-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Age-Dependent Brain Gene Expression and Copy Number Anomalies in Autism Suggest Distinct Pathological Processes at Young Versus Mature Ages
Autism is a highly heritable neurodevelopmental disorder, yet the genetic underpinnings of the disorder are largely unknown. Aberrant brain overgrowth is a well-replicated observation in the autism literature; but association, linkage, and expression studies have not identified genetic factors that explain this trajectory. Few studies have had sufficient statistical power to investigate whole-genome gene expression and genotypic variation in the autistic brain, especially in regions that display the greatest growth abnormality. Previous functional genomic studies have identified possible alterations in transcript levels of genes related to neurodevelopment and immune function. Thus, there is a need for genetic studies involving key brain regions to replicate these findings and solidify the role of particular functional pathways in autism pathogenesis. We therefore sought to identify abnormal brain gene expression patterns via whole-genome analysis of mRNA levels and copy number variations (CNVs) in autistic and control postmortem brain samples. We focused on prefrontal cortex tissue where excess neuron numbers and cortical overgrowth are pronounced in the majority of autism cases. We found evidence for dysregulation in pathways governing cell number, cortical patterning, and differentiation in young autistic prefrontal cortex. In contrast, adult autistic prefrontal cortex showed dysregulation of signaling and repair pathways. Genes regulating cell cycle also exhibited autism-specific CNVs in DNA derived from prefrontal cortex, and these genes were significantly associated with autism in genome-wide association study datasets. Our results suggest that CNVs and age-dependent gene expression changes in autism may reflect distinct pathological processes in the developing versus the mature autistic prefrontal cortex. Our results raise the hypothesis that genetic dysregulation in the developing brain leads to abnormal regional patterning, excess prefrontal neurons, cortical overgrowth, and neural dysfunction in autism.
Published in the journal: Age-Dependent Brain Gene Expression and Copy Number Anomalies in Autism Suggest Distinct Pathological Processes at Young Versus Mature Ages. PLoS Genet 8(3): e32767. doi:10.1371/journal.pgen.1002592
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002592Summary
Autism is a highly heritable neurodevelopmental disorder, yet the genetic underpinnings of the disorder are largely unknown. Aberrant brain overgrowth is a well-replicated observation in the autism literature; but association, linkage, and expression studies have not identified genetic factors that explain this trajectory. Few studies have had sufficient statistical power to investigate whole-genome gene expression and genotypic variation in the autistic brain, especially in regions that display the greatest growth abnormality. Previous functional genomic studies have identified possible alterations in transcript levels of genes related to neurodevelopment and immune function. Thus, there is a need for genetic studies involving key brain regions to replicate these findings and solidify the role of particular functional pathways in autism pathogenesis. We therefore sought to identify abnormal brain gene expression patterns via whole-genome analysis of mRNA levels and copy number variations (CNVs) in autistic and control postmortem brain samples. We focused on prefrontal cortex tissue where excess neuron numbers and cortical overgrowth are pronounced in the majority of autism cases. We found evidence for dysregulation in pathways governing cell number, cortical patterning, and differentiation in young autistic prefrontal cortex. In contrast, adult autistic prefrontal cortex showed dysregulation of signaling and repair pathways. Genes regulating cell cycle also exhibited autism-specific CNVs in DNA derived from prefrontal cortex, and these genes were significantly associated with autism in genome-wide association study datasets. Our results suggest that CNVs and age-dependent gene expression changes in autism may reflect distinct pathological processes in the developing versus the mature autistic prefrontal cortex. Our results raise the hypothesis that genetic dysregulation in the developing brain leads to abnormal regional patterning, excess prefrontal neurons, cortical overgrowth, and neural dysfunction in autism.
Introduction
Clinical and subclinical manifestations of autism begin during the first years of life [1]. Neuroimaging studies of living infants and young children with autism have revealed abnormal brain overgrowth in the majority of cases, particularly in prefrontal, temporal and amygdala regions [2]–[13]. These studies have also shown abnormal functional asymmetry and activation in the cortex and cerebellum [14]–[16]. Children with autism have 67% excess neuron numbers in the prefrontal cortex, a substantial pathology that points to disruption of early developmental mechanisms that govern neuron numbers, and an 18% increase in brain weight at autopsy [17]. However, by late adolescence and early adulthood, the autistic brain commonly displays neuron loss and cortical thinning and is no longer enlarged [2], [8], [13], [18]. Therefore, the underlying developmental molecular defects in the majority of autistic cases seem likely to be most evident at younger rather than older ages.
The average age of individuals studied in more than fifty postmortem analyses of the autistic brain is 22 years [19], making it difficult if not impossible to comprehensively assess early developmental molecular pathologies associated with autism. In studies combining both young and adult autistic brain tissue, transcript and protein expression patterns suggest that cortical patterning, synaptic [20], apoptotic [21], immune [20], [22], and inflammatory [23] aberrances in addition to dysregulation of neurotransmitter systems [24] may be involved in autism. None of these molecular pathologies at older ages, however, can explain the 67% excess prefrontal neuron numbers and brain enlargement at younger ages in autism.
Thus, there seems to be a gap between neuroanatomical and cellular abnormalities reported for autism at younger ages and molecular pathologies in autism at older ages. Molecular pathologies specifically present at younger ages in autism are largely unknown and unexplored. Also, whether some molecular abnormalities detected in the older autistic brain reflect conditions unique to the older brain and whether others are common to both younger and older autistic brains are unknown. Genome-wide analyses of the genes, pathways and processes that exhibit dysregulation specifically in the young autistic brain have not been pursued to a great degree. This is particularly true for the prefrontal and temporal cortex, where abnormalities of growth and function during early development are pronounced and likely to contribute significantly to social, communication, language and emotional deficits associated with autism. Additionally, although copy number variation (CNV) has been hypothesized to play an important role in the pathogenesis of autism [25], [26] and may underlie important differences in gene expression, CNVs identified in brain tissue from individuals with autism have not been examined in relation to aberrant brain gene expression from the same tissue samples.
We examined the linked hypotheses that underlying neuroanatomical differences between younger and adult individuals with autism are a result of differences in molecular pathology. We hypothesize that some molecular pathologies at younger ages in autism may provide insight into the very early neural developmental processes that lead to the disorder. To do so, we identified abnormal genetic pathways in the young autistic brain, determined expression patterns that distinguished the young from the adult autistic brain and identified evidence for gene expression dysregulation that is age-independent using genome-wide expression and genotyping techniques. We found age-dependent gene expression differences between young and adult autistic brains as well as rare and common genetic variants associated with autism that have important roles in neurodevelopment.
Results
First, we analyzed genome-wide expression of 57 frozen samples of dorsolateral prefrontal cortex (DLPFC, BA 9/46; Table S1) using a DASL-based platform on the Illumina Human-Ref8 v3 microarray [27], [28]. Thirty-three samples in total passed quality control measures (Text S1): 16 of these samples were from young postmortem males (2–14 y; autism = 9, control = 7) and 17 were from adult males (15–56 y, autism = 6, control = 11). Though RNA Integrity Numbers (RIN) are not predictive of array quality [29], multiple RIN assessments were used to determine RNA quality (Table S1). We found no significant differences between the RINs of the autism and control groups (t = 0.16, DF = 20, p = 0.87).
Two-way analysis of variance (ANOVA) and post-hoc pair-wise ANOVA-based comparisons were performed to identify genes exhibiting expression differences in young autistic vs. young control DLPFC compared to adult autistic vs. adult control DLPFC. An overall ANOVA-based F-test p-value<0.05 [empirical false discovery rate (FDR), not threshold, FDR = 0.27] was used to identify genes showing an effect of interaction between age and diagnosis for further analyses. From the two-way ANOVA results, we attempted to tease out expression differences between: 1) young autism, 2) young control, 3) adult autism and 4) adult control groups.
Of the genes exhibiting an effect of interaction from ANOVA-based F-tests with p-values<0.05, 102 genes resulted in ANOVA-based post-hoc contrasts between young autistic and control groups with p-value<0.05 (Figure S1). Seven hundred thirty six genes resulted in an ANOVA-based post-hoc contrast between adult autistic and control cases (p-value<0.05). Finally, an overall ANOVA-based F-test p-value<0.05 (empirical FDR = 0.13) was performed to assess the main effect of diagnosis across all autistic and control cases independent of age. Two thousand seventeen genes resulted in this contrast with p-value<0.05. Genes common between the three comparisons are shown in Figure S1.
Genes exhibiting a diagnosis main effect and a post-hoc pairwise contrast (e.g., autism vs. control, adult vs. young; p-value<0.05) were further subjected to enrichment analyses in the MetaCore software suite (MetaCore from GeneGo Inc.) as well as the online enrichment program DAVID [30], [31]. By focusing on genes exhibiting a diagnosis main effect, we explored pathological mRNA expression in autism irrespective of age. qPCR validation verified expression differences on a subset of the differentially expressed genes (Figure S2).
Genes regulating cell number, genetic integrity, and neural patterning are dysregulated in young autistic cases
Since early brain overgrowth and the clinical onset of autistic symptoms occur at young ages in autism, we focused on unique gene expression differences between autistic and control cases below the age of 14 years to identify genes that may be dysregulated early in autism pathogenesis. One hundred two genes were differentially expressed in young autistic cases compared with the young control group and exhibited a diagnosis x age group interaction effect (p-value<0.05, empirical FDR = 0.27; Table S2). Many of these genes were previously identified autism candidate loci based on the literature (Table S3).
Pathway enrichment analyses of these 102 genes via the MetaCore software suite (defining an enriched pathway as having an enrichment p-value<0.05 and empirical FDR<0.1) suggested that DNA damage-response, cell cycle and apoptosis-related MetaCore pathways (‘M-Pathways’) were significantly altered (Table 1, Young autism versus young control map folders). Key players such as BRCA1 and CHK2 were downregulated. Most significantly dysregulated M-Pathways in this category included the DNA-damage-induced response and role of NFBD1 in DNA damage response. A Development-Neurogenesis Process Network, Development-Neurogenesis (p = 1.09E-03, 6/192 network objects), was also significantly altered.
Tab. 1. MetaCore Pathway Map Folders (left) and M-Pathways (right) in three ANOVA-based analyses. 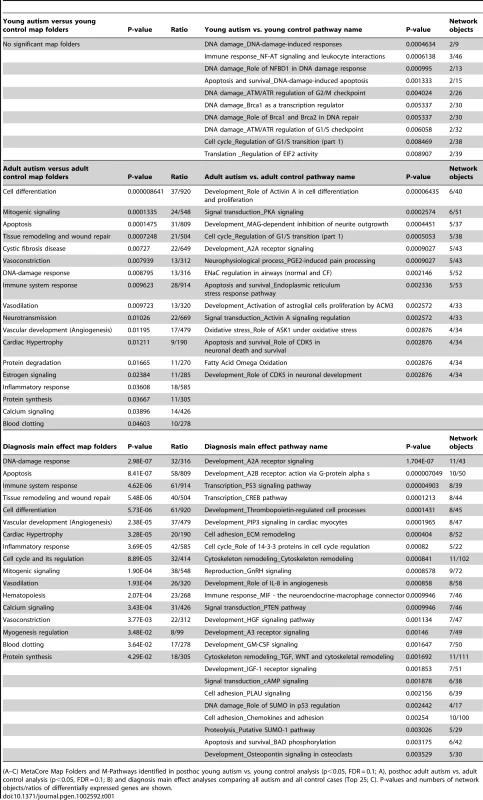
(A–C) MetaCore Map Folders and M-Pathways identified in posthoc young autism vs. young control analysis (p<0.05, FDR = 0.1; A), posthoc adult autism vs. adult control analysis (p<0.05, FDR = 0.1; B) and diagnosis main effect analyses comparing all autism and all control cases (Top 25; C). P-values and numbers of network objects/ratios of differentially expressed genes are shown. We additionally annotated all 102 genes using DAVID and identified overlapping sets of 12, 19, 7 and 16 genes involved in DNA damage/cell cycle, apoptosis, immune signaling and neurogenesis and neural development (Figure 1), respectively. Of the 7 immune response genes, 4 (FAS, BCL3, GREM1 and FOSL2) were also found to contribute to apoptosis. Neurogenesis and neural development pathways included the WNT pathway and were driven by dysregulation of the WNT3 gene. WNT pathway genes are known to regulate cell proliferation, cell fate and patterning during embryogenesis [32]. Downstream components of the WNT pathway, such as Dvl1, are also known to regulate social behavior in mouse models [33]. In addition, we found significant downregulation of genes involved in neural patterning and differentiation, such as FGF1, HOXD1, NDE1, NODAL, PCSK6 and GREM1 (Figure 1, Table S2).
Fig. 1. Dysregulated gene expression in developmental M-Pathways and Process Networks in young autistic prefrontal cortex. 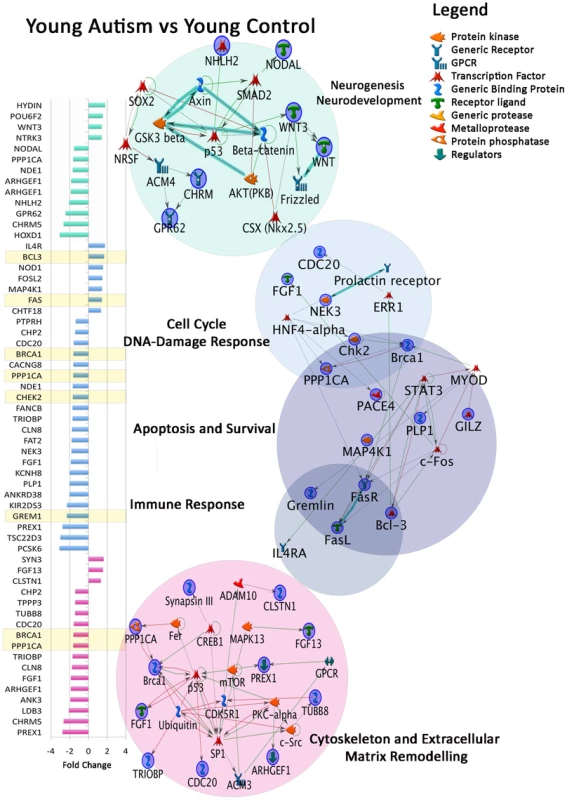
From differentially expressed genes of young autistic vs. young control cases (Table S2), networks were created using MetaCore Network Analysis. Colors on fold change graph correspond to circles on the right depicting network maps in each category. Yellow = differentially expressed genes in two or more functional domains. Overlapping circles = differentially expressed genes common to two domains. Expression anomalies of genes involved in DNA damage, cell cycle and apoptosis may contribute to abnormal brain growth by increasing production or reducing elimination of neurons during development. Dysregulated neural patterning and differentiation genes may lead to abnormal cellular organization and cytoarchitecture. For example, HOX and DLX family genes play important roles in vertebrate patterning and are important for neuronal subtype differentiation [34]. Moreover, NODAL controls dorsal mesoderm induction, anterior patterning and initiation of left-right asymmetry during gastrulation [35]. These findings suggest that in the young autistic brain, genetic regulation of cell number, genetic integrity and neural patterning is disturbed.
Genes regulating signaling, repair, and response pathways are dysregulated in adult autistic cases
As a complement to the analyses of the young autistic brain, we compared the genes dysregulated in young autistic cases to those dysregulated in adult autistic cases. We identified genes using the same ANOVA diagnosis x age interaction effect p-value criterion but with emphasis on genes differentially expressed in adult autistic brains relative to adult control brains (defined as cases ≥15 years of age). Seven hundred thirty six genes were differentially expressed based on this analysis (Figure S1, Table S4). These genes were also analyzed with MetaCore for functional enrichment. The 3 most significant MetaCore Map Folders (‘Map Folders’: a label MetaCore provides to sets of genes with an overarching function of pathway participation) included cell differentiation, mitogenic signaling and apoptosis genes (Table 1, Adult autism versus adult control map folders, Figure 2A; p<0.05, FDR<0.1). Dysregulated pathways specific for the adult brain included multiple signaling and remodeling functions in neurons and glia (Table 1, Adult autism versus adult control map folders). M-Pathway categories that were dysregulated in adults but not young cases included development, signaling and oxidative stress pathways (Table 1, Figure 2B).
Fig. 2. Dysregulated gene expression in top three Map Folders in adult autistic prefrontal cortex and differentially affected M-Pathways of young and adult autistic cases. 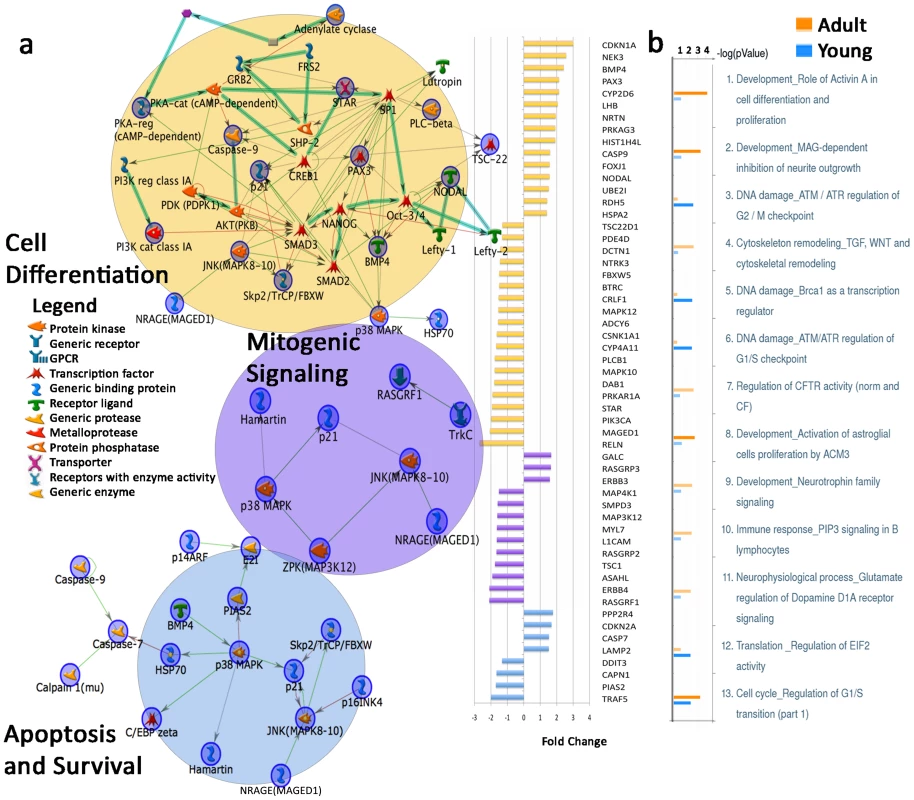
(A) Graph on the right shows fold change of genes in cell differentiation, mitogenic signaling, and apoptosis and survival Map Folders. Colors on fold change graph correspond to circles on the left depicting network maps in each category. From each category of differentially expressed genes in adult autistic vs. adult control posthoc comparison (Table S4), gene networks on the left were created using MetaCore Network Analysis. (B) Top differentially affected M-pathway comparisons of dysregulated genes between adult and young autistic cases. Bars represent significance of listed pathways. Orange = adult; blue = young; faded color = FDR>0.1 or p>0.05. The Cell Differentiation Map Folder included significantly dysregulated genes RELN, BTRC, BMP4, MAPK10 and NTRK3 (Figure 2A). This Map Folder also included suggested dysregulation of the ‘Activin A in Cell Differentiation and Proliferation’ pathway, which involved the genes LHB, NODAL, STAR, CDKN1A, PRKAR1A and ADCY6. Genes playing multiple functions in all three top map folders included MAPK12, CDKN1A, NTRK3, PRKAR1A, PIK3CA, CASP9, MAPK10, ADCY6 and MAGED1 (Figure 2A). Notably, Tissue Remodeling and Wound Repair-related genes also exhibited dysregulation in adult autistic cases (Table 1, Adult autism versus adult control map folders).
These analyses suggest that in the adult autistic brain, cell differentiation, mitogenic, apoptotic and remodeling and repair functions could be components of recovery responses, in accordance with previous reports [22], [23], [36]. They could, however, also be signatures of ongoing reparatory neurogenesis processes [37]. For example, the Activin A signaling pathway is expressed by neurons following injury and is essential for adult neurogenesis [38], [39]. BTRC inhibits the beta-catenin (CTNNB1) pathway [40], which in adult animals is upregulated after insults such as seizures [41] and promotes adult neurogenesis [42]. Furthermore, BMP4 expressed in adult subventricular zones serves to inhibit neurogenesis [43]. Thus, it appears that aberrant signaling and repair processes may distinguish adult autistic cases from young cases.
Genes important in development were dysregulated across both young and adult autistic cases
Finally, we examined genes showing a main effect of diagnosis to identify differentially expressed between autistic and control cases independent of age (i.e., we did not confine attention to genes exhibiting diagnosis x age interaction effects or age-specific effects). More than 2000 such genes were detected based on a simple contrast between autism and control brains (p<0.05, empirical FDR = 0.13; Figure S1, Table S5). Enrichment analyses of these genes using MetaCore suggested that seventeen Map Folders were altered in all autistic brains (p<0.05, FDR = 0.1; Table 1, Diagnosis main effect map folders). The three most significant Map Folders included DNA-damage response, apoptosis and immune system response functions (Figure 3, Figure S3). In addition, the top 25 M-Pathways (Table 1, Diagnosis main effect map folders, Table S6) included cell cycle [14-3-3 (YWHAZ), CDC25A, CDCD25C, ATRX], proliferation [CTNNB1 (beta-catenin), FSHB, PRKACB, PRKCZ], apoptosis (BAD, CASP8, CASP10, MDM2), cytoskeleton and extracellular matrix remodeling (ErbB4, MMP2, NID1, TIMP1, COL4A3) and growth and development [RELN, ROBO1, ADORA2A, p21 (CDKN1A), 14-3-3, HGF, FGFRL1, TSC1] functions. Specifically, the p53 signaling pathway and the PTEN pathway were among these dysregulated M-Pathways (Table 1, Diagnosis main effect map folders).
Fig. 3. Dysregulated gene expression in top two MetaCore Map Folders in autism independent of age. 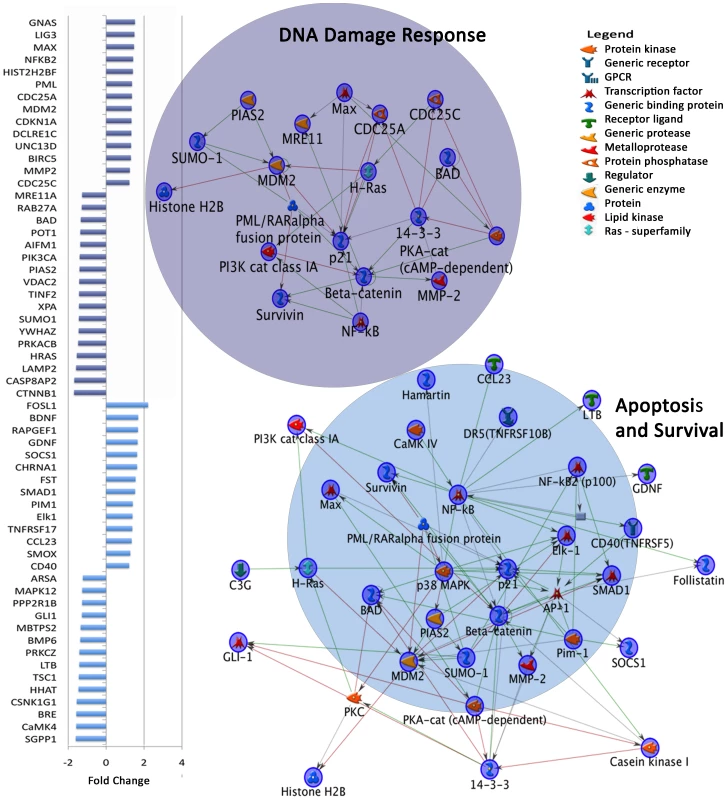
Graph on left shows fold change of genes in DNA damage response and apoptosis and survival Map Folders. The third most significant map folder is depicted in Figure S3. Colors on fold change graph correspond to circles on the left depicting network maps in each category. From each category of differentially expressed genes in the all autistic vs. all control comparison (Table S5), networks were created using MetaCore Network Analysis. A number of genes in these M-Pathways have known functions in neuronal development: PTEN signaling regulates proliferation [44] and is associated with macrocephaly in autism [45], [46]. TSC1 is mutated in tuberous sclerosis, acts downstream of PTEN in the mTOR pathway [47] and affects cortical lamination, neuronal migration and axon pathfinding [48]. CTNNB1, a key member of the WNT pathway, regulates cerebral cortical size. CTNNB1 transgenic mice have enlarged and folded cortices [49]. Furthermore, RELN is critical for human neuronal migration [50] and has been previously linked to autism [51]. Genes differentiating autistic cases from controls independent of age have important developmental, immune and cytoskeletal remodeling functions.
Finally, given the novel cytoskeletal age-independent M-Pathways identified in this analysis, we revisited the 102 significant genes identified in the young autism vs. control analysis. We found 17 cytoskeletal and matrix remodeling genes (Figure 1). These analyses support a role for cytoskeletal dysregulation in young autistic brains as well. Cytoskeletal elements have been linked to defects in neuronal migration in other neurodevelopmental disorders such as lissencephaly [52].
Comparison with a published functional genomic study shows overlap in repair and immune response genes
Due to commonalities between previously identified candidate loci and the genes we found to exhibit expression differences between young autism vs. control, we also investigated similarities between the genes we identified and those identified in a recent functional genomic study. We compared genes showing a main effect of diagnosis in the present study with differentially expressed genes implicated recently by Voineagu et al. [20].
Twenty-five probes detected as p<0.05 in comparing autism and control cases and 21,564 probes detected as p>0.05 in both studies were identified as overlapping (p<0.0001). Among the probes detected at p<0.05 were 6 genes important for Tissue Remodeling and Wound Repair (p = 1.622E-4, FDR<0.05) and 7 genes important for Immune System Response (p = 4.772E-4, FDR<0.05). Ultimately, we found some consistency between genes detected in our analyses with those of a previous functional genomic study, particularly within domains of repair and immune response.
Genetic variation may underlie cell cycle and cytoskeleton gene dysregulation
To further investigate the findings of dysregulated genetic functions in young autistic cases particularly, we determined whether CNVs in autistic cases were distinct from those in controls. We genotyped prefrontal cortex samples from 55 of the 57 total autistic and control cases in our study. After quality control, CNV enrichment was analyzed in 30 DLPFC cases from male and female autistic and control cases (Table S1) using PennCNV with GC adjustment and CNVision programs [53], [54]. Nearly all (>99%) brain-derived CNVs in autistic and control cases were deletions (Table S7, Table S8). There were no differences between autistic and control cases in numbers of CNVs, numbers of genes per CNV or average CNV size (Figure S4).
Restricting analysis to male cases, genes identified in the autistic group were enriched in cell cycle (mitosis and S phase, including BUBR1, Cyclin B, BRCA1, RAD51, CRM1, RFC2 and LIS1) and cytoskeleton (spindle and cytoplasmic microtubules, including CLIP170, Tubulin alpha, Dynein light and heavy chains, DNAL1 and ROD) GeneGO Process Networks. No significant enrichment was identified in the gene content of controls (Figure 4). Similar network enrichment was noted in male autistic cases after filtering out common, non-pathogenic CNVs (Materials and Methods), while no significant network enrichment was found in controls (Figure 4). Notably, BRCA1 expression was found to be dysregulated in the young autistic brain (Figure 1) and predicted as deleted in the CNV analysis (Table S7). Subsequent enrichment analysis of CNVs present in male and female autistic and control cases yielded similar results (Figure S4). These results are consistent with the above described gene expression results showing abnormal expression of cell cycle and developmental pathways in the autistic cases.
Fig. 4. MetaCore process networks and network maps in autism based on gene deletions located in CNVs from DLPFC, and genetic association results. 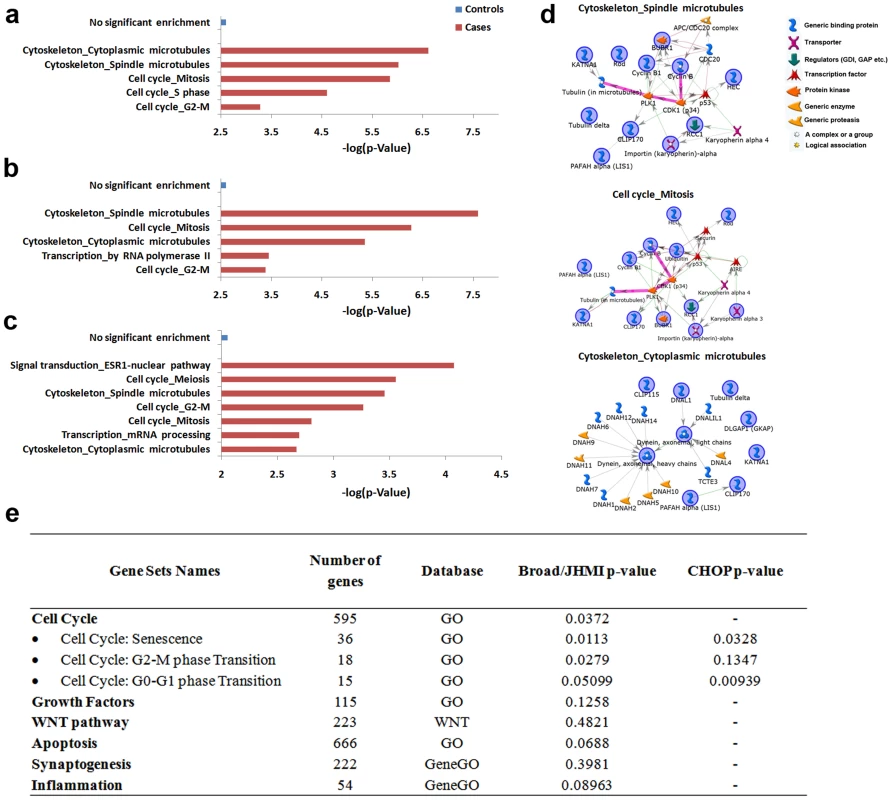
Autistic cases had significant enrichment of MetaCore process networks in genes contained within total CNVs (A) and filtered CNVs (i.e., not present in the Database of Genomic Variants) using PennCNV (B) and CNVision (C). Blue bars = controls; red bars = autistic cases. (D) MetaCore network analysis of the top three enriched MetaCore process networks in (B). Symbols in (D) represent gene types or associations. Genes with blue circles were identified autistic CNVs; genes without circles were summoned by the database to complete network. Pink lines = canonical pathway connections. (E) Gene sets tested in set-based association analysis using PLINK in Broad/JHMI and CHOP datasets, number of genes in each set, database from which genes were taken and p-values of associations. To extend these genotypic findings and specifically test whether common variants of cell cycle genes are associated with autism, we determined whether common variants in processes involved in cell cycle and other hypothesized regulatory processes were associated with autism using two previously analyzed SNP datasets: one from the Autism Genetic Resource Exchange (AGRE) and National Institutes of Mental Health genotyped at the Broad Institute and Johns Hopkins Medical Institute (Broad/JHMI) and another from AGRE genotyped at the Children's Hospital of Philadelphia (CHOP) [55], [56]. Set-based association analysis was applied to the Broad/JHMI dataset (Materials and Methods). Four sets of genes postulated to be involved in brain overgrowth (cell cycle, WNT pathway, growth factors and apoptosis) were used as experimental sets for analysis, while other postulated pathological processes such as synaptogenesis and inflammation were used as controls.
We found nominal associations of only cell cycle-related genes with ASD (Figure 4, p = 0.037). Cell cycle genes were then subdivided into twenty-one subgroups. Genes regulating cell cycle senescence (p = 0.011), the G2-M phase transition (p = 0.028) and the G0-G1 phase transition (p = 0.051) in particular were associated with autism in the Broad/JHMI dataset (Figure 4). Because these analyses were not statistically corrected for the number of pathways considered, the findings were replicated for the three significant subcategories (p<0.05) of cell cycle genes using the independent CHOP dataset (Figure 4). Two of the subcategories (senescence and G0-G1 phase transition) were replicated (p<0.0328 and p<0.0093 respectively), while the third (G2-M phase transition) was not. These results suggest that abnormal expression of cell cycle and developmental pathways may originate from genetic variation.
Discussion
Using genome-wide expression and CNV detection techniques, we discovered specific developmental pathways and processes in the prefrontal cortex that are significantly abnormal in younger autistic cases, other non-developmental processes that are dysregulated in older autism cases and, finally, processes common across younger and older ages in autism. Age-dependent gene expression alterations may underlie the differences in cellular and neuroanatomical pathologies between young and adult ages in autism that have been previously reported in MRI and postmortem research [13], [19]. In addition, we uncovered possible genotypic sources of developmental abnormalities in autism.
The young autistic prefrontal cortex displays dysregulated developmental genes
It is widely held that maldevelopment of the prefrontal cortex, in conjunction with several other cortical and subcortical regions, plays a pivotal role in the social, communication, cognitive and emotion processing deficits in autism. In the present study, the young autistic prefrontal cortex displayed disturbances in critical developmental pathways and processes that govern cell number, cortical patterning and differentiation, including those regulating proliferation, cell cycle, DNA damage response and apoptosis and survival. We hypothesize that such dysregulation could be the basis of the 67% excess neuron numbers also reported for prefrontal cortex in children with autism [17]. The top dysregulated pathway map in the young autistic brain was the A2A receptor signaling pathway (Table 1, Diagnosis main effect map folders). Adenosine receptors play important roles for both brain development and function including the regulation of neuronal stem cell proliferation (via nitric oxide signaling) [57], synaptic plasticity, motor function, cognition and emotion-related behaviors. This pathway has been a therapeutic target for studies of other complex neurologic and psychiatric disorders [58].
We also found strong evidence of abnormalities in cortical patterning pathways that regulate normal anterior/posterior and dorsal/ventral patterning as well as right/left asymmetry. At young ages in autism, MRI evidence indicates disruption of normal cortical patterning with gray matter enlargement greatest in anterior dorsolateral prefrontal cortex and least in posterior occipital and ventral orbital prefrontal cortex [3], [6], [19]. It will be important to further investigate the possible role that genetic dysregulation of neural patterning may play in causing abnormal left/right functional and structural asymmetries in addition to abnormal anterior/posterior and dorsal/ventral gradients that have been reported in autistic infants, children and adults [3], [6], [14], [15], [19], [XPATH ERROR: unknown variable "next".]. Dysregulation of proliferation, cell cycle and apoptosis may be involved in early brain overgrowth in autism and excess neuron numbers in the prefrontal cortex [17] and could therefore contribute to dysfunction at the neural systems level [19], [26], [60], [61]
The adult autism prefrontal cortex displays arrested growth and degeneration
In contrast to the younger autistic brain [62], the adult autistic brain shows evidence of neuron loss, reduced or arrested growth, cortical thinning and possible degeneration. It has been speculated that such pathologies may be related directly or indirectly to the earlier pathological overgrowth. It is known that the functions of genes are modulated by the age of the organism, such that dysregulation of the same gene in both young and adult autistic cases may have differing consequences. Genes such as BMP4 and BTRC, which have important functions in neurodevelopment, may regulate recovery functions such as neurogenesis in the adult brain [63]–[65]. Furthermore, we found that genes involved in cell differentiation, including RELN, BMP4, NODAL and NTRK3, and tissue remodeling and wound repair are dysregulated in adults. Though the literature on adult neurogenesis and cell differentiation is sparse, recent studies propose that the brain activates these mechanisms in response to injury in the neocortex [66], [67]. In the context of brain overgrowth, these data suggest the hypothesis that reactive reorganization of functional genetic networks and processes may occur in the adult autistic brain. Such functional reorganization of genetic modules or networks has recently been described for systems that regulate DNA-damage responses [68].
Age-independent differential expression represents pathological developmental processes
To complement analyses of age-specific expression pathology in autism, we also identified gene expression abnormalities in autism that were age independent. Among the most abnormally expressed genes were those that regulate DNA-damage response, apoptosis and immune system response functions. These genetic systems play important roles during development of the central nervous system [69]–[71] and suggest that the brain in autism arises from defects during this critical period. Several lines of evidence indicate that immune system responses closely participate in the neuropathogenesis of ASD both at young and adult ages. Although little is known about this role, both inflammatory and autoimmune mechanisms are necessary for normal neurodevelopment and are found to be altered in ASD [72]. Microglia activation and increases in inflammatory cytokine and chemokine production have direct effects on neuronal development by affecting cellular proliferation, migration and differentiation as well as synapse formation [72]. For example, TNF-α can modulate neuronal cell proliferation or cell death and plays an important role in synaptic pruning [73], [74]. In addition, auto-antibodies may affect receptor function, activate neuronal and glial cells and induce cellular damage or death [72].
More work remains to be done, however, to uncover the significance of these pathways in the postnatal brain. These results may point to mechanisms in the brain that are continuously pathological throughout the lifespan in autism and suggest possible candidates for genetic therapies.
Genes in common with a previous genetic study support pathway results
While large sample in vivo studies, such as Pinto et al. [26], have identified potential functional genomic abnormalities in autism, biological validation requires postmortem studies, such as the present one and Voineagu et al. [20]. The comparison of findings between the present study and Voineagu et al. [20] demonstrates a consistent overlap involving several pathways including repair - and immune-related processes.
We speculate that the aberrances in these genes, which may be distinct from individual to individual, may collectively underlie the repair or response mechanisms in the mature autistic brain. Commonalities across autistic brains may converge at the level of disturbances in these genetic pathways, and monitoring most commonly affected pathways across functional genomic studies may help subgroup different pathogenic mechanisms.
Though these genetic pathways with high impact on autism susceptibility may be similar between studies, there are also copious pathways that are disparate. The differences in the results between our study and Voineagu et al. [20] may be due to genetic heterogeneity, different criteria for sample selection and age and gender distribution. For example, although our studies assayed a similar number of postmortem cases, our sample consisted of all male autistic and control cases, while that of Voineagu et al. [20] consisted of 36% female autistic and 6% female control cases. Also, to identify gene expression abnormalities in the young autistic brain, we compared expression in the young autistic brain to the young control brain, while Voineagu et al. [20] lacked young controls. Thus, some effects that we observed may not have been identified in Voineagu et al. [20] because of differences in study design.
Copy number anomalies in autism
Our analyses using mRNA and DNA from the same cortices suggest that a large and heterogeneous array of genes and gene expression abnormalities regulate multiple foundational prenatal processes critical to cortex formation. Although DNA defects vary from autistic case to case, the diverse genetic deletions seem to underlie a relatively common biological theme, hitting a shared set of gene pathways that impact cell cycle, DNA damage detection and repair, migration, neural patterning and cell differentiation. The set of functional gene pathways identified by our direct analyses of autistic brain tissue are consistent with those identified by CNV pathway enrichment analyses in living autistic patients [26].
Conclusion
In this study, we found evidence for distinct gene expression anomalies in the prefrontal cortex of young autistic individuals that may underlie structural and functional maldevelopment of this and other association cortices. Our evidence shows that some important gene expression abnormalities in the prefrontal cortex change with age in autism and such changes may be related to findings in other studies that report a shift in morphology and function in the adult autistic brain. Enrichment of CNVs and association analysis of cell cycle gene sets, in accordance with early expression defects of cell cycle mechanisms, suggest that genetic variation may contribute to this dysregulation. We also found that irrespective of age, the expression of atypical developmental processes distinguish the autistic brain from that of control individuals. The modulation of these expression mechanisms may underlie the abnormal brain growth trajectory in autism. Further knowledge of the specific developmental neurobiological mechanisms behind the age-dependent anomalies reported here could point to distinct early developmental processes that lead to autism, uncover mechanisms that respond to early pathologies in the mature brain and suggest novel molecular targets for prevention strategies and treatment over the course of the disorder.
Materials and Methods
Gene expression analysis
Ethics statement
All cases are deceased and are deidentified by the brain banks where tissue was obtained. However, the same human protections procedures were employed as for live subjects. Research procedures employed in this study were approved by the institutional review board of the University of California, San Diego (protocol number 091205).
RNA extraction and quantification
A full description of the methods is provided in Text S1. Extraction of total RNA from 5–10 mg of frozen brain tissue from grey and white matter of prefrontal cortex of 57 postmortem autism and control cases aged 2–56 years was performed using the Ambion MELT kit according to manufacturer's instructions. Select samples were analyzed using Bioanalyzer (Agilent) according to the manufacturer's protocol for quality control and quantification (Table S1). RNA from remaining samples was quantified using a NanoDrop spectrophotometer.
Microarray processing
Four hundred fifty ng of total RNA from each of 57 cases was submitted to Illumina, Inc. (San Diego, CA) for DASL-based labeling and hybridization to the Illumina HumanRef8 v3 microarray. Using biotinylated random primers and oligo-dT, 200 ng RNA was converted to cDNA. The biotinylated cDNA was then immobilized to a streptavidin-coated solid support and annealed by 24526 pairs of oligonucleotides (18626 genes). Following extension and ligation, the ligated oligonucleotides were PCR-amplified with biotinylated or fluorophore-labeled universal primers and captured using streptavidin paramagnetic beads. Finally, the products were washed and denatured before hybridization to the BeadChips at 58°C for 16 hours. A BeadArray Reader extracted images and read fluorescence intensities, and all data was uploaded into BeadStudio software for quality control and processing.
Data preprocessing
Raw data exported from Illumina's BeadStudio underwent outlier removal, log2 transformation and quantile normalization as implemented in the lumi package for R/Bioconductor [75]. To simultaneously remove variance attributed to batch and seizures, we performed batch and covariate correction using ComBat [76]. Multivariate Distance Matrix Regression [77] with 10,000 permutations confirmed that variance attributable to batch and seizures were decreased following correction. Detailed information regarding the methodology for normalizing gene expression data and removing outliers is provided in Chow et al. [78].
Statistical analysis
Filtering and differential expression analyses were performed using BRBArrayTools (http://linus.nci.nih.gov/BRBArrayTools.html). Filtering was performed based on variance and minimum intensity. Probes with <20% of expression values having at least a 1.5-fold up - or down-regulation from the gene's median value across cases, and probes with a minimum log intensity of less than 15 were excluded.
We identified 1086 probes with an interaction effect of age and diagnosis by performing a two-way analysis of variance with 2 levels of diagnosis (autism and control) and 2 levels of categorical age (2–14 years and 15–56 years, n = 33). Interaction probes of p<0.05, corresponding to an empirical FDR of 0.27 [79], were selected for posthoc analysis to examine which differences were driven by the young autism and young control groups and the adult autism and adult control groups by t-tests (p<0.05) to investigate gene expression changes in the young and adult autistic brain. Probes exhibiting a main effect of diagnosis across age groups were also identified (p<0.05, FDR = 0.13).
Enrichment analysis
Enrichment analyses were performed using the MetaCore software suite (www.genego.com/metacore.php) and DAVID (http://david.abcc.ncifcrf.gov/) to examine the biological and functional relevance of differentially expressed genes. These genes were uploaded into MetaCore and filtered for known expression specific to the brain or the fetal brain. The default background gene list was used for all enrichment analyses. All results met threshold of corrected p<0.05 and FDR<0.1 [80]. Gene references used to confirm categorizations by MetaCore are listed in Table SXPATH ERROR: unknown variable "checknextn".XPATH ERROR: unknown variable "checknextn".
Independent validation of microarray results
RNA from 1 male autistic and 1 control case both 31 years old were analyzed using SYBR green RT-PCR to validate the intensity values detected by microarray of 19 genes (Figure S2). Primer3 software [81] was used to design primers across splice junctions to produce amplicons of ∼200 bp.
One mg of total RNA was used for cDNA synthesis using random hexamers and AMV reverse transcriptase. An equivalent of 50 ng of RNA was processed by qPCR using Roche's LightCycler rapid thermal cycler system (Roche Diagnostics Ltd, Lewes, UK) according to the manufacturer's instructions in a 96-well, 10 µL format using standard PCR conditions. One µL of cDNA template, 250 nM of forward and reverse primer and 5 µL of PCR Master Mix (Roche) were mixed for each reaction.
We took the geometric mean of all reference genes and the difference between this mean and the average intensity of experimental genes to find the delta Ct for each experimental gene. Subsequently, log2 fold change was assessed using -(T-C) where T = delta Ct of gene of the autistic case and C = delta Ct of gene of the control case. Using Spearman's rank correlation, the log2 fold changes of these 19 genes across qPCR and microarray platforms were found to be correlated at R = 0.78 (p = 0.000075, DF = 17; Figure S2).
Comparison between studies
Unique ProbeIDs of differentially expressed in the ‘initial’ dataset from Voineagu et al. [20] (file = ‘nature10110-s3.xls’) were compared with the differential expression gene list in the present study comparing all autism and control cases. Genes overlapping in each study with the present one were subjected to enrichment analysis in MetaCore.
Copy Number Variation (CNV) detection and association analysis
Fifty-five samples were genotyped on the Illumina 660 Bead Array (Illumina Inc., San Diego, California) and SNP calls were made using the Illumina Genome Studio software.
CNV calls were first obtained using the PennCNV software (http://www.openbioinformatics.org/penncnv/) implementing the wave adjustment procedure via the “–gcmodel” argument [53] and then using the CNVision pipeline [54] to select for high confidence CNV calls.
We first analyzed only male cases that passed QC (12 autism and 12 controls; Figure 4) and identified a total of about 850 CNVs. Known nonpathogenic regions reported in the Database of Genomic Variants (http://projects.tcag.ca/cgi-bin/variation/gbrowse/hg18) were filtered without upper size limits. No large-size CNVs (>1 Mb) were identified in single cases that could potentially drive the gene enrichment results. We next analyzed males and female cases together (14 autism and 16 controls; Figure S4) using the same parameters and identified a total of about 1300 CNVs and then proceeded with the filtering of common regions. We also excluded the possibility of a biased CNV detection given the small sample size (Text S1 and Figure S4).
Due to possible high false positive rates, we re-analyzed all cases using CNVision, a recently described analysis pipeline [54] (www.cnvision.org) that merges results of PennCNV, QuantiSNP and GNOSIS. The analysis of the male cases yielded about 350 CNVs (11 autism and 13 controls). Gene enrichment was performed considering both the gene content of gene-rich CNVs and the nearest gene at the 5 - and 3-prime end of gene-desert CNVs. As performed with PennCNV alone, we next analyzed male and female cases together but were unable to compare the gene enrichment of the two categories due to the significant difference in the number of cases that passed QC (12 autism versus 19 controls).
GO enrichment of the gene content of these regions was also performed using the MetaCore software suite (FDR<0.01 and p<0.05). GeneGO Network Processes of each of these gene lists are reported in Figure 4 and Figure S4.
Gene association analysis
For gene association analysis we utilized the AGRE-NIMH Broad/Johns Hopkins Medical Institute (Broad/JHMI) sample as our experimental dataset using original quality control filters and the Children's Hospital of Philadelphia (CHOP) sample dataset as a replication sample, as previously described [56].
Gene sets were created using Gene Ontology (GO; http://www.geneontology.org/), GeneGO (http://www.genego.com/metacore.php) and the Wnt homepage (http://www.stanford.edu/group/nusselab/cgi-bin/wnt/). We used PLINK [82] (http://pngu.mgh.harvard.edu/~purcell/plink/) to retrieve the SNPs within a window of 20 kb around each gene and to perform set-based association analysis.
Significantly associated pathways (p<0.05) in the cell cycle gene set were then broken down into 21 subsets of genes denoting specific phases of the cell cycle using Ingenuity and set-based tests were again performed on the experimental dataset. Subsets that were found to be significant in the Broad/JHMI sample were then tested on the CHOP sample for replication using the same set-based analysis.
Supporting Information
Zdroje
1. OzonoffSHeungKByrdRHansenRHertz-PicciottoI 2008 The onset of autism: patterns of symptom emergence in the first years of life. Autism research: official journal of the International Society for Autism Research 1 320 328
2. CourchesneEKarnsCMDavisHRZiccardiRCarperRA 2001 Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology 57 245 254
3. CarperRAMosesPTigueZDCourchesneE 2002 Cerebral lobes in autism: early hyperplasia and abnormal age effects. NeuroImage 16 1038 1051
4. SparksBFFriedmanSDShawDWAylwardEHEchelardD 2002 Brain structural abnormalities in young children with autism spectrum disorder. Neurology 59 184 192
5. SchumannCMHamstraJGoodlin-JonesBLLotspeichLJKwonH 2004 The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. The Journal of neuroscience : the official journal of the Society for Neuroscience 24 6392 6401
6. CarperRACourchesneE 2005 Localized enlargement of the frontal cortex in early autism. Biological psychiatry 57 126 133
7. HazlettHCPoeMGerigGSmithRGProvenzaleJ 2005 Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Archives of general psychiatry 62 1366 1376
8. RedcayECourchesneE 2005 When is the brain enlarged in autism? A meta-analysis of all brain size reports. Biological psychiatry 58 1 9
9. MunsonJDawsonGAbbottRFajaSWebbSJ 2006 Amygdalar volume and behavioral development in autism. Archives of general psychiatry 63 686 693
10. SchumannCMBarnesCCLordCCourchesneE 2009 Amygdala enlargement in toddlers with autism related to severity of social and communication impairments. Biological psychiatry 66 942 949
11. SchumannCMBlossCSBarnesCCWidemanGMCarperRA 2010 Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. The Journal of neuroscience : the official journal of the Society for Neuroscience 30 4419 4427
12. HazlettHCPoeMDGerigGStynerMChappellC 2011 Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch Gen Psychiatry 68 467 476
13. CourchesneECampbellKSolsoS 2011 Brain growth across the life span in autism: age-specific changes in anatomical pathology. Brain Res 1380 138 145
14. RedcayECourchesneE 2008 Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2–3-year-old children with autism spectrum disorder. Biological psychiatry 64 589 598
15. EylerLPierceK*CourchesneE* in press A Failure of Left Temporal Cortex to Specialize for Language is an Early Emerging and Fundamental Property of Autism. Brain
16. PierceKEylerL 2011 Structural and Functional Brain Development in ASD: The Impact of Early Brain Overgrowth and Considerations for Treatment. FeinD The Neuropsychology of Autism New York, NY Oxford University Press 407 450
17. CourchesneEMoutonPRCalhounMESemendeferiKAhrens-BarbeauC 2011 Neuron number and size in prefrontal cortex of children with autism. JAMA 306 2001 2010
18. StanfieldACMcIntoshAMSpencerMDPhilipRGaurS 2008 Towards a neuroanatomy of autism: a systematic review and meta-analysis of structural magnetic resonance imaging studies. Eur Psychiatry 23 289 299
19. CourchesneEPierceKSchumannCMRedcayEBuckwalterJA 2007 Mapping early brain development in autism. Neuron 56 399 413
20. VoineaguIWangXJohnstonPLoweJKTianY 2011 Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 474 380 384
21. Araghi-NiknamMFatemiSH 2003 Levels of Bcl-2 and P53 are altered in superior frontal and cerebellar cortices of autistic subjects. Cellular and molecular neurobiology 23 945 952
22. GarbettKEbertPJMitchellALintasCManziB 2008 Immune transcriptome alterations in the temporal cortex of subjects with autism. Neurobiology of disease 30 303 311
23. VargasDLNascimbeneCKrishnanCZimmermanAWPardoCA 2005 Neuroglial activation and neuroinflammation in the brain of patients with autism. Annals of neurology 57 67 81
24. OblakALGibbsTTBlattGJ 2010 Decreased GABA(B) Receptors in the Cingulate Cortex and Fusiform Gyrus in Autism. Journal of neurochemistry 114 1414 1423
25. SebatJLakshmiBMalhotraDTrogeJLese-MartinC 2007 Strong association of de novo copy number mutations with autism. Science (New York, NY) 316 445 449
26. PintoDPagnamentaATKleiLAnneyRMericoD 2010 Functional impact of global rare copy number variation in autism spectrum disorders. Nature 466 368 372
27. AprilCKlotzleBRoyceTWickham-GarciaEBoyaniwskyT 2009 Whole-genome gene expression profiling of formalin-fixed, paraffin-embedded tissue samples. PLoS ONE 4 e8162 doi:10.1371/journal.pone.0008162
28. FanJ-BYeakleyJMBibikovaMChudinEWickhamE 2004 A versatile assay for high-throughput gene expression profiling on universal array matrices. Genome research 14 878 885
29. AbramovitzMOrdanic-KodaniMWangYLiZCatzavelosC 2008 Optimization of RNA extraction from FFPE tissues for expression profiling in the DASL assay. BioTechniques 44 417 423
30. HuangDWShermanBTLempickiRA 2009 Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic acids research 37 1 13
31. HuangDWShermanBTLempickiRA 2009 Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols 4 44 57
32. FreeseJLPinoDPleasureSJ 2010 Wnt signaling in development and disease. Neurobiology of disease 38 148 153
33. LijamNPaylorRMcDonaldMCrawleyJDengC 1997 Social Interaction and Sensorimotor Gating Abnormalities in Mice Lacking Dvl1. Cell 90 895 905
34. WigleJTEisenstatDD 2008 Homeobox genes in vertebrate forebrain development and disease. Clinical genetics 73 212 226
35. SchierAF 2009 Nodal morphogens. Cold Spring Harbor perspectives in biology 1 a003459
36. ChenZPalmerTD 2008 Cellular repair of CNS disorders: an immunological perspective. Human molecular genetics 17 R84 92
37. LieDCSongHColamarinoSAMingG-lGageFH 2004 NEUROGENESIS IN THE ADULT BRAIN: New Strategies for Central Nervous System Diseases
38. LaiMGluckmanPDragunowMHughesPE 1997 Focal brain injury increases activin betaA mRNA expression in hippocampal neurons. Neuroreport 8 2691 2694
39. AgetaHMurayamaAMigishimaRKidaSTsuchidaK 2008 Activin in the brain modulates anxiety-related behavior and adult neurogenesis. PLoS ONE 3 e1869 doi:10.1371/journal.pone.0001869
40. LatresEChiaurDSPaganoM 1999 The human F box protein beta-Trcp associates with the Cul1/Skp1 complex and regulates the stability of beta-catenin. Oncogene 18 849 854
41. MadsenT 2003 Chronic electroconvulsive seizure up-regulates β-catenin expression in rat hippocampus: role in adult neurogenesis. Biological Psychiatry 54 1006 1014
42. KuwabaraTHsiehJMuotriAYeoGWarashinaM 2009 Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nature neuroscience 12 1097 1105
43. LimDATramontinADTrevejoJMHerreraDGGarcía-VerdugoJM 2000 Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron 28 713 726
44. GroszerMEricksonRScripture-AdamsDDLescheRTrumppA 2001 Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science (New York, NY) 294 2186 2189
45. ButlerMGDasoukiMJZhouX-PTalebizadehZBrownM 2005 Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. Journal of medical genetics 42 318 321
46. PageDTKutiOJPrestiaCSurM 2009 Haploinsufficiency for Pten and Serotonin transporter cooperatively influences brain size and social behavior. Proceedings of the National Academy of Sciences of the United States of America 106 1989 1994
47. HayN 2005 The Akt-mTOR tango and its relevance to cancer. Cancer cell 8 179 183
48. OrlovaKACrinoPB 2010 The tuberous sclerosis complex. Annals of the New York Academy of Sciences 1184 87 105
49. ChennAWalshCA 2002 Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science (New York, NY) 297 365 369
50. TissirFGoffinetAM 2003 Reelin and brain development. Nature reviews Neuroscience 4 496 505
51. PersicoAMD'AgrumaLMaioranoNTotaroAMiliterniR 2001 Reelin gene alleles and haplotypes as a factor predisposing to autistic disorder. Molecular psychiatry 6 150 159
52. Wynshaw-BorisAPramparoTYounYHHirotsuneS 2010 Lissencephaly: mechanistic insights from animal models and potential therapeutic strategies. Seminars in cell & developmental biology 21 823 830
53. WangKLiMHadleyDLiuRGlessnerJ 2007 PennCNV: an integrated hidden Markov model designed for high-resolution copy number variation detection in whole-genome SNP genotyping data. Genome research 17 1665 1674
54. SandersSJErcan-SencicekAGHusVLuoRMurthaMT 2011 Multiple Recurrent De Novo CNVs, Including Duplications of the 7q11.23 Williams Syndrome Region, Are Strongly Associated with Autism. Neuron 70 863 885
55. WangKZhangHMaDBucanMGlessnerJT 2009 Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature 459 528 533
56. WeissLAArkingDEDalyMJChakravartiA 2009 A genome-wide linkage and association scan reveals novel loci for autism. Nature 461 802 808
57. CarreiraBPMorteMIInacioACostaGRosmaninho-SalgadoJ 2010 Nitric oxide stimulates the proliferation of neural stem cells bypassing the epidermal growth factor receptor. Stem Cells 28 1219 1230
58. WeiCJLiWChenJF 2011 Normal and abnormal functions of adenosine receptors in the central nervous system revealed by genetic knockout studies. Biochim Biophys Acta 1808 1358 1379
59. PierceKEylerL 2011 Structural and Functional Brain Development In Autism: The Impact of Early Brain Overgrowth and Considerations for Treatment. FeinDeborah Oxford University Press, The Neuropsychology of Autism 407 450
60. CourchesneEPierceK 2005 Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Current opinion in neurobiology 15 225 230
61. CourchesneEPierceK 2005 Brain overgrowth in autism during a critical time in development: implications for frontal pyramidal neuron and interneuron development and connectivity. Int J Dev Neurosci 23 153 170
62. CourchesneECampbellKSolsoS 2010 Brain Growth Across the Life Span in Autism: Age-Specific Changes in Anatomical Pathology. Brain research
63. BonaguidiMAPengCYMcGuireTFalcigliaGGobeskeKT 2008 Noggin expands neural stem cells in the adult hippocampus. J Neurosci 28 9194 9204
64. GajeraCREmichHLioubinskiOChristABeckervordersandforth-BonkR 2010 LRP2 in ependymal cells regulates BMP signaling in the adult neurogenic niche. J Cell Sci 123 1922 1930
65. FrankCLGeXXieZZhouYTsaiLH 2010 Control of activating transcription factor 4 (ATF4) persistence by multisite phosphorylation impacts cell cycle progression and neurogenesis. J Biol Chem 285 33324 33337
66. ZhangCWuHZhuXWangYGuoJ 2011 Role of transcription factors in neurogenesis after cerebral ischemia. Rev Neurosci 22 457 465
67. OhiraK 2011 Injury-induced neurogenesis in the mammalian forebrain. Cell Mol Life Sci 68 1645 1656
68. IdekerTDutkowskiJHoodL 2011 Boosting signal-to-noise in complex biology: prior knowledge is power. Cell 144 860 863
69. BoulangerLMShatzCJ 2004 Immune signalling in neural development, synaptic plasticity and disease. Nat Rev Neurosci 5 521 531
70. O'DriscollMJeggoPA 2008 The role of the DNA damage response pathways in brain development and microcephaly: insight from human disorders. DNA Repair (Amst) 7 1039 1050
71. KimWRSunW 2011 Programmed cell death during postnatal development of the rodent nervous system. Dev Growth Differ 53 225 235
72. OnoreCCareagaMAshwoodP 2011 The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun
73. WideraDMikenbergIElversMKaltschmidtCKaltschmidtB 2006 Tumor necrosis factor alpha triggers proliferation of adult neural stem cells via IKK/NF-kappaB signaling. BMC Neurosci 7 64
74. StellwagenDMalenkaRC 2006 Synaptic scaling mediated by glial TNF-alpha. Nature 440 1054 1059
75. DuPKibbeWALinSM 2008 lumi: a pipeline for processing Illumina microarray. Bioinformatics (Oxford, England) 24 1547 1548
76. JohnsonWELiCRabinovicA 2007 Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics (Oxford, England) 8 118 127
77. ZapalaMASchorkNJ 2006 Multivariate regression analysis of distance matrices for testing associations between gene expression patterns and related variables. Proceedings of the National Academy of Sciences of the United States of America 103 19430 19435
78. ChowMWinnMLiHAprilCBarnesCC in press Genome-wide Brain Gene Expression Microarray Data Preprocessing and Quality Control Scheme using the Illumina DASL Assay
79. BenjaminiYHochbergY 1995 Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. J R Statist Soc 57 289 300
80. FalconSGentlemanR 2007 Using GOstats to test gene lists for GO term association. Bioinformatics (Oxford, England) 23 257 258
81. RozenSSkaletskyH 2003 Primer3 on the WWW for General Users and for Biologist Programmers. Methods 132
82. PurcellSNealeBToddbrownKThomasLFerreiraM 2007 PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. The American Journal of Human Genetics 81 559 575
Štítky
Genetika Reprodukčná medicína
Článek Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1Článek Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / TranscriptionČlánek Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding DomainČlánek Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin ComplexesČlánek An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood ObesityČlánek Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAsČlánek Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2012 Číslo 3- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
- Genomic Analysis of the Hydrocarbon-Producing, Cellulolytic, Endophytic Fungus
- Networks of Neuronal Genes Affected by Common and Rare Variants in Autism Spectrum Disorders
- Akirin Links Twist-Regulated Transcription with the Brahma Chromatin Remodeling Complex during Embryogenesis
- Too Much Cleavage of Cyclin E Promotes Breast Tumorigenesis
- Imprinted Genes … and the Number Is?
- Genetic Architecture of Highly Complex Chemical Resistance Traits across Four Yeast Strains
- Exploring the Complexity of the HIV-1 Fitness Landscape
- MNS1 Is Essential for Spermiogenesis and Motile Ciliary Functions in Mice
- A Fundamental Regulatory Mechanism Operating through OmpR and DNA Topology Controls Expression of Pathogenicity Islands SPI-1 and SPI-2
- Evidence for Positive Selection on a Number of MicroRNA Regulatory Interactions during Recent Human Evolution
- Variation in Modifies Risk of Neonatal Intestinal Obstruction in Cystic Fibrosis
- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Critical Evaluation of Imprinted Gene Expression by RNA–Seq: A New Perspective
- A Meta-Analysis and Genome-Wide Association Study of Platelet Count and Mean Platelet Volume in African Americans
- Mouse Genetics Suggests Cell-Context Dependency for Myc-Regulated Metabolic Enzymes during Tumorigenesis
- Transcriptional Control in Cardiac Progenitors: Tbx1 Interacts with the BAF Chromatin Remodeling Complex and Regulates
- Synthetic Lethality of Cohesins with PARPs and Replication Fork Mediators
- APOBEC3G-Induced Hypermutation of Human Immunodeficiency Virus Type-1 Is Typically a Discrete “All or Nothing” Phenomenon
- Interpreting Meta-Analyses of Genome-Wide Association Studies
- Error-Prone ZW Pairing and No Evidence for Meiotic Sex Chromosome Inactivation in the Chicken Germ Line
- -Dependent Chemosensory Functions Contribute to Courtship Behavior in
- Diverse Forms of Splicing Are Part of an Evolving Autoregulatory Circuit
- Phenotypic Plasticity of the Drosophila Transcriptome
- Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1
- Precocious Metamorphosis in the Juvenile Hormone–Deficient Mutant of the Silkworm,
- Igf1r Signaling Is Indispensable for Preimplantation Development and Is Activated via a Novel Function of E-cadherin
- Accurate Prediction of Inducible Transcription Factor Binding Intensities In Vivo
- Mitochondrial Oxidative Stress Alters a Pathway in Strongly Resembling That of Bile Acid Biosynthesis and Secretion in Vertebrates
- Mammalian Neurogenesis Requires Treacle-Plk1 for Precise Control of Spindle Orientation, Mitotic Progression, and Maintenance of Neural Progenitor Cells
- Tcf7 Is an Important Regulator of the Switch of Self-Renewal and Differentiation in a Multipotential Hematopoietic Cell Line
- REST–Mediated Recruitment of Polycomb Repressor Complexes in Mammalian Cells
- Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / Transcription
- Age-Dependent Brain Gene Expression and Copy Number Anomalies in Autism Suggest Distinct Pathological Processes at Young Versus Mature Ages
- A Genome-Wide Association Study Identifies Variants Underlying the Shade Avoidance Response
- -by- Regulatory Divergence Causes the Asymmetric Lethal Effects of an Ancestral Hybrid Incompatibility Gene
- Genome-Wide Association and Functional Follow-Up Reveals New Loci for Kidney Function
- A Natural System of Chromosome Transfer in
- Cell Size and the Initiation of DNA Replication in Bacteria
- Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding Domain
- Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin Complexes
- Temporal Transcriptional Profiling of Somatic and Germ Cells Reveals Biased Lineage Priming of Sexual Fate in the Fetal Mouse Gonad
- Rapid Analysis of Genome Rearrangements by Multiplex Ligation–Dependent Probe Amplification
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- The Atypical Calpains: Evolutionary Analyses and Roles in Cellular Degeneration
- The Silkworm Coming of Age—Early
- Development of a Panel of Genome-Wide Ancestry Informative Markers to Study Admixture Throughout the Americas
- Balanced Codon Usage Optimizes Eukaryotic Translational Efficiency
- The Min System and Nucleoid Occlusion Are Not Required for Identifying the Division Site in but Ensure Its Efficient Utilization
- Neurobeachin, a Regulator of Synaptic Protein Targeting, Is Associated with Body Fat Mass and Feeding Behavior in Mice and Body-Mass Index in Humans
- Statistical Analysis of Readthrough Levels for Nonsense Mutations in Mammalian Cells Reveals a Major Determinant of Response to Gentamicin
- Gene Reactivation by 5-Aza-2′-Deoxycytidine–Induced Demethylation Requires SRCAP–Mediated H2A.Z Insertion to Establish Nucleosome Depleted Regions
- The miR-35-41 Family of MicroRNAs Regulates RNAi Sensitivity in
- Genetic Basis of Hidden Phenotypic Variation Revealed by Increased Translational Readthrough in Yeast
- An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood Obesity
- Modelling Human Regulatory Variation in Mouse: Finding the Function in Genome-Wide Association Studies and Whole-Genome Sequencing
- Novel Loci for Adiponectin Levels and Their Influence on Type 2 Diabetes and Metabolic Traits: A Multi-Ethnic Meta-Analysis of 45,891 Individuals
- Polycomb-Like 3 Promotes Polycomb Repressive Complex 2 Binding to CpG Islands and Embryonic Stem Cell Self-Renewal
- Insulin/IGF-1 and Hypoxia Signaling Act in Concert to Regulate Iron Homeostasis in
- EMF1 and PRC2 Cooperate to Repress Key Regulators of Arabidopsis Development
- Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAs
- Contrasted Patterns of Molecular Evolution in Dominant and Recessive Self-Incompatibility Haplotypes in
- A Machine Learning Approach for Identifying Novel Cell Type–Specific Transcriptional Regulators of Myogenesis
- Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
- Nos2 Inactivation Promotes the Development of Medulloblastoma in Mice by Deregulation of Gap43–Dependent Granule Cell Precursor Migration
- Intracranial Aneurysm Risk Locus 5q23.2 Is Associated with Elevated Systolic Blood Pressure
- Heritability and Genetic Correlations Explained by Common SNPs for Metabolic Syndrome Traits
- A Genome-Wide Association Study of Nephrolithiasis in the Japanese Population Identifies Novel Susceptible Loci at 5q35.3, 7p14.3, and 13q14.1
- DNA Damage in Nijmegen Breakage Syndrome Cells Leads to PARP Hyperactivation and Increased Oxidative Stress
- DNA Resection at Chromosome Breaks Promotes Genome Stability by Constraining Non-Allelic Homologous Recombination
- Genetic Analysis of Floral Symmetry in Van Gogh's Sunflowers Reveals Independent Recruitment of Genes in the Asteraceae
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Promoter Nucleosome Organization Shapes the Evolution of Gene Expression
- The Nucleoside Diphosphate Kinase Gene Acts as Quantitative Trait Locus Promoting Non-Mendelian Inheritance
- The Ciliogenic Transcription Factor RFX3 Regulates Early Midline Distribution of Guidepost Neurons Required for Corpus Callosum Development
- Phosphorylation of the RNA–Binding Protein HOW by MAPK/ERK Enhances Its Dimerization and Activity
- A Genome-Wide Scan of Ashkenazi Jewish Crohn's Disease Suggests Novel Susceptibility Loci
- Parkinson's Disease–Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria
- LMW-E/CDK2 Deregulates Acinar Morphogenesis, Induces Tumorigenesis, and Associates with the Activated b-Raf-ERK1/2-mTOR Pathway in Breast Cancer Patients
- Mapping the Hsp90 Genetic Interaction Network in Reveals Environmental Contingency and Rewired Circuitry
- Autoregulation of the Noncoding RNA Gene
- The Human Pancreatic Islet Transcriptome: Expression of Candidate Genes for Type 1 Diabetes and the Impact of Pro-Inflammatory Cytokines
- Spo0A∼P Imposes a Temporal Gate for the Bimodal Expression of Competence in
- Antagonistic Regulation of Apoptosis and Differentiation by the Cut Transcription Factor Represents a Tumor-Suppressing Mechanism in
- A Downstream CpG Island Controls Transcript Initiation and Elongation and the Methylation State of the Imprinted Macro ncRNA Promoter
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



