-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Comprehensive Analysis Reveals Dynamic and Evolutionary Plasticity of Rab GTPases and Membrane Traffic in
Cellular sophistication is not exclusive to multicellular organisms, and unicellular eukaryotes can resemble differentiated animal cells in their complex network of membrane-bound structures. These comparisons can be illuminated by genome-wide surveys of key gene families. We report a systematic analysis of Rabs in a complex unicellular Ciliate, including gene prediction and phylogenetic clustering, expression profiling based on public data, and Green Fluorescent Protein (GFP) tagging. Rabs are monomeric GTPases that regulate membrane traffic. Because Rabs act as compartment-specific determinants, the number of Rabs in an organism reflects intracellular complexity. The Tetrahymena Rab family is similar in size to that in humans and includes both expansions in conserved Rab clades as well as many divergent Rabs. Importantly, more than 90% of Rabs are expressed concurrently in growing cells, while only a small subset appears specialized for other conditions. By localizing most Rabs in living cells, we could assign the majority to specific compartments. These results validated most phylogenetic assignments, but also indicated that some sequence-conserved Rabs were co-opted for novel functions. Our survey uncovered a rare example of a nuclear Rab and substantiated the existence of a previously unrecognized core Rab clade in eukaryotes. Strikingly, several functionally conserved pathways or structures were found to be associated entirely with divergent Rabs. These pathways may have permitted rapid evolution of the associated Rabs or may have arisen independently in diverse lineages and then converged. Thus, characterizing entire gene families can provide insight into the evolutionary flexibility of fundamental cellular pathways.
Published in the journal: Comprehensive Analysis Reveals Dynamic and Evolutionary Plasticity of Rab GTPases and Membrane Traffic in. PLoS Genet 6(10): e32767. doi:10.1371/journal.pgen.1001155
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001155Summary
Cellular sophistication is not exclusive to multicellular organisms, and unicellular eukaryotes can resemble differentiated animal cells in their complex network of membrane-bound structures. These comparisons can be illuminated by genome-wide surveys of key gene families. We report a systematic analysis of Rabs in a complex unicellular Ciliate, including gene prediction and phylogenetic clustering, expression profiling based on public data, and Green Fluorescent Protein (GFP) tagging. Rabs are monomeric GTPases that regulate membrane traffic. Because Rabs act as compartment-specific determinants, the number of Rabs in an organism reflects intracellular complexity. The Tetrahymena Rab family is similar in size to that in humans and includes both expansions in conserved Rab clades as well as many divergent Rabs. Importantly, more than 90% of Rabs are expressed concurrently in growing cells, while only a small subset appears specialized for other conditions. By localizing most Rabs in living cells, we could assign the majority to specific compartments. These results validated most phylogenetic assignments, but also indicated that some sequence-conserved Rabs were co-opted for novel functions. Our survey uncovered a rare example of a nuclear Rab and substantiated the existence of a previously unrecognized core Rab clade in eukaryotes. Strikingly, several functionally conserved pathways or structures were found to be associated entirely with divergent Rabs. These pathways may have permitted rapid evolution of the associated Rabs or may have arisen independently in diverse lineages and then converged. Thus, characterizing entire gene families can provide insight into the evolutionary flexibility of fundamental cellular pathways.
Introduction
Cells can respond to and shape their environments by taking up and releasing macromolecules. Both in - and outbound transport is facilitated by a network of membrane-bound compartments that represent one of the hallmarks of eukaryotic cells [1]. Traffic through the network is highly regulated, and a convergence of data from structural, functional, and evolutionary studies demonstrate that gene families encode proteins functioning as conserved specificity determinants for endocytic and exocytic compartments [2], [3]. They do so primarily by controlling the formation, targeting and fusion of vesicles that transport cargo between compartments [4]. One family of key determinants are monomeric GTPases called Rabs, which function as molecular switches by interacting with membrane bilayers and diverse protein effectors in cycles controlled by GTP binding and hydrolysis [5].
A fundamental aspect of Rab function is that multiple Rabs, encoded as a gene family, are co-expressed within a single cell, and the individual family members are each targeted to a small subset of membrane compartments, where they interact with unique effectors [6]. In this manner, a cohort of Rabs can coordinately regulate a pathway consisting of sequential and distinct trafficking events [7]. It is therefore likely that gene duplications within the Rab family, followed by diversification into functionally-distinct variants, were key steps in the evolution of eukaryotic membrane complexity [8], [9]. This model is strongly supported by the tendency of Rabs associated with specific organelles, across a wide range of eukaryotes, to be most closely related to one another. For example, the Rab1 clade has remained highly conserved at the sequence level among all eukaryotic kingdoms; the corresponding proteins, where they have been characterized, are all associated with traffic between the endoplasmic reticulum and cis-Golgi [10]. These experimental findings coupled with phylogenetic parsimony reveal that Rab1 was already a determinant for this step in an early eukaryotic ancestor. It follows that the analysis of lineage-specific expansions or losses in conserved Rab clades can provide insights into the range of evolutionary paths that have led to modern cells.
Because of their central importance, Rabs have been studied in a variety of model and non-model organisms. As expected, the number of Rabs in an organism is generally related to organismal complexity [11], [12] including tissue-specific expression in multicellular organisms [13]. However, a surprising result emerging from several recently-sequenced genomes is that some unicellular organisms express a cohort of Rabs whose number equals or exceeds the 63 Rabs in humans [14]. An extreme example is the parasite Entamoeba histolytica, whose genome encodes 91 Rabs [15]. The sheer number of Rabs hints at an interesting discordance between organismal simplicity and cellular complexity, the latter facilitated at least in part by extensive, lineage-restricted Rab expansions. However, the roles of most Entamoeba Rabs are unknown, and the large Rab number in Entamoeba could endow the cell with flexibility, rather than structural complexity, if different subsets of the Entamoeba Rabs are expressed under the widely disparate conditions that are cyclically encountered by a parasite.
Ciliates have classically been recognized as unicellular organisms of great structural complexity including a wealth of membrane-bound compartments [16]–[22]. Recent sequencing of two ciliate genomes revealed that these organisms are similarly gene-rich [23], [24]. The free-living ciliate Tetrahymena thermophila has more than 20,000 genes and preliminary analysis suggested that these included 70 Rabs, raising the same issues discussed above for Entamoeba [23]. To shed light on the nature and evolution of unicellular cell complexity, we have taken a whole genome approach by considering the entire set of Rabs in Tetrahymena, including phylogenetic analysis, expression profiling, and localization of GFP-tagged variants in living cells. While many of the cellular pathways illuminated by this study represent ancient, highly conserved functions, the unbiased nature of our survey also uncovered a surprising level of both evolutionary and cell-stage flexibility within Rab GTPases, key determinants of membrane traffic.
Results
Tetrahymena encodes a set of predicted Rabs as extensive as those in many multicellular organisms
Beginning with amino acid sequences of human Rabs, we identified 56 Rabs in the ciliate Tetrahymena thermophila. This does not include seventeen putative Rabs that were reported in a previous survey [23], because these were found to lack the specific C-terminal residues to which prenyl residues are attached in authentic Rabs. These 17 predicted genes should therefore be reassigned as Rab-like proteins [25]. We then analyzed all putative Tetrahymena Rabs, including 3 that were not detected in the previous survey, for the presence of five Rab-specific motifs: IGVDF, KLQIW, RFRSIT, YYRGA and LVYDIT [26]. While many of the Tetrahymena Rabs have diverged to various degrees at some of these consensus sites, these genes are nonetheless clearly more similar to Rabs than to any other class of small GTPase, i.e., Ras, Rho/Rac, Sar/Arf or Ran (Figure S1; GenBank ID numbers provided in Table S1). Consistent with this, BLAST searches based on any of the 56 Rabs identified only other Rabs as best hits.
Tetrahymena, although a single-celled organism, therefore has a somewhat larger set of Rabs than in D. melanogaster or C. elegans, and a much larger set than is present in model fungi (S. cerevisiae or S. pombe) or parasitic protists such as T. brucei or P. falciparum, the latter belonging to the clade most closely related to Ciliates, the Apicomplexans(Table 1). The number of Rabs is similar, however, to those reported for some other single-celled organisms, such as T. vaginalis. The large number of predicted Rabs suggests that some protists maintain networks of membrane compartments that are at least similar in complexity to those in multicellular organisms. However, no comprehensive analysis of the Rabs in any of these organisms has been reported, and the large number of Rabs could reflect alternative expression of paralogs to optimize a relatively simple network of compartments for changes in environmental conditions or lifestages.
Tab. 1. The genomes of some unicellular organisms encode large numbers of predicted Rabs, similar to or exceeding those of multicellular organisms. 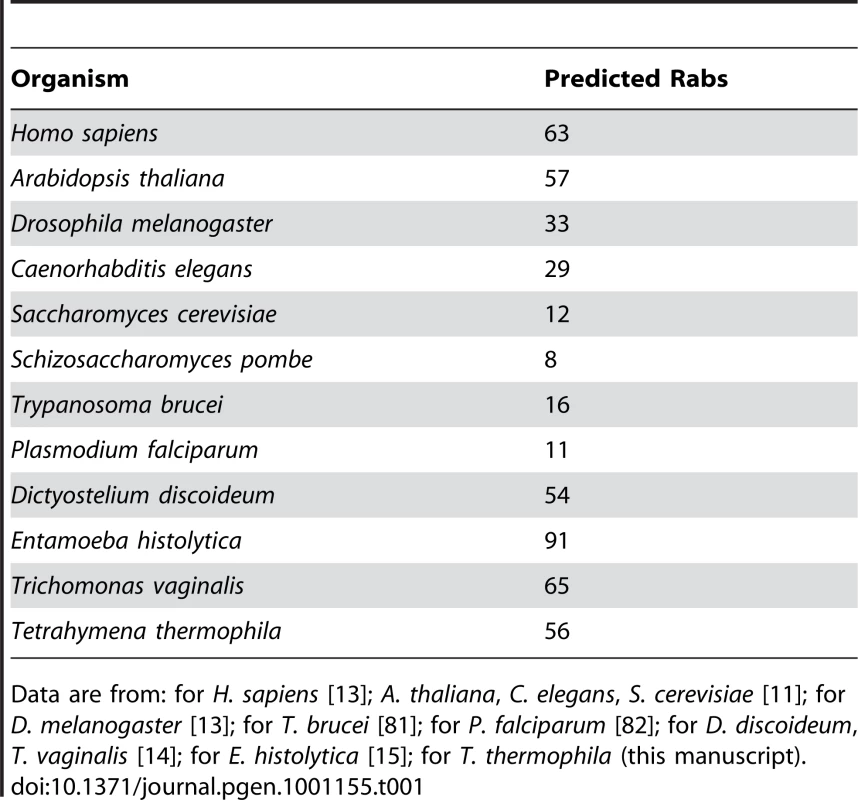
Data are from: for H. sapiens [13]; A. thaliana, C. elegans, S. cerevisiae [11]; for D. melanogaster [13]; for T. brucei [81]; for P. falciparum [82]; for D. discoideum, T. vaginalis [14]; for E. histolytica [15]; for T. thermophila (this manuscript). Concurrent expression of most Tetrahymena Rabs indicates a large number of concurrent pathways
The transcript abundance of all Tetrahymena genes has been measured via whole genome microarrays, using mRNA samples derived from growing (medium and high density) and stationary cultures in growth medium, 7 successive time points during starvation, and 10 successive time points during conjugation [27]. This dataset is particularly useful since transcription plays a central role in the control of differential gene expression in Tetrahymena [28]. Using the publicly accessible database at http://tged.ihb.ac.cn, we found that the transcript abundance of all 56 Rabs is above background and, strikingly, 86% are in the highest-expressing class of genes in this organism [27]. Therefore, none is likely to be a pseudogene. Importantly, 94% of the Rabs are transcribed in growing cell cultures, indicating that membrane traffic in growing Tetrahymena involves more than 50 distinct Rab proteins. Thus the large number of Rab genes is likely to reflect a large number of pathways of membrane traffic that function concurrently in a complex cell, rather than a small number of pathways that are controlled by alternative Rab isoforms in a stage-specific fashion. This analysis also suggested that the majority of Tetrahymena Rabs could be meaningfully localized in cells from growing cultures.
Both morphological as well as some molecular studies have revealed extensive changes in membrane traffic when Tetrahymena are starved [29]–[32], and additional changes during conjugation including the elaboration of a cell-cell fusion zone and the creation of some, and loss of other, nuclei [33]–[35]. The expression data suggest that these changes involve two different phenomena with regard to Rab expression (Table S1). First, RabsD16 and D12 are not expressed in growing cultures but are greatly induced at specific stages in starvation or conjugation: the former at 6h of starvation, the latter most dramatically at 9h of starvation and at the beginning of conjugation (Figure S2). Secondly, many Rabs that are expressed in growing cells are redeployed in starvation or conjugation. In particular, many of the Tetrahymena Rabs that are expressed in growing cells are also expressed in starved or conjugating cells, and indeed most show even higher transcript levels in one of these conditions (65% in starved, and 27% in conjugating cells). However, because average translation efficiency is much greater in growing than in starved cells [36], the changes in transcript levels under different culture conditions do not necessarily reflect corresponding protein abundance. We therefore looked for peaks of Rab expression within each growth condition, as an indication that some Rabs might be determinants of precise stage-specific structures in starvation or conjugation. Strikingly, 20 Rabs show a discrete >2-fold expression peak at a distinct time point during starvation (Rabs D22, D16, D21, all near S6, i.e., the 6h starvation timepoint) or during conjugation (C0, i.e., the beginning of conjugation: Rabs D29, D7; C2, i.e, the 2h conjugation time point: Rabs 11A, D26, D33, D24, D7, D23, D31, D38, 21; C4: Rabs D35, 11C; C6: Rabs 4A, D13; C14: RabD34; C16: Rabs D21, D15; C18: Rabs D22, D16)(Table S1). The preponderance of relative Rab expression peaks at C2 corresponds with extensive membrane restructuring during pair formation. In addition, many Rabs show a large expression peak at S0; however, this time point is difficult to interpret because it corresponds to a change in the culture medium.
Many Rabs can be assigned to known pathways of membrane traffic based on localization of GFP-fusions
Figure 1 gives an overview of known or inferred pathways of membrane traffic in Tetrahymena, as well as those in a generalized mammalian cell. To identify Tetrahymena Rabs dedicated to specific pathways or structures, we expressed them individually as GFP-tagged proteins and determined the localization in living Tetrahymena. GFP-Rab expression was induced at the lowest level permitting clear visualization, and confirmed using Western blotting of Tetrahymena lysates (Figure S3, with two exceptions for Rabs expressed at low levels). For practical reasons, most Rabs were localized solely in growing cultures. In preliminary studies, we found evidence that Rabs whose endogenous expression was largely limited to starvation or conjugation were de-localized when expressed in growing cells (Figure S2), and therefore we did not include that small set of Rabs in this study. We captured time-lapse and/or full z-stacks of cells expressing each of the Rabs in living, immobilized cells. Movies of all Rabs described in this paper are publicly accessible at tetrahymenacell.uchicago.edu. A number of the Rabs were seen to associate with more than one structure (Table S2). In these cases we considered the stronger signal as primary, and the data below are organized based on these primary signals. In rare cases, we saw equally strong labeling of two structures.
Fig. 1. Pathways of membrane traffic in Tetrahymena and in animal cells. 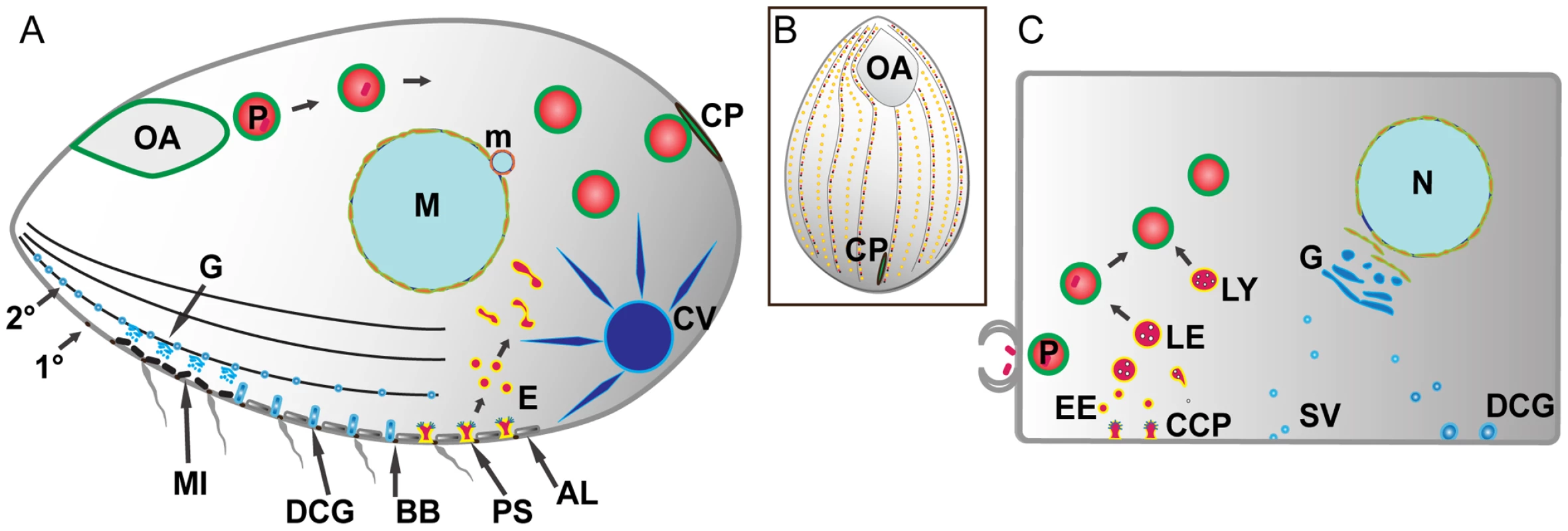
A. Tetrahymena cell cartoon. Cell length is ∼50µM; anterior to the left. A prominent pathway of phagocytosis begins at the anterior oral apparatus (OA), resulting in phagosomes (P) that eventually egest undigested material at the cytoproct (CP). The OA and CP exist as single-copy structures at fixed positions. Clathrin-mediated endocytosis occurs at multiple, regularly-spaced invaginations called parasomal sacs (PS), giving rise to endocytic vesicles (E). These endocytic vesicles coalesce in tubulovesicular endosomes in the cell posterior. Protein secretion occurs via the endoplasmic reticulum (not shown) and Golgi (G), which is present as single cisterna or short stacks near the cell periphery, close to mitochondria (MI). Some of the secretory cargo is packaged into dense core granules (DCG), which dock and subsequently undergo exocytosis at sites on 1° and 2° meridians (1° and 2°), which are cytoskeletal “ribs”. A prominent organelle, whose membrane dynamics are poorly understood, is the water-pumping contractile vacuole (CV). Other structures detailed in the Tetrahymena cell: AL: alveolae: a calcium-storage compartment. BB: ciliary basal bodies. M: macronucleus (polyploidy vegetative nucleus). m: micronucleus (diploid germline nucleus). B. All surface features in Tetrahymena except the OA and CP are repeated at fixed positions in a grid that can be defined by the array of 1° and 2° meridians. C. Analogous structures and pathways are diagrammed in a generalized mammalian cell. The abbreviations are the same as in the Tetrahymena cell with the additional structures: CCP: clathrin-coated pit. EE: early endosomes. LE: late endosomes. LY: lysosomes. SV: secretory vesicles. An active pathway of phagocytosis involves a large subset of the Tetrahymena Rabs
Ciliates like Tetrahymena can feed via the phagocytic uptake of bacteria or other particulate matter via a cavity at the cell anterior called the oral apparatus (Figure 2A) [37], [38]. Bacteria are swept into the oral apparatus by cilia at the oral apparatus rim, and ingested at the base. Following ingestion, the bacteria are digested via phagosome maturation resembling that in mammalian cells, including fusion of phagosomes with both early and late endocytic compartments. The final stage of phagosome maturation, which has no precise equivalent in mammalian cells, entails docking and fusion with a structure at the cell cortex called the cytoproct, which results in the egestion of any residual material in the phagosome lumen. Egestion is followed by a burst of membrane retrieval, in a process (documented by EM in another ciliate, Paramecium micronucleatum) that results in the rapid appearance of endosomes in the cytoproct region. These endosomes appear to be transported anteriorly along cytoplasmic microtubules, where they may contribute membrane during the formation of phagocytic vesicles from the oral apparatus [39].
Fig. 2. Rabs associated with phagocytosis. 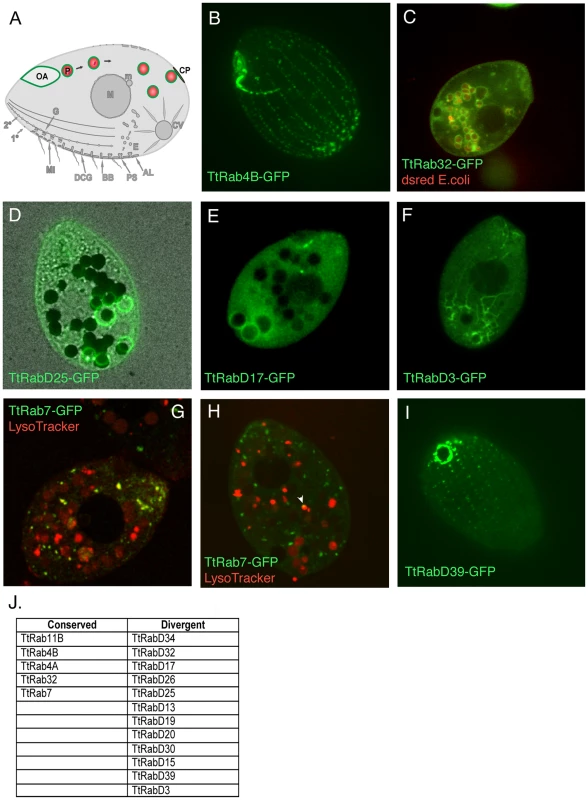
A. Phagosomes (P) form at the oral apparatus (OA), undergo maturation and transport in the cytoplasm in a process involving membrane fusion and fission, and eventually fuse with the plasma membrane at the cytoproct (CP). This is followed by membrane retrieval and transport of endosomes back to the oral apparatus. B–I. All panels are confocal images of live cells following induction of GFP-Rab expression for 2 hours in S media, unless otherwise indicated. GFP-TtRabs are associated with distinct structures: (B) oral apparatus; (C) all phagosomes; (D) selected phagosomes; (E) cytoproct-associated phagosomes; (F) cytoplasmic microtubules extending from posterior phagosomes to the cell anterior. Phagosomes in C–F contain either dsRed-expressing E. coli (red) or india ink particles (black). G. For cells in SPP medium, LysoTracker (red) labels both phagosomes (larger red vacuoles) and smaller vesicles (red and yellow) that are likely to be lysosomes. TtRab7 colocalizes with the latter, which are concentrated in the anterior cytoplasm. H. In cells cultured in S medium, the colocalization of GFP-TtRab7 with LysoTracker is limited chiefly to very small puncta at the periphery of phagosomes (arrowhead). I. cytoproct. The Tetrahymena shown are ∼50×20µM. J. The full set of TtRabs associated with phagocytosis. Remarkably, almost 1/3rd of the Tetrahymena Rabs were found to be associated with some aspect of the phagocytic pathway (examples shown in Figure 2; full set in Figure S4). Five of these are localized primarily to structures at or near the oral apparatus, while eleven are localized to phagosomes that can be unambiguously identified in cells that have ingested either fluorescent bacteria or India ink (Figure S4) (Table 2). Some GFP-Rab fusion proteins are associated with the entire cohort of phagosomes in a cell (Figure 2C), but many of the Rabs are associated with only a subset of the phagosomes (Figure 2D), which may correspond to stages in maturation. Consistent with the specialization of the cytoproct as a zone of membrane fusion and retrieval, three of the phagosome Rabs are restricted to phagosomes that are localized at or near the cytoproct (Figure 2E), and time-lapse movies of cells expressing these GFP-Rab fusions allowed us to directly visualize phagosome egestion and retrieval (Videos S1, S2, S3, S4).
Tab. 2. Summary of all TtRabs based on primary localization pattern. 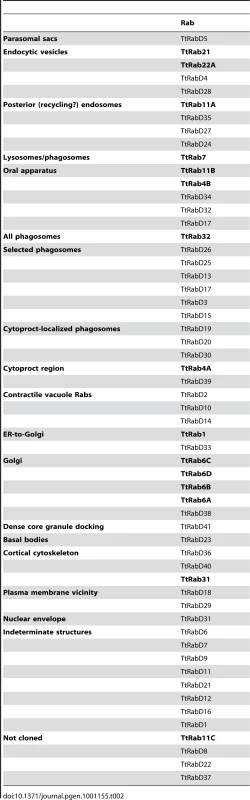
TtRab7, the Rab7 homolog, was previously associated with phagosomes in a proteomics study [37]. We found that this protein localizes both to lysosomes (as defined by co-labeling with LysoTracker) and to small puncta on the surface of phagosomes (Figure 2G and 2H).
TtRabD17 localizes to phagosomes in the vicinity of the cytoproct but also localizes strongly to the oral apparatus, suggesting the possibility of long-distance transport between these structures. Consistent with this, the live imaging of TtRabD3 provided a stunning illustration of transport over 40–50µM between late phagosomes, in the extreme posterior of the cell, and the cell anterior (Figure 2F, Video S5). Inferring from the EM studies in Paramecium, this transport is likely to involve endosomes generated during membrane retrieval following phagosome egestion, which are transported to the oral apparatus. The localization of RabsD17 and D3 suggests the possibility that some Rabs could contribute to coherence within a multi-step pathway by functioning at both early and late steps. Finally, one Rab is localized at the cytoproct itself (Figure 2I).
Endocytosis is associated with expansions in both conserved and divergent Rabs
Tetrahymena has a classical endocytic pathway involving clathrin - and dynamin-dependent formation of small endocytic vesicles, which arise from hundreds of depressions at the plasma membrane called parasomal sacs [40]. These structures occur at regularly-spaced sites along well-defined ciliary rows, also called 1° meridians (Figure 1A and 1B) [41]. The endosomes arising from parasomal sacs can be labeled with the styryl dye FM1-43 [42]. There may also be other pathways of uptake from the plasma membrane, as revealed by residual FM1-43 uptake under conditions of clathrin or dynamin inhibition [42], but this has not been rigorously examined.
We identified endosome-associated GFP-Rabs as those Rabs showing significant colocalization with the styryl dye FM4-64, after first confirming that FM4-64 labels the identical compartments as FM1-43 (Figure S5A). In total, nine of the Rabs in Tetrahymena co-localized with FM4-64 (examples in Figure 3; full set in Figure S6). Only a subset of these Rabs were related to endocytic or endocytic/recycling Rabs from other organisms (Figure 3E). The Rabs show a variable level of overlap with the endocytic tracer, consistent with the idea that multiple, sequential compartments are labeled (Figure S5C). Moreover, the endocytic Rabs can be divided into three broad classes. The 1st group, a single Rab, labels what are likely to be parasomal sacs (Figure 3B). The 2nd group labels small vesicles that are widely distributed throughout the cell (Figure 3C). The 3rd group labels larger vesicles or tubular structures that are concentrated in the cell posterior, often in clumps. Based on previous studies, these are likely to represent a compartment to which FM1-43 gains access several minutes post internalization, and which may function as recycling endosomes (Figure 3D).
Fig. 3. Rabs associated with endocytosis. 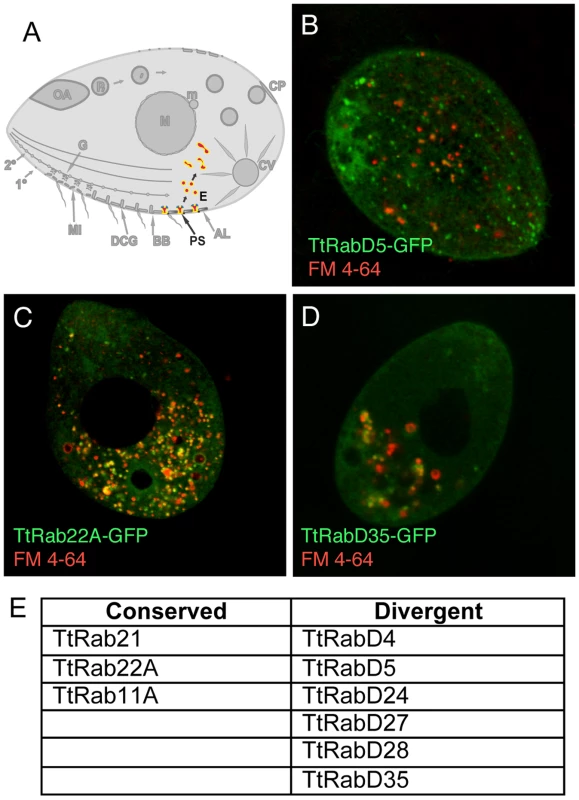
A. Clathrin-mediated vesicle formation at parasomal sacs (PS) generates endocytic vesicles (E) that coalesce in clumps of tubulovesicular structures at the cell posterior, which may function as recycling endosomes. FM4-64 (red) functions as an endocytic tracer. All panels are confocal images of live cells following induction of GFP-Rab expression for 2 hours in S media, unless otherwise indicated. B. TtRabD5 localizes to parasomal sacs. C. TtRab22A localizes to small abundant cytoplasmic vesicles. D. TtRabD35 localizes to large endosomes toward the cell posterior. E. The full set of TtRabs associated with endocytosis. A relatively small group of Rabs appears associated with the outbound secretory pathway
Tetrahymena secrete newly synthesized proteins via at least 3 recognized pathways beginning with translocation into the endoplasmic reticulum (ER). The Tetrahymena ER is present as both a cortical as well as cytoplasmic reticulum, while the Golgi are present in multiple copies as small stacks of cisterna that are adjacent to mitochondria, close to the cell periphery [43] (Figure 4A). The 3 known secretory pathways are functionally equivalent to known pathways in mammalian cells: a pathway of rapid constitutive secretion, a pathway of secretion via exocytic fusion of lysosomes, and regulated exocytosis from dense core granule-like bodies called mucocysts. However, the only exocytic sites or secretory carriers that have been directly visualized in Tetrahymena are the dense core granules, which dock and undergo exocytic fusion along both the 1° and intervening 2° meridians, at junctions between adjacent alveoli [44].
Fig. 4. Rabs associated with secretion. 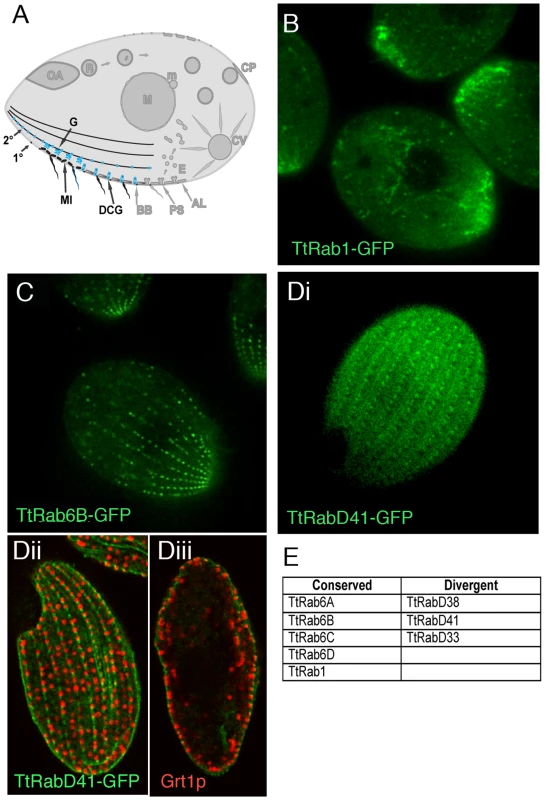
A. Known compartments in the secretory pathway. The endoplasmic reticulum in Tetrahymena may also include vesicular forms [32] (not shown). Golgi (G) are present as many individual cisterna or short stacks near mitochondria (MI) at the cell periphery. The most prominent secretory vesicles are dense core granules (DCGs), which after maturing in a post-Golgi pathway are transported to stable docking sites along 1° and 2° meridians, where they undergo exocytosis upon extracellular stimulation. B–D. All panels are confocal images of live cells following induction of GFP-Rab expression for 2 hours in S media, unless otherwise indicated. B. TtRab1 is predicted to function in ER-to-Golgi traffic. C. TtRab6B shows the expected distribution for a Golgi-localized Rab. Di. TtRabD41 localizes to puncta at 1° and 2° meridians. Dii and Diii. (Confocal slices of fixed, immunostained cells) TtRabD41 (green) shows extensive colocalization with a DCG marker, Grt1p (red). Dii: optical slice of cell surface; Diii: optical slice through cell midbody. E. The full set of TtRabs associated with secretion. Tetrahymena has a single, very highly expressed member of the Rab1 clade, associated with ER-to-Golgi traffic. This association is robust in Bayesian and Neighbor Joining trees, but not Maximum Likelihood (Figure S9). TtRab1 shows a complex localization pattern, labeling mobile puncta that are primarily concentrated at the anterior end of the cell, and tubular or reticular structures both in the anterior and posterior of the cell (Figure 4B). A 2nd, divergent Rab shows similar localization (Figure S7B).
Five Rabs, four of which belong to a conserved Golgi-associated clade, appear to localize to large puncta in a loose meridional array expected for Golgi stacks, and similar to that of a putative Golgi marker, Cda12p [45] (Figure 4C; Figure S7C, S7D, S7E; Video S7). Also consistent with the known location of Golgi, these puncta are positioned near but not at the cell periphery.
TtRabD41 localizes to regularly-spaced puncta at both 1° and 2° meridians (Figure 4Di) and appears to co-localize with the secretory granule marker Grt1p [46], in a pattern suggesting that this Rab may localize to granule docking sites (Figure 4Dii and 4Diii). Since the Rab is also present at cortical sites between granules, it may also localize to unoccupied docking sites.
A divergent set of Rabs is associated with a specialized organelle, the contractile vacuole
The contractile vacuole functions to collect water from the cytoplasm and pump it to the cell exterior, to maintain osmotic balance. The complex structure includes a contractile bladder, from which water-collecting tubules extend into the cytoplasm (Figure 5A) [47]. Three divergent and unrelated Tetrahymena Rabs primarily labeled the contractile vacuole (Figure 5F). TtRabD14 labels large vesicles that are clearly associated with the contractile vacuole but distinct from the central bladder, while TtRabD10 labels tubular extensions thereof (Figure 5B and 5C). TtRabD2 shows diffuse labeling always centered on the contractile vacuole (Figure 5D). In addition, several other GFP-Rabs show secondary contractile vacuole localization (Table S2).
Fig. 5. Rabs associated with the contractile vacuole. 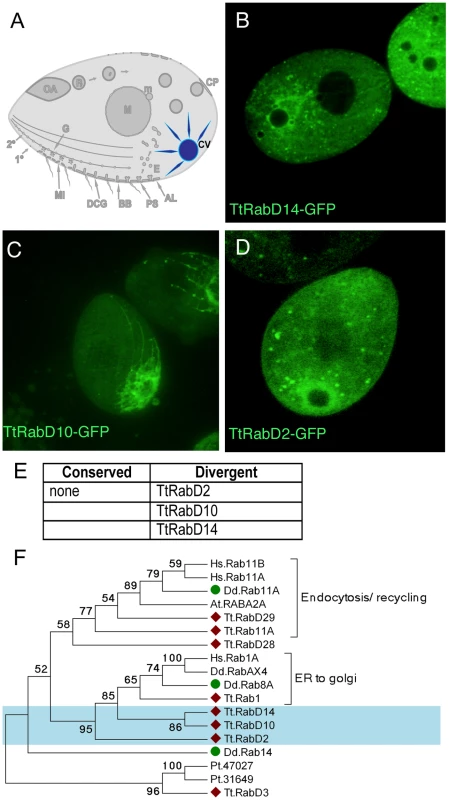
A. The contractile vacuole (CV) collects water through a set of radial arms that connect to a central bladder, from which water is pumped to the cell exterior during periodic contractions, via pores (not shown) on the cell cortex. B–D. All panels are confocal images of live cells following induction of GFP-Rab expression for 2 hours, unless otherwise indicated. Three Rabs, each with a distinct distribution, are primarily localized to the contractile vacuole. E. The full set of TtRabs associated primarily with the contractile vacuole; other Rabs showing 2° localization to the contractile vacuole are listed in Table S2. F. Maximum likelihood tree showing the relationship between the Tetrahymena contractile vacuole Rabs, 3 Dictyostelium contractile vacuole Rabs [79], and other Tetrahymena Rabs that align with the Dictyostelium contractile vacuole Rabs. Tetrahymena Rabs are labeled with diamonds; Tetrahymena contractile vacuole Rabs are shaded blue; Dictyostelium contractile vacuole Rabs are labeled with green circles. In all cases, the Tetrahymena Rabs most closely related to Dictyostelium contractile vacuole Rabs are not themselves associated with the contractile vacuole. Active water pumping is required for all cells that lack cell walls and inhabit fresh water, and contractile vacuoles are accordingly found in diverse unicellular lineages. In Dictyostelium discoideum, a slime mold very distantly related from Ciliates, the Rabs associated with the contractile vacuole have been described [48] (and refs therein). Based on phylogenetic analysis, none of the Tetrahymena contractile vacuole Rabs appears orthologous to any of the functionally related Dictyostelium Rabs (Figure 5F).
The remaining Tetrahymena Rabs, including proteins that localize to the nuclear envelope, cell cortex, ciliary basal bodies, and numerous other structures, are illustrated in Figure S8.
Tetrahymena Rab phylogeny helps to elucidate the origins of membrane traffic
We used maximum likelihood, Bayesian, and neighbor-joining methods to understand the relatedness of the Tetrahymena Rabs to those in other lineages (Figure S9). Some of the bootstrap values for nodes linking the Ciliate sequences with those of other lineages were low, as expected in light of the known deep evolutionary divergence between the Alveolates (Ciliates, Dinoflagellates, Apicomplexans) and other lineages [49]. Nonetheless, these reconstructions produced strong evidence that 15 of the Tetrahymena Rabs align in clades with Rabs in animals and other lineages, and can therefore be considered as highly conserved. The conserved Tetrahymena Rabs fall within six predicted functional groups (Figure 6). Five of these clades were previously defined, by comparison of many eukaryotic genomes, to belong to a core set of eight whose wide distribution in existing eukaryotes implies that all may have been present in the last common eukaryotic ancestor [11]. The Tetrahymena Rabs in this group are predicted, based on sequence similarity, to be associated with ER-to-golgi traffic (intermediate compartment) (1 Rab), endocytosis (3 Rabs), endocytic recycling (5 Rabs), late endocytosis (1 Rab), and retrograde golgi traffic (4 Rabs). Tetrahymena does not appear to have representatives within three remaining core Rab clades. Two of these, including human Rabs 8,10,13, 19 and 30, are associated with Golgi traffic. The third clade is associated with regulated exocytosis, and includes human Rabs 3 and 27.
Fig. 6. A subset of the TtRabs is highly conserved. 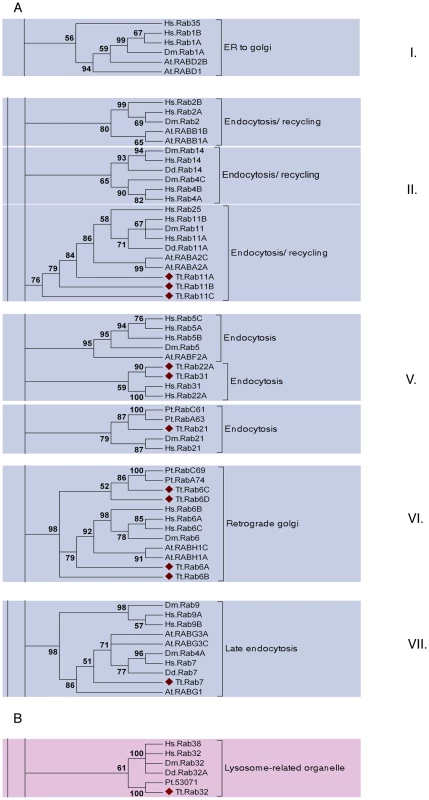
Fifteen (27%) of the Tetrahymena Rabs align within highly-conserved clades, together with Rabs of known function in other lineages. Shown are panels from the Maximum Likelihood tree in Figure S9, highlighting the 6 clades containing 12 of the conserved Tetrahymena Rabs (marked with diamonds). Three additional Rabs were judged to be conserved because they aligned within these conserved clades using both Neighbor-Joining and Bayesian methods (Figure S9): they are TtRab1 (ER-to-Golgi), TtRab4A and TtRab4B (Endocytosis/Recycling). A. Five of the 6 clades (ER to golgi, endocytosis/recycling, endocytosis, retrograde golgi and late endocytosis) belong to a group of 8 Rab clades that have been identified as a core group that is broadly conserved among eukaryotes. The core groups have been numbered following [11]. B. The sixth conserved clade, not previously considered as a core group, contains Rabs in Opisthokonts (H. sapiens, D. melanogaster), Amoebozoans (Dictyostelium discoideum) and Chromalveolates (Tetrahymena thermophila and Paramecium tetraurelia). Animal Rabs in this clade are associated with lysosome-related organelles [80], while the Dictyostelium Rab has not been functionally characterized. Strikingly, a single Tetrahymena Rab does not belong to the previously defined core set, but nonetheless aligns with a clade with representatives in both Opisthokonts (H. sapiens, D. melanogaster) and Amoebozoa (D. discoideum) (Figure 6B). The animal Rabs in this group, which are all associated with transport of lysosome-related organelles, all share a WDIAGQE motif, which is also found in both the Dictyostelium and Tetrahymena members but not in Rabs from any other clade [50]. We propose that this clade, with representatives from at least three deeply divergent lineages, represents a previously unrecognized core Rab clade in eukaryotes.
Sequence-conserved Rabs, while generally being associated with the predicted pathways, show significant exceptions that may reveal new pathways
One feature that emerged from comparison of phylogenetic assignments and localization data was that some highly conserved Rabs failed to show clear association with the expected compartments. In particular, four of the putative endosomal Rabs showed little or no colocalization with FM4-64. Three of these were localized to structures associated with the phagocytic pathway. Two of these, 11B and 4B, labeled highly mobile puncta at the oral apparatus, whose lack of overlap with FM 4-64 indicates that these are not vesicles pinching off from the plasma membrane (Figure S4A, S4D). This is graphically reinforced by time-lapse movies of cells expressing TtRab11B-GFP, which show that labeled puncta, which are likely to be vesicles, are traveling toward the oral apparatus along a cytoplasmic fiber (Video S6), where they may be delivering bulk membrane required for phagocytosis [39]. TtRab4A labels vesicles that accumulate near the cytoproct, which is a zone where end-stage phagosomes fuse and eject undigested contents [51]. The fourth Rab in this group, TtRab31, localized to puncta along cortical meridians (Figure S8A).
Discussion
While the initial annotation of the Tetrahymena macronuclear genome provided a rough sketch of the genetic underpinnings of cellular complexity, our focused analysis of a single gene family illuminates the pathways of membrane traffic in these cells, including their dynamics and evolutionary origins. T. thermophila encodes at least 56 Rabs and 17 Rab-like proteins, the latter lacking the residues required for prenylation [52]. Additional Tetrahymena Rabs may have escaped detection if extreme sequence divergence precluded their detection by our homology-based approach or if those genes were present on remaining gaps in the sequenced genome, although EST-based evidence suggests that few highly expressed genes can be present in such gaps [53].
More than 90% of the Tetrahymena Rabs are expressed under typical growth conditions. This suggests that the size of the gene family reflects the need to specify a large set of concurrent steps, assuming that most Rabs serve unique functions. While four conserved Rabs showed similar expression patterns as well as localization to putative Golgi, few other Rabs appeared to share identical localization patterns, and even Rabs associated with a single structure often showed subtle differences in distribution. These observations are also consistent with the high degree of sequence divergence between family members. For example, with the exception of a single pair of 84% identical Rabs that may be related by gene conversion (TtRabD21 and TtRabD22), there are few sequence-similar Rabs in Tetrahymena. Moreover, even pairs such as Rabs D5 and D17, which are 70% sequence identical, show clearly distinct patterns of localization as well as expression.
Roughly 27% of the Tetrahymena Rabs aligned with orthologs in other eukaryotic lineages. The modest fraction may reflect a relatively fast rate of evolution of genes in Ciliates relative to other organisms [54], potentially compounded by limitations on phylogenetic analysis due to the paucity of sequenced ciliate genomes and the absence of any sequenced genome from the sister Dinoflagellate clade. However, non-Ciliate protists with large Rab cohorts show a similar preponderance of divergent Rabs: 51/65 in T. vaginalis, and 69/91 in E. histolytica [14], [15]. In comparison, the paralogous radiation within Rabs of animals and plants resulted primarily in expansions of conserved Rab clades: 35 of 60 H. sapiens Rabs are conserved, as are 48 of 57 in A. thaliana [55]. These disparities may suggest that multicellularity, when it arose in a lineage, imposed subsequent constraints on the evolution of intracellular membrane traffic.
Using Rab sequences mined from divergent eukaryotic genomes, it was deduced that an ancestral eukaryotic cell contained Rabs corresponding to eight core pathways [11]. We used phylogenetic analysis to gauge the allotment of the 15 sequence-conserved Tetrahymena Rabs to these pathways. Surprisingly, one of the conserved Rabs did not fall into any of the proposed core clades, but nonetheless aligned with Rabs in both animals and Dictyostelium, with which it also shared a unique motif [50]. The animal Rabs in this clade are associated with transport of lysosome-related organelles, while the Tetrahymena protein localized to phagosomes. Because the related Dictyostelium Rab has not been characterized, there are not yet sufficient data to infer an ancestral function for what we propose to be a deeply conserved clade. The remaining 14 conserved Rabs in Tetrahymena fall within five of the proposed core pathways [11]: ER-to-golgi (I),endocytosis/recycling (II), endocytosis (III), retrograde golgi (VI) and late endocytosis (VII). The localization data suggest that paralogs within the endocytic and endocytic/recycling clades are likely to reflect multiple specialized pathways, while the paralogs within the retrograde Golgi clade may potentially reflect a dosage requirement. The idea that endocytic pathways have undergone greater expansion and diversification over evolutionary time, compared with some other pathways, was also inferred from SNARE phylogeny [56],[57]. Tetrahymena appear to have lost the core clades corresponding to golgi-related (IV and VIII), and regulated exocytosis (III) pathways. Such lineage-restricted loss of specific core Rabs has previously been noted, for example in fungi [58]. Since Tetrahymena has a prominent pathway of stimulus-dependent protein release, the absence of Rabs in the conserved regulated exocytosis clade is consistent with an independent origin of secretory granules in ciliates [59]. Tetrahymena lacks an ortholog of human Rab8, which is required for ciliogenesis [60]. A similar function may be played by a divergent Rab in ciliates, although we did not identify such a Rab in this survey.
Dynamic flexibility of Rab GTPases
Growing vs. starved Tetrahymena differ in pathways involved in feeding and secretion, while conjugation brings pronounced changes to the cell cortex, and to nuclear and other organelles [22]. We found that membrane traffic in starvation and conjugation chiefly involve the same set of Rabs that are expressed in growing cells. However, several Rabs are exclusively expressed in growth and/or starvation, some showing dramatic expression peaks at distinct stages.
To understand the contributions of Rabs to cellular plasticity on an evolutionary timescale, one pertinent question is whether specific subsets of Rabs arose in Ciliates. Among the non-conserved Rabs, a subset of 14 aligned using all tree-building methods into seven clades containing only other Ciliate genes (Figure 7). Four of these clades are entirely composed of T. thermophila genes, suggesting four relatively recent paralogous expansions. In contrast, the remaining T. thermophila Rabs aligned in 3 clades with Rabs in Paramecium tetraurelia. These 14 Rabs may tentatively be considered as lineage-restricted. One interesting question is whether Ciliate-restricted Rabs are preferentially associated with pathways that arose in this lineage. Four of the currently recognized lineage-restricted Rabs localize to the oral apparatus, contractile vacuole, or cytoproct, which are structures that are likely to have undergone extensive elaboration, at a minimum, in the Ciliate lineage. The remainder localize to endosomes, the cell cortex, phagosomes, and as-yet undefined structures.
Fig. 7. At least fourteen Tetrahymena Rabs appear lineage-restricted. 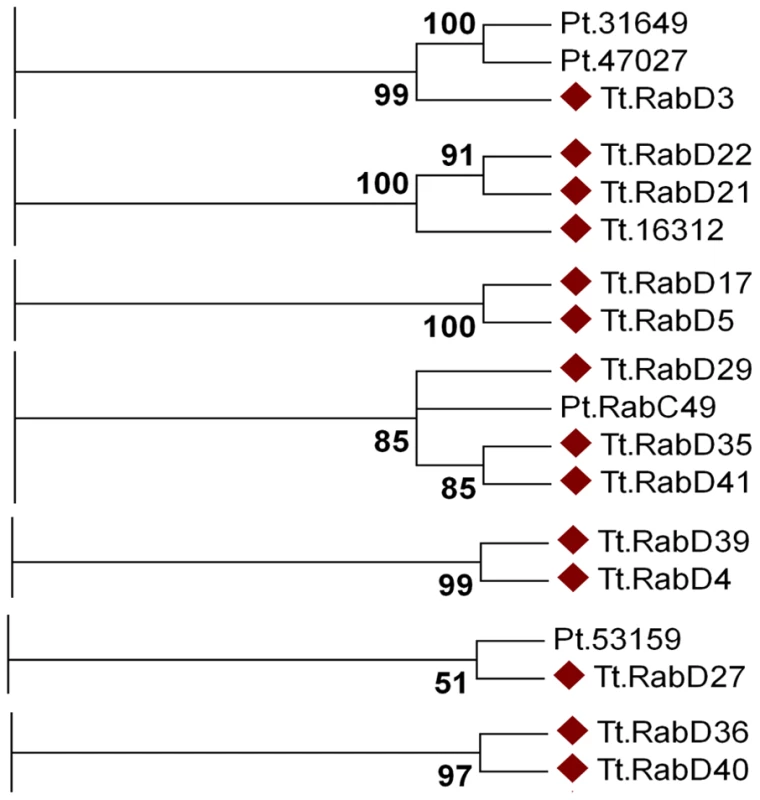
Shown are panels from the Maximum likelihood tree in Figure S9, highlighting the 7 clades containing lineage-specific Tetrahymena Rabs (marked with diamonds). Three clades also contain Paramecium Rabs. The dataset also allowed us to ask whether structures or pathways that appear deeply conserved have conserved Rab determinants. In humans, 18 Rabs are associated with phagosomes, while 11 Tetrahymena Rabs were found on phagosomes. Only two of these Tetrahymena Rabs are orthologous to those in animals, TtRabs 32 and 7 (Figure 2J). TtRab32 belongs to the Rab32/38 clade, whose members in animals are most strongly linked with transport of lysosome-related organelles. Similarly, Rab7 in both humans and Tetrahymena is associated with lysosomes as well as phagosomes. Therefore, most phagosomal Rabs in humans and Tetrahymena are unrelated, and the related Rabs are primarily associated with lysosomes. This implies either that mechanisms of phagosome maturation arose independently in animals and ciliates, or that this set of Rabs evolved under relatively few constraints in one or both lineages. In this regard, it is noteworthy that some phagosomal Rabs may have arisen within ciliates. The inference that phagosomes in Tetrahymena may have evolved separately from those in animals is consistent with the finding that phagosome-associated syntaxins in another Ciliate, Paramecium tetraurelia, form a lineage-restricted clade [61]. Similarly, we found no relatedness between Rabs associated with the contractile vacuole in Tetrahymena and in Dictyostelium. These results suggest that, in some cases, the similar cellular structures or pathways in different lineages do not primarily reflect constraints on an inherited ancestral pathway but rather parallel selective pressures leading via innovation to similar outcomes. This would depend, in the context of this paper, on Rabs that evolve novel functions, of which there are several good illustrations in the Tetrahymena cohort. First, several phylogenetically conserved Rabs were not associated with the predicted compartments, a finding that also underscores the importance of doing phylogenetic and localization analysis in parallel. Secondly, there is evidence for rapid Rab evolution within the lineage-restricted Rabs. Some lineage-restricted Rabs appear to have retained similar functions, judging by the cortical localization of TtRabs D41, D29 and D35. In contrast, TtRabs D39 and D4 are nearest neighbors in a single clade, but the former localizes to the cytoproct and oral primordium while the latter is found at endosomes.
In summary, analysis of the large Rab gene family in Tetrahymena has provided an extensive set of new molecular markers for studies in this organism, and has provided insights into cellular and evolutionary aspects of membrane plasticity. Though Ciliate genes may be prone to undergoing fast evolution [54], the observation that Ciliate and non-Ciliate protists show comparable ratios of conserved to divergent Rabs suggests that many of our observations are generalizable. This work sets the stage for functional analysis of informative Rab family members in this organism. More broadly, Rabs belong to a small set of proteins that, as products of large gene families, act as compartmental determinants of membrane traffic. The availability of sequenced genomes from divergent lineages highlights the need for combined approaches such as those taken here, to understand the consequences of gene family expansions and the evolutionary flexibility that is built into fundamental cell biological features, such as the complex network of membrane trafficking pathways that are crucial for homeostasis and signaling.
Materials and Methods
Rab identification and cloning
Beginning with amino acid sequences of known human Rabs (accession numbers in [11]), we previously identified 70 putative T. thermophila Rabs by tblastn searches of the Macronuclear (Mac) genome, using the primary Tetrahymena hits as queries to detect additional Tetrahymena Rabs [23]. We subsequently annotated all genes using ESTs corresponding to 45 Tetrahymena Rabs for which ESTs became available during genome refinement [53], aligning the ESTs with genomic sequences as well as the predicted mRNAs using Muscle (MUltiple Sequence Comparison by Log-Expectation: http://www.ebi.ac.uk/Tools/muscle/index.html), and viewing the results using Seaview (http://pbil.univ-lyon1.fr/software/seaview.html). Confirmed mRNA sequences were translated (including 5′ and 3′ UTRs) in 3 frames to establish the ORF encoding the conserved Rab motifs and predict the start sites, which in general lay shortly upstream of the 1st conserved motif. Stop codons were identified as the 1st in-frame UGA (the sole stop codon used in T. thermophila [62]. In this process, we identified 3 Rabs not detected in earlier work, adjusted several gene predictions, and disqualified 17 previously identified Rabs that were determined to be Rab-like. Forward and reverse primers (Table S3) were designed to initiate from start and stop codons. All Rab genes were PCR amplified from genomic DNA isolated by phenol-chloroform extraction from strain CU428.1, using Pfu Ultra polymerase (Agilent Technologies, Santa Clara, CA), and confirmed by sequencing. GenBank ID numbers of all genes are listed in Table S1.
Phylogenetic tree-building methods
We used three different tree-building methods (maximum likelihood [63], Bayesian [64], [65] and neighbor-joining [66]) including all predicted Rabs from T. thermophila and H. sapiens and selected Rabs from D. melanogaster, A. thaliana, D. discoideum and P. tetraurelia. The basic tree topology presented in this manuscript is supported by all three distinct algorithms. In cases where detailed topological conclusions are supported by 2 out of 3 approaches, this is specified in the text. Protein sequences were aligned using Muscle [67] (http://www.ebi.ac.uk/Tools/muscle/index.html), and gaps and the C-terminal hypervariable region of each Rab (∼30–80 residues) were manually removed from the multiple alignment using Seaview [68] (http://pbil.univ-lyon1.fr/software/seaview.html). The output from alignment and gap removal were 167 total aligned sites, which were then used as input for bootstrap analysis using Seqboot from Phylip [69] (http://evolution.genetics.washington.edu/phylip.html). 100 bootstraps were run. The bootstrapped outfile was used as the input for a maximum likelihood test using the Phyml program (http://atgc.lirmm.fr/phyml/), using the WAG substitution model. For Bayesian analysis, the bootstrapped outfile from Seqboot was used (in Nexus format) to run an analysis using MrBayes (http://mrbayes.csit.fsu.edu/) for 100,000 generations, with sampling every 100 generations.
For Tetrahymena Rabs that failed to align with any Rabs in H. sapiens, S. cerevisiae, or A. thaliana, we sought orthologs from other species using tblastn. The top-scoring putative homologs were then used in reverse BLAST searches to screen the Tetrahymena genome. If the original Tetrahymena Rab gene was the top-scoring hit in this reciprocal BLAST, the genes were considered potential orthologs and added to the large phylogenetic tree analysis. The chief outcome of this approach was addition of Paramecium genes to the phylogenies.
Rab nomenclature
Conserved Rabs were named according to their human ortholog. Divergent Rabs were numbered D1–D41.
Transcription profiling
The expression profiles of all Tetrahymena Rabs in growing, starved and conjugating cultures were downloaded from the whole genome expression database at http://tged.ihb.ac.cn/ [27].
Expression of Rab-GFPs
The Gateway (Invitrogen) system was used to engineer each Rab as a GFP (Green Fluorescent Protein) fusion. First, PCR-amplified Rab genes were TOPO cloned (Invitrogen) into the pENTR-D-TOPO entry vector. CACC was added to each forward primer in order to allow directional cloning into pENTR-D. The pENTR clones were sequenced and the genes recombined using the Clonase reaction into the target Gateway-based T. thermophila expression vector pIGF-GTW, a gift from Doug Chalker [70]. We used site-specific mutagenesis to change the GFP gene in pIGF-GTW to the monomeric variant (A206K) [71]. Genes subcloned into pIGF-GTW are fused at their C-terminus, via a linker sequence, to the GFP gene, with the fusion under the transcriptional control of the cadmium-inducible MTT1 promoter [72]. When introduced into Tetrahymena, the vector is maintained as an extrachromosomal Mac plasmid and confers paromomycin resistance. TtRabD37 could not be cloned into pENTR; in addition, TtRab11C could not be expressed in Tetrahymena as a protein of the predicted size.
Cell culture and transformation
T. thermophila strains were provided by Peter Bruns and Donna Cassidy-Hanley (Cornell University)(CU428.1) and Eduardo Orias (UC Santa Barbara)(B2086). Cells were grown at 30°C in SPP media (1% proteose peptone, 0.2% dextrose, 0.1% yeast extract, 0.009% ferric EDTA) with shaking. To reduce autofluorescence background in food vacuoles when imaging cells expressing GFP-tagged proteins, the cells were transferred to S medium (0.2% yeast extract plus 0.003% iron EDTA) for 2h prior to imaging. For experiments requiring starvation (also at 30°), cells were transferred to DMC, a one-tenth dilution of Dryl's (1.7 mM sodium citrate, 1mM NaH2PO4, 1mM Na2HPO4, 0.5 mM CaCl2) supplemented with an additional 0.1 mM MgCl2 and 0.5 mM CaCl2.
Transformation of GFP fusions was by conjugant electroporation [73]. Briefly, T. thermophila strains CU428 and B2086, after 10h of conjugation, were combined with 20µg of DNA and electroporated; after 1d 100 µg/ml paromomycin sulfate was added to select for transformants, which were picked at 5 d. In general, the transformants were maintained at room temperature by weekly transfer in 24-well plates in SPP with paromomycin, and showed stable expression of the Rab-GFP for at least 6 weeks and often longer, depending upon the particular Rab. Transformants were stored in parallel in tube cultures (in 2% proteose peptone, at room temperature, no paromomycin), and showed stable Rab-GFP expression for 2–3 months.
Live cell microscopy
The GFP-transgenes were induced with the lowest level of CdCl2 (0.25–1µg/ml, determined empirically for each strain) that produced a discernible localization pattern. We analyzed 4 independent lines per transformation. The rare cases where differences were seen were due to variation in levels of what appeared to be diffuse cytoplasmic fluorescence. For imaging, transformants were grown overnight in SPP media and then transferred, 2h before imaging, to S medium at room temperature containing 0.25–1µg/ml CdCl2. Cells induced in DMC required lower levels of CdCl2 (0.1–0.25µg/ml).
Microscopy was performed at room temperature. For initial visualization, live GFP-Rab-expressing Tetrahymena cells were imaged on an Olympus DSU spinning disk inverted confocal microscope with a 100× objective. Cells were immobilized by mixing 1∶1 with either 6% polyethylene oxide (PEO: MW = ca.900,000), or 6–10% poly(ethylene glycol)-polyalanine (2000–1500) diblock copolymer solutions that undergo sol-to-gel transition as the temperature increases [74], both made in S media. Images were acquired with an EM-CCD Hamamatsu camera and SlideBook acquisition software. Unless indicated, figures are a projection of a z-stack of the entire cell or the cell mid-section. The Z projections and color channel merges, as well as adjustments to brightness and contrast, were made using the public domain NIH ImageJ program (http://rsbweb.nih.gov/ij/). Each image shown is representative of the majority of cells expressing that Rab-GFP. A fraction of the cells appeared to contain multivesicular phagosomes, which accumulated FM4-64 in cells exposed to this endocytic tracer (see below) and may be a response to stress. This fraction increased with time under the coverslip and was particularly evident in PEO-immobilized samples. Both for microscopy and for quantitative analysis, we chose cells with few or no such structures.
Time-lapse and/or image stack movies of all Rabs, as well as additional images of some Rabs showing colocalization (see below), are accessible at tetrahymenacell.uchicago.edu.
Labeling endocytic and phagocytic compartments with FM 4-64, India ink, or DsRed-bacteria
Tetrahymena transformants were incubated for 5min with 5µM FM 4-64 dye [75] (Invitrogen) (excitation/emission maxima ∼515/640 nm). The cells were then pelleted and resuspended in ∼40µl of supernatant, and immobilized as above for microscopy for up to 2h. In all cases, Rabs that showed colocalization with FM4-64 showed this pattern within 10 min. Cells were imaged on a Leica TCS SP2 AOBS laser scanning confocal microscope with LCS Leica confocal software with simultaneous capture in the green (495–520 nm) and red (656–746 nm) channels. Images shown are single slices for clarity. As above, adjustments in brightness and contrast were conducted in ImageJ. Phagosomes were labeled with india ink by incubating Tetrahymena for 1–2 h prior to imaging with 2.5% v/v india ink in S medium. The cells were simultaneously induced with CdCl2 (for 2h) to induce transgene expression. Alternatively, phagosomes were labeled by incubating Tetrahymena in S medium for 2h with E. coli expressing dsRed-Express2 [76] (gift of R. Strack and B. Glick, U. Chicago)(adding 0.5% v/v of an overnight culture), with simultaneous induction of Rab-GFP expression. Cells were imaged using a Zeiss Axioplan2 upright widefield microscope with a Hamamatsu camera and Axiovision software (using a 63× objective).
Labeling acidic compartments with Lysotracker
Lysosomes and phagosomes were labeled in cells expressing TtRab7-GFP by addition of 0.05% (v/v) LysoTracker (Invitrogen) for 90min to cells in SPP at 22°C, in which Rab-GFP expression was induced for a total of 150min with 0.5µg/ml CdCl2. The cells were pelleted and then resuspended in PEO for immobilization and microscopy. Cells were imaged using a Zeiss LSM-510 laser scanning confocal microscope with LSM 5 software.
Image quantification
Overlap quantification (e.g., a Rab-GFP and FM4-64) was performed using NIH ImageJ, based on the method described in [77]. Briefly, pixels of interest were identified by generating a mask for each channel to eliminate background signal. Single focal plane images were used and we defined a threshold value in red and green channels separately that included only the brightest labeled structures. Overlap was defined as the percentage of total signal ‘intensity’ present in shared pixels. To recover intensity lost in the creation of the masks, we modified the binarized masks using subtract and invert functions in ImageJ to regain the green and/or red intensity values above threshold. To calculate overlap for the green signal we used the ratio of the average intensity of the green pixels in the AND mask over that of the green mask. We did the converse for the red signal. For each line, at least 4 cells were analyzed.
Immunofluorescence
Secretory granules (mucocysts) were immunostained with the 4D11 or 5E9 monoclonal antibodies as described in [78], except fixation was at room temperature rather than on ice. Basal bodies were stained using the 20H5 anti-centrin antibody (gift of Jeff Salisbury, Mayo Clinic), with Texas Red-conjugated goat anti-mouse 2° antibody (Invitrogen). Immunostained cells were mounted with 0.1µM Trolox to inhibit bleaching and imaged the Leica SP2 laser scanning confocal microscope as described above.
Cell lysates and western blotting
Whole cell lysates were prepared by 10% trichloroacetic acid precipitation (∼1×106 cells in SPP per sample) and then dissolved in SDS-PAGE sample buffer. For western blots, 2×105 cell equivalents per sample were resolved by SDS-PAGE, transferred to 0.45µM nitrocellulose (General Electric Water and Process Technologies), probed with polyclonal anti-GFP antibody at 1∶1,000 dilution (Invitrogen) and Alexa 680 anti-rabbit antibody (Invitrogen) at 1∶5,000 dilution, and imaged using the Li-Cor Odyssey protocol and scanner (http://www.licor.com).
Supporting Information
Zdroje
1. DerbyMC
GleesonPA
2007 New insights into membrane trafficking and protein sorting. Int Rev Cytol 261 47 116
2. MartensS
McMahonHT
2008 Mechanisms of membrane fusion: disparate players and common principles. Nat Rev Mol Cell Biol 9 543 556
3. DacksJB
FieldMC
2007 Evolution of the eukaryotic membrane-trafficking system: origin, tempo and mode. J Cell Sci 120 2977 2985
4. BonifacinoJS
GlickBS
2004 The mechanisms of vesicle budding and fusion. Cell 116 153 166
5. SegevN
2001 Ypt/rab gtpases: regulators of protein trafficking. Sci STKE 2001 RE11
6. SegevN
2001 Ypt and Rab GTPases: insight into functions through novel interactions. Curr Opin Cell Biol 13 500 511
7. MarkgrafDF
PeplowskaK
UngermannC
2007 Rab cascades and tethering factors in the endomembrane system. FEBS Lett 581 2125 2130
8. DacksJB
PedenAA
FieldMC
2009 Evolution of specificity in the eukaryotic endomembrane system. Int J Biochem Cell Biol 41 330 340
9. Pereira-LealJB
TeichmannSA
2005 Novel specificities emerge by stepwise duplication of functional modules. Genome Res 15 552 559
10. DhirV
GouldingD
FieldMC
2004 TbRAB1 and TbRAB2 mediate trafficking through the early secretory pathway of Trypanosoma brucei. Mol Biochem Parasitol 137 253 265
11. Pereira-LealJB
SeabraMC
2001 Evolution of the Rab family of small GTP-binding proteins. J Mol Biol 313 889 901
12. BockJB
MaternHT
PedenAA
SchellerRH
2001 A genomic perspective on membrane compartment organization. Nature 409 839 841
13. ZhangJ
SchulzeKL
HiesingerPR
SuyamaK
WangS
2007 Thirty-one flavors of Drosophila rab proteins. Genetics 176 1307 1322
14. LalK
FieldMC
CarltonJM
WarwickerJ
HirtRP
2005 Identification of a very large Rab GTPase family in the parasitic protozoan Trichomonas vaginalis. Mol Biochem Parasitol 143 226 235
15. Saito-NakanoY
LoftusBJ
HallN
NozakiT
2005 The diversity of Rab GTPases in Entamoeba histolytica. Exp Parasitol 110 244 252
16. AllenRD
FokAK
2000 Membrane trafficking and processing in Paramecium. Int Rev Cytol 198 277 318
17. AllenRD
SchroederCC
FokAK
1992 Endosomal system of Paramecium: coated pits to early endosomes. J Cell Sci 101 Pt 2 449 461
18. AllenRD
StaehelinLA
1981 Digestive system membranes: freeze-fracture evidence for differentiation and flow in Paramecium. J Cell Biol 89 9 20
19. SedarAW
PorterKR
1955 The fine structure of cortical components of Paramecium multimicronucleatum. J Biophys Biochem Cytol 1 583 604
20. ThompsonGAJr
NozawaY
1977 Tetrahymena: a system for studying dynamic membrane alterations within the eukaryotic cell. Biochim Biophys Acta 472 55 92
21. AllenRD
1978 Membranes of ciliates: ultrastructure, biochemistry and fusion. Membrane Fusion Amsterdam Elsevier/North-Holland Biomed. Press 657 763
22. FrankelJ
2000 Cell biology of Tetrahymena thermophila. Methods Cell Biol 6299432807 27 125
23. EisenJA
CoyneRS
WuM
WuD
ThiagarajanM
2006 Macronuclear genome sequence of the ciliate Tetrahymena thermophila, a model eukaryote. PLoS Biol 4 e286 doi:10.1371/journal.pbio.0040286
24. AuryJM
JaillonO
DuretL
NoelB
JubinC
2006 Global trends of whole-genome duplications revealed by the ciliate Paramecium tetraurelia. Nature 444 171 178
25. AdhiamboC
BlisnickT
ToutiraisG
DelannoyE
BastinP
2009 A novel function for the atypical small G protein Rab-like 5 in the assembly of the trypanosome flagellum. J Cell Sci 122 834 841
26. Pereira-LealJB
SeabraMC
2000 The mammalian Rab family of small GTPases: definition of family and subfamily sequence motifs suggests a mechanism for functional specificity in the Ras superfamily. J Mol Biol 301 1077 1087
27. MiaoW
XiongJ
BowenJ
WangW
LiuY
2009 Microarray analyses of gene expression during the Tetrahymena thermophila life cycle. PLoS ONE 4 e4429 doi:10.1371/journal.pone.0004429
28. StargellLA
KarrerKM
GorovskyMA
1990 Transcriptional regulation of gene expression in Tetrahymena thermophila. Nucl Acids Res 18 6637 6639
29. NelsenEM
DebaultLE
1978 Transformation in Tetrahymena pyriformis: description of an inducible phenotype. J Protozool 25 113 119
30. BannoY
SasakiN
NozawaY
1987 Secretion heterogeneity of lysosomal enzymes in Tetrahymena pyriformis. Exp Cell Res 170 259 268
31. MadingerCL
CollinsK
FieldsLG
TaronCH
BennerJS
2010 Constitutive secretion in Tetrahymena thermophila. Eukaryot Cell 9 674 681
32. RahamanA
EldeNC
TurkewitzAP
2008 A dynamin-related protein required for nuclear remodeling in Tetrahymena. Curr Biol 18 1227 1233
33. MartindaleDW
AllisCD
BrunsPJ
1982 Conjugation in Tetrahymena thermophila. A temporal analysis of cytological stages. Exp Cell Res 140 227 236
34. OriasJD
HamiltonEP
OriasE
1983 A microtubule meshwork associated with gametic pronucleus transfer across a cell-cell junction. Science 222 181 184
35. NelsenEM
WilliamsNE
YiH
KnaakJ
FrankelJ
1994 “Fenestrin” and conjugation in Tetrahymena thermophila. J Eukaryot Microbiol 41 483 495
36. CalzoneFJ
AngererRC
GorovskyMA
1983 Regulation of protein synthesis in Tetrahymena. Quantitative estimates of the parameters determining the rates of protein synthesis in growing, starved and starved-deciliated cells. J Biol Chem 258 6887 6898
37. JacobsME
DeSouzaLV
SamaranayakeH
PearlmanRE
SiuKW
2006 The Tetrahymena thermophila phagosome proteome. Eukaryot Cell 5 1990 2000
38. NilssonJR
1979 Phagotrophy in Tetrahymena.
LevandowskyM
HutnerSH
Biochemistry and Physiology of Protozoa New York Academic Press 339 379
39. AllenRD
FokAK
1980 Membrane recycling and endocytosis in Paramecium confirmed by horseradish peroxidase pulse-chase studies. J Cell Sci 45 131 145
40. NilssonJR
Van DeursB
1983 Coated pits and pinocytosis in Tetrahymena. J Cell Sci 63 209 222
41. AllenRD
1967 Fine structure, reconstruction and possible functions of components of the cortex of Tetrahymena pyriformis. J Protozool 14 553 565
42. EldeNC
MorganG
WineyM
SperlingL
TurkewitzAP
2005 Elucidation of Clathrin-Mediated Endocytosis in Tetrahymena Reveals an Evolutionarily Convergent Recruitment of Dynamin. PLoS Genet 1 e52 doi:10.1371/journal.pgen.0010052
43. KurzS
TiedtkeA
1993 The Golgi apparatus of Tetrahymena thermophila. J Eukaryot Microbiol 40 10 13
44. TurkewitzAP
2004 Out with a bang! Tetrahymena as a model system to study secretory granule biogenesis. Traffic 5 63 68
45. ZweifelE
SmithJ
RomeroD
GiddingsTHJr
WineyM
2009 Nested genes CDA12 and CDA13 encode proteins associated with membrane trafficking in the ciliate Tetrahymena thermophila. Eukaryot Cell 8 899 912
46. BowmanGR
EldeNC
MorganG
WineyM
TurkewitzAP
2005 Core Formation and the Acquisition of Fusion Competence are Linked During Secretory Granule Maturation in Tetrahymena. Traffic 6 303 323
47. CameronIL
BurtonAL
1969 On the cycle of the water expulsion vesicle in the ciliate Tetrahymena pyriformis. Trans Am Microsc Soc 88 386 393
48. DuF
EdwardsK
ShenZ
SunB
De LozanneA
2008 Regulation of contractile vacuole formation and activity in Dictyostelium. Embo J 27 2064 2076
49. TekleYI
GrantJR
KovnerAM
TownsendJP
KatzLA
2010 Identification of new molecular markers for assembling the eukaryotic tree of life. Mol Phylogenet Evol
50. NorianL
DragoiIA
O'HalloranT
1999 Molecular characterization of rabE, a developmentally regulated Dictyostelium homolog of mammalian rab GTPases. DNA Cell Biol 18 59 64
51. AllenRD
WolfRW
1979 Membrane recycling at the cytoproct of Tetrahymena. J Cell Sci 35 217 227
52. CetkovicH
MikocA
MullerWE
GamulinV
2007 Ras-like small GTPases form a large family of proteins in the marine sponge Suberites domuncula. J Mol Evol 64 332 341
53. CoyneRS
ThiagarajanM
JonesKM
WortmanJR
TallonLJ
2008 Refined annotation and assembly of the Tetrahymena thermophila genome sequence through EST analysis, comparative genomic hybridization, and targeted gap closure. BMC Genomics 9 562
54. ZufallRA
McGrathCL
MuseSV
KatzLA
2006 Genome architecture drives protein evolution in ciliates. Mol Biol Evol 23 1681 1687
55. RutherfordS
MooreI
2002 The Arabidopsis Rab GTPase family: another enigma variation. Curr Opin Plant Biol 5 518 528
56. KienleN
KloepperTH
FasshauerD
2009 Phylogeny of the SNARE vesicle fusion machinery yields insights into the conservation of the secretory pathway in fungi. BMC Evol Biol 9 19
57. KienleN
KloepperTH
FasshauerD
2009 Differences in the SNARE evolution of fungi and metazoa. Biochem Soc Trans 37 787 791
58. Pereira-LealJB
2008 The Ypt/Rab family and the evolution of trafficking in fungi. Traffic 9 27 38
59. EldeNC
LongM
TurkewitzAP
2007 A role for convergent evolution in the secretory life of cells. Trends Cell Biol 17 157 164
60. NachuryMV
LoktevAV
ZhangQ
WestlakeCJ
PeränenJ
2007 A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129 1201 1213
61. KissmehlR
SchildeC
WassmerT
DanzerC
NuehseK
2007 Molecular identification of 26 syntaxin genes and their assignment to the different trafficking pathways in Paramecium. Traffic 8 523 542
62. HorowitzS
GorovskyMA
1985 An unusual genetic code in nuclear genes of Tetrahymena. Proc Natl Acad Sci U S A 82 2452 2455
63. GuindonS
GascuelO
2003 A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52 696 704
64. HuelsenbeckJP
RonquistF
2001 MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17 754 755
65. RonquistF
HuelsenbeckJP
2003 MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19 1572 1574
66. FelsensteinJ
2006 Accuracy of coalescent likelihood estimates: do we need more sites, more sequences, or more loci? Mol Biol Evol 23 691 700
67. EdgarRC
2004 MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32 1792 1797
68. GouyM
GuindonS
GascuelO
2010 SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol 27 221 224
69. FelsensteinJ
1989 PHYLIP - Phylogeny Inference Package (Version 3.2). Cladistics 5 164 166
70. MaloneCD
FalkowskaKA
LiAY
GalantiSE
KanuruRC
2008 Nucleus-specific importin alpha proteins and nucleoporins regulate protein import and nuclear division in the binucleate Tetrahymena thermophila. Eukaryot Cell 7 1487 1499
71. ZachariasDA
ViolinJD
NewtonAC
TsienRY
2002 Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science 296 913 916
72. ShangY
SongX
BowenJ
CorstanjeR
GaoY
2002 A robust inducible-repressible promoter greatly facilitates gene knockouts, conditional expression, and overexpression of homologous and heterologous genes in Tetrahymena thermophila. Proc Natl Acad Sci U S A 99 3734 3739
73. GaertigJ
GorovskyMA
1992 Efficient mass transformation of Tetrahymena thermophila by electroporation of conjugants. Proc Natl Acad Sci USA 89 9196 9200
74. ChoiYY
JooMK
SohnYS
JeongB
2008 Significance of secondary structure in nanostructure formation and thermosensitivity of polypeptide block copolymers. Soft Matter 4 2383 2387
75. VidaTA
EmrSD
1995 A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol 128 779 792
76. StrackRL
StronginDE
BhattacharyyaD
TaoW
BermanA
2008 A noncytotoxic DsRed variant for whole-cell labeling. Nat Methods 5 955 957
77. ZhaoX
ClaudeA
ChunJ
ShieldsDJ
PresleyJF
2006 GBF1, a cis-Golgi and VTCs-localized ARF-GEF, is implicated in ER-to-Golgi protein traffic. J Cell Sci 119 3743 3753
78. BowmanGR
TurkewitzAP
2001 Analysis of a mutant exhibiting conditional sorting to dense core secretory granules in Tetrahymena thermophila. Genetics 159 1605 1616
79. HarrisE
YoshidaK
CardelliJ
BushJ
2001 Rab11-like GTPase associates with and regulates the structure and function of the contractile vacuole system in dictyostelium. J Cell Sci 114 3035 3045
80. WasmeierC
RomaoM
PlowrightL
BennettDC
RaposoG
2006 Rab38 and Rab32 control post-Golgi trafficking of melanogenic enzymes. J Cell Biol 175 271 281
81. AckersJP
DhirV
FieldMC
2005 A bioinformatic analysis of the RAB genes of Trypanosoma brucei. Mol Biochem Parasitol 141 89 97
82. QuevillonE
SpielmannT
BrahimiK
ChattopadhyayD
YeramianE
2003 The Plasmodium falciparum family of Rab GTPases. Gene 306 13 25
Štítky
Genetika Reprodukčná medicína
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2010 Číslo 10- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- FSHD: A Repeat Contraction Disease Finally Ready to Expand (Our Understanding of Its Pathogenesis)
- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- The Meiotic Recombination Checkpoint Suppresses NHK-1 Kinase to Prevent Reorganisation of the Oocyte Nucleus in
- Actin Depolymerizing Factors Cofilin1 and Destrin Are Required for Ureteric Bud Branching Morphogenesis
- DSIF and RNA Polymerase II CTD Phosphorylation Coordinate the Recruitment of Rpd3S to Actively Transcribed Genes
- Continuous Requirement for the Clr4 Complex But Not RNAi for Centromeric Heterochromatin Assembly in Fission Yeast Harboring a Disrupted RITS Complex
- Genome-Wide Association Study of Blood Pressure Extremes Identifies Variant near Associated with Hypertension
- The Cytosine Methyltransferase DRM2 Requires Intact UBA Domains and a Catalytically Mutated Paralog DRM3 during RNA–Directed DNA Methylation in
- β-Actin and γ-Actin Are Each Dispensable for Auditory Hair Cell Development But Required for Stereocilia Maintenance
- Genetic Association Study Identifies as a Risk Gene for Idiopathic Dilated Cardiomyopathy
- Evidence for a Xer/ System for Chromosome Resolution in Archaea
- Four Novel Loci (19q13, 6q24, 12q24, and 5q14) Influence the Microcirculation
- Lifespan Extension by Preserving Proliferative Homeostasis in
- Ancient and Recent Adaptive Evolution of Primate Non-Homologous End Joining Genes
- Loss of the p53/p63 Regulated Desmosomal Protein Perp Promotes Tumorigenesis
- Altering a Histone H3K4 Methylation Pathway in Glomerular Podocytes Promotes a Chronic Disease Phenotype
- Characterization of LINE-1 Ribonucleoprotein Particles
- Conserved Genes Act as Modifiers of Invertebrate SMN Loss of Function Defects
- Alternative Splicing at a NAGNAG Acceptor Site as a Novel Phenotype Modifier
- Tight Regulation of the Gene of the KplE1 Prophage: A New Paradigm for Integrase Gene Regulation
- Conjugative DNA Transfer Induces the Bacterial SOS Response and Promotes Antibiotic Resistance Development through Integron Activation
- Nasty Viruses, Costly Plasmids, Population Dynamics, and the Conditions for Establishing and Maintaining CRISPR-Mediated Adaptive Immunity in Bacteria
- Stress-Induced Activation of Heterochromatic Transcription
- H3K27me3 Profiling of the Endosperm Implies Exclusion of Polycomb Group Protein Targeting by DNA Methylation
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
- Characterising and Predicting Haploinsufficiency in the Human Genome
- Dual Functions of ASCIZ in the DNA Base Damage Response and Pulmonary Organogenesis
- Pervasive Cryptic Epistasis in Molecular Evolution
- Transition from Positive to Neutral in Mutation Fixation along with Continuing Rising Fitness in Thermal Adaptive Evolution
- Comprehensive Analysis Reveals Dynamic and Evolutionary Plasticity of Rab GTPases and Membrane Traffic in
- Regulates Tissue-Specific Mitochondrial DNA Segregation
- Role for the Mammalian Swi5-Sfr1 Complex in DNA Strand Break Repair through Homologous Recombination
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Genome-Wide Identification of Targets and Function of Individual MicroRNAs in Mouse Embryonic Stem Cells
- Common Genetic Variants and Modification of Penetrance of -Associated Breast Cancer
- Allele-Specific Down-Regulation of Expression Induced by Retinoids Contributes to Climate Adaptations
- Simultaneous Disruption of Two DNA Polymerases, Polη and Polζ, in Avian DT40 Cells Unmasks the Role of Polη in Cellular Response to Various DNA Lesions
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



