-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
The Williams-Beuren Syndrome—A Window into Genetic Variants Leading to the Development of Cardiovascular Disease
article has not abstract
Published in the journal: The Williams-Beuren Syndrome—A Window into Genetic Variants Leading to the Development of Cardiovascular Disease. PLoS Genet 8(2): e32767. doi:10.1371/journal.pgen.1002479
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1002479Summary
article has not abstract
Background
Williams-Beuren Syndrome (WBS) arises when there is a genomic microdeletion at human chromosome 7q11.23 (Mouse 5G2), resulting in various cardiovascular, developmental, metabolic, and mental disorders [1]. Cardiovascular complications from WBS are a frequent cause of death. The deleted region is predisposed to non-allelic homologous recombination (NAHR) due to the presence of repetitive DNA regions called segmental duplications. WBS can result in deletions of up to 1.83 Mb in a region containing roughly 28 genes that includes the gene ELN encoding the tissue structural protein elastin [2]. Many of the cardiovascular features of WBS can be partially explained by elastin defects. WBS individuals have a combined prevalence of cardiovascular disease of 84% that includes supra - and sub-aortic stenosis (SVAS); aortic, pulmonary, and mitral valvular disease; aortic coarctation; hypertension; and, less commonly, myocardial infarction [2], [3]. These defects have also been found in mouse models [4]. Elastin not only provides “elastic” support for vessels, it also serves as a negative regulator for smooth muscle cell proliferation. WBS patients are also at high risk for hypertension and Eln+/− mice are hypertensive [1], [4]. In 2006, Del Campo et al. recognized that hemizygosity of the NCF1 gene as a result of the largest recognized WBS microdeletion—about 1.83 Mb—decreases the risk for hypertension in WBS patients compared to those possessing the more common smaller deletion (1.55 Mb) not incorporating NCF1 (Figure 1, top) [5]. NCF1 codes for p47phox, a critical subunit in the assembly of NADPH oxidase (NOX); homozygous deficiency accounts for 20% of patients with chronic granulomatous disease, a disorder associated with repeated infections due to an inability to kill bacteria. p47phox is a major effector of angiotensin II (AngII), as demonstrated by a lack of elevation in blood pressure of Ncf1−/− mice [6]. In this issue of PLoS Genetics, Campuzano and colleagues replicate the WBS cardiovascular phenotype in a WBS mouse model with and without a deletion of the Ncf1 gene [7]. Their study has broad implications for our understanding of hypertension - and reactive oxygen species (ROS)-related cardiovascular disease.
Fig. 1. Genotype and phenotype of human and murine Williams-Beuren Syndrome. 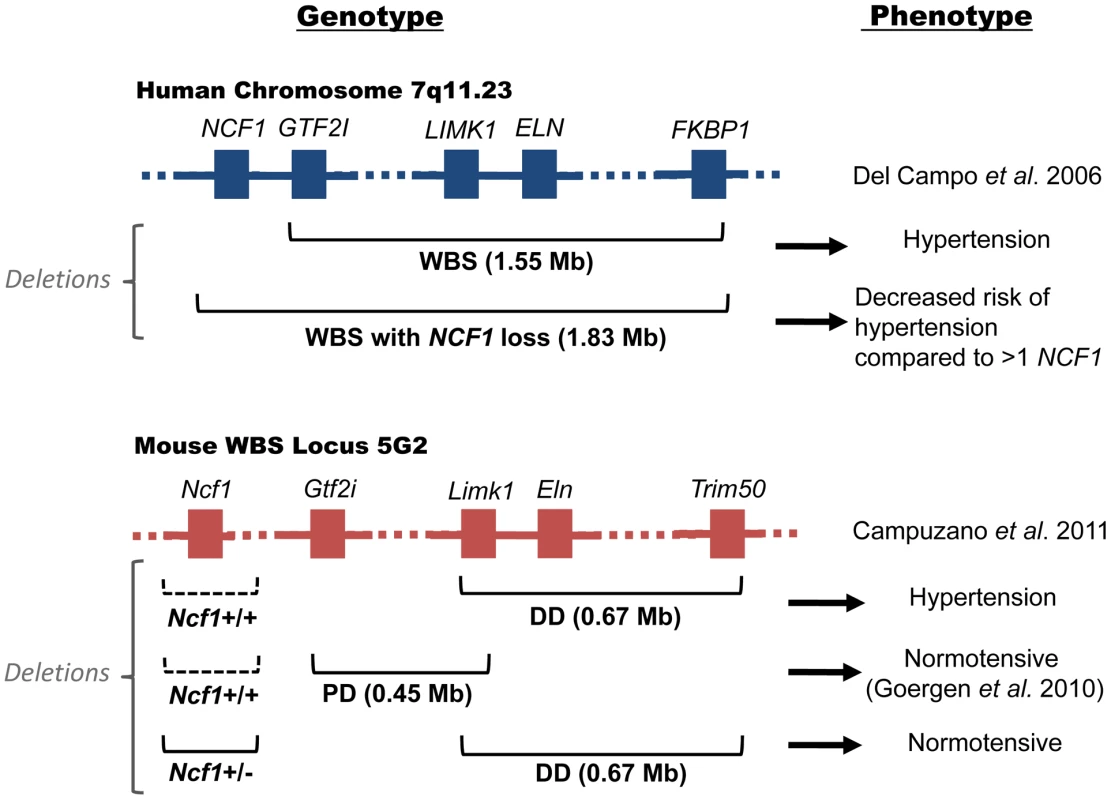
Top: human WBS locus; Bottom: murine WBS locus; Left: genotypes; Right: phenotypes. In the human WBS locus, deletion of ELN is associated with hypertension. WBS patients with deletions incorporating loss of NCF1 (1.83 Mb) are at lower risk for hypertension compared to patients with the more common WBS deletion (1.55 Mb). In the murine WBS locus, the DD mouse with a 0.67 Mb deletion containing Eln is hypertensive. It contains both Ncf1 alleles (dotted lines indicate that the gene is present). Mice with an adjacent PD deletion are normotensive [4]. Hypertension in the DD mouse is corrected when mated with Ncf1+/− mice. A Genetic Basis for Hypertension in WBS
The investigation of Campuzano et al. [7] determined whether oxidative stress is a feature of WBS mice and whether genetic or pharmacologic reduction of NOX activity reduces oxidative stress and ameliorates hypertension associated with these mice. Vascular NOX production of superoxide has an important role in various cardiovascular pathologies, including hypertension. Superoxide antagonizes the vasculo-protective molecule nitric oxide (NO) either through direct interaction with NO or through oxidation of the endothelial NO-synthase (eNOS) enzyme co-factor tetrahydrobiopterin. Campuzano et al. observed that WBS mice called “DD”, with a 0.67 Mb deletion from Limk1 to Trim50 (that contains Eln), manifest a cardiovascular phenotype including hypertension with elevated angiotensinogen (Agt), renin (Ren), and angiotensin converting enzyme (Ace) mRNA throughout life (Figure 1, bottom) [7]. Another WBS syndrome mouse called “PD” with a 0.45 Mb deletion from Gtf2i to Limk1 (not containing Eln) is normotensive (Figure 1, bottom) [4]. These data suggest that in WBS the absence of the elastin gene is necessary for hypertension. However, the DD condition was reversed when the mice were mated with Ncf1+/− mice, indicating that the presence of the Ncf1 gene also contributes to the observed hypertension. As predicted, AngII levels in the DD/Ncf1+/− mice were reduced with respect to the DD mice alone, but elevated with respect to wild type mice. These combined studies indicate that both the Eln gene deletion and the Ncf1 gene presence contribute to observed murine hypertension. It is of note that vascular pathologies such as supravalvular aortic stenosis were also corrected in DD mice partially depleted of the Ncf1 gene.
Role of ROS in Hypertension
In addition to elevated AngII levels, DD mice have elevated protein nitrosylation and superoxide anions as determined by dihydroethidium fluorescence in the ascending aorta. In contrast, DD/Ncf1+/− mice have reduced ROS in their aorta. These observations are consistent with other rodent hypertensive phenotypes [8], [9]. The fact that genetic reduction of ROS-producing mechanisms occurred in the DD/Ncf1+/− mice suggested that pharmacologic treatment to reduce ROS may also be helpful in treating DD mouse hypertension. Treatment of DD mice with the broad antioxidant apocynin and losartan (an angiotensin receptor type 1 antagonist) independently lowered blood pressure in DD mice and, together, their effect was additive. Blood pressure control was associated with reduced vessel ROS and lowered plasma AngII levels. Further, the anti-ROS therapies reduced the degree of anatomical changes in cardiac hypertrophy and vascular elastic fiber fragmentation. These pharmacologic studies suggest an alternative approach to treating the hypertensive phenotype in WBS patients. Currently, the most common treatments for WBS-induced hypertension are beta-blockers and calcium channel blockers. Using the present WBS mouse studies as an insight, the combined use of a specific antioxidant with losartan appears mechanistically more rational.
Use of Antioxidants in Hypertension
The use of antioxidants for the treatment of vascular oxidant stress associated with cardiovascular disease has been questioned after failed clinical trials using non-specific antioxidants such as vitamins C and E [10]. Recognizing specific etiologies of superoxide in hypertension suggests that past antioxidant clinical trials were not well targeted to superoxide or hydrogen peroxide. To date, ROS-specific treatments such as the superoxide dismutase mimetic tempol have not been used in clinical trials. Tempol has been partially successful in animal models to treat hypertension [8], [11], [12]. When tempol is tagged with a mitochondrial-specific tag, the drug mitoTEMPO has been used to successfully treat hypertension in two rodent models [8], [9]. Campuzano et al. [7] also used the antioxidant apocynin to treat hypertension and both pre - and post-natal SVAS in the WBS mouse model. Although the specificity of apocynin for NOX is controversial, when it is delivered clinically by inhalation, it lowers an exhaled breath marker for ROS and increases a nitric oxide marker [13], [14].
ROS in Thrombosis
In addition to hypertension and developmental structural defects in the cardiovascular system, increased vascular ROS may increase arterial thrombosis risk. Several animal models have been associated with increased vascular ROS and higher arterial thrombosis risk. Both heme oxygenase I–deleted mice and prolylcarboxypeptidase-deficient mice have increased vascular ROS and reduced arterial thrombosis occlusion times [9], . Vascular ROS is associated with uncoupled eNOS, inactivated thrombomodulin, and increased endothelial cell tissue factor and plasminogen activator inhibitor 1 [9], [16]. In the case of prolylcarboxypeptidase deficiency, in vivo treatment with apocynin or tempol resulted in correction of thrombosis risk and reduction in tissue ROS. Myocardial infarction and stroke are uncommon in WBS patients, probably due to the overriding risk posed by their severe cardiovascular developmental abnormalities. However, we would predict that the DD mice would be prothrombotic on ROS-generating thrombosis models, and specific antioxidants to superoxide or hydrogen peroxide would reduce any thrombosis risk, in addition to ameliorating hypertension.
Future Directions
The studies by Campuzano et al. [7] on WBS show a double-hit genetic basis for ROS - and hypertension-related cardiovascular disease. They indicate that both pharmacologic and genetic targets could be used to manage the specific manifestations of WBS, and hypertension in general. Further, we believe that these approaches may also be useful in reducing arterial thrombosis risk in susceptible populations.
Zdroje
1. PoberBR 2010 Williams-Beuren syndrome. N Engl J Med 362 239 252
2. PoberBRJohnsonMUrbanZ 2008 Mechanisms and treatment of cardiovascular disease in Williams-Beuren Syndrome. J Clin Invest 118 1606 1615
3. Del PasquaARinelliGToscanoAIacobelliRDigilioC 2009 New findings concerning cardiovascular manifestations emerging from long-term follow-up of 150 patients with the Williams-Beuren-Beuren Syndrome. Cardiol Young 19 563 567
4. GoergenCJLiHHFranckeUTaylorCA 2011 Induced chromosome deletion in a Williams-Beuren Syndrome mouse model causes cardiovascular sbnormalities. J Vasc Res 48 119 129
5. Del CampoMAntonellAMaganoLFMuñozFJFloresR 2006 Hemizygosity at the NCF1 gene in patients with Williams-Beuren syndrome decreases their risk of hypertension. Am J Hum Genet 78 533 542
6. LandmesserUSpiekermannSDikalovSTatgeHWilkeR 2002 Vascular oxidative stress and endothelial dysfunction in patients with chronic heart failure: role of xanthine-oxidase and extracellular superoxide dismutase. Circulation 106 3073 3078
7. CampuzanoVSeguraMTerradoVSánchez-RodríguezCCoustestM 2012 Reduction of NADPH-oxidase activity ameliorates the cardiovascular phenotype in a mouse model of Williams-Beuren syndrome. PLoS Genet 8 e1002458 doi:10.1371/journal.pgen.1002458
8. DikalovaAEBikineyevaATBudzynKNazarewiczRRMcCannL 2010 Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res 107 106 116
9. AdamsGNLaRuschGAStavrouEZhouYNiemanMT 2011 Murine prolylcarboxypeptidase depletion induces vascular dysfunction with hypertension and faster arterial thrombosis. Blood 117 3929 3937
10. Kris-EtheronPMLichtensteinAHHowardBVSteinbergDWiztumJL 2004 Antioxidant vitamin supplements and cardiovascular disease. Circulation 110 637 641
11. SimonsenURodriguez-RodriguezRDalsgaardTBuusNHStankeviciusE 2009 Novel approaches to improving endothelium-dependent nitric oxide-mediated vasodilation. Pharmacol Rep 61 105 115
12. HoffmannDSWeydertCJLazartiguesEKutschekeWJKienzleMF 2008 Chronic tempol prevents hypertension, proteinuria, and poor feto-placental outcomes in BPH/5 mouse model of preeclampsia. Hypertension 51 1058 1065
13. HeumüllerSWindSBarbosa-SicardESchmidtHHBusseR 2008 Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51 211 217
14. StefanskaJSokolowskaMSarniakAWlodarczykADoniecZ 2010 Apocynin decreases hydrogen peroxide and nitrate concentrations in exhaled breath in healthy subjects. Pulm Pharmacol Ther 23 48 54
15. TrueALOliveMBoehmMSanHWestrickRJ 2007 Heme oxygenase-1 deficiency accelerates formation of arterial thrombosis through oxidative damage to the endothelium, which is rescued by inhaled carbon monoxide. Circ Res 101 893 901
16. GlaserCBMorserJClarkeJHBlaskoEMcLeanK 1992 Oxidation of a specific methionine in thrombomodulin by activated neutrophil products blocks cofactor activity. J Clin Invest 90 2565 2573
Štítky
Genetika Reprodukčná medicína
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2012 Číslo 2- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Upsetting the Dogma: Germline Selection in Human Males
- A Strong Deletion Bias in Nonallelic Gene Conversion
- Positive Selection for New Disease Mutations in the Human Germline: Evidence from the Heritable Cancer Syndrome Multiple Endocrine Neoplasia Type 2B
- Genome-Wide Association Study in East Asians Identifies Novel Susceptibility Loci for Breast Cancer
- Mixed Effects Modeling of Proliferation Rates in Cell-Based Models: Consequence for Pharmacogenomics and Cancer
- Reduction of NADPH-Oxidase Activity Ameliorates the Cardiovascular Phenotype in a Mouse Model of Williams-Beuren Syndrome
- Genome-Wide Association Study Identifies Chromosome 10q24.32 Variants Associated with Arsenic Metabolism and Toxicity Phenotypes in Bangladesh
- Structural Basis of Transcriptional Gene Silencing Mediated by MOM1
- Genomic Restructuring in the Tasmanian Devil Facial Tumour: Chromosome Painting and Gene Mapping Provide Clues to Evolution of a Transmissible Tumour
- Genome-Wide Association Study Identifies Novel Loci Associated with Circulating Phospho- and Sphingolipid Concentrations
- Contrasting Properties of Gene-Specific Regulatory, Coding, and Copy Number Mutations in : Frequency, Effects, and Dominance
- The Origin and Nature of Tightly Clustered Deletions in Precursor B-Cell Acute Lymphoblastic Leukemia Support a Model of Multiclonal Evolution
- Ultrafast Evolution and Loss of CRISPRs Following a Host Shift in a Novel Wildlife Pathogen,
- Phosphorylation of Chromosome Core Components May Serve as Axis Marks for the Status of Chromosomal Events during Mammalian Meiosis
- Psoriasis Patients Are Enriched for Genetic Variants That Protect against HIV-1 Disease
- A Pathogenic Mechanism in Huntington's Disease Involves Small CAG-Repeated RNAs with Neurotoxic Activity
- The Mitochondrial Chaperone Protein TRAP1 Mitigates α-Synuclein Toxicity
- Homeobox Genes Critically Regulate Embryo Implantation by Controlling Paracrine Signaling between Uterine Stroma and Epithelium
- Developmental Transcriptional Networks Are Required to Maintain Neuronal Subtype Identity in the Mature Nervous System
- Down-Regulating Sphingolipid Synthesis Increases Yeast Lifespan
- Gene Expression and Stress Response Mediated by the Epigenetic Regulation of a Transposable Element Small RNA
- Loss of Tgif Function Causes Holoprosencephaly by Disrupting the Shh Signaling Pathway
- Sequestration of Highly Expressed mRNAs in Cytoplasmic Granules, P-Bodies, and Stress Granules Enhances Cell Viability
- Discovery of a Modified Tetrapolar Sexual Cycle in and the Evolution of in the Species Complex
- The Role of Glypicans in Wnt Inhibitory Factor-1 Activity and the Structural Basis of Wif1's Effects on Wnt and Hedgehog Signaling
- Nondisjunction of a Single Chromosome Leads to Breakage and Activation of DNA Damage Checkpoint in G2
- A Regulatory Network for Coordinated Flower Maturation
- Coexpression Network Analysis in Abdominal and Gluteal Adipose Tissue Reveals Regulatory Genetic Loci for Metabolic Syndrome and Related Phenotypes
- Diced Triplets Expose Neurons to RISC
- The Williams-Beuren Syndrome—A Window into Genetic Variants Leading to the Development of Cardiovascular Disease
- The Empirical Power of Rare Variant Association Methods: Results from Sanger Sequencing in 1,998 Individuals
- Systematic Detection of Epistatic Interactions Based on Allele Pair Frequencies
- Familial Identification: Population Structure and Relationship Distinguishability
- Raf1 Is a DCAF for the Rik1 DDB1-Like Protein and Has Separable Roles in siRNA Generation and Chromatin Modification
- Loss of Function of the Cik1/Kar3 Motor Complex Results in Chromosomes with Syntelic Attachment That Are Sensed by the Tension Checkpoint
- Computational Prediction and Molecular Characterization of an Oomycete Effector and the Cognate Resistance Gene
- The Dynamics and Prognostic Potential of DNA Methylation Changes at Stem Cell Gene Loci in Women's Cancer
- GTPase Activity and Neuronal Toxicity of Parkinson's Disease–Associated LRRK2 Is Regulated by ArfGAP1
- Evaluation of the Role of Functional Constraints on the Integrity of an Ultraconserved Region in the Genus
- Neurophysiological Defects and Neuronal Gene Deregulation in Mutants
- Genetic and Functional Analyses of Mutations Suggest a Multiple Hit Model of Autism Spectrum Disorders
- Negative Supercoiling Creates Single-Stranded Patches of DNA That Are Substrates for AID–Mediated Mutagenesis
- Rewiring of PDZ Domain-Ligand Interaction Network Contributed to Eukaryotic Evolution
- The Eph Receptor Activates NCK and N-WASP, and Inhibits Ena/VASP to Regulate Growth Cone Dynamics during Axon Guidance
- Repression of a Potassium Channel by Nuclear Hormone Receptor and TGF-β Signaling Modulates Insulin Signaling in
- The Retrohoming of Linear Group II Intron RNAs in Occurs by Both DNA Ligase 4–Dependent and –Independent Mechanisms
- Cell Lineage Analysis of the Mammalian Female Germline
- Association of a Functional Variant in the Wnt Co-Receptor with Early Onset Ileal Crohn's Disease
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- Gene Expression and Stress Response Mediated by the Epigenetic Regulation of a Transposable Element Small RNA
- Contrasting Properties of Gene-Specific Regulatory, Coding, and Copy Number Mutations in : Frequency, Effects, and Dominance
- Homeobox Genes Critically Regulate Embryo Implantation by Controlling Paracrine Signaling between Uterine Stroma and Epithelium
- Nondisjunction of a Single Chromosome Leads to Breakage and Activation of DNA Damage Checkpoint in G2
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



