytotoxicity. Cytotoxicity was calculated as the percentage of total LDH release after complete cell lysis. Error bars indicate standard deviation (based on three independent experiments). Complementation of the glpQ mutation
In order to exclude the possibility that the phenotypes observed with the glpQ mutant are due to a polar effect on the downstream alaS gene, we compared the alaS transcript levels in the wild type strain and the glpQ mutant. We observed fourfold increased amounts of alaS mRNA levels in the glpQ mutant, most likely due to the presence of a strong promoter in the transposon (data not shown). The expression of the alaS gene in the glpQ mutant strongly suggests that the observed phenotypes are the result of the glpQ disruption rather than of a polar effect. However, to provide unequivocal evidence for the implication of GlpQ in the growth phenotype as well as in hydrogen peroxide production and cytotoxicity, we performed a complementation assay. For this purpose, the M. pneumoniae glpQ gene with its own promoter was cloned into the integrative vector pMTnTetM438 and introduced into the chromosome of the glpQ mutant GPM81 (for details, see “Materials and Methods”). The resulting complementation strain GPM92 and the isogenic glpQ mutant GPM93 carrying the empty vector integrated into the chromosome were analysed for growth in the presence of glucose and glycerol, for hydrogen peroxide formation and for cytotoxicity. As shown in Figure 4A, the complemented mutant grew in the presence of glucose as the wild type strain. In contrast, the control strain carrying the empty vector grew slowly in the presence of glucose and was in this respect indistinguishable from the original glpQ mutant. These data clearly establish that the growth defect of the glpQ mutant in Hayflick medium containing glucose is a specific result of the glpQ inactivation. Similar results were observed for hydrogen peroxide production. Ectopic expression of the glpQ gene in the mutant strain restored the wild type phenotype, i.e. strong hydrogen peroxide formation in the presence of GPC. Again, the empty vector did not alter the phenotype of the glpQ mutant (see Figure 5). Finally, we assessed the cytotoxicity of the complemented strain towards HeLa cells. As one would expect from the restoration of hydrogen peroxide production upon complementation, the complemented mutant GPM92 was toxic for the HeLa cells, whereas the mutant carrying the control vector was not (Figure 6). In conclusion, ectopic expression of glpQ complemented all mutant phenotypes thus demonstrating, that the active glycerophosphodiesterase GlpQ is indeed essential for hydrogen peroxide production in the presence of the major substrate glycerophosphocholine and for cytotoxicity of M. pneumoniae.
The role of GlpQ in gene expression
As reported above, the glpQ mutant exhibits multiple phenotypes related to motility, metabolism, and pathogenicity. We asked therefore whether some of the effects are due to changes in the proteome of the glpQ mutant GPM81. To answer this question, we compared the total protein profiles of the wild type strain and the glpQ and mpn566 mutants, GPM81 and GPM82, respectively, after growth in glucose and glycerol. While the protein patterns in the mpn566 mutant were indistinguishable from the wild type strain under both conditions, several differences were noted for the glpQ mutant.
To identify those proteins that exhibit altered accumulation in the glpQ mutant, the total proteins of the wild type and the glpQ mutant strains were identified by mass spectrometry. For the protein extracts from glucose-grown cells, 532 different proteins were identified. This corresponds to about 77% of the theoretical proteome of M. pneumoniae. In the presence of glycerol, 473 proteins corresponding to 69% of the theoretical proteome were identified. The differences in protein expression between glucose - and glycerol-grown cells as well as proteins that could not be detected at all are summarized in Tables S1 and S2. A detailed list of the differences of the protein profiles between the wild type strain and the glpQ mutant is presented in Tables S3 and S4. As expected, the GlpQ protein was detected in the protein extracts of the wild type strain but not in those of the glpQ mutant strain. In glucose-grown cells, 33 and 21 proteins were in elevated and reduced amounts, respectively, in the glpQ mutant. The strongest increase was observed for the glycerol facilitator GlpF and the uncharacterized lipoprotein MPN162. A strongly reduced accumulation was observed for the lipoprotein MPN506. In the presence of glycerol, five induced and five repressed proteins were detected (see Table S4). Five proteins were subject to a identical regulation under both conditions (Table 1).
Tab. 1. Proteins with GlpQ-dependent expression pattern. 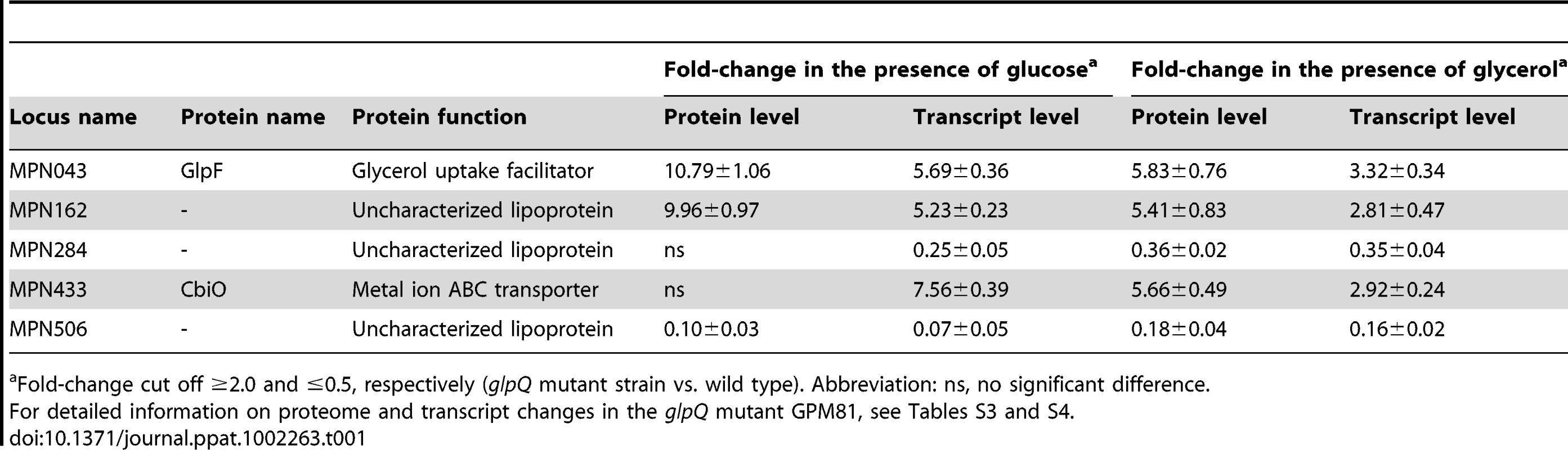
Fold-change cut off ≥2.0 and ≤0.5, respectively (glpQ mutant strain vs. wild type). Abbreviation: ns, no significant difference. It has been shown before that changes at the proteome level may result from altered gene expression or from changes in protein stability [30], [31]. Therefore, we studied the expression of the genes corresponding to the most prominently regulated proteins and of genes encoding potential regulators, transport systems and potential pathogenicity factors. For this purpose, we isolated RNA from cultures grown in modified Hayflick medium supplemented with glucose and performed slot blot analyses (Figures 7 and S3).
Fig. 7. Transcription analysis of GlpQ-dependent genes in M. pneumoniae. 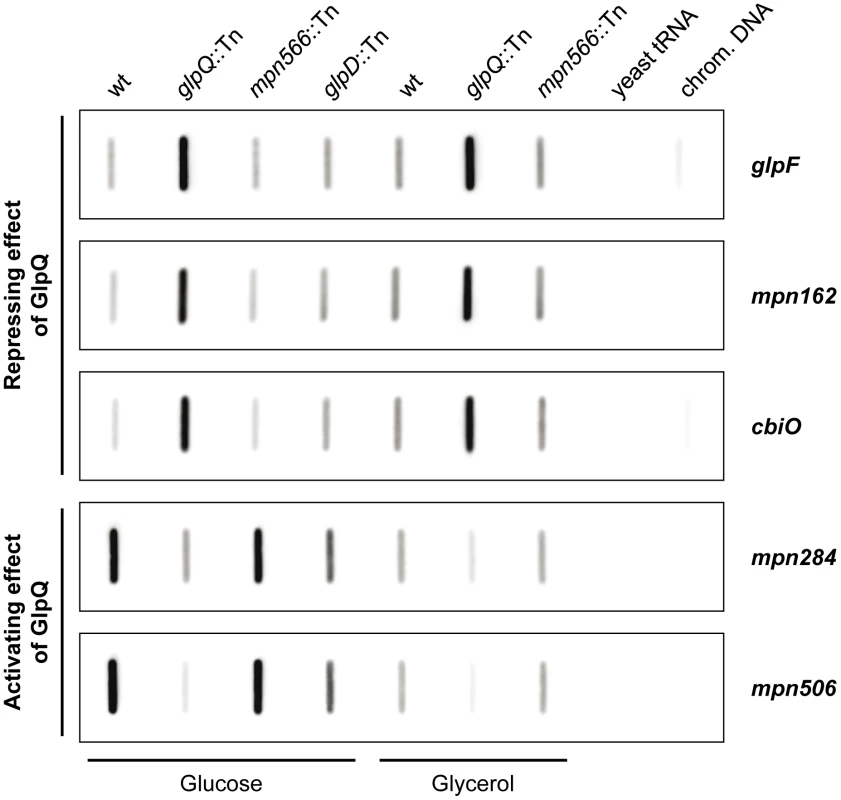
Slot blots were performed with whole RNA extracts of M. pneumoniae wild type (wt), glpQ::Tn, mpn566::Tn, and glpD::Tn (control) mutant strains grown in modified Hayflick medium containing either glucose or glycerol as sole carbon source (1% [wt/vol]). A dilution series of RNA extracts was blotted onto a positively charged nylon membrane and probed with a DIG-labeled riboprobe specific for an internal part of a particular open reading frame. Names of riboprobes are given next to each blot. Signals obtained with 1 µg of RNA are shown. Yeast tRNA and M. pneumoniae chromosomal DNA served as controls. For detailed information on differences of transcript levels see Table 1, S3, and S4. These studies demonstrated that the regulation of the glycerol facilitator GlpF and the lipoproteins MPN162 and MPN506 occurs at the level of transcription (Table 1). Moreover, our results confirmed the higher expression of glpF and mpn162 and the repression of mpn506 in the glpQ mutant. For the other proteins that were induced in the presence of glucose, with exception of plsC and mpn566 (nearly two-fold higher transcript levels), similar accumulation of the mRNAs compared to the protein amount was observed (Table S3 and Figure S3). In contrast, for the proteins that were present in reduced amounts in glucose-grown cells, no changes of the corresponding mRNAs were observed for all transport proteins. Interestingly, the lipoprotein MPN083 showed a similar pattern at the level of transcription as the induced proteins and the ribonucleoside-diphosphate reductase (encoded by nrdFIE) was the only protein with reduced mRNA amounts, however changes in transcript level were not significant (Table S3 and Figure S3).
Identification of a potential target site for GlpQ-dependent regulation
The proteome and transcription analyses identified three genes that are significantly regulated - either induced or repressed - in a GlpQ-dependent manner. An inspection of the upstream region of these genes revealed the presence of a common palindromic DNA motif (Figure 8). To exclude the possibility that this motif is randomly distributed in the genome of M. pneumoniae because of the extremely AT-rich consensus sequence, we tested its presence in the genome using the GLAM2SCAN algorithm [32]. In nine cases (matching score cut-off ≥30), this potential motif was located upstream of open reading frames, among them the three genes mentioned above. Therefore, the expression of the remaining six genes was tested by slot blot analysis, and for two of these genes, cbiO and mpn284, a significant accumulation and reduction of the mRNA, respectively, was observed (data not shown; Figure 7). Interestingly, the corresponding proteins, a subunit of a putative metal ion ABC transporter CbiO and the uncharacterized lipoprotein MPN284 were found to be present in higher or lower amounts in the glpQ mutant in glycerol-grown cell. Thus, there is a very good agreement between the regulatory effect of GlpQ at the proteome level, the regulation at the level of transcription, and the presence of the cis-acting element.
Fig. 8. Transcriptional organization of GlpQ-dependent genes in M. pneumoniae. 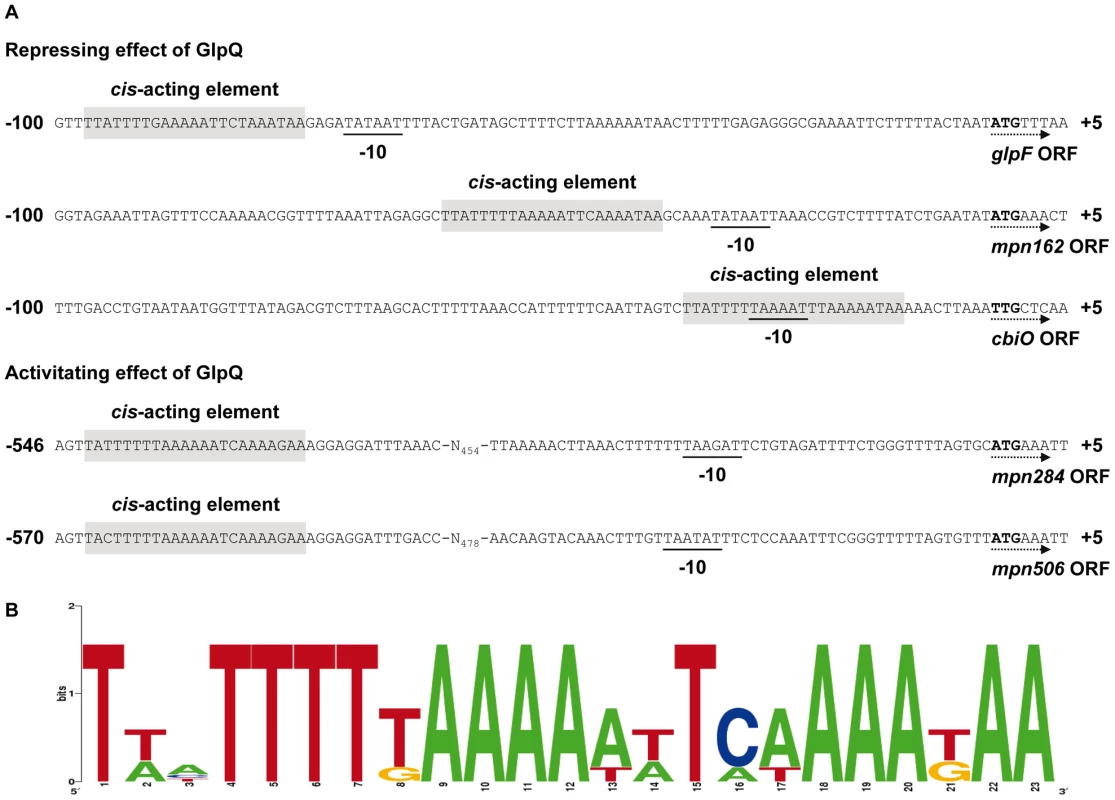
(A) Nucleotide sequence of promoter regions of M. pneumoniae glpF (mpn043), mpn162, cbiO (mpn433), mpn284, and mpn506 genes. Promoter sequences are numbered relative to the 5’ end. Directions of the open reading frames (ORF) are indicated by dotted arrows and predicted ATG/TTG start codons are highlighted by bold type. The respective -10 motifs are underlined. GlpQ-dependent cis-acting elements (palindromic DNA motifs) are indicated by grey shading. (B) Consensus sequence of the GlpQ-dependent palindromic DNA motif in M. pneumoniae. The sequence logo was created using WebLogo v2.8.2 [50] based on all five palindromic DNA motifs mentioned in A. Discussion
Two cytotoxicity factors are known in M. pneumoniae: The formation of hydrogen peroxide and the CARDS toxin that possesses ADP-ribosyltransferase activity [11], [33]. This work establishes that the glycerophosphodiesterase GlpQ of M. pneumoniae is essential for cytotoxicity of these bacteria. This is in excellent agreement with previous reports that carbon metabolism is intimately linked to virulence in pathogenic bacteria, including M. pneumoniae and other mollicutes [1], [2], [8]. The utilization of glycerol and phospholipids plays a particularly important role in the virulence of Mycoplasma species: Hydrogen peroxide, the major cytotoxic substance produced by these bacteria, is generated as a product of glycerol metabolism, and both glpD and glpQ mutants are severely affected in pathogenicity [10, this work]. In M. mycoides, pathogenicity is associated with the presence of a highly efficient ABC transporter for glycerol. Non-pathogenic strains of M. mycoides rely on the less efficient glycerol facilitator for glycerol uptake [34].
In M. pneumoniae, GlpQ is not only important for virulence but also for growth in the commonly used medium in the laboratory, i.e. Hayflick medium with glucose as the added carbon source (see Figure 4A). This observation is in good agreement with a recent analysis of the M. pneumoniae metabolism that suggested that glycerol is essential for growth of M. pneumoniae [35]. Accordingly, no difference between the wild type strain and the glpQ mutant was observed during growth in the presence of glycerol (see Figure 4B). Therefore, it is tempting to speculate that some glycerophosphodiesters in the Hayflick medium support growth.
In addition to GlpQ, M. pneumoniae encodes a second paralogous protein. However, as shown in this work, this protein does not exhibit enzymatic activity nor does the inactivation of the corresponding gene (mpn566) cause any detectable phenotype. This lack of detectable activity of MPN566 is easily explained by the lack of conservation of amino acid residues that are essential for the activity as a glycerophosphodiesterase. Interestingly, a very similar arrangement with two glpQ-like genes is also present in M. genitalium and Mycoplasma alligatoris. Based on the conservation of the catalytically important residues (see Figure S1), there is an active and an inactive enzyme in M. genitalium, as observed here for M. pneumoniae. In M. alligatoris, both potential glycerophosphodiesterases contain all the important amino acids suggesting that both proteins are enzymatically active. It is tempting to speculate that the possession of two active glycerophosphodiesterases is related to the fact that M. alligatoris is the only mollicute that obligatorily causes fatal infections [36]. In the syphilis spirochaete, Treponema pallidum, one glpQ-like gene is present; however, the encoded protein is not active as a glycerophosphodiesterase. Again, the inactivity is most likely caused by the lack of conservation of functionally important amino acids [37], [38]. The presence of inactive GlpQ-like proteins in several pathogens, including a spirochaete and M. genitalium, the bacterium with the smallest genome, suggests that these proteins have other functions that have yet to be identified. Unfortunately, the experiments reported in this study did not give any hints as to a putative function of MPN566.
Many proteins have activities in addition to their primary functions. On one hand, this allows gene duplication and specialization to non-related functions of similar proteins. On the other hand, a protein may acquire a second useful activity and act as a so-called moonlighting protein [39]. The former is very common and might apply to the putative functional specialization of GlpQ and MPN566. In contrast, the latter phenomenon is true for all trigger enzymes that measure the availability of their respective metabolites and transduce this information to the regulatory machinery of the cell. In mammals, a glycerophosphodiesterase controls the development of skeletal muscles independent from its enzymatic activity [40]. Our results suggest that GlpQ might also have such a second activity. Indeed, the expression of the glycerol facilitator GlpF, a lipoprotein, and the ATP-binding subunit of a metal ion ABC transporter are strongly overexpressed in the glpQ mutant, whereas two uncharacterized lipoproteins are less expressed in the mutant. Interestingly, the genes that appear to be repressed by GlpQ are more strongly transcribed in the presence of glycerol as the carbon source (as compared to glucose). In contrast, the two lipoprotein genes mpn284 and mpn506 that require GlpQ for expression are only weakly expressed in the presence of glycerol, but they are strongly induced if glucose is used as the carbon source. These observations might be explained as follows: In the presence of glucose, only little glycerol or glycerol-3-phosphate (the product of the reaction catalyzed by GlpQ) is present in the cell. Free GlpQ might then directly bind DNA or trigger the DNA-binding activity of another, yet unknown transcription factor, resulting in repression or activation of the two sets of genes. In the presence of glycerol, glycerol-3-phosphate would be formed due to the activity of glycerol kinase, and this metabolite might then prevent GlpQ from its regulatory activity. As a result, those genes that are subject to GlpQ-dependent repression (glpF, mpn162, and cbiO) are stronger expressed than in the presence of glucose, whereas the GlpQ-activated genes (mpn284, mpn506) would be less expressed. Finally, in the glpQ mutant, the former set of GlpQ-repressed genes is highly constitutively expressed, and only a very low level of transcription can be detected for the two GlpQ-dependent lipoprotein genes. Since glycerol-3-phosphate is the product of the glycerophosphodiesterase reaction, this metabolite is an excellent candidate for detection by GlpQ. Moreover, the glpQ gene is constitutively expressed and the GlpQ protein was detected in M. pneumoniae cells irrespective of the carbon source used in similar amounts in this study [41, this study]. Thus, GlpQ is available in the cell under all conditions to cause regulation. In a recent study on the phosphoproteome of M. pneumoniae, phosphorylation of GlpQ was observed [42]; however, no precise phosphorylation site could be detected and predicted, respectively. Therefore, the functional relevance of this modification remains unknown so far.
As observed for several other transcription regulators and trigger enzymes, GlpQ exerts both an activating and repressing effect on gene expression. The location of the putative cis-acting element correlates perfectly with the regulatory effect: Those genes that seem to be repressed by GlpQ-dependent manner have this element overlapping or in the very close vicinity of the -10 region of the promoters. This element is the only conserved promoter element in M. pneumoniae and it is sufficient for transcription initiation [30], [41], [43]. Binding of GlpQ or of a transcription factor that is controlled by GlpQ would prevent a productive interaction with RNA polymerase and therefore cause transcription repression. On the other hand, the cis-acting elements that may be involved in the regulation of the GlpQ-activated genes are located upstream of the promoters. This is usually the case for binding sites of transcription activators and fits perfect with the observed regulation.
Our future work will focus on the elucidation of the mechanism(s) by which GlpQ controls gene expression. Moreover, we will address the functions of the lipoproteins that are subject to glycerol - and GlpQ-dependent regulation.
Materials and Methods
Bacterial strains and growth conditions
The M. pneumoniae strains used in this study were M. pneumoniae M129 (ATCC 29342) in the 32nd broth passage, and its isogenic mutant derivatives GPM52 (glpD::mini-Tn, GmR) [10], GPM81 (glpQ::mini-Tn, GmR), and GPM82 (mpn566::mini-Tn, GmR). M. pneumoniae was grown at 37°C in 150 cm2 tissue culture flasks containing 100 ml of modified Hayflick medium as described previously [29]. Carbon sources were added to a final concentration of 1% (w/v). Growth curves were obtained by determining the wet weight of M. pneumoniae cultures as described previously [29]. Strains harboring transposon insertions were cultivated in the presence of 80 µg/ml gentamicin and/or 2 µg/ml tetracycline as required. Escherichia coli DH5α and BL21(DE3)/pLysS [44] were used as host for cloning and recombinant protein expression, respectively.
The sequences of the oligonucleotides used in this study are listed in Table S5.
Construction of a glpQ complementation strain
To achieve complementation of the glpQ mutant, we constructed strain GPM92 as follows: The M. pneumoniae glpQ gene including its own promoter was amplified using the primer pair SS245/SS267. The PCR product was digested with EcoRI and XhoI and cloned between the EcoRI/SalI sites of the integrative plasmid pMTnTetM438 [45]. The resulting plasmid, pGP695, was introduced by electroporation into the genome of the M. pneumoniae glpQ mutant GPM81. As a control, we transformed GPM81 with the empty vector pMTnTetM438. The resulting strain was M. pneumoniae GPM93. To exclude multiple insertions of the integrative plasmids in the two constructed strains, we performed Southern blot analyses with both mutants using a probe specific for the tetracycline resistance gene. In both cases, unique insertion events were detected.
M. pneumoniae chromosomal DNA was prepared as described previously [28]. Finally, digests of chromosomal DNA were separated using 1% agarose gels and transferred onto a positively charged nylon membrane (Roche Diagnostics) [44] and probed with Digoxigenin labeled riboprobes obtained by in vitro transcription with T7 RNA polymerase (Roche Diagnostics) using PCR-generated fragments as templates. Primer pairs for the amplification of glpQ, mpn566, aac-ahpD, and tet gene fragments were SS42/SS43, SS44/SS45, SH62/SH63, and SS272/SS273, respectively (Table S5). The reverse primers contained a T7 RNA polymerase recognition sequence. In vitro RNA labeling, hybridisation and signal detection were carried out according to the manufacturer’s instructions (DIG RNA labeling Kit and detection chemicals; Roche Diagnostics).
Production and analysis of recombinant putative glycerophosphodiesterases
The M. pneumoniae genes encoding proteins similar to glycerophosphodiesterases (glpQ and mpn566) were amplified with chromosomal DNA as the template and the primer pairs SS34/SS35 and SS39/SS40, respectively. The PCR products were digested with SacI and BamHI and cloned into the expression vector pGP172 that allows the fusion of the target proteins to a Strep-tag at their N-terminus [46]. The resulting plasmids were pGP1018 and pGP1020. Since the glpQ gene contains three TGA codons that are recognized as stop codons in E. coli, these codons were replaced by TGG specifying tryptophan as in M. pneumoniae. For this purpose we applied the multiple mutation reaction [47] using the phosphorylated mutagenesis primers SS36, SS37, and SS38 and the external primers SS34 and SS35. The PCR product was digested and cloned into pGP172 as described above. The resulting expression vector was pGP1019. The plasmids pGP1019 and pGP1020 allowed the purification of the putative M. pneumoniae glycerophosphodiesterases (GlpQ and MPN566) carrying an N-terminal Strep-tag.
A mutant variant of MPN566 was obtained by the multiple mutation reaction using pGP1020 as the template and the phosphorylated mutagenesis primers SS192, SS193, and SS194 and the external primers SS39 and SS40. The PCR product was cloned into pGP172 as described above and the resulting plasmid was pGP661.
The putative glycerophosphodiesterases were overexpressed in E. coli BL21(DE3)/pLysS. Expression was induced by the addition of IPTG (final concentration 1 mM) to exponentially growing cultures (OD600 of 0.8). Cells were lysed using a french press (20.000 p.s.i., 138,000 kPa, two passes, Spectronic Instruments, UK). After lysis the crude extracts were centrifuged at 15,000 g for 60 min. The crude extract was passed over a Streptactin column (IBA, Göttingen, Germany). The recombinant proteins were eluted with desthiobiotin (IBA, final concentration 2.5 mM).
After elution the fractions were tested for the desired protein using 12% SDS-PAGE. Only fractions that contained the desired protein in apparent homogeneity (content of the specific protein >95%) were used for further experiments. The relevant fractions were combined and dialyzed overnight. Protein concentration was determined according to the method of Bradford using the Bio-Rad dye-binding assay where Bovine serum albumin served as the standard.
Glycerophosphodiesterase activity was measured in a coupled spectrophotometric assay as described previously [48]. The enzyme assay is based on the formation of glycerol-3-phosphate and the subsequent oxidation by the glycerol-3-phosphate dehydrogenase and the formation of NADH. Briefly, 5 µg of glycerophosphodiesterase were incubated with 20 U of rabbit muscle glycerol-3-phosphate dehydrogenase (Sigma) in a 0.9 M glycine-hydrazine buffer containing 0.5 mM glycerophosphodiester and 0.5 mM NAD+ in a volume of 1 ml. Divalent cations were added as indicated. NADH formation was determined photospectrometrically at 340 nm.
Determination of in vivo hydrogen peroxide production
The hydrogen peroxide production in M. pneumoniae was determined using the Merckoquant peroxide test (Merck, Darmstadt, Germany) as previously described [10]. Briefly, growing cells were resuspended in assay buffer and after incubation for 1 h at 37°C, glucose, glycerol, glycerol-3-phosphate or glycerophosphodiesters (final concentration 100 µM) were added to one aliquot. An aliquot without any added carbon source served as the control. The test strips were dipped into the suspensions for 1 s and subsequently read.
Preparation and analysis of whole cell extracts
Whole cell extracts of the different M. pneumoniae strains were prepared as described previously [31]. In order to analyze the complete proteome, 15 µg of the cell extracts were separated by one-dimensional 12% SDS-PAGE and the gels subsequently stained with Coomassie Brillant Blue R250 dye (Serva). For protein identification, each running lane was cut out into 15 pieces followed by a separate analysis by mass spectrometry. The proteome analyses were performed in triplicate.
Gel pieces were washed twice with 200 µl 20 mM NH4HCO3/30% (v/v) acetonitrile for 30 min, at 37°C and dried in a vacuum centrifuge (Concentrator 5301, Eppendorf). Trypsin solution (10 ng/µl trypsin in 20 mM ammonium bicarbonate) was added until gel pieces stopped swelling and digestion was allowed to proceed for 16 to 18 hours at 37°C. Peptides were extracted from gel pieces by incubation in an ultrasonic bath for 15 min in 20 µl HPLC grade water and transferred into micro vials for mass spectrometric analysis.
The tryptic digested proteins obtained from the one-dimensional SDS PAGE gel pieces were subjected to a reversed phase column chromatography (Waters BEH 1.7 µm, 100-µm i.d.×100 mm, Waters Corporation, Milford, Mass., USA) operated on a nanoACQUITY UPLC (Waters Corporation, Milford, Mass., USA). Peptides were first concentrated and desalted on a trapping column (Waters nanoACQUITY UPLC column, Symmetry C18, 5 µm, 180 µm × 20 mm, Waters Corporation, Milford, Mass., USA) for 3 min at a flow rate of 1 ml/min with 0.1% acetic acid. Subsequently the peptides were eluted and separated with a non-linear 80-min gradient from 5–60% acetonitrile in 0.1% acetic acid at a constant flow rate of 400 nl/min. MS and MS/MS data were acquired with the LTQ Orbitrap mass spectrometer (Thermo Fisher, Bremen, Germany) equipped with a nanoelectrospray ion source. After a survey scan in the Orbitrap (r = 30,000), MS/MS data were recorded for the five most intensive precursor ions in the linear ion trap. Singly charged ions were not taken into account for MS/MS analysis.
Tandem mass spectra were extracted using Sorcerer v3.5 (Sage-N Research). All MS/MS samples were analyzed using SEQUEST (Thermo Fisher Scientific, San Jose, CA, USA; version 2.7, revision 11). Database searching was performed against a target decoy database of M. pneumoniae with added common laboratory contaminant proteins. Cleavage specificity for full tryptic cleavage and a maximum of 2 missed cleavages was assumed. SEQUEST was run with a fragment ion mass tolerance of 1.00 Da and a parent ion tolerance of 10 ppm. Oxidation of methionine (+15.99492 Da) and phosphorylation of serine/threonine/tyrosine (+79.966331 Da) were specified in SEQUEST as variable modifications. Proteins were identified by at least two peptides applying a stringent SEQUEST filter (Xcorr vs. charge state: 1.8 for singly, 2.2 for doubly, 3.3 for triply, and 3.5 for higher charged ions). To address protein amount differences between the M. pneumoniae wild type and mutant strains, fold-changes were calculated by comparing number of assigned spectra for each protein (mutant vs. wild type strain).
Analysis of mRNA amounts
Preparation of total M. pneumoniae RNA was done as previously described [29]. For slot blot analysis, serial twofold dilutions of the RNA extract in 10x SSC (2 µg–0.25 µg) were blotted onto a positively charged nylon membrane using a PR 648 Slot Blot Manifold (Amersham Biosciences). Equal amounts of yeast tRNA (Roche) and M. pneumoniae chromosomal DNA served as controls. DIG-labelled riboprobes were obtained by in vitro transcription from PCR products that cover ORF internal sequences using T7 RNA polymerase (Roche). The reverse primers used to generate the PCR products contained a T7 promoter sequence (Table S5). The quantification was performed using the Image J software v1.44c [49].
HeLa cell cytotoxicity assay
Infection of HeLa cell cultures with M. pneumoniae cells was done as described previously [10], [31]. After four days upon infection, HeLa cells cultures were stained with crystal violet and photographed. Additionally, lactate dehydrogenase (LDH) release of HeLa cell cultures after 2 h of infection was used as an index of cytotoxicity. LDH release was measured with the CytoTox 96 Non-Radioactive Cytotoxicity Assay (Promega) according to the manufacturer’s instructions. Results are expressed as cytotoxicity calculated as the percentage of total LDH release after cell lysis with the lysis buffer provided in the kit. The cytotoxicity assays were performed in triplicate.
Scanning electron microscopy
After growing of M. pneumoniae cultures in 5 ml volumes to mid-log, the cells were scraped off and passed ten times through a syringe. Then, 20 µl of this cell suspension were inoculated to 2 ml of Hayflick medium in a Lab-Tek chamber slide (Nunc). After growing cells to mid-log phase, the medium was removed and the cells were washed three times with PBS and fixed with 1% glutaraldehyde for 1 h. The samples were washed three times with PBS and then dehydrated sequentially with 30, 50, 70, 90, and 100% ethanol for 10 min each. Immediately, the critical point dried of samples was performed (K850 critical point drier; Emitech Ashfort, United Kingdom) and sputter coated with 20 nm of gold. Samples were observed using a Hitachi S-570 (Tokyo, Japan) scanning electron microscope.
Microcinematography
After passing through a syringe cells grown in a 5 ml culture, 20 µl of disaggregated cells were inoculated to 2 ml of modified Hayflick medium including 3% gelatine in 14 mm glass bottom culture dishes plates (MatTek). Cell movement was examined at 37°C using a Nikon Eclipse TE 2000-E microscope, and images were captured at intervals of 2 s for a total of 2 min with a digital sight DS-SMC Nikon camera controlled by NIS-Elements BR software. Tracks from 50 individual motile cells corresponding to 2 min of observation and 2 separated experiments were analyzed to determine the gliding velocity and gliding motile patterns.
Supporting Information
Zdroje
1. SonensheinAL 2007 Control of key metabolic intersections in Bacillus subtilis. Nat Rev Microbiol 5 917 927
2. GörkeBStülkeJ 2008 Carbon catabolite repression in bacteria: Many ways to make the most out of nutrients. Nat Rev Microbiol 6 613 624
3. EisenreichWDandekarTHeesemannJGoebelW 2010 Carbon metabolism of intracellular bacterial pathogens and possible links to virulence. Nat Rev Microbiol 8 40 412
4. WaitesKBTalkingtonDF 2004 Mycoplasma pneumoniae and its role as human pathogen. Clin Microbiol Rev 17 697 728
5. TsiodrasSKelesidisIKelesidisTStamboulisEGiamarellouH 2005 Central nervous system manifestations of Mycoplasma pneumoniae infection. J Infect 51 343 354
6. NaritaM 2009 Pathogenesis of neurogenic manifestations of Mycoplasma pneumoniae infection. Pediatr Neurol 41 159 166
7. NaritaM 2010 Pathogenesis of extrapulmonary manifestations of Mycoplasma pneumoniae infection with special reference to pneumonia. J Infect Chemother 16 162 169
8. HalbedelSHamesCStülkeJ 2007 Regulation of carbon metabolism in the mollicutes and its relation to virulence. J Mol Microbiol Biotechnol 12 147 154
9. VeldhuizenRNagKOrgeigSPossmayerF 1998 The role of lipids in pulmonary surfactant. Biochim Biophys Acta 1408 90 108
10. HamesCHalbedelSHoppertMFreyJStülkeJ 2009 Glycerol metabolism is important for cytotoxicity of Mycoplasma pneumoniae. J Bacteriol 191 747 753
11. SomersonNLWallsBEChanockRM 1965 Hemolysin of Mycoplasma pneumoniae: Tentative identification as a peroxide. Science 150 226 228
12. PiloPVileiEMPeterhansEBonvin-KlotzLStoffelMH 2005 A metabolic enzyme as a primary virulence factor of Mycoplasma mycoides subsp. mycoides small colony. J Bacteriol 187 6824 6831
13. BischofDFVileiEMFreyJ 2009 Functional and antigenic properties of GlpO from Mycoplasma mycoides subsp. mycoides SC: Characterization of a flavine adenine dinucleotide-binding site deletion mutant. Vet Res 40 35
14. OhshimaNYamashitaSTakahashiNKuroishiCShiroY 2008 Escherichia coli cytosolic glycerophosphodiester phosphodiesterase (UgpQ) requires Mg2+, Co2+, or Mn2+ for its enzyme activity. J Bacteriol 190 1219 1223
15. WongKKKwanHS 1992 Transcription of glpT of Escherichia coli K12 is regulated by anaerobiosis and Fnr. FEMS Microbiol Lett 73 15 18
16. NilssonRPBeijerLRutbergB 1994 The glpT and glpQ genes of the glycerol regulon in Bacillus subtilis. Microbiology 140 723 730
17. AntelmannHScharfCHeckerM 2000 Phosphate starvation-inducible proteins of Bacillus subtilis: Proteomics and transcriptional analysis. J Bacteriol 182 4478 4490
18. BlenckeHMHomuthGLudwigHMäderUHeckerM 2003 Transcriptional profiling of gene expression in response to glucose in Bacillus subtilis: Regulation of the central metabolic pathways. Metab Eng 5 133 149
19. FanXGoldfineHLysenkoEWeiserJN 2001 The transfer of choline from the host to the bacterial cell surface requires glpQ in Haemophilus influenzae. Mol Microbiol 41 1029 1036
20. ForsgrenARiesbeckKJansonH 2008 Protein D of Haemophilus influenzae: A protective nontypeable H. influenzae antigen and a carrier for pneumococcal conjugate vaccines. Clin Infect Dis 46 726 731
21. SchwanTGBattistiJMPorcellaSFRaffelSJSchrumpfME 2003 Glycerol-3-phosphate acquisition in spirochaetes: Distribution and biological activity of glycerophosphodiester phosphodiesterase (GlpQ) among Borrelia species. J Bacteriol 185 1346 1356
22. CommichauFMStülkeJ 2008 Trigger enzymes: Bifunctional proteins active in metabolism and in controlling gene expression. Mol Microbiol 67 692 702
23. BeinertHKennedyMCStoutCD 1996 Aconitase as iron-sulfur protein, enzyme, and iron-regulatory protein. Chem Rev 96 2335 2374
24. ZhuWBeckerDF 2003 Flavin redox state triggers conformational changes in the PutA protein from Escherichia coli. Biochemistry 42 5469 5477
25. SchmalischMHBachemSStülkeJ 2003 Control of the Bacillus subtilis antiterminator protein GlcT by phosphorylation. Elucidation of the phosphorylation chain leading to inactivation of GlcT. J Biol Chem 51108-51115
26. CommichauFMHerzbergCTripalPValeriusOStülkeJ 2007 A regulatory protein-protein interaction governs glutamate biosynthesis in Bacillus subtilis: The glutamate dehydrogenase RocG moonlights in controlling the transcription factor GltC. Mol Microbiol 65 642 654
27. HalbedelSStülkeJ 2007 Tools for the genetic analysis of Mycoplasma. Int J Med Microbiol 297 37 44
28. HalbedelSBusseJSchmidlSRStülkeJ 2006 Regulatory protein phosphorylation in Mycoplasma pneumoniae: A PP2C-type phosphatase serves to dephosphorylate HPr(Ser-P). J Biol Chem 281 26253 26259
29. HalbedelSHamesCStülkeJ 2004 In vivo activity of enzymatic and regulatory components of the phosphoenolpyruvate:sugar phosphotransferase system in Mycoplasma pneumoniae. J Bacteriol 186 7936 7943
30. HalbedelSEilersHJonasBBusseJHeckerM 2007 Transcription in Mycoplasma pneumoniae: Analysis of the promoters of the ackA and ldh genes. J Mol Biol 371 596 607
31. SchmidlSRGronauKHamesCBusseJBecherD 2010 The stability of cytadherence proteins in Mycoplasma pneumoniae requires activity of the protein kinase PrkC. Infect Immun 78 184 192
32. FrithMCSaundersNFKobeBBaileyTL 2008 Discovering sequence motifs with arbitrary insertions and deletions. PLoS Comput Biol 4 e1000071
33. KannanTRBasemanJB 2006 ADP-ribosylating and vacuolating cytotoxin of Mycoplasma pneumoniae represents unique virulence determinant among bacterial pathogens. Proc Natl Acad Sci U S A 103 6724 6729
34. VileiEMFreyJ 2001 Genetic and biochemical characterization of glycerol uptake in Mycoplasma mycoides subsp. mycoides SC: Its impact on H2O2 production and virulence. Clin Diagn Lab Immunol 8 85 92
35. YusEMaierTMichalodimitrakisKvan NoortVYamadaT 2009 Impact of genome reduction on bacterial metabolism and its regulation. Science 326 1263 1268
36. BrownDRFarleyJMZacherLACarltonJMClippingerTL 2001 Mycoplasma alligatoris sp. nov., from American alligators. Int J Syst Evol Microbiol 51 419 424
37. ShevchenkoDVAkinsDRRobinsonEJLiMShevchenkoOV 1997 Identification of homologs for thioredoxin, peptidyl prolyl cis-trans isomerase, glcerophosphodiester phosphodiesterase in outer membrane fractions of Treponema pallidum, the syphilis spirochaete. Infect Immun 65 4179 4189
38. StebeckCEShafferJMArrollTWLukehartSAvan VoohisWC 1997 Identification of the Treponema pallidum subsp. pallidum glcerophosphodiester phosphodiesterase homologue. FEMS Microbiol Lett 154 303 310
39. JefferyCJ 1999 Moonlighting proteins. Trends Biochem Sci 24 8 11
40. OkazakiYOhshimaNYoshizawaIKameiYMarrigioS 2010 A novel glycerophosphodiester phosphodiesterase, GDE5, controls skeletal muscle development via a non-enzymatic mechanism. J Biol Chem 285 27652 27663
41. GüellMvan NoortVYusEChenWHLeigh-BellJ 2009 Transcriptome complexity in a genome-reduced bacterium. Science 328 1268 1271
42. SchmidlSRGronauKPietackNHeckerMBecherD 2010 The phosphoproteome of the minimal bacterium Mycoplasma pneumoniae: Analysis of the complete known Ser/Thr kinome suggests the existence of novel kinases. Mol Cell Proteomics 9 1228 1242
43. Waldo RH3rdPophamPLRomero-ArroyoCEMothershedEALeeKK 1998 Transcriptional analysis of the hmw gene cluster of Mycoplasma pneumonia. J Bacteriol 181 4978 4985
44. SambrookJFritschEFManiatisT 1989 Molecular cloning: A laboratory manual, 2nd ed. N.Y Cold Spring Harbor Laboratory, Cold Spring Harbor
45. PichOQBurgosRPlanellRQuerolEPiñolJ 2006 Comparative analysis of antibiotic resistance gene markers in Mycoplasma genitalium: Application to studies of the minimal gene complement. Microbiology 152 519 527
46. MerzbacherMDetschCHillenWStülkeJ 2004 Mycoplasma pneumoniae HPr kinase/phosphorylase: Assigning functional roles to the P-loop and the HPrK/P signature sequence motif. Eur J Biochem 271 367 374
47. HamesCHalbedelSStülkeJ 2005 Multiple-mutation reaction: A method for simultaneous introduction of multiple mutations into the glpK gene of Mycoplasma pneumoniae. Appl Environ Microbiol 71 4097 4100
48. LarsonTJEhrmannMBoosW 1983 Periplasmic glycerophosphodiester phosphodiesterase of Escherichia coli, a new enzyme of the glp regulon. J Biol Chem 258 5428 5432
49. AbramoffMDMagelhaesPJRamSJ 2004 Image processing with ImageJ. Biophotonics Int 11 36 42
50. CrooksGEHonGChandoniaJMBrennerSE 2004 WebLogo: A sequence logo generator. Genome Res 14 1188 1190
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage EgressČlánek An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes byČlánek Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine ScrapieČlánek Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2011 Číslo 9- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity
- Envelope Deglycosylation Enhances Antigenicity of HIV-1 gp41 Epitopes for Both Broad Neutralizing Antibodies and Their Unmutated Ancestor Antibodies
- Co-opts GBF1 and CERT to Acquire Host Sphingomyelin for Distinct Roles during Intracellular Development
- Nrf2, a PPARγ Alternative Pathway to Promote CD36 Expression on Inflammatory Macrophages: Implication for Malaria
- Robust Antigen Specific Th17 T Cell Response to Group A Streptococcus Is Dependent on IL-6 and Intranasal Route of Infection
- Targeting of a Chlamydial Protease Impedes Intracellular Bacterial Growth
- The Protease Cruzain Mediates Immune Evasion
- High-Resolution Phenotypic Profiling Defines Genes Essential for Mycobacterial Growth and Cholesterol Catabolism
- Plague and Climate: Scales Matter
- Exhausted CD8 T Cells Downregulate the IL-18 Receptor and Become Unresponsive to Inflammatory Cytokines and Bacterial Co-infections
- Maturation-Induced Cloaking of Neutralization Epitopes on HIV-1 Particles
- Murine Gamma-herpesvirus Immortalization of Fetal Liver-Derived B Cells Requires both the Viral Cyclin D Homolog and Latency-Associated Nuclear Antigen
- Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression
- Strain Specific Resistance to Murine Scrapie Associated with a Naturally Occurring Human Prion Protein Polymorphism at Residue 171
- Development of a Transformation System for : Restoration of Glycogen Biosynthesis by Acquisition of a Plasmid Shuttle Vector
- Monalysin, a Novel -Pore-Forming Toxin from the Pathogen Contributes to Host Intestinal Damage and Lethality
- Host Phylogeny Determines Viral Persistence and Replication in Novel Hosts
- BC2L-C Is a Super Lectin with Dual Specificity and Proinflammatory Activity
- Expression of the RAE-1 Family of Stimulatory NK-Cell Ligands Requires Activation of the PI3K Pathway during Viral Infection and Transformation
- Structure of the Vesicular Stomatitis Virus N-P Complex
- HSV Infection Induces Production of ROS, which Potentiate Signaling from Pattern Recognition Receptors: Role for S-glutathionylation of TRAF3 and 6
- The Human Papillomavirus E6 Oncogene Represses a Cell Adhesion Pathway and Disrupts Focal Adhesion through Degradation of TAp63β upon Transformation
- Analysis of Behavior and Trafficking of Dendritic Cells within the Brain during Toxoplasmic Encephalitis
- Exposure to the Viral By-Product dsRNA or Coxsackievirus B5 Triggers Pancreatic Beta Cell Apoptosis via a Bim / Mcl-1 Imbalance
- Multidrug Resistant 2009 A/H1N1 Influenza Clinical Isolate with a Neuraminidase I223R Mutation Retains Its Virulence and Transmissibility in Ferrets
- Structure of Herpes Simplex Virus Glycoprotein D Bound to the Human Receptor Nectin-1
- Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion
- Complex Recombination Patterns Arising during Geminivirus Coinfections Preserve and Demarcate Biologically Important Intra-Genome Interaction Networks
- An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by
- Non-Lytic, Actin-Based Exit of Intracellular Parasites from Intestinal Cells
- The Fecal Viral Flora of Wild Rodents
- The General Transcriptional Repressor Tup1 Is Required for Dimorphism and Virulence in a Fungal Plant Pathogen
- Interferon Regulatory Factor-1 (IRF-1) Shapes Both Innate and CD8 T Cell Immune Responses against West Nile Virus Infection
- A Small Non-Coding RNA Facilitates Bacterial Invasion and Intracellular Replication by Modulating the Expression of Virulence Factors
- Evaluating the Sensitivity of to Biotin Deprivation Using Regulated Gene Expression
- The Motility of a Human Parasite, , Is Regulated by a Novel Lysine Methyltransferase
- Phosphodiesterase-4 Inhibition Alters Gene Expression and Improves Isoniazid – Mediated Clearance of in Rabbit Lungs
- Restoration of IFNγR Subunit Assembly, IFNγ Signaling and Parasite Clearance in Infected Macrophages: Role of Membrane Cholesterol
- Protease ROM1 Is Important for Proper Formation of the Parasitophorous Vacuole
- The Regulated Secretory Pathway in CD4 T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse
- Rerouting of Host Lipids by Bacteria: Are You CERTain You Need a Vesicle?
- Transmission Characteristics of the 2009 H1N1 Influenza Pandemic: Comparison of 8 Southern Hemisphere Countries
- Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine Scrapie
- Sequential Bottlenecks Drive Viral Evolution in Early Acute Hepatitis C Virus Infection
- Genomic Insights into the Origin of Parasitism in the Emerging Plant Pathogen
- Genomic and Proteomic Analyses of the Fungus Provide Insights into Nematode-Trap Formation
- Influenza Virus Ribonucleoprotein Complexes Gain Preferential Access to Cellular Export Machinery through Chromatin Targeting
- Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
- Protease-Sensitive Conformers in Broad Spectrum of Distinct PrP Structures in Sporadic Creutzfeldt-Jakob Disease Are Indicator of Progression Rate
- Vaccinia Virus Protein C6 Is a Virulence Factor that Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7
- c-di-AMP Is a New Second Messenger in with a Role in Controlling Cell Size and Envelope Stress
- Structural and Functional Studies on the Interaction of GspC and GspD in the Type II Secretion System
- APOBEC3A Is a Specific Inhibitor of the Early Phases of HIV-1 Infection in Myeloid Cells
- Impairment of Immunoproteasome Function by β5i/LMP7 Subunit Deficiency Results in Severe Enterovirus Myocarditis
- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Tri6 Is a Global Transcription Regulator in the Phytopathogen
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- The Next Opportunity in Anti-Malaria Drug Discovery: The Liver Stage
- Significant Effects of Antiretroviral Therapy on Global Gene Expression in Brain Tissues of Patients with HIV-1-Associated Neurocognitive Disorders
- Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in by a Modified Competence Stimulating Peptide
- A Novel Metal Transporter Mediating Manganese Export (MntX) Regulates the Mn to Fe Intracellular Ratio and Virulence
- Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control
- Hsp90 Governs Dispersion and Drug Resistance of Fungal Biofilms
- Secretion of Genome-Free Hepatitis B Virus – Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
- Membrane Remodeling by the Double-Barrel Scaffolding Protein of Poxvirus
- A Diverse Population of Molecular Type VGIII in Southern Californian HIV/AIDS Patients
- Disruption of TLR3 Signaling Due to Cleavage of TRIF by the Hepatitis A Virus Protease-Polymerase Processing Intermediate, 3CD
- Quantitative Analyses Reveal Calcium-dependent Phosphorylation Sites and Identifies a Novel Component of the Invasion Motor Complex
- Discovery of the First Insect Nidovirus, a Missing Evolutionary Link in the Emergence of the Largest RNA Virus Genomes
- Old World Arenaviruses Enter the Host Cell via the Multivesicular Body and Depend on the Endosomal Sorting Complex Required for Transport
- Exploits a Unique Repertoire of Type IV Secretion System Components for Pilus Assembly at the Bacteria-Host Cell Interface
- Recurrent Signature Patterns in HIV-1 B Clade Envelope Glycoproteins Associated with either Early or Chronic Infections
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



