-
Články
- Časopisy
- Kurzy
- Témy
- Kongresy
- Videa
- Podcasty
Igf1r Signaling Is Indispensable for Preimplantation Development and Is Activated via a Novel Function of E-cadherin
Insulin-like growth factor I receptor (Igf1r) signaling controls proliferation, differentiation, growth, and cell survival in many tissues; and its deregulated activity is involved in tumorigenesis. Although important during fetal growth and postnatal life, a function for the Igf pathway during preimplantation development has not been described. We show that abrogating Igf1r signaling with specific inhibitors blocks trophectoderm formation and compromises embryo survival during murine blastocyst formation. In normal embryos total Igf1r is present throughout the membrane, whereas the activated form is found exclusively at cell contact sites, colocalizing with E-cadherin. Using genetic domain switching, we show a requirement for E-cadherin to maintain proper activation of Igf1r. Embryos expressing exclusively a cadherin chimera with N-cadherin extracellular and E-cadherin intracellular domains (NcEc) fail to form a trophectoderm and cells die by apoptosis. In contrast, homozygous mutant embryos expressing a reverse-structured chimera (EcNc) show trophectoderm survival and blastocoel cavitation, indicating a crucial and non-substitutable role of the E-cadherin ectodomain for these processes. Strikingly, blastocyst formation can be rescued in homozygous NcEc embryos by restoring Igf1r signaling, which enhances cell survival. Hence, perturbation of E-cadherin extracellular integrity, independent of its cell-adhesion function, blocked Igf1r signaling and induced cell death in the trophectoderm. Our results reveal an important and yet undiscovered function of Igf1r during preimplantation development mediated by a unique physical interaction between Igf1r and E-cadherin indispensable for proper receptor activation and anti-apoptotic signaling. We provide novel insights into how ligand-dependent Igf1r activity is additionally gated to sense developmental potential in utero and into a bifunctional role of adhesion molecules in contact formation and signaling.
Published in the journal: Igf1r Signaling Is Indispensable for Preimplantation Development and Is Activated via a Novel Function of E-cadherin. PLoS Genet 8(3): e32767. doi:10.1371/journal.pgen.1002609
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002609Summary
Insulin-like growth factor I receptor (Igf1r) signaling controls proliferation, differentiation, growth, and cell survival in many tissues; and its deregulated activity is involved in tumorigenesis. Although important during fetal growth and postnatal life, a function for the Igf pathway during preimplantation development has not been described. We show that abrogating Igf1r signaling with specific inhibitors blocks trophectoderm formation and compromises embryo survival during murine blastocyst formation. In normal embryos total Igf1r is present throughout the membrane, whereas the activated form is found exclusively at cell contact sites, colocalizing with E-cadherin. Using genetic domain switching, we show a requirement for E-cadherin to maintain proper activation of Igf1r. Embryos expressing exclusively a cadherin chimera with N-cadherin extracellular and E-cadherin intracellular domains (NcEc) fail to form a trophectoderm and cells die by apoptosis. In contrast, homozygous mutant embryos expressing a reverse-structured chimera (EcNc) show trophectoderm survival and blastocoel cavitation, indicating a crucial and non-substitutable role of the E-cadherin ectodomain for these processes. Strikingly, blastocyst formation can be rescued in homozygous NcEc embryos by restoring Igf1r signaling, which enhances cell survival. Hence, perturbation of E-cadherin extracellular integrity, independent of its cell-adhesion function, blocked Igf1r signaling and induced cell death in the trophectoderm. Our results reveal an important and yet undiscovered function of Igf1r during preimplantation development mediated by a unique physical interaction between Igf1r and E-cadherin indispensable for proper receptor activation and anti-apoptotic signaling. We provide novel insights into how ligand-dependent Igf1r activity is additionally gated to sense developmental potential in utero and into a bifunctional role of adhesion molecules in contact formation and signaling.
Introduction
The ultimate goal of the mammalian preimplantation development is the formation of a hollow shaped embryo called blastocyst, crucial for all stages of subsequent development. It is generated by a highly organized interplay of multiple signaling pathways guiding development from a fertilized egg to an 128-cell staged embryo. At the end of this important process three distinct cell lineages are established – the epiblast, that will give rise to the embryo proper, the primitive endoderm, that forms some of the extraembryonic membranes and the trophectoderm (TE) that contributes to the placenta [1], [2]. In mice, at embryonic day (E)4.5 after segregation of the early lineages is completed the blastocyst hatches from its glycoprotein envelop (zona pellucida) in order to invade the uterine epithelium and implant. Besides the orchestrated interplay of transcription factor networks that regulate expression of Oct4, Cdx2, Nanog, Gata4 and Gata6, indispensable for correct lineage segregation [3]–[7], the formation of a proper blastocyst strongly depends on tightly controlled cell adhesion mainly mediated by E-cadherin (E-cad, also known as Cdh1) [8]. Mice deficient for E-cad (Cdh1) are incapable of forming a proper trophectodermal epithelium [9], [10]. Compaction, however, is accomplished by residual maternally provided gene expression and is lost upon maternal/zygotic E-cad (Cdh1) depletion [11], [12]. In contrast, N-cadherin (N-cad, also known as Cdh2), another crucial member of classical cadherins is first detected after implantation and its gene ablation demonstrates distinct functions as well. N-cad (Cdh2) expression is initiated when the first mesoderm cells start to emerge at the primitive streak during gastrulation [8]. Although the mesoderm is properly formed in N-cad (Cdh2)-deficient mice, patterning of the somites and the neural tube is severely affected, and embryos die due to a heart defect [13]. Interestingly, the cardiac phenotype is rescued by ectopic expression of E-cad (Cdh1) in the developing heart, indicating that the adhesive function of cadherins is at least in part interchangeable [14]. In agreement with this finding, E-cad and N-cad have similar properties in the degree of conservation of amino acids (aa), in mediating homophilic adhesion, and in binding to the same intracellular interaction partners, such as β-catenin, Plakoglobin and p120ctn [15], [16]. However, E-cad (Cdh1) and N-cad (Cdh2) are usually expressed in a mutually exclusive pattern and induce different cellular properties. Cell polarity and an epithelial sessile shape are established in cells that express E-cad (Cdh1), whereas cell migration can be induced in cells that gain N-cad (Cdh2) expression [17], [18]. This phenomenon reflects the contribution of cadherins to epithelial-mesenchymal transition during gastrulation, as well as during carcinogenesis [8], [19], [20]. Detailed knowledge about how the unique properties of the two related cadherins are translated in molecular terms is still limited.
By ectopically switching cadherin expression from E-cad to N-cad using a previously reported gene replacement approach, we were able to maintain cell adhesion and analyze specific and unique molecular features of either E-cad or N-cad [21]. Interestingly, embryos carrying two N-cad knock-in alleles in the E-cad (Cdh1) locus (N-cad ki/ki) were not able to form a proper TE and died within the zona pellucida, similar to E-cad−/− embryos. Since control embryos that harbor an HA-tagged E-cad (Cdh1) cDNA knock-in allele were able to form blastocysts and implant properly, the result of N-cad ki/ki embryos suggests that E-cad has a unique function during blastocyst formation [21], [22]. However, how this crucial and unique function of E-cad is implemented and why it cannot be replaced by N-cad is a complete enigma.
The insulin-like growth factor I receptor (Igf1r) belongs to the protein family of receptor tyrosine kinases and is mainly activated by Igf1 and Igf2 acting in an autocrine and paracrine manner. The downstream signaling cascade regulates proliferation, differentiation, metabolism and survival of most cell types during fetal growth and postnatal life [23], [24]. Blocking kinase activity by either loss-of-function mutation of the receptor or of both ligands result in reduced body weight and size combined with multiple defects including muscle dystrophy and impaired survival of newborn pups [25], [26]. The Igf1/Igf2/Igf1r axis provides growth promoting, anti-apoptotic functions in almost all tissues and organs and treatment of mouse preimplantation embryos with Igf1 enhanced blastocyst formation in vitro by supporting PI3K/Akt activity [27], [28]. However, detailed knowledge about a role of this pathway during preimplantation development is lacking.
Here, we further addressed the question about the unique function of E-cad by replacing its expression with chimeric cadherin genes using similar knock-in approaches as for N-cad ki/+ mice and identified a novel fundamental and cell-adhesion independent function of E-cad in promoting cell survival of the TE by facilitating Igf1r activity.
Results
Generation of mice expressing chimeric cadherins under the control of the E-cad (Cdh1) locus
To elucidate the function of E-cad during TE formation, the protein was divided into two parts, its N-terminal extracellular adhesive region and its C-terminal transmembrane and intracellular portion, the latter of which mediates its interaction with catenins. These regions were combined with the matching portions of the N-cad molecule to generate artificial chimeric cadherins (Figure 1A). Cloned cDNAs encoding EcNc (corresponding to aa 1–710 of the E-cad precursor peptide and 725–906 of N-cad) and NcEc (aa 1–724 of N-cad and 711–884 of E-cad) were fused to a sequence encoding an HA-tag and inserted into the E-cad (Cdh1) locus to replace E-cad as described previously (Figure 1B–1D) [21], [22], [29]. For both EcNc and NcEc approaches, two independent ES-cell clones were used to generate the corresponding knock-in strains. Proper expression of the chimeric molecules was confirmed on mRNA level in ES cells and by immunofluorescence and immunohistochemistry of embryos after deletion of the selection cassette. RNA levels of the two knock-in alleles were comparable to the amount of N-cad ki and EcHA transcripts (Figure S1A). Distribution of both chimeric proteins completely overlapped with endogenous E-cad staining in TE and inner cell mass (ICM) cells of heterozygous preimplantation embryos (Figure 1E) and accurately recapitulated E-cad (Cdh1) expression in the epithelia of post-implantation stages (Figure S1B). The analysis confirmed successful gene replacement and correct spatiotemporal expression of both knock-in alleles.
Fig. 1. Generation of EcNc and NcEc cadherin proteins expressed in the E-cad (Cdh1) locus. 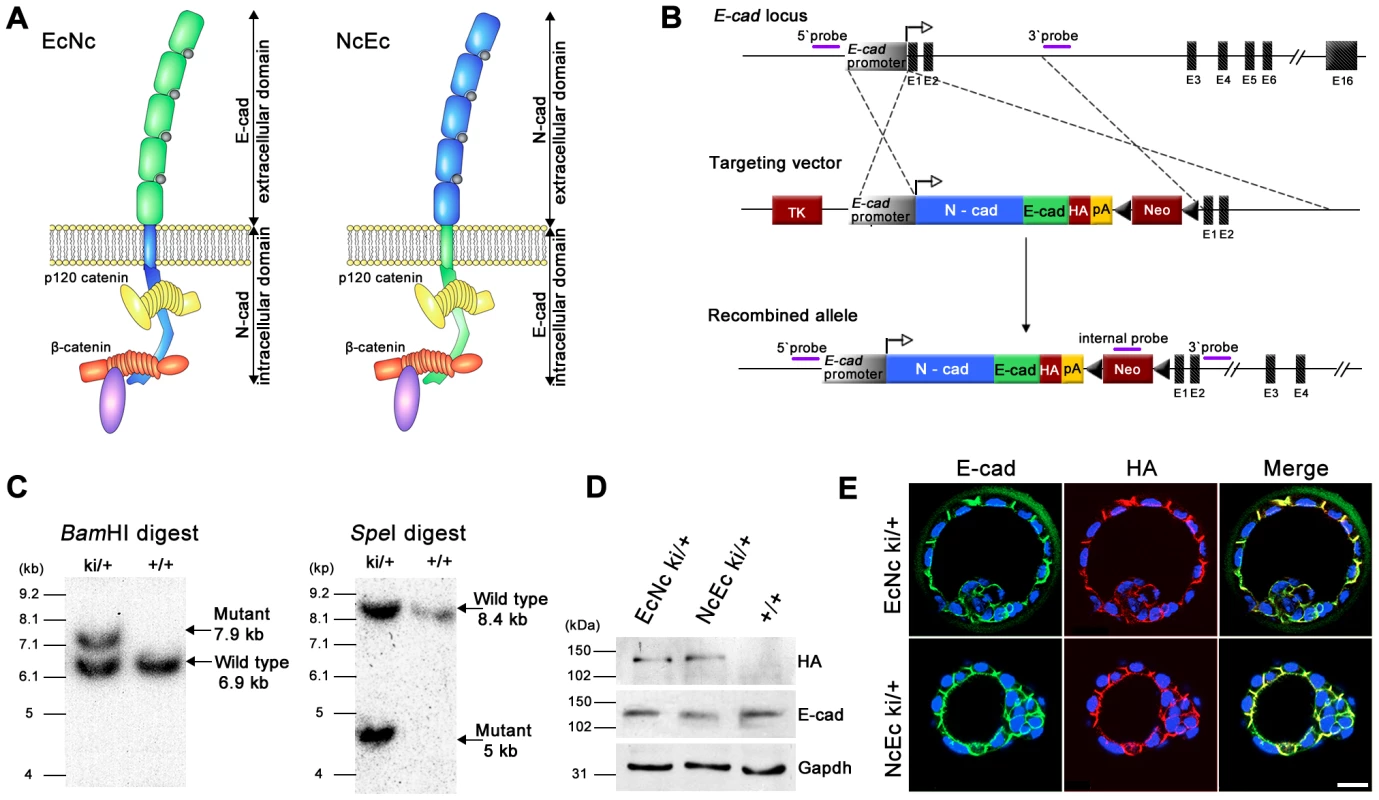
(A) Schematic representation of EcNc and NcEc protein structure in their cadherin-catenin complex (adapted from [8]). (B) Gene targeting strategy and the resultant knock-in allele, representatively shown for NcEc; TK, HSV::tk negative selection cassette; HA, haemagglutinin tag; pA, SV40 polyadenylation signal; Neo, neomycin resistance cassette, flanked by loxP sites (black triangles). (C) Southern blot analysis of obtained ES cell clones using the 5′ probe. (D) Expression of the knock-in alleles in ES cells after removal of the neomycin cassette reveals equal expression of both HA-tagged proteins. (E) Immunofluorescence labeling of EcNc (upper) and NcEc (lower panel) showing complete overlap of anti-HA and anti-E-cad staining in heterozygous E3.5 blastocysts in confocal optical sections. Scale bar, 25 µm. Homozygous mutant NcEc embryos fail to form an intact TE layer
Similar to the N-cad ki/+ mice, no phenotype was detected in heterozygous NcEc or EcNc animals, indicating that the chimeric proteins did not interfere with E-cad-mediated adhesion. At E2.5, EcNc and NcEc homozygous embryos were observed in a 24-h time-lapse experiment to monitor blastocyst formation. Both homozygous mutants underwent compaction normally and were indistinguishable from their heterozygous littermates (Figure 2A and 2C, 0–6 h). EcNc homozygous mutants (EcNc ki/ki), which express the cadherin with the adhesive domain of E-cad, properly segregated the TE from the ICM cells and formed a blastocoel cavity similar to control embryos (Figure 2A, 6–24 h, and 2E). However, the TE was more fragile as pulsing caused by sequential expansion and collapse was observed more frequently in EcNc homozygous mutants than in control littermates (Figure 2A and Video S1). Proper protein localization to the basolateral membranes of TE cells was confirmed by immunofluorescence (Figure 2B). In contrast, homozygous NcEc mutants (NcEc ki/ki), which express the cadherin with the extracellular domain of N-cad, were incapable of establishing a blastocyst. Cells on the outside were shuffled around, rounded up, vacuolated and became scattered at the surface of the embryo, whereas the control littermates formed blastocysts during the 24-h time-lapse recording (Figure 2C, 2F and Video S2). Confocal optical plane sections of immunofluorescently labeled embryos revealed that the NcEc protein was evenly distributed on the scattered cells on the surface (Figure 2D, yellow arrow). The results indicated that TE and blastocoel cavity formation required the presence of the extracellular domain of E-cad. However, the reduced stability of the TE layer in homozygous EcNc mutants showed that the intracellular domain of the E-cad molecule also contributed to the process and has an important function that cannot be performed by N-cad.
Fig. 2. Homozygous NcEc embryos fail to form a functional trophectoderm. 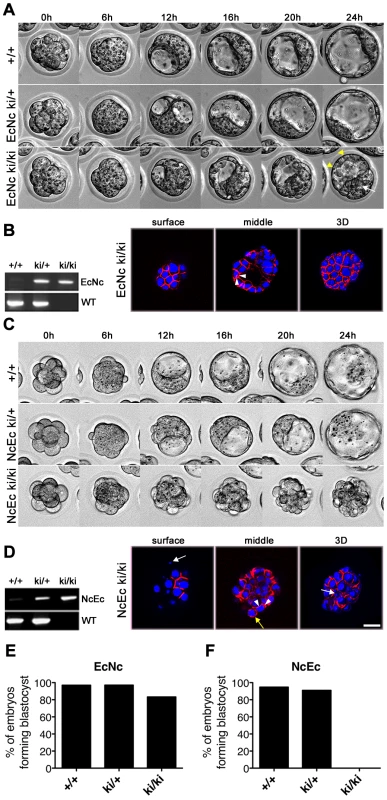
(A) Still images from a twenty-four-hour time-lapse recording of in vitro cultured embryos from heterozygous EcNc intercrosses at E2.5. All embryos properly compact and separate the ICM (white arrow) from the TE (yellow arrowheads). (B) PCR genotyping of embryos in (A) and HA.11 immunofluorescence labeling of EcNc protein given in surface and medial optical sections, as well as z-stack 3D reconstructions, demonstrating the proper distribution of EcNc at the basolateral membrane (arrowheads). (C) Still images from a twenty-four-hour time-lapse recording of in vitro cultured embryos from heterozygous NcEc intercrosses at E2.5. Compaction is accomplished in all genotypes, but NcEc homozygous embryos cannot form a TE layer. (D) Similar analysis of NcEc homozygous embryos as shown in (B). Although NcEc is located mainly at basolateral sites (arrowheads), a uniform distribution was found in several outer cells (yellow arrow), and fragmented nuclei (white arrows) were also detected. (E,F) Percentage of embryos that formed a proper blastocyst in time-lapse experiments and during in vitro culture for EcNc (E, n = 33 wt, n = 101 ki/+ and n = 48 ki/ki) and NcEc mutants (F, n = 58 wt, n = 156 ki/+ and n = 56 ki/ki) in >10 independent experiments. Scale bar, 25 µm. Key features required for blastocyst formation are correctly distributed in homozygous NcEc embryos
To confirm that lineage segregation, cell polarity and expression of molecules playing a key role during the cavitation process were not affected in NcEc homozygous embryos, we analyzed the expression of specific marker genes by immunofluorescence labeling. No difference in the expression or localization of essential proteins was found in NcEc embryos, indicating that the ICM and the TE were correctly specified and that the cavitation machinery was present (Figure 3A and 3B). Cell polarity was correctly established based on detection of apical staining of ZO-1 and Ezrin and basolateral localization of the chimeric cadherins and Na+/K+-ATPase (Figure 3C). In contrast to E-cad−/−, both EcNc ki/ki and NcEc ki/ki embryos show proper expression and membrane localization of β-catenin, Plakoglobin and p120ctn (Figure S2A). They were connected to both chimeric cadherin molecules in a similar manner as detected by immunoprecipitation (Figure S2B). In addition, embryo-derived homozygous TE cells differentiated into trophoblast giant cells, and ES cells showed proper adhesive colony formation. Similar differentiation capacities were observed for EcNc ki/ki and NcEc ki/ki genotypes, in stark contrast to Ecad−/− ES cells (Figure S3). This indicated that cell polarity, adhesion, cadherin complex composition and the cavitation machinery are well established in both homozygous mutants.
Fig. 3. Markers for lineage specification, cell polarity, and vectorial fluid flow are correctly expressed and localized in NcEc homozygous mutants. 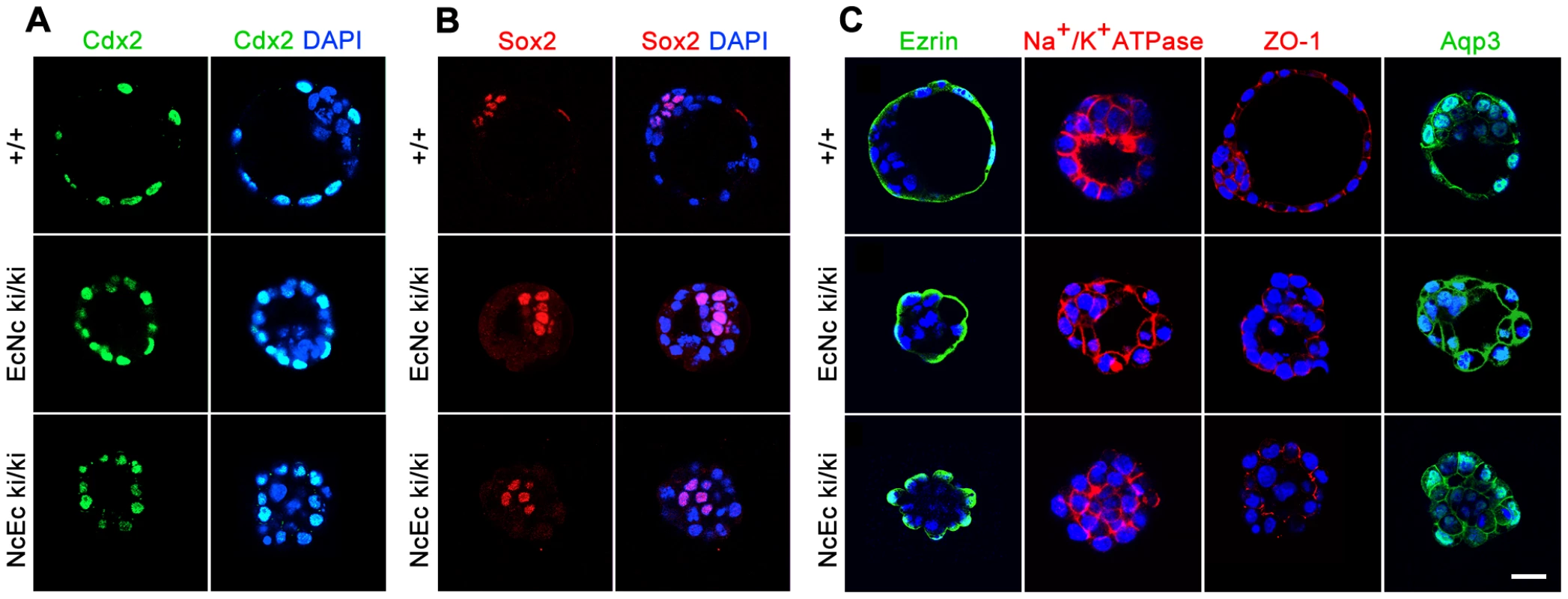
(A) Proper segregation of outer TE cells is shown by anti-Cdx2 labeling in wt, EcNc and NcEc homozygous mutants at E3.5. (B) Wildtype, EcNc and NcEc homozygous mutant embryos labeled for the ICM cell marker Sox2 in inner cells, showing ICM cell specification and its localization inside NcEc homozygous embryos. (C) Ezrin and Na+/K+-ATPase staining to verify apical-basal polarity with same distribution in wt, EcNc and NcEc homozygous mutants at the apical and basolateral membrane. Correct sealing of the TE layer is indicated by the presence and proper localization of the tight junctional component ZO-1 at apical sites of lateral TE membranes in wt, EcNc and NcEc homozygous embryos. Key components required for vectorial fluid transport are shown by the presence of Na+/K+-ATPase and Aqp3. In embryos of all genotypes, this expression is detected in the outer cells, without any obvious differences in expression between the embryos. Hence, the first lineage segregation is specified correctly, and proteins that are essential for the TE formation process and its function are present and properly localized in NcEc homozygous mutants. Nuclei were labeled with DAPI (blue). Scale bar, 25 µm. Absence of the extracellular domain of E-cad leads to the induction of apoptosis in TE cells
One major difference between the NcEc ki/ki embryos and the EcNc ki/ki embryos was that the failure of proper TE formation in NcEc mutants was accompanied by cell scattering and vacuolation in the outside cells, both of which indicate the induction of programmed cell death (PCD). To verify an aberrant induction of PCD in NcEc mutants, embryos were labeled for cleaved Caspase 3, a general marker for the activation of apoptosis. A substantial increase in number of Caspase 3-positive cells was detected in the TROMA-1 labeled outer cells of the mutants (Figure 4A and 4B). In contrast, no apoptosis was found in the TE cells of homozygous EcNc embryos or control littermates. Moreover, EcNc ki/ki embryos did not show a delayed onset of apoptosis as identified in prolonged embryo cultures for additional 24 h, indicating that in these mutants TE was not prone to PCD (Figure S4B and S4C). Interestingly, the induction of PCD in homozygous NcEc mutants was phenocopied if wildtype (wt) embryos were incubated with staurosporine, a bacterial-derived alcaloid which activates PCD by inducing Caspase 3. Treating wt embryos with 50 nM staurosporine severely compromised blastocyst formation (Figure S5A, S5B and S5D). This result strongly indicated that in NcEc mutants, the fragile equilibrium between cell survival and cell death was shifted towards apoptosis due to the misexpression of the NcEc chimeric cadherin in the E-cad (Cdh1) expression domain. Moreover, since increased PCD was not detected in EcNc mutants, we concluded that replacing the extracellular domain of E-cad with N-cad specifically caused this imbalance. In the following experiments, we sought to re-establish this fine-tuned equilibrium to promote cell survival.
Fig. 4. Increased apoptosis is detected in the outer cells of homozygous NcEc embryos and is blocked by iloprost treatment, rescuing blastocyst formation. 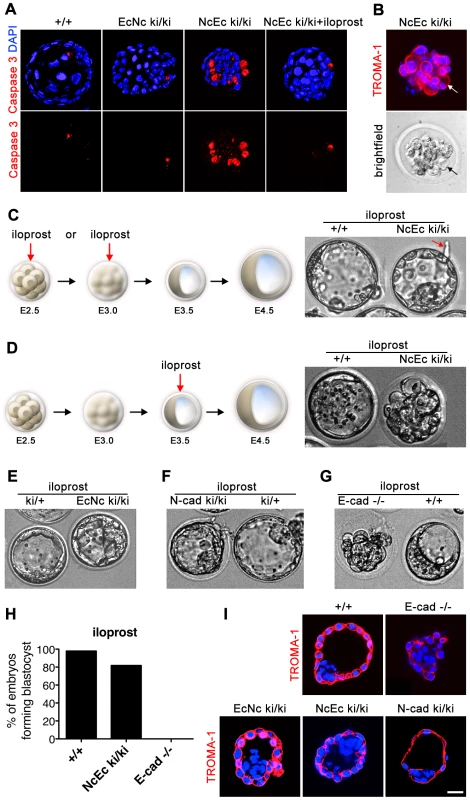
(A) Labeling of cleaved and activated Caspase 3 (red) shows only one apoptotic cell in the ICM of control and EcNc ki/ki embryos, whereas the outer cells of NcEc homozygous mutant embryos display a substantial increase in Caspase 3-positive cells. Cleavage of Caspase 3 was not detected upon iloprost treatment (1 µM) (B) Cell blebbing and vacuolization is detected in TROMA-1 positive outer cells of NcEc ki/ki embryos demonstrating induction of PCD in cells destined to become TE (arrow). (C) Treatment of NcEc ki/ki embryos with 1 µM iloprost at the precompacted or compacted morula stage (E2.25–E3.0) observed by time-lapse microscopy rescues the TE formation defect and hatching is initiated (arrow). (D) Treatment with iloprost at a later time-point (E3.5) did not rescue the phenotype. (E) One representative frame of a time-lapse recording of iloprost-treated EcNc ki/ki embryos. The formation of the blastocyst is marginally improved. (F) Treatment of N-cad ki/ki embryos with iloprost resulted in a rescue similar to that for NcEc homozygous mutants. (G) E-cad-null embryos were not rescued upon treatment with iloprost. (H) Percentage of iloprost-treated embryos that formed a proper blastocyst in time-lapse experiments and during in vitro culture for wt (n = 65), NcEc homozygous mutants (n = 33) and E-cad−/− (n = 15) in >5 independent experiments. (I) With the exception of E-cad−/− embryos, proper specification of the rescued TE of iloprost-treated homozygous mutants was confirmed by cytokeratin 8 expression, which is restricted to TE cells (TROMA-1, red). Scale bar, 25 µm. Blocking PCD rescues the blastocyst formation defect observed in NcEc ki/ki mutants
Proper preimplantation development in mice and humans relies on the orchestrated program of various growth factors and small secreted molecules, such as prostaglandins, which are produced by the embryo and the oviduct, [30], [31]. In vitro, activation of prostacyclin-dependent signaling enhances embryo survival and hatching of mouse, human and pig embryos by suppressing Caspase 3 activation via PPARδ and 14-3-3ε and by blocking cytochrome C release from the mitochondria [31]. Here, we used iloprost, a stable synthetic analogue of prostacyclin (PGI2) and analyzed the effect on blastocyst formation in NcEc homozygous mutants [32], [33]. Strikingly, if homozygous NcEc mutants were cultured between E2.5 and E3.5 in the presence of 1 µM iloprost, an accurate blastocoel cavity formed within the 24-h time-lapse recording (Figure 4C, 4D, 4H; Figure S5C; Video S3). Hence, blocking Caspase 3-mediated activation of PCD rescued this phenotype. We re-investigated N-cad ki/ki embryos that also failed to form a TE under standard conditions [21]. Interestingly, treatment of those embryos rescued blastocyst formation in a similar fashion, whereas there was only a moderate effect on homozygous EcNc mutants, resulting in enhanced stability of the TE (Figure 4E, 4F and Video S4). In contrast, blastocyst formation was not rescued in in vitro cultured E-cad-null embryos, presumably due to the entire absence of cadherin-mediated adhesion, which is indispensable for TE formation (Figure 4G, 4H and Video S5). The proper spatial organization and the epithelial nature of the TE in iloprost-stimulated mutants were confirmed by TROMA-1 staining. An intact TE layer of TROMA-1-positive cells that was correctly separated from TROMA-1-negative ICM cells was detected in homozygous NcEc and N-cad ki/ki mutants (Figure 4I). Manipulation of other branches of the PCD pathway resulted similarly in rescue of NcEc embryos. Either blocking p53 by cyclic pifithrin alpha (cPFT) or directly inhibiting Caspase 3 by Z-DEVD-FMK enabled both TE formation and the maintenance of blastocyst integrity (Figure S4A). These results indicated that prosurvival cues need to be active during blastocyst formation and that in homozygous NcEc mutants the shifted balance of cell survival and PCD was artificially returned to its equilibrium by inhibiting the apoptotic program at different levels. However, this rescue was only possible in a cadherin-mediated manner since blocking apoptosis rescued only cadherin-expressing embryos.
Activation of insulin-like growth factor I receptor (Igf1r) signaling by excess Igf1 rescues blastocyst formation in NcEc embryos
Since all heterozygous mutant mice analyzed here did not show defects and developed normally until adulthood, a dominant effect of an inappropriate cadherin in TE cells is very unlikely. This was confirmed by analysis of compound NcEc/EcNc mice that show proper blastocyst formation capacity (Figure S4E). When searching for a putative prosurvival signal that is triggered by the presence of the extracellular domain of E-cad, we focused on receptor tyrosine kinase (RTK) signaling cascades. In specific cellular contexts, cadherins interact with RTKs like Egfr and Fgfr2 and thus modulate downstream signaling activities [34]–[36]. Since incubation with bFGF did not improve blastocyst formation (data not shown), and mutations in Egfr do not reveal TE defects [37], we focused on Igf1r-mediated signaling. In previous studies, Igf1 enhanced blastocyst formation by providing a survival signal through PI3K/Akt [27], [28]. In agreement with these data, blocking Igf1r signaling in wt embryos at the morula stage with a specific inhibitor (Tyrphostin AG1024) induced cell fragmentation of outer cells and blocked TE formation (Figure S5E). When NcEc homozygous mutant embryos were treated with 100 ng/ml Igf1 for 24 h and recorded with time-lapse microscopy, these embryos formed a stable TE and even initiated hatching at the end of the recording (Figure 5A, 5F; Figure S4A, S4B; Video S6). In agreement with our previous results, Igf1-mediated rescue was observed in homozygous NcEc and N-cad ki/ki mutants but not in E-cad−/− embryos (Figure 5C–5F and Videos S8 and S9). To rule out the possibility that Igf1 simply delayed the induction of PCD we generated prolonged embryonic cultures of NcEc ki/ki. After initial 24 h incubation, embryos were transferred to fresh medium and kept for additional 24 h in the incubator. In the presence of Igf1 NcEc ki/ki embryos formed a stable TE without showing indications of apoptosis (Figure S4B). Thus, the treatment of NcEc ki/ki embryos with either Igf1, iloprost or cPFT rescued apoptosis as indicated by absence of active Caspase 3 staining (Figure S4D).
Fig. 5. Artificial increase of Igf1 levels during in vitro culture rescues blastocyst formation suggesting Igf1 signaling as the endogenous prosurvival stimulus. 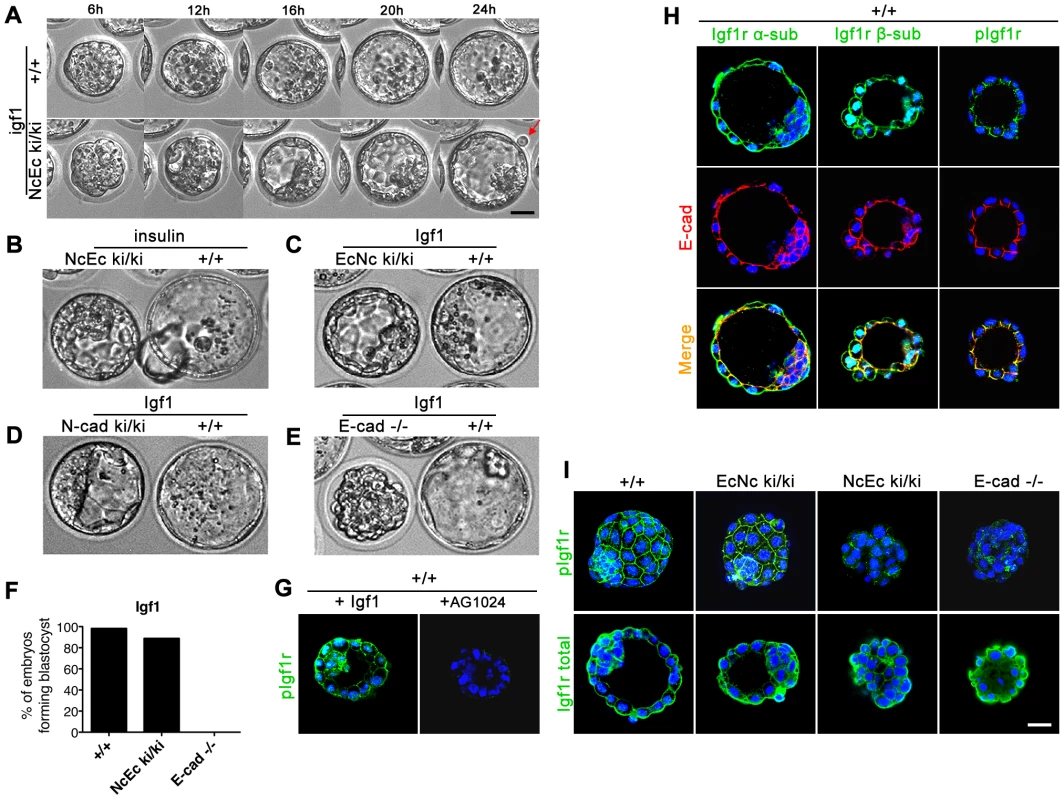
(A) Wildtype and homozygous NcEc mutant embryos were incubated in the presence of 100 ng/ml Igf1 and recorded in 15-min intervals for 24 h. Images are displayed for 6-h intervals. Mutant embryos form a proper blastocyst similar to their control littermates and initiation of hatching is observed (arrow). (B) Insulin treatment rescues TE formation in a similar but milder fashion compared to Igf1 treatment. (C) Incubation of homozygous EcNc mutant embryos with Igf1 did not result in significant changes in blastocyst formation. (D) N-cad ki/ki embryos formed a blastocyst in the presence of Igf1. (E) E-cad−/− embryos were not rescued by Igf1 treatment. (F) Percentage of Igf1-treated embryos that formed a proper blastocyst in time-lapse experiments and during in vitro culture for wt (n = 31), NcEc homozygous mutants (n = 18) and E-cad−/− (n = 13) in >5 independent experiments. (G) Incubation of wt embryos with 100 ng/ml Igf1 or 10 µm Tyrphostin AG1024, a specific Igf1r inhibitor, induced hyperactivation or absence of Igf1r activation, respectively, demonstrating specificity of both the anti-Igf1r antibody and the inhibitor. Nuclear staining of the β-subunit and the phosphorylated form of Igf1r is additionally detected in the nucleus and is increasing upon Igf1 treatment as observed previously [65]. (H) The activated form of Igf1r showed protein colocalization with E-cad in the TE cells of wt embryos. An antibody detecting the α- or the β-subunit of Igf1r (total Igf1r) showed localization of Igf1r throughout the membrane, partially overlapping with E-cad (left and middle panel, respectively). A complete overlap of the activated phosphorylated form of the receptor (pIgf1r) and E-cad labeling at lateral cell-cell contact sites was detected in the TE (right panel). (I) Igf1r was hypoactivated in NcEc- and E-cad-null embryos. Immunofluorescence labeling of pIgf1r and total Igf1r showed comparable intensities of activated Igf1r in wt and EcNc embryos, whereas a substantial reduction was found in NcEc and E-cad−/− embryos. Total Igf1r levels were unaffected. Scale bar, 25 µm. In utero as well as in vitro, preimplantation embryos receive insulin (Ins1) - and insulin receptor (Insr)-mediated signals [23]. Thus, homozygous mutants were incubated with 25 µg/ml insulin to determine whether this pathway contributes to cell survival. Although there was a significant improvement in the formation of a blastocoel cavity, the effect was modest in comparison to Igf1 treatment (Figure 5B and Video S7). This result revealed that Igf1 and the activation of its receptor Igf1r plays a crucial role during preimplantation development and the receptor kinase activity provides the endogenous survival signal in wt embryos that is blocked or attenuated in NcEc and N-cad ki/ki mutants.
E-cadherin interacts with Igf1r and is required for efficient receptor activation to mediate the survival of TE cells
Our previous analysis indicated a functional link between E-cad and Igf1r. In vitro, a direct interaction between these two proteins was observed in MCF-7 cells [38], [39], but whether this interaction also influences Igf1r activity in a ligand-dependent or ligand-independent manner is unknown. To further study the putative role of Igf1r in preimplantation development and whether Igf1r kinase activity is facilitated by E-cad to regulate survival of TE cells, we first analyzed the expression of Igf1r and the amount of its activated form (pIgf1r). In wt embryos, the receptor was detected in preimplantation stages. It localized to basolateral membranes and additionally to the apical membrane of TE cells, showing a partial overlap with E-cad at cell contact sites (Figure 5H and Figure S5F). Interestingly, analysis of pIgf1r with a phospho-specific antibody revealed that the receptor was only activated at cell contact sites that showed substantial overlap with anti-E-cad staining at lateral membranes (Figure 5H and Figure S5F). Treatment of wt embryos with Igf1 hyperactivated Igf1r resulting in ectopic pIgf1r detection at apical sites, whereas blocking of receptor activation by Tyrphostin AG1024 abolished pIgf1r detection (Figure 5G and Figure S5F). Strikingly, and in contrast to wt or EcNc embryos, NcEc and E-cad−/− embryos showed weaker or absent pIgf1r staining intensities, although the overall amount of Igf1r was not changed (Figure 5I). Moreover, treatment of wt embryos with a chelating agent, like EGTA to deplete Ca2+-ions and to interfere with cadherin conformation and function [40] led to a decrease in pIgf1r levels mimicking the lack of Igf1r activation of homozygous NcEc mutant embryos (Figure S5G). These results suggested that the activity of the receptor is reduced in NcEc mutants due to lack of facilitation by interaction of E-cad and Igf1r. To test this idea, we performed a Duolink proximity ligation assay, a fluorescence-based method to show protein-protein interaction in situ. As a control, we analyzed the known interaction between E-cad and β-catenin in wt blastocysts, which showed fluorescent signals at sites of interaction at basolateral membranes as expected (Figure 6A, 6). Analysis of the interaction of E-cad and Igf1r by Duolink revealed fluorescent labeling in wt blastocysts. In agreement with the cellular distribution of pIgf1r, interaction was detected at lateral membranes (Figure 6A, 1). A similar assay in wt embryos using an antibody against pIgf1r gave a comparable result, suggesting that E-cad interacts with the activated form of the receptor or that only E-cad-bound receptor becomes activated (Figure 6A, 4). However, In NcEc ki/ki embryos only a reduced and almost absent signal was present, although anti-pIgf1r and anti-E-cad antibodies were able to detected both proteins. The analysis demonstrated a lack of interaction in NcEc ki/ki embryos in agreement with our hypothesis (Figure 6A, 3). No signal was obtained in E-cad-null embryos (Figure 6A, 2, 5). In a second approach complexes between endogenously expressed E-cad and Igf1r were analyzed by anti-E-cad immunoprecipitation (IP). Wt and NcEc ki/ki trophoblast stem cells (TS cells) were isolated from blastocyst outgrowths and from ES cells after transient induction of ectopic Cdx2 expression, respectively. Binding of E-cad to Igf1r was identified upon co-immunoprecipitation of lysates from wt TS cell lysates (Figure 6B, upper panel). Two additional fragments of lower molecular weight were specifically co-precipitated and detected by two individual anti-Igf1r antibodies (Figure 6B and data not shown). The additional bands were very faint in the input samples. They presumably represent γ-secretase processed forms of the receptor as observed previously [41] generated upon activation and are enriched in the immunoprecipitation (Figure 6B, upper panel). In contrast to that, no interaction was seen when chimeric NcEc was precipitated with the same anti-E-cad antibody from lysates of NcEc ki/ki TS cells. Specific Igf1r signals were not detectable in IgG and E-cad IPs. Our data suggest that E-cad and Igf1r interact in the TE at sites of cell-cell contact. This interaction is indispensable for TE formation via facilitation of RTK signaling activity, which in turn promotes cell survival and keeps apoptosis at bay.
Fig. 6. E-cad interacts with Igf1r and increases receptor activity in TE cells. 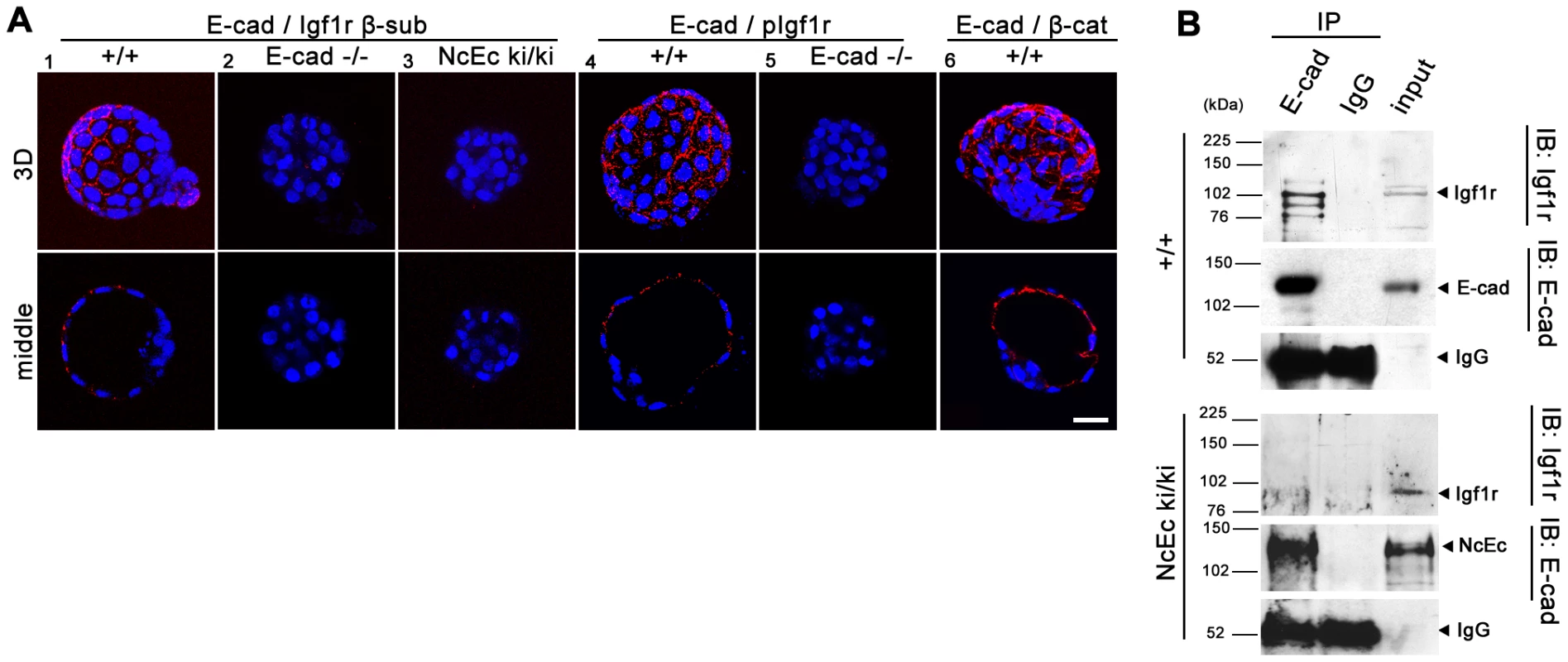
(A) A proximity ligation assay (Duolink) examining wt (1, 4, 6), E-cad-null (2, 5) and NcEc homozygous embryos (3). Red dotted fluorescence indicates sites of interaction of analyzed proteins as indicated in optical sections (lower row) and 3D reconstructions (upper row). The E-cad-Igf1r interaction was detected by antibodies against the β-subunit of Igf1r and the intracellular domain of E-cad, which also binds to the NcEc protein. In wt embryos, fluorescent dots indicating sites of protein-protein interactions, were found at cell-cell contact sites (1), whereas E-cad-null embryos that contained only residual maternally derived E-cad and NcEc homozygous embryos did not show a fluorescence signal, although both anti-Igf1r and anti-E-cad antibodies detect the β-subunit of Igf1r and NcEc, respectively in NcEc homozygous mutant embryos (2, 3). Similar results were obtained in a Duolink assay using anti-E-cad (intracellular domain) and anti-pIgf1r (activated form) antibodies (4, 5). The known interaction between E-cad and β-catenin gave a punctuate pattern at basolateral membranes in cells of wt embryos as control (6). (B) The interaction between E-cad and Igf1r analyzed by co-immunoprecipitation experiments in wt and NcEc ki/ki TS cells. Immunoprecipitation with anti-E-cad and IgG control antibodies displayed a specific interaction of E-cad to coprecipitated Igf1r (total) in wt TS cells (upper panel) whereas the receptor was not co-immunoprecipitated with the NcEc protein in NcEc ki/ki TS cells (lower panel). 5% input was loaded in the last lane. Scale bar, 25 µm. Discussion
Cadherins are bona fide adhesion molecules that are involved in clustering cells of the same type together. Additionally, a role in cadherin-mediated RTK signal transduction through their interactions with different receptors has been suggested. These interactions either attenuate or enhance RTK activation in a ligand-dependent or ligand-independent manner [34]–[36], [42], [43]. The Igf/insulin-like growth factor I receptor axis controls growth, differentiation and cell survival and comprises Igf1, Igf2 and insulin as ligands and Igf1r, Igf2r and insulin receptor (Insr) [23]. The activity of this pathway is further regulated by the Igf1-binding proteins Igfbp3, Igfbp4 and Igfbp5 [44], [45]. In addition, Igf1 binds to Insr and insulin to Igf1r with lower affinity, and Igf2 signaling is transduced through both Igf1r and Insr simultaneously [25]. In contrast, the major role of Igf2r is to attenuate Igf1 and Igf2 signals since it lacks a kinase domain [46]. During preimplantation development, Igf1r, Igf2r and Insr are expressed, and Igf1 and insulin are provided both maternally and zygotically [47]. Treatment of embryos in vitro with Igf1 enhances embryo viability via mitogenic and anti-apoptotic responses, indicating that Igf1 has a role in providing survival signals [27], [28], [30]. In this study, we unraveled a link between E-cad and Igf1r that promotes cell survival in the TE. Our data indicate a previously unknown function of Igf1r during preimplantation development. Furthermore, full activation of the Igf1r kinase domain likely requires physical interaction to the extracellular domain of E-cad. Abrogating cadherin function by conformational changes upon Ca2+-withdrawal [40], [48] results in reduced phosphorylation of Igf1r. If E-cad is replaced by either N-cad or a chimera harboring the extracellular domain of N-cad, no interaction of the two proteins is detected, and Igf1r is only inefficiently activated. Consequently, the balance between cell survival and cell death is shifted towards PCD, and embryos cannot form a functional TE. Homozygous NcEc and N-cad ki/ki embryos are rescued by an excess of Igf1 ligand because the activity of the receptor is artificially raised to normal levels. However, a full rescue of blastocyst formation is possible only if cadherin-mediated cell adhesion is also present (Figure 7). We hypothesize that E-cad, in addition to its role in mediating homophilic cell adhesion, has a novel function during preimplantation development and triggers the survival signal initiated by Igf1/Igf1r activation.
Fig. 7. A model of the molecular pathways that are involved in TE survival but are blocked in homozygous NcEc and N-cad ki/ki mutants. 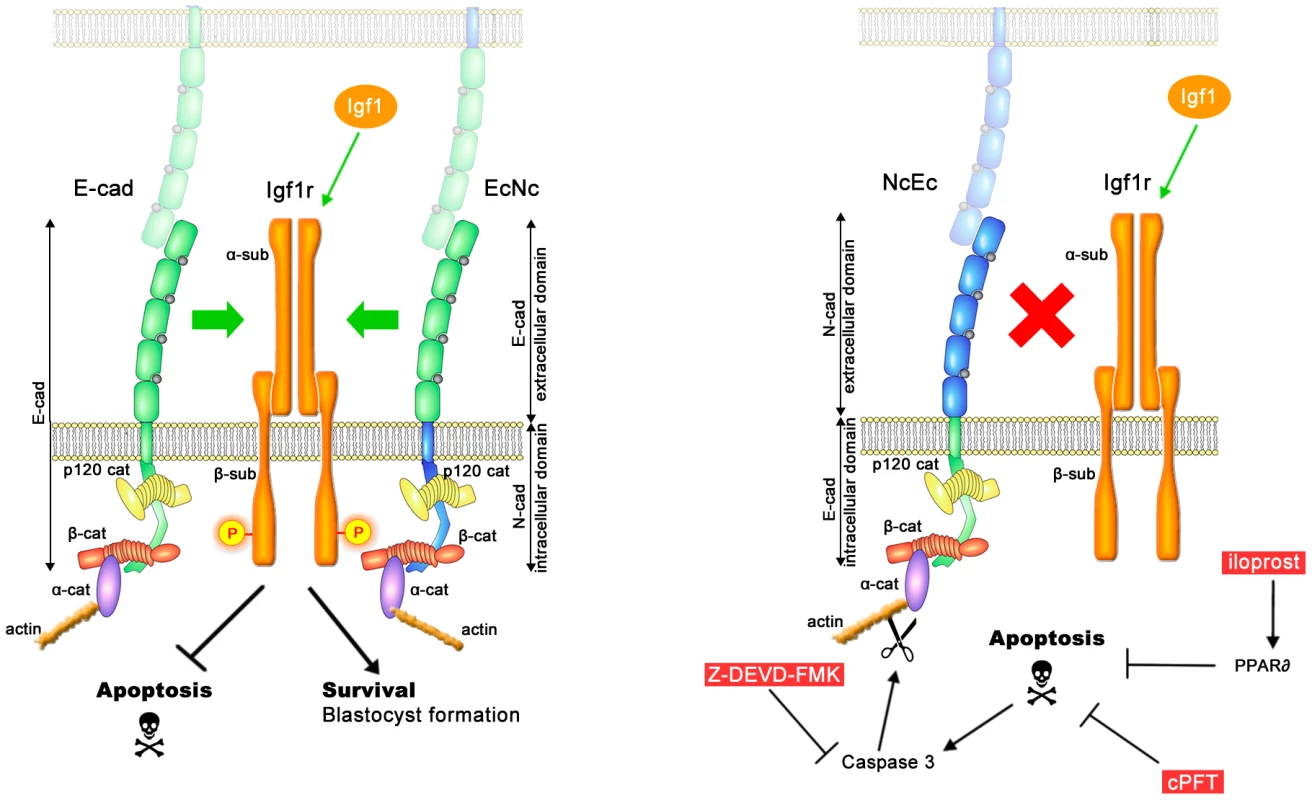
In the presence of full-length E-cad or of the E-cad extracellular domain in EcNc embryos interaction of cadherins with Igf1r is occurring. This enables proper activation of Igf1r upon Igf1 signaling (phosphorylation), which supplies survival signals and blocks PCD (left panel). In the absence of E-cad cell adhesion is maintained in presence of NcEc or N-cad, but both proteins are incapable of interacting with Igf1r. As a consequence of the uncoupled interaction, Igf1r is not fully activated, prosurvival signals are lacking and apoptotic pathways reach the threshold levels for PCD induction (right panel). In the presence of cadherin-mediated adhesion (in homozygous NcEc and N-cad ki/ki, but not in E-cad-null embryos), apoptotic pathways can be blocked only by external cues (red boxes), which inhibit PCD at different levels and thereby rescue TE formation. According to this model E-cad is required for providing survival cues via the extracellular domain in addition to its role in cell adhesion. Mice in which components of the Igf axis are knocked out have been described by several groups, and deficiencies in this axis lead to reductions in body size and weight [44]. Igf1 mutants are severely affected and display muscle dystrophies, with only 5% of offspring reaching adulthood, and these mice are infertile [26]. Although single, double and even triple knockouts of components of the Igf axis do not show preimplantation defects [25], [46], certain combinations of mutations always result in infertility. These mice may not show preimplantation defects, due to residual maternal activity: that is, the mice still receive Igf/insulin signals from maternal tissues, and they are also provided with maternal mRNA and protein for the receptors of the Igf axis during oocyte maturation. A combined maternal/zygotic loss-of function experiment has not been performed yet to show whether the complete depletion of ligands and receptors results in TE formation defects. By treatment with Tyrphostin AG1024 we here targeted Igf1r and Insr of both maternal and zygotic origin. This simultaneous blocking of entire downstream signaling results in the induction of apoptosis. In addition to previous observations, our data support the importance of Igf1r signaling already during preimplantation development, since also E-cad dependent loss of Igf1r activation results in inefficient maintenance of survival signals. Analyses of loss - and gain-of-function mutations are indicating a general underlying mechanism that controls cell survival. Cells isolated from Igf2-null or Igfr1-null animals display increased apoptosis as indicated by the small body size [25], [49]. In contrast, overexpression of Igf1 or Igf2 led to a decrease in apoptosis, observed as non-involuting mammary glands and pancreas hyperplasia, indicating a dramatic shift towards cell survival [50], [51]. Combined with our new findings Igf1 has a general role in controlling cell survival and cell death, a function that is active during preimplantation development as well.
Cadherin-mediated modulation of RTKs has been previously shown for Egfr and Fgfr2 [34], [35], [42], and it may be an intrinsic property of RTKs that they need to be clustered by or interact with cadherins to be efficiently activated. Interestingly, soluble E-cad isolated from the serum of cancer patients blocks apoptosis via activation of Egfr in MDCK cells [52]. This suggests a comparable role of the E-cad/Egfr interaction to that found in our analysis for Igf1r. It is tempting to speculate that during preimplantation development control of PCD by Igf1r is regulated in an E-cad-dependent manner and has an important function. The coupling of both proteins may act as a sensor to eliminate embryos that are unable to manage the crucial step during the morula to blastocyst transition, in which the embryo switches from depending on pyruvate to glucose [53]. This switch is correlated with increasing demands for ATP within the embryo, to support Na+/K+-ATPase activity, and is mediated by Igf1r [54]. In line of our hypothesis, the fragile balance between survival and apoptosis is linked to an interaction of Igf1r and E-cad, which acts as a checkpoint to assess the viability of the embryos. Saving nutrients and energy by eliminating abnormal embryos at an early stage is a favored strategy. Our analysis suggests a hitherto unknown preimplantation checkpoint that couples integrity of the TE to embryo survival. Additionally, there is evidence that connects E-cad and the regulation of the PCD-cell survival balance in other tissues. Hyperactivated Igf1r in the mammary gland shifted the balance towards cell survival [50], whereas an opposite effect that promotes apoptosis was observed after E-cad (Cdh1) depletion. During lactation milk production is hampered due to precocious involution, resulting in a shift towards PCD [55]. It will be interesting to address whether loss of E-cad also impairs proper Igf1r function in the mammary gland by a similar mechanism as described here.
Although our data are in favor of this model, we cannot fully exclude a different mechanism and contribution of secondary effects. Many RTKs utilize co-receptors like integrins or adhesion molecules, such as CD44 for proper function [56]. In our mutants adhesion and cell polarity may be altered resulting in improper localization of Igf1r. However, staining for total Igf1r and cell polarity markers of NcEc ki/ki mutants indicated that Igf1r expression and protein localization were not changed and the TE cells still maintain apical-basal polarity. Nevertheless, secondary effects may occur due to reduced cell adhesion based on the artificial nature of the chimeric cadherins and/or to altered connection to the cytoskeleton, essential for proper adhesion. This may differ between the two molecules EcNc and NcEc and may have escaped from our analysis. Interestingly, our data suggest that in addition to the extracellular domain the intercellular domain of E-cad contributes to TE formation and normal development as well since the EcNc homozygous mutants are incapable of hatching. Unique molecular features may reside in differential affinities to bind to β-catenin or other interacting proteins, as has been suggested previously [57]. E-cad and N-cad differ in their interactions with p120ctn isoforms, which may influence p120ctn-mediated small GTPase activity and the flexibility of adherens junctions [58]. Very likely, unique intracellular interaction partners exist for both cadherins but need to be identified in future experiments.
Our study has unraveled a novel role of Igf1r activity and a crucial mechanism that provides a link of how E-cad may control the balance between cell survival and PCD. Igf1r activation is essential to promote cell survival in the TE lineage, but requires E-cad-mediated facilitation of the signal via protein interaction. Unraveling the role of this function and its implication for morphogenesis and differentiation will have a significant impact on our understanding of cadherin-mediated signaling during embryogenesis and in human diseases, such as cancer.
Materials and Methods
Ethics statement
Animal husbandry and all experiments were performed according to the German Animal Welfare guidelines and approved by the local authorities.
Generation of knock-in mice and genotyping
cDNAs of E-cad and N-cad were used to generate coding sequences for chimeric proteins EcNc and NcEc corresponding to the extracellular domain of E-cad (amino acids 1–710) fused to intracellular domain including transmembrane portion of N-cad (aa 725–906) and aa 1–724 of N-cad fused to aa 711–884 of E-cad, respectively. Both sequences were combined with a C-terminal HA-tag and inserted into the ATG codon of the previously described targeting vector (pBluescriptII, Stratagene) using standard molecular cloning techniques [21], [22]. Homologous recombination and analysis of surviving ES cells by Southern blot was done as described [21], [22]. Two independent clones were used for injection into blastocysts to generate chimeric mice. After backcrossing to Zp3-cre mice to delete the neomycin resistance cassette during oocyte maturation EcNc and NcEc heterozygous mouse lines were established [59], backcrossed to C57BL/6 and inter se to obtain homozygous mutant embryos. Genotyping was performed by PCR using tail biopsies, yolk sacs or entire embryos with the following primers: wt allele (Ecad5′UTR_s, CCC AAG AAC TTC TGC TAG AC/Ecad1_as, TAC GTC CGC GCT ACT TCA), EcNc and NcEc alleles (ENcad3′_s, AAG CTG GCG GAC ATG TAC/Ecad1_as), EcNc (Ecad_s, ATC GCC ACA CTC AAA GTG/Ncad1_as, CTG TGG CTC AGC ATG GAT), NcEc (Ncad_s, TGG AAG CTG GTA TCT ATG/Ecad2_as, TCA TCA GGA TTG GCA GGA), Ncad ki (Ecad5′UTR_s/Ncad2_as, TGG CAA GTT GTC TAG GGA).
Embryo time-lapse microscopy and treatments
Preimplantation embryos were isolated by flushing the oviducts or uteri with M2 medium, transferred into 10 µl KSOM droplets covered with mineral oil (Fluka). Time-lapse microscopy was performed as described with minor modifications using a Zeiss Axiovert 200 M microscope equipped with Narishige manipulators, Incubator XL and Tempcontrol together with a humidifier connected to a heating stage E100 (Zeiss) at 37°C and 7.5% CO2 [21], [22]. Embryos were photographed every 15 min for 24 h. Zeiss AxioVision ver. 4.8 software and Uniblitz shutters were used for the acquisition of time-lapse images. Embryos were treated with specific inhibitors and growth hormones as indicated in the following concentrations: 1 µM iloprost (Cayman chemical), 30 µM cPFTalpha (Sigma), 50 µM Z-DEVD-FMK (Enzo), 1–50 nM staurosporine (Enzo), 100 ng/ml Igf1 (eBioscience), 25 µg/ml insulin (Sigma), 10 µM Tyrphostin AG1024 (Alexis biochemicals), 2 mM EGTA. Each experiment was repeated at least five times.
Immunofluorescence labeling and confocal microscopy
After isolation embryos were washed in PBT (0.05% Tween/PBS) and fixed with 2% PFA/PBS for 10 min. Cellular permeabilization was carried out for 5 min with 0.3% Triton X-100/PBS and embryos were incubated in primary antibody in 2.5% BSA/PBT for 2 h to overnight at room temperature. Subsequently, alexa488 or alexa594-conjugated secondary antibodies were applied for 1 h. Embryos were stained with DAPI to visualize nuclei (1∶1000, Invitrogen) and mounted in PBS droplets covered with mineral oil in glass bottom petri dishes (Willco wells). Confocal microscopy was performed using Leica TCS SP2 laser scan head attached to a Leica DM IRE2 inverted microscope. Images were processed using IMARIS software (Bitplane). Antibodies: anti-E-cadherin (intracellular), anti-N-cadherin, anti-β-catenin, anti-Plakoglobin, (BD Bioscience), HA.11 (Covance), anti-Ezrin, anti-cleaved Caspase 3 (Cell Signaling), anti-E-cadherin (extracellular, gp84) [60], TROMA-1 [61], anti-p120ctn, anti-ZO-1 (Zymed), anti-Na+/K+-ATPase (Millipore), anti-AQP3, anti-Sox2 (Calbiochem), anti-Oct4 (Santa Cruz), anti-Nanog [62], anti-Cdx2 (Biogenex), anti-Igf1r (α-subunit, abcam), anti-Igf1r (β-subunit, Cell Signaling), anti-pIgf1r (abcam).
Duolink assay
The Duolink assay (Olink Bioscience) was performed according to the manufacturers instructions in 10 µl droplets covered with mineral oil at 37°C.
Immunoblotting and immunoprecipitation
Immunoblotting and immunoprecipitation (IP) was performed as described for ES cell lysates (500 ng protein, 500 ng antibody) or with minor modifications for TS cells [22]. Briefly, TS cells were stimulated with 50 ng/ml Igf1 for 10 min, incubated in crosslinking buffer (6 mM KCl, 2 mM Bissulfosuccinimidyl suberate/PBS) for 30 min at 4°C, followed by quenching in 100 mM Glycine/PBS and harvested in lysis buffer (20 mM Tris-HCl pH 7.9, 137 mM NaCl, 2 mM MgCl2, 5 mM EDTA, 1 mM EGTA, 1% Triton X-100, 10% Glycerol, 10 mM Na3VO4, 10 mM NaF, 1× Complete protease inhibitor, Roche, 1 mM PMSF) [43]. For IP, 2 mg of protein were incubated overnight at 4°C using 1 µg anti-E-cad (BD) antibody and 25 µl slurry of protein-G coupled Dynabeads (Invitrogen). After washing in washing buffer (25 mM Tris-HCl pH 7.4, 100 mM NaCl, 0.1% Tween-20) IP samples were separated by 8–10% SDS-PAGE. Proteins of the IP or from whole cell lysates were transferred to nitrocellulose membranes using a semi-dry electroblotter (Biorad) with 200 mA for 45 min. After blocking (2% dry milk in TBS/0.1% Tween-20) for 30 min, membranes were incubated with antibody solution for 2 h to overnight and subsequently with a secondary horseradish peroxidase-conjugated antibody for 1 h. Anti-Igf1r (β-subunit), anti-Igf1r (total, α-IR3, Calbiochem), anti-E-cad (BD) and anti-Gapdh (Calbiochem) were used. Proteins were detected by soaking membranes in ECL Plus (GE Healthcare) exposed to autoradiography films.
ES/TS-cell derivation and teratoma formation
Generation of homozygous EcNc and NcEc ES cells was performed as described previously using morulae or blastocysts from heterozygous intercrosses, plated on mytomycin-treated embryonic fibroblasts [22]. Alkaline phosphatase (AP) staining of isolated ES cells was used to verify undifferentiated pluripotent status. Cells were fixed in 4% PFA/PBS for 15 min, washed two times with PBS and incubated for 30 min in AP staining solution (25 mM Tris-maleic acid pH 9.0, 0.4 mg/ml α-naphtyl phosphate, 1 mg/ml Fast Red TR Salt, 8 mM MgCl2, 0.01% Na-deoxycholate, 0.02% NP40). Wt TS cells were generated similar to ES cells by blastocyst outgrowth in RPMI 1640, 20% FCS, 2 mM glutamine, 1 mM pyruvate, 50 µg/ml penicillin/streptomycin, 100 µM β-mercaptoethanol, 25 ng/ml FGF4 (Sigma), 1 µg/ml Heparin as described [63]. NcEc ki/ki TS cells were derived from transdifferentiated ES cells by stable transfection of an inducible Cdx2 plasmid (Cdx2ER) and treated with 1 µg/ml 4-OH-tamoxifen for 10 days [64]. Teratoma formation was induced by subcutaneous injection of 1×107 trypsinized ES cells into BALB/c nude mice as described [21], [22].
Paraffin embedding and immunohistochemistry
Specimen were fixed at 4°C overnight in 4% PFA/PBS and dehydrated in 30%, 50%, 70%, 100% Ethanol/PBS series for 1 h each, followed by two 10 min incubations in 100% Xylene before transferring them to paraffin overnight. Casted into paraffin blocks samples were sectioned using a RM2155 microtome (Leica) at 7 µm and stored at 4°C until further processing. Hematoxylin/eosin (H&E) staining and immunohistochemistry was carried out as described previously using epitope retrieval by 20 min boiling in Tris-EDTA pH 9.0 buffer [22], [57].
RNA isolation and quantitative RT–PCR
Analysis of transcripts of the knock-in alleles was carried out as described previously [22]. For detection of individual transgenic transcripts the following primers were used to detect sequences of: 5′ E-cad wt or knock-in (5′EcadUPL_s, AGT GTT TGC TCG GCG TCT/5′EcadUPL_as, GCA AAG CCA TGA GGA GAC C); 3′ E-cad knock-in (3′EcadUPL_s, CAC CCC CTT ACG ACT CTC TG/HA-UPL_as, GAC GTC ATA AGG ATA TCC AGC A); 5′ N-cad knock-in (5′NcadKIUPL_s, CCA TGG CCA CTA GTA TGT GC/5′NcadKIUPL_as, AAT TTC ACC AGA AGC CTC CA); 3′ N-cad knock-in (3′NcadKIUPL_s, GGC CTT AAA GCT GCT GAC AA/3′NcadKIUPL_as, AAC CAT TAT AAG CTG CAA TAA ACA A); Actb (bActUPL_s, AAG GCC AAC CGT GAA AAG AT/bActUPL_as, GTG GTA CGA CCA GAG GCA TAC).
Supporting Information
Zdroje
1. CockburnKRossantJ 2010 Making the blastocyst: lessons from the mouse. J Clin Invest 120 995 1003
2. RossantJTamPP 2009 Blastocyst lineage formation, early embryonic asymmetries and axis patterning in the mouse. Development 136 701 713
3. AvilionAANicolisSKPevnyLHPerezLVivianN 2003 Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev 17 126 140
4. ChazaudCYamanakaYPawsonTRossantJ 2006 Early lineage segregation between epiblast and primitive endoderm in mouse blastocysts through the Grb2-MAPK pathway. Dev Cell 10 615 624
5. NicholsJZevnikBAnastassiadisKNiwaHKlewe-NebeniusD 1998 Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95 379 391
6. NishiokaNInoueKAdachiKKiyonariHOtaM 2009 The Hippo Signaling Pathway Components Lats and Yap Pattern Tead4 Activity to Distinguish Mouse Trophectoderm from Inner Cell Mass. Dev Cell 16 398 410
7. StrumpfDMaoCAYamanakaYRalstonAChawengsaksophakK 2005 Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development 132 2093 2102
8. StemmlerMP 2008 Cadherins in development and cancer. Mol Biosyst 4 835 850
9. LarueLOhsugiMHirchenhainJKemlerR 1994 E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci U S A 91 8263 8267
10. RiethmacherDBrinkmannVBirchmeierC 1995 A targeted mutation in the mouse E-cadherin gene results in defective preimplantation development. Proc Natl Acad Sci U S A 92 855 859
11. De VriesWNEvsikovAVHaacBEFancherKSHolbrookAE 2004 Maternal beta-catenin and E-cadherin in mouse development. Development 131 4435 4445
12. StephensonROYamanakaYRossantJ 2010 Disorganized epithelial polarity and excess trophectoderm cell fate in preimplantation embryos lacking E-cadherin. Development 137 3383 3391
13. RadiceGLRayburnHMatsunamiHKnudsenKATakeichiM 1997 Developmental defects in mouse embryos lacking N-cadherin. Dev Biol 181 64 78
14. LuoYFerreira-CornwellMBaldwinHKostetskiiILenoxJ 2001 Rescuing the N-cadherin knockout by cardiac-specific expression of N - or E-cadherin. Development 128 459 469
15. AberleHButzSStappertJWeissigHKemlerR 1994 Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci 107 Pt 12 3655 3663
16. ReynoldsABDanielJMcCreaPDWheelockMJWuJ 1994 Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol 14 8333 8342
17. LiGSatyamoorthyKHerlynM 2001 N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res 61 3819 3825
18. VleminckxKVakaetLJrMareelMFiersWvan RoyF 1991 Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 66 107 119
19. WheelockMJShintaniYMaedaMFukumotoYJohnsonKR 2008 Cadherin switching. J Cell Sci 121 727 735
20. NietoMA 2009 Epithelial-Mesenchymal Transitions in development and disease: old views and new perspectives. Int J Dev Biol 53 1541 1547
21. KanNGStemmlerMPJunghansDKanzlerBde VriesWN 2007 Gene replacement reveals a specific role for E-cadherin in the formation of a functional trophectoderm. Development 134 31 41
22. StemmlerMPBedzhovI 2010 A Cdh1HA knock-in allele rescues the Cdh1-/ - phenotype but shows essential Cdh1 function during placentation. Dev Dyn 239 2330 2344
23. AllanGJFlintDJPatelK 2001 Insulin-like growth factor axis during embryonic development. Reproduction 122 31 39
24. NakaeJKidoYAcciliD 2001 Distinct and overlapping functions of insulin and IGF-I receptors. Endocr Rev 22 818 835
25. LiuJPBakerJPerkinsASRobertsonEJEfstratiadisA 1993 Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75 59 72
26. Powell-BraxtonLHollingsheadPWarburtonCDowdMPitts-MeekS 1993 IGF-I is required for normal embryonic growth in mice. Genes Dev 7 2609 2617
27. LinTCYenJMGongKBHsuTTChenLR 2003 IGF-1/IGFBP-1 increases blastocyst formation and total blastocyst cell number in mouse embryo culture and facilitates the establishment of a stem-cell line. BMC Cell Biol 4 14
28. RileyJKCarayannopoulosMOWymanAHChiMMoleyKH 2006 Phosphatidylinositol 3-kinase activity is critical for glucose metabolism and embryo survival in murine blastocysts. J Biol Chem 281 6010 6019
29. StemmlerMPHechtAKemlerR 2005 E-cadherin intron 2 contains cis-regulatory elements essential for gene expression. Development 132 965 976
30. HardyKSpanosS 2002 Growth factor expression and function in the human and mouse preimplantation embryo. J Endocrinol 172 221 236
31. PakrasiPLJainAK 2008 Cyclooxygenase-2-derived endogenous prostacyclin reduces apoptosis and enhances embryo viability in mouse. Prostaglandins Leukot Essent Fatty Acids 79 27 33
32. HuangJCWunWSGoldsbyJSWunICNoorhasanD 2007 Stimulation of embryo hatching and implantation by prostacyclin and peroxisome proliferator-activated receptor delta activation: implication in IVF. Hum Reprod 22 807 814
33. KimJSChaeJISongBSLeeKSChooYK 2010 Iloprost, a prostacyclin analogue, stimulates meiotic maturation and early embryonic development in pigs. Reprod Fertil Dev 22 437 447
34. Fedor-ChaikenMHeinPWStewartJCBrackenburyRKinchMS 2003 E-cadherin binding modulates EGF receptor activation. Cell Commun Adhes 10 105 118
35. SuyamaKShapiroIGuttmanMHazanRB 2002 A signaling pathway leading to metastasis is controlled by N-cadherin and the FGF receptor. Cancer Cell 2 301 314
36. HoschuetzkyHAberleHKemlerR 1994 Beta-catenin mediates the interaction of the cadherin-catenin complex with epidermal growth factor receptor. J Cell Biol 127 1375 1380
37. ThreadgillDWDlugoszAAHansenLATennenbaumTLichtiU 1995 Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science 269 230 234
38. CanoniciASteelantWRigotVKhomitch-BaudABoutaghou-CheridH 2008 Insulin-like growth factor-I receptor, E-cadherin and alpha v integrin form a dynamic complex under the control of alpha-catenin. Int J Cancer 122 572 582
39. GuvakovaMASurmaczE 1997 Overexpressed IGF-I receptors reduce estrogen growth requirements, enhance survival, and promote E-cadherin-mediated cell-cell adhesion in human breast cancer cells. Exp Cell Res 231 149 162
40. PokuttaSHerrenknechtKKemlerREngelJ 1994 Conformational changes of the recombinant extracellular domain of E-cadherin upon calcium binding. Eur J Biochem 223 1019 1026
41. McElroyBPowellJCMcCarthyJV 2007 The insulin-like growth factor 1 (IGF-1) receptor is a substrate for gamma-secretase-mediated intramembrane proteolysis. Biochem Biophys Res Commun 358 1136 1141
42. PeceSGutkindJS 2000 Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell-cell contact formation. J Biol Chem 275 41227 41233
43. QianXKarpovaTSheppardAMMcNallyJLowyDR 2004 E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J 23 1739 1748
44. BelfioreAFrascaFPandiniGSciaccaLVigneriR 2009 Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev 30 586 623
45. FengZLevineAJ 2010 The regulation of energy metabolism and the IGF-1/mTOR pathways by the p53 protein. Trends Cell Biol 20 427 434
46. LouviAAcciliDEfstratiadisA 1997 Growth-promoting interaction of IGF-II with the insulin receptor during mouse embryonic development. Dev Biol 189 33 48
47. KaneMTMorganPMCoonanC 1997 Peptide growth factors and preimplantation development. Hum Reprod Update 3 137 157
48. GumbinerBM 2005 Regulation of cadherin-mediated adhesion in morphogenesis. Nature Reviews Molecular Cell Biology 6 622 634
49. LammGMChristoforiG 1998 Impairment of survival factor function potentiates chemotherapy-induced apoptosis in tumor cells. Cancer Res 58 801 807
50. NeuenschwanderSSchwartzAWoodTLRobertsCTJrHennighausenL 1996 Involution of the lactating mammary gland is inhibited by the IGF system in a transgenic mouse model. J Clin Invest 97 2225 2232
51. PetrikJPellJMAranyEMcDonaldTJDeanWL 1999 Overexpression of insulin-like growth factor-II in transgenic mice is associated with pancreatic islet cell hyperplasia. Endocrinology 140 2353 2363
52. IngeLJBarweSPD'AmbrosioJGopalJLuK 2011 Soluble E-cadherin promotes cell survival by activating epidermal growth factor receptor. Exp Cell Res 317 838 848
53. LeeseHJBartonAM 1984 Pyruvate and glucose uptake by mouse ova and preimplantation embryos. J Reprod Fertil 72 9 13
54. PantaleonMKayePL 1996 IGF-I and insulin regulate glucose transport in mouse blastocysts via IGF-I receptor. Molecular Reproduction and Development 44 71 76
55. BoussadiaOKutschSHierholzerADelmasVKemlerR 2002 E-cadherin is a survival factor for the lactating mouse mammary gland. Mech Dev 115 53 62
56. PontaHShermanLHerrlichPA 2003 CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 4 33 45
57. LibusovaLStemmlerMPHierholzerASchwarzHKemlerR 2010 N-cadherin can structurally substitute for E-cadherin during intestinal development but leads to polyp formation. Development 137 2297 2305
58. SeidelBBraegSAdlerGWedlichDMenkeA 2004 E - and N-cadherin differ with respect to their associated p120ctn isoforms and their ability to suppress invasive growth in pancreatic cancer cells. Oncogene 23 5532 5542
59. LewandoskiMWassarmanKMMartinGR 1997 Zp3-cre, a transgenic mouse line for the activation or inactivation of loxP-flanked target genes specifically in the female germ line. Curr Biol 7 148 151
60. VestweberDKemlerR 1984 Rabbit antiserum against a purified surface glycoprotein decompacts mouse preimplantation embryos and reacts with specific adult tissues. Exp Cell Res 152 169 178
61. KemlerRBruletPSchnebelenMTGaillardJJacobF 1981 Reactivity of monoclonal antibodies against intermediate filament proteins during embryonic development. J Embryol Exp Morphol 64 45 60
62. MesserschmidtDMKemlerR 2010 Nanog is required for primitive endoderm formation through a non-cell autonomous mechanism. Dev Biol 344 129 137
63. TanakaSKunathTHadjantonakisAKNagyARossantJ 1998 Promotion of trophoblast stem cell proliferation by FGF4. Science 282 2072 2075
64. NiwaHToyookaTShimosatoDStrumpfDTakahashiK 2005 Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell 123 917 929
65. AleksicTChitnisMMPerestenkoOVGaoSThomasPH 2010 Type 1 insulin-like growth factor receptor translocates to the nucleus of human tumor cells. Cancer Res 70 6412 6419
Štítky
Genetika Reprodukčná medicína
Článek Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1Článek Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / TranscriptionČlánek Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding DomainČlánek Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin ComplexesČlánek An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood ObesityČlánek Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAsČlánek Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
Článok vyšiel v časopisePLOS Genetics
Najčítanejšie tento týždeň
2012 Číslo 3- Gynekologové a odborníci na reprodukční medicínu se sejdou na prvním virtuálním summitu
- Je „freeze-all“ pro všechny? Odborníci na fertilitu diskutovali na virtuálním summitu
-
Všetky články tohto čísla
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
- Genomic Analysis of the Hydrocarbon-Producing, Cellulolytic, Endophytic Fungus
- Networks of Neuronal Genes Affected by Common and Rare Variants in Autism Spectrum Disorders
- Akirin Links Twist-Regulated Transcription with the Brahma Chromatin Remodeling Complex during Embryogenesis
- Too Much Cleavage of Cyclin E Promotes Breast Tumorigenesis
- Imprinted Genes … and the Number Is?
- Genetic Architecture of Highly Complex Chemical Resistance Traits across Four Yeast Strains
- Exploring the Complexity of the HIV-1 Fitness Landscape
- MNS1 Is Essential for Spermiogenesis and Motile Ciliary Functions in Mice
- A Fundamental Regulatory Mechanism Operating through OmpR and DNA Topology Controls Expression of Pathogenicity Islands SPI-1 and SPI-2
- Evidence for Positive Selection on a Number of MicroRNA Regulatory Interactions during Recent Human Evolution
- Variation in Modifies Risk of Neonatal Intestinal Obstruction in Cystic Fibrosis
- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Critical Evaluation of Imprinted Gene Expression by RNA–Seq: A New Perspective
- A Meta-Analysis and Genome-Wide Association Study of Platelet Count and Mean Platelet Volume in African Americans
- Mouse Genetics Suggests Cell-Context Dependency for Myc-Regulated Metabolic Enzymes during Tumorigenesis
- Transcriptional Control in Cardiac Progenitors: Tbx1 Interacts with the BAF Chromatin Remodeling Complex and Regulates
- Synthetic Lethality of Cohesins with PARPs and Replication Fork Mediators
- APOBEC3G-Induced Hypermutation of Human Immunodeficiency Virus Type-1 Is Typically a Discrete “All or Nothing” Phenomenon
- Interpreting Meta-Analyses of Genome-Wide Association Studies
- Error-Prone ZW Pairing and No Evidence for Meiotic Sex Chromosome Inactivation in the Chicken Germ Line
- -Dependent Chemosensory Functions Contribute to Courtship Behavior in
- Diverse Forms of Splicing Are Part of an Evolving Autoregulatory Circuit
- Phenotypic Plasticity of the Drosophila Transcriptome
- Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1
- Precocious Metamorphosis in the Juvenile Hormone–Deficient Mutant of the Silkworm,
- Igf1r Signaling Is Indispensable for Preimplantation Development and Is Activated via a Novel Function of E-cadherin
- Accurate Prediction of Inducible Transcription Factor Binding Intensities In Vivo
- Mitochondrial Oxidative Stress Alters a Pathway in Strongly Resembling That of Bile Acid Biosynthesis and Secretion in Vertebrates
- Mammalian Neurogenesis Requires Treacle-Plk1 for Precise Control of Spindle Orientation, Mitotic Progression, and Maintenance of Neural Progenitor Cells
- Tcf7 Is an Important Regulator of the Switch of Self-Renewal and Differentiation in a Multipotential Hematopoietic Cell Line
- REST–Mediated Recruitment of Polycomb Repressor Complexes in Mammalian Cells
- Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / Transcription
- Age-Dependent Brain Gene Expression and Copy Number Anomalies in Autism Suggest Distinct Pathological Processes at Young Versus Mature Ages
- A Genome-Wide Association Study Identifies Variants Underlying the Shade Avoidance Response
- -by- Regulatory Divergence Causes the Asymmetric Lethal Effects of an Ancestral Hybrid Incompatibility Gene
- Genome-Wide Association and Functional Follow-Up Reveals New Loci for Kidney Function
- A Natural System of Chromosome Transfer in
- Cell Size and the Initiation of DNA Replication in Bacteria
- Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding Domain
- Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin Complexes
- Temporal Transcriptional Profiling of Somatic and Germ Cells Reveals Biased Lineage Priming of Sexual Fate in the Fetal Mouse Gonad
- Rapid Analysis of Genome Rearrangements by Multiplex Ligation–Dependent Probe Amplification
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- The Atypical Calpains: Evolutionary Analyses and Roles in Cellular Degeneration
- The Silkworm Coming of Age—Early
- Development of a Panel of Genome-Wide Ancestry Informative Markers to Study Admixture Throughout the Americas
- Balanced Codon Usage Optimizes Eukaryotic Translational Efficiency
- The Min System and Nucleoid Occlusion Are Not Required for Identifying the Division Site in but Ensure Its Efficient Utilization
- Neurobeachin, a Regulator of Synaptic Protein Targeting, Is Associated with Body Fat Mass and Feeding Behavior in Mice and Body-Mass Index in Humans
- Statistical Analysis of Readthrough Levels for Nonsense Mutations in Mammalian Cells Reveals a Major Determinant of Response to Gentamicin
- Gene Reactivation by 5-Aza-2′-Deoxycytidine–Induced Demethylation Requires SRCAP–Mediated H2A.Z Insertion to Establish Nucleosome Depleted Regions
- The miR-35-41 Family of MicroRNAs Regulates RNAi Sensitivity in
- Genetic Basis of Hidden Phenotypic Variation Revealed by Increased Translational Readthrough in Yeast
- An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood Obesity
- Modelling Human Regulatory Variation in Mouse: Finding the Function in Genome-Wide Association Studies and Whole-Genome Sequencing
- Novel Loci for Adiponectin Levels and Their Influence on Type 2 Diabetes and Metabolic Traits: A Multi-Ethnic Meta-Analysis of 45,891 Individuals
- Polycomb-Like 3 Promotes Polycomb Repressive Complex 2 Binding to CpG Islands and Embryonic Stem Cell Self-Renewal
- Insulin/IGF-1 and Hypoxia Signaling Act in Concert to Regulate Iron Homeostasis in
- EMF1 and PRC2 Cooperate to Repress Key Regulators of Arabidopsis Development
- Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAs
- Contrasted Patterns of Molecular Evolution in Dominant and Recessive Self-Incompatibility Haplotypes in
- A Machine Learning Approach for Identifying Novel Cell Type–Specific Transcriptional Regulators of Myogenesis
- Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
- Nos2 Inactivation Promotes the Development of Medulloblastoma in Mice by Deregulation of Gap43–Dependent Granule Cell Precursor Migration
- Intracranial Aneurysm Risk Locus 5q23.2 Is Associated with Elevated Systolic Blood Pressure
- Heritability and Genetic Correlations Explained by Common SNPs for Metabolic Syndrome Traits
- A Genome-Wide Association Study of Nephrolithiasis in the Japanese Population Identifies Novel Susceptible Loci at 5q35.3, 7p14.3, and 13q14.1
- DNA Damage in Nijmegen Breakage Syndrome Cells Leads to PARP Hyperactivation and Increased Oxidative Stress
- DNA Resection at Chromosome Breaks Promotes Genome Stability by Constraining Non-Allelic Homologous Recombination
- Genetic Analysis of Floral Symmetry in Van Gogh's Sunflowers Reveals Independent Recruitment of Genes in the Asteraceae
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Promoter Nucleosome Organization Shapes the Evolution of Gene Expression
- The Nucleoside Diphosphate Kinase Gene Acts as Quantitative Trait Locus Promoting Non-Mendelian Inheritance
- The Ciliogenic Transcription Factor RFX3 Regulates Early Midline Distribution of Guidepost Neurons Required for Corpus Callosum Development
- Phosphorylation of the RNA–Binding Protein HOW by MAPK/ERK Enhances Its Dimerization and Activity
- A Genome-Wide Scan of Ashkenazi Jewish Crohn's Disease Suggests Novel Susceptibility Loci
- Parkinson's Disease–Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria
- LMW-E/CDK2 Deregulates Acinar Morphogenesis, Induces Tumorigenesis, and Associates with the Activated b-Raf-ERK1/2-mTOR Pathway in Breast Cancer Patients
- Mapping the Hsp90 Genetic Interaction Network in Reveals Environmental Contingency and Rewired Circuitry
- Autoregulation of the Noncoding RNA Gene
- The Human Pancreatic Islet Transcriptome: Expression of Candidate Genes for Type 1 Diabetes and the Impact of Pro-Inflammatory Cytokines
- Spo0A∼P Imposes a Temporal Gate for the Bimodal Expression of Competence in
- Antagonistic Regulation of Apoptosis and Differentiation by the Cut Transcription Factor Represents a Tumor-Suppressing Mechanism in
- A Downstream CpG Island Controls Transcript Initiation and Elongation and the Methylation State of the Imprinted Macro ncRNA Promoter
- PLOS Genetics
- Archív čísel
- Aktuálne číslo
- Informácie o časopise
Najčítanejšie v tomto čísle- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
Prihlásenie#ADS_BOTTOM_SCRIPTS#Zabudnuté hesloZadajte e-mailovú adresu, s ktorou ste vytvárali účet. Budú Vám na ňu zasielané informácie k nastaveniu nového hesla.
- Časopisy



