-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Extracellular Matrix Dynamics in Hepatocarcinogenesis: a Comparative Proteomics Study of Transgenic and Null Mouse Models
We are reporting qualitative and quantitative changes of the extracellular matrix (ECM) and associated receptor proteomes, occurring during the transition from liver fibrosis and steatohepatitis to hepatocellular carcinoma (HCC). We compared two mouse models relevant to human HCC: PDGFC transgenic (Tg) and Pten null mice, models of disease progression from fibrosis and steatohepatitis to HCC. Using mass spectrometry, we identified in the liver of both models proteins for 26 collagen-encoding genes, providing the first evidence of expression at the protein level for 16 collagens. We also identified post-transcriptional protein variants for six collagens and lysine hydroxylation modifications for 14 collagens. Tumor-associated collagen proteomes were similar in both models with increased expression of collagens type IV, VI, VII, X, XIV, XV, XVI, and XVIII. Splice variants for Col4a2, Col6a2, Col6a3 were co-upregulated while only the short form of Col18a1 increased in the tumors. We also identified tumor specific increases of nidogen 1, decorin, perlecan, and of six laminin subunits. The changes in these non-collagenous ECM proteins were similar in both models with the exception of laminin β3, detected specifically in the Pten null tumors. Pdgfa and Pdgfc mRNA expression was increased in the Pten null liver, a possible mechanism for the similarity in ECM composition observed in the tumors of both models. In contrast and besides the strong up-regulation of integrin α5 protein observed in the liver tumors of both models, the expression of the six other integrins identified was specific to each model, with integrins α2b, α3, α6, and β1 up-regulated in Pten null tumors and integrins α8 and β5 up-regulated in the PDGFC Tg tumors. In conclusion, HCC–associated ECM proteins and ECM–integrin networks, common or specific to HCC subtypes, were identified, providing a unique foundation to using ECM composition for HCC classification, diagnosis, prevention, or treatment.
Published in the journal: Extracellular Matrix Dynamics in Hepatocarcinogenesis: a Comparative Proteomics Study of Transgenic and Null Mouse Models. PLoS Genet 7(6): e32767. doi:10.1371/journal.pgen.1002147
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002147Summary
We are reporting qualitative and quantitative changes of the extracellular matrix (ECM) and associated receptor proteomes, occurring during the transition from liver fibrosis and steatohepatitis to hepatocellular carcinoma (HCC). We compared two mouse models relevant to human HCC: PDGFC transgenic (Tg) and Pten null mice, models of disease progression from fibrosis and steatohepatitis to HCC. Using mass spectrometry, we identified in the liver of both models proteins for 26 collagen-encoding genes, providing the first evidence of expression at the protein level for 16 collagens. We also identified post-transcriptional protein variants for six collagens and lysine hydroxylation modifications for 14 collagens. Tumor-associated collagen proteomes were similar in both models with increased expression of collagens type IV, VI, VII, X, XIV, XV, XVI, and XVIII. Splice variants for Col4a2, Col6a2, Col6a3 were co-upregulated while only the short form of Col18a1 increased in the tumors. We also identified tumor specific increases of nidogen 1, decorin, perlecan, and of six laminin subunits. The changes in these non-collagenous ECM proteins were similar in both models with the exception of laminin β3, detected specifically in the Pten null tumors. Pdgfa and Pdgfc mRNA expression was increased in the Pten null liver, a possible mechanism for the similarity in ECM composition observed in the tumors of both models. In contrast and besides the strong up-regulation of integrin α5 protein observed in the liver tumors of both models, the expression of the six other integrins identified was specific to each model, with integrins α2b, α3, α6, and β1 up-regulated in Pten null tumors and integrins α8 and β5 up-regulated in the PDGFC Tg tumors. In conclusion, HCC–associated ECM proteins and ECM–integrin networks, common or specific to HCC subtypes, were identified, providing a unique foundation to using ECM composition for HCC classification, diagnosis, prevention, or treatment.
Introduction
Cirrhosis, the result of end-stage fibrosis, and steatohepatitis are common pre-neoplastic conditions associated with hepatocarcinogenesis [1]. It is therefore important to understand the mechanisms leading to the transition from fibrosis and steatosis to HCC. Mice with liver-specific transgenic (Tg) expression of platelet-derived growth factor-C (PDGFC) represent a relevant model for such a study as members of the PDGF family play major roles in angiogenesis and fibrosis [2], [3]. Moreover, these mice develop liver fibrosis resembling human alcoholic and nonalcoholic fatty liver disease, which precedes development of HCC [4], [5]. Another relevant model is mice with liver specific deletion of the phosphoinositide 3-kinase (PI3K)/phosphatase and tensin homolog (Pten). PTEN loss of function in hepatocytes leads to steatohepatitis, fibrosis and HCC later in life [6], [7]. While the liver tumors in PDGFC Tg mice show characteristics of HCC, the tumors in the Pten null model present a mixed phenotype of HCC and cholangiocarcinoma [8], [9]. Up to 40% of human HCCs potentially arise from progenitor-like tumor initiating cells and tend to have a more aggressive phenotype [10]. In addition, the presence of intermediate cells co-expressing both hepatocyte and biliary markers is associated with HCC occurrence [11] and acquisition of cholangiocarcinoma-like expression traits plays a critical role in the heterogeneous progression of HCC [12]. It is therefore of particular relevance to compare liver proteome changes in both the PDGFC Tg and the Pten null models.
Through mass-spectrometry-based profiling of the liver tissues collected at different disease stages in these two mouse models, we have characterized changes in the liver proteome occurring in fibrotic and steatotic tissue, as well as in tumors. We previously reported that the extensive mass-spectrometry-based approach we used in this study reaches depth and allows for quantitative estimates of protein abundance [13]. Changes in specific protein families or networks can be characterized as shown here for proteins of the extracellular matrix (ECM) and their receptors. The ECM is a key component of the microenvironment that is in immediate contact with the tumor cells and is a critical source for growth, survival, motility and angiogenic factors that significantly affect tumor biology and progression. In addition, cell adhesion to the ECM through integrins and other cell surface receptors triggers intracellular signaling pathways that can regulate cell cycle progression, migration and differentiation. While hepatic ECM has been extensively studied in the context of liver fibrosis, little attention has been given to the role of the ECM in the early steps of hepatocarcinogenesis. Therefore, delineating and comparing the liver proteome changes of ECM components in two mouse models of liver cancer represents a unique and important contribution to our understanding of the molecular mechanisms of early hepatocarcinogenesis, and to ongoing efforts to identify novel diagnostic and therapeutic targets.
Results
Tumor Development in the PDGFC Tg and Pten Null Mice and Liver Proteome Profiling
The liver specific PDGFC Tg and Pten null mouse models reproduce the steps of HCC development observed in humans progressing from steatohepatitis and fibrosis to hepatocyte dysplasia and tumorigenesis. These events are associated with significant modification of the stroma and associated extracellular matrix. Preceding tumor development, there is accumulation of collagen fibers in the liver of these mice (Figure 1A). Steatosis is also particularly pronounced in the Pten null liver as shown by the accumulation of lipid droplets (Figure 1A). Interestingly, the phenotypes of the tumors are different in these two models with characteristics of HCC in the PDGFC Tg model and with mixed cell characteristics of HCC and cholangiocarcinoma in the Pten null mice (Figure 1B). Extensive mass spectrometry analysis following a multi-dimensional protein separation strategy composed of two-dimensional HPLC followed by SDS-PAGE was applied as described [13] to livers collected from these two models at the fibrosis and steatosis stage as well as on small HCCs. For each sample group, livers from three or four mice were pooled. A total of 10,707 protein isoforms, products of 8,278 individual genes were identified with high confidence. For each identified protein, protein abundance was calculated using the frequency of tandem mass spectra assigned to that protein. We previously reported that this label-free approach provides a good estimate of protein abundance in liver [13].
Fig. 1. Collagen deposition and tumor morphology in PDGFC Tg and Pten null liver. 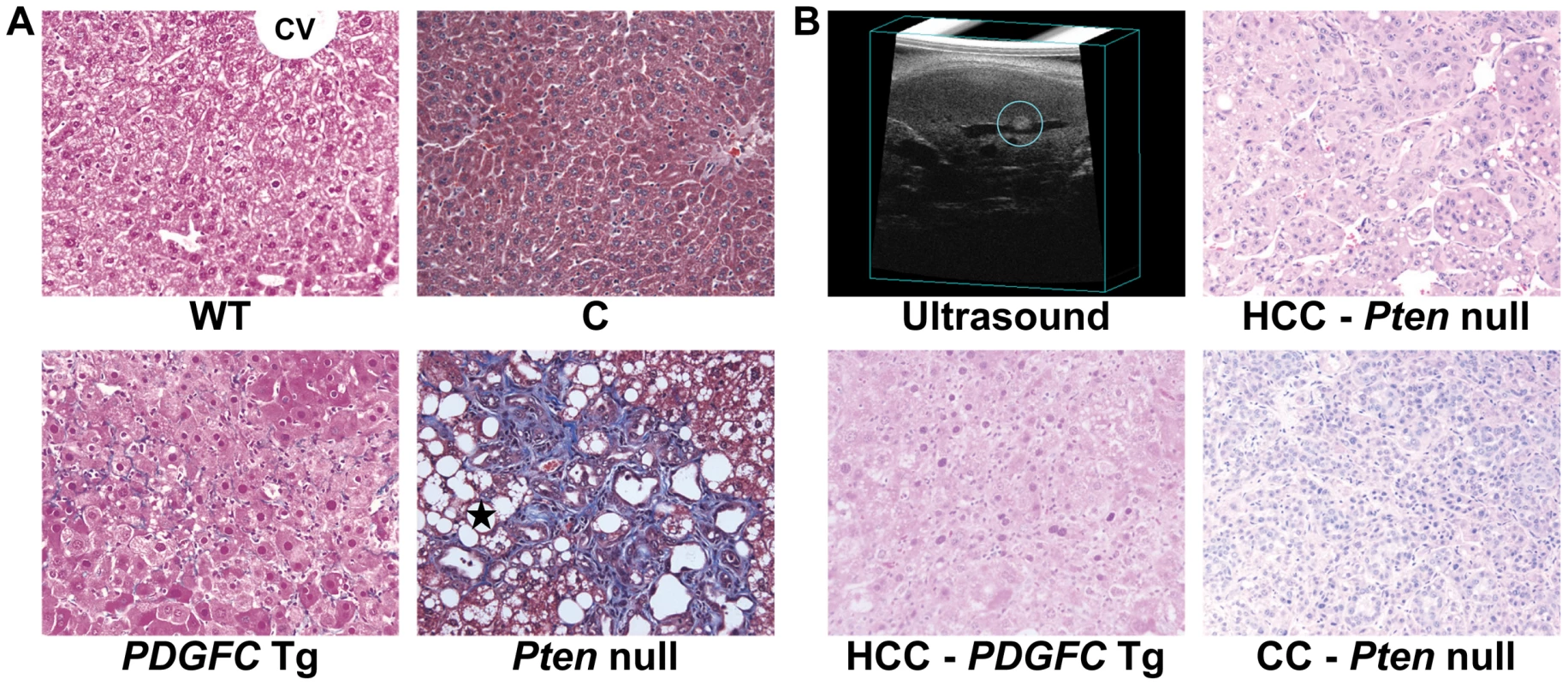
A) Masson's trichrome stained liver sections showing hepatic pericellular collagen deposition in 6-month-old PDGFC Tg and Pten null mice compared to WT littermates and control mice. Black star: lipid droplets in Pten null livers; CV: central vein. B) H&E stained liver sections showing HCC formation in a 8-month-old PDGFC Tg mouse detected by ultrasound, and HCC and CC formation in a 9-month-old Pten null mouse. Magnification: ×200. Collagen Protein Identification and Quantification
Out of the 44 alpha chains of the murine collagen family, a total of 26, corresponding to 16 collagen types, were identified: COL1A1, COL1A2, COL2A1, COL3A1, COL4A1, COL4A2, COL4A3, COL4A4, COL4A5, COL4A6, COL5A1, COL5A2, COL5A3, COL6A1, COL6A2, COL6A3, COL7A1, COL8A1, COL10A1, COL14A1, COL15A1, COL16A1, COL18A1, COL22A1, COL27A1, COL28A1 (Table 1). All 26 collagens were identified with ProteinProphet scores of 0.97 or higher corresponding to a false discovery rate of 0.003 and all 26 collagens were identified in both mouse models. For 16 of these 26 collagens, this study represents to date the first evidence at the protein level (www.uniprot.org). The information on protein annotation, peptide numbers and sequences is summarized in Table S1. As expected, collagens types I and III were predominant in abundance. Among the remaining collagens, collagens types IV, V and VI were the most abundant. Significant changes in abundance during disease progression were observed for all 26 collagen proteins. In human liver, type I and III collagen levels increase up to eight times during fibrogenesis, with a significantly higher increase of type I collagen than of type III collagen. Similarly, type I and III collagen levels strongly increased in the fibrotic liver of 2-month-old PDGFC Tg mice with a significantly higher increase of COL1A1 and COL1A2 than of COL3A1 (Figure 2A). Eight collagens of lower abundance were also up-regulated in the PDGFC Tg fibrotic liver. These included: COL2A1, COL5A1, COL5A2, COL5A3, COL8A1, COL22A1, COL27A1 and COL28A1 (Figure 2B).
Fig. 2. Collagen proteins up-regulated in PDGFC Tg fibrotic liver. 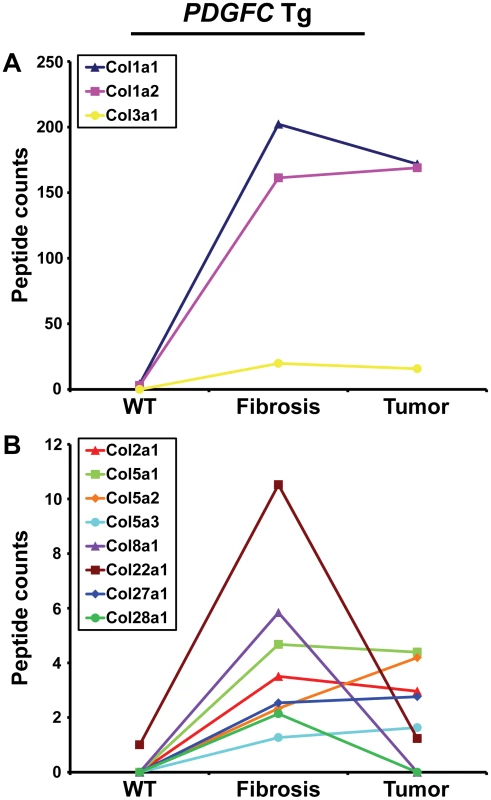
The figure includes the collagens identified as up-regulated in PDGFC Tg fibrotic liver compared to PDGFC Tg tumors and WT liver. For each protein, the abundance is shown as the total number of tandem mass spectra assigned to that protein. The collagens of high abundance are shown in panel A and those of lower abundance are shown in panel B. Tab. 1. Collagen proteins identified in PDGFC Tg and Pten null liver. 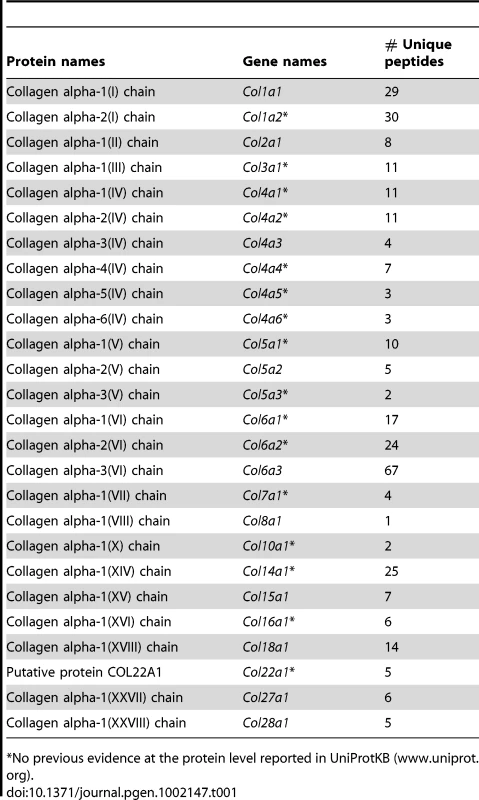
*No previous evidence at the protein level reported in UniProtKB (www.uniprot.org). Collagen Protein Abundance Changes in Tumors
The protein abundance of the remaining 15 identified collagens increased in the tumors of 8-month old PDGFC Tg mice as shown in Figure 3A for the proteins of moderate to high abundance (COL4A1, COL4A2, COL6A1, COL6A2, COL6A3, COL14A1 and COL18A1) and in Figure 3B for the proteins of lower abundance (COL4A3, COL4A4, COL4A5, COL4A6, COL7A1, COL10A1, COL15A1 and COL16A1). The same abundance changes were observed for these 15 collagens in the tumors of 9-month old Pten null mice (Figure 3C, 3D). In both models, the tumor-associated abundance increase was particularly significant for collagens type IV, VI, XIV, XV and XVI. Validation at the transcript level was performed for Col4a2 and Col15a1. Col4a2 mRNA was strongly up-regulated in tumors of both models with 10.8-fold increase (p = 0.008) in PDGFC Tg mice (Figure 4A) and 4.8-fold increase (p = 0.002) in Pten null mice (Figure 4B). Col4a2 mRNA expression was also significantly higher in tumors compared to adjacent tissue of both models (p = 0.01 in PDGFC Tg mice and p = 0.001 in Pten null mice). Col15a1 mRNA was not detected in control liver tissues and was only weakly expressed in fibrotic and steatotic liver in both models. Its expression was significantly increased in tumors in both models with 6.8-fold increase (p = 0.009) in PDGFC Tg mice (Figure 4C) and 6.3-fold increase (p = 0.003) in Pten null mice (Figure 4D). Col15a1 mRNA expression was also significantly higher in tumors compared to adjacent tissue in both models (p = 0.04 in PDGFC Tg mice and p = 0.02 in Pten null mice).
Fig. 3. Collagen proteins up-regulated in PDGFC Tg and Pten null tumors. 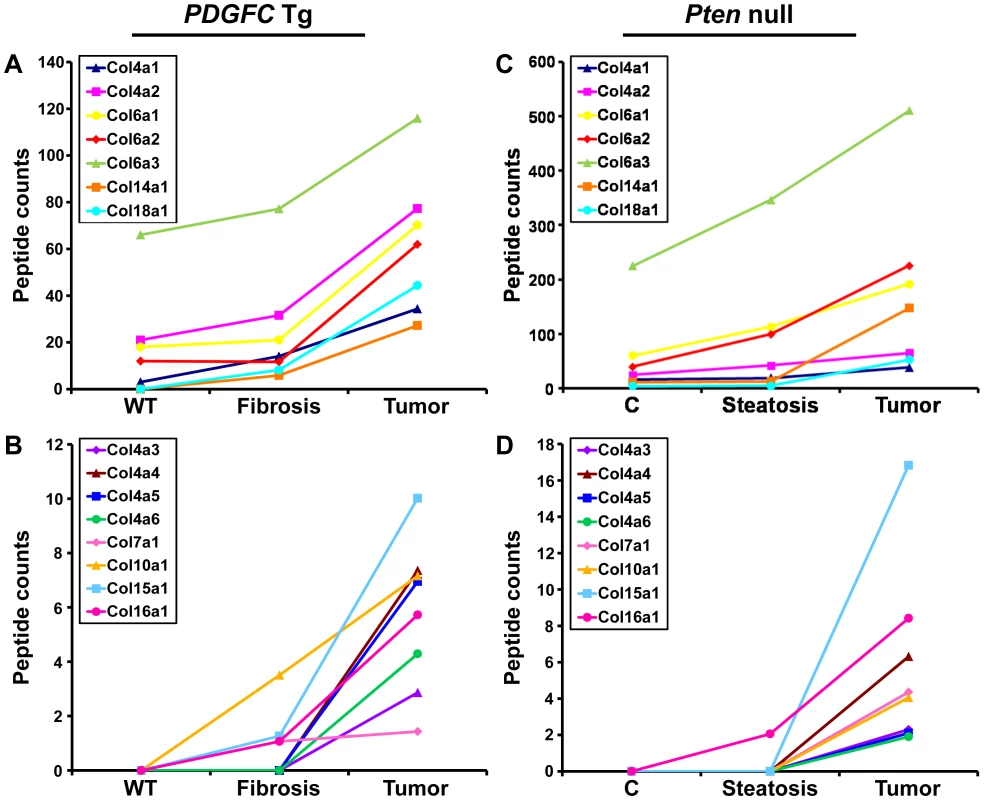
A,B) The figure includes the collagens identified as up-regulated in PDGFC Tg tumors compared to PDGFC Tg fibrotic and WT liver. C,D) The figure includes the collagens identified as up-regulated in Pten null tumors compared to Pten null steatotic and control liver. For each protein, the abundance is shown as the total number of tandem mass spectra assigned to that protein. The collagens of high abundance are shown in panels A and C and those of lower abundance are shown in panels B and D. Fig. 4. Up-regulation of Col4a2 and Col15a1 mRNAs in PDGFC Tg and Pten null tumors. 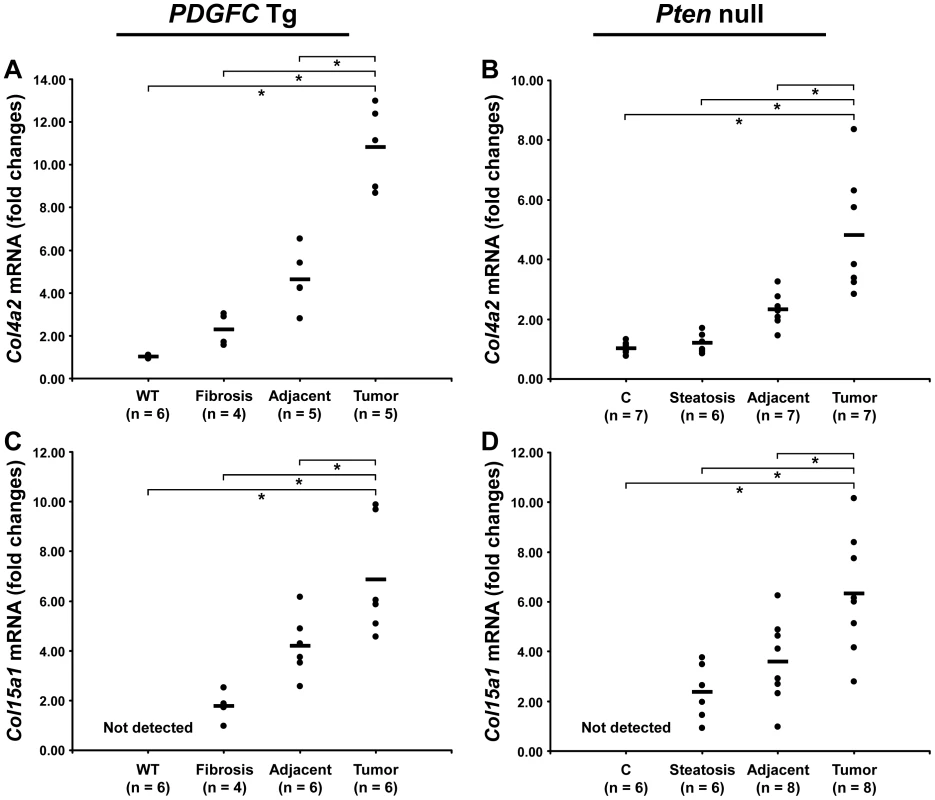
A,C) Expression of Col4a2 and Col15a1 mRNAs was measured by quantitative PCR in PDGFC Tg fibrotic liver, in PDGFC Tg tumor and adjacent tissue, and in age-matched WT liver. B,D) Similarly, expression of Col4a2 and Col15a1 mRNAs was measured in Pten null steatotic liver, in Pten null tumor and adjacent tissue, and in age-matched control liver. Expression in the disease groups is represented as fold changes over the mean of expression in the control groups. Collagen Post-Transcriptional Variants
Peptides specific to post-transcriptional variants were identified for Col1a1, Col6a2, Col6a3 and Col18a1 (Figure 5). These variants result from alternative splicing for Col1a1, Col6a2 and Col6a3 and from alternative promoter usage for Col18a1. Validation at the transcript level was performed for Col6a2 and Col18a1 using primers specific to the post-transcriptional variants. Col6a2 canonical mRNA was strongly up-regulated in tissue adjacent to tumors in both models with 23.4-fold increase (p = 0.01) in PDGFC Tg mice (Figure 6A) and 6.0-fold increase (p = 0.02) in Pten null mice (Figure 6B). A correlated up-regulation was observed for Col6a2 splice variant with 8.5-fold increase (p = 0.01) in PDGFC Tg mice (Figure 6C) and 3.4-fold increase (p = 0.02) in Pten null mice (Figure 6D). Col18a1 canonical mRNA also called NC1-764, was unchanged in liver tissue and tumors of PDGFC Tg mice (Figure 7A) and decreased in tumors of Pten null mice (5.5-fold, p = 0.002) (Figure 7B). In contrast, Col18a1 variant NC1-301 strongly increased in tumors in both models with 32.8-fold increase (p = 0.01) in PDGFC Tg mice (Figure 7C) and 118.7-fold increase (p = 0.0006) in Pten null mice (Figure 7D). Col18a1 variant NC1-301 also strongly increased in adjacent tissue in both models with 16.9-fold increase (p = 0.01) in PDGFC Tg mice (Figure 7C) and 50.7-fold increase (p = 0.001) in Pten null mice (Figure 7D).
Fig. 5. Identification of peptides specific to post-transcriptional variants for Col1a1, Col6a2, Col6a3, and Col18a1. 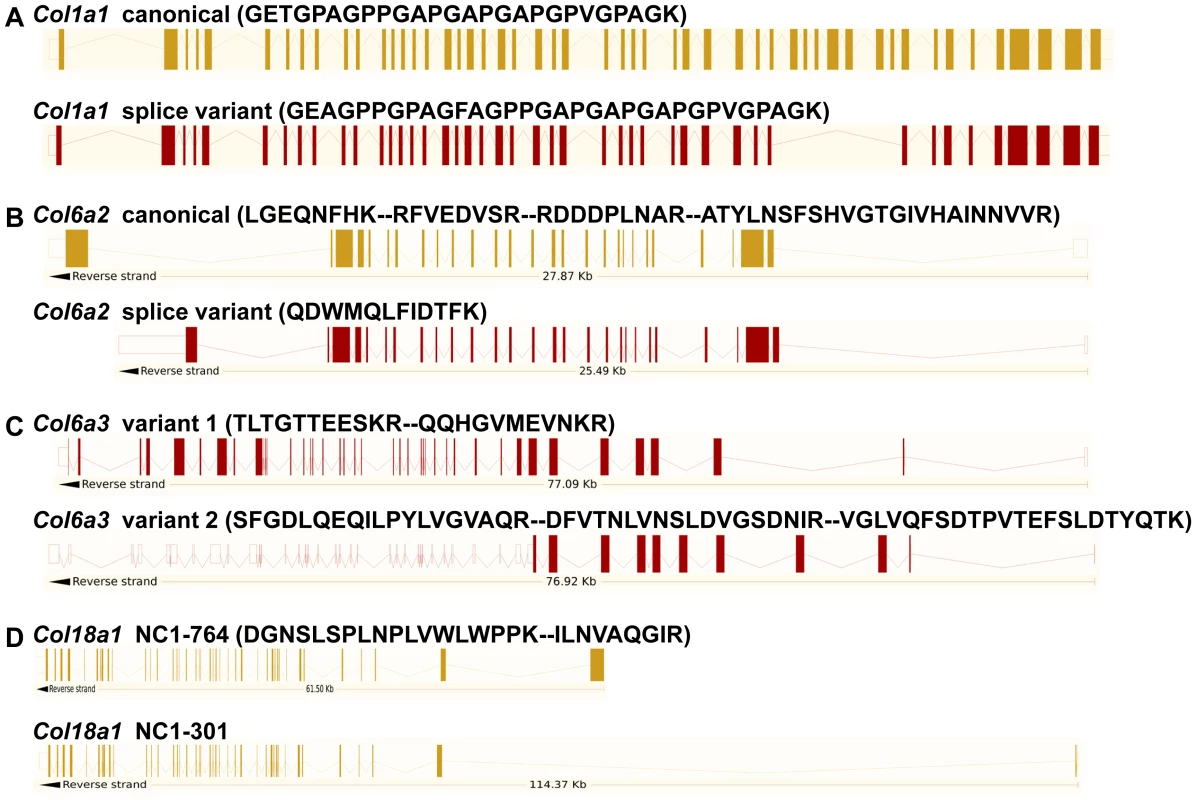
The figure shows the exon structures of post-transcriptional variants for Col1a1, Col6a2, Col6a3 and Col18a1 and the associated specific peptides identified by mass spectrometry in the PDGFC Tg and Pten null liver. Fig. 6. Expression of Col6a2 mRNA variants upon disease progression in PDGFC Tg and Pten null liver. 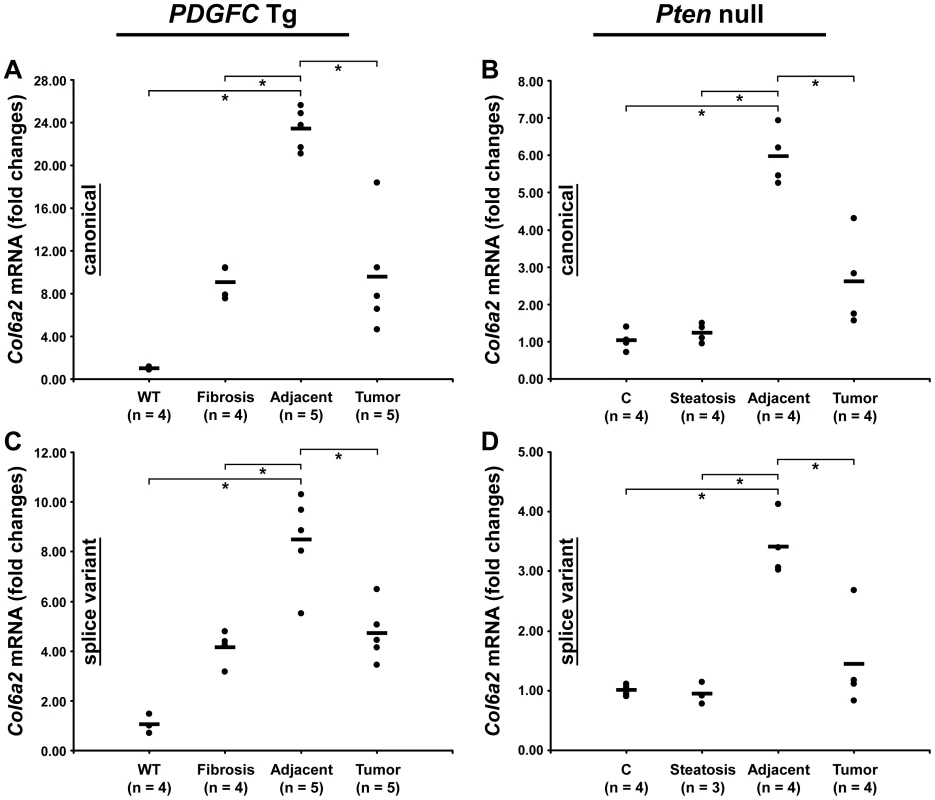
A,C) Expression of Col6a2 mRNA splice variants was measured by quantitative PCR in PDGFC Tg fibrotic liver, in PDGFC Tg tumor and adjacent tissue and in age-matched WT liver. B,D) Similarly, expression of Col6a2 mRNA splice variants was measured in Pten null steatotic liver, in Pten null tumor and adjacent tissue and in age-matched control liver. Expression in the disease groups is represented as fold changes over the mean of expression in the control groups. Fig. 7. Expression of Col18a1 mRNA variants upon disease progression in PDGFC Tg and Pten null liver. 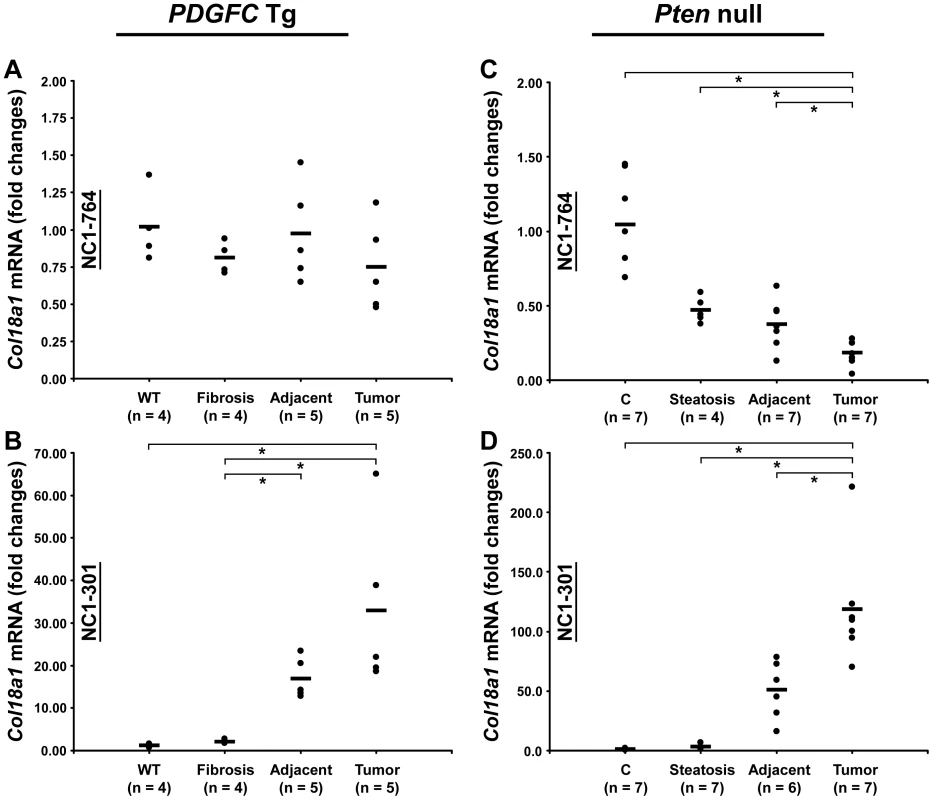
A,C) Expression of Col18a1 mRNA variants NC1-764 and NC1-301 was measured by quantitative PCR in PDGFC Tg fibrotic liver, in PDGFC Tg tumor and adjacent tissue and in age-matched WT liver. B,D) Similarly, expression of Col18a1 mRNA variants NC1-764 and NC1-301 was measured in Pten null steatotic liver, in Pten null tumor and adjacent tissue and in age-matched control liver. Expression in the disease groups is represented as fold changes over the mean of expression in the control groups. Lysine Hydroxylation of Collagens
Lysine hydroxylation is a well-known post-translational modification of type I, III and V collagens and contributes to matrix remodeling and stiffening. We investigated whether lysine hydroxylation occurs on other collagens and changes in abundance during tumor development, by researching the mass spectrometry raw data using criteria allowing for the identification of lysine hydroxylation modifications. Extensive lysine hydroxylation modification was observed as expected for COL1A1, COL1A2, COL3A1 and COL5A1 with 9, 12, 7 and 5 modified lysine residues identified, respectively. Other collagens with modified lysine residues included all six type IV collagens, COL6A2, COL16A1 and COL27A1 (Table 2). The lysine hydroxylation status was particularly high for COL3A1 with 94% of identified peptides presenting with lysine modifications in both PDGFC Tg fibrotic liver and Pten null steatotic liver; and with 100% of identified peptides presenting with lysine modifications in the tumors collected from both models (Figure 8). The lysyl hydroxylation status of COL1A1 and COL1A2 also slightly increased in the tumors compared to the fibrotic and steatotic livers in both models increasing from 33% to 43% for COL1A1 and from 21% to 25% for COL1A2 in PDGFC Tg mice and increasing from 27% to 37% for COL1A1 and from 15% to 17% for COL1A2 in Pten null mice (Figure 8). Inversely, the lysyl hydroxylation status of COL6A2 slightly decreased in the tumors compared to the fibrotic and steatotic livers in both models decreasing from 29% to 17% in PDGFC Tg mice and from 15% to 13% in Pten null mice (Figure 8). For the type IV collagens, the hydroxylation status was below 5%. Low hydroxylation was also found for COL5A1, COL5A2, COL16A1 and COL27A1.
Fig. 8. Percentage of peptides with lysine hydroxylation identified for COL1A1, COL1A2, COL3A1, and COL6A2. 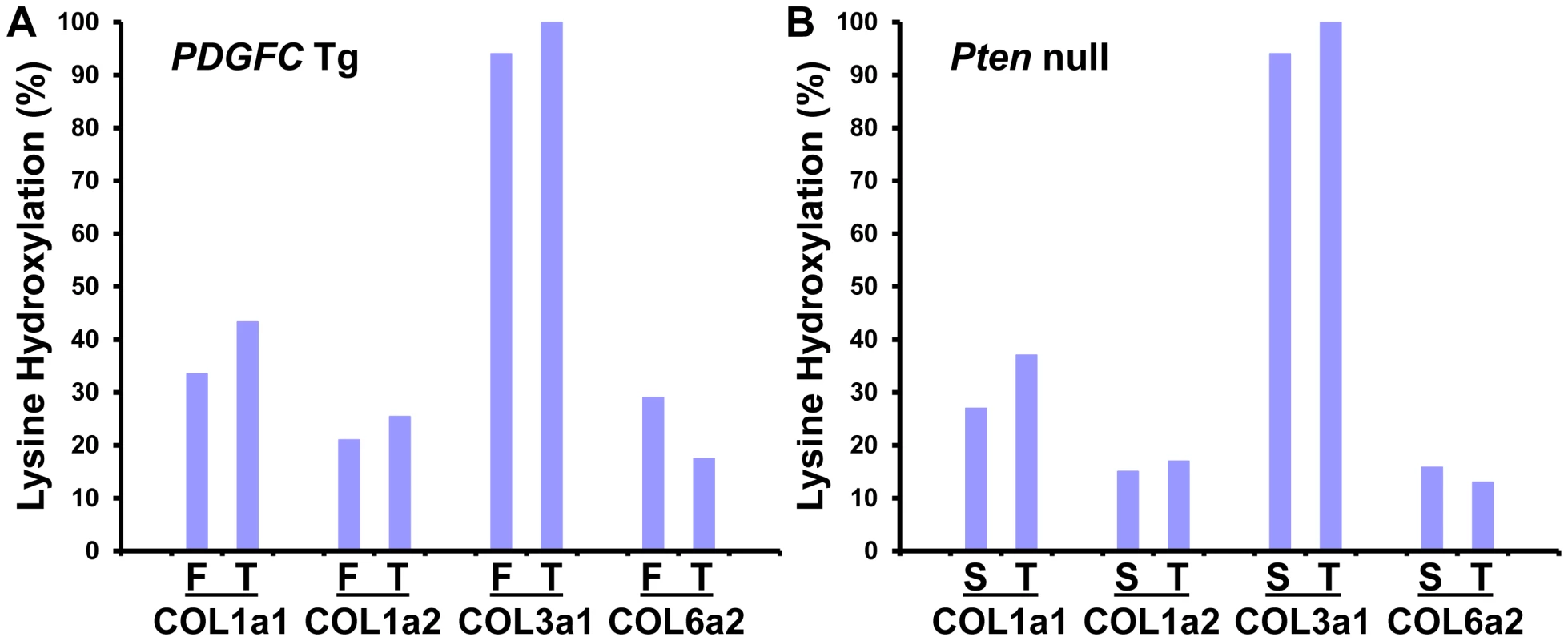
In A) PDGFC Tg fibrotic (F) and tumors (T) and in B) Pten null steatotic liver (S) and tumors (T). Tab. 2. Collagens with lysine hydroxylation modifications identified in <i>PDGFC</i> Tg and <i>Pten</i> null liver. 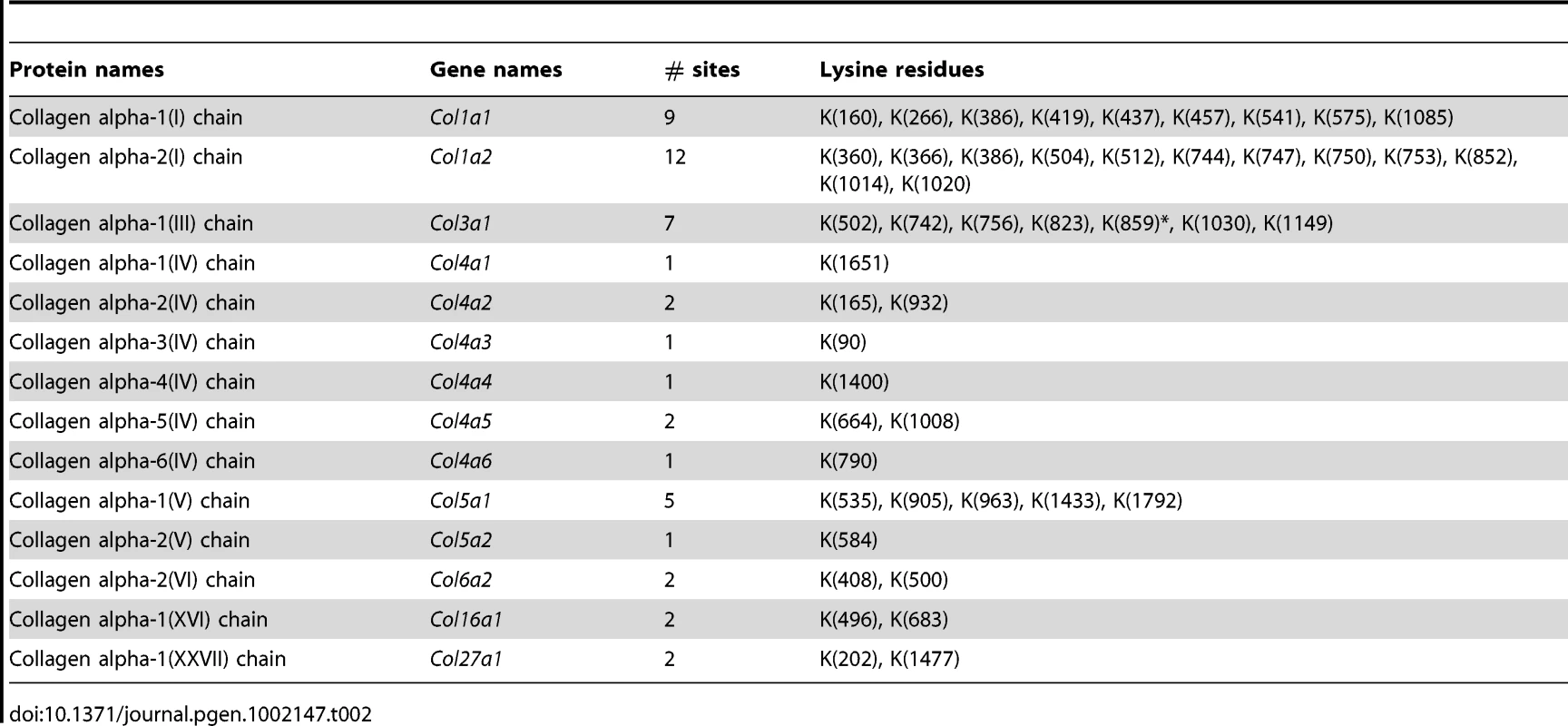
Non-Collagenous ECM Components
The ECM is also composed of non-collagenous proteins such as laminins. Laminins are large macromolecules constituted by the association of one α, one β and one γ chain. Laminin α5, laminin β2 and laminin γ1 were up-regulated in the tumors of both mouse models (Figure 9), suggesting that laminin 521 (previously called laminin 11) is the most abundant laminin in HCC. Laminin β3 and laminin γ2 were specifically up-regulated in Pten null tumors while laminin β1 was specifically up-regulated in PDGFC Tg tumors (Figure 9). Perlecan, also known as HSPG2, decorin and nidogen 1 were up-regulated in tumors of both models. Validation at the transcript level was performed for laminin α5 and nidogen 1. Laminin α5 mRNA was only weakly expressed in fibrotic and steatotic liver in both models but was significantly up-regulated in tumors in both models (6.4-fold (p = 0.01) in PDGFC Tg mice and 10.5-fold (p = 0.0002) in Pten null mice) (Figure 10). Laminin α5 mRNA expression was also significantly higher in tumors compared to adjacent tissues, in both models (p = 0.02 in PDGFC Tg mice and p = 0.03 in Pten null mice) (Figure 10). Nidogen 1 mRNA was increased by 7.2-fold (p = 0.008) and by 8.9-fold (p = 0.0003) in tumors from PDGFC Tg and Pten null mice, respectively (Figure 11A, 11B). Similarly, nidogen 1 protein was increased by 6.1-fold (p = 0.01) and by 15.3-fold (p = 0.001) in tumors from PDGFC Tg and Pten null mice, respectively (Figure 11C, 11D).
Fig. 9. Non-collagenous ECM proteins up-regulated in PDGFC Tg and Pten null tumors. 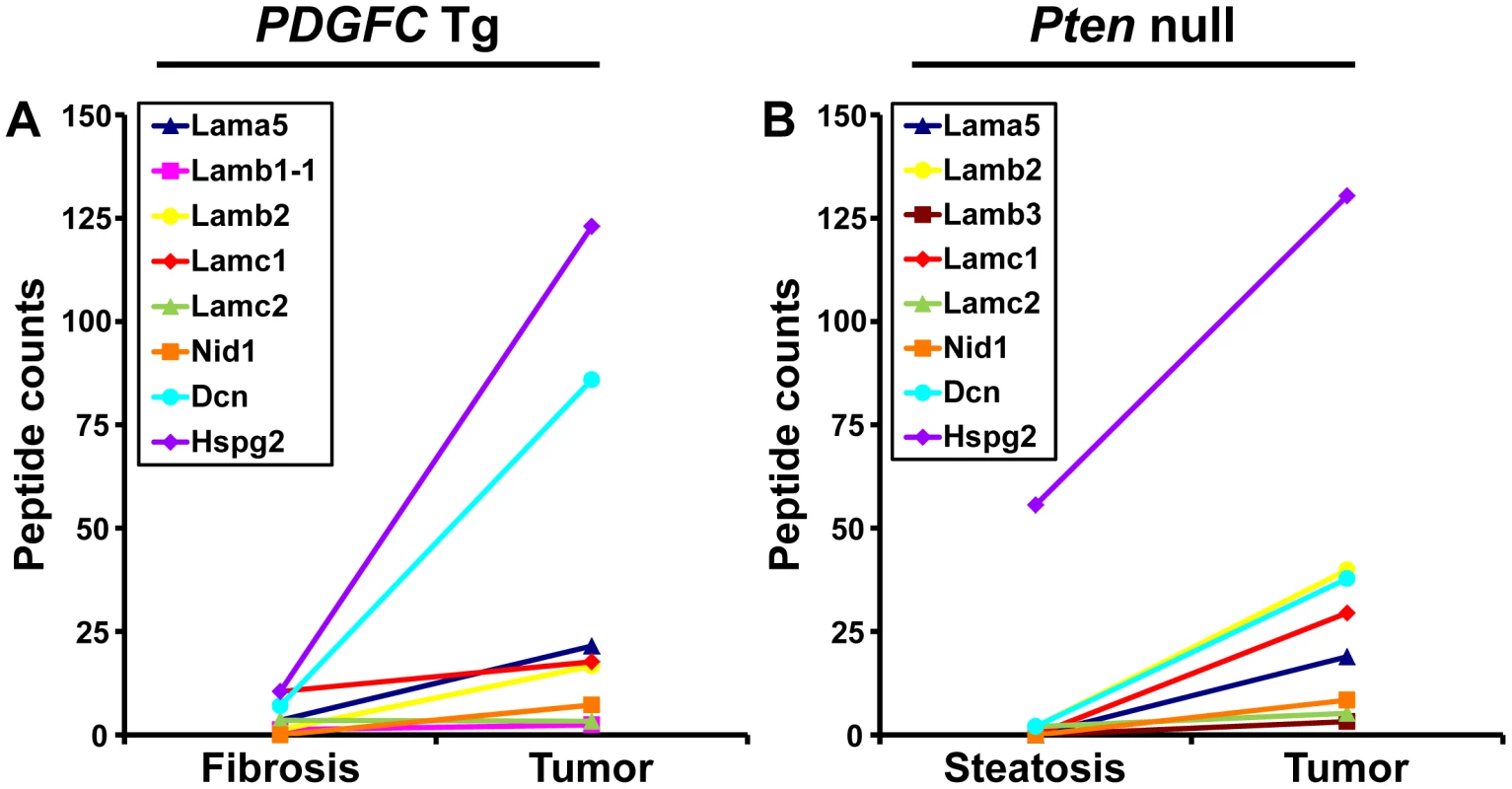
A) The figure includes the non-collagenous ECM proteins identified as up-regulated in PDGFC Tg tumors compared to PDGFC Tg fibrotic liver. B) The figure includes the non-collagenous ECM proteins identified as up-regulated in Pten null tumors compared to Pten null steatotic liver. For each protein, the abundance is shown as the total number of tandem mass spectra assigned to that protein. Fig. 10. Up-regulation of laminin α5 mRNA in PDGFC Tg and Pten null tumors. 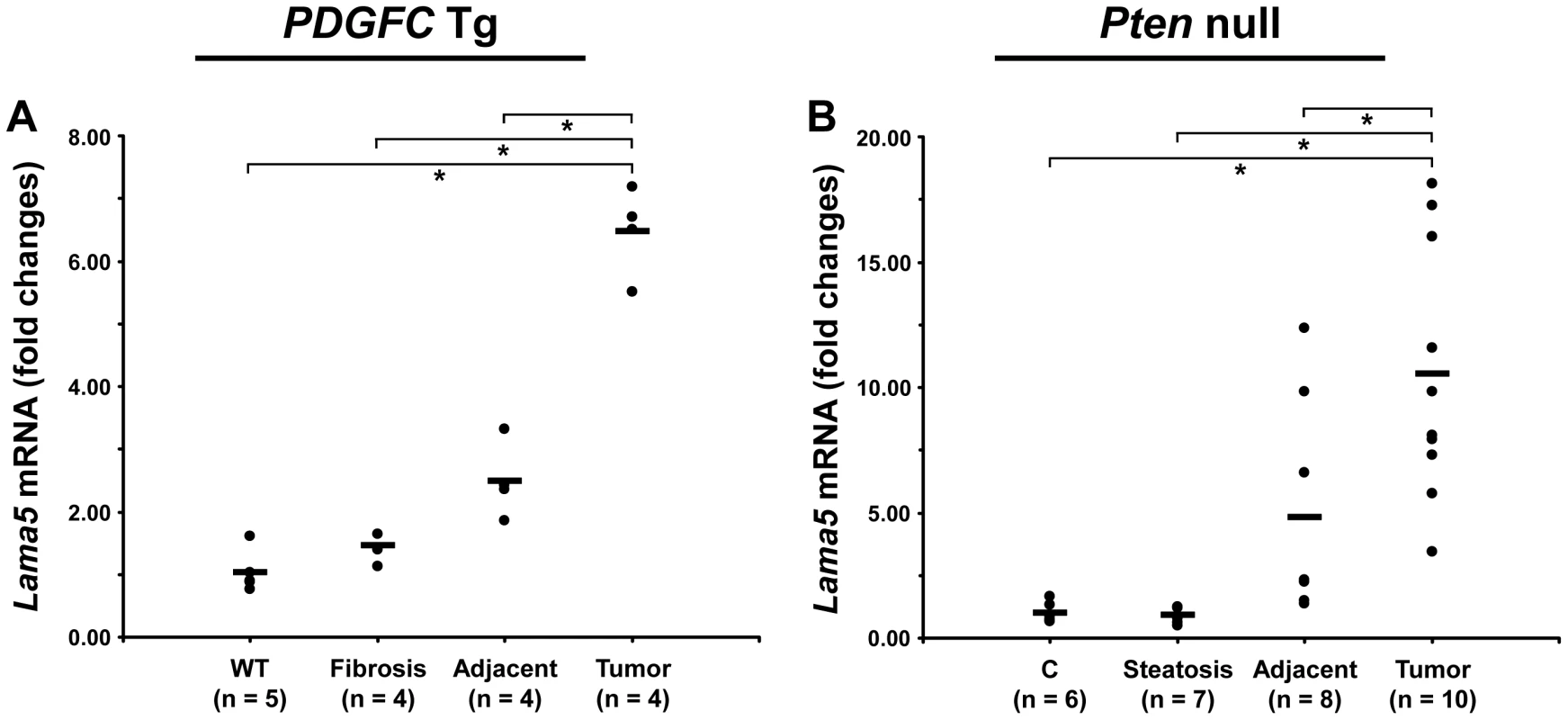
A) Expression of laminin α5 (Lama5) mRNA was measured by quantitative PCR in PDGFC Tg fibrotic liver, in PDGFC Tg tumor and adjacent tissue, and in age-matched WT liver. B) Similarly, expression of laminin α5 (Lama5) mRNA was measured in Pten null steatotic liver, in Pten null tumor and adjacent tissue, and in age-matched control liver. Expression in the disease groups is represented as fold changes over the mean of expression in the control groups. Fig. 11. Up-regulation of nidogen 1 mRNA and protein in PDGFC Tg and Pten null tumors. 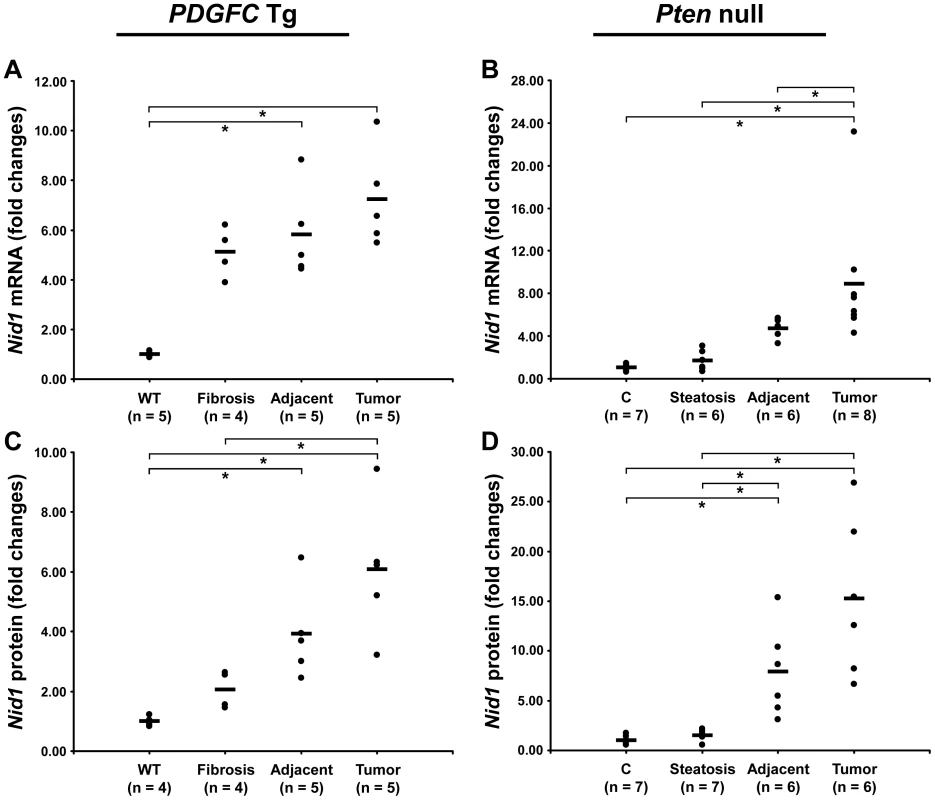
A,C) Expression of nidogen 1 mRNA and protein was measured by quantitative PCR and Western-blot, in PDGFC Tg fibrotic liver, in PDGFC Tg tumor and adjacent tissue and in age-matched WT liver. B,D) Similarly, expression of nidogen 1 mRNA and protein was measured in Pten null steatotic liver, in Pten null tumor and adjacent tissue, and in age-matched control liver. Expression in the disease groups is represented as fold changes over the mean of expression in the control groups. PDGF Expression in Pten Null Mice
Because of the similarity in ECM composition in both models, we investigated whether PDGF ligands were up-regulated in Pten null liver. While Pdgfb mRNA was undetected, both Pdgfa and Pdgfc mRNAs were up-regulated in Pten null tumors by 3.0-fold (p = 0.0007 and p = 0.002, respectively) and in adjacent tissue by 2-fold (p = 0.003 and p = 0.02, respectively) (Figure 12). The up-regulation of both PDGFA and PDGFC may therefore explain the common ECM changes observed in the PDGFC Tg and Pten null tumors and adjacent tissue.
Fig. 12. Up-regulation of Pdgfa and Pdgfc mRNAs in Pten null tumors. 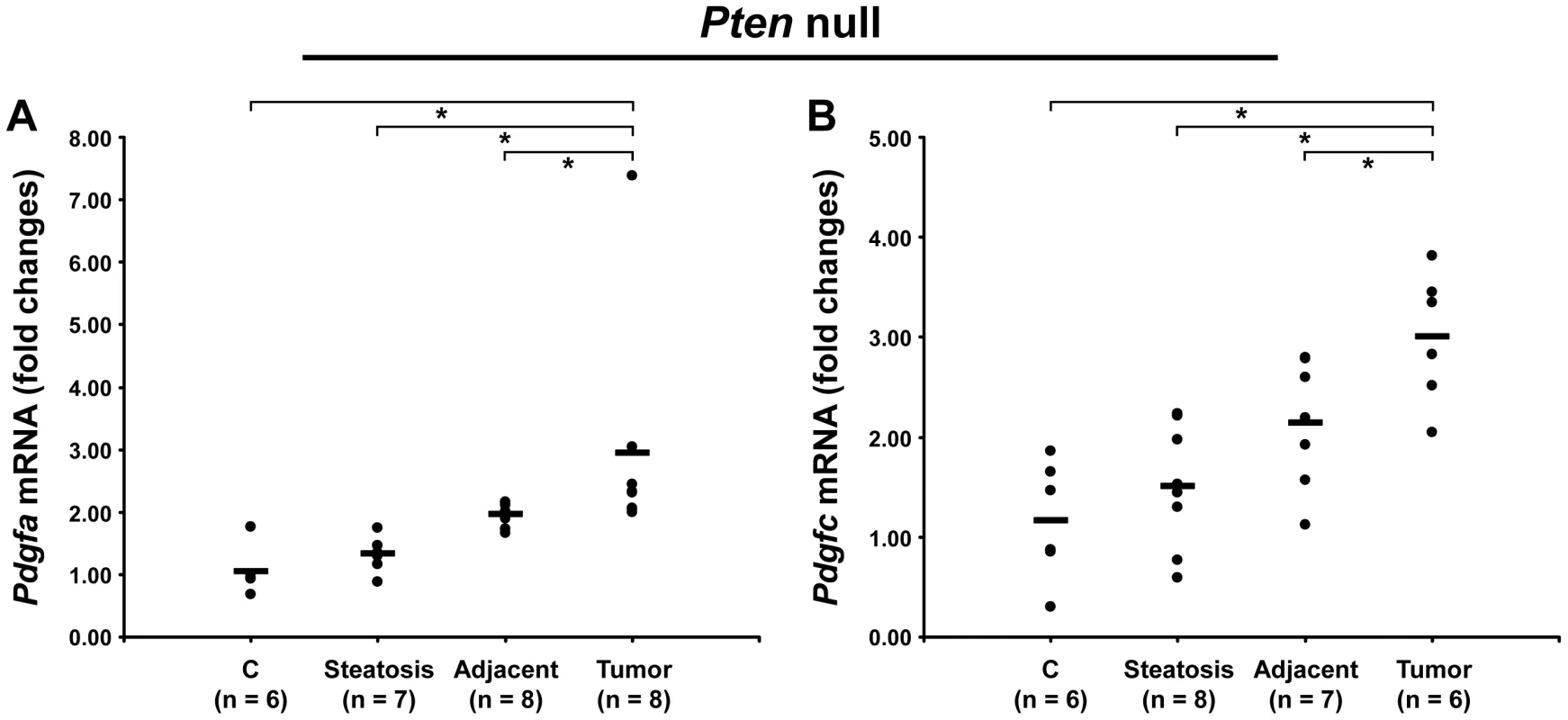
Expression of A) Pdgfa mRNA and B) Pdgfc mRNA was measured in Pten null steatotic liver, in Pten null tumor and adjacent tissue, and in age-matched control liver. Expression in the disease groups is represented as fold changes over the mean of expression in the control groups. ECM Receptors
Cell-ECM interactions are largely mediated through receptors called integrins made up of α and β chains. While integrin α5 was the most abundant and commonly up-regulated integrin chain in the tumors of both mouse models, the pattern of the other identified integrins was significantly different between the two models (Figure 13). Integrins α2b, α3 and β1 were specific to Pten null tumors while integrins α8 and β5 were specific to PDGFC Tg tumors. The up-regulation of integrin α6 and of CD44 was also much stronger in Pten null tumors compared to PDGFC Tg tumors. The differential expression of integrins α6 and α8 was further validated. Integrin α6 mRNA was increased by 39.0-fold (p = 0.001) in Pten null tumors and by 11.6-fold (p = 0.002) in PDGFC Tg tumors (Figure 14A, 14B). Integrin α8 mRNA was increased in PDGFC Tg liver tissue at all disease stages by 16.2 - to 24.0-fold (Figure 14C) but remained unchanged in Pten null liver (Figure 14D).
Fig. 13. CD44 and integrin proteins up-regulated in PDGFC Tg and Pten null tumors. 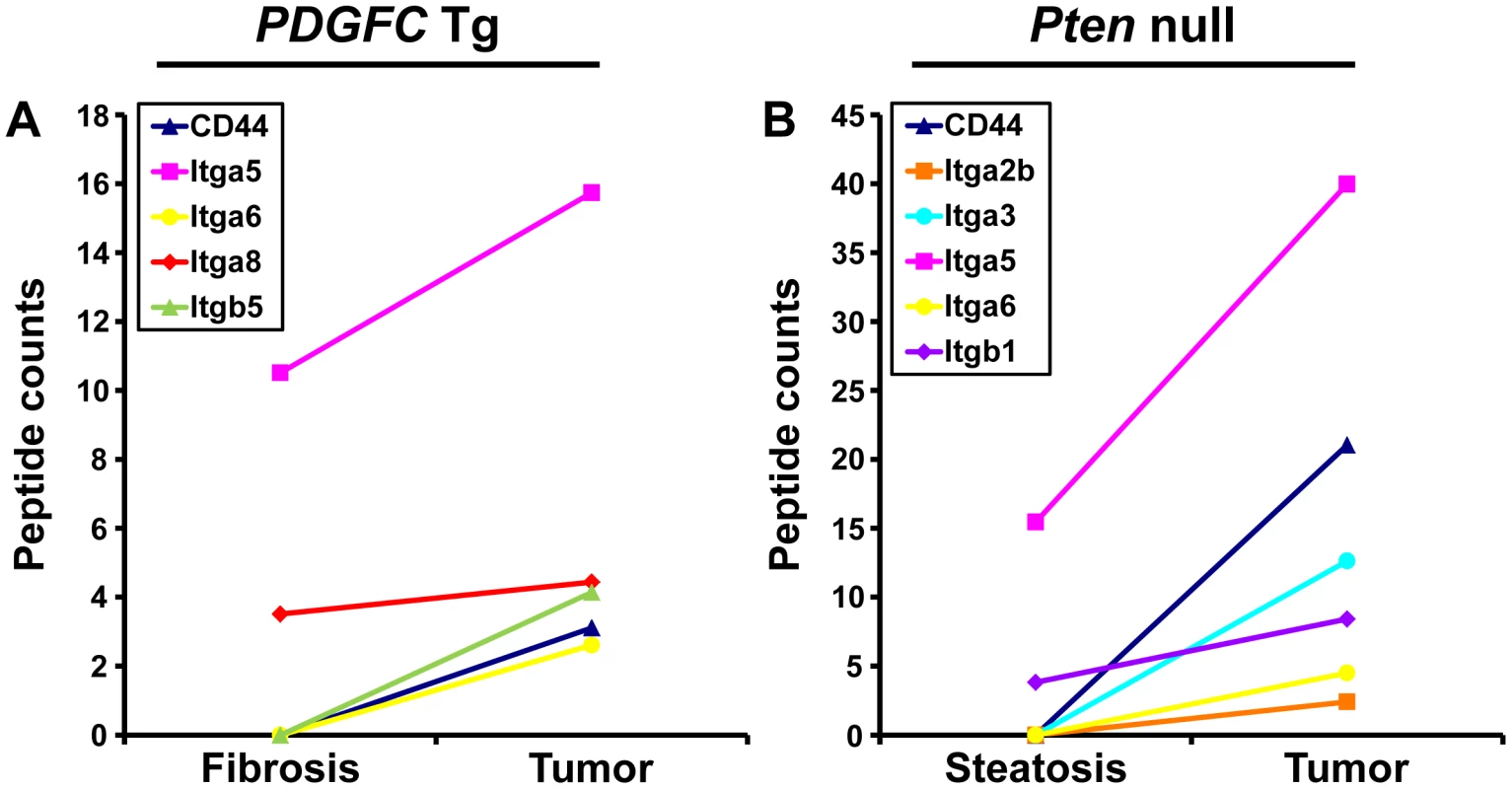
A) The figure includes CD44 and the integrin subunits identified up-regulated in PDGFC Tg tumors compared to PDGFC Tg fibrotic liver. B) The figure includes CD44 and the integrin subunits identified as up-regulated in Pten null tumors compared to Pten null steatotic liver. For each protein, the abundance is shown as the total number of tandem mass spectra assigned to that protein. Fig. 14. Expression of integrin α6 and α8 mRNAs upon disease progression in PDGFC Tg and Pten null liver. 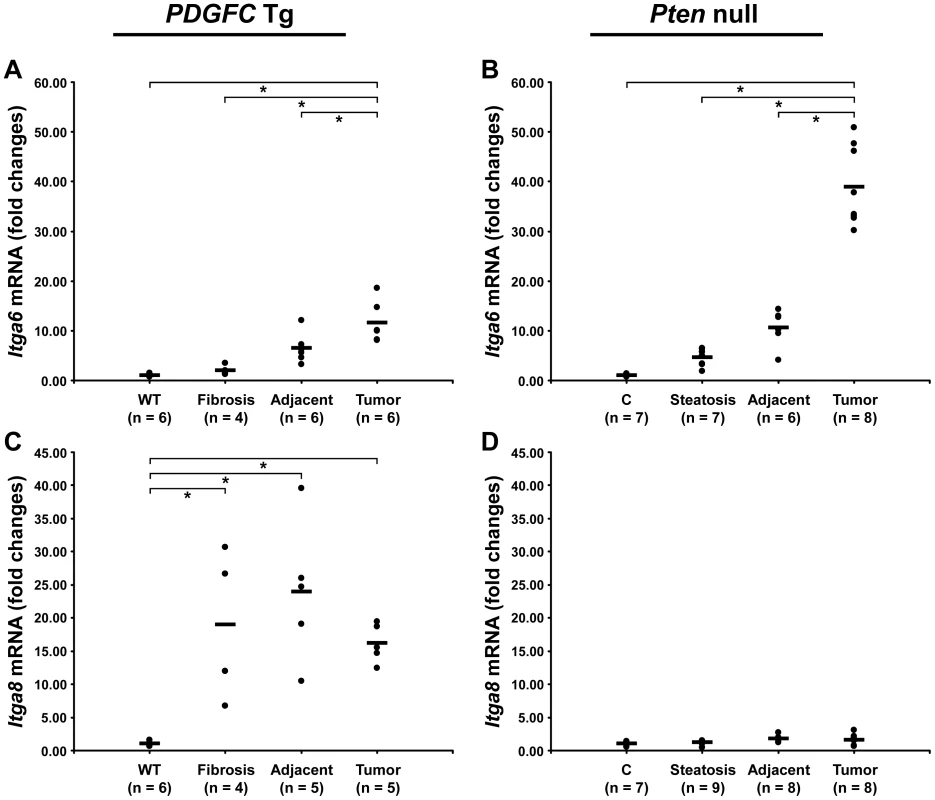
A,C) Expression of Itga6 and Itga8 mRNAs was measured by quantitative PCR in PDGFC Tg fibrotic liver, in PDGFC Tg tumor and adjacent tissue, and in age-matched WT liver. B,D) Similarly, expression of Itga6 and Itga8 mRNAs was measured in Pten null steatotic liver, in Pten null tumor and adjacent tissue, and in age-matched control liver. Expression in the disease groups is represented as fold changes over the mean of expression in the control groups. In summary (Figure 15), this study identified collagens type IV, VI, VII, X, XIV, XV, XVI and the short variant of COL18A1, NC1-301, as tumor-associated collagens in HCC. Laminin 521 was the most abundant laminin in HCC and integrin α5 the most abundant integrin subunit. High ratios of COL18A1 variant NC1-301 over COL18A1 variant NC1-764, high ratios of integrin α6 over integrin α8 and high levels of integrin α3 were specifically observed in the Pten null tumors.
Fig. 15. Schema summarizing the ECM protein components and their receptors identified as up-regulated in PDGFC Tg and Pten null tumors. 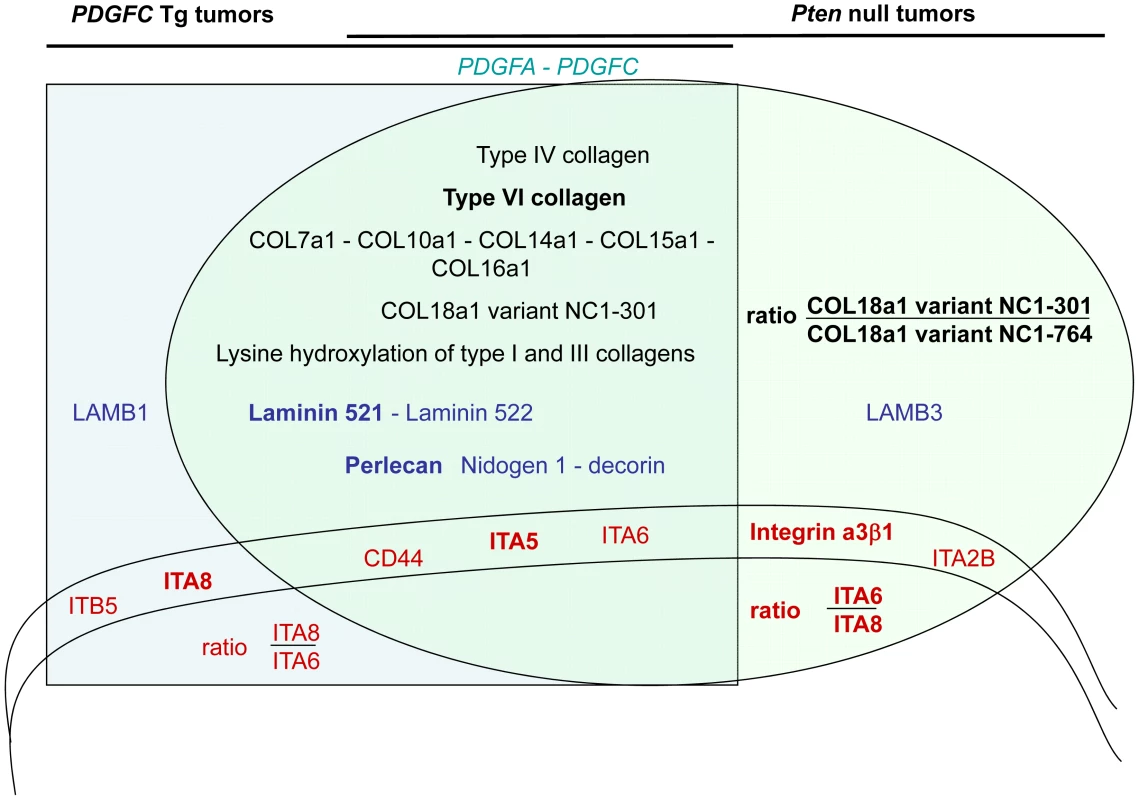
All ECM proteins and associated receptors identified as up-regulated in the mice tumors are shown as follows: in black for collagens, in blue for non-collagenous ECM proteins and in red for ECM receptors. Their position in the graph indicates whether they were commonly identified in the tumors of both mouse models (overlapping section between the light green oval representing the Pten null tumors and the light blue square representing the PDGFC Tg tumors) or identified specifically in one tumor type (light green section for Pten null tumors and light blue section for PDGFC Tg tumors). These two latter non-overlapping sections of the graph also indicate ratios of selected proteins that may have utility in discriminating between the PDGFC Tg and the Pten null tumors. The proteins in bold are those identified with higher abundance. Discussion
The microenvironment can have profound influences on cellular behavior, survival and growth of developing tumor cells [14]. Increased rigidity of the ECM is commonly associated with HCC [15] and ECM deposition and matrix remodeling has been shown to affect liver progenitor cell expansion [16]. We characterized and compared ECM protein changes occurring during tumor development in the PDGFC Tg mouse model, a model of HCC associated with fibrosis and angiogenesis [4] and in the Pten null mouse model, a model of liver tumors of mixed cholangio - and hepatocytic features [6]–[8], [17]. This study represents the most comprehensive analysis of the ECM and associated receptor proteome reported to date, and demonstrates the utility of mass spectrometry-based approaches to study gene families with extensive sequence homology, post-transcriptional and post-translational regulations. It is also the first study to analyze and compare proteome changes occurring during the transition from fibrosis and steatosis to HCC in two mouse models.
Collagens, the most abundant structural components of the ECM are homo - and heterotrimeric molecules whose subunits, the alpha chains, are distinct gene products. Forty-four different alpha chains have been sequenced, several of them being differentially spliced, which adds to the diversity of the collagen family. To date, 28 different combinations of the alpha chains (collagen types I–XXVIII) have been identified or predicted to exist (www.uniprot.org). While only ten collagen types have been described in the liver [15], this extensive proteomic study resulted in the identification of 16 types. Fibril-forming types I and III collagens are predominantly synthesized by hepatic stellate cells and are used as markers for liver fibrogenesis. In the fibrotic liver of PDGFC Tg mice, type I and III collagen levels strongly increased with a significantly higher increase of type I collagen than of type III collagen changing the I/III ratio from 1∶1 in the healthy liver to over 2∶1, as observed in human fibrotic liver. Collagens type V (COL5A1, COL5A2 and COL5A3) and type II (COL2A1), the other fibril-forming collagens, were also up-regulated in the fibrotic liver of the PDGFC Tg mice. This ECM composition in the fibrotic liver of PDGFC Tg mice is consistent with a signature of activated hepatic stellate cells, a hallmark of PDGFC Tg mice [4]. Out of the 26 alpha chains identified, 15 were up-regulated in tumors of both models. These include the six alpha chains of collagen IV, the three alpha chains of collagen VI, COL7A1, COL10A1, COL14A1, COL15A1, COL16A1 and COL18A1. Collagen VI, a component of microfibrillar structures in many tissues, is a heterotrimer with the chain composition (6a1)(6a2)(6a3). Type VI collagen binds cells and may be involved in cell migration and differentiation and embryonic development. All collagen VI subunits, including splice variants for COL6A2 and COL6A3, were up-regulated in the tumors and adjacent tissue of both models. The most abundant structural component of basement membranes is collagen IV. The six different alpha chains 4a1–4a6 were up-regulated in the tumors of both mouse models. Besides the heterotrimeric molecule (4a1)2(4a2) composed of the two most abundant collagen IV subunits, the other combinations between alpha chains, particularly those including the subunits of minor abundance, are not yet established. Whereas COL4A1 and COL4A2 are found in all basement membranes studied, COL4A3, COL4A4 and COL4A5 are found only in a subset of basement membranes and are always found together [18]. Strong deposition of collagen type IV was described in dysplastic areas and small HCCs in human cirrhotic livers indicative of early events in hepatocarcinogenesis [19]. The multiplexin collagens XV and XVIII are also localized to basement membranes. COL15A1 was the collagen alpha chain that showed the stronger up-regulation in both the Pten null and PDGFC Tg tumors.
COL18A1 was also up-regulated in the mice tumors. Interestingly, increases in this protein correlated with the increase of a specific isoform of Col18a1 mRNA, isoform NC1-301, resulting from alternative promoter usage. NC1-301 mRNA was increased by over 30-fold in PDGFC Tg tumors and over 100-fold in Pten null tumors. In contrast, the canonical Col18a1 mRNA, NC1-764, was unchanged or slightly decreased in the PDGFC Tg tumors and strongly decreased in Pten null tumors. It was previously reported that NC1-764 mRNA expression decreases in advanced HCCs [20] and that cholangiocarcinoma cells expressed NC1-301 which was deposited in tumor basement membrane [21]. This is in good agreement with the changes we observed in both COL18A1 isoforms in the mice tumors, with a greater ratio NC1-301/NC1-764 in Pten null tumors compared to PDGFC Tg tumors. These results suggest that the ratio of COL18A1 isoforms could directly correlate with the expansion of intermediate cells co-expressing both hepatocytes and biliary markers. Finally, collagen VII, the main constituent of anchoring fibrils, was also up-regulated in tumors of both models. It has been reported that human epidermal cells devoid of collagen VII did not form tumors in mice, whereas those retaining the specific N-terminal NC1 domain were tumorigenic [22].
Other glycoproteins in basement membranes such as laminins and nidogen 1 increased in the mice tumors. Nidogens are believed to connect laminin and collagen IV networks, hence stabilizing the basement membrane structure and appear critical for anchoring other components such as perlecan. At present, five laminin α (α1–α5), three β (β1–β3) and three γ (γ1–γ3) chains and 16 trimers have been characterized in mouse and human [23]. Based on the chain identification, laminins 511 or 521 (previously called laminin 10/11) and laminin 522 are likely up-regulated in PDGFC Tg and Pten null tumors. It was reported that laminins containing the α5 chain serve as functional regulators of HCC progression [24]. Up-regulation of laminin β3, a major component of laminin 332 (previously called laminin 5) was observed specifically in Pten null tumors. Interestingly, laminin β3 was reported up-regulated in cholangiocarcinoma cell lines compared to HCC cell lines [25] and laminin 332 is present in almost all intrahepatic cholangiocarcinoma cases [26].
The global expression of the ECM in liver during tumor development results from the combined expression profiles of tumor cells, stromal cells, and non-tumor hepatocytes. Activated hepatic stellate cells and myofibroblasts express a wide spectrum of ECM molecules but an important fraction of ECM is also synthesized by other liver cells, notably sinusoidal and portal endothelia, bile duct epithelia and hepatocytes [27], [28]. While this study increases our knowledge of HCC-specific matrix composition, future studies should focus on the cellular distribution of the described proteins. Overall, beside a subset of laminins, the ECM changes were remarkably similar in the tumors and adjacent tissues of both mouse models, suggesting a common molecular and cellular mechanism. We therefore investigated the possibility that PDGF factors were up-regulated in Pten null mice. While Pdgfb mRNA was undetected in Pten null liver, both Pdgfa and Pdgfc transcripts were increased in Pten null tumors and adjacent tissues. We also observed an up-regulation of both PDGF receptor alpha and PDGF receptor beta in Pten null tumors and adjacent tissues (LB, personal communication).
Most cell types have the ability to bind to the surrounding ECM and certain ECM components can transmit signals to cells via transmembrane receptors [29]. Such matrix sensors are mainly integrins. Integrins comprise a large family of cell surface glycoproteins which consist of alpha and beta subunits and that regulate cell adhesion, migration, proliferation and apoptosis [30]. There are 18 α and 8 β subunits, each of which can bind to several partners giving rise to at least 24 distinct integrin heterodimers with different functions and ligand binding activities. Laminins are ligands for both α6β1 and α3β1 integrins. These integrins were specifically up-regulated in Pten null tumors. Interestingly, laminin 511 modulates human embryonic stem cell aggregation and adherence through binding of the α6β1 integrin receptor highly expressed in the membranes of undifferentiated stem cells [31], [32]. It was also reported that oval cells express integrin α6 [33]. Similarly, laminin 332 and integrin α3 were co-up-regulated in Pten null tumors. It was reported that cells lacking integrin α3 do not proliferate in response to laminin 332 treatment [34]. Altogether, these results suggest that laminin 332/integrin α3–induced HCC growth and that laminin 511-integrin α6β1 interaction is specific to Pten null tumors.
The identified HCC–associated ECM and integrin components could play an important role in the promotion of the early steps of hepatocarcinogenesis, providing a foundation for novel strategies to prevent, diagnose and treat HCC. Inhibiting the expression of ECM components or blocking their interactions with signaling integrins could serve as a means for establishing a non-permissive microenvironment that may prevent tumor development. Integrin inhibitors such as humanized antibodies or blocking peptides against integrin α5β1 are currently under clinical investigation. Our results suggest that these novel drugs should be evaluated for the treatment of HCC. In addition, integrin α3β1-laminin 332 and integrin α6β1-laminin 511 networks may be promising targets to prevent laminin-tumor cell interaction in HCC with dysregulated PTEN function.
Methods
Mouse Samples
The PDGFC Tg mice used for this study were previously described [4]. Liver tissue samples were collected by necropsy from 1.5-month old PDGFC Tg mice with hepatic fibrosis, 8-month old PDGFC Tg mice with small HCCs and from 1.5-month and 8-month old wild-type controls. Mice carrying Pten conditional knockout alleles were crossed with an Albumin (Alb)-Cre-transgenic mouse. The Alb-Cre-transgenic mice were genotyped using Cre specific primers. For this model, control animals are PtenloxP/loxP; Alb-Cre−. Liver tissue samples were collected by necropsy from 6-month old Pten null mice with steatosis, 9-month old Pten null mice with small HCCs and from 6-month and 9-month old control mice. All tissues were immediately snap-frozen in liquid nitrogen or fixed in 10% neutral buffered formalin overnight, processed to paraffin blocks, sectioned, and stained with hematoxylin/eosin or Masson's trichrome by using standard techniques. This study was carried out in strict accordance with the regulations of the U.S. National Institutes of Health. All of the work with animals was performed in adherence to the “Guide for the Care and Use of Laboratory Animals” published by the U.S. National Research Council, including the use of appropriate anesthesia whenever recommended by these guidelines. The protocol was approved and reviewed annually by the Institutional Animal Care and Use Committee of the Fred Hutchinson Cancer Research Center (File #1662). Every effort was made to minimize the number of animals required for the study and to minimize the pain and discomfort experienced.
Protein Extraction and Separation
Liver tissues from three or four mice in each study group were separately ground on dry ice and subsequently pooled. Proteins were extracted twice from 40 mg of each pooled group in 1 ml lysis buffer (5 M urea, 2 M thiourea, 2% w/v n-Octyl-β-D-Glucopyranoside, 40 mM Tris and 1 mM phenylmethylsulfonyl fluoride). Following centrifugation at 16,100×g at 4°C for 1 hr, the pellet fraction was solubilized in Laemmli buffer and the proteins from the supernatant were separated using the Alliance 2-D Bioseparations System (Waters Corporation, Milford, MA) as previously described [13]. Briefly, an anion exchange column, BioSuite Q 10 µm, (Waters Corporation, Milford, MA) was used for the first dimension. Eight stepwise gradients were performed consisting of 0, 100 mM, 200 mM, 350 mM, 500 mM, 650 mM, 800 mM and 1000 mM NaCl. The reversed phase columns, Symmetry300 C4 3.5 µm, (Waters Corporation, Milford, MA) were used for separation of the fractions obtained from the first dimension steps. Two reversed phase columns were switched through the column selector. A total of ∼260 fractions was collected for each pooled group. Some adjacent fractions were combined leading to a final number of 34 samples for each pooled group. All fractions were lyophilized and resuspended in Laemmli buffer. Proteins obtained from the 2-D HPLC separations were further separated by 12% SDS PAGE. Gel pieces were combined into 37 individual samples for each pooled group according to protein size and abundance, dehydrated with 100% acetonitrile and dried using a speed vacuum. Gel pieces were incubated with 10 µl of 6.7 ng/µl trypsin in digestion buffer overnight at 37°C. The reaction was stopped with 15 µl of extraction buffer (2% formic acid/3% acetonitrile) and the supernatants were collected.
Mass Spectrometry
The generated peptide samples were desalted using Symmetry C18 de-salting columns (Waters Corporation, Milford, MA) and subjected in duplicate to nanoflow LC-MS/MS analysis with a nano-UPLC system (Waters Corporation, Milford, MA) coupled to a hybrid 7-Tesla linear ion-trap Fourier-transform ion cyclotron resonance mass spectrometer (LTQ-FT, Thermo Scientific, Waltham, MA). Peptides were separated on a reversed phase column (75 µm×250 mm) packed with Magic C18AQ (5-µm 100 Å resin; Michrom Bioresources, Auburn, CA) and directly mounted on the electrospray ion source. We used a 60 min gradient from 10% to 40% acetonitrile in 0.1% formic acid at a flow rate of 300 nl/min. A spray voltage of 1600 V was applied. The LTQ-FT instrument was operated in the data-dependent mode, switching automatically between MS survey scans in the FTICR (target value 1,600,000, resolution 100,000, and injection time 1.5 s) with MS/MS spectra acquisition in the linear ion trap. The five most intense ions from the FT full scan were selected for fragmentation in the linear ion trap by collision-induced dissociation with a normalized collision energy of 30% at a target value of 10,000 (injection time 400 ms). Selected ions were dynamically excluded for 60 s. The absolute average mass accuracy for the parent ion was <5 ppm.
Proteomic Data Analysis
Acquired data were processed using the X!Tandem search algorithm [35] and PeptideProphet and ProteinProphet statistical tools [36], [37]. The tandem mass spectra were searched against the mouse International Protein Index protein sequence database (IPI, version 3.34, http://www.ebi.ac.uk/IPI/). The following search criteria were used in all cases: trypsin specificity, 2.5 Da of mass accuracy for the parent ion and methionine oxidation as a variable modification and when specified, lysine oxidation was added as a variable modification. Relative abundance scores were calculated for individual proteins based on total peptide counts normalized to account for the total amount of protein in the mixture.
Quantitative PCR
Total RNA was extracted from individual liver tissue samples and purified using the Trizol reagent (Invitrogen, Carlsbad, CA). RNA samples were then submitted to DNAse digestion, reverse transcription using random hexamers and real-time PCR using specific primers listed in Table S2. For each sample, the cDNA equivalent to 1 ug total RNA per 20 µl reaction was amplified with the iCycler MyiQ using SYBR Green Supermix and analyzed by MyiQ software (Bio-Rad Laboratories, Hercules, CA) and relative quantification of RNA expression was calculated with the 2−ΔΔCt method. Actin quantification was used for normalization. The specificity of qPCR products was confirmed by melting curve analysis and gel-based analysis of the PCR products.
Western Blotting
Proteins from individual liver tissues were extracted from 100 mg of liver, in 1 ml lysis buffer consisting of 5 M urea, 2 M thiourea, 2% w/v n-Octyl-β-D-Glucopyranoside (Sigma-Aldrich, St Louis, MO), 50 mM Tris (Fisher Scientific) and 1 mM phenylmethylsulfonyl fluoride (GE Healthcare, Little Chalfont, UK). The homogenate was centrifuged at 19,000× g at 4°C for 1 hour and supernatant was collected. Proteins (40 ug) were loaded onto 12% SDS PAGE gels and immunoblotting was performed using rat monoclonal anti-Nidogen 1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at 1/100 dilution. Immunoreactivity was revealed by enhanced chemiluminescence using ECL kit (GE Healthcare, Little Chalfont, UK) and quantification was performed using ImageJ (http://rsbweb.nih.gov/ij/).
Supporting Information
Zdroje
1. El-SeragHBRudolphKL 2007 Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132 2557 2576
2. ReigstadLJVarhaugJELillehaugJR 2005 Structural and functional specificities of PDGF-C and PDGF-D, the novel members of the platelet-derived growth factors family. FEBS J 272 5723 5741
3. BatallerRBrennerDA 2005 Liver fibrosis. J Clin Invest 115 209 218
4. CampbellJSHughesSDGilbertsonDGPalmerTEHoldrenMS 2005 Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. Proc Natl Acad Sci U S A 102 3389 3394
5. FaustoNCampbellJS 2010 Mouse models of hepatocellular carcinoma. Semin Liver Dis 30 87 98
6. HorieYSuzukiAKataokaESasakiTHamadaK 2004 Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest 113 1774 1783
7. StilesBWangYStahlABassilianSLeeWP 2004 Liver-specific deletion of negative regulator Pten results in fatty liver and insulin hypersensitivity [corrected]. Proc Natl Acad Sci U S A 101 2082 2087
8. XuXKobayashiSQiaoWLiCXiaoC 2006 Induction of intrahepatic cholangiocellular carcinoma by liver-specific disruption of Smad4 and Pten in mice. J Clin Invest 116 1843 1852
9. RountreeCBSenadheeraSMatoJMCrooksGMLuSC 2008 Expansion of liver cancer stem cells during aging in methionine adenosyltransferase 1A-deficient mice. Hepatology 47 1288 1297
10. RoskamsT 2006 Liver stem cells and their implication in hepatocellular and cholangiocarcinoma. Oncogene 25 3818 3822
11. ZiolMNaultJCAoutMBargetNTepperM 2010 Intermediate hepatobiliary cells predict an increased risk of hepatocarcinogenesis in patients with hepatitis C virus-related cirrhosis. Gastroenterology 139 335 343 e332
12. WooHGLeeJHYoonJHKimCYLeeHS 2010 Identification of a cholangiocarcinoma-like gene expression trait in hepatocellular carcinoma. Cancer Res 70 3034 3041
13. LaiKKKolippakkamDBerettaL 2008 Comprehensive and quantitative proteome profiling of the mouse liver and plasma. Hepatology 47 1043 1051
14. HynesRO 2009 The extracellular matrix: not just pretty fibrils. Science 326 1216 1219
15. SchuppanDRuehlMSomasundaramRHahnEG 2001 Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis 21 351 372
16. Van HulNKAbarca-QuinonesJSempouxCHorsmansYLeclercqIA 2009 Relation between liver progenitor cell expansion and extracellular matrix deposition in a CDE-induced murine model of chronic liver injury. Hepatology 49 1625 1635
17. GaliciaVAHeLDangHKanelGVendryesC 2010 Expansion of hepatic tumor progenitor cells in Pten-null mice requires liver injury and is reversed by loss of AKT2. Gastroenterology
18. ThornerPSZhengKKalluriRJacobsRHudsonBG 1996 Coordinate gene expression of the alpha3, alpha4, and alpha5 chains of collagen type IV. Evidence from a canine model of X-linked nephritis with a COL4A5 gene mutation. J Biol Chem 271 13821 13828
19. Le BailBFaouziSBoussarieLBalabaudCBioulac-SageP 1997 Extracellular matrix composition and integrin expression in early hepatocarcinogenesis in human cirrhotic liver. J Pathol 181 330 337
20. QuelardDLavergneEHendaouiIElamaaHTiirolaU 2008 A cryptic frizzled module in cell surface collagen 18 inhibits Wnt/beta-catenin signaling. PLoS ONE 3 e1878 doi:10.1371/journal.pone.0001878
21. MussoOTheretNHeljasvaaraRRehnMTurlinB 2001 Tumor hepatocytes and basement membrane-producing cells specifically express two different forms of the endostatin precursor, collagen XVIII, in human liver cancers. Hepatology 33 868 876
22. Ortiz-UrdaSGarciaJGreenCLChenLLinQ 2005 Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science 307 1773 1776
23. AumailleyMBruckner-TudermanLCarterWGDeutzmannREdgarD 2005 A simplified laminin nomenclature. Matrix Biol 24 326 332
24. KikkawaYSudoRKonJMizuguchiTNomizuM 2008 Laminin alpha 5 mediates ectopic adhesion of hepatocellular carcinoma through integrins and/or Lutheran/basal cell adhesion molecule. Exp Cell Res 314 2579 2590
25. SrisomsapCSawangareetrakulPSubhasitanontPChokchaichamnankitDChiablaemK 2010 Proteomic studies of cholangiocarcinoma and hepatocellular carcinoma cell secretomes. J Biomed Biotechnol 2010 437143
26. OkamuraNYoshidaMShibuyaASugiuraHOkayasuI 2005 Cellular and stromal characteristics in the scirrhous hepatocellular carcinoma: comparison with hepatocellular carcinomas and intrahepatic cholangiocarcinomas. Pathol Int 55 724 731
27. WellsRG 2008 Cellular sources of extracellular matrix in hepatic fibrosis. Clin Liver Dis 12 759 768, viii
28. FriedmanSL 2008 Mechanisms of hepatic fibrogenesis. Gastroenterology 134 1655 1669
29. HeinoJKapylaJ 2009 Cellular receptors of extracellular matrix molecules. Curr Pharm Des 15 1309 1317
30. HynesRO 2002 Integrins: bidirectional, allosteric signaling machines. Cell 110 673 687
31. EvseenkoDSchenke-LaylandKDravidGZhuYHaoQL 2009 Identification of the critical extracellular matrix proteins that promote human embryonic stem cell assembly. Stem Cells Dev 18 919 928
32. RodinSDomogatskayaAStromSHanssonEMChienKR 2010 Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat Biotechnol 28 611 615
33. HoppoTFujiiHHiroseTYasuchikaKAzumaH 2004 Thy1-positive mesenchymal cells promote the maturation of CD49f-positive hepatic progenitor cells in the mouse fetal liver. Hepatology 39 1362 1370
34. BergaminiCSgarraCTrerotoliPLupoLAzzaritiA 2007 Laminin-5 stimulates hepatocellular carcinoma growth through a different function of alpha6beta4 and alpha3beta1 integrins. Hepatology 46 1801 1809
35. CraigRBeavisRC 2004 TANDEM: matching proteins with tandem mass spectra. Bioinformatics 20 1466 1467
36. NesvizhskiiAIKellerAKolkerEAebersoldR 2003 A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75 4646 4658
37. KellerANesvizhskiiAIKolkerEAebersoldR 2002 Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem 74 5383 5392
Štítky
Genetika Reprodukčná medicína
Článek Local Absence of Secondary Structure Permits Translation of mRNAs that Lack Ribosome-Binding SitesČlánek Independent Chromatin Binding of ARGONAUTE4 and SPT5L/KTF1 Mediates Transcriptional Gene SilencingČlánek Trade-Off between Bile Resistance and Nutritional Competence Drives Diversification in the Mouse GutČlánek FGF Signaling Regulates the Number of Posterior Taste Papillae by Controlling Progenitor Field SizeČlánek Mammalian BTBD12 (SLX4) Protects against Genomic Instability during Mammalian SpermatogenesisČlánek Identification of Nine Novel Loci Associated with White Blood Cell Subtypes in a Japanese PopulationČlánek Differential Effects of and Risk Variants on Association with Diabetic ESRD in African AmericansČlánek Dynamic Chromatin Localization of Sirt6 Shapes Stress- and Aging-Related Transcriptional Networks
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2011 Číslo 6- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Local Absence of Secondary Structure Permits Translation of mRNAs that Lack Ribosome-Binding Sites
- Statistical Inference on the Mechanisms of Genome Evolution
- Revisiting Heterochromatin in Embryonic Stem Cells
- A Two-Stage Meta-Analysis Identifies Several New Loci for Parkinson's Disease
- Identification of a Sudden Cardiac Death Susceptibility Locus at 2q24.2 through Genome-Wide Association in European Ancestry Individuals
- Genomic Prevalence of Heterochromatic H3K9me2 and Transcription Do Not Discriminate Pluripotent from Terminally Differentiated Cells
- Epistasis between Beneficial Mutations and the Phenotype-to-Fitness Map for a ssDNA Virus
- Recurrent Chromosome 16p13.1 Duplications Are a Risk Factor for Aortic Dissections
- Telomere DNA Deficiency Is Associated with Development of Human Embryonic Aneuploidy
- Genome-Wide Association Study of White Blood Cell Count in 16,388 African Americans: the Continental Origins and Genetic Epidemiology Network (COGENT)
- Unexpected Role for DNA Polymerase I As a Source of Genetic Variability
- Transportin-SR Is Required for Proper Splicing of Genes and Plant Immunity
- How Chromatin Is Remodelled during DNA Repair of UV-Induced DNA Damage in
- Independent Chromatin Binding of ARGONAUTE4 and SPT5L/KTF1 Mediates Transcriptional Gene Silencing
- Two Evolutionary Histories in the Genome of Rice: the Roles of Domestication Genes
- Natural Allelic Variation Defines a Role for : Trichome Cell Fate Determination
- Multiple Common Susceptibility Variants near BMP Pathway Loci , , and Explain Part of the Missing Heritability of Colorectal Cancer
- Pathogenic Mechanism of the FIG4 Mutation Responsible for Charcot-Marie-Tooth Disease CMT4J
- A Functional Variant in Promoter Modulates Its Expression and Confers Disease Risk for Systemic Lupus Erythematosus
- Drift and Genome Complexity Revisited
- Chromosomal Macrodomains and Associated Proteins: Implications for DNA Organization and Replication in Gram Negative Bacteria
- Trade-Off between Bile Resistance and Nutritional Competence Drives Diversification in the Mouse Gut
- Pathways of Distinction Analysis: A New Technique for Multi–SNP Analysis of GWAS Data
- Web-Based Genome-Wide Association Study Identifies Two Novel Loci and a Substantial Genetic Component for Parkinson's Disease
- Chk2 and p53 Are Haploinsufficient with Dependent and Independent Functions to Eliminate Cells after Telomere Loss
- Exome Sequencing Identifies Mutations in High Myopia
- Distinct Functional Constraints Partition Sequence Conservation in a -Regulatory Element
- CorE from Is a Copper-Dependent RNA Polymerase Sigma Factor
- A Single Sex Pheromone Receptor Determines Chemical Response Specificity of Sexual Behavior in the Silkmoth
- FGF Signaling Regulates the Number of Posterior Taste Papillae by Controlling Progenitor Field Size
- Maps of Open Chromatin Guide the Functional Follow-Up of Genome-Wide Association Signals: Application to Hematological Traits
- Increased Susceptibility to Cortical Spreading Depression in the Mouse Model of Familial Hemiplegic Migraine Type 2
- Differential Gene Expression and Epiregulation of Alpha Zein Gene Copies in Maize Haplotypes
- Parallel Adaptive Divergence among Geographically Diverse Human Populations
- Genetic Analysis of Genome-Scale Recombination Rate Evolution in House Mice
- Mechanisms for the Evolution of a Derived Function in the Ancestral Glucocorticoid Receptor
- Mammalian BTBD12 (SLX4) Protects against Genomic Instability during Mammalian Spermatogenesis
- Interferon Regulatory Factor 8 Regulates Pathways for Antigen Presentation in Myeloid Cells and during Tuberculosis
- High-Resolution Analysis of Parent-of-Origin Allelic Expression in the Arabidopsis Endosperm
- Specific SKN-1/Nrf Stress Responses to Perturbations in Translation Elongation and Proteasome Activity
- Graded Nodal/Activin Signaling Titrates Conversion of Quantitative Phospho-Smad2 Levels into Qualitative Embryonic Stem Cell Fate Decisions
- Genome-Wide Analysis Reveals PADI4 Cooperates with Elk-1 to Activate Expression in Breast Cancer Cells
- Trait Variation in Yeast Is Defined by Population History
- Meiosis-Specific Loading of the Centromere-Specific Histone CENH3 in
- A Genome-Wide Survey of Imprinted Genes in Rice Seeds Reveals Imprinting Primarily Occurs in the Endosperm
- Multiple Regulatory Mechanisms to Inhibit Untimely Initiation of DNA Replication Are Important for Stable Genome Maintenance
- SIRT1 Promotes N-Myc Oncogenesis through a Positive Feedback Loop Involving the Effects of MKP3 and ERK on N-Myc Protein Stability
- Bacteriophage Crosstalk: Coordination of Prophage Induction by Trans-Acting Antirepressors
- Role of the Single-Stranded DNA–Binding Protein SsbB in Pneumococcal Transformation: Maintenance of a Reservoir for Genetic Plasticity
- Genomic Convergence among ERRα, PROX1, and BMAL1 in the Control of Metabolic Clock Outputs
- Genome-Wide Association of Bipolar Disorder Suggests an Enrichment of Replicable Associations in Regions near Genes
- Identification of Nine Novel Loci Associated with White Blood Cell Subtypes in a Japanese Population
- DNA Ligase III Promotes Alternative Nonhomologous End-Joining during Chromosomal Translocation Formation
- Differential Effects of and Risk Variants on Association with Diabetic ESRD in African Americans
- Finished Genome of the Fungal Wheat Pathogen Reveals Dispensome Structure, Chromosome Plasticity, and Stealth Pathogenesis
- Dynamic Chromatin Localization of Sirt6 Shapes Stress- and Aging-Related Transcriptional Networks
- Extracellular Matrix Dynamics in Hepatocarcinogenesis: a Comparative Proteomics Study of Transgenic and Null Mouse Models
- Integrating 5-Hydroxymethylcytosine into the Epigenomic Landscape of Human Embryonic Stem Cells
- Vive La Différence: An Interview with Catherine Dulac
- Multiple Loci Are Associated with White Blood Cell Phenotypes
- Nuclear Accumulation of Stress Response mRNAs Contributes to the Neurodegeneration Caused by Fragile X Premutation rCGG Repeats
- A New Mutation Affecting FRQ-Less Rhythms in the Circadian System of
- Cryptic Transcription Mediates Repression of Subtelomeric Metal Homeostasis Genes
- A New Isoform of the Histone Demethylase JMJD2A/KDM4A Is Required for Skeletal Muscle Differentiation
- Genetic Determinants of Lipid Traits in Diverse Populations from the Population Architecture using Genomics and Epidemiology (PAGE) Study
- A Genome-Wide RNAi Screen for Factors Involved in Neuronal Specification in
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Recurrent Chromosome 16p13.1 Duplications Are a Risk Factor for Aortic Dissections
- Statistical Inference on the Mechanisms of Genome Evolution
- Genome-Wide Association Study of White Blood Cell Count in 16,388 African Americans: the Continental Origins and Genetic Epidemiology Network (COGENT)
- Chromosomal Macrodomains and Associated Proteins: Implications for DNA Organization and Replication in Gram Negative Bacteria
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



