Detection of unstable carotid plaque in ischemic stroke prevention
Authors:
P. Kešnerová 1,2; D. Viszlayová 3,4,5; D. Školoudík 2,6
Authors‘ workplace:
Neurologická klinika 2. LF UK a FN Motol, Praha
1; Neurologická klinika 1. LF UK a VFN v Praze
2; Neurologická klinika FN a FSVaZ UKF, Nitra
3; Neurologická klinika LF UP, Olomouc
4; Neurologická klinika LF UK, Hradec Králové
5; Centrum vědy a výzkumu, Fakulta zdravotnických věd, UP Olomouc
6
Published in:
Cesk Slov Neurol N 2018; 81(4): 378-391
Category:
Minimonography
doi:
https://doi.org/10.14735/amcsnn2018378
Overview
Carotid stenosis is well-known risk factor for ischaemic stroke or transient ischaemic attack. Plaque features, such as intraplaque haemorrhage, neovascularization, large lipid core, thin or ruptured fibrous cap or inflammation seems to play important role in plaque instability and are related to higher risk of stroke. At present, no single modality is able to measure accurately the degree of stenosis and to detect all vulnerable plaque features simultaneously. For detailed plaque morphology, only the combination of methods mentioned below is providing the accurate assessment.
Computed tomography (CT) is widely available and often first imaging modality used, with strength to detect plaque calcifications, ulceration and degree of stenosis but differentiation between lipids pools and intraplaque haemorrhage is low. Magnetic resonance imaging (MRI) can identify most of plaque unstable features but time constraints, movement artefacts and absence of unified procedure are complications for wider use. Positron emission tomography (PET) is en effective tool to detect metabolic processes in the plaque mainly the inflammation but spatial resolution is imprecise. Ultrasound (US) is widely available, low-cost tool for monitoring plaque development especially if 3D mode applied but for plaque characteristics differentiation is suboptimal. Future development of US methods, i.e. computerized analysis of Gray scale and contrast enhancement improves the US potential significantly.
Key words:
atherosclerosis – unstable plaque – carotid artery – stroke – ultrasound
The author declares he has no potential conflicts of interest concerning drugs, products, or services used in the study.
The Editorial Board declares that the manuscript met the ICMJE “uniform requirements” for biomedical papers.
Introduction
Carotid atherosclerotic disease is well-known and important cause of stroke and represents one of the main goals in stroke prevention. Atheromatous mass in carotid arteries contributes by different mechanisms at least to 10–30% of ischaemic strokes and transient ischaemic attacks (TIAs). At present, besides clinical symptoms, the severity of carotid stenosis is the leading parameter affecting the patient’s treatment including the decision on surgical procedures. This paradigm that has been used for decades has been questioned many times during last years, and there is growing evidence that composition and character of carotid plaque plays key role in its stability and ipsilateral stroke risk based on the presence of the plaque.
For the reasons mentioned above, over the past few decades we targeted carotid diagnostic efforts on methods evaluating the diameter of the stenosis with highest accuracy. Success at stenosis quantification clarified that the severity of vessel narrowing is not the only stroke risk factor and that there is not a linear relationship between the carotid stenosis percentage and the risk of an ipsilateral stroke, especially in asymptomatic carotid stenosis [1]. Current guidelines, based on large studies, recommend interventional treatment in severe symptomatic stenosis [2]. However, clinical practice and research based on many small studies shows that stroke occurs in stenosis moderate and even low-grade [3], and the amount of stroke-free patients with asymptomatic high-grade stenosis during long term follow-up is considerable. Important role plays pathophysiology of stenosis, development of collaterals and also, best medical treatment (BMT) [4]. These facts advocate the need of re-considering diagnostic criteria for carotid plaques with a high risk of stroke [5], 6].
This need has led to much detailed exploration of the plaque surface and volume in order to measure plaque integrally (which improves the quantification of the plaque as a whole and not only by stenosis definition that refers simply to the narrowest part of the lumen), and immense effort has been focused on plaque composition analysis (revealing plaque characteristics which determine vulnerability or instability of the plaque and incorporate qualitative parameters of the plaque).
Definition of “vulnerable” or “unstable” plaque has been subject to many research activities and a topic of many discussions. According to the standard in this research field, the histological findings from carotid endarterectomy (CEA) are analysed and subsequently compared with the collection of previously performed in-vivo imaging modalities. Despite undoubted success in defining some risky features of the plaque, we have to be aware that important data are missing as the specimens obtained are mainly from symptomatic patients with moderate to severe symptomatic stenosis (with quite variable interval between surgical plaque removal and ischaemic event that can influence result of histopathology findings significantly) [7] or severe asymptomatic stenosis.
Nevertheless, several morphological characteristics that can identify high-risk carotid plaques have been proposed and verified. These include especially ulceration on the surface of the plaque, lipid rich necrotic core (LRNC), thin or ruptured fibrous cap (FC), intraplaque haemorrhage (IPH), plaque neovascularization and inflammation [8–11].
In the development and progression of atherosclerotic disease, the severity of the stenosis, tandem stenosis, progression of total plaque area (TPA) or total plaque volume (TPV) resp. have been proposed as significant plaque modulation characteristics [12–15].
In assessing the overall risk potential of the plaque, we should also evaluate emboligenic potential of the plaque by detecting microembolic signals (MES) on transcranial Doppler monitoring (TCD) [16,17] as well as examine brain tissue for signs of cerebral embolism [18,19].
Advanced diagnostic tools that would enable to detect the features of an unstable plaque would also help to specify the etiology of ischaemic events. This is especially relevant for cryptogenic strokes incidence of which is estimated to 30% of all ischaemic strokes. There is a relevant presumption that part of these events will be attributed to non-stenosing carotid disease with the presence of unstable plaque. Pilot studies show this could be up to one third of all ischaemic events without established etiology but large multicentric trials are needed to verify this hypothesis [20,21].
Detection of plaque vulnerable characteristics, plaque changes in time as well as emboligenic potential of the plaque are the target of diagnostic efforts to discover potentially unstable plaque stage that optimally should result in changes in therapy with the aim to stabilise or to eliminate such a plaque and therefore to reduce stroke risk. The methods, available in clinical practice nowadays, have different potential to reveal these plaque features. At this article we provide overview of those routinely available: CT, MRI, US and its different modalities – two-dimensional (2D) and three-dimensional (3D) US, contrast-enhanced ultrasound (CEUS) and TCD, and PET with specified diagnostic advantages and limitations of each of them in the detection of vulnerable plaque characteristics and in monitoring the plaque dynamics. Marginally we mention some of the experimental methods such as intravascular ultrasound (IVUS), optical coherence tomography (OCT), micro-optical coherence tomography(μOCT) and near infrared spectroscopy (NIRS) to provide comprehensive overview of the imaging methods of atherosclerotic carotid disease.
Finally, the therapeutics guidelines are mentioned with suggestion of how unstable plaque detection could change the decision-making process in clinical practice. Detailed analysis of carotid plaques could help to optimize the therapeutic approach of both directions: to refine patient selection suitable for carotid revascularization as well as to identify those who would benefit more from a conservative approach [6].
In this review, we summarize current state-of-the-art imaging modalities for carotid atherosclerotic disease with emphasize to those methods that can be translated to clinical practice. Special attention is paid to those that can be used as a monitoring tool on regular basis as one of the most important aspect of the carotid plaque evaluating is its change in time. After all those years of enormous research interest the truth is that we know little about causes of initial plaque formation, neither the prediction of plaque development, and its transformation from stable to unstable one, is satisfactorily explained. This knowledge still remains quite a challenge for future research activities.
Computed tomography
Technical design
For plaque detection, there are two dominant CTA techniques available: conventional single source energy CT (also called multidetector-row CT; MDCT) and dual source energy CT (DSCT). Common for both techniques is the necessity of administration of contrast agent for proper plaque visualization and the usage of x-ray attenuation that is quantified in Hounsfield units (HU) and is represented on the final images in shades of gray. The difference is in the energy and number of used sources and in the post-procedural imaging.
On the conventional CT, the single x-ray energy provides representation of the objects based on the linear attenuation coefficient of each of the anatomical structures and is independent of the material density and the mass-attenuation coefficient. This brings limits to material specification.
On DSCT, we achieve two different HUs from the same anatomic structure as two different x-ray energies are applied to allow the analysis of energy-dependent changes in the attenuation of different material. Material specification is therefore much detailed as the attenuation difference of high and low energy reveals more nuances in tissue characteristics. Typical example of this is differentiation between residua of iodine contrast and calcium that may not be easily recognizable on conventional CT as they demonstrate similar attenuation. On DSCT, we obtain attenuation difference as the attenuation of iodine increases more markedly than that of calcium. This principle enables the specification of the displayed features. Another great advantage of DSCT mode is the bone subtraction providing better vasculature visualization [22].
Both CT modalities allow multiplanar reconstruction in all three planes, high spatial resolution and accurate identification of a plaque especially after the contrast bolus enhancement [23].
Third technique that is using x-ray in plaque imaging is digital subtraction angiography (DSA). However, despite being often used in cerebrovascular imaging, plaque visualization is limited to surface irregularities and quantification of stenosis degree but at this point, CTA also provides this information and is more suitable for screening due to its lower invasivity [24]. DSA does not demonstrate plaque morphology in more detail, it is limited to 2D imaging and in detecting vulnerable plaque features it is the marginal method, also for its time and x-ray intensity.
Unstable plaque detection
CTA has been a validated tool for assessment of the degree of carotid stenosis, with ability to image all the vessels from the aortic arch to the intracranial vasculature, including tortuosity and other anatomical abnormities. In the plaque features imaging, CT is increasingly being explored especially due its wide availability in a routine practice. Hounsfield density is used to distinguish between tissues.
CT is considered to be a very sensitive method to identify calcification [25], which is detectable as a high-density signal within the plaque. The higher amount of calcium contained, the more stable is a plaque believed to be. MDCT can reliably detect calcification, with average 250 HU,
in differentiation from iodine contrast DSCT is helpful as mentioned above [22].
Very good results are seen in the detection of plaque surface ulcers with as high resolutions as 1mm [26], and CT correlates well with DSA. MDCT detects ulcers with moderate to good sensitivity (60–94%) and specificity (70–99%), also in comparison with histology [27].
Fissured FC is believed to be associated with the vulnerable character of the plaque and the contrast enhancement is observed in plaques with fibrous surface discontinuity. In Saba et al study an association between fissured FC and contrast plaque enhancement was confirmed (κ = 0.855) [25, 28].
With further plaque characteristics, IPH, neovascularization, LRNC and fibrous tissues are detectable as the structures with low HU but significant overlap between these hypodense structures is observed which limits the use of this method in plaque analysis. However, CTA showed good correlation with histologic examination for large lipid cores (κ = 0.796) and large hemorrhages (κ = 0.72) [25] with high interobserver agreement. In general, lower density corresponds to less stable plaques and dynamic assessment of contrast seems to be very valuable technique to evaluate plaque stability on MDCTA. An increase of density in HU from the early to the delayed phase indicates plaque stability with more fibrous tissue and less LRNC, IPH, and neovascularization [23], probably due to a faster washout from the unstable plaque.
Limitations of the method
Two of the main disadvantages are generally present to CT examination: 1. the need to administer an intravenous contrast agent with iodine, potential allergic reaction and nephrotoxicity; 2. radiation exposure. In plaque analysis, limits derive from artefacts based on calcification presence and insufficient resolution to hypodense structures, mainly smaller IPH, LRNC and fibrous tissue [25]. This favours CT as a very good tool for acute diagnostic method with currently existing limitations to vulnerable plaque evaluation. Despite some studies showing plaque composition and changes in time can be monitored using MDCT [29], for reasons mentioned above we don’t consider CT as an optimal routine monitoring tool for plaque dynamics.
Future goals
DSCT with bone removal algorithm seems to be quite promising in soft tissue analysis. Incorporating contrast enhancements phases into the protocol is a small step to be made in CT examination process but would bring valuable information in plaque characterization. MDCT 3D geometry assessment may contribute information about various hemodynamic factors, such as shear stress which seems to have important role in plaque dynamics but little is known about pathophysiological mechanism [27].
Magnetic resonance imaging
Technical design
MRI is often considered as a gold standard for plaque analysis and its role is well established. Good sensitivity and specificity of the method for the assessment of plaque morphology, composition and volume is well known, and in detection of plaque vulnerable characteristics, multiple pulse sequences are recommended. The most commonly used are the T1-weighted and T2-weighted fast spin-echo sequences and proton density weighted (PDW) imaging and these techniques are excellent for high spatial resolution. Double inversion recovery or also called “black blood imaging” provides a high contrast between vessel wall and the lumen. We achieve high signal-to-noise ratio in all these modalities but prolonged scanning times are the cost and motion artefacts occur [26].
MR image can be manipulated by changing parameters and in order to shorten the scanning time, and different techniques are applied.
For all sequences, fat suppression technique is used to maximally eliminate signals from subcutaneous fat and also to facilitate the distinction between LRNC and IPH [30].
MRI contrast enhanced (CE) imaging helps to identify some of the plaque components precisely and most commonly gadolinium (Gd) chelates are administered. On T1-weighted images, differentiation between vascular areas with enhancement, i.e. fibrous tissue, from those avascular ones, such as LRNC, IPH and thrombus, is clearly visible but the mechanisms underlying Gd enhancement of carotid plaques are complex.
Gd is known to distribute to the extracellular extravascular space. Thus, the increased enhancement may be due to the increased perfusion and permeability, increased extracellular volume, and/or decreased washout, and in carotid plaques is indirectly attributed to the pathophysiological processes such as neovascularization or inflammation and to vessel wall composition and organization [31].
Unstable plaque detection
Great potential of MRI to identify vulnerable plaque components was confirmed in many studies. High soft tissue contrast, reproducibility and high interobserver agreement ensured this non-invasive diagnostic tool significant position in plaque composition analysis.
One of the most investigated plaque components is undoubtedly IPH. It can be detected with sensitivity of 82–92% and specificity of 74–100% resp. [32, 33]. Combination of T1, T2-weighted and PDW images helps determine the age of the thrombus. Fresh IPH is hyperintense on T1 and hypointense/isointense on T2 and PDW sequences. Recent IPH (1–6 weeks) is hyperintense on all contrast weightings (Fig. 1, 2). Old IPH (≤6 weeks) is hypointense on all contrast weightings. IPH was also found to be in strong correlation with ischaemic symptoms regardless of the degree of stenosis [3] with hazard ratio 4.59 (95% CI 2.91–7.24) [15] to 9.8 (95% CI 1.3–75.1; p = 0.03) [34].
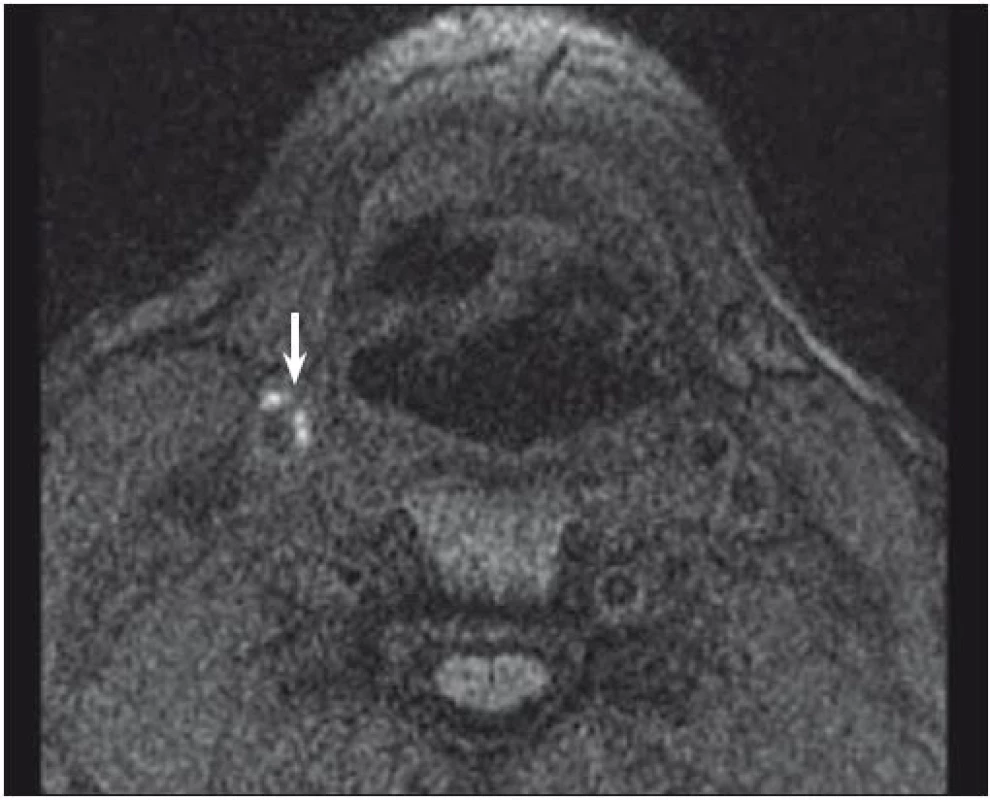
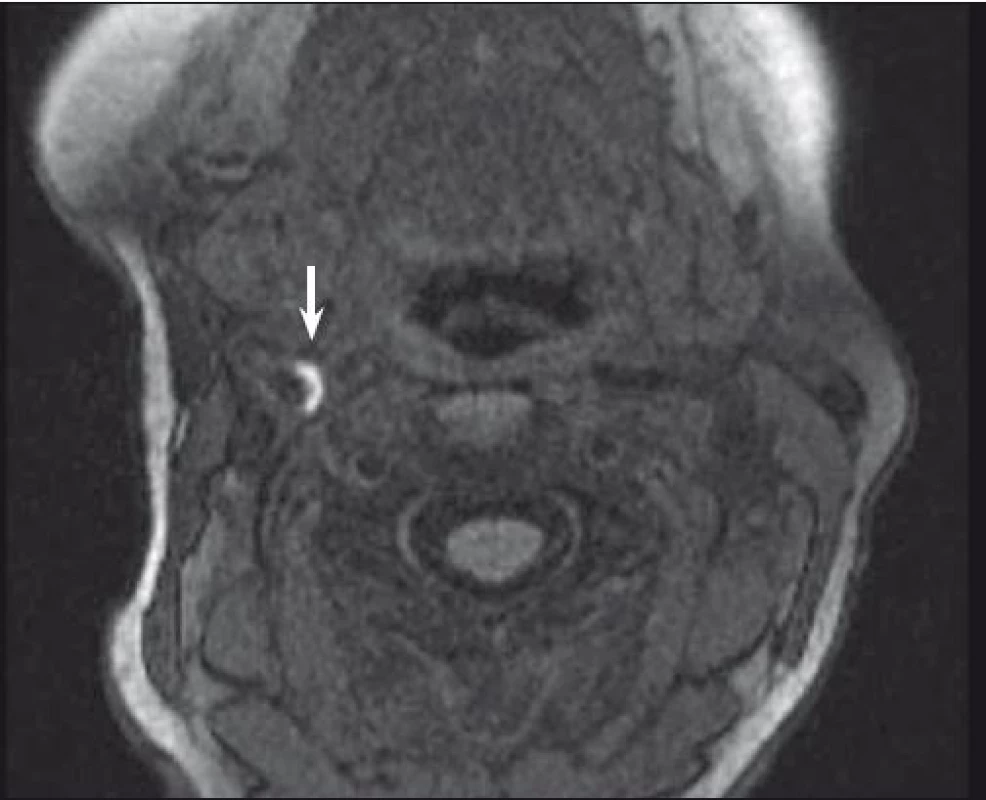
IPH differentiation from LRNC is also the dominance of this method. LRNC itself can be detected on MRI with a sensitivity of 82–100% and specificity of 40–100% [27,33]. As IPH is often dispersed within the lipid wards, sequence combination again is needed to differentiate these two pathologies – while IPH is hyperintense on T1 and time-of-flight (TOF – which is based on the principle of flow-related enhancement and stationary tissues in an imaged volume becoming magnetically saturated by multiple repetitive pulses), LRNC is hyperintense only on T1 and isointense on TOF images [35]. Not only detecting LRNC within the plaque but also its quantification seems to have clinical impact as the increasing volume of the lipid areas may govern the risk of the future surface disruption as found in the moderate carotid stenosis [36]. Strong correlation of IPH and LRNC with histopathology findings was proved [33,35].
Neovascularization and inflammation are two entities associated with plaque vulnerability and detectable on the T1 postcontrast enhancement with prevalence of neovascularization in 97% and of inflammation in 87% [31]. Chan et al aimed to assess the degree of inflammatory process in plaques by using antibody-conjugated superparamagnetic iron oxide (SPIO) particles as an MRI probe and symptomatic plaques were distinguished from asymptomatic ones by the degree of inflammation. In this small study, MR contrast effect was significantly correlated with the degree of the plaque inflammation (r = 0.64, p < 0.001) [37]. Dynamic contrast enhanced MRI was used to quantify plaque enhancement and thus neovascularity and inflammation, and strong correlation was proved [38].
MRI is a very good tool also in detecting plaque ulcerations in MRA mode. On all contrast weightings (T1, T2, PDW, TOF and CE T1 sequences) ulcers appear as a surface disruption and adding black-blood AG helps to reach the sensitivity and specificity of MRI to 80% and 70% resp. [36].
FC can be identified as a hypointense linear structure at T1, T2 and PDW sequences and its absence indicates either thinning or rupture of the fibrin plaque cover [32].
Calcifications appears hypointense on all contrast weighted images and can be detected by MRI with high sensitivity and specificity [32,35].
MRI plaque imaging has proved capability to identify high-risk plaques in symptomatic as well as asymptomatic patients independently of the degree of stenosis [15] and may improve stratification of patients with increased risk of future cerebrovascular ipsilateral ischaemic event [39].
Undeniable advantage of MRI is also the fact that the brain tissue lesions can be evaluated together with the plaque examination. Plaque instability, namely IPH, was found to be associated with white matter lesions in symptomatic carotid stenosis [40] and the impact of an unstable plaque on the brain tissue can be rated when a brain scan is performed. Plaque MRI categorization in asymptomatic carotid stenosis was suggested and high-risk lesion types were identified: only plaques with unstable features (presence of LRNC, fibrous tissue with possible calcification, surface defect, haemorrhage or thrombus) showed a significant association with future ischaemic cerebral events [39]. Such stratification may improve selection criteria for invasive therapy in the future.
Limitations of the method
Important disadvantages of MRI include long procedure time with high susceptibility to motion artefacts, high cost of the examination and relatively low availability which is even more striking in plaque analysis. Current clinical practise does not provide specified protocols on MRI plaque scanning and these are usually available only in specialized centres. Automated tools to describe plaque compositions are also unavailable and due to manual processing plaque composition description is a subject to high inter - and intraobserver variability. The process of MRI plaque scanning is time consuming and it is not a routine matter for radiologists which creates limitation to MRI implementation as a routine risk stratification tool [27].
Also, commonly available MRI scanners are of intensity 1,5T and the distinction between plaque components is rather challenging. Application of stronger magnetic field seems to be of better diagnostic accuracy and is under investigation [32,41].
Future goals
As mentioned above, one of undisputable advantages of MR imaging is its ability to scan multiple structures in single time examination. Therefore, combination of brain, plaque and lumen imaging might be considered for future complex neuroimaging scanning.
Recently a simultaneous non-contrast angiography and intraplaque haemorrhage (SNAP) MRI technique was proposed to detect both luminal stenosis and IPH in a single scan [42].
Improvement in resolution by using 3T and 7T scanners [41,43,44] and development of automated software to overcome human error in the evaluation of plaque components is another target of many research activities [45]. New biomarkers that target specific molecules detectable in plaque transformation, such as remodelling or inflammation, are of scientific interest. Functional molecular MRI probes are proposed, and consists usually of dual antibody-conjugated SPIO or other contrast agent which enhance specific molecules or cells [46], for example fibrin, elastin or inflammatory markers such as VCAM-1 or E-selectin. Thus, it is possible to detect unstable plaque features on molecular level with high spatial resolution and also to quantify the degree of inflammatory process [37]. These novel imaging methods for atherosclerosis are currently reserved to ex vivo imaging and further development for translation to clinical practice is needed.
Positron emisson tomography
Technical design
PET is the main molecular imaging technique as single emission computed tomography (SPECT) has much lower spatial resolution and is not that much relevant for plaque imaging. Principle of PET is in detection of gamma radiation emitted from a radiopharmaceutical injected intravenously and dispersed in metabolically active areas. Insufficient spatial resolution of PET ranges between 3 and 5 mm and is being corrected by using hybrid scanners with CT or more recently with MRI [47]. The most explored radionuclide tracer in atherosclerotic disease is 18-F-fluorodeoxyglucose (18FDG, FDG), which is partially metabolized through glycolysis and serves as a marker of inflammation or hypoxia. Following the FDG administration, image acquisition is performed after 60.–90. min. Tracer dispersion in the blood volume may result in a poor contrast resolution for such a small structure as carotid plaque, more remarkably in an early imaging, and optimal timing for plaque visualization is subject of many debates. In a smaller study, Blomberg et al. found that delayed 18FDG PET/CT imaging at 180. min improves quantitation of atherosclerotic plaque inflammation over standardly advocated scans at 90. min [48]. Reproducibility of the method was scored very high with favourable inter - and intraobserver agreement [49].
Unstable plaque detection
The main advantage of PET over other existing imaging techniques is the potential to target inflammation directly by FDG. Several studies reported significant correlation between the FDG concentration and the histopathological findings of macrophage infiltration (high standardized uptake value; SUV plaques) [50,51]. Inflammation-related FDG uptake was also found to be associated with the risk of ischaemic events, independent of the degree of stenosis [50,52].
For other plaque vulnerable characteristics PET itself lacks the anatomic precision and only the combination with CT or MRI adds the morphological value. Nevertheless, weak correlation with CT and MRI findings in LRNC, calcification or fibrous tissue, was found [53], neither was strongly correlating the FDG uptake and plaque neovascularization [29].
However, potential of PET is worth mentioning, since metabolism and plaque pathophysiological mechanism can be explored in vivo and this method is well established and clinically available.
Limitations of the method
As mentioned above, poor spatial resolution, inability to visualize neovascularization, LRNC, IPH or fibrin defects are significant limits [26]. The examination is expensive, availability of PET scanners is low and the burden of radioactivity is considerable. Contraindications and limits of CT or MRI have to be counted as well. Patient preparation and diet may influence FDG uptake. To stage atherosclerosis, serial imaging is required. Exposure to radiation, especially if PET performed simultaneously with CT, currently precludes the feasibility of frequent serial imaging. Hybrid PET/MRI and PET/CT imaging also require accurate image co-registration between molecular and morphological modality, especially when evaluating small structures such as carotid plaques. Patient motion between image acquisitions can result in misalignment that can bias attenuation correction, precluding accurate co-localization, and image interpretation [47].
Future goals
Combination of PET with MRI seems to have a potential in identifying of some worrisome plaque morphological features and in detection of vessel wall [27]. Algorithms for lowering radiation exposure to allow serial imaging are being developed.
New tracers are probably the most studied field. Numerous of those are probed in processes of thrombosis, neoangiogenesis, lipid accumulation, microcalcifications and others. 18F-sodium fluoride (18F-NaF) was found to have a potential as a tool to detect ruptured and high-risk coronary plaque and also to target microcalcifications. Small deposits of calcium within the plaque signalize plaque progression and the degree a plaque is prone to rupture [54]. Studies for carotid arteries are needed [55]. In non-FDG imaging, the vulnerable rupture-prone plaque is studied in the relation to imaging of matrix metalloproteinases (MMP) [56]. Another novel biomarker of plaque vulnerability could be[18F]-galacto-RGD PET/CT imaging of αvβ3 molecule as this integrin is expressed by macrophages and angiogenic endothelial cells in atherosclerotic lesions and thus is a marker of plaque inflammation and neoangiogenesis [57]. PET/CT biomarkers offer the chance to stage atherosclerotic lesions and asses different aspects of a plaque progression, but at present, these tracers are still in very early development and wider application is a matter of future research [47].
Duplex sonography
Technical design
Last, but not least, US is probably the clinically most used imaging technique in the plaque diagnostic and the method of choice for regular screening and initial evaluation of the atherosclerotic carotid disease. Wide availability, low cost and perfect tolerance by patient with no major side effects are undisputable advantages of this long established modality [58]. It is the only method that provides real time imaging with the assessment of hemodynamic parameters.
The transducer probe generates and receives sound waves using a piezoelectric effect, frequencies range typically from 4–14 MHz and the linear array probe is the most suitable for carotid imaging. Images are obtained in the longitudinal and axial section and there are few imaging techniques available. Overview and description of each technique is provided in relation to vulnerable plaque imaging.
Unstable plaque detection
B-mode
B-mode (brightness mode or two dimensional) can visualize a number of anatomical features of the plaque and of the arterial wall. B-mode images are primarily used for assessing the echogenicity of tissues and conventionally, the echogenicity of sternocleidomastoid muscle is used as the reference value. Inter - and intraobserver agreement is one the main limitations of the method and post-processing techniques are applied to correct for the human error [59]. The aim is to differentiate echolucent plaques (associated with lipid and haemorrhagic content) from echogenic (corresponding to the content of fibrin and calcium), believed to be the stable structures. Gray scale median (GSM) is a computerised measurement of the plaque echogenicity and correlates inversely with the risk factors for cardiovascular (CV) disease. There is not a clear consensus on cut-off values for vulnerable plaque definition due to the different software applications for GSM but low numbers correspond to echolucent plaques [60]. Low echogenicity is associated with an increased risk of ischaemic events in symptomatic [60] and in asymptomatic patients with carotid stenosis and low GSM is a strong predictor of future strokes [61]. However, as obvious from the definition, GSM provides the average value on plaque echogenicity and lacks the ability to evaluate critical plaque histological components and their distribution [62].
Pixel distribution analysis (PDA) exceeds this problem by attributing characteristic echogenicity to the key components of atherosclerotic plaques and maps the architectural features of the plaque burden (LRNC, IPH, calcium and fibromuscular tissue) not only by location but also by size [63]. This noninvasive evaluation of the plaque may have important clinical impact as the large juxtaluminal black area (JBA), containing lipids, thrombus or necrotic mass, is linearly related to the risk of stroke [64] and these unstable parts may be vulnerable to endovascular catheter [62]. This mechanism could predispose to atheroembolization during carotid stenting (CAS) and there are studies where the incidence of embolization after CAS is higher compared to CEA [65]. Therefore, plaque characterization can be used in the risk stratification models, including management of invasive procedures [64].
In 2D mode, the FC appears as a linear echogenic structure on the plaque surface. Measurement of FC thickness is a standard part of ultrasonic examination in order to detect thinning or defects in FC since both of these are included among the risk factors for the stroke. Integrated ultrasonic backscatter (IBS) was suggested as an effective tool in detection of FC thinning as IBS was lower in the thin fibrous cap than in the thick lesion (–10.9 ± 6.4 vs. –2.4 ± –6.2 dB; p < 0.001) and a correlation with histology findings was found. IBS may help to characterize atherosclerotic lesions in the carotid artery as thin FC is frequently associated with unstable plaque [66].
Ulcers are defined as the irregularities of the plaque surface and the conventional criteria stipulate a concavity larger than 2 × 2 mm with a well defined back wall and flow reversal within the recess. Muraki et al suggested new more sensitive criteria with ulcer specification as a concavity in the plaque with the basal border echo weaker than that of the adjacent plaque surface, regardless of the size. These suggested parameters were compared to surgical and histologic findings. The sensitivity and specificity of the conventional criteria were 35.7% and 75.0%, resp., and those of new criteria were 85.7% and 81.3%, resp. [67]. Irregularity of plaque surface was evaluated on US B-mode in the association to the risk of ischaemic stroke. Presence of irregular plaque (vs. no plaque) was independently associated with ischaemic stroke (hazard ratio 3.1; 95% CI 1.1–8.5) [68].
Calcifications are the most echogenic structures within the plaque and good correlation was proved by GSM and PDA detection [60,63] and with histology findings. Problems may occur in heavily calcified plaques when acoustic shadowing may limit the evaluation plaque characteristics.
IPH is detectable within the plaque burden as an echolucent structure (Fig. 3, 4) and its origin is traced from neovessel leakage. If plaque fissuring occurs, blood clot may form a thrombus detectable on a plaque surface, which appears also echolucent in 2D mode. The accuracy of detection of the IPH or thrombus on B-mode imaging has the sensitivity of 80–90% and the specificity of 80–91% [27], and the presence of those two features is correlated to plaque vulnerability [69].
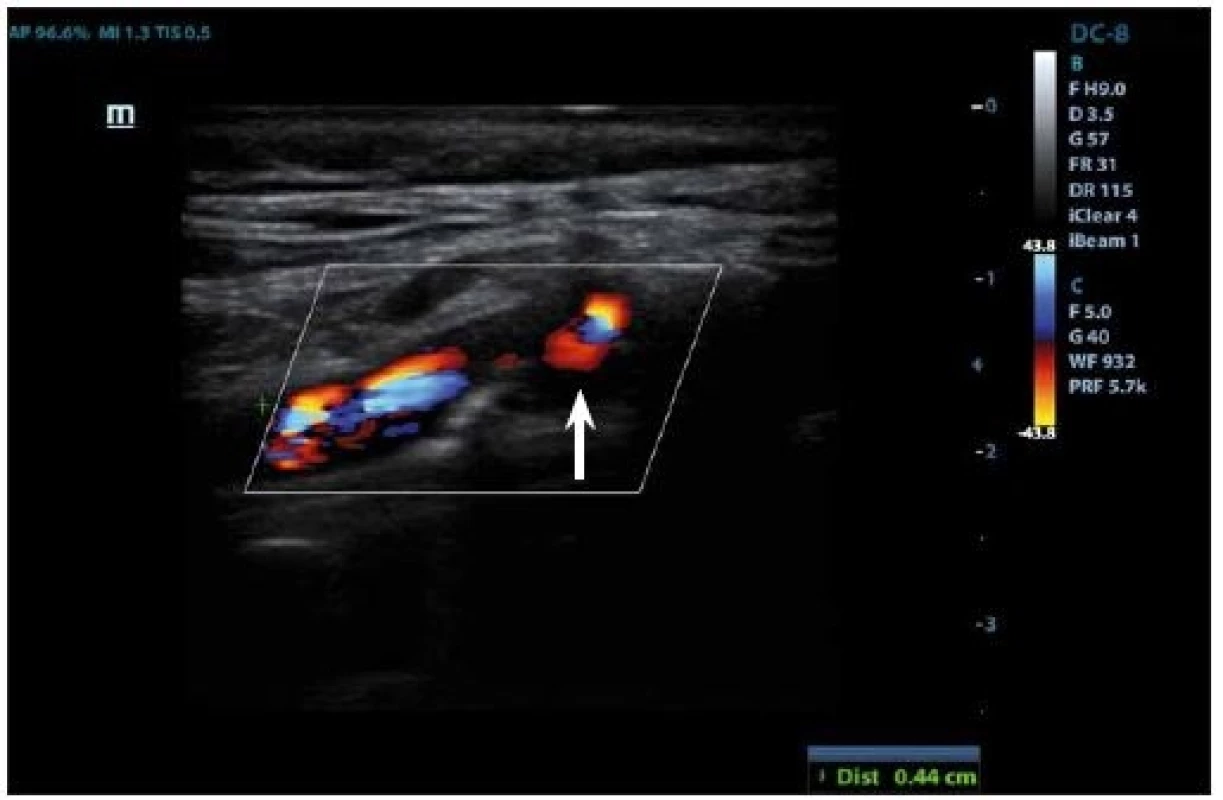
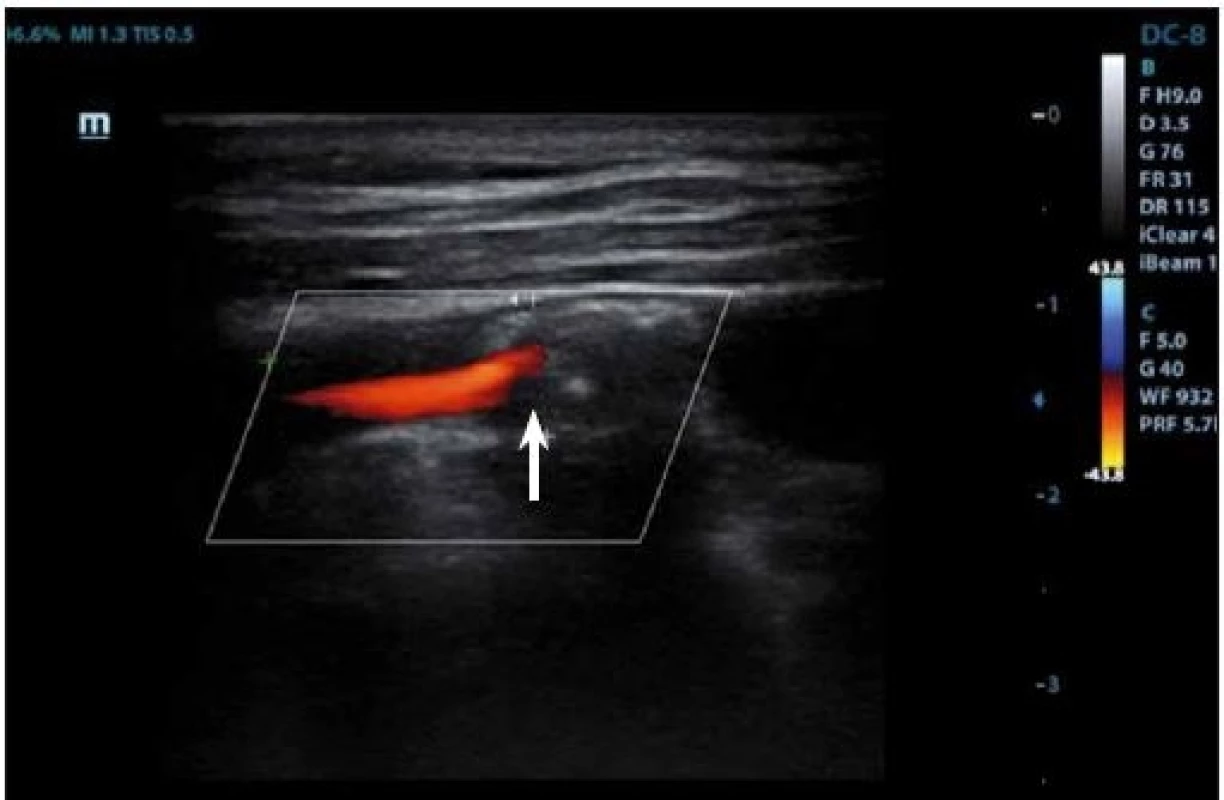
Neovascularization is another blood-filled element that indicates plaque instability and can be found at the adventitial side of the arterial wall. Its origin is due to formation of new microvasculature in the plaque and is the sign of plaque growth and development. Neovascularization on B-mode appears also as an echolucent area and in superposition of color-coded Doppler imaging or power mode, blood flow may be detected. However, dominant method to reveal newly formed vasculature is contrasted enhancement and will be described below. Inflammation of the plaque will be mentioned in the chapter about CEUS for the same reason, as this feature shows no specific signs on 2D US mode.
LRNC is another echolucent structure and looks similar to IPH. Distinguishing those two histopathological features on B-mode imaging seems to be quite a challenge and is not the dominance of this method. Nevertheless, lipid contained within the plaques are well known risk factors [70] of ischaemic events as well as IPH [69] and are important parts of the echolucent plaque areas.
JBA were evaluated in their size and relevance to the future risk of stroke in a population with asymptomatic carotid stenosis and strong correlation was found [64]. The size of lipid core seems critical for the stability of the plaque [70] and atheroma vulnerability to rupture is increased in the presence of a large lipid core as found in MRI studies [11]. For the evaluation of plaque on unenhanced ultrasonography we can conclude that detection of echolucency of the plaque provides negative predictive information beyond luminal stenosis [71]. The adjusted relative risk for cerebrovascular events in subjects with echolucent plaques is 4.6 (95% CI 1.1–18.9), with significant linear progressive trend for higher risk with increasing plaque echolucency [72].
Contrast enhanced ultrasound
CEUS uses an intravenous microbubble contrast agent, which is dispersed strictly intravasculary. This allows assessing a vessel lumen and neovascularization within the plaque accurately. Specific pulse sequences are needed to supress the signal from tissue within the image and accentuate the signal from the intravascular bubbles [26]. CEUS identifies important luminal plaque characteristics and, uniquely, the presence of an intraplaque neovascularization (IPN). IPN has been associated with an increased inflammation, IPH and matrix degradation, all of which are associated with an increased plaque vulnerability [73].
Contrast microbubbles detected within the plaque indicate neovascularization and quantitative methods to measure contrast enhancement are established. In the study evaluating vulnerable plaque by CEUS in correlation with histology, symptoms and CT, decibel enhancement (dB-E) was used. A higher dB-E was significantly associated with thinner FC (< 200 μm; p = 0.01) and greater inflammatory infiltrate (p = 0.03). Preoperative ipsilateral embolic lesions at CT were correlated with higher dB-E (p = 0.01) [74]. Several biomarkers reflecting inflammatory or proteolytic activity have been known to represent plaque vulnerability. In some small studies, significant association of MMP-9 (p = 0.021; 95% CI 1.002–1.027) was shown with IPN detected by CEUS [75]. Serum C-reactive protein levels were positively correlated with the degree of carotid plaque enhancement corresponding to IPN (Rs = 0.69; p < 0.01) [76]. Thus, combination of biomarkers screening and intraplaque neovascularization detected by the CEUS may allow more accurate evaluation of the plaque stability [76].
Delayed imaging is used to detect plaque inflammation and surface disruption. Macrophages are known to phagocytose the contrast agent and the retention of microbubbles on plaque surface can be visualized [77]. This observation has been described as a late phase enhancement and can be identified objectively by imaging plaque 6 min after the contrast administration [78].
Ulceration are easily detectable as CEUS provides good delineation between plaque and lumen border and in comparison with 2D mode and color Doppler US, CEUS has the higher sensitivity, specificity and intra - and interobserver agreement [79]. Because of this higher sensitivity to luminal surface changes, CEUS is capable of detecting more subclinical carotid atherosclerosis than conventional ultrasound and changes the risk category as estimated by a traditional risk stratification model [80]. High reliability of CEUS is proved in distinguishing between the severe carotid stenosis and the occlusion in correlation with other methods [81].
Three-dimensional ultrasound imaging
3D US imaging uses a special 3D probe and combines series of 2D US cross-sectional slices, collated in computer and reconstructed in 3D volume. The operator’s hand and patient’s head is held steady to prevent movement artefacts. Images are acquired through the entire longitudinal and axial plane and converted into 3D reconstructions [62]. TPV is calculated.
3D US seems to be a promising technique to reliably measure TPV and current evidence supports the good reproducibility of the 3D US for the evaluation of carotid plaque volume [82]. This method is safe, eligible for serial testing and 3D technique reduces the operator variability, which is one of the main limits of 2D US mode. Manual segmentation may be time consuming, therefore semi-automated algorithms are being developed to shorten the time of examination [62].
Plaques grow along the artery 2.4 times faster than they thicken [83] and progress and regress circumferentially. As the changes of the plaque occur in all three dimensions, evaluating plaques in 2D imaging methods does not seem sufficient anymore. Another advantage of 3D imaging is an improved detection of the plaque surface. 3D analysis provides detection of TPA as well as detailed imaging of plaque ulcers. These were defined as a continuous and distinct depression into the plaque with a volume of 1 mm³ or more. Ulcers with volume more then 5 mm³ were associated with higher rate of ischaemic cerebral and CV events [84].
For assessment of the response to an antiatherosclerotic therapy, different predictors of CV outcomes were compared.
Progression/regression of intima-medial thickness (IMT), TPA, and TPV were measured with 3D US and followed in patients annually for 5 years. Progression of TPV predicted stroke, death, TIA or any CV event (p = 0.001). Progression of TPA weakly predicted stroke, death or TIA but not CV event (p = 0.143); change in IMT did not predict stroke, death or TIA or any CV event (p = 0.455) [14]. TPA was also found to be stronger predictor than IMT for the first-ever ischaemic stroke [13]. Measurement of TPV is superior to both IMT and TPA, so that 3D plaque assessment seems highly promising for the clinical practice in the future.
Elastography
Hemodynamic forces acting on the plaque and viscoelastic mechanical properties are biomechanical factors that are hypothesized to play a role in increasing the risk of a plaque rupture. US elastography or strain imaging is a method that aims to quantify the deformation that plaque burden undergoes in response to internal and external mechanical forces. This method has been validated on other structures, mainly parenchymatous organs, but in carotid plaque its clinical application is a bit of a challenge as the structure is too small and respiratory plus cardio-induced movements interfere. Data are acquired in a longitudinal and an axial plane using radiofrequency or B-mode images and analysis is performed. In a smaller study, echolucent plaques demonstrated high strain and high heterogeneity of lateral displacement of the radial motion of the artery [62]. Another dataset suggests that unstable plaque tends to be more elastic and heterogeneous [85]. High-risk plaques were identified on MRI and measured by elastography in another research group and high absolute strain was correlated to the plaque with vulnerable features [86]. These findings indicate that unstable plaques tend to be more elastic and may be prone to rupture. However, further evaluation of the method is needed.
Transcranial colour coded duplex sonography and transcranial Doppler
Transcranial colour coded duplex sonography and TCD are not precisely the methods eligible to visualize carotid plaque itself but provide an interesting way for remote monitoring of the plaque emboligenic potential.
Usually, this method is performed on the same machine, as are the other ultrasound techniques, and is fast, noninvasive and highly beneficial in evaluating carotid plaque risky behaviour as microembolic signals (MES) are well-known sign of unstable plaques [16]. Sector-array probe of low frequencies (2–4 MHz) are used. Standard image acquisition is through transtemporal, transforaminal and transorbital acoustic windows and the middle cerebral artery is the most preferred to monitor MES from ICA.
In The Asymptomatic Carotid Emboli Study (ACES), TCD was used as a tool to identify patients with asymptomatic carotid stenosis who might benefit from surgery. MES detection was found as a suitable method to detect high-risk plaques with emboligenic potential. The absolute annual risk of an ipsilateral stroke was 3.62% in patients with embolic signals and 0.70% in those without [17]. Topakian et al suggested that combination of MES detection together with plaque echolucency allows for a greater prediction than either measure alone and identifies a high-risk group with an annual stroke risk of 8%, and a low-risk group with a risk of < 1% per annum. This risk stratification may prove useful in the selection of patients with ACS for endarterectomy [87]. This is even further supported by the fact that MES significantly diminishes after CEA [88].
Limitations of the method
Each of the presented US methods has certain disadvantages. In B-mode, dependence on the operator skills is considerable and intra - and interobserver agreement was not rated as optimal in many studies [27]. Variations in vascular anatomy, body habitus or vessel wall may limit the ultrasonic beam input [73], furthermore, heavily calcified plaques cause the acoustic shadowing which complicates the examination. An important disadvantage of US is limited ability to characterize plaque composition [89,90], mainly in the differentiation between IPH and LRNC, which both appears as an echolucent structure [27]. Ulceration detection was found of low sensitivity in some studies [28].
In CEUS, several limitations are due to the necessary use of contrast agent. These microbubble-based products are contraindicated in patients known to have right-to-left shunts, allergy, severe pulmonary hypertension, uncontrolled systemic hypertension, unstable angina, endocarditis and adult respiratory distress syndrome [62]. Also, phenomenon of pseudoenhacement may lead to overinterpretation of more distant vessel wall enhancement due to the increased signal in the vessel wall furthest from the probe [91].
In the 3D US imaging, financial and time requirements may partially inhibit the transition to clinical practice. Furthermore, data on the accuracy of the 3D ultrasound plaque characterization with histological validation are limited [82]. Although there are high hopes in 3D US plaque analysis for the future, so far it remains a research tool.
Future goals
Research efforts are ongoing to overcome above-mentioned limitations. 2D B-mode imaging with adding GSM, PDA or other advanced texture analysis improve the evaluation of the plaque composition and architecture. Standardized computer assessment may help to reduce the interobserver variability.
Broadening CEUS into a common clinical practice brings the advantage of detailed description of the plaque luminal surface, neovascularization and inflammation especially with delayed imaging. The use of the late phase enhancement also offers the opportunity to develop new contrast agents that would combine immunohistochemistry visualization of the plaque and could generate molecular images of the carotid plaque. These tracers could focus on further detection of the plaque inflammation if using P-selectin or vascular cell molecule 1 antibodies, or could target IPN within the plaque if vascular endothelial growth factor 1 would be involved [92].
3D US provides further details on plaque surface and uniquely, the volumetric assessment. Development in the area of the computer analysis may provide even more comprehensive information on the plaque tissue. 3D protocols to quantify plaque composition are being developed and tested and seem to be promising as highly reliable method in detection of plaque vulnerable features [62], also if combined with the plaque volume measurements [93].
Shear stress is a known local modulator of plaque location, and is associated with changes in local plaque composition and with the local metalloproteinase-2 (MMP-2) activity [94]. US elastography brings new way to measure plaque response to the mechanical forces and may detect plaque prone-to-rupture status. Studies on mechanical plaque properties with acoustic radiation force impulse were applied successfully in a small number of patients undergoing CEA [95].
Combination of methods is another way of improving plaque vulnerability diagnostic. Future research will focus on the utility of 3D CEUS for the detection of IPN and quantification of plaque burden simultaneously to multiply the analysis refinement effect [73].
Other methods
Several invasive procedures that are not part of a routine clinical practice are about to be mentioned as these could bring new insight into plaque analysis and are subject of research interest. However, the reference will be only marginal for the benefit of keeping this review comprehensive.
IVUS uses the catheter with miniature transducer of high frequency in the range of 20–50 MHz. In combination with PDA, a map of the constituent histological components of the plaque is obtained and transformed into color-coded map representing a virtual histology. In the Carotid Artery Plaque Virtual Histology Evaluation (CAPITAL) study a strong correlation was found between virtual histology IVUS plaque characterization and the true histological examination of the plaque following endarterectomy, particularly in the "vulnerable" plaque types [96].
NIRS is capable of characterizing the chemical composition of the arterial wall. Infrared light is emitted into the tissue and the proportion of the reflected light over the wide range of optical wavelength is measured. NIRS was successfully validated against the histology for vulnerable plaque detection in human coronaries.
OCT is an optical analog of IVUS with about 10-fold higher spatial resolution of ~10 μm. OCT imaging is based on light polarization characteristics and uses a single fiberoptic wire that emits light and captures the reflection. Both IVUS and OCT demonstrated high sensitivity and specificity for unstable plaque characterization. Micro OCT with spatial resolution of < 1 μm was developed in order to visualize microstructures of the plaque. IVUS and OCT allow detecting macrophage density, changes in FC thickness and some other delicate plaque features. In case of μOCT, cellular components of the plaque can be visualized. Monitoring of those underlying biological processes of atherosclerosis may allow refinement and expansion of the definition of vulnerable plaque [97].
However, due to invasive character and the low clinical applicability of these procedures those are suitable mainly for scientific purposes and their further description is beyond the scope of this review.
Imaging modalities and therapeutical managment
A new concept of Treating arteries instead of risk factors has been brought to practice [98]. It is a well-known fact that the BMT is efficient in the plaque remodelling and lowering the risk of stroke [4]. Plaque progression is estimated in 50% of cases without medical treatment while only 25% of plaques do regress. After implementation of therapy, this ratio reverses [98]. There are several suggested parameters to measure size of a plaque and mainly TPA and TPV were found to be the most accurate in the correlation of CV risk [13,99] and should be monitored during treatment. Not only the plaque burden but also its composition may change under drug influence and so the suggestion that we should be able to monitor the effect of antisclerotic therapy routinely is highly relevant.
This brings important question whether the optimal monitoring tool is available. As analysed in this article, the diagnostic potential varies with the methods, and it is equally true for their monitoring capabilities. Consequently, it is essential to ask which method could be useful in identifying revascularization candidates.
CT is not an optimal monitoring tool, especially if it is to be repeated as radiation dose and renal load with a contrast agent is considerable. However, in few studies performed, MDCTA was found as an efficient and accurate method to detect changes in the carotid plaque burden and also in the plaque composition. Patients were monitored for 5 years and the plaque burden development was found to be a heterogeneous and slow process [29]. MDCTA is usually one of the initial acute examinations and is therefore taken into diagnostic consideration when candidates for recanalization are selected. Though this is quite a frequent situation in a routine clinical practice, plaque morphology is not the leading parameter of the choice and larger studies that are based on vulnerable plaque imaging by CT are needed.
MRI has been used to evaluate the efficacy of pharmacological therapy in improving plaque stability relatively frequently and was found to be good monitoring tool. In NIA plaque study, MRI-measured regression of carotid atherosclerosis induced by statins with and without niacin was performed and the conclusion was that the treatment in both groups resulted in significant atherosclerosis reduction with no major difference in niacin subgroup [100]. In another trial, the effects of rosuvastatin on 1,5T MRI was investigated and the evidence that statin therapy may have a beneficial effect on plaque volume and composition, was confirmed [101]. MRI was also found to be useful in identifying surgical candidates. In symptomatic low-grade carotid stenosis, MRI confirmed vulnerable plaque, and high rate of stroke recurrence that was refractory to aggressive medical treatment was observed. In these cases, carotid endarterectomy was performed and surgery was found to significantly reduce the subsequent ischaemic events [102]. High intraplaque signal on MR TOF indicates vulnerable plaque and CAS performed in these patients was associated with increased rates of adverse events compared to CEA. Treatment selection by plaque imaging was the only factor identified as an independent predictor of periprocedural events in this study [103].
PET with FDG tracer was used as a monitor tool in the study with pioglitazone. This drug was the first hypoglycaemic agent confirmed as effective in suppressing inflammation of the atherosclerotic plaque in patients with impaired glucose tolerance or in diabetic patients independent of glucose lowering effect [104]. Some other drugs have been investigated through this molecular imaging tool, including hypolipidemics. High-dose atorvastatin produced significant rapid dose-dependent reductions in FDG uptake that may represent changes in atherosclerotic plaque inflammation [105]. PET is emerging as a potential tool to evaluate metabolic changes in response to medical treatment. At present, no data from large studies using PET as a tool to select candidates for revascularization procedures, are known [26].
US role in plaque monitoring is invincible. Fast, noninvasive and highly informative modality with arbitrary amount of repetition is undoubtedly to be the method of choice not only for plaque dynamics but also for the efficacy of therapeutic procedures. In B-mode imaging, more studies have been conducted. Plaque echolucency evaluated by grey scale densitometry decreased on high-dose atorvastatin [106]. Among patients with moderate carotid stenosis, an aggressive atorvastatin regimen enhanced carotid plaque echogenicity [107]. In CEUS, IPN and its development seems to be a potential target for monitoring. In a study for identifying high-risk patients with coronary artery disease, subgroup analysis showed that carotid CEUS-assessed neovascularization regressed in 46% of plaques in patients during 6 months of statin treatment [108]. 3D US imaging is suggested for risk assessment of plaques with high interobserver reliability [109]. 3D plaque volume measurement can show large effects of drug therapy on atherosclerosis in 3 months, especially significant trend in plaque regression on atorvastatin [110]. Role of transcranial colour coded duplex sonography in risk stratification was already mentioned. MES detection is a strong marker of plaque instability and is far more frequent in symptomatic carotid stenosis than asymptomatic patients [19]. Combination of transcranial colour coded duplex sonography with B-mode verified increase in US method efficiency [87] and other combination variants are being tested. For selecting revascularization candidates, US seems as a promising and widely available tool. So far, reliability of US to detect unstable plaque features need to be verified in large studies. Nevertheless, currently available data offer great hopes for US future in carotid monitoring.
Discussion
The estimation of carotid atherosclerotic disease only by the degree of stenosis is clearly insufficient at present and clinical imperative to obtain additional information on the plaque characteristic is undeniable. Plaque unstable features, such as increasing volume, changes on the plaque surface including thinning FC or ulceration, LRNC and its size and location, IPH, IPN, thrombus and inflammation are the ones most explored. The attempts to find a single diagnostic modality able to display all of those are the subject of many research efforts.
CT is promising for detection of surface irregularities and ulceration, excellent for calcification and good at revealing further plaque characteristics, like IPH, neovascularization and LRNC but differentiation between those is misleading. Late phase modality seems to indicate less stable plaques and dynamic protocols are being tested.
MRI has the most potential to identify vulnerable morphology of the plaque and most of its characteristics. Excellency in IPH detection, including the evaluation of the thrombus age, and its differentiation from LRNC was proven many times. IPN and inflammation can be reached with postcontrast enhancement and ulceration is visible on all contrast weightings.
PET has a major role in detecting inflammation and plaque remodelling but resolution of anatomical structures is low.
US is a rapidly evolving and increasingly expanding method with new modalities refined in the detection of an unstable plaque. We can detect most of the plaque characteristics and insufficient resolution of some of them can be refined using advanced methods like CEUS (with excellent detection of IPN and inflammation in late phase imaging), 3D US (with measurement of plaque volume and dynamics monitoring) or computerized measurement of plaque echogenicity. Especially mapping of plaque architecture including JBA detection can influence choice of invasive procedure significantly. Real-time imaging with hemodynamic evaluation and non-invasivity makes US the leading method for serial testing. MES detection is another method that can indicate plaque unstability and plaque emboligenic signals are another important criteria in invasive stenosis treatment.
Invasive techniques, like IVUS, OCT or NIRS and their combination are valuable research tools but these are not eligible for clinical practice due to their invasivity. However, these techniques may have the potential to assist in planning endovascular procedures.
Serial in vivo imaging of atherosclerosis is important for understanding plaque progression and is potentially useful in predicting CV events and monitoring treatment efficacy. One time imaging cannot asses the development of a plaque in time as atherosclerosis is a complex and slow process and the success of the treatment effect should be monitored [98]. Only this way we can assess the effective treatment and the duration of a drug administration necessary to reach the plaque stabilization. For example, the duration of the lipid lowering therapy using statins was questioned and the time to produce regression of plaques was in average 19.7 months [111]. If BMT is not effective, regular monitoring may help to specify the timing for surgical procedure. Screening for asymptomatic carotid artery stenosis in the general population and selected subsets of patients is recommended [112] and serial monitoring tool is needed. Plaque characterization is probably the chief advantage of MRI but at this point, US is the leading method that is likely to remain in general use for studies of atherosclerosis [89].
In the future, advanced imaging of each method is expected to provide more detailed morphological information about the carotid plaque. Molecular imaging with new tracers for CEUS and PET, texture analysis for B-mode US, further 3D US analysis, dynamic protocols in contrast enhanced modalities or carotid vessel wall imaging at 7T MRI are some of the research interests and their correlation with histological findings is essential to further develop these techniques.
Conclusion
Although many of aforementioned imaging modalities hold the promise for future, at present, there is no single imaging technique that can reliably identify the vulnerable plaque and only combination of methods can bring complex plaque evaluation. To date, the decision on CEA is mainly based on the degree of the stenosis but additional plaque characteristics might be better predictors of stroke, allowing for more precise selection of patients for surgery - studies in this field are ongoing [113].
Large multicentre studies are needed to determine whether and how much can be plaque imaging superior to using only the degree of stenosis for selecting candidates for invasive therapy. We can conclude with confidence that plaque burden analysis could help to pave the road to personalized risk assessment and allow for individualization of care in order to reduce the risk of ischaemic stroke originating from carotid vulnerable plaque.
List of abbreviations
18FDG – 18F-fluorodeoxyglucose
18F-NaF – 18F-natriumfluorid
2D – two-dimensional
3D – three-dimensional
ACES – Asymptomatic Carotid Emboli Study
BMT – best medical treatment
CAPITAL – Carotid Artery Plaque Virtual Histology Evaluation
CAS – carotid stenting
CEA – carotid endarterectomy
CE MRI – contrast enhanced - magnetic resonance imaging
CEUS – contrast enhanced ultrasound
CV – cardiovascular
dB-E – decibel enhancement
DSA – digital subtraction angiography
DSCT – dual source CT
FC – fibrous cup
FDG – F-fluorodeoxyglukóza
Gd – Gadolinium
GSM – gray scale median
HU – Hounsfield unit
IBS – integrated ultrasonic backscatter
ICA – internal carotid artery
IMT – intima-medial thickness
IPH – intraplaque haemorrhage
IPN – intraplaque neovascularization
IVUS – intravascular ultrasound
JBA – juxtaluminal black area
LRNC – lipid rich necrotic core
MD CT – multidetector-row CT
MES – microembolic signals
MMP – matrix metalloproteinases.
NIRS – near infrared spectroscopy
OCT – optical coherence tomography
PDA – pixel distribution analysis
PDW – proton density weighted
SNAP – simultaneous non-contrast angiography and intraplaque haemorrhage
SPECT – single emission computed tomography
SPIO – superparamagnetic iron oxide
SUV – standardized uptake value
TBR – target-to-background ratio
TCD – transcranial Doppler
TIA – transient ischaemic attacks
TOF – time-of-flight
TPA – total plaque area
TPV – total plaque volume
μOCT – micro-optical coherence tomography
Accepted for review: 18. 4. 2018
Accepted for print: 25. 6. 2018
This work was supported by Ministry of Health of the Czech Republic grants No.16-29148A, 16-30965A and 17-31016A.
MUDr. Petra Kešnerová
Department of Neurology
Second Faculty of Medicine
Charles University and University Hospital Motol
V Úvalu 84
150 06 Praha 5
Czech Republic
e-mail: kesnerova.petra@gmail.com
Sources
1. Touzé E. Natural history of asymptomatic carotid artery stenosis. Rev Neurol (Paris) 2008; 164(10): 793–800. doi: 10.1016/ j.neurol.2008.07.005.
2. Kernan WN, Ovbiagele B, Black HR et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/ American Stroke Association. Stroke 2014; 45(7): 2160–2236. doi: 10.1161/ STR.0000000000000024.
3. Altaf N, Daniels L, Morgan PS et al. Detection of intraplaque hemorrhage by magnetic resonance imaging in symptomatic patients with mild to moderate carotid stenosis predicts recurrent neurological events. J Vasc Surg 2008; 47(2): 337–342. doi: 10.1016/ j.jvs.2007.09.064.
4. Marquardt L, Geraghty OC, Mehta Z et al. Low risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment: a prospective, population-based study. Stroke 2010; 41(1): e11–e17. doi: 10.1161/ STROKEAHA.109.561837.
5. Mossa-Basha M, Wasserman BA. Low-grade carotid stenosis: implications of MR imaging. Neuroimaging Clin N Am 2016; 26(1): 129–145. doi: 10.1016/ j.nic.2015.09.010.
6. Paraskevas KI, Spence JD, Veith FJ et al. Identifying which patients with asymptomatic carotid stenosis could benefit from intervention. Stroke 2014; 45(12): 3720–3724. doi: 10.1161/ STROKEAHA.114.006912.
7. Kolodgie FD, Yahagi K, Mori H et al. High-risk carotid plaque: lessons learned from histopathology. Semin Vasc Surg 2017; 30(1): 31–43. doi: 10.1053/ j.semvascsurg.2017.04.008.
8. Fleiner M, Kummer M, Mirlacher M et al. Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation 2004; 110(18): 2843–2850. doi: 10.1161/ 01.CIR.0000146787.16297.E8.
9. Hellings WE, Peeters W, Moll FL et al. Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: a prognostic study. Circulation 2010; 121(17): 1941–1950. doi: 10.1161/ CIRCULATIONAHA.109.887497.
10. Fisher M, Paganini-Hill A, Martin A et al. Carotid plaque pathology: thrombosis, ulceration, and stroke pathogenesis. Stroke 2005; 36(2): 253–257. doi: 10.1161/ 01.STR.0000152336.71224.21.
11. Wasserman BA, Sharrett AR, Lai S et al. Risk factor associations with the presence of a lipid core in carotid plaque of asymptomatic individuals using high-resolution MRI: the multi-ethnic study of atherosclerosis (MESA). Stroke 2008; 39(2): 329–335. doi: 10.1161/ STROKEAHA.107.498634.
12. Naylor AR, Schroeder TV, Sillesen H. Clinical and imaging features associated with an increased risk of late stroke in patients with asymptomatic carotid disease. Eur J Vasc Endovasc Surg 2014; 48(6): 633–640. doi: 10.1016/ j.ejvs.2014.08.017.
13. Mathiesen EB, Johnsen SH, Wilsgaard T et al. Carotid plaque area and intima-media thickness in prediction of first-ever ischemic stroke: a 10-year follow-up of 6584 men and women: the Tromsø Study. Stroke 2011; 42(4): 972–998. doi: 10.1161/ STROKEAHA.110.589754.
14. Wannarong T, Parraga G, Buchanan D et al. Progression of carotid plaque volume predicts cardiovascular events. Stroke 2013; 44(7): 1859–1865. doi: 10.1161/ STROKEAHA.113.001461.
15. Gupta A, Baradaran H, Schweitzer AD et al. Carotid plaque MRI and stroke risk: a systematic review and meta-analysis. Stroke 2013; 44(11): 3071–3077. doi: 10.1161/ STROKEAHA.113.002551.
16. Spence JD. Transcranial Doppler monitoring for microemboli: a marker of a high-risk carotid plaque. Semin Vasc Surg 2017; 30(1): 62–66. doi: 10.1053/ j.semvascsurg.2017.04.011.
17. Markus HS, King A, Shipley M et al. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol 2010 : 9(7): 663–671. doi: 10.1016/ S1474-4422(10)70120-4.
18. Redgrave JN, Coutts SB, Schulz UG et al. Systematic review of associations between the presence of acute ischemic lesions on diffusion-weighted imaging and clinical predictors of early stroke risk after transient ischemic attack. Stroke 2007; 38(5): 1482–1488. doi: 10.1161/ STROKEAHA.106.477380.
19. Ritter MA, Dittrich R, Thoenissen N et al. Prevalence and prognostic impact of microembolic signals in arterial sources of embolism. A systematic review of the literature. J Neurol 2008; 255(7): 953–961. doi: 10.1007/ s00415-008-0638-8.
20. Freilinger TM, Schindler A, Schmidt C et al. Prevalence of nonstenosing, complicated atherosclerotic plaques in cryptogenic stroke. JACC Cardiovasc Imaging 2012; 5(4): 397–405. doi: 10.1016/ j.jcmg.2012.01.012.
21. Bayer-Karpinska A, Schwarz F, Wollenweber FA et al. The carotid plaque imaging in acute stroke (CAPIAS) study: protocol and initial baseline data. BMC Neurol 2013; 13 : 201. doi: 10.1186/ 1471-2377-13-201.
22. Das M, Braunschweig T, Mühlenbruch G et al. Carotid plaque analysis: comparison of dual-source computed tomography (CT) findings and histopathological correlation. Eur J Vasc Endovasc Surg 2009; 38(1): 14–19. doi: 10.1016/ j.ejvs.2009.03.013.
23. Horie N, Morikawa M, Ishizaka S et al. Assessment of carotid plaque stability based on the dynamic enhancement pattern in plaque components with multidetector CT angiography. Stroke 2012; 43(2): 393–398. doi: 10.1161/ STROKEAHA.111.635953.
24. Josephson SA, Bryant SO, Mak HK et al. Evaluation of carotid stenosis using CT angiography in the initial evaluation of stroke and TIA. Neurology 2004; 63(3): 457–460.
25. Wintermark M, Jawadi SS, Rapp JH et al. High-resolution CT imaging of carotid artery atherosclerotic plaques. AJNR Am J Neuroradiol 2008; 29(5): 875–882. doi: 10.3174/ ajnr.A0950.
26. Brinjikji W, Huston J 3rd, Rabinstein AA et al. Contemporary carotid imaging: from degree of stenosis to plaque vulnerability. J Neurosurg 2016; 124(1): 27–42. doi: 10.3171/ 2015.1.JNS142452.
27. Huibers A, de Borst GJ, Wan S et al. Non-invasive Carotid Artery Imaging to Identify the Vulnerable Plaque: Current Status and Future Goals. Eur J Vasc Endovasc Surg 2015; 50(5): 563–572. doi: 10.1016/ j.ejvs.2015.06.113.
28. Saba L, Tamponi E, Raz E et al. Correlation between fissured fibrous cap and contrast enhancement: preliminary results with the use of CTA and histologic validation. AJNR Am J Neuroradiol 2014; 35(4): 754–759. doi: 10.3174/ ajnr.A3759.
29. van Gils MJ, Vukadinovic D, van Dijk AC et al. Carotid atherosclerotic plaque progression and change in plaque composition over time: a 5-year follow-up study using serial CT angiography. AJNR Am J Neuroradiol 2012; 33(7): 1267–1273. doi: 10.3174/ ajnr.A2970.
30. Watanabe Y, Nagayama M. MR plaque imaging of the carotid artery. Neuroradiology 2010; 52(4): 253–274. doi: 10.1007/ s00234-010-0663-z.
31. Millon A, Boussel L, Brevet M et al. Clinical and histological significance of gadolinium enhancement in carotid atherosclerotic plaque. Stroke 2012; 43(11): 3023–3028. doi: 10.1161/ STROKEAHA.112.662692.
32. Cai JM, Hatsukami TS, Ferguson MS et al. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation 2002; 106(11): 1368–1373.
33. Puppini G, Furlan F, Cirota N et al. Characterisation of carotid atherosclerotic plaque: comparison between magnetic resonance imaging and histology. Radiol Med 2006; 111(7): 921–930. doi: 10.1007/ s11547-006-0091-7.
34. Saam T, Hetterich H, Hoffmann V et al. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol 2013; 62(12): 1081–1091. doi: 10.1016/ j.jacc.2013.06.015.
35. den Hartog AG, Bovens SM, Koning W et al. Current status of clinical magnetic resonance imaging for plaque characterisation in patients with carotid artery stenosis. Eur J Vasc Endovasc Surg 2013; 45(1): 7–21. doi: 10.1016/ j.ejvs.2012.10.022.
36. Underhill HR, Yuan C, Yarnykh VL. Predictors of surface disruption with MR imaging in asymptomatic carotid artery stenosis. AJNR Am J Neuroradiol 2010; 31(3): 487–493. doi: 10.3174/ ajnr.A1842.
37. Chan JM, Monaco C, Wylezinska-Arridge M et al. Imaging of the vulnerable carotid plaque: biological targeting of inflammation in atherosclerosis using iron oxide particles and MRI. Eur J Vasc Endovasc Surg 2014; 47(5): 462–469. doi: 10.1016/ j.ejvs.2014.01.017.
38. Gaens ME, Backes WH, Rozel S et al. Dynamic contrast-enhanced MR imaging of carotid atherosclerotic plaque: model selection, reproducibility, and validation. Radiology 2013; 266(1): 271–279. doi: 10.1148/ radiol.12120499.
39. Esposito-Bauer L, Saam T, Ghodrati I et al. MRI plaque imaging detects carotid plaques with a high risk for future cerebrovascular events in asymptomatic patients. PLoS One 2013; 8(7): e67927. doi: 10.1371/ journal.pone.0067927.
40. Altaf N, Morgan PS, Moody A et al. Brain white matter hyperintensities are associated with carotid intraplaque hemorrhage. Radiology 2008; 248(1): 202–209. doi: 10.1148/ radiol.2481070300.
41. den Hartog AG, Bovens SM, Koning W et al. PLACD-7T Study: Atherosclerotic Carotid Plaque Components Correlated with Cerebral Damage at 7 Tesla Magnetic Resonance Imaging. Curr Cardiol Rev 2011; 7(1): 28–34. doi: 10.2174/ 157340311795677743.
42. Wang J, Börnert P, Zhao H et al. Simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation. Magn Reson Med 2013; 69(2): 337–345. doi: 10.1002/ mrm.24254.
43. de Rotte AA, Koning W, den Hartog AG et al. 7.0 T MRI detection of cerebral microinfarcts in patients with a symptomatic high-grade carotid artery stenosis. J Cereb Blood Flow Metab 2014; 34(10): 1715–1719. doi: 10.1038/ jcbfm.2014.141.
44. de Rotte AA, Koning W, Truijman MT et al. Seven-tesla magnetic resonance imaging of atherosclerotic plaque in the significantly stenosed carotid artery: a feasibility study. Invest Radiol 2014; 49(11): 749–757. doi: 10.1097/ RLI.0000000000000079.
45. van’t Klooster R, de Koning PJ, Dehnavi RA et al. Auto-matic lumen and outer wall segmentation of the carotid artery using deformable three-dimensional models in MR angiography and vessel wall images. J Magn Reson Imaging 2012; 35(1): 156–165. doi: 10.1002/ jmri.22809.
46. Makowski MR, Botnar RM. MR imaging of the arterial vessel wall: molecular imaging from bench to bedside. Radiology 2013; 269(1): 34–51. doi: 10.1148/ radiol.13102336.
47. Cocker MS, Mc Ardle B, Spence JD et al. Imaging atherosclerosis with hybrid [18F]fluorodeoxyglucose positron emission tomography/ computed tomography imaging: what Leonardo da Vinci could not see. J Nucl Cardiol 2012; 19(6): 1211–1225. doi: 10.1007/ s12350-012-9631-9.
48. Blomberg BA, Thomassen A, Takx RA et al. Delayed ¹⁸F-fluorodeoxyglucose PET/ CT imaging improves quantitation of atherosclerotic plaque inflammation: results from the CAMONA study. J Nucl Cardiol 2014; 21(3): 588–597. doi: 10.1007/ s12350-014-9884-6.
49. Rudd JH, Myers KS, Bansilal S et al. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol 2007; 50(9): 892–896. doi: 10.1016/ j.jacc.2007.05.024.
50. Tawakol A, Migrino RQ, Bashian GG et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol 2006; 48(9): 1818–1824. doi: 10.1016/ j.jacc.2006.05.076.
51. Masteling MG, Zeebregts CJ, Tio RA et al. High-resolution imaging of human atherosclerotic carotid plaques with micro 18F-FDG PET scanning exploring plaque vulnerability. J Nucl Cardiol 2011; 18(6): 1066–1075. doi: 10.1007/ s12350-011-9460-2.
52. Marnane M, Merwick A, Sheehan OC et al. Carotid plaque inflammation on 18F-fluorodeoxyglucose positron emission tomography predicts early stroke recurrence. Ann Neurol 2012; 71(5): 709–718. doi: 10.1002/ ana.23553.
53. Kwee RM, Teule GJ, van Oostenbrugge RJ et al. Multimodality imaging of carotid artery plaques: 18F-fluoro-2-deoxyglucose positron emission tomography, computed tomography, and magnetic resonance imaging. Stroke 2009; 40(12): 3718–3724. doi: 10.1161/ STROKEAHA.109.564088.
54. Joshi NV, Vesey AT, Williams MC et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet 2014; 383(9918): 705–713. doi: 10.1016/ S0140-6736(13)61754-7.
55. Derlin T, Wisotzki C, Richter U et al. In vivo imaging of mineral deposition in carotid plaque using 18F-sodium fluoride PET/ CT: correlation with atherogenic risk factors. J Nucl Med 2011; 52(3): 362–368. doi: 10.2967/ jnumed.110.081208.
56. Hermann S, Starsichova A, Waschkau B et al. Non-FDG imaging of atherosclerosis: will imaging of MMPs assess plaque vulnerability? J Nucl Cardiol 2012; 19(3): 609–617. doi: 10.1007/ s12350-012-9553-6.
57. Beer AJ, Pelisek J, Heider P et al. PET/ CT imaging of integrin αvβ3 expression in human carotid atherosclerosis. JACC Cardiovasc Imaging 2014; 7(2): 178–187. doi: 10.1016/ j.jcmg.2013.12.003.
58. Lammie GA, Wardlaw J, Allan P et al. What pathological components indicate carotid atheroma activity and can these be identified reliably using ultrasound? Eur J Ultrasound 2000; 11(2): 77–86.
59. Kyriacou EC, Pattichis C, Pattichis M et al. A review of noninvasive ultrasound image processing methods in the analysis of carotid plaque morphology for the assessment of stroke risk. IEEE Trans Inf Technol Biomed 2010; 14(4): 1027–1038. doi: 10.1109/ TITB.2010.2047649.
60. Grønholdt ML, Nordestgaard BG, Schroeder TV et al. Ultrasonic echolucent carotid plaques predict future strokes. Circulation 2001; 104(1): 68–73.
61. Nicolaides AN, Kakkos SK, Kyriacou E et al. Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) Study Group. Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J Vasc Surg 2010; 52(6): 1486-1496. doi: 10.1016/ j.jvs.2010.07.021.
62. Cires-Drouet RS, Mozafarian M, Ali A et al. Imaging of high-risk carotid plaques: ultrasound. Semin Vasc Surg 2017; 30(1): 44–53. doi: 10.1053/ j.semvascsurg.2017.04.010.
63. Lal BK, Hobson RW 2nd, Hameed M et al. Noninvasive identification of the unstable carotid plaque. Ann Vasc Surg 2006; 20(2): 167–174. doi: 10.1007/ s10016-006-9000-8.
64. Kakkos SK, Griffin MB, Nicolaides AN et al. The size of juxtaluminal hypoechoic area in ultrasound images of asymptomatic carotid plaques predicts the occurrence of stroke. J Vasc Surg 2013; 57(3): 609–618. doi: 10.1016/ j.jvs.2012.09.045.
65. Roubec M, Kuliha M, Jonszta T et al. Detection of intracranial arterial stenosis using transcranial color-coded duplex sonography, computed tomographic angiography, and digital subtraction angiography. J Ultrasound Med 2011; 30(8): 1069–1075.
66. Waki H, Masuyama T, Mori H et al. Ultrasonic tissue characterization of the atherosclerotic carotid artery: histological correlates or carotid integrated backscatter. Circ J 2003; 67(12): 1013–1016.
67. Muraki M, Mikami T, Yoshimoto T et al. New criteria for the sonographic diagnosis of a plaque ulcer in the extracranial carotid artery. AJR Am J Roentgenol 2012; 198(5): 1161–1166. doi: 10.2214/ AJR.11.7018.
68. Prabhakaran S, Rundek T, Ramas R et al. Carotid plaque surface irregularity predicts ischemic stroke: the northern Manhattan study. Stroke 2006; 37(11): 2696–2701. doi: 10.1161/ 01.STR.0000244780.82190.a4.
69. Salem MK, Bown MJ, Sayers RD et al. Identification of patients with a histologically unstable carotid plaque using ultrasonic plaque image analysis. Eur J Vasc Endovasc Surg 2014; 48(2): 118–125. doi: 10.1016/ j.ejvs.2014.05.015.
70. Davies MJ, Richardson PD, Woolf N et al. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J 1993; 69(5): 377–381.
71. Gupta A, Kesavabhotla K, Baradaran H et al. Plaque echolucency and stroke risk in asymptomatic carotid stenosis: a systematic review and meta-analysis. Stroke 2015; 46(1): 91–97. doi: 10.1161/ STROKEAHA.114.006091.
72. Mathiesen EB, Bønaa KH, Joakimsen O. Echolucent plaques are associated with high risk of ischemic cerebrovascular events in carotid stenosis: the tromsø study. Circulation 2001; 103(17): 2171–2175.
73. Saha SA, Gourineni V, Feinstein SB. The use of contrast-enhanced ultrasonography for imaging of carotid atherosclerotic plaques: current evidence, future directions. Neuroimaging Clin N Am 2016; 26(1): 81–96. doi: 10.1016/ j.nic.2015.09.007.
74. Faggioli GL, Pini R, Mauro R et al. Identification of carotid ‘vulnerable plaque’ by contrast-enhanced ultrasonography: correlation with plaque histology, symptoms and cerebral computed tomography. Eur J Vasc Endovasc Surg 2011; 41(2): 238–248. doi: 10.1016/ j.ejvs.2010.11.002.
75. Kim HS, Woo JS, Kim BY et al. Biochemical and clinical correlation of intraplaque neovascularization using contrast-enhanced ultrasound of the carotid artery. Atherosclerosis 2014; 233(2): 579–583. doi: 10.1016/ j.atherosclerosis.2014.01.042.
76. Chang X, Feng J, Ruan L et al. Positive correlation between neovascularization degree of carotid atherosclerosis determined by contrast-enhanced ultrasound and level of serum C-reactive protein. Vasa 2015; 44(3): 187–194. doi: 10.1024/ 0301-1526/ a000429.
77. Lindner JR, Dayton PA, Coggins MP et al. Noninvasive imaging of inflammation by ultrasound detection of phagocytosed microbubbles. Circulation 2000; 102(5): 531–538.
78. Owen DR, Shalhoub J, Miller S et al. Inflammation within carotid atherosclerotic plaque: assessment with late-phase contrast-enhanced US. Radiology 2010; 255(2): 638–644. doi: 10.1148/ radiol.10091365.
79. ten Kate GL, van Dijk AC, van den Oord SC et al. Usefulness of contrast-enhanced ultrasound for detection of carotid plaque ulceration in patients with symptomatic carotid atherosclerosis. Am J Cardiol 2013; 112(2): 292–298. doi: 10.1016/ j.amjcard.2013.03.028.
80. van den Oord SC, ten Kate GL, Sijbrands EJ et al. Effect of carotid plaque screening using contrast-enhanced ultrasound on cardiovascular risk stratification. Am J Cardiol 2013; 111(5): 754–759. doi: 10.1016/ j.amjcard.2012.11.033.
81. Hammond CJ, McPherson SJ, Patel JV et al. Assessment of apparent internal carotid occlusion on ultrasound: prospective comparison of contrast-enhanced ultrasound, magnetic resonance angiography and digital subtraction angiography. Eur J Vasc Endovasc Surg 2008; 35(4): 405–412. doi: 10.1016/ j.ejvs.2007.12.008.
82. Makris GC, Lavida A, Griffin M et al. Three-dimensional ultrasound imaging for the evaluation of carotid atherosclerosis. Atherosclerosis 2011; 219(2): 377–383. doi: 10.1016/ j.atherosclerosis.2011.05.006.
83. Spence JD, Eliasziw M, DiCicco M et al. Carotid plaque area: a tool for targeting and evaluating vascular preventive therapy. Stroke 2002; 33(12): 2916–2922.
84. Kuk M, Wannarong T, Beletsky V et al. Volume of carotid artery ulceration as a predictor of cardiovascular events. Stroke 2014; 45(5): 1437–1441. doi: 10.1161/ STROKEAHA.114.005163.
85. Zhang Q, Li C, Zhou M et al. Quantification of carotid plaque elasticity and intraplaque neovascularization using contrast-enhanced ultrasound and image registration-based elastography. Ultrasonics 2015; 62 : 253–262. doi: 10.1016/ j.ultras.2015.05.025.
86. Huang C, Pan X, He Q et al. ultrasound-based carotid elastography for detection of vulnerable atherosclerotic plaques validated by magnetic resonance imaging. Ultrasound Med Biol 2016; 42(2): 365–377. doi: 10.1016/ j.ultrasmedbio.2015.09.023.
87. Topakian R, King A, Kwon SU et al. Ultrasonic plaque echolucency and emboli signals predict stroke in asymptomatic carotid stenosis. Neurology 2011; 77(8): 751–758. doi: 10.1212/ WNL.0b013e31822b00a6.
88. van Zuilen EV, Moll FL, Vermeulen FE et al. Detection of cerebral microemboli by means of transcranial Doppler monitoring before and after carotid endarterectomy. Stroke 1995; 26(2): 210–213.
89. Spence JD. Determinants of carotid plaque burden. Atherosclerosis 2016; 255 : 122–123. doi: 10.1016/ j.atherosclerosis.2016.10.045.
90. Tegos TJ, Sohail M, Sabetai MM et al. Echomorphologic and histopathologic characteristics of unstable carotid plaques. AJNR Am J Neuroradiol 2000; 21(10): 1937–1944.
91. ten Kate GL, Renaud GG, Akkus Z et al. Far-wall pseudoenhancement during contrast-enhanced ultrasound of the carotid arteries: clinical description and in vitro reproduction. Ultrasound Med Biol 2012; 38(4): 593–600. doi: 10.1016/ j.ultrasmedbio.2011.12.019.
92. Alonso A, Artemis D, Hennerici MG. Molecular imaging of carotid plaque vulnerability. Cerebrovasc Dis 2015; 39(1): 5–12. doi: 10.1159/ 000369123.
93. van Engelen A, Wannarong T, Parraga G et al Three-dimensional carotid ultrasound plaque texture predicts vascular events. Stroke 2014; 45(9): 2695–2701. doi: 10.1161/ STROKEAHA.114.005752.
94. Krams R, Cheng C, Helderman F et al. Shear stress is associated with markers of plaque vulnerability and MMP-9 activity. EuroIntervention 2006; 2(2): 250–256.
95. Czernuszewicz TJ, Homeister JW, Caughey MC et al. Non-invasive in vivo characterization of human carotid plaques with acoustic radiation force impulse ultrasound: comparison with histology after endarterectomy. Ultrasound Med Biol 2015; 41(3): 685–697. doi: 10.1016/ j.ultrasmedbio.2014.09.016.
96. Diethrich EB, Pauliina Margolis M, Reid DB et al. Virtual histology intravascular ultrasound assessment of carotid artery disease: the Carotid Artery Plaque Virtual Histology Evaluation (CAPITAL) study. J Endovasc Ther 2007; 14(5): 676–686. doi: 10.1177/ 152660280701400512.
97. Celeng C, Takx RA, Ferencik M et al. Non-invasive and invasive imaging of vulnerable coronary plaque. Trends Cardiovasc Med 2016; 26(6): 538–547. doi: 10. 1016/ j.tcm.2016.03.005.
98. Spence JD, Hackam DG. Treating arteries instead of risk factors: a paradigm change in management of atherosclerosis. Stroke 2010; 41(6): 1193–1199. doi: 10.1161/ STROKEAHA.110.577973.
99. Spence JD, Parraga G. Three-simensional ultrasound of carotid plaque. Neuroimaging Clin N Am 2016; 26(1): 69–80. doi: 10.1016/ j.nic.2015.09.006.
100. Sibley CT, Vavere AL, Gottlieb I et al. MRI-measured regression of carotid atherosclerosis induced by statins with and without niacin in a randomised controlled trial: the NIA plaque study. Heart 2013; 99(22): 1675–1680. doi: 10.1136/ heartjnl-2013-303926.
101. Underhill HR, Yuan C, Zhao XQ et al. Effect of rosuvastatin therapy on carotid plaque morphology and composition in moderately hypercholesterolemic patients: a high-resolution magnetic resonance imaging trial. Am Heart J 2008; 155(3): 584.e1–584.e8. doi: 10.1016/ j.ahj.2007.11.018.
102. Yoshida K, Sadamasa N, Narumi O et al. Symptomatic low-grade carotid stenosis with intraplaque hemorrhage and expansive arterial remodeling is associated with a high relapse rate refractory to medical treatment. Neurosurgery 2012; 70(5): 1143–1150. doi: 10.1227/ NEU.0b013e31823fe50b.
103. Yoshimura S, Yamada K, Kawasaki M et al. Selection of carotid artery stenting or endarterectomy based on magnetic resonance plaque imaging reduced periprocedural adverse events. J Stroke Cerebrovasc Dis 2013; 22(7): 1082–1087. doi: 10.1016/ j.jstrokecerebrovasdis.2012.07.018.
104. Mizoguchi M, Tahara N, Tahara A et al. Pioglitazone attenuates atherosclerotic plaque inflammation in patients with impaired glucose tolerance or diabetes a prospective, randomized, comparator-controlled study using serial FDG PET/ CT imaging study of carotid artery and ascending aorta. JACC Cardiovasc Imaging 2011; 4(10): 1110–1118. doi: 10.1016/ j.jcmg.2011.08.007.
105. Tawakol A, Fayad ZA, Mogg R et al. Intensification of statin therapy results in a rapid reduction in atherosclerotic inflammation: results of a multicenter fluorodeoxyglucose-positron emission tomography/ computed tomography feasibility study. J Am Coll Cardiol 2013; 62(10): 909–917. doi: 10.1016/ j.jacc.2013.04.066.
106. Della-Morte D, Moussa I, Elkind MS et al. The short-term effect of atorvastatin on carotid plaque morphology assessed by computer-assisted gray-scale densitometry: a pilot study. Neurol Res 2011; 33(9): 991–994. doi: 10.1179/ 1743132811Y.0000000039.
107. Kadoglou NP, Sailer N, Moumtzouoglou A et al. Aggressive lipid-lowering is more effective than moderate lipid-lowering treatment in carotid plaque stabilization. J Vasc Surg 2010; 51(1): 114–121. doi: 10.1016/ j.jvs.2009.07.119.
108. Deyama J, Nakamura T, Takishima I et al. Contrast-enhanced ultrasound imaging of carotid plaque neovascularization is useful for identifying high-risk patients with coronary artery disease. Circ J 2013; 77(6): 1499–1507.
109. Bar M, Roubec M, Farana R et al. Inter-rater reliability of carotid atherosclerotic plaque quantification by 3-dimensional sonography. J Ultrasound Med 2014; 33(7): 1273–1278. doi: 10.7863/ ultra.33.7.1273.
110. Ainsworth CD, Blake CC, Tamayo A et al. 3D ultrasound measurement of change in carotid plaque volume: a tool for rapid evaluation of new therapies. Stroke 2005; 36(9): 1904–1909. doi: 10.1161/ 01.STR.0000178543.19433.20.
111. Noyes AM, Thompson PD. A systematic review of the time course of atherosclerotic plaque regression. Atherosclerosis 2014; 234(1): 75–84. doi: 10.1016/ j.atherosclerosis.2014.02.007.
112. Qureshi AI, Alexandrov AV, Tegeler CH et al. Guidelines for screening of extracranial carotid artery disease: a statement for healthcare professionals from the multidisciplinary practice guidelines committee of the American Society of Neuroimaging; cosponsored by the Society of Vascular and Interventional Neurology. J Neuroimaging 2007; 17(1): 19–47. doi: 10.1111/ j.1552-6569.2006.00085.x.
113. Truijman MT, Kooi ME, van Dijk AC et al. Plaque At RISK (PARISK): prospective multicenter study to improve diagnosis of high-risk carotid plaques. Int J Stroke 2014; 9(6): 747–754. doi: 10.1111/ ijs.12167.
Labels
Paediatric neurology Neurosurgery NeurologyArticle was published in
Czech and Slovak Neurology and Neurosurgery

2018 Issue 4
- Advances in the Treatment of Myasthenia Gravis on the Horizon
- Memantine in Dementia Therapy – Current Findings and Possible Future Applications
- Memantine Eases Daily Life for Patients and Caregivers
-
All articles in this issue
- Detection of unstable carotid plaque in ischemic stroke prevention
- Aggressive treatment of intracerebral hemorrhage with lowering of blood pressure and indication of surgery - YES
- Aggressive treatment of intracerebral hemorrhage with lowering of blood pressure and indication of surgery - NO
- Aggressive treatment of intracerebral hemorrhage with lowering of blood pressure and indication of surgery - COMMENT
- Treatment targeted for B lymphocytes – significant progress in the treatment of multiple sclerosis
- Possibilities of regulation of neuroimmune and neuroendocrine processes using physiotherapy
- Parietal atrophy score on magnetic resonance imaging of the brain in normally aging people
- Imaging of peripheral nerves using diffusion tensor imaging and MR tractography
- The differences in clinical, radiological and treatment modalities of cervical intramedullary arachnoid cysts and cervical syringomyelia – report of 12 cases
- The relationship between tinnitus intensity and the degree of sensorineural hearing loss from the aspect of contribution of hyperbaric oxygen therapy
- Antiplatelet and anticoagulant therapy in carotid endarterectomies
- A comparison of efficacy of subcutaneous interferon β-1a 44 μg, dimethyl fumarate and fingolimod in the real-life clinical practise – a multicenter observational study
- A set of pictures with the opposite difficulty of naming
- Pharyngo-cervico-brachial variant of Guillain-Barré syndroma
- Bilateral abducens nerve palsy after head and cervical spinal injury
- Late-onset Huntington’s disease – an overlooked diagnosis
- Early postoperative complications after elective degenerative lumbar spine surgery in elderly patients
- Orolingual bradykinin angioedema after tissue plasminogen activator in acute stroke – treatment with or without C1-esterase inhibitor
- Czech and Slovak Neurology and Neurosurgery
- Journal archive
- Current issue
- About the journal
Most read in this issue
- Antiplatelet and anticoagulant therapy in carotid endarterectomies
- Bilateral abducens nerve palsy after head and cervical spinal injury
- Imaging of peripheral nerves using diffusion tensor imaging and MR tractography
- Late-onset Huntington’s disease – an overlooked diagnosis
