-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Theory and Empiricism in Virulence Evolution
article has not abstract
Published in the journal: Theory and Empiricism in Virulence Evolution. PLoS Pathog 10(10): e32767. doi:10.1371/journal.ppat.1004387
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004387Summary
article has not abstract
Dobzhansky famously wrote, “Nothing in biology makes sense except in the light of evolution.” Given the importance of viral evolution to disease emergence, pathogenesis, drug resistance, and vaccine efficacy, it has been well studied by theoreticians and experimentalists. Indeed, as the highly theoretical concepts of quasispecies and error catastrophe gained mainstream attention over the last thirty years, notions of viral populations and viral evolution became almost inseparable for many virologists. In contrast, a large body of theoretical work on the evolution of virulence has yet to gain traction in the virology community. Our purpose here is to offer a brief introduction to virulence theory, explain some of its strengths and weaknesses, and suggest how theory might be united with empiric data. While we focus our discussion on viruses, many of the concepts presented are similarly applicable to other prokaryotic and eukaryotic pathogens.
What Is Virulence and Does It Evolve?
The generic term “virulence” has many meanings. The fact that empiricists and theorists have different meanings of virulence is not necessarily a problem—understanding the evolution of virulence under any definition would be useful. In the realm of existing theory, it often means mortality—an increased death rate of the infected host. In theoretical models, this narrow framing is convenient; dead hosts do not transmit, so the outcome of virulence has an easily quantified dynamical consequence. Mortality is also universal, and its use as a virulence measure allows for comparative modeling across different systems. However, experimentalists often use sub-lethal measures of virulence, such as weight loss, behavioral change, or damage to a specific organ. As we will show below, such measures are often difficult to incorporate into models of virulence evolution and have led to a gap between theorists and empiricists.
Virulence by most any definition is clearly evolvable; viruses that are serially passaged in laboratory animal experiments will often become more virulent in that host [1]. A “natural experiment” in virulence evolution followed two separate introductions of myxoma virus into rabbit populations in Australia and France in the 1950s. While rabbits infected with this virus initially exhibited mortality rates of >99%, the virus eventually became less virulent [2]. The recent experimental adaptation of H5N1 influenza viruses for respiratory droplet transmission raised fears that increased virulence would accompany selection for transmission [3], [4]. Virulence theory seeks to understand what social and ecological factors drive the evolution of higher and lower virulence, with the hope that predictive models will enable rational virulence management [5].
How Has the Evolution of Virulence Been Modeled?
Early theories of virulence suggested that pathogens would evolve to avirulent commensals since harming the host would be a poor long-term survival strategy. This view was challenged in the mid-20th century as evolutionary biologists and population geneticists considered how competition among different strains of a given pathogen would influence the evolution of virulence (see [6] for an excellent historical review). Here, the superiority of one strain over another would depend on its ability to replicate within a host, the length of time that the host is infected (recovery rate), and successful transmission to a new host. These measures of pathogen fitness are easily integrated into a single term, the basic reproductive number (R0), which was modeled by Anderson and May [7] as:
R0 gives us a measure of fitness, but not of its evolution. There are several ways to model evolution on this scaffold, and the choice of model is critical. If each parameter in the formula were to evolve independently of the others, a virus could increase its fitness (R0) by simply evolving any or all of the following: a lower host mortality rate, a lower recovery rate (longer infectious period), and a higher transmission rate. Instead, most models assume that a subset of these parameters is coupled in a “trade-off.” A trade-off is a genetic constraint that reduces the dimensionality of evolutionary models by forcing one parameter to change with another. Pathogens are assumed to evolve to an optimal balance of these factors subject to the constraints of the trade-off. This balance is often represented graphically as a maximum value on a trade-off curve (Figure 1A, 1B). For example, gains in transmission rate influence virulence by increasing either host mortality or host recovery rates at the population level. Conversely, reducing the length of an infection either by death or pathogen clearance will limit transmission. The most common trade-off, explored in many models, is between transmission and host mortality.Fig. 1. Trade-off models for virulence evolution. 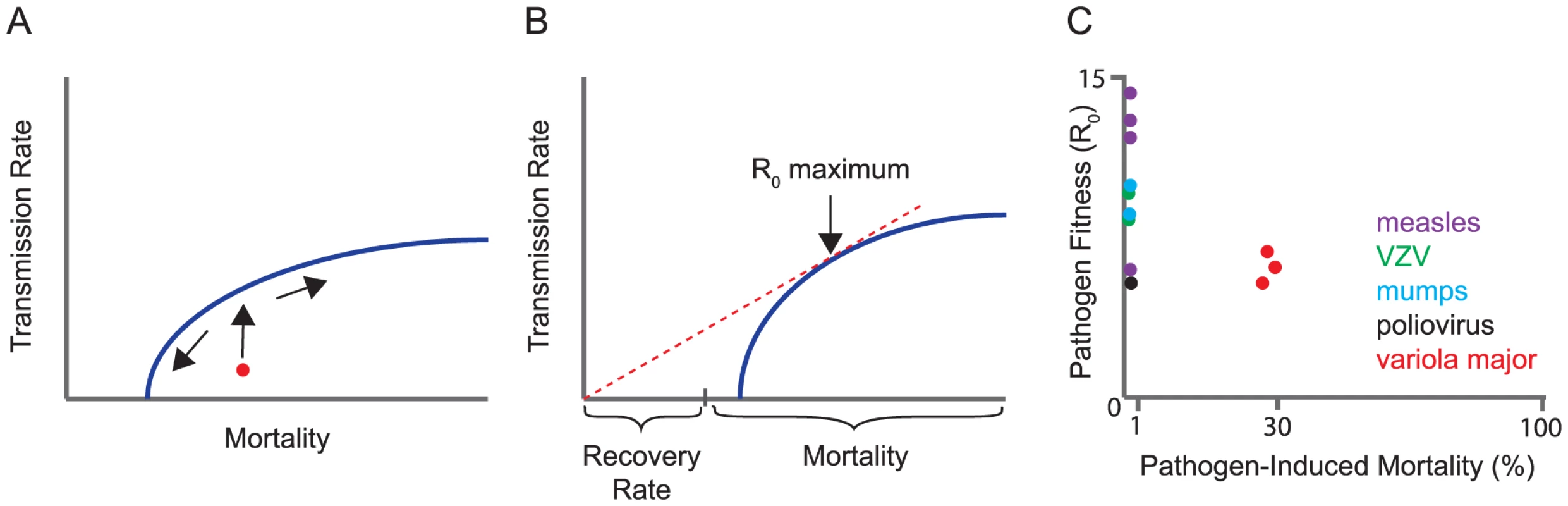
(A) A typical trade-off curve for virulence evolution, in this case between transmission rate and host mortality. The trade-off curve is a boundary on the mortality rate and transmission rate that the parasite can evolve. If the characteristics of the pathogen initially lie underneath the trade-off curve (red circle), the early evolution will be toward the boundary, and then along it, shown by arrows. (B) Parasite fitness (R0) is proportional to the ratio of transmission rate over the sum of recovery rate and mortality rate. By displacing the trade-off curve along the horizontal axis by an amount equal to the rate of recovery, the R0 of any point on the trade-off curve is simply the slope of the line from the origin to the point. The maximum R0 is thus achieved at the tangent of a line through the origin, as shown. This figure illustrates how the choice of the trade-off function affects what can be concluded about the evolution of virulence (mortality). Although recovery rate affects the optimum parasite fitness (R0), recovery is unaffected by evolution when it is not part of the trade-off (as shown here). However, if the trade-off instead was between transmission and recovery (swapping recovery and mortality rates on the x-axis), evolution of parasite fitness would affect only the rate of host recovery, and there would be no predictions about host mortality. (C) Data on R0 and pre-vaccination mortality rates for the viruses indicated (bottom) are abstracted from [16], [17] and references therein. VZV, Varicella zoster virus. What Data Support the Existence of a Transmission-Mortality Trade-Off?
Early attempts to validate the transmission-mortality trade-off examined how host mortality limits transmission. Here, the existence of a trade-off can be inferred merely by comparing variants of the same virus that differ in their rates of transmission to see whether mortality also varies—or vice versa. Well-recognized cases in which higher transmission appears to have been linked to higher mortality include feline calicivirus, myxoma virus, H5N2 influenza in avian species, and perhaps smallpox (variola major and variola minor) [2], [8], [9]. Live, attenuated virus vaccines may provide circumstantial evidence for the trade-off model, since they are only rarely transmissible and are much less virulent than their wild-type counterparts. However, the manner in which attenuated vaccines are typically generated makes it difficult to use these data to interpret virulence evolution models. Given the careful work that is required to observe viral variants differing in host mortality and the further difficulty in assessing their relative transmission rates, we know little about the shape of the trade-off curves and the location of the maxima for these viruses. Thus, it is certainly plausible that the paucity of documented trade-off variants is due to insufficient observations rather than their absence.
Is the Transmission-Mortality Trade-Off Broadly Applicable?
Whereas some evidence supports a transmission-mortality trade-off, other observations do not. The most straightforward interpretation of models for the evolution of fitness suggest that a pathogen's R0 at an evolutionary equilibrium would entail at least a modest level of disease-induced mortality (see arrow, Figure 1B). The reasoning is that if host mortality is very low, the denominator of R0 is dominated by the recovery rate. In a transmission-mortality trade-off, evolution should proceed until further gains in transmission are offset by increases in mortality. For a large number of infectious diseases, including many common and highly transmissible human viral infections, the case fatality rate is indeed very low, 0.001 to 0.01 (Figure 1C). Although the case fatality rate does not strictly coincide with the mortality rate in the R0 formula above, in these cases a low case fatality rate implies a low mortality rate. Modest increases in transmission should be possible and almost unconditionally beneficial to pathogen fitness, and yet have not been observed. Rather, the relatively high fitness (R0) and low mortality rate for many viruses suggests that a factor other than host mortality is limiting further transmission gains. It is also difficult to apply the trade-off model to many viral infections in which the majority of individuals are asymptomatic and yet efficiently shed virus. On balance, it is hard to reconcile low case-fatality rates of many human viruses with the main prediction of the trade-off model—that there is an optimum at which viral transmission is offset by host mortality.
If Host Mortality Is Frequently Not the Factor Limiting Higher Transmission Rates, What Is?
The principle that natural selection on infectious agents will favor between-host transmission seems well founded, and nearly everything in evolution involves a trade-off. The choice of which trade-off function to use (e.g., transmission-mortality or transmission-recovery) is thus absolutely critical to understanding the evolution of virulence; yet, we have little empirical understanding of the trade-offs involved. While it made for more quantitative and precise models, the early focus on host mortality obscured the importance of recovery rate and other sublethal measures of virulence as limiting factors for transmission. In sublethal infections, host control will place a boundary on viral replication, which will tend to reduce the length of an infection (increasing recovery rate), and therefore limit transmission. Given the diversity in “life history” among viruses, we suspect that there will be many viral and host factors at play with one or several being limiting for a given agent.
Intrinsic limits on pathogen replication and spread
Trade-off models assume that, aside from the trade-off, a virus can evolve to infinite extremes, from arbitrarily low levels of transmission to arbitrarily high ones. Yet viral evolution is bounded; there must be a maximal rate of viral entry, replication, assembly, and spread within a given tissue or host. A virus near this bound cannot do any better—it will not be able to evolve higher transmission or virulence, even if selection favors an increase. We know little about predicting these evolutionary boundaries, but experimental adaptations commonly reveal their existence [10].
Host immunity
Invading pathogens are rapidly sensed by the immune system, and inducible immune effectors are perhaps the most significant barriers to intra - and inter-host spread. Both innate and adaptive immune responses will clearly limit viral replication and transmission. Peak viral loads—and transmission—are often observed just prior to the onset of symptoms, a surrogate marker for the inflammatory response. Further evidence for the importance of immune control as a rate-limiting factor in transmission is the prolonged, often asymptomatic, shedding of viruses in immunodeficient hosts. The relationship between viral replication, immunity, and pathogenesis is clearly a complex one, because stronger immune responses will limit the transmission of some viruses and increase the immunopathologic manifestations, or virulence, of others. Of the evolutionary models that incorporate host immunity as a limiting factor, several invoke trade-offs [11].
How Can Empiric Data Lead to Better Models of Virulence Evolution?
While the transmission-mortality trade-off perhaps applies to a subset of pathogens, the complex intra-host dynamics of many infectious diseases make it poorly generalizable. We believe that more empiric work is needed on the relationships between transmission, virulence, and recovery rate. These data will define the mechanistic nature of the trade-offs, if any, that are specific to a given pathogen and will ultimately lead to better models. For example, population-level studies of chronic human immunodeficiency virus infection suggest that intermediate viral loads maximize transmission potential, reflecting a potential trade-off between the transmission and the duration of asymptomatic infection [12]. There is also a clear need for comparative analysis of transmission and virulence among strains of a given pathogen. In the H5N1 influenza system, one could use ancestral and evolved strains to examine how virulence was affected by selection for increased airborne transmission [3], [4]. Experimental work may also elucidate how heterogeneity in host immune function influences the evolution of virulence and transmission [13]. Finally, the virology literature is replete with studies of interactions between virus and cell. This type of work would go far toward elucidating the evolution of virulence if those studies also addressed the likely consequences of virus–host dynamics for transmission. More refined datasets will enable heavily parameterized, multiscale computational models that describe cell–cell and tissue-specific viral transit, ultimately leading to viral release from the host [14], [15]. The time is ripe to bring this new dimension of evolutionary virology into the fold, and models based on empiric data will allow for better identification and control of emerging and rapidly evolving pathogens.
Zdroje
1. EbertD (1998) Experimental evolution of parasites. Science 282 : 1432–1435.
2. KerrPJ (2012) Myxomatosis in Australia and Europe: a model for emerging infectious diseases. Antiviral Res 93 : 387–415 doi:10.1016/j.antiviral.2012.01.009
3. HerfstS, SchrauwenEJA, LinsterM, ChutinimitkulS, de WitE, et al. (2012) Airborne transmission of influenza A/H5N1 virus between ferrets. Science 336 : 1534–1541 doi:10.1126/science.1213362
4. ImaiM, WatanabeT, HattaM, DasSC, OzawaM, et al. (2012) Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486 : 420–428 doi:10.1038/nature10831
5. Dieckmann U, Metz JAJ, Sabelis MW, editors (2005) Adaptive Dynamics of Infectious Diseases. First edition. New York: Cambridge University Press. 1 pp.
6. AlizonS, HurfordA, MideoN, Van BaalenM (2009) Virulence evolution and the trade-off hypothesis: history, current state of affairs and the future. J Evol Biol 22 : 245–259 doi:10.1111/j.1420-9101.2008.01658.x
7. AndersonRM, MayRM (1982) Coevolution of hosts and parasites. Parasitology 85(Pt 2): 411–426.
8. CoyneKP, JonesBRD, KiparA, ChantreyJ, PorterCJ, et al. (2006) Lethal outbreak of disease associated with feline calicivirus infection in cats. Vet Rec 158 : 544–550.
9. van der GootJA, de JongMCM, KochG, Van BovenM (2003) Comparison of the transmission characteristics of low and high pathogenicity avian influenza A virus (H5N2). Epidemiol Infect 131 : 1003–1013.
10. BullJJ, BadgettMR, SpringmanR, MolineuxIJ (2004) Genome properties and the limits of adaptation in bacteriophages. Evolution 58 : 692–701.
11. AlizonS (2008) Transmission-recovery trade-offs to study parasite evolution. Am Nat 172: E113–E121 doi:10.1086/589892
12. FraserC, HollingsworthTD, ChapmanR, de WolfF, HanageWP (2007) Variation in HIV-1 set-point viral load: epidemiological analysis and an evolutionary hypothesis. Proc Natl Acad Sci USA 104 : 17441–17446 doi:10.1073/pnas.0708559104
13. KubinakJL, PottsWK (2013) Host resistance influences patterns of experimental viral adaptation and virulence evolution. Virulence 4 : 410–418 doi:10.4161/viru.24724
14. OriveME, StearnsMN, KellyJK, BarfieldM, SmithMS, et al. (2005) Viral infection in internally structured hosts. I. Conditions for persistent infection. J Theor Biol 232 : 453–466 doi:10.1016/j.jtbi.2004.08.023
15. KellyJK, WilliamsonS, OriveME, SmithMS (2003) Linking dynamical and population genetic models of persistent viral infection. Am Nat 162 : 14–28 doi:10.1086/375543
16. Anderson RM, May RM (1992) Infectious Diseases of Humans. First edition. Oxford: Oxford University Press. 768 pp.
17. Atkinson W, Wolfe C, Hamborsky J, editors (2011) Epidemiology and Prevention of Vaccine-Preventable Diseases. Atlanta: Department of Health and Human Services, Centers for Disease Control and Prevention.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článek Identification of the Microsporidian as a New Target of the IFNγ-Inducible IRG Resistance SystemČlánek Human Cytomegalovirus Drives Epigenetic Imprinting of the Locus in NKG2C Natural Killer CellsČlánek APOBEC3D and APOBEC3F Potently Promote HIV-1 Diversification and Evolution in Humanized Mouse ModelČlánek Role of Non-conventional T Lymphocytes in Respiratory Infections: The Case of the Pneumococcus
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2014 Číslo 10- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Theory and Empiricism in Virulence Evolution
- -Related Fungi and Reptiles: A Fatal Attraction
- Adaptive Prediction As a Strategy in Microbial Infections
- Antimicrobials, Stress and Mutagenesis
- A Novel Function of Human Pumilio Proteins in Cytoplasmic Sensing of Viral Infection
- Social Motility of African Trypanosomes Is a Property of a Distinct Life-Cycle Stage That Occurs Early in Tsetse Fly Transmission
- Autophagy Controls BCG-Induced Trained Immunity and the Response to Intravesical BCG Therapy for Bladder Cancer
- Identification of the Microsporidian as a New Target of the IFNγ-Inducible IRG Resistance System
- mRNA Structural Constraints on EBNA1 Synthesis Impact on Antigen Presentation and Early Priming of CD8 T Cells
- Infection Causes Distinct Epigenetic DNA Methylation Changes in Host Macrophages
- Neutrophil Crawling in Capillaries; A Novel Immune Response to
- Live Attenuated Vaccine Protects against Pulmonary Challenge in Rats and Non-human Primates
- The ESAT-6 Protein of Interacts with Beta-2-Microglobulin (β2M) Affecting Antigen Presentation Function of Macrophage
- Characterization of Uncultivable Bat Influenza Virus Using a Replicative Synthetic Virus
- HIV Acquisition Is Associated with Increased Antimicrobial Peptides and Reduced HIV Neutralizing IgA in the Foreskin Prepuce of Uncircumcised Men
- Uses a Unique Ligand-Binding Mode for Trapping Opines and Acquiring A Competitive Advantage in the Niche Construction on Plant Host
- Involvement of a 1-Cys Peroxiredoxin in Bacterial Virulence
- Ethanol Stimulates WspR-Controlled Biofilm Formation as Part of a Cyclic Relationship Involving Phenazines
- Densovirus Is a Mutualistic Symbiont of a Global Crop Pest () and Protects against a Baculovirus and Bt Biopesticide
- Insights into Intestinal Colonization from Monitoring Fluorescently Labeled Bacteria
- Mycobacterial Antigen Driven Activation of CD14CD16 Monocytes Is a Predictor of Tuberculosis-Associated Immune Reconstitution Inflammatory Syndrome
- Lipoprotein LprG Binds Lipoarabinomannan and Determines Its Cell Envelope Localization to Control Phagolysosomal Fusion
- Dampens the DNA Damage Response
- MicroRNAs Suppress NB Domain Genes in Tomato That Confer Resistance to
- Novel Cyclic di-GMP Effectors of the YajQ Protein Family Control Bacterial Virulence
- Vaginal Challenge with an SIV-Based Dual Reporter System Reveals That Infection Can Occur throughout the Upper and Lower Female Reproductive Tract
- Detecting Differential Transmissibilities That Affect the Size of Self-Limited Outbreaks
- One Small Step for a Yeast - Microevolution within Macrophages Renders Hypervirulent Due to a Single Point Mutation
- Expression Profiling during Arabidopsis/Downy Mildew Interaction Reveals a Highly-Expressed Effector That Attenuates Responses to Salicylic Acid
- Human Cytomegalovirus Drives Epigenetic Imprinting of the Locus in NKG2C Natural Killer Cells
- Interaction with Tsg101 Is Necessary for the Efficient Transport and Release of Nucleocapsids in Marburg Virus-Infected Cells
- The N-Terminus of Murine Leukaemia Virus p12 Protein Is Required for Mature Core Stability
- Sterol Biosynthesis Is Required for Heat Resistance but Not Extracellular Survival in
- Allele-Specific Induction of IL-1β Expression by C/EBPβ and PU.1 Contributes to Increased Tuberculosis Susceptibility
- Host Cofactors and Pharmacologic Ligands Share an Essential Interface in HIV-1 Capsid That Is Lost upon Disassembly
- APOBEC3D and APOBEC3F Potently Promote HIV-1 Diversification and Evolution in Humanized Mouse Model
- Structural Basis for the Recognition of Human Cytomegalovirus Glycoprotein B by a Neutralizing Human Antibody
- Systematic Analysis of ZnCys Transcription Factors Required for Development and Pathogenicity by High-Throughput Gene Knockout in the Rice Blast Fungus
- Epstein-Barr Virus Nuclear Antigen 3A Promotes Cellular Proliferation by Repression of the Cyclin-Dependent Kinase Inhibitor p21WAF1/CIP1
- The Host Protein Calprotectin Modulates the Type IV Secretion System via Zinc Sequestration
- Cyclophilin A Associates with Enterovirus-71 Virus Capsid and Plays an Essential Role in Viral Infection as an Uncoating Regulator
- A Novel Alpha Kinase EhAK1 Phosphorylates Actin and Regulates Phagocytosis in
- The pH-Responsive PacC Transcription Factor of Governs Epithelial Entry and Tissue Invasion during Pulmonary Aspergillosis
- Sensing of Immature Particles Produced by Dengue Virus Infected Cells Induces an Antiviral Response by Plasmacytoid Dendritic Cells
- Co-opted Oxysterol-Binding ORP and VAP Proteins Channel Sterols to RNA Virus Replication Sites via Membrane Contact Sites
- Characteristics of Memory B Cells Elicited by a Highly Efficacious HPV Vaccine in Subjects with No Pre-existing Immunity
- HPV16-E7 Expression in Squamous Epithelium Creates a Local Immune Suppressive Environment via CCL2- and CCL5- Mediated Recruitment of Mast Cells
- Dengue Viruses Are Enhanced by Distinct Populations of Serotype Cross-Reactive Antibodies in Human Immune Sera
- CD4 Depletion in SIV-Infected Macaques Results in Macrophage and Microglia Infection with Rapid Turnover of Infected Cells
- A Sialic Acid Binding Site in a Human Picornavirus
- Contact Heterogeneity, Rather Than Transmission Efficiency, Limits the Emergence and Spread of Canine Influenza Virus
- Myosins VIII and XI Play Distinct Roles in Reproduction and Transport of
- HTLV-1 Tax Stabilizes MCL-1 via TRAF6-Dependent K63-Linked Polyubiquitination to Promote Cell Survival and Transformation
- Species Complex: Ecology, Phylogeny, Sexual Reproduction, and Virulence
- A Critical Role for IL-17RB Signaling in HTLV-1 Tax-Induced NF-κB Activation and T-Cell Transformation
- Exosomes from Hepatitis C Infected Patients Transmit HCV Infection and Contain Replication Competent Viral RNA in Complex with Ago2-miR122-HSP90
- Role of Non-conventional T Lymphocytes in Respiratory Infections: The Case of the Pneumococcus
- Kaposi's Sarcoma-Associated Herpesvirus Induces Nrf2 during Infection of Endothelial Cells to Create a Microenvironment Conducive to Infection
- A Relay Network of Extracellular Heme-Binding Proteins Drives Iron Acquisition from Hemoglobin
- Glutamate Secretion and Metabotropic Glutamate Receptor 1 Expression during Kaposi's Sarcoma-Associated Herpesvirus Infection Promotes Cell Proliferation
- Reduces Malaria and Dengue Infection in Vector Mosquitoes and Has Entomopathogenic and Anti-pathogen Activities
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Novel Cyclic di-GMP Effectors of the YajQ Protein Family Control Bacterial Virulence
- MicroRNAs Suppress NB Domain Genes in Tomato That Confer Resistance to
- The ESAT-6 Protein of Interacts with Beta-2-Microglobulin (β2M) Affecting Antigen Presentation Function of Macrophage
- Characterization of Uncultivable Bat Influenza Virus Using a Replicative Synthetic Virus
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy




