-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Social Motility of African Trypanosomes Is a Property of a Distinct Life-Cycle Stage That Occurs Early in Tsetse Fly Transmission
African trypanosomes, single-celled parasites that cause human sleeping sickness and Nagana in animals, are transmitted by tsetse flies. Bloodstream form trypanosomes ingested by tsetse differentiate into procyclic forms in the midgut lumen of the insect. Successful transmission to a new mammalian host requires at least two migrations within the fly: one from the midgut lumen to the ectoperitrophic space, and a subsequent migration from the ectoperitrophic space to the salivary glands. Procyclic forms can exhibit social motility, a form of coordinated movement, on semi-solid surfaces. While social motility in bacteria is linked to virulence, the biological significance for trypanosomes is unknown. We demonstrate that social motility is a property of early procyclic forms, which are equivalent to the forms present during the first week of fly infection. In contrast, late procyclic forms characteristic for established infections are deficient for social motility. Our findings link social motility to a biological process, confirm that early and late procyclic forms are distinct life-cycle stages and imply that genes essential for social motility will be of key importance in fly transmission. We suggest that using the social motility assay as a surrogate for fly experiments should enable many more laboratories to examine this aspect of parasite transmission.
Published in the journal: Social Motility of African Trypanosomes Is a Property of a Distinct Life-Cycle Stage That Occurs Early in Tsetse Fly Transmission. PLoS Pathog 10(10): e32767. doi:10.1371/journal.ppat.1004493
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004493Summary
African trypanosomes, single-celled parasites that cause human sleeping sickness and Nagana in animals, are transmitted by tsetse flies. Bloodstream form trypanosomes ingested by tsetse differentiate into procyclic forms in the midgut lumen of the insect. Successful transmission to a new mammalian host requires at least two migrations within the fly: one from the midgut lumen to the ectoperitrophic space, and a subsequent migration from the ectoperitrophic space to the salivary glands. Procyclic forms can exhibit social motility, a form of coordinated movement, on semi-solid surfaces. While social motility in bacteria is linked to virulence, the biological significance for trypanosomes is unknown. We demonstrate that social motility is a property of early procyclic forms, which are equivalent to the forms present during the first week of fly infection. In contrast, late procyclic forms characteristic for established infections are deficient for social motility. Our findings link social motility to a biological process, confirm that early and late procyclic forms are distinct life-cycle stages and imply that genes essential for social motility will be of key importance in fly transmission. We suggest that using the social motility assay as a surrogate for fly experiments should enable many more laboratories to examine this aspect of parasite transmission.
Introduction
Various sub-species of the protozoan parasite Trypanosoma brucei cause sleeping sickness in humans and Nagana in domestic animals. Irrespective of their mammalian host range, all these parasites are dependent on tsetse flies for their transmission. Two features enable trypanosomes to establish chronic infections in the mammalian host - their ability to evade the immune response by periodic switching of their variant surface glycoprotein (VSG) coat (reviewed in [1]) and a quorum-sensing mechanism that drives the differentiation of proliferating slender bloodstream forms to non-dividing stumpy forms, thus limiting the parasitaemia [2], [3]. Stumpy-inducing factor (SIF) is a small molecule (<500 Da) produced by the slender forms; its chemical identity is not known. Stumpy forms are pre-adapted for further differentiation and, following ingestion by the tsetse fly, differentiate into early procyclic forms in the lumen of the insect midgut [4]. In addition to changes in morphology and metabolism, differentiation involves the replacement of the VSG coat by two insect-specific coat proteins, GPEET and EP procyclin. At the beginning of tsetse infection procyclic forms can have two fates: they can be eliminated by the fly or they can migrate across/around the peritrophic matrix and colonise the ectoperitrophic space [5]. Once the infection is established, it is characterised by late procyclic forms that express high levels of EP, but are negative for GPEET. GPEET is not required for migration to the ectoperitrophic space, since deletion mutants can establish infections at normal rates [6].
Early and late procyclic forms can be maintained in axenic culture. Addition of glycerol to the culture medium prolongs the expression of GPEET; once glycerol is removed, the cells undergo a transient growth arrest and GPEET is repressed within a few days [4], [7]. Different trypanosome stocks vary in the relative amounts of GPEET or EP that they express in culture [8]. In contrast to what is observed in tsetse, GPEET-negative cells can revert to being GPEET-positive in culture, for example in response to glucose depletion [9] or by an unknown mechanism that is independent of glycerol [10]. To complete the cycle in the fly, parasites must migrate from the midgut, via the proventriculus, to the salivary glands. This migration constitutes a major bottleneck in the life cycle [11]. Once they reach the salivary glands trypanosomes attach to the epithelia and proliferate as epimastigote forms, finally giving rise to infectious metacyclic forms that can infect a new mammalian host.
Unicellular organisms can function as multicellular communities that exchange signals with each other and move in a coordinated manner. This is particularly well described for bacteria, which can form biofilms, communicate by quorum sensing and exhibit adventurous or social motility (SoMo) [12]–[15]. These types of concerted behaviour have implications for virulence and present potential targets for new classes of antimicrobial drugs. In contrast to what is known about social behaviour in prokaryotes, there is considerably less information on social interactions between unicellular eukaryotes. While several species of fungi are capable of forming biofilms [16], studies of swarming motility have focused almost exclusively on the free-living social amoeba Dicytostelium discoideum [17], [18]. In general, unicellular parasites tend to be studied as isolated entities or as organisms that need to perceive and interact with their hosts, with relatively little attention being paid to how they communicate with each other [19].
Procyclic forms of T. b. brucei exhibit SoMo when plated on a semi-solid surface, in a manner reminiscent of swarming bacteria [20]. Parasites first grow at the site of inoculation, and then form radial protrusions or “fingers” that extend outwards. Independent communities are able to sense each other and reorganise group movement to prevent contact. Migration on plates is abolished if the trypanosomes have a dysfunctional flagellum [20] or other motility defects [21]. It has been hypothesised that the social motility observed on plates might reflect one of the migration steps within the fly vector, either from the midgut lumen to the ectoperitrophic space, or from the ectoperitophic space to the salivary glands [20]. By using a series of mutants that had previously been characterised in tsetse, we show that SoMo is unrelated to the parasites' ability to establish salivary gland infections. Instead, it is a property of the early procyclic form, which is found in the first few days after transmission of bloodstream forms to the tsetse fly. We have also identified several new markers in addition to GPEET that are differentially expressed in early and late procyclic culture forms, and verified their differential expression in tsetse flies. Taken together, this confirms that early and late procyclic forms are distinct life-cycle stages with specific expression profiles and characteristics and links SoMo to an early event in the colonisation of the tsetse midgut.
Results
The time-point of migration correlates with the density of the inoculum
As a first step we optimised the plating protocol for the fly-transmissible strain AnTat 1.1. The main differences from the previously published protocol [20] are that we used SDM79 rather than SM as the medium and cells were not preincubated with ethanol before plating. In addition, low melting temperature agarose was replaced by normal agarose, rendering the plates more robust. While establishing the SoMo assay we observed that the time-point when radial protrusions formed differed between experiments. To test if the cell density influenced the assay, different numbers of cells were pipetted onto the plates (Figure 1). When 8×105 cells were plated in a volume of 5 µl, fingers were already visible after 24 hours. Cells plated at a density of 4×105 or 2×105 cells in 5 µl showed SoMo after 48 or 72 hours, respectively. It was reported previously that the doubling time of trypanosomes on plates is 24 h [20]. This suggests that the cells reach a threshold number of approximately 1.6×106 before migration starts. We observed that when communities were plated on their own, the radial projections always grew in a clockwise direction (Figure 1, 72 h). This directionality was overridden, however, when cells sensed and avoided neighbouring communities (Figure 2).
Fig. 1. The time-point of migration is density dependent. 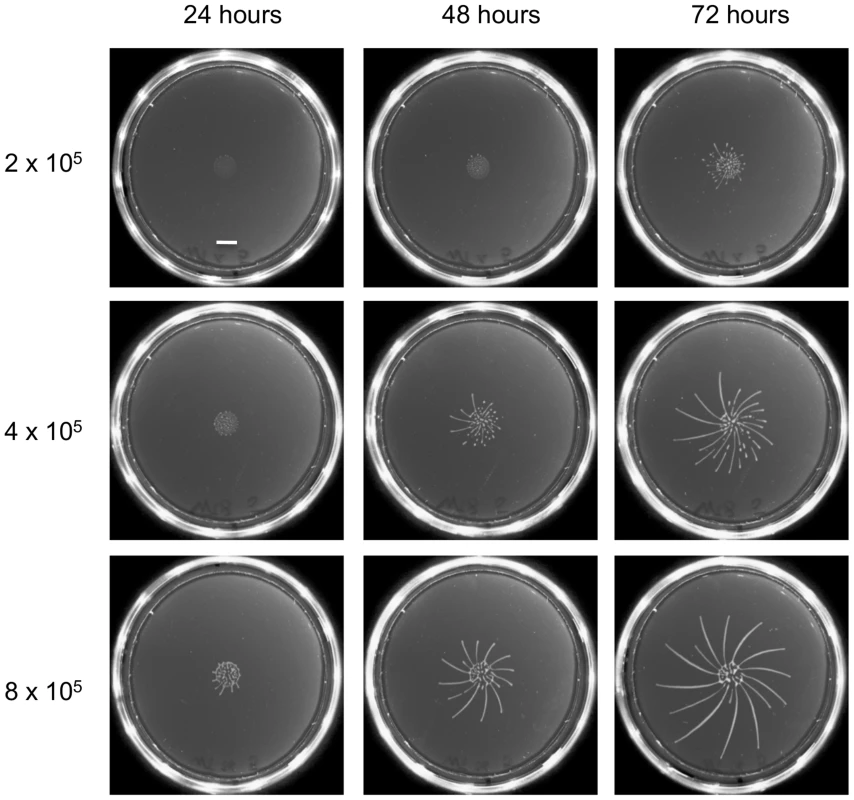
Different numbers of early procyclic forms of AnTat 1.1 were resuspended in 5 µl and pipetted onto 0.4% agarose plates. Photographs of the plates were taken every 24 h. The scale bar is 1 cm. Fig. 2. Only GPEET-positive cells exhibit SoMo. 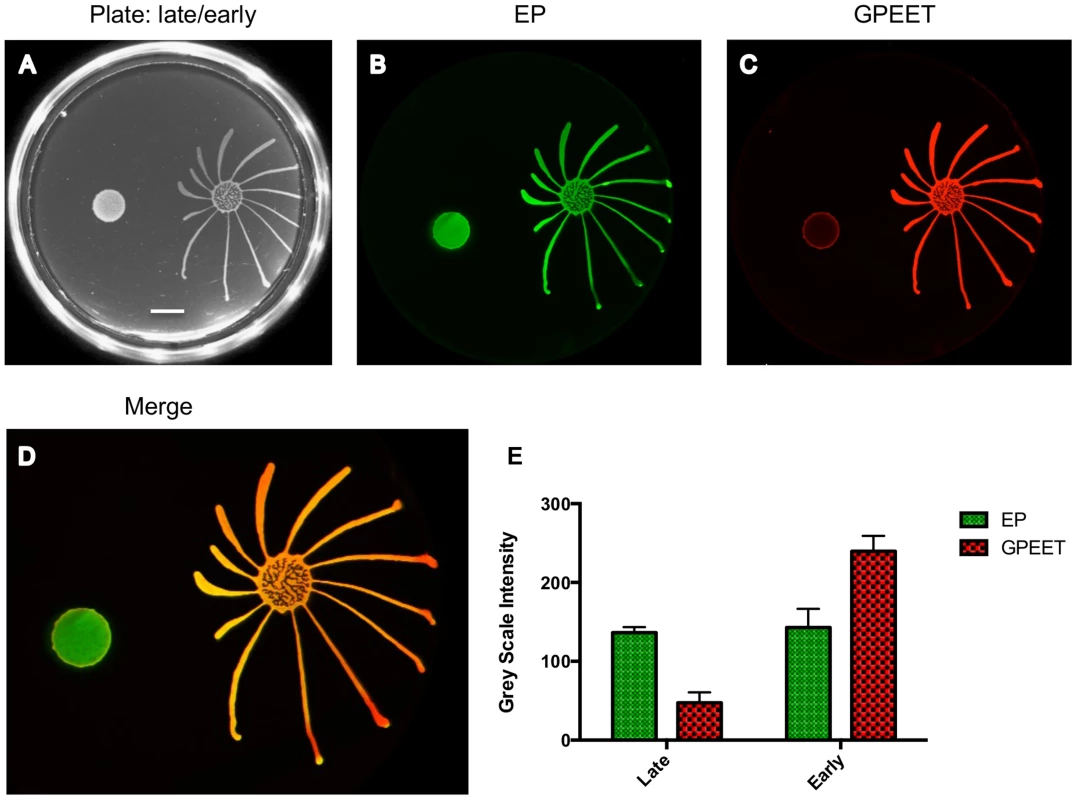
2×105 procyclic forms growing in medium with or without 20 mM glycerol were inoculated on an agarose plate containing 20 mM glycerol. (A) Photograph of the community 5 days post plating. Scale bar is 1 cm. (B–D) A community lift incubated with α-EP and α-GPEET antibodies. (E) Quantification of signal intensities. The intensity of EP is comparable between the early and the late procyclic communities. GPEET is predominantly expressed in the early, migrating colony. The error bar shows the mean standard deviation of seven individual areas. Social motility is a property of early procyclic forms
When we tested a variety of mutants, the high frequency of clones that were SoMo-negative, coupled with the observation that some addback mutants gave inconsistent results, made us suspect that a factor unrelated to the genotype might be influencing the outcome. We have shown previously that culture conditions can influence GPEET expression [22]. When we monitored the expression of GPEET, we found that 3 cultures that were SoMo-positive were all GPEET-positive and conversely, 4 cultures that were SoMo-negative were all negative for GPEET.
We then systematically examined SoMo of early and late procyclic forms. For these experiments we derived early procyclic forms from bloodstream forms and let them differentiate into late procyclic forms by removal of glycerol. When these cultures were seeded onto plates containing glycerol, both early and late procyclic forms grew and formed colonies at the inoculation site, but only the former produced migrating fingers (Figure 2). It has previously been shown that glycerol alone does not trigger the reversion of late to early procyclic forms in liquid culture [4]. Nevertheless, to be sure that the status of the cells had not changed on the plates, a “community lift” was performed. This entails placing a nitrocellulose filter on the plate; when the filter is removed, the cells adhere to it and can be labelled with antibodies. Incubation of the filter with antibodies against GPEET and EP confirmed that the early procyclic forms were positive for both, as expected, and that most cells in the colony of late procyclic forms were negative for GPEET. Some GPEET-positive cells can always be detected in cultures without glycerol [7]; these are visible as a narrow ring at the edge of the colony in Figure 2. It is not clear if the few early procyclic forms actively migrate to the border of the colony or if cells at the edge are more likely to revert to expressing GPEET. Although late procyclic forms do not show SoMo, they are recognised by early procyclic forms, which react by changing their direction of migration (Figure 2).
GPEET is not essential for social motility
GPEET is the major surface protein of early procyclic forms. To test if it was required for SoMo we used the ΔGPEET deletion mutant previously generated in our laboratory [6]. Since these cells lack a marker for early procyclic forms, we once again took bloodstream forms and triggered them to differentiate to procyclic forms. In common with its wild-type parent, ΔGPEET was SoMo-positive as long as it was cultured in the presence of glycerol and became SoMo-negative after being transferred to glycerol-free medium (Figure 3A). In order to track the differentiation status of ΔGPEET, it was transformed with a reporter construct in which the GFP coding region is fused to the GPEET 3′ untranslated region [23]. This regulatory sequence ensures that expression of GFP mimics that of GPEET, and indicates whether or not a cell is still an early procyclic form. A community lift using an anti-GFP antibody revealed once again that only the early procyclic forms migrate while the late, GFP-negative cells stay at the point of inoculation (Figure 3B). In addition to migrating, ΔGPEET cells are still capable of recognising and reacting to other trypanosome communities (Figure 3A).
Fig. 3. GPEET is not essential for SoMo. 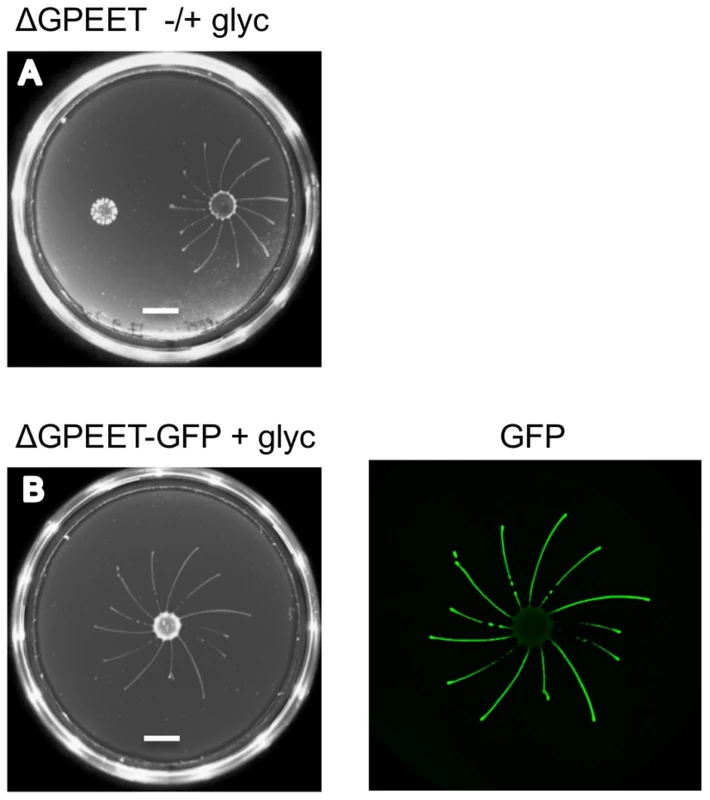
(A) 2×105 ΔGPEET cells, cultured with or without glycerol, were inoculated onto a plate containing 20 mM glycerol. Four days post plating only the cells cultured in medium containing glycerol showed SoMo. The scale bar is 1 cm. (B) 4×105 ΔGPEET/GFP cells, cultured in medium containing glycerol, were inoculated onto a plate containing 20 mM glycerol. Four days post plating a community lift was stained with α-GFP antibody. Other markers differentially expressed in early and late procyclic forms
In culture, early and late procyclic forms are morphologically indistinguishable. Since GPEET was the only known marker for early procyclic forms at the beginning of this study, we used SILAC to identify additional proteins that were differentially expressed between GPEET-positive and GPEET-negative cells. Two independent experiments identified a limited number of candidates that were significantly different in at least one experiment (≥2-fold; Figures 4 and 5; Table S2). Of the differentially regulated proteins, three examples were encoded by related genes. These were the calflagins (Tb-44, Tb-24 and Tb-17), the two hexokinases (HK1 and HK2) and three adenylate cyclases. The members of a protein family could not be identified unequivocally as they contained identical peptides that are randomly assigned during mapping. Lacking antibodies that discriminated between isoforms, we analysed the transcripts for unique signatures. In the case of the adenylate cyclases (Tb927.5.285b, Tb927.5.320 and Tb 927.5.330 - here designated AC330, AC320 and AC285b) differences in their 3′ untranslated regions, allowed AC330 to be distinguished from AC320/285b by Northern blot analysis (Figure 6A). Both were differentially expressed, with AC330 up 9-fold in early procyclic forms and AC320/285b up 6.25-fold in late procyclic forms. Thus, the changes in protein levels detected by SILAC are probably an under-estimate for the individual proteins. Since an antiserum was available against calflagins, we monitored expression of these proteins by immunofluorescence. This revealed that calflagin-positive cells were always also positive for GPEET (Figure 6B). In addition, we performed quantitative RT-PCR to measure transcript levels in early and late procyclic forms (Figure 6C). This confirmed the differential expression at the level of mRNA for the adenylate cyclases and HK1/HK2. HK1 mRNA was expressed 4-fold more in early procyclic forms while HK2 was up-regulated 2-fold in late procyclic forms. In contrast to what was observed by immunofluorescence and SILAC, calflagin transcripts were down-regulated only 2-fold in late procyclic forms, suggesting that there is an additional level of regulation. Finally, we tested the mRNA levels of a set of putative pteridine transporters (PPT: Tb927.1.2850, Tb927.1.2880), which we have observed to be up-regulated (at least transiently) during differentiation of early to late procyclic forms; these were increased 4.6-fold in late procyclic forms. In summary, although no other gene is as tightly regulated as GPEET, we have identified several additional differentially regulated transcripts/proteins in early and late procyclic forms.
Fig. 4. Proteins up-regulated in early procyclic forms. 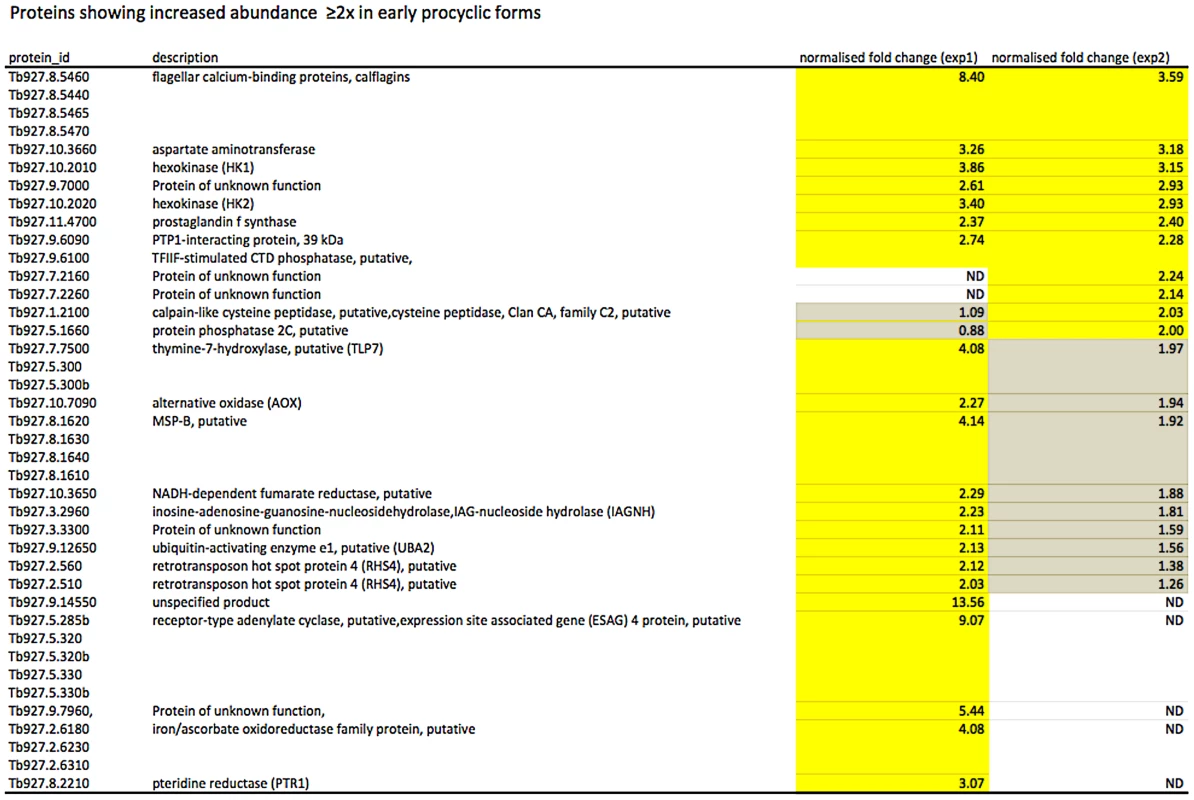
Yellow: proteins showing ≥2-fold increase in abundance. Grey: ≤2-fold change in abundance. N.D. not detected. Biological replicates (exp1 and exp2) were performed. Fig. 5. Proteins up-regulated in late procyclic forms. 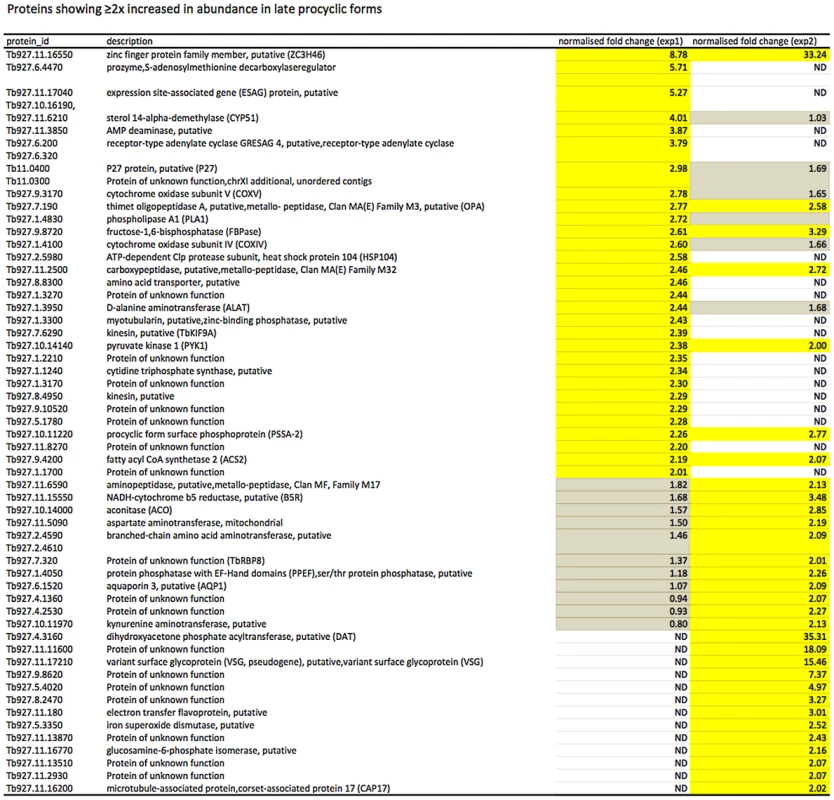
Yellow: proteins showing ≥2-fold increase in abundance. Grey: ≤2-fold change in abundance. N.D. not detected. Biological replicates (exp1 and exp2) were performed. Fig. 6. Differential expression of markers in early and late procyclic culture forms. 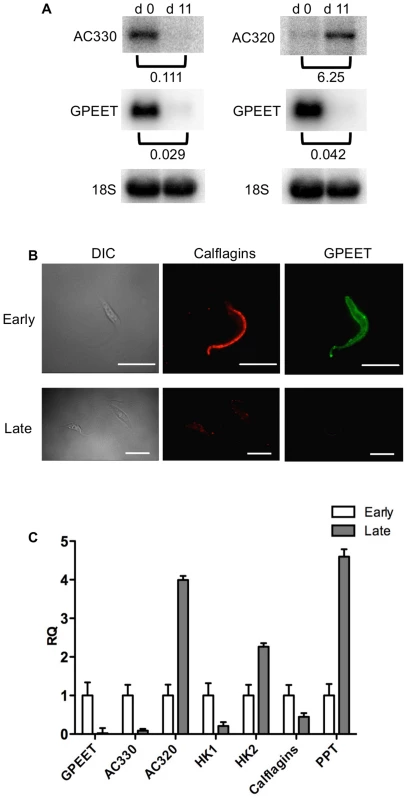
(A) Northern blot analysis of adenylate cyclases Tb927.5.330 (AC 330) and Tb.927.5.320/285b (AC 320). GPEET was used to verify that the cultures correspond to early and late procyclic forms, respectively. Signals were quantified using a PhosphoImager and normalised against 18S rRNA as described previously [47]. Signals in early procyclic forms were set at 1. (B) Immunofluorescence analysis reveals that GPEET and calflagin are co-expressed by early procyclic culture forms and are not detectable in late procyclic culture forms. Scale bar: 10 µm. (C) Quantitative RT-PCR performed using RNA from early procyclic culture forms and late procyclic culture forms 11 days after removal of glycerol from the medium [4], [7]. RQ: Relative quantification. Expression levels in early procyclic forms are set at 1. α-tubulin was used to normalise mRNA levels. Error bars are ΔCt standard errors. AC 330: Tb927.5.330 3′ UTR; AC 320: Tb927.5.320/285b 3′ UTR; HK1: 3′ UTR of Tb927.10.2010: HK2: 3′ UTR of Tb927.10.2020; Calflagin: coding region of Tb927.8.5460, Tb927.8.5440, Tb927.8.5465, Tb927.8.5470. PPT: coding region of Tb927.1.2850, Tb927.1.2880 (putative pteridine transporters). It has been shown previously that expression of the GPEET transcript and protein in the fly mirrors that of cells differentiating from early to late procyclic forms in culture [4], [22], [24]. To test if the new markers that we identified were similarly regulated in vivo, tsetse flies were infected and trypanosomes were harvested 3 and 12 days post infection. Figure 7A shows the co-expression of GPEET and calflagin in early procyclic forms isolated from fly midguts at day 3 and the repression of both proteins by day 12. Quantitative RT-PCR (Figure 7B) showed the same profiles that were observed in culture, with GPEET, AC330 and HK1 being more highly expressed in early procyclic forms and AC320, HK2 and PPT being more highly expressed in late procyclic forms. Taken together, these data convincingly show that early procyclic forms in culture are equivalent to the procyclic forms early in infection and late procyclic forms correspond to those in established infections.
Fig. 7. Differential expression of early and late procyclic form markers in tsetse flies. 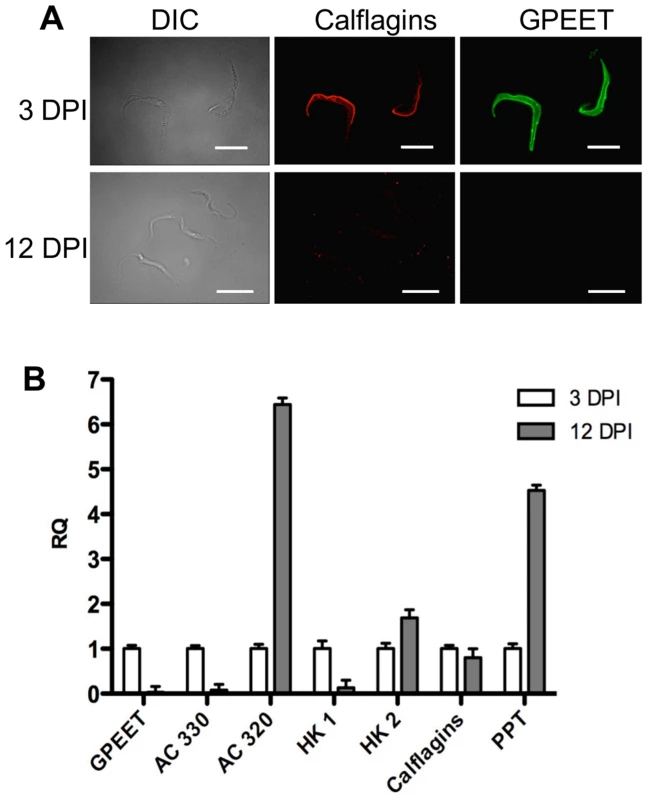
A. GPEET and calflagin are co-expressed by early procyclic forms 3 days post infection (DPI), but neither is detectable in late procyclic forms 12 DPI. Trypanosomes were isolated from tsetse fly midguts, fixed with formaldehyde and glutaraldehyde and permeabilised with Triton-×100. Immunofluorescence was performed with anti-GPEET and anti-calflagin antisera. Scale bar: 10 µm. B. Quantitative RT-PCR was performed with RNA isolated from infected tsetse flies 3 and 12 days post infection. Gene designations are the same as for Figure 6. RQ: relative quantification. α-tubulin was used to normalise mRNA levels. Error bars are ΔCt standard errors. Mutants with a defect in salivary gland infection are SoMo-positive
Despite the lack of SoMo by late procyclic forms, it is possible that it plays a role in migration of proventricular forms across the cardia to the tsetse salivary glands. To test this hypothesis we used a series of deletion mutants with defects in salivary gland infection. Our previous studies have implicated at least two proteins in the establishment of mature salivary gland infections, mitogen-activated kinase kinase 1 (MKK1; [25]) and the surface protein PSSA-2 [26]. Parasites lacking MKK1 were completely unable to establish salivary gland infections and parasites lacking PSSA-2 showed reductions in the prevalence and intensity of infections. A procyclin null mutant, lacking all EP and GPEET genes (Δproc), also showed a defect in colonisation of the salivary glands [6]. ΔMKK1 and ΔPSSA-2 infect the midgut at normal rates and intensities [25], [26], while Δproc establishes heavy infections at about half the rate of its wild-type parent [6]. MKK1 AND PSSA-2 knockouts were plated as early and late procyclic forms; in the case of Δproc only early procyclic forms, derived directly from bloodstream forms, were tested (Figure 8). In all cases, the early procyclic forms were positive for SoMo and were also able to sense and avoid the communities of late procyclic forms on the same plate.
Fig. 8. Deletion mutants with defects in salivary gland infection rates are SoMo-positive. 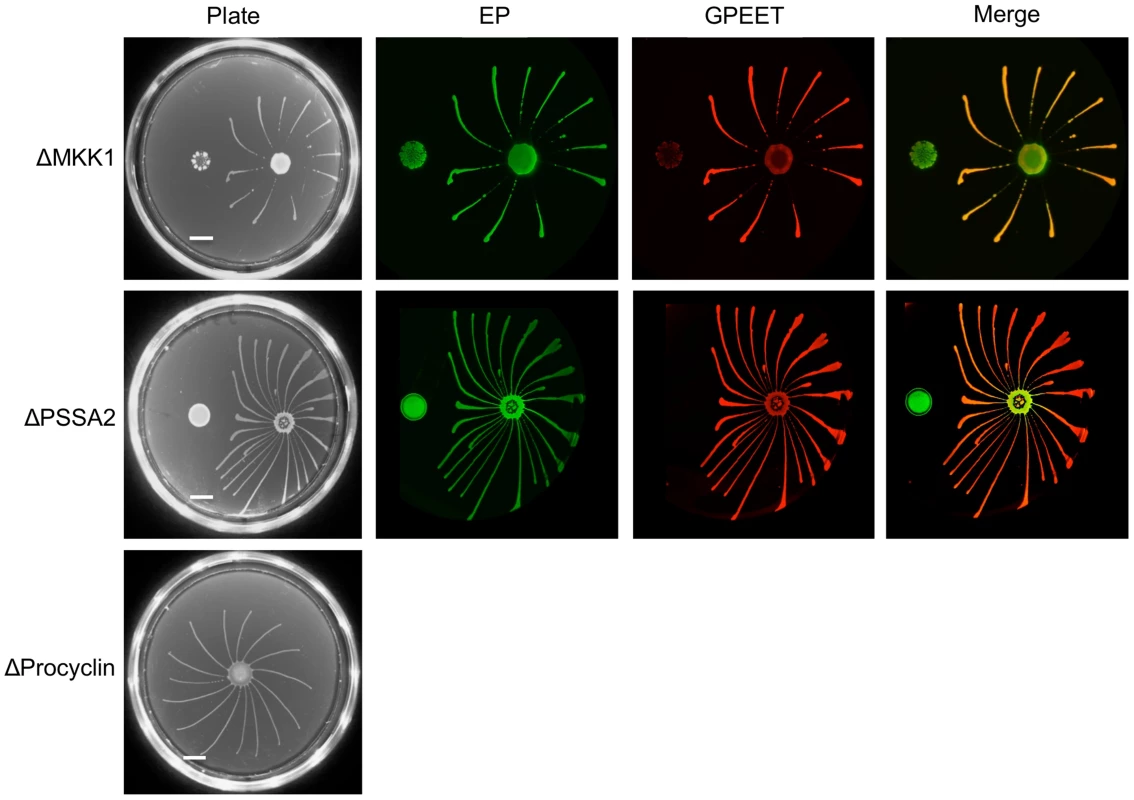
A and B. MKK1 and PSSA-2 deletion mutants were cultured with or without glycerol. 4×105 cells from each culture were inoculated onto plates containing 20 mM glycerol. The scale bar is 1 cm. Four days post plating community lifts were incubated with α-EP and α-GPEET antibodies. C. 2×105 cells of the procyclin null mutant (Δproc), obtained by differentiation of bloodstream forms, were inoculated onto a 0.4% agarose plate containing 20 mM glycerol. A photograph was taken 4 days post plating. Discussion
Early procyclic forms - defined as GPEET-positive cells - are detected in the midgut of tsetse flies in the first week after uptake of bloodstream form trypanosomes [4], while establishment of a persistent infection correlates with differentiation to late (GPEET-negative) procyclic forms. We have discovered that SoMo is a property of early procyclic forms and that late procyclic forms are consistently SoMo-negative. There are several indications that SoMo reflects the migration of trypanosomes from the midgut lumen to the ectoperitrophic space in the first days of fly infection rather than the subsequent migration from the ectoperitrophic space to the salivary glands. First and foremost, SoMo is restricted to early procyclic forms whereas late procyclic forms, which are forerunners of the forms migrating to the salivary glands, are SoMo-negative. Second, the timing of the switch from early to late procyclic forms [4] correlates with the appearance of parasites in the ectoperitrophic space [5]. Third, SoMo is independent of GPEET, as is colonisation of the ectoperitrophic space [4]. Furthermore, three mutants (Δproc, ΔPSSA-2 and ΔMKK1) that show normal colonisation of the midgut, but defects in colonisation of the salivary glands [6], [25], [26] are SoMo-positive as early procyclic forms. While it might be argued that these mutants have other defects, such as an inability to penetrate the proventriculus or to differentiate to epimastigote forms, in no case does the mutation impair SoMo by early procyclic forms.
Social interactions between bacteria are known to involve outer membrane proteins [27]. Despite being the major surface glycoproteins of procyclic forms, and present in several million copies, neither GPEET nor EP procyclin is required by trypanosomes for SoMo. It is known, however, that procyclin null mutants export free GPI anchors to their surface [28], and these might compensate for the loss of procyclins. The insect-stage specific transmembrane protein PSSA-2 [26], which shows increased expression in late procyclic forms (Table 2), is also dispensable for SoMo.
In this study we identified additional proteins and transcripts that are differentially expressed in these two life-cycle stages, both in culture and in the fly. Like GPEET, calflagins are expressed by early procyclic forms, but are down-regulated in late procyclic forms. It was recently shown by Emmer and coworkers that calflagins are expressed by bloodstream and procyclic forms [29]. However, when Kolev et al. induced differentiation from procyclic to epimastigote and metacyclic forms by overexpression of RBP6 [30], the procyclic forms in their cultures were heterogeneous with respect to calflagin expression, suggesting that they were a mixed population of early and late forms. Calflagins were not detected in epimastigotes but were re-expressed by metacyclic forms in culture [30] and in the fly salivary glands [31]. Guided by SILAC, we also identified two pairs of proteins, HK1/HK2 and AC330/AC320, whose transcripts are reciprocally expressed in early and late procyclic forms. This is similar to the situation that is seen with GPEET and EP3 [4], [24], [32]. Given that we only detected about 1300 proteins by mass spectrometry in the two experiments (Table S2), we do not claim that the list of differentially regulated proteins is complete, and indeed we suspect that there might be other sets of proteins that are reciprocally regulated without a discernible net change. Moreover, there might also be post-translational modifications or non-peptide moieties that are stage-specific. For example, the activity of the kinase that phosphorylates GPEET is restricted to early procyclic forms [33]. It is also known that early procyclic forms of T. congolense preferentially express PRS, a protease-resistant surface glycoconjugate [34], [35], although an equivalent molecule has not been reported for T. brucei.
Our experiments show that the parasites need to reach a threshold concentration on plates before they start to migrate. Although the number of parasites in the midgut lumen is significantly lower during the early days of infection [5], [36], the three-dimensional structure of the midgut and host-derived factors might contribute to the response. Moreover, local accumulation of parasites, for example at the peritrophic matrix, could condition the micro-environment in a manner conducive to SoMo. In addition to migrating, early procyclic forms have the capacity to recognise and be repelled by communities of early or late procyclic forms. At present we can only speculate about the significance of such repellents, but one possibility is that they are used by late procyclic forms to prevent or reduce superinfection by a second strain of trypanosomes. Mixed infections of tsetse can be detected in the field [37], [38], but there is no information on whether flies acquire the parasites simultaneously or sequentially from infected mammals.
Although the differentiation of early to late procyclic forms in the tsetse fly is irreversible, it should always be borne in mind that trypanosomes can switch between these two life-cycle stages in liquid culture [22]. The fact that these can change over time has important implications for the interpretation of results. In particular, before it can be concluded that a specific gene is required for SoMo, it is essential to determine whether the parasites are early or late procyclic forms.
In conclusion, our findings add further credence to the designation of early and late procyclic forms as two distinct life cycle stages with different biological properties. Since early procyclic forms are only detected in the first week of tsetse fly infection [4], [32], this strongly suggests that SoMo reflects an early event in the colonisation of the insect host. It also implies that genes that are important for SoMo will also play a role in the establishment of a midgut infection. Using the SoMo assay as a surrogate for fly experiments would enable many more laboratories to examine this aspect of parasite transmission. In addition, related parasites such as the South American trypanosomes and Leishmania, which are also transmitted by insects, may be amenable to such studies.
Materials and Methods
Ethics statement
No vertebrate animals were used in this study. Bloodstream form trypanosomes were obtained from frozen stabilates stored in liquid nitrogen. Antibodies were obtained from cell culture supernatants or from pre-existing sources of serum.
Trypanosomes
The pleomorphic strain AnTat 1.1 [39], [40] and genetically manipulated derivatives of it were used in this study. The deletion mutants ΔPSSA-2 [26], ΔMKK1 [25], ΔGPEET and Δproc [6] have all been described previously. Procyclic forms were cultured in SDM79 [41] supplemented with 10% heat inactivated foetal bovine serum (iFBS). The medium for early procyclic forms was also supplemented with 20 mM glycerol [4].
Infection of tsetse flies and RNA isolation
Pupae of Glossina morsitans morsitans were obtained from the Department of Entomology, Slovak Academy of Science (Bratislava). Teneral flies were infected with early procyclic forms during the first blood meal as described [42]. Flies were dissected and total RNA was isolated from midguts using standard procedures [43]. Fifty midguts were collected 3 days post infection for RNA isolation and immunofluorescence analysis. Approximately 10–15 infected midguts were collected for analysis at days 12 or 13 post infection.
Differentiation
Bloodstream forms obtained from frozen stabilates (500 µl blood plus 500 µl HMI-9) were centrifuged and resuspended in SDM79 supplemented with 10% iFBS and 20 mM glycerol. Differentiation to early procyclic forms was induced by the addition of 6 mM cis-aconitate and a temperature shift from 37° to 27° [44]. Early procyclic forms were cultured in the same medium [4]. To trigger differentiation to late procyclic forms, early procyclic forms were transferred to SDM79, 10% iFBS without glycerol, as described previously [4].
Plasmids and generation of stable transformants
Stable transformation of procyclic form trypanosomes was performed as described [26]. To generate the ΔGPEET/GFP-GPEET cell line, which expresses GFP under the control of the GPEET promoter and 3′ UTR, ΔGPEET [6] was stably transformed with the plasmid pCorleone-GFP/GPEET-blast [23]. When linearised with Spe I, this plasmid integrates upstream of a procyclin locus.
Plates
The protocol to produce plates was adapted from [20]. Plates were always used within 24 h. Briefly, 36 ml SDM79 supplemented with 10% iFBS were pre-warmed to 42°; for plates containing glycerol, 400 µl of a 2M glycerol stock was added. 4 ml agarose (Promega V3125; 4% w/v in water) was added to the pre-warmed medium and the resulting 0.4% agarose medium was immediately poured into Petri dishes with a diameter of 85 mm, 10 ml per dish. The open plates were then air-dried for 1 hour in a laminar flow cabinet. Cells from an exponentially growing culture were centrifuged briefly and resuspended in the residual medium at a density of 3–4×107 cells ml−1. Five µl were spotted onto the surface of the agarose, the Petri dish was sealed with Parafilm and incubated at 27°. All experiments were performed at least twice and there were no incongruent results. It should be noted, however, that the number of spokes produced by a given clone can vary between experiments.
Community lifts
A nitrocellulose filter (Whatman Protran BA 85) was laid carefully on top of the cells on the agarose plate and incubated for 5 minutes at room temperature. The filter was then removed and air-dried for 15 minutes. The membrane was blocked in PBS containing 5% (w/v) defatted milk for 1 h at 4°, after which the primary antibodies were added at the appropriate dilution and incubated for 1 h at room temperature. The following primary antibodies were used: TBRP1/247 mouse α-EP 1∶500 [45], K1 rabbit α-GPEET 1∶1000 [42] and mouse α-GFP (Roche, 1∶2000). After incubation with the primary antibodies the membrane was washed 3 times in TBS Tween, then incubated with secondary antibodies (in PBS 5% milk) for 1 h at room temperature. The following secondary antibodies were used at a dilution of 1∶10000: goat α-mouse IRDye 800CW (LI-COR Biosciences) and goat α-rabbit IRDye 680LT (LI-COR Biosciences). The membrane was washed 3 times in TBS Tween and then scanned on a LI-COR Odyssey Infrared Imager model 9120, using Odyssey Application Software, Version 3.0.21.
Imaging and image processing
Images from plates were made with a Nikon MH-56 digital camera. To quantify the intensity of the community lifts a grey scale image of the membranes was exported from the Odyssey Application Software and analysed with ImageJ 1.46r. Seven individual areas were analysed for each value. The values were subtracted from 255 to obtain a maximum intensity of 255 and a minimum intensity of 1. The graphs were generated with Prism6.
Stable isotope labeling by amino acids in culture (SILAC) and mass spectrometry
Late procyclic forms were derived from early procyclic forms by removal of glycerol in two independent experiments. Pairs of early and late procyclic forms were adapted to SDM80 supplemented with 10% dialysed foetal bovine serum. The medium for early procyclic forms was supplemented with 20 mM glycerol. SILAC and mass spectrometry analyses were performed as described [46] at the Mass Spectrometry and Proteomics Facility, Department of Clinical Research, University of Bern.
Northern blot analysis and qRT-PCR
The isolation of RNA from early and late procyclic culture forms and northern blot analysis were performed as described [6]. Purified RNA was subjected to DNAse treatment prior to cDNA synthesis. Reverse transcription was performed using an Omniscript RT kit (Quiagen, Switzerland) according to the manufacturer's instructions with random hexamers as primers. PCR primers are shown in Table S1. qPCR was performed using MESA GREEN qPCR MasterMix Plus for SYBR Assay (Eurogentec) in the ABI Prism 7000 Sequence Detection System (Applied Biosystems). Specificity of the reactions was confirmed by agarose gel electrophoresis and melting temperature analysis. The data were analysed using 7000 System SDS software v1.2 (Applied Biosytems). Two biological replicates were analysed independently. Within an experiment, technical triplicates were run in parallel.
Immunofluorescence
Cells were washed twice with PBS and spread on a coverslip to let them settle down for 10 minutes. The cells were fixed with 4% paraformaldehyde and 0.1% glutaraldehyde in PBS for 15 minutes, then permeabilised with 0.2% Triton X-100 and blocked with 2% BSA/PBS. The primary antiserum, rabbit K1 anti-GPEET was diluted 1∶1000 [42] and the calflagin mouse antiserum (a gift from David Engman), was diluted 1∶500 [29]. The secondary antibodies Alexa Fluor 488 goat anti-rabbit and Cy3 goat anti-mouse (Invitrogen) were diluted 1∶1000 in 2% BSA/PBS. Images were taken with a Leica DFC360FX monochrome CCD (charge-coupled-device) camera mounted on a Leica DM5500 B microscope with a 100× oil immersion objective and analysed using LAS AF software (Leica).
Supporting Information
Zdroje
1. SchwedeA, CarringtonM (2010) Bloodstream form Trypanosome plasma membrane proteins: antigenic variation and invariant antigens. Parasitology 137 : 2029–2039.
2. ReunerB, VassellaE, YutzyB, BoshartM (1997) Cell density triggers slender to stumpy differentiation of Trypanosoma brucei bloodstream forms in culture. Molecular and biochemical parasitology 90 : 269–280.
3. VassellaE, ReunerB, YutzyB, BoshartM (1997) Differentiation of African trypanosomes is controlled by a density sensing mechanism which signals cell cycle arrest via the cAMP pathway. Journal of cell science 110 (Pt 21): 2661–2671.
4. VassellaE, Den AbbeeleJV, ButikoferP, RenggliCK, FurgerA, et al. (2000) A major surface glycoprotein of Trypanosoma brucei is expressed transiently during development and can be regulated post-transcriptionally by glycerol or hypoxia. Genes Dev 14 : 615–626.
5. GibsonW, BaileyM (2003) The development of Trypanosoma brucei within the tsetse fly midgut observed using green fluorescent trypanosomes. Kinetoplastid Biol Dis 2 : 1.
6. VassellaE, OberleM, UrwylerS, RenggliCK, StuderE, et al. (2009) Major surface glycoproteins of insect forms of Trypanosoma brucei are not essential for cyclical transmission by tsetse. PLoS One 4: e4493.
7. KnüselS, RoditiI (2013) Insights into the regulation of GPEET procyclin during differentiation from early to late procyclic forms of Trypanosoma brucei. Molecular and biochemical parasitology 191 : 66–74.
8. RoditiI, FurgerA, RueppS, SchurchN, ButikoferP (1998) Unravelling the procyclin coat of Trypanosoma brucei. Molecular and biochemical parasitology 91 : 117–130.
9. MorrisJC, WangZ, DrewME, EnglundPT (2002) Glycolysis modulates trypanosome glycoprotein expression as revealed by an RNAi library. The EMBO journal 21 : 4429–4438.
10. VassellaE, ProbstM, SchneiderA, StuderE, RenggliCK, et al. (2004) Expression of a major surface protein of Trypanosoma brucei insect forms is controlled by the activity of mitochondrial enzymes. Molecular biology of the cell 15 : 3986–3993.
11. OberleM, BalmerO, BrunR, RoditiI (2010) Bottlenecks and the maintenance of minor genotypes during the life cycle of Trypanosoma brucei. PLoS pathogens 6: e1001023.
12. ShapiroJA (1998) Thinking about bacterial populations as multicellular organisms. Annual review of microbiology 52 : 81–104.
13. FraserGM, HughesC (1999) Swarming motility. Current opinion in microbiology 2 : 630–635.
14. HarsheyRM (2003) Bacterial motility on a surface: many ways to a common goal. Annual review of microbiology 57 : 249–273.
15. BasslerBL, LosickR (2006) Bacterially speaking. Cell 125 : 237–246.
16. BlankenshipJR, MitchellAP (2006) How to build a biofilm: a fungal perspective. Current opinion in microbiology 9 : 588–594.
17. FirtelRA, MeiliR (2000) Dictyostelium: a model for regulated cell movement during morphogenesis. Current opinion in genetics & development 10 : 421–427.
18. ShaulskyG, KessinRH (2007) The cold war of the social amoebae. Current biology: CB 17: R684–692.
19. LopezMA, NguyenHT, OberholzerM, HillKL (2011) Social parasites. Current opinion in microbiology 14 : 642–648.
20. OberholzerM, LopezMA, McLellandBT, HillKL (2010) Social motility in African trypanosomes. PLoS Pathog 6: e1000739.
21. FreireER, VashishtAA, MalvezziAM, ZuberekJ, LangousisG, et al. (2014) eIF4F-like complexes formed by cap-binding homolog TbEIF4E5 with TbEIF4G1 or TbEIF4G2 are implicated in post-transcriptional regulation in Trypanosoma brucei. RNA
22. VassellaE, ProbstM, SchneiderA, StuderE, RenggliCK, et al. (2004) Expression of a major surface protein of Trypanosoma brucei insect forms is controlled by the activity of mitochondrial enzymes. Mol Biol Cell 15 : 3986–3993.
23. Schumann BurkardG, KaserS, de AraujoPR, SchimanskiB, NaguleswaranA, et al. (2013) Nucleolar proteins regulate stage-specific gene expression and ribosomal RNA maturation in Trypanosoma brucei. Molecular microbiology 88 : 827–840.
24. UrwylerS, VassellaE, Van Den AbbeeleJ, RenggliCK, BlundellP, et al. (2005) Expression of procyclin mRNAs during cyclical transmission of Trypanosoma brucei. PLoS Pathog 1: e22.
25. MorandS, RenggliCK, RoditiI, VassellaE (2012) MAP kinase kinase 1 (MKK1) is essential for transmission of Trypanosoma brucei by Glossina morsitans. Mol Biochem Parasitol 186 : 73–76.
26. FragosoCM, Schumann BurkardG, OberleM, RenggliCK, HilzingerK, et al. (2009) PSSA-2, a membrane-spanning phosphoprotein of Trypanosoma brucei, is required for efficient maturation of infection. PLoS One 4: e7074.
27. NudlemanE, WallD, KaiserD (2005) Cell-to-cell transfer of bacterial outer membrane lipoproteins. Science 309 : 125–127.
28. VassellaE, ButikoferP, EngstlerM, JelkJ, RoditiI (2003) Procyclin null mutants of Trypanosoma brucei express free glycosylphosphatidylinositols on their surface. Molecular biology of the cell 14 : 1308–1318.
29. EmmerBT, DanielsMD, TaylorJM, EptingCL, EngmanDM (2010) Calflagin inhibition prolongs host survival and suppresses parasitemia in Trypanosoma brucei infection. Eukaryot Cell 9 : 934–942.
30. KolevNG, Ramey-ButlerK, CrossGA, UlluE, TschudiC (2012) Developmental progression to infectivity in Trypanosoma brucei triggered by an RNA-binding protein. Science 338 : 1352–1353.
31. RotureauB, SubotaI, BuissonJ, BastinP (2012) A new asymmetric division contributes to the continuous production of infective trypanosomes in the tsetse fly. Development 139 : 1842–1850.
32. Acosta-SerranoA, VassellaE, LinigerM, Kunz RenggliC, BrunR, et al. (2001) The surface coat of procyclic Trypanosoma brucei: programmed expression and proteolytic cleavage of procyclin in the tsetse fly. Proc Natl Acad Sci U S A 98 : 1513–1518.
33. SchlaeppiAC, MalherbeT, BütikoferP (2003) Coordinate expression of GPEET procyclin and its membrane-associated kinase in Trypanosoma brucei procyclic forms. The Journal of biological chemistry 278 : 49980–49987.
34. BütikoferP, VassellaE, BoschungM, RenggliCK, BrunR, et al. (2002) Glycosylphosphatidylinositol-anchored surface molecules of Trypanosoma congolense insect forms are developmentally regulated in the tsetse fly. Molecular and biochemical parasitology 119 : 7–16.
35. UtzS, RoditiI, Kunz RenggliC, AlmeidaIC, Acosta-SerranoA, et al. (2006) Trypanosoma congolense procyclins: unmasking cryptic major surface glycoproteins in procyclic forms. Eukaryotic cell 5 : 1430–1440.
36. Van Den AbbeeleJ, ClaesY, van BockstaeleD, Le RayD, CoosemansM (1999) Trypanosoma brucei spp. development in the tsetse fly: characterization of the post-mesocyclic stages in the foregut and proboscis. Parasitology 118 (Pt 5): 469–478.
37. StevensJR, Mathieu-DaudeF, McNamaraJJ, MizenVH, NzilaA (1994) Mixed populations of Trypanosoma brucei in wild Glossina palpalis palpalis. Tropical medicine and parasitology: official organ of Deutsche Tropenmedizinische Gesellschaft and of Deutsche Gesellschaft fur Technische Zusammenarbeit 45 : 313–318.
38. MacLeodA, TurnerCM, TaitA (1999) A high level of mixed Trypanosoma brucei infections in tsetse flies detected by three hypervariable minisatellites. Molecular and biochemical parasitology 102 : 237–248.
39. DelauwMF, PaysE, SteinertM, AertsD, Van MeirvenneN, et al. (1985) Inactivation and reactivation of a variant-specific antigen gene in cyclically transmitted Trypanosoma brucei. EMBO J 4 : 989–993.
40. Le RayD, BarryJD, EastonC, VickermanK (1977) First tsetse fly transmission of the “AnTat” serodeme of Trypanosoma brucei. Ann Soc Belg Med Trop 57 : 369–381.
41. BrunR, Schonenberger (1979) Cultivation and in vitro cloning or procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Short communication. Acta Trop 36 : 289–292.
42. RueppS, FurgerA, KurathU, RenggliCK, HemphillA, et al. (1997) Survival of Trypanosoma brucei in the tsetse fly is enhanced by the expression of specific forms of procyclin. J Cell Biol 137 : 1369–1379.
43. ChomczynskiP, SacchiN (1987) Single-step RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anan Biochem 162 : 156–159.
44. BrunR, SchonenbergerM (1981) Stimulating effect of citrate and cis-Aconitate on the transformation of Trypanosoma brucei bloodstream forms to procyclic forms in vitro. Z Parasitenkd 66 : 17–24.
45. RichardsonJP, BeecroftRP, TolsonDL, LiuMK, PearsonTW (1988) Procyclin: an unusual immunodominant glycoprotein surface antigen from the procyclic stage of African trypanosomes. Mol Biochem Parasitol 31 : 203–216.
46. GunasekeraK, WüthrichD, Braga-LagacheS, HellerM, OchsenreiterT (2012) Proteome remodelling during development from blood to insect-form Trypanosoma brucei quantified by SILAC and mass spectrometry. BMC Genomics 13 : 556.
47. FlückC, SalomoneJY, KurathU, RoditiI (2003) Cycloheximide-mediated accumulation of transcripts from a procyclin expression site depends on the intergenic region. Mol Biochem Parasitol 127 : 93–97.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Identification of the Microsporidian as a New Target of the IFNγ-Inducible IRG Resistance SystemČlánek Human Cytomegalovirus Drives Epigenetic Imprinting of the Locus in NKG2C Natural Killer CellsČlánek APOBEC3D and APOBEC3F Potently Promote HIV-1 Diversification and Evolution in Humanized Mouse ModelČlánek Role of Non-conventional T Lymphocytes in Respiratory Infections: The Case of the Pneumococcus
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2014 Číslo 10- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Theory and Empiricism in Virulence Evolution
- -Related Fungi and Reptiles: A Fatal Attraction
- Adaptive Prediction As a Strategy in Microbial Infections
- Antimicrobials, Stress and Mutagenesis
- A Novel Function of Human Pumilio Proteins in Cytoplasmic Sensing of Viral Infection
- Social Motility of African Trypanosomes Is a Property of a Distinct Life-Cycle Stage That Occurs Early in Tsetse Fly Transmission
- Autophagy Controls BCG-Induced Trained Immunity and the Response to Intravesical BCG Therapy for Bladder Cancer
- Identification of the Microsporidian as a New Target of the IFNγ-Inducible IRG Resistance System
- mRNA Structural Constraints on EBNA1 Synthesis Impact on Antigen Presentation and Early Priming of CD8 T Cells
- Infection Causes Distinct Epigenetic DNA Methylation Changes in Host Macrophages
- Neutrophil Crawling in Capillaries; A Novel Immune Response to
- Live Attenuated Vaccine Protects against Pulmonary Challenge in Rats and Non-human Primates
- The ESAT-6 Protein of Interacts with Beta-2-Microglobulin (β2M) Affecting Antigen Presentation Function of Macrophage
- Characterization of Uncultivable Bat Influenza Virus Using a Replicative Synthetic Virus
- HIV Acquisition Is Associated with Increased Antimicrobial Peptides and Reduced HIV Neutralizing IgA in the Foreskin Prepuce of Uncircumcised Men
- Uses a Unique Ligand-Binding Mode for Trapping Opines and Acquiring A Competitive Advantage in the Niche Construction on Plant Host
- Involvement of a 1-Cys Peroxiredoxin in Bacterial Virulence
- Ethanol Stimulates WspR-Controlled Biofilm Formation as Part of a Cyclic Relationship Involving Phenazines
- Densovirus Is a Mutualistic Symbiont of a Global Crop Pest () and Protects against a Baculovirus and Bt Biopesticide
- Insights into Intestinal Colonization from Monitoring Fluorescently Labeled Bacteria
- Mycobacterial Antigen Driven Activation of CD14CD16 Monocytes Is a Predictor of Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome
- Lipoprotein LprG Binds Lipoarabinomannan and Determines Its Cell Envelope Localization to Control Phagolysosomal Fusion
- Dampens the DNA Damage Response
- MicroRNAs Suppress NB Domain Genes in Tomato That Confer Resistance to
- Novel Cyclic di-GMP Effectors of the YajQ Protein Family Control Bacterial Virulence
- Vaginal Challenge with an SIV-Based Dual Reporter System Reveals That Infection Can Occur throughout the Upper and Lower Female Reproductive Tract
- Detecting Differential Transmissibilities That Affect the Size of Self-Limited Outbreaks
- One Small Step for a Yeast - Microevolution within Macrophages Renders Hypervirulent Due to a Single Point Mutation
- Expression Profiling during Arabidopsis/Downy Mildew Interaction Reveals a Highly-Expressed Effector That Attenuates Responses to Salicylic Acid
- Human Cytomegalovirus Drives Epigenetic Imprinting of the Locus in NKG2C Natural Killer Cells
- Interaction with Tsg101 Is Necessary for the Efficient Transport and Release of Nucleocapsids in Marburg Virus-Infected Cells
- The N-Terminus of Murine Leukaemia Virus p12 Protein Is Required for Mature Core Stability
- Sterol Biosynthesis Is Required for Heat Resistance but Not Extracellular Survival in
- Allele-Specific Induction of IL-1β Expression by C/EBPβ and PU.1 Contributes to Increased Tuberculosis Susceptibility
- Host Cofactors and Pharmacologic Ligands Share an Essential Interface in HIV-1 Capsid That Is Lost upon Disassembly
- APOBEC3D and APOBEC3F Potently Promote HIV-1 Diversification and Evolution in Humanized Mouse Model
- Structural Basis for the Recognition of Human Cytomegalovirus Glycoprotein B by a Neutralizing Human Antibody
- Systematic Analysis of ZnCys Transcription Factors Required for Development and Pathogenicity by High-Throughput Gene Knockout in the Rice Blast Fungus
- Epstein-Barr Virus Nuclear Antigen 3A Promotes Cellular Proliferation by Repression of the Cyclin-Dependent Kinase Inhibitor p21WAF1/CIP1
- The Host Protein Calprotectin Modulates the Type IV Secretion System via Zinc Sequestration
- Cyclophilin A Associates with Enterovirus-71 Virus Capsid and Plays an Essential Role in Viral Infection as an Uncoating Regulator
- A Novel Alpha Kinase EhAK1 Phosphorylates Actin and Regulates Phagocytosis in
- The pH-Responsive PacC Transcription Factor of Governs Epithelial Entry and Tissue Invasion during Pulmonary Aspergillosis
- Sensing of Immature Particles Produced by Dengue Virus Infected Cells Induces an Antiviral Response by Plasmacytoid Dendritic Cells
- Co-opted Oxysterol-Binding ORP and VAP Proteins Channel Sterols to RNA Virus Replication Sites via Membrane Contact Sites
- Characteristics of Memory B Cells Elicited by a Highly Efficacious HPV Vaccine in Subjects with No Pre-existing Immunity
- HPV16-E7 Expression in Squamous Epithelium Creates a Local Immune Suppressive Environment via CCL2- and CCL5- Mediated Recruitment of Mast Cells
- Dengue Viruses Are Enhanced by Distinct Populations of Serotype Cross-Reactive Antibodies in Human Immune Sera
- CD4 Depletion in SIV-Infected Macaques Results in Macrophage and Microglia Infection with Rapid Turnover of Infected Cells
- A Sialic Acid Binding Site in a Human Picornavirus
- Contact Heterogeneity, Rather Than Transmission Efficiency, Limits the Emergence and Spread of Canine Influenza Virus
- Myosins VIII and XI Play Distinct Roles in Reproduction and Transport of
- HTLV-1 Tax Stabilizes MCL-1 via TRAF6-Dependent K63-Linked Polyubiquitination to Promote Cell Survival and Transformation
- Species Complex: Ecology, Phylogeny, Sexual Reproduction, and Virulence
- A Critical Role for IL-17RB Signaling in HTLV-1 Tax-Induced NF-κB Activation and T-Cell Transformation
- Exosomes from Hepatitis C Infected Patients Transmit HCV Infection and Contain Replication Competent Viral RNA in Complex with Ago2-miR122-HSP90
- Role of Non-conventional T Lymphocytes in Respiratory Infections: The Case of the Pneumococcus
- Kaposi's Sarcoma-Associated Herpesvirus Induces Nrf2 during Infection of Endothelial Cells to Create a Microenvironment Conducive to Infection
- A Relay Network of Extracellular Heme-Binding Proteins Drives Iron Acquisition from Hemoglobin
- Glutamate Secretion and Metabotropic Glutamate Receptor 1 Expression during Kaposi's Sarcoma-Associated Herpesvirus Infection Promotes Cell Proliferation
- Reduces Malaria and Dengue Infection in Vector Mosquitoes and Has Entomopathogenic and Anti-pathogen Activities
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Novel Cyclic di-GMP Effectors of the YajQ Protein Family Control Bacterial Virulence
- MicroRNAs Suppress NB Domain Genes in Tomato That Confer Resistance to
- The ESAT-6 Protein of Interacts with Beta-2-Microglobulin (β2M) Affecting Antigen Presentation Function of Macrophage
- Characterization of Uncultivable Bat Influenza Virus Using a Replicative Synthetic Virus
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



