-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Developmental Transcriptional Networks Are Required to Maintain Neuronal Subtype Identity in the Mature Nervous System
During neurogenesis, transcription factors combinatorially specify neuronal fates and then differentiate subtype identities by inducing subtype-specific gene expression profiles. But how is neuronal subtype identity maintained in mature neurons? Modeling this question in two Drosophila neuronal subtypes (Tv1 and Tv4), we test whether the subtype transcription factor networks that direct differentiation during development are required persistently for long-term maintenance of subtype identity. By conditional transcription factor knockdown in adult Tv neurons after normal development, we find that most transcription factors within the Tv1/Tv4 subtype transcription networks are indeed required to maintain Tv1/Tv4 subtype-specific gene expression in adults. Thus, gene expression profiles are not simply “locked-in,” but must be actively maintained by persistent developmental transcription factor networks. We also examined the cross-regulatory relationships between all transcription factors that persisted in adult Tv1/Tv4 neurons. We show that certain critical cross-regulatory relationships that had existed between these transcription factors during development were no longer present in the mature adult neuron. This points to key differences between developmental and maintenance transcriptional regulatory networks in individual neurons. Together, our results provide novel insight showing that the maintenance of subtype identity is an active process underpinned by persistently active, combinatorially-acting, developmental transcription factors. These findings have implications for understanding the maintenance of all long-lived cell types and the functional degeneration of neurons in the aging brain.
Published in the journal: Developmental Transcriptional Networks Are Required to Maintain Neuronal Subtype Identity in the Mature Nervous System. PLoS Genet 8(2): e32767. doi:10.1371/journal.pgen.1002501
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002501Summary
During neurogenesis, transcription factors combinatorially specify neuronal fates and then differentiate subtype identities by inducing subtype-specific gene expression profiles. But how is neuronal subtype identity maintained in mature neurons? Modeling this question in two Drosophila neuronal subtypes (Tv1 and Tv4), we test whether the subtype transcription factor networks that direct differentiation during development are required persistently for long-term maintenance of subtype identity. By conditional transcription factor knockdown in adult Tv neurons after normal development, we find that most transcription factors within the Tv1/Tv4 subtype transcription networks are indeed required to maintain Tv1/Tv4 subtype-specific gene expression in adults. Thus, gene expression profiles are not simply “locked-in,” but must be actively maintained by persistent developmental transcription factor networks. We also examined the cross-regulatory relationships between all transcription factors that persisted in adult Tv1/Tv4 neurons. We show that certain critical cross-regulatory relationships that had existed between these transcription factors during development were no longer present in the mature adult neuron. This points to key differences between developmental and maintenance transcriptional regulatory networks in individual neurons. Together, our results provide novel insight showing that the maintenance of subtype identity is an active process underpinned by persistently active, combinatorially-acting, developmental transcription factors. These findings have implications for understanding the maintenance of all long-lived cell types and the functional degeneration of neurons in the aging brain.
Introduction
Tremendous progress has been made in delineating the transcriptional mechanisms that diversify neuronal subtype identities during development. Spatiotemporally-patterned transcription factor cascades act within increasingly diversified progenitor populations to specify postmitotic neuron subtype fate. Within those postmitotic neurons, subtype-specific sets of transcription factors act combinatorially to differentiate subtype identity by initiating expression of the genes that define subtype form and function [1], [2], [3]. These so-called terminal differentiation genes include subtype-specific neuropeptides, neurotransmitter enzymes and ion channels [4]. Developmental transcriptional cascades are progressive and typically nonlinear; many transcription factors act at multiple levels, they exhibit extensive cross-regulation, and their expression undergoes considerable refinement in developing postmitotic neurons [1], [5], [6], [7]. Here, we apply the term ‘subtype transcription network’ to refer to the transcription factors that direct subtype specification and differentiation, their cross-regulatory relationships (or configuration), and the manner in which they direct the expression of subtype-specific sets of terminal differentiation genes.
After subtype-specific gene expression profiles are established by differentiation, continued neuronal function throughout life depends upon their maintenance of subtype gene expression profiles. However, we currently have only a rudimentary understanding of the mechanisms of long-term, subtype-specific gene maintenance. Two extreme models would posit that subtype identity is either actively maintained by persistent subtype transcription network activity or passively maintained by for example stabilized chromatin structure, independent of a subtype transcription network. Here, we test the active model in two Drosophila neuronal subtypes to address the following largely unanswered questions: Do developmental subtype transcription networks persist in adult neurons or are they dispensed with? Are they required to maintain the expression of subtype-specific sets of terminal differentiation genes? If they are required, does maintenance of terminal differentiation genes require the same complex combinatorial codes of transcription factors as for their initiation, or a simplified code involving fewer transcription factors? Finally, do persisting developmental transcription factors retain the same cross-regulatory relationships that regulated their expression during development?
We model these questions in Drosophila Tv1 and Tv4 neurons. There are six clusters of Tv neurons in the Drosophila ventral nerve cord, each comprising four distinct subtypes (Tv1–Tv4). Tv1 and Tv4 express subtype-specific terminal differentiation genes, the neuropeptides Nplp1 (Tv1) and FMRFa (Tv4) and the neuropeptide amidase PHM (peptidylglycine alpha-hydroxylating monooxygenase; in Tv1/Tv4). Using these genes as markers for subtype-specific differentiation, previous work had revealed the elaborate subtype transcription networks that direct Tv1/4 subtype specification and differentiation [6], [8], [9], [10], [11], [12], [13]. The expression of FMRFa [14], Nplp1 and PHM (herein) are stably maintained in Tv1/4 neurons throughout Drosophila life. Thus, our detailed understanding of their subtype-specific initiation in the embryo provides an ideal background to investigate how such terminal differentiation genes, and hence subtype identity, are maintained in the adult. We previously established that persistent retrograde BMP signaling is required to initiate and maintain FMRFa in Tv4 neurons [14]. Here, we examined the adult function of Tv1 and Tv4 subtype transcription networks in the maintenance of Nplp1, FMRFa and PHM. We further examined whether the cross-regulatory interactions observed between the Tv1/Tv4 network transcription factors during development were maintained in adults. We found that each subtype transcription network is largely retained in adult Tv neurons and is required to actively maintain subtype-specific gene expression. Thus, the combinatorial transcription codes for subtype-specific gene expression are not ‘simplified’ or dispensed with for maintenance. Further, we find that certain critical developmental cross-regulatory interactions between transcription factors are no longer utilized in adults for transcription factor maintenance. Thus, we observe a post-developmental switch to a distinct maintenance configuration between individual transcription factors. Collectively, these data provide novel insight relevant to understanding how long-lived cell types maintain their subtype identity.
Results
Genetic analysis has defined cascades of transcription factors that specify Tv1 and Tv4 neuron fates and then differentiates their subtype-specific terminal differentiation gene expression profiles (Figure 1A, 1B) [6], [8], [9], [10], [11], [12]. Tv1–4 neurons are born sequentially from the NB5-6T neuroblast lineage within an expression window of the ‘temporal’ transcription factors castor (cas) and grainy head (grh) [6], [15]. These specify Tv subtype generation together with collier (col), squeeze (sqz) and nab [6], [9], [12], [16]. Within postmitotic Tv1 neurons, ap, eya, dimmed (dimm) and col then differentiate Tv1 identity in part by initiating Nplp1 expression [9]. In Tv4 neurons ap, eya, dimm, dachshund (dac), sqz and grh act combinatorially with target-derived BMP signaling to differentiate Tv4 identity, in part by initiating FMRFa expression [6], [10], [11], [12], [17], [18], [19]. Also, in both Tv1 and Tv4, dimm acts independently of other regulators to induce expression of the neuropeptide amidase, PHM [10], [20]. We refer to these two transcription factor cascades as the Tv1 and Tv4 ‘subtype transcription networks’ (outlined in Figure 1A, 1B). Genetic analysis has placed these transcription factors into two partially overlapping categories; those that are necessary for directing the specification, or generation, of Tv subtypes around the time of postmitotic neuron birth, and those that thereafter direct differentiation of Tv subtypes by initiating subtype-specific terminal differentiation gene expression. Tv1 neurons are not specified (or generated) in cas or col mutants, whereas eya, ap, col and dimm all act combinatorially thereafter to initiate Nplp1 expression [6], [9]. Tv4 neurons are not specified in cas, col or grh mutants. In sqz and nab mutants, Tv4 neurons are not specified in a segment-specific manner [6], [12]. Thereafter, eya, ap, sqz, dimm, dac, grh and BMP signaling all appear to combinatorially initiate FMRFa expression.
Fig. 1. Adult Tv1 and Tv4 neurons maintain Nplp1 and FMRFa and a subset of embryonic transcription factors. 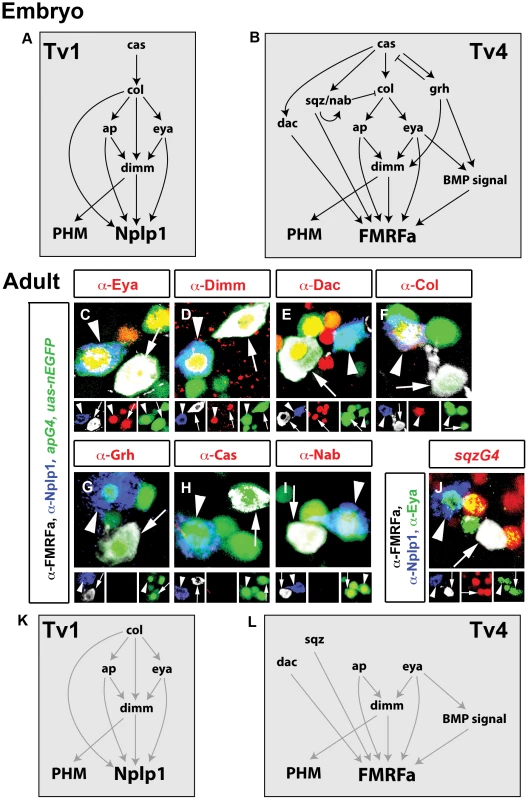
(A–B) Outline of transcription factor network configuration during specification and differentiation of Tv1 (A) and Tv4 (B) neurons at embryonic stages. Tv1 expresses Nplp1 (blue). Tv4 expresses FMRFa (red). Tv1 and Tv4 neurons both express PHM. Arrows represent known regulatory relationships. (C–J) Representative images from Tv clusters in adults (green) in thoracic hemisegments 1 and 3. Tv4 neurons (arrows) express FMRFa (white). Tv1 neurons (arrowheads) express Nplp1 (blue). Tv1 and Tv4 retain expression of transcription factors ap (C–I, green), eya (C red, J green) and dimm (D, red). Tv1 neurons also retains col (F, red). Tv4 neurons also retain dac (E, red) and sqz (J, red). Tv1 and Tv4 do not express transcription factors grh (G, red), cas (H red) or nab (I, red) in adults. Genotype: (C–I) FMRFa-LacZ,apGal4/+; UAS-nEGFP/+. (J) UAS-nEGFP/+; sqzGal4/+. (K,L) Outline of transcription factors present in fully differentiated adult Tv1 (K) and Tv4 (L) neurons. Grey arrows indicate known developmental interactions between transcription factors in embryonic Tv cluster neurons that, herein, we test in the adult. Subtype transcription network retention in adult Tv1 and Tv4 neurons
We previously reported that adult Tv4 neurons maintain FMRFa, ap and eya expression, as well as retrograde BMP-signaling. We also demonstrated that FMRFa maintenance in adult Tv4 neurons requires persistent retrograde BMP-signaling [14]. Here, we determined the adult expression of the other transcriptional regulators implicated in Tv1 and Tv4 development (Figure 1C–1J). We found that adult Tv4 neurons retained ap, eya, dimm, dac and sqz (Figure 1C–1E, 1J), but no longer expressed grh, cas or nab (Figure 1G–1I). Additionally, adult Tv1 neurons retained expression of Nplp1, as well as ap, eya, dimm and col (Figure 1C, 1D, 1F). Previously, cas expression was shown to be lost in all Tv neurons prior to neuropeptide initiation [9], and here we find that it does not become re-expressed in adult Tv1 or Tv4 neurons (Figure 1H). These data are summarized (Figure 1K, 1L). Further analysis found that the adult complement of transcription factors was established by the start of the L1 larva stage (Figure S1), shortly after Tv1/4 terminal differentiation.
Which transcription factors persist in adult neurons is intriguing. All those previously implicated in the postmitotic differentiation of Tv1 and/or Tv4 neurons persist. In contrast, transcription factors that act within the neuroblast and newborn postmitotic neuron to specify the fate of Tv1 (cas) or Tv4 (cas, col, grh, nab) neurons are not retained in the adult. The exceptions to this are col and sqz. Both are implicated in Tv subtype specification, but it is notable that both transcription factors have also been implicated, by loss and gain of function genetics, as part of the combinatorial transcription factor codes that initiate Nplp1 or FMRFa expression [6], [9], [12]. Thus, we find that only regulators implicated in postmitotic subtype differentiation are maintained in adult neurons.
Conditional dsRNAi knockdown in adult Tv neurons
To test the function of each transcription factor in maintaining terminal differentiation gene expression in Tv1 and Tv4 neuronal subtypes, we used apGAL4 (except where noted) to express UAS-dsRNAi transgenes (abbreviated to dsRNAi) targeted to each transcription factor. We also overexpressed UAS-Dicer2 in all experiments to enhance dsRNAi efficacy [21]. To selectively induce dsRNAi in adults, we utilized the TARGET system wherein a temperature-sensitive GAL4-repressor GAL80 (GAL80TS) controls the activity of GAL4 [22]. Flies were raised at 18°C to allow functional GAL80TS to repress GAL4 activity throughout development. Then at adult day 1 (A1), flies were switched to 29°C and kept at that temperature for the remainder of each experiment. At this temperature, GAL80TS becomes dysfunctional and thus GAL4 is allowed to induce dsRNAi expression (Figure 2A) [14].
Fig. 2. Transcription factors ap, eya, sqz are required for persistent FMRFa expression in the adult Tv4 neuron. 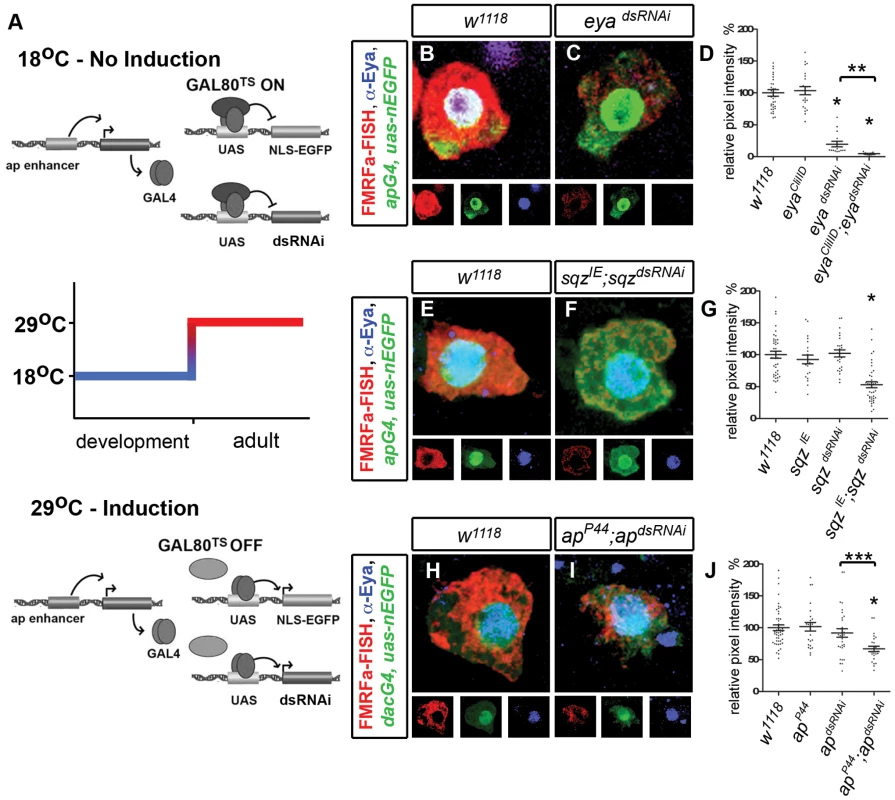
(A) Cartoon illustrating adult induction of dsRNAi constructs in adult Tv neurons using the TARGET system. (B,C, E,F, H,I) Images of adult Tv4 neurons expressing FMRFa transcript (red), apGal4,UAS-nlsEGFP (green) and Eya (blue) after A15 (B–G) or A20 (H–J) of dsRNAi expression at 29°C. (B,C) Eya immunoreactivity is eliminated and FMRFa is downregulated in eyadsRNAi (C) compared to w1118 control (B). (E,F) FMRFa is downregulated in sqzIE,sqzdsRNAi (F) compared to w1118 control (E). (H,I) FMRFa is downregulated in apP44;apdsRNAi (I) compared to w1118 control (H). (D,G,J) Quantification of FMRFa transcript in individual adult Tv4 neurons at A15 (D,G) or A20 (J) at 29°C. (D) * p<0.0001 eyadsRNAi (n = 15) and eyadsRNAi; eyaCliIID (n = 13) compared to w1118 (n = 35) and eyaCliIID controls. ** p<0.0001 compared to eyadsRNAi alone. (G) * p<0.0001 sqzIE; sqzdsRNAi (n = 39) compared to w1118 (n = 40), sqzIE (n = 21), and sqzdsRNAi (n = 25) controls. (J) * p<0.0001 apP44; apdsRNAi (n = 25) compared to w1118 (n = 47) and apP44 (n = 25) controls. *** p<0.005 compared to apdsRNAi (n = 32) alone. Genotypes: w1118 (UAS-dicer2/+; apGal4/+; tub-Gal80TS, UAS-nEGFP) (A–C) eyaCliIID (UAS-dicer2/+; apGal4/eyaCliIID; tub-Gal80TS, UAS-nEGFP/+); eyadsRNAi (UAS-dicer2/UAS-eyadsRNAi; apGal4/+; tub-Gal80TS, UAS-nEGFP/+); eyadsRNAi; eyaCliIID (UAS-dicer2/UAS-eyadsRNAi; apGal4/eyaCliIID; tub-Gal80TS, UAS-nEGFP/+). (E–G) sqzIE (UAS-dicer2/+; apGal4/sqzIE; tub-Gal80TS, UAS-nEGFP/+); sqzdsRNAi (UAS-dicer2/+; apGal4/+; tub-Gal80TS, UAS-nEGFP/UAS-sqzdsRNAi); sqzIE; sqzdsRNAi (UAS-dicer2/+; apGal4/sqzIE; tub-Gal80TS, UAS-nEGFP/UAS-sqzdsRNAi). (H–I) apP44 (UAS-dicer2/+; apGal4/apP44; tub-Gal80TS, UAS-nEGFP/+); apdsRNAi (UAS-dicer2/+; apGal4/+; tub-Gal80TS, UAS-nEGFP/UAS-apdsRNAi); apP44; apdsRNAi (UAS-dicer2/+; apGal4/apP44; tub-Gal80TS, UAS-nEGFP/UAS-apdsRNAi). eya, ap, and sqz are required for FMRFa maintenance in adults
We induced eyadsRNAi expression at adult day 1 (A1) and quantified FMRFa transcript levels by fluorescent in situ hybridization, relative to the mean of controls. In adults, eyadsRNAi dramatically reduced FMRFa transcript to 19.7±4.4% of control (p<0.0001) by adult day A15 (Figure 2B–2D). We observed a similar downregulation in immunoreactivity to the mature amidated FMRFa peptide (Table S1). To confirm dsRNAi specificity, we tested for enhancement of the FMRFa phenotype when expressing eyadsRNAi in an eya heterozygous background. Indeed, we found that this further reduced FMRFa transcript to 4.6±0.5% of control by A15 (p<0.0001 to eyadsRNAi alone or eya heterozygosity alone) (Figure 2D). Eya immunoreactivity was eliminated in all cases (Table S4). Previous studies demonstrated that FMRFa is severely downregulated in eya mutants by late embryogenesis [11]. Our data now show that FMRFa maintains this critical dependence on eya in adults.
We next tested the role of apterous (ap). As apGAL4 is a strong hypomorphic ap allele [23], we used dacGAL4 to express apdsRNAi in wildtype and heterozygous ap backgrounds. We observed a significant downregulation of FMRFa transcript when expressing apdsRNAi in ap heterozygotes, falling to 66.8±4.2% of control (p<0.0001 from control or ap heterozygote alone, and p<0.005 from apdsRNAi alone). No loss of FMRFa was observed in either ap heterozygotes or apdsRNAi alone (Figure 2H–2J). Similar results were obtained for downregulation of the mature FMRFa amidated peptide (Table S1). We also examined FMRFa expression using the strong apGAL4 driver to overexpress apdsRNAi, and observed a significant reduction of mature amidated FMRFa peptide to 40.0±2.2% of control by A20 (w1118 control n = 40, apdsRNAi n = 38; p<0.0001). Finally, we also confirmed that an apdsRNAi targeting different ap sequences also significantly downregulated FMRFa (Table S2). The downregulation of FMRFa that we observed in adults is comparable to that reported for embryonic ap null mutants [12], [17]. Thus, we conclude that ap maintains a persistent role in FMRFa regulation. We were unable to determine the extent of Ap knockdown by either apdsRNAi transgene due to a lack of suitable Ap-specific antibodies. Therefore, we tested apdsRNAi efficacy by examining another ap phenotype. In ap mutants, the wings fail to form [24]. We found that expression of apdsRNAi in the developing wing, using apGAL4, could precisely phenocopy this ap phenotype (Figure S2). Thus, we conclude that apdsRNAi is specific and highly effective. However, as we could not directly quantify Ap downregulation in Tv neurons, we cannot formally discount the possibility that FMRFa would be further downregulated if Ap were entirely eliminated.
To test the role of sqz in adult Tv4 neurons, we expressed sqzdsRNAi at A1 and observed a partial downregulation of FMRFa expression in sqz heterozygotes to 53.0±4.8% of control (p<0.0001 from control, sqz heterozygote or sqzdsRNAi alone) (Figure 2E–2G). Similar results were obtained for immunoreactivity to the mature amidated FMRFa peptide (Table S1). Previous reports established that FMRFa is partially downregulated in embryonic sqz mutants [12]. Thus, our data indicate that sqz maintains its partial requirement for FMRFa expression. Due to ubiquitous but weak Sqz expression in the thoracic nerve cord, we were not able to adequately quantitate Sqz downregulation in Tv neurons. Thus, we do not discount the possibility that Sqz may not have been entirely eliminated, and therefore we may be underestimating its effect on FMRFa expression. Taken together, our data demonstrate that eya, ap and sqz are required to maintain wildtype FMRFa levels in the adult.
dimm maintains FMRFa peptide processing
We induced dimmdsRNAi at A1 and found that immunoreactivity to the mature amidated FMRFa peptide was rapidly and profoundly reduced by dimmdsRNAi to 24.0±3.2% of control by A10 (p<0.0001), and this was enhanced to 9.8±1.6% of control in dimm heterozygotes (p<0.0001 to control and dimm heterozygotes, p<0.001 to dimmdsRNAi alone) (Figure 3A–3C, Figure S3). Immunoreactivity to Dimm demonstrated that it had been eliminated (Table S4). In contrast, FMRFa transcript in adults was downregulated in dimm heterozygotes to 67.1±2.9% of control at A20 (p<0.0001) (Figure 3D). Similar effects were observed using a dimmdsRNAi that targets different dimm sequences (Table S2). It is notable that downregulation of the transcript was only observed after 20 days of dimmdsRNAi induction but the peptide was profoundly reduced after only 10 days of induction. In late Stage 17 embryonic dimm mutants, immunoreactivity to the mature amidated FMRFa peptide was profoundly reduced, but the extent to which FMRFa transcript was affected had not been quantified [10], [18]. Here, we find that FMRFa transcript was only modestly downregulated in late Stage 17 embryonic dimm mutants to 71.6±3.9% of controls (wild type control n = 54, dimm mutant n = 34 (p<0.0001)). Thus, we conclude that dimm retains its role in the initiation and maintenance of both FMRFa transcript and mature peptide. Why is the mature peptide more responsive to dimmdsRNAi than is the transcript? We postulated that this was due to dimm's regulation of proprotein convertases and peptide amidases in secretory neurons, both of which are required to process the FMRFa prepropeptide into amidated neuropeptides [10], [20], [25]. We tested this in adults by examining expression of peptidylglycine α-hydroxylating monooxygenase after dimmdsRNAi induction (PHM). Confirming our hypothesis, dimmdsRNAi entirely eliminated PHM immunoreactivity in Tv4 neurons (Figure 3E, 3F). Thus, the maintenance of neuropeptide-processing enzyme expression and biosynthesis of the amidated FMRFa peptide is highly dependent upon persistent dimm function in adult Tv neurons.
Fig. 3. dimm maintains peptidergic phenotype and dac has an enhanced maintenance function. 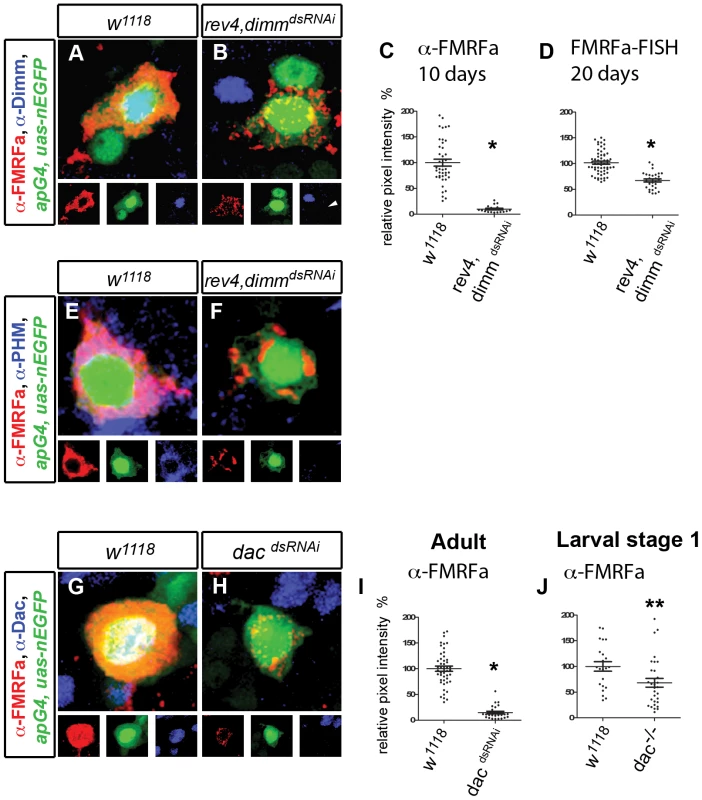
(A,B) Representative images of adult Tv4 neurons expressing FMRFa peptide (red), apGal4,UAS-nlsEGFP (green) and Dimm (blue) at A10 at 29°C. FMRFa is downregulated and Dimm is lost in rev4,dimmdsRNAi (B) compared to w1118 control (A). (C,D) Quantification of FMRFa peptide at A10 (C) and FMRFa transcript at A20 (D) in individual adult Tv4 neurons at 29°C. (C)* p<0.0001 rev4,dimmdsRNAi (n = 19) compared to w1118 control (n = 42). (D)* p<0.0001 rev4,dimmdsRNAi (n = 30) compared to w1118 control (n = 58). (E,F) Representative images of Tv4 neurons expressing mature FMRFa peptide (red), apGal4,UAS-nlsEGFP (green) and PHM (blue) in adult Tv4 neurons at A10 at 29°C. PHM is lost in rev4,dimmdsRNAi (n = 26) (F) compared to w1118 control (n = 30) (E). (G,H) Images of adult Tv4 neurons expressing FMRFa peptide (red), apGal4,UAS-nEGFP (green) and Dac (blue) at A10 at 29°C. FMRFa is downregulated and Dac immunoreactivity is lost in dacdsRNAi (H) compared to w1118 control (G). (I,J) Quantification of FMRFa peptide in individual adult Tv4 neurons at A10 at 29°C (I), and in L1 larval Tv4 neurons in dac null mutants. (J) * p<0.0001 dacdsRNAi (n = 26) compared to w1118 control (n = 47). (J) ** P = 0.02 dac−/− (n = 31) compared to w1118 control (n = 22). Genotypes: w1118 (UAS-dicer2/+; apGal4/+; tub-Gal80TS, UAS-nEGFP/+); rev4,dimmdsRNAi (UAS-dicer2/+; apGal4/rev4, UAS-dimmdsRNAi; tub-Gal80TS, UAS-nEGFP/+); dacdsRNAi (UAS-dicer2/+; apGal4/UAS-dacdsRNAi; tub-Gal80TS, UAS-nEGFP/+); dac−/− (dac3/dacDf(3L)EXEL 7066). dac is necessary for FMRFa maintenance
Previous studies found that FMRFa was only modestly downregulated in dac mutants during development [11]. In confirmation, we found here that in L1 larvae, FMRFa immunofluorescence per Tv4 neuron was 68.3±8.5% of control (Figure 3J; P<0.02). We tested dac function in adults and found that dacdsRNAi dramatically downregulated FMRFa immunoreactivity in adults to 14.8±2.5% of controls, as early as A10 (p<0.0001) (Figure 3G–3I). Correspondingly, FMRFa transcript was reduced to 24.9±4.2% of controls (p<0.0001 to controls), and this was enhanced in dac heterozygotes to 6.5±0.8% (p<0.001 to dacdsRNAi alone) (Figure S3). Notably, by A15, FMRFa peptide and transcript were entirely eliminated (not shown). In all cases, we found that Dac immunoreactivity was eliminated (Table S4). Moreover, similar effects were observed using a dacdsRNAi that targets different dac sequences (Figure S2). Thus, dac appears to be unique amongst the Tv4 subtype transcription network factors in that it assumes an increasingly essential role in maintenance compared to developmental initiation.
Post-developmental changes to Tv4 subtype transcription network configuration
The Tv4 subtype transcription network acts through hierarchical and feedforward transcription factor activity, which we refer to here as the network's configuration (summarized in Figure 1A, 1B) [6], [10]. Initiation of grh, dac, sqz and col requires transient cas activity [6]. Expression of ap and eya requires transient col expression [9]. The induction of dimm then requires eya, ap and grh [6], [9], [10]. BMP signaling is dependent upon eya [11]. Finally, ap, eya, dimm, dac, sqz, grh and BMP signaling are all required for FMRFa initiation [6], [11], [12], [17], [18]. This cascade represents a progressive and dynamic set of interactions during Tv4 neuron specification and differentiation. However, for long-term maintenance of subtype gene expression, the subtype transcription network presumably resolves into a stable configuration. As grh, cas and col are lost by early L1 (Figure S1), network configuration must change as the remaining transcription factors become independent of those that initiated their expression. However, we wished to ask whether the developmental cross-regulatory interactions between the persisting transcription factors are retained in the adult to help stabilize the network post-developmentally. Thus, we examined the configuration (cross-regulatory interactions) of all Tv4 subtype transcription network factors.
BMP signaling in embryonic Tv4 neurons is dramatically reduced in eya mutants [11]. We expressed eyadsRNAi in adults until A15 and found that nuclear pMad, an indicator of BMP activity [12], was significantly downregulated to 47.9%±2.9 of control (Figure 4A, 4B, 4D). As BMP signaling is required for FMRFa expression in embryos and adults, we asked whether eya-dependence of FMRFa in adults is due to reduced BMP signaling. To do this, we simultaneously expressed eyadsRNAi and restored BMP signaling, using constitutively-activated type I BMP-receptors, thickveins and saxophone. Even though nuclear pMad was robustly activated in all Tv neurons, eyadsRNAi-induced FMRFa downregulation was not rescued (Figure 4C, 4E). Thus, in adults, eya independently maintains both BMP signaling and FMRFa expression.
Fig. 4. Changes in Tv4 network configuration for maintenance. 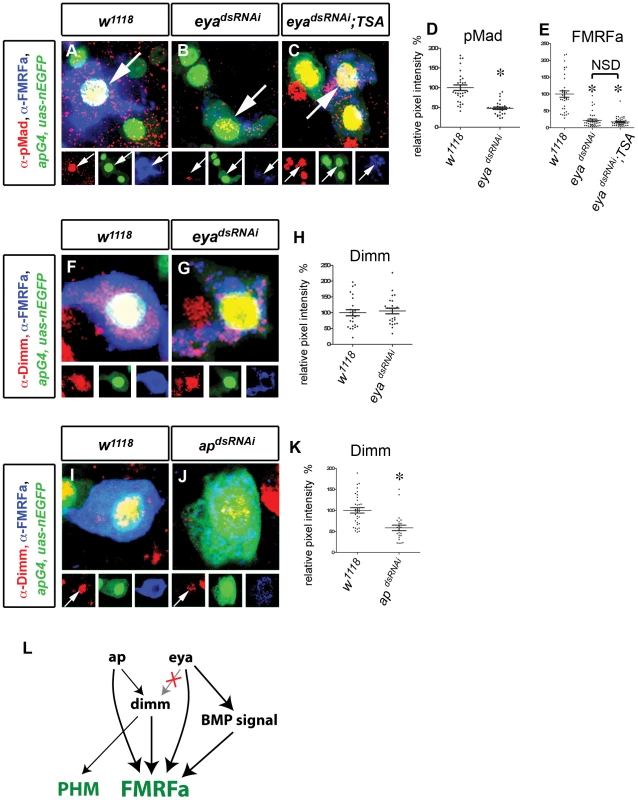
(A–E) eya regulates FMRFa independently of BMP signaling. (A,B) Nuclear pMad accumulation (red) in Tv4 (arrowhead) was downregulated in eyadsRNAi flies (B) compared to w1118 control (A). (C) Expression of eyadsRNAi in the presence of constitutively-activated Thickveins and Saxophone BMP type I receptors (TSA) activated pMad accumulation (red) in all Tv neurons (including Tv4; arrow) but failed to rescue FMRFa (blue) compared to w1118 control. (D) Quantification of pMad immunoreactivity in Tv4 nucleus in w1118 and eyadsRNAi flies. * p<0.0001 eyadsRNAi (n = 30) compared to w1118 control (n = 30). (E) Quantification of FMRFa immunoreactivity in Tv4 in w1118, eyadsRNAi and eyadsRNAi; TSA. * p<0.0001 eyadsRNAi (n = 38) and eyadsRNAi; TSA (n = 42) compared to w1118 control (n = 34). NSD, no significant difference between eyadsRNAi and eyadsRNAi; TSA. (F–H) eya does not regulate dimm in adult Tv4. dimm (red) in Tv neurons (blue) is maintained in eyadsRNAi (G) compared to w1118 control (F). (H) Quantification of Dimm immunoreactivity in Tv4. There is no significant difference between eyadsRNAi (n = 24) and w1118 (n = 25) controls (p = 0.67). (I–K) ap regulates dimm in adult Tv4. dimm (red) in Tv neurons (blue) is downregulated in apdsRNAi (J) compared to w1118 control (I). (K) Quantification of Dimm immunoreactivity in Tv4. * p<0.0001 apdsRNAi (n = 24) compared to w1118 (n = 35). (L) Model depicting regulatory configuration of ap, eya, dimm and BMP signaling from embryonic to adult Tv4. The dependence of dimm on eya expression is not maintained (X). Genotypes: w1118 (A,D, F,H, I,J) (UAS-dicer2/+; apGal4/+; tub-Gal80TS, UAS-nEGFP/+); eyadsRNAi flies (B,D,E, G,H) (UAS-dicer2/UAS-eyadsRNAi; apGal4/+; tub-Gal80TS, UAS-nEGFP/+); eyadsRNAi; TSA flies (C,E) (UAS-dicer2/UAS-eyadsRNAi; apGal4/UAS-tkvA, UAS-saxA; tub-Gal80TS, UAS-nEGFP/+). apdsRNAi flies (J),K (UAS-dicer2/+; apGal4; tub-Gal80TS, UAS-nEGFP/UAS-apdsRNAi). In the embryo, initiation of dimm expression in Tv4 is absolutely dependent upon eya and grh [6], [9] and partially dependent upon ap [10]. As grh is not expressed in adult Tv4 neurons, dimm maintenance must become independent of grh. However, as eya and ap are retained, we tested their role in dimm maintenance. We expressed apdsRNAi in adults until A20 using apGAL4 (a strong hypomorphic allele) and found that Dimm immunoreactivity was significantly downregulated to 58.5%±6.6 of control (Figure 4I–4K). In contrast, we found that Dimm expression in adult Tv4 neurons was entirely unaffected by eyadsRNAi (Figure 4F–4H). These data indicate that Dimm becomes independent of eya in adult Tv4 neurons, even though eya expression persists in adults and eya is absolutely required for the initiation of dimm expression [9]. We conclude that maintenance of dimm remains dependent on ap but becomes independent of eya and grh post-developmentally.
We also examined all other potential cross-regulatory relationships within the Tv4 subtype transcription network (Table S3), but found no instances of a transcription factor requiring the presence of another for its maintenance.
Maintenance of subtype transcription network output, but not configuration, in mature Tv1 neurons
Is the maintained role found for the Tv4 subtype transcription network common to other subtype transcription networks? To determine this, we examined the output and configuration of the adult Tv1 subtype transcription network. In the neuroblast lineage that gives rise to the Tv1 neuron, cas induces col. Upon birth of the postmitotic Tv1 neuron col initiates eya and ap expression. Initiation of dimm is then absolutely dependent on each of col, ap and eya. Then all four regulators are required for Nplp1 initiation [6], [9].
In adult Tv1 neurons, col, eya, ap and dimm were maintained (Figure 1). Induction of coldsRNAi [9] at A1 reduced Nplp1 to 10.7±1.7% of control by A15 (Figure 5A–5C). We also found that induction of apdsRNAi, eyadsRNAi or dimmdsRNAi significantly reduced Nplp1 expression levels to 22.7±2.7%, 45.3±4.2% and 19.0±2.4% of control, respectively (all p<0.0001 to control) (Figure 5G, 5J, 5O). We verified that Col, Eya and Dimm immunoreactivity in Tv1 were eliminated by their respective dsRNAi (Table S4). In addition, we found that dimmdsRNAi also eliminated PHM expression in Tv1 neurons (Figure 5N). Thus, the Tv1 subtype transcription network is required to maintain the expression of Tv1-specific terminal differentiation gene expression.
Fig. 5. Transcriptional regulation of Nplp1 in adult Tv1 neurons. 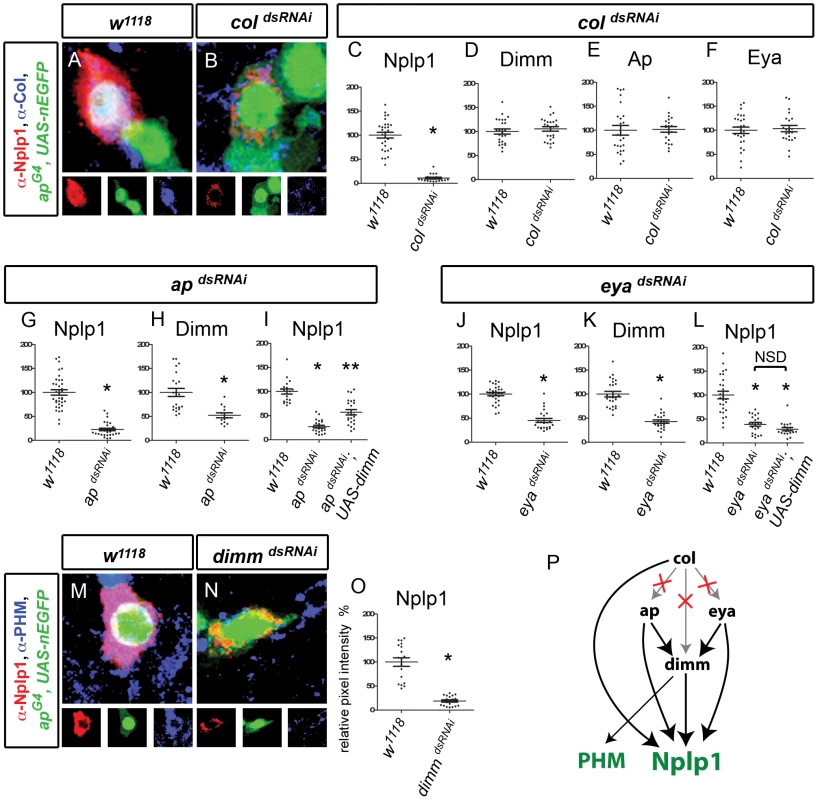
(A–F) coldsRNAi downregulated Nplp1 but not ap, eya, or dimm in adult Tv1. (A,B) Expression of Nplp1 (red), apGal4,UAS-nEGFP (green) and Col (blue) in adult Tv4 neurons at A10 at 29°C. Col expression is lost and Nplp1 is downregulated in coldsRNAi (B) compared to w1118 control (A). (C) Quantification of Nplp1 immunoreactivity in w1118 (n = 29) and coldsRNAi (n = 20) at A10 at 29°C. (D–F) coldsRNAi did not affect Dimm immunoreactivity (D), apGal4,UAS-nEGFP fluorescence (E) or Eya immunoreactivity (F) compared to w1118 at A10 at 29°C. coldsRNAi (D n = 24; E n = 22; F n = 21). w1118 control (D n = 25; E n = 27; F n = 24). (G–I) apdsRNAi significantly reduced Nplp1 (G) and Dimm (H) immunoreactivity compared to w1118 at A20 at 29°C. (G) * p<0.0001 apdsRNAi (n = 30) compared to w1118 control (n = 36). (H) * p<0.001 apdsRNAi (n = 13) compared to w1118 control (n = 22) (I) Dimm restoration (UAS-dimm) in apdsRNAi background only partially rescued Nplp1 downregulation at A15 at 29°C. * p<0.0001 apdsRNAi (n = 22) compared to w1118 control (n = 19). ** p<0.0001 apdsRNAi;UAS-dimm (n = 23) compared to apdsRNAi and w1118 controls. (J–L) eyadsRNAi significantly reduced Nplp1 (J) and Dimm (K) immunoreactivity compared to w1118 at A10 at 29°C. (L) Dimm restoration (UAS-dimm) in eyadsRNAi background failed to rescue Nplp1 immunoreactivity at A15 at 29°C. * p<0.0001 eyadsRNAi and eyadsRNAi; UAS-dimm (n = 19) compared to w1118 control. NSD, no significant difference between eyadsRNAi and eyadsRNAi;UAS-dimm. eyadsRNAi (J n = 23; K n = 23; L n = 22). w1118 control (J n = 30; K n = 25; L n = 26). (M–O) dimmdsRNAi downregulated Nplp1 and PHM in adult Tv1. (M,N) Tv1 neurons expressing Nplp1 (red), apGal4,UAS-nlsEGFP (green) and PHM (blue) in adult Tv4 neurons at A10 at 29°C. Nplp1 is downregulated and PHM is lost in dimmdsRNAi (n = 18) (M) compared to w1118 control (n = 15) (N). (O) dimmdsRNAi significantly reduced Nplp1 immunoreactivity compared to w1118 at A10 at 29°C. * p<0.0001 dimmdsRNAi (n = 19) compared to w1118 control (n = 18). (P) Model depicting regulation of Nplp1 and PHM and the configuration changes between ap, eya, dimm and col from embryonic to adult Tv1. Notably, col no longer regulates dimm, eya or ap expression (denoted by X). Genotypes: w1118 (UAS-dicer2/+; apGal4/+; tub-Gal80TS, UAS-nEGFP/+); coldsRNAi (UAS-dicer2/+; apGal4/+; tub-Gal80TS, UAS-nEGFP/UAS-coldsRNAi); apdsRNAi (UAS-dicer2/+; apGal4/+; tub-Gal80TS, UAS-nEGFP/UAS-apdsRNAi); apdsRNAi; UAS-dimm (UAS-dicer2/+; apGal4/UAS-dimm; tub-Gal80TS, UAS-nEGFP/UAS-apdsRNAi); eyadsRNAi (UAS-dicer2/UAS-eyadsRNAi; apGal4/+; tub-Gal80TS, UAS-nEGFP/+); eyadsRNAi; UAS-dimm (UAS-dicer2/UAS-eyadsRNAi; apGal4/UAS-dimm; tub-Gal80TS, UAS-nEGFP/+); dimmdsRNAi (UAS-dicer2/+; apGal4/UAS-eyadsRNAi; tub-Gal80TS, UAS-nEGFP/+); rev4,dimmdsRNAi (UAS-dicer2/+; apGal4/rev4, UAS-dimmdsRNAi; tub-Gal80TS, UAS-nEGFP/+). Next, we examined the configuration of the adult Tv1 subtype transcription network. Intriguingly, even through col is essential for eya, ap and dimm expression in the embryo, coldsRNAi had no effect on dimm, ap, or eya expression in Tv1 (Figure 5D–5F). In contrast, expression of either apdsRNAi or eyadsRNAi led to a significant downregulation of Dimm levels in Tv1 to 52.4±5.1% and 42.9±3.7% (Figure 5H, 5K). These data are highly intriguing. The loss of col-dependence of ap, eya and dimm on col was unexpected, notably because Nplp1 retains its col-dependence. Moreover, it is intriguing that dimm retains its eya-dependence in adult Tv1 neurons, but not in adult Tv4 neurons. Thus, the cross-regulatory interactions between persistent transcription factors can be significantly altered after the process of differentiation.
Genetic studies in the embryo had established that col, ap, eya and dimm act non-redundantly to initiate Nplp1 expression during development [6]. We tested whether these transcription factors also act non-redundantly in the adult. As coldsRNAi dramatically downregulated Nplp1 but did not affect ap, eya or dimm, we conclude that col acts non-redundantly in this case. However, dimm is dependent on both ap and eya in Tv1. Therefore, to test for redundancy between these transcription factors, we restored dimm (UAS-dimm) in either apdsRNAi or eyadsRNAi backgrounds. UAS-dimm expression was found to only partially rescue Nplp1 expression in an apdsRNAi background, from 22.7±2.7% to 57.0±5.4% (p<0.0001 compared to apdsRNAi and also w1118 control) (Figure 5I). However, UAS-dimm expression failed to rescue Nplp1 expression in an eyadsRNAi background (Figure 5L). Thus, as during development, all regulators are required combinatorially for normal Nplp1 expression in adult Tv1 neurons.
Adult neurons respond predictively to ectopic reconstitution of subtype transcription network activity
During development, late-acting subtype transcription networks can override earlier-acting transcriptional codes to dominantly activate ectopic expression of their target genes and/or subtype identity [6]. For example, in embryos, overexpression of col in all Tv neurons ectopically activated Nplp1 in Tv4, presumably reconstituting the col/ap/eya/dimm Tv1 subtype transcription network (Baumgardt et al., 2007). Interestingly, this did not disrupt native FMRFa expression in Tv4 neurons, nor its known subtype transcription network profile. Here, we verify these data in embryos (Figure 6A, 6C). Additionally, we demonstrate here that the reciprocal subtype transcription network reconstitution, dac and BMP activation in all Tv neurons, is sufficient to initiate FMRFa expression in embryonic Tv1 neurons. Interestingly, we found that this also occurred without a concomitant disruption of Nplp1 expression in Tv1 (Figure 6A, 6B).
Fig. 6. Differentiation networks have different abilities to activate ectopic gene expression in adult neurons. 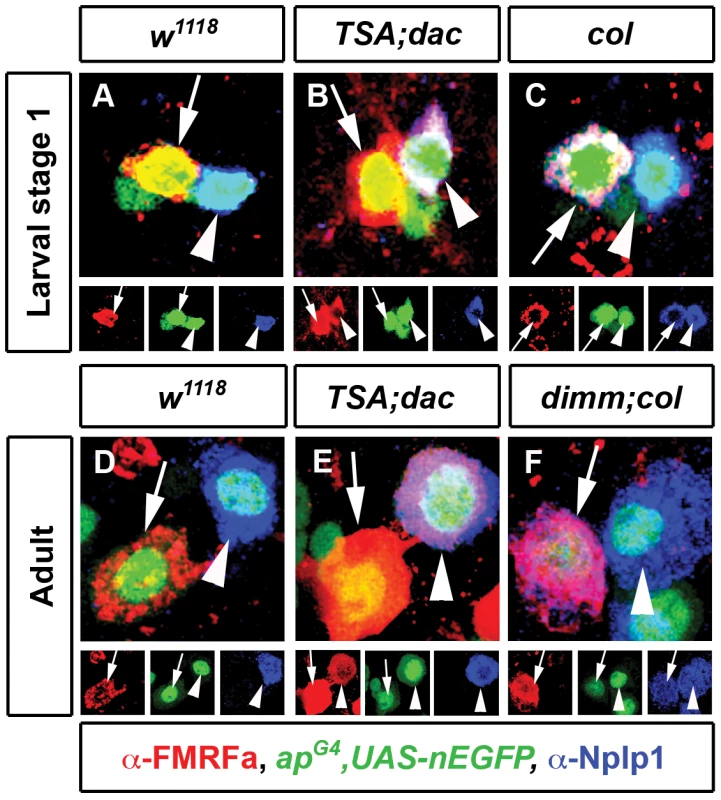
(A–F) Representative images of Tv4 neurons (arrows) and Tv1 neurons (arrowheads) at larval stage 1 (A–C) and adults (D–F). (B,E) Induction of ectopic FMRFa expression (arrowhead, red) in Tv1 (identified with Nplp1, blue) after misexpression of dac and constitutively-activated Thickveins and Saxophone BMP receptors (TSA) in embryos (B n = 33) and for 5 days in adults (E n = 31). (C) Misexpression of col in embryonic Tv neurons using apGal4 initiates ectopic Nplp1 expression (arrow, blue) in Tv4 neurons (identified with FMRFa, red) (C n = 24). (F) Misexpression of col and upregulation of dimm for 5 days in adults using apGal4 initiates ectopic Nplp1 expression (arrow, blue) in Tv4 neurons (identified with FMRFa, red) (F n = 42). Genotypes: (A–C) Larval stage 1 w1118 (apGal4/+; UAS-nEGFP/+); TSA;dac (apGal4/UAS-tkvA, UAS-saxA; UAS-nEGFP/UAS-dac); col (apGal4/UAS-col; UAS-nEGFP/+). (D-F) Adult w1118 (apGal4/+; tub-Gal80TS, UAS-nEGFP/+); TSA;dac (apGal4/UAS-tkvA, UAS-saxA; tub-Gal80TS, UAS-nEGFP/UAS-dac); dimm;col (apGal4/UAS-col; tub-Gal80TS, UAS-nEGFP/UAS-dimm). We tested whether Tv1 and Tv4 subtype transcription networks retained this capacity in adult neurons. This would prove that subtype transcription networks are capable of inducing expression of their pertinent target gene in a mature cell that never had expressed that gene. We found that activation of BMP signaling and dac in adult Tv1 neurons for 5 days robustly activated ectopic FMRFa expression in 100% of Tv1 neurons (Figure 6D, 6E), without affecting Nplp1 in Tv1. We next ectopically expressed col in adult Tv neurons, but this failed to induce ectopic Nplp1 expression (n = 21 Tv4 neurons) (data not shown). However, co-expression of col and dimm in adult Tv neurons was sufficient to trigger ectopic Nplp1 expression in 100% of Tv4 neurons (n = 42) (Figure 6D, 6F). These data show that subtype transcription networks are sufficient to initiate pertinent target gene expression, even in adult neurons that had never expressed the gene.
Discussion
Our data provide novel insight supporting the view of Blau and Baltimore [26] that cellular differentiation is a persistent process that requires active maintenance, rather than being passively ‘locked-in’ or unalterable. We make two primary findings regarding the long-term maintenance of neuronal identity. First, we find that all known developmental transcription factors acting in postmitotic Tv1 and Tv4 neurons to initiate the expression of subtype terminal differentiation genes are then persistently required to maintain their expression. Second, we found that key developmental cross-regulatory relationships that initiated the expression of certain transcription factors were no longer required for their maintained expression in adults. Notably, we found this to be the case even between transcription factors whose expression persists in adults.
In this study, all transcription factors implicated in the initiation of subtype-specific neuropeptide expression in Tv1 and Tv4 neurons were found to maintain subtype terminal differentiation gene expression in adults (summarized in Figure 7). In Tv1, col, eya, ap and dimm are required for Nplp1initiation during development (Figure 1A). In this study, knockdown of each transcription factor in adult Tv1 neurons was shown to dramatically downregulate Nplp1. In Tv4 neurons, FMRFa initiation during development requires eya, ap, sqz, dac, dimm and retrograde BMP signaling (Figure 1B). Together with our previous work showing that BMP signaling maintains FMRFa expression in adults [14], we now demonstrate that all six regulatory inputs are required for FMRFa maintenance. Most transcription factors, except for dac, also retained their relative regulatory input for FMRFa and Nplp1 expression. In addition, individual transcription factors also retained their developmental subroutines. For example, as found during development [10], [11], [20], dimm was required in adults to maintain PHM (independently of other regulators) and FMRFa/Nplp1 expression (combinatorially with other regulators).
Fig. 7. Summary of changes in subtype transcription network configuration between initiation and maintenance of subtype identity. 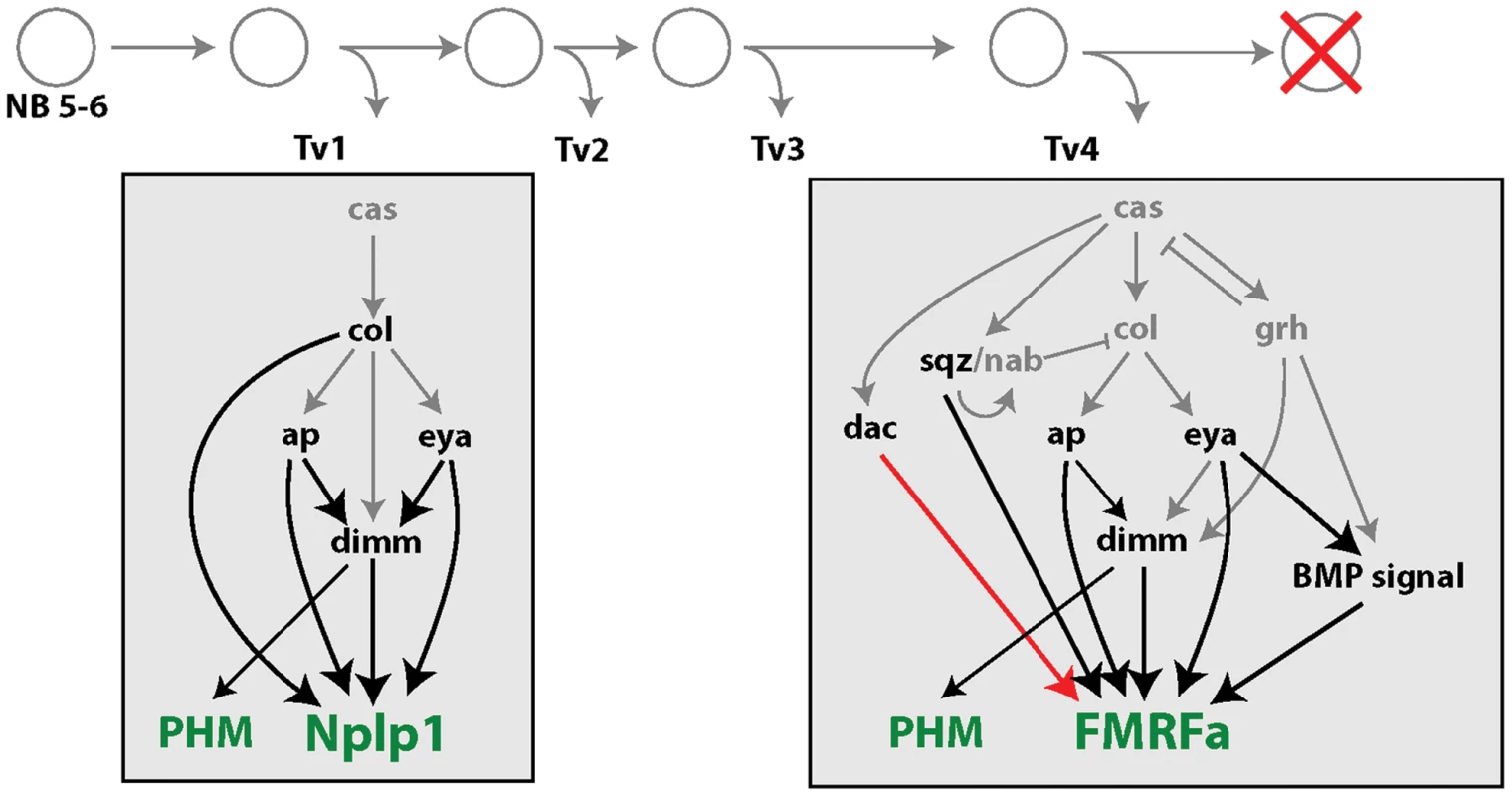
Following terminal differentiation, Tv1 and Tv4 maintain expression of terminal differentiation genes (green text). Tv1 (PHM and Nplp1). Tv4 (PHM and FMRFa). The expression of transcription factors in grey text is lost by larval stages, all transcription factors in black text are retained in adult Tv1/4 neurons. Notably, all black transcription factors have been implicated in differentiation of subtype-specific neuropeptide expression in postmitotic Tv1/4 neurons. In contrast, all grey transcription factors have been implicated in the specification of Tv neuron subtype fates, and not in direct differentiation of subtype neuropeptide gene expression. All remaining transcription factors are required for maintained Nplp1 and FMRFa expression (black arrows), but their cross-regulatory relationships are mostly lost in adults (grey arrows). The only transcription factors that maintain their embryonic configuration are ap and eya's requirement for dimm in Tv1, and eya's continued regulation of active BMP signaling in Tv4. Dac plays an enhanced role in FMRFa expression in adult Tv4 neurons (red arrow). The few genetic studies that test a persistent role for developmental transcription factors support their role in initiating and maintaining terminal differentiation gene expression. In C. elegans, where just one or two transcription factors initiate most neuronal subtype-specific terminal differentiation genes, they then also appear to maintain their target terminal differentiation genes. In ASE and dopaminergic neurons respectively, CHE-1 and AST-1 initiate and maintain expression of pertinent subtype-specific terminal differentiation genes [27], [28]. In vertebrate neurons, where there is increased complexity in the combinatorial activity of transcription factors in subtype-specific gene expression, certain transcription factors have been demonstrated to be required for maintenance of subtype identity. These are Hand2 that initiates and maintains tyrosine hydroxylase and dopa β-hydroxylase expression in mouse sympathetic neurons [29], Pet-1, Gata3 and Lmx1b for serotonergic marker expression in mouse serotonergic neurons [30], [31], and Nurr1 for dopaminergic marker expression in murine dopaminergic neurons [32].
However, while these studies confirm a role for certain developmental transcription factors in subtype maintenance, it had remained unclear whether the elaborate developmental subtype transcription networks, that mediate neuronal differentiation in Drosophila and vertebrates, are retained in their entirety for maintenance, or whether they become greatly simplified. Our analysis of all known subtype transcription network factors in Tv1 and Tv4 neurons now indicates that the majority of a developmental subtype transcription network is indeed retained and required for maintenance. Why would an entire network of transcription factors be required to maintain subtype-specific gene expression? The combinatorial nature of subtype-specific gene expression entails cooperative transcription factor binding at clustered cognate DNA sequences and/or synergism in their activation of transcription. In such cases, our data would indicate that this is not dispensed with for maintaining terminal differentiation gene expression in mature neurons.
How the transcription factors of the subtype transcription networks are maintained is less well understood. An elegant model has emerged from studies in C. elegans, wherein transcription factors stably auto-maintain their own expression and can then maintain the expression of subtype terminal differentiation genes [33]. The transcription factor CHE-1 is a key transcription factor that initiates and maintains subtype identity in ASE neurons. CHE-1 binds to a cognate DNA sequence motif (the ASE motif) in most terminal differentiation genes expressed in ASE neurons, as well as in its own cis-regulatory region. Notably, a promoter fusion of the che-1 transcription factor failed to express in che-1 mutants, indicative of CHE-1 autoregulation [27], [34]. Similar observations were made for AST-1 [28], and for the cooperatively-acting TTX-3 and CEH-10 transcription factors in AIY neurons [35]. Thus, subtype maintenance in C. elegans is anchored by auto-maintenance of the transcription factors that initiate and maintain terminal differentiation gene expression.
In contrast, all available evidence in Tv1 and Tv4 neurons fails to support such an autoregulatory mechanism. An ap reporter (apC-τ-lacZ) is expressed normally in ap mutants [12], [36], and in this study apdsRNAi was not found to alter apGAL4 reporter activity (Figure 2I). Moreover, col transcription was unaffected in col mutants that express a non-functional Col protein [9]. This leaves unresolved the question of how the majority of the transcription factors are stably maintained. For transcription factors that are initiated by transiently expressed inputs, a shift to distinct maintenance mechanisms have been invoked and in certain cases shown [35]. In this study, this was found for the loss of cas expression in Tv1 (required for col initiation) and the loss of cas, col and grh in Tv4 (required for eya, ap, dimm, sqz, dac initiation). However, we were surprised to find that the cross-regulatory relationships between persistently-expressed transcription factors were also significantly altered in adults. Notably, eya initiated but did not maintain dimm in Tv4. In Tv1, col initiated but did not maintain eya, ap or dimm. This was particularly unexpected as eya remained critical for FMRFa maintenance and col remained critical for Nplp1 maintenance. Indeed, although we tested for cross-regulatory interactions between all transcription factors in both the Tv1 and Tv4 subtype transcription networks, only Dimm was found to remain dependent upon its developmental input; Eya and Ap in Tv1 as well as Ap in Tv4. However, even in this case, the regulation of Dimm was changed; it no longer required eya in Tv4, and in Tv1 it no longer required col, in spite of the fact that both col and eya are retained in these neurons. We anticipate that such changes in transcription factor cross-regulatory relationships will be found in other Drosophila and vertebrate neurons, which exhibit high complexity in their subtype transcription networks [1], [5]. Indeed, recent evidence has found that in murine serotonergic neurons, the initiation of Pet-1 requires Lmx-1b, but ablation of Lmx-1b in adults did not perturb the maintenance of Pet-1 expression [31].
We are pursuing the potential role of autoregulation for the other factors in the Tv1/Tv4 subtype transcription networks. However, we consider there to be three additional, potentially overlapping, models for subtype transcription network maintenance. First, regulators may act increasingly redundantly upon one another. Second, unknown regulators may become increasingly sufficient for transcription factor maintenance. Third, transcription factors may be maintained by dedicated maintenance mechanisms, as has been shown for the role of trithorax group genes in the maintenance of Hox genes and Engrailed [37], [38]. Moreover, chromatin modification is undoubtedly involved and likely required to maintain high-level transcription of Tv transcription factors as well as FMRFa, Nplp1 and PHM. However, the extent to which these are instructive as opposed to permissive has yet to be established [39]. In this light, it is intriguing that MYST-HAT complexes, in addition to the subtype transcription factors Che-1 and Die-1, are required for maintenance of ASE-Left subtype identity in C. elegans [31].
Taken together, our studies have identified two apparent types of maintenance mechanism that are operational in adult neurons. On one hand, there are sets of genes that are maintained by their initiating set of transcription factors. These include the terminal differentiation genes and the transcription factor dimm. On the other, most transcription factors appear to no longer require regulatory input from their initiating transcription factor(s). Further work will be required to better understand whether these differences represent truly distinct modes of gene maintenance or reflect the existence of yet unidentified regulatory inputs onto these transcription factors. One issue to consider here is that the expression of certain terminal differentiation genes in neurons, but perhaps not subtype transcription factors, can be plastic throughout life, with changes commonly occurring in response to a developmental switch or physiological stimulus [40], [41], [42]. Thus, terminal differentiation genes may retain complex transcriptional control in order to remain responsive to change. It is notable, however, that FMRFa, Nplp1 and PHM appear to be stably expressed at high levels in Tv1/4 neurons, and we have not found any conditions that alter their expression throughout life. Thus, we consider these to be stable terminal differentiation genes akin to serotonergic or dopaminergic markers in their respective neurons that define those cells' functional identity and, where tested, are actively maintained by their developmental inputs [30], [32]. Tv1/4 neurons undoubtedly express a battery of terminal differentiation genes, and sets of unknown transcription factors are likely required for their subtype-specific expression. We consider subtype transcription networks to encompass all regulators required for differentiating the expression of all subtype-specific terminal differentiation genes. Further, we view differentiation of subtype identity as the completion of a multitude of distinct gene regulatory events in which each gene is regulated by a subset of the overall subtype transcription network. As highly restricted terminal differentiation genes expressed in Tv1 and Tv4 neurons, we believe that Nplp1, FMRFa and PHM provide a suitable model for the maintenance of overall identity, with the understanding that other unknown terminal differentiation genes expressed in Tv1 and Tv4 may not be perturbed by knockdown of the transcription factors tested herein. In the future, it will be important to incorporate a more comprehensive list of regulators and terminal differentiation genes for each neuronal subtype. However, we believe that the principles uncovered here for FMRFa, Nplp1 and PHM maintenance will hold for other terminal differentiation genes.
Finally, we propose that the active mechanisms utilized for maintenance of subtype differentiation represent an Achilles heel that renders long-lived neurons susceptible to degenerative disorders. Nurr1 ablation in adult mDA neurons reduced dopaminergic markers and promoted cell death [32]. Notably, Nurr1 mutation is associated with Parkinson's disease [43], and its downregulation is observed in Parkinson's disease mDA neurons [44]. Adult mDA are also susceptible to degeneration in foxa2 heterozygotes, another regulator of mDA neuron differentiation that is maintained in adult mDA neurons [45]. Studies in other long-lived cell types draw similar conclusions. Adult conditional knockout of Pdx1 reduced insulin and β-cell mass [46], [47] and, importantly, heterozygosity for Pdx1 leads to a rare monogenic form of non-immune diabetes, MODY4 [48]. Similarly, NeuroD1 haploinsufficiency is linked to MODY6 [49] and adult ablation of NeuroD in β-islet cells results in β-cell dysfunction and diabetes [50]. These data, together with our results here, underscore the need to further explore the transcriptional networks that actively maintain subtype identity, and hence the function, of adult and aging cells.
Materials and Methods
Fly stocks
Flies were maintained on standard cornmeal food and maintained at stable temperatures in environment rooms set at 70% humidity at 18°C, 25°C or 29°C.
Fly strains
apterousmd544 (referred to as apGAL4); apP44; sqzIE; sqzGAL4; UAS-thickveins activated (UAS-tkvA); UAS-saxophone activated (UAS-saxA) [12]; dacGAL4 [51]; rev4; UAS-dimm [18]; dac3 [52]; ; eyaCliIID [53]; grhGAL4 [6]. tubP>GAL80TS (temperature-sensitive GAL80 under the control of the Drosophila tubulin 84B promoter) (McGuire et al., 2003); UAS-nEGFP (nuclear localized EGFP); UAS-dicer2 [21].
dsRNAi lines
Strains used for primary data: UAS-col#24E [9]; UAS-apdsRNAi 8376R-2; UAS-dacdsRNAi 4952R-2; UAS-sqzdsRNAi 5557R-2 (NIG-FLY); UAS-dimmdsRNAi GD44470; UAS-eyadsRNAi GD43911 (VDRC). Strains used secondarily to verify data: UAS-dimmdsRNAi KK103356; UAS-eyadsRNAi 108071KK (VDRC); UAS-eyadsRNAi JF03160; UAS-dacdsRNAi JF02322 (TRiP).
Spatial and temporal regulation of transgene expression using the TARGET system
Flies for TARGET-mediated transgene induction were generated by crossing utility flies (UAS-dicer2/UAS-dicer2; apGal4; tubP>GAL80TS, UAS-nEGFP/SM6-TM6,Tb) or (UAS-dicer/UAS-dicer; dacGal4; tubP>GAL80TS, UAS-nEGFP/SM6-TM6,Tb) to UAS-dsRNAi (experimental group) or w1118 flies (control). Experiments were performed on resulting progeny bearing appropriate genotypes (screened by loss of SM6-TM6, Tb balancer chromosome). All experimental and control flies were raised at 18°C until eclosion (hatching from the pupal case). On adult day 1 (A1), flies were switched to 29°C for the duration of the induction period indicated. Throughout the text, we present dsRNAi data for the induction period at which we observe the maximal phenotype of Nplp1 and FMRFa expression. Further details for each dsRNAi are provided in the text.
Antibodies
Primary antibodies
Sheep anti-digoxygenin (1∶1500; Roche); rabbit anti-GFP (1∶100; A6455 Invitrogen); rabbit anti-FMRFa (1∶1000; T-4757 Peninsula Labs); mouse anti-β-Galactosidase (1∶100; 40-1a); chicken anti-Nplp1 (1∶1000), guinea pig anti-Dimm (1∶1000) and rat anti-Cas (1∶1000) (gifts from S. Thor, Linkoping U, Sweden); guinea pig anti-Collier (1∶1000; gift from Adrian Moore, RIKEN, Japan); rabbit anti-pMad (1∶100; cell signaling); rabbit anti-nab (gift from Fernando Jimenez Diaz-Benjumea, Universidad Autónoma de Madrid, Spain); mouse anti-Eya (1∶100; clone 10H6) and mouse anti-Dac (1∶2; Mab Dac 2–3) (both from Developmental Studies Hybridoma Bank; Iowa U. Iowa).
Secondary antibodies
Donkey anti-sheep Alexa 555 (1∶10; Invitrogen, Carlsbad, USA); donkey anti-mouse Cy5 and donkey anti-rabbit Cy2 (1∶200; Jackson Immunoresearch, West Grove, USA). Antisense DIG-RNA Probe: DIG-Uracil tagged RNA probes were generated using T3 RNA polymerase from clone RH03963 (DGRC: Drosophila Genomic Resource Centre, Indiana, USA) containing a 1584 bp FMRFa cDNA (using the Roche DIG-U-RNA Labelling Kit). Probe synthesis was confirmed using gel electrophoresis.
Multiplex fluorescent in situ hybridization (FISH) and immunohistochemistry
Standard in situ and immunohistochemistry protocols were carried out as described [14]. All tissues compared for fluorescence intensity were processed at the same time using the same aliquots of all solutions under the same conditions. They were then mounted on the same slide and confocal settings were calibrated to control staining levels.
Image and statistical analysis
All images acquired on an Olympus FV1000 confocal microscope. Fluorescent intensity of individual neurons was measured using Image J (US National Institutes of Health). The mean pixel intensity for each neuron was measured from compressed Z-slices, and corrected for background fluorescence. Analysis was performed on every identifiable Tv1 and Tv4 neuron in segments T1 and T3. The resulting value for each Tv neuron was then incorporated as a single datum point towards the mean intensity for each experiment. Each datum point is represented as a percentage of the mean of the w1118 control for that experiment. Data are presented as Mean ± SEM. Representative images of Tv neurons that were directly compared in figures were processed in an identical way, simultaneously, using Adobe Photoshop CS4. Normally distributed unpaired data were compared using a two-tailed T-test assuming equal variance, to identify significant differences between means. All statistical analysis and graphs data were performed using Prism 5 software. (Graphpad).
Supporting Information
Zdroje
1. di SanguinettoSADasenJSArberS 2008 Transcriptional mechanisms controlling motor neuron diversity and connectivity. Curr Opin Neurobiol 18 36 43
2. LandgrafMThorS 2006 Development of Drosophila motoneurons: specification and morphology. Semin Cell Dev Biol 17 3 11
3. HobertO 2010 Neurogenesis in the nematode Caenorhabditis elegans. WormBook 1 24
4. HobertOCarreraIStefanakisN 2010 The molecular and gene regulatory signature of a neuron. Trends Neurosci 33 435 445
5. AlavianKNScholzCSimonHH 2008 Transcriptional regulation of mesencephalic dopaminergic neurons: the full circle of life and death. Mov Disord 23 319 328
6. BaumgardtMKarlssonDTerrienteJDiaz-BenjumeaFJThorS 2009 Neuronal subtype specification within a lineage by opposing temporal feed-forward loops. Cell 139 969 982
7. HabenerJFKempDMThomasMK 2005 Minireview: transcriptional regulation in pancreatic development. Endocrinology 146 1025 1034
8. KarlssonDBaumgardtMThorS 2010 Segment-specific neuronal subtype specification by the integration of anteroposterior and temporal cues. PLoS Biol 8 e1000368 doi:10.1371/journal.pbio.1000368
9. BaumgardtMMiguel-AliagaIKarlssonDEkmanHThorS 2007 Specification of neuronal identities by feedforward combinatorial coding. PLoS Biol 5 e37 doi:10.1371/journal.pbio.0050037
10. AllanDWParkDSt PierreSETaghertPHThorS 2005 Regulators acting in combinatorial codes also act independently in single differentiating neurons. Neuron 45 689 700
11. Miguel-AliagaIAllanDWThorS 2004 Independent roles of the dachshund and eyes absent genes in BMP signaling, axon pathfinding and neuronal specification. Development 131 5837 5848
12. AllanDWSt PierreSEMiguel-AliagaIThorS 2003 Specification of neuropeptide cell identity by the integration of retrograde BMP signaling and a combinatorial transcription factor code. Cell 113 73 86
13. KarlssonDBaumgardtMThorS Segment-specific neuronal subtype specification by the integration of anteroposterior and temporal cues. PLoS Biol 8 e1000368 doi:10.1371/journal.pbio.1000368
14. EadeKTAllanDW 2009 Neuronal phenotype in the mature nervous system is maintained by persistent retrograde bone morphogenetic protein signaling. J Neurosci 29 3852 3864
15. BrodyTOdenwaldWF 2005 Regulation of temporal identities during Drosophila neuroblast lineage development. Curr Opin Cell Biol 17 672 675
16. Terriente FelixJMagarinosMDiaz-BenjumeaFJ 2007 Nab controls the activity of the zinc-finger transcription factors Squeeze and Rotund in Drosophila development. Development 134 1845 1852
17. BenvenisteRJThorSThomasJBTaghertPH 1998 Cell type-specific regulation of the Drosophila FMRF-NH2 neuropeptide gene by Apterous, a LIM homeodomain transcription factor. Development 125 4757 4765
18. HewesRSParkDGauthierSASchaeferAMTaghertPH 2003 The bHLH protein Dimmed controls neuroendocrine cell differentiation in Drosophila. Development 130 1771 1781
19. MarquesGHaerryTECrottyMLXueMZhangB 2003 Retrograde Gbb signaling through the Bmp type 2 receptor wishful thinking regulates systemic FMRFa expression in Drosophila. Development 130 5457 5470
20. ParkDShaferOTShepherdSPSuhHTriggJS 2008 The Drosophila basic helix-loop-helix protein DIMMED directly activates PHM, a gene encoding a neuropeptide-amidating enzyme. Mol Cell Biol 28 410 421
21. DietzlGChenDSchnorrerFSuKCBarinovaY 2007 A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448 151 156
22. McGuireSEMaoZDavisRL 2004 Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE 2004 pl6
23. O'KeefeDDThorSThomasJB 1998 Function and specificity of LIM domains in Drosophila nervous system and wing development. Development 125 3915 3923
24. ButterworthFMKingRC 1965 The developmental genetics of apterous mutants of Drosophila melanogaster. Genetics 52 1153 1174
25. HewesRSGuTBrewsterJAQuCZhaoT 2006 Regulation of secretory protein expression in mature cells by DIMM, a basic helix-loop-helix neuroendocrine differentiation factor. J Neurosci 26 7860 7869
26. BlauHMBaltimoreD 1991 Differentiation requires continuous regulation. J Cell Biol 112 781 783
27. EtchbergerJFLorchASleumerMCZapfRJonesSJ 2007 The molecular signature and cis-regulatory architecture of a C. elegans gustatory neuron. Genes Dev 21 1653 1674
28. FlamesNHobertO 2009 Gene regulatory logic of dopamine neuron differentiation. Nature 458 885 889
29. SchmidtMLinSPapeMErnsbergerUStankeM 2009 The bHLH transcription factor Hand2 is essential for the maintenance of noradrenergic properties in differentiated sympathetic neurons. Dev Biol 329 191 200
30. LiuCMaejimaTWylerSCCasadesusGHerlitzeS 2010 Pet-1 is required across different stages of life to regulate serotonergic function. Nat Neurosci 13 1190 1198
31. SongNNXiuJBHuangYChenJYZhangL 2011 Adult raphe-specific deletion of Lmx1b leads to central serotonin deficiency. PLoS ONE 6 e15998 doi:10.1371/journal.pone.0015998
32. KadkhodaeiBItoTJoodmardiEMattssonBRouillardC 2009 Nurr1 is required for maintenance of maturing and adult midbrain dopamine neurons. J Neurosci 29 15923 15932
33. HobertO 2011 Maintaining a memory by transcriptional autoregulation. Curr Biol 21 R146 147
34. EtchbergerJFFlowersEBPooleRJBashllariEHobertO 2009 Cis-regulatory mechanisms of left/right asymmetric neuron-subtype specification in C. elegans. Development 136 147 160
35. BertrandVHobertO 2009 Linking asymmetric cell division to the terminal differentiation program of postmitotic neurons in C. elegans. Dev Cell 16 563 575
36. LundgrenSECallahanCAThorSThomasJB 1995 Control of neuronal pathway selection by the Drosophila LIM homeodomain gene apterous. Development 121 1769 1773
37. MihalyJBargesSSiposLMaedaRCleardF 2006 Dissecting the regulatory landscape of the Abd-B gene of the bithorax complex. Development 133 2983 2993
38. BreenTRChinwallaVHartePJ 1995 Trithorax is required to maintain engrailed expression in a subset of engrailed-expressing cells. Mech Dev 52 89 98
39. PtashneM 2007 On the use of the word ‘epigenetic’. Curr Biol 17 R233 236
40. XuPVan SlambrouckCBerti-MatteraLHallAK 2005 Activin induces tactile allodynia and increases calcitonin gene-related peptide after peripheral inflammation. J Neurosci 25 9227 9235
41. SprecherSGDesplanC 2008 Switch of rhodopsin expression in terminally differentiated Drosophila sensory neurons. Nature 454 533 537
42. BorodinskyLNRootCMCroninJASannSBGuX 2004 Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature 429 523 530
43. GrimesDAHanFPanissetMRacachoLXiaoF 2006 Translated mutation in the Nurr1 gene as a cause for Parkinson's disease. Mov Disord 21 906 909
44. LeWPanTHuangMXuPXieW 2008 Decreased NURR1 gene expression in patients with Parkinson's disease. J Neurol Sci 273 29 33
45. KittappaRChangWWAwatramaniRBMcKayRD 2007 The foxa2 gene controls the birth and spontaneous degeneration of dopamine neurons in old age. PLoS Biol 5 e325 doi:10.1371/journal.pbio.0050325
46. HollandAMGonezLJNaselliGMacdonaldRJHarrisonLC 2005 Conditional expression demonstrates the role of the homeodomain transcription factor Pdx1 in maintenance and regeneration of beta-cells in the adult pancreas. Diabetes 54 2586 2595
47. LottmannHVanselowJHessabiBWaltherR 2001 The Tet-On system in transgenic mice: inhibition of the mouse pdx-1 gene activity by antisense RNA expression in pancreatic beta-cells. J Mol Med 79 321 328
48. AhlgrenUJonssonJJonssonLSimuKEdlundH 1998 beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev 12 1763 1768
49. MaleckiMTJhalaUSAntonellisAFieldsLDoriaA 1999 Mutations in NEUROD1 are associated with the development of type 2 diabetes mellitus. Nat Genet 23 323 328
50. GuCSteinGHPanNGoebbelsSHornbergH 2010 Pancreatic beta cells require NeuroD to achieve and maintain functional maturity. Cell Metab 11 298 310
51. HeanueTAReshefRDavisRJMardonGOliverG 1999 Synergistic regulation of vertebrate muscle development by Dach2, Eya2, and Six1, homologs of genes required for Drosophila eye formation. Genes Dev 13 3231 3243
52. MardonGSolomonNMRubinGM 1994 dachshund encodes a nuclear protein required for normal eye and leg development in Drosophila. Development 120 3473 3486
53. PignoniFHuBZavitzKHXiaoJGarrityPA 1997 The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell 91 881 891
Štítky
Genetika Reprodukčná medicína
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2012 Číslo 2- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Upsetting the Dogma: Germline Selection in Human Males
- A Strong Deletion Bias in Nonallelic Gene Conversion
- Positive Selection for New Disease Mutations in the Human Germline: Evidence from the Heritable Cancer Syndrome Multiple Endocrine Neoplasia Type 2B
- Genome-Wide Association Study in East Asians Identifies Novel Susceptibility Loci for Breast Cancer
- Mixed Effects Modeling of Proliferation Rates in Cell-Based Models: Consequence for Pharmacogenomics and Cancer
- Reduction of NADPH-Oxidase Activity Ameliorates the Cardiovascular Phenotype in a Mouse Model of Williams-Beuren Syndrome
- Genome-Wide Association Study Identifies Chromosome 10q24.32 Variants Associated with Arsenic Metabolism and Toxicity Phenotypes in Bangladesh
- Structural Basis of Transcriptional Gene Silencing Mediated by MOM1
- Genomic Restructuring in the Tasmanian Devil Facial Tumour: Chromosome Painting and Gene Mapping Provide Clues to Evolution of a Transmissible Tumour
- Genome-Wide Association Study Identifies Novel Loci Associated with Circulating Phospho- and Sphingolipid Concentrations
- Contrasting Properties of Gene-Specific Regulatory, Coding, and Copy Number Mutations in : Frequency, Effects, and Dominance
- The Origin and Nature of Tightly Clustered Deletions in Precursor B-Cell Acute Lymphoblastic Leukemia Support a Model of Multiclonal Evolution
- Ultrafast Evolution and Loss of CRISPRs Following a Host Shift in a Novel Wildlife Pathogen,
- Phosphorylation of Chromosome Core Components May Serve as Axis Marks for the Status of Chromosomal Events during Mammalian Meiosis
- Psoriasis Patients Are Enriched for Genetic Variants That Protect against HIV-1 Disease
- A Pathogenic Mechanism in Huntington's Disease Involves Small CAG-Repeated RNAs with Neurotoxic Activity
- The Mitochondrial Chaperone Protein TRAP1 Mitigates α-Synuclein Toxicity
- Homeobox Genes Critically Regulate Embryo Implantation by Controlling Paracrine Signaling between Uterine Stroma and Epithelium
- Developmental Transcriptional Networks Are Required to Maintain Neuronal Subtype Identity in the Mature Nervous System
- Down-Regulating Sphingolipid Synthesis Increases Yeast Lifespan
- Gene Expression and Stress Response Mediated by the Epigenetic Regulation of a Transposable Element Small RNA
- Loss of Tgif Function Causes Holoprosencephaly by Disrupting the Shh Signaling Pathway
- Sequestration of Highly Expressed mRNAs in Cytoplasmic Granules, P-Bodies, and Stress Granules Enhances Cell Viability
- Discovery of a Modified Tetrapolar Sexual Cycle in and the Evolution of in the Species Complex
- The Role of Glypicans in Wnt Inhibitory Factor-1 Activity and the Structural Basis of Wif1's Effects on Wnt and Hedgehog Signaling
- Nondisjunction of a Single Chromosome Leads to Breakage and Activation of DNA Damage Checkpoint in G2
- A Regulatory Network for Coordinated Flower Maturation
- Coexpression Network Analysis in Abdominal and Gluteal Adipose Tissue Reveals Regulatory Genetic Loci for Metabolic Syndrome and Related Phenotypes
- Diced Triplets Expose Neurons to RISC
- The Williams-Beuren Syndrome—A Window into Genetic Variants Leading to the Development of Cardiovascular Disease
- The Empirical Power of Rare Variant Association Methods: Results from Sanger Sequencing in 1,998 Individuals
- Systematic Detection of Epistatic Interactions Based on Allele Pair Frequencies
- Familial Identification: Population Structure and Relationship Distinguishability
- Raf1 Is a DCAF for the Rik1 DDB1-Like Protein and Has Separable Roles in siRNA Generation and Chromatin Modification
- Loss of Function of the Cik1/Kar3 Motor Complex Results in Chromosomes with Syntelic Attachment That Are Sensed by the Tension Checkpoint
- Computational Prediction and Molecular Characterization of an Oomycete Effector and the Cognate Resistance Gene
- The Dynamics and Prognostic Potential of DNA Methylation Changes at Stem Cell Gene Loci in Women's Cancer
- GTPase Activity and Neuronal Toxicity of Parkinson's Disease–Associated LRRK2 Is Regulated by ArfGAP1
- Evaluation of the Role of Functional Constraints on the Integrity of an Ultraconserved Region in the Genus
- Neurophysiological Defects and Neuronal Gene Deregulation in Mutants
- Genetic and Functional Analyses of Mutations Suggest a Multiple Hit Model of Autism Spectrum Disorders
- Negative Supercoiling Creates Single-Stranded Patches of DNA That Are Substrates for AID–Mediated Mutagenesis
- Rewiring of PDZ Domain-Ligand Interaction Network Contributed to Eukaryotic Evolution
- The Eph Receptor Activates NCK and N-WASP, and Inhibits Ena/VASP to Regulate Growth Cone Dynamics during Axon Guidance
- Repression of a Potassium Channel by Nuclear Hormone Receptor and TGF-β Signaling Modulates Insulin Signaling in
- The Retrohoming of Linear Group II Intron RNAs in Occurs by Both DNA Ligase 4–Dependent and –Independent Mechanisms
- Cell Lineage Analysis of the Mammalian Female Germline
- Association of a Functional Variant in the Wnt Co-Receptor with Early Onset Ileal Crohn's Disease
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Gene Expression and Stress Response Mediated by the Epigenetic Regulation of a Transposable Element Small RNA
- Contrasting Properties of Gene-Specific Regulatory, Coding, and Copy Number Mutations in : Frequency, Effects, and Dominance
- Homeobox Genes Critically Regulate Embryo Implantation by Controlling Paracrine Signaling between Uterine Stroma and Epithelium
- Nondisjunction of a Single Chromosome Leads to Breakage and Activation of DNA Damage Checkpoint in G2
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



