-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Genome-Wide Association Study in East Asians Identifies Novel Susceptibility Loci for Breast Cancer
Genetic factors play an important role in the etiology of both sporadic and familial breast cancer. We aimed to discover novel genetic susceptibility loci for breast cancer. We conducted a four-stage genome-wide association study (GWAS) in 19,091 cases and 20,606 controls of East-Asian descent including Chinese, Korean, and Japanese women. After analyzing 690,947 SNPs in 2,918 cases and 2,324 controls, we evaluated 5,365 SNPs for replication in 3,972 cases and 3,852 controls. Ninety-four SNPs were further evaluated in 5,203 cases and 5,138 controls, and finally the top 22 SNPs were investigated in up to 17,423 additional subjects (7,489 cases and 9,934 controls). SNP rs9485372, near the TGF-β activated kinase (TAB2) gene in chromosome 6q25.1, showed a consistent association with breast cancer risk across all four stages, with a P-value of 3.8×10−12 in the combined analysis of all samples. Adjusted odds ratios (95% confidence intervals) were 0.89 (0.85–0.94) and 0.80 (0.75–0.86) for the A/G and A/A genotypes, respectively, compared with the genotype G/G. SNP rs9383951 (P = 1.9×10−6 from the combined analysis of all samples), located in intron 5 of the ESR1 gene, and SNP rs7107217 (P = 4.6×10−7), located at 11q24.3, also showed a consistent association in each of the four stages. This study provides strong evidence for a novel breast cancer susceptibility locus represented by rs9485372, near the TAB2 gene (6q25.1), and identifies two possible susceptibility loci located in the ESR1 gene and 11q24.3, respectively.
Published in the journal: Genome-Wide Association Study in East Asians Identifies Novel Susceptibility Loci for Breast Cancer. PLoS Genet 8(2): e32767. doi:10.1371/journal.pgen.1002532
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002532Summary
Genetic factors play an important role in the etiology of both sporadic and familial breast cancer. We aimed to discover novel genetic susceptibility loci for breast cancer. We conducted a four-stage genome-wide association study (GWAS) in 19,091 cases and 20,606 controls of East-Asian descent including Chinese, Korean, and Japanese women. After analyzing 690,947 SNPs in 2,918 cases and 2,324 controls, we evaluated 5,365 SNPs for replication in 3,972 cases and 3,852 controls. Ninety-four SNPs were further evaluated in 5,203 cases and 5,138 controls, and finally the top 22 SNPs were investigated in up to 17,423 additional subjects (7,489 cases and 9,934 controls). SNP rs9485372, near the TGF-β activated kinase (TAB2) gene in chromosome 6q25.1, showed a consistent association with breast cancer risk across all four stages, with a P-value of 3.8×10−12 in the combined analysis of all samples. Adjusted odds ratios (95% confidence intervals) were 0.89 (0.85–0.94) and 0.80 (0.75–0.86) for the A/G and A/A genotypes, respectively, compared with the genotype G/G. SNP rs9383951 (P = 1.9×10−6 from the combined analysis of all samples), located in intron 5 of the ESR1 gene, and SNP rs7107217 (P = 4.6×10−7), located at 11q24.3, also showed a consistent association in each of the four stages. This study provides strong evidence for a novel breast cancer susceptibility locus represented by rs9485372, near the TAB2 gene (6q25.1), and identifies two possible susceptibility loci located in the ESR1 gene and 11q24.3, respectively.
Introduction
Breast cancer is one of the most common malignancies diagnosed among women worldwide, including those living in East Asian countries. Genetic factors play an important role in the etiology of both sporadic and familial breast cancer [1]. In the past two decades, more than 1,000 reports have been published addressing the association between variants in candidate genes and breast cancer risk. However, only a few genetic risk factors have been confirmed for this common malignancy [2]. Recent genome-wide association studies (GWAS) have identified approximately 20 common genetic susceptibility loci for breast cancer [3]–[14]. However, these newly-identified genetic factors, along with known high-penetrance breast cancer susceptibility genes explain less than 30% of the heritability for this cancer [2], [15]. Furthermore, most GWAS were conducted among women of European ancestry, and many of the variants discovered in European-ancestry populations showed only a weak or no association with breast cancer in other ethnic groups [16], [17]. For example, only 8 of 12 breast cancer risk SNPs identified in women of European ancestry were directly replicated in Chinese population [18]. Therefore, GWAS conducted in non-European women are needed to fully uncover the genetic basis for breast cancer susceptibility. Herein, we report results from a large GWAS of breast cancer conducted in East Asian women.
Results
A total of 19,091 female breast cancer cases and 20,606 female controls—including 23,891 Chinese, 11,907 Korean and 3,809 Japanese women—were included in the present study (Table 1). In Stage I, we analyzed 690,947 SNPs in 2,918 breast cancer cases and 2,324 community controls recruited from studies conducted in Shanghai, China (Figure 1, Text S1). Top 5,365 SNPs were investigated in Stage IIa including 1,613 Chinese cases and 1,800 Chinese controls recruited from studies conducted in Shanghai, China. Of the SNPs evaluated, 68 SNPs showed an association with breast cancer risk at P≤0.05 with the same direction as observed in Stage I. We performed a meta-analysis for the remaining 4,913 SNPs with data available from both Stage IIa and Stage IIb (2,359 Korean cases and 2,052 Korean controls). Twenty-six SNPs showed an association with breast cancer risk with Pmeta≤0.05 and the association was consistent among Stages I, IIa and IIb. These SNPs, along with the 68 SNPs mentioned above, were selected for Stage III replication in 4,712 cases and 4,496 controls. Finally, based on the results of the first three stages, 22 top SNPs were selected for Stage IV evaluation in 7,489 cases and 9,934 controls.
Fig. 1. Overview of the study design. 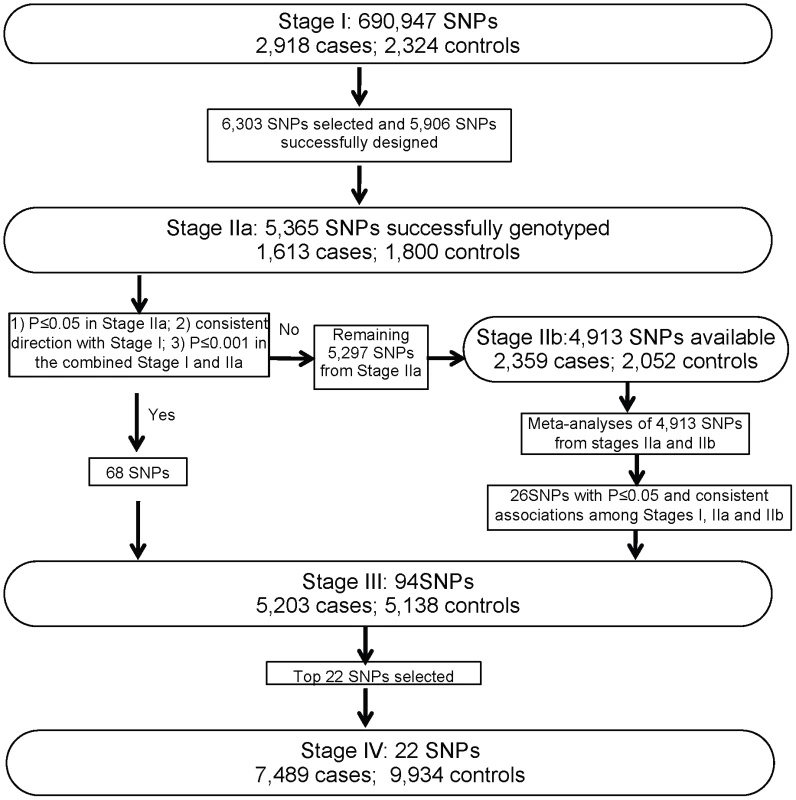
Tab. 1. Selected characteristics of studies participating in the Asia Breast Cancer Consortium. 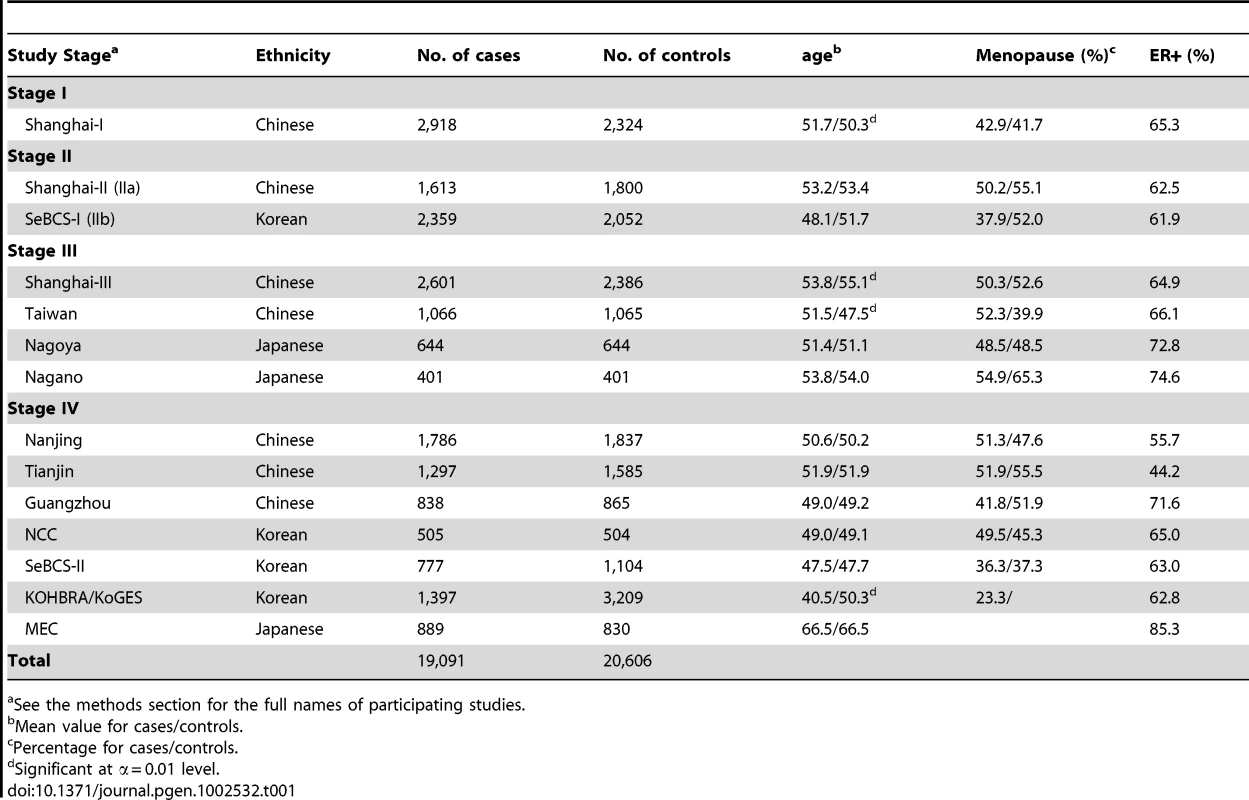
See the methods section for the full names of participating studies. SNP rs9485372 showed a statistically significant association with breast cancer risk in each of the four stages (Table 2). The OR (95% CI) per A allele was 0.88 (0.81–0.95), 0.86 (0.81–0.92), 0.94 (0.88–1.00) and 0.90 (0.85–0.94), respectively, for stages I to IV. The association with this SNP was remarkably consistent across all but one small study (Figure 2A). Pooled analysis of samples from all studies produced OR (95% CI) of 0.90 (0.87–0.92) and P-value of 3.8×10−12, which is substantially lower than the conventional genome-wide significance level of 5×10−8 based on conservative Bonferroni adjustment of multiple comparisons at α = 0.05, providing strong evidence for an association of this SNP with breast cancer risk.
Fig. 2. ORs per risk allele and 95% CIs for breast cancer associated with three SNPs by study site and ethnicity. 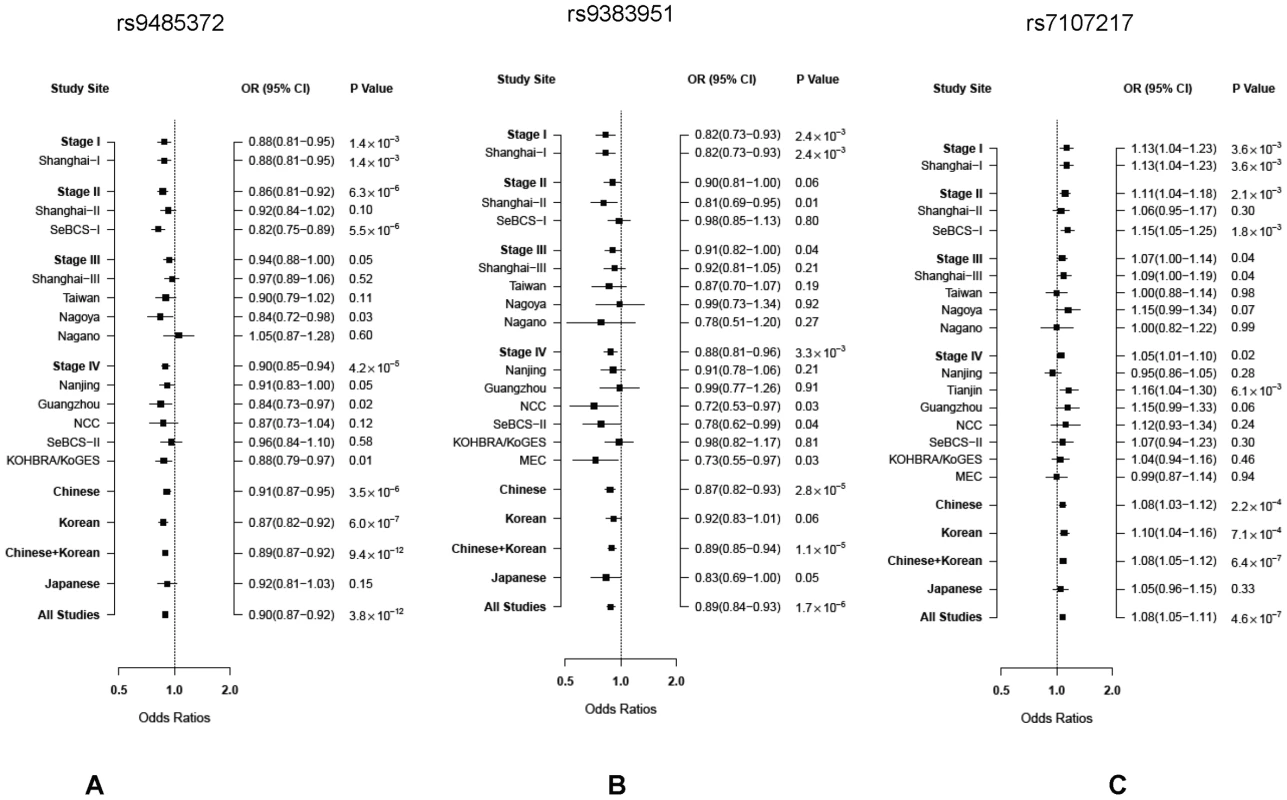
A: rs9485372, B: rs9383951; and C: rs7107217. Tab. 2. Summary of results for the three SNPs showing a statistically or marginally significant association in all four stages with breast cancer risk, the Asia Breast Cancer Consortium. 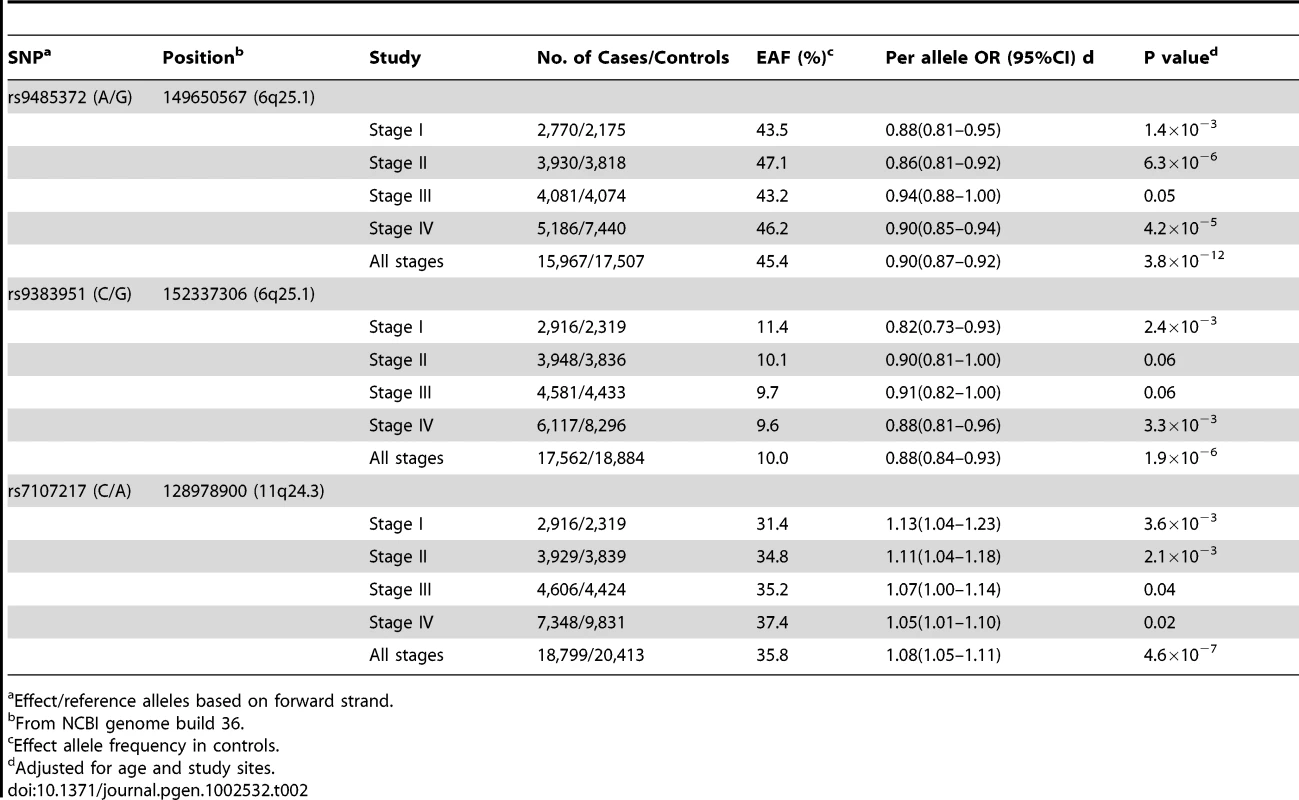
Effect/reference alleles based on forward strand. Two other SNPs, rs9383951 and rs7107217, were also consistently replicated in each of the three replication sets. The C allele of rs9383951 was associated with decreased risk with OR (95% CI) of 0.82 (0.73–0.93), 0.90 (0.81–1.00), 0.91 (0.82–1.00), and 0.88 (0.81–0.96), respectively, for stages I to IV (Table 2). The P-value reached 1.9×10−6 in the pooled analysis of samples from all four stages. For SNP rs7107217, the ORs (95% CI) per C allele were 1.13 (1.04–1.23), 1.11 (1.04–1.18), 1.07 (1.00–1.14) and 1.05 (1.01–1.10), respectively, for stages I to IV, respectively (Table 2). Analyses with all subjects combined showed OR (95% CI) of 1.08 (1.05–1.11) and P value of 4.6×10−7. Again, the association of breast cancer risk with these two SNPs was very consistent across the vast majority of participating studies (Figure 2B and 2C).
Stratified analyses showed that the associations with these three SNPs were consistent in all three East Asian populations, although the association for SNPs rs9485372 and rs7107217 was not significant for Japanese subjects, probably due to a small sample size (Table 3). Associations of these three SNPs with breast cancer risk were similar when stratified by menopausal or estrogen receptor status and none of the heterogeneity tests was statistically significant (Table S1). No significant interaction was observed with other risk factors (Table S1). After adjusted for the top 5 or 10 principal components, the results did not change significantly (Table S2).
Tab. 3. Association of SNPs with breast cancer risk by ethnic groups, the Asia Breast Cancer Consortium. 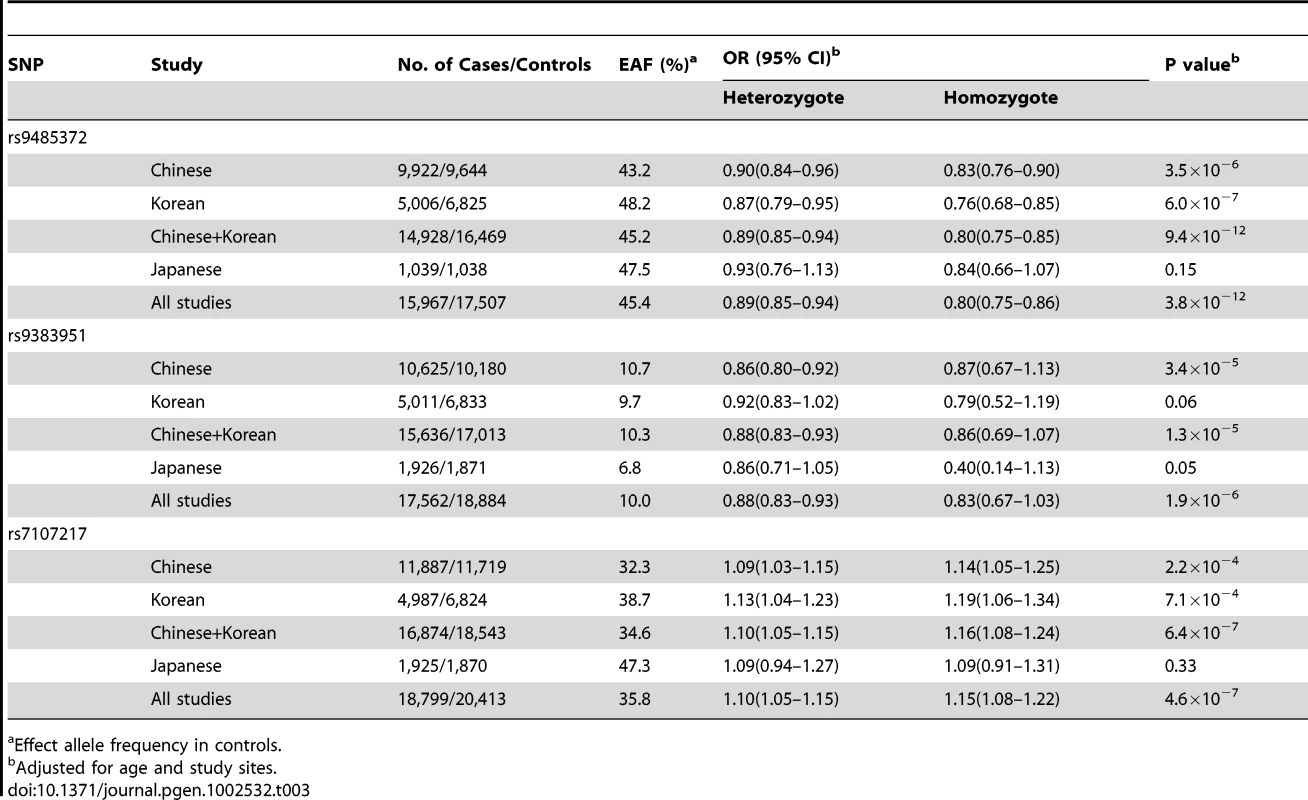
Effect allele frequency in controls. Both SNPs rs9485372 and rs9383951 are located at chromosome 6q25.1, approximately 2.34 Mb and 350 kb from the SNP rs2046210 that we previously reported for breast cancer risk [8]. None of these three SNPs, however, are in LD (r2<0.1) in any of the three populations (Asian, European and Africans) as determined using data generated in the HapMap or any of the study populations included in the current study (Table S3 and Figure S1). In an analysis including all 30,153 subjects who were genotyped for three SNPs in 6q25.1, all three SNPs remained strongly associated with breast cancer risk after mutual adjustment of the other 2 SNPs with P values of 1.4×10−12, 1.3×10−4, and 6.0×10−39 for SNPs rs9485372, rs9383951 and rs2046210, respectively (Table S4). No significant interaction was observed for these three SNPs (Table S5). We also created a genetic risk score (GRS) to evaluate the combined effect of three SNPs located in 6q25.1 (Table S6). Compared with women carrying 0–1 risk variants, women carrying 6 variants had over two-fold increased risk with an OR (95% CI) of 2.36 (1.89–2.96) and a P value of 1.3×10−47.
A total of 376 SNPs were successfully imputed in the LD blocks including rs2046210 and rs9485372 and the whole ESR1 gene with RSQ≥0.3 and minor allele frequency (MAF)≥0.05. Among them, 27 SNPs showed an association with breast cancer risk with P≤0.05 after adjusted for age, rs9485372, rs9383951 and rs2046210 (Table S7). With the exception of rs4591859 and rs7776340 in the locus of rs2046210 and rs7768330 in the locus of rs9383921, all other SNPs are in the same LD block within the ESR1 gene (Figure S2). No additional SNP in the rs9485372 locus showed an association with breast cancer risk at p<0.05 after adjusted for rs9485372, rs2046210, and rs9383921.
Discussion
In this large GWAS conducted in East-Asian women including 19,091 cases and 20,606 controls, we provided strong evidence for a novel breast cancer susceptibility locus represented by rs9485372 and suggestive evidence for two other loci, represented by SNPs rs9383951 and rs7107217.
We previously reported a genetic susceptibility locus at 6q25.1, represented by rs2046210, for breast cancer risk [8]. The newly identified SNPs, rs9485372 and rs9383951, also are located at chromosome 6q25.1. However, these three SNPs are not in LD and are thus representing independent breast cancer susceptibility loci. All of them were associated with breast cancer risk after mutual adjustment of the other two SNPs. SNP rs9485372 is approximately 31 Kb upstream of the TGF-β activated kinase 1/MAP3K7 binding protein 2 (TAB2) gene (Figure 3). The protein encoded by this gene is an activator of MAP3K7/TAK1, which is required for the IL-1 induced activation of NF-κB and MAPK8/JNK. The TGF-β pathway plays a major role in breast cancer development and progression [19]. The MAP kinases pathway is critical in regulating cell growth and cell death [20] and may contribute to the development of cancer [20]. Furthermore, the TAB2 protein is required for DNA damage-induced TAK1 activation, suggesting that TAB2 may play a role in DNA damage repair [21]. Other genes in the region identified in the study included SUMO4, LATS1, PPIL4, and UST. However, given the proximity of the TAB2 gene with rs9485372 and the important role of this gene in breast carcinogenesis, it is possible that the association between rs9485372 and breast cancer risk may be mediated through the TAB2 gene. It is also possible that the association may be mediated through regulating the ESR1 gene, located approximately 2.5 Mb from rs9485372. This possibility was highlighted by a recent study showing that several open reading frames in the 6q25.1 regions co-expressed with ESR1 [22]. Further research is warranted to clarify the mechanism of the association identified in the study.
Fig. 3. A regional plot of the −log10P-values for SNPs at 6q25.1. 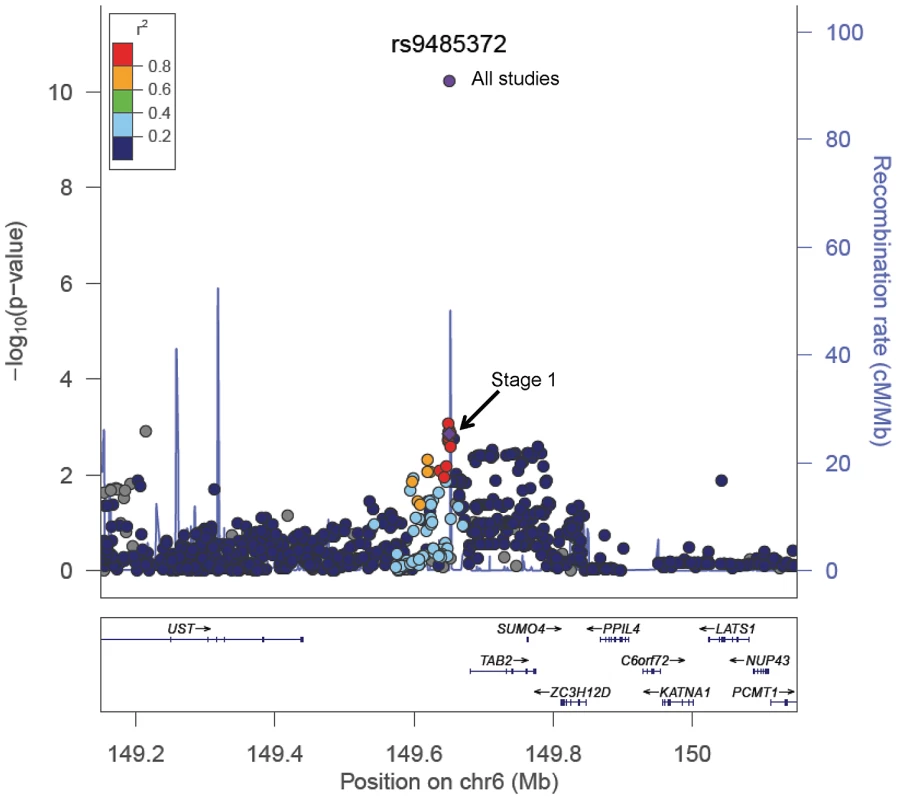
The LD is estimated using data from HapMap Asian population. Also shown are the SNP Build 36 coordinates in kilobases (Kb), recombination rates in centimorgans (cM) per megabase (Mb) and genes in the region (below) based on the March 2006 UCSC genome browser assembly. SNP rs9383951 is located in intron 5 of the ESR1 gene, an important gene that has been documented to play a key role in breast cancer development and progression. Previous candidate gene studies have extensively evaluated two SNPs, rs2234693 (Pvull) and rs9340799 (XbaI), in the ESR1 gene in relation to breast cancer risk; the results, however, have been inconsistent [2]. Neither rs2234693 nor rs9340799 are in LD (r2<0.01) with the SNPs discovered in the present study. To follow-up the lead from our previous study reporting a susceptibility locus at 6q25.1 for breast cancer [8], two recent studies conducted among women of European descent identified rs3757318 and rs9397435 in relation to breast cancer risk [11], [23]. These two SNPs are in strong LD (r2>0.6 in Asians) with the SNP (rs2046210) we previously reported at 6q25.1 in East Asians but not in other populations. Again, these two SNPs are not in LD (r2<0.01 in Asian, European and African populations) with rs9383951 and rs9485372 identified in this study. Although the association with rs9383951 did not reach the conventional genome-wide significance, the fact that this SNP is located in the ESR1 gene strongly suggests a true association of this SNP with breast cancer risk.
SNP rs7107217 also showed a consistent association in all four stages, although the pooled P-value did not reach the conventional genome-wide significance level. This SNP is located at 11q24.3, 152 Kb downstream of the BARX2 gene and 212 Kb upstream of the TMEM45B gene (Figure S3). BARX2 is a homeobox gene for which the mouse ortholog has been shown to influence cellular processes that control cell adhesion and cytoskeleton remodeling. It has been shown, BARX2 and estrogen receptor-alpha (ESR1) coordinately regulate the production of alternatively spliced ESR1 isoforms and control breast cancer cell growth and invasion [24]. BARX2 also acts in a tumor suppressor and loss of heterozygosity of this gene, lead to poorer survival in patients with ovarian cancer [25].
It could be ideal to increase the sample size in the discovery stage and simplify the replication stages of the study. However, like many other consortium projects, financial constraints and some logistical issues prevented us for achieving the maximum statistical power. Nevertheless, with approximately 40,000 cases and controls, our study represents the largest breast cancer genetic association study in East Asian women. This consortium will continue to provide valuable resources to identify additional novel susceptibility loci for breast cancer.
In summary, in this large GWAS conducted in East Asia women, we provided convincing evidence for an association with a novel independent susceptibility locus located at 6q25.1, near the TAB2 gene. Our study also suggests that genetic variants in the ESR1 gene and chromosome 11q24.3 may be related to breast cancer risk. Given that multiple independent breast cancer susceptibility loci have identified in our studies and studies conducted by others in 6q25.1 that harbors the ESR1 gene, it is possible that 6q25.1 may represent an important region for breast cancer susceptibility.
Methods
Study populations
Included in this consortium project were 19,091 cases and 20,606 controls from 14 studies (Table 1). Detailed descriptions of these participating studies and demographic characteristics of study participants are provided in Text S1. Briefly, the consortium included 23,981 Chinese women, 11,907 Korean women, 3,809 Japanese women. The Chinese women were from 8 studies: Shanghai [n = 13,642, Shanghai Breast Cancer Study, Shanghai Breast Cancer Survival Study (SBCSS), Shanghai Endometrial Cancer Study (SECS), Shanghai Women Health Study (SWHS)] [8], , Nanjing (n = 3,623) [27], Tianjin (n = 2,882) [28], Taiwan (n = 2,131) [29], and Guangzhou (n = 1,703). The Korean women were from four studies [Seoul Breast Cancer Study (SeBCS) (n = 6,292) [30], Korea NCC (n = 1,009), KoGES (n = 3,209) [31], and KOHBRA (n = 1,397) [32]]. The Japanese women were from three studies conducted in Hawaii and Los Angeles [n = 1,719; Multiethnic Cohort Study (MEC) [33]], Nagoya (n = 1,288) [34], and Nagano (n = 802) [35] (Table 1). Approval was granted from relevant institutional review boards in all study sites; all included subjects gave informed consent.
Genotyping methods
The Genotyping protocol for Stage I has been described previously [8]. Briefly, the initial 300 subjects were genotyped using the Affymetrix GeneChip Mapping 500K Array Set. The remaining 4,985 subjects were genotyped using the Affymetrix Genome-Wide Human SNP Array 6.0. We included one negative control and at least three positive quality control (QC) samples from the Coriell Cell Repositories (http://ccr.coriell.org/) in each of the 96-well plates for Affymetrix SNP Array 6.0 genotyping. A total of 273 positive QC samples were successfully genotyped, and the average concordance rate was 99.9% with a median value of 100%. The sex of all study samples was confirmed to be female. Genetically identical, unexpected duplicated samples were excluded, as were close relatives with a pair-wise proportion of identify-by-descent (IBD) estimate greater than 0.25. All samples with a call rate<95% were excluded. The SNPs were excluded if: (i) MAF<1%, (ii) call rate<95%, or (iii) genotyping concordance rate<95% in quality control samples. The final dataset included 2,918 cases and 2,324 controls for 690,947 markers. There are 21,223 SNPs that were on Affymetrix 500K Array Set but not on the Affymetrix SNP Array 6.0. These SNPs were excluded. SNPs on the Affymetrix 6.0 array but not on the Affymetrix 500k array were treated as missing data for those samples genotyped on using the Affymetrix 500k array. Similar results were obtained after excluding women genotyped by Affymetrix 500K Array Set from the analyses.
Genotyping for Stage IIa was completed using the Illumina iSelect platform. To compare the consistency between the Affymetrix and Illumina iSelect platforms, we also included 43 samples from Stage I that were genotyped by Affymetrix SNP 6.0. Similar to the QC procedures used in Stage I, the following criteria were used to exclude samples: (i) call rate<95%; or (ii) unexpected duplicated samples based on IBD estimate. SNPs were excluded if: (i) call rate<95%, or (ii) genotyping concordance rate<95% in quality control samples when compared with Affymetrix 6.0 data. After QC, the mean concordance rate was 99.85% between Illumina iSelect and Affymetrix 6.0 genotyping.
Data for the SNPs analyzed in Stage IIb were extracted from the Korean GWAS genotyped using the Affymetrix Genome-Wide Human SNP Array 6.0 chip. A total of 30 QC samples were successfully genotyped, and the concordance rate was 99.83%. The sex of all samples was confirmed to be female. The SNPs were excluded if: (1) genotype call rate<95%, (2) MAF<1% in either the cases or controls, (3) deviation from HWE at P-value<10−6, and (4) poor cluster plot in either the cases or controls.
Genotyping for Stage III and all samples from Koreans in Stage IV was completed using the iPLEX Sequenom MassArray platform in the Vanderbilt Molecular Epidemiology Laboratory. Included in each 96-well plate as QC samples were one negative control (water), two blinded duplicates, and two samples from the HapMap project. To compare the consistency between the Affymetrix and Sequenom platforms, we also genotyped 45 samples included in Stage I. The mean concordance rate was 99.67% for the blind duplicates, 98.88% for HapMap samples, and 99.52% between Sequenom and Affymetrix 6.0 genotyping. Data quality from the Hong Kong study was low and thus data from the study were excluded for the current analysis. Genotyping for two Chinese studies (Nanjing and Guangzhou) in Stage IV was completed using the iPLEX Sequenom MassArray platform at the Fudan University, Shanghai, China. Blind duplicate QC samples were included and the mean concordance rate was 98.70%. Genotyping for the Tianjin study in Stage IV was performed using TaqMan assays. Genotyping assay protocols were developed and validated at the Vanderbilt Molecular Epidemiology Laboratory, and TaqMan genotyping assay reagents were provided to investigators of the Tianjin study (Tianjin Cancer Institute and Hospital). For the MEC study, data for the three SNPs presented in this study were extracted from the GWA scan data generated using Illumina 660W. For SNPs not included on the chip, imputed data using HapMap as reference were extracted. Genotype frequencies for SNP rs9485372 deviated from HWE in controls (P = 0.004), therefore, this SNP was excluded in data analyses. Not all SNPs for Stage IV were genotyped in all studies included in Stage IV due to genotyping failure or the use of different genotyping platforms (Table S8).
SNP selection for replication
SNP selection for Stage II replication: Promising SNPs were selected for replication in Stage II based on the following criteria: 1) minor allele frequency (MAF)≥5%; 2) P<0.02 in Stage I; 3) Hardy-Weinberg equilibrium (HWE) test P>1.0×10−6 in controls; 4) not in strong linkage disequilibrium (LD) (r2<0.5) with any of the previously confirmed breast cancer genetic risk variants or SNPs evaluated in our previous studies [8], [12]; and 5) high genotyping quality as indicated by very clear genotyping clusters checked manually. When multiple SNPs are in LD with r2≥0.5, one SNP with the lowest P-value was selected. In total, 6,303 SNPs were selected for replication. A total of 5,906 SNPs (93.7%) were successfully designed by Illumina and included in the iSelect array. After stringent QC procedures, data from 5,365 SNPs were considered high quality for association analyses in Stage IIa, which include 1,613 breast cancer patients and 1,800 controls recruited from Shanghai studies.
SNP selection for Stage III replication: Among the 5,365 SNPs successfully genotyped in Stage IIa, 68 SNPs were selected for Stage III replication in an independent set of 5,203 cases and 5,138 controls recruited from Shanghai and several other East Asian populations (Table 1 and Text S1). The selection criteria are: 1) an association with breast cancer risk in Stage IIa with P≤0.05; 2) the direction of the association consistent in both stages; and 3) P≤0.001 in the merged data of Stage I and IIa. During the course of Stage III genotyping, genome-wide association scan data from 2,359 cases and 2,052 controls were obtained from the Seoul Breast Cancer GWAS (Stage IIb). Therefore, we performed a meta-analysis of Stage IIa and IIb data. Of the 5,297 SNPs which were not selected initially for Stage III replication based on Stage IIa data alone, data were available for 4,913 SNPs in Stage IIb. Meta-analyses of these 4,913 SNPs from Stage IIa and IIb yielded 26 additional SNPs that showed an association at P≤0.05 and in the same direction among stages I, IIa, and IIb. These 26 SNPs were then added to the list of SNPs to be genotyped in Stage III.
SNP selection for Stage IV replication: Based on the results of the first three stages, 22 top SNPs were selected for Stage IV evaluation and genotyped in up to 17,423 additional subjects (7,489 cases and 9,934 controls) (Table 1 and Text S1).
Statistical analyses
Case-control differences in selected demographic characteristics and major risk factors were evaluated using t-tests (for continuous variables) and Chi-square tests (for categorical variables). Associations between SNPs and breast cancer risk were assessed using odds ratios (ORs) and 95% confidence intervals (CIs) derived from logistic regression models. ORs were estimated for heterozygote and homozygote for the variant allele compared with homozygotes for the common allele. ORs were also estimated for the variant allele based on a log-additive model and adjusted for age, and study site, when appropriate. Stratified analyses by ethnicity, menopausal status, and estrogen receptor (ER) status were carried out. PLINK version 1.06 was used to analyze genome-wide data obtained in Stage I and the replication data in Stage IIa. Results from Stage IIb were also obtained from PLINK version 1.06. Meta-analyses of Stage IIa and Stage IIb were performed using a weighted z-statistics method, where weights were proportional to the square root of the number of individuals in each sample and standardized such that the weights added up to one. The z-statistic summarizes the magnitude and direction of the effect relative to the reference allele. An overall z-statistic and p value were then calculated from the weighted average of the individual statistics. Calculations were implemented in the METAL package (http://www.sph.umich.edu/csg/abecasis/Metal). Individual data were obtained from each study for Stage IV SNPs for a pooled analysis, which were conducted using SAS, version 9.2, with the use of two-tailed tests.
We first investigated the population structure by estimating inflation factor λ using all 690,947 SNPs SNPs that passed the QC. The inflation factor λ was estimated to be 1.042, suggesting that any population substructure, if present, should not have any appreciable effect on the results. Among the final 690,947 SNPs obtained in Stage I after QC, we generated a list of 196,471 SNPs with pairwise LD<0.2 by using plink (http://pngu.mgh.harvard.edu/~purcell/plink/). Then, principal components were estimated based on these 196,471 SNPs using EIGENSTRAT [36]. We then drew a plot for all Stage I and HapMap II subjects based on the first two principal components (Figure 4). All study participants in Stage I were clustered very closely with HapMap Asians. The first 5 or 10 principal components were adjusted in the logistic regression analyses for evaluating associations of SNPs and breast cancer risk.
Fig. 4. Principal Component Analysis (PCA) based on the first two eigenvectors obtained by PCA. 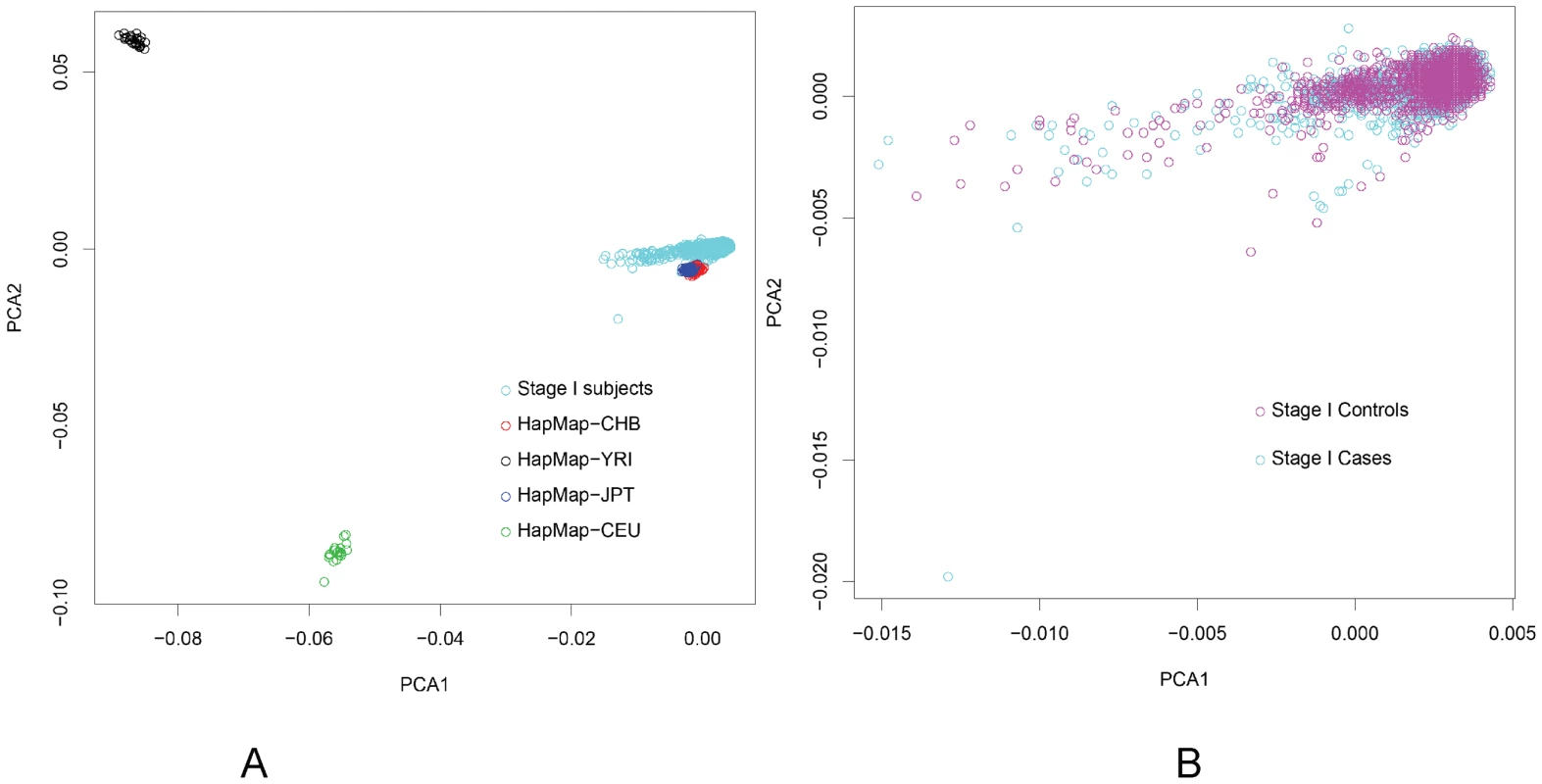
A: all individuals from Stage I and HapMap; B: breast cancer cases and controls from Stage I. To evaluate the combined effect of SNPs located in chromosome 6q25.1 on breast cancer risk, we created a genetic risk score (GRS) by summing the number (0–2) of risk alleles that each woman carried for each of the three SNPs, including rs9383951, rs9485372, rs2046210. The GRS was constructed among those who had complete data for all three SNPs. We also did imputation using MACH (http://www.sph.umich.edu/csg/abecasis/MACH/index.html) with HapMap II Asian data as reference. LD structure was estimated from the flanking 100 kb of these three SNPs and the ESR1 gene using data from HapMap II Asians (Figure S1). All SNPs in the LD blocks including rs9485372, rs2046210 and rs9383951 and SNPs inside the ESR1 gene were analyzed in relation to breast cancer risk with age, rs9485372, rs9383951 and rs2046210 adjusted.
Supporting Information
Zdroje
1. NathansonKLWoosterRWeberBL 2001 Breast cancer genetics: what we know and what we need. Nat Med 7 552 556
2. ZhangBBeeghly-FadielALongJZhengW 2011 Genetic variants associated with breast-cancer risk: comprehensive research synopsis, meta-analysis, and epidemiological evidence. Lancet Oncol 12 477 488
3. EastonDFPooleyKADunningAMPharoahPDThompsonD 2007 Genome-wide association study identifies novel breast cancer susceptibility loci. Nature 447 1087 1093
4. HunterDJKraftPJacobsKBCoxDGYeagerM 2007 A genome-wide association study identifies alleles in FGFR2 associated with risk of sporadic postmenopausal breast cancer. Nat Genet 39 870 874
5. StaceySNManolescuASulemPRafnarTGudmundssonJ 2007 Common variants on chromosomes 2q35 and 16q12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet 39 865 869
6. GoldBKirchhoffTStefanovSLautenbergerJVialeA 2008 Genome-wide association study provides evidence for a breast cancer risk locus at 6q22.33. Proc Natl Acad Sci U S A 105 4340 4345
7. StaceySNManolescuASulemPThorlaciusSGudjonssonSA 2008 Common variants on chromosome 5p12 confer susceptibility to estrogen receptor-positive breast cancer. Nat Genet 40 703 706
8. ZhengWLongJGaoYTLiCZhengY 2009 Genome-wide association study identifies a new breast cancer susceptibility locus at 6q25.1. Nat Genet 41 324 328
9. ThomasGJacobsKBKraftPYeagerMWacholderS 2009 A multistage genome-wide association study in breast cancer identifies two new risk alleles at 1p11.2 and 14q24.1 (RAD51L1). Nat Genet 41 579 584
10. AhmedSThomasGGhoussainiMHealeyCSHumphreysMK 2009 Newly discovered breast cancer susceptibility loci on 3p24 and 17q23.2. Nat Genet 41 585 590
11. TurnbullCAhmedSMorrisonJPernetDRenwickA 2010 Genome-wide association study identifies five new breast cancer susceptibility loci. Nat Genet 42 504 507
12. LongJCaiQShuXOQuSLiC 2010 Identification of a functional genetic variant at 16q12.1 for breast cancer risk: results from the Asia Breast Cancer Consortium. PLoS Genet 6 e1001002 doi:10.1371/journal.pgen.1001002
13. AntoniouACWangXFredericksenZSMcGuffogLTarrellR 2010 A locus on 19p13 modifies risk of breast cancer in BRCA1 mutation carriers and is associated with hormone receptor-negative breast cancer in the general population. Nat Genet % 19
14. FletcherOJohnsonNOrrNHoskingFJGibsonLJ 2011 Novel breast cancer susceptibility locus at 9q31.2: results of a genome-wide association study. J Natl Cancer Inst 103 425 435
15. FletcherOHoulstonRS 2010 Architecture of inherited susceptibility to common cancer. Nat Rev Cancer 10 353 361
16. ZhengWCaiQSignorelloLBLongJHargreavesMK 2009 Evaluation of 11 breast cancer susceptibility loci in African-American women. Cancer Epidemiol Biomarkers Prev 18 2761 2764
17. ZhengWWenWGaoYTShyrYZhengY 2010 Genetic and clinical predictors for breast cancer risk assessment and stratification among Chinese women. J Natl Cancer Inst 102 972 981
18. LongJShuXOCaiQGaoYTZhengY 2010 Evaluation of breast cancer susceptibility loci in Chinese women. Cancer Epidemiol Biomarkers Prev 19 2357 2365
19. BensonJR 2004 Role of transforming growth factor beta in breast carcinogenesis. Lancet Oncol 5 229 239
20. DavisRJ 2000 Signal transduction by the JNK group of MAP kinases. Cell 103 239 252
21. HinzMStilmannMArslanSCKhannaKKDittmarG 2010 A cytoplasmic ATM-TRAF6-cIAP1 module links nuclear DNA damage signaling to ubiquitin-mediated NF-kappaB activation. Mol Cell 40 63 74
22. DunbierAKAndersonHGhazouiZLopez-KnowlesEPancholiS 2011 ESR1 Is Co-Expressed with Closely Adjacent Uncharacterised Genes Spanning a Breast Cancer Susceptibility Locus at 6q25.1. PLoS Genet 7 e1001382 doi:10.1371/journal.pgen.1001382
23. StaceySNSulemPZanonCGudjonssonSAThorleifssonG 2010 Ancestry-shift refinement mapping of the C6orf97-ESR1 breast cancer susceptibility locus. PLoS Genet 6 e1001029 doi:10.1371/journal.pgen.1001029
24. StevensTAMeechR 2006 BARX2 and estrogen receptor-alpha (ESR1) coordinately regulate the production of alternatively spliced ESR1 isoforms and control breast cancer cell growth and invasion. Oncogene 25 5426 5435
25. SellarGCLiLWattKPNelkinBDRabiaszGJ 2001 BARX2 induces cadherin 6 expression and is a functional suppressor of ovarian cancer progression. Cancer Res 61 6977 6981
26. GaoYTShuXODaiQPotterJDBrintonLA 2000 Association of menstrual and reproductive factors with breast cancer risk: results from the Shanghai Breast Cancer Study. Int J Cancer 87 295 300
27. LiangJChenPHuZZhouXChenL 2008 Genetic variants in fibroblast growth factor receptor 2 (FGFR2) contribute to susceptibility of breast cancer in Chinese women. Carcinogenesis 29 2341 2346
28. ZhangLGuLQianBHaoXZhangW 2009 Association of genetic polymorphisms of ER-alpha and the estradiol-synthesizing enzyme genes CYP17 and CYP19 with breast cancer risk in Chinese women. Breast Cancer Res Treat 114 327 338
29. DingSLYuJCChenSTHsuGCKuoSJ 2009 Genetic variants of BLM interact with RAD51 to increase breast cancer susceptibility. Carcinogenesis 30 43 49
30. ChoiJYLeeKMParkSKNohDYAhnSH 2005 Association of paternal age at birth and the risk of breast cancer in offspring: a case control study. BMC Cancer 5 143
31. ChoYSGoMJKimYJHeoJYOhJH 2009 A large-scale genome-wide association study of Asian populations uncovers genetic factors influencing eight quantitative traits. Nat Genet 41 527 534
32. HanSAParkSKHyunASHyukLMNohDY 2011 The Korean Hereditary Breast Cancer (KOHBRA) Study: Protocols and Interim Report. Clin Oncol (R Coll Radiol)
33. KolonelLNHendersonBEHankinJHNomuraAMWilkensLR 2000 A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol 151 346 357
34. HamajimaNMatsuoKSaitoTHiroseKInoueM 2001 Gene-environment Interactions and Polymorphism Studies of Cancer Risk in the Hospital-based Epidemiologic Research Program at Aichi Cancer Center II (HERPACC-II). Asian Pac J Cancer Prev 2 99 107
35. ItohHIwasakiMHanaokaTKasugaYYokoyamaS 2009 Serum organochlorines and breast cancer risk in Japanese women: a case-control study. Cancer Causes Control 20 567 580
36. PriceALPattersonNJPlengeRMWeinblattMEShadickNA 2006 Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet 38 904 909
Štítky
Genetika Reprodukčná medicína
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2012 Číslo 2- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Upsetting the Dogma: Germline Selection in Human Males
- A Strong Deletion Bias in Nonallelic Gene Conversion
- Positive Selection for New Disease Mutations in the Human Germline: Evidence from the Heritable Cancer Syndrome Multiple Endocrine Neoplasia Type 2B
- Genome-Wide Association Study in East Asians Identifies Novel Susceptibility Loci for Breast Cancer
- Mixed Effects Modeling of Proliferation Rates in Cell-Based Models: Consequence for Pharmacogenomics and Cancer
- Reduction of NADPH-Oxidase Activity Ameliorates the Cardiovascular Phenotype in a Mouse Model of Williams-Beuren Syndrome
- Genome-Wide Association Study Identifies Chromosome 10q24.32 Variants Associated with Arsenic Metabolism and Toxicity Phenotypes in Bangladesh
- Structural Basis of Transcriptional Gene Silencing Mediated by MOM1
- Genomic Restructuring in the Tasmanian Devil Facial Tumour: Chromosome Painting and Gene Mapping Provide Clues to Evolution of a Transmissible Tumour
- Genome-Wide Association Study Identifies Novel Loci Associated with Circulating Phospho- and Sphingolipid Concentrations
- Contrasting Properties of Gene-Specific Regulatory, Coding, and Copy Number Mutations in : Frequency, Effects, and Dominance
- The Origin and Nature of Tightly Clustered Deletions in Precursor B-Cell Acute Lymphoblastic Leukemia Support a Model of Multiclonal Evolution
- Ultrafast Evolution and Loss of CRISPRs Following a Host Shift in a Novel Wildlife Pathogen,
- Phosphorylation of Chromosome Core Components May Serve as Axis Marks for the Status of Chromosomal Events during Mammalian Meiosis
- Psoriasis Patients Are Enriched for Genetic Variants That Protect against HIV-1 Disease
- A Pathogenic Mechanism in Huntington's Disease Involves Small CAG-Repeated RNAs with Neurotoxic Activity
- The Mitochondrial Chaperone Protein TRAP1 Mitigates α-Synuclein Toxicity
- Homeobox Genes Critically Regulate Embryo Implantation by Controlling Paracrine Signaling between Uterine Stroma and Epithelium
- Developmental Transcriptional Networks Are Required to Maintain Neuronal Subtype Identity in the Mature Nervous System
- Down-Regulating Sphingolipid Synthesis Increases Yeast Lifespan
- Gene Expression and Stress Response Mediated by the Epigenetic Regulation of a Transposable Element Small RNA
- Loss of Tgif Function Causes Holoprosencephaly by Disrupting the Shh Signaling Pathway
- Sequestration of Highly Expressed mRNAs in Cytoplasmic Granules, P-Bodies, and Stress Granules Enhances Cell Viability
- Discovery of a Modified Tetrapolar Sexual Cycle in and the Evolution of in the Species Complex
- The Role of Glypicans in Wnt Inhibitory Factor-1 Activity and the Structural Basis of Wif1's Effects on Wnt and Hedgehog Signaling
- Nondisjunction of a Single Chromosome Leads to Breakage and Activation of DNA Damage Checkpoint in G2
- A Regulatory Network for Coordinated Flower Maturation
- Coexpression Network Analysis in Abdominal and Gluteal Adipose Tissue Reveals Regulatory Genetic Loci for Metabolic Syndrome and Related Phenotypes
- Diced Triplets Expose Neurons to RISC
- The Williams-Beuren Syndrome—A Window into Genetic Variants Leading to the Development of Cardiovascular Disease
- The Empirical Power of Rare Variant Association Methods: Results from Sanger Sequencing in 1,998 Individuals
- Systematic Detection of Epistatic Interactions Based on Allele Pair Frequencies
- Familial Identification: Population Structure and Relationship Distinguishability
- Raf1 Is a DCAF for the Rik1 DDB1-Like Protein and Has Separable Roles in siRNA Generation and Chromatin Modification
- Loss of Function of the Cik1/Kar3 Motor Complex Results in Chromosomes with Syntelic Attachment That Are Sensed by the Tension Checkpoint
- Computational Prediction and Molecular Characterization of an Oomycete Effector and the Cognate Resistance Gene
- The Dynamics and Prognostic Potential of DNA Methylation Changes at Stem Cell Gene Loci in Women's Cancer
- GTPase Activity and Neuronal Toxicity of Parkinson's Disease–Associated LRRK2 Is Regulated by ArfGAP1
- Evaluation of the Role of Functional Constraints on the Integrity of an Ultraconserved Region in the Genus
- Neurophysiological Defects and Neuronal Gene Deregulation in Mutants
- Genetic and Functional Analyses of Mutations Suggest a Multiple Hit Model of Autism Spectrum Disorders
- Negative Supercoiling Creates Single-Stranded Patches of DNA That Are Substrates for AID–Mediated Mutagenesis
- Rewiring of PDZ Domain-Ligand Interaction Network Contributed to Eukaryotic Evolution
- The Eph Receptor Activates NCK and N-WASP, and Inhibits Ena/VASP to Regulate Growth Cone Dynamics during Axon Guidance
- Repression of a Potassium Channel by Nuclear Hormone Receptor and TGF-β Signaling Modulates Insulin Signaling in
- The Retrohoming of Linear Group II Intron RNAs in Occurs by Both DNA Ligase 4–Dependent and –Independent Mechanisms
- Cell Lineage Analysis of the Mammalian Female Germline
- Association of a Functional Variant in the Wnt Co-Receptor with Early Onset Ileal Crohn's Disease
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Gene Expression and Stress Response Mediated by the Epigenetic Regulation of a Transposable Element Small RNA
- Contrasting Properties of Gene-Specific Regulatory, Coding, and Copy Number Mutations in : Frequency, Effects, and Dominance
- Homeobox Genes Critically Regulate Embryo Implantation by Controlling Paracrine Signaling between Uterine Stroma and Epithelium
- Nondisjunction of a Single Chromosome Leads to Breakage and Activation of DNA Damage Checkpoint in G2
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



