-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Infection Is Associated with Impaired Hepatic Dimethylarginine Dimethylaminohydrolase Activity and Disruption of Nitric Oxide Synthase Inhibitor/Substrate Homeostasis
During a malaria infection, the vascular endothelium becomes more adhesive, permeable, and prone to trigger blood clotting. These changes help the parasite adhere to blood vessels, but endanger the host by obstructing blood flow through small vessels. Endothelial nitric oxide (NO) would normally counteract these pathological changes, but NO signalling is diminished malaria. NO synthesis is inhibited by asymmetric dimethylarginine (ADMA), a methylated derivative of arginine that is released during normal protein turnover. We found the ratio of ADMA to arginine to be elevated in Gambian children with severe malaria, a metabolic disturbance known to inhibit NO synthesis. ADMA was associated with markers of endothelial activation and impaired tissue perfusion. In parallel experiments using mice, the enzyme responsible for metabolizing ADMA, dimethylarginine dimethylaminohydrolase (DDAH), was inactivated after infection with a rodent malaria. Based on these studies, we propose that decreased metabolism of ADMA by DDAH might contribute to the elevated ADMA/arginine ratio observed during an acute episode of malaria. Strategies to preserve or increase DDAH activity might improve NO synthesis and help to prevent the vascular manifestations of severe malaria.
Published in the journal: Infection Is Associated with Impaired Hepatic Dimethylarginine Dimethylaminohydrolase Activity and Disruption of Nitric Oxide Synthase Inhibitor/Substrate Homeostasis. PLoS Pathog 11(9): e32767. doi:10.1371/journal.ppat.1005119
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1005119Summary
During a malaria infection, the vascular endothelium becomes more adhesive, permeable, and prone to trigger blood clotting. These changes help the parasite adhere to blood vessels, but endanger the host by obstructing blood flow through small vessels. Endothelial nitric oxide (NO) would normally counteract these pathological changes, but NO signalling is diminished malaria. NO synthesis is inhibited by asymmetric dimethylarginine (ADMA), a methylated derivative of arginine that is released during normal protein turnover. We found the ratio of ADMA to arginine to be elevated in Gambian children with severe malaria, a metabolic disturbance known to inhibit NO synthesis. ADMA was associated with markers of endothelial activation and impaired tissue perfusion. In parallel experiments using mice, the enzyme responsible for metabolizing ADMA, dimethylarginine dimethylaminohydrolase (DDAH), was inactivated after infection with a rodent malaria. Based on these studies, we propose that decreased metabolism of ADMA by DDAH might contribute to the elevated ADMA/arginine ratio observed during an acute episode of malaria. Strategies to preserve or increase DDAH activity might improve NO synthesis and help to prevent the vascular manifestations of severe malaria.
Introduction
Current estimates of world-wide mortality due to malaria range from 367,000 to 755,000 deaths per year, mostly in African children [1,2]. Prompt treatment with parenteral artesunate improves survival in children with severe malaria but mortality remains high in those presenting with complications such as coma or acidosis [3]. Development of effective therapies for these patients will require improved understanding of the pathophysiology of severe malaria.
Patients with severe malaria exhibit impaired endothelium-dependent vasodilation [4] and reduced nitrite and nitrate concentrations in plasma and urine [5], indicating decreased nitric oxide synthesis. Impaired NO signalling has been implicated in microcirculatory dysfunction [6], loss of blood-brain barrier integrity [7,8] and cytoadherence of infected erythrocytes to the vascular endothelium in mice [9]. Similar pathology has been directly observed in human malaria, but the importance of NO signalling in these processes is less certain [10–13]. NO production by nitric oxide synthase (NOS) is dependent in part on the relative bioavailability of arginine, the NOS substrate, and asymmetric dimethylarginine (ADMA), an endogenous NOS inhibitor released during hydrolysis of proteins that have been methylated by protein arginine methyltransferase [14–16] (Fig 1). By inhibiting NOS, ADMA not only causes vasoconstriction, increased blood pressure, increased systemic vascular resistance and decreased forearm blood flow in vivo [17–19], but also affects adhesion, inflammation, thrombosis, barrier integrity, motility, growth and repair in vitro [20–31]–endothelial functions that are relevant to the pathophysiology of malaria.
Fig. 1. DDAH regulates NO synthesis via ADMA metabolism. 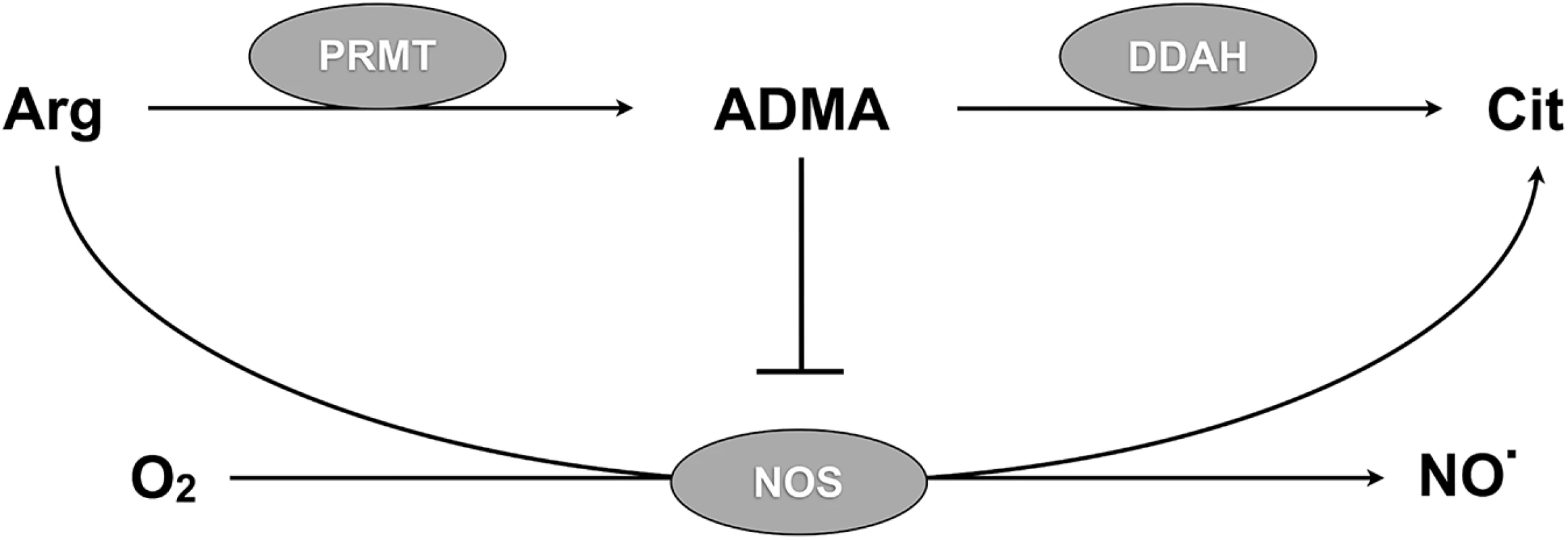
Protein arginine methyltransferases (PRMTs) methylate arginine (Arg) residues on proteins to form asymmetric dimethylarginine (ADMA). Proteolysis releases free ADMA that inhibits nitric oxide synthase (NOS). Dimethylarginine dimethylaminohydrolase (DDAH) metabolizes free ADMA to citrulline (Cit) that can be recycled to arginine. Inactivation of DDAH leads to accumulation of ADMA, inhibition of endothelial NO synthesis, and endothelial dysfunction. Dimethylarginine dimethylaminohydrolase 1 (DDAH1) metabolizes ADMA at a rate inversely proportional to arginine concentration [32] and thus stabilizes the ratio of ADMA to arginine when arginine levels vary [33,34]. In Gambian children, an intronic DDAH1 polymorphism is associated with susceptibility to severe malaria [35], raising the possibility that DDAH1 might be functionally linked to disrupted ADMA/arginine homeostasis and impaired NO synthesis in severe malaria. In this study, we identify dysregulation of ADMA/arginine homeostasis in Gambian children with severe malaria and hypothesize that ADMA clearance is impaired by hepatic DDAH1 inactivation. To test this hypothesis, we infected mice with P. berghei ANKA and assessed changes in DDAH1 expression, protein levels and activity in hepatic tissue, a major site of ADMA metabolism [29,36–38].
Results
ADMA/Arginine Homeostasis Is Disrupted in Gambian Children with Severe Malaria
We determined ADMA and arginine concentrations in blood plasma obtained from Gambian children with severe or uncomplicated malaria at initial presentation and 28 days later. Healthy afebrile aparasitemic Gambian children served as an additional control group. Baseline characteristics of the study populations are presented in Table 1.
Tab. 1. Baseline clinical characteristics of the study population. 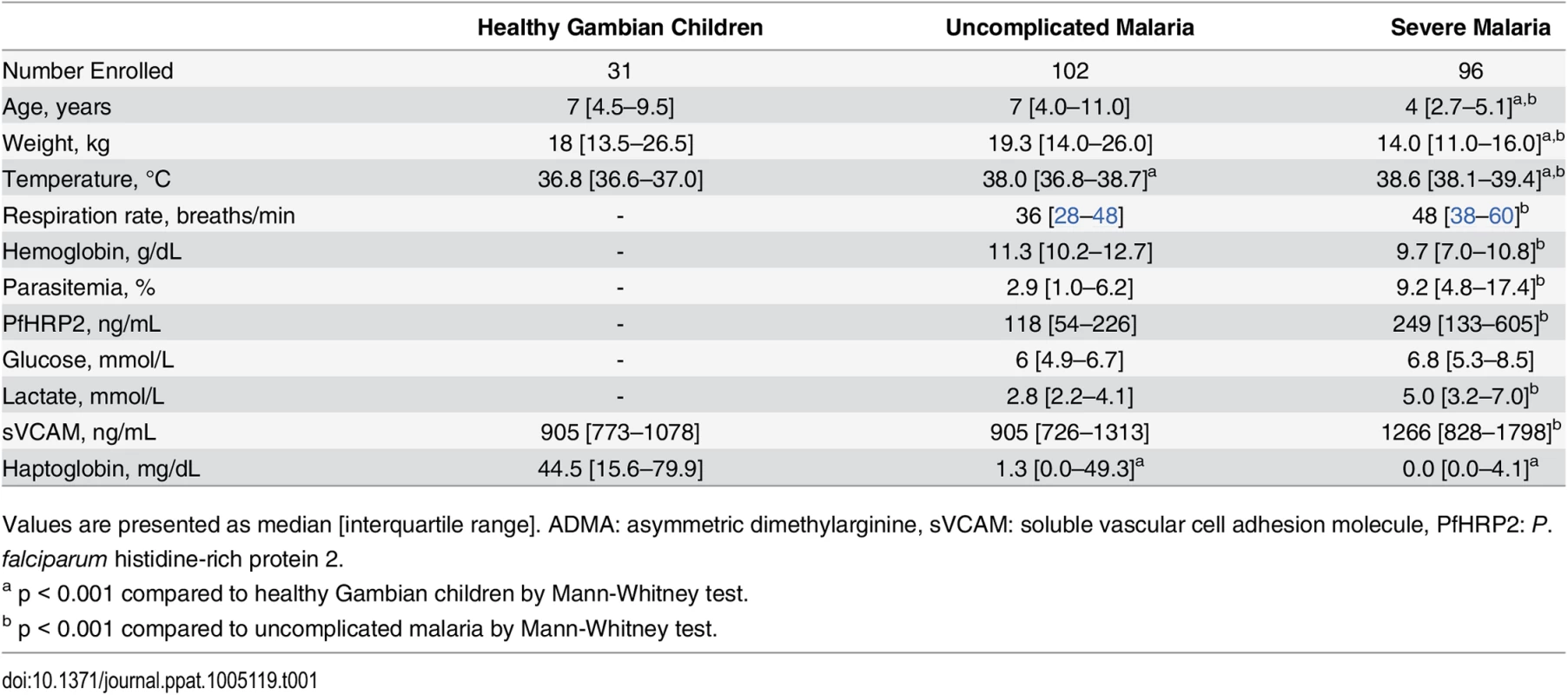
Values are presented as median [interquartile range]. ADMA: asymmetric dimethylarginine, sVCAM: soluble vascular cell adhesion molecule, PfHRP2: P. falciparum histidine-rich protein 2. Plasma ADMA was lower in children with severe malaria (median [IQR]: 0.40 [0.30–0.51] μmol/L) or uncomplicated malaria (0.40 [0.33–0.47] μmol/L) compared to healthy children (0.61 [0.56–0.69] μmol/L, p < 0.0001 vs. uncomplicated; p < 0.0001 vs. severe; Fig 2 and Table 2). ADMA remained low at the 28-day follow-up visit for patients recovered from malaria. Plasma arginine was profoundly depleted in children with severe malaria compared to children with uncomplicated malaria or healthy children (severe malaria: 31.7 [23.0–40.6] μmol/L; uncomplicated malaria: 45.0 [35.4–55.7] μmol/L, p < 0.0001 vs severe; healthy: 88.7 [79.3–102.5] μmol/L, p < 0.0001 vs severe; Fig 2 and Table 2). By the 28-day follow up visit, plasma arginine concentration increased to 56.7 [42.1–78.9] μmol/L among children who recovered from severe malaria and to 70.8 [58.6–85.1] μmol/L among children who recovered from uncomplicated malaria (p < 0.0001 vs acute, Fig 2), but remained lower than the arginine concentration observed in healthy Gambian children (88.7 μmol/L; p < 0.0001 for either comparison).
Fig. 2. The ADMA/arginine ratio is acutely elevated in African children with severe malaria. 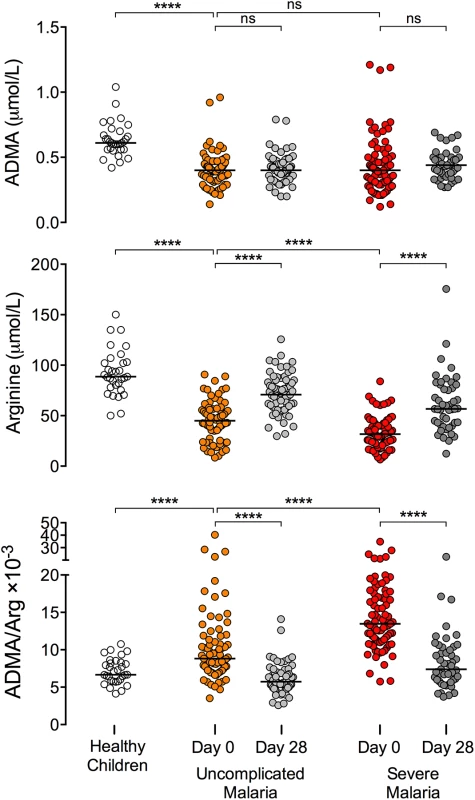
ADMA and arginine concentrations were measured in plasma samples collected at the time of presentation (Day 0) and at follow-up visits 28 days later (Day 28) in children with WHO-defined uncomplicated malaria or severe malaria. Healthy Gambian children served as a reference group. Wilcoxon test was used for pair-wise comparison of admission and day 28 mesurements within individuals (47 paired observations from patients with severe malaria; 65 paired observations from patients with uncomplicated malaria). Mann-Whitney test was used to compare patients with severe malaria (n = 81) versus uncomplicated malaria (n = 75) and to compare patients with uncomplicated malaria versus healthy children (n = 31). Each horizontal line depicts the group median. **** p < 0.0001; ns p > 0.05. Tab. 2. ADMA and arginine concentrations in plasma. 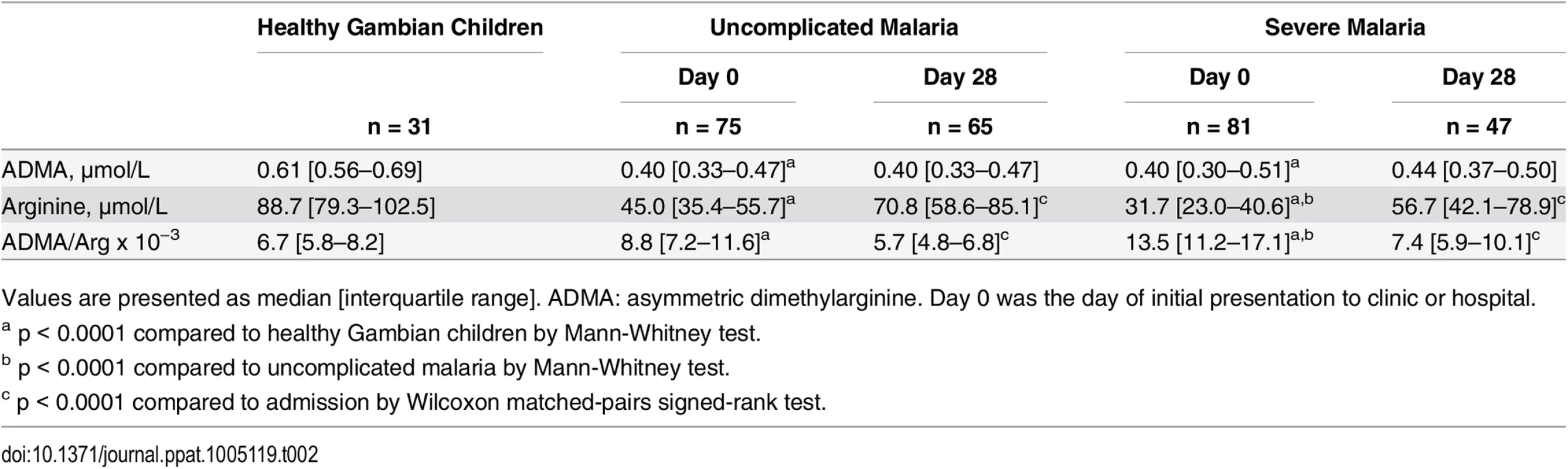
Values are presented as median [interquartile range]. ADMA: asymmetric dimethylarginine. Day 0 was the day of initial presentation to clinic or hospital. ADMA is a competitive inhibitor of NOS, and the ratio of ADMA to arginine determines NOS activity [39]. The ratio of ADMA to arginine was elevated among children with severe malaria (13.5 [11.2–17.1] ×10−3) compared to children with uncomplicated malaria (8.8 [7.2–11.6] ×10−3, p < 0.0001) or healthy children (6.7 [5.8–8.2] ×10−3, p < 0.0001, Fig 2). After recovery from severe malaria, the ADMA/arginine ratio returned to the level observed in healthy Gambian children (recovered from severe malaria: 7.4 [5.9–10.1] ×10−3, p < 0.0001 vs. acute; p = 0.25 vs. healthy children; Fig 2 and Table 2). Thus elevation of the ADMA/arginine ratio appears to be an acute metabolic disturbance associated with a symptomatic episode of malaria (modeled in S2 Fig).
Relationships between Arginine Metabolites and Biomarkers of Malaria Severity
In healthy Gambian children, plasma ADMA concentration was correlated with plasma arginine concentration (r = 0.43, p < 0.05); children with lower arginine tended to have lower ADMA (S1 Fig). In children with uncomplicated malaria, the correlation was stronger (r = 0.59, p < 0.0001) and the slope of the linear regression was steeper (p < 0.0001 vs healthy; S1 Fig). In children with severe malaria, the correlation was stronger (r = 0.77, p < 0.0001) and the slope of the linear regression was steeper still (p < 0.0001 vs uncomplicated; S1 Fig). At the day 28 follow up visit, the relationship between ADMA and arginine (ie, the slopes of the linear regressions) had returned to normal (S1 Fig), though the absolute levels of ADMA and arginine remained lower than in health children.
Lactate is a biomarker of impaired tissue perfusion that is associated with mortality from severe malaria in children [40–43]. In our study, lactate (median [IQR]) was elevated in children with severe malaria (5.0 [3.2–7.0] mmol/L) compared to children with uncomplicated malaria (2.8 [2.2–4.1] mmol/L, p < 0.0001, Table 1). Lactate correlated positively with ADMA among children with severe malaria (r = 0.34, p = 0.004, S3 Fig), implying that tissue perfusion was impaired among those with higher ADMA levels. Lactate did not correlate with arginine (r = 0.16, p = 0.20, S3 Fig) but did correlate with the ADMA/arginine ratio (r = 0.28, p = 0.02, Table 3). In multiple linear regression analysis using ADMA and arginine as explanatory variables, ADMA was positively related to lactate (β = 0.758, p = 0.002) while arginine was negatively and non-significantly related to lactate (β = -0.393, p = 0.09; Table 4).
Tab. 3. Correlation of ADMA with biomarkers of anemia, hemolysis, parasite biomass, endothelial activity, and tissue perfusion among children with severe malaria. 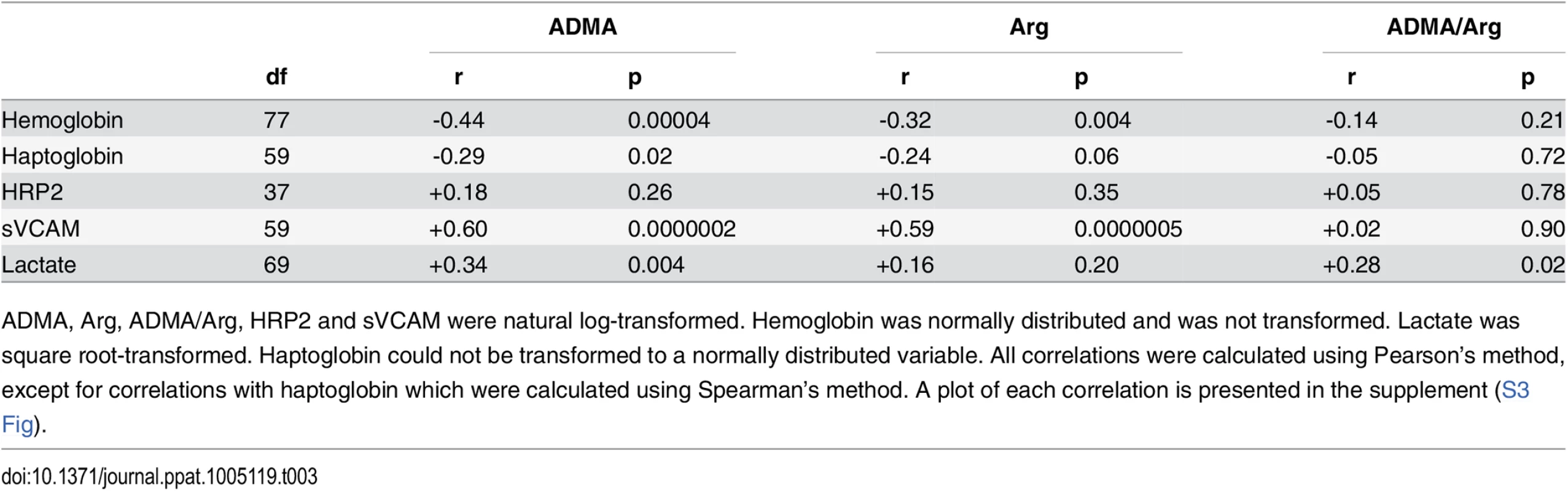
ADMA, Arg, ADMA/Arg, HRP2 and sVCAM were natural log-transformed. Hemoglobin was normally distributed and was not transformed. Lactate was square root-transformed. Haptoglobin could not be transformed to a normally distributed variable. All correlations were calculated using Pearson’s method, except for correlations with haptoglobin which were calculated using Spearman’s method. A plot of each correlation is presented in the supplement (S3 Fig). Tab. 4. Multiple linear regression analysis of the relationships between ADMA and arginine and hemoglobin, HRP2, sVCAM, or lactate. 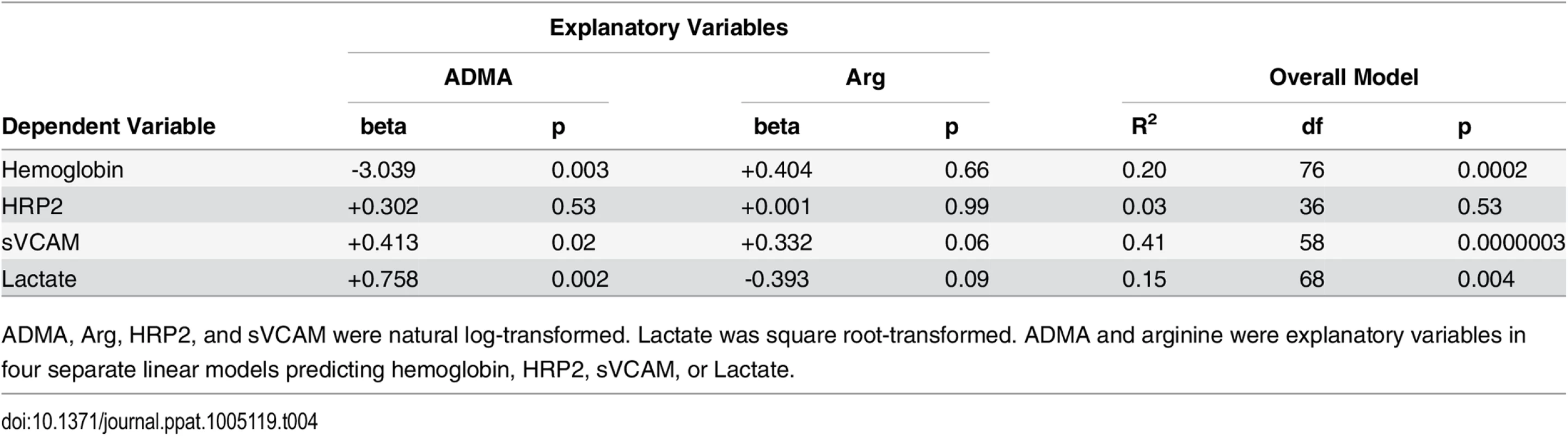
ADMA, Arg, HRP2, and sVCAM were natural log-transformed. Lactate was square root-transformed. ADMA and arginine were explanatory variables in four separate linear models predicting hemoglobin, HRP2, sVCAM, or Lactate. We measured soluble vascular cell adhesion molecule (sVCAM) as a biomarker of endothelial activation. Plasma sVCAM was elevated in children with severe malaria at the time of admission compared to children with uncomplicated malaria or healthy children (severe malaria admission: 1266 [828–1798] ng/mL; acute uncomplicated: 905 [726–1313] ng/mL, p < 0.05 vs severe, healthy children: 905 [773–1078] ng/mL, p < 0.05 vs severe, Table 1). sVCAM returned to normal at day 28 among children who had severe malaria (841 [655–1147] ng/mL, p < 0.001 vs admission). In contrast, the sVCAM level of children with uncomplicated malaria was similar to the level measured in healthy children (p = 0.84). Endothelial activation appears to be a distinctive feature of acute severe malaria.
Soluble VCAM was positively correlated with plasma ADMA in severe malaria patients (r = 0.60, p < 0.0001, Table 3 and S3 Fig). This observation suggests that ADMA, a NOS inhibitor, is associated with endothelial activation and release of sVCAM into circulation. sVCAM was also positively correlated with arginine (r = 0.59, p < 0.0001, Table 3 and S3 Fig). In multiple linear regression analysis, ADMA (β = +0.413, p = 0.02) was more significantly related to sVCAM levels than was arginine (β = +0.332, p = 0.06; Table 4).
Haptoglobin becomes depleted from plasma during acute intravascular hemolysis [44]. Haptoglobin was low at the time of admission in children with severe malaria or uncomplicated malaria (severe: 0 [0–4.1] mg/dL; uncomplicated: 1.3 [0–49.3] mg/dL), and increased by the 28-day follow up visit (severe day 28 : 13.8 [1.2–44.3] md/dL, p < 0.0001 vs admission; uncomplicated day 28 : 18.7 [0.2–59.4] mg/dL, p < 0.07 vs admission). Admission haptoglobin values, but not day 28 values, were significantly lower than in healthy children (44.5 [15.6–79.9] mg/dL).
Because haptoglobin was undetectable in many children with severe malaria, we analyzed the correlation with ADMA and arginine using Spearman’s method. The correlation between haptoglobin and ADMA was weak (r = -0.29, p = 0.02, Table 3 and S3 Fig), and weaker still with arginine (r = -0.24, p = 0.06, Table 3 and S3 Fig). There were however, moderate negative correlations with hemoglobin, a measure of anemia that may be partially reflective of hemolysis (correlation with Hb and ADMA: r = -0.44, p <0.0001; Hb and Arg r = -0.32, p = 0.004; Table 3 and S3 Fig). Multiple linear regression analysis again revealed that this correlation was primarily due to the association of hemoglobin with ADMA (β = -3.039, p = 0.003) and not with arginine (β = +0.404, p = 0.66; Table 4).
P. falciparum histidine-rich protein 2 (PfHRP2), a circulating marker of parasite biomass, was higher in children with severe malaria compared to uncomplicated malaria (severe: 249 [133–605] ng/mL vs uncomplicated: 118[54–226] ng/mL, p = 0.0001, Table 1). However, PfHRP2 was not correlated with ADMA or arginine (Table 3; S3 Fig).
Plasmodium berghei Infection Alters Plasma ADMA/Arginine Homeostasis and Blood Nitrite Levels
To determine whether P. berghei infection was associated with systemic changes in ADMA and arginine, we analyzed plasma from P. berghei ANKA-infected mice. Similar to Gambian children with malaria, plasma ADMA concentrations were lower in infected animals compared to uninfected controls on day 6 post-inoculation (0.44 [0.40–0.49] vs. 0.59 [0.54–0.63] μmol/L, p < 0.0001, Fig 3A). Arginine concentrations were also lower in infected mice compared to uninfected controls (46.7 [39.2–53.3] vs. 78.1 [66.5–101.9] μmol/L, p<0.0001, Fig 3B). Arginine decreased to a greater extent than ADMA, resulting in an increased ratio of ADMA to arginine among infected mice (9.83 [8.65–12.49] ×10−3 vs. 7.10 [5.94–8.81] ×10−3, p<0.0001, Fig 3C). The murine findings recapitulated our observations in Gambian children with malaria.
Fig. 3. (A-D) Plasmodium berghei ANKA infection increases the plasma ratio of ADMA to arginine in mice. 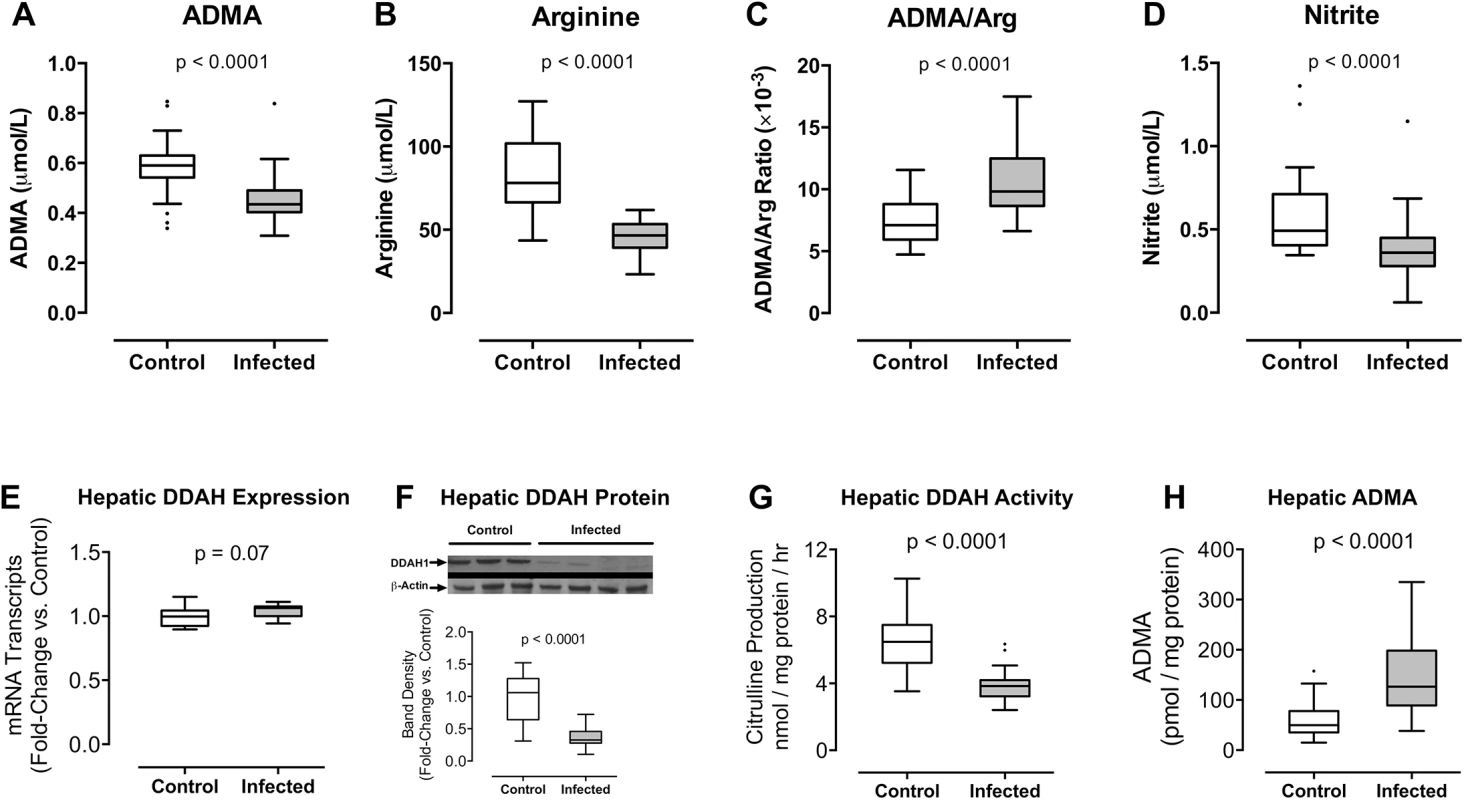
HPLC was used to determine (A) ADMA, (B) arginine concentrations, and (C) ADMA/Arg ratio in plasma samples; (D) gas phase chemiluminescent assay was used to determine nitrite concentration in blood. Blood was obtained from mice 6 days after inoculation with P. berghei ANKA (n = 23) and from uninfected control mice (n = 28) in 3 independent experiments. (E-H) Plasmodium berghei ANKA infection decreases hepatic DDAH activity in mice. (E) Quantitative RT-PCR was performed to assess hepatic Ddah1 expression 6 days after inoculation with P. berghei ANKA. Values were normalized to Gapdh mRNA transcripts and expressed as fold-change vs. control values. Liver samples were obtained from 12 control mice and 12 infected mice representing 2 independent inoculation experiments. (F) Western blot was used to detect hepatic DDAH1 protein (38 kDa) in liver tissue obtained from mice 6 days after inoculation with P. berghei ANKA and from uninfected control mice. β-actin (42 kDa) was used as an internal control. Densitometry was used to quantify DDAH1 band density normalized to β-actin and expressed as fold-change vs. control values. Data are pooled from 12 control mice and 12 infected mice representing 3 independent experiments. (G) DDAH activity was assessed by quantification of L-citrulline production by liver homogenates in the presence of saturating concentrations of ADMA substrate (2.5 mM). L-citrulline production was calculated on a per-hour basis and normalized to protein content. (H) Intracellular hepatic ADMA was assessed by HPLC in liver homogenates and normalized to protein content. Liver samples were collected from mice 6 days after inoculation with P. berghei ANKA (n = 25) and from uninfected control mice (n = 28). Results were pooled from 3 independent experiments. Boxes indicate median, 25th and 75th percentiles. Values greater than 1.5 times the IQR are plotted as individual points (Tukey’s method). Mann-Whitney test was used to compare groups. Whole blood nitrite is reflective of NOS activity [45]. We determined nitrite concentrations in whole blood samples from P. berghei ANKA-infected mice and uninfected controls using a gas-phase chemiluminescent assay. Whole blood nitrite was decreased in infected mice (0.36 [0.28–0.45] μmol/L) compared with uninfected controls (0.49 [0.41–0.71] μmol/L, p = 0.0001, Fig 3D), suggesting that P berghei ANKA infection causes a decrease in systemic NO production in mice.
Plasmodium berghei Infection Decreases DDAH1 Abundance in Hepatic Tissue
DDAH1 is highly active in the liver and plays a key role in regulating circulating levels of ADMA [29,33,36–38]. To determine whether severe malaria affects hepatic DDAH1 function, we assessed hepatic Ddah1 gene expression and protein levels in liver tissue from C57BL/6 mice 6 days after inoculation with P. berghei ANKA. Using quantitative RT-PCR to assess hepatic Ddah1 mRNA transcript number relative to Gapdh, we found that Ddah1 gene expression was not changed by P. berghei ANKA infection (median [IQR] fold change: 1.1 [1.0–1.1], p = 0.07, Fig 3E). In contrast, Western blot analysis revealed a decrease in hepatic DDAH1 protein from P. berghei ANKA-infected mice compared to uninfected control mice (median [IQR] fold change: 0.33 [0.28–0.46], p < 0.0001, Fig 3F). These data demonstrate that P. berghei ANKA infection decreases DDAH1 protein abundance by a post-transcriptional mechanism.
Plasmodium berghei Infection Decreases DDAH1 Metabolism of ADMA to Citrulline
To determine the functional impact of hepatic DDAH1 inactivation by Plasmodium infection, we quantified ADMA clearance in liver homogenates by measuring the rate of de novo citrulline production in the presence of saturating concentrations of ADMA substrate (assay validation presented in S5 Fig). Hepatic ADMA clearance was lower in mice infected with P. berghei ANKA compared with uninfected controls (infected: 3.83 [3.22–4.19] nmol citrulline × mg protein-1 × hr-1 vs uninfected control: 6.48 [5.23–7.49] nmol citrulline × mg protein-1 × hr-1, p < 0.0001, Fig 3G). To assess the impact of P. berghei infection on hepatic ADMA metabolism, we determined intracellular ADMA concentrations in PBS-perfused liver samples from infected and control mice. ADMA was increased in liver tissue from infected mice compared with uninfected controls (infected: 126.5 [88.9–198.2] pmol/mg protein vs uninfected control: 49.7 [35.3–77.8] pmol/mg protein, p < 0.0001, Fig 3H).
We calculated the correlation between hepatic DDAH activity and hepatic ADMA concentration in healthy mice and found a positive correlation (r = 0.46, p = 0.01), i.e., hepatic DDAH activities were greater in mice that had higher tissue levels of ADMA. This may reflect induction of DDAH activity in response to tissue levels of ADMA. Among P. berghei-infected mice, the correlation was similar (r = 0.42, p = 0.04) though the tissue levels of ADMA were higher, and the DDAH activities were lower than in uninfected mice (S4 Fig and S1 Table). The partial correlation coefficient between hepatic DDAH activity and hepatic ADMA concentration, accounting for infection status, remained positive (rpart = 0.34, p = 0.01). We also calculated the correlation between hepatic DDAH activity and plasma ADMA/Arginine ratio in mice, and found a negative trend, i.e., mice with lower DDAH activity tended to have higher ADMA/Arginine ratios in plasma (rpart = -0.23, p = 0.09; S4 Fig and S1 Table).
Discussion
We have analyzed ADMA and arginine concentrations in plasma from children with severe malaria to determine whether malaria is associated with disruption of ADMA/arginine homeostasis. We found that children with acute severe malaria have uncompensated hypoargininemia, i.e., low arginine with an elevated ADMA/arginine ratio. The hypoargininemia persisted over the 28 days of follow-up, while the ratio of ADMA to arginine returned to normal. We interpret this as a transient inability to metabolize ADMA at a sufficient rate to compensate for low arginine during acute infection. Although plasma ADMA levels were below normal in patients with severe malaria, ADMA was positively correlated with lactate, a biomarker of severity, and sVCAM, a biomarker of endothelial activation, suggesting that higher ADMA levels are associated with adverse pathophysiologic changes. DDAH1 metabolizes ADMA, so we examined changes in DDAH1 activity in mice infected with a Plasmodium berghei ANKA, a model of severe malaria. P berghei infection caused inactivation of hepatic DDAH1, accumulation of intracellular ADMA in liver tissue, elevation of the ADMA to arginine ratio in plasma, and decreased levels of nitrite in blood. Although these findings in the mouse model cannot be directly extrapolated to human malaria, it raises the possibility that the elevated ADMA/arginine ratio observed in children with severe malaria could be due in part to inactivation of DDAH1.
Our results extend upon a previous report of ADMA and arginine levels in Tanzanian children. Weinberg et al. observed ADMA/arginine ratios of 13.2 [11.1–16.4] ×10−3 in cerebral malaria, 12.3 [10.0–15.1] ×10−3 in non-cerebral severe malaria, 12.6 [10.7–15.1] ×10−3 in moderately severe malaria and 7.1 [5.8–9.0] ×10−3 in healthy children [46]. These values are consistent with the ADMA/arginine ratios observed in our study. We found the ADMA/arginine ratio to be significantly greater in children with severe malaria compared to children with uncomplicated malaria, while Weinberg et al found no differences among the ADMA/arginine ratios of cerebral malaria, non-cerebral severe malaria and moderately severe malaria groups. This discrepancy may be explained by the increased severity of the moderately severe malaria group in Weinberg, et al. that differed from our uncomplicated group by the inclusion of patients who could not tolerate oral medication [46]. As a result, these children may have had greater dietary insufficiency of arginine and arginine precursors than our group of uncomplicated malaria patients. Although the plasma arginine concentration was lower in Gambian children with severe malaria compared to the Tanzanian children with cerebral malaria, the rise from admission to day 28 in the Gambian children (31.7 to 56.7 umol/L, an increase of 25 umol/L) was similar to the rise from admission to day 7 in the Tanzanian children (45 umol/l to 70 umol/L, an increase of 25 umol/L).
In both Gambian and Tanzanian children with severe malaria, ADMA and the ADMA/Arg ratio were each correlated with lactate. This could be mediated through the vasoconstrictive or pro-adhesive effects of ADMA on vascular endothelium especially in the setting of hypoarginemia, with subsequent impairment of tissue perfusion leading to anaerobic glycolysis and lactate generation. Inter-individual differences in hepatic blood flow could also be responsible for the strong correlation between ADMA and lactate, since the clearance of each is dependent on hepatic perfusion. Impaired perfusion of liver tissue has been observed in adults with severe malaria [47] and could limit hepatic clearance of plasma ADMA [33,36–38].
In both Gambian and Tanzanian children with severe malaria, ADMA was correlated with biomarkers of endothelial activation (sVCAM and Angiopoietin-2, respectively). This could be through direct effects of ADMA on endothelial cells [48] or via the pro-adhesive effects of ADMA on circulating immune cells that interact with endothelium [49,50].
P. falciparum histidine-rich protein 2 (PfHRP2) has been previously assessed as a quantitative marker of parasite biomass [51,52]. PfHRP2 did not correlate significantly with ADMA, arginine or the ADMA/arginine ratio among children with severe or uncomplicated malaria (Table 3). Our findings are in agreement with the prior study [46] and together suggest that in children host ADMA metabolism is not determined by parasite biomass.
Arginine depletion and disruption of ADMA/arginine homeostasis have also been observed in adults with moderately severe and severe malaria [53]. In contrast to African children, Indonesian adults with severe malaria demonstrated elevated ADMA [53]. The apparent discrepancy in plasma ADMA in children and adults with severe malaria might be explained by differences in severe malaria pathophysiology observed in older versus younger patients [54]. Changes in plasma ADMA also differ between children and adults with acute sepsis; compared to age-matched healthy controls, ADMA was decreased in pediatric sepsis, but studies of adult sepsis found ADMA to be either unchanged or increased [55–58].
Disruption of ADMA/arginine homeostasis in children with severe malaria could be due to increased protein methylation, accelerated proteolysis of methylated proteins or impaired clearance of free ADMA. One might expect plasma ADMA to be directly elevated during a severe malaria infection, due to the combination of increased release of ADMA from erythrocytes undergoing hemolysis [59] and the impaired activity of hepatic DDAH that we present here. Instead, we observed lower plasma ADMA concentrations in both human and mouse malaria, consistent with prior measurements in children with malaria [46]. This could be due to increased uptake of ADMA from plasma into cellular compartments as has been observed in vitro after LPS, TNF or IL-1 stimulation [60]. In addition, plasma ADMA was strongly correlated with plasma arginine in our study, suggesting that arginine deficiency might lead to lower plasma ADMA. Mechanisms that could potentially link plasma ADMA to plasma arginine are inadequately understood, but might include the requirement for protein-incorporated arginine as the substrate for PRMTs that generate ADMA [16], upregulation of the cationic transporters that allow both ADMA and arginine to cross cell membranes [61,62], and negative feedback of arginine on DDAH activity [32].
DDAH1 is known to regulate ADMA/arginine homeostasis: heterozygous knock-out of DDAH1 in mice increased plasma ADMA, decreased NO-dependent vasodilation, and elevated blood pressure [63]. Conversely, transgenic over-expression of DDAH1 decreased plasma ADMA concentrations, increased urinary nitrites/nitrates and decreased blood pressure [64]. A second DDAH isoform (DDAH2) has been identified [65], but in contrast to DDAH1, suppression of DDAH2 expression did not result in altered plasma ADMA concentrations [34]. The liver expresses DDAH1 [65] and metabolic tracer studies identified the liver as a major site for clearance of circulating ADMA [37]. Induction of DDAH1 expression in liver significantly lowered plasma ADMA [33]. Endothelial cell-specific knock-out of DDAH1 revealed hepatic DDAH1 expression not only in hepatic endothelial cells but also in hepatocytes [29,66], which appear to be primarily responsible for systemic ADMA metabolism. Moreover, DDAH1 may regulate the release of ADMA from non-endothelial cell sources that affect local ADMA levels and vascular function. Patients with hepatic failure had elevated plasma levels of ADMA [38,67,68] that decreased after liver transplantation [67]. Conversely, patients with acute rejection of their liver graft had elevated ADMA compared to patients without episodes of rejection [67]. Taken together, results from human patients and animal studies implicate hepatic DDAH1 as a key regulator of circulating ADMA. In severe malaria, renal insufficiency [17], in addition to the hepatic DDAH1 dysfunction we present here, could contribute to dysregulation of ADMA/arginine homeostasis.
Plasmodium infection appears to accelerate the degradation of DDAH1 protein in hepatic tissue. Oxidative stress is a potential trigger of DDAH degradation that is present during malaria infection [69,70]. Overexpression of the p22phox subunit of NADPH oxidase in smooth muscle cells increased oxidative stress, decreased DDAH protein levels, decreased DDAH activity and caused accumulation of both intracellular and extracellular ADMA [71]. Treatment of p22phox-transfected smooth muscle cells with the proteasome inhibitor epoxomicin raised DDAH protein concentrations and reduced intracellular ADMA, demonstrating that oxidative modification of DDAH protein may target it for degradation by the proteasome. Plasmodium infection causes oxidative stress in liver tissue of mice [72], which could be sufficient to accelerate the degradation of DDAH1 in liver endothelium.
The liver may be exposed to reactive oxygen species generated by the increased populations of neutrophils and pigment-laden monocytes found in the hepatic vasculature during malaria infection [72,73]. Increased cell-free heme due to hemolysis promotes neutrophil infiltration and resulting liver damage [72], raising the possibility that hemolysis may contribute to hepatic DDAH dysfunction. Hemolysis may also result in direct release of ADMA into circulation. Human erythrocytes contain total (free plus protein-incorporated) ADMA concentrations in the range of 47.85 ± 1.68 μmol/L, extrapolated from a concentration of 15.95 ± 0.56 μmol/L reported for hydrolysates of erythrocyte samples diluted 1 : 3 in water [59]. Rat erythrocytes contain a similar concentration of total ADMA, estimated to be 40.6 ± 7.2 μmol/L [74]. Following hemolysis, methylated erythrocyte proteins are exposed to proteases that disproportionately release ADMA relative to arginine [74,75]. Thus hemolysis could both increase ADMA release and inhibit ADMA clearance by promoting DDAH degradation.
Elevation of ADMA relative to arginine favors NOS inhibition because ADMA is a competitive inhibitor of NOS [14]. ADMA also competes with arginine for cellular uptake, which could limit arginine availability for NO synthesis [61]. The intracellular concentration of ADMA in endothelial cells is approximately 10-fold higher than extracellular levels, reaching a concentration of 3–5 μmol/L which is near the Ki of eNOS [76,77]. Even small elevations in extracellular ADMA concentration to 2 umol/L had profound effects on brain NOS activity and gene expression profiles of endothelial cells in culture or in mice [78,79]. Impaired NO synthesis has been implicated in impaired vasoregulation, loss of blood-brain barrier integrity and cytoadherence of parasitized erythrocytes to the vascular endothelium during severe malaria. In a mouse model, treatment with an NO-donor improved cerebral microcirculation, reduced cerebral hemorrhages and prevented blood-brain barrier break-down [6,7]. NO synthase inhibition by ADMA downregulates tight junction protein expression [24], which may explain the beneficial effect of NO on endothelial barrier integrity. Impaired NO synthesis is associated with increased adhesion molecule expression [80] and L-NAME (a synthetic NOS inhibitor) increased cytoadherence of parasitized red blood cells to vascular endothelial cells in vitro [9]. Thus, impaired NO signaling may contribute to microhemorrhage, vascular leak and sequestration of parasitized red blood cells observed in children with fatal cerebral malaria [13]. Taken together, these findings suggest that disruption of ADMA/arginine homeostasis could contribute to severe malaria pathogenesis by inhibiting NO synthesis.
Therapeutic strategies that preserve or enhance DDAH activity during Plasmodium infection are needed to establish a causal relationship between DDAH degradation and disruption of ADMA/arginine homeostasis. While restoring ADMA/Arginine homeostasis might be necessary to improve endothelial NO synthesis, it might not be sufficient: impaired endothelial NO synthesis is likely to be limited by arginine deficiency and oxidation of tetrahydrobiopterin. NO that is produced will have a limited half-life due to reactions with cell free hemoglobin, superoxide, and other radicals that have increased abundance during malaria infection.
In summary, through clinical observational studies of Gambian children and controlled experiments mice, we have identified hepatic DDAH dysfunction as a potential mechanism disturbing ADMA/Arginine homeostasis and limiting nitric oxide synthesis in severe malaria.
Methods
Ethics Statement
Patient enrollment and sample collection were conducted following ethical review and approval by the Gambian Government/MRC Joint Ethics Committee and the Ethics Committee of the London School of Hygiene & Tropical Medicine (SCC 670, SCC 1002, SCC 1003, SCC 1077 & SCC 1113 [healthy children]). Analysis of plasma samples and de-identified clinical data at the National Institutes of Health was exempted from further ethical review by the NIH Office of Subjects Research (Exemption #5161). Written informed consent was provided by a parent or guardian on behalf of children enrolled in the study. Families who declined to participate were provided standard medical care.
Animal studies (described in detail in the supplementary information, S1 Methods) were specifically approved by the National Institutes of Allergy and Infectious Diseases (NIAID) Animal Care and Use Committee (ACUC) under the protocol identification LMVR 18E. The NIAID ACUC complies with the U.S. Government Principles for the Utilization and Care of Vertebrate Animals, the Public Health Service (PHS) Policy on Humane Care and Use of Laboratory Animals, and the Animal Welfare Act.
Human Subjects and Sample Collection
Children with severe malaria or uncomplicated malaria were enrolled at health centers in a peri-urban area around Fajara, The Gambia as previously described [81]. Enrollment sites included the Royal Victoria Teaching Hospital, the Brikama Health Centre, the MRC Fajara Gate Clinic and the Jammeh Foundation for Peace Hospital in Serekunda.
Acute uncomplicated malaria was defined as asexual P. falciparum parasitemia of >5000 parasites/μl detected by slide microscopy with an episode of fever (temperature >37.5°C) within the previous 48 hrs and the absence of severe criteria. Acute severe malaria was defined as parasitemia of >5000 parasites/μl, a history of fever and one or more of the following: severe anemia (Hb < 6g/dl), severe acidosis (serum lactate >7 mmol/L), cerebral malaria (Blantyre coma score 2 or less in the absence of hypoglycemia or hypovolemia with the coma lasting for at least 2 hrs), and severe prostration (inability to sit unsupported in children >6 months or inability to suck in children <6 months). Patients with severe malaria were admitted and treated with quinine, and patients with uncomplicated malaria were treated with chloroquine plus sulfadoxine-pyrimethamine according to Gambian Government Treatment Guidelines [82].
Ninety-six children with severe malaria and 102 children with uncomplicated malaria were enrolled during the 2005–2008 malaria seasons. Four milliliters of blood were collected in heparinized vacutainers (BD) at the time of initial presentation and at a follow-up visit 28 days later. Blood samples were immediately refrigerated, placed on ice for transport, and processed within 2 hours of collection. Plasma was frozen at -80°C. Three patients with severe malaria died and 65 completed follow-up visits; plasma sample were available from 47 of them. Eighty-five patients with uncomplicated malaria completed follow-up visits; plasma samples were available from 65 of them. Thirty-one healthy, afebrile, aparasitemic Gambian children of similar ages were also studied at a single visit.
Clinical Laboratory Methods
P. falciparum parasitemia was determined by bright-field microscopy of giemsa-stained blood smears. 50 fields were counted at high power. Full blood counts were obtained with an automated instrument (Clinical Diagnostics solutions, Inc., Fort Lauderdale, FL, USA). Lactate was measured with a handheld Lactate Pro device (Arkray, Edina, MN, USA). Soluble VCAM (sVCAM) and plasma haptoglobin concentrations were determined by ELISA (sVCAM: R&D Systems, Minneapolis, MN, USA; haptoglobin: Alpco, Salem, NH, USA) according to the manufacturer’s instructions.
P. falciparum HRP2 was measured in duplicate in plasma by ELISA (Cellabs) according to the manufacturer’s instructions. A standard curve was constructed using serial dilutions of the PfHRP2 standard and run with every plate. Laboratory staff were unaware of clinical status of the subjects.
Determination of ADMA and Arginine in Human and Mouse Plasma
Each plasma sample was diluted in PBS containing NG-monoethyl-L-arginine (MEA) as an internal standard before undergoing solid-phase extraction (Oasis MCX 96-well μElution Plate, Waters Corporation, Milford, MA, USA). The eluted cationic amino acids were dried, resuspended in water, and derivatized with ortho-phthalaldehyde (OPA) in 3-mercaptopropionic acid. Derivatized samples were separated by reverse-phase liquid chromatography over a 1×100 mm C18(2) column (Phenomenex, Torrance, CA, USA) and fluorescence detected at excitation and emission wavelengths of 340nm and 455nm, respectively. Concentrations were determined by integrating peak area with reference to the internal standard (MEA) and daily external standards (Arg, ADMA, and MEA).
Statistical Analyses
Data are expressed as median and inter-quartile range (IQR). Statistical analyses were performed with GraphPad Prism 6.02 software and the R computing environment. P-values of less than 0.05 were considered significant. Mann-Whitney test was used to compare median values between healthy children and children with uncomplicated or severe malaria. Wilcoxon matched-pairs signed rank test was used to compare acute and recovery values in children with uncomplicated or severe malaria. Correlations were calculated on transformed data using Pearson’s correlation test, except in the case of correlation with haptoglobin in which Spearman’s method was used. When two separate groups were combined, partial correlations were calculated. Multiple linear regression analysis was used to assess the independent contributions of ADMA or arginine to correlations with hemoglobin, HRP2, sVCAM, or lactate. In these linear models, arginine and ADMA were the explanatory variables, and the direction, strength and significance of the association was assessed by the beta value and p-value. Mann-Whitney test was used to compare values between infected mice and uninfected controls. Data are available in supplemental files S1 Data and S2 Data.
Supporting Information
Zdroje
1. World Health Organization. World malaria report 2014 [Internet]. 2014. Available: http://apps.who.int/iris/bitstream/10665/144852/2/9789241564830_eng.pdf?ua=1
2. Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. The Lancet. 2012;379 : 413–431. doi: 10.1016/S0140-6736(12)60034-8
3. Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A, Chhaganlal KD, et al. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet. 2010;376 : 1647–57. doi: 10.1016/S0140-6736(10)61924-1 21062666
4. Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, McNeil YR, et al. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med. 2007;204 : 2693–704. 17954570
5. Anstey NM, Weinberg JB, Hassanali MY, Mwaikambo ED, Manyenga D, Misukonis MA, et al. Nitric oxide in Tanzanian children with malaria: inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J Exp Med. 1996;184 : 557–67. 8760809
6. Cabrales P, Zanini GM, Meays D, Frangos JA, Carvalho LJ. Nitric oxide protection against murine cerebral malaria is associated with improved cerebral microcirculatory physiology. J Infect Dis. 2011;203 : 1454–63. doi: 10.1093/infdis/jir058 21415018
7. Gramaglia I, Sobolewski P, Meays D, Contreras R, Nolan JP, Frangos JA, et al. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat Med. 2006;12 : 1417–1422. doi: 10.1038/nm1499 17099710
8. Serghides L, Kim H, Lu Z, Kain DC, Miller C, Francis RC, et al. Inhaled nitric oxide reduces endothelial activation and parasite accumulation in the brain, and enhances survival in experimental cerebral malaria. PLoS One. 2011;6: e27714. doi: 10.1371/journal.pone.0027714 22110737
9. Serirom S, Raharjo WH, Chotivanich K, Loareesuwan S, Kubes P, Ho M. Anti-adhesive effect of nitric oxide on Plasmodium falciparum cytoadherence under flow. Am J Pathol. 2003;162 : 1651–60. 12707049
10. Dondorp AM, Ince C, Charunwatthana P, Hanson J, Kuijen A van, Faiz MA, et al. Direct In Vivo Assessment of Microcirculatory Dysfunction in Severe Falciparum Malaria. J Infect Dis. 2008;197 : 79–84. doi: 10.1086/523762 18171289
11. Yeo TW, Lampah DA, Kenangalem E, Tjitra E, Price RN, Anstey NM. Impaired skeletal muscle microvascular function and increased skeletal muscle oxygen consumption in severe falciparum malaria. J Infect Dis. 2013;207 : 528–536. doi: 10.1093/infdis/jis692 23162136
12. Dorovini-Zis K, Schmidt K, Huynh H, Fu W, Whitten RO, Milner D, et al. The neuropathology of fatal cerebral malaria in malawian children. Am J Pathol. 2011;178 : 2146–2158. doi: 10.1016/j.ajpath.2011.01.016 21514429
13. Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NG, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10 : 143–5. 14745442
14. Cardounel AJ, Cui H, Samouilov A, Johnson W, Kearns P, Tsai AL, et al. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J Biol Chem. 2007;282 : 879–87. doi: 10.1074/jbc.M603606200 17082183
15. Kakimoto Y, Akazawa S. Isolation and Identification of Ng,Ng - and Ng,N’g-Dimethylarginine, Nε-Mono-, Di-, and Trimethyllysine, and Glucosylgalactosyl - and Galactosyl-δ-hydroxylysine from Human Urine. J Biol Chem. 1970;245 : 5751–5758. 5472370
16. Lee HW, Kim S, Paik WK. S-Adenosylmethionine:protein-arginine methyltransferase. Purification and mechanism of the enzyme. Biochemistry (Mosc). 1977;16 : 78–85. doi: 10.1021/bi00620a013
17. Vallance P, Leone A, Calver A, Collier J, Moncada S. Accumulation of an endogenous inhibitor of nitric oxide synthesis in chronic renal failure. Lancet. 1992;339 : 572–575. 1347093
18. Achan V, Broadhead M, Malaki M, Whitley G, Leiper J, MacAllister R, et al. Asymmetric Dimethylarginine Causes Hypertension and Cardiac Dysfunction in Humans and Is Actively Metabolized by Dimethylarginine Dimethylaminohydrolase. Arterioscler Thromb Vasc Biol. 2003;23 : 1455–1459. doi: 10.1161/01.ATV.0000081742.92006.59 12805079
19. Kielstein JT, Impraim B, Simmel S, Bode-Böger SM, Tsikas D, Frölich JC, et al. Cardiovascular Effects of Systemic Nitric Oxide Synthase Inhibition With Asymmetrical Dimethylarginine in Humans. Circulation. 2004;109 : 172–177. doi: 10.1161/01.CIR.0000105764.22626.B1 14662708
20. Chen M, Li Y, Yang T, Wang Y, Bai Y, Xie X. ADMA induces monocyte adhesion via activation of chemokine receptors in cultured THP-1 cells. Cytokine. 2008;43 : 149–159. doi: 10.1016/j.cyto.2008.05.001 18617418
21. Zhang G, Bai Y, Chen M, Shi R, Jiang D, Fu Q, et al. Asymmetric dimethylarginine induces TNF-alpha production via ROS/NF-kappaB dependent pathway in human monocytic cells and the inhibitory effect of reinioside C. Vascul Pharmacol. 2008;48 : 115–21. doi: 10.1016/j.vph.2008.01.004 18295546
22. Jiang D, Cao Y, Xin H, Li X, Luo Z, Li Y. Asymmetric dimethylarginine induces tissue factor expression in monocytes via NF-kappaB-dependent pathway: Role in acute coronary syndromes. Atherosclerosis. 2009;205 : 554–60. doi: 10.1016/j.atherosclerosis.2008.12.024 19167713
23. Zhang Q, Chen N, Qiu W, Xu X, Wang D, Tsao PS, et al. Asymmetric dimethylarginine impairs fibrinolytic activity in human umbilical vein endothelial cells via p38 MAPK and NF-κB pathways. Thromb Res. 2011;128 : 42–46. doi: 10.1016/j.thromres.2011.02.013 21429569
24. Wojciak-Stothard B, Torondel B, Zhao L, Renné T, Leiper JM. Modulation of Rac1 Activity by ADMA/DDAH Regulates Pulmonary Endothelial Barrier Function. Mol Biol Cell. 2009;20 : 33–42. doi: 10.1091/mbc.E08-04-0395 18923147
25. Fiedler L, Wojciak-Stothard B. The DDAH/ADMA pathway in the control of endothelial cell migration and angiogenesis. Biochem Soc Trans. 2009;37 : 1243–7. doi: 10.1042/BST0371243 19909255
26. Wojciak-Stothard B, Torondel B, Tsang L, Fleming I, Fisslthaler B, Leiper J, et al. The ADMA/DDAH pathway is a critical regulator of endothelial cell motility. J Cell Sci. 2007;120 : 929–42. doi: 10.1242/jcs.002212 17327280
27. Achan V, Ho H, Heeschen C, Stuehlinger M, Jang J, Kimoto M, et al. ADMA regulates angiogenesis: genetic and metabolic evidence. Vasc Med Lond Engl. 2005;10 : 7–14.
28. Fiedler L, Bachetti T, Leiper J, Zachary I, Chen L, Renné T, et al. The ADMA/DDAH pathway regulates VEGF-mediated angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29 : 2117–24. doi: 10.1161/ATVBAHA.109.194035 19778944
29. Dowsett L, Piper S, Slaviero A, Dufton N, Wang Z, Boruc O, et al. Endothelial DDAH1 is an Important Regulator of Angiogenesis but Does Not Regulate Vascular Reactivity or Hemodynamic Homeostasis. Circulation. 2015; doi: 10.1161/CIRCULATIONAHA.114.015064 25910799
30. Zhang P, Xu X, Hu X, Wang H, Fassett J, Huo Y, et al. DDAH1 deficiency attenuates endothelial cell cycle progression and angiogenesis. PloS One. 2013;8: e79444. doi: 10.1371/journal.pone.0079444 24260221
31. Konishi H, Sydow K, Cooke J. Dimethylarginine dimethylaminohydrolase promotes endothelial repair after vascular injury. J Am Coll Cardiol. 2007;49 : 1099–105. doi: 10.1016/j.jacc.2006.10.068 17349891
32. Wang J, Sim AS, Wang XL, Wilcken DEL. L-arginine regulates asymmetric dimethylarginine metabolism by inhibiting dimethylarginine dimethylaminohydrolase activity in hepatic (HepG2) cells. Cell Mol Life Sci CMLS. 2006;63 : 2838–2846. doi: 10.1007/s00018-006-6271-8 17075694
33. Hu T, Chouinard M, Cox AL, Sipes P, Marcelo M, Ficorilli J, et al. Farnesoid X Receptor Agonist Reduces Serum Asymmetric Dimethylarginine Levels through Hepatic Dimethylarginine Dimethylaminohydrolase-1 Gene Regulation. J Biol Chem. 2006;281 : 39831–39838. doi: 10.1074/jbc.M606779200 17065154
34. Wang D, Gill PS, Chabrashvili T, Onozato ML, Raggio J, Mendonca M, et al. Isoform-specific regulation by N(G),N(G)-dimethylarginine dimethylaminohydrolase of rat serum asymmetric dimethylarginine and vascular endothelium-derived relaxing factor/NO. Circ Res. 2007;101 : 627–635. doi: 10.1161/CIRCRESAHA.107.158915 17673667
35. Jallow M, Teo YY, Small KS, Rockett KA, Deloukas P, Clark TG, et al. Genome-wide and fine-resolution association analysis of malaria in West Africa. Nat Genet. 2009;41 : 657–665. doi: 10.1038/ng.388 19465909
36. Siroen MPC, van der Sijp JRM, Teerlink T, van Schaik C, Nijveldt RJ, van Leeuwen PAM. The human liver clears both asymmetric and symmetric dimethylarginine. Hepatology. 2005;41 : 559–565. doi: 10.1002/hep.20579 15726655
37. Nijveldt RJ, Teerlink T, Siroen MPC, van Lambalgen AA, Rauwerda JA, van Leeuwen PAM. The liver is an important organ in the metabolism of asymmetrical dimethylarginine (ADMA). Clin Nutr Edinb Scotl. 2003;22 : 17–22.
38. Nijveldt RJ, Teerlink T, Siroen MPC, van der Hoven B, Prins HA, Wiezer MJ, et al. Elevation of asymmetric dimethylarginine (ADMA) in patients developing hepatic failure after major hepatectomy. JPEN J Parenter Enteral Nutr. 2004;28 : 382–387. 15568284
39. Cardounel AJ, Cui H, Samouilov A, Johnson W, Kearns P, Tsai AL, et al. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J Biol Chem. 2007;282 : 879–87. doi: 10.1074/jbc.M603606200 17082183
40. Taylor TE, Borgstein A, Molyneux ME. Acid-base status in paediatric Plasmodium falciparum malaria. Q J Med. 1993;86 : 99–109. 8464997
41. Krishna S, Waller DW, ter Kuile F, Kwiatkowski D, Crawley J, Craddock CF, et al. Lactic acidosis and hypoglycaemia in children with severe malaria: pathophysiological and prognostic significance. Trans R Soc Trop Med Hyg. 1994;88 : 67–73. 8154008
42. Waller D, Krishna S, Crawley J, Miller K, Nosten F, Chapman D, et al. Clinical features and outcome of severe malaria in Gambian children. Clin Infect Dis. 1995;21 : 577–87. 8527547
43. Planche T, Agbenyega T, Bedu-Addo G, Ansong D, Owusu-Ofori A, Micah F, et al. A Prospective Comparison of Malaria with Other Severe Diseases in African Children: Prognosis and Optimization of Management. Clin Infect Dis. 2003;37 : 890–897. doi: 10.1086/377536 13130399
44. Blumberg BS, Kuvin SF, Robinson JC, Teitelbaum JM, Contacos PG. Alterations in haptoglobin levels. JAMA. 1963;184 : 1021–1023. 13971874
45. Kleinbongard P, Dejam A, Lauer T, Rassaf T, Schindler A, Picker O, et al. Plasma nitrite reflects constitutive nitric oxide synthase activity in mammals. Free Radic Biol Med. 2003;35 : 790–796. doi: 10.1016/S0891-5849(03)00406-4 14583343
46. Weinberg JB, Yeo TW, Mukemba JP, Florence SM, Volkheimer AD, Wang H, et al. Dimethylarginines: endogenous inhibitors of nitric oxide synthesis in children with falciparum malaria. J Infect Dis. 2014; jiu156. doi: 10.1093/infdis/jiu156 24620026
47. Molyneux ME, Looareesuwan S, Menzies IS, Grainger SL, Phillips RE, Wattanagoon Y, et al. Reduced Hepatic Blood Flow and Intestinal Malabsorption in Severe Falciparum Malaria. Am J Trop Med Hyg. 1989;40 : 470–476. 2729505
48. Jiang J-L, Wang S, Li N-S, Zhang X-H, Deng H-W, Li Y-J. The inhibitory effect of simvastatin on the ADMA-induced inflammatory reaction is mediated by MAPK pathways in endothelial cells. Biochem Cell Biol Biochim Biol Cell. 2007;85 : 66–77. doi: 10.1139/o06-146
49. Chan JR, Böger RH, Bode-Böger SM, Tangphao O, Tsao PS, Blaschke TF, et al. Asymmetric Dimethylarginine Increases Mononuclear Cell Adhesiveness in Hypercholesterolemic Humans. Arterioscler Thromb Vasc Biol. 2000;20 : 1040–1046. doi: 10.1161/01.ATV.20.4.1040 10764670
50. Böger R, Bode-Böger S, Tsao P, Lin P, Chan J, Cooke J. An endogenous inhibitor of nitric oxide synthase regulates endothelial adhesiveness for monocytes. J Am Coll Cardiol. 2000;36 : 2287–95. 11127475
51. Dondorp AM, Desakorn V, Pongtavornpinyo W, Sahassananda D, Silamut K, Chotivanich K, et al. Estimation of the Total Parasite Biomass in Acute Falciparum Malaria from Plasma PfHRP2. PLoS Med. 2005;2: e204. doi: 10.1371/journal.pmed.0020204 16104831
52. Cunnington AJ, Bretscher MT, Nogaro SI, Riley EM, Walther M. Comparison of parasite sequestration in uncomplicated and severe childhood Plasmodium falciparum malaria. J Infect. 2013;67 : 220–230. doi: 10.1016/j.jinf.2013.04.013 23623771
53. Yeo TW, Lampah DA, Tjitra E, Gitawati R, Darcy CJ, Jones C, et al. Increased asymmetric dimethylarginine in severe falciparum malaria: association with impaired nitric oxide bioavailability and fatal outcome. PLoS Pathog. 2010;6: e1000868. doi: 10.1371/journal.ppat.1000868 20421938
54. Idro R, Jenkins NE, Newton CR. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol. 2005;4 : 827–40. 16297841
55. Weiss SL, Haymond S, Ranaivo HR, Wang D, De Jesus VR, Chace DH, et al. Evaluation of asymmetric dimethylarginine, arginine, and carnitine metabolism in pediatric sepsis. Pediatr Crit Care Med July 2012. 2012;13. doi: 10.1097/PCC.0b013e318238b5cd
56. Brenner T, Fleming TH, Rosenhagen C, Krauser U, Mieth M, Bruckner T, et al. L-Arginine and Asymmetric Dimethylarginine Are Early Predictors for Survival in Septic Patients with Acute Liver Failure. Mediators Inflamm. 2012;2012: e210454. doi: 10.1155/2012/210454
57. O’Dwyer MJ, Dempsey F, Crowley V, Kelleher DP, McManus R, Ryan T. Septic shock is correlated with asymmetrical dimethyl arginine levels, which may be influenced by a polymorphism in the dimethylarginine dimethylaminohydrolase II gene: a prospective observational study. Crit Care Lond Engl. 2006;10: R139. doi: 10.1186/cc5053
58. Davis JS, Darcy CJ, Yeo TW, Jones C, McNeil YR, Stephens DP, et al. Asymmetric Dimethylarginine, Endothelial Nitric Oxide Bioavailability and Mortality in Sepsis. PLoS ONE. 2011;6: e17260. doi: 10.1371/journal.pone.0017260 21364995
59. Davids M, van Hell AJ, Visser M, Nijveldt RJ, van Leeuwen PAM, Teerlink T. Role of the human erythrocyte in generation and storage of asymmetric dimethylarginine. AJP Heart Circ Physiol. 2012;302: H1762–H1770. doi: 10.1152/ajpheart.01205.2011
60. Bogle RG, MacAllister RJ, Whitley GS, Vallance P. Induction of NG-monomethyl-L-arginine uptake: a mechanism for differential inhibition of NO synthases? Am J Physiol-Cell Physiol. 1995;269: C750–C756.
61. Closs EI, Basha FZ, Habermeier A, Forstermann U. Interference of L-arginine analogues with L-arginine transport mediated by the y+ carrier hCAT-2B. Nitric Oxide. 1997;1 : 65–73. doi: 10.1006/niox.1996.0106 9701046
62. Hatzoglou M, Fernandez J, Yaman I, Closs E. Regulation of cationic amino acid transport: the story of the CAT-1 transporter. Annu Rev Nutr. 2004;24 : 377–99. doi: 10.1146/annurev.nutr.23.011702.073120 15459982
63. Leiper J, Nandi M, Torondel B, Murray-Rust J, Malaki M, O’Hara B, et al. Disruption of methylarginine metabolism impairs vascular homeostasis. Nat Med. 2007;13 : 198–203. doi: 10.1038/nm1543 17273169
64. Dayoub H, Achan V, Adimoolam S, Jacobi J, Stuehlinger MC, Wang B, et al. Dimethylarginine Dimethylaminohydrolase Regulates Nitric Oxide Synthesis Genetic and Physiological Evidence. Circulation. 2003;108 : 3042–3047. doi: 10.1161/01.CIR.0000101924.04515.2E 14638548
65. Leiper JM, Santa Maria J, Chubb A, MacAllister RJ, Charles IG, Whitley GS, et al. Identification of two human dimethylarginine dimethylaminohydrolases with distinct tissue distributions and homology with microbial arginine deiminases. Biochem J. 1999;343 Pt 1 : 209–214. 10493931
66. Hu X, Xu X, Zhu G, Atzler D, Kimoto M, Chen J, et al. Vascular Endothelial-Specific Dimethylarginine Dimethylaminohydrolase-1–Deficient Mice Reveal That Vascular Endothelium Plays an Important Role in Removing Asymmetric Dimethylarginine. Circulation. 2009;120 : 2222–2229. doi: 10.1161/CIRCULATIONAHA.108.819912 19917889
67. Siroen MPC, Warlé MC, Teerlink T, Nijveldt RJ, Kuipers EJ, Metselaar HJ, et al. The transplanted liver graft is capable of clearing asymmetric dimethylarginine. Liver Transplant Off Publ Am Assoc Study Liver Dis Int Liver Transplant Soc. 2004;10 : 1524–1530. doi: 10.1002/lt.20286
68. Mookerjee RP, Dalton RN, Davies NA, Hodges SJ, Turner C, Williams R, et al. Inflammation is an important determinant of levels of the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine (ADMA) in acute liver failure. Liver Transpl. 2007;13 : 400–405. doi: 10.1002/lt.21053 17318866
69. Griffiths MJ, Ndungu F, Baird KL, Muller DPR, Marsh K, Newton CRJC. Oxidative stress and erythrocyte damage in Kenyan children with severe Plasmodium falciparum malaria. Br J Haematol. 2001;113 : 486–491. doi: 10.1046/j.1365-2141.2001.02758.x 11380421
70. Rubach MP, Mukemba J, Florence S, Lopansri BK, Hyland K, Volkheimer AD, et al. Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Oxidized Biopterins in Pediatric Falciparum Malaria: Association with Disease Severity. PLoS Pathog. 2015;11: e1004655. doi: 10.1371/journal.ppat.1004655 25764173
71. Luo Z, Teerlink T, Griendling K, Aslam S, Welch WJ, Wilcox CS. Angiotensin II and NADPH Oxidase Increase ADMA in Vascular Smooth Muscle Cells. Hypertension. 2010;56 : 498–504. doi: 10.1161/HYPERTENSIONAHA.110.152959 20696982
72. Dey S, Bindu S, Goyal M, Pal C, Alam A, Iqbal MS, et al. Impact of Intravascular Hemolysis in Malaria on Liver Dysfunction INVOLVEMENT OF HEPATIC FREE HEME OVERLOAD, NF-κB ACTIVATION, AND NEUTROPHIL INFILTRATION. J Biol Chem. 2012;287 : 26630–26646. doi: 10.1074/jbc.M112.341255 22696214
73. Seydel KB, Milner DA, Kamiza SB, Molyneux ME, Taylor TE. The Distribution and Intensity of Parasite Sequestration in Comatose Malawian Children. J Infect Dis. 2006;194 : 208–215. doi: 10.1086/505078 16779727
74. Billecke SS. Contribution of whole blood to the control of plasma asymmetrical dimethylarginine. AJP Heart Circ Physiol. 2006;291: H1788–H1796. doi: 10.1152/ajpheart.00066.2006
75. Davids M, van Hell AJ, Visser M, Nijveldt RJ, van Leeuwen PAM, Teerlink T. Role of the human erythrocyte in generation and storage of asymmetric dimethylarginine. Am J Physiol Heart Circ Physiol. 2012;302: H1762–1770. doi: 10.1152/ajpheart.01205.2011 22367507
76. Teerlink T, Luo Z, Palm F, Wilcox C. Cellular ADMA: regulation and action. Pharmacol Res Off J Ital Pharmacol Soc. 2009;60 : 448–60. doi: 10.1016/j.phrs.2009.08.002
77. Masuda H, Goto M, Tamaoki S, Azuma H. Accelerated intimal hyperplasia and increased endogenous inhibitors for NO synthesis in rabbits with alloxan-induced hyperglycaemia. Br J Pharmacol. 1999;126 : 211–218. doi: 10.1038/sj.bjp.0702298 10051138
78. Faraci FM, Brian JE, Heistad DD. Response of cerebral blood vessels to an endogenous inhibitor of nitric oxide synthase. Am J Physiol. 1995;269: H1522–1527. 7503244
79. Smith C, Anthony S, Hubank M, Leiper J, Vallance P. Effects of ADMA upon gene expression: an insight into the pathophysiological significance of raised plasma ADMA. PLoS Med. 2005;2: e264. doi: 10.1371/journal.pmed.0020264 16190779
80. De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA, et al. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest. 1995;96 : 60–8. 7542286
81. Walther M, Jeffries D, Finney OC, Njie M, Ebonyi A, Deininger S, et al. Distinct roles for FOXP3 and FOXP3 CD4 T cells in regulating cellular immunity to uncomplicated and severe Plasmodium falciparum malaria. PLoS Pathog. 2009;5: e1000364. doi: 10.1371/journal.ppat.1000364 19343213
82. Guidelines for the Management of Malaria. Banjul, The Gambia: Ministry of Health and Social Welfare; 2005.
Štítky
Hygiena a epidemiológia Infekčné lekárstvo Laboratórium
Článok vyšiel v časopisePLOS Pathogens
Najčítanejšie tento týždeň
2015 Číslo 9- Parazitičtí červi v terapii Crohnovy choroby a dalších zánětlivých autoimunitních onemocnění
- Očkování proti virové hemoragické horečce Ebola experimentální vakcínou rVSVDG-ZEBOV-GP
- Koronavirus hýbe světem: Víte jak se chránit a jak postupovat v případě podezření?
-
Všetky články tohto čísla
- Ross River Virus: Many Vectors and Unusual Hosts Make for an Unpredictable Pathogen
- Distinct but Spatially Overlapping Intestinal Niches for Vancomycin-Resistant and Carbapenem-Resistant
- Intracellular Survival of Depends on Uptake and Degradation of Extracellular Matrix Glycosaminoglycans by Macrophages
- Type IX Secretion Substrates Are Cleaved and Modified by a Sortase-Like Mechanism
- Structural and Functional Characterization of Anti-A33 Antibodies Reveal a Potent Cross-Species Orthopoxviruses Neutralizer
- Suppression of a Natural Killer Cell Response by Simian Immunodeficiency Virus Peptides
- Inhibition of Translation Initiation by Protein 169: A Vaccinia Virus Strategy to Suppress Innate and Adaptive Immunity and Alter Virus Virulence
- Enteropathogenic Uses NleA to Inhibit NLRP3 Inflammasome Activation
- Flavodoxin-Like Proteins Protect from Oxidative Stress and Promote Virulence
- Cullin4 Is Pro-Viral during West Nile Virus Infection of Mosquitoes
- The NLRP3 Inflammasome and IL-1β Accelerate Immunologically Mediated Pathology in Experimental Viral Fulminant Hepatitis
- DYRK2 Negatively Regulates Type I Interferon Induction by Promoting TBK1 Degradation via Ser527 Phosphorylation
- A KSHV microRNA Directly Targets G Protein-Coupled Receptor Kinase 2 to Promote the Migration and Invasion of Endothelial Cells by Inducing CXCR2 and Activating AKT Signaling
- The Operon Essential for Biofilm and Rugose Colony Development in
- ADAP2 Is an Interferon Stimulated Gene That Restricts RNA Virus Entry
- The Role of the Antiviral APOBEC3 Gene Family in Protecting Chimpanzees against Lentiviruses from Monkeys
- The Deacetylase Sirtuin 1 Regulates Human Papillomavirus Replication by Modulating Histone Acetylation and Recruitment of DNA Damage Factors NBS1 and Rad51 to Viral Genomes
- Experimental Malaria in Pregnancy Induces Neurocognitive Injury in Uninfected Offspring via a C5a-C5a Receptor Dependent Pathway
- Intrahepatic Transcriptional Signature Associated with Response to Interferon-α Treatment in the Woodchuck Model of Chronic Hepatitis B
- Adipose Tissue Is a Neglected Viral Reservoir and an Inflammatory Site during Chronic HIV and SIV Infection
- Infection Is Associated with Impaired Hepatic Dimethylarginine Dimethylaminohydrolase Activity and Disruption of Nitric Oxide Synthase Inhibitor/Substrate Homeostasis
- Conserved Motifs within Hepatitis C Virus Envelope (E2) RNA and Protein Independently Inhibit T Cell Activation
- The RelA/SpoT Homolog and Stringent Response Regulate Survival in the Tick Vector and Global Gene Expression during Starvation
- Hybridization in Parasites: Consequences for Adaptive Evolution, Pathogenesis, and Public Health in a Changing World
- KSHV Latency Locus Cooperates with Myc to Drive Lymphoma in Mice
- Immunostimulatory Defective Viral Genomes from Respiratory Syncytial Virus Promote a Strong Innate Antiviral Response during Infection in Mice and Humans
- Retraction: Extreme Resistance as a Host Counter-counter Defense against Viral Suppression of RNA Silencing
- Appetite for a Foodborne Infection
- Here I Am, Despite Myself
- Microbial Regulation of p53 Tumor Suppressor
- Fiat Luc: Bioluminescence Imaging Reveals In Vivo Viral Replication Dynamics
- Knocking on Closed Doors: Host Interferons Dynamically Regulate Blood-Brain Barrier Function during Viral Infections of the Central Nervous System
- Rapid Lymphatic Dissemination of Encapsulated Group A Streptococci Lymphatic Vessel Endothelial Receptor-1 Interaction
- Simian Immunodeficiency Virus Infection of Chimpanzees () Shares Features of Both Pathogenic and Non-pathogenic Lentiviral Infections
- Epicellular Apicomplexans: Parasites “On the Way In”
- The Depsipeptide Romidepsin Reverses HIV-1 Latency
- Skin-Derived C-Terminal Filaggrin-2 Fragments Are -Directed Antimicrobials Targeting Bacterial Replication
- Type IV Pili Composed of Sequence Invariable Pilins Are Masked by Multisite Glycosylation
- Heterosexual Transmission of Subtype C HIV-1 Selects Consensus-Like Variants without Increased Replicative Capacity or Interferon-α Resistance
- Prevention of Influenza Virus-Induced Immunopathology by TGF-β Produced during Allergic Asthma
- Global Analysis of Mouse Polyomavirus Infection Reveals Dynamic Regulation of Viral and Host Gene Expression and Promiscuous Viral RNA Editing
- Modulation of the Host Lipid Landscape to Promote RNA Virus Replication: The Picornavirus Encephalomyocarditis Virus Converges on the Pathway Used by Hepatitis C Virus
- Intrinsic MyD88-Akt1-mTOR Signaling Coordinates Disparate Tc17 and Tc1 Responses during Vaccine Immunity against Fungal Pneumonia
- PLOS Pathogens
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Epicellular Apicomplexans: Parasites “On the Way In”
- Fiat Luc: Bioluminescence Imaging Reveals In Vivo Viral Replication Dynamics
- Knocking on Closed Doors: Host Interferons Dynamically Regulate Blood-Brain Barrier Function during Viral Infections of the Central Nervous System
- A KSHV microRNA Directly Targets G Protein-Coupled Receptor Kinase 2 to Promote the Migration and Invasion of Endothelial Cells by Inducing CXCR2 and Activating AKT Signaling
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



